Covalent organic frameworks in heterogeneous catalysis: recent advances and future perspective
Ziad
Alsudairy
,
Normanda
Brown
,
Allea
Campbell
,
Abrianna
Ambus
,
Bianca
Brown
,
Kayla
Smith-Petty
and
Xinle
Li
 *
*
Department of Chemistry, Clark Atlanta University, Atlanta, Georgia 30314, USA. E-mail: xli1@cau.edu
First published on 19th April 2023
Abstract
Catalysis is ubiquitous in ∼90% of chemical manufacturing processes and contributes up to 35% of global GDP. Hence, the development of advanced catalytic systems is of utmost importance for academia, industry, and government. Covalent organic frameworks (COFs) are a rapidly emerging class of crystalline porous materials that precisely integrate organic monomer units into extended periodic networks, offering a propitious platform for heterogeneous catalysis due to salient structural merits of ultralow density, high crystallinity, permanent porosity, structural tunability, functional diversity, and synthetic versatility. The past decade has witnessed an upsurge of interest in COFs for heterogeneous catalysis and this trend is expected to continue. In this review, we briefly introduce COF chemistry concerning the design principles, growth mechanism, and cutting-edge advances in structural evolution, linkage chemistry, and facile synthesis. We then scrutinize four leading design strategies for COF catalysts, namely pristine COFs with catalytically active backbones, COFs as hosts for the inclusion of catalytic species, COF-based heterostructures, and COF-derived carbons for thermo-, photo-, and electrocatalysis. Next, we overview the most recent advances (mainly from 2020 to 2023) of COFs in heterogeneous catalysis, along with their fundamentals and advantages. Finally, we outline the current challenges and offer our perspectives on the future directions of COFs for heterogeneous catalysis.
1. Introduction
Catalysis, which was first scientifically defined by Ostwald in 1894 as the “acceleration of a slow chemical process by the presence of a foreign material”, holds paramount significance in both fundamental research and industrial applications. From an economic perspective, catalysis contributes to over 35% of the world's gross domestic product (GDP), and every major innovation in catalysis has profoundly advanced global economic success.1 From an environmental perspective, the urgent need to address the energy crisis and unprecedented global warming necessitates the development of novel catalytic technology to sustainably produce commodity chemicals and achieve carbon neutrality by the mid-21st century.2 From a scientific perspective, catalysis research remains at the forefront of chemistry, physics, biology, chemical engineering, and materials science, expanding scientific understanding and resulting in nearly 20 Nobel Prizes in the field of catalysis, from the first one on fundamental catalysis principles (Wilhelm Ostwald, 1909) to the most recent one on asymmetric organocatalysis (Benjamin List and David MacMillan, 2021).At the core of catalysis science, heterogeneous catalysis has garnered enormous attention because of its low cost, eco-benignity, high activity, selectivity, and stability, enabling facile catalyst recovery, regeneration, and sustainable manufacturing.3 As a result, it plays an integral role in producing over 80% of chemical products worldwide. In the pursuit of developing high-performing heterogeneous catalysts, a broad array of porous catalytic solids has been developed, such as zeolites, mesoporous silica, activated carbons, metal–organic frameworks (MOFs), molecular cages, polymers of intrinsic microporosity (PIM), and porous aromatic frameworks (PAFs). Decades of research on these materials have furnished numerous porous catalysts that are of substantial scientific and industrial interest.
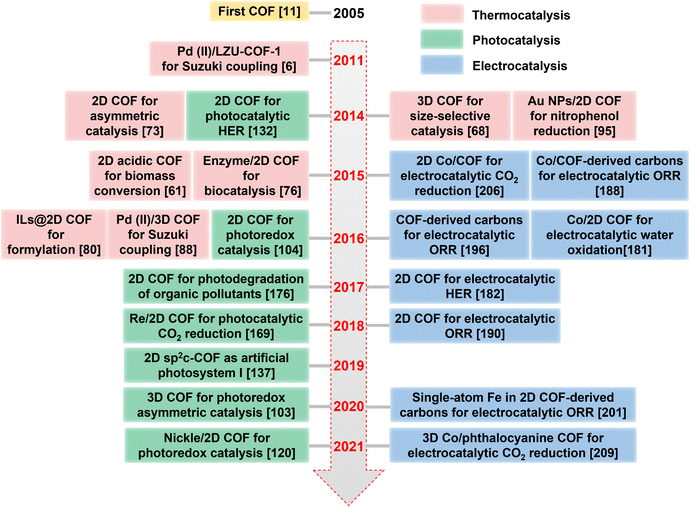
Covalent organic frameworks (COFs) are two- or three-dimensional (2D or 3D) crystalline porous solids solely composed of organic building blocks via strong covalent bonds.4 Since their discovery in 2005, COFs have been in the limelight due to their eminent structural merits, such as ultralow density, high crystallinity, permanent porosity, skeleton modularity, and synthetic versatility, which underpin their broad applications in catalysis, gas storage, separation, chemo-sensing, water harvesting, drug delivery, optoelectronics, and many more.5 The field of COFs has been rapidly expanding, as evidenced by the exponential increase in annual publications in recent years (blue bar in Fig. 1). The research on COF-based heterogeneous catalysis began in 2011, with the pioneering work on COF-supported Pd(II) for the Suzuki–Miyaura coupling reaction.6 This seminal work sparked a surge of research interest in the use of COFs for heterogeneous catalysis over the past decade, as reflected by the steadily increasing annual publications (red bar in Fig. 1). Scheme 1 illustrates the timeline of notable advances in COF-based heterogeneous catalysis. The first thermocatalysis by pristine COF, metal nanoparticle/COF, and 3D COF catalysts was initiated in 2011, January 2014, and February 2014, respectively. In 2014, the use of COFs was extended to asymmetric and tandem catalysis. Meanwhile, the first COF-mediated photocatalysis for hydrogen evolution was reported. With the pressing need to address the energy and environmental crisis, COFs emerged as promising catalysts for electrocatalytic CO2 reduction, biomass conversion, and biocatalysis in 2015. In 2017, COFs showed great promise in the photodegradation of water contaminants. Thereafter, the scope of catalytic reactions in COF-based thermo-, photo-, and electrocatalysis, as well as the structural diversity of COF catalysts, have expanded considerably, further underscoring the enormous potential of this rapidly evolving field.
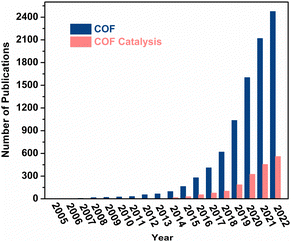 | ||
| Fig. 1 Annual publications using the terms “covalent organic frameworks” and “covalent organic frameworks catalysis” from SciFinder. Data retrieved 31 December 2022. | ||
Compared to other porous solids such as MOFs, carbons, and zeolites, COFs offer a unique set of advantages as heterogeneous catalysts: (i) the exceptional structural tunability and synthetic versatility of COFs lead to a near-infinite variety of COF catalysts with high activity, selectivity, and durability; (ii) the atomically precise and regular structures of COFs enable the subtle modulation of catalytic properties at the atomic level and establish clear structure–catalysis relationships, thereby facilitating the mechanism-guided design and improvement of heterogeneous catalysts; (iii) the superb chemical stabilities of COFs ensure durable catalytic performance and catalyst recovery even under rigorous conditions; (iv) the permanent porosity and ordered nanochannels of COFs promote the mass diffusion of substrates and their ready access to active sites; (v) the conserved COF pores with amenable microenvironment such as molecular functionality, size, and chirality bestow catalytic reactions with high chemo-, size-, regio-, and enantio-selectivity; (vi) the spatial arrangement of multiple catalytic sites within a single COF solid synergistically facilitate cooperative catalysis and/or tandem reactions; (vii) the ease of hybridizing COFs with other catalytically active materials allows the design of well-defined heterostructures with inherited merits from parent materials and enhanced catalytic efficiency; (viii) the ultralight COFs permit high gravimetric performance in catalysis. Taken together, these unparalleled structural merits collectively underpin the surging exploitations of COFs in heterogeneous catalysis.
To avoid duplicating several recent reviews on COF catalysis in 2020,7–10 here we aim to spotlight the most recent advances in COF catalysis with a particular focus on novel COFs, outstanding catalytic performance, as well as advantages of COFs over benchmark porous solids and homogeneous catalysts. In doing so, we primarily review publications from 2020 to 2023 while acknowledging significant prior research. Firstly, we outline the design principles, growth mechanisms, and cutting-edge research frontiers of COFs. Next, we highlight the chronological milestones in the field of COF catalysis. We scrutinize leading design strategies of COF catalysts including manipulating COFs backbones, using COFs as hosts for the embedment of catalytic species, creating COF-based heterostructures, and pyrolyzing COFs into carbons. We then provide a brief overview of COFs in thermo-, photo- and electrocatalysis using up-to-date examples. Finally, we discuss current challenges and prospects for the application of COFs in heterogeneous catalysis. We anticipate that this review will stimulate further scientific endeavors in this intriguing field.
2. Chemistry of COFs
Assembling discrete molecular units into artificial high-ordered organic polymers has long been a substantial challenge. In 2005, Yaghi and co-workers overcame this impasse and successfully synthesized the first two COFs (COF-1 and COF-5) through the self-condensation of boronic acids or the co-condensation of boronic acids with catechols.11 COFs set them apart from other established porous materials, such as carbons, zeolites, mesoporous silica, MOFs, and amorphous porous polymers, due to their hallmark features, including high crystallinity, ultralow density (e.g., 0.106 g cm−3 for TUS-64),12 large surface areas (e.g., 5083 m2 g−1 for DBA-3D-COF-1),13 adjustable pore aperture (e.g., 10.0 nm for TDCOF-3),14 excellent thermal stability (e.g., 600 °C for COF-5),11 high carrier mobility (e.g., 35.4 cm2 V−1 s−1 for NiPc-NH-CoPcF8 COF),15 bespoke structural backbones, and versatile synthesis methods. These unique features make COFs highly promising for diverse niche applications, particularly in heterogeneous catalysis.2.1 Design principles of COFs
COFs, often described as “Molecular Legos” and covalent analogs of MOFs, can be predicably designed by judiciously selecting geometrically defined monomers in terms of topology, dimensionality, and functionality. Depending on the extension of the covalent connectivity, COFs can be classified as 2D and 3D COFs. 2D COFs use rigid building blocks with reactive sites in a specific geometry to direct the growth of polygon skeletons via geometry matching, culminating in nine topologies, including trigonal, tetragonal, rhombic, hexagonal, and Kagome shapes. Hexagonal 2D COFs can be commonly designed using the [C3 + C2], [C2 + C2 + C2], and [C3 + C3] monomer combination, while tetragonal 2D COFs are generated using [C4 + C2] and [C4 + C4] combinations. To enhance the topological diversity and impart hierarchical porosity to 2D COFs, novel topologies such as heteropore COFs can be designed using desymmetrized monomers16 and multi-component polymerization.17 Unlike 2D COFs which typically demand planar building units, most 3D COFs require a tetrahedral (Td) building block to extend the covalent connectivity in 3D. The assembly of a Td unit with C1, C2, C3, C4, and Td-symmetric building blocks yields various 3D COF topologies such as ctn (cubic-C3N4), dia (diamond), bor (boracite), pts (platinum sulfide), lon (lonsdaleite), ljh (Luojia hill), and rra topologies.18 Beyond traditional Td monomers, high-connectivity monomers such as trigonal prism-shaped organic cage,19 6-connected (D3h) unit,20 octahedral unit,21 and 8-connected B4P4O12 cube22 result in unusual 3D COF nets such as acs, stp, ceq, hea, soc, and bcu. A comprehensive topology diagram to guide the design of COFs is available in several prior reviews.23–252.2 Growth mechanism of COFs: advancing the mechanistic understanding of COF formation
Despite enormous advances in the synthesis and application of COFs, in-depth mechanistic studies of COF formation far lag behind. It is generally accepted that dynamic covalent chemistry is essential to impart error-correction and defect-healing during COF crystallization, ultimately reaching the thermodynamic minimum of the system.26 In 2014, Dichtel and co-workers conducted the first investigation into the COF growth mechanism using a prototypical boronate ester-linked COF-5 in a homogeneous solution. They found the nanocrystalline COF formed initially and then irreversibly precipitated. Later in 2016, they disclosed that imine COF first formed as an amorphous polyimine that gradually transformed into crystalline networks.27 This amorphous-to-crystalline transformation was further corroborated by in situ X-ray scattering analysis and the facile synthesis of crystalline COFs from their amorphous counterparts.28 In 2020, the groups of Dichtel and Marder further improved this mechanistic understanding. They demonstrated that certain 2D imine COFs formed as crystalline sheets within just 60 seconds and then rearranged into networks with greater 3D order.29 Recently in 2022, Zhao and co-workers investigated the growth process of 2D imine COFs using mass spectroscopy and density functional theory (DFT) calculations.30 They proposed a template growth mechanism, in which existing COF surfaces direct the assembly and pre-arrangement of monomers/oligomers, which are crucial to the formation of crystalline 2D COFs. This clear mechanistic insight facilitated the growth and resolution of a single-crystalline 2D COF by continuous rotation electron diffraction for the first time. However, most mechanistic studies have focused on boronate ester- or imine-linked COFs, while the growth mechanism of COFs with other linkage types remains scarcely explored.2.3 Structural evolution of COFs: moving from predominant 2D to 3D networks
2D COFs crystallize to form layered networks and stack through non-covalent forces, whereas 3D COFs are wholly held together by covalent bonds with interpenetrated channels. Despite the extensive study of 2D COFs, 3D COFs are of particular interest owing to ultralow density (as low as 0.106 g cm−3), large surface areas (up to 5083 m2 g−1), hierarchical porosity, multifarious functionalities, and abundant open sites. However, the exploration of 3D COFs has been limited by major bottlenecks, such as limited building blocks, arduous structural determination, and crystallization issues, resulting in only ∼120 structures since 2007.31 To expand the structural complexity and diversity of reticular chemistry, 3D COFs have become appealing synthetic targets in recent years and have been discussed in several latest reviews (2022).32,33 Enormous progress has been achieved in the following aspects: (i) diversifying topology of 3D COFs by using high-connectivity monomers and controlling the alignment of building units;34 (ii) rapid synthesis of 3D COFs at ambient temperature in ionic liquids (ILs);35 (iii) growing single-crystalline 3D COFs using the modulator approach36 or supercritical fluid as reaction mediums;37 (iv) atomic-resolution structural determination of 3D COFs with the aid of single-crystal X-ray diffraction, 3D electron diffraction, and cryo-continuous rotation electron diffraction;38 (v) diverse applications in heterogeneous catalysis, polarized optics, selective gas adsorption, lithium–sulfur batteries, ion separation, and white light-emitting diodes. To fully realize the potential of 3D COFs, future research should focus on developing 3D COFs with easily accessible building blocks, unprecedented topologies, facile synthesis, minimal interpenetration, strong stability, and atomic-level structural elucidation.2.4 Linkage chemistry of COFs: towards crystalline, robust, and π-conjugated COFs
Linkages, which connect the discrete building blocks to form the extended framework, are immensely crucial for the emergent crystallinity, function, and stability of COFs. Reversible linkages, such as imine and boronate ester, have dominated the COF field due to their ability to ensure the dynamic healing of structural defects. However, dynamic linkages limit COF stability and hinder their practical implementations. Moreover, 2D COFs with imine and boronic ester linkages display inferior in-plane π-conjugation, compromising their optoelectronic properties. To upend the stability-crystallinity dichotomy in COFs and enhance the in-plane π-conjugation, considerable efforts have been devoted to developing novel linkages that simultaneously impart COFs with high crystallinity, full π-conjugation, and superb chemical stability,39 making linkage chemistry a frontier in the COF community. COF linkages have expanded rapidly and span boronate ester, triazine, imine, imide, hydrazone, imidazole, urea, ester, ether, aminal, pyrimidazole, indazole, cyanurate, etc. COFs with novel robust linkages are mainly developed via the following routes:40 (i) the confined synthesis of COFs on the metal surface or at the liquid–air and liquid/liquid interfaces;41 (ii) single-step reactions with limited reversibility;42,43 (iii) reversible–irreversible multicomponent reactions;44 (iv) postsynthetic modification (PSM) or exchange of labile imine linkages.45,46 The booming linkage chemistry of COFs not only broadens the scope of reticular chemistry but also furnishes novel COFs with highly sought-after properties, including pronounced stability, improved optoelectronic properties, and unprecedented functionalities.2.5 Synthetic strategy of COFs: towards rapid, facile, green, and ambient synthesis
COF synthesis has dominantly relied on the solvothermal protocol, which often involves lengthy reaction times (3–9 d), sealed pressurized vessels under vacuum or inert conditions, the use of hazardous organic solvents, and elevated synthetic temperatures (80–200 °C), all of which limit the full potential of COFs. As such, the rapid, facile, green, and ambient synthesis of high-quality COFs has been extensively pursued in recent years. These viable strategies mainly include: (i) employing new energy sources such as mechanical agitation,47 ultrasound,48 microwave,49 and electron beam50 to replace conventional thermal heating; (ii) developing novel catalysts such as metal triflates,51,52 and Brønsted base53 instead of traditional acetic acid; (iii) deploying new reaction mediums such as water,54 ILs,35 CO2/water,55 and ZnCl2/eutectic salt56 in place of noxious organic solvents; (iv) restricting the bond rotation in monomers to minimize structural errors in COF formation;57 (v) using supercritical CO2 instead of vacuum activation to minimize the structural collapse of COFs during the workup process.58 Two recent reviews on the expeditious synthesis (2020),59 and ambient synthesis of COFs (2021)60 offer more details on these developments. The significant progress in COF synthesis calls for continued scientific efforts towards the rapid synthesis of COFs with broad generality, pronounced stability, scalability, mild reaction condition, and a clear growth mechanism.3. General design principles of COF catalysts
COFs offer a versatile material platform to design tailor-made heterogenous catalysts for thermo-, photo-, and electrocatalysis. The immense structural and functional diversity empowers the precise installation of targeted catalytic sites into COFs, resulting in solids with tailored catalytic activities. To date, four prevalent strategies exist for the fabrication of COF catalysts (Scheme 2). First, the COF backbone, including the linkage and functionalized pore walls, can serve as intrinsic catalytic centers for thermocatalysis. Additionally, the ordered columnar π-arrays in 2D COFs facilitate the transport of exciton, electron, and charge carriers, enabling the design of metal-free COF catalysts in photo- and electrocatalysis (Scheme 2A). Second, the well-defined regular pores of COFs enable the inclusion of diverse catalysts, ranging from single atoms, metal complexes, and clusters, to metal nanoparticles and enzymes. Much more attractive, the atomically precise pore environment, such as pore size, shape, functionality, chirality, and wettability, can be accurately modulated to steer the catalytic behaviors of encapsulated catalysts at the atomic level, resulting in optimal catalytic performances (Scheme 2B). Third, COFs can be hybridized with diverse functional materials and combine the structural attributes of each component. The resultant COF-based heterostructures, exemplified by ILs/COF, MOF/COF, and graphene/COF hybrids, hold enormous prospects in heterogeneous catalysis due to their synergistically augmented catalytic properties over the individual components (Scheme 2C). Finally, to address the limitations of inferior stability and conductivity in pristine COFs, COF-derived carbons can be readily formed via pyrolysis and have gained growing popularity in electrocatalysis under aqueous acidic/alkaline environments (Scheme 2D).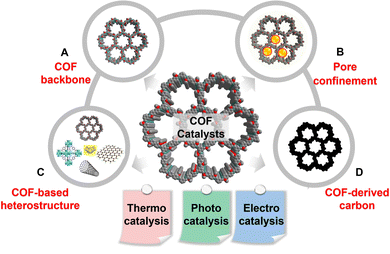 | ||
| Scheme 2 Leading design principles of COF catalysts for thermo-, photo-, and electrocatalysis: (A) COF backbone, (B) pore confinement, (C) COF-based heterostructure, and (D) COF-derived carbon. | ||
4. Thermocatalysis in COFs
4.1 Advantages of COF in thermocatalysis
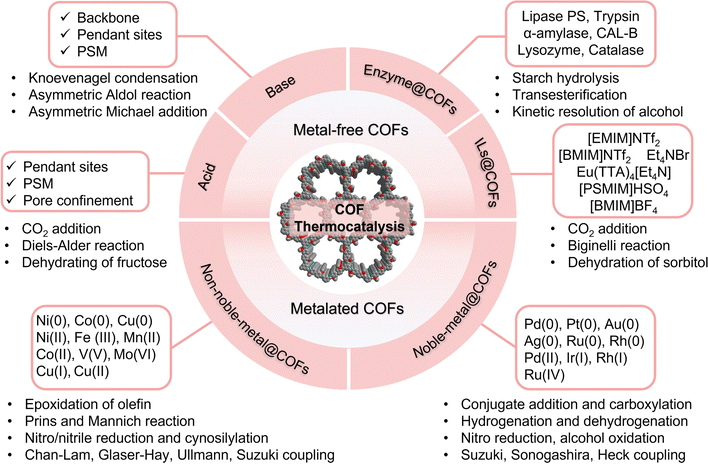
COFs have emerged as compelling heterogeneous catalysts for thermocatalysis due to their ability to combine the merits of both homogeneous and heterogeneous catalysts, resulting in high reactivity, selectivity, and recyclability. Notably, COF catalysts possess the following unique features: (i) the highly adaptable COFs enable the incorporation of target-specific active sites onto the backbone through de novo synthesis, PSM, and pore encapsulation, giving rise to a wide range of heterogeneous catalysts; (ii) the conserved pore spaces of COFs induce nano-confinement effects to enhance catalysis; (iii) the high porosity and regular nanochannels facilitate the adsorption and enrichment of substrates, promoting the catalytic site-reactant interaction and maximizing the catalytic efficiency; (iv) the thermal and chemical stability of COFs ensures durable catalytic performance, a prerequisite for real-world application; (v) the precise and tailorable structure of COFs enable the understanding of the underlying catalytic mechanism through structure–catalysis correlations, accelerating the design of on-demand heterogeneous catalysts.In the following section, we have classified COF catalysts into three categories: metal-free COFs (acid, base, enzyme/COF, ILs/COF), non-noble metal/COFs, and noble metal/COFs, as illustrated in Scheme 3. We will briefly discuss the primary design strategies of COF catalysts in thermocatalysis and scrutinize pertinent examples reported recently.
4.2 Metal-free COFs for thermocatalysis
COF with intrinsic acidic backbones. COF catalysts with intrinsic acidic backbones can be synthesized de novo by using monomers containing acidic sites. The first example of using an acidic COF as a Brønsted acid solid catalyst was demonstrated by Zhao and co-workers in 2015, in which they synthesized a sulfonic acid-containing 2D COF for the dehydration of fructose to 5-hydroxymethylfurfural.61 Following the analogous strategy, Dong and co-workers recently developed an acidic chiral 2D COF, (R)-CuTAPBP-COF, by linking an (R)-1,1'-binaphthol (BINOL)-phosphate monomer bearing Lewis acidic BINOL and Brønsted phosphoric acid sites with a Cu(II)-porphyrin monomer in 2022.62 Thanks to its inherent Brønsted/Lewis acidic sites and chiral pore confinement, the resulting COF displayed high activity (98%), enantioselectivity (95% ee), and broad substrate scope in the asymmetric α-benzylation of aldehydes with alkyl halides via photothermal conversion, outperforming the homogeneous Cu(II)-TAPP and (R)-BINOLPA-DA catalysts. A leaching test and recycling experiment confirmed the heterogeneity nature of (R)-CuTAPBP-COF, as the COF catalyst retained high activity and structural integrity after 5 catalytic cycles. Furthermore, (R)-CuTAPBP-COF was capable of catalyzing gram-scale enantioselective a-benzylation of propanal in high yield (93%) and enantioselectivity (93%), revealing its immense potential in practical catalysis. Notably, switching the chirality of the COF catalyst to (S)-CuTAPBP-COF led to a drastic change in the chirality of the product, suggesting that the COF catalyst can provide exquisite control over the catalytic outcomes in heterogeneous asymmetric catalysis.
Compared to 2D COFs, 3D acidic COFs are of great interest but have been less studied. In 2021, Cui and co-workers developed a pair of chiral 3D COFs, CCOF-15, and CCOF-16 (Fig. 2A), which contain acidic BINOL sites. These COFs were prepared by Schiff-base condensation of tetra(p-aminophenyl)methane and two BINOL-based chiral linkers with different lengths. The resulting isoreticular chiral COFs were highly crystalline with 9-fold and 11-fold interpenetrated diamondoid frameworks. The periodic arrangement of BINOL Brønsted acid sites within the 3D COFs engendered much stronger Brønsted acidity than those of homogenous acidic monomers, as confirmed by the Hammett indicator method. CCOF-15 with smaller pores was highly effective in the asymmetric acetalization of aldehydes and anthranilamides, with yields of up to 91% and 97% ee after 24 hours at 40 °C. In contrast, CCOF-16 with larger channels manifested much lower enantioselectivity. Furthermore, free homogeneous BINOL monomers exhibited no enantioselectivity and/or low activity, highlighting the superiority of chiral COFs over homogeneous counterparts in asymmetric catalysis. To account for the catalytic difference, DFT calculations indicated that 3D COFs catalysts provide preferential secondary interactions between the substrate and framework, leading to enantioselectivities that are otherwise not accessible in homogeneous systems. Despite the remarkable progress made, the de novo synthesis of novel acidic COFs may still encounter challenges in crystallization. Moreover, the limited availability of acidic monomers could pose a significant barrier to the wide use of these materials.
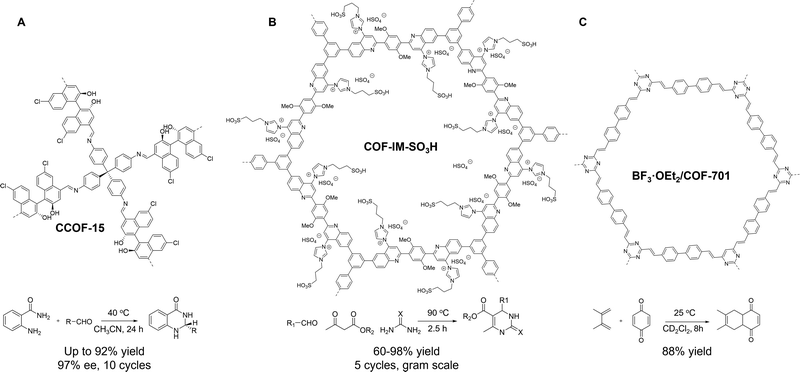 | ||
| Fig. 2 Schematics of (A) 3D CCOF-15 with chiral acidic linkers, (B) COF-IM-SO3H made via PSM, (C) BF3·OEt2/COF-701 as solid acids for thermocatalysis. | ||
Acidic COFs via PSM. To overcome the drawbacks mentioned above, the PSM strategy provides a facile way to incorporate acidic sites onto the backbone of pre-formed COFs.63 Instead of attaching acid sites into the organic linkers, Dong and co-workers introduced an imidazole group in the linkage of a quinoline-linked COF via a one-pot multicomponent reaction, wherein imine was formed first, succeeded by Povarov reaction between the imine and 1-vinylimidazole.64 The imidazole was then post-modified with 1,3-propane sultone to yield a sulfonic acid-decorated COF-IM-SO3H (Fig. 2B), which served as an efficient Brønsted acid catalyst for the Biginelli reaction under solventless conditions. COF-IM-SO3H achieved high yields, turnover frequency, recyclability (5 cycles), and wide substrate scope, placing it among the best catalysts for heterogeneous Biginelli reactions. The outstanding activity was attributed to the densely distributed sulfonic acid sites and the large surface area of COF-IM-SO3H. Importantly, a fixed-bed reactor based on the COF-IM-SO3H/chitosan aerogel was designed for a gram-scale Biginelli reaction and achieved a 93% isolated yield of the target product (73.9 g) within 6 hours, demonstrating the tremendous potential of COFs in the practical catalysis. Grafting acidic sites directly onto COF linkages via multicomponent reaction not only reinforces the chemical stability but also tailors the pore surface of COFs for targeted catalysis,65 which merits more scientific attention in the future.
Acidic COFs via pore confinement. The design of solid acid catalysts can be achieved by encapsulating acidic species inside the pores of porous materials.66 In a pioneering work (2019), Yaghi and co-workers demonstrated the immobilization of a strong Lewis acid, BF3·OEt2, into an unsubstituted sp2 carbon-conjugated COF (sp2c-COF, COF-701, Fig. 2C), which was synthesized by the acid-catalyzed Aldol condensation between 2,4,6-trimethyl-1,3,5-triazine and 4,4′-biphenyldicarbaldehyde.67 The sp2 C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C linkages substantially improved the chemical stability of COF-701, enabling the non-covalent inclusion of BF3·OEt2 inside the COF pore with the perseverance of crystallinity. BF3·OEt2/COF-701 retained 86% of the activity of free BF3·OEt2 in a benchmark Diels–Alder reaction. However, this strategy remains relatively unexplored, as encapsulating acids may impair the structural integrity of COF hosts due to their dynamic linkages. To tackle this challenge, researchers can either use more chemically robust COFs as hosts or weaker acids. Further studies in this direction hold great promise for designing more efficient and stable solid acids.
C linkages substantially improved the chemical stability of COF-701, enabling the non-covalent inclusion of BF3·OEt2 inside the COF pore with the perseverance of crystallinity. BF3·OEt2/COF-701 retained 86% of the activity of free BF3·OEt2 in a benchmark Diels–Alder reaction. However, this strategy remains relatively unexplored, as encapsulating acids may impair the structural integrity of COF hosts due to their dynamic linkages. To tackle this challenge, researchers can either use more chemically robust COFs as hosts or weaker acids. Further studies in this direction hold great promise for designing more efficient and stable solid acids.
COFs with intrinsic basic backbones. COFs with intrinsic basicity can be conveniently designed de novo using N-containing building blocks. The inbuilt N-rich linkages (e.g., imine and amine) and aromatic skeleton (e.g., pyridine and porphyrin) can act as basic sites for thermocatalysis.68,69 For example, in 2021, Beyzavi and co-workers used H3PO3 as a bifunctional catalytic/reducing agent to prepare a 2D amine-linked porphyrin COF (COF-366-R, Fig. 3A) by the tandem condensation-reduction reaction between 5,10,15,20-tetra(4-aminophenyl)porphyrin and terephthalaldehyde.70 Thanks to the improved chemical stability and Lewis basicity endowed by amine linkage, COF-366-R catalyzed Knoevenagel condensation between benzaldehyde and malononitrile with good yields (62–97%), wide substrate scope, and superb recyclability, outperforming its imine COF analog, which structurally degraded under identical condition. Apart from using the Schiff-base linkage as a basic site, the N-doped aromatic skeleton can offer a viable option. In 2022, Zhang and co-workers constructed two pyridine-containing sp2c-COFs by a quaternization-promoted Knoevenagel condensation of 2,4,6-trimethylpyridine (TMP) and aryl aldehydes in the presence of acylating reagents, which converted TMP into electron-withdrawing alkylated pyridinium for efficient polycondensation.71 The resultant sp2c-COFs are highly crystalline and porous (1915 m2 g−1 and 1345 m2 g−1). Importantly, the abundant pyridine sites within the backbone of sp2c-COFs enabled active esterification of salicylic acid and acetic anhydride with high yields, selectivity, and recyclability (5 cycles). Notably, sp2c-COFs afforded higher yields than those of the pyridine-based small molecule catalysts and amorphous polymer counterparts. Furthermore, the catalytic use of two sp2c-COFs can be extended to the esterification of essential pharmaceutical intermediates with high yields and recyclability.
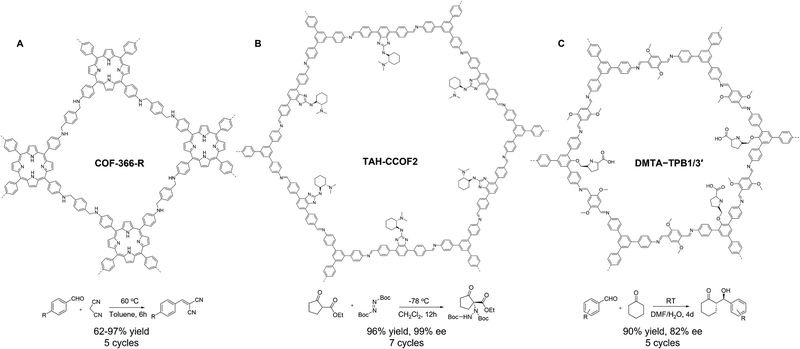 | ||
| Fig. 3 Schematics of (A) COF-366-R, (B) TAH-CCOF2, (C) DMTA-TPB1/3′ as solid bases for thermocatalysis. | ||
COFs with intrinsic basic pendant groups. In addition to the intrinsic basicity arising from the COF backbone, pendant groups in organic linkers can also introduce basicity. This strategy has been widely used for the construction of chiral COFs as solid bases in asymmetric catalysis, which is of utter interest to fundamental synthetic chemistry and the pharmaceutical industry. In 2019, Wang and co-workers disclosed a divergent strategy to construct a series of chiral 2D COFs with analogous structures but diverse functionalities.72 The key to this strategy lies in the design of a highly modular platform molecule, 4,7-dibromo-2-chloro-1H-benzo[d]imidazole, which underwent various organic transformations to generate a library of ditopic aldehydes with diverse chiral functionalities. The covalent assembly of ditopic aldehyades and tritopic 1,3,5-tris(4-aminophenyl)-benzene gave rise to nine chiral COFs bearing basic pendant groups, which were implemented as solid bases for asymmetric amination reaction. COFs with pendant tertiary-amine groups (TAH-CCOF2, Fig. 3B) and H-bonding sites manifested high yield (96%), exceptional enantioselectivity (99% ee), good recyclability (7 cycles), and broad substrate scope, outperforming the homogeneous controls. This superior catalytic performance was ascribed to the weakened intermolecular H-bonding facilitated by the COF catalyst. This approach significantly expands the structural diversity of chiral COFs and offers an auspicious platform to precisely correlate COF structure to asymmetric catalysis at the atomic level.
Basic COFs via PSM. The aforementioned de novo synthesis of COFs with monomers bearing bulky basic functionalities can be challenging, as these monomers may hinder the crystallization of COFs or be incompatible with COF synthesis. To circumvent this dilemma, basicity can be introduced into COFs through the PSM approach.73 In 2017, Cui and co-workers prepared a series of multivariate imine COFs by condensing bare triamine, tert-butyloxycarbonyl (Boc)-protected triamine, and dialdehyde. Upon the post-deprotection to remove the N-Boc and Me protecting groups in the triamines, a series of 2D chiral COFs bearing L-pyrrolidine and L-imidazolidines were derived.74 In contrast, de novo synthesis using unprotected triamines failed to crystallize COFs. The resultant COF solid bases (Fig. 3C) were highly active and selective in three asymmetric reactions, including aminooxylation, Aldol condensation, and Diels–Alder reaction, surpassing their homogeneous analogs in terms of stereoselectivity and diastereoselectivity. Moreover, these COFs were highly recyclable, retaining their activity and enantioselectivity for up to 5 cycles.
Traditional immobilization of enzymes in COFs typically suffers from drawbacks such as insufficient conformational freedom, slow mass transfer, low loading, and high leaching. To this end, Chen and co-workers (2020) designed a series of customizable hollow COF capsules for enzyme immobilization.78 They first encapsulated enzymes (BSA and catalase) within digestible MOF cores (ZIF-90), accompanied by the ambient growth of hydrazone COF shells (COF-42). The resulting COF capsules were further modified by chemically etching the MOF cores under an acidic phosphate buffer solution to unleash the enzymes within the hollow COF capsule. Importantly, the pore sizes, thickness, and dimensions of COF shells can be facilely tuned. In addition, the novel COF capsules provided commodious environments for the optimal and robust enzymatic activities of the encapsulated enzyme, exceeding that of benchmark porous materials such as SBA-15.
In 2022, the same group devised a facile strategy to fabricate robust immobilized biocatalysts via in situ assemblies of lipase with COFs under ambient conditions.79 They added the enzyme solution to an aqueous solution containing COF precusors and then introduced an acetic acid catalyst, which resulted in the rapid synthesis of enzyme@COF composites at room temperature. Impressively, this method was eco-friendly (aqueous medium), expeditious (10–30 minutes), scalable (∼2.3 g per reaction), and highly amenable to plentiful enzyme/COF systems. More importantly, the obtained lipase/COF biocatalysts displayed salient reactivity and reusability (15 cycles) towards the hydrolysis of Aspirin methyl ester, superior to free enzymes or traditional lipase/MOF systems. This strategy shows tremendous potential for industrial enzyme applications.
In 2020, Cai and co-workers grafted an IL, 1-alkyl-3-methylimidazolium bromide onto the pore walls of 2D porphyrin COF via a post-nucleophilic substitution reaction between phenolic hydroxyl-decorated COF and 1-alkyl-3-methylimidazolium.821H NMR spectroscopy indicated that ∼16 mol% 1-methylimidazole was grafted onto the COF. The resulting imidazolium-salt-functionalized COFs were highly active (91–95% conversion) and selective in the cyclization reaction of CO2 and various substituted epoxides at 120 °C, 1.0 MPa CO2, which outperformed the non-functionalized COF and blank sample. Moreover, the COF catalyst can be reused for 5 consecutive cycles with retained porosity. Aside from CO2 epoxidation, ILs/COFs composites were also effective in biomass upgrading. In 2021, Zhang and co-workers developed a series of ILs/COFs composites (PIL–COFs) via the post-modification of vinyl-decorated multivariate COFs with 4-vinyl benzyl chloride, followed by quaternization with tertiary amines.83 The resultant PIL–COFs with tunable amounts of basic ILs retained crystallinity and porosity. When used to catalyze the dehydration of sorbitol into isosorbide at 140 °C, PIL–COF-0.33 outperformed individual components and previously reported mesoporous PIL counterparts in terms of yields and catalytic efficiency, presumably due to uniform pore sizes and flexible linear catalytic chains. In addition, the robust PIL–COF-0.33 can be facilely recovered and reused for 10 cycles without significant loss of reactivity. Despite these advances, the use of ILs/COFs composites in heterogeneous catalysis is still in its infancy. As a result, expanding the ILs/COFs portfolio and catalytic scope will be crucial in unlocking their enormous potential in heterogenous catalysis.
4.3 Metalated COFs for thermocatalysis
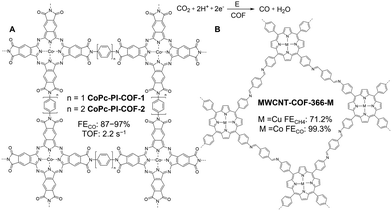
Homogeneous metal complexes are indisputably essential catalysts but are plagued by difficulty to reuse and rapid inter-molecular deactivation. To overcome these limitations, the immobilization of homogeneous complex catalysts into porous materials has been a theme of extensive research. COFs, which possess open pore spaces and periodically arranged metal chelating sites, such as nitrogen, thiol, hydroxyl, N-heterocyclic-carbene, and alkynl, have emerged as promising scaffolds for encapsulating metal species, leading to numerous metalated COFs as heterogenous catalysts for thermocatalysis (Scheme 3). Depending on the precious nature of encapsulated metals, we have categorized metalated COFs into two main types, i.e., non-noble metal/COF and noble metal/COF.| COFs | Linkage | Metal | Reaction | Condition | Yield | Recyclability | Year | Ref. |
|---|---|---|---|---|---|---|---|---|
| CCOF 19 | Imine | Ir | Asymmetric hydrogenation of quinolines | RT, toluene | 72 h, up to 98%, up to 98% ee | 10 Cycles | 2023 | 89 |
| 1 bar H2 | ||||||||
| CCOF 20 | Ru | Asymmetric hydrogenation of β-keto ester | 80 °C, EtOH, 2 bar H2 | 5 d, up to 98%, up to 99.9% ee | ||||
| Bth-Tp-COF | Hydrazone | Pd(II) | Suzuki–Miyaura coupling | 100 °C, dioxane/H2O | 20 min, 95–99% | 5 Cycles | 2023 | 90 |
| IISERP-COF15 | Imine | Cu NPs | Ullmann coupling | 135 °C, DMF | 24 h, 80–100% | 3 Cycles | 2022 | 85 |
| TTA-DFB | Imine | Pd(II) | Suzuki–Miyaura coupling | 150 °C, p-xylene | 2 h, 97% | 3 Cycles | 2022 | 91 |
| (S)-NHC-Au-SA-COF | Imine | Au(I) | Oxidation–Aldol asymmetric relay reaction | RT, air, toluene | 36 h, 69–90%, 91–95% ee | 5 Cycles | 2022 | 92 |
| Py-COF | Imine | Pd NPs | Hydrogenation | 40 °C, EtOH, 10 bar H2 | 0.75–10 h, 52–99% | 4 Cycles | 2022 | 100 |
| TpBpy | Keto-enamine | Ag NPs | Carboxylation of phenylacetylene with CO2 | 60 °C, CO2, DMSO | 6 h, 93% | 5 Cycles | 2021 | 96 |
| NHC-COF | Imine | Au(I) | Carboxylation of phenylacetylene with CO2 | 60 °C, H2O | 20 h, 99% | 5 Cycles | 2020 | 93 |
| COF-DB | Hydrazone | Pd(II) | Suzuki–Miyaura coupling | 80 °C, EtOH-H2O | 0.5 h, 95–99% | 4 Cycles | 2020 | 94 |
| Phos-COF-1 | Imine | Au NPs | Nitrophenol reduction | RT, NaBH4, H2O | 2 h, 99% | 5 Cycles | 2020 | 97 |
| TpBD-Me2 | Keto-enamine | RuO2 NPs | Formic acid dehydrogenation | 125–200 °C | 50% | 120 °C for 25 h | 2020 | 98 |
| COF-QA | Hydrazone | Pd NPs | Suzuki–Miyaura coupling | 50 °C, air H2O | 6 h, >99% | 10 Cycles | 2020 | 99 |
| DBA-COF-5 | Azine | Ni(0) | Aryl C–S bond cleavage | 130 °C, toluene | 14 h, 2–87% | 5 Cycles | 2020 | 86 |
| LZU-COF | Imine | Fe(III) | Alcoholysis of epoxides | RT, CH3OH | 15 min, > 99% | 5 Cycles | 2020 | 87 |
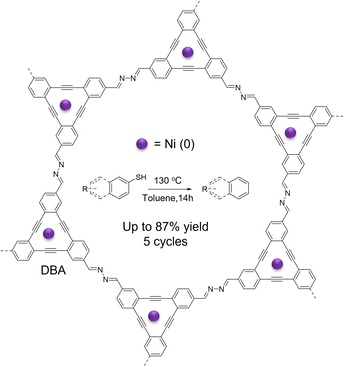
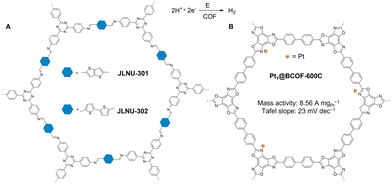
In 2020, McGrier and co-workers synthesized a 2D azine-linked COF with triangular macrocycle dehydrobenzoannulene (DBA) units, which acted as the solitary binding site to complex with Ni(0) through a simple solution impregnation method (Fig. 4).86 The resulting Ni-DBA-COF contained up to 8.56 wt% of Ni while retaining its crystallinity and porosity. Notably, Ni-DBA-COF reductively cleaved the C–S bond of several aryl thioethers at 140 °C, exhibiting yields of 22–87%, broad functional group tolerance, and high recyclability up to 5 cycles. Importantly, these activities were better or comparable to homogeneous catalyst Ni-DBA and previously reported Ni-based homogeneous catalysts. However, the laborious synthesis of DBA limits its wide application. To facilely encapsulate metal species in COFs, Tang and co-workers prepared a defective 2D imine COF (COF-LZU1) by introducing a monodentate monomer, protocatechualdehyde, into the skeleton of COF through a facile one-pot condensation.87 The resulting defective COF-LZU1-OH contained abundant free hydroxy groups that can chelate with active Fe(III). The metaled COF-OFe was used to catalyze the alcoholysis of epoxides, showing a high yield of >99% in just 15 minutes at room temperature and a remarkable TOF of 154 h−1. The strategy paves a facile route to incorporating desired chelating sites into COFs to immobilize metal complexes for heterogeneous catalysis.
Apart from metal complexes, metal nanoparticles (NPs) are of paramount fundamental and applied interest in heterogeneous catalysis. The use of COFs as support for metal NPs has gained increasing interest due to the precise control of the size, dispersion, and surrounding environment of NPs for efficient and synergistic catalysis.95 Various noble metal NPs including Au, Ag, Pd, Pd, Rh, Ru, and Ir NPs have been immobilized within COFs for a wide range of catalytic reactions, such as formic acid dehydrogenation, nitrophenol reduction, carboxylation, conjugate addition, Suzuki, Sonogashira, and Heck coupling reactions (Scheme 3 and Table 1).96–98 In the pursuit of more active noble metal NPs/COF catalysts, Dong and co-workers encapsulated small Pd NPs (∼2.4 nm) into paraffin-chain quaternary ammonium salt-decorated hydrazone COF (Pd/COF-QA, Fig. 5) via an in situ reduction approach, in which the free-end formamide groups on COF served as reducing agent.99 Pd NPs/COF-QA functioned as a highly active phase transfer catalyst for the aqueous Suzuki–Miyaura coupling reaction at 50 °C for 6 hours, outperforming Pd NPs supported by quaternary ammonium-free COF and COF with a shorter quaternary ammonium chain. The heterogeneity of Pd NPs/COF-QA was confirmed by the leaching and recycling tests (10 cycles). Remarkably, Pd/COF-QA represents a rare heterogeneous catalyst that displayed high activity (99%) for the challenging chlorobenzene-based Suzuki–Miyaura coupling reaction at mild conditions. Of particular interest, Pd NPs/COF-QA/chitosan aerogel monolith retained high activity (88%) and cyclic stability (3 cycles) in a continuous gram-scale coupling reaction between chlorobenzene and phenylboronic acid, revealing its great potential for practical catalysis.
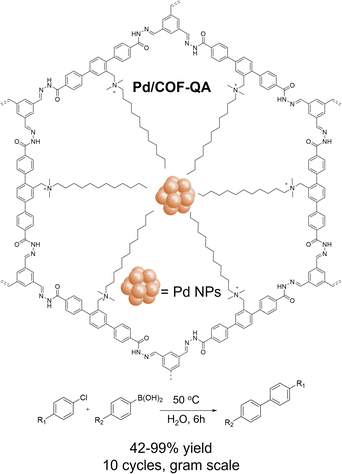 | ||
| Fig. 5 Schematic of paraffin-chain quaternary ammonium salt-decorated COF supported Pd NPs for challenging chlorobenzene-based Suzuki–Miyaura coupling reaction. | ||
COF hosts not only confine the size of encapsulated metal NPs but also considerably influence their catalytic efficiency through non-covalent interactions. In 2022, Yang and co-workers immobilized high loading (24–26%) ultrafine Pd NPs (∼1.7 nm) into three 2D imine COFs with different conjugated skeletons.100In situ FT-IR spectra of CO adsorption studies and XPS analysis revealed similar surface electronic/geometric structures of Pd NPs in the three COFs. Despite the similarities, Pd NPs supported by a pyrene-based 2D COF (Pd/Py-COF) showed a 3–10-fold enhanced activity in the hydrogenation of acetophenone compared to Pd NPs in COFs with no pyrene structs. Combined experimental results and DFT calculation disclosed that the strong π–π interaction of acetophenone and pyrene motifs could drastically reduce the activation barrier in the rate-determining step and thus boost catalysis. Such promotion effect of π–π interactions empowered Pd/Py-COF with high activity and cyclability (3 cycles) in the hydrogenation of aromatic compounds bearing various polar groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, NO2, C
O, NO2, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). This work underlines the pivotal role of non-covalent interaction between metal NPs and COF hosts in promoting activity in heterogenous catalysis.
N). This work underlines the pivotal role of non-covalent interaction between metal NPs and COF hosts in promoting activity in heterogenous catalysis.
5. Photocatalysis in COFs
5.1. Advantages of COFs in photocatalysis
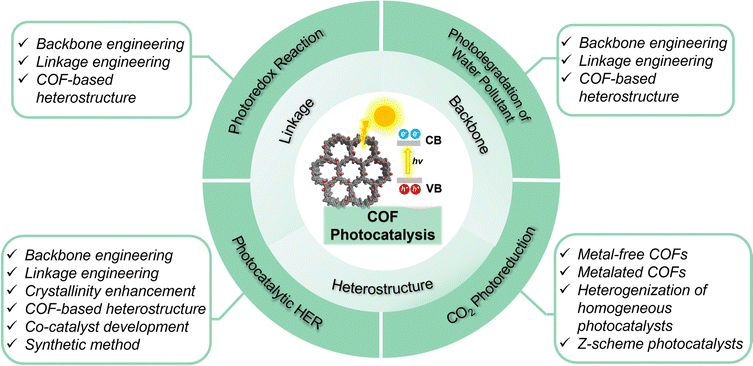
Photocatalysis enables the conversion of inexhaustible solar energy into clean fuel and/or fine chemicals, representing a promising solution to alleviating severe environmental pollution and the energy crisis nowadays. Upon absorption of light with higher energy than that of the band gap, photocatalysts generate electron–hole (e−–h+) pairs, which migrate to the catalyst surface and catalyze reduction and oxidation reactions, respectively. In comparison to other photocatalysts like inorganic semiconductors, MOFs, and π-conjugated polymers, COFs are advantageous due to intriguing structural merits in several aspects:101,102 (i) The structure and optical band gap of COF photocatalysts can be precisely adjusted to maximize light harvesting, promote charge separation, migration, and transport, and enhance photocatalytic efficiency; (ii) the high porosity of COFs promotes the diffusion of substrates, sensitizers, and sacrificial agents; (iii) the long-range order of COFs facilitates charge transport, suppresses recombination of charge carriers, and minimizes charge trapping at defect sites; (iv) the synergistic hybridization of COFs with co-catalysts, photosensitizers, inorganic semiconductors, and MOFs leads to unique heterostructures with improved photocatalytic efficiency; (v) the enormous linkage diversity bestows COF with superb photochemical stability, which is the premise to the design of durable photocatalyst. (vi) The unequivocal structure-photocatalysis correlation in COFs shed light on the fundamental mechanism behind photocatalytic processes.In this section, we will scrutinize the leading design strategies of COF photocatalysts through the engineering of linkage, backbone, and heterostructures (Scheme 4). Moreover, we will survey the up-to-date advances in four prevalent photocatalytic processes: photoredox reaction, photocatalytic hydrogen evolution, CO2 reduction, and photodegradation of water pollutants.
5.2. COFs for photoredox catalysis
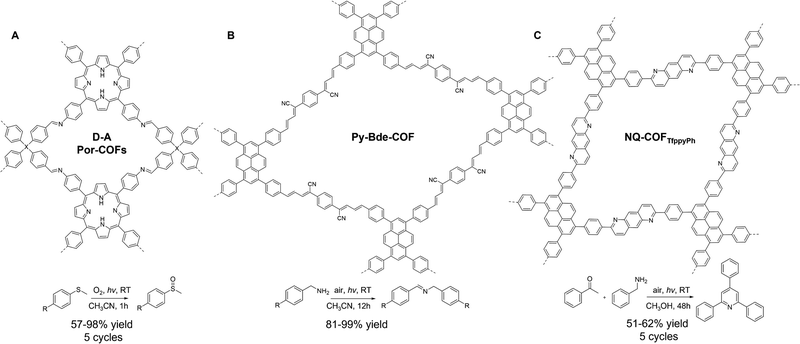
Visible-light photoredox catalysis has emerged as a frontier in organic synthesis, offering an eco-benign, sustainable, and effective means of producing valuable molecules.103 The photoinduced electrons and holes generated in photoredox catalysis are harnessed as reactive species, such as superoxide radical anion O2˙− and single oxygen 1O2, which function as potent oxidants for light-driven oxidation reactions. The first report of using COFs for photoredox catalysis dates back to 2016, when a 2D hydrazone COF was utilized in a visible-light mediated aerobic cross-dehydrogenative-coupling reaction.104 Since then, considerable progress has been made in the design of COF photoredox catalysts through the backbone, linkage, and heterostructure engineering (Table 2 and Scheme 4).105–107| Photocatalyst | Linkage | Light irradiation | Catalysis | Condition | Activity | Year | Ref. |
|---|---|---|---|---|---|---|---|
| TpAzo | Keto-enamine | 467 nm | C–H borylation | CH3CN–H2O | 16 h | 2023 | 105 |
| N2, RT | 18–96% | ||||||
| NiCN (Pyrene-based) | Olefin | 370 nm | Borylation and trifluoromethylation | CH3CN | 18 h | 2023 | 106 |
| RT | 60–92% | ||||||
| Hex-Aza-COF-3 | Phenazine | White LED | Oxidative [3+2] cycloaddition | CH3CN | 9 h | 2023 | 113 |
| RT | 83–95% | ||||||
| PTBC-Por COF | Imine | 400–780 nm | Oxidation of sulfides | CH3CN–MeOH | 1 h | 2022 | 112 |
| O2, RT | 91–98% | ||||||
| LZU-191 | Oxazole | 440 nm | Oxidation of methylphenylsulfide | CH3OH | 2 h | 2022 | 114 |
| O2, RT | 57–95% | ||||||
| TA-Por-sp2-COF | Olefin | White LED | Oxidation of benzylamine | CH3CN | 2 h | 2022 | 115 |
| RT, air | >99% | ||||||
| Py-Bde-COF | Buta-1,3-diene | White LED | Oxidation of benzylamine | CH3CN | 12 h | 2022 | 118 |
| RT, air | 82–99% | ||||||
| NQ-COFTfppyPh | Quinoline | 460–465 nm | Oxidation of benzylamines and aryl ketones | CH3OH | 48 h | 2022 | 119 |
| RT | 51–62% | ||||||
| Ni–Ir@TpBpy COF | Keto-enamine | 427 nm | C–N cross-coupling | CH3CN | 24 h | 2022 | 121 |
| 40 °C | 75–94% | ||||||
| Tp-AcrCOF-Ni | Keto-enamine | 440 nm | C–N cross-coupling | DMAc | 16–72 h | 2022 | 122 |
| RT | 66–93% | ||||||
| Ti-COF-1 (3D) | Imine | λ > 420 nm | Meerwein addition | CH3CN | 12 h | 2021 | 21 |
| RT | 49–75% | ||||||
| 2D-PN-1 | Imine | 440 nm | Radical ring-opening polymerization | THF | 48 h | 2021 | 107 |
| 3D-PN-1 | 10 °C | 42–98% | |||||
| FEAx-COF | Imine | 463 nm | Oxidation of aryl alcohol | CH3CN–H2O | 17 h | 2021 | 108 |
| O2, 45 °C | 44% | ||||||
| Por-BC-COF | Imine | 400–830 nm | Oxidation of benzylamine | CH3CN | 70 min | 2021 | 109 |
| Air, RT | 97% | ||||||
| Ace-COF-Ni | Imine | 420–430 nm | S–C cross-coupling | CH3CN | 24 h | 2021 | 120 |
| Ar, RT | 67–96% | ||||||
| Ir,Ni@Phen-COF | Imine | 450 nm | Csp3–Csp2 cross-coupling | Acetone–MeOH | 24 h | 2021 | 123 |
| Ar, RT | 34–99% | ||||||
| COF-UARK-49-Pt(II) | Imine | Blue LED | Decarboxylative difluoroalkylation | CH3CN | 3.5 h | 2021 | 124 |
| RT | 64–87% | ||||||
| OH-TFP-TTA | Imine | 530 nm | Reductive dehalogenation | CH3CN | 18 h | 2020 | 110 |
| RT | 90% | ||||||
| DhaTph-Zn | Imine | λ > 420 nm | Oxidation of α-terpinene | CH3CN | 4 h | 2020 | 125 |
| O2, RT | 99% | ||||||
| NH2-MIL-125@TAPB-PDA-3 | Imine | λ > 420 nm | Oxidation of benzyl alcohol | CH3CN | 30 h | 2020 | 128 |
| O2, RT | 94.7% |
In addition to the multicomponent de novo synthesis, intriguing COF linkages can be obtained via PSM of labile imine linkages. In 2022, Xiang and co-workers demonstrated that unsubstituted quinoline-linked COFs (NQ-COFs) could be obtained via a postsynthetic Rhodium-catalyzed annulation of imine COFs (Fig. 6C).119 This imine linkage conversion retained the crystallinity and porosity of NQ-COFs while significantly boosting the chemical stability. Moreover, the enhanced π-conjugation empowered NQ-COFs with improved light absorption and charge carrier transfer efficiency, making them highly active, generalizable, and durable heterogeneous catalysts for the photocatalytic synthesis of 2,4,6-triphenylpyridines, benzimidazole, and sulfoxide derivatives at room temperature. Notably, NQ-COFs displayed higher activity than their imine- and substituted quinoline-linked COF counterparts, underscoring the pivotal role of COF linkages in photoredox catalysis. These remarkable findings highlight the need for continued efforts towards the development of new linkages in COF catalysts, which not only advance the field of reticular chemistry but also engender unprecedented photocatalytic functions.
In addition to transition metal/COF photocatalysts, MOF/COF heterostructures have been extensively utilized for photocatalysis since 2017,126 due to their inherited structural merits from the parent reticular materials and improved charge separation efficiency.127 For instance, in 2020, a core–shell (Ti)-NH2-MIL-125/TAPB-PDA COF heterostructure was prepared through a seed growth approach,128 in which the imine TAPB-PDA COF nucleated and grew on the MOF seeds. Remarkably, the band gap and photocurrent density of COF/MOF heterostructures can be facilely regulated by varying the content of COFs. With an optimal thickness of the COF shell, this composite was employed for the photocatalytic oxidation of aromatic alcohols at ambient temperature, showing excellent activity, high selectivity, wide substrate scope, and recyclability (5 cycles), far superior to those of the parent (Ti)-NH2-MIL-125, TAPB-PDA COF, and physically blended MOF/COF. The remarkable photocatalytic performance was attributed to the enhanced visible-light absorption and suppressed recombination of charge carriers endowed by the unique COF/MOF heterostructure. Aside from aryl alcohol oxidation, a Pd-doped core–shell Pd/TiATA MOF/COF-LZU-1 heterostructure was developed and effectively catalyzed the olefin hydrogenation and dehydrogenation of ammonia borane under visible-light irradiation.129
5.3. COFs for photocatalytic hydrogen evolution reaction (HER)
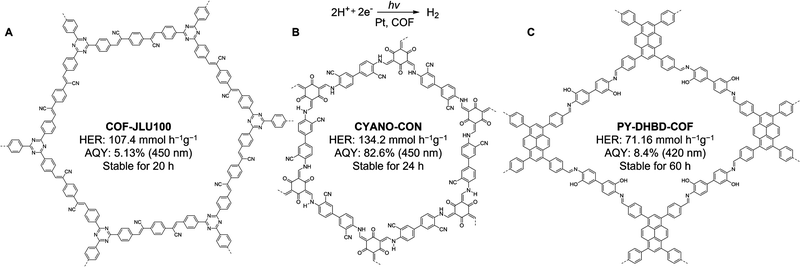
Hydrogen (H2) production using photocatalysis is a promising alternative to fossil fuels and can achieve near-zero emissions from energy consumption while fostering environmental sustainability. Efficient artificial photocatalysts are crucial to improve the efficiency of photocatalytic water splitting, which is an “uphill” chemical reaction.130 In this process, a photocatalyst absorbs light to engender electron–hole pairs, which are then separated and migrate to the catalyst surface. Photogenerated electrons reduce the protons from H2O to yield H2, while the holes oxidize water to generate O2. Owing to the abovementioned structural uniqueness, COFs have aroused enormous research attention as organic photocatalysts for the hydrogen evolution reaction (HER). As illustrated in Fig. 7, there has been a remarkable increase in H2 evolution activity recently, from the initial 1.97 mmol g−1 h−1 in 2014 to benchmark 134.2 mmol g−1 h−1.131 Photocatalytic HER is arguably among the most studied reactions in COF photocatalysis and has been extensively explored in recent years (Table 3).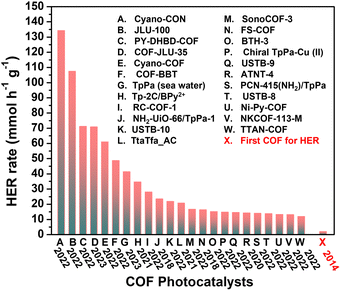 | ||
| Fig. 7 Representative COF photocatalysts with exceptional H2 evolution rates (>11 mmol g−1 h−1) using Pt or Cu(II) as co-catalyst under visible-light irradiation. Corresponding references are summarised in Table 3. | ||
| Photocatalyst | Linkage | Band gap (eV) | SED | Co-catalyst | HER (mmol h−1 g−1) | AQY (%) | Year | Ref. |
|---|---|---|---|---|---|---|---|---|
| a SED: sacrificial electron donor; AQY: apparent quantum yield; TEA: triethylamine; AA: ascorbic acid; SA: sodium ascorbate; TEOA: triethanolamine. b The photocatalysis was performed in seawater. | ||||||||
| COF-JLU35 | Olefin | 1.85 | AA | 1%Pt | 70.8 | 3.21 (500 nm) | 2023 | 135 |
| ZnP-Pz-PEO-COF | Imine | 1.45 | Lactic acid | [Mo3S13]2− | 10.7 | 5.3 (500 nm) | 2023 | 154 |
| TpPa | Keto-enamine | 2.04 | AA | 0.5%Pt | 41.3 | 0.54 (TpPa 450 nm) | 2023b | 163 |
| TpBd | 2.21 | 21.7 | ||||||
| TpTPy | 2.32 | 14.9 | ||||||
| CYANO-CON | Keto-enamine | 2.17 | AA | 1%Pt | 134.2 | 82.6 (450 nm) | 2022 | 152 |
| CYANO-COF | 60.9 | |||||||
| COF-JLU100 | Olefin | 1.95 | TEOA | 12%Pt | 107.4 | 5.13 (450 nm) | 2022 | 138 |
| PY-DHBD-COF | Imine | 2.28 | AA | 3%Pt | 71.2 | 8.4 (420 nm) | 2022 | 153 |
| COF-BBT | Keto-enamine | 2.0 | AA | 2.4%Pt | 48.7 | 6.8 (420 nm) | 2022 | 143 |
| RC-COF-1 | Keto-enamine | 1.87 | AA | 3%Pt | 27.98 | 6.39 (420 nm) | 2022 | 139 |
| USTB-8 | Imine | 2.60 | AA | 3%Pt | 13.7 | 0.68 (420 nm) | 2022 | 144 |
| USTB-9 | 2.20 | 14.6 | ||||||
| USTB-10 | 2.17 | 21.8 | ||||||
| SonoCOF-3 | Imine | 2.46 | AA | 8%Pt | 16.6 | 3.71 (420 nm) | 2022 | 162 |
| BTH-3 | Olefin | 1.42 | AA | 8%Pt | 15.1 | 0.792 (420 nm) | 2022 | 151 |
| TpPa-Cu(II) | Keto-enamine | 1.72 | L-cysteine | 10.51%Cu(II) | 14.72 | 0.78 (600 nm) | 2022 | 157 |
| PdTCPP-PCN-415(NH2)/TpPa | Keto-enamine | 1.72 | AA | 1.2%Pt | 13.98 | 5.7 (450 nm) | 2022 | 161 |
| Ni-Py-COF | Imine | 1.82 | AA | 14%Pt | 13.2 | 4.28 (420 nm) | 2022 | 145 |
| NKCOF-113-M | Olefin | 2.3 | TEOA | 5%Pt | 13.1 | 52.6 (475 nm) | 2022 | 146 |
| TTAN-COF | Olefin | 2.66 | AA | 1.6%Pt | 11.94 | / | 2022 | 136 |
| BT-PyBuOH COF | Imine | 2.36 | AA | 2%Pt | 11.28 | 4.88 (420 nm) | 2022 | 140 |
| TtaTfa_AC | Protonated Imine | 1.90 | AA | 8%Pt | 20.7 | 1.43 (450 nm) | 2021 | 133 |
| CTF film | Triazine | / | TEOA | 2%Pt | 10.2 | 0.11 (420 nm) | 2021 | 134 |
| Tp-2C/BPy2+-COF | Keto-enamine | <2.2 | AA | 3%Pt | 34.6 | 6.93 (420 nm) | 2021 | 147 |
| NKCOF-108 | Imine | 1.82 | AA | 5%Pt | 11.6 | 2.96 (520 nm) | 2021 | 148 |
| Tp(BT0.05 TP0.95)-COF | Keto-enamine | 2.18 | AA | 5%Pt | 9.84 | 2.34 (420 nm) | 2021 | 149 |
| Py-ClTP-BT-COF | Imine | 1.78 | AA | 5%Pt | 8.87 | 8.45 (420 nm) | 2020 | 150 |
| NTU-BDA-THTA/NH2-Ti3C2Tx | Keto-enamine | 2.09 | AA | 3%Pt | 14.2 | 9.75 (500 nm) | 2020 | 158 |
TiO2-TpPa-1-COF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
Keto-enamine | 2.15 | SA | 3%Pt | 11.2 | 7.6 (420 nm) | 2020 | 159 |
To develop COF photocatalysts that meet the requirements of low cost, good durability, and high solar-to-H2 conversion efficiency, it is essential to maximize photon absorption, decrease the optical band gap, and improve charge carrier separation. To accomplish this, various strategies have been employed through the precise engineering of COF structures concerning the linkage, crystallinity, backbone, heterostructure, co-catalyst, and synthetic methods.
Until now, noble Pt has been the dominant co-catalysts in COF-based photocatalytic HER, so there is a dire necessity to develop low-cost co-catalysts for sustainable and economical photocatalysis.154 While some progress has been made with the use of noble metal-free co-catalysts, such as chloro(pyridine)cobaloxime (2017)155 and nickel-thiolate hexameric cluster (2019),156 their photocatalytic HER activity is far from satisfactory, with rates of only 0.782 mmol g−1 h−1 and 0.941 mmol g−1 h−1, respectively. A breakthrough was made in 2022, when Guo and co-workers showed that a well-known chiral 2D β-ketoenamine-linked COF (TpPa-1) in combination with a single atom Cu(II) as an electron transfer mediator and L-/D-cysteine as a sacrificial donor led to a record-high photocatalytic HER activity of 14.72 mmol h−1 g−1,157 significantly outperforming all previous non-noble metal/COF photocatalytic systems. This unique photocatalytic system involved a cascade process, in which Cu(II) is reduced to Cu(I) in a dark reaction by L-cysteine, followed by a photochemical process for hole extraction using Cu(I). Moreover, DFT calculations revealed the electron transfer from the Tp-Cu(I) complex to the Tp moiety. The remarkable photocatalytic efficiency was attributed to the improved hole extraction kinetics, effective accumulation, and transfer of photoexcited electrons, and reduced energy barriers of H2 evolution due to the well-aligned COF layers. These studies on earth-abundant co-catalyst development uncover the vast potential of low-cost COF catalytic systems for solar-to-H2 conversion.
5.4. COFs for CO2 photoreduction
Artificially reducing excessive CO2 into hydrocarbon fuels using ubiquitous solar light represents an emerging approach to simultaneously address the worsening global warming and the energy crisis. In a typical CO2 photoreduction process, CO2 molecules are firstly adsorbed onto the photocatalyst, which generates electron–hole pairs upon excitation of suitable light. The photoexcited electrons migrate to catalytic sites and reduce the adsorbed CO2 to value-added carbon-based products such as CO, HCOOH, CH3OH, CH4, etc., Meanwhile, the holes oxidize H2O to generate O2. To facilitate the photoreduction of CO2 which has a high dissociation energy, it is essential to enhance CO2 adsorption, lower the band gap of photocatalysts, and promote the migration of photoexcited electrons. Since 2018, COFs have been demonstrated to be promising electrocatalysts for CO2 photoreduction, with CO as the dominant carbonaceous product. In comparison to metal-free COFs, metalated COFs typically exhibit superior activity in CO2 photoreduction.Not surprisingly, the utilization of 3D COFs for CO2 photoreduction remains elusive. In a recent study in 2022, a 3D imine COF (TAPP-HFPB-COF) adopting an unprecedented she net was synthesized by the condensation of D4h-symmetric 5,10,15,20-tetra(4-aminophenyl)porphyrin and D3d-symmetric hexa(4-formylphenyl)benzene.165 The resulting TAPP-HFPB-COF displayed high crystallinity, large surface area (1060 m2 g−1), superb chemical stability, more accessible porphyrin sites than 2D COFs, broad light absorption ranging from 300 to 700 nm, and well-aligned energy levels, making it an attractive photocatalyst for CO2 reduction. The metal-free TAPP-HFPB-COF acted as a photocatalyst for CO2 reduction, producing CO and CH4 at rates of 128 μmol g−1 h−1 and 5 μmol g−1 h−1, respectively. Additionally, it showed a high CO/CH4 selectivity of 96%, outperforming the control porphyrin molecular analog and 2D porphyrin COF. The isotopic test using 13CO2 as the reactant produced 13CO, indicating the CO is indeed from the reduction of CO2. Albeit the preliminary promise, the inferior activity of metal-free COFs substantially hinders their widespread use.
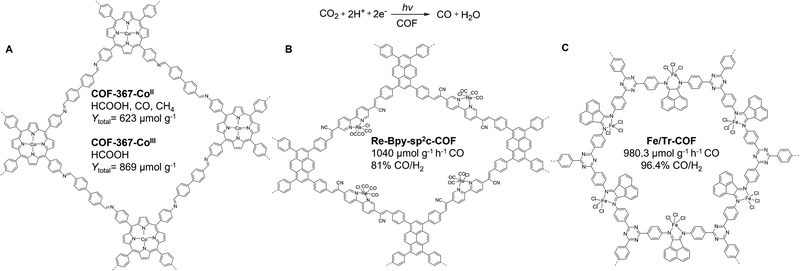 | ||
| Fig. 9 Schematics of (A) COF-367-CoII and CoIII, (B) Re-Bpy-sp2c-COF, and (C) Fe/Tr-COF for CO2 photoreduction under visible-light irradiation. | ||
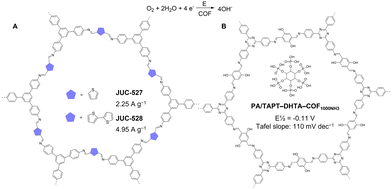
Very recently in 2023, Fang, Jiang, and co-workers extended this strategy to 3D porphyrin COFs (JUC-640-M, M = Co, Ni, or H), which were prepared by condensing the 6-connected 2,3,6,7,14,15-hexa(4′-formylphenyl) triptycene with 5,10,15,20-tetrakis(4-aminophenyl)porphyrin bearing different metal sites.167 The resulting JUC-640-M has a non-interpenetrated stp net, a record-low density of 0.106 g cm−3, ultra-large interconnected pore size of 4.6 nm, a large surface area of 2204 m2 g−1, and abundant exposed porphyrin sites of 0.845 mmol g−1, which endowed JUC-640-M with vast potential in CO2 photoreduction. Using 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]imidazole as the sacrificial agent and Ru(bpy)3Cl2·6H2O as the photosensitizer, JUC-640-Co exhibited a record-high CO production rate (15.1 mmol g−1 h−1) and selectivity (94.4%), rendering it the most active photocatalyst among reported COF-based materials for CO2 photoreduction. Moreover, JUC-640-Co displayed high photocatalytic stability after 5 cycles. The Gibbs free-energy diagram from DFT calculations revealed that the high photocatalytic activity of JUC-640-Co could be attributed to the much lower free-energy barriers of Co-porphyrin compared to its Ni-counterparts. Despite the great promise of metalloporphyrin COFs for CO2 photoreduction,168 the arduous synthesis of metalloporphyrin monomers necessitates the development of cost-effective COF catalysts for CO2 photoreduction.
In addition to expensive noble metal complexes, earth-abundant metal catalysts are of great interest for their economic and environmental advantages in CO2 photoreduction. In 2022, Hou and co-workers reported a universal synthetic strategy to anchor multiple single-atom metal sites (e.g., Fe, Co, Ni, Zn, Cu, and Mn) onto the backbone of a triazine-based 2D COF (Tr-COF), which was prepared by condensing 4,4′4′′-(1,3,5-triazine-2,4,6-triyl)trianiline and ace-naphthenequinone monomers (Fig. 9C).171 The facile solution impregnation method enabled the integration of various metal ions into the Tr-COF with the retention of porosity and crystallinity. X-Ray absorption near-edge structure (XANES) analysis confirmed the atomically dispersed Fe sites in Tr-COF. Steady-state photoluminescence spectra together with ultra-fast femtosecond time-resolved transient absorption spectroscopy indicated the suppressed e−–h+ recombination in Fe/Tr-COF compared to Tr-COF. Notably, the Fe/Tr-COF afforded a high CO generation rate of 980.3 μmol g−1 h−1 and 96.4% CO/H2 selectivity for CO2 photoreduction, which outperformed the bare Tr-COF significantly. This exceptional catalytic performance was primarily due to the synergy between single-atom metal sites and the COF host, which reduced the energy barriers of COOH* intermediates formation and improved CO2 adsorption, activation, and CO desorption.
5.5. COFs for photodegradation of water contaminants
Photocatalysis has been long recognized as a promising tool for sustainable environmental remediation.130 In recent years, COFs have emerged as potential catalysts for the photocatalytic degradation of aqueous pollutants, providing a feasible solution to mitigate severe water pollution. Upon photoexcitation, photoactive and hydrolytically stable COFs generate highly oxidative holes to degrade organic contaminants including dyes, pesticides, pharmaceuticals, and agrochemicals in sewage. Additionally, the photoexcited electrons can reduce noxious high-valence metal ions such as Cr(VI). Since the earliest report in 2017,176 a broad array of COF photocatalysts has been developed for water depollution using bare COFs and COF-based heterostructures.6. Electrocatalysis in COFs
6.1. Advantages of COFs in electrocatalysis
The urgent goal of achieving carbon neutrality by mid-century in response to global warming and energy shortage demands advanced energy conversion and storage, making electrocatalysis a potent option for this goal. To fabricate high-performance electrocatalysts, high intrinsic electrical conductivity, large surface area, exposed catalytic sites, and profound stabilities are imperative. Like the advantages of using COFs for thermocatalysis and photocatalysis, the ordered π-skeletons, large surface area, tunable pore metrics, precise pore environment, and homogeneous dispersion of active sites endow COFs with high catalytic efficiency in various electrocatalytic reactions in acidic/alkaline media.181 COF-based electrocatalysts are categorized as follows: metal-free heteroatom-doped COFs, metalated COFs, COF-based heterostructures, and COF-derived carbons (Scheme 5). In this section, we will elaborate on the leading design principles of COF electrocatalysts and highlight their most recent progress in electrocatalytic HER, oxygen reduction reaction, and CO2 reduction.6.2 COFs for electrocatalytic HER
Electrocatalytic HER can convert electricity into clean H2 on a large scale and is of immense significance for addressing the pressing energy crisis. However, HER requires a large driving overpotential to overcome the energy barrier of water splitting, which can be lowered with the aid of state-of-the-art Pt catalysts. Nevertheless, the scarcity of Pt hinders its extensive application. Consequently, pursuing alternate electrocatalysts combing both low cost and high efficiency has been the subject of extensive research. Since 2017, COFs have gained increasing popularity for electrocatalytic HER and COF catalysts can be designed by using bare heteroatom-doped COFs, metalated COFs, and COF-based heterostructures.6.3 COFs for electrocatalytic oxygen reduction reaction (ORR)
Electrocatalytic ORR is of prime importance for electrochemical energy conversion and storage, as it is ubiquitous in proton exchange membrane fuel cells and metal-air batteries. To accelerate the kinetics of ORR, a wide range of electrocatalysts have been developed, including noble metals, transition metal chalcogenides, single-atom catalysts, conjugated porous polymers, carbonaceous materials, and MOFs. Since 2015, COFs and their derivatives have drawn extensive attention in electrocatalytic ORR,188 which can be rationally designed by using metal-free COFs, metalated COFs, metal-free COF-derived carbons, and single-atom metal in COF-derived carbons.6.4 COFs for CO2 electroreduction
The conversion of CO2 into value-added carbonaceous products via electroreduction under mild operating conditions has been recognized as a sustainable strategy to achieve carbon neutrality. However, a significant obstacle to the broad use of CO2 electroreduction is the competitive HER in aqueous electrolytes, which leads to low energy efficiency and poor selectivity for CO2 electroreduction. To address this issue, massive efforts have been focused on enhancing CO2 reduction while simultaneously inhibiting competitive HER, leading to the development of a wide range of active, selective, and durable electrocatalysts, such as metal NPs, metal dichalcogenide, molecular catalysts, and MOFs. Since the first attempt in 2015, COFs have attracted enormous attention for CO2 electroreduction recently (Table 4). COF-based electrocatalysts can be synthesized by incorporating active metal sites like metalloporphyrin and metallophthalocyanines into the skeletons of COFs and hybridizing COFs with conductive carbon materials. As of now, most COF-based electrocatalysts produce CO as the primary product via a two-electron transfer pathway, with few deeply reduced multi-carbon (C2+) products observed.204| Photocatalyst | Linkage | Electrolyte | Potential (V vs. RHE) | FEco (%) | TOF | J CO (mA m−2) | Tafel slope (mV dec−1) | Year | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a FE: faradaic efficiency; TOF: turnover of frequency. J: partial current density. b The main product is CH4. c Photo-coupled electrocatalysts. | |||||||||
| 3D-Por(Co/H)-COF | Imine | 0.5 M KHCO3 | −0.6 | 92.4 | 4610 h−1 (−1.1 V) | −15.5 (−1.1 V) | — | 2022 | 208 |
| CoPc-PI-COF-3 (3D) | Imide | 0.5 M KHCO3 | −0.9 | 96 | 0.6 s−1 | −31.7 | 117 | 2022 | 209 |
| MWCNT-Por-COF-Co | Imine | 0.5 M KHCO3 | −0.6 | 99.3 | 70.6 s−1 (−1.0 V) | −18.77 | 319 | 2022 | 221 |
| MCH-3 (Cu-COF-366-OH@MOF) | Imine | 1 M KOH | −1.0 | 76.7b | — | −398.1 | — | 2022 | 222 |
| CoPc-PI-COF-1 | Imide | 0.5 M KHCO3 | −0.7 | 93 | 2.2 s−1 | −9.4 | 95 | 2021 | 207 |
| −0.9 | 95 | 4.9 s−1 | −21.2 | ||||||
| CoPc-PI-COF-2 | |||||||||
| −0.7 | 95 | 1.9 s−1 | −6.2 | 105 | |||||
| −0.9 | 92 | 5.0 s−1 | −16.6 | ||||||
| Cu-Tph-COF-Dct | Imine | 1 M KOH | −0.9 | 80b | — | −175.2 | — | 2021 | 210 |
| AAn-COF-Cu (NF) | Keto-enamine | 1 M KOH | −0.9 | 77b | — | −128.1 | 379.7 | 2021 | 211 |
| −47.1 | 311.2 | ||||||||
| −1.0 | 61b | ||||||||
| OH-AAn-COF-Cu (HT) | |||||||||
| COFbpyMn | Imine | 0.5 M KHCO3 | −1.34 | 55 | 805 h−1 | −15 | — | 2021 | 218 |
| TAPP(Co)-B18C6-COF | Imine | 0.5 M KHCO3 | −0.7 | 93.3 | 696 h−1 | −9.45 | 173 | 2021 | 219 |
| −0.9 | 84.4 | 1267 h−1 | |||||||
| NiPc-COF | Phenazine | 0.5 M KHCO3 | −0.9 | 99.1 | 2155 h−1 | −35 (−1.1 V) | 117 | 2020 | 212 |
| Co-TTCOF | Imine | 0.5 M KHCO3 | −0.7 | 91.3 | 267 h−1 | −1.84 | 237 | 2020 | 213 |
| TT-Por(Co)-COF | Imine | 0.5 M KHCO3 | −0.6 | 91.4 | 481 h−1 (-0.7 V) | −7.28 | — | 2020 | 214 |
| CoPc-PDQ-COF | Phenazine | 0.5 M KHCO3 | −0.66 | 96 | 11412 h−1 | −49.4 | 112 | 2020 | 215 |
| NiPc-TFPN COF | Ether | 0.5 M KHCO3 | −0.9 | 99.8 | 490 h−1 | −14.1 | 209.9 | 2020c | 216 |
| CoPc-TFPN COF | −0.9 | 96.1 | 369 h−1 | −10.6 | 570 | ||||
Apart from CNTs, a honeycomb-like MOF/COF heterostructure (MCH-1-4) was designed by growing COF-366-OH-Cu layers on the surface of (Zr)-UiO-66-NH2 MOF in 2022.222 The high porosity, structural regularity, unique honeycomb morphology of MOFs, and abundant Cu-N4 catalytic centers in MCH-3 facilitated the CO2 adsorption, activation, and reduction into a rare CH4 product, showing an outstanding current density (398.1 mA cm−2) and a higher CH4 faradaic efficiency (76.7%) than that of bare COF (37.5%), and MOF (15.9%), the physically blended mixture (38.0%), and COF/MOF with no honeycomb morphology (47.7%). DFT calculations corroborated the crucial role of (Zr)-UiO-66-NH2 in CO2 adsorption, activation, intermediate stabilization, and overcoming the energy barrier of the rate-determining step in CO2 electroreduction.
7. Summary and outlook
COFs are a rapidly developing class of porous materials that offer an advantageous catalytic platform over traditional porous solids. Their unique combination of crystallinity, porosity, structural tunability, functional diversity, and durability render them a game-changer in heterogeneous catalysis. In this review, we have shed light on the chemistry of COFs and highlighted the current research frontiers in structural evolution, linkage chemistry, and expeditious synthesis. We have surveyed the core design principles of COF catalysts (Scheme 2) and spotlighted the most recent advances (2020–2023) in COF-based heterogeneous catalysis. In thermocatalysis (Scheme 3), COF catalysts are designed by de novo synthesis using monomers with catalytically active sites, PSM strategy, encapsulating catalytic species within COFs, and creating COF-based heterostructures. These catalysts have revealed excellent activity, selectivity, and recyclability in diverse chemical reactions, including cross-coupling reactions, redox reactions, biomass conversion, addition reactions, etc. In photocatalysis (Scheme 4), COF photocatalysts are judiciously fabricated by using bare COFs with desired linkage and backbone, immobilizing homogeneous photocatalysts in COFs, and creating heterojunction in COFs. The emergent photocatalysts exhibit high yet controllable catalytic performance in photoredox catalysis, HER, CO2 photoreduction, and aqueous pollutant degradation. Lastly, although in infancy, various COF electrocatalysts (Scheme 5), such as heteroatom-doped bare COFs, metalated COFs, COF-based heterostructures, and COF-derived carbons, have demonstrated enormous prospects in electrocatalytic applications including HER, ORR, and CO2 reduction.Despite a decade of research, COF catalysis is still in its nascent stage and calls for more scientific efforts to fully harness the potential of COF catalysts. Several unsettled challenges are yet to tackle and are listed below.
(i). The utilization of 2D COFs dominates the field of heterogeneous catalysis, while 3D COFs remain largely underexplored, despite their intriguing properties that are apt for catalysis. Hence, exploring the potential of 3D COF catalysis is of vast scientific significance and highly desired.
(ii). Developing COF catalysts that can maintain long-term stability under stringent conditions remains a grand challenge. Emerging robust COFs such as sp2c-COFs and aryl ether-linked COFs feature extraordinary chemical stabilities in drastic chemical environments and hold immense potential in practical catalysis.
(iii). The predominant solvothermal synthesis of COFs is a major hurdle to their practical use due to the slow reaction times, cumbersome conditions, and deficient scalability. Therefore, it is of utmost interest to develop facile, rapid, green, economical, and scalable methods for synthesizing COF catalysts.
(iv). The intrinsic micro- and mesopores in COFs can restrict the mass transport and diffusion of substrates during catalysis. Therefore, there is a growing interest in the development of COFs with hierarchical porosity, which are expected to improve catalytic efficiency and merit further exploration.
(v). To meet industrial needs, shaping the brittle COF powders into processable solids, such as thin films, monoliths, pellets, gels, and foams may result in unique catalytic outcomes. However, this area of study remains scarcely explored thus far.
(vi). The catalytic reaction scope of COF catalysts is rather limited when compared to MOFs. The potential of COFs in highly sought-after catalytic processes such as gas-phase reaction, continuous flow catalysis, biomass valorization, cascade catalysis, C–H activation, selective methane oxidation, and nitrogen fixation remains largely unexplored. Therefore, it is crucial to invest significant efforts in expanding the application of COF catalysts in these cutting-edge catalytic reactions.
(vii). An in-depth understanding of catalytic mechanisms is imperative for the rational design and improvement of COF catalysts. Therefore, a synergistic combination of computational, in situ spectroscopic methods (e.g., IR, Raman, X-ray absorption spectroscopy, etc.) and sophisticated characterization techniques can collectively unravel underlying catalytic mechanisms by dint of monitoring the progression of the reaction, uncovering real active sites, clarifying reaction intermediates, and deconvoluting the reaction pathway.
Addressing the challenges outlined above will not only unleash the immense potential of COFs in heterogeneous catalysis, but also exert profound impacts on other niche applications. Looking forward, future research should prioritize addressing these knowledge gaps by developing COF catalysts with an unrivaled combination of high reactivity, superb selectivity, pronounced stability, broad scope, facile synthesis, and clarified mechanism. Despite tremendous challenges ahead, we foresee that COFs will continue to trigger ever-increasing research efforts in heterogeneous catalysis, ultimately contributing to the global efforts to combat the energy and ecological crisis.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors acknowledge the support from the U.S. Department of Energy Early Career Award (DE-SC0022000), the National Science Foundation HBCU-UP-RIA program (2100360), and the PREM program (DMR-2122147). Z. A. acknowledges the support from Qassim University. Finally, X. L. is grateful for his daughter, Aria Li's birth during the preparation of this review.Notes and references
- F. Zaera, The New Materials Science of Catalysis: Toward Controlling Selectivity by Designing the Structure of the Active Site, J. Phys. Chem. Lett., 2010, 1, 621–627 CrossRef CAS
.
- F. Wang, J. D. Harindintwali, Z. Yuan, M. Wang, F. Wang, S. Li, Z. Yin, L. Huang, Y. Fu and L. Li, Technologies and perspectives for achieving carbon neutrality, Innovation, 2021, 2, 100180 CAS
.
- R. Schlögl, Heterogeneous catalysis, Angew. Chem., Int. Ed., 2015, 54, 3465–3520 CrossRef PubMed
.
- C. S. Diercks and O. M. Yaghi, The atom, the molecule, and the covalent organic framework, Science, 2017, 355, eaal1585 CrossRef PubMed
.
- K. Geng, T. He, R. Liu, S. Dalapati, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang and D. Jiang, Covalent organic frameworks: design, synthesis, and functions, Chem. Rev., 2020, 120, 8814–8933 CrossRef CAS PubMed
.
- S.-Y. Ding, J. Gao, Q. Wang, Y. Zhang, W.-G. Song, C.-Y. Su and W. Wang, Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki–Miyaura coupling reaction, J. Am. Chem. Soc., 2011, 133, 19816–19822 CrossRef CAS PubMed
.
- R. K. Sharma, P. Yadav, M. Yadav, R. Gupta, P. Rana, A. Srivastava, R. Zbořil, R. S. Varma, M. Antonietti and M. B. Gawande, Recent development of covalent organic frameworks (COFs): synthesis and catalytic (organic-electro-photo) applications, Mater. Horiz., 2020, 7, 411–454 RSC
.
- J. Guo and D. Jiang, Covalent organic frameworks for heterogeneous catalysis: principle, current status, and challenges, ACS Cent. Sci., 2020, 6, 869–879 CrossRef CAS PubMed
.
- Y. Yusran, H. Li, X. Guan, Q. Fang and S. Qiu, Covalent organic frameworks for catalysis, EnergyChem, 2020, 2, 100035 CrossRef
.
- Y. Zhi, Z. Wang, H. L. Zhang and Q. Zhang, Recent progress in metal-free covalent organic frameworks as heterogeneous catalysts, Small, 2020, 16, 2001070 CrossRef CAS PubMed
.
- A. P. Cote, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Porous, crystalline, covalent organic frameworks, Science, 2005, 310, 1166–1170 CrossRef CAS PubMed
.
- Y. Zhao, S. Das, T. Sekine, H. Mabuchi, T. Irie, J. Sakai, D. Wen, W. Zhu, T. Ben and Y. Negishi, Record Ultralarge-Pores, Low Density Three-Dimensional Covalent Organic Framework for Controlled Drug Delivery, Angew. Chem., Int. Ed., 2023, 135, e202300172 Search PubMed
.
- L. A. Baldwin, J. W. Crowe, D. A. Pyles and P. L. McGrier, Metalation of a Mesoporous Three-Dimensional Covalent Organic Framework, J. Am. Chem. Soc., 2016, 138, 15134–15137 CrossRef CAS PubMed
.
- Z. Mu, Y. Zhu, B. Li, A. Dong, B. Wang and X. Feng, Covalent Organic Frameworks with Record Pore Apertures, J. Am. Chem. Soc., 2022, 144, 5145–5154 CrossRef CAS PubMed
.
- Y. Yue, H. Li, H. Chen and N. Huang, Piperazine-Linked Covalent Organic Frameworks with High Electrical Conductivity, J. Am. Chem. Soc., 2022, 144, 2873–2878 CrossRef CAS PubMed
.
- R.-R. Liang and X. Zhao, Heteropore covalent organic frameworks: a new class of porous organic polymers with well-ordered hierarchical porosities, Org. Chem. Front., 2018, 5, 3341–3356 RSC
.
- N. Huang, L. Zhai, D. E. Coupry, M. A. Addicoat, K. Okushita, K. Nishimura, T. Heine and D. Jiang, Multiple-component covalent organic frameworks, Nat. Commun., 2016, 7, 12325 CrossRef PubMed
.
- S. J. Lyle, P. J. Waller and O. M. Yaghi, Covalent organic frameworks: organic chemistry extended into two and three dimensions, Trends Chem., 2019, 1, 172–184 CrossRef CAS
.
- Q. Zhu, X. Wang, R. Clowes, P. Cui, L. Chen, M. A. Little and A. I. Cooper, 3D Cage COFs: A Dynamic Three-Dimensional Covalent Organic Framework with High-Connectivity Organic Cage Nodes, J. Am. Chem. Soc., 2020, 142, 16842–16848 CrossRef CAS PubMed
.
- C. Yu, H. Li, Y. Wang, J. Suo, X. Guan, R. Wang, V. Valtchev, Y. Yan, S. Qiu and Q. Fang, Three-Dimensional Triptycene-Functionalized Covalent Organic Frameworks with hea Net for Hydrogen Adsorption, Angew. Chem., Int. Ed., 2022, 61, e202117101 CAS
.
- H.-S. Lu, W.-K. Han, X. Yan, C.-J. Chen, T. Niu and Z.-G. Gu, A 3D Anionic Metal Covalent Organic Framework with soc Topology Built from an Octahedral TiIV Complex for Photocatalytic Reactions, Angew. Chem., Int. Ed., 2021, 60, 17881–17886 CrossRef CAS PubMed
.
- C. Gropp, T. Ma, N. Hanikel and O. M. Yaghi, Design of higher valency in covalent organic frameworks, Science, 2020, 370, eabd6406 CrossRef CAS PubMed
.
- N. Huang, P. Wang and D. Jiang, Covalent organic frameworks: a materials platform for structural and functional designs, Nat. Rev. Mater., 2016, 1, 1–19 Search PubMed
.
- D. Jiang, Covalent organic frameworks: an amazing chemistry platform for designing polymers, Chem, 2020, 6, 2461–2483 CAS
.
- R. Liu, K. T. Tan, Y. Gong, Y. Chen, Z. Li, S. Xie, T. He, Z. Lu, H. Yang and D. Jiang, Covalent organic frameworks: an ideal platform for designing ordered materials and advanced applications, Chem. Soc. Rev., 2021, 50, 120–242 RSC
.
- J. Hu, S. K. Gupta, J. Ozdemir and H. Beyzavi, Applications of dynamic covalent chemistry concept toward tailored covalent organic framework nanomaterials: A review, ACS Appl. Nano Mater., 2020, 3, 6239–6269 CrossRef CAS PubMed
.
- B. J. Smith, A. C. Overholts, N. Hwang and W. R. Dichtel, Insight into the crystallization of amorphous imine-linked polymer networks to 2D covalent organic frameworks, Chem. Commun., 2016, 52, 3690–3693 RSC
.
- Z. Xiong, B. Sun, H. Zou, R. Wang, Q. Fang, Z. Zhang and S. Qiu, Amorphous-to-Crystalline Transformation: General Synthesis of Hollow Structured Covalent Organic Frameworks with High Crystallinity, J. Am. Chem. Soc., 2022, 144, 6583–6593 CrossRef CAS PubMed
.
- C. Feriante, A. M. Evans, S. Jhulki, I. Castano, M. J. Strauss, S. Barlow, W. R. Dichtel and S. R. Marder, New mechanistic insights into the formation of imine-linked two-dimensional covalent organic frameworks, J. Am. Chem. Soc., 2020, 142, 18637–18644 CrossRef CAS PubMed
.
- C. Kang, K. Yang, Z. Zhang, A. K. Usadi, D. C. Calabro, L. S. Baugh, Y. Wang, J. Jiang, X. Zou and Z. Huang, Growing single crystals of two-dimensional covalent organic frameworks enabled by intermediate tracing study, Nat. Commun., 2022, 13, 1370 CrossRef CAS PubMed
.
- X. Guan, F. Chen, Q. Fang and S. Qiu, Design and applications of three dimensional covalent organic frameworks, Chem. Soc. Rev., 2020, 49, 1357–1384 RSC
.
- X. Guan, Q. Fang, Y. Yan and S. Qiu, Functional regulation and stability engineering of three-dimensional covalent organic frameworks, Acc. Chem. Res., 2022, 55, 1912–1927 CrossRef CAS PubMed
.
- X. Guan, F. Chen, S. Qiu and Q. Fang, Three-Dimensional Covalent Organic Frameworks: From Synthesis to Applications, Angew. Chem., Int. Ed., 2023, 62, e202213203 CrossRef CAS PubMed
.
- H. L. Nguyen, C. Gropp, Y. Ma, C. Zhu and O. M. Yaghi, 3D Covalent Organic Frameworks Selectively Crystallized through Conformational Design, J. Am. Chem. Soc., 2020, 142, 20335–20339 CrossRef CAS PubMed
.
- X. Guan, Y. Ma, H. Li, Y. Yusran, M. Xue, Q. Fang, Y. Yan, V. Valtchev and S. Qiu, Fast, ambient temperature and pressure ionothermal synthesis of three-dimensional covalent organic frameworks, J. Am. Chem. Soc., 2018, 140, 4494–4498 CrossRef CAS PubMed
.
- T. Ma, E. A. Kapustin, S. X. Yin, L. Liang, Z. Zhou, J. Niu, L.-H. Li, Y. Wang, J. Su, J. Li, X. Wang, W. D. Wang, W. Wang, J. Sun and O. M. Yaghi, Single-crystal X-ray diffraction structures of covalent organic frameworks, Science, 2018, 361, 48–52 CrossRef CAS PubMed
.
- L. Peng, Q. Guo, C. Song, S. Ghosh, H. Xu, L. Wang, D. Hu, L. Shi, L. Zhao, Q. Li, T. Sakurai, H. Yan, S. Seki, Y. Liu and D. Wei, Ultra-fast single-crystal polymerization of large-sized covalent organic frameworks, Nat. Commun., 2021, 12, 5077 CrossRef CAS PubMed
.
- B. Gui, H. Ding, Y. Cheng, A. Mal and C. Wang, Structural design and determination of 3D covalent organic frameworks, Trends Chem., 2022, 4, 437–450 CrossRef CAS
.
- F. Haase and B. V. Lotsch, Solving the COF trilemma: towards crystalline, stable and functional covalent organic frameworks, Chem. Soc. Rev., 2020, 49, 8469–8500 RSC
.
- X. Li, S. Cai, B. Sun, C. Yang, J. Zhang and Y. Liu, Chemically robust covalent organic frameworks: progress and perspective, Matter, 2020, 3, 1507–1540 CrossRef
.
- Y. Jin, Y. Hu, M. Ortiz, S. Huang, Y. Ge and W. Zhang, Confined growth of ordered organic frameworks at an interface, Chem. Soc. Rev., 2020, 49, 4637–4666 RSC
.
- B. Zhang, M. Wei, H. Mao, X. Pei, S. A. Alshmimri, J. A. Reimer and O. M. Yaghi, Crystalline dioxin-linked covalent organic frameworks from irreversible reactions, J. Am. Chem. Soc., 2018, 140, 12715–12719 CrossRef CAS PubMed
.
- X. Li, H. Wang, H. Chen, Q. Zheng, Q. Zhang, H. Mao, Y. Liu, S. Cai, B. Sun and C. Dun, Dynamic covalent synthesis of crystalline porous graphitic frameworks, Chem, 2020, 6, 933–944 CAS
.
- S. Yang, C. Yang, C. Dun, H. Mao, R. S. H. Khoo, L. M. Klivansky, J. A. Reimer, J. J. Urban, J. Zhang and Y. Liu, Covalent Organic Frameworks with Irreversible Linkages via Reductive Cyclization of Imines, J. Am. Chem. Soc., 2022, 144, 9827–9835 CrossRef CAS PubMed
.
- L. Cusin, H. Peng, A. Ciesielski and P. Samorì, Chemical conversion and locking of the imine linkage: enhancing the functionality of covalent organic frameworks, Angew. Chem., Int. Ed., 2021, 133, 14356–14370 CrossRef
.
- X. Li, C. Zhang, S. Cai, X. Lei, V. Altoe, F. Hong, J. J. Urban, J. Ciston, E. M. Chan and Y. Liu, Facile transformation of imine covalent organic frameworks into
ultrastable crystalline porous aromatic frameworks, Nat. Commun., 2018, 9, 2998 CrossRef PubMed
.
- S. T. Emmerling, L. S. Germann, P. A. Julien, I. Moudrakovski, M. Etter, T. Friščić, R. E. Dinnebier and B. V. Lotsch, In situ monitoring of mechanochemical covalent organic framework formation reveals templating effect of liquid additive, Chem, 2021, 7, 1639–1652 CAS
.
- W. Zhao, P. Yan, B. Li, M. Bahri, L. Liu, X. Zhou, R. Clowes, N. D. Browning, Y. Wu and J. W. Ward, Accelerated Synthesis and Discovery of Covalent Organic Framework Photocatalysts for Hydrogen Peroxide Production, J. Am. Chem. Soc., 2022, 144, 9902–9909 CrossRef CAS PubMed
.
- Z. Alsudairy, N. Brown, C. Yang, S. Cai, F. Akram, A. Ambus, C. Ingram and X. Li, Facile Microwave-Assisted Synthesis of 2D Imine-Linked Covalent Organic Frameworks for Exceptional Iodine Capture, Precis. Chem., 2023 DOI:10.1021/prechem.3c00006
.
- M. Zhang, J. Chen, S. Zhang, X. Zhou, L. He, M. V. Sheridan, M. Yuan, M. Zhang, L. Chen and X. Dai, Electron beam irradiation as a general approach for the rapid synthesis of covalent organic frameworks under ambient conditions, J. Am. Chem. Soc., 2020, 142, 9169–9174 CrossRef CAS PubMed
.
- M. Matsumoto, R. R. Dasari, W. Ji, C. H. Feriante, T. C. Parker, S. R. Marder and W. R. Dichtel, Rapid, low temperature formation of imine-linked covalent organic frameworks catalyzed by metal triflates, J. Am. Chem. Soc., 2017, 139, 4999–5002 CrossRef CAS PubMed
.
- T. Feng, B. Sun, Q. Hao, J. Li, Y. Xu, H. Shang and D. Wang, Ambient synthesis of metal–covalent organic frameworks with Fe-iminopyridine linkages, Chem. Commun., 2022, 58, 8830–8833 RSC
.
- L. Zhang, R. Liang, C. Hang, H. Wang, L. Sun, L. Xu, D. Liu, Z. Zhang, X. Zhang, F. Chang, S. Zhao and W. Huang, A facile solution-phase synthetic approach for constructing phenol-based porous organic cages and covalent organic frameworks, Green Chem., 2020, 22, 2498–2504 RSC
.
- J. Á. Martín-Illán, D. Rodríguez-San-Miguel, C. Franco, I. Imaz, D. Maspoch, J. Puigmartí-Luis and F. Zamora, Green synthesis of imine-based covalent organic frameworks in water, Chem. Commun., 2020, 56, 6704–6707 RSC
.
- F. Zhang, J. Zhang, B. Zhang, X. Tan, D. Shao, J. Shi, D. Tan, L. Liu, J. Feng, B. Han, G. Yang, L. Zheng and J. Zhang, Room-Temperature Synthesis of Covalent Organic Framework (COF-LZU1) Nanobars in CO2/Water Solvent, ChemSusChem, 2018, 11, 3576–3580 CrossRef CAS PubMed
.
- J. Maschita, T. Banerjee, G. Savasci, F. Haase, C. Ochsenfeld and B. V. Lotsch, Ionothermal Synthesis of Imide-Linked Covalent Organic Frameworks, Angew. Chem., Int. Ed., 2020, 59, 15750–15758 CrossRef CAS PubMed
.
- X. Li, J. Qiao, S. W. Chee, H.-S. Xu, X. Zhao, H. S. Choi, W. Yu, S. Y. Quek, U. Mirsaidov and K. P. Loh, Rapid, Scalable Construction of Highly Crystalline Acylhydrazone Two-Dimensional Covalent Organic Frameworks via Dipole-Induced Antiparallel Stacking, J. Am. Chem. Soc., 2020, 142, 4932–4943 CrossRef CAS PubMed
.
- C. H. Feriante, S. Jhulki, A. M. Evans, R. R. Dasari, K. Slicker, W. R. Dichtel and S. R. Marder, Rapid Synthesis of High Surface Area Imine-Linked 2D Covalent Organic Frameworks by Avoiding Pore Collapse During Isolation, Adv. Mater., 2020, 32, 1905776 CrossRef CAS PubMed
.
- X. Li, C. Yang, B. Sun, S. Cai, Z. Chen, Y. Lv, J. Zhang and Y. Liu, Expeditious synthesis of covalent organic frameworks: a review, J. Mater. Chem. A, 2020, 8, 16045–16060 RSC
.
- A. R. Bagheri and N. Aramesh, Towards the room-temperature synthesis of covalent organic frameworks: a mini-review, J. Mater. Sci., 2021, 56, 1116–1132 CrossRef CAS
.
- Y. Peng, Z. Hu, Y. Gao, D. Yuan, Z. Kang, Y. Qian, N. Yan and D. Zhao, Synthesis of a sulfonated two-dimensional covalent organic framework as an efficient solid acid catalyst for biobased chemical conversion, ChemSusChem, 2015, 8, 3208–3212 CrossRef CAS PubMed
.
- H.-C. Ma, Y.-N. Sun, G.-J. Chen and Y.-B. Dong, A BINOL-phosphoric acid and metalloporphyrin derived chiral covalent organic framework for enantioselective α-benzylation of aldehydes, Chem. Sci., 2022, 13, 1906–1911 RSC
.
- J. L. Segura, S. Royuela and M. M. Ramos, Post-synthetic modification of covalent organic frameworks, Chem. Soc. Rev., 2019, 48, 3903–3945 RSC
.
- B.-J. Yao, W.-X. Wu, L.-G. Ding and Y.-B. Dong, Sulfonic acid and ionic liquid functionalized covalent organic framework for efficient catalysis of the Biginelli reaction, J. Org. Chem., 2021, 86, 3024–3032 CrossRef CAS PubMed
.
- Q. Guan, L.-L. Zhou and Y.-B. Dong, Construction of Covalent Organic Frameworks via Multicomponent Reactions, J. Am. Chem. Soc., 2023, 145, 1475–1496 CrossRef CAS PubMed
.
- B. Zhang, X. Li, J. Chen, T. Liu, A. Cruz, Y. Pei, M. Chen, X. Wu and W. Huang, Tandem Synthesis of ε-Caprolactam from Cyclohexanone by an Acidified Metal-organic Framework, ChemCatChem, 2021, 13, 3084–3089 CrossRef CAS
.
- H. Lyu, C. S. Diercks, C. Zhu and O. M. Yaghi, Porous crystalline olefin-linked covalent organic frameworks, J. Am. Chem. Soc., 2019, 141, 6848–6852 CrossRef CAS PubMed
.
- Q. Fang, S. Gu, J. Zheng, Z. Zhuang, S. Qiu and Y. Yan, 3D microporous base-functionalized covalent organic frameworks for size-selective catalysis, Angew. Chem., Int. Ed., 2014, 126, 2922–2926 CrossRef
.
- D. B. Shinde, S. Kandambeth, P. Pachfule, R. R. Kumar and R. Banerjee, Bifunctional covalent organic frameworks with two dimensional organocatalytic micropores, Chem. Commun., 2015, 51, 310–313 RSC
.
- J. Hu, F. Zanca, G. J. McManus, I. A. Riha, H. G. T. Nguyen, W. Shirley, C. G. Borcik, B. J. Wylie, M. Benamara and R. D. Van Zee, Catalyst-Enabled In Situ Linkage Reduction in Imine Covalent Organic Frameworks, ACS Appl. Mater. Interfaces, 2021, 13, 21740–21747 CrossRef CAS PubMed
.
- F. Meng, S. Bi, Z. Sun, D. Wu and F. Zhang, 2, 4, 6-Trimethylpyridine-Derived Vinylene-Linked Covalent Organic Frameworks for Confined Catalytic Esterification, Angew. Chem., Int. Ed., 2022, 61, e202210447 CAS
.
- L.-K. Wang, J.-J. Zhou, Y.-B. Lan, S.-Y. Ding, W. Yu and W. Wang, Divergent Synthesis of Chiral Covalent Organic Frameworks, Angew. Chem., Int. Ed., 2019, 58, 9443–9447 CrossRef CAS PubMed
.
- H. Xu, X. Chen, J. Gao, J. Lin, M. Addicoat, S. Irle and D. Jiang, Catalytic covalent organic frameworks via pore surface engineering, Chem. Commun., 2014, 50, 1292–1294 RSC
.
- J. Zhang, X. Han, X. Wu, Y. Liu and Y. Cui, Multivariate Chiral Covalent Organic Frameworks with Controlled Crystallinity and Stability for Asymmetric Catalysis, J. Am. Chem. Soc., 2017, 139, 8277–8285 CrossRef CAS PubMed
.
- J. P. Richard, Enzymatic Rate Enhancements: A Review and Perspective, Biochem, 2013, 52, 2009–2011 CrossRef CAS PubMed
.
- S. Kandambeth, V. Venkatesh, D. B. Shinde, S. Kumari, A. Halder, S. Verma and R. Banerjee, Self-templated chemically stable hollow spherical covalent organic framework, Nat. Commun., 2015, 6, 6786 CrossRef CAS PubMed
.
- F. L. Oliveira, A. S. de França, A. M. de Castro, R. O. Alves de Souza, P. M. Esteves and R. S. B. Gonçalves, Enzyme immobilization in covalent organic frameworks: Strategies and applications in biocatalysis, ChemPlusChem, 2020, 85, 2051–2066 CrossRef CAS PubMed
.
- M. Li, S. Qiao, Y. Zheng, Y. H. Andaloussi, X. Li, Z. Zhang, A. Li, P. Cheng, S. Ma and Y. Chen, Fabricating covalent organic framework capsules with commodious microenvironment for enzymes, J. Am. Chem. Soc., 2020, 142, 6675–6681 CrossRef CAS PubMed
.
- Y. Zheng, S. Zhang, J. Guo, R. Shi, J. Yu, K. Li, N. Li, Z. Zhang and Y. Chen, Green and Scalable Fabrication of High-Performance Biocatalysts Using Covalent Organic Frameworks as Enzyme Carriers, Angew. Chem., Int. Ed., 2022, 61, e202208744 CrossRef CAS PubMed
.
- B. Dong, L. Wang, S. Zhao, R. Ge, X. Song, Y. Wang and Y. Gao, Immobilization of ionic liquids to covalent organic frameworks for catalyzing the formylation of amines with CO2 and phenylsilane, Chem. Commun., 2016, 52, 7082–7085 RSC
.
- Y. Zhang, M.-X. Wu, G. Zhou, X.-H. Wang and X. Liu, A Rising Star from Two Worlds: Collaboration of COFs and ILs, Adv. Funct. Mater., 2021, 31, 2104996 CrossRef CAS
.
- J. Qiu, Y. Zhao, Z. Li, H. Wang, Y. Shi and J. Wang, Imidazolium-Salt-Functionalized Covalent Organic Frameworks for Highly Efficient Catalysis of CO2 Conversion, ChemSusChem, 2019, 12, 2421–2427 CAS
.
- Y.-R. Du, B.-H. Xu, J.-S. Pan, Y.-W. Wu, X.-M. Peng, Y.-F. Wang and S.-J. Zhang, Confinement of Brønsted acidic ionic liquids into covalent organic frameworks as a catalyst for dehydrative formation of isosorbide from sorbitol, Green Chem., 2019, 21, 4792–4799 RSC
.
- D. Chakraborty, D. Mullangi, C. Chandran and R. Vaidhyanathan, Nanopores of a Covalent Organic Framework: A Customizable Vessel for Organocatalysis, ACS Omega, 2022, 7, 15275–15295 CrossRef CAS PubMed
.
- C. Chandran, H. D. Singh, L. S. Leo, P. Shekhar, D. Rase, D. Chakraborty, C. P. Vinod and R. Vaidhyanathan, A covalent organic framework with electrodeposited copper nanoparticles – a desirable catalyst for the Ullmann coupling reaction, J. Mater. Chem. A, 2022, 10, 15647–15656 RSC
.
- W. K. Haug, E. R. Wolfson, B. T. Morman, C. M. Thomas and P. L. McGrier, A nickel-doped dehydrobenzoannulene-based two-dimensional covalent organic framework for the reductive cleavage of inert aryl C–S bonds, J. Am. Chem. Soc., 2020, 142, 5521–5525 CrossRef CAS PubMed
.
- Y. Shi, X. Zhang, H. Liu, J. Han, Z. Yang, L. Gu and Z. Tang, Metalation of Catechol-Functionalized Defective Covalent Organic Frameworks for Lewis Acid Catalysis, Small, 2020, 16, 2001998 CrossRef CAS PubMed
.
- R. S. B. Gonçalves, A. B. V. de Oliveira, H. C. Sindra, B. S. Archanjo, M. E. Mendoza, L. S. A. Carneiro, C. D. Buarque and P. M. Esteves, Heterogeneous Catalysis by Covalent Organic Frameworks (COF): Pd(OAc)2@COF-300 in Cross-Coupling Reactions, ChemCatChem, 2016, 8, 743–750 CrossRef
.
- Z. Zheng, C. Yuan, M. Sun, J. Dong, Y. Liu and Y. Cui, Construction of Monophosphine–Metal Complexes in Privileged Diphosphine-Based Covalent Organic Frameworks for Catalytic Asymmetric Hydrogenation, J. Am. Chem. Soc., 2023, 145, 6100–6111 CrossRef CAS PubMed
.
- Y. Nailwal, M. A. Addicoat, M. Gaurav and S. K. Pal, Role of Intralayer Hydrogen Bonding in the Fast Crystallization of the Hydrazone-Linked Nanoporous Covalent Organic Framework for Catalytic Suzuki–Miyaura Cross-Coupling Reactions, ACS Appl. Nano Mater., 2023, 6, 1714–1723 CrossRef CAS
.
- C. Krishnaraj, H. S. Jena, K. S. Rawat, J. Schmidt, K. Leus, V. Van Speybroeck and P. Van Der Voort, Linker Engineering of 2D Imine Covalent Organic Frameworks for the Heterogeneous Palladium-Catalyzed Suzuki Coupling Reaction, ACS Appl. Mater. Interfaces, 2022, 14, 50923–50931 CrossRef CAS PubMed
.
- Y. Li, J.-M. Wang, J.-L. Kan, F. Li, Y. Dong and Y.-B. Dong, Combination of a Metal-N-Heterocyclic-Carbene Catalyst and a Chiral Aminocatalyst within a Covalent Organic Framework: a Powerful Cooperative Approach for Relay Asymmetric Catalysis, Inorg. Chem., 2022, 61, 2455–2462 CrossRef CAS PubMed
.
- Y. Li, Y. Dong, J.-L. Kan, X. Wu and Y.-B. Dong, Synthesis and catalytic properties of metal–N-heterocyclic-carbene-decorated covalent organic framework, Org. Lett., 2020, 22, 7363–7368 CrossRef CAS PubMed
.
- C. Qian, W. Zhou, J. Qiao, D. Wang, X. Li, W. L. Teo, X. Shi, H. Wu, J. Di and H. Wang, Linkage engineering by harnessing supramolecular interactions to fabricate 2D hydrazone-linked covalent organic framework platforms toward advanced catalysis, J. Am. Chem. Soc., 2020, 142, 18138–18149 CrossRef CAS PubMed
.
- P. Pachfule, S. Kandambeth, D. Díaz Díaz and R. Banerjee, Highly stable covalent organic framework–Au nanoparticles hybrids for enhanced activity for nitrophenol reduction, Chem. Commun., 2014, 50, 3169–3172 RSC
.
- L. Zhang, R. Bu, X.-Y. Liu, P.-F. Mu and E.-Q. Gao, Schiff-base molecules and COFs as metal-free catalysts or silver supports for carboxylation of alkynes with CO2, Green Chem., 2021, 23, 7620–7629 RSC
.
- R. Tao, X. Shen, Y. Hu, K. Kang, Y. Zheng, S. Luo, S. Yang, W. Li, S. Lu, Y. Jin, L. Qiu and W. Zhang, Phosphine-Based Covalent Organic Framework for the Controlled Synthesis of Broad-Scope Ultrafine Nanoparticles, Small, 2020, 16, 1906005 CrossRef CAS PubMed
.
- L. P. L. Gonçalves, D. B. Christensen, M. Meledina, L. M. Salonen, D. Y. Petrovykh, E. Carbó-Argibay, J. P. S. Sousa, O. S. G. P. Soares, M. F. R. Pereira, S. Kegnæs and Y. V. Kolen'ko, Selective formic acid dehydrogenation at low temperature over a RuO2/COF pre-catalyst synthesized on the gram scale, Catal. Sci. Technol., 2020, 10, 1991–1995 RSC
.
- J.-C. Wang, C.-X. Liu, X. Kan, X.-W. Wu, J.-L. Kan and Y.-B. Dong, Pd@COF-QA: a phase transfer composite catalyst for aqueous Suzuki–Miyaura coupling reaction, Green Chem., 2020, 22, 1150–1155 RSC
.
- M. Guo, S. Jayakumar, M. Luo, X. Kong, C. Li, H. Li, J. Chen and Q. Yang, The promotion effect of π–π interactions in Pd NPs catalysed selective hydrogenation, Nat. Commun., 2022, 13, 1770 CrossRef CAS PubMed
.
- S. Liu, M. Wang, Y. He, Q. Cheng, T. Qian and C. Yan, Covalent organic frameworks towards photocatalytic applications: Design principles, achievements, and opportunities, Coord. Chem. Rev., 2023, 475, 214882 CrossRef CAS
.
- Y.-N. Gong, X. Guan and H.-L. Jiang, Covalent organic frameworks for photocatalysis: Synthesis, structural features, fundamentals and performance, Coord. Chem. Rev., 2023, 475, 214889 CrossRef CAS
.
- X. Kang, X. Wu, X. Han, C. Yuan, Y. Liu and Y. Cui, Rational synthesis of interpenetrated 3D covalent organic frameworks for asymmetric photocatalysis, Chem. Sci., 2020, 11, 1494–1502 RSC
.
- W. Liu, Q. Su, P. Ju, B. Guo, H. Zhou, G. Li and Q. Wu, A Hydrazone-Based Covalent Organic Framework as an Efficient and Reusable Photocatalyst for the Cross-Dehydrogenative Coupling Reaction of N-Aryltetrahydroisoquinolines, ChemSusChem, 2017, 10, 664–669 CrossRef CAS PubMed
.
- A. Basak, S. Karak and R. Banerjee, Covalent Organic Frameworks as Porous Pigments for Photocatalytic Metal-Free C–H Borylation, J. Am. Chem. Soc., 2023, 145, 7592–7599 CrossRef CAS PubMed
.
- Y. Fan, D. W. Kang, S. Labalme, J. Li and W. Lin, Enhanced Energy Transfer in A π-Conjugated Covalent Organic Framework Facilitates Excited-State Nickel Catalysis, Angew. Chem., Int. Ed., 2023, 135, e202218908 CrossRef
.
- K. Wang, X. Kang, C. Yuan, X. Han, Y. Liu and Y. Cui, Porous 2D and 3D Covalent Organic Frameworks with Dimensionality-Dependent Photocatalytic Activity in Promoting Radical Ring-Opening Polymerization, Angew. Chem., Int. Ed., 2021, 60, 19466–19476 CrossRef CAS PubMed
.
- S. Trenker, L. Grunenberg, T. Banerjee, G. Savasci, L. M. Poller, K. I. Muggli, F. Haase, C. Ochsenfeld and B. V. Lotsch, A flavin-inspired covalent organic framework for photocatalytic alcohol oxidation, Chem. Sci., 2021, 12, 15143–15150 RSC
.
- H. He, X. Fang, D. Zhai, W. Zhou, Y. Li, W. Zhao, C. Liu, Z. Li and W. Deng, A Porphyrin-Based Covalent Organic Framework for Metal-Free Photocatalytic Aerobic Oxidative Coupling of Amines, Chem. – Eur. J., 2021, 27, 14390–14395 CrossRef CAS PubMed
.
- H. Liu, C. Li, H. Li, Y. Ren, J. Chen, J. Tang and Q. Yang, Structural engineering of two-dimensional covalent organic frameworks for visible-light-driven organic transformations, ACS Appl. Mater. Interfaces, 2020, 12, 20354–20365 CrossRef CAS PubMed
.
- J. Zhao, J. Ren, G. Zhang, Z. Zhao, S. Liu, W. Zhang and L. Chen, Donor-Acceptor Type Covalent Organic Frameworks, Eur. J. Chem., 2021, 27, 10781–10797 CrossRef CAS PubMed
.
- H. Shan, D. Cai, X. Zhang, Q. Zhu, P. Qin and J. Baeyens, Donor–acceptor type two-dimensional porphyrin-based covalent organic framework for visible-light-driven heterogeneous photocatalysis, J. Chem. Eng., 2022, 432, 134288 CrossRef CAS
.
- P. T. Parvatkar, S. Kandambeth, A. C. Shaikh, I. Nadinov, J. Yin, V. S. Kale, G. Healing, A. H. Emwas, O. Shekhah, H. N. Alshareef, O. F. Mohammed and M. Eddaoudi, A Tailored COF for Visible-Light Photosynthesis of 2,3-Dihydrobenzofurans, J. Am. Chem. Soc., 2023, 145, 5074–5082 CrossRef CAS PubMed
.
- C.-J. Wu, X.-Y. Li, T.-R. Li, M.-Z. Shao, L.-J. Niu, X.-F. Lu, J.-L. Kan, Y. Geng and Y.-B. Dong, Natural Sunlight Photocatalytic Synthesis of Benzoxazole-Bridged Covalent Organic Framework for Photocatalysis, J. Am. Chem. Soc., 2022, 144, 18750–18755 CrossRef CAS PubMed
.
- X. Liu, R. Qi, S. Li, W. Liu, Y. Yu, J. Wang, S. Wu, K. Ding and Y. Yu, Triazine–Porphyrin-Based Hyperconjugated Covalent Organic Framework for High-Performance Photocatalysis, J. Am. Chem. Soc., 2022, 144, 23396–23404 CrossRef CAS PubMed
.
- X. Li, sp2 carbon-conjugated covalent organic frameworks: synthesis, properties, and applications, Mater. Chem. Front., 2021, 5, 2931–2949 RSC
.
- S. Xu, M. Richter and X. Feng, Vinylene-Linked Two-Dimensional Covalent Organic Frameworks: Synthesis and Functions, Acc. Chem. Res., 2021, 2, 252–265 CAS
.
- Y. Su, B. Li, H. Xu, C. Lu, S. Wang, B. Chen, Z. Wang, W. Wang, K.-I. Otake, S. Kitagawa, L. Huang and C. Gu, Multi-Component Synthesis of a Buta-1,3-diene-Linked Covalent Organic Framework, J. Am. Chem. Soc., 2022, 144, 18218–18222 CrossRef CAS PubMed
.
- X. Zhao, H. Pang, D. Huang, G. Liu, J. Hu and Y. Xiang, Construction of Ultrastable Nonsubstituted Quinoline-Bridged Covalent Organic Frameworks via Rhodium-Catalyzed Dehydrogenative Annulation, Angew. Chem., Int. Ed., 2022, 61, e202208833 CAS
.
- H. Chen, W. Liu, A. Laemont, C. Krishnaraj, X. Feng, F. Rohman, M. Meledina, Q. Zhang, R. Van Deun, K. Leus and P. Van Der Voort, A Visible-Light-Harvesting Covalent Organic Framework Bearing Single Nickel Sites as a Highly Efficient Sulfur–Carbon Cross-Coupling Dual Catalyst, Angew. Chem., Int. Ed., 2021, 60, 10820–10827 CrossRef CAS PubMed
.
- A. Jati, K. Dey, M. Nurhuda, M. A. Addicoat, R. Banerjee and B. Maji, Dual Metalation in a Two-Dimensional Covalent Organic Framework for Photocatalytic C–N Cross-Coupling Reactions, J. Am. Chem. Soc., 2022, 144, 7822–7833 CrossRef CAS PubMed
.
- M. Traxler, S. Gisbertz, P. Pachfule, J. Schmidt, J. Roeser, S. Reischauer, J. Rabeah, B. Pieber and A. Thomas, Acridine-Functionalized Covalent Organic Frameworks (COFs) as Photocatalysts for Metallaphotocatalytic C−N Cross-Coupling, Angew. Chem., Int. Ed., 2022, 61, e202117738 CrossRef CAS PubMed
.
- A. López-Magano, B. Ortín-Rubio, I. Imaz, D. Maspoch, J. Alemán and R. Mas-Ballesté, Photoredox Heterobimetallic Dual Catalysis Using Engineered Covalent Organic Frameworks, ACS Catal., 2021, 11, 12344–12354 CrossRef PubMed
.
- Z. Almansaf, J. Hu, F. Zanca, H. R. Shahsavari, B. Kampmeyer, M. Tsuji, K. Maity, V. Lomonte, Y. Ha, P. Mastrorilli, S. Todisco, M. Benamara, R. Oktavian, A. Mirjafari, P. Z. Moghadam, A. R. Khosropour and H. Beyzavi, Pt(II)-Decorated Covalent Organic Framework for Photocatalytic Difluoroalkylation and Oxidative Cyclization Reactions, ACS Appl. Mater. Interfaces, 2021, 13, 6349–6358 CrossRef CAS PubMed
.
- Y. Qian, D. Li, Y. Han and H.-L. Jiang, Photocatalytic Molecular Oxygen Activation by Regulating Excitonic Effects in Covalent Organic Frameworks, J. Am. Chem. Soc., 2020, 142, 20763–20771 CrossRef CAS PubMed
.
- Y. Peng, M. Zhao, B. Chen, Z. Zhang, Y. Huang, F. Dai, Z. Lai, X. Cui, C. Tan and H. Zhang, Hybridization of MOFs and COFs: A New Strategy for Construction of MOF@COF Core–Shell Hybrid Materials, Adv. Mater., 2018, 30, 1705454 CrossRef PubMed
.
- Z. Chen, X. Li, C. Yang, K. Cheng, T. Tan, Y. Lv and Y. Liu, Hybrid porous crystalline materials from metal organic frameworks and covalent organic frameworks, Adv. Sci., 2021, 8, 2101883 CrossRef CAS PubMed
.
- G. Lu, X. Huang, Y. Li, G. Zhao, G. Pang and G. Wang, Covalently integrated core-shell MOF@COF hybrids as efficient visible-light-driven photocatalysts for selective oxidation of alcohols, J. Energy Chem., 2020, 43, 8–15 CrossRef
.
- D. Sun, S. Jang, S.-J. Yim, L. Ye and D.-P. Kim, Metal Doped Core–Shell Metal–Organic Frameworks@Covalent Organic Frameworks (MOFs@COFs) Hybrids as a Novel Photocatalytic Platform, Adv. Funct. Mater., 2018, 28, 1707110 CrossRef
.
- A. Sudhaik, P. Raizada, T. Ahamad, S. M. Alshehri, V.-H. Nguyen, Q. Van Le, S. Thakur, V. K. Thakur, R. Selvasembian and P. Singh, Recent advances in cellulose supported photocatalysis for pollutant mitigation: A review, Int. J. Biol. Macromol., 2023, 226, 1284–1308 CrossRef CAS PubMed
.
- Y. Li, X. Song, G. Zhang, L. Wang, Y. Liu, W. Chen and L. Chen, 2D Covalent Organic Frameworks Toward Efficient Photocatalytic Hydrogen Evolution, ChemSusChem, 2022, 15, e202200901 CAS
.
- L. Stegbauer, K. Schwinghammer and B. V. Lotsch, A hydrazone-based covalent organic framework for photocatalytic hydrogen production, Chem. Sci., 2014, 5, 2789–2793 RSC
.
- J. Yang, A. Acharjya, M.-Y. Ye, J. Rabeah, S. Li, Z. Kochovski, S. Youk, J. Roeser, J. Grüneberg, C. Penschke, M. Schwarze, T. Wang, Y. Lu, R. van de Krol, M. Oschatz, R. Schomäcker, P. Saalfrank and A. Thomas, Protonated Imine-Linked Covalent Organic Frameworks for Photocatalytic Hydrogen Evolution, Angew. Chem., Int. Ed., 2021, 60, 19797–19803 CrossRef CAS PubMed
.
- X. Hu, Z. Zhan, J. Zhang, I. Hussain and B. Tan, Immobilized covalent triazine frameworks films as effective photocatalysts for hydrogen evolution reaction, Nat. Commun., 2021, 12, 6596 CrossRef CAS PubMed
.
- Z. Li, T. Deng, S. Ma, Z. Zhang, G. Wu, J. Wang, Q. Li, H. Xia, S.-W. Yang and X. Liu, Three-Component Donor−π–Acceptor Covalent–Organic Frameworks for Boosting Photocatalytic Hydrogen Evolution, J. Am. Chem. Soc., 2023 DOI:10.1021/jacs.2c11893
.
- Y. Yang, N. Luo, S. Lin, H. Yao and Y. Cai, Cyano Substituent on the Olefin Linkage: Promoting Rather than Inhibiting the Performance of Covalent Organic Frameworks, ACS Catal., 2022, 12, 10718–10726 CrossRef CAS
.
- Y. Zhao, H. Liu, C. Wu, Z. Zhang, Q. Pan, F. Hu, R. Wang, P. Li, X. Huang and Z. Li, Fully Conjugated Two-Dimensional sp2-Carbon Covalent Organic Frameworks as Artificial Photosystem I with High Efficiency, Angew. Chem., Int. Ed., 2019, 58, 5376–5381 CrossRef CAS PubMed
.
- S. Ma, T. Deng, Z. Li, Z. Zhang, J. Jia, Q. Li, G. Wu, H. Xia, S.-W. Yang and X. Liu, Photocatalytic Hydrogen Production on a sp2-Carbon-Linked Covalent Organic Framework, Angew. Chem., Int. Ed., 2022, 61, e202208919 CAS
.
- W. Zhang, L. Chen, S. Dai, C. Zhao, C. Ma, L. Wei, M. Zhu, S. Y. Chong, H. Yang, L. Liu, Y. Bai, M. Yu, Y. Xu, X.-W. Zhu, Q. Zhu, S. An, R. S. Sprick, M. A. Little, X. Wu, S. Jiang, Y. Wu, Y.-B. Zhang, H. Tian, W.-H. Zhu and A. I. Cooper, Reconstructed covalent organic frameworks, Nature, 2022, 604, 72–79 CrossRef CAS PubMed
.
- A. M. Elewa, A. F. M. El-Mahdy, A. E. Hassan, Z. Wen, J. Jayakumar, T.-L. Lee, L.-Y. Ting, I. M. A. Mekhemer, T.-F. Huang, M. H. Elsayed, C.-L. Chang, W.-C. Lin and H.-H. Chou, Solvent polarity tuning to enhance the crystallinity of 2D-covalent organic frameworks for visible-light-driven hydrogen generation, J. Mater. Chem. A, 2022, 10, 12378–12390 RSC
.
- J. Yang, F. Kang, X. Wang and Q. Zhang, Design strategies for improving the crystallinity of covalent organic frameworks and conjugated polymers: a review, Mater. Horiz., 2022, 9, 121–146 RSC
.
- R. Chen, Y. Wang, Y. Ma, A. Mal, X.-Y. Gao, L. Gao, L. Qiao, X.-B. Li, L.-Z. Wu and C. Wang, Rational design of isostructural 2D porphyrin-based covalent organic frameworks for tunable photocatalytic hydrogen evolution, Nat. Commun., 2021, 12, 1354 CrossRef CAS PubMed
.
- W. Huang, Y. Hu, Z. Qin, Y. Ji, X. Zhao, Y. Wu, Q. He, Y. Li, C. Zhang, J. Lu and Y. Li, Highly crystalline and water-wettable benzobisthiazole-based covalent organic frameworks for enhanced photocatalytic hydrogen production, Natl. Sci. Rev., 2022, 10 CAS
.
- W. Li, X. Ding, B. Yu, H. Wang, Z. Gao, X. Wang, X. Liu, K. Wang and J. Jiang, Tuning Molecular Chromophores of Isoreticular Covalent Organic Frameworks for Visible Light-Induced Hydrogen Generation, Adv. Funct. Mater., 2022, 32, 2207394 CrossRef CAS
.
- L. Sun, M. Lu, Z. Yang, Z. Yu, X. Su, Y.-Q. Lan and L. Chen, Nickel Glyoximate Based Metal–Covalent Organic Frameworks for Efficient Photocatalytic Hydrogen Evolution, Angew. Chem., Int. Ed., 2022, 61, e202204326 CAS
.
- Z. Zhao, X. Chen, B. Li, S. Zhao, L. Niu, Z. Zhang and Y. Chen, Spatial Regulation of Acceptor Units in Olefin-Linked COFs toward Highly Efficient Photocatalytic H2 Evolution, Adv. Sci., 2022, 9, 2203832 CrossRef CAS PubMed
.
- Z. Mi, T. Zhou, W. Weng, J. Unruangsri, K. Hu, W. Yang, C. Wang, K. A. I. Zhang and J. Guo, Covalent Organic Frameworks Enabling Site Isolation of Viologen-Derived Electron-Transfer Mediators for Stable Photocatalytic Hydrogen Evolution, Angew. Chem., Int. Ed., 2021, 60, 9642–9649 CrossRef CAS PubMed
.
- Z. Zhao, Y. Zheng, C. Wang, S. Zhang, J. Song, Y. Li, S. Ma, P. Cheng, Z. Zhang and Y. Chen, Fabrication of Robust Covalent Organic Frameworks for Enhanced Visible-Light-Driven H2 Evolution, ACS Catal., 2021, 11, 2098–2107 CrossRef CAS
.
- T. Zhou, X. Huang, Z. Mi, Y. Zhu, R. Wang, C. Wang and J. Guo, Multivariate covalent organic frameworks boosting photocatalytic hydrogen evolution, Polym. Chem., 2021, 12, 3250–3256 RSC
.
- W. Chen, L. Wang, D. Mo, F. He, Z. Wen, X. Wu, H. Xu and L. Chen, Modulating benzothiadiazole-based covalent organic frameworks via halogenation for enhanced photocatalytic water splitting, Angew. Chem., Int. Ed., 2020, 132, 17050–17057 CrossRef
.
- Y. Wang, W. Hao, H. Liu, R. Chen, Q. Pan, Z. Li and Y. Zhao, Facile construction of fully sp2-carbon conjugated two-dimensional covalent organic frameworks containing benzobisthiazole units, Nat. Commun., 2022, 13, 100 CrossRef CAS PubMed
.
- C. Li, J. Liu, H. Li, K. Wu, J. Wang and Q. Yang, Covalent organic frameworks
with high quantum efficiency in sacrificial photocatalytic hydrogen evolution, Nat. Commun., 2022, 13, 2357 CrossRef CAS PubMed
.
- Y. Li, L. Yang, H. He, L. Sun, H. Wang, X. Fang, Y. Zhao, D. Zheng, Y. Qi, Z. Li and W. Deng, In situ photodeposition of platinum clusters on a covalent organic framework for photocatalytic hydrogen production, Nat. Commun., 2022, 13, 1355 CrossRef CAS PubMed
.
- T. He, W. Zhen, Y. Chen, Y. Guo, Z. Li, N. Huang, Z. Li, R. Liu, Y. Liu and X. Lian, Integrated interfacial design of covalent organic framework photocatalysts to promote hydrogen evolution from water, Nat. Commun., 2023, 14, 329 CrossRef CAS PubMed
.
- K. Gottschling, G. Savasci, H. Vignolo-González, S. Schmidt, P. Mauker, T. Banerjee, P. Rovó, C. Ochsenfeld and B. V. Lotsch, Rational Design of Covalent Cobaloxime–Covalent Organic Framework Hybrids for Enhanced Photocatalytic Hydrogen Evolution, J. Am. Chem. Soc., 2020, 142, 12146–12156 CrossRef CAS PubMed
.
- H. N. Kagalwala, E. Gottlieb, G. Li, T. Li, R. Jin and S. Bernhard, Photocatalytic Hydrogen Generation System Using a Nickel-Thiolate Hexameric Cluster, Inorg. Chem., 2013, 52, 9094–9101 CrossRef CAS PubMed
.
- W. Weng and J. Guo, The effect of enantioselective chiral covalent organic frameworks and cysteine sacrificial donors on photocatalytic hydrogen evolution, Nat. Commun., 2022, 13, 5768 CrossRef CAS PubMed
.
- H. Wang, C. Qian, J. Liu, Y. Zeng, D. Wang, W. Zhou, L. Gu, H. Wu, G. Liu and Y. Zhao, Integrating Suitable Linkage of Covalent Organic Frameworks into Covalently Bridged Inorganic/Organic Hybrids toward Efficient Photocatalysis, J. Am. Chem. Soc., 2020, 142, 4862–4871 CrossRef CAS PubMed
.
- C.-C. Li, M.-Y. Gao, X.-J. Sun, H.-L. Tang, H. Dong and F.-M. Zhang, Rational combination of covalent-organic framework and nano TiO2 by covalent bonds to realize dramatically enhanced photocatalytic activity, Appl. Catal., B, 2020, 266, 118586 CrossRef CAS
.
- F.-M. Zhang, J.-L. Sheng, Z.-D. Yang, X.-J. Sun, H.-L. Tang, M. Lu, H. Dong, F.-C. Shen, J. Liu and Y.-Q. Lan, Rational Design of MOF/COF Hybrid Materials for Photocatalytic H2 Evolution in the Presence of Sacrificial Electron Donors, Angew. Chem., Int. Ed., 2018, 57, 12106–12110 CrossRef CAS PubMed
.
- C.-X. Chen, Y.-Y. Xiong, X. Zhong, P. C. Lan, Z.-W. Wei, H. Pan, P.-Y. Su, Y. Song, Y.-F. Chen, A. Nafady, S. Sirajuddin and S. Ma, Enhancing Photocatalytic Hydrogen Production via the Construction of Robust Multivariate Ti-MOF/COF Composites, Angew. Chem., Int. Ed., 2022, 61, e202114071 CAS
.
- W. Zhao, P. Yan, H. Yang, M. Bahri, A. M. James, H. Chen, L. Liu, B. Li, Z. Pang, R. Clowes, N. D. Browning, J. W. Ward, Y. Wu and A. I. Cooper, Using sound to synthesize covalent organic frameworks in water, Nat. Syn., 2022, 1, 87–95 CrossRef
.
- Q. Yue, G. Li, P. Fu, B. Meng, F. Ma, Y. Zhou and J. Wang, In-situ polarization of covalent organic frameworks in seawater enables enhanced photocatalytic hydrogen evolution under visible-light irradiation, Nano Res., 2023, 1–9 Search PubMed
.
- L. Peng, S. Chang, Z. Liu, Y. Fu, R. Ma, X. Lu, F. Zhang, W. Zhu, L. Kong and M. Fan, Visible-light-driven photocatalytic CO2 reduction over ketoenamine-based covalent organic frameworks: role of the host functional groups, Catal. Sci. Technol., 2021, 11, 1717–1724 RSC
.
- X. Xu, P. Cai, H. Chen, H.-C. Zhou and N. Huang, Three-Dimensional Covalent Organic Frameworks with she Topology, J. Am. Chem. Soc., 2022, 144, 18511–18517 CrossRef CAS PubMed
.
- Y.-N. Gong, W. Zhong, Y. Li, Y. Qiu, L. Zheng, J. Jiang and H.-L. Jiang, Regulating Photocatalysis by Spin-State Manipulation of Cobalt in Covalent Organic Frameworks, J. Am. Chem. Soc., 2020, 142, 16723–16731 CrossRef CAS PubMed
.
- J. Ding, X. Guan, J. Lv, X. Chen, Y. Zhang, H. Li, D. Zhang, S. Qiu, H.-L. Jiang and Q. Fang, Three-Dimensional Covalent Organic Frameworks with Ultra-Large Pores for Highly Efficient Photocatalysis, J. Am. Chem. Soc., 2023, 145, 3248–3254 CrossRef CAS PubMed
.
- M. Lu, J. Liu, Q. Li, M. Zhang, M. Liu, J.-L. Wang, D.-Q. Yuan and Y.-Q. Lan, Rational Design of Crystalline Covalent Organic Frameworks for Efficient CO2 Photoreduction with H2O, Angew. Chem., Int. Ed., 2019, 58, 12392–12397 CrossRef CAS PubMed
.
- S. Yang, W. Hu, X. Zhang, P. He, B. Pattengale, C. Liu, M. Cendejas, I. Hermans, X. Zhang, J. Zhang and J. Huang, 2D Covalent Organic Frameworks as Intrinsic Photocatalysts for Visible Light-Driven CO2 Reduction, J. Am. Chem. Soc., 2018, 140, 14614–14618 CrossRef CAS PubMed
.
- Z. Fu, X. Wang, A. M. Gardner, X. Wang, S. Y. Chong, G. Neri, A. J. Cowan, L. Liu, X. Li, A. Vogel, R. Clowes, M. Bilton, L. Chen, R. S. Sprick and A. I. Cooper, A stable covalent organic framework for photocatalytic carbon dioxide reduction, Chem. Sci., 2020, 11, 543–550 RSC
.
- L. Ran, Z. Li, B. Ran, J. Cao, Y. Zhao, T. Shao, Y. Song, M. K. H. Leung, L. Sun and J. Hou, Engineering Single-Atom Active Sites on Covalent Organic Frameworks for Boosting CO2 Photoreduction, J. Am. Chem. Soc., 2022, 144, 17097–17109 CrossRef CAS PubMed
.
- W. Zhang, A. R. Mohamed and W. J. Ong, Z-scheme photocatalytic systems for carbon dioxide reduction: where are we now?, Angew. Chem., Int. Ed., 2020, 59, 22894–22915 CrossRef CAS PubMed
.
- A. Kumar, P. Raizada, P. Singh, R. V. Saini, A. K. Saini and A. Hosseini-Bandegharaei, Perspective and status of polymeric graphitic carbon nitride based Z-scheme photocatalytic systems for sustainable photocatalytic water purification, J. Chem. Eng., 2020, 391, 123496 CrossRef CAS
.
- M. Zhang, M. Lu, Z.-L. Lang, J. Liu, M. Liu, J.-N. Chang, L.-Y. Li, L.-J. Shang, M. Wang, S.-L. Li and Y.-Q. Lan, Semiconductor/Covalent-Organic-Framework Z-Scheme Heterojunctions for Artificial Photosynthesis, Angew. Chem., Int. Ed., 2020, 59, 6500–6506 CrossRef CAS PubMed
.
- L. Wang, G. Huang, L. Zhang, R. Lian, J. Huang, H. She, C. Liu and Q. Wang, Construction of TiO2-covalent organic framework Z-Scheme hybrid through coordination bond for photocatalytic CO2 conversion, J. Energy Chem., 2022, 64, 85–92 CrossRef CAS
.
- S. He, Q. Rong, H. Niu and Y. Cai, Construction of a superior visible-light-driven photocatalyst based on a C3N4 active centre-photoelectron shift platform-electron withdrawing unit triadic structure covalent organic framework, Chem. Commun., 2017, 53, 9636–9639 RSC
.
- S. He, B. Yin, H. Niu and Y. Cai, Targeted synthesis of visible-light-driven covalent organic framework photocatalyst via molecular design and precise construction, Appl. Catal., B, 2018, 239, 147–153 CrossRef CAS
.
- Y. Yang, H. Niu, L. Xu, H. Zhang and Y. Cai, Triazine functionalized fully conjugated covalent organic framework for efficient photocatalysis, Appl. Catal., B, 2020, 269, 118799 CrossRef CAS
.
- W. Chen, Z. Yang, Z. Xie, Y. Li, X. Yu, F. Lu and L. Chen, Benzothiadiazole functionalized D–A type covalent organic frameworks for effective photocatalytic reduction of aqueous chromium(vi), J. Mater. Chem. A, 2019, 7, 998–1004 RSC
.
- Y. Deng, Z. Zhang, P. Du, X. Ning, Y. Wang, D. Zhang, J. Liu, S. Zhang and X. Lu, Embedding Ultrasmall Au Clusters into the Pores of a Covalent Organic Framework for Enhanced Photostability and Photocatalytic Performance, Angew. Chem., Int. Ed., 2020, 59, 6082–6089 CrossRef CAS PubMed
.
- H. B. Aiyappa, J. Thote, D. B. Shinde, R. Banerjee and S. Kurungot, Cobalt-Modified Covalent Organic Framework as a Robust Water Oxidation Electrocatalyst, Chem. Mater., 2016, 28, 4375–4379 CrossRef CAS
.
- S. Bhunia, S. K. Das, R. Jana, S. C. Peter, S. Bhattacharya, M. Addicoat, A. Bhaumik and A. Pradhan, Electrochemical Stimuli-Driven Facile Metal-Free Hydrogen Evolution from Pyrene-Porphyrin-Based Crystalline Covalent Organic Framework, ACS Appl. Mater. Interfaces, 2017, 9, 23843–23851 CrossRef CAS PubMed
.
- S. Ruidas, B. Mohanty, P. Bhanja, E. S. Erakulan, R. Thapa, P. Das, A. Chowdhury, S. K. Mandal, B. K. Jena and A. Bhaumik, Metal-Free Triazine-Based 2D Covalent Organic Framework for Efficient H2 Evolution by Electrochemical Water Splitting, ChemSusChem, 2021, 14, 5057–5064 CrossRef CAS PubMed
.
- Y. Ma, Y. Fu, W. Jiang, Y. Wu, C. Liu, G. Che and Q. Fang, Excellent electrocatalytic performance of metal-free thiophene–sulfur covalent organic frameworks for hydrogen evolution in alkaline medium, J. Mater. Chem. A, 2022, 10, 10092–10097 RSC
.
- Y. Zhao, Y. Liang, D. Wu, H. Tian, T. Xia, W. Wang, W. Xie, X.-M. Hu, X. Tian and Q. Chen, Ruthenium Complex of sp2 Carbon-Conjugated Covalent Organic Frameworks as an Efficient Electrocatalyst for Hydrogen Evolution, Small, 2022, 18, 2107750 CrossRef CAS PubMed
.
- J.-Y. Yue, X.-L. Ding, L.-P. Song, Y.-T. Wang, P. Yang, Y. Ma and B. Tang, Pd(II) functionalized vinylene-linked covalent organic frameworks for acidic electrocatalytic hydrogen evolution reaction, Microporous Mesoporous Mater., 2022, 344, 112169 CrossRef CAS
.
- J.-M. Seo, H.-J. Noh, J.-P. Jeon, H. Kim, G.-F. Han, S. K. Kwak, H. Y. Jeong, L. Wang, F. Li and J.-B. Baek, Conductive and Ultrastable Covalent Organic Framework/Carbon Hybrid as an Ideal Electrocatalytic Platform, J. Am. Chem. Soc., 2022, 144, 19973–19980 CrossRef CAS PubMed
.
- W. Ma, P. Yu, T. Ohsaka and L. Mao, An efficient electrocatalyst for oxygen reduction reaction derived from a Co-porphyrin-based covalent organic framework, Electrochem. Commun., 2015, 52, 53–57 CrossRef CAS
.
- D. Li, C. Li, L. Zhang, H. Li, L. Zhu, D. Yang, Q. Fang, S. Qiu and X. Yao, Metal-Free Thiophene-Sulfur Covalent Organic Frameworks: Precise and Controllable Synthesis of Catalytic Active Sites for Oxygen Reduction, J. Am. Chem. Soc., 2020, 142, 8104–8108 CrossRef CAS PubMed
.
- S. Wu, M. Li, H. Phan, D. Wang, T. S. Herng, J. Ding, Z. Lu and J. Wu, Toward Two-Dimensional π-Conjugated Covalent Organic Radical Frameworks, Angew. Chem., Int. Ed., 2018, 57, 8007–8011 CrossRef CAS PubMed
.
- Z. Liang, H.-Y. Wang, H. Zheng, W. Zhang and R. Cao, Porphyrin-based frameworks for oxygen electrocatalysis and catalytic reduction of carbon dioxide, Chem. Soc. Rev., 2021, 50, 2540–2581 RSC
.
- Z. Lei, F. W. Lucas, E. C. Moya, S. Huang, Y. Rong, A. Wesche, P. Li, L. Bodkin, Y. Jin and A. Holewinski, Highly stable dioxin-linked metallophthalocyanine covalent organic frameworks, Chin. Chem. Lett., 2021, 32, 3799–3802 CrossRef CAS
.
- P. Peng, L. Shi, F. Huo, S. Zhang, C. Mi, Y. Cheng and Z. Xiang, In Situ Charge Exfoliated Soluble Covalent Organic Framework Directly Used for Zn–Air Flow Battery, ACS Nano, 2019, 13, 878–884 CrossRef CAS PubMed
.
- J.-Y. Yue, Y.-T. Wang, X. Wu, P. Yang, Y. Ma, X.-H. Liu and B. Tang, Two-dimensional porphyrin covalent organic frameworks with tunable catalytic active sites for the oxygen reduction reaction, Chem. Commun., 2021, 57, 12619–12622 RSC
.
- Y.-B. Huang, P. Pachfule, J.-K. Sun and Q. Xu, From covalent–organic frameworks to hierarchically porous B-doped carbons: a molten-salt approach, J. Mater. Chem. A, 2016, 4, 4273–4279 RSC
.
- X. Zhuang, W. Zhao, F. Zhang, Y. Cao, F. Liu, S. Bi and X. Feng, A two-dimensional conjugated polymer framework with fully sp2-bonded carbon skeleton, Polym. Chem., 2016, 7, 4176–4181 RSC
.
- Q. Xu, Y. Tang, X. Zhang, Y. Oshima, Q. Chen and D. Jiang, Template Conversion of Covalent Organic Frameworks into 2D Conducting Nanocarbons for Catalyzing Oxygen Reduction Reaction, Adv. Mater., 2018, 30, 1706330 CrossRef PubMed
.
- T. Jiang, W. Jiang, Y. Li, Y. Xu, M. Zhao, M. Deng and Y. Wang, Facile regulation of porous N-doped carbon-based catalysts from covalent organic frameworks nanospheres for highly-efficient oxygen reduction reaction, Carbon, 2021, 180, 92–100 CrossRef CAS
.
- J. Ning, Y. Gao, X. Cao, H. Wei, B. Wang and L. Hao, Substituent engineering of covalent organic frameworks modulates the crystallinity and electrochemical reactivity, J. Energy Chem., 2022, 65, 490–496 CrossRef CAS
.
- V. Hasija, S. Patial, P. Raizada, A. Aslam Parwaz Khan, A. M. Asiri, Q. Van Le, V.-H. Nguyen and P. Singh, Covalent organic frameworks promoted single metal atom catalysis: Strategies and applications, Coord. Chem. Rev., 2022, 452, 214298 CrossRef CAS
.
- S. Wei, Y. Wang, W. Chen, Z. Li, W.-C. Cheong, Q. Zhang, Y. Gong, L. Gu, C. Chen, D. Wang, Q. Peng and Y. Li, Atomically dispersed Fe atoms anchored on COF-derived N-doped carbon nanospheres as efficient multi-functional catalysts, Chem. Sci., 2020, 11, 786–790 RSC
.
- M. Zhou, M. Liu, Q. Miao, H. Shui and Q. Xu, Synergetic Pt Atoms and Nanoparticles Anchored in Standing Carbon-Derived from Covalent Organic Frameworks for Catalyzing ORR, Adv. Mater. Interfaces, 2022, 9, 2201263 CrossRef CAS
.
- S. Yang, X. Li, T. Tan, J. Mao, Q. Xu, M. Liu, Q. Miao, B. Mei, P. Qiao, S. Gu, F. Sun, J. Ma, G. Zeng and Z. Jiang, A fully-conjugated covalent organic framework-derived carbon supporting ultra-close single atom sites for ORR, Appl. Catal., B, 2022, 307, 121147 CrossRef CAS
.
- Y. Fan, M. Chen, N. Xu, K. Wang, Q. Gao, J. Liang and Y. Liu, Recent progress on covalent organic framework materials as CO2 reduction electrocatalysts, Front. Chem., 2022, 10, 882 Search PubMed
.
- C. S. Diercks, S. Lin, N. Kornienko, E. A. Kapustin, E. M. Nichols, C. Zhu, Y. Zhao, C. J. Chang and O. M. Yaghi, Reticular Electronic Tuning of Porphyrin Active Sites in Covalent Organic Frameworks for Electrocatalytic Carbon Dioxide Reduction, J. Am. Chem. Soc., 2018, 140, 1116–1122 CrossRef CAS PubMed
.
- S. Lin, C. S. Diercks, Y. B. Zhang, N. Kornienko, E. M. Nichols, Y. Zhao, A. R. Paris, D. Kim, P. Yang, O. M. Yaghi and C. J. Chang, Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water, Science, 2015, 349, 1208–1213 CrossRef CAS PubMed
.
- B. Han, X. Ding, B. Yu, H. Wu, W. Zhou, W. Liu, C. Wei, B. Chen, D. Qi, H. Wang, K. Wang, Y. Chen, B. Chen and J. Jiang, Two-Dimensional Covalent Organic Frameworks with Cobalt(II)-Phthalocyanine Sites for Efficient Electrocatalytic Carbon Dioxide Reduction, J. Am. Chem. Soc., 2021, 143, 7104–7113 CrossRef CAS PubMed
.
- S.-Y. Chi, Q. Chen, S.-S. Zhao, D.-H. Si, Q.-J. Wu, Y.-B. Huang and R. Cao, Three-dimensional porphyrinic covalent organic frameworks for highly efficient electroreduction of carbon dioxide, J. Mater. Chem. A, 2022, 10, 4653–4659 RSC
.
- B. Han, Y. Jin, B. Chen, W. Zhou, B. Yu, C. Wei, H. Wang, K. Wang, Y. Chen and B. Chen, Maximizing Electroactive Sites in a Three-Dimensional Covalent Organic Framework for Significantly Improved Carbon Dioxide Reduction Electrocatalysis, Angew. Chem., Int. Ed., 2022, 61, e202114244 CAS
.
- Y. R. Wang, H. M. Ding, X. Y. Ma, M. Liu, Y. L. Yang, Y. Chen, S. L. Li and Y. Q. Lan, Imparting CO2 Electroreduction Auxiliary for Integrated Morphology Tuning and Performance Boosting in a Porphyrin-based Covalent Organic Framework, Angew. Chem., Int. Ed., 2022, 134, e202114648 Search PubMed
.
- M. Liu, Y.-R. Wang, H.-M. Ding, M. Lu, G.-K. Gao, L.-Z. Dong, Q. Li, Y. Chen, S.-L. Li and Y.-Q. Lan, Self-assembly of anthraquinone covalent organic frameworks as 1D superstructures for highly efficient CO2 electroreduction to CH4, Sci. Bull., 2021, 66, 1659–1668 CrossRef CAS PubMed
.
- M. D. Zhang, D. H. Si, J. D. Yi, S. S. Zhao, Y. B. Huang and R. Cao, Conductive Phthalocyanine-Based Covalent Organic Framework for Highly Efficient Electroreduction of Carbon Dioxide, Small, 2020, 16, e2005254 CrossRef PubMed
.
- H. J. Zhu, M. Lu, Y. R. Wang, S. J. Yao, M. Zhang, Y. H. Kan, J. Liu, Y. Chen, S. L. Li and Y. Q. Lan, Efficient electron transmission in covalent organic framework nanosheets for highly active electrocatalytic carbon dioxide reduction, Nat. Commun., 2020, 11, 497 CrossRef CAS PubMed
.
- Q. Wu, M.-J. Mao, Q.-J. Wu, J. Liang, Y.-B. Huang and R. Cao, Construction of Donor–Acceptor Heterojunctions in Covalent Organic Framework for Enhanced CO2 Electroreduction, Small, 2021, 17, 2004933 CrossRef CAS PubMed
.
- N. Huang, K. H. Lee, Y. Yue, X. Xu, S. Irle, Q. Jiang and D. Jiang, A stable and conductive metallophthalocyanine framework for electrocatalytic carbon dioxide reduction in water, Angew. Chem., Int. Ed., 2020, 132, 16730–16736 Search PubMed
.
- M. Lu, M. Zhang, C.-G. Liu, J. Liu, L.-J. Shang, M. Wang, J.-N. Chang, S.-L. Li and Y.-Q. Lan, Stable Dioxin-Linked Metallophthalocyanine Covalent Organic Frameworks (COFs) as Photo-Coupled Electrocatalysts for CO2 Reduction, Angew. Chem., Int. Ed., 2021, 60, 4864–4871 CrossRef CAS PubMed
.
- H. Liu, J. Chu, Z. Yin, X. Cai, L. Zhuang and H. Deng, Covalent Organic Frameworks Linked by Amine Bonding for Concerted Electrochemical Reduction of CO2, Chem, 2018, 4, 1696–1709 CAS
.
- G. C. Dubed Bandomo, S. S. Mondal, F. Franco, A. Bucci, V. Martin-Diaconescu, M. A. Ortuño, P. H. van Langevelde, A. Shafir, N. López and J. Lloret-Fillol, Mechanically Constrained Catalytic Mn(CO)3Br Single Sites in a Two-Dimensional Covalent Organic Framework for CO2 Electroreduction in H2O, ACS Catal., 2021, 11, 7210–7222 CrossRef CAS
.
- S. An, C. Lu, Q. Xu, C. Lian, C. Peng, J. Hu, X. Zhuang and H. Liu, Constructing Catalytic Crown Ether-Based Covalent Organic Frameworks for Electroreduction of CO2, ACS Energy Lett., 2021, 6, 3496–3502 CrossRef CAS
.
- Y. Song, J.-J. Zhang, Z. Zhu, X. Chen, L. Huang, J. Su, Z. Xu, T. H. Ly, C.-S. Lee, B. I. Yakobson, B. Z. Tang and R. Ye, Zwitterionic ultrathin covalent organic polymers for high-performance electrocatalytic carbon dioxide reduction, Appl. Catal., B, 2021, 284, 119750 CrossRef CAS
.
- H. Dong, M. Lu, Y. Wang, H.-L. Tang, D. Wu, X. Sun and F.-M. Zhang, Covalently anchoring covalent organic framework on carbon nanotubes for highly efficient electrocatalytic CO2 reduction, Appl. Catal., B, 2022, 303, 120897 CrossRef CAS
.
- Y.-L. Yang, Y.-R. Wang, L.-Z. Dong, Q. Li, L. Zhang, J. Zhou, S.-N. Sun, H.-M. Ding, Y. Chen, S.-L. Li and Y.-Q. Lan, A Honeycomb-Like Porous Crystalline Hetero-Electrocatalyst for Efficient Electrocatalytic CO2 Reduction, Adv. Mater., 2022, 34, 2206706 CrossRef CAS PubMed
.
| This journal is © the Partner Organisations 2023 |