Seaweed-based polysaccharides – review of extraction, characterization, and bioplastic application
Lakshmi
Krishnan†
a,
Nandhini
Ravi†
a,
Anjon
Kumar Mondal†
a,
Farjana
Akter†
a,
Manoj
Kumar†
ab,
Peter
Ralph†
a and
Unnikrishnan
Kuzhiumparambil†
 *a
*a
aClimate Change Cluster, University of Technology Sydney, Ultimo, NSW 2007, Australia. E-mail: unnikrishnan.kuzhiumparambil@uts.edu.au
bSchool of Health, Medical and Applied Sciences, Coastal Marine Ecosystems Research Centre, Central Queensland University, Australia
First published on 25th March 2024
Abstract
Seaweed biomass is gaining industrial and economic momentum as a renewable feedstock for high-value products, including nutraceuticals, value-added chemicals and bioplastics. These organisms can be sustainably cultivated on an industrial scale, provide new opportunities for decreasing our dependency on fossil feedstocks, and potentially contribute to climate mitigation while reducing pressure on land resources. Seaweed generally possesses a high polysaccharide content, making it an alternative source to crop plants for bioplastic production. Polysaccharides like alginate, carrageenan, ulvan, fucoidan and laminarin are good candidates as bioplastic components due to their compatibility with various matrices and film-forming abilities. A wide range of applications are already demonstrated for these polysaccharides in biomedical areas, food packaging, functional foods, nutraceuticals and the pharmaceutical industry. Composites and blends of these polysaccharides with other polymers, additives, and fillers have been reported as a solution to improve the mechanical properties and, in some cases, to combine desired attributes strategically. This review examines seaweed-derived polysaccharides, their extraction and characterization techniques, application and performance as bioplastics, blends and composites, and material characteristics.
1. Introduction
Seaweeds are macroscopic marine algae. Seaweeds are broadly classified into red (Rhodophyta), brown (Phaeophyta), and green (Chlorophyta) algae.1 The major macromolecular components of macroalgae/seaweed are polysaccharides, lipids, cellulose, proteins, minerals and polyphenols. The percentage of the components varies with the species. There have been extensive studies on the therapeutic properties of the seaweed-derived polysaccharides and many biomedical applications have been demonstrated.2–6 Polysaccharides form a part of the structural component of the macroalgal cell wall and can be extracted using various techniques. The most critical polysaccharides derived from these seaweeds are alginate, carrageenan, ulvan, fucoidan and laminarin. Seaweed cultivation has gained interest as an industrial crop over the past decade.Recently, there has been a growing emphasis on the circular bioeconomy, which centres around the sustainable enhancement and conversion of biomass within production chains. This involves turning agro-industrial wastes into high-value products and utilizing renewable resources to create products with significant added value. The exploration of versatile and eco-friendly photosynthetic organisms, such as algae, holds great promise in advancing the development of these closed-loop systems.7
Developing value-added products from seaweeds plays a crucial role in contributing to zero carbon emissions and UN sustainability goals. The extracted polysaccharides have functional groups like sulphate, carboxyl, and hydroxyl, which helps them bind with biological components. Many of these polysaccharides show good gelling behaviour, have excellent biocompatibility, and possess antimicrobial and hypoallergenic traits. The anionic polysaccharides are particularly interesting in wound dressing applications as they do not bind well with serum proteins and hence avoid forming aggregates.3
Plastic-based products have become inevitable in day-to-day life. Increasing plastic consumption leads to the bigger problem of white pollution from piling plastic debris, which is a grave environmental problem. Bioplastics made from bio-based renewable resources provide a more sustainable commercial plastic life cycle as part of a circular economy. Bioplastics derived from agro-polymers, such as starch and lignocellulosic waste, offer a renewable and cost-effective resource, forming odorless, colourless, and transparent biofilms. However, their limitations, including poor water and gas barrier properties, a low melting point, lower mechanical strength, and potential impacts on the human food chain, along with an extended production time, have spurred the search for alternative renewable sources.8,9 Macroalgae (seaweeds) surpass the aforementioned sources due to their high biomass production, cost-effectiveness, non-competition for land and fertilizers, ease of cultivation in natural ocean environments, and year-round harvestability. Seaweeds find wide applications in food technology, biotechnology, microbiology, and medicine. Although these applications are beyond the scope of this review, readers can refer to other sources10,11 for up-to-date information.
The use of seaweeds in the plastic industry is emerging as a potential renewable candidate, providing non-toxic, environmentally friendly, and cost-effective bioplastics with qualities comparable in terms of tensile strength and chemical resistance. These unique properties position seaweeds as a new and alternative source for bioplastics in today's world.9 Beyond their biodegradability, seaweed-derived biofilms possess antimicrobial properties due to a variety of phenolic compounds. These biofilms are proposed for application in the food packaging industry to prolong the shelf life of products by inhibiting the growth of foodborne pathogens.
Investigating seaweed as a bioresource for plastics and materials alike is of great advantage for the following reasons: (i) replacing conventional plastics with biodegradable and compostable systems for short-lifespan applications reduces waste management stress; (ii) sustainably sourced biomaterials can replace non-replenishable fossil-based sourcing; (iii) seaweeds are known to be carbon sinks that help to reduce global warming; (iv) seaweed farming can bring additional income for marginal communities in coastal regions; (v) it provides an alternate source of biomass for making bioplastics and hence not competing with food sources like corn or potato; and (vi) the land, water and nutrient requirements for this cultivation are much less than for traditional methods.1,12
Polysaccharides present in seaweed can be in the form of stored mass, structural, or present as mucilage in intercellular locations.1 The structural polysaccharides could be cellulose or hemicellulose found in the cell walls. Food storage in seaweeds is in the form of polysaccharides such as fucoidans, laminarin, and starch. The mucilage type are generally located between the plant cells. They are usually slimy in character with the function of storing water. Examples of these polysaccharides include xylan, porphyran, carrageenan, agar, fucans, and alginates. The capability of these polysaccharides to be used in the bio-plastics industry has been extensively demonstrated.1 The different polysaccharides and the major molecules present in brown, green and red seaweed are provided in Table 1. Extracted polysaccharides generally have inferior mechanical and permeation characteristics compared with petroleum-based plastics.3,4,6 This limitation is overcome by modification of the structure, blending with other polymers, and preparing composites with suitable fillers and additives.
| Seaweed | Polysaccharides | Major molecule |
|---|---|---|
| Brown | Laminaran | (1,3)-β-D-Glucose and (1,6)-β-D-glucose branch unit mannitol |
| Fucoidan | β-(1,4)-D-Mannuronic acid and 3-D-xyosyl-L-fucose-4-sulfate | |
| (1,2)- α-L-Fucose-L-sulfate | ||
| (1,4)-D-Galactose and L-fucosyl-3-sulfate branch units-D-xylose, D-galactose and D-mannose | ||
| Alginate | β-(1,4)-D-Mannuronic acid and α-(1,4)-L-guluronic acid | |
| Green | Ulvan | β-D-Glucuronosyl-(1,4)-α-L-rhamnose 3-sulfate |
| Mannan | Mannose (C-2 epimer of glucose) | |
| Xylan | β(1,4)-D-Xylose | |
| Starch | α-D-Glucose | |
| 20% amylose, 80–90% amylopectin | ||
| Red | Carrageenans | α(1,3)-Galactose acid and β-(1,4,3,6)-anhydro-D-galactose |
| Floridean starch | α-D-Glucose, amylopectin | |
| Xylan | β(1,4)-D-Xylose | |
| Agar | D-Galactose acid and (3,6)-anhydro-L-galactose | |
| Mannan | Mannose (C-2 epimer of glucose) |
The efficiency of any process is assessed using green metrics, which incorporated measurements of material efficiency, economic evaluation, total energy input, and land use. The environmental impact of chemical processes is commonly assessed using two widely accepted, straightforward metrics. The first is the E factor, which is the mass ratio of waste to the desired product. The second is the atom economy, also known as atom utilization, which is defined as the percentage ratio of the molecular weight of the desired product to the total molecular weights of all the substances produced.13,14
This review aims to collate the techniques used to extract and characterize high-value polysaccharides from macroalgae. A novel approach of this paper is that it covers the use of green solvents utilized for the extraction of seaweed polysaccharides. Another novelty is that the challenges faced by the bioplastics and the future perspective of these bioplastics have been illustrated in this paper. A wide range of applications of bioplastics obtained from a diverse variety of polysaccharides has been outlined. The techno-economic aspects and life cycle assessment of seaweed-based bioplastics have also been covered briefly in this review paper.
2. Extraction technologies of macroalgal polysaccharides
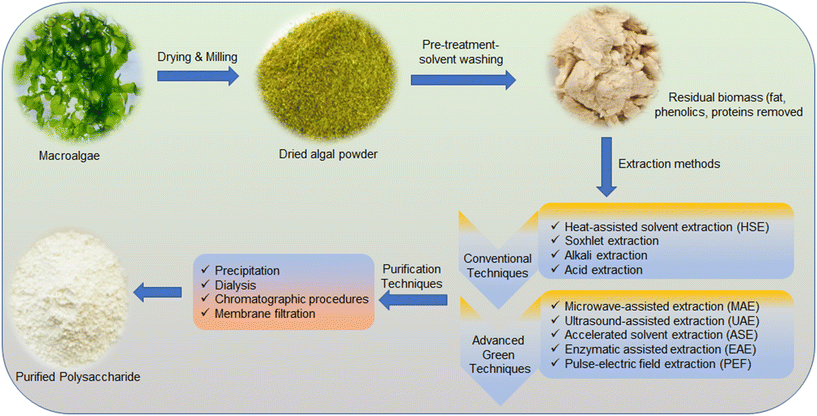
Macroalgal polysaccharides (MP) have attracted significant attention from the scientific community due to their unique properties such as biological activities, film-formation, high potential for functional food, and biotechnological and biomedical applications.15 A general summary of processes to obtain high-purity MP from algal biomass is summarized in Fig. 1. The first step comprises the preparation of the macroalgal biomass, which includes washing to remove salt and impurities, drying or freeze-drying, and crushing to convert the biomass to a powder. The next step is pre-treatment to remove interfering compounds, such as pigments, lipids, and low molecular compounds.16 In general, solvents or a combination of solvents with different polarities that do not affect MP's structural integrity are used for pre-treatment.17 Lower-polarity solvents like chloroform are used to remove lipids; semi-polar solvents such as methanol, ethanol and acetone are used to remove pigments; whereas water is used to extract high-polarity molecules, such as minerals, monosaccharides and proteins.16 Then, polysaccharides are extracted by using both conventional and advanced green techniques.The amount, location and composition of MP are influenced by the raw materials and their harvesting conditions, including location, season and species. However, other factors, such as extraction methods and conditions, also need to be considered.18
2.1 Conventional techniques
Heat-assisted solvent extraction (HSE) and Soxhlet extraction are the most common conventional techniques used in laboratory research and industrial applications. Hot water is generally used as a solvent to extract polysaccharides from macroalgae.19 Tiwari et al. used the HSE technique, which involves temperature and agitation to increase the solubility of the targeted compounds in the solvent.20 According to Huang's group, the pre-treated sample was mixed with 95% ethanol (w/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to remove unwanted materials, then centrifuged and washed with water and kept in the water bath at 85 °C to extract the polysaccharides.21 Other literature described the use of a mixture of solvents, such as methanol and dichloromethane (1
10) to remove unwanted materials, then centrifuged and washed with water and kept in the water bath at 85 °C to extract the polysaccharides.21 Other literature described the use of a mixture of solvents, such as methanol and dichloromethane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), and hydrochloric solutions (PH: 1.5) for the extraction.22,23 The extraction time varies from several hours to 48 h, while the temperature ranges from ambient temperature to ≥100 °C. In contrast, the Soxhlet extraction technique is categorized by a continuous flow over the biomass, which increases the amount of product compared with HSE.20 In this technique, hot water is the most used solvent, similar to HSE. However, to precipitate the polysaccharides, calcium chloride can be used.24 Due to the continuous flow of solvents, the extraction time is significantly reduced in Soxhlet extraction.
1, v/v), and hydrochloric solutions (PH: 1.5) for the extraction.22,23 The extraction time varies from several hours to 48 h, while the temperature ranges from ambient temperature to ≥100 °C. In contrast, the Soxhlet extraction technique is categorized by a continuous flow over the biomass, which increases the amount of product compared with HSE.20 In this technique, hot water is the most used solvent, similar to HSE. However, to precipitate the polysaccharides, calcium chloride can be used.24 Due to the continuous flow of solvents, the extraction time is significantly reduced in Soxhlet extraction.
Besides HSE and Soxhlet extraction, alkali and acid extraction techniques have also been employed to extract MP. In alkali and acid extraction techniques, HCl and NaOH are generally used to isolate polysaccharides from the macroalgae. The H+ and OH− of the acid and base interfere with the hydrogen linkage of the polysaccharides, releasing polysaccharides into the solvent.25,26 Zhao et al. reported alkali extraction, where the dried macroalgae were treated with 0.3 mol L−1 NaOH solution for 3 h at ambient temperature with the algae and a solvent ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/v).27 However, Chi et al. reported 0.5 M NaOH with a ratio of 1
10 (w/v).27 However, Chi et al. reported 0.5 M NaOH with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 for 3 h at 60 °C.28 The residue was filtered and neutralised with 0.1 M HCl. Many researchers employed acid extraction methods combining different times and temperatures to isolate polysaccharides. Liu et al. treated macroalgae using 0.1 N HCl in a 1
40 for 3 h at 60 °C.28 The residue was filtered and neutralised with 0.1 M HCl. Many researchers employed acid extraction methods combining different times and temperatures to isolate polysaccharides. Liu et al. treated macroalgae using 0.1 N HCl in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (w/v) algae:solvent ratio at room temperature for 4 h to extract the polysaccharide.26 Chi et al. also isolated polysaccharides from macroalgae with 0.1 M HCl in the ratio of 1
30 (w/v) algae:solvent ratio at room temperature for 4 h to extract the polysaccharide.26 Chi et al. also isolated polysaccharides from macroalgae with 0.1 M HCl in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/v) at 80 °C for 1 h. After filtration, the polysaccharide was treated with 6 N NaOH to neutralize the acid.28 It was found that the alkali method is less effective and has a lower yield than the acid method.28
20 (w/v) at 80 °C for 1 h. After filtration, the polysaccharide was treated with 6 N NaOH to neutralize the acid.28 It was found that the alkali method is less effective and has a lower yield than the acid method.28
There are several disadvantages associated with the use of conventional techniques of extraction. A longer process time, large quantity of solvent consumption, and degradation of extracted compounds due to high-temperature operation are some of the few issues reported.
2.2 Advanced green techniques
Several advanced techniques have been employed to develop a more effective extraction process for MP. Microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), accelerated solvent extraction (ASE), enzymatic-assisted extraction (EAE) and pulse-electric field extraction (PEF) are some of the advanced green extraction techniques used as described below.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) have also been reported.32 Carrageenan from Solieria chordalis (Rhodophyceae) has been successfully extracted by using MAE technique with a maximum yield of 29.3% at 90 °C for 10 minutes.33 Yuan et al. reported polysaccharides from Ulva prolifera by MAE using HCl in 15 min. The highest yield of 36.4% was achieved with 0.01 M HCl at 120 °C.34
5) have also been reported.32 Carrageenan from Solieria chordalis (Rhodophyceae) has been successfully extracted by using MAE technique with a maximum yield of 29.3% at 90 °C for 10 minutes.33 Yuan et al. reported polysaccharides from Ulva prolifera by MAE using HCl in 15 min. The highest yield of 36.4% was achieved with 0.01 M HCl at 120 °C.34
After the initial extraction, the crude polysaccharide solution is treated with ethanol or calcium chloride at different temperatures and concentrations to precipitate the polysaccharides.55,56 Then, the polysaccharides are purified using various techniques, including further precipitation, dialysis, chromatographic procedures and membrane filtration.57,58
3. Comparison of extraction techniques
Because of the variations in experimental conditions (different instrumental parameters, time, solvents) and the variety of targeted MP in the published literature (as summarized in Table 2), it is difficult to make a direct comparison among extraction techniques. Wang et al. employed MAE and UAE to extract fucoidan from S. siliquosum using water as a solvent. MAE showed an extraction yield of 6.94%, while UAE is less effective with a rate of 4.78%.38 Yaich et al. utilized an acidic solution (pH 1.5–2) to extract ulvan from U. lactuca using EAE and conventional HAE. EAE achieved a significantly higher extraction yield of 17.15%, while the range of yield was between 3.04% and 13.04% in conventional HAE.23 A recent study compared different techniques to recover fucoidan from A. nodosum. The conventional HAE showed a much higher extraction yield of 11.9%, while MAE, UAE and EAE showed 5.71, 4.56 and 3.89%, respectively.31 Alboofetileh et al. employed water as a solvent to compare the recovery of fucoidans from N. zanardinii using HAE, ASE, MAE, UAE and EAE. Conventional HAE showed a yield of 5.2%, while ASE and MAE presented higher yields of 13.1 and 6.17%, respectively. Depending on the applied enzyme in EAE, the rate was between 5.58 to 4.36% and UAE was less effective, with a rate of 3.51%.30| Name of organisms and method | Pre-treatment | Extraction condition | Purification | Percentage of recovery | Ref. |
|---|---|---|---|---|---|
| Abbreviations: TP: total polysaccharides; Ulv: ulvan; Fuc: fucoidan; Alg: alginate; Carr: carrageenan; Lam: laminarin; pr: precipitation; RT: room temperature; EtOH: ethanol; MeOH: methanol; AcO: Acetone; d-DW: defatted dry weight; DW: dry weight; DES: deep eutectic solvents; IEC: ion-exchange chromatography. | |||||
| Heat-assisted extraction (HAE) | |||||
| T. ornata | 95% EtOH | H2O, 95 °C, 3 h | EtOH pr. | TP | 19 |
| U. lactuca | — | H2O-HCl (pH 1.5, 2), 80 and 90 °C, 1 h | EtOH pr. | Ulv. (3.04–13.06%) | 23 |
| N. zanardinii | 85% EtOH, RT, overnight | H2O, 65 °C, 3 h (×2) | CaCl2 pr., EtOH pr. | Fuc. (5.2%) | 30 |
| A. nodosum | 80% EtOH, RT, 20 h; 70 °C, 5 h | 0.01 M HCl, 70 °C, 3 h | CaCl2 pr., EtOH pr., filtration | Fuc. (11.9%) | 31 |
| A. nodosum, L. hyperborea | — | 0.1 M HCl, 70 °C 150 min; H2O, 70 °C 150 min | EtOH pr., dialysis | Lam. (4.3%; 4.6%) | 55 |
| G. persica, E. intestinalis | 85% EtOH, RT, overnight | H2O, 65 °C, 2 h | EtOH pr., filtration | TP | 59 |
| C. langsdorfii | 96% EtOH, 40 °C, 24 h | 0.1 M HCl, RT, 3 h | EtOH pr., dialysis, IEC | Alg. (13.3% d-DW); Lam. | 60 |
| (0.4% d-DW); Fuc. | |||||
| (3.0–46.0% d-DW) | |||||
| Soxhlet extraction | |||||
| F. evanescens, F. serratus, D. foeniculaceus, S. latissimi, F. vesiculosus, L. digitata | 99% EtOH | 2% CaCl2, 85 °C, 2 h | EtOH pr., dialysis | Fuc. (10.9–31.7% DW) | 24 |
| H. cornea, S. fluitans, R. pseudopalmata, S. filiformis | AcO, MeOH | H2O, 90 °C, 2 h | EtOH pr. | TP | 61 |
| T. ornata | — | Hot H2O | — | TP (12%) | 62 |
| Microwave-assisted extraction (MAE) | |||||
| N. zanardinii | 85% EtOH, RT, overnight | H2O, 90 °C, 700 W, 20 min (×2) | CaCl2 pr., EtOH pr. | Fuc. (6.17%) | 30 |
| A. nodosum | 80% EtOH, RT, 20 h; 70 °C, 5 h | 0.01 M HCl, 90 °C, 15 min | CaCl2 pr., EtOH pr. | Fuc. (5.71%) | 31 |
| U. prolifera | — | 0.01–0.1 M HCl, 90–120 °C, 500 W, 15 min | Neutralization with NaOH, EtOH pr. | TP | 34 |
| S. siliquosum | 95% EtOH, 4 h | H2O, 750 W, 10 min | Dialysis, EtOH pr., IEC | Fuc. (6.94%) | 38 |
| S. ceylonensis, U. lactuca, D. antarctica, G. lemaneiformis | H2O, 70 °C, 500 W, 50 min | EtOH pr., IEC | TP (11.09; 13.32; 12.49; 14.21% DW) | 63 | |
| Ultrasound-assisted extraction (UAE) | |||||
| N. zanardinii | 85% EtOH, RT, overnight | H2O, 55 °C, 20 kHz, 200 W (×2) | CaCl2 pr., EtOH pr. | Fuc. (3.51%) | 30 |
| A. nodosum | 80% EtOH, RT, 20 h//70 °C, 5 h | 0.01M HCl, RT, 20 kHz; 35 min | CaCl2 pr., EtOH pr. | Fuc. (4.56%) | 31 |
| S. siliquosum | 95% EtOH, 4 h | H2O, RT, 21–25 kHz, 950 W, 10 min | Dialysis, EtOH pr., IEC | Fuc. (4.78%) | 38 |
| K. alvarezii, E. denticulatum | 80% EtOH, RT, overnight | H2O, 90 °C, 25 kHz, 150 W, 15–30 min | Hot filtration | Carr. (50%; 55% DW) | 39 |
| A. nodosum, L. hyperborea | — | 0.1 M HCl//H2O, RT, 750 W, 15 min | EtOH pr.//dialysis (10 kDa) | Lam. (6.24%//5.97%) | 55 |
| U. intestinalis | 80% EtOH, RT, overnight (×2) | H2O, 66 °C, 53 kHz, 180 W, 40 min | EtOH pr. | Ulv. (8.30%) | 64 |
| Silvetia compressa | — | 25% EtOH, 57.5 °C, 30 min | MeOH pr. | TP (25.13%) | 65 |
| Accelerated solvent extraction (ASE) | |||||
| N. zanardinii | 85% EtOH, RT, overnight | H2O, 150 °C, 10 min, ×2 | CaCl2 pr., EtOH pr. | Fuc. (13.1% DW) | 30 |
| K. alvarezii | — | H2O, (60–180 °C), 50 bar, 5 min | 2-Propanol pr. | k-Carr. (55–70%) | 45 |
| S. japonica | — | H2O-DES (50–70%), 100–150 °C, 5–50 bar, 10–25 min | CaCl2 pr., EtOH pr. | Alg. (5–27.2%) | 66 |
| Fuc. (5–14.9%) | |||||
| Enzyme-assisted extraction (EAE) | |||||
| U. lactuca | — | Protease, cellulase | Dialysis, EtOH pr. | Ulv. (17.14%) | 23 |
| pH 7, 50 °C, 2 h | |||||
| N. zanardinii | 85% EtOH, RT, overnight | Alcalase, pH 8, 50 °C, 24 h, Flavourzyme, pH 7, 50 °C, 24 h | CaCl2 pr., EtOH pr. | Fuc. (5.58%), (4.36%) | 30 |
| A. nodosum | 80% EtOH, RT, 20 h; 70 °C, 5 h | Cellulase, pH 4.5, 50 °C, 24 h | CaCl2 pr., EtOH pr. | Fuc. (3.89%) | 31 |
| C. fragile | — | Celluclast, pH 4.5, 50 °C, 24 h | EtOH pr. | TP (55.78%) | 67 |
Published reports have shown that advanced techniques are more effective, achieving higher yields than conventional extraction. However, the optimization of different operating parameters (time, temperature and solvent) and selection of extraction techniques are vital to achieve maximum output.
4. Green solvents for seaweed polysaccharide extraction
In the realm of seaweed biomass processing, conventional organic solvents pose challenges such as low extraction and processing efficiency, inadequate selectivity for target compounds, extended processing time, variable operational requisites, and substantial demands for both water and solvent volumes. Additionally, these solvents are volatile, toxic, and non-recyclable, raising environmental and health risks. Such concerns highlight sustainability, safety, and product integrity issues. In response, the surge in eco-friendly practices has spotlighted green solvents like ionic liquids (ILs) and deep eutectic solvents (DES), offering a fresh avenue for sustainable chemistry. These innovative solvents enhance efficiency, reduce resource usage, and align with environmentally conscious processes, heralding a transformative paradigm in seaweed biomass processing. Moreover, the physiochemical properties of both bio-solvents are highly tunable, which makes them “designer solvents” adaptable to a plethora of techniques and technologies.684.1 Ionic liquids
Ionic liquids (ILs) are composed of ions and exhibit a melting point lower than room temperature or even below the boiling point. These substances possess attributes like non-flammability, low vapor pressure, excellent thermal and electrochemical stability, along with features such as high conductivity, remarkable solute stabilization, and recyclability. Over time, numerous strategies have been investigated for the extraction of various biomolecules, including proteins, pigments, and phenolics from diverse seaweed sources using ILs.69 However, the application of ILs for the extraction and purification of polysaccharides has seen limited exploration in a few select studies.For the establishment of an eco-friendly polysaccharide industry derived from seaweed, meticulous operational control is essential. This entails minimizing chemical usage and optimizing chemical and solvent recycling during the polysaccharide isolation process, signifying a firm commitment to sustainable and responsible resource management. The idea of extracting seaweed polysaccharides using green solvents such as ILs was not considered until Prasad et al. (2009) showcased the capacity of 1-butyl-3-methylimidazolium chloride (BMIMCl) to create robust ion gels with diverse carrageenans and their cellulose composites. This pivotal demonstration foresaw the potential of ILs in extracting polysaccharides from seaweed sources; however their biocompatibility remained questionable. Hence, in many applications bio-based nontoxic ionic liquids (bio-ILs) are preferred considering their inherit properties of biodegradability, non-toxicity and biocompatibility.70,71
Trivedi and Kumar (2014) extracted agarose from Gracilaria dura, a red seaweed, by subjecting the biomass to 1-ethyl-3-methylimidazolium acetate treatment under microwave conditions followed by agarose precipitation in methanol. However, this process involved multiple processing and purification stages.72 Subsequently, Sharma et al. (2015) introduced a more streamlined approach: a single-step selective precipitation of agarose using choline laurate-based bio-ILs (4.0% w/w). This method in an energy-efficient process directly extracted agarose from the hot seaweed extract of G. dura, yielding 14% w/w without purifying agar and freeze thawing. A notable advantage of this method was the recycling and reuse of bio-ILs for successive rounds of agarose isolation, without compromising the yield or quality of the agarose.73 In a similar strategy, Das et al. (2021) preferentially coagulated κ-carrageenan from the aqueous extract of Kappaphycus alvarezii by using the bio-ILs choline capriate and choline laurate.74 In another work, coupling IL 1-butyl-3-methylimidazolium acetate (BMIMA) with subcritical water extraction (SWE) resulted in >78% yield of high purity κ-carrageenan from K. alvarezii when compared with conventional alkaline treatments (55.3%).45
A diverse array of ILs, spanning switchable, distillable, low-viscosity, and blended compositions, in addition to ILs enriched with oligo-ethylene glycol (EG) chains, have been explored comprehensively. These ILs have been rigorously evaluated for their effectiveness in hydrolysing and dissolving seaweed biomass, facilitating the extraction of carbohydrates and sugars from a variety of seaweed species across different genera.75–78 These investigations involved subjecting seaweed biomass to direct treatment with ILs at elevated temperatures (90–160 °C) over extended operational periods (3–360 minutes). Notably, within the context of hydrolysis and dissolution employing ILs, a single study has successfully demonstrated a remarkable recovery of IL to 92% through precipitation.75
The application of ILs has further extended to the modification of silica particles for use in size exclusion chromatography, coupled with a refractive index detector. This innovative technique was employed to determine the presence of specific polysaccharides, including fucoidan, alginic acid, and laminarin, derived from Undaria pinnatifida.79 In a more recent context, proteins and carbohydrates from Ulva lactuca were extracted under non-denaturing conditions using IL-ethyl methyl imidazolium dibutyl phosphate ([Emim][DBP]) by means of IL-assisted mechanical shear. This process was followed by two-phase partitioning or ultrafiltration, resulting in extraction yields of 80.4% for proteins and 30.7% for carbohydrates, with an impressive 85.7% recovery of the IL.80
4.2 Deep eutectic solvents
While ILs possess remarkable properties, their “green” credentials often come into question due to concerns over toxicity, poor biodegradability, biocompatibility, and sustainability. In 2003 Abbott and colleagues introduced a novel category of solvents termed deep eutectic solvents (DESs).81 These solvents consist of hydrogen bond donors (HBDs) and acceptors (HBAs) which, when combined at precise ratios, yield a viscous liquid system at room temperature with a significantly lower melting point than that of each individual HBD and HBA species.82 The formation of these eutectic systems is thought to be driven by hydrogen bonding interactions. Notably, certain researchers distinguish natural DESs (NADESs) from standard DESs when the constituent species are primary metabolites such as amino acids, organic acids, sugars, or choline derivatives.83DESs are cousins of ILs, offering many shared benefits, including minimal vapor pressure, elevated boiling points, a broad electrochemical window, and the potential for efficient recycling. However, unlike traditional ILs, DESs do not solely consist of ionic species; rather, they can be derived from non-ionic components. Notably, DESs are often recognized for their cost-effectiveness and ease of preparation in contrast to ILs, as well as their marked biodegradability and low toxicity.82 The synthesis of DESs relies on vacuum evaporation, freeze-drying, grinding and heating and stirring methods. Moreover, the manipulation of the ratio between the two components (HBD and HBA) enables the fine-tuning of thermodynamic attributes such as polarity and conductivity within the eutectic system. This distinctive ability renders DESs task-specific solvents, as highlighted by Yu et al. (2022).84
Type III DES comprising ChCl as an HBA is one of the simplest and highly biocompatible among the four DES categories,85 most used in seaweed polysaccharide extraction studies. Das et al. (2016) explored diverse aqueous and non-aqueous ChC –urea/glycerol/ethylene glycol-based DESs for the extraction of κ-carrageenan. The highest yield of κ-carrageenan (60%) achieved using a 10% hydrated ChCl:glycerol DES surpassed the 36% yield obtained in conventional alkali methods. Nevertheless, it is worth noting that residual traces of the DES detected in the extracted carrageenan contributed to a slight softening of the resulting gels. Intriguingly, these gels exhibited greater viscoelastic properties, indicating potential advantages of this DES-based extraction approach.74
Researchers employed Box–Behnken response surface modelling to optimize alginate and fucoidan extraction from brown seaweeds using deep eutectic solvents (DESs) paired with subcritical water extraction (SWE) or microwave-assisted water extraction (MWE). Seven type III DESs, combined with SWE at 150 °C, 20 bar pressure, 70% water content, and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 S
36 S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratio, yielded high alginate (28% DW) and fucoidan (15% DW) from defatted Saccharina japonica.86 Similarly, a DES (choline chloride
L ratio, yielded high alginate (28% DW) and fucoidan (15% DW) from defatted Saccharina japonica.86 Similarly, a DES (choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-butanediol; 1
1,4-butanediol; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) with MWE at 168 °C and 32% water content produced potent fucoidan extracts from Fucus vesiculosus, exhibiting strong antioxidant and antiproliferative effects.87 A DES consisting of choline chloride and 1.2-propanediol (1
5) with MWE at 168 °C and 32% water content produced potent fucoidan extracts from Fucus vesiculosus, exhibiting strong antioxidant and antiproliferative effects.87 A DES consisting of choline chloride and 1.2-propanediol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) with a 30% water content, a 1
2) with a 30% water content, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 S
30 S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratio, and ultrasonication (30 min, 40% amplitude) at 70 °C rapidly extracted polysaccharides with an 11% yield from Sargassum horneri.88
L ratio, and ultrasonication (30 min, 40% amplitude) at 70 °C rapidly extracted polysaccharides with an 11% yield from Sargassum horneri.88
Deep eutectic solvents (DESs) offer a sustainable and cost-effective approach for creating functionalized graphene nano-polymeric sheets directly from seaweed through pyrolysis conditions.89,90 Furthermore, DESs have gained attention for their utility in molecular imprinting (MIP) processes, enabling a selective recognition of seaweed polysaccharides and imprinting on specific sites with matching size, shape, and functional groups. Initially, Li and Row (2017) postulated the potential of hybrid magnetic imprinted polymers (HMIPs) modified by DESs, employing three templates for the swift purification of monosaccharides (fucose, galactose, and mannose) from seaweed.91 Later, Li et al. (2019) prepared modified MIPs using DESs and ILs as packing materials for the solid-phase extraction (SPE) of fucoidan and laminarin. MIPs modified with DESs, specifically choline chloride and urea, exhibited outstanding extraction efficiencies for fucoidan (95.5%) and laminarin (87.6%) from marine kelp.92 Similarly, Li et al. (2022) successfully synthesized MIPs based on 4-vinylbenzyltrimethylammonium chloride (VBTAC) and 4-vinylbenzoic acid (VBA) DESs as dual functional monomers, enabling the specific recognition and extraction of laminarin.92
These discoveries underscore the potential of DES-based extraction methods as eco-friendly and cost-efficient solvent alternatives within green chemistry. Nevertheless, our knowledge regarding their toxicological characteristics and biodegradability remains limited, especially concerning their impact on aquatic invertebrates.93 Thus far, Type III DESs (used in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio) have demonstrated biodegradability ranging from 75% to 91% within a four-week period.94
2 molar ratio) have demonstrated biodegradability ranging from 75% to 91% within a four-week period.94
Recently, Sanches et al., (2023) conducted an extensive ecotoxicological assessment of fifteen DESs, employing various marine and freshwater bioassays. Their findings indicated a general absence of toxicity in all the tested samples. However, it is essential to consider that natural ecosystems are complex, with both living (biotic) and non-living (abiotic) factors interacting. Chemical compounds like ionic liquids (ILs) and deep eutectic solvents (DESs) can exhibit synergistic effects. Therefore, continuous monitoring of their ecological impact remains crucial, especially given the rising predicted environmental concentrations (PECs) resulting from increased industrial use.95
5. Structural characterization methods
5.1 Structural characterization of algal polysaccharides
Structural characterization is performed after the extraction, purification, and isolation of algal polysaccharides from seaweeds. Structural characterization includes monomer composition analysis using chromatographic techniques, functional group identification by FTIR (Fourier transform infrared spectroscopy), and structure elucidation by NMR (nuclear magnetic resonance) spectroscopy. Yaich et al. reported that an extraction temperature below 160 °C caused moisture volatilization and resulted in yield loss during ulvan extraction from Ulva lactuca.96 To analyse for the presence of sulphate content, a turbidimetric assay is performed. This colorimetric analysis combined with other spectroscopic methods (FTIR, Raman scattering, NMR) provides essential information on the structure of extracted algal polysaccharides.97Another critical parameter for structural analysis is molecular weight determination. This analysis is done by size exclusion chromatography (SEC) equipped with a refractive index or UV-detector. SEC can measure the molecular weight of all algal polysaccharides-carrageenans, agar, ulvan, and alginate as well as particle size distribution.98,99 For monomer composition analysis, several chromatographic techniques such as liquid chromatography (LC), high-performance liquid chromatography (HPLC), gas chromatography (GC) equipped with refractive index, and mass spectrometer detection are used.100 GC is used to characterize volatile compounds (such as alditol) in polysaccharides, such as in ulvan.63
Complete structural analysis requires Fourier transform infrared spectroscopy (FTIR) coupled with nuclear magnetic resonance (NMR) spectroscopy. FTIR spectroscopy is performed within the 500–4000 cm−1 absorption band. Some characteristic bands for algal polysaccharides derived from seaweeds are 3200–3400 cm−1 representing O–H vibrations, 2927–2891 cm−1 corresponding to C–H stretching, 1638–1597 cm−1 representing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, a 1247–1257 cm−1 absorption band suggesting S
O stretching, a 1247–1257 cm−1 absorption band suggesting S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond vibration and 1134–1150 cm−1 representing the stretching vibration of C–H bonds.63 A characteristic band for agarose and carrageenan is found at 928–933 cm−1, corresponding to 3,6-anhydro-galactose residues.1011H- and 13C-NMR data provide information on the conformation of the individual components, molar ratios, and content of each component in the carrageenan mixture/blends.177
O bond vibration and 1134–1150 cm−1 representing the stretching vibration of C–H bonds.63 A characteristic band for agarose and carrageenan is found at 928–933 cm−1, corresponding to 3,6-anhydro-galactose residues.1011H- and 13C-NMR data provide information on the conformation of the individual components, molar ratios, and content of each component in the carrageenan mixture/blends.177
Sometimes it is challenging to analyse 1H-NMR spectra due to extensive peaks overlapping, especially for heterogenous polysaccharides. On the other hand, 13C-NMR spectra offer advantages because of the high quantum of information obtained. Anomeric carbons appear in the range of 90–110 ppm. Unlike 1H-NMR spectrum, 13C-NMR spectra show a wide range of displacement values and less overlapping of signals. The composition of the carrageenan blend can be determined quantitatively by the intensity of the anomeric carbon resonances of both monomeric units of carrageenan.178 However, 13C-NMR is a low-sensitivity technique. It requires a high-concentration sample (7–10%). For viscous polysaccharides like agar, and carrageenan, a 13C experiment takes 12–18 h to achieve a rational signal-to-noise ratio.
Currently, some 2D NMR techniques, such as analysis of the seaweed polysaccharides’ structural skeleton, heteronuclear single quantum coherence (HSQC) and heteronuclear multiple bond correlation (HMBC), are effective methods for investigating the structures of monomers and glycosidic linkage types.102
Analysis of the chain structure of polysaccharides includes periodate oxidation, enzymatic hydrolysis, and Smith degradation reactions. A modified methylation process, more straightforward than other reactions, is followed for monosaccharide linkage analysis.103 The first step is the methylation of the polysaccharides; later, dialysis and freeze-drying are performed to recover the product. The next step is hydrolysis by TFA, followed by acetylation after reduction by borohydride to yield partially methylated monosaccharide derivatives (PMAA). Then the derivatives are analysed by GC-MS, and the composition and linkage pattern are analysed by comparing the spectral data with the Carbohydrate Research Centre Spectral Database-PMAA.103,104,179 For example, the α, ı, and pyruvated carrageenan content and position of sulphate esters in the carrageenan from the red alga Sarconema filiforme were determined by linkage analysis by GC-MS.105 Similarly, the nature of the glycosidic linkages and sulphate positions in the extracted sulphated polysaccharides from Ulva pertusa were elucidated by methylation analysis using GC-MS.106
5.2 Bioplastics from algal polysaccharides and structural characterization
Algal polysaccharides have been shown as a prospective renewable biomass source to produce bioplastic to combat the challenges posed by conventional plastic usage. Algae polysaccharides, such as carrageenan, ulvan, starch, and PHAs, have been extensively studied for bioplastic production. To achieve the best bioplastic quality, especially in the medical, food, and pharmaceutical industries, the structural features of the key ingredients, algal polysaccharides, must be customized. Standardizing the growth conditions of the algae, harvesting, environment-friendly extraction procedures, and a complete characterization including structural, physicochemical, thermal, and rheological properties characterization using cutting-edge technologies would be a promising strategy for achieving higher quality bioplastic production and hence reduce the pollution from petroleum-based plastics.5.3 Characterization by molecular techniques and morphology analysis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) starch = 2
starch = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).108 This shifting explains that the –OH groups of starch interact with k-carrageenan ends and form hydrogen bridges. Davoodi et al. compared the FTIR spectra of Ulva intestinalis sulphated polysaccharide films after adding different plasticizers at different concentrations.109 The spectra reveal essential information about the interactions between the polymer and plasticizers, i.e., glycerol as a plasticizer increases the hydrophilicity of the biofilms more than PEG, which can be confirmed by comparing the absorbance of –OH functional groups from the FTIR spectra. Moreover, the absorbance of –OH functional groups increases with increasing concentration of the plasticizers, indicating an increasing number of –OH groups and increasing the water absorption capacity of the polymer blend.
1).108 This shifting explains that the –OH groups of starch interact with k-carrageenan ends and form hydrogen bridges. Davoodi et al. compared the FTIR spectra of Ulva intestinalis sulphated polysaccharide films after adding different plasticizers at different concentrations.109 The spectra reveal essential information about the interactions between the polymer and plasticizers, i.e., glycerol as a plasticizer increases the hydrophilicity of the biofilms more than PEG, which can be confirmed by comparing the absorbance of –OH functional groups from the FTIR spectra. Moreover, the absorbance of –OH functional groups increases with increasing concentration of the plasticizers, indicating an increasing number of –OH groups and increasing the water absorption capacity of the polymer blend.
In a study by Zhang et al., a sodium alginate–pullulan composite was blended with capsaicin, and the FTIR spectroscopy results showed that the addition of capsaicin shifted the stretching vibration of the –OH groups from 3340 cm−1 to 3369 cm−1, meaning that the hydrogen bonds in Na alginate + pullulan + capsaicin are weaker than the bonds present in the Na-alginate + pullulan composite.110 Also, the asymmetric and symmetric stretching vibrations of COO− remained the same in the Na-alginate + pullulan and Na-alginate + pullulan + capsaicin composites. Therefore, capsaicin dispersed in the actual composite (Na-alginate + pullulan) without any chemical interaction. Table 3 describes the band assignments of the algal-based polymers and their composites from several studies.
| Algal-based polymer | Blending material | Band characteristics of blends | Ref. |
|---|---|---|---|
| Carrageenan | PEG | • Galactose-4-sulfate (840–896 cm−1) | 107 |
| • 3,6-Anhydrogalactose unit (900–930 cm−1) | |||
| • OH stretching (3200–3600 cm−1) | |||
| • CH stretching (2800–2900 cm−1) | |||
| Starch | • OH stretching (3100–3500 cm−1) | 108 | |
| • Carbohydrate characteristic region (750–1300 cm−1) | |||
| • C–O stretching band after interaction of carrageenan and starch (1200–1300 cm−1) | |||
| • C–O–H stretching at (1151–1162 cm−1) | |||
| • Ester sulphate groups in carrageenan (1220 cm−1) | |||
| Ca-alginate | • Sulphate stretch of S–O from carrageenan (1228 cm−1) | 131 | |
| • Asymmetric and symmetric vibrations of COO−1 from Ca-alginate at 1416 cm−1 and 1595 cm−1 | |||
| ι/κ/λ at different concentrations | • Stretching (3500–3000 cm−1) | 132 | |
• O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O asymmetric stretching at 1250–1290 cm−1 O asymmetric stretching at 1250–1290 cm−1 |
|||
• S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O symmetric stretching at 1190 cm−1 O symmetric stretching at 1190 cm−1 |
|||
• C–O–C asymmetric stretching 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 501–1160 cm−1 501–1160 cm−1 |
|||
| • C–O–C stretching in 3,6 anhydro-galactose unit 920–930 cm−1 | |||
| • C–O–S stretching in (1–3) D-galactose at 854 cm−1 | |||
| Agar | Chitosan, glycerol | • OH stretching (3000–3500 cm−1) | 116 |
| • Alkane CH groups from agar and glycerol (2900−1) | |||
| • Amide-I and amide-II stretching functional groups from chitosan (1640 cm−1 and 1562 cm−1) | |||
| • Bending of aliphatic C–O (1370 cm−1) | |||
| • Bending of alkane C–C (1150 cm−1) | |||
| Agar | Starch, maltodextrin | • Free, inter-, intramolecular –OH groups at 3000–3700 cm−1 | 133 |
| • C–H stretching regarding CH2 deformation due to increased agar concentration at 2930 cm−1 | |||
| • Coupling of C–O, C–C and O–H stretching vibration and C–O–C glycosidic linkage asymmetric vibration attributes at 1080 and 1150 cm−1, respectively | |||
| Agar | Glycerol | • 3,6-Anhydro-galactose unit at 930 and 1070 cm−1 | 134 |
| • C–H stretching at 2850–3000 cm−1 | |||
| • OH stretching at 3200–3500 cm−1 | |||
| Ulvan | PEG, glycerol | • Presence of OH groups at 3265.1 cm−1 | 109 |
| • C–H stretching vibration at 2933.53 cm−1 | |||
• Stretching of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and COO− groups shows peaks at 1500–1700 cm−1 O and COO− groups shows peaks at 1500–1700 cm−1 |
|||
| Alginate | Gelatine, tea polyphenol | • Stretching vibration of N–H/O–H present in amide shows band at 3301.67 cm−1 | 135 |
| • Band of C–H and -NH2 stretching in amide at 2939.59 cm−1 | |||
• C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration in amide at 1641.68 cm−1 O stretching vibration in amide at 1641.68 cm−1 |
|||
| • Stretching vibration of C–N and bending vibration of N–H present in amide shown at 1547.66 cm−1 | |||
| Alginate | Essential oils | • Asymmetric stretching vibration of COO− at 1600–1602 cm−1 | 122 |
| • Deformation vibration of C–OH at 1403–1415 cm−1 | |||
| • Stretching vibration of C–O present in uronic acid at 929 cm−1 | |||
| • Presence of α-L-guluronic acid at 869 cm−1 | |||
| • Presence of mannuronic acid residue at 815 cm−1 | |||
| Alginate | Carboxymethyl cellulose, glycerol, collagen powder | The following bands were common for the films having blended materials at different concentrations: | 136 |
| • OH, stretching peak at 3295 cm−1 | |||
| • Asymmetric and symmetric vibrations of COO−1 at 1619 and 1420 cm−1 | |||
| • Presence of 3,6-glycosidic bridge at 1400 cm−1 | |||
| • C-4 absorption peak at 1070 cm−1 and COC absorption peak at 900 cm−1 confirm the presence of galactose |
Investigation of the feasibility of nanoparticle addition into algal polysaccharides to improve bio-nanocomposite film properties can be performed by SEM. For example, the addition of ZnO/CuO in carrageenan-based nanocomposites caused small pits on the surface.112 This may trap air into the surface and make it hydrophobic. On the other hand, the addition of SiO2 nanoparticles in carrageenan-based biofilms made the surface smoother.113 Therefore, the distribution of suspension of nanoparticles within the matrix, interactions between the added components (nanoparticles/plasticizers) with the algal-based polymers, and properties like hydrophilicity, transparency/opacity can be investigated by SEM.
5.4 Mechanical properties
Mechanical properties are one of the most important indicators of the core performance of algae-based bioplastics. Physical properties frequently measured are tensile strength, elongation at break and Young's modulus to assess the stress/compression applied on a thin film and study the deformation mechanism.However, some studies reported increased concentrations of plasticizing agents to decrease TS and EAB. Fransiska et al. reported that the TS and EAB were reduced by 10% with the increasing concentration of chitosan in agar bioplastic.116
In alginate-based bioplastic, castor oil's incorporation initially increased the alginate's tensile strength due to the formation of hydrogen bonds and electrostatic attraction between the castor oil and alginate. However, further increasing the concentration (beyond 1%) decreased the TS value due to the ionic interaction of the castor oil with the Ca2+ ions present in the alginate and the formation of the crosslinked network.117
5.5 Physical properties
Optical property is also a function of the property and concentration of algal polymers and blending agents. The colour of films is measured by a colorimeter using the parameters L* (between black to white), a* (between red to green), and b* (between yellow to blue). L* indicates brightness. According to Mahcene et al., a biofilm made of Na-alginate blended with essential oils showed a higher value of L* than only Na-alginate biofilm, meaning that the colour of the biofilm is transparent.122 This report indicates that the homogenization and proper incorporation of blending materials into the polymer matrix affect the optical properties, which can be confirmed by SEM analysis.
5.6 Barrier properties characterization
Barrier properties refer to the material's ability to resist the absorption of light, moisture, and oxygen. These properties come from the accumulation of the individual films used in the construction of the packaging. Barrier properties – water and oxygen permeability – are significant if the material's intended use is packaging.| Bioplastic film samples | Water solubility (%) | Oxygen permeability (×10−4 cm3 mm m−2 d−1 kPa−1) | Water vapour permeability (×10−10 g H2O per m s per Pa) | Light transmission, T600, % | Opacity (UA per mm) | Ref. |
|---|---|---|---|---|---|---|
| Semirefined K-carrageenan (SRC) | 42.8 | 1.82 | 3.26 | — | 4.82 | 137 |
| SRC with 20% glycerol | 44.4 | 1.84 | 3.30 | — | 4.47 | 137 |
| SRC with 20% sorbitol | 42.6 | 0.89 | 3.26 | — | 3.83 | 137 |
| Alginate with chitosan | 89.53 | 5.53 | 6.1 | 21.45 | 6.28 | 138 |
| Alginate with chitosan and fucoidan | 76.15 | 8.56 | 9.22 | 12.05 | 6.44 | 138 |
| Alginate with chitosan fucoidan and calcium ions138 | 68.65 | 8.18 | 8.92 | 12.50 | 6.65 | 138 |
| Ulvan with 1% glycerol | 97.36 | — | 2.41 | 7.05 | 0.12 | 114 |
| Ulvan with 1% sorbitol | 76.81 | — | 1.54 | 6.14 | 0.13 | 114 |
5.7 Thermal properties
6. Alginate-based bioplastics
Alginate, being the most abundant marine polysaccharide, with its characteristic gelling properties is evidenced to be a likely ingredient for synthetically modified biomaterials. A combination of alginate with other natural and synthetic polymers can be looked upon to alter the structure and properties of the resulting alginate-based bioplastic. Zia et al. have elaborated the use of polysaccharides in numerous applications due to their unique characteristics, such as biodegradability, structural diversity, biocompatibility, non-toxicity, abundance, and specific bioactive properties. Polyurethanes/alginate hydrogels, elastomers, and nanocomposites systems showing uniqueness in their properties are proving alginates to be effective polymers worthy of further research and development.139Sodium alginate is one such edible polysaccharide that could form films with the following properties: flexibility, gloss, water solubility, microbial resistance, low permeability to oxygen and vapours, and tasteless or odourless. Sodium alginate is an effective alternative for packaging in the food industry and also finds usage in medicine and pharmaceutics. Increased usage of plastics is causing an environmental problem in the form of waste disposal behaviour. Laboratory research has confirmed that one solution to this is using edible bio-based polymer packaging materials.140 Gheorghita et al. (2019) have recommended that further research can be carried out in food packaging films or foils to improvise certain features, such as maintaining or increasing the shelf life of the packed food products.140
Alginate is a phycocolloid extracted from brown algae. It consists of two 1,4-linked uronic acids: α-L-guluronic acid and β-D-mannuronic acid. The water-insoluble property is one of the important characteristics of alginates, which occurs through the formation of water-insoluble gels in the presence of cations such as calcium ions (Ca2+). This is attributed to the strong cross-linking interaction between the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of guluronic acid with Ca2+.141 Jost and Reinelt have discussed about the crosslinking of alginates done in two ways: by diffusion and by internal setting. In the diffusion method, the cast alginate films are immersed in a solution of polyvalent ions. The polyvalent ions diffuse into the alginate matrix and initiate crosslinking. In the internal method, the polyvalent ions are combined in the alginate matrix formulation in an inactive form. The crosslinking is then initiated by a release mechanism brought about by a pH shift before casting the films.142
O groups of guluronic acid with Ca2+.141 Jost and Reinelt have discussed about the crosslinking of alginates done in two ways: by diffusion and by internal setting. In the diffusion method, the cast alginate films are immersed in a solution of polyvalent ions. The polyvalent ions diffuse into the alginate matrix and initiate crosslinking. In the internal method, the polyvalent ions are combined in the alginate matrix formulation in an inactive form. The crosslinking is then initiated by a release mechanism brought about by a pH shift before casting the films.142
6.1 Plasticization
Plasticization is defined as the softening and thereby improvement of the flexibility of a polymer by the addition of a plasticizer. Alginate films have great potential for food packaging use but exhibit highly moisture-sensitive, absorbing characteristics. Alginate is commonly extracted from species of brown algae; however, Lim et al. conducted a study on the extraction of alginate from Sargassum siliquosum J. Agardh. Extraction processes with and without formaldehyde pre-treatment were carried out. The casting of the polymer solution was done to produce the cast film. The films cast were subjected to calcium chloride immersion for 1 minute. The extraction yield was higher in the case of formaldehyde pre-treatment (33.75 ± 1.21%) than without formaldehyde pre-treatment (26.91 ± 1.59%). A statistical model was developed to determine the bioplastic film composition as 2 g of extracted alginate, 15% sorbitol, followed by 75% calcium chloride immersion treatment. This model predicted the optimised properties of the bioplastic film as: 33.9 MPa tensile strength, 3.58% elongation at break, 2.63 × 10−10 g Pa−1 s−1 m−1 water vapour permeability, and 33.73% water solubility.141Plasticizers such as diethyl phthalate, triacetin, tributyl citrate, or triethyl citrate can reduce the alginate matrix's water permeability and susceptibility to moisture in environmental applications. Thin films plasticized with glycerol were compared with films plasticized with tributyl citrate (TC). Also, thin films plasticized with varying proportions of glycerol and tributyl citrate were studied.143 When Paixão et al. performed the moisture absorption tests, the films plasticized with pure TC were found to be less water soluble and performed well in the protection against dissolution. In contrast, the films plasticized with glycerol exhibited a high degree of dissolution. The tensile strength of the films with pure glycerol and pure TC were 103.1 ± 19.3 MPa and 146.8 ± 20.0 MPa, respectively. This increase in tensile strength could result from a greater interaction between the chains in the form of secondary bonds. The tensile strength, however, decreased as the glycerol proportion increased. The elongation at break for the films plasticized with pure TC and pure glycerol was 3.3 ± 1.6% and 6.05 ± 2.4%, respectively.143
Crosslinking alginate can be done by two methods: internal method and diffusion. Jost and Reinelt chose the internal method of crosslinking alginate for the preparation of alginate films with the help of crosslinking agents: CaCl2 + Na2-ethylene diamine tetra-acetic acid (EDTA) complex, calcium hydrogen phosphate (CaHPO4) and calcium carbonate (CaCO3) being used in the formulation of alginate solution. Glucono δ-lactone (GDL) was used to lower the system's pH and thereby initiate a controlled release of Ca2+.142
The process of film formation is found to influence the film performance due to the relaxation of the polymeric chains during the process. Yeom and Lee developed membranes of sodium alginate crosslinked with glutaraldehyde (GA) using the solution method, in which acetone (non-solvent of sodium alginate) is used. It is found that with a GA content above 5 vol%, stable membranes are obtained by this method. Crosslinking helps reduce both the solubility in water and the stability of the membrane toward water. The crosslinked membrane is validated for excellent performance during the pervaporation separation of ethanol–water mixtures containing 70–90 wt% ethanol content at temperatures between 40 to 80 °C.144
6.2 Composites of alginate
Alginate films when incorporated with certain materials, that is, either polymers or non-polymers, can exhibit enhanced properties. This enhancement in properties arises due to the formation of chemical and physical bonds between the different constituents in the composite. Hu et al. incorporated silver nanoparticles into alginate/polycaprolactone composite fibres which have been shown to have applications in wound dressings. Silver nanoparticles inhibited the growth of microorganisms, including Gram-positive and Gram-negative bacteria. Calcium chloride is also used to incorporate crosslinking in the composite fibres; in addition, calcium ions when released into the wound help to accelerate coagulation. Wound healing studies confirmed that composite fibres accelerate wound closure and assist in the formation of collagen.145Extensive research is attempted on polyurethanes to make them a green and sustainable material. Polyurethanes are conventionally produced by polymerizing polyols with poly-isocyanates under controlled reaction conditions. Polyols provide heat resistance, spatial stability, and physical cross-linking properties, while poly-isocyanates provide elasticity in polyurethane chains. Fossil derivatives are mainly used as polyurethane raw materials.146 To replace the fossil-based derivatives, renewable resources such as natural oils and cellulose are being looked upon as a substitute to obtain bio-based polyurethanes. Almost all vegetable oils, except castor oil, must be chemically modified to introduce hydroxyl groups that need to be present in polyols to synthesize polyurethanes. Additives such as graphene oxide are incorporated to introduce secondary polymer chains in the polyurethane matrix, thus improving the properties of the resulting polymer composite. When added to bio-based polyurethane dispersions, castor oil incorporates the waterborne property of the prepared polyurethane-based films. 2,2-Dimethylolbutanoic acid is used as a chain extender. The prepared composite films from polyurethane and sodium alginate showed an increase in tensile strength and storage moduli with increased sodium alginate content, whereas a decrease in elongation at break occurred with increased sodium alginate content. Wang et al. attributed the property enhancements to the increased crosslinking and the development of interpenetrating networks with sodium alginate molecules.146
The properties of fibre-reinforced composites can be improved by subjecting the fibres to surface treatment. Khan et al. subjected calcium alginate fibres to silane treatment by exposure to vinyl triethoxy silane. Reductions in the fibers’ hydrophilic nature and improvements in the mechanical and interfacial properties of the fibre-reinforced composites are achieved by silane treatment of the fibres. These silane-treated and untreated calcium alginate fibres are reinforced in a polypropylene (PP) matrix by compression moulding. The mechanical properties of the silane-treated fibre-reinforced composites are improved relative to the untreated fibre-reinforced composites. Degradation tests of the composites in soil confirmed that the silane-treated fibre-reinforced composites retained 84% of their original strength. The interfacial shear strength of the silane-treated fibre-reinforced composites is twice that of the untreated fibre-reinforced composites, confirming enhanced compatibility between the silane-treated calcium alginate fibre and the PP matrix.147
When a polymer matrix is reinforced with fibres, the mechanical properties of the resulting composite are found to be enhanced. The strong hydrophilic nature of calcium alginate fibres can be overcome by reinforcing them in a polypropylene matrix, thereby developing calcium alginate fibre-reinforced polypropylene composites. Khan et al. reported that the mechanical properties of the composites possess greater improvement than the matrix polypropylene. From the degradation tests, it is found that the composites lose 25% of their mechanical properties, thus confirming their biodegradability.148
The flame-retardancy characteristic of alginate is explored and studied. When coated on polyester fabric, a flame-retardant system consisting of phosphorus, nitrogen, silicon, and calcium alginate exhibits flame retardancy and anti-dripping properties. The coated fabric surface produced a thick intumescent char layer upon burning. Wang et al. found that the flame-retardant system displayed a good smoke suppression efficacy, which is also confirmed by thermogravimetric analysis and cone calorimetry testing. The limiting oxygen index value improved from 19.2 ± 0.2% to 32.5 ± 0.3% during the combustion of the coated polyester fabric.149
A polymer matrix composite incorporated with naturally sourced flame retardant materials is tested for its flame retardancy performance. Pallmann et al. developed an intumescent flame retardant (IFR) material from polypropylene by integrating it with naturally sourced flame retardants such as phosphorylated sodium alginate, dipentaerythritol, and ammonium polyphosphate. These flame retardants are incorporated into polypropylene by the method of melt blending. The mechanisms of the gas phase and condensed phase synergistic flame-retardant ability during combustion and degradation of the flame-retardant polypropylene make this system an effective IFR. This IFR system improves thermal stability by significantly slowing thermal degradation. This IFR system will find application in the automotive industry and the structure of electronic devices.150
Biocompatible scaffolds were designed using alginate and synthetic polymers, developed through 3D printing and tested for their biocompatibility. Kim et al. developed PCL/alginate fibrous scaffolds through a combination of electrospinning and rapid prototyping method developments. The improved hydrophilic and water absorption properties were attributed to the alginate fibres, while the enhanced mechanical properties in the developed scaffolds were attributed to the polycaprolactone. Even a small alginate content (5%) contributed to the outstanding performance of these scaffolds in terms of cell viability and proliferation. It has been found that the developed PCL/alginate fibrous composite scaffold could be appropriate for the rejuvenation of various hard tissues.151
The antimicrobial property was incorporated to polymer fabrics by the addition of alginate or other materials, and the mechanism/performance of antimicrobial property was explored. Marković et al. prepared polypropylene fabrics to be antimicrobial by incorporating alginate biopolymer and nanoparticles of copper oxides. Alginate is bound to the surface of polypropylene fabric by corona discharge, after which the carboxyl groups of alginates are used to bind with Cu2+ ions by immersing in copper(II) sulphate solution. The developed composite fabric developed outstanding antimicrobial resistance to Gram-negative bacteria E. coli, Gram-positive bacteria S. aureus and yeast C. albicans. The composite fabric also proved not to be cytotoxic to human keratinocyte cells.152
The change in morphology of the composite fibre is found to be dependent on the composition of alginate in the spinning solution. Lee and Lyoo prepared an organic–metallic nanocomposite consisting of PVA, SA and silver nanoparticles by directly mixing and electrospinning the nanocomposite into fibres. When PVA with the degree of polymerization of 1700 is kept fixed, and by increasing the content of SA in a spinning solution, it is found that the obtained morphology transformed from fine uniform fibre to beaded fibre. It was shown that the intermolecular interaction by the hydrogen bonding of PVA with SA increased the spinnability, forming a uniform and continuous nanocomposite fibre. Micrographs confirmed the presence of silver nanoparticles in spherical form with a diameter ∼50 ± 20 nm and were present with uniform dispersion in the PVA/SA matrix.153
6.3 Blends of alginate
Blends of alginate are obtained by physically mixing two or more different polymers together; they may be miscible or immiscible with each other. No chemical bonds are formed between the individual constituents of the blend.The wound healing property of alginate is explored by blending it with gelatin. Alginate fibres, when used in wound dressings, exhibit two properties: an in situ moist healing environment and the consequent high absorbency of the wound dressings. Gelatin can also be blended with alginate to make the wound dressing economical. Fan et al. prepared gelatin blend fibres in combination with alginate and tested for their performance compared with pure alginate fibres. The blended fibres were named following their gelatin content of 10, 30, 50 and 70 wt% as AG10, AG30, AG50 and AG70, respectively. Pure alginate fibres and gelatin fibres were named AL and G, respectively. The crystallinity of the blended fibres was in the order of G > AG70 > AG50 > AG30 > AG10 > AL, implying that the crystallinity decreased with an increase in alginate content in the blend fibres. This also signifies good miscibility between alginate and gelatin. The thermal stability was also of the order G > AG70 > AG50 > AG30 > AG10 > AL, suggesting that the intermolecular interaction improved the thermal stability of the blended fibres. When the blend's gelatin content is 30 wt%, the blended fibre exhibited a dry tensile strength of 12.31 cN/tex and an elongation of 21.3%. The water retention property increased as the amount of gelatin was increased in the alginate/gelatin blended fibre. This increase in water retention property is due to gelatin's hydrophilic nature compared with calcium alginate.154
Alginate nanofibres are found to be spinnable by blending with selective polymer materials. Safi et al. demonstrated the spinnability of sodium alginate, sodium alginate/polyvinyl alcohol, and sodium alginate/polyethylene oxide. Sodium alginate is easily soluble in water, so its aqueous solution is not spinnable into ultrafine nanofibers. To make the sodium alginate electro-spinnable, polymer additives like polyvinyl alcohol (PVA) and polyethylene oxide (PEO) solutions are mixed with sodium alginate aqueous solution. SEM analysis showed the electro-spun nanofibers of a sodium alginate/PVA system of volume ratio 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 and a sodium alginate/PEO system of volume ratio 50
30 and a sodium alginate/PEO system of volume ratio 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 having diameters of 118.3 nm and 99.1 nm, respectively. The rheological studies confirm the dependence of spinnability and fibre morphology on solution viscosity or the sodium alginate to polymer additive blend ratio. Hydrogen bond interactions between the hydroxyl groups of PVA (or ether oxygen of PEO) and the hydroxyl groups of sodium alginate were confirmed from the FTIR analysis.155
50 having diameters of 118.3 nm and 99.1 nm, respectively. The rheological studies confirm the dependence of spinnability and fibre morphology on solution viscosity or the sodium alginate to polymer additive blend ratio. Hydrogen bond interactions between the hydroxyl groups of PVA (or ether oxygen of PEO) and the hydroxyl groups of sodium alginate were confirmed from the FTIR analysis.155
Films of alginate which are water insoluble in nature are designed and investigated for their performance in food packaging. Gohil investigated the nature of films made of pectin and sodium alginate blends for their water vapor permeation rate and mechanical properties. A water insolubility property is incorporated into films by subjecting the cast films to CaCl2 treatment, by which physical crosslinking between the molecules of both polymers occurs. Comparing the change in mechanical properties in cast films before and after CalCl2 treatment, it is found that the molecular mobility reduced and the molecular network structure changed, causing a drastic change. It is also observed that an increase in pectin content in the blends showed a rise in film flexibility. Attempts were made to improve water barrier properties by coating a thin layer of an edible beeswax-based formulation. Such bilayer films exhibited a better water vapor permeation rate than non-edible Cellophane films.156
A blend membrane used for the selective separation of solution mixtures is designed and tested. Yeom and Lee developed a blend membrane based on sodium alginate (SA), a rigid polymer, and polyvinyl alcohol (PVA), a flexible polymer, for the pervaporation separation of ethanol–water mixtures. During pervaporation, the flexible PVA component helps maintain the consistency of membrane performance, while the rigid SA component exhibited an increased separation factor by the relaxation of polymeric chains.157
Numerous patents on alginate blends have been reported especially in the fields of wound healing, drug encapsulation, extrusion and 3D printing. A polymer blend consisting of a combination of lichenan, alginate and solvents of polymers has been developed for the cosmetics, food packaging, or wound dressings in the patent WO 2006/125857 A1.158 A chitosan–alginate scaffold system has been designed and fabricated for use in anticancer therapeutic drug development in the patent US 9,157,908 B2.159 A biocompatible polymer blend consisting of alginate had been developed for use as wound dressings and drug encapsulation; the manufacturing methods of such polymers in order to control the mechanical properties have also been disclosed in the patent US 2010/0254900 A1.160 A highly flowable biodegradable polymer combination has been formulated that can be processed by extrusion and injection moulding and has been designed in the patent 5,523,293.161 An alginate-based material had been dispensed into a three-dimensional object by layer manufacturing in the patent US 2005/0087902 A1.162
Flame-retardant alginate blend fibres are developed for their use as filling materials in toys. Commonly used filling materials are polyester fibres, yet they show melt-dripping and flammable characteristics. To produce flame-retardant fibres, Li et al. combined flame-retardant alginate fibres with polyester fibres. When tested in the vertical flame test, the blend containing 20 wt% alginate fibres exhibited fire extinguishment without any melt-dripping. In the cone calorimetry test, the mixture with 50 wt% alginate displayed a notable decrease in smoke and heat release compared with the blend containing 20 wt% alginate fibres. When these blended fibres are exposed to heating, it is found that the alginate fibres decompose prematurely and then delay the weight loss of the polyester fibres. These blended fibres are also found to exhibit good flame-retardant properties in the vapor and condensed phases. These blend fibres show potential applications in filling materials in furniture, toys, and clothing.163 The various forms and applications of alginate-based systems are compiled in Table 5.
| Bioplastic combination | Application | Properties investigated | Ref. |
|---|---|---|---|
| Alginate extracted from Sargassum siliquosum J. Agardh (cross-linked with Ca2+ during the extraction process) | Thin film – packaging material | Mechanical properties, water vapour permeability, water solubility | 141 |
| Alginate cross-linked with EDTA + CaCl2, CaHPO4, and CaCO3 | Thin film – packaging material | Crosslinking reactivity, transparency, morphology, mechanical, oxygen transmission, water vapor transmission properties | 142 |
| Alginate plasticized with tributyl citrate | Thin film – food packaging application | Water vapour permeability, mechanical properties, opacity | 143 |
| Sodium alginate cross-linked with glutaraldehyde | Membrane – used for pervaporation | Pervaporation process, swelling properties | 144 |
| Alginate and polycaprolactone | Fibres – as multifunctional wound dressings | Thermal properties, wound healing, and blood clotting studies | 145 |
| Castor-oil based waterborne polyurethane with sodium alginate | Waterborne films – coatings, wound dressing, drug delivery | Thermal, mechanical, water resistance properties | 146 |
| Calcium alginate fibres reinforced in polypropylene matrix | Natural fibre-reinforced composites | Mechanical, interfacial and degradation (in soil) properties | 147 |
| Calcium alginate fibres and polypropylene | Fibre-reinforced composite – automobiles, buildings | Mechanical properties, single-fibre fragmentation test | 148 |
| Alginate coated on polyester fabric which is then soaked in 10% CaCl2 solution for 30 minutes | Flame retardant coating on polyester fabric | Morphology, element distribution, flammability, thermal, combustion properties | 149 |
| Polypropylene, phosphorylated sodium alginate, dipentaerythritol and ammonium polyphosphate | Flame retardant sheets – automotive industry and electronic devices | Chemical structures, thermal properties, and degradation mechanisms | 150 |
| Polycaprolactone, alginate fibres | Fibre-reinforced composite scaffolds – tissue engineering | Morphology, hydrophilic, mechanical, cell viability and proliferation properties | 151 |
| Polypropylene fabricate, alginate and nanoparticles of copper oxide | Antimicrobial polypropylene nonwoven fabric – healthcare products | Morphology, wettability, antimicrobial, cytotoxic properties | 152 |
| Alginate-gelatine blend cross-linked with Ca2+ | Fibres – as wound dressings | Morphology, crystallinity, thermal, mechanical and water retention properties | 154 |
| Sodium alginate/PVA blend and sodium alginate/PEO blend | Ultrafine nanofibers – biomedical applications | Spinnability, SEM morphology, FTIR | 155 |
| Polyvinyl alcohol, sodium alginate and silver nanoparticles | Nanocomposite fibres – wound dressings, optical information storage | Morphology, chemical composition | 153 |
| Pectin, sodium alginate and blends of both | Food packaging films | Mechanical properties, water barrier properties | 156 |
| Blend of sodium alginate and polyvinyl alcohol | Membrane – used for pervaporation | Pervaporation process, swelling properties | 157 |
| Blend of alginate and polyester | Fibres – as filling materials in furniture, toys, and clothing | Thermal, flammability properties | 163 |
| Polyvinyl alcohol, chitosan, and sodium alginate | Composite membranes – for shape memory applications | Physical, mechanical, shape memory properties | 164 |
6.4 Drawbacks of alginate-based bioplastics
The efficiency of the alginate extraction process can vary based on the pre-treatment methods applied. For instance, the use of formaldehyde pre-treatment in the extraction process has been shown to increase the extraction yield, but the overall environmental and health impacts of such pre-treatments need to be considered. Alginate-based films may have limitations in terms of mechanical strength. The tensile strength and elongation properties of alginate films are crucial for their performance in various applications, and efforts are needed to optimize these properties for specific uses. Alginate films tend to absorb moisture, and the plasticization process, involving the use of plasticizers like glycerol, can impact the water permeability and susceptibility to moisture. Balancing plasticization for flexibility without compromising moisture resistance is a challenge. Alginate-based composites, such as those blended with polyurethanes or other polymers, may face challenges related to shelf life, especially if the degradation rates of individual components differ significantly. This can impact the overall stability and performance of the composite over time. The crosslinking of alginate, whether through diffusion or internal setting, depends on external factors like polyvalent ions. Controlling these factors during processing is crucial for achieving the desired properties, and variations can affect the final product's characteristics. While alginate has been explored for flame retardancy, achieving optimal performance without compromising other properties can be challenging. The balance between flame retardancy and mechanical strength needs careful consideration.7. Carrageenan-based bioplastics
Combinations of carrageenan with other natural and synthetic polymers are obtained as carrageenan-based bioplastics. These bioplastics are found to have a wide range of applications ranging from food and pharmaceuticals, to therapeutic drugs. Carrageenan is usually derived from a class of red seaweed (Rhodophycae). It is a safe and natural polysaccharide approved to be used as a food additive. Applications of carrageenan in the food and pharmaceutical industries are well reported.116 Applications include viscosity modifiers, stabilizing agents, and emulsifiers. Carrageenans are of three types (λ, ί and κ) depending on the 3,6-anhydro-α-D-galactopyranosyl and the sulphate ester content.165 The three types of carrageenan have different gelling, solubility, and mechanical properties. They also differ in the ions and the ion-holding positions. K-Carrageenan has more tendency to have monovalent ions like (K+), but iota-carrageenan tends to have divalent ions (e.g., Ca2+).166–168 It is a biologically inert substance with good biocompatibility and is compostable.169,170 The use of carrageenan is well-explored in the pharmaceutical industry as a binder matrix in preparing tablets. It is used as a binder in the extrusion of active ingredients. The gelling efficiency of carrageenan helps to manipulate the viscosity and rheology of therapeutic drugs and hence makes it an ideal candidate for controlled drug-release systems.171Film formability and fibre formability have been explored with carrageenan-based formulations. Many researchers have prepared carrageenan-based films and have conducted detailed characterisations and property evaluations.166,172–175 The K-carrageenan has better film-forming properties than the λ and ί forms, with comparatively better moisture barrier and mechanical properties. K-Carrageenan films have shown moisture permeability of 1.87 × 10−10 ng m m−2 s−1 Pa−1 and tensile strength of 22–32 MPa.170,176 Efforts have also been made to make pure carrageenan fibres.177
Some carrageenan films needed modifications to achieve mechanical and water-vapor barrier properties for commercial applications.169 Later studies have focussed on chemical modifications and blending with other materials to enhance carrageenan's desirable properties. The major routes of property enhancements are achieved by (a) plasticization, (b) composite making and (c) blending with other materials.
7.1 Plasticization
Carrageenan films when mixed with plasticizers such as glycerol, sorbitol and polyethylene glycol have proved to have improved mechanical and water resistance properties. Unmodified carrageenan film has been shown to exhibit good tensile strength with a tensile strength value of close to 70 MPa, but the films are very brittle. Low molecular weight additives are added to improve the intermolecular slippage and motion within the matrix. This will improve the ductility and elongation of the products. The plasticizer's impact on improving the mechanical properties depends on its compatibility with the matrix, the dosage, and the chemical structure. The type and optimum dosage of the additives are critical to improving the desired properties of the carrageenan film.178,179 Property variations with plasticizers such as glycerol, sorbitol, and polyethylene glycol-300 in the range up to sixty percent were studied. The thickness and moisture content of the films increased significantly with the plasticizer concentration. Films incorporating sorbitol showed the least moisture absorption but had better tensile strength. In an interesting study, the red seaweed Kappaphycus alvarezii was plasticized with polyethylene glycol and sheets were prepared.179Carrageenan plasticized with glycerol was evaluated for its performance in comparison with alginate plasticized with glycerol. Paula et al. investigated the mechanical and physical properties of carrageenan/glycerol blends as edible film. Kappa-carrageenan, iota-carrageenan, and alginate were examined as probable candidates. It was demonstrated that κ-carrageenan showed better mechanical properties and water permeability than ι-carrageenan and alginate-based films. Optical clarity was good with alginate-based films compared with both carrageenan types.172,173
7.2 Composites of carrageenan
Whole seaweed when reinforced with nanoparticles was investigated for its antibacterial property in comparison with commercial carrageenan reinforced with such nanoparticles. Sudhakar et al. have proposed a process where the whole seaweed is used to make nanocomposites with zinc oxide, copper oxide, and silicon dioxide nanoparticles. These composites were then compared with the respective nanocomposites made from commercial carrageenan. It was observed that the antibacterial activity was good for all the nanocomposites under investigation. It could be inferred that the mixtures based on whole seaweed performed better than those made from commercial carrageenan alone.180Modification of the water absorption property of carrageenan films was possible by reinforcing certain materials in the right quantity. The water absorption characteristics of carrageenan are a problem when used for packaging applications. Modifying carrageenan with water-repellent groups and additives can help improve the hydrophobicity.172,174 Nano-clay-incorporated semi-refined carrageenan composites were prepared and then laminated with a polycaprolactone sheet. This combination was shown to give desirable moisture resistance and mechanical properties.181
The mechanism of water-repellent properties in modified carrageenan composites was studied. Dogaru et al. studied nanocomposite formulations with various concentrations of κ-carrageenan and bentonite nano-clay. The morphological and structural features, interactions, and sorption properties of the nano-composites were evaluated. It is believed that physical bonding was enabled between the carrageenan and nano-clay by hydrogen bonding. This impairs the chances of moisture adsorption into the composite.169
Incorporating microbial resistance in carrageenan films for its use in food packaging has been attempted and reported. Composites based on carrageenan have been investigated as potential candidates as wrappers in the food industry.165,182 They are of great interest if microbial protection can be further improved by appropriate blending and composite preparation. This can be achieved by blending antimicrobial or oxidation retarders into the carrageenan films. This will deter bacterial growth, discolouration caused by enzymes, nutrition loss, and oxidation on the carrageenan films. These measures will in turn help to improve the shelf life of the food that is wrapped with these films. In one experiment, grapefruit seed extracts are considered an antibacterial additive. The extract is added to carrageenan solutions at different concentrations and film is cast. Mechanical, antibacterial, and physical parameters characterize the films. It has been shown that these composite films do exhibit good antimicrobial activity against the common microorganisms that affect packaged food.165
Carrageenan composites with antimicrobial and biocompatibility are designed to be used as wound dressing materials. K-Carrageenan blended with potassium sorbate has been shown to have good antimicrobial activity. Hence it can improve the longevity of the products packed in it and makes them safer for consumption.183 Incorporating halloysite–silver nanoparticles into the carrageenan matrix has improved the system's mechanical strength and UV barrier properties. The films have good antimicrobial properties as well. It was observed that sodium dodecyl sulphate helps to improve the incorporation of the silver nanoparticles in the carrageenan films. The better dispersion of nano-particles leads to good mechanical properties, optical clarity, and antibacterial activity of the above film.184 Innovative bioactive composite scaffolds from k-carrageenan blended with silk fibroin have been developed by Nourmohammadi et al. This composite can be used in bone regeneration applications.185 Carrageenan composites with cellulose have also been studied in detail. These show good bio-compatibility, moisture retention capacity, and mechanical strength to be considered as wound dressing materials.3,186
7.3 Blends of carrageenan
Blending biopolymers with other polymer matrices could be used to tailor the desired properties for specific applications. Carrageenan was mixed with cassava starch in an extruder to form a bioplastic with better mechanical properties. Carrageenan in this starch bioplastic positively influences the tensile strength and modulus. The mechanical properties also increased as the carrageenan percentage in the blend increased.176 Moustafa et al. studied K-carrageenan and potato starch blends for making hydrogels. This blend is shown to have good hydrogel properties, especially the ability to absorb water. Hence, this could be a non-toxic, biodegradable, and safe material for manufacturing toys.187 K-Carrageenan can absorb extracellular fluids and could be applied in wound dressing hydrogels. To improve the degradation of carrageenan, it can be blended with a synthetic polymer such as polyvinyl alcohol, polyvinyl acetate, polyvinyl pyrrolidone, and polyethylene oxide. This will help improve the mechanical properties, water absorption, and shelf life.4,188 Wound dressing formulations based on carrageenan and carboxy methyl cellulose have been made from hydrogels prepared by blending them with polyethylene oxide. This wound dressing has shown the capability to clot blood and adhere to platelets. This dressing system has shown a similar bleeding time compared with the commercially available dressing made from chitosan. In addition, they have demonstrated reduced inflammations and observed lesser depositions of grain-like structures on the dressing.4 An interesting work is the preparation of carrageenan fibres by a wet spinning technique. In this method, carrageenan reacts with epoxy chloropropane for improved properties.177 Boateng et al. prepared solvent-cast wound dressings formulations by blending polyethylene oxide and carrageenan. In one experiment, the dressing system had streptomycin incorporated in this film. Another experiment contained diclofenac similarly. The dressing can help to reduce bacterial infection by the antimicrobial action of streptomycin and further improve the efficacy with diclofenac. As an anti-inflammatory, diclofenac will help alleviate the pain and reduce the local swelling around the wound.189Carrageenan/polyvinyl alcohol composite was found to exhibit enhanced elongation when reinforced with bio-polyol. Carrageenan/polyvinyl alcohol-based blends have many applications.190,191 A flexible carrageenan–polyvinyl alcohol film was uniquely prepared using a bio-polyol called liquefied banana pseudo-stem as a plasticizer and common cations (Ca2+ or K+) as cross-linkers. The elongation of the composite film is significantly increased by up to 440% when adding three percent bio-polyol. This renewable and degradable organic–inorganic hybrid film has the potential for packaging applications.192
To make films, blended films can be prepared from K-carrageenan, PVA and red pitaya flesh extract (RPFE). RPFE is found to be rich in betacyanins and is known to be sensitive to pH variations. It is an effective antimicrobial agent and antioxidant. These features can make it a good additive in packaging film for the food industry. The optical properties, barrier properties against light, water retention, and mechanical properties were assessed and reported. The films have shown good sensitivity to pH and ammonia.193
Improving the electrical conductivity of carrageenan is attempted by reinforcing it with certain materials to find their use in energy storage applications. Polysaccharides like carrageenan have good compatibility with salts and hence can be candidates in developing electrically conductive materials. Carrageenan is abundant with functional groups like –OH (hydroxyl). This can facilitate carrageenan to form networks with various compounds and form polyelectrolytes.174,194,195 The conductivity of these films has been reported (10−5 to 10−3 S cm−1). Iota-carrageenan matrix has been complexed with poly(ortho-phenylenediamine-co-aniline). These composites can have potential applications as sensors, capacitors, and other energy storage devices.191 Biopolymer blends based on k-carrageenan and carboxymethyl cellulose derived from kenaf fibres were shown to have a conductivity of 3.25 × 10−4 S cm−1. This composite can form a polyelectrolyte and could also be evaluated for energy storage devices.196
The spinnability of carrageenan into fibres has been explored. An interesting work is the preparation of carrageenan fibres by the wet-spinning technique. The carrageenan fibres were reacted with epoxy chloropropane for improved properties. This helped enhance the carrageenan fibers’ mechanical properties.177 A biopolymer composite film containing kappa-carrageenan, PVP (polyvinyl pyrrolidone), and polyethylene glycol (PEG) was formulated. These films were compared with the ones mixed with silver nanoparticles. The silver nanoparticles make the films moisture-resistant and give better tensile strength.197 The electrical and mechanical properties of a film prepared by the evaporative casting of a carrageenan/carbon nanotube/glycerine system have been evaluated. This film has been tested as a gas sensor and has shown some positive indications of its application.168Table 6 gives a concise picture of various carrageenan application sectors, especially bioplastics.
| Bioplastic combination | Application | Properties investigated | Ref. |
|---|---|---|---|
| Carrageenan/grapefruit seed extract | Active packaging | Antimicrobial | 165 |
| Carrageenan/carbon nanotube/glycerine | Gas-sensing films | Mechanical properties, sensory properties | 168 |
| K-Carrageenan/bentonite nano-clay | Biopolymer films and coating | Water absorption and mechanical properties | 169 |
| K-Carrageenan/nano-clay/Zataria multiflora/glycerol | Active food packaging | Antimicrobial | 170 |
| Kappa-carrageenan or iota-carrageenan or alginate with glycerol | Edible films | Moisture absorption and mechanical characteristics | 173 |
| Carrageenan/cassava starch | Films and moulded articles | Water permeability and mechanical properties | 176 |
| Carrageenan/epoxy chloropropane | Fibres | Mechanical properties and structure | 177 |
| K-Carrageenan with glycerol/sorbitol and PEG | Packaging films | Mechanical and moisture-absorbing properties | 178 |
| Kappaphycus alvarezii biomass based | Films | Oxygen and moisture permeation, mechanical characteristics | 179 and 180 |
| Semirefined carrageenan | Packaging film | Moisture barrier, mechanical properties | 181 |
| Carrageenan/essential oils | Antimicrobial and antioxidant edible films | Moisture permeability, transparency, antioxidant characteristics | 182 |
| K-Carrageenan/potassium sorbate | Antimicrobial food packaging | Controlled release of additives | 183 |
| Kappa-carrageenan/silk fibroin | Bone scaffolds | Compressive strength, bone apatite formation | 185 |
| Carrageenan/cellulose, carrageenan/carboxy methyl cellulose/polyethylene oxide | Wound dressing materials | Biocompatibility, water retention capacity and mechanical properties | 3, 4 and 186 |
| K-Carrageenan/potato starch | Biodegradable water-absorbent children's toys | Hydrogel properties, mechanical properties | 187 |
| Carrageenan/polyethylene oxide/streptomycin/diclofenac | Wound dressing films | Mechanical, mucoadhesive and drug-release characteristics | 189 |
| i-Carrageenan/poly(ortho-phenylenediamine-co-aniline) | Energy storage devices | Ionic and electrical conductivity | 191 |
| Carrageenan/PVA/bio-plasticizer | Packaging | Hydrophobicity and mechanical properties | 192 and 193 |
| k-Carrageenan/carboxymethyl cellulose | Polymer electrolytes | Electrical and mechanical properties | 196 |
| K-Carrageenan/polyvinyl pyrrolidone/PEG/silver nanoparticles | Biomedical applications | Fungicide activity, mechanical properties | 197 |
| Carrageenan with glycerol | Edible coating | Mechanical properties, moisture permeability | 198 and 199 |
| K-Carrageenan composites with pectin and mica flakes | Biodegradable film | Barrier properties to water vapour and gases | 200 |
| Carrageenan/rice starch | Biodegradable films | Mechanical and water vapour permeability | 201 |
| i-Carrageenan/rice starch/stearic acid/glycerol | Fruit and vegetable packaging | Physical, mechanical and barrier properties | 202 |
7.4 Drawbacks of carrageenan-based bioplastics
Unmodified carrageenan films, although exhibiting good tensile strength, can be brittle. This brittleness may restrict their use in applications where flexibility and impact resistance are essential. Some carrageenan films may require additional modifications to meet the mechanical and water-vapor barrier properties necessary for commercial applications. This could involve chemical modifications or blending with other materials, which may add complexity to the manufacturing process. The mechanical and water resistance properties of carrageenan films are often improved by the addition of plasticizers such as glycerol, sorbitol, and polyethylene glycol. However, the effectiveness of plasticizers depends on factors such as compatibility with the matrix, dosage, and chemical structure. Finding the optimal conditions for plasticizer incorporation can be challenging. The three main types of carrageenan (λ, ί, and κ) exhibit different properties. While K-carrageenan is reported to have better film-forming properties, moisture barrier, and mechanical properties, the choice of carrageenan type adds complexity to the material selection process, and the desired properties may vary. Different applications may demand specific properties, and achieving a balance between the various desirable characteristics can be challenging. For instance, films suitable for food packaging may require different properties compared with those intended for drug delivery systems.8. Bioplastics from ulvan
Ulvan is a versatile polysaccharide with immense potential. Its biological activities and physicochemical properties are found to be dependent on the molecular size and degree of branching. Recently, its physicochemical and rheological properties have been tuned to see its use in biomaterials, drug delivery and tissue engineering applications. The widespread usage of plastic in recent decades has caused environmental pollution, as packaging waste alone contributes to one-third of plastic waste. Sustainable alternatives to plastic are being sourced, for which algae are emerging for their potential to be used for bioplastic production. This sustainable approach to using algae in producing bioplastics for packaging applications minimizes waste generation, resource consumption, and combating greenhouse gas emissions.203Ulvan is a polysaccharide extracted from the seaweeds of the Ulva genus consisting of sulphated rhamnose and glucuronic and iduronic acids. The extraction yield depends on the conditions followed in each process and the stages of the extraction procedure. A lower pH results in increased ulvan selectivity, higher extraction temperatures permit further ulvan dissolution and increasing the duration of extraction increases the yield. From the data obtained in the literature, the optimized conditions to achieve a high yield are 80–90 °C, 2–4.5 pH, and 1–3 hours duration.204 To date, ulvan is used in particles, membranes, 3D porous structures, nanofibers, and hydrogels. Ulvan is either used as is or modified by chemical modification, crosslinking, and complexation.205,206
8.1 Plasticization with ulvan
The effect of the plasticizer used for film formulations on the properties of the ulvan films is analysed. Edible films of semi-refined carrageenan, ulvan polysaccharides and glycerol can be utilized in food packaging. The addition of glycerol improved the physicochemical and mechanical properties of the film required for food packaging.207 Film formulations made of ulvan polysaccharide are found to be successful irrespective of the extraction procedure. But it is found that specific characteristics such as optical, thermal, structural, physicochemical, barrier, mechanical, and antioxidant characteristics are affected by the extraction conditions and several types and concentrations of plasticizer used. The effects of glycerol, sorbitol, and enzymatic-chemical extract on the characteristics of the films have been analysed.208,209 Glycerol and polyethylene glycol were chosen, of which the film containing 30% polyethylene glycol exhibited greater tensile strength and lower permeability to water vapor and oxygen.210 Composite films and laminates of ulvan, polyvinyl alcohol, glycerol, and starch have been produced. Glycerol, when added to contents of 10–20%, contributes to the flexibility of the laminates. The biodegradability of the formulated laminate is carried out, from which it is observed that 80% mineralization in 100 days is achieved.211Ulvan is water-soluble but can be made water-insoluble by crosslinking. Such ulvan crosslinked membranes proved their outstanding water uptake ability (until 1800% of its original dry weight) and improved mechanical performance (1.76 MPa). Ulvan has been evaluated for its use as drug delivery device, where nearly 49% of the drug had been steadily released initially, followed by a sustained slow release for 14 days.212
8.2 Composites of ulvan
Ulvan films are synthesized by incorporating silver nanoparticles to make the films antimicrobial for food packaging applications. These bio-nanocomposite films are found to demonstrate a good barrier to water vapour permeability. These films show good antimicrobial properties against Gram-negative and Gram-positive bacteria.213 The incorporation of ulvan into cellulose films had shown an improvement in film thickness, water solubility, and water vapor permeability, and good barrier properties against UV and visible light but decreased oxygen permeability. The antioxidant properties and thermal stability had improved significantly at higher contents of ulvan.214Wound dressing materials designed from ulvan formulations are designed and reported. Chitosan–ulvan hydrogels containing epidermal growth factor (EGF) and cellulose nanocrystals (CNCs) as reinforcement are designed as wound dressings. The nanocrystal reinforcement reduced the porous microstructure from a pore size of 237 ± 59 μm to 53 ± 16 μm, which in turn increased the mechanical stress curve from 0.57 MPa to 1.2 MPa, as well as the swelling, and thermal behaviour. The addition of EGF is found to encourage the recovery of skin wounds. In addition, the formulated hydrogel possesses non-toxic behaviour and good cell viability.215
8.3 Blends of ulvan
Biological scaffolds are designed from a combination of ulvan and chitosan. Scaffolds made of either ulvan or chitosan or a combination of ulvan and chitosan are found to exhibit cytocompatibility. It is found that membranes made of ulvan/chitosan sustained the human cell viability and morphology and also encouraged the attachment and proliferation of 7F2 osteoblasts.216 Complex films made of chitosan and ulvan by ionic crosslinking are found to demonstrate antioxidant and whitening properties. The ionic crosslinking agent used here is tripolyphosphate, which is found to improve the tensile properties of the chitosan/ulvan complex films. These films are biocompatible with normal human cells and toxic to melanoma cancer cells, demonstrating cell selectivity that could be attributed to generating reactive oxygen species.217 Scaffolds made of ulvan and chitosan are treated with alkaline phosphatase to deposit calcium phosphate minerals on the surface. The scaffolds with deposited minerals are non-toxic and are found to encourage cell adhesion and differentiation.218Patents on the usage of ulvan as a bioplastic material and paper have been reported. A bioplastic material composed of ulvan blend had been produced by a reactive extrusion process in the patent WO 2016/090511 A1. An algae powder with a reduced content of protein had been prepared from Ulva species algae biomass and a bioplastic material had been formulated from such a powder in the patent WO 2017/046356 A1.219 Paper pulp had been prepared from Ulva species seaweed powder by extracting cellulose and using the same with wood cellulose to manufacture paper in the patent WO 2012/114045A1.220
The non-cytotoxic nature of ulvan/chitosan blend is evaluated to allow its usage in biomedical applications. Ulvan-based hydrogels have been developed, which displayed thermo-gelling behaviour. At 30–31 °C, sol–gel transition occurs, thus confirming their usage as in situ hydrogels in biomedical applications.221 Carboxymethylation is done on ulvan/chitosan powder to render it highly acidic, which finds application as a bone cement. This blend possesses enhanced mechanical performance and is found to be non-cytotoxic.222 The areas of applications of ulvan-based bioplastics are summarized in Table 7.
| Bioplastic combination | Application | Properties investigated | Ref. |
|---|---|---|---|
| Semi-refined carrageenan, ulvan, acetic acid, glycerol | Edible films – food packaging | Viscoelastic, moisture content, opacity, antioxidant, mechanical properties | 207 |
| Ulvan, glycerol or sorbitol | Films – food packaging | Optical, thermal, structural and antioxidant properties | 208 |
| Ulvan, glycerol or sorbitol | Films – food packaging | Physico-chemical, optical, barrier and mechanical properties | 209 |
| Ulva, glycerol, polyethylene glycol | Film – food packaging | Mechanical, water vapor permeability, oxygen permeability, transparency, physicochemical, morphology | 210 |
| Ulva, polyvinyl alcohol, glycerol, starch | Films – agriculture and food packaging | Mechanical, thermal, morphology, mineralization studies | 211 |
| Ulvan, butanediol diglycidyl ether, sodium hydroxide | Wound dressing and drug delivery applications | Morphology, mechanical, water uptake, drug release properties | 212 |
| Ulvan, silver nanoparticles | Bio-nanocomposite film – food packaging | Morphology, physicochemical, water vapour permeability, antimicrobial properties | 213 |
| Ulva, cellulose, polyvinyl alcohol, glycerol | Composite film – food packaging | Physicochemical, water vapor permeability, oxygen permeability, antioxidant properties | 214 |
| Chitosan, ulvan, cellulose nanocrystals, epidermal growth factor | Hydrogel – wound dressing | Crystal structure, morphology, porosity, mechanical, thermal, in vitro cytocompatibility | 215 |
| Ulvan, chitosan, ulvan/chitosan | Membranes – scaffold materials | Morphology, cellular compatibility | 216 |
| Chitosan/ulvan, tripolyphosphate, glycerol, chlorophyll | Film – biomedical applications | Tensile mechanical, physicochemical, crystalline, swelling, biocompatibility, antioxidant properties | 217 |
| Ulvan, chitosan, alkaline phosphatase | Polyelectrolyte complex – scaffolds | Morphology, thermal, crystal structure, rheological, biological properties | 218 |
| Ulvan, poly(N-isopropylacrylamide) | Hydrogels – biomedical applications | Thermal, rheological properties | 221 |
| Ulvan/chitosan, monochloroacetic acid | Powder – bone cement | Structural, mechanical, bioactivity, in vitro cytotoxicity | 222 |
8.4 Drawbacks of ulvan-based bioplastics
The properties of ulvan, including its biological activities and physicochemical characteristics, are highly dependent on the extraction conditions. Variations in pH, temperature, and extraction duration can influence the yield and specific characteristics of ulvan. The properties of ulvan-based films, such as optical, thermal, structural, physicochemical, barrier, mechanical, and antioxidant characteristics, are influenced by the type and concentration of the plasticizers used. Although ulvan can be crosslinked to improve its mechanical performance, there may still be limitations in achieving mechanical strength comparable to certain synthetic materials. The mechanical properties of ulvan-based materials may need to be carefully balanced for different applications. Ulvan is naturally water-soluble, which may limit its use in certain applications where water resistance is crucial. However, efforts have been made to make ulvan water-insoluble through crosslinking, presenting a potential solution. The extraction yield of ulvan can be sensitive to various factors, and achieving a high yield may require careful control of the extraction conditions. This sensitivity can impact the scalability and cost-effectiveness of ulvan extraction processes.9. Bioplastics from fucoidans
Fucoidans are found to be antiviral and anticoagulant in nature which makes them a good candidate for biomedical applications. Fucoidans are generally extracted from brown algae and some echinoderms. They are natural polysaccharides consisting of sulphated α-L-fucose components and varying amounts of other monosaccharides, uronic acids, and proteins.223,224 The composition of fucoidans can vary with the species of macroalgae they are extracted from.223 Fucoidans exhibit antiviral, anticoagulant, and antitumor properties, and others of clinical interest.223–225 Fucoidans have good water solubility, and compatibility with human tissues, and are a safe product.226,227 They are not cytotoxic components, especially with other commercial antiviral products. This makes them highly acceptable in many therapeutic uses.223,226 However, fucoidan is not known to form gels or films by itself. Films and other therapeutic applications are developed by blending with other polymers and binders.1389.1 Blends of fucoidans
Blending materials is a plausible solution to bring in the positive elements of the mixture. A remarkably successful film was prepared from the blending of fucoidan, chitosan polyvinyl alcohol and ampicillin, which shows excellent cell regeneration properties. The increased concentration of biopolymers in this system helps to facilitate the proliferation of cells more efficiently. The polyvinyl alcohol makes the film more stable and controls the water absorption and solubility. The chitosan and fucoidan in the blend facilitate the porous structure of the system. This microstructure is critical to help the cells to adhere to the surface and grow.226Patents on fucoidan-based bioplastic and an edible fucoidan-based film have been reported. Bloom Holdings have patented several formulations and processes to effectively use fucoidan in polyurethane, ethylene vinyl acetate and other plastic products. The anti-microbial, anti-fungal, anti-viral and fire-retardant activities of fucoidan can be incorporated into thermoplastics and foams.228,229 Gomaa et al. prepared an edible composition with chitosan, alginate and fucoidan. This film was demonstrated to have better oxygen and moisture permeation resistance. This film also showed good antioxidant properties. Adding fucoidan and/or calcium ions improved the water resistance in the blend with alginate and chitosan. This also helped to make thicker films feasible. The films exhibited better permeation resistance to moisture and oxygen permeability.138,230
Fucoidan and chitosan when combined together exhibited good characteristics suitable for drug release applications. Nakamura et al. prepared a hydrogel by blending chitosan with fucoidan. This blend was investigated for fibroblast growth factor (FGF-2) drug release characteristics. The bioplastic matrix was used as a carrier for FGF-2, which can bind with heparin. The hydrogel was made into an injectable form with FGF-2 particles and used for the controlled release of FGF-2. It was observed that this hydrogel system is stable and extended the half-life time of the fibroblast growth factor.231
9.2 Drawbacks of fucoidan-based bioplastics
Fucoidans are not known to form gels or films. This limitation requires additional processing steps, such as blending with other polymers and binders, to create functional films or matrices for specific applications. This additional step may complicate the manufacturing process. While fucoidans are generally considered safe and compatible with human tissues, the biocompatibility of the blended materials needs to be thoroughly evaluated. The introduction of additional polymers, binders, or drugs in the blend may impact the overall biocompatibility of the composite material. The use of fucoidans in biomedical applications may be subject to regulatory approval, especially when incorporated into blended materials intended for therapeutic use. Meeting the regulatory standards for safety and efficacy is a critical consideration for the successful translation of fucoidan-based products. The composition of fucoidans can vary depending on the species of macroalgae from which they are extracted. This variability may introduce challenges in standardizing the properties of fucoidan-based blends, making it important to carefully select and characterize the source of the fucoidans. While the mentioned film blend with fucoidan, chitosan, polyvinyl alcohol, and ampicillin showed excellent cell regeneration properties, the long-term stability and performance of such films under different conditions need to be thoroughly assessed. Factors such as degradation, mechanical strength, and interaction with the environment should be considered.10. Laminarin-based bioplastics
Laminarin exhibits low molecular weight, low viscosity, and ready solubility in water which makes it a preferred constituent in many bioplastic formulations. Laminarin is generally extracted from brown algae and comprises glucose units with low molecular weight.232,233β(1,3) glycosidic bonds link the glucose units, while some β(1,6) side-chain branches are also in the structure. This low molecular weight composition leads to laminarin solutions of very low viscosity. Laminarin has good solubility in water and some organic solvents. This also explains why they are readily biodegradable in various environments. These properties make them attractive additives in many bioplastic investigations.232–235 Laminarin is nontoxic and has anti-oxidant, antitumor and many therapeutic properties. Reports of anti-inflammatory and anticoagulant activities are available.6,55,236,237 Blends and composites based on laminarin have been studied to understand their applications in packaging and medical products.237,238 Fish gelatine/laminarin blend films have been investigated for food packaging applications. K-Carrageenan/laminarin film mixed with nano-curcumin has been prepared and studied for food packaging applications. This system has shown extended shelf life for fish fillets when used as active packaging.238 A summary of applications is given in Table 8.| Bioplastic combination | Application | Properties investigated | Ref. |
|---|---|---|---|
| Fucoidan/chitosan/polyvinyl alcohol/ampicillin | Cell regeneration | Swelling/antimicrobial/water resistance | 163 |
| Alginate/fucoidan/chitosan | Edible films | Barrier properties/water absorption | 165 and 168 |
| Polyurethane/EVA/fucoidan | Foams | Anti-microbial/antifungal | 166 and 167 |
| Fucoidan/chitosan hydrogel | Controlled drug release | Fibroblast growth factor (FGF)-2 immobilization | 169 |
| Fish gelatine/laminarin | Packaging films | Antimicrobial activity | 176 |
10.1 Drawbacks of laminarin-based bioplastics
Films with low viscosity may have challenges in maintaining physical integrity, especially under stress or load. The low molecular weight and viscosity of laminarin may result in films or products with limited mechanical strength and poor tensile properties. This limitation can impact the range of applications, especially in scenarios where robust and durable materials are required, such as in certain packaging applications. While the solubility of laminarin in water and some organic solvents is beneficial for processing, it can also pose challenges in certain applications. For instance, the susceptibility to dissolution in water may limit the use of laminarin-based materials in environments with high humidity or when exposure to water is undesirable. While laminarin has been studied for various applications, each application may present unique challenges. For instance, the use of laminarin in food packaging, as mentioned in the example of fish gelatine/laminarin blend films, may require addressing specific issues such as moisture resistance, mechanical strength, and compatibility with food products.11. Techno-economic aspects and life cycle assessment of seaweed-based bioplastics
Bio-based and biodegradable plastics constitute 58.1% of the overall bioplastic market. The global bioplastics and biopolymers market is anticipated to surge from USD 10.7 billion in 2021 to USD 29.7 billion by 2026, exhibiting a compound annual growth rate (CAGR) of 22.7%. Presently, the cost varies between USD 2 per kg and USD 6 per kg, compared with the USD 1 per kg to USD 2 per kg range of its petrochemical counterparts. Concerning land use for the cultivation of feedstock for bioplastic production, it is minimal, accounting for only 0.015% of the total arable land worldwide (1.4 billion ha). Projections indicate a rise to 1.1 million ha (0.02% of total area) by 2025. Addressing the end-of-life (EOL) phase is crucial in life cycle assessment (LCA).Mechanical recycling emerges as the most environmentally suitable disposal method for bioplastics, boasting the least carbon footprint. In comparison, the anaerobic digestion, landfilling, composting, and incineration of 1 kg PLA generate 2.2 kg, 1.6 kg, 1.5 kg, and 1.7 kg of CO2, respectively, while mechanical recycling produces only 0.62 kg CO2. Mechanical recycling is cost-effective, emits no noxious gases (unlike incineration), and avoids issues like the leachate seepage and uncontrolled methane release associated with landfilling. For effective anaerobic digestion, bioplastics should ideally degrade within 15–30 days; PHB meets this criterion, whereas PVA, PLA, and PCL do not. Algae, often considered waste, could be a valuable resource when converted into new-generation bioplastics. Developing predictive models like BioWin and the Biodegradability Evaluation and Simulation System (BESS) can guide consumers toward the best bioplastic based on application and available EOL options.239
A SWOT analysis of seaweed-based bioplastics is shown in Fig. 2. Seaweed-based bioplastics offer a promising array of advantages specific to their unique properties and applications. With seaweed's abundance and renewability, these bioplastics present a sustainable alternative to traditional plastics. Their biodegradability and low environmental impact address pressing concerns regarding plastic pollution. Seaweed's inherent functional properties make it suitable for various applications, ranging from food packaging to biomedical materials. Additionally, seaweed-based bioplastics have the potential for value-added products, as they can be enriched with bioactive compounds. Government support and funding further propel research and development in this field, aligning with global sustainability initiatives.
However, several challenges must be navigated for the widespread adoption of seaweed-based bioplastics. These include the limited infrastructure for cultivation and processing, technological hurdles in scaling production, and higher costs associated with manufacturing compared with conventional plastics. Overcoming perceptions and educating consumers about the benefits of seaweed-based bioplastics is essential for market acceptance. Yet, opportunities abound for further research and innovation, collaboration across sectors, market growth, and diversification into new applications. Mitigating environmental risks, addressing competition from conventional plastics, navigating regulatory landscapes, ensuring supply chain resilience, and adapting to technological and market uncertainties are crucial considerations for the industry's sustainable advancement.
The primary tool for evaluating the environmental impact of both bioplastics and conventional plastics is life cycle assessment (LCA) or cradle-to-grave analysis. Protocols have been established for conducting LCA studies on specific bioplastics, such as PLA, currently in the market. These studies often compare the LCA of bioplastics with that of fossil-fuel plastics like polyethylene and PET. A recent study using the LCA cradle-to-grave analysis demonstrated a significant reduction in greenhouse gases when 20% of PET bottles were substituted with PLA bottles during bottle manufacturing. Another study using the Global Warming Potential (GWP) guide showed that substituting petroleum-based plastics with bioplastics could reduce greenhouse gas emissions. LCA also serves as a valuable means of identifying the most effective methods for managing and disposing of bioplastic waste. Incineration or landfilling of bioplastic products has been revealed, through LCA, as an inadequate alternative. Following the LUC emissions principle has confirmed the reliability of bioplastics as an excellent substitute for petroleum-based plastics in waste management. Compared with conventional petroleum-derived plastics, the use of PLA and thermoplastic starch significantly reduces carbon dioxide emissions, with the former achieving a reduction of 50–70%. Similarly, bio-urethanes and poly(trimethyleneterephthalate) (PTT) exhibit 36% and 44% lower greenhouse gas emissions than their petroleum-derived counterparts. To enhance the intelligent management of bioplastic waste, it is proposed that the reduction of greenhouse gas emissions should aim for zero LUC emissions. Future studies should concentrate on conducting individual LCAs for the expanding range of bioplastics.240
Carbon balance estimates for the recirculation of co-products from the alginate extraction step into the bioplastic film have been reported. A scenario based modelling provide insight into the carbon flow within various scenarios. In the no-incineration composting scenario (BASE), where alginate is the sole seaweed component in the bioplastic and accounts for 0.44 kg C in 1 kg of bioplastic, 44% of the carbon is biogenic. When cellulose and glycerol are externally added, 69% of the carbon in the seaweed contributes to seaweed residues. The cellulose incineration composting scenario (CELL) demonstrates the recirculation of alginate and cellulose from the seaweed to the bioplastic film, avoiding 12.5% of carbon in cellulose nanofibers. The cellulose and mannitol incineration composting scenario (MANN) reveals that the mannitol in seaweed is lower than the glycerol required for bioplastics. Incorporation of mannitol to the bioplastic film avoids 5% of the carbon from glycerol. PLA filler pellet incineration composting scenarios PLA5 and PLA30 show how seaweed residues in the base scenario are later used to substitute 5% or 30% of PLA, integrating more original seaweed biomass into the products. Overall, the carbon balance results indicate that recirculating cellulose and mannitol by substituting PLA in scenarios CELL, MANN, PLA5, and PLA30 converts more of the originally absorbed seaweed carbon into products, reducing the need for external carbon inputs. The BASE scenario requires the most external carbon inputs. The contribution analysis of the Global Warming (GW) impact across all life cycle stages of 1 kg of bioplastic film reveals that scenarios with the highest impact are BASE, CELL, and MANN with incineration end-of-life (EoL), having impacts of 3.72 kg CO2-eq., 3.79 kg CO2-eq., and 3.77 kg CO2-eq., respectively. The scenarios with the lowest impacts are PLA substitution scenarios with composting EoL, with PLA30 substitution having the lowest impact at 2.3 kg CO2-eq. and PLA5 at 2.53 kg CO2-eq., closely followed by BASE composting at 2.56 kg CO2-eq. Film fabrication contributes the most to the impact in all cases, primarily due to the production of glycerol with an impact of 2.11 kg CO2-eq. In all scenarios, the GW impacts of the offshore farm are significantly low, attributed to the carbon uptake in seaweed cultivation.241
Micro- or macro-algae, particularly seaweed, are renewable resources that do not compete with land-based food resources. Seaweed production costs, especially in Asian countries, are expected to be lower due to the availability of low-cost manpower and shorter harvest times (30–45 days) for certain marine macroalgae like Kappaphycus sp. and Gracilaria sp. Bio-based plastics derived from algae feedstock include hybrid plastics, cellulose-based plastics, polylactic acid, and bio-polyethylene. Economically, directly utilizing seaweeds for bioplastic production provides a cost advantage, whereas processing costs are higher for PLA, PHA, and PHB production. Small- and medium-scale industries involved in agar, alginate, and carrageenan production from seaweeds have significant opportunities for small startups. The econometrics of bioplastic production from algal biomass, along with its production costs, have been evaluated at the lab scale, considering factors such as seaweed quantity, plasticizers, water, electricity, and heat. Notably, the manpower cost for producing specific-sized bioplastic films was not included in the assessment. The cost of Kappaphycus alvarezii is higher compared with other Indian native species, selling at around Rs. 100–130 per kg dry weight, while G. edulis and G. dura are sold at around Rs. 35–40 per kg dry weight.179
12. Challenges and future prospect
Elevated production costs, amounting to twice those of traditional synthetic plastics, pose a significant challenge. Incomplete degradation under various conditions necessitates a thorough investigation to identify optimal solutions for addressing negative ecological impacts, with a particular emphasis on mitigating greenhouse gas emissions, including methane. Enhancing the pathways of algae-based biopolymers, currently marked by high costs and low efficiency, is crucial for ensuring a sustainable future. Inadequate information on bioplastic labels from producers further complicates the understanding and acceptance of these materials. Poor water-resistance properties may hinder the practical applications of bioplastics, requiring further attention and improvement in this aspect.242There is a need for further exploration into strategies that minimize the use of additives, as their current usage renders the product unsuitable for applications in the medical and food packaging sectors. Additionally, there is a call for innovative, cost-effective, and more efficient conversion technologies. The life cycle assessment (LCA) of bioplastics remains an under-researched area that demands a deeper understanding, particularly when adopting a gate-to-grave approach. The development of bioplastics designed to degrade effectively under mild conditions can significantly broaden their applicability. Economic constraints stand out as a pivotal factor influencing the upscaling of the successful laboratory-scale technologies mentioned earlier.239
There has been significant investigation into the various types, benefits, and potential of bioplastics derived from renewable sources such as edible crops and waste. However, there remains ample room for further investigation, particularly in the context of algae-based bioplastics production. Continuous research and progress in this field are essential for advancing the realm of algal-based bioplastics. Each step in the process requires thorough exploration to guarantee the optimal output of algal-based bioplastic at the lowest possible cost.243
While bioplastic extracted from algae is in its early stages of development, achieving commercialization remains a goal for industry experts. The genetic engineering of algae to enhance biopolymer accumulation and production is anticipated to play a pivotal role. Presently, some companies produce plastics labeled as biodegradable, utilizing materials like PHA, PLA, low-density polyethylene (LDPE), high-density polyethylene (HDPE), and bio-based polymer blends that are both degradable and recyclable. Seaweeds, in combination with safe and commercially viable plasticizers, offer a promising avenue for producing 100% biodegradable single-use plastics.244
13. Conclusions
Algal systems are emerging as a sustainable resource for biomass that is widely investigated for various industrial applications. A major field of research and development is in the manufacturing of bioplastics. This will help reduce our reliance on fossil fuels and create compostable and biodegradable systems. The polymer industry is expected to take a large volume of algal biomass along this route, helping carbon sequestration and creating good economic opportunities for coastal communities. Seaweed components like polysaccharides, lipids, proteins, and cellulose have been used to develop plastics; polysaccharides are of prime importance as the percentage extracted can be economically viable. The major seaweed-derived polysaccharides are alginate, carrageenan, ulvan, fucoidan and laminarin. These are reported to be suitable for bioplastic development due to their compatibility with various matrices and film-forming abilities. This review collates the extraction characterisation and applications of these polysaccharides, especially bioplastics. A wide range of applications are demonstrated for these polysaccharides in biomedical products, food packaging, functional foods, nutraceuticals and the pharmaceutical industry. These systems are combined with other polymers and additives for the desired properties. We can certainly see that seaweed derivatives, especially polysaccharides, will grow in economic importance in the coming days.Author contributions
All authors contributed equally.Conflicts of interest
There are no conflicts to declare.References
- C. Lim, S. Yusoff, C. G. Ng, P. E. Lim and Y. C. Ching, Bioplastic made from seaweed polysaccharides with green production methods, J. Environ. Chem. Eng., 2021, 9(5), 105895 CrossRef CAS.
- E. M. Cabral, M. Oliveira, J. R. M. Mondala, J. Curtin, B. K. Tiwari and M. Garcia-Vaquero, Antimicrobials from Seaweeds for Food Applications, Mar. Drugs, 2021, 19(4), 211 CrossRef CAS PubMed.
- H. P. S. Abdul Khalil, C. K. Saurabh, Y. Y. Tye, T. K. Lai, A. M. Easa and E. Rosamah, et al., Seaweed based sustainable films and composites for food and pharmaceutical applications: A review, Renewable Sustainable Energy Rev., 2017, 77, 353–362 CrossRef CAS.
- B. J. D. Barba, C. T. Aranilla, L. S. Relleve, V. R. C. Cruz, J. R. Vista and L. V. Abad, Hemostatic granules and dressing prepared from formulations of carboxymethyl cellulose, kappa-carrageenan and polyethylene oxide crosslinked by gamma radiation, Radiat. Phys. Chem., 2018, 144, 180–188 CrossRef CAS.
- G. El-Fawal, Preparation, characterization and antibacterial activity of biodegradable films prepared from carrageenan, J. Food Sci. Technol., 2014, 51(9), 2234–2239 CrossRef CAS PubMed.
- M. Garcia-Vaquero, G. Rajauria, J. V. O'Doherty and T. Sweeney, Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification, Food Res. Int., 2017, 99(Pt 3), 1011–1020 CrossRef CAS PubMed.
- F. Frongia, L. Arru, M. R. Cramarossa and L. Forti, Microalgae potential in the capture of CO2 emission, Acta Innovations, 2021,(41), 19–27 Search PubMed.
- J.-G. Rosenboom, R. Langer and G. Traverso, Bioplastics for a circular economy, Nat. Rev. Mater., 2022, 7(2), 117–137 CrossRef PubMed.
- S. Lomartire, J. C. Marques and A. M. M. Gonçalves, An overview of the alternative use of seaweeds to produce safe and sustainable bio-packaging, Appl. Sci., 2022, 12(6), 3123 CrossRef CAS.
- S. Polat, M. Trif, A. Rusu, V. Šimat, M. Čagalj and G. Alak, et al., Recent advances in industrial applications of seaweeds, Crit. Rev. Food Sci. Nutr., 2023, 63(21), 4979–5008 CrossRef CAS PubMed.
- S. Lomartire and A. M. M. Gonçalves, An overview of potential seaweed-derived bioactive compounds for pharmaceutical applications, Mar. Drugs, 2022, 20(2), 141 CrossRef CAS PubMed.
- H. L. Kite-Powell, E. Ask, S. Augyte, D. Bailey, J. Decker and C. A. Goudey, et al., Estimating production cost for large-scale seaweed farms, J. Appl. Phycol., 2022, 3(1), 435–445 CrossRef.
- C. T. Matos, L. Gouveia, A. R. C. Morais, A. Reis and R. Bogel-Łukasik, Green metrics evaluation of isoprene production by microalgae and bacteria, Green Chem., 2013, 15(10), 2854–2864 RSC.
- R. A. Sheldon and J. P. M. Sanders, Toward concise metrics for the production of chemicals from renewable biomass, Catal. Today, 2015, 239, 3–6 CrossRef CAS.
- L. Gomez, C. Alvarez, M. Zhao, U. Tiwari, J. Curtin and M. Garcia-Vaquero, et al., Innovative processing strategies and technologies to obtain hydrocolloids from macroalgae for food applications, Carbohydr. Polym., 2020, 248, 116784 CrossRef CAS PubMed.
- A. Dobrinčić, S. Balbino, Z. Zorić, S. Pedisić, D. Bursać Kovačević and I. Elez Garofulić, et al., Advanced technologies for the extraction of marine brown algal polysaccharides, Mar. Drugs, 2020, 18(3), 168 CrossRef PubMed.
- C. M. P. G. Dore, M. Alves, L. S. E. P. Will, T. G. Costa, D. A. Sabry and L. A. R. de Souza Rêgo, et al., A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects, Carbohydr. Polym., 2013, 91(1), 467–475 CrossRef CAS PubMed.
- P. Otero, M. Carpena, P. Garcia-Oliveira, J. Echave, A. Soria-Lopez and P. Garcia-Perez, et al., Seaweed polysaccharides: Emerging extraction technologies, chemical modifications and bioactive properties, Crit. Rev. Food Sci. Nutr., 2021, 1–29 Search PubMed.
- M. Bhardwaj, T. Padmavathy, S. Mani, R. Malarvizhi, V. K. Sali and H. R. Vasanthi, Sulfated polysaccharide from Turbinaria ornata suppress lipopolysaccharide-induced inflammatory response in RAW 264.7 macrophages, Int. J. Biol. Macromol., 2020, 164, 4299–4305 CrossRef CAS PubMed.
- M. Garcia-Vaquero, G. Rajauria and B. Tiwari, Conventional extraction techniques: Solvent extraction, in Sustainable seaweed technologies, Elsevier, 2020, pp. 171–189 Search PubMed.
- C.-Y. Huang, S.-J. Wu, W.-N. Yang, A.-W. Kuan and C.-Y. Chen, Antioxidant activities of crude extracts of fucoidan extracted from Sargassum glaucescens by a compressional-puffing-hydrothermal extraction process, Food Chem., 2016, 197, 1121–1129 CrossRef CAS PubMed.
- M. D. Abid, S. Lajili, H. H. Ammar, D. Cherif, N. Eltaief and H. Majdoub, et al., Chemical and biological properties of sodium alginates isolated from tow brown algae Dictyopteris Membranaceae and Padina Pavonica, Trends J. Sci. Res., 2019, 4(2), 62–67 CrossRef.
- H. Yaich, A. B. Amira, F. Abbes, M. Bouaziz, S. Besbes and A. Richel, et al., Effect of extraction procedures on structural, thermal and antioxidant properties of ulvan from Ulva lactuca collected in Monastir coast, Int. J. Biol. Macromol., 2017, 105(Pt 2), 1430–1439 CrossRef CAS PubMed.
- K. S. Bittkau, S. Neupane and S. Alban, Initial evaluation of six different brown algae species as source for crude bioactive fucoidans, Algal Res., 2020, 45, 101759 CrossRef.
- Z. Zhang, X. Wang, M. Zhao, S. Yu and H. Qi, The immunological and antioxidant activities of polysaccharides extracted from Enteromorpha linza, Int. J. Biol. Macromol., 2013, 57, 45–49 CrossRef CAS PubMed.
- Y. Liu, X. Wu, W. Jin and Y. Guo, Immunomodulatory effects of a low-molecular weight polysaccharide from Enteromorpha prolifera on RAW 264.7 macrophages and cyclophosphamide-induced immunosuppression mouse models, Mar. drugs, 2020, 18(7), 340 CrossRef CAS PubMed.
- S. Zhao, Q. Sun, Y. Gu, W. Yang, Y. Chen and J. Lin, et al., Enteromorpha prolifera polysaccharide based coagulant aid for humic acids removal and ultrafiltration membrane fouling control, Int. J. Biol. Macromol., 2020, 152, 576–583 CrossRef CAS PubMed.
- Y. Chi, Y. Li, G. Zhang, Y. Gao, H. Ye and J. Gao, et al., Effect of extraction techniques on properties of polysaccharides from Enteromorpha prolifera and their applicability in iron chelation, Carbohydr. Polym., 2018, 181, 616–623 CrossRef CAS PubMed.
- I. Michalak and K. Chojnacka, Algal extracts: Technology and advances, Eng. Life Sci., 2014, 14(6), 581–591 CrossRef CAS.
- M. Alboofetileh, M. Rezaei, M. Tabarsa, M. Rittà, M. Donalisio and F. Mariatti, et al., Effect of different non-conventional extraction methods on the antibacterial and antiviral activity of fucoidans extracted from Nizamuddinia zanardinii, Int. J. Biol. Macromol., 2019, 124, 131–137 CrossRef CAS PubMed.
- C. L. Okolie, B. Mason, A. Mohan, N. Pitts and C. C. Udenigwe, The comparative influence of novel extraction technologies on in vitro prebiotic-inducing chemical properties of fucoidan extracts from Ascophyllum nodosum, Food Hydrocolloids, 2019, 90, 462–471 CrossRef CAS.
- X.-c. Shang, D. Chu, J.-x. Zhang, Y.-f. Zheng and Y. Li, Microwave-assisted extraction, partial purification and biological activity in vitro of polysaccharides from bladder-wrack (Fucus vesiculosus) by using deep eutectic solvents, Sep. Purif. Technol., 2021, 259, 118169 CrossRef CAS.
- R. Boulho, C. Marty, Y. Freile-Pelegrín, D. Robledo, N. Bourgougnon and G. Bedoux, Antiherpetic (HSV-1) activity of carrageenans from the red seaweed Solieria chordalis (Rhodophyta, Gigartinales) extracted by microwave-assisted extraction (MAE), J. Appl. Phycol., 2017, 29(5), 2219–2228 CrossRef CAS.
- Y. Yuan, X. Xu, C. Jing, P. Zou, C. Zhang and Y. Li, Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: Functional properties and bioactivities, Carbohydr. Polym., 2018, 181, 902–910 CrossRef CAS PubMed.
- A. Silva, S. A. Silva, M. Carpena, P. Garcia-Oliveira, P. Gullón and M. F. Barroso, et al., Macroalgae as a source of valuable antimicrobial compounds: Extraction and applications, Antibiotics, 2020, 9(10), 642 CrossRef CAS PubMed.
- F. Adam, M. Abert-Vian, G. Peltier and F. Chemat, “Solvent-free” ultrasound-assisted extraction of lipids from fresh microalgae cells: a green, clean and scalable process, Bioresour. Technol., 2012, 114, 457–465 CrossRef CAS PubMed.
- A.-M. Cikoš, S. Jokić, D. Šubarić and I. Jerković, Overview on the application of modern methods for the extraction of bioactive compounds from marine macroalgae, Mar. Drugs, 2018, 16(10), 348 CrossRef PubMed.
- S.-H. Wang, C.-Y. Huang, C.-Y. Chen, C.-C. Chang, C.-Y. Huang and C.-D. Dong, et al., Isolation and purification of brown algae fucoidan from Sargassum siliquosum and the analysis of anti-lipogenesis activity, Biochem. Eng. J., 2021, 165, 107798 CrossRef CAS.
- L. Youssouf, L. Lallemand, P. Giraud, F. Soule, A. Bhaw-Luximon and O. Meilhac, et al., Ultrasound-assisted extraction and structural characterization by NMR of alginates and carrageenans from seaweeds, Carbohydr. Polym., 2017, 166, 55–63 CrossRef CAS PubMed.
- Y. Kumar, S. Singhal, A. Tarafdar, A. Pharande, M. Ganesan and P. C. Badgujar, Ultrasound assisted extraction of selected edible macroalgae: Effect on antioxidant activity and quantitative assessment of polyphenols by liquid chromatography with tandem mass spectrometry (LC-MS/MS), Algal Res., 2020, 52, 102114 CrossRef.
- S. U. Kadam, C. P. O'Donnell, D. K. Rai, M. B. Hossain, C. M. Burgess and D. Walsh, et al., Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound assisted extraction, characterization and bioactivity, Mar. drugs, 2015, 13(7), 4270–4280 CrossRef CAS PubMed.
- C. Jacobsen, A.-D. M. Sørensen, S. L. Holdt, C. C. Akoh and D. B. Hermund, Source, extraction, characterization, and applications of novel antioxidants from seaweed, Annu. Rev. Food Sci. Technol., 2019, 10, 541–568 CrossRef CAS PubMed.
- K. Duarte, C. I. L. Justino, A. M. Gomes, T. Rocha-Santos and A. C. Duarte, Green analytical methodologies for preparation of extracts and analysis of bioactive compounds, Comprehensive analytical chemistry, Elsevier, 2014, vol. 65, pp. 59–78 Search PubMed.
- P. S. Saravana, Y.-J. Cho, Y.-B. Park, H.-C. Woo and B.-S. Chun, Structural, antioxidant, and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction, Carbohydr. Polym., 2016, 153, 518–525 CrossRef CAS PubMed.
- C. R. N. Gereniu, P. S. Saravana and B.-S. Chun, Recovery of carrageenan from Solomon Islands red seaweed using ionic liquid-assisted subcritical water extraction, Sep. Purif. Technol., 2018, 196, 309–317 CrossRef CAS.
- S. M. Zakaria and S. M. M. Kamal, Subcritical water extraction of bioactive compounds from plants and algae: applications in pharmaceutical and food ingredients, Food Eng. Rev., 2016, 8(1), 23–34 CrossRef CAS.
- M. Puri, D. Sharma and C. J. Barrow, Enzyme-assisted extraction of bioactives from plants, Trends Biotechnol., 2012, 30(1), 37–44 CrossRef CAS PubMed.
- H. P. S. Khalil, T. K. Lai, Y. Y. Tye, S. Rizal, E. W. N. Chong and S. W. Yap, et al., A review of extractions of seaweed hydrocolloids: Properties and applications, eXPRESS Polym. Lett., 2018, 12(4), 296–317 CrossRef.
- I. Michalak, A. Dmytryk, A. Śmieszek and K. Marycz, Chemical characterization of Enteromorpha prolifera extract obtained by enzyme-assisted extraction and its influence on the metabolic activity of Caco-2, Int. J. Mol. Sci., 2017, 18(3), 479 CrossRef PubMed.
- W. Wijesinghe and Y.-J. Jeon, Enzyme-assistant extraction (EAE) of bioactive components: a useful approach for recovery of industrially important metabolites from seaweeds: a review, Fitoterapia, 2012, 83(1), 6–12 CrossRef CAS PubMed.
- A. Silva, S. A. Silva, M. Carpena, P. Garcia-Oliveira, P. Gullon and M. F. Barroso, et al., Macroalgae as a Source of Valuable Antimicrobial Compounds: Extraction and Applications, Antibiotics, 2020, 9(10), 642 CrossRef CAS PubMed.
- H.-J. La, G.-G. Choi, C. Cho, S.-H. Seo, A. Srivastava and B.-H. Jo, et al., Increased lipid productivity of Acutodesmus dimorphus using optimized pulsed electric field, J. Appl. Phycol., 2016, 28(2), 931–938 CrossRef CAS.
- D. Carullo, B. D. Abera, A. A. Casazza, F. Donsì, P. Perego and G. Ferrari, et al., Effect of pulsed electric fields and high pressure homogenization on the aqueous extraction of intracellular compounds from the microalgae Chlorella vulgaris, Algal Res., 2018, 31, 60–69 CrossRef.
- M. Prabhu, A. Chemodanov, R. Gottlieb, M. Kazir, O. Nahor and M. Gozin, et al., Starch from the sea: the green macroalga Ulva ohnoi as a potential source for sustainable starch production in the marine biorefinery, Algal Res., 2019, 37, 215–227 CrossRef.
- S. U. Kadam, B. K. Tiwari and C. P. O'Donnell, Extraction, structure and biofunctional activities of laminarin from brown algae, Int. J. Food Sci. Technol., 2015, 50(1), 24–31 CrossRef CAS.
- H. Hadj Ammar, S. Lajili, R. Ben Said, D. Le Cerf, A. Bouraoui and H. Majdoub, Physico-chemical characterization and pharmacological evaluation of sulfated polysaccharides from three species of Mediterranean brown algae of the genus Cystoseira, Daru, J. Pharm. Sci., 2015, 23(1), 1–8 CrossRef PubMed.
- Q. Cong, H. Chen, W. Liao, F. Xiao, P. Wang and Y. Qin, et al., Structural characterization and effect on anti-angiogenic activity of a fucoidan from Sargassum fusiforme, Carbohydr. Polym., 2016, 136, 899–907 CrossRef CAS PubMed.
- S. J. Lim, W. M. W. Aida, M. Y. Maskat, S. Mamot, J. Ropien and D. M. Mohd, Isolation and antioxidant capacity of fucoidan from selected Malaysian seaweeds, Food Hydrocolloids, 2014, 42, 280–288 CrossRef CAS.
- S. V. Safavi, A. A. Kenari, M. Tabarsa and M. Esmaeili, Effect of sulfated polysaccharides extracted from marine macroalgae (Ulva intestinalis and Gracilariopsis persica) on growth performance, fatty acid profile, and immune response of rainbow trout (Oncorhynchus mykiss), J. Appl. Phycol., 2019, 31, 4021–4035 CrossRef CAS.
- T. I. Imbs, S. P. Ermakova, O. S. Malyarenko, V. V. Isakov and T. N. Zvyagintseva, Structural elucidation of polysaccharide fractions from the brown alga Coccophora langsdorfii and in vitro investigation of their anticancer activity, Carbohydr. Polym., 2016, 135, 162–168 CrossRef CAS PubMed.
- G. Bedoux, E. Caamal-Fuentes, R. Boulho, C. Marty, N. Bourgougnon and Y. Freile-Pelegrín, et al., Antiviral and cytotoxic activities of polysaccharides extracted from four tropical seaweed species, Nat. Prod. Commun., 2017, 12(6), 1934578X1701200602 CrossRef.
- A. Subash, G. Veeraraghavan, V. K. Sali, M. Bhardwaj and H. R. Vasanthi, Attenuation of inflammation by marine algae Turbinaria ornata in cotton pellet induced granuloma mediated by fucoidan like sulphated polysaccharide, Carbohydr. Polym., 2016, 151, 1261–1268 CrossRef CAS PubMed.
- J. He, Y. Xu, H. Chen and P. Sun, Extraction, structural characterization, and potential antioxidant activity of the polysaccharides from four seaweeds, Int. J. Mol. Sci., 2016, 17(12), 1988 CrossRef PubMed.
- F. Rahimi, M. Tabarsa and M. Rezaei, Ulvan from green algae Ulva intestinalis: Optimization of ultrasound-assisted extraction and antioxidant activity, J. Appl. Phycol., 2016, 28, 2979–2990 CrossRef CAS.
- B. Vázquez-Rodríguez, J. A. Gutiérrez-Uribe, M. Antunes-Ricardo, L. Santos-Zea and L. E. Cruz-Suárez, Ultrasound-assisted extraction of phlorotannins and polysaccharides from Silvetia compressa (Phaeophyceae), J. Appl. Phycol., 2020, 32, 1441–1453 CrossRef.
- P. S. Saravana, Y.-N. Cho, H.-C. Woo and B.-S. Chun, Green and efficient extraction of polysaccharides from brown seaweed by adding deep eutectic solvent in subcritical water hydrolysis, J. Cleaner Prod., 2018, 198, 1474–1484 CrossRef CAS.
- L. Wang, J. Y. Oh, J. G. Je, T. U. Jayawardena, Y.-S. Kim and J. Y. Ko, et al., Protective effects of sulfated polysaccharides isolated from the enzymatic digest of Codium fragile against hydrogen peroxide-induced oxidative stress in in vitro and in vivo models, Algal Res., 2020, 48, 101891 CrossRef.
- W. Kunz and K. Häckl, The hype with ionic liquids as solvents, Chem. Phys. Lett., 2016, 661, 6–12 CrossRef CAS.
- M. Martins and S. P. M. Ventura, Emerging seaweed extraction techniques using ionic liquids, in Sustainable Seaweed Technologies, 2020, pp. 287–311 Search PubMed.
- R. A. Sequeira, J. Bhatt and K. Prasad, Recent Trends in Processing of Proteins and DNA in Alternative Solvents: A Sustainable Approach, Sustainable Chem., 2020, 1(2), 116–137 CrossRef.
- A. K. Das, R. A. Sequeira, T. K. Maity and K. Prasad, Bio-ionic liquid promoted selective coagulation of κ-carrageenan from Kappaphycus alvarezii extract, Food Hydrocolloids, 2021, 111, 106382 CrossRef CAS.
- T. J. Trivedi and A. Kumar, Efficient Extraction of Agarose from Red Algae Using Ionic Liquids, Green Sustainable Chem., 2014, 04(04), 190–201 CrossRef.
- M. Sharma, J. Prakash Chaudhary, D. Mondal, R. Meena and K. Prasad, A green and sustainable approach to utilize bio-ionic liquids for the selective precipitation of high purity agarose from an agarophyte extract, Green Chem., 2015, 17(5), 2867–2873 RSC.
- A. K. Das, M. Sharma, D. Mondal and K. Prasad, Deep eutectic solvents as efficient solvent system for the extraction of kappa-carrageenan from Kappaphycus alvarezii, Carbohydr. Polym., 2016, 136, 930–935 CrossRef CAS PubMed.
- R. Pezoa-Conte, A. Leyton, I. Anugwom, S. von Schoultz, J. Paranko and P. Mäki-Arvela, et al., Deconstruction of the green alga Ulva rigida in ionic liquids: Closing the mass balance, Algal Res., 2015, 12, 262–273 CrossRef.
- L. B. Malihan, N. Mittal, G. M. Nisola, T. G. Weldemhret, H. Kim and W.-J. Chung, Macroalgal biomass hydrolysis using dicationic acidic ionic liquids, J. Chem. Technol. Biotechnol., 2017, 92(6), 1290–1297 CrossRef CAS.
- L. B. Malihan, G. M. Nisola, N. Mittal, J. G. Seo and W.-J. Chung, Blended ionic liquid systems for macroalgae pretreatment, Renewable Energy, 2014, 66, 596–604 CrossRef CAS.
- D.-H. Park and G.-T. Jeong, Production of Reducing Sugar from Macroalgae Saccharina japonica Using Ionic Liquid Catalyst, Korean Chem. Eng. Res., 2013, 51(1), 106–110 CrossRef CAS.
- Y. R. Lee, W. Ma and K. H. Row, Determination of Polysaccharides in Undaria pinnatifida by Ionic Liquid-Modified Silica Gel Size Exclusion Chromatography, Anal. Lett., 2018, 51(13), 1999–2012 CrossRef CAS.
- E. Suarez Garcia, C. F. Miranda, M. T. Cesario, R. H. Wijffels, C. van den Berg and M. H. M. Eppink, Ionic Liquid-Assisted Selective Extraction and Partitioning of Biomolecules from Macroalgae, ACS Sustainable Chem. Eng., 2023, 11(5), 1752–1762 CrossRef CAS PubMed.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Novel solvent properties of choline chloride/urea mixtures, Chem. Commun., 2003,(1), 70–71 RSC.
- C. McReynolds, A. Adrien, N. Castejon and S. C. M. Fernandes, Green in the deep blue: deep eutectic solvents as versatile systems for the processing of marine biomass, Green Chem. Lett. Rev., 2022, 15(2), 383–404 CrossRef CAS.
- Y. H. Choi, J. van Spronsen, Y. Dai, M. Verberne, F. Hollmann and I. W. Arends, et al., Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology?, Plant Physiol., 2011, 156(4), 1701–1705 CrossRef CAS PubMed.
- D. Yu, Z. Xue and T. Mu, Deep eutectic solvents as a green toolbox for synthesis, Cell Rep. Phys. Sci., 2022, 3(4), 100809 CrossRef CAS.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Deep eutectic solvents (DESs) and their applications, Chem. Rev., 2014, 114(21), 11060–11082 CrossRef CAS PubMed.
- W. Zhang, S. Cheng, X. Zhai, J. Sun, X. Hu and H. Pei, et al., Green and Efficient Extraction of Polysaccharides From Poria cocos F.A. Wolf by Deep Eutectic Solvent, Nat. Prod. Commun., 2020, 15(2), 1–10 CrossRef.
- X.-c. Shang, D. Chu, J.-x. Zhang, Y.-f. Zheng and Y. Li, Microwave-assisted extraction, partial purification and biological activity in vitro of polysaccharides from bladder-wrack (Fucus vesiculosus) by using deep eutectic solvents, Sep. Purif. Technol., 2021, 259, 118169 CrossRef CAS.
- J. Nie, D. Chen and Y. Lu, Deep Eutectic Solvents Based Ultrasonic Extraction of Polysaccharides from Edible Brown Seaweed Sargassum horneri, J. Mar. Sci. Eng., 2020, 8(6), 440 CrossRef.
- D. Mondal, M. Sharma, C.-H. Wang, Y.-C. Lin, H.-C. Huang and A. Saha, et al., Deep eutectic solvent promoted one step sustainable conversion of fresh seaweed biomass to functionalized graphene as a potential electrocatalyst, Green Chem., 2016, 18(9), 2819–2826 RSC.
- M. Sharma, D. Mondal, N. Singh, K. Upadhyay, A. Rawat and R. V. Devkar, et al., Seaweed-Derived Nontoxic Functionalized Graphene Sheets as Sustainable Materials for the Efficient Removal of Fluoride from High Fluoride Containing Drinking Water, ACS Sustainable Chem. Eng., 2017, 5(4), 3488–3498 CrossRef CAS.
- X. Li and K. H. Row, Application of novel ternary deep eutectic solvents as a functional monomer in molecularly imprinted polymers for purification of levofloxacin, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2017, 1068–1069, 56–63 CAS.
- G. Li, Y. Dai, X. Wang and K. Row, Molecularly Imprinted Polymers Modified by Deep Eutectic Solvents and Ionic Liquids with Two Templates for the Simultaneous Solid-Phase Extraction of Fucoidan and Laminarin from Marine Kelp, Anal. Lett., 2018, 52(3), 511–525 CrossRef.
- D. Lapena, D. Errazquin, L. Lomba, C. Lafuente and B. Giner, Ecotoxicity and biodegradability of pure and aqueous mixtures of deep eutectic solvents: glyceline, ethaline, and reline, Environ. Sci. Pollut. Res. Int., 2021, 28(7), 8812–8821 CrossRef CAS PubMed.
- I. Juneidi, M. Hayyan and M. A. Hashim, Evaluation of toxicity and biodegradability for cholinium-based deep eutectic solvents, RSC Adv., 2015, 5(102), 83636–83647 RSC.
- M. Vieira Sanches, R. Freitas, M. Oliva, A. Mero, L. De Marchi and A. Cuccaro, et al., Are natural deep eutectic solvents always a sustainable option? A bioassay-based study, Environ. Sci. Pollut. Res. Int., 2023, 30(7), 17268–17279 CrossRef CAS PubMed.
- H. Yaich, A. B. Amira, F. Abbes, M. Bouaziz, S. Besbes and A. Richel, et al., Effect of extraction procedures on structural, thermal and antioxidant properties of ulvan from Ulva lactuca collected in Monastir coast, Int. J. Biol. Macromol., 2017, 105, 1430–1439 CrossRef CAS PubMed.
- B. E. Abdelmalek, A. Sila, F. Krichen, W. Karoud, O. Martinez-Alvarez and S. Ellouz-Chaabouni, et al., Sulfated polysaccharides from Loligo vulgaris skin: Potential biological activities and partial purification, Int. J. Biol. Macromol., 2015, 72, 1143–1151 CrossRef CAS PubMed.
- M. A. Jmel, N. Anders, G. B. Messaoud, M. N. Marzouki, A. Spiess and I. Smaali, The stranded macroalga Ulva lactuca as a new alternative source of cellulose: extraction, physicochemical and rheological characterization, J. Cleaner Prod., 2019, 234, 1421–1427 CrossRef CAS.
- C. Rochas and M. Lahaye, Average molecular weight and molecular weight distribution of agarose and agarose-type polysaccharides, Carbohydr. Polym., 1989, 10(4), 289–298 CrossRef CAS.
- N. Wahlström, U. Edlund, H. Pavia, G. Toth, A. Jaworski and A. J. Pell, et al., Cellulose from the green macroalgae Ulva lactuca: isolation, characterization, optotracing, and production of cellulose nanofibrils, Cellulose, 2020, 27(7), 3707–3725 CrossRef.
- Y. Freile-Pelegrín, J. Azamar and D. Robledo, Preliminary characterization of carrageenan from the red seaweed Halymenia floresii, J. Aquat. Food Prod. Technol., 2011, 20(1), 73–83 CrossRef.
- P. Li, S. Wen, K. Sun, Y. Zhao and Y. Chen, Structure and bioactivity screening of a low molecular weight ulvan from the green alga ulothrix flacca, Mar. Drugs, 2018, 16(8), 281 CrossRef PubMed.
- A. Usov, Structural analysis of red seaweed galactans of agar and carrageenan groups, Food Hydrocolloids, 1998, 12(3), 301–308 CrossRef CAS.
- J. T. Kidgell, M. Magnusson, R. de Nys and C. R. Glasson, Ulvan: A systematic review of extraction, composition and function, Algal Res., 2019, 39, 101422 CrossRef.
- S. Kumar, G. K. Mehta, K. Prasad, R. Meena and A. K. Siddhanta, Chemical investigation of carrageenan from the red alga Sarconema filiforme (Gigartinales, Rhodophyta) of Indian waters, Nat. Prod. Commun., 2011, 6(9), 1934578X1100600928 CrossRef.
- M. Tabarsa, S.-J. Lee and S. You, Structural analysis of immunostimulating sulfated polysaccharides from Ulva pertusa, Carbohydr. Res., 2012, 361, 141–147 CrossRef CAS PubMed.
- M. P. Sudhakar, D. Magesh Peter and G. Dharani, Studies on the development and characterization of bioplastic film from the red seaweed (Kappaphycus alvarezii), Environ. Sci. Pollut. Res., 2021, 28(26), 33899–33913 CrossRef CAS PubMed.
- M. Moustafa, M. A. Abu-Saied, T. H. Taha, M. Elnouby, E. A. El Desouky and S. Alamri, et al., Preparation and Characterization of Super-Absorbing Gel Formulated from κ-Carrageenan–Potato Peel Starch Blended Polymers, Polymers, 2021, 13(24), 4308 CrossRef CAS PubMed.
- M. N. Davoodi, J. M. Milani and R. Farahmandfar, Preparation and characterization of a novel biodegradable film based on sulfated polysaccharide extracted from seaweed Ulva intestinalis, Food Sci. Nutr., 2021, 9(8), 4108–4116 CrossRef CAS PubMed.
- S. Zhang, F. Wei and X. Han, An edible film of sodium alginate/pullulan incorporated with capsaicin, New J. Chem., 2018, 42(21), 17756–17761 RSC.
- A. A. Abdillah and A. L. Charles, Characterization of a natural biodegradable edible film obtained from arrowroot starch and iota-carrageenan and application in food packaging, Int. J. Biol. Macromol., 2021, 191, 618–626 CrossRef CAS PubMed.
- A. A. Oun and J.-W. Rhim, Carrageenan-based hydrogels and films: Effect of ZnO and CuO nanoparticles on the physical, mechanical, and antimicrobial properties, Food Hydrocolloids, 2017, 67, 45–53 CrossRef CAS.
- C. Cheng, S. Chen, J. Su, M. Zhu, M. Zhou and T. Chen, et al., Recent advances in carrageenan-based films for food packaging applications, Front. Nutr., 2022, 9, 1004588 CrossRef PubMed.
- M. Guidara, H. Yaich, S. Benelhadj, Y. D. Adjouman, A. Richel and C. Blecker, et al., Smart ulvan films responsive to stimuli of plasticizer and extraction condition in physico-chemical, optical, barrier and mechanical properties, Int. J. Biol. Macromol., 2020, 150, 714–726 CrossRef CAS PubMed.
- Influence of carrageenan on the mechanical strength of starch bioplastic formed by extrusion process, in IOP Conference Series: Materials Science and Engineering, ed. H. Suryanto, A. Rahmawan, R. Sahana, M. Muhajir and U. Yanuhar, 2019, IOP Publishing Search PubMed.
- Physical properties of bioplastic agar/chitosan blend, in IOP Conference Series: Earth and Environmental Science, ed. D. Fransiska, T. Wahyuni, H. Irianto, P. Priambudi, A. Abdullah and R. Nissa, et al., 2022, IOP Publishing Search PubMed.
- M. S. A. Aziz, H. E. Salama and M. W. Sabaa, Biobased alginate/castor oil edible films for active food packaging, LWT–Food Sci. Technol., 2018, 96, 455–460 CrossRef.
- L. E. Abugoch, C. Tapia, M. C. Villamán, M. Yazdani-Pedram and M. Díaz-Dosque, Characterization of quinoa protein–chitosan blend edible films, Food Hydrocolloids, 2011, 25(5), 879–886 CrossRef CAS.
- S. Galus and A. Lenart, Development and characterization of composite edible films based on sodium alginate and pectin, J. Food Eng., 2013, 115(4), 459–465 CrossRef CAS.
- A. I. Balqis, M. N. Khaizura, A. Russly and Z. N. Hanani, Effects of plasticizers on the physicochemical properties of kappa-carrageenan films extracted from Eucheuma cottonii, Int. J. Biol. Macromol., 2017, 103, 721–732 CrossRef PubMed.
- Q. Tong, Q. Xiao and L. T. Lim, Effects of glycerol, sorbitol, xylitol and fructose plasticisers on mechanical and moisture barrier properties of pullulan–alginate–carboxymethylcellulose blend films, Int. J. Food Sci. Technol., 2013, 48(4), 870–878 CrossRef CAS.
- Z. Mahcene, A. Khelil, S. Hasni, P. K. Akman, F. Bozkurt and K. Birech, et al., Development and characterization of sodium alginate based active edible films incorporated with essential oils of some medicinal plants, Int. J. Biol. Macromol., 2020, 145, 124–132 CrossRef CAS PubMed.
- A. Sadeghi-Varkani, Z. Emam-Djomeh and G. Askari, Physicochemical and microstructural properties of a novel edible film synthesized from Balangu seed mucilage, Int. J. Biol. Macromol., 2018, 108, 1110–1119 CrossRef CAS PubMed.
- R. Gheribi, L. Puchot, P. Verge, N. Jaoued-Grayaa, M. Mezni and Y. Habibi, et al., Development of plasticized edible films from Opuntia ficus-indica mucilage: A comparative study of various polyol plasticizers, Carbohydr. Polym., 2018, 190, 204–211 CrossRef CAS PubMed.
- G. I. Olivas and G. V. Barbosa-Cánovas, Alginate–calcium films: Water vapor permeability and mechanical properties as affected by plasticizer and relative humidity, LWT–Food Sci. Technol., 2008, 41(2), 359–366 CrossRef CAS.
- T. Huq, S. Salmieri, A. Khan, R. A. Khan, C. Le Tien and B. Riedl, et al., Nanocrystalline cellulose (NCC) reinforced alginate based biodegradable nanocomposite film, Carbohydr. Polym., 2012, 90(4), 1757–1763 CrossRef CAS PubMed.
- J. A. Sirviö, A. Kolehmainen, H. Liimatainen, J. Niinimäki and O. E. Hormi, Biocomposite cellulose-alginate films: Promising packaging materials, Food Chem., 2014, 151, 343–351 CrossRef PubMed.
- T. Wittaya, Influence of type and concentration of plasticizers on the properties of edible film from mung bean proteins, Curr. J. Appl. Sci. Technol., 2013, 13(1), 51–58 Search PubMed.
- . Antifungal poly (lactic acid) films containing thymol and carvone, MATEC web of conferences, K.Boonruang, W. Chinsirikul, B. Hararak, N. Kerddonfag and V. Chonhenchob, 2016, EDP Sciences.
- E. Chiellini, P. Cinelli, V. I. Ilieva and M. Martera, Biodegradable thermoplastic composites based on polyvinyl alcohol and algae, Biomacromolecules, 2008, 9(3), 1007–1013 CrossRef CAS PubMed.
- Z. Ye, P. Ma, M. Tang, X. Li, W. Zhang and X. Hong, et al., Interactions between calcium alginate and carrageenan enhanced mechanical property of a natural composite film for general packaging application, Polym. Bull., 2017, 74(8), 3421–3429 CrossRef CAS.
- S. Nanaki, E. Karavas, L. Kalantzi and D. Bikiaris, Miscibility study of carrageenan blends and evaluation of their effectiveness as sustained release carriers, Carbohydr. Polym., 2010, 79(4), 1157–1167 CrossRef CAS.
- P. Wongphan and N. Harnkarnsujarit, Characterization of starch, agar and maltodextrin blends for controlled dissolution of edible films, Int. J. Biol. Macromol., 2020, 156, 80–93 CrossRef CAS PubMed.
- A. Awadhiya, S. Tyeb, K. Rathore and V. Verma, Agarose bioplastic–based drug delivery system for surgical and wound dressings, Eng. Life Sci., 2017, 17(2), 204–214 CrossRef CAS PubMed.
- L. Dou, B. Li, K. Zhang, X. Chu and H. Hou, Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols, Int. J. Biol. Macromol., 2018, 118, 1377–1383 CrossRef CAS PubMed.
- D. Ma, Y. Jiang, S. Ahmed, W. Qin and Y. Liu, Physical and antimicrobial properties of edible films containing Lactococcus lactis, Int. J. Biol. Macromol., 2019, 141, 378–386 CrossRef CAS PubMed.
- A. Farhan and N. M. Hani, Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol, Food Hydrocolloids, 2017, 64, 48–58 CrossRef CAS.
- M. Gomaa, A. F. Hifney, M. A. Fawzy and K. M. Abdel-Gawad, Use of seaweed and filamentous fungus derived polysaccharides in the development of alginate-chitosan edible films containing fucoidan: Study of moisture sorption, polyphenol release and antioxidant properties, Food Hydrocolloids, 2018, 82, 239–247 CrossRef CAS.
- K. M. Zia, F. Zia, M. Zuber, S. Rehman and M. N. Ahmad, Alginate based polyurethanes: A review of recent advances and perspective, Int. J. Biol. Macromol., 2015, 79, 377–387 CrossRef CAS PubMed.
- R. Gheorghita, G. Gutt and S. Amariei, The Use of Edible Films Based on Sodium Alginate in Meat Product Packaging: An Eco-Friendly Alternative to Conventional Plastic Materials, Coatings, 2020, 10(2), 166 CrossRef CAS.
- J.-Y. Lim, S.-L. Hii, S.-Y. Chee and C.-L. Wong, Sargassum siliquosum J. Agardh extract as potential material for synthesis of bioplastic film, J. Appl. Phycol., 2018, 30(6), 3285–3297 CrossRef CAS.
- V. Jost and M. Reinelt, Effect of Ca2 + induced crosslinking on the mechanical and barrier properties of cast alginate films, J. Appl. Polym. Sci., 2018, 135(5), 45754 CrossRef.
- L. C. Paixão, I. A. Lopes, A. K. D. Barros Filho and A. A. Santana, Alginate biofilms plasticized with hydrophilic and hydrophobic plasticizers for application in food packaging, J. Appl. Polym. Sci., 2019, 136(48), 48263 CrossRef.
- C. K. Yeom and K. H. Lee, Characterization of Sodium Alginate Membrane Crosslinked with Glutaraldehyde in Pervaporation Separation, J. Appl. Polym. Sci., 1997, 67, 209–219 CrossRef.
- W. W. Hu and Y. T. Lin, Alginate/polycaprolactone composite fibers as multifunctional wound dressings, Carbohydr. Polym., 2022, 289, 119440 CrossRef CAS PubMed.
- X. Wang, Y. Zhang, H. Liang, X. Zhou, C. Fang and C. Zhang, et al., Synthesis and properties of castor oil-based waterborne polyurethane/sodium alginate composites with tunable properties, Carbohydr. Polym., 2019, 208, 391–397 CrossRef CAS PubMed.
- A. Khan, T. Huq, M. Saha, R. A. Khan, M. A. Khan and M. A. Gafur, Effect of Silane Treatment on the Mechanical and Interfacial Properties of Calcium Alginate Fiber Reinforced Polypropylene Composite, J. Compos. Mater., 2010, 44(24), 2875–2886 CrossRef CAS.
- R. A. Khan, M. A. Khan, H. U. Zaman, M. Mollah, M. N. Khan and A. Khan, et al., Thermomechanical and Interfacial Properties of Calcium Alginate Fiber-Reinforced Polypropylene Composites, Polym.-Plast. Technol. Eng., 2010, 49(4), 325–331 CrossRef CAS.
- H. Wang, L. Sun, S. Wang, Y. Li, X. Jin and W. He, et al., A novel flame-retardant system toward polyester fabrics: flame retardant, anti-dripping and smoke suppression, J. Polym. Res., 2022, 29(5), 180 CrossRef CAS.
- J. Pallmann, Y. L. Ren, B. Mahltig and T. G. Huo, Phosphorylated sodium alginate/APP/DPER intumescent flame retardant used for polypropylene, J. Appl. Polym. Sci., 2019, 136(29), 47794 CrossRef.
- M. S. Kim and G. Kim, Three-dimensional electrospun polycaprolactone (PCL)/alginate hybrid composite scaffolds, Carbohydr. Polym., 2014, 114, 213–221 CrossRef CAS PubMed.
- D. Marković, H.-H. Tseng, T. Nunney, M. Radoičić, T. Ilic-Tomic and M. Radetić, Novel antimicrobial nanocomposite based on polypropylene non-woven fabric, biopolymer alginate and copper oxides nanoparticles, Appl. Surf. Sci., 2020, 527, 146829 CrossRef.
- Y. J. Lee and W. S. Lyoo, Preparation and characterization of high-molecular-weight atactic poly(vinyl alcohol)/sodium alginate/silver nanocomposite by electrospinning, J. Polym. Sci., Part B: Polym. Phys., 2009, 47(19), 1916–1926 CrossRef CAS.
- L. Fan, Y. Du, R. Huang, Q. Wang, X. Wang and L. Zhang, Preparation and characterization of alginate/gelatin blend fibers, J. Appl. Polym. Sci., 2005, 96(5), 1625–1629 CrossRef CAS.
- S. Safi, M. Morshed, S. A. Hosseini Ravandi and M. Ghiaci, Study of electrospinning of sodium alginate, blended solutions of sodium alginate/poly(vinyl alcohol) and sodium alginate/poly(ethylene oxide), J. Appl. Polym. Sci., 2007, 104(5), 3245–3255 CrossRef CAS.
- R. M. Gohil, Synergistic blends of natural polymers, pectin and sodium alginate, J. Appl. Polym. Sci., 2011, 120(4), 2324–2336 CrossRef CAS.
- C. K. Yeom and K. H. Lee, Characterization of Sodium Alginate and Poly (vinyl alcohol) Blend Membranes in Pervaporation Separation, J. Appl. Polym. Sci., 1998, 67, 949–959 CrossRef CAS.
- M. Reijonen , Tapio, inventorComposition and Manufacturing Process of Cetraria Islandica based polymer blend 2006.
- M. Zhang, F. Kievit, M. C.-h Leung and S. Florczyk, inventorsChitosan-Alginate Scaffold Cell Culture System and related methods 2015.
- P. G. Campbell, L. E. Weiss and J. Smith, inventorsBiocompatible polymers and Methods of use2010.
- J.-L. J. S. Wang , inventorSoy Protein-based Thermoplastic composition for preparing molded articles 1996.
- I. Farr, H. Hovagimian and T. M. Lambright, inventorsAlginate-based materials, methods of application thereof, and systems for using the Alginate-based materials 2005.
- P. Li, Q.-Z. Wang, B. Wang, Y.-Y. Liu, Y.-J. Xu and Y. Liu, et al., Blending alginate fibers with polyester fibers for flame-retardant filling materials: Thermal decomposition behaviors and fire performance, Polym. Degrad. Stab., 2021, 183, 109470 CrossRef CAS.
- J. M. Yang, P. K. Panda, C. J. Jie, P. Dash and Y. H. Chang, Poly (vinyl alcohol)/chitosan/sodium alginate composite blended membrane: Preparation, characterization, and water–induced shape memory phenomenon, Polym. Eng. Sci., 2022, 62(5), 1526–1537 CrossRef CAS.
- P. Kanmani and J. W. Rhim, Development and characterization of carrageenan/grapefruit seed extract composite films for active packaging, Int. J. Biol. Macromol., 2014, 68, 258–266 CrossRef CAS PubMed.
- C. Zhang, P. L. Show and S. H. Ho, Progress and perspective on algal plastics - A critical review, Bioresour. Technol., 2019, 289, 121700 CrossRef CAS PubMed.
- A. Aldalbahi, Nano-composite materials from carrageenan conducting polymers and carbon nanotubes, University of Wollogong, 2012 Search PubMed.
- A. Aldalbahi, J. Chu and P. Feng, In Het Panhuis M. Conducting composite materials from the biopolymer kappa-carrageenan and carbon nanotubes, Beilstein J. Nanotechnol., 2012, 3, 415–427 CrossRef PubMed.
- B. I. Dogaru, B. Simionescu and M. C. Popescu, Synthesis and characterization of kappa-carrageenan bio-nanocomposite films reinforced with bentonite nanoclay, Int. J. Biol. Macromol., 2020, 154, 9–17 CrossRef CAS PubMed.
- A. Nouri, M. Tavakkoli Yaraki, A. Lajevardi, T. Rahimi, M. Tanzifi and M. Ghorbanpour, An investigation of the role of fabrication process in the physicochemical properties of κ-carrageenan-based films incorporated with Zataria multiflora extract and nanoclay, Food Packag. Shelf Life, 2020, 23, 100435 CrossRef.
- L. Li, R. Ni, Y. Shao and S. Mao, Carrageenan and its applications in drug delivery, Carbohydr. Polym., 2014, 103, 1–11 CrossRef CAS PubMed.
- S. Lomartire, J. C. Marques and A. M. M. Gonçalves, An Overview of the Alternative Use of Seaweeds to Produce Safe and Sustainable Bio-Packaging, Appl. Sci., 2022, 12(6), 3123 CrossRef CAS.
- G. A. Paula, N. M. B. Benevides, A. P. Cunha, A. V. de Oliveira, A. M. B. Pinto and J. P. S. Morais, et al., Development and characterization of edible films from mixtures of κ-carrageenan, ι-carrageenan, and alginate, Food Hydrocolloids, 2015, 47, 140–145 CrossRef CAS.
- B. B. Sedayu, M. J. Cran and S. W. Bigger, A Review of Property Enhancement Techniques for Carrageenan-based Films and Coatings, Carbohydr. Polym., 2019, 216, 287–302 CrossRef CAS PubMed.
- E. Tavassoli-Kafrani, H. Shekarchizadeh and M. Masoudpour-Behabadi, Development of edible films and coatings from alginates and carrageenans, Carbohydr. Polym., 2016, 137, 360–374 CrossRef CAS PubMed.
- H. Suryanto, A. R. Solichin, R. T. Sahana, M. Muhajir and U. Yanuhar, Influence of Carrageenan on the Mechanical Strength of Starch Bioplastic Formed by Extrusion, IOP Conf. Ser.: Mater. Sci. Eng., 2019, 494, 012075 CAS.
- W. Zhang, Z. Xue, M. Yan, J. Liu and Y. Xia, Effect of epichlorohydrin on the wet spinning of carrageenan fibers under optimal parameter conditions, Carbohydr. Polym., 2016, 150, 232–240 CrossRef CAS PubMed.
- A. M. Ili Balqis, M. A. R. Nor Khaizura, A. R. Russly and Z. A. Nur Hanani, Effects of plasticizers on the physicochemical properties of kappa-carrageenan films extracted from Eucheuma cottonii, Int. J. Biol. Macromol., 2017, 103, 721–732 CrossRef CAS PubMed.
- M. P. Sudhakar, D. Magesh Peter and G. Dharani, Studies on the development and characterization of bioplastic film from the red seaweed (Kappaphycus alvarezii), Environ. Sci. Pollut. Res. Int., 2021, 28(26), 33899–33913 CrossRef CAS PubMed.
- M. P. Sudhakar, S. Venkatnarayanan and G. Dharani, Fabrication and characterization of bio-nanocomposite films using kappa-Carrageenan and Kappaphycus alvarezii seaweed for multiple industrial applications, Int. J. Biol. Macromol., 2022, 219, 138–149 CrossRef CAS PubMed.
- B. B. Sedayu, M. J. Cran and S. W. Bigger, Improving the moisture barrier and mechanical properties of semi–refined carrageenan films, J. Appl. Polym. Sci., 2020, 137, e49238 CrossRef.
- S. Shojaee-Aliabadi, H. Hosseini, M. A. Mohammadifar, A. Mohammadi, M. Ghasemlou and S. M. Hosseini, et al., Characterization of kappa-carrageenan films incorporated plant essential oils with improved antimicrobial activity, Carbohydr. Polym., 2014, 101, 582–591 CrossRef CAS PubMed.
- J. H. Choi, W. Y. Choi, D. S. Cha, M. J. Chinnan, H. J. Park and D. S. Lee, et al., Diffusivity of potassium sorbate in κ-carrageenan based antimicrobial film, LWT–Food Sci. Technol., 2005, 38(4), 417–423 CrossRef CAS.
- S. Saedi, M. Shokri and J.-W. Rhim, Preparation of carrageenan-based nanocomposite films incorporated with functionalized halloysite using AgNP and sodium dodecyl sulfate, Food Hydrocolloids, 2020, 106, 105934 CrossRef CAS.
- J. Nourmohammadi, F. Roshanfar and M. Farokhi, Haghbin Nazarpak M. Silk fibroin/kappa-carrageenan composite scaffolds with enhanced biomimetic mineralization for bone regeneration applications, Mater. Sci. Eng., C, 2017, 76, 951–958 CrossRef CAS PubMed.
- K. Prasad, Y. Kaneko and J. Kadokawa, Novel gelling systems of kappa-, iota- and lambda-carrageenans and their composite gels with cellulose using ionic liquid, Macromol. Biosci., 2009, 9(4), 376–382 CrossRef CAS PubMed.
- M. Moustafa, M. E. A. E. D. Elnouby, S. Alamri, M. A. A. Saied and T. H. Taha, et al., Preparation and Characterization of Super-Absorbing Gel Formulated from kappa-Carrageenan-Potato Peel Starch Blended Polymers, Polymers, 2021, 13(24), 4308 CrossRef CAS PubMed.
- C. Tranquilan-Aranillaa, F. Yoshii, A. M. D. Rosa and K. Makuuchi, Kappa-carrageenan-polyethylene oxide hydrogel blends prepared by gamma irradiation, Radiat. Phys. Chem., 1999, 127–131 CrossRef.
- J. S. Boateng, H. V. Pawar and J. Tetteh, Polyox and carrageenan based composite film dressing containing anti-microbial and anti-inflammatory drugs for effective wound healing, Int. J. Pharm., 2013, 441(1–2), 181–191 CrossRef CAS PubMed.
- M. Shahbazi, G. Rajabzadeh, A. Rafe, R. Ettelaie and S. J. Ahmadi, The physico-mechanical and structural characteristics of blend film of poly (vinyl alcohol) with biodegradable polymers as affected by disorder-to-order conformational transition, Food Hydrocolloids, 2016, 60, 393–404 CrossRef CAS.
- J. L. Olmedo-Martínez and A. Vega-Rios, Armando Zaragoza-Contreras E. Influence of iota-carrageenan on the morphology and electrical properties of poly(ortho-phenylenediamine) based copolymers, Synth. Met., 2019, 258, 116192 CrossRef.
- F. Meng, Y. Zhang, Z. Xiong, G. Wang, F. Li and L. Zhang, Mechanical, hydrophobic and thermal properties of an organic-inorganic hybrid carrageenan-polyvinyl alcohol composite film, Composites, Part B, 2018, 143, 1–8 CrossRef CAS.
- X. Yao, J. Liu, H. Hu, D. Yun and J. Liu, Development and comparison of different polysaccharide/PVA-based active/intelligent packaging films containing red pitaya betacyanins, Food Hydrocolloids, 2022, 124, 107305 CrossRef CAS.
- A. K. Arof, N. E. A. Shuhaimi, N. A. Alias, M. Z. Kufian and S. R. Majid, Application of chitosan/iota-carrageenan polymer electrolytes in electrical double layer capacitor (EDLC), J. Solid State Electrochem., 2010, 14(12), 2145–2152 CrossRef CAS.
- H. Ueno and M. Kaneko, Investigation of a nanostructured polysaccharide solid medium for electrochemistry, J. Electroanal. Chem., 2004, 568, 87–92 CrossRef CAS.
- S. Rudhziah, M. S. A. Rani, A. Ahmad, N. S. Mohamed and H. Kaddami, Potential of blend of kappa-carrageenan and cellulose derivatives for green polymer electrolyte application, Ind. Crops Prod., 2015, 72, 133–141 CrossRef CAS.
- M. M. Fouda, M. R. El-Aassar, G. F. El Fawal, E. E. Hafez, S. H. Masry and A. Abdel-Megeed, k-Carrageenan/poly vinyl pyrollidone/polyethylene glycol/silver nanoparticles film for biomedical application, Int. J. Biol. Macromol., 2015, 74, 179–184 CrossRef CAS PubMed.
- H. M. Hamzah, A. Osman and C. P. Tan, Mohamad Ghazali F. Carrageenan as an alternative coating for papaya (Carica papaya L. cv. Eksotika), Postharvest Biol. Technol., 2013, 75, 142–146 CrossRef CAS.
- R. E. Cian, P. R. Salgado, S. R. Drago and A. N. Mauri, Effect of glycerol and Ca+2 addition on physicochemical properties of edible carrageenan/porphyran-based films obtained from the red alga, Pyropia columbina, J. Appl. Phycol., 2014, 27(4), 1699–1708 CrossRef.
- V. D. Alves, N. Costa and I. M. Coelhoso, Barrier properties of biodegradable composite films based on kappa-carrageenan/pectin blends and mica flakes, Carbohydr. Polym., 2010, 79(2), 269–276 CrossRef CAS.
- V. Rajasekar, P. Karthickumar, A. H. R. Rose, N. Manimmehalai and D. Subhasri, Development and characterization of biodegradable film from marine red seaweed (Kappaphycus alvarezii), Pigm. Resin Technol., 2022, 52(4), 478–489 CrossRef.
- R. Thakur, B. Saberi, P. Pristijono, J. Golding, C. Stathopoulos and C. Scarlett, et al., Characterization of rice starch-iota-carrageenan biodegradable edible film. Effect of stearic acid on the film properties, Int. J. Biol. Macromol., 2016, 93(Pt A), 952–960 CrossRef CAS PubMed.
- X. Y. Yap, L. T. Gew, M. Khalid and Y.-Y. Yow, Algae-Based Bioplastic for Packaging: A Decade of Development and Challenges (2010–2020), J. Polym. Environ., 2022, 31, 833–851 CrossRef.
- F. R. Cindana Mo’o, G. Wilar, H. P. Devkota and N. Wathoni, Ulvan, a Polysaccharide from Macroalga Ulva sp.: A Review of Chemistry, Biological Activities and Potential for Food and Biomedical Applications, Appl. Sci., 2020, 10(16), 5488 CrossRef.
- L. A. Tziveleka, E. Ioannou and V. Roussis, Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: A review, Carbohydr. Polym., 2019, 218, 355–370 CrossRef CAS PubMed.
- D. S. Lakshmi, S. Sankaranarayanan, T. K. Gajaria, G. Li, W. Kujawski and J. Kujawa, et al., A Short Review on the Valorization of Green Seaweeds and Ulvan: FEEDSTOCK for Chemicals and Biomaterials, Biomolecules, 2020, 10(7), 991 CrossRef CAS PubMed.
- A. Ramu Ganesan, M. Shanmugam and R. Bhat, Producing novel edible films from semi refined carrageenan (SRC) and ulvan polysaccharides for potential food applications, Int. J. Biol. Macromol., 2018, 112, 1164–1170 CrossRef CAS PubMed.
- M. Guidara, H. Yaich, A. Richel, C. Blecker, S. Boufi and H. Attia, et al., Effects of extraction procedures and plasticizer concentration on the optical, thermal, structural and antioxidant properties of novel ulvan films, Int. J. Biol. Macromol., 2019, 135, 647–658 CrossRef CAS PubMed.
- M. Guidara, H. Yaich, S. Benelhadj, Y. D. Adjouman, A. Richel and C. Blecker, et al., Smart ulvan films responsive to stimuli of plasticizer and extraction condition in physico-chemical, optical, barrier and mechanical properties, Int. J. Biol. Macromol., 2020, 150, 714–726 CrossRef CAS PubMed.
- M. N. Davoodi, J. M. Milani and R. Farahmandfar, Preparation and characterization of a novel biodegradable film based on sulfated polysaccharide extracted from seaweed Ulva intestinalis, Food Sci. Nutr., 2021, 9(8), 4108–4116 CrossRef CAS PubMed.
- E. Chiellini, P. Cinelli, V. Ilieva and M. Martera, Biodegradable Thermoplastic Composites Based on Polyvinyl Alcohol and Algae, Biomacromolecules, 2008, 9, 1007–1013 CrossRef CAS PubMed.
- A. Alves, E. D. Pinho, N. M. Neves, R. A. Sousa and R. L. Reis, Processing ulvan into 2D structures: cross-linked ulvan membranes as new biomaterials for drug delivery applications, Int. J. Pharm., 2012, 426(1–2), 76–81 CrossRef CAS PubMed.
- H. H. Amin, Safe Ulvan Silver Nanoparticles Composite Films for Active Food Packaging, Am. J. Biochem. Biotechnol., 2021, 17(1), 28–39 CrossRef CAS.
- M. Gomaa, A. A. Al-Badaani, A. F. Hifney and M. S. Adam, Utilization of cellulose and ulvan from the green seaweed Ulva lactuca in the development of composite edible films with natural antioxidant properties, J. Appl. Phycol., 2022, 34(5), 2615–2626 CrossRef CAS.
- K. Mariia, M. Arif, J. Shi, F. Song, Z. Chi and C. Liu, Novel chitosan-ulvan hydrogel reinforcement by cellulose nanocrystals with epidermal growth factor for enhanced wound healing: In vitro and in vivo analysis, Int. J. Biol. Macromol., 2021, 183, 435–446 CrossRef CAS PubMed.
- G. Toskas, S. Heinemann, C. Heinemann, C. Cherif, R. D. Hund and V. Roussis, et al., Ulvan and ulvan/chitosan polyelectrolyte nanofibrous membranes as a potential substrate material for the cultivation of osteoblasts, Carbohydr. Polym., 2012, 89(3), 997–1002 CrossRef CAS PubMed.
- T.-M. Don, L.-M. Liu, M. Chen and Y.-C. Huang, Crosslinked complex films based on chitosan and ulvan with antioxidant and whitening activities, Algal Res., 2021, 58, 102423 CrossRef.
- M. Dash, S. K. Samal, A. Morelli, C. Bartoli, H. A. Declercq and T. E. L. Douglas, et al., Ulvan-chitosan polyelectrolyte complexes as matrices for enzyme induced biomimetic mineralization, Carbohydr. Polym., 2018, 182, 254–264 CrossRef CAS PubMed.
- P. Lavoisier, R. Pierre and M. Benoit, inventorsMethod for preparing an algae powder with reduced protein content and bioplastic composition formulated from such a powder patentWO 2017/046356 A1, 2017.
- G. Depres, J.-M. Vau and M. Pottier, inventorsMethods for preparing paper pulp and for manufacturing paper from seaweed powder patentWO 2012/114045 A1, 2012.
- A. Morelli, M. Betti, D. Puppi and F. Chiellini, Design, preparation and characterization of ulvan based thermosensitive hydrogels, Carbohydr. Polym., 2016, 136, 1108–1117 CrossRef CAS PubMed.
- A. A. Barros, A. Alves, C. Nunes, M. A. Coimbra, R. A. Pires and R. L. Reis, Carboxymethylation of ulvan and chitosan and their use as polymeric components of bone cements, Acta Biomater., 2013, 9(11), 9086–9097 CrossRef CAS PubMed.
- B. Li, F. Lu, X. Wei and R. Zhao, Fucoidan: structure and bioactivity, Molecules, 2008, 13(8), 1671–1695 CrossRef CAS PubMed.
- M. I. Bilan, N. E. Ustuzhanina, A. S. Shashkov, A. I. Usov, A. A. Grachev and N. E. Nifantiev, Structure of a fucoidan from the brown seaweed Fucus e anescens C.Ag, Carbohydr. Res., 2002, 337, 719–730 CrossRef CAS PubMed.
- K. Ozaltin, M. Lehocky, P. Humpolicek, J. Pelkova, A. Di Martino and I. Karakurt, et al., Anticoagulant Polyethylene Terephthalate Surface by Plasma-Mediated Fucoidan Immobilization, Polymers, 2019, 11(5), 750 CrossRef CAS PubMed.
- A. Bernal-Ballen, J. A. Lopez-Garcia and K. Ozaltin, (PVA/Chitosan/Fucoidan)-Ampicillin: A Bioartificial Polymeric Material with Combined Properties in Cell Regeneration and Potential Antibacterial Features, Polymers, 2019, 11(8), 1325 CrossRef CAS PubMed.
- P. Manivasagan, G. Hoang, M. Santha Moorthy, S. Mondal, V. H. Minh Doan and H. Kim, et al., Chitosan/fucoidan multilayer coating of gold nanorods as highly efficient near-infrared photothermal agents for cancer therapy, Carbohydr. Polym., 2019, 211, 360–369 CrossRef CAS PubMed.
- R. Falken , inventor; Bloom Holdings, LLC, assignee. <US20170142978A1.pdf>2017.
- R. H. Robert Falken and Z. Ashton, inventor < US20170183469A1.pdf>2017.
- K. Y. Perera, S. Sharma, D. Pradhan, A. K. Jaiswal and S. Jaiswal, Seaweed Polysaccharide in Food Contact Materials (Active Packaging, Intelligent Packaging, Edible Films, and Coatings), Foods, 2021, 10(9), 2088 CrossRef CAS PubMed.
- S. Nakamura, M. Nambu, T. Ishizuka, H. Hattori, Y. Kanatani and B. Takase, et al., Effect of controlled release of fibroblast growth factor-2 from chitosan/fucoidan micro complex-hydrogel on in vitro and in vivo vascularization, J. Biomed. Mater. Res., Part A, 2008, 85(3), 619–627 CrossRef PubMed.
- A. M. S. Costa, J. M. M. Rodrigues, M. M. Perez-Madrigal, A. P. Dove and J. F. Mano, Modular Functionalization of Laminarin to Create Value-Added Naturally Derived Macromolecules, J. Am. Chem. Soc., 2020, 142(46), 19689–19697 CrossRef CAS PubMed.
- S. Becker, A. Scheffel, M. F. Polz and J. H. Hehemann, Accurate Quantification of Laminarin in Marine Organic Matter with Enzymes from Marine Microbes, Appl. Environ. Microbiol., 2017, 83(9), e03389–16 CrossRef CAS PubMed.
- S. Becker, J. Tebben, S. Coffinet, K. Wiltshire, M. H. Iversen and T. Harder, et al., Laminarin is a major molecule in the marine carbon cycle, Proc. Natl. Acad. Sci. U. S. A., 2020, 117(12), 6599–6607 CrossRef CAS PubMed.
- R. V. Menshova, S. P. Ermakova, S. D. Anastyuk, V. V. Isakov, Y. V. Dubrovskaya and M. I. Kusaykin, et al., Structure, enzymatic transformation and anticancer activity of branched high molecular weight laminaran from brown alga Eisenia bicyclis, Carbohydr. Polym., 2014, 99, 101–109 CrossRef CAS PubMed.
- S. U. Kadam, C. P. O'Donnell, D. K. Rai, M. B. Hossain, C. M. Burgess and D. Walsh, et al., Laminarin from Irish Brown Seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound Assisted Extraction, Characterization and Bioactivity, Mar. Drugs, 2015, 13(7), 4270–4280 CrossRef CAS PubMed.
- Y. Li, Y. Zheng, Y. Zhang, Y. Yang, P. Wang and B. Imre, et al., Brown Algae Carbohydrates: Structures, Pharmaceutical Properties, and Research Challenges, Mar. Drugs, 2021, 19(11), 620 CrossRef CAS PubMed.
- R. S. Matche, G. J. Anup and G. Mrudula, Development of Biodegradable Films from Marine Ingredients Incorporated with Natural Antimicrobial Agents for Food Packaging, J. Packag. Technol. Res., 2020, 4(1), 45–55 CrossRef.
- N. Nanda and N. Bharadvaja, Algal bioplastics: current market trends and technical aspects, Clean Technol. Environ. Policy, 2022, 24(9), 2659–2679 CrossRef PubMed.
- G. Atiwesh, A. Mikhael, C. C. Parrish, J. Banoub and T. T. Le, Environmental impact of bioplastic use: A review, Heliyon, 2021, 7(9), e07918 CrossRef CAS PubMed.
- M. Ayala, M. Thomsen and M. Pizzol, Life Cycle Assessment of pilot scale production of seaweed-based bioplastic, Algal Res., 2023, 71, 103036 CrossRef.
- Y. Arora, S. Sharma and V. Sharma, Microalgae in Bioplastic Production: A Comprehensive Review, Arabian J. Sci. Eng., 2023, 48(6), 7225–7241 CrossRef CAS PubMed.
- W. Y. Cheah, A. C. Er, K. Aiyub, N. H. Mohd Yasin, S. L. Ngan and K. W. Chew, et al., Current status and perspectives of algae-based bioplastics: A reviewed potential for sustainability, Algal Res., 2023, 71, 103078 CrossRef.
- M. P. Sudhakar, R. Maurya, S. Mehariya, O. P. Karthikeyan, G. Dharani and K. Arunkumar, et al., Feasibility of bioplastic production using micro- and macroalgae- A review, Environ. Res., 2024, 240(Pt 2), 117465 CrossRef CAS PubMed.
Footnote |
| † All authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |