Selective neodymium recovery from model permanent magnets using cost-effective organic acid systems†
Cristina
Pozo-Gonzalo‡§
 *a,
Rabeeh
Golmohammadzadeh¶||
*a,
Rabeeh
Golmohammadzadeh¶||
 a,
Munkhshur
Myekhlai||
a,
Henrique
Bastos
a,
Munkhshur
Myekhlai||
a,
Henrique
Bastos
 a,
Glen B.
Deacon
a,
Glen B.
Deacon
 b and
Anthony E.
Somers
b and
Anthony E.
Somers
 a
a
aInstitute for Frontier Materials, Deakin University, Geelong, Victoria 3200, Australia. E-mail: cpg@deakin.edu.au; cpozo@icb.csic.es
bSchool of Chemistry, Monash University, Melbourne 3800, Australia
First published on 26th January 2024
Abstract
Recovery of critical metals from waste is becoming very important to bridge the gap between the limited natural resources available and their ever-increasing demand. One such vital metal is neodymium (Nd), which plays an essential role in advancing sustainable clean energy technologies. Therefore, in this work, the key parameters to selectively recover Nd over iron (Fe) from their oxides, as model systems were investigated. By investigating the effect of key parameters, we aim to understand the underpinning science principles necessary for the safe and efficient recovery of critical metals from secondary sources. A series of deep eutectic solvents, consisting of a hydrogen bond donor (HBD), lactic acid or acetic acid, and hydrogen bond acceptor (HBA), guanidine hydrochloride (GUC), have been investigated in HBA–HBD combinations, and individually in the presence of water to determine the role of the HBD and HBA towards Nd and Fe leaching efficiency and selectivity. The combination of GUC with HBDs was less beneficial for the leaching of Nd2O3, with a maximum value of 78% in comparison with the individual systems, in the absence of GUC, which demonstrated a maximum dissolution of 95%. Among the different combinations, the acetic acid aqueous solution led to the highest dissolution efficiency and selectivity, probably due to the high basicity and strong stability constants for Nd-acetate complexes. Other parameters, such as the impact of the molar ratio Nd2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe2O3 were also explored, and a synergetic effect that promotes Nd2O3 solubility at 1
Fe2O3 were also explored, and a synergetic effect that promotes Nd2O3 solubility at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Nd
1 Nd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe weight ratio is observed across the samples. However, when increasing the amount of Fe2O3 in the mixture to simulate realistic ratios present in spent magnets, selectivity is strongly affected, and only the acetic acid solution is capable of selectively dissolving Nd2O3 with a separation factor of up to 5038, which is higher than the current state of the art (1608). Finally, the acetic acid concentration was also studied as a factor to assess its effect on selectivity while also reducing cost.
Fe weight ratio is observed across the samples. However, when increasing the amount of Fe2O3 in the mixture to simulate realistic ratios present in spent magnets, selectivity is strongly affected, and only the acetic acid solution is capable of selectively dissolving Nd2O3 with a separation factor of up to 5038, which is higher than the current state of the art (1608). Finally, the acetic acid concentration was also studied as a factor to assess its effect on selectivity while also reducing cost.
1. Introduction
Rare earth elements (REEs), especially neodymium (Nd), are essential for a wide array of applications due to their unique magnetic, catalytic and optical properties.1,2 However, there are environmental issues with REE extraction from mined ore bodies as current methods are energy intensive, require large volumes of kerosene and typically produce large amounts of highly acidic and radioactive waste.1,3 More recently there have also been increasing concerns around the guaranteed supply of REEs, with China controlling 70% of their primary production.1,3,4 An ever-increasing demand for REEs in clean energy technologies, in conjunction with these issues related to primary extraction has triggered the urgent need to extract and recover such critical metals from secondary waste resources.1,3REEs find applications in a wide range of fields, including permanent magnets, catalysts, defence technologies, medical equipment, rechargeable batteries, and water treatment technologies.5 In the context of the green energy economy, one of the most significant applications of REEs is in permanent magnets. These magnets are a crucial part of wind turbines, electric vehicles and advanced electronics due to their strong magnetic properties.6–8 The Nd-based permanent magnets consist of approximately 30 wt% Nd and 65 wt% iron (Fe) and a small amount of other metals ranging from Pr, Dy, Al, Fe, Co, Ni, Cu, B.6–8 It is estimated that the demand for REEs will increase 40-fold from 2015 to 2030 just to meet the need for magnets in wind turbines.9,10 Therefore, there is a need to recycle the vast amount of waste magnets to avert a REE supply crisis.
To date, different approaches have been used to recover REEs from waste NdFeB magnets, including pyrometallurgy,11 hydrometallurgy,12–14 biohydrometallurgy,15 liquid metal extraction,16 hydrogen decrepitation,17 and chemical vapour transport techniques.18,19 These methods consume large amounts of energy, operate at high pressure or use volatile solvents such as kerosene and strong acids such as sulfuric, nitric, and hydrochloric acids, which cause detrimental environmental issues by generating a large amount of secondary aqueous waste. Thus, from an environmental perspective it is important to develop green solvents for recovering REEs while maintaining the leaching efficiency. An emerging family of solvents known as deep eutectic solvents (DESs), which have an abnormally deep melting point depression at the eutectic composition, was first introduced by Abbott et al. in 2001.20,21 These systems contain a hydrogen bond acceptor (HBA) and a hydrogen bond donor (HBD), with a commonly used example being choline chloride (HBA) with urea (HBD). However, we would like to highlight that DES-like systems have also been reported in the literature as mixtures of HBAs and HBDs without investigating the impact on the mixture's melting point and thus their use is not limited to the eutectic composition.
Generally due to their low cost, safety and ease of manufacture, DES-like systems have gained significant attention in a variety of fields, including electrodeposition, organic extraction or metal oxide dissolution.22–25 Lately, DESs have shown efficiency in safe and effective selective leaching of REEs from used magnets.26–30 Among those used, DESs based on organic acids have been effective in enhancing metal dissolution due to increased acidity, as well as coordination by HBA and HBD ligands.26,28 Riaño et al.26 dissolved NdFeB magnets in a DES consisting of choline chloride and lactic acid with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. All metals present (e.g. Fe, Nd and other REEs) in the magnet were dissolved in the DES with more than 90% dissolution efficiency under the leaching conditions of a 1
2. All metals present (e.g. Fe, Nd and other REEs) in the magnet were dissolved in the DES with more than 90% dissolution efficiency under the leaching conditions of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 solid-to-liquid ratio at 70 °C for 12 h. The high leaching ability of this DES was attributed to the protons present in lactic acid, together with the coordination abilities of lactate and chloride anions. However, subsequent steps involving an organic extraction were required to separate the metals in the leachate with an additional economic cost.
50 solid-to-liquid ratio at 70 °C for 12 h. The high leaching ability of this DES was attributed to the protons present in lactic acid, together with the coordination abilities of lactate and chloride anions. However, subsequent steps involving an organic extraction were required to separate the metals in the leachate with an additional economic cost.
More recently, Liu et al.28 developed a family of DESs containing guanidine hydrochloride and found that a DES composed of guanidine hydrochloride–lactic acid (GUC–lactic acid) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio could achieve selective leaching of Nd versus Fe. In this work, Nd and Fe oxides were initially studied, to mimic the composition of a roasted NdFeB magnet and were dissolved in the DES at 50 °C for 24 h with a 1
2 molar ratio could achieve selective leaching of Nd versus Fe. In this work, Nd and Fe oxides were initially studied, to mimic the composition of a roasted NdFeB magnet and were dissolved in the DES at 50 °C for 24 h with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 solid-to-liquid ratio, resulting in the selective leaching of Nd (solubility of 86.2% for Nd and 1.4% for Fe). After determining the optimal leaching conditions with the model oxides, a roasted magnet was leached in the 1
50 solid-to-liquid ratio, resulting in the selective leaching of Nd (solubility of 86.2% for Nd and 1.4% for Fe). After determining the optimal leaching conditions with the model oxides, a roasted magnet was leached in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 solution of GUC–lactic acid with a 1
2 solution of GUC–lactic acid with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 solid-to-liquid ratio at 40 °C for 6 h and reached an even higher solubility for Nd (95.0%) while also showing low solubility for Fe (1.0%). The reason behind the improved efficiency and selectivity of Nd2O3 in the 1
10 solid-to-liquid ratio at 40 °C for 6 h and reached an even higher solubility for Nd (95.0%) while also showing low solubility for Fe (1.0%). The reason behind the improved efficiency and selectivity of Nd2O3 in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 GUC–lactic acid for the magnet material as compared to the model oxide mixtures was not unravelled in this work. Comparing both these research works, from Riaño and Liu, is interesting that a difference in selectivity is observed by changing the HBA from ChCl to GUC; however, it is important to highlight that apart from the nature of the HBA, the experimental leaching conditions also differ between the reported research works and these can also play a major role on leaching efficiency. Although these results are encouraging there is still a need for an in depth understanding of key chemical features of the electrolyte composition and process conditions that enable efficient and selective recovery of Nd from other metals.
2 GUC–lactic acid for the magnet material as compared to the model oxide mixtures was not unravelled in this work. Comparing both these research works, from Riaño and Liu, is interesting that a difference in selectivity is observed by changing the HBA from ChCl to GUC; however, it is important to highlight that apart from the nature of the HBA, the experimental leaching conditions also differ between the reported research works and these can also play a major role on leaching efficiency. Although these results are encouraging there is still a need for an in depth understanding of key chemical features of the electrolyte composition and process conditions that enable efficient and selective recovery of Nd from other metals.
Thus, due to their selective leaching efficiency determined in recent studies, we have prepared GUC-based systems, consisting of GUC paired with the organic acids, lactic acid and acetic acid, in line with the principles of green chemistry (e.g. less hazardous reagents) and guided by and guided by these recent results. Based on the literature,31 an alkaline baking process has been demonstrated to convert magnets into Nd2O3 and Fe2O3, removing B as sodium borate and with the other elements present in very minor amounts that are unlikely to affect selective dissolution of Nd and Fe. Therefore, our study is designed to explore the mechanisms underlying the dissolution behaviour of Nd2O3 and Fe2O3, which could be applied after such a baking process. We explored the dissolution characteristics of individual neodymium oxide (Nd2O3) and iron(III) oxide (Fe2O3), and mixtures of these metal oxides in different mass ratios.
To investigate the fundamental factors influencing leaching and selectivity for these systems, a mixture of HBA and HBD was employed as a dissolution agent, along with the individual constituents of HBA and HBD as control systems. This approach holds value for enhancing the understanding of the broader chemistry pertaining to rare earth materials and their recovery.
Herein, it is proposed that by controlling the solvent acidity and metal complexation in the solvent, the selective leaching of Nd2O3 over Fe2O3 could be achieved due to their different basicity. This systematic study solely focuses on the selective dissolution of Nd2O3 and Fe2O3 using safe and affordable solvents.
2. Results and discussion
2.1. Leaching behaviour of Nd2O3 and Fe2O3 separately in different solvent mixtures
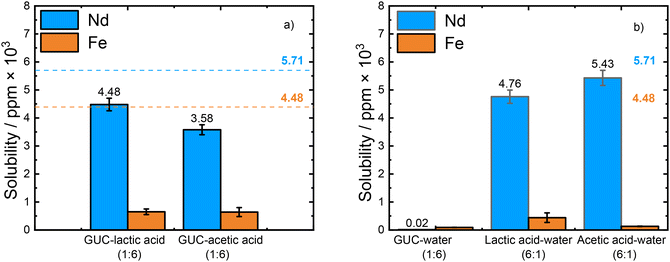
Nd2O3 and Fe2O3 are the main components in spent magnets after demagnetisation, calcination, and alkaline baking, as reported in the literature,10,31 so it is crucial to understand their distinct dissolution properties in the mixtures of HBA–HBD. Therefore, a series of HBA–HBDs composed of guanidine hydrochloride (GUC), as a hydrogen bond acceptor (HBA) and lactic acid or acetic acid as hydrogen bond donors (HBDs) were prepared (Table 1). We hypothesized that the HBA and HBD combinations may be more acidic than the individual systems, which would increase their capacity to break the metal–oxide (M–O) bond, as reported in the literature.32To explore the dissolution properties of individual Nd2O3 and Fe2O3 in the mixtures of HBA–HBD, 10 mg of Nd2O3 or Fe2O3 was dissolved in 1.5 mL of solvent at 50 °C for 24 h while stirring (500 rpm). Fig. 1a exhibits the solubility of Nd and Fe from the individual, separate oxides in the mixtures of HBA–HBD – GUC–lactic acid and GUC–acetic acid – with molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, respectively. The amount of organic acid in the mixtures was higher in this work than in the literature28 to favour metal oxide dissolution.
6, respectively. The amount of organic acid in the mixtures was higher in this work than in the literature28 to favour metal oxide dissolution.
Comparing the two DES-like systems, the highest dissolution of Nd2O3 (4.48 × 103 ppm, 78.3%) was obtained in GUC–lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), and the values are comparable to recently published studies.26,28 Liu et al. has investigated a series of DESs by varying the nature of the HBAs and HBDs and found that GUC–lactic acid (1
6), and the values are comparable to recently published studies.26,28 Liu et al. has investigated a series of DESs by varying the nature of the HBAs and HBDs and found that GUC–lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) showed the highest dissolution amongst their DES series. When tested at 50 °C for 24 h in a shaking bath with a solid-to-liquid ratio of 1
2) showed the highest dissolution amongst their DES series. When tested at 50 °C for 24 h in a shaking bath with a solid-to-liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 the GUC–lactic acid (1
50 the GUC–lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) dissolved 86.2% Nd2O3, while only 0.85% of Fe2O3 was dissolved.28 Notably, in our work, while GUC–lactic acid (1
2) dissolved 86.2% Nd2O3, while only 0.85% of Fe2O3 was dissolved.28 Notably, in our work, while GUC–lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) also showed a low dissolution of Fe2O3 (0.65 × 103 ppm, 14.5%), this is higher than the work from Liu et al. (0.85%).28 This could possibly be due to a different solid-to-liquid ratio, stirring method and the ratio of HBA–HBD mixtures. However, in Liu's study both metal oxides were present at the same time in the leaching dissolution.
6) also showed a low dissolution of Fe2O3 (0.65 × 103 ppm, 14.5%), this is higher than the work from Liu et al. (0.85%).28 This could possibly be due to a different solid-to-liquid ratio, stirring method and the ratio of HBA–HBD mixtures. However, in Liu's study both metal oxides were present at the same time in the leaching dissolution.
When comparing the DES-like systems studied here it can be seen that similar amounts of Fe2O3 (∼0.64 × 103 ppm, 14.3%) were dissolved while Nd2O3 was more soluble in GUC–lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) (4.48 × 103 ppm, 78.3%) than in GUC–acetic acid (1
6) (4.48 × 103 ppm, 78.3%) than in GUC–acetic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) (3.58 × 103 ppm, 62.6%). Overall, both DES-like solvents dissolved high amounts of Nd2O3 but to different degrees while dissolving significantly less Fe2O3. Therefore, the role of the individual HBA and HBDs on the dissolution of individual Nd2O3 and Fe2O3 were further investigated, by combining each component with water; HBA–water (1
6) (3.58 × 103 ppm, 62.6%). Overall, both DES-like solvents dissolved high amounts of Nd2O3 but to different degrees while dissolving significantly less Fe2O3. Therefore, the role of the individual HBA and HBDs on the dissolution of individual Nd2O3 and Fe2O3 were further investigated, by combining each component with water; HBA–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) and HBDs–water (6
6) and HBDs–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), respectively. Thus, this study will provide detailed information of the DES performance versus the individual components.
1), respectively. Thus, this study will provide detailed information of the DES performance versus the individual components.
The molar ratio of the mixtures was chosen in accordance with the molar ratio of the mixtures of HBA–HBD in this work. Fig. 1b shows the solubilities of the individual Nd2O3 and Fe2O3 in GUC–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), lactic acid–water (6
6), lactic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and acetic acid–water (6
1), and acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), respectively. GUC–water (1
1), respectively. GUC–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) only dissolved 0.02 × 103 ppm of Nd2O3 and 0.09 × 103 ppm of Fe2O3, which is less than 2.0% of the metal oxides. These results are similar to the previous study published by Liu et al.28 in which GUC dissolved less than 1.0% of Nd2O3 and Fe2O3. The reason for its poor dissolving ability can be due to GUC being a weak Brønsted acid that cannot break these M–O bonds.28
6) only dissolved 0.02 × 103 ppm of Nd2O3 and 0.09 × 103 ppm of Fe2O3, which is less than 2.0% of the metal oxides. These results are similar to the previous study published by Liu et al.28 in which GUC dissolved less than 1.0% of Nd2O3 and Fe2O3. The reason for its poor dissolving ability can be due to GUC being a weak Brønsted acid that cannot break these M–O bonds.28
In contrast, both lactic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and acetic acid–water (6
1) and acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) dissolved a greater amount of Nd2O3 (lactic acid–water (6
1) dissolved a greater amount of Nd2O3 (lactic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 4.76 × 103 ppm, 83.3% and acetic acid–water (6
1): 4.76 × 103 ppm, 83.3% and acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 5.43 × 103 ppm, 95.0%) than both the GUC–acid mixtures and the GUC–water mixture. Interestingly, both mixtures also showed very low dissolution for Fe2O3 (lactic acid–water (6
1): 5.43 × 103 ppm, 95.0%) than both the GUC–acid mixtures and the GUC–water mixture. Interestingly, both mixtures also showed very low dissolution for Fe2O3 (lactic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 0.44 × 103 ppm, 10.0% and acetic acid–water (6
1): 0.44 × 103 ppm, 10.0% and acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 0.13 × 103 ppm, 2.9%). While effective leaching of both Nd and Fe from spent NdFeB magnets with high solubilities (more than 99.0%) has been reported using 1.0 M acetic acid, no selectivity was observed in the dissolution of Nd over Fe.33 This means that an additional separation process is required to separate Nd from Fe. However, according to Yoon et al. grinding and roasting the magnet between 400–500 °C led to the generation of only Nd2O3 and Fe2O3 and under these conditions 94.2% of Nd, which is between our results, was selectively recovered from Fe (∼1.0%).34
1): 0.13 × 103 ppm, 2.9%). While effective leaching of both Nd and Fe from spent NdFeB magnets with high solubilities (more than 99.0%) has been reported using 1.0 M acetic acid, no selectivity was observed in the dissolution of Nd over Fe.33 This means that an additional separation process is required to separate Nd from Fe. However, according to Yoon et al. grinding and roasting the magnet between 400–500 °C led to the generation of only Nd2O3 and Fe2O3 and under these conditions 94.2% of Nd, which is between our results, was selectively recovered from Fe (∼1.0%).34
Our results indicate that although lactic and acetic acid are both weak acids, they are strong enough to break the Nd–O bond in Nd2O3. It is interesting to note that by changing the HBA GUC to water, the leaching ability of Nd2O3 is increased by a magnitude up to 1.5 for acetic acid and the dissolution values of the lactic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) are much greater than the ones from the literature, where only 18% leaching efficiency was obtained.28 This could be of interest for commercial applications as it has the potential to reduce both the cost as well as the environmental impact of the process.
1) are much greater than the ones from the literature, where only 18% leaching efficiency was obtained.28 This could be of interest for commercial applications as it has the potential to reduce both the cost as well as the environmental impact of the process.
The dissolution of Nd2O3 should require the breaking of the M–O bond, driven by the pKa values of the acid, leading to the solvation of Nd3+ in solution. However, in a second stage the complexation of the Nd3+ will take place in the bulk of the solution. Thus, the improved solubilities observed in the water-based systems in comparison with the GUC-based systems could possibly be due to the nature and stability of the subsequent Nd complex formed. For instance, the removal of chloride from the system will change the ligand of Nd from chloride to acetate or lactate, which could lead to more stable and hence soluble species.35 It has been found that the solvent nature affects the formation of metal complexes, resulting in different coordination numbers and ligands.35–38 For instance, Amphlett et al.35 recently reported the effect of HBDs (e.g. ethylene glycol, urea, and lactic acid) in choline chloride-based DESs on the coordination environment of lanthanides (Ln), including Nd, using different spectroscopic techniques. The interaction between HBD and HBA in the DES affects the coordination of Nd which resulted in different complex types forming. It is well known that the most stable complexes of lanthanides is through the coordination of an oxygen;36 however as reported by Amphlett et al., other coordination chemistries are also possible. For instance, they found that Ln were coordinated by the –OH group of the ethylene glycol in ChCl–ethylene glycol, by the carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in ChCl–urea, and interestingly through the Cl− in the case of ChCl–lactic acid, instead of through the carboxyl group (COOH) of the lactic acid. The latter was attributed to the repulsion between the choline and lactic acid moieties, and hence forming the [LnClx]3−x complex.28 This could be translated to our system where [NdClx]3−x complexes are more likely to form in the GUC-based systems, while Nd(CH3CHOHCOO)x and Nd(CH3COO)x complexes, for lactic and acetic acid respectively, are more energetically favourable to form in the absence of Cl− in the water systems. To justify this hypothesis, it is important to discuss complex stability.
O) in ChCl–urea, and interestingly through the Cl− in the case of ChCl–lactic acid, instead of through the carboxyl group (COOH) of the lactic acid. The latter was attributed to the repulsion between the choline and lactic acid moieties, and hence forming the [LnClx]3−x complex.28 This could be translated to our system where [NdClx]3−x complexes are more likely to form in the GUC-based systems, while Nd(CH3CHOHCOO)x and Nd(CH3COO)x complexes, for lactic and acetic acid respectively, are more energetically favourable to form in the absence of Cl− in the water systems. To justify this hypothesis, it is important to discuss complex stability.
There is a direct relationship between Gibbs free energy of formation (ΔfG°) and the complex stability constant (Kf) as shown in eqn (1):
ΔfG° = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kf Kf | (1) |
The stability constants of the Nd–lactate complex for one to three ligands (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kf1, 2, 3) are 4.0, 2.3, and 1.7, while for Nd–acetate these complex constants are 2.6, 2.1, and 1.9
Kf1, 2, 3) are 4.0, 2.3, and 1.7, while for Nd–acetate these complex constants are 2.6, 2.1, and 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 and these are all considerably higher than that of the Nd–chloride complex (log
39 and these are all considerably higher than that of the Nd–chloride complex (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kf1 = 0.06).40 From the Kf1 perspective (coordination with only 1 ligand), the values for lactate are larger than those for acetate because of a chelation effect of the extra OH group in lactic acid, while acetate coordinate acts as a monodentate. However, Nd has a coordination number of 8–9
Kf1 = 0.06).40 From the Kf1 perspective (coordination with only 1 ligand), the values for lactate are larger than those for acetate because of a chelation effect of the extra OH group in lactic acid, while acetate coordinate acts as a monodentate. However, Nd has a coordination number of 8–9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41,42 therefore more than one ligand is required to fill the Nd coordination sphere. Therefore, attention must be paid to Kf2 and Kf3 which are similar or even slightly more stable in the case of acetate.
41,42 therefore more than one ligand is required to fill the Nd coordination sphere. Therefore, attention must be paid to Kf2 and Kf3 which are similar or even slightly more stable in the case of acetate.
On the other hand, the solubility of Fe2O3 in lactic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was found to be 0.44 × 103 ppm, (10.0%) which is about three times higher than that in acetic acid–water (6
1) was found to be 0.44 × 103 ppm, (10.0%) which is about three times higher than that in acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (0.13 × 103 ppm, 2.9%). The poor solubility of Fe in the organic acid solutions may be due to the Fe–O bond strength in Fe2O3, which requires higher energy to break than Nd2O3. However, there is also a possibility that the oxide dissociates in the acid solutions and the resulting Fe(CH3COO)3 or Fe(CH3CHOHCOO)3 complexes formed precipitate out of the solution. XRD measurements were performed on the Fe2O3 samples before and after leaching in acetic acid–water (6
1) (0.13 × 103 ppm, 2.9%). The poor solubility of Fe in the organic acid solutions may be due to the Fe–O bond strength in Fe2O3, which requires higher energy to break than Nd2O3. However, there is also a possibility that the oxide dissociates in the acid solutions and the resulting Fe(CH3COO)3 or Fe(CH3CHOHCOO)3 complexes formed precipitate out of the solution. XRD measurements were performed on the Fe2O3 samples before and after leaching in acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to determine the composition of the solid Fe compound filtered out of the leachate (Fig. 2). There was no noticeable change in the XRD patterns of Fe2O3 before and after leaching, indicating that acetic acid–water (6
1) to determine the composition of the solid Fe compound filtered out of the leachate (Fig. 2). There was no noticeable change in the XRD patterns of Fe2O3 before and after leaching, indicating that acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is unable to break the Fe–O bond in Fe2O3. Both samples are also closely matched to the peaks of Fe2O3 from the standard (JCPDS no. 04-022-0741).
1) is unable to break the Fe–O bond in Fe2O3. Both samples are also closely matched to the peaks of Fe2O3 from the standard (JCPDS no. 04-022-0741).
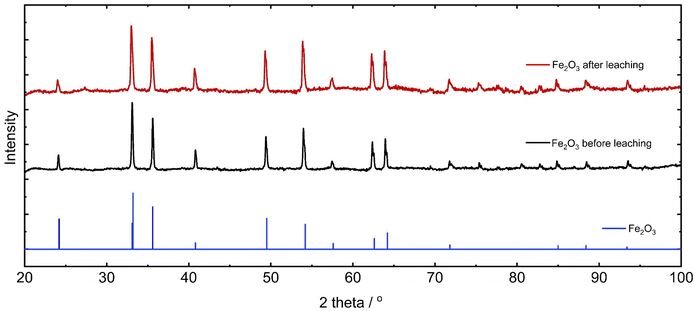 | ||
| Fig. 2 XRD patterns of Fe2O3 before (black) and after (red) leaching, and α-Fe2O3 standard (JCPDS no. 04-022-0741) (blue). | ||
Although replacing GUC with water improved the leaching efficiency of Nd2O3, that is not the case for Fe2O3 with its solubility decreasing from 14.5% in the DES-like mixtures to 10.0% in the lactic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and to 2.9% in acetic acid–water (6
1) and to 2.9% in acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
Overall, for these separate oxide leaching experiments, Nd2O3 was consistently dissolved at high levels between ∼63% to 95% (e.g. GUC–acetic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6): 62.6%, GUC–lactic acid (1
6): 62.6%, GUC–lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6): 78.3%, lactic acid–water (6
6): 78.3%, lactic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 83.3%, and acetic acid–water (6
1): 83.3%, and acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 95.0%), whereas the solubilities of Fe2O3 exhibited poor solubilities from ∼3% to 14% (GUC–lactic acid (1
1): 95.0%), whereas the solubilities of Fe2O3 exhibited poor solubilities from ∼3% to 14% (GUC–lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6): 14.5%, GUC–acetic acid (1
6): 14.5%, GUC–acetic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6): 14.3%, lactic acid–water (6
6): 14.3%, lactic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 9.3%, and acetic acid–water (6
1): 9.3%, and acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 2.9%). Only the GUC–water (1
1): 2.9%). Only the GUC–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) mixture dissolves either of Nd2O3 or Fe2O3 in a very small percentage (∼2%).
6) mixture dissolves either of Nd2O3 or Fe2O3 in a very small percentage (∼2%).
The greater solubility of Nd2O3 than Fe2O3 is likely due to the following reasons: (i) Nd exhibits a lower electronegativity than Fe (1.14 vs. 1.83) and a less stable lone pair, which increases its basicity28,43 and (ii) the Fe–O bond is stronger than Nd–O due to its smaller atomic radius (atomic radius of Fe is 126 pm and Nd is 229 pm, respectively) and stronger lattice energy (Nd2O3 – 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 736 kJ mol−1, Fe2O3 – 14309 kJ mol−1).28,43
736 kJ mol−1, Fe2O3 – 14309 kJ mol−1).28,43
Solubilities may change in a mixture of metals compared to their individual dissolution properties. Thus, understanding the change in solubility patterns of the mixed oxides is an important step to achieve selective and effective Nd leaching from used permanent magnets, considering that both Nd and Fe will be present.
2.2 Dissolution of the Nd2O3 and Fe2O3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 1 wt%) mixture in different solvent mixtures
1 wt%) mixture in different solvent mixtures
This set of experiments was conducted to comprehend the dissolution properties of Nd2O3 and Fe2O3 mixtures in the solvents used in the previous section. Also, any possible synergistic effect on the dissolution properties of having a mixture of Nd2O3 and Fe2O3 present is explored, which, to the best of our knowledge, has not yet been investigated. Mixtures of Nd2O3 and Fe2O3 with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio (5 mg
1 weight ratio (5 mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
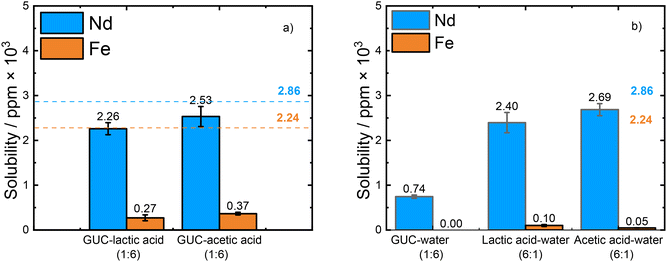
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mg) in 1.5 mL of the solvent mixtures were prepared to maintain the same total solid-to-liquid ratio as in the previous experiments. Fig. 3 shows the dissolution results for these mixed oxides in the DES-like systems (Fig. 3a) and the separate DES components mixed with water (Fig. 3b). When both oxides were present in solution, the mixtures containing GUC presented an enhancement of leaching efficiency in different degrees. GUC–acetic acid (1
5 mg) in 1.5 mL of the solvent mixtures were prepared to maintain the same total solid-to-liquid ratio as in the previous experiments. Fig. 3 shows the dissolution results for these mixed oxides in the DES-like systems (Fig. 3a) and the separate DES components mixed with water (Fig. 3b). When both oxides were present in solution, the mixtures containing GUC presented an enhancement of leaching efficiency in different degrees. GUC–acetic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) showed a solubility of 83.8% (2.53 × 103 ppm) for Nd2O3, which is greater than when Nd2O3 was the only oxide in the same solvent (62.6% in GUC–acetic acid (1
6) showed a solubility of 83.8% (2.53 × 103 ppm) for Nd2O3, which is greater than when Nd2O3 was the only oxide in the same solvent (62.6% in GUC–acetic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6)). This suggests that the presence of Fe2O3 may enhance the dissolution of Nd2O3. Similarly, for GUC–water (1
6)). This suggests that the presence of Fe2O3 may enhance the dissolution of Nd2O3. Similarly, for GUC–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) the dissolution of Nd2O3 improves significantly for the mixed oxides (0.74 × 103 ppm, 26.0%), compared to when Fe2O3 is absent from the mixture (0.02 × 103 ppm, <2.0%), showing again a possible synergistic effect of Fe in the dissolution of Nd. In the case of GUC–lactic acid (1
6) the dissolution of Nd2O3 improves significantly for the mixed oxides (0.74 × 103 ppm, 26.0%), compared to when Fe2O3 is absent from the mixture (0.02 × 103 ppm, <2.0%), showing again a possible synergistic effect of Fe in the dissolution of Nd. In the case of GUC–lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) there is a very small increase in Nd leaching efficiency from 78.3% to 79.1% when adding Fe2O3 to the mixture, which could be related to experimental error.
6) there is a very small increase in Nd leaching efficiency from 78.3% to 79.1% when adding Fe2O3 to the mixture, which could be related to experimental error.
The dissolution of Nd2O3 in the water based solutions remained almost the same in the presence or absence of Fe2O3 in the mixture (2.40 × 103 ppm, 83.8% in lactic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and 2.69 × 103 ppm, 94.0% in acetic acid–water (6
1), and 2.69 × 103 ppm, 94.0% in acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Fig. 1 and 3) and all maintained lower Fe2O3 solubility. This could be due to the different concentration of the metal oxides (5 mg for mixed metal oxides vs. 10 mg in the case of individual metal oxides) in solution which could affect the dissolution mechanism or the joint effect of both metal oxides in the mixture. Since the increase in solubility is not extended across the different mixtures under study, we can confirm that such synergistic effect seen for GUC based systems is not just due to a lower effective solid-to-liquid ratio for the Nd oxide in these mixed oxide experiments.
1), Fig. 1 and 3) and all maintained lower Fe2O3 solubility. This could be due to the different concentration of the metal oxides (5 mg for mixed metal oxides vs. 10 mg in the case of individual metal oxides) in solution which could affect the dissolution mechanism or the joint effect of both metal oxides in the mixture. Since the increase in solubility is not extended across the different mixtures under study, we can confirm that such synergistic effect seen for GUC based systems is not just due to a lower effective solid-to-liquid ratio for the Nd oxide in these mixed oxide experiments.
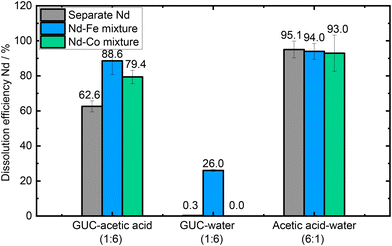
To confirm if the enhanced solubility of Nd2O3 is due to the presence of another metal oxide or Fe2O3 in particular, the iron oxide was replaced by cobalt(II,III) oxide (Co3O4), which has similar basicity, while maintaining the other experimental conditions. The GUC–acetic acid (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and GUC–water (1
1) and GUC–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) solvents were chosen because Nd solubility increased more significantly in those solvents in the presence of Fe2O3. One of the other solvents, acetic acid–water (6
6) solvents were chosen because Nd solubility increased more significantly in those solvents in the presence of Fe2O3. One of the other solvents, acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), in which Nd solubility was not affected by the presence of Fe2O3 was chosen for comparison.
1), in which Nd solubility was not affected by the presence of Fe2O3 was chosen for comparison.
We can observe that the presence of Co3O4 together with Nd2O3 in both GUC-based solutions lead to lower solubility of Nd in comparison with that observed with Fe2O3, being more drastic for the GUC–water solution (Fig. 4). This result demonstrates that Fe2O3 has an active role in the solubility of Nd2O3.
In the GUC–acetic acid (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), when either Fe2O3 or Co3O4 was present in solution with Nd2O3, Nd solubility was increased by 1.4 (Nd2O3
1), when either Fe2O3 or Co3O4 was present in solution with Nd2O3, Nd solubility was increased by 1.4 (Nd2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe2O3 mixture: 2.53 × 103 ppm, 83.8% and Nd2O3
Fe2O3 mixture: 2.53 × 103 ppm, 83.8% and Nd2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co3O4 mixture: 2.27 × 103 ppm, 79.4%) in comparison to Nd solubility from the separate Nd2O3 solution (3.58 × 103 ppm, 62.6%). On the other hand, in GUC–water (1
Co3O4 mixture: 2.27 × 103 ppm, 79.4%) in comparison to Nd solubility from the separate Nd2O3 solution (3.58 × 103 ppm, 62.6%). On the other hand, in GUC–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), Nd2O3 did not dissolve in the presence of Co3O4 (not detected by ICP-MS), which is comparable to the low Nd solubility (0.02 × 103 ppm of Nd2O3, 0.3%) from separate Nd2O3 and lower than Nd solubility (0.74 × 103 ppm of Nd2O3, 26.0%) from the solution with Nd2O3 and Fe2O3 present. Those results show that the enhancement in solubility is due to the nature of the metal oxide present in the mixture. Both Fe2O3 and Co3O4 are known catalysts, for example in the case of the oxygen reduction reaction;44,45 however in this case it seems likely that Fe2O3 has a more pronounced effect.
6), Nd2O3 did not dissolve in the presence of Co3O4 (not detected by ICP-MS), which is comparable to the low Nd solubility (0.02 × 103 ppm of Nd2O3, 0.3%) from separate Nd2O3 and lower than Nd solubility (0.74 × 103 ppm of Nd2O3, 26.0%) from the solution with Nd2O3 and Fe2O3 present. Those results show that the enhancement in solubility is due to the nature of the metal oxide present in the mixture. Both Fe2O3 and Co3O4 are known catalysts, for example in the case of the oxygen reduction reaction;44,45 however in this case it seems likely that Fe2O3 has a more pronounced effect.
Interestingly, acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) dissolved almost the same amount of Nd from the three samples (93.0–95.1%). These findings are significant because they demonstrate that the Nd solubility can be tuned by the presence of less soluble metal oxides together with Nd2O3 and the chemical composition of the solvent. This is of interest in the recycling of permanent magnets or other end of life devices where selective dissolution of valuable metals is highly sought.
1) dissolved almost the same amount of Nd from the three samples (93.0–95.1%). These findings are significant because they demonstrate that the Nd solubility can be tuned by the presence of less soluble metal oxides together with Nd2O3 and the chemical composition of the solvent. This is of interest in the recycling of permanent magnets or other end of life devices where selective dissolution of valuable metals is highly sought.
The solubility of Fe2O3 in the mixture with Nd2O3 exhibited the following trend: GUC–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) (not detected) < acetic acid–water (6
6) (not detected) < acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 0.05 × 103 ppm, 2.0% < GUC–lactic acid (1
1): 0.05 × 103 ppm, 2.0% < GUC–lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6): 0.27 × 103 ppm, 12.1% < GUC–acetic acid (1
6): 0.27 × 103 ppm, 12.1% < GUC–acetic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6): 0.36 × 103 ppm, 16.3% < lactic acid–water (6
6): 0.36 × 103 ppm, 16.3% < lactic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 0.67 × 103 ppm, 29.9%. This lower solubility of Fe2O3 could be attributed to its basicity and Fe–O strength as mentioned previously.
1): 0.67 × 103 ppm, 29.9%. This lower solubility of Fe2O3 could be attributed to its basicity and Fe–O strength as mentioned previously.
Overall, the selective dissolution can be demonstrated by the separation factors of Nd when dissolved in mixtures with Fe, as shown in Table 2. A similar selective dissolution was present in GUC–lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) and GUC–acetic acid (1
6) and GUC–acetic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) (SF of 27.5 and 26.6, respectively), but some selectivity is lost when lactic acid–water (6
6) (SF of 27.5 and 26.6, respectively), but some selectivity is lost when lactic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is used (SF = 12.1). Nonetheless, this system showed lower Nd selectivity compared to another GUC
1) is used (SF = 12.1). Nonetheless, this system showed lower Nd selectivity compared to another GUC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactic acid system reported in the literature (1
lactic acid system reported in the literature (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio) (SF = 440), however as mentioned before the working conditions are different.28 The best system for selective Nd dissolution was acetic acid–water (6
2 molar ratio) (SF = 440), however as mentioned before the working conditions are different.28 The best system for selective Nd dissolution was acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which had a significantly higher SF of 767.7, one order of magnitude larger compared to all other solvent mixtures. The selectivity for GUC–water (1
1), which had a significantly higher SF of 767.7, one order of magnitude larger compared to all other solvent mixtures. The selectivity for GUC–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) cannot be calculated accurately as no Fe was detected by ICP.
6) cannot be calculated accurately as no Fe was detected by ICP.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe2O3 mixtures (1
Fe2O3 mixtures (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt%), respectively, using different solvent mixtures
1 wt%), respectively, using different solvent mixtures
The results exhibited that, except for GUC–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), both HBD–HBA mixtures and acid–water (6
6), both HBD–HBA mixtures and acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixtures could effectively dissolve Nd2O3 from both individual oxides and mixtures. It is noteworthy that both excellent selectivity (SFNd/Fe: 767.7) and efficiency of Nd2O3 solubility (94.0%) were achieved in acetic acid–water (6
1) mixtures could effectively dissolve Nd2O3 from both individual oxides and mixtures. It is noteworthy that both excellent selectivity (SFNd/Fe: 767.7) and efficiency of Nd2O3 solubility (94.0%) were achieved in acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
2.3 Dissolution of the Nd2O3 and Fe2O3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 7 wt%) mixture
7 wt%) mixture
Although the dissolution properties of a Nd2O3 and Fe2O3 mixture were investigated in the previous section, the ratio of metal oxides should be consistent with a chemical composition of NdFeB magnets to implement this process on waste magnets and fully explore the selectivity of acetic acid. For this purpose, a mixture of Nd2O3 (1.25 mg) and Fe2O3 (8.75 mg) with the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 wt% (based on the permanent magnet delivered to us) was dissolved in the DES-like mixtures; GUC–lactic acid (1
7 wt% (based on the permanent magnet delivered to us) was dissolved in the DES-like mixtures; GUC–lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), GUC–acetic acid (1
6), GUC–acetic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), and also in the acetic acid–water (6
6), and also in the acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture due to its promising results. The experimental conditions were maintained from the previous leaching experiments.
1) mixture due to its promising results. The experimental conditions were maintained from the previous leaching experiments.
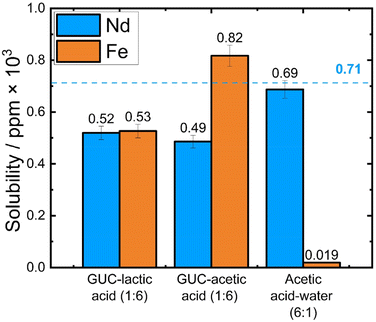
As shown in Fig. 5, all three solvents dissolved Nd2O3 effectively, with values ranging from 68.0–96.2% (e.g. 0.52 × 103 ppm (72.7%) in GUC–lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), 0.49 × 103 ppm (68.0%) in GUC–acetic acid (1
6), 0.49 × 103 ppm (68.0%) in GUC–acetic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), and 0.46 × 103 ppm (96.2%) in acetic acid–water (6
6), and 0.46 × 103 ppm (96.2%) in acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)). Compared to the 1
1)). Compared to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 oxide mixture, the solubilities of Nd2O3 when increasing the amount of Fe2O3 in the mixture are less favourable, particularly in the case of the GUC-based mixtures. This decrease in the catalytic effect of Fe2O3 previously discussed could be explained since normally small amounts of catalyst are required, therefore increasing its amount will not be translated into better dissolution efficiencies. In the acetic acid–water (6
1 oxide mixture, the solubilities of Nd2O3 when increasing the amount of Fe2O3 in the mixture are less favourable, particularly in the case of the GUC-based mixtures. This decrease in the catalytic effect of Fe2O3 previously discussed could be explained since normally small amounts of catalyst are required, therefore increasing its amount will not be translated into better dissolution efficiencies. In the acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as previously observed, no significant changes are observed based on the presence or absence of metal oxides as well as their concentrations.
1) as previously observed, no significant changes are observed based on the presence or absence of metal oxides as well as their concentrations.
The solubilities of Fe2O3 in GUC–lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) and GUC–acetic acid (1
6) and GUC–acetic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), while maintaining a similar low percentage dissolved as for the 1
6), while maintaining a similar low percentage dissolved as for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 experiment, showed a higher overall level of Fe present in terms of ppm (0.53 × 103 ppm, 13.4% and 0.82 × 103 ppm, 20.9% respectively). From Fig. 5 and Table 3 we can see that the separation factors of Nd from Fe using the GUC–lactic acid (1
1 experiment, showed a higher overall level of Fe present in terms of ppm (0.53 × 103 ppm, 13.4% and 0.82 × 103 ppm, 20.9% respectively). From Fig. 5 and Table 3 we can see that the separation factors of Nd from Fe using the GUC–lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) and GUC–acetic acid (1
6) and GUC–acetic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) systems were lower in this ratio. Curiously, the selectivity Nd
6) systems were lower in this ratio. Curiously, the selectivity Nd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe has been markedly increased in the acetic acid–water (6
Fe has been markedly increased in the acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) system (SF of 5038, from 768 in a 1
1) system (SF of 5038, from 768 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt% metal mixture, Tables 2 and 3), further corroborating the potential of this system for selective Nd dissolution, the highest dissolution efficiency in this experimental series (96.2%).
1 wt% metal mixture, Tables 2 and 3), further corroborating the potential of this system for selective Nd dissolution, the highest dissolution efficiency in this experimental series (96.2%).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe2O3 mixtures (1
Fe2O3 mixtures (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 wt%), respectively, using different solvent mixtures
7 wt%), respectively, using different solvent mixtures
| Solvent mixture | L Nd % | L Fe % | SFNd/Fe |
|---|---|---|---|
GUC–lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
72.7 | 13.4 | 17.2 |
GUC–acetic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
68.0 | 20.9 | 8.0 |
Acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
96.2 | 0.5 | 5037.8 |
These results indicate that the solvent mixture of acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is a promising candidate for selectively dissolving Nd2O3 due to the difference in the dissolution abilities of Fe2O3 and Nd2O3. Among previous studies that have used acetic acid solution to dissolve NdFeB magnets, Yoon et al. achieved selective recovery of Nd (SFNd/Fe: 1608) from the magnet after roasting,34 which is lower than our results.
1) is a promising candidate for selectively dissolving Nd2O3 due to the difference in the dissolution abilities of Fe2O3 and Nd2O3. Among previous studies that have used acetic acid solution to dissolve NdFeB magnets, Yoon et al. achieved selective recovery of Nd (SFNd/Fe: 1608) from the magnet after roasting,34 which is lower than our results.
Other researchers were unable to achieve a selective dissolution, with Fe dissolved in a range between 21–38%, which required additional separation.10,33,46–48 The difference in Nd selectivity may be due to the different composition, chemistries and phases of the Nd and Fe in the spent NdFeB magnets due to different pre-treatments. The most favourable scenario is for Fe and Nd to be in their oxide form (Fe2O3 and Nd2O3) so leaching and selectivity of Nd may be maximised.
2.4 Dissolution of Nd2O3 in acetic acid solutions with different concentrations
The current concentration of the acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
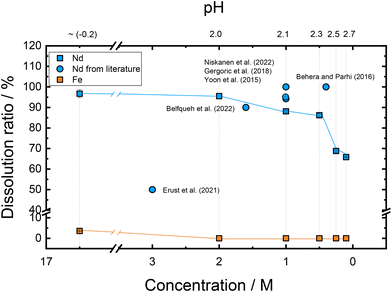
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (16.5 M) mixture studied in this work is higher than both the stoichiometry ratio of acid to metal oxide (6 mol CH3COOH per 1 mol Nd2O3, Nd2O3 + 6CH3COOH = 2Nd(CH3COO)3 + 3H2O) and the concentration of acetic acid solutions used in previous studies10,33,34,46–50 which are in the range 0.4–3.0 M. Fig. 6 shows the Nd leaching efficiencies reported in the previous studies from the literature that used acetic acid for dissolving Nd from NdFeB magnets and metal oxides in different leaching conditions.10,33,34,46–49 In our study leaching experiments for Fe2O3 and Nd2O3 in a weight ratio of 1
1) (16.5 M) mixture studied in this work is higher than both the stoichiometry ratio of acid to metal oxide (6 mol CH3COOH per 1 mol Nd2O3, Nd2O3 + 6CH3COOH = 2Nd(CH3COO)3 + 3H2O) and the concentration of acetic acid solutions used in previous studies10,33,34,46–50 which are in the range 0.4–3.0 M. Fig. 6 shows the Nd leaching efficiencies reported in the previous studies from the literature that used acetic acid for dissolving Nd from NdFeB magnets and metal oxides in different leaching conditions.10,33,34,46–49 In our study leaching experiments for Fe2O3 and Nd2O3 in a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 in acetic acid–water ranging in concentration from 16.5 M to 0.1 M were conducted under the same experimental conditions (Fig. 6). Just a note that the initial molar ratio of acetic acid–water (6
7 in acetic acid–water ranging in concentration from 16.5 M to 0.1 M were conducted under the same experimental conditions (Fig. 6). Just a note that the initial molar ratio of acetic acid–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was chosen according to the ratios of HBA and HBD in this work. The Nd2O3 over Fe2O3 weight ratios in this study were chosen based on the highest dissolution and selectivity observed in our study for the acetic acid
1) was chosen according to the ratios of HBA and HBD in this work. The Nd2O3 over Fe2O3 weight ratios in this study were chosen based on the highest dissolution and selectivity observed in our study for the acetic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixture.
water mixture.
It was observed that the solubility of Nd2O3 decreased from 96.7 to 65.8% when the acetic acid concentration reduced from 16.5 to 0.1 M. However, a leaching efficiency as high as 95.5% is maintained when acid concentration is reduced to 2.0 M. Therefore, it is shown that under these leaching conditions (solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio: 1
liquid ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150, T = 50 °C, 24 h, 500 rpm), acetic acid concentration could be reduced to 2.0 M without significantly affecting the Nd2O3 leaching efficiency. Note that the selectivity of Nd2O3 was improved with the reduced concentration of acetic acid solutions (0.1–2.0 M), by dissolving smaller and smaller amount of Fe2O3 which were beyond the detection limit for the ICP-MS (<0.5% of Fe2O3, Fig. 6).
150, T = 50 °C, 24 h, 500 rpm), acetic acid concentration could be reduced to 2.0 M without significantly affecting the Nd2O3 leaching efficiency. Note that the selectivity of Nd2O3 was improved with the reduced concentration of acetic acid solutions (0.1–2.0 M), by dissolving smaller and smaller amount of Fe2O3 which were beyond the detection limit for the ICP-MS (<0.5% of Fe2O3, Fig. 6).
Previous studies attained Nd dissolution efficiencies ranging from 99.99% to 50% in acetic acid solutions by varying the concentration from 3.0 to 0.4 M. Interestingly, by using low concentrations of acetic acid (0.4–1.6 M) higher Nd solubilities (>90.0%)10,33,34,46–49 were achieved (Fig. 6), whereas the lower Nd efficiency (50%) was achieved when using the higher concentration of acetic acid (3 M). Nevertheless, the different leaching efficiencies can be attributed to their respective leaching conditions, including phase composition of the NdFeB spent magnets attained from the pre-treatment, leaching temperature, time, solid-to-liquid ratio and stirring speed. Due to the differences in experimental conditions, it is difficult to draw too many conclusions from the effect of acid concentration.
Here, we achieved a solubility of 95.5% (0.68 × 103 ppm) for Nd2O3 in 2.0 M acetic acid with a high Nd selectivity (Fe2O3 solubility < 0.5%, SF > 4223) (process temperature 50 °C) from a model mixed oxide solution, whereas Yoon et al.34 attained high solubility (94.2%) and also selectivity (Fe solubility: 1.0%, SF of 1608) of Nd from NdFeB magnet scraps after grinding and roasting to their oxides using a 1.0 M acetic acid solution (process temperature 90 °C). The results showed that under these moderate leaching conditions, acetic acid could be diluted to 2.0 M or lower without affecting the leaching efficiency of Nd2O3 while maintaining high selectivity.
2.5 Alignment with green chemistry principles
Throughout our manuscript, we have addressed several green chemistry principles, as outlined in the ACS“12 Principles of Green Chemistry”,51 including waste prevention, designing safer chemicals (minimizing toxicity and maintaining function and efficiency) and design for energy efficiency. In this section we will conclude and summarise the main points that are aligned with the 12 principles of Green Chemistry.Our new system (diluted acetic acid (2.0 M)) demonstrates selectivity specific to Nd without requiring additional steps for metal separation. This exemplifies the green advantage of our approach by minimizing the quantity of chemicals used and thereby reducing waste generation through prevention. Additionally, while in our study we initially considered some of the DES components reported in the literature (e.g. lactic acid and guanidine hydrochloride), acetic acid was also included as a hydrogen bond donor system in the mixture.
Such a simple, economical and green system as diluted acetic acid (designing safer chemicals) has shown an ability to selectively dissolve Nd over Fe with a leaching efficiency of 95.5% and a separation factor (SF) of 4223. This value is considerably higher than those reported in the literature (SF: 1608)34 and therefore aligned with the principle of Green Chemistry; design for energy efficiency. Our use of acetic acid in the separation of Nd and Fe is certainly greener than the use of versatic acid to separate Nd and Fe after the alkaline baking method31 given the preparative method used for versatic acid.52
Finally, to further enhance the green contribution of this manuscript, we also observed the beneficial and synergetic effect of iron oxide in the mixtures to improve the dissolution of Nd (design for energy efficiency and prevention). This is quite exciting as this means that common impurities present in for instance, magnets, could be beneficial for selective Nd dissolution but also minimise the need of additional chemicals to enable a purification and separation process to eliminate Fe completely from the starting material.
3. Conclusions
The dissolution properties of individual Nd2O3 and Fe2O3 and their metal oxide mixtures with different weight ratios were explored using a series of hydrogen bond acceptor (HBA) and hydrogen bond donors (HBD) with molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 composed of guanidine hydrochloride (GUC) as HBA and lactic acid, and acetic acid as HBDs, as well as the individual HBDs and HBA in water. In summary, the leaching ability of Nd2O3 was increased by a factor 1.5 times in water-based systems compared to GUC-based systems. We concluded that the presence of chloride in the GUC-based mixtures led to the formation of the chloride complex ([NdClx]3−x) which is less stable (log
6 composed of guanidine hydrochloride (GUC) as HBA and lactic acid, and acetic acid as HBDs, as well as the individual HBDs and HBA in water. In summary, the leaching ability of Nd2O3 was increased by a factor 1.5 times in water-based systems compared to GUC-based systems. We concluded that the presence of chloride in the GUC-based mixtures led to the formation of the chloride complex ([NdClx]3−x) which is less stable (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kf1 = 0.06) than the acetate Nd(CH3CHOHCOO)x or lactate Nd(CH3COO)x complexes, which result in more stable (log
Kf1 = 0.06) than the acetate Nd(CH3CHOHCOO)x or lactate Nd(CH3COO)x complexes, which result in more stable (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kf1, 2, 3 = 4.0, 2.3, and 1.7 and log
Kf1, 2, 3 = 4.0, 2.3, and 1.7 and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kf1, 2, 3 = 2.6, 2.1, and 1.9, respectively) and soluble species.
Kf1, 2, 3 = 2.6, 2.1, and 1.9, respectively) and soluble species.
Overall, it was found that metal-complexation and metal oxide basicity were the determining factors in the leaching efficiency. Nd2O3 was found more soluble in weaker acids than Fe2O3 due to its basicity and weaker lattice energy (Nd2O3 – 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 736 kJ mol−1, Fe2O3 – 14309 kJ mol−1) and the mixture acetic acid
736 kJ mol−1, Fe2O3 – 14309 kJ mol−1) and the mixture acetic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (6
water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) led to best leaching and selectivity of Nd2O3 over Fe2O3.
1) led to best leaching and selectivity of Nd2O3 over Fe2O3.
In mixed oxide systems composed of Nd2O3 and Fe2O3, some significant differences were observed in comparison with the individual metal oxide dissolution pattern. It was also found that the presence of the Fe2O3 in the mixture enhanced the dissolution of Nd2O3, depending on the amount of Fe2O3, especially in the case of GUC-based systems which was attributed to the Fe2O3 catalytic effect with a separation factor up to 5038.
However, for acetic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (6
water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) the presence or absence of other metal oxides (Fe2O3 and Co3O4) did not affect the leaching efficiency of Nd2O3 and led to the highest efficiency and selectivity values of the systems studied. Finally, the concentration of acetic acid was varied and with diluted acetic acid (2.0 M), it was found that Nd leaching efficiency (95.5%) could be maintained at a similar level to that achieved in concentrated acetic acid, while maintaining high Nd selectivity (separation factor > 4223).
1) the presence or absence of other metal oxides (Fe2O3 and Co3O4) did not affect the leaching efficiency of Nd2O3 and led to the highest efficiency and selectivity values of the systems studied. Finally, the concentration of acetic acid was varied and with diluted acetic acid (2.0 M), it was found that Nd leaching efficiency (95.5%) could be maintained at a similar level to that achieved in concentrated acetic acid, while maintaining high Nd selectivity (separation factor > 4223).
Therefore, by modifying the chemical composition of the mixtures (e.g. HBD, HBA, water, metal oxide) it is possible to tune the dissolution efficiency and selectivity of metal oxides. This systematic study is of great interest for the selective dissolution of valuable and critical metals from model systems using less hazardous reagents and we anticipate scope to apply the knowledge reported in this work (i.e., metal speciation, electrolyte design) to procedures for other critical metals (notably Co, Ni, Li).
Author contributions
Cristina Pozo-Gonzalo supervision, project joint conception, results discussion, revision of intemediate draft versions and final draft preparation. Rabeeh Golmohammadzadeh, project joint conception, co-experiment design, conducting experiments and initial data analysis by chemical leaching and ICP, preparation of first draft, discussion and revision, Munkhshur Myekhlai, leaching experiments, XRD, preparation of intermediate draft versions, Henrique Bastos, leaching experiments and ICP, discussions and revision of manuscript. Glen B. Deacon, discussion and revised manuscript. Anthony E. Somers, supervision, discussion and revised manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
CPG acknowledges the Australian Research Council (ARC) Centre for Training Centre for Future Energy Storage Technologies (storEnergy) (IC180100049) for their funding. The authors thanks AMF magnetics (https://magnet.com.au/) for partly funding the project and materials supply.References
- K. Binnemans, P. McGuiness and P. T. Jones, Nat. Rev. Mater., 2021, 6, 459–461 CrossRef CAS.
- Y. Fujita, S. K. McCall and D. Ginosar, MRS Bull., 2022, 47, 283–288 CrossRef CAS.
- K. M. Goodenough, F. Wall and D. Merriman, Nat. Resour. Res., 2017, 27, 201–216 CrossRef.
- K. Periyapperuma, L. Sanchez-Cupido, J. M. Pringle and C. Pozo-Gonzalo, Sustainable Chem., 2021, 2, 550–563 CrossRef CAS.
- V. Balaram, Geosci. Front., 2019, 10, 1285–1303 CrossRef CAS.
- J. H. Rademaker, R. Kleijn and Y. Yang, Environ. Sci. Technol., 2013, 47, 10129–10136 CrossRef CAS PubMed.
- B. Sprecher, R. Kleijn and G. J. Kramer, Environ. Sci. Technol., 2014, 48, 9506–9513 CrossRef CAS PubMed.
- Q. Liu, K. Sun, X. Ouyang, B. Sen, L. Liu, T. Dai and G. Liu, Environ. Sci. Technol., 2022, 56, 11807–11817 CrossRef CAS PubMed.
- G. Corbetta, A. Ho, I. Pineda, K. Ruby, L. Van de Velde and J. Bickley, Wind in Power, European Wind Energy Association, 2015 Search PubMed.
- J. Niskanen, M. Lahtinen and S. Perämäki, Clean. Eng. Technol., 2022, 10, 100544 CrossRef.
- M. Firdaus, M. A. Rhamdhani, Y. Durandet, W. J. Rankin and K. McGregor, J. Sustain. Metall., 2016, 2, 276–295 CrossRef.
- M. A. R. Önal, C. R. Borra, M. Guo, B. Blanpain and T. Van Gerven, J. Sustain. Metall., 2015, 1, 199–215 CrossRef.
- M. K. Jha, A. Kumari, R. Panda, J. Rajesh Kumar, K. Yoo and J. Y. Lee, Hydrometallurgy, 2016, 161, 77–101 CrossRef CAS.
- H.-S. Yoon, C.-J. Kim, K.-W. Chung, S.-D. Kim, J.-Y. Lee and J. R. Kumar, Hydrometallurgy, 2016, 165, 27–43 CrossRef CAS.
- R. Auerbach, K. Bokelmann, R. Stauber, O. Gutfleisch, S. Schnell and S. Ratering, Miner. Eng., 2019, 134, 104–117 CrossRef CAS.
- T. H. Okabe, O. Takeda, K. Fukuda and Y. Umetsu, Mater. Trans., 2003, 44, 798–801 CrossRef CAS.
- M. Zakotnik, I. R. Harris and A. J. Williams, J. Alloys Compd., 2008, 450, 525–531 CrossRef CAS.
- T. Uda, Mater. Trans., 2002, 43, 55–62 CrossRef CAS.
- M. Itoh, K. Miura and K.-I. Machida, J. Alloys Compd., 2009, 477, 484–487 CrossRef CAS.
- A. P. Abbott, G. Capper, D. L. Davies, H. L. Munro, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2001, 2010–2011, 10.1039/b106357j.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71, 10.1039/b210714g.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232–1285 CrossRef CAS PubMed.
- D. Yu, Z. Xue and T. Mu, Chem. Soc. Rev., 2021, 50, 8596–8638 RSC.
- Z. Yuan, H. Liu, W. F. Yong, Q. She and J. Esteban, Green Chem., 2022, 24, 1895–1929 RSC.
- A. Zhu, X. Bian, W. Han, D. Cao, Y. Wen, K. Zhu and S. Wang, Resour., Conserv. Recycl., 2023, 188, 106690 CrossRef CAS.
- S. Riaño, M. Petranikova, B. Onghena, T. Vander Hoogerstraete, D. Banerjee, M. R. S. Foreman, C. Ekberg and K. Binnemans, RSC Adv., 2017, 7, 32100–32113 RSC.
- W. Chen, J. Jiang, X. Lan, X. Zhao, H. Mou and T. Mu, Green Chem., 2019, 21, 4748–4756 RSC.
- C. Liu, Q. Yan, X. Zhang, L. Lei and C. Xiao, Environ. Sci. Technol., 2020, 54, 10370–10379 CrossRef CAS PubMed.
- Q. Yan, C. Liu, X. Zhang, L. Lei and C. Xiao, ACS Sustainable Chem. Eng., 2021, 9, 8507–8514 CrossRef CAS.
- I. M. Pateli, A. P. Abbott, K. Binnemans and N. Rodriguez Rodriguez, RSC Adv., 2020, 10, 28879–28890 RSC.
- M. A. R. Önal, S. Riaño and K. Binnemans, Hydrometallurgy, 2020, 191, 105213 CrossRef.
- A. Skulcova, A. Russ, M. Jablonski and J. Sima, BioResources, 2018, 13, 5042–5051 CAS.
- S. S. Behera and P. K. Parhi, Sep. Purif. Technol., 2016, 160, 59–66 CrossRef CAS.
- H.-S. Yoon, C.-J. Kim, K. Chung, S. Jeon, I. Park, K. Yoo and M. Jha, Metals, 2015, 5, 1306–1314 CrossRef.
- J. T. M. Amphlett, Y. Lee, W. Yang, D. Kang, N. E. Sung, J. Park, E. C. Jung and S. Choi, ACS Omega, 2022, 7, 921–932 CrossRef CAS PubMed.
- G. Tian, L. R. Martin and L. Rao, Inorg. Chem., 2010, 49, 10598–10605 CrossRef CAS PubMed.
- O. Gutten and L. Rulisek, Inorg. Chem., 2013, 52, 10347–10355 CrossRef CAS PubMed.
- S. Tang, M. Zhang and M. Guo, ACS Sustainable Chem. Eng., 2022, 10, 975–985 CrossRef CAS.
- R. H. Karraker, Retrospective Theses and Dissertations, Iowa State University of Science and Technology, Ames, IA, USA, 1969, pp. 16–18 Search PubMed.
- C. H. Gammons, S. A. Wood and A. E. Williams-Jones, Geochim. Cosmochim. Acta, 1996, 60, 4615–4630 CrossRef CAS.
- L. Sanchez-Cupido, J. M. Pringle, A. I. Siriwardana, M. Hilder, M. Forsyth and C. Pozo-Gonzalo, ACS Sustainable Chem. Eng., 2020, 8, 14047–14057 CrossRef CAS.
- K. Periyapperuma, J. M. Pringle, L. Sanchez-Cupido, M. Forsyth and C. Pozo-Gonzalo, Green Chem., 2021, 23, 3410–3419 RSC.
- A. Ding, C. Liu, X. Zhang, L. Lei and C. Xiao, Environ. Sci. Technol., 2022, 56, 4404–4412 CrossRef CAS PubMed.
- K. Shimizu, L. Sepunaru and R. G. Compton, Chem. Sci., 2016, 7, 3364–3369 RSC.
- F. T. Haase, A. Bergmann, T. E. Jones, J. Timoshenko, A. Herzog, H. S. Jeon, C. Rettenmaier and B. R. Cuenya, Nat. Energy, 2022, 7, 765–773 CrossRef CAS.
- M. Gergoric, C. Ravaux, B.-M. Steenari, F. Espegren and T. Retegan, Metals, 2018, 8, 721 CrossRef.
- S. S. Behera, S. K. Panda, D. Mandal and P. K. Parhi, Hydrometallurgy, 2019, 185, 61–70 CrossRef CAS.
- S. Belfqueh, A. Seron, S. Chapron, G. Arrachart and N. Menad, J. Rare Earths, 2023, 41, 621–631 CrossRef CAS.
- C. Erust, A. Akcil, A. Tuncuk, H. Deveci and E. Y. Yazici, Miner. Process. Extr. Metall. Rev., 2021, 42, 90–101 CrossRef CAS.
- X. Li and K. Binnemans, Chem. Rev., 2021, 121, 4506–4530 CrossRef CAS PubMed.
- J. C. W. Paul and T. Anastas, Green Chemisty: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed.
- M. Fefer, J. Am. Oil Chemists. Soc., 1978, 55, 342A–345A CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3gc04800d |
| ‡ Current address: Instituto de Carboquímica (ICB-CSIC), C/Miguel Luesma Castán, 4, 50018, Zaragoza, Spain. |
| § Current address: Research and Development Agency of Aragon (ARAID) Foundation, Zaragoza, Spain. |
| ¶ Current address: Environment Protection Authority Victoria, EPA Science, Centre for Applied Sciences, Ernest Jones Drive, Macleod, Melbourne, Victoria, 3085, Australia. |
| || Author equal contribution. |
| This journal is © The Royal Society of Chemistry 2024 |