Metal-free semiconductors for visible-light-induced carbocarboxylation of styrenes with aliphatic redox-active esters and CO2†
Hao
Hou
a,
Meizhen
Luo
a,
Senmao
Zhai
a,
Tao
Yuan
ab,
Meifang
Zheng
 *a and
Sibo
Wang
*a and
Sibo
Wang
 *a
*a
aState Key Laboratory of Photocatalysis on Energy and Environment, College of Chemistry, Fuzhou University, Fuzhou 350116, P. R. China. E-mail: mfzheng@fzu.edu.cn; sibowang@fzu.edu.cn
bCollege of Chemical Engineering, Fuzhou University, Fuzhou 350116, P. R. China
First published on 21st December 2023
Abstract
A sustainable system for the carbocarboxylation of olefins with redox-active esters and CO2via visible-light-induced photoredox catalysis has been developed using metal-free boron carbonitride (BCN) as the photocatalyst. The scheme introduces versatile alkyl groups (including primary, secondary, and tertiary) and CO2 into the C–C double bond in a fully regioselective manner to produce valuable carboxylic derivatives with excellent yields.
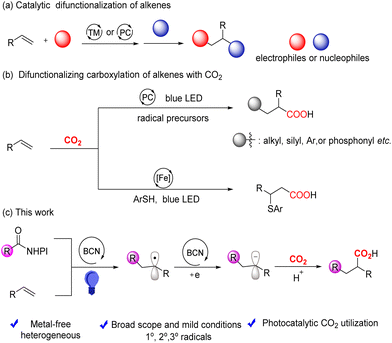
The vicinal difunctionalization of olefins has emerged as a powerful synthetic method as it enables the increase of molecular complexity from readily available building blocks in one step.1 Versatile electrophilic and nucleophilic reagents have been forged to form new C–C or C–X bonds in molecules by transition metal-catalyzed difunctionalization reactions (Scheme 1a).2 The incorporation of carbon dioxide (CO2) into olefins to generate value-added chain carboxylic acids is a hot research topic, because CO2 is a sustainable, low-cost, abundant and non-toxic C1 synthon (Scheme 1b).3,4 The boom is assisting the development of photochemistry, which enables previously redox-challenging reactions via a single electron transfer (SET) process with visible light.5 Recently, the groups of Yu, Martin, Wu, etc.6–9 have developed the regioselective carboxylation of activated alkenes with transition metal-containing complexes or organic dyes as photosensitizers, and introduced CO2 and diverse radical precursors, including aryl halides, H–P(O) compounds, alkyl silicon reagents, etc., into the alkenes forming valuable carboxylic derivatives.
The recycling and cost issues of those homogeneous photosensitizers inspire the idea of heterogeneous photocatalysis, which emerges as a growing technique for organic synthesis and exhibits superior advantages such as recyclability, good stability, and facile separation of the products from the reaction mixture.10 Therefore, efforts have been dedicated to building Earth-abundant element-containing heterogeneous semiconductor systems for catalysis and organic synthesis.11 Metal-free semiconductors, including graphitic carbon nitrides (g-CN),12 covalent organic frameworks (COFs),13 and conjugated microporous polymers (CMPs),14 could function as photocatalysts for cycloaddition, cross-coupling, hydrogenation and so on.15–17 Boron carbonitrides (BCNs) are non-metallic two-dimensional semiconductor materials, composed of the elements boron, carbon and nitrogen, featuring the advantages of easily available precursors, simple synthetic operation, and a large open surface area with abundant active sites for catalysis performance.18,19 They are visible light-responsive with a tunable band gap (0–5.5 eV), which depends on the carbon content incorporated into the boron nitride (BN) frameworks, allowing extended utilization of light energy for artificial photosynthesis.20–23 To date, they have rarely been used in direct carboxylation with CO2, with just one exception of hydrocarboxylation of alkenes.24 Therefore, we herein design the carbocarboxylation of alkenes using the surface catalytic process of BCN (Scheme 1c).
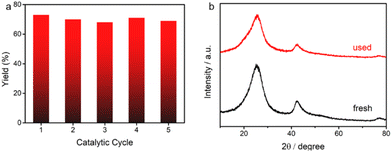
The reaction started with 4-cyanostyrene 1a and cyclohexyl N-(acyloxy)phthalimide 2a as the model substrates under atmospheric pressure of CO2 with 450 nm LEDs at 25 °C in the solvent N,N-dimethylacetamide (DMA) (Table 1, entry 1). Metal-based Ru(bpy)3Cl2·6H2O and fac-Ir(ppy)3 were less efficient than BCN for this transformation (entries 2 and 3). Other solvents depressed the yield of 3aa (entries 4 and 5). The yield of 3aa declined when using Et3N and DABCO instead of DIPEA as the sacrificial agent (entries 6 and 7). We also screened a range of bases, among which CsF gave the optimal yield of 78% (Table S1 in the ESI†). Notably, product 3aa was not detected in the absence of either BCN or light, indicating the necessity of these factors (entries 9 and 10). Gratifyingly, when recycled BCN was tested under the standard reaction conditions, it exhibited conserved catalytic performance (Scheme 2a and b), which demonstrated good stability of the BCN photocatalyst during prolonged light exposure. It was further verified by the similar patterns of XRD, DRS, SEM, and TEM characterization studies of fresh and used BCN (Fig. S13 and S14†).
| Entry | Variation from standard conditions | Yield of 3aab [%] |
|---|---|---|
| a Standard conditions: 1a (0.1 mmol), 2a (0.15 mmol), BCN1000 (10 mg), CsF (0.2 mmol), DIPEA (0.3 mmol), DMA (1.0 mL), CO2 (1 atm), 450 nm blue LEDs, 25 °C, quenched with 2N HCl (aq.). “N.D.” is short for “not detected”. b Yield was determined by 1H NMR with dibromomethane as the internal standard. c Isolated yield. | ||
| 1 | None | 78 (73c) |
| 2 | Ru(bpy)3Cl26H2O used as the PC | 14 |
| 3 | fac-Ir(ppy)3 used as the PC | 28 |
| 4 | DMF instead of DMA | 54 |
| 5 | MeCN instead of DMA | N.D. |
| 6 | DABCO instead of DIPEA | 14 |
| 7 | Et3N instead of DIPEA | 32 |
| 8 | Without CsF | 30 |
| 9 | Without light | N.D. |
| 10 | Without PC | N.D. |
| 11 | Without DIPEA | N.D. |
| 12 | Without CO2 | Trace |
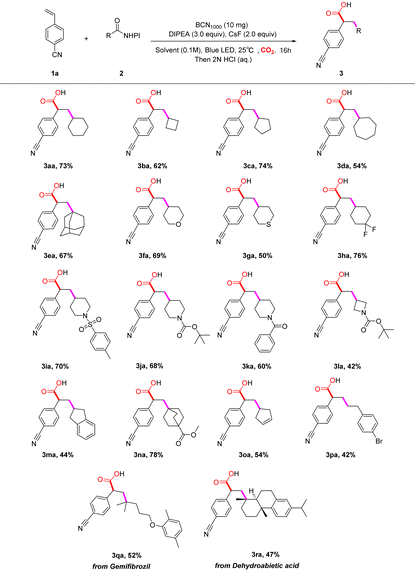
Having identified the optimized reaction conditions, we then surveyed the reaction scope of aliphatic N-(acyloxy)phthalimide redox-active esters (RAEs) 2 with 1a (Table 2). A variety of secondary aliphatic acid esters were efficient substrates to afford the desired products. Specifically, the generation of secondary alkyl radicals from cyclobutyl, cyclopentyl, cyclohexyl, and cycloheptyl RAEs formed the desired products in moderate to good yields (3aa–3da). The reaction with a sterically-hindered tertiary adamantanyl RAE was feasible with a 67% yield of 3ea. In addition, secondary alkyl RAEs containing biologically-active pyran, thiane, and piperidine motifs25 underwent the reaction smoothly, affording the corresponding products in moderate yields (3fa, 3ga and 3ia–3la). Furthermore, RAE derivatives with difluoromethyl or alkenyl substituents were also well tolerated under the reaction conditions, delivering the desired products 3ha and 3oa smoothly. Of note, tertiary aliphatic esters could also benefit from the established strategy. The protocol is also feasible for a polycyclic [2.2.2]bicyclooctane-containing ester (3na). In particular, the primary alkyl derivative could undergo the photocatalytic SET process forming a primary alkyl radical and the subsequent corresponding products (3pa). The heterogeneous photocatalysis system could be applied to the derivatives of RAEs containing a gemfibrozil fragment or an abietic acid fragment, for this transformation, producing the products 3qa and 3ra.
| a Standard conditions: 1a (0.1 mmol), 2 (0.15 mmol), BCN1000 (10 mg), CsF (0.2 mmol), DIPEA (0.3 mmol), DMA (1.0 mL), CO2 (1 atm), 450 nm blue LEDs, 25 °C, for 16 h, quenched with 2N HCl (aq.). Isolated yields. |
|---|
|
We further screened the scope of alkenes (Table 3). A series of substituted styrenes with sensitive substituents, such as the trifluoromethyl group, cyano group and ester group, could be accommodated and they afforded the corresponding products in moderate to good yields (3aa–3af). Notably, polyfluorine-substituted styrene could also be converted to difunctionalized product 3ag with a yield of 70%. 1,1-Diarylethylenes and α-substituted styrenes could also result in the target compounds (3ah, 3ak and 3al) with quaternary carbon centers under the standard reaction conditions. Remarkably, unconjugated alkenyl and alkynyl substitutions were compatible in this protocol (3ai and 3aj). Cyclodecanal-derived styrene underwent the process smoothly with moderate yield (3am). Satisfactorily, isobornyl and menthol derivatives underwent the carbocarboxylation successfully, giving the products in moderate yields (3an and 3ao). Moreover, methacrylate could also be converted to the corresponding product (3ap).
| a Standard conditions: 1 (0.1 mmol), 2a (0.15 mmol), BCN1000 (10 mg), CsF (0.2 mmol), DIPEA (0.3 mmol), DMA (1.0 mL), CO2 (1 atm), 450 nm blue LEDs, 25 °C, for 16 h, quenched with 2N HCl (aq.). Isolated yields. |
|---|

|
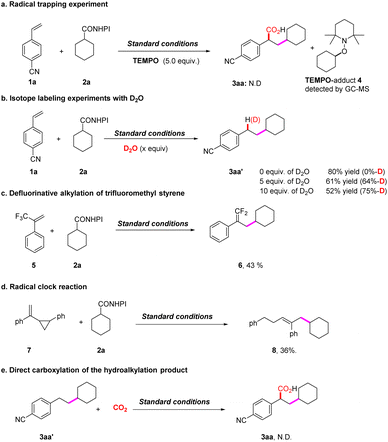
Additional experiments were performed to gain insight into the mechanism (Scheme 3). The Stern–Volmer quenching plots exhibited that the excited BCN was quenched by RAE 2a or DIPEA rather than 4-cyanostyrene 1a (see the ESI, Fig. S6–S9†). With the addition of 2,2,6,6-tetramethyl-piperidinyloxyl (TEMPO), 3aa was not detected (Scheme 3a), and the cyclohexyl radical–TEMPO adduct 4 was detected. Furthermore, the radical clock experiment of (1-(2-phenylcyclopropyl)vinyl)benzene 7 with 2a resulted in the ring-opening product 8, verifying the formation of the carbon radical intermediate in the process (Scheme 3d).9 When D2O was added, up to 75% deuterium incorporation of 3aa′ was observed (Scheme 3b). Furthermore, the gem-difluoro alkene 6 was detected when using trifluoromethylalkenes 5 and cyclohexyl RAE 2a as the substrates, which was consistent with the radical/polar crossover defluorinative alkylation pathway (Scheme 3c). The results indicate the existence of a benzylic carbon anion intermediate. Since 3aa was not detected when 3aa′ was used, the possibility that 3aa′ was the intermediate was ruled out (Scheme 3e).
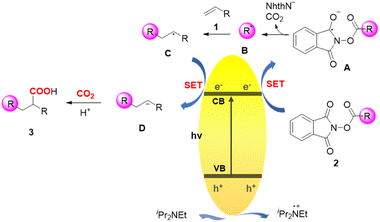
Based on the mechanistic studies and literature precedents,26 a mechanism for BCN-catalyzed carbocarboxylation of alkenes was proposed (Scheme 4). Initially, a photogenerated electron–hole pair is generated from the excited state BCN upon visible light irradiation. N-Hydroxyphthalimide esters 2 are then reduced by the photogenerated electron forming the phthalimide ester radical, which decomposes to the NHPI anion, CO2, and alkyl radical B. Meanwhile, the photogenerated hole is consumed by DIPEA. The following radical addition of intermediate B to alkene 1 affords benzylic radical C, which subsequently is reduced by the photogenerated electron to give the benzylic carbanion D through the single electron transfer pathway. Nucleophilic addition of intermediate D to CO2 and subsequent protonation furnish the final carboxylic product 3.
Conclusions
In summary, a ternary metal-free heterogeneous photoredox system was introduced into the carbocarboxylation of olefins with CO2 and aliphatic redox-active esters in one pot with visible light. This strategy has the advantages of wide substrate scope, mild reaction conditions, low photocatalyst loading and recyclability. The protocol presented the potential practicality of CO2 fixation in fine chemical synthesis via heterogeneous catalysis.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was financially supported by the National Key Technologies R & D Program of China (2021YFA1502100), the National Natural Science Foundation of China (22071026, 22032002, 22372035, 22302039, and 22302041), the China Postdoctoral Science Foundation (2023M740638), and the State-Sponsored Postdoctoral Researcher Program (GZB20230142).References
- For selected reviews, see: (a) S. O. Badir and G. A. Molander, Chem, 2020, 6, 1327–1339 CrossRef CAS PubMed; (b) R. K. Dhungana, S. Kc, P. Basnet and R. Giri, Chem. Rec., 2018, 18, 1314–1340 CrossRef CAS PubMed; (c) R. Giri and S. Kc, J. Org. Chem., 2018, 83, 3013–3022 CrossRef CAS PubMed.
- (a) L. Guo, M. Yuan, Y. Zhang, F. Wang, S. Zhu, O. Gutierrez and L. Chu, J. Am. Chem. Soc., 2020, 142, 20390–20399 CrossRef CAS PubMed; (b) C. C. C. J. Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062–5085 CrossRef PubMed; (c) Z.-L. Li, G.-C. Fang, Q.-S. Gu and X.-Y. Liu, Chem. Soc. Rev., 2020, 49, 32–48 RSC; (d) L. Liao, R. Jana, K. B. Urkalan and M. S. Sigman, J. Am. Chem. Soc., 2011, 133, 5784–5787 CrossRef CAS PubMed; (e) X. H. Ouyang, R. J. Song, M. Hu, Y. Yang and J. H. Li, Angew. Chem., Int. Ed., 2016, 55, 3187–3191 CrossRef CAS PubMed; (f) X. Qi and T. Diao, ACS Catal., 2020, 10, 8542–8556 CrossRef CAS PubMed; (g) L. Wu, F. Wang, X. Wan, D. Wang, P. Chen and G. Liu, J. Am. Chem. Soc., 2017, 139, 2904–2907 CrossRef CAS PubMed.
- For selected reviews, see: (a) W. Kleij, M. North and A. Urakawa, ChemSusChem, 2017, 10, 1036–1038 CrossRef PubMed; (b) Q. Liu, L. Wu, R. Jackstell and M. Beller, Nat. Commun., 2015, 6, 5933 CrossRef PubMed; (c) Y.-X. Luan and M. Ye, Tetrahedron Lett., 2018, 59, 853–861 CrossRef CAS; (d) C.-K. Ran, Y.-N. Niu, L. Song, M.-K. Wei, Y.-F. Cao, S.-P. Luo, Y.-M. Yu, L.-L. Liao and D.-G. Yu, ACS Catal., 2021, 12, 18–24 CrossRef; (e) A. Tortajada, F. Juliá-Hernández, M. Börjesson, T. Moragas and R. Martin, Angew. Chem., Int. Ed., 2018, 57, 15948–15982 CrossRef CAS PubMed; (f) M. Xu, A. R. Jupp, M. S. E. Ong, K. I. Burton, S. S. Chitnis and D. W. Stephan, Angew. Chem., Int. Ed., 2019, 58, 5707–5711 CrossRef CAS PubMed; (g) C. S. Yeung, Angew. Chem., Int. Ed., 2019, 58, 5492–5502 CrossRef CAS PubMed; (h) J.-H. Ye, T. Ju, H. Huang, L.-L. Liao and D.-G. Yu, Acc. Chem. Res., 2021, 54, 2518–2531 CrossRef CAS PubMed.
- (a) Z. Zhang, L. Gong, X.-Y. Zhou, S.-S. Yan, J. Li and D.-G. Yu, Acta Chim. Sin., 2019, 77, 783–793 CrossRef CAS; (b) W. Zhang, Z. Chen, Y.-X. Jiang, L.-L. Liao, W. Wang, J.-H. Ye and D.-G. Yu, Nat. Commun., 2023, 14, 3529 CrossRef CAS PubMed; (c) B. Zhang, Y. Yi, Z.-Q. Wu, C. Chen and C. Xi, Green Chem., 2020, 22, 5961–5965 RSC; (d) C. Zhou, M. Li, J. Sun, J. Cheng and S. Sun, Org. Lett., 2021, 23, 2895–2899 CrossRef CAS PubMed.
- (a) M. D. Kärkäs, J. A. Porco and C. R. J. Stephenson, Chem. Rev., 2016, 116, 9683–9747 CrossRef PubMed; (b) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed; (c) N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075–10166 CrossRef CAS PubMed; (d) C.-S. Wang, P. H. Dixneuf and J.-F. Soulé, Chem. Rev., 2018, 118, 7532–7585 CrossRef CAS PubMed.
- (a) J.-H. Ye, M. Miao, H. Huang, S.-S. Yan, Z.-B. Yin, W.-J. Zhou and D.-G. Yu, Angew. Chem., Int. Ed., 2017, 56, 15416–15420 CrossRef CAS PubMed; (b) L.-L. Liao, G.-M. Cao, Y.-X. Jiang, X.-H. Jin, X.-L. Hu, J. J. Chruma, G.-Q. Sun, Y.-Y. Gui and D.-G. Yu, J. Am. Chem. Soc., 2021, 143, 2812–2821 CrossRef CAS PubMed; (c) Y.-N. Niu, X.-H. Jin, L.-L. Liao, H. Huang, B. Yu, Y.-M. Yu and D.-G. Yu, Sci. China: Chem., 2021, 64, 1164–1169 CrossRef CAS; (d) J.-P. Yue, J.-C. Xu, H.-T. Luo, X.-W. Chen, H.-X. Song, Y. Deng, L. Yuan, J.-H. Ye and D.-G. Yu, Nat. Catal., 2023, 6, 959–968 CrossRef CAS; (e) W. Zhang, Z. Chen, Y.-X. Jiang, L.-L. Liao, W. Wang, J.-H. Ye and D.-G. Yu, Nat. Commun., 2023, 14, 3529 CrossRef CAS PubMed; (f) L. Song, W. Wang, J.-P. Yue, Y.-X. Jiang, M.-K. Wei, H.-P. Zhang, S.-S. Yan, L.-L. Liao and D.-G. Yu, Nat. Catal., 2022, 5, 832–838 CrossRef CAS; (g) T. Ju, Y.-Q. Zhou, K.-G. Cao, Q. Fu, J.-H. Ye, G.-Q. Sun, X.-F. Liu, L. Chen, L.-L. Liao and D.-G. Yu, Nat. Catal., 2021, 4, 304 CrossRef CAS; (h) Q. Fu, Z.-Y. Bo, J.-H. Ye, T. Ju, H. Huang, L.-L. Liao and D.-G. Yu, Nat. Commun., 2019, 10, 3592 CrossRef PubMed.
- V. R. Yatham, Y. Shen and R. Martin, Angew. Chem., Int. Ed., 2017, 56, 10915–10919 CrossRef CAS PubMed.
- (a) J. Hou, A. Ee, H. Cao, H. W. Ong, J. H. Xu and J. Wu, Angew. Chem., Int. Ed., 2018, 57, 17220–17224 CrossRef CAS PubMed; (b) J. Hou, A. Ee, W. Feng, J. H. Xu, Y. Zhao and J. Wu, J. Am. Chem. Soc., 2018, 140, 5257–5263 CrossRef CAS PubMed.
- (a) H. Wang, Y. Gao, C. Zhou and G. Li, J. Am. Chem. Soc., 2020, 142, 8122–8129 CrossRef CAS PubMed; (b) C. Zhou, X. Wang, L. Yang, L. Fu and G. Li, Green Chem., 2022, 24, 6100–6107 RSC; (c) J. Bai, M. Li, C. Zhou, Y. Sha, J. Cheng, J. Sun and S. Sun, Org. Lett., 2021, 23, 9654–9658 CrossRef CAS PubMed; (d) Y. Yi, Z. Fan and C. Xi, Green Chem., 2022, 24, 7894–7899 RSC.
- (a) A. Jati, S. Dam, S. Kumar, K. Kumar and B. Maji, Chem. Sci., 2023, 14, 8624–8634 RSC; (b) L. L. Mao and H. Cong, ChemSusChem, 2017, 10, 4461–4464 CrossRef CAS PubMed; (c) K. Murugesan, A. Sagadevan, L. Peng, O. Savateev and M. Rueping, ACS Catal., 2023, 13(20), 13414–13422 CrossRef CAS; (d) B. Pieber, M. Shalom, M. Antonietti, P. H. Seeberger and K. Gilmore, Angew. Chem., Int. Ed., 2018, 57, 9976–9979 CrossRef CAS PubMed.
- (a) B. Su, M. Zheng, W. Lin, X. Lu, D. Luan, S. Wang and X. W. Lou, Adv. Energy Mater., 2023, 13, 2203290 CrossRef CAS; (b) G. Chen, F. Wei, Z. Zhou, B. Su, C. Yang, X. Lu, S. Wang and X. Wang, Sustainable Energy Fuels, 2023, 7, 381–388 RSC.
- (a) S. Mazzanti, B. Kurpil, B. Pieber, M. Antonietti and A. Savateev, Nat. Commun., 2020, 11, 1387 CrossRef CAS PubMed; (b) F. Su, S. C. Mathew, L. Möhlmann, M. Antonietti, X. Wang and S. Blechert, Angew. Chem., Int. Ed., 2010, 50, 657–660 CrossRef PubMed.
- (a) Q. Li, J. Wang, Y. Zhang, L. Ricardez-Sandoval, G. Bai and X. Lan, ACS Appl. Mater. Interfaces, 2021, 13, 39291–39303 CrossRef CAS PubMed; (b) Z. Li, Y. Zhi, P. Shao, H. Xia, G. Li, X. Feng, X. Chen, Z. Shi and X. Liu, Appl. Catal., B, 2019, 245, 334–342 CrossRef CAS.
- S. Han, Z. Li, S. Ma, Y. Zhi, H. Xia, X. Chen and X. Liu, J. Mater. Chem. A, 2021, 9, 3333–3340 RSC.
- (a) C. Yang, R. Li, K. A. I. Zhang, W. Lin, K. Landfester and X. Wang, Nat. Commun., 2020, 11, 1329 CrossRef PubMed; (b) Y. Zhao and M. Antonietti, Angew. Chem., Int. Ed., 2017, 56, 9336–9340 CrossRef CAS PubMed.
- (a) M. Yang, R. Lian, X. Zhang, C. Wang, J. Cheng and X. Wang, Nat. Commun., 2022, 13, 4900 CrossRef CAS PubMed; (b) S. Das, K. Murugesan, G. J. V. Rodríguez, J. Kaur, J. P. Barham, A. Savateev, M. Antonietti and B. König, ACS Catal., 2021, 11, 1593–1603 CrossRef CAS; (c) W. Zhang, J. Tang, W. Yu, Q. Huang, Y. Fu, G. Kuang, C. Pan and G. Yu, ACS Catal., 2018, 8, 8084–8091 CrossRef CAS; (d) P. T. Parvatkar, S. Kandambeth, A. C. Shaikh, I. Nadinov, J. Yin, V. S. Kale, G. Healing, A.-H. Emwas, O. Shekhah, H. N. Alshareef, O. F. Mohammed and M. Eddaoudi, J. Am. Chem. Soc., 2023, 145, 5074–5082 CrossRef CAS PubMed.
- (a) J. Khamrai, I. Ghosh, A. Savateev, M. Antonietti and B. König, ACS Catal., 2020, 10, 3526–3532 CrossRef CAS; (b) C. Qiu, Y. Sun, Y. Xu, B. Zhang, X. Zhang, L. Yu and C. Su, ChemSusChem, 2021, 14, 3344–3350 CrossRef CAS PubMed; (c) J. Ding, F. Li, J. Zhang, H. Qi, Z. Wei, C. Su, H. B. Yang, Y. Zhai and B. Liu, Adv. Mater., 2023, e202306480 Search PubMed.
- For selected reviews, see: (a) Y. Fang and X. Wang, Angew. Chem., Int. Ed., 2017, 56, 15506–15518 CrossRef CAS PubMed; (b) Z. Luo, Y. Fang, M. Zhou and X. Wang, Angew. Chem., Int. Ed., 2019, 58, 6033–6037 CrossRef CAS PubMed; (c) Y. Wang, H. Li, J. Yao, X. Wang and M. Antonietti, Chem. Sci., 2011, 2, 446–450 RSC; (d) M. Zhang, Z. Luo, M. Zhou, C. Huang and X. Wang, Sci. China Mater., 2015, 58, 867–876 CrossRef CAS; (e) M. Zheng, W. Cai, Y. Fang and X. Wang, Nanoscale, 2020, 12, 3593–3604 RSC; (f) M. Zhou, P. Yang, S. Wang, Z. Luo, C. Huang and X. Wang, ChemSusChem, 2018, 11, 3949–3955 CrossRef CAS PubMed.
- (a) L. Chen, M. Zhou, Z. Luo, M. Wakeel, A. M. Asiri and X. Wang, Appl. Catal., B, 2019, 241, 246–255 CrossRef CAS; (b) M. Zhou, Z. Chen, P. Yang, S. Wang, C. Huang and X. Wang, Appl. Catal., B, 2020, 276, 118916 CrossRef CAS.
- For selected reviews, see: T. Yuan, M. Zheng, M. Antonietti and X. Wang, Chem. Sci., 2021, 12, 6323–6332 RSC.
- L. Chen and X. Wang, Chem. Commun., 2017, 53, 11988–11991 RSC.
- M. Zhou, S. Wang, P. Yang, C. Huang and X. Wang, ACS Catal., 2018, 8, 4928–4936 CrossRef CAS.
- (a) J. Shi, T. Yuan, R. Wang, M. Zheng and X. Wang, Green Chem., 2021, 23, 3945–3949 RSC; (b) J. Shi, T. Yuan, M. Zheng and X. Wang, ACS Catal., 2021, 11, 3040–3047 CrossRef CAS; (c) T. Yuan, M. Zheng, M. Antonietti and X. Wang, Chem. Sci., 2021, 12, 6323–6332 RSC; (d) T. Yuan, S. W. Huan, B. Q. Ge, S. Lin, M. F. Zheng and X. C. Wang, CCS Chem., 2022, 4, 2597–2603 CrossRef CAS; (e) T. Yuan, L. Sun, Z. Wu, R. Wang, X. Cai, W. Lin, M. Zheng and X. Wang, Nat. Catal., 2022, 5, 1157–1168 CrossRef CAS.
- T. Yuan, Z. Wu, S. Zhai, R. Wang, S. Wu, J. Cheng, M. Zheng and X. Wang, Angew. Chem., Int. Ed., 2023, 62, e202304861 CrossRef CAS PubMed.
- (a) Y. Wang, W.-X. Zhang and Z. Xi, Chem. Soc. Rev., 2020, 49, 5810–5849 RSC; (b) M. Baumann and I. R. Baxendale, J. Org. Chem., 2013, 9, 2265–2319 Search PubMed.
- (a) Z. Hong, W. K. Chong, A. Y. R. Ng, M. Li, R. Ganguly, T. C. Sum and H. S. Soo, Angew. Chem., Int. Ed., 2019, 58, 3456–3460 CrossRef CAS PubMed; (b) T. Shi, K. Sun, X. L. Chen, Z. X. Zhang, X. Q. Huang, Y. Y. Peng, L. B. Qu and B. Yu, Adv. Synth. Catal., 2020, 362, 2143–2149 CrossRef; (c) P. Yang, L. Wang, H. Zhuzhang, R. Wang, M.-M. Titirici and X. Wang, Appl. Catal., B, 2019, 256, 117794–111780 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3gc04450e |
| This journal is © The Royal Society of Chemistry 2024 |