Open Access Article
Open Access ArticleGreen ammonia synthesis from stationary NOx emission sources on a catalytic lean NOx trap†
Frea
Van Steenweghen
 ,
Lander
Hollevoet
,
Lander
Hollevoet
 and
Johan A.
Martens
and
Johan A.
Martens
 *
*
Surface Chemistry and Catalysis: Characterisation and Application Team (COK-KAT), KU Leuven, Leuven BE-3001, Belgium. E-mail: johan.martens@kuleuven.be
First published on 6th February 2024
Abstract
A process for producing ammonia out of NOx from hydrogen engine flue gases is proposed. NOx is captured on a lean NOx trap (LNT) and catalytically reduced with hydrogen to ammonia (NOCCRA). The energy requirement is similar to that of Haber–Bosch processes. NOCCRA is attractive for decentralised green NH3 production.
Ammonia (NH3) is the base chemical for producing N-fertilisers, explosives, and nitrogen-containing organic chemicals, and its use as an energy vector is emerging. The global NH3 market is expected to continue growing in the next decennium.1–6 NH3 is mainly produced from nitrogen gas (N2) and hydrogen gas (H2) with Haber–Bosch (HB) processes, at a production volume of 185 M tonnes in 2020.3,7 Fossil hydrocarbon-based HB is responsible for nearly 2% of the global anthropogenic CO2 emission.2,3,8 Green ammonia synthesis avoiding the use of fossil carbon is one of the big challenges preoccupying the scientific community.9
The natural gas-based HB process in which N2 from air is reduced with H2 from steam methane reforming (SMR) is very energy-efficient, with an energy cost as low as 0.48 MJ molNH3−1.2,5,6 The use of natural gas as an energy and H-atom source entails a CO2 emission of ca. 1.6 tonne per tonne of NH3 produced.5,6 An obvious way of rendering NH3 synthesis more sustainable is by using green instead of grey hydrogen. An HB plant, running with hydrogen from water electrolysis using renewable electricity, has an estimated energy cost of ca. 0.65–0.70 MJ molNH3−1.2,10
Powering the HB process with renewable energy is challenging because of the large scale at which the process is cost-effective. The economy of scale of HB results from the need for a reaction pressure of 10–40 MPa at 400–650 °C. Such reaction conditions impose a requirement of continuous operation3,11,12 which does not align well with variable renewable electricity supply.2,6 Furthermore, the application on land of N-fertiliser of ammonia and ammonium nitrate (NH4NO3) derived from it is highly decentralised.13 This makes small-scale ammonia production under mild reaction conditions with flexible production schemes, despite a slightly higher energy cost, a viable alternative to the centralised HB process.1,3,14 Local production complementing centralised production could solve supply chain problems and price volatility, especially for remote farming areas.15
Several concepts for the synthesis of ammonia from atmospheric N2 using renewable energy sources have been proposed, like (i) direct electrocatalytic reduction,16,17 (ii) plasma-enabled synthesis,18 and (iii) chemical looping,19,20 as documented in reviews.4,6,21 Electrocatalytic N2 reduction suffers from very low yield.22 Plasma processes have an energy cost many times higher than those of HB processes.23 Chemical looping is facing some challenges related to mass transfer, cyclability, material volumes and cost.21,24 An additional option for producing ammonia is to extract and convert N-sources contained in side products and waste streams from the agro-industry, contributing in this way to N-circularity.25–27
The N2 molecule is very difficult to activate for chemical reaction. Oxidation of N2 molecules to nitrogen oxides (NOx) or nitrates (NO3−) leads to more reactive N-species. The dissociation energy of the N–O bond in the NO molecule of 204 kJ mol−1 (ref. 28) is much lower than the energy needed for cleaving the triple N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond in the N2 molecule (942 kJ mol−1 (ref. 29)).30 In an approach proposed earlier30,31 atmospheric N2 is oxidised using a plasma process.27,30 The downside of oxidising first and performing the reduction to ammonia in a second step is the need for more hydrogen molecules per ammonia molecule (eqn (1) compared to 2). Nevertheless, producing ammonia according to the reaction of eqn (1) could be attractive because of the less severe reaction conditions.11,25,30,31
N bond in the N2 molecule (942 kJ mol−1 (ref. 29)).30 In an approach proposed earlier30,31 atmospheric N2 is oxidised using a plasma process.27,30 The downside of oxidising first and performing the reduction to ammonia in a second step is the need for more hydrogen molecules per ammonia molecule (eqn (1) compared to 2). Nevertheless, producing ammonia according to the reaction of eqn (1) could be attractive because of the less severe reaction conditions.11,25,30,31
| 2NO + 5H2 → 2NH3 + 2H2O | (1) |
| N2 + 3H2 → 2NH3 | (2) |
| 4NO + 4NH3 + O2 → 4N2 + 6H2O | (3) |
| 2NO2 + 4NH3 + O2 → 3N2 + 6H2O | (4) |
| NO + NO2 + 2NH3 → 2N2 + 3H2O | (5) |
| NH2CO2NH2 + H2O → 2NH3 + CO2 | (6) |
One of the commercial NOx abatement technologies for lean-burn automobile combustion engines is the lean NOx trap (LNT) also known as the NOx storage and reduction (NSR) system or NOx adsorber.41 The washcoat of an LNT monolith typically consists of platinum and barium oxide supported on alumina (Pt–Ba/Al2O3).42,43 An internal combustion engine equipped with LNT is alternatingly run on lean and rich fuel mixtures. During the lean phase, NOx is trapped on the LNT and converted to barium nitrate (Ba(NO3)2) (eqn (7)). During the rich phase, the LNT is regenerated by chemical reduction of the trapped nitrates with reductants produced in the engine (eqn (8)).42 Among H2, CO and hydrocarbon reductants in the exhaust from rich fuel mixtures, at low temperatures H2 is most reactive.44
In the pollution abatement application, N2 is the desired reaction product (eqn (8)). NH3 is a reaction intermediate in the two-step formation of N2 (eqn (9) and (10)), while N2O is an unwanted by-product (eqn (11)).43,45–48 The reaction of eqn (10) is slow and rate determining for the formation of N2 at temperatures below 150 °C.48 Limiting the conversion of Ba(NO3)2 to the first step (eqn (9)) is a way to produce ammonia. High NH3-selectivity of nitrate reduction on LNT catalysts in the range of 75–90% has been reported.47–52 Provided green hydrogen is available, the ammonia produced in this way on an LNT can be qualified as “green”.
| BaO + 2NO2 + ½O2 → Ba(NO3)2 | (7) |
| Ba(NO3)2 + 5H2 → N2 + BaO + 5H2O | (8) |
| Ba(NO3)2 + 8H2 → 2NH3 + BaO + 5H2O | (9) |
| 3Ba(NO3)2 + 10NH3 → 8N2 + BaO + 15H2O | (10) |
| Ba(NO3)2 + 4H2 → N2O + BaO + 4H2O | (11) |
Flue gases of stationary emission sources are considered as a NOx source for a NOx capture and catalytic reduction to ammonia process, NOCCRA. The use of NOx emission sources for ammonia synthesis has three major advantages: (i) the use of an inexpensive source of NOx which otherwise would entail a cost of elimination, (ii) resolving the NOx emission issue of flue gases, and (iii) in contrast to state-of-the-art NOx abatement technologies, NH3 is now produced instead of consumed for reducing NOx to N2.
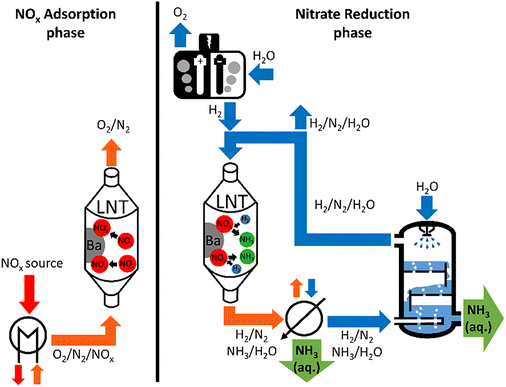
NOx-containing flue gas is the feed of the NOCCRA process (Fig. 1). It is sent over an LNT to selectively chemisorb the NOx. During the subsequent reduction phase, nitrate molecules stored on the LNT are reduced with green H2 from an electrolyser to ammonia which desorbs spontaneously. NH3 is separated from the outlet gas stream by phase transfer to an aqueous solution in a washing column. In this way, residual H2 remaining in the gas phase can be recovered and reused. The LNT alternates between phases of NOx trapping and chemical reduction. For achieving continuous ammonia production with NOCCRA at least two LNT units, alternating between NOx adsorption and NH3 formation, are needed.
Experimental details of the laboratory setup are provided in the ESI (sections 1 and 2†). Pt/Ba/Al2O3 catalyst pellets were prepared as described earlier.31 The Pt/Ba/Al2O3 weight ratios were 1/20/100. The performance of Pt/Ba/Al2O3 pellets in a NOCCRA cycle was investigated in a tubular flow reactor described earlier.31 More information about the automated reactor set-up can be found in ref. 53. A flue gas mimic composed of 200 ppm NO, 5% O2 and 1.5% H2O in N2 carrier gas was fed to the reactor at a gas flow rate of 0.1 mL h−1 g−1 for 250 s. Chemical reduction of adsorbed NOx was performed using a gas mixture of 5% H2 with 1.5% H2O in N2 carrier gas at the same gas feed rate and temperature during 1800 s. The temperature was varied in the range of 75–200 °C. The operating pressure was always atmospheric.
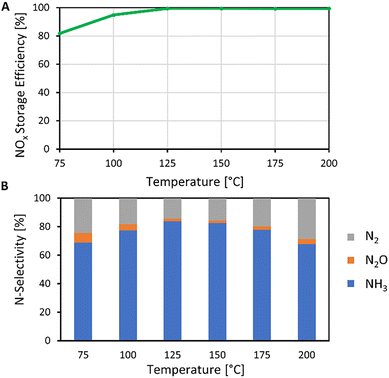
At temperatures above 100 °C all NOx in the feed was captured on the Pt/Ba/Al2O3 LNT material (Fig. 2A). The NH3-selectivity showed a maximum of 84% at 125 °C (Fig. 2B). The N2O-selectivity showed a minimum of 1.7% at 150 °C. Over the investigated reaction temperature range, the N2O-concentration in the product attained an average of ca. 0.4 ppm, which is four orders of magnitudes lower than the typical N2O concentration in the flue gas of nitric acid plants.54,55 These results confirm the feasibility of producing ammonia from flue gas on an LNT.
NOx adsorption and its reduction to NH3 using hydrogen are exothermic processes.42 It is envisioned to use hot flue gas as a feed such that NOCCRA will be self-sufficient in terms of heating. Energy is consumed in the production of hydrogen gas, needed for reducing the adsorbed NOx. The NOCCRA energy consumption essentially depends on the energy efficiency of the electrolyser and the NH3-selectivity of nitrate reduction reached on the LNT. As excess, unconverted H2 is separated from the produced NH3 in the washing column and recirculated in the NOCCRA process, near-complete utilization of H2 can be achieved. Stoichiometrically 4 moles of H2 are needed for producing 1 mol of NH3 (eqn (8)). Hence, assuming 100% NH3-selectivity of the barium nitrate reduction reaction, the hydrogen production in an electrolyser with an efficiency of 70%56 would need 1.37 MJ per mol NH3 (estimations detailed in the ESI, section 3†). At an NH3-selectivity of 84% reached experimentally at 125 °C (Fig. 2B), the energy consumption amounts to 1.50 MJ molNH3−1. In earlier work, a related process called PNOCRA (“plasma nitrogen oxidation coupled with catalytic reduction to ammonia”) was proposed, in which the feed consists of NOx generated from air by a plasma reactor.30,31 The plasma process producing NOx requires energy, lifting the total energy cost of ammonia production with PNOCRA to 2.10 MJ molNH3−1.31
Natural gas-based HB processes are run at an energy cost of 0.47–0.71 MJ molNH3−1.2,5 Green HB processes in which H2 is generated by H2O electrolysis have energy costs of 0.65–0.70 MJ molNH3−1.2,10 While NOCCRA is more energy demanding, its benefits are rather indirect. Besides producing ammonia, NOCCRA can be considered to be a depollution technique for NOx emissions. State-of-the-art NOx emission abatement techniques, such as NH3-SCR and SNCR, consume NH3, while NOCCRA produces it. NH3-SCR and SNCR require 1 mol of NH3 to eliminate 1 mol of NOx.37,38 In this way, the NOCCRA process saves 1 mol of NH3 and generates 1 mol of NH3. Taking this benefit into consideration, the energy requirement of NOCCRA is reduced to 0.75 MJ molNH3−1, making it more competitive with HB processes (Fig. 3).
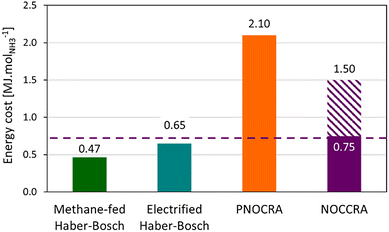 | ||
| Fig. 3 Energy requirement [MJ molNH3−1] of the ammonia synthesis processes: natural gas-based Haber–Bosch,2 electrified Haber–Bosch,2 PNOCRA31 and NOCCRA with and without accounting for the savings of NH3 by avoiding the need for an S(N)CR process for NOx abatement. | ||
Besides the operating cost of which energy is the largest share, the installation cost of NOCCRA determines the economic viability. In this early stage of research and development, a detailed techno-economic analysis would be inaccurate. Qualitative comparison of NOCCRA with competing processes hints at its potential for practical application. NOCCRA, being an integration of green ammonia production and NOx abatement, intrinsically has the potential to lower the equipment cost. Compared to the HB process for ammonia synthesis, NOCCRA operates at much lower pressures and temperatures. Long lifetimes are predicted for the lean NOx trap of NOCCRA based on experience in the automotive industry, but the downside of this benefit is the use of a noble metal-based catalyst (Pt).
In the NOCCRA process, ammonia is recovered at the outlet of the LNT using a gas scrubber (Fig. 1). The scrubber can be run using water, sulphuric acid (H2SO4) or nitric acid (HNO3) to produce ammonium sulphate ((NH4)2SO4) or ammonium nitrate (NH4NO3), respectively.3 The product is directly applicable as fertiliser. Concentration of the ammonia is not needed for that application, implying no additional energy cost.57 In this manner, NOCCRA also avoids corrosive substances like anhydrous ammonia, which enables the use of low-cost materials for reactors and conducts.
Further potential advantages of NOCCRA are related to the intermittent character of renewable energy supply. An electrified HB process will produce green NH3 in large-scale, highly centralised production facilities,58 which require a steady supply of vast amounts of green electricity. However, when green electricity is intermittently available, and when NOx emissions are present, NOCCRA becomes attractive for the distributed small- and medium-scale production of NH3-based fertilisers.16,17 Intermittent green hydrogen production and storage are easy to accommodate in small plants running a NOCCRA process. Given that locally produced NH3 with NOCCRA is not intended to be stored over long periods nor transported over long distances, it can be applied directly to fields via fertigation, or irrigation with fertiliser solutions.11,59 The commercial use of LNT technology in the automotive industry provides evidence for scalability.60
One limitation of NOCCRA is the need for a local NOx source. Stationary sources are qualified, while mobile applications such as automobiles would be impractical due to the need for on-board ammonia storage. Another limitation is that agricultural activities need to be present within the vicinity of the NOx source to provide a market for the ammonia fertiliser product.
The exhaust from fossil fuel-based combustion processes contains sulphur oxides (SOx) which are poisons of LNT catalysts so that they require periodic regeneration.61,62 The transition towards green energy carriers, such as hydrogen gas, offers a futureproof perspective overcoming this sulphur problem. Combustion of hydrogen causes thermal NOx formation, but the absence of CO2 and SOx in the exhaust gas makes it an ideal inlet gas feed for the NOCCRA process.63,64
The potential of NOCCRA for distributed production of ammonia is illustrated by the following case of a hydrogen-fuelled combustion engine for electricity generation.65 Small-scale internal engines, such as gas turbines or combined heat and power systems, with a capacity of 100 kW and powered by hydrogen gas at 100%, have a flue gas flow rate of 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 m3 h−1.66 Flue gases of such hydrogen engines typically contain NOx at an average concentration of 500 ppm.65 Such a NOx source represents a potential NH3 production capacity of 62.5 tonnes per year, assuming an NH3-selectivity of 84% (Fig. 2B). This local NH3 production by one industrial NOCCRA plant would meet the yearly fertiliser demand of ca. 730 ha of cropland,13 assuming an average nitrogen fertiliser demand of 86 kgN ha−1 in the European Union67 (estimation provided in the ESI, section 4†).
400 m3 h−1.66 Flue gases of such hydrogen engines typically contain NOx at an average concentration of 500 ppm.65 Such a NOx source represents a potential NH3 production capacity of 62.5 tonnes per year, assuming an NH3-selectivity of 84% (Fig. 2B). This local NH3 production by one industrial NOCCRA plant would meet the yearly fertiliser demand of ca. 730 ha of cropland,13 assuming an average nitrogen fertiliser demand of 86 kgN ha−1 in the European Union67 (estimation provided in the ESI, section 4†).
Conclusion & future perspectives
A novel green ammonia production process, called NOCCRA (NOx capture & catalytic reduction to ammonia) based on stationary NOx emissions, water and renewable electricity is proposed. The NOCCRA process works with alternating phases of NOx storage and catalytic reduction of temporarily stored NOx with green hydrogen to NH3 on a lean NOx trap such as those employed for exhaust gas purification in automotive applications. Experimentally, a NOx storage efficiency of almost 100% and an NH3-selectivity of 84% were achieved with an LNT catalyst composed of Pt/Ba/Al2O3 at atmospheric pressure and a temperature of 125 °C.The energy cost of ammonia production with NOCCRA is estimated at 1.50 MJ molNH3−1. Considering that NOCCRA avoids NH3 consumption for abating NOx emissions the net energy cost becomes competitive with electrified HB processes. The NOCCRA process serves as both a green ammonia production method and a NOx abatement technique, consolidating two distinct processes into a single one, thereby offering potential advantages in terms of reduced installation cost.
NOCCRA enables the small-scale decentralised production of green ammonium nitrate fertiliser in remote farming areas. NOCCRA uses intermittently available renewable energy sources. A convincing example of a hydrogen engine from which the NOx emission is used to produce green ammonium nitrate is presented.
Some scientific challenges remain to make NOCCRA fully competitive, including better matching of the two phases of the NOCCRA cycle and catalyst development to enhance the NH3-selectivity and productivity of the precious metal catalyst. Platinum is a high-cost precious metal, and therefore, replacing it with Earth abundant metals would reduce the cost of the NOCCRA process.68
Author contributions
Van Steenweghen Frea: investigation, data curation, visualization, writing – original draft. Hollevoet Lander: conceptualization, formal analysis, writing – review & editing. Martens Johan: supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
F. V. S. acknowledges Research Foundation Flanders (FWO) for an FWO-SB fellowship (No. 1S58723N). J. A. M. acknowledges the Flemish Government for long-term structural funding (Methusalem). This work was supported by the Flemish Industrial Research fund (IOF) (project No. C3/20/067). The table of contents has been designed using a picture captured by Johannes Plenio, acquired from the online platform Pexels.References
- M. Wang, M. A. Khan, I. Mohsin, J. Wicks, A. H. Ip, K. Z. Sumon, C. T. Dinh, E. H. Sargent, I. D. Gates and M. G. Kibria, Energy Environ. Sci., 2021, 14, 2535–2548 RSC.
- C. Smith, A. K. Hill and L. Torrente-Murciano, Energy Environ. Sci., 2020, 13, 331–344 RSC.
- International Energy Agency, Ammonia Technology Roadmap Towards more sustainable nitrogen fertiliser production, 2021 Search PubMed.
- S. Ghavam, M. Vahdati, I. A. G. Wilson and P. Styring, Front. Energy Res., 2021, 1–19 Search PubMed.
- J. Brightling, Johnson Matthey Technol. Rev., 2018, 62, 32–47 CrossRef CAS.
- D. R. MacFarlane, P. V. Cherepanov, J. Choi, B. H. R. Suryanto, R. Y. Hodgetts, J. M. Bakker, F. M. Ferrero Vallana and A. N. Simonov, Joule, 2020, 4, 1186–1205 CrossRef CAS.
- International Renewable Energy Agency (IRENA) and Ammonia Energy Association (AEA), Innovation outlook: Renewable Ammonia, Abu Dhabi, 2022 Search PubMed.
- J. Humphreys, R. Lan and S. Tao, Adv. Energy Sustainability Res., 2021, 2, 2000043 CrossRef CAS.
- M. Jain, R. Muthalathu and X.-Y. Wu, iScience, 2022, 25, 104724 CrossRef CAS PubMed.
- L. Wang, M. Xia, H. Wang, K. Huang, C. Qian, C. T. Maravelias and G. A. Ozin, Joule, 2018, 2, 1055–1074 CrossRef CAS.
- C. Butler, Y. Fan, S. Grewal and L. R. Winter, ACS Sustainable Chem. Eng., 2023, 11, 5803–5818 CrossRef CAS.
- A. Dechany, K. Van Geem and J. Proost, Curr. Opin. Chem. Eng., 2023, 40, 100915 CrossRef.
- J. von Braun and A. Mirzabaev, Small Farms: Changing Structures and Roles in Economic Development, Zentrum fur Entwicklungsforschung (ZEF), Bonn, 2015, vol. 31 Search PubMed.
- T. Brown, The capital intensity of small-scale ammonia plants, 2018, https://www.ammoniaenergy.org/articles/the-capital-intensity-of-small-scale-ammonia-plants/, (accessed 27 October 2023) Search PubMed.
- J. Osorio-Tejada, N. N. Tran and V. Hessel, Sci. Total Environ., 2022, 826, 154162 CrossRef CAS PubMed.
- G. Qing, R. Ghazfar, S. T. Jackowski, F. Habibzadeh, M. M. Ashtiani, C.-P. Chen, M. R. Smith and T. W. Hamann, Chem. Rev., 2020, 120, 5437–5516 CrossRef CAS PubMed.
- M. Jewess and R. H. Crabtree, ACS Sustainable Chem. Eng., 2016, 4, 5855–5858 CrossRef CAS.
- P. Peng, P. Chen, C. Schiappacasse, N. Zhou, E. Anderson, D. Chen, J. Liu, Y. Cheng, R. Hatzenbeller, M. Addy, Y. Zhang, Y. Liu and R. Ruan, J. Cleaner Prod., 2018, 177, 597–609 CrossRef CAS.
- J. M. McEnaney, A. R. Singh, J. A. Schwalbe, J. Kibsgaard, J. C. Lin, M. Cargnello, T. F. Jaramillo and J. K. Nørskov, Energy Environ. Sci., 2017, 10, 1621–1630 RSC.
- W. Gao, J. Guo, P. Wang, Q. Wang, F. Chang, Q. Pei, W. Zhang, L. Liu and P. Chen, Nat. Energy, 2018, 3, 1067–1075 CrossRef CAS.
- S. Yu, T. Xiang, N. S. Alharbi, B. A. Al-aidaroos and C. Chen, Chin. J. Chem. Eng., 2023, 62, 65–113 CrossRef.
- S. Giddey, S. P. S. Badwal and A. Kulkarni, Int. J. Hydrogen Energy, 2013, 38, 14576–14594 CrossRef CAS.
- L. Hollevoet, M. de Ras, M. Roeffaers, J. Hofkens and J. A. Martens, ACS Energy Lett., 2020, 5, 1124–1127 CrossRef CAS.
- S. Brown and J. Hu, Chem. Eng. Sci., 2023, 280, 119063 CrossRef CAS.
- R. Daiyan, T. Tran-Phu, P. Kumar, K. Iputera, Z. Tong, J. Leverett, M. H. A. Khan, A. Asghar Esmailpour, A. Jalili, M. Lim, A. Tricoli, R. S. Liu, X. Lu, E. Lovell and R. Amal, Energy Environ. Sci., 2021, 14, 3588–3598 RSC.
- J. Long, S. Chen, Y. Zhang, C. Guo, X. Fu, D. Deng and J. Xiao, Angew. Chem., Int. Ed., 2020, 59, 9711–9718 CrossRef CAS PubMed.
- J. Lim, C. A. Fernández, S. W. Lee and M. C. Hatzell, ACS Energy Lett., 2021, 6, 3676–3685 CrossRef CAS.
- Y. Yu, C. Wang, Y. Yu, Y. Wang and B. Zhang, Sci. China: Chem., 2020, 63, 1469–1476 CrossRef CAS.
- H. Liu, Chin. J. Catal., 2014, 35, 1619–1640 CrossRef CAS.
- L. Hollevoet, F. Jardali, Y. Gorbanev, J. Creel, A. Bogaerts and J. A. Martens, Angew. Chem., Int. Ed., 2020, 59, 23825–23829 CrossRef CAS PubMed.
- L. Hollevoet, E. Vervloessem, Y. Gorbanev, A. Nikiforov, N. De Geyter, A. Bogaerts and J. A. Martens, ChemSusChem, 2022, 15, 10 CrossRef PubMed.
- Environmental Protection Agency (EPA), Alternative control techniques document - NOx emissions from cement manufacturing, 1994 Search PubMed.
- European Environmental Agency (EEA), Nitrogen oxides (NOx) emissions, 2021, https://www.eea.europa.eu/data-and-maps/indicators/eea-32-nitrogen-oxides-nox-emissions-1/assessment.2010-08-19.0140149032-3, (accessed 15 January 2023).
- Environmental Protection Agency (EPA), Nitrogen Oxides (NOx), Why and How They Are Controlled, 1999 Search PubMed.
- B. W. Doyle and C. Solt, NOx Emissions Control from Stationary Sources, 2014, vol. 215 Search PubMed.
- Z. Zhu and B. Xu, Purification Technologies for NOx Removal from Flue Gas: A Review, MDPI, 2022, vol. 9 Search PubMed.
- J. L. Sorrels, D. D. Randall, K. S. Schaffner and C. Richardson Fry, Chapter 2 Selective Catalytic Reduction, 2019 Search PubMed.
- J. L. Sorrels, D. D. Randall, C. R. Fry and K. S. Schaffner, Chapter 1 - Selective Noncatalytic Reduction, 2019 Search PubMed.
- Manufacturers of Emission Controls Association (MECA), Emission Control Technology for Stationary Internal Combustion Engines, Arlington, 2015 Search PubMed.
- Environmental Protection Agency (EPA), Section 4 NO Controls x Section 4.2 NO Post-Combustion x, 2002 Search PubMed.
- P. Granger and V. I. Parvulescu, Catalytic NOx abatement systems for mobile sources: From three-way to lean burn after-treatment technologies, 2011, vol. 111 Search PubMed.
- P. Forzatti, L. Castoldi, L. Lietti, I. Nova and E. Tronconi, in Past and Present in DeNOx Catalysis, ed. P. Granger and V. Pârvulescu, Elsevier Science, 1st edn, 2007, vol. 418, pp. 175–208 Search PubMed.
- G. Liu and P. X. Gao, Catal. Sci. Technol., 2011, 1, 552–568 RSC.
- A. Reihani, B. Corson, J. W. Hoard, G. B. Fisher, E. Smirnov, D. Roemer, J. Theis and C. Lambert, SAE Int. J. Engines, 2016, 9, 1630–1641 CrossRef.
- D. Mráček, P. Kočí, J. S. Choi and W. P. Partridge, Appl. Catal., B, 2016, 182, 109–114 CrossRef.
- A. S. Kota, D. Luss and V. Balakotaiah, Chem. Eng. J., 2015, 262, 541–551 CrossRef CAS.
- P. Forzatti, L. Lietti and N. Gabrielli, Appl. Catal., B, 2010, 99, 145–155 CrossRef CAS.
- L. Lietti, I. Nova and P. Forzatti, J. Catal., 2008, 257, 270–282 CrossRef CAS.
- C. D. DiGiulio, J. A. Pihl, J.-S. Choi, J. E. Parks, M. J. Lance, T. J. Toops and M. D. Amiridis, Appl. Catal., B, 2014, 147, 698–710 CrossRef CAS.
- B. M. Shakya, M. P. Harold and V. Balakotaiah, Chem. Eng. J., 2013, 230, 584–594 CrossRef CAS.
- R. D. Clayton, M. P. Harold, V. Balakotaiah and C. Z. Wan, Appl. Catal., B, 2009, 90, 662–676 CrossRef CAS.
- F. Van Steenweghen, L. Hollevoet and J. A. Martens, Catal. Today, 2023, 114499 CrossRef.
- M. De Prins, E. Verheyen, A. Hoffmann, G. Vanbutsele, S. P. Sree, S. Kerkhofs, L. Van Tendeloo, F. W. Schütze and J. Martens, J. Catal., 2020, 390, 224–236 CrossRef CAS.
- M. Kamphus, Emission monitoring in nitric acid plants, 2014 Search PubMed.
- Environmental Protection Agency (EPA), Available and Emerging Technologies for Reducing Greenhouse Gas Emissions from the Nitric Acid Production Industry, North Carolina, 2010 Search PubMed.
- J. Brauns and T. Turek, Processes, 2020, 8, 248 CrossRef CAS.
- European Commission, Large Volume Inorganic Chemicals - Ammonia, Acids and Fertilisers, 2007 Search PubMed.
- C. Philibert, Renewable Energy for Industry: From green energy to green materials and fuels, Paris, 2017 Search PubMed.
- U. Kafkafi and J. Tarchitzky and Impr. Point 44, Fertigation: a tool for efficient fertilizer and water management, International fertilizer industry Association, Paris, 1st edn, 2011, vol. 123 Search PubMed.
- W. A. Majewski, NOx Adsorber Applications, 2020, https://dieselnet.com/tech/cat_nox-trap_applications.php, (accessed 16 January 2024) Search PubMed.
- J. Li, J. R. Theis, W. Chun, C. T. Goralski, R. J. Kudla, W. L. Watkins and R. H. Hurley, Sulfur Poisoning and Desulfation of the Lean NOx Trap, 2001 Search PubMed.
- C. Lijuan, L. Zhijun, H. Li, S. Boxi, Z. Lingya and W. Yue, Energy Procedia, 2019, 158, 4383–4388 CrossRef.
- C. Douglas, B. Emerson, T. Lieuwen, T. Martz and R. Steele, NO x Emissions from Hydrogen-Methane Fuel Blends, 2022 Search PubMed.
- A. C. Lewis, Environ. Sci.: Atmos., 2021, 1, 201–207 CAS.
- M. Ilbas, I. Yilmaz and Y. Kaplan, Int. J. Hydrogen Energy, 2005, 30, 1139–1147 CrossRef CAS.
- R. Noroozian and P. Asgharian, in Distributed Generation Systems, Elsevier, 2017, pp. 149–219 Search PubMed.
- University of Oxford, Fertilizer use per hectare of cropland, 2020, https://ourworldindata.org/grapher/fertilizer-per-hectare?country=OWID_WRL~CHN~USA~GBR~IND~BRA~NGA~European+Union~GHA~ECU, (accessed 16 March 2023) Search PubMed.
- J. Li, W. Watkins, C. Goralski and H. Gandhi, Catalyst composition for use in a Lean NOx Trap and method of using, U.S Pat., 7749474, 2010 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental method and additional results. See DOI: https://doi.org/10.1039/d3gc04432g |
| This journal is © The Royal Society of Chemistry 2024 |