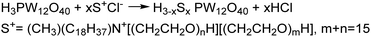Ionic liquid-stabilized metal oxoclusters: from design to catalytic application
Yunxiang
Qiao†
 a,
Enting
Shi†
b,
Xinjia
Wei
b and
Zhenshan
Hou
a,
Enting
Shi†
b,
Xinjia
Wei
b and
Zhenshan
Hou
 *b
*b
aMax-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
bState Key Laboratory of Green Chemical Engineering and Industrial Catalysis, Research Institute of Industrial Catalysis, School of Chemistry & Molecular Engineering, East China University of Science and Technology, Shanghai 200237, People's Republic of China. E-mail: houzhenshan@ecust.edu.cn; Tel: +86 21 64251686
First published on 5th April 2024
Abstract
Metal oxoclusters constitute a dynamic and expanding domain of research with versatile applications in catalysis, materials science, medicine, and beyond. Particular attention has been given to their potential utility due to their distinctive properties. The incorporation of ionic liquid (IL) environments into metal oxocluster (MOC) systems offers numerous advantages, including higher stability, tunable coordination to metal sites, and the capability to dissolve diverse substrates for catalytic applications. Although IL-stabilized MOCs (IL–MOCs) are still in the developmental phase, their potential to revolutionize catalysis is substantial. As research progresses, IL–MOCs will likely play an increasingly important role in catalysis. This perspective article focuses on the preparation methods of IL–MOCs, how the MOCs are stabilized/regulated by ILs or even PIL (polymeric ILs), as well as their catalytic applications, including oxidation, epoxidation, oxidative desulfurization, biomass oxidation, hydroxylation of benzene, acid catalysis, CO2 activation and utilization, carbonylation, hydrogenation/hydrolysis, electrochemistry, etc. IL–MOCs have played a crucial role in the development of sustainable chemistry in the light of the potential opportunities and challenges. The unique advantages make IL–MOCs outstanding candidates for industrial catalysts.
1. Introduction
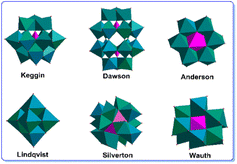
Metal oxoclusters (MOCs) are a diverse and fascinating class of inorganic compounds composed of metal atoms (typically transition metals) bonded to oxygen atoms. These clusters can be found in nature, but they can also be synthesized in the laboratory. Characterized as molecular oxide species, MOCs have discrete chemical formulas and can be isolated as pure compounds. They are soluble in water or some organic solvents and have a wide range of compositions and structures, shapes and sizes, and are composed of a wide range of different metal cations. Distinguished by their unique structures and properties, MOCs can be tailored for a wide range of specific applications, including catalysis,1 materials science,2–4 and biomedical applications.5,6 In catalysis, they are able to serve as catalysts in practical processes like the production of fuels and fine chemicals.7 Moreover, MOCs are under investigation as potential materials for batteries, solar cells, and other electronic devices in materials science.8 Additionally, they have shown to have antiviral, antibacterial, and anticancer activity, expanding their potential applications in the biomedical field.9,10 In summary, MOCs offer versatility in terms of their compositions and structures, making them highly adaptable for specific applications across different scientific and technological domains.Common examples of MOCs include polyoxometalates (POMs), MOCs with organic ligands (e.g. ILs) or metalloenzymes etc. POMs, a specific class of MOCs, are composed of early transition metals, such as molybdenum, tungsten, and vanadium, in their highest oxidation states.11–13 Various POM structures have been determined, with the most known ones being the Keggin, Dawson, Anderson, Lindqvist, Siverton and Wauth structures, etc. (Fig. 1). For instance, Keggin-POM clusters, featuring 12 metal atoms and 40 oxygen atoms, while Lindqvist-POM clusters, comprising 6 metal atoms and 18 oxygen atoms, both show a cage-like structure and share similar applications.
On the other hand, ionic liquids (ILs) are a class of non-volatile, non-flammable, and tunable solvents, which are made up of ions. They can be tailored to have specific properties, such as polarity, viscosity, and acidity, making them unique media for a wide variety of catalytic reactions and the synthesis of a wide range of MOCs. IL-stabilized MOCs (IL–MOCs) are a new class of catalysts that have shown great promise for a variety of applications in catalysis, electrochemistry, energy production, and materials science.14–17 One of the key advantages of IL–MOCs is their high catalytic activity. This is due to the unique structure of these catalysts with the presence of the exposed metal sites, which allows for close contact between the highly exposed metal atoms and the reactants, thus facilitating the transfer of electrons and the formation of chemical bonds, which are essential for catalysis. ILs could not only stabilize MOCs, but also regulate MOCs through a combination of interactions, e.g. ionic and electrostatic forces, tuning of hydrophilicity or hydrophobicity, and facilitating redox processes. The careful selection of IL components and design considerations can tailor the IL environment to enhance the stability and catalytic properties of MOCs in catalytic applications.
Several research groups actively work on the use of IL–MOCs. In recent years, our group not only designed different IL–MOCs (especially POM-based ILs, POM–ILs), but also utilized them for catalytic epoxidation, oxidation, esterification, and carbonylation/carboxylation, etc. This perspective article aims to provide a brief overview of the development and catalytic applications of IL–MOCs, with a focus on the reports from our own research group and recent contributions from the other groups. Herein, we also briefly discuss the advantages, opportunities, challenges, and the developmental direction of IL–MOCs for the catalytic applications.
2. The synthesis routes for IL–MOCs
The synthesis of IL–MOCs involves the combination of MOCs with ILs.17–19 It's essential to consider factors such as solubility, stability, and compatibility between the MOC and IL during the synthesis process. Additionally, the choice of synthesis method depends on the properties desired for the IL–MOC hybrids and the nature of the MOC and IL involved, and it can in turn influence the structure and properties of the resulting IL–MOCs. The general synthesis approaches of IL–MOCs mainly include ion exchange, direct mixing or dissolution, in situ synthesis, self-assembly strategy, etc.2.1 Ion exchange
The ion exchange method for IL–MOC synthesis involves subjecting a preformed IL to ion exchange with a MOC anion, which replaces some or all of the counterions in the IL, resulting in the formation of IL–MOCs with MOC as the anion of the new IL. This simple method just consists of mixing the MOC (normally dissolved in water) with the organic salt dissolved in water or in a miscible organic solvent (often an alcohol) in the right stoichiometry. After a certain time of reaction whilst stirring, the two phases are separated sometimes with the help of centrifugation when emulsions are obtained. The heavy phase is normally the IL–MOC product which precipitates or forms a new liquid phase. After several times of washing, the resulting IL–MOC hybrids are dried and analyzed. As a typical example, the first reported POM–IL was prepared through partial exchange of the protons in heteropoly acid (HPA) by polyethylene glycol (PEG)-containing quaternary ammonium, yielding a liquid derivative of the POM with similar properties to other ILs (Scheme 1), such as a low melting point, comparable viscosity, and high conductivity.20The more widely researched examples are the anion-exchange of PILs by POM anions, a process similar to that of monomeric ILs, except the cation is PIL.21–23 PILs are the polymeric products of IL monomers, composed of polymeric backbones with IL species in each repeating unit, forming a network structure.24 Mixing a PIL solution in a miscible organic solvent (often ethanol) with a MOC aqueous solution forms the desired PIL–MOC, which can be separated normally by filtration. As such, PIL–MOCs in this article refer to MOCs serving as anions of PILs. PILs exhibit properties similar to traditional ILs but possess additional characteristics stemming from their polymeric nature. These materials often feature high thermal stability, tunable mechanical properties, compatibility with various solvents and substrates, and more easily tunable amphiphilicity, etc. These characteristics make them similar to, but slightly different from monomeric IL–MOCs. PIL–MOCs are also widely used in various catalytic reactions, which is illustrated in the following sections.
2.2 Direct mixing or dissolution
This is a straightforward method where metal oxides are mixed or dissolved directly in an IL. The choice of IL depends on the desired properties of the resulting IL–MOCs. A specific example is the usage of protonated betaine bis(trifluoromethylsulfonyl)imide [Hbet][NTf2] as a versatile task-specific IL that can be used for the selective solubilization of metal oxides and metal salts due to its acidic functional groups.25,26 The zwitterionic nature of the betaine ligand in combination with the weakly coordination ability of [NTf2]− anion facilitates the formation of metal complexes into oligonuclear and polynuclear metal complexes. Crystal structures of six metal complexes, cobalt(II), nickel(II), manganese(II), zinc(II), silver(I), and lead(II) compounds, were obtained after solubilizing metal oxides in [Hbet][NTf2] in the presence of water.26 The resulting crystals exhibited rich structural chemistry, including trimeric units for the cobalt(II) compound, tetrameric units for the manganese(II) and zinc(II) compounds, pentameric units for the nickel(II), an oxo–hydroxo-cluster formation for the lead(II) compound, and polymeric units for the silver(I) compound.2.3 In situ generation
MOCs can be generated in situ within an IL. The addition of a metal salt and a suitable oxidizing agent to an IL leads to the formation of MOCs within the IL medium, in which ILs act as both solvents and charge-compensating species. Noticeably, the reaction temperature can be increased further owing to the unique characteristics of ILs as high thermal stability and nearly non-volatility. The first member of a new family of POM–ILs, demonstrated by Rickert et al.,27,28 was prepared by pairing Keggin or Lindqvist POM anions with tetraalkylphosphonium cations. Other examples are: [EMIm]4[[beta]-Mo8O26] (EMIm is 1-ethyl-3-methylimidazolium) could be obtained from the IL [EMIm]BF4,29 [EMIm]8Na9[WFe9(μ3-O)3(μ2-OH)6O4H2O(SiW9O34)3]·7H2O and [EMIm]4[SiMo12O40]·12H2O could be successfully produced in the IL [EMIM]Br.30 Additionally, crystal-phase polyoxomolybdate compounds such as [BMIm]3NH4[Mo8O26], [BMIm]4[PMoVMo11O40] and [BMIm]3[PMo12O40] (BMIm = 1-butyl-3-methylimidazolium) were synthesized in situ using [BMIm][BF4] and simple molybdate salts.31 Detailed information on these in situ synthesis examples is shown in Table 1.| IL–MOCs | POM precursor | IL | T/°C | t | Ref. |
|---|---|---|---|---|---|
| (R3R′P)2W6O19 | Na2WO4 | R3R′PBr | — | — | 27 and 28 |
| [EMIm]4[β-Mo8O26] | Na2MoO4·2H2O | [EMIm]BF4 | 170 | 24 h | 29 |
| [EMIm]8Na9[WFe9(μ3-O)3(μ2-OH)6O4H2O(SiW9O34)3]·7H2O | Na10[SiW9O34]·16H2O, Fe(NO3)3·9H2O | [EMIm]Br | 150 | Three days | 30 |
| [EMIm]4[SiMo12O40]·12H2O | Na2MoO4·2H2O, Na2SiO3·9H2O | [EMIM]Br | 150 | Three days | 30 |
| [BMIm]3NH4[Mo8O26] (crystal) | Ammonium molybdate, La(OH)3, 2-pyridinecarboxylic acid | [BMIm][BF4] | 140 | Six days | 31 |
| [BMIm]4[PMoVMo11O40] (crystal) | (NH4)6Mo7O24·4H2O, La(OH)3, NH4H2PO4 | [BMIm][BF4] | 135 | Six days | 31 |
| [BMIm]3[PMo12O40] (crystal) | Sodium molybdate, copper chloride, phosphoric acid, water | [BMIm][BF4] | 150 | Seven days | 31 |
2.4 Self-assembly strategy
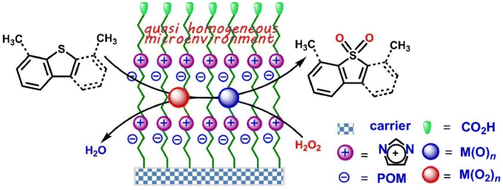
The self-assembly strategy of IL–MOC involves the spontaneous arrangement of MOCs within an IL environment, which is also known as amphiphile self-assembly media.32–34 This process is governed by the specific interactions between the MOC anions and the IL cations, e.g. covalent and non-covalent (electrostatic, hydrophobic and solvophobic) interactions.35 The same principle also applied to PILs, taking composite POM/PIL/Gr as an example, it is with monodisperse POM clusters immobilized by PIL been successfully supported on graphene (Gr) through ionic self-assembly (Fig. 2).36 This synthesized composite shares a similar morphology to the original graphene, with POM anionic clusters being monodispersed on graphene, which endowed the catalyst with a higher specific surface area, large mesopore size, and dramatically improved accessibility of POMs. PILs play the linking role in bonding with POMs via multivalent electrostatic interactions and combining with graphene through a series of non-covalent interactions. The reason why self-assembly may occur is due to the multi-charged PIL cations and POM anions, which attracted each other because of opposite charges and resulted in the compact aggregations, as well as the highly negative-charged graphene surface under non-acidic conditions. Notably, neither the precise control nor toxic organic solvent were necessary for this process.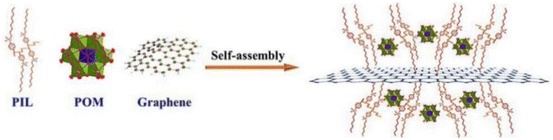 | ||
| Fig. 2 Schematic diagram of preparation principle to the POM/PIL/Gr hybrids. Adapted from ref. 36 with permission from Elsevier, copyright 2020. | ||
3. How the MOCs are stabilized/regulated by ILs
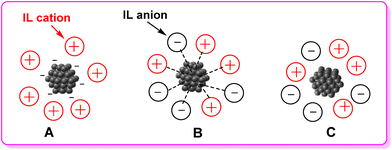
MOCs are a broad class of inorganic compounds with nanosized dimensions. However, they can be unstable and prone to degradation. Thus the stabilization of MOCs is essential to prevent their aggregation and maintain their unique properties for various applications, which often involves careful control over the synthesis, as well as the use of compatible solvents, counterions,37 ligands,38 support materials39 or surface modifications.40 ILs have proved to be able to stabilize a wide range of MOCs via the following ways.One way is by forming hybrids with MOCs that act directly as ILs’ anions.17 The unique combination of IL cations and MOC anions can be used to tune the activity and selectivity of IL–MOC/PIL–MOC catalysts. This type of hybrids is the focus of this perspective article, especially their design and catalytic application under green chemistry conditions (Scheme 2A).
Another way is by forming strong bonds with the metal atoms.41–43 ILs are composed of cations and anions. The specific charge distribution on IL components can interact with charged sites on MOCs through electrostatic forces, and also their polar functional groups, such as nitrogen, oxygen, and sulfur atoms. This coordination can help to protect the MOCs from the environment, strengthen the bonds within the MOCs, and make them more stable, thus preventing them from decomposition (Scheme 2B).
To some extent, the universal solvent property of ILs can be ascribed to the strong bonds formed between the ILs and MOCs. So-called task-specific ILs, which are ILs with a functional group covalently tethered to the cation or anion, are being developed to increase the solubility of metal compounds in these solvents. The functional group that has the ability to coordinate with the metal ion, preferably as a bidentate or a polydentate ligand, facilitates the dissolution of MOCs. Taking deprotonated betaine Me3N+CH2COO− as an example, the carboxylate group on one end of the molecule can act as a bridging ligand providing super-exchange pathways between metal ions, while the large trimethylammonium part on the other end behaves like a spacer that can separate adjacent chains.26 And the protonated betaine (also called betainium) exhibits a higher acidity (pKa 1.83) than alkanoic acids like acetic acid (pKa 4.75) or propionic acid (pKa 4.87), it is the higher acidity of the betainium ion improved an enhanced solubilizing ability for MOCs.
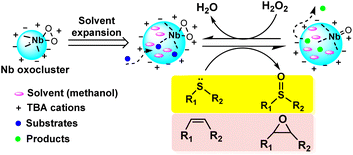
Yet another way is ILs can dissolve MOCs that are insoluble in traditional solvents and prevent them from aggregating.44–46 ILs have the ability to dissolve a wide range of compounds, polar and non-polar, which is why they are often referred to as “universal solvents.” This ability to dissolve a wide range of compounds makes ILs ideal for stabilizing MOCs, which can be quite complex and diverse in their structure and composition. Thus, ILs can provide a solvation environment that prevents the MOCs from aggregating and further improves the stability of the cluster (Scheme 2C).
Overall, ILs could stabilize the MOCs through ionic or electronic interactions, or by solubilization. In a sense, ILs could also regulate the stability and structural integrity as well as the catalytic activity of the MOCs within the IL environment. On one hand, ILs can be tailored to have varying degrees of hydrophilicity or hydrophobicity by selecting specific cation and anion combinations. This allows for the adjustment of the IL environment to match the hydrophilic or hydrophobic nature of MOCs, optimizing their interactions. On the other hand, ILs with appropriate structures can enhance the accessibility of catalytic active sites on MOCs. The unique architecture of ILs can act as a scaffold, providing an environment that facilitates interactions between reactants and the catalytic centers of MOCs. Furthermore, ILs often exhibit unique thermophysical properties, such as low volatility and high thermal stability. These characteristics can create a stable environment for MOCs, influencing their behavior, especially in high-temperature or high-pressure catalytic reactions.
4. Classification of IL–MOCs
IL–MOCs can be categorized based on their metal oxide compositions and structural arrangements. In the following, we will briefly introduce (1) peroxometalate anion-based (W, Nb, Ta, V, Ti etc.) ILs, (2) POM anion-based ILs, including Keggin and Lindqvist structure POM-based ILs, (3) a specific category of POM–ILs: POM–IL-anchored single-atom catalysts, and (4) another distinct category of POM–ILs: supported POM–IL. Table 2 provides examples of IL–MOCs, illustrating their diverse applications and structural characteristics.| Type of MOCs | IL–MOCs | Application | Ref. |
|---|---|---|---|
| Peroxotungstate anion-based IL | [HDIm]2[{W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O(O2)2}2(μ–O)] O(O2)2}2(μ–O)] |
Epoxidation | 47 |
| MNP-[HDMIM]2[W2O11], MNP-[SDMIM]2[W2O11] | Epoxidation | 48 | |
| Imidazolium–tungsten peroxo complex | Oxidative desulfurization | 49 and 50 | |
| [BPy]4W10O32, [BMIm]4W10O32 | Alcohol oxidation | 51 | |
| (P6,6,6,14)4[W10O32] | Olefin/alcohol oxidation | 52 and 53 | |
| (P6,6,6,14)4[W10O32] | Selenoether oxidation | 54 | |
| Peroxoniobate anion-based ILs | [DMIm]0.1·Nb2O7·2H2O; [TTA]0.08·Nb2O7·2H2O. | Epoxidation | 55 |
[A+][Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O(O–O)(OH)2] (A+ = ammonium cation) O(O–O)(OH)2] (A+ = ammonium cation) |
Epoxidation | 56 | |
| [TBA][NbO(OH)2(R)] (TBA = tetrabutylammonium; R = lactic acid, glycolic acid, malic acid) | Epoxidation | 57 | |
| [TBA][LA]-stabilized Nb oxoclusters | Epoxidation & sulfide oxidation | 58 | |
| Nb-OC@TBAF-0.5 | Epoxidation | 59 | |
| 18-Crown-6 and ammonium peroxoniobate (NH4–Nb) | Epoxidation | 60 | |
| Peroxotantalum anion-based ILs | [P4,4,4,n]3[Ta(O)3(η-O2)], P4,4,4,n = quaternary phosphonium cation, n = 4, 8, and 14 | Epoxidation | 61 |
| [P4,4,4,4]3[Ta(η2-O2)3(CO4)] | Epoxidation | 62 | |
| Peroxovanadium anion-based ILs | ([TBA][Pic])-stabilized vanadium oxo-clusters (Pic = picolinate ions) | Alkane oxidation | 63 |
| [BIm][Pic](Bim = 1-butylimidazolium)-stabilized vanadium oxo-cluster | Selective oxidative cleavage of β-O-4 lignin model compounds | 64 | |
| Peroxotitanium anion-based ILs | Ti oxo-HSO4 | Oxidative desulfurization | 65 |
| Keggin structure POM-based IL | [PEG-containing quaternary ammonium][PW12O40] | Electrochemistry | 20 |
| [Tetraalkylphosphonium] Keggin | Electrochemistry | 27 and 28 | |
| [MIMPS]3[PW12O40], [PyPS]3[PW12O40], [TEAPS]3[PW12O40] | Esterification | 66 | |
| [BMIm]3[PW12O40] | Epoxidation | 67 | |
| [Dopy]3[PW12O40] | Epoxidation | 68 | |
| [DEDSA]3[PW12O40] [DEDSA]3[PMo12O40] | Oxidation of alcohols | 69 | |
| Chiral IL-[PW12O40] | Asymmetric oxidation | 70 | |
| [Im-PEG-Im]1.5[PW12O40] | Oxidation of benzyl alcohol | 71 | |
| [Im-PEG-Im][HPW12O40] | Esterification | 72 | |
| [Im-PEG-Im]1.5[PO4(W(O)(O2)2)4] | Epoxidation | 73 | |
| [BMIm]3[PO4(W(O)(O2)2)4] | Oxidation of alcohols | 74 | |
| Ni[MIMPSH][PW12O40] | Acetalization of benzaldehyde with ethylene glycol | 75 | |
| [n-C16H33N-(CH3)3]4Na3PW11O39 | Epoxidation | 76 | |
| [C12MIm]5PTiW11O40, [CTA]5PTiW11O40, [TBA]5PTiW11O40 | Epoxidation | 77 | |
| (ODA)4PMo11VO40 | Oxidation of benzyl alcohol | 78 | |
| [MIMPS]NH4−NPMo11VO40 (N = 1–4), [PyPS]3HPMo11VO40, [TEAPS]3HPMo11VO40, and [BMIm]3HPMo11VO40 | Cellulose into formic acid | 79 | |
| [HC4Im]3PMo12O40, [HC4Im]5PV2Mo10O40, [HC2Im]5PV2Mo10O40, [HC4Im]4SiMo12O40 | Lignin depolymerization | 80 | |
| [PyBS]5PV2Mo10O40 | Cellobiose to formic acid and levulinic acid | 81 | |
| Pyridinium cation-PMo10V2O40 | Alkane oxidation | 82 | |
| [BMIm]4[Ti(H2O)TiMo11O39] | Degradation of polyethylene terephthalate | 83 | |
| [BMIm]4[Ti(H2O)TiMo11O39] | Transesterification | 84 | |
| Co4PW–PDDVAC (a porous POM-based composite) | Isolation of proteinase K | 85 | |
| Lindqvist structure | [Tetraalkylphosphonium] Lindqvist | Electrochemistry | 28 |
| [TBA]2Mo6O19 and [TBA]2W6O19 | Electrolytes | 86 | |
| [tBu-Hptz]2[Mo6O19] | Epoxidation | 87 | |
| POM–IL-anchored single-atom | [(n-C4H9)4N]6[SiW11O39Pd], [(n-C4H9)4N]5[PW11O39Pd] | Hydrocarboxylation of olefins | 88 |
| [TOMA6SiW11O39Ru(dmso)], TOMA = methyltrioctylammonium | N-Formylation of amines with CO2 and H2 | 89 | |
| Supported POM–IL | [α-PW12O40]3− immobilized on IL-modified polystyrene resin beads (PS–IL–PW) | Alcohol oxidation | 90 |
| [PW12O40]3− immobilized on dual amino-functionalized IL-modified MIL-101(Cr) | Alcohol oxidation | 91 | |
| PEG–PW11–CMC (carboxymethyl cellulose) | Epoxidation | 92 | |
| P8W48@PIL–G | Electrocatalysts | 93 and 94 | |
| AILs/POM/UiO-66-2COOH | Esterification | 95 | |
| PMo10V2-ILs@MIL-100(Fe) | Epoxidation | 96 | |
4.1 Peroxometalate anion-based ILs
The investigation on peroxotungstate anion-based ILs represents an early research area of POM anion-based ILs. For instance, the combination of tungsten peroxo complex and imidazolium cation, with49,50 or without47,48 bidentate picolinato ligand, has been extensively studied and proved to be efficient catalysts for different chemical transformations.Peroxoniobates, a noteworthy class of MOC materials, have gained attention for their structural diversity and high nucleophilicity, making them promising catalyst candidates for environment-friendly applications.97 Consequently, a series of Nb-based ILs have been constructed in Hou's group, such as niobium peroxides modified with IL-type 1-dodecyl-3-methylimidazolium hydroxide,55 the first proposed novel Nb-based IL, assigned as [A+][Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O(O–O)(OH)2] (A+ = ammonium cation),56 and a series of Nb-based ILs coordinated by organic carboxylic acid,57 and then a new class of carboxylate ILs-stabilized Nb oxoclusters58 were reported. Moreover, Nb oxoclusters can also be stabilized/modified by readily available organic fluoride salts.59 Furthermore, supramolecular complexations between 18-crown-6 and ammonium peroxoniobate can lead to the supramolecular IL catalysts with suitable hydrophobicity by a direct mixing of 18-crown-6 and NH4–Nb ((NH4)3[Nb(O2)4]) in methanol, which provides a much simpler approach, as compared with that of the previous Nb-based ILs.60
O(O–O)(OH)2] (A+ = ammonium cation),56 and a series of Nb-based ILs coordinated by organic carboxylic acid,57 and then a new class of carboxylate ILs-stabilized Nb oxoclusters58 were reported. Moreover, Nb oxoclusters can also be stabilized/modified by readily available organic fluoride salts.59 Furthermore, supramolecular complexations between 18-crown-6 and ammonium peroxoniobate can lead to the supramolecular IL catalysts with suitable hydrophobicity by a direct mixing of 18-crown-6 and NH4–Nb ((NH4)3[Nb(O2)4]) in methanol, which provides a much simpler approach, as compared with that of the previous Nb-based ILs.60
Hou's group also pioneered the development of early examples of ILs based on monomeric peroxotantalate anions.61 Similar to other active POM–ILs, the Ta-based ILs ([P4,4,4,n]3[Ta(O)3(η-O2)], P4,4,4,n = quaternary phosphonium cation, n = 4, 8, and 14) underwent a structural transformation in the presence of H2O2. Pressurization of [P4,4,4,4]3[Ta(O)3(η2-O2)] with CO2 in the presence of H2O2 produced a new class of Ta-based peroxocarbonate IL ([P4,4,4,4]3[Ta(η2-O2)3(CO4)]), which was found to be more superior towards epoxidation to the monomeric peroxotantalate analogs, and the transformation between Ta-peroxocarbonate and peroxotantalate anions was completely reversible, leading to an excellent regeneration of IL catalysts.62
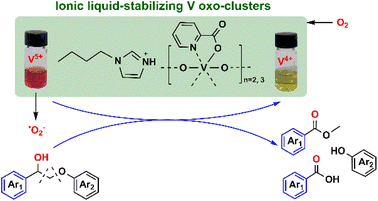
Vanadium oxoclusters have been synthesized through the condensation of peroxovanadium species in the presence of the functionalized IL [TBA][Pic] (TBA = tetrabutylammonium; Pic = picolinate ions) with tunable IL/V molar ratios.63 Similarly, imidazolium IL [BIm][Pic] (BIm = 1-butyl-imidazolium)-stabilized vanadium oxocluster catalysts were prepared as well.64
Ti oxoclusters stabilized by carboxylic acid-functionalized imidazolium-based IL were also fabricated via a solvothermal method.65 Characterization indicated that Ti oxoclusters (Ti oxo-HSO4) existed in the form of subnanosized structure, uniformly dispersed with an average particle size of ca. 1 nm due to the protection role of the ILs. It was found that the coordination of the carboxylic acid functional group of IL with Ti sites could prevent the Ti oxo-HSO4 from aggregation, and on the other hand, HSO4− could play an additional role in stabilizing the subnanosized Ti oxo-HSO4 cluster via electrostatic interaction.
4.2 POM anion-based IL
The Keggin structure, characterized by α-Keggin anions with a general formula of [XM12O40]n−, represents a prominent form for HPAs, where X is the heteroatom, M is the addendum atom (typically Mo or W), and O denotes oxygen. Self-assembling in acidic aqueous solutions, the Keggin structure is widely employed in POM catalysts. The first POM–IL hybrid, prepared via ion exchange method as illustrated in Section 2.1, is composed of a PEG-containing quaternary ammonium cation and 12-tungstophosphoric anion.20 Subsequently, a series of POM–ILs with PW12O403− anion but with different cations, such as pyridinium, phosphonium, ammonium, imidazolium, PEG-containing alkylimidazolium, and even chiral ILs were reported.66–71 Partial exchange of the protons in H3PW12O40 by a PEG-2000 chain-functionalized alkylimidazolium chloride yielded acidic [IM-PEG-IM][HPW12O40].72 Furthermore, complexes pairing the [PO4(W(O)(O2)2)4]3− anion, the well-known and most important active species in Ishii–Venturello system, with imidazolium cations were reported as well.73,74A series of transition-metal (M = Mn, Fe, Co, Ni, Cu) ion-exchanged H3PW12O40-based IL catalysts were synthesized by integrating metal and methylimidazolium propyl sulfobetaine (MIMPS) zwitterionic IL precursors onto H3PW12O40.75 Hybrids comprising a lacunary-type phosphotungstate anion [PW11O39]7− and (PEG-containing) ammonium cations were also investigated.76 Additionally, Ti-substituted POMs ([C12MIm]5PTiW11O40, [CTA]5PTiW11O40, and [TBA]5PTiW11O40) were synthesized and characterized.77 Keggin type H5PV2Mo10O40 and H4PVMo11O40-based POM–ILs were investigated as well due to their wide and high catalytic activity.78,79,81
Combining a Keggin [PW12O40]3− (or Lindqvist ([W6O19]2−)) anion with an appropriate tetraalkylphosphonium can produce POM-based ILs, exhibiting comparable conductivity and viscosity to a previously described inorganic–organic POM–IL hybrid but possess enhanced thermal stability.28 Furthermore, [TBTP]4PW11VO40 and [TBTP]4PMo11VO40 (TBTP = tributyl-tradecylphosphonium), composed of Keggin POMs and tetraalkylphosphonium cation have been synthesized.98 They show layered structures due to the self-assembly of cations and anions through electrostatic interactions and van der Waals forces, and especially they also display reversible phase transformation process below 100 °C. It was indicated that the oxidability of the Mo-containing POM is greater than that of the W-containing analog from the electrochemical study, which provides valuable insight into the further development of POM–ILs.
4.3 POM–IL-single atom catalysts (POM–IL–SACs)
The concept of single-atom catalysts (SACs) is highly regarded within heterogeneous catalysis due to their ability to achieve maximum metal utilization. POMs with precisely known composition and structure, as well as a limited number of sites to anchor atomically dispersed metals, are reported to be good support material, and the resulting POM-supported SACs (POM–SACs) serve as valuable models for investigating the complex catalytic systems at the atomic level,89–92,99 facilitating in situ spectroscopic studies of single atom sites during reactions. Moreover, they contribute to a deeper understanding of the structure–activity relationship and the dynamic changes in active sites during catalysis. POM clusters play a dual role as ligands and supports in the precise construction of SACs, enabling control over coordination states and active site loading. Additionally, the integration of ILs could help to isolate and stabilize SACs on solid support materials through interactions. Thus, POM–IL–SACs function as a hybrid catalyst, exhibiting catalytic activity akin to that of single-atom catalysts, while benefiting from the advantages of both homogeneous and heterogeneous catalysis.Besides recent advancements in POM–SACs, several POM–IL–SACs systems were reported also in Hou's group, such as POM–IL–Rh SAC,100 POM–IL Pd SAC,88 and POM–IL–Ru SAC89 catalytic systems. In these materials, the isolated single metal atoms were proved to be incorporated into the POM structure according to analysis. The bulky IL cations, on one hand, function as stabilizers of the single atoms and thus avoid the usage of the organic phosphine ligands; on the other hand, they prevent the aggregation and leaching of the isolated metal atoms. These POM–IL–SACs displayed a unique “pseudo-liquid-phase” behavior and showed efficient activity for catalytic carbonylation reactions. The detailed discussion of Rh-catalyzed hydroformylation to produce aldehydes, Pd-catalyzed hydrocarboxylation to yield carboxylic acids, and Ru-catalyzed N-formylation of CO2 to generate N-formyl compounds will be given in Section 5.4.
4.4 Supported POM–IL hybrids
Based on the recognized advantages of POM–ILs, it is anticipated that the self-assembly of POM–ILs on certain support materials would yield robust hybrids with enhanced chemical properties. IL-modified polystyrene resin beads proved to be a suitable support for POM.90 Zhang and Keita et al. demonstrated the functionalization of graphene with POMs associated with PIL objects.93,94 The one-pot synthesis of the P8W48 ([H7P8W48O184]33−)@PIL–graphene tri-component hybrids was performed by visible light irradiation of a mixture of the desired amounts of P8W48 and PIL in the presence of graphite oxide. The P8W48 serves as both an efficient graphite oxide reductant and a stabilizer. Due to the strong adsorption of P8W48 on the resulting graphene sheets, water-dispersible P8W48@graphene hybrids were obtained without the need for surfactant or polymeric stabilizers. On the other hand, metal–organic frameworks (MOFs), which are inorganic–organic hybrid porous crystalline materials, were incorporated with POM–ILs due to their large surface area, highly-ordered structure, regular and accessible pores, as well as versatile architecture.95,96 For instance, H3PW12O40 could be immobilized into the nanocages of a dual amino-functionalized IL-modified MIL-101(Cr) framework under mild conditions.91 UiO-66-2COOH MOFs were also functionalized with Keggin-type POM acids and further incorporated with sulfonated acidic ILs by pairing POM anions with functionalized IL cations.95 Furthermore, a series of ILs-functionalized POM-based MOF hybrids (POMs–ILs@MOFs) via encapsulating ILs within POMs@MOFs were reported.96 According to characterizations, POMs were successfully immobilized in MOF cages, with the structure of MOFs retained after immobilization.5. The catalytic application of IL–MOCs
MOCs are widely recognized for their superior catalytic efficiency in both academic and industrial sectors compared to traditional homogeneous catalysts. The selection of an appropriate IL and its specific properties is crucial, considering the characteristics of MOCs and the intended application. It is imperative to verify the stability and compatibility of the IL with the clusters through comprehensive experimentation and characterizations. ILs provide a versatile and effective method for stabilizing MOCs in diverse research and industrial applications. The following outlines specific examples of IL–MOCs for catalytic applications.5.1 The catalytic oxidation
Selective or partial oxidation of organic compounds is one of the most important chemical processes due to its industrial significance.101 Since conventional oxidation reaction often suffers from challenges in achieving selective oxidation, leading to the formation of undesired by-products and reduced overall efficiency, selectively producing the specific products is both challenging and crucial. The application of IL–MOCs to oxidation catalysis is inspired by their attractive properties, such as adjustable structure, acidity and redox properties, inherent resistance to oxidative decomposition, high thermal and chemical stability, etc.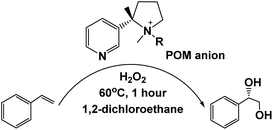 | ||
| Fig. 3 The combination of the POM with the chiral IL for the asymmetric styrene oxidation with H2O2. Adapted from ref. 70 with permission from Elsevier, copyright 2020. | ||
Various IL–MOCs, such as peroxotungstate anion- and Keggin structure-based POM–ILs, have shown particular efficiency in catalyzing the epoxidation of olefins. Most of them show reaction-induced phase-separation properties with the system transitioning from a tri-phase to an emulsion and eventually self-precipitating at the reaction's end, facilitating catalyst recovery and reuse.73 To better reuse the hybrid material, the POM–ILs could be immobilized on support materials to get magnetically separable catalysts exhibiting constant activity over at least ten cycles in the epoxidation of olefins with H2O2.48
Ti-substituted POMs ([C12mim]5PTiW11O40, [CTA]5PTiW11O40, and [TBA]5PTiW11O40) were also efficient for catalyzing the epoxidation of various olefins and the heterogeneous nature of catalysis in ethyl acetate media was confirmed by a hot catalyst filtration test.77 It was found the organic countercations significantly affected the catalytic activity, and the stable Ti-based peroxo structure serves as the active species, allowing for multiple catalytic runs without notable loss of activity.
Similar high activity also applies to polyoxoniobate-based POM–ILs. Niobium peroxides modified with IL-type 1-dodecyl-3-methylimidazolium hydroxide demonstrated excellent yields (80–99%) for the epoxidation of allylic alcohols to their epoxides even if the reaction was performed without any other solvent under ice bath conditions.55 The first proposed novel Nb-based POM–IL, designated as [A+][Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O(O–O)(OH)2] (A+ = ammonium cation), showed excellent catalytic activity and recyclability in epoxidizing various allyl alcohols under solvent-free, ice bath conditions using H2O2 as the oxidant.56 It was observed that the formation of an [Nb(O–O)2(OOH)2]− active center promoted the whole reaction through a homogeneous process and also facilitated the recyclability. To broaden the substrate scope from allyl alcohols to more challenging-to-convert olefins, a series of Nb-based POM–ILs coordinated by organic carboxylic acids were designed.57 Subsequently, a new class of carboxylate ILs-stabilized Nb oxoclusters was reported.58 They had an average particle size of 2–3 nm and exhibited extremely high catalytic activity for the epoxidation of olefins and allyl alcohols, using only 50 ppm of catalyst (0.065 mol%), with turnover number (TON) values exceeding 1000, outperforming the same transition metal catalyst (Scheme 3). Furthermore, organic fluoride salts were found to be able to stabilize or modify Nb oxoclusters due to their strong coordination interaction with Nb sites.59 The F atoms also form a hydrogen-bond interaction with the –OH group of the allylic alcohol, favoring the epoxidation reaction. Notably, the supramolecular complexations between ammonium peroxoniobate and 18-crown-6 were designed, demonstrating high catalytic activity and recyclability for olefin epoxidation.60 These supramolecular IL catalysts provide easy access to hydrophobic substrates and represent a novel approach to selective olefin epoxidation.
O(O–O)(OH)2] (A+ = ammonium cation), showed excellent catalytic activity and recyclability in epoxidizing various allyl alcohols under solvent-free, ice bath conditions using H2O2 as the oxidant.56 It was observed that the formation of an [Nb(O–O)2(OOH)2]− active center promoted the whole reaction through a homogeneous process and also facilitated the recyclability. To broaden the substrate scope from allyl alcohols to more challenging-to-convert olefins, a series of Nb-based POM–ILs coordinated by organic carboxylic acids were designed.57 Subsequently, a new class of carboxylate ILs-stabilized Nb oxoclusters was reported.58 They had an average particle size of 2–3 nm and exhibited extremely high catalytic activity for the epoxidation of olefins and allyl alcohols, using only 50 ppm of catalyst (0.065 mol%), with turnover number (TON) values exceeding 1000, outperforming the same transition metal catalyst (Scheme 3). Furthermore, organic fluoride salts were found to be able to stabilize or modify Nb oxoclusters due to their strong coordination interaction with Nb sites.59 The F atoms also form a hydrogen-bond interaction with the –OH group of the allylic alcohol, favoring the epoxidation reaction. Notably, the supramolecular complexations between ammonium peroxoniobate and 18-crown-6 were designed, demonstrating high catalytic activity and recyclability for olefin epoxidation.60 These supramolecular IL catalysts provide easy access to hydrophobic substrates and represent a novel approach to selective olefin epoxidation.
 | ||
| Scheme 3 The proposed reaction route for selective oxidation of sulfides and olefins with IL-stabilized Nb oxocluster catalysts. Adapted from ref. 58 with permission from Wiley-VCH Verlag GmbH, Weinheim, copyright 2019. | ||
Recently, the Lindqvist-type POM [tBu-Hptz]2[Mo6O19] (tBu-Hptz = 2-tert-butyl-5-(2-pyridyl)tetrazole) was studied as epoxidation catalysts using readily available and relatively eco-friendly hydroperoxide oxidants (hydrogen peroxide and tert-butyl hydroperoxide).87 This POM acted as a homogeneous catalyst and could be recycled by employing an IL solvent. It was found that the type of anion of the IL could affect the catalytic performance. Besides, the presence of water in the ILs (in different amounts) is often unavoidable, which may also influence the level of hydration of the anions, the strength of the anion–cation interactions, and the viscosity of the IL medium. On the other hand, one cannot exclude the possibility that ion-exchange might occur between [tBu-Hptz]2[Mo6O19] and the IL, depending on the type of IL.
Meanwhile, the POM-supported IL@core–shell polymer nanoparticles (PS–ImCn–PW4) have been established to offer an efficient Pickering emulsion catalytic system (Scheme 4).102 A series of PS–ImCn–PW4 nanospheres were prepared with cross-linked polystyrene as the core and POM–IL as the shell. Allyl chloride and H2O2 can be emulsified by these nanospheres. The surface properties of the nanospheres can be modulated by changing the carbon chain length of IL, which in turn affects the properties and catalytic performance of the Pickering emulsion. Interestingly, C2–C6 can stabilize O/W-type emulsion, while C8–C12 refers to W/O-type emulsion. This catalytic system can be used for the epoxidation of allyl chloride with low-concentration (9.6 wt%) H2O2 under solvent-free conditions.
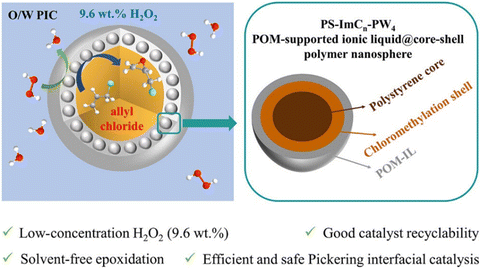 | ||
| Scheme 4 Epoxidation of allyl chloride with H2O2 on PS–ImCn–PW4 catalyst. Adapted from ref. 102 with permission from American Chemical Society, copyright 2023. | ||
Overall, the effectiveness of POM–IL-catalyzed epoxidation reactions stems from the cooperative interactions between POMs and ILs, enabling a catalytic system that is efficient, selective, and environmentally friendly.
For example, pairing the task-specifically designed IL-cation of N,N-bis-2-aminoethyl-4,4-bipyridinium with the Keggin-structured vanadium-substituted POM anion [PMo10V2O40]5− can result in organic–inorganic hybrid catalyst, which is a catalytically active and recyclable catalyst for cyclohexane oxidation with H2O2, giving KA oil (a mixture of cyclohexanol and cyclohexanone).82 Another example of alkane oxidation using IL–MOCs was reported in Hou's group. Vanadium oxoclusters were synthesized through the condensation of peroxovanadium species in the presence of a functionalized IL [TBA][Pic] (Pic = picolinate ions) with adjustable IL/V molar ratios.63 The picolinate anion of the IL acted as a ligand to stabilize the oligomeric vanadium oxocluster by coordinating with the vanadium sites, while the cation of the IL played a dual role in charge balancing and fine-tuning the miscibility of the oxocluster in solvents. The molar ratio of IL to V influenced the oligovanadate structure, with the appropriate ratio forming active trimer and dimer configurations. These vanadium oxoclusters exhibited remarkable efficiency as catalysts for the oxidation of cyclohexane, utilizing H2O2 as the oxidant. Notably, a significant 30% total yield of KA oil was achieved without the need of additional co-catalysts, and the catalyst demonstrated recyclability due to the incorporation of the IL component. Further studies on the reaction mechanism indicated that the HO˙ radicals are formed from the adduct of H2O2 and vanadium complex, and then the HO˙ radicals react with alkanes to afford the oxidation products (Fig. 4).
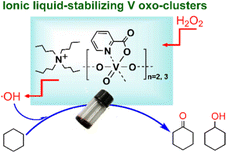 | ||
| Fig. 4 IL-stabilized vanadium oxo-clusters catalyzing alkane oxidation. Adapted from ref. 63 with permission from Royal Society of Chemistry, copyright 2020. | ||
Looking forward, IL–MOC holds promise for alkane oxidation reactions which involve in electron transfer processes, while POMs are well known for their superior redox properties. In the presence of H2O2 or molecular O2, the oxidation of alkanes often proceeds via a radical mechanism. POMs could affect reaction kinetics and selectivity by regulating radical pathway of alkane oxidation. Further research to fully understand these mechanisms and optimize POM utilization in alkane oxidation is still underway.
Early in 2005, the catalytic efficiency of POM–IL [BMIm]3[PO4(W(O)(O2)2)4] in alcohol oxidation using IL [BMIm][BF4] as a solvent was reported.74 The system exhibited several advantages, including a homogeneous reaction mixture, high product yields, minimal solvent consumption, facile product separation, and the ability to recover and recycle the catalyst without a significant reduction in product yield. Later, Keggin-type H3PW12O40-based di-imidazolium IL hybrids were prepared and exhibited reaction-controlled phase transfer properties with the catalysts precipitated from the aqueous system upon the consumption of H2O2 and could be reused several times without loss of activity and selectivity.71 Similar POM–ILs [DEDSA]3[PW12O40] and [DEDSA]3[PMo12O40] (DEDSA = diethyldisulphoammonium) with solvent-responsive self-separative characteristic were investigated for the oxidation of organic alcohols using hydrogen peroxide as an oxidizing agent.69 Due to the varied solubility of the hybrids in different solvents, they can effectively catalyze the oxidation reaction in homogenous conditions and then switch back to the heterogeneous system, finally self-precipitate upon the addition of a suitable solvent at the end of the reaction. This special property makes this method an environment-friendly and simpler way for the oxidation of alcohols to aldehydes and ketones. Additionally, [PW12O40]3− immobilized on IL-modified polystyrene resin beads,90 and into the nanocages of a dual amino-functionalized IL-modified MIL-101(Cr) framework were reported.91 In the latter case, the dual amino-functionalized IL not only enhances the oxidant accessibility due to the formed hydrogen bonds but also results in increased thermal stability.
V-containing Keggin H4PMo11VO40 was functionalized with ammonium cations with different carbon-chain lengths to form a series of POM-based amphiphilic catalysts.78 Their catalytic activities were evaluated in the selective oxidation of benzyl alcohol to benzaldehyde using H2O2 under organic solvent-free conditions. Among the investigated catalysts, (ODA)4PMo11VO40 (ODA: octadecylmethylammonium) exhibited the highest catalytic efficiency for the selective oxidation with a maximum 61% conversion of benzyl alcohol and a 99% selectivity of benzaldehyde, also offering excellent reusability. Besides, the complexes of decatungstate combined with N-butylpyridinium ([BPy]4W10O32) and 1-butyl-3-methylimidazolium ([BMIm]4W10O32) were synthesized in high yields directly from bisulfate-based ILs and used as novel catalysts in the selective oxidation of alcohols to aldehydes or ketones with H2O2.51 Combined with phosphorous cation, the hybrid (P6,6,6,14)4[W10O32] also demonstrated to be a very promising and recyclable catalytic material for alcohol oxidation with acids as the main products under fixed reaction conditions.52,53
POM–IL (P6,6,6,14)4[W10O32] was also proved to be an efficient catalyst for oxidizing perfluoroalkyl selenoethers.54 A series of perfluoroalkyl selenoxides, selenones, and selenoximines were prepared, with the separation and characterization of enantiomers in some cases. The use of Oxone® as an oxidant allowed the production of perfluoroalkyl selenoxides with electron-withdrawing or donating substituents in different positions (ortho, meta, or para). The reaction also works with various perfluoroalkyl chains and substituents with satisfactory to excellent yields. Overall, the use of POM–IL as a catalyst provides access to the underexplored perfluoroalkyl selenoxides. This study represents the first comprehensive report on the preparation of the perfluorinated selenoximine family with good yields.
Thioether oxidation was achieved by carboxylate IL-stabilized Nb oxoclusters.58 These clusters, dispersed in polar organic solvents, exhibited “pseudo” liquid phase behavior, enhancing substrate accessibility to catalytic sites. Specifically, [TBA][lactate]-stabilized Nb oxoclusters demonstrated exceptional activity, with TONs more than 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 at catalyst loading as low as 0.0033 mol% (1 ppm), which is superior to other Nb-based catalysts. The clusters’ flexibility and swelling behavior in polar solvents facilitated substrate access and product diffusion, showcasing their potential as high-performance catalysts for thioether oxidation and beyond.
000 at catalyst loading as low as 0.0033 mol% (1 ppm), which is superior to other Nb-based catalysts. The clusters’ flexibility and swelling behavior in polar solvents facilitated substrate access and product diffusion, showcasing their potential as high-performance catalysts for thioether oxidation and beyond.
It has been demonstrated that POMs can effectively promote the dissolution of wood in ILs while concurrently reducing the lignin content in the resultant cellulose-rich pulp.103,104 It's noteworthy that POMs do not completely degrade lignin into low molecular weight fragments, and they can be reoxidized under mild conditions in IL, albeit with some loss in POM and activity. Importantly, different POM species affect significantly the efficiency of delignification. For instance, [BMIm]3[PW12O40] not only dissolves approximately 30 wt% of cellulosic biomass but also converts biomass into commodity monosaccharides like glucose and xylose.105 Some examples of biomass oxidation using IL–MOCs as catalysts are shown in Table 3.
| IL–MOCs | Solvent | Starting material | Products | Ref. |
|---|---|---|---|---|
| [EMIm]4H[PV2Mo10O40] | [EMIm]OAc | Lignocellulosic biomass | Cellulose-rich pulp | 103 |
| [BMIm]3[PW12O40] | None | Biomass | Monosaccharides such as glucose and xylose | 105 |
| H5PV2Mo10O40 | [HC4Im][HSO4] | Lignin samples extracted from pine and willow | Vanillin and syringaldehyde | 106 |
| [HC4Im]3PMo12O40, [HC4Im]5PV2Mo10O40, [HC2Im]5PV2Mo10O40, [HC4Im]4SiMo12O40 | Acidic IL | Alkali lignin | Various aromatic compounds | 80 |
| HPMoV–C4C1Im–SO3H | DMSO | 2-Phenoxy-1-phenylethanol | Benzoic acid and phenol | 107 |
| [C2COOHC1IM]4H[PV2Mo10O40] | Deionized water | Carbohydrates | Glycolic acid | 108 |
| BetH5V2Mo18 | None | Native lignocellulose of pinewood and poplar wood | Ethyl vanillate, ethyl syringate, vanillin, and syringaldehyde | 109 |
| [BIm][Pic]-stabilized V2O5 | MeOH | β-O-4 lignin model compounds | Phenols, eaters, and acids | 64 |
| [BSmIm]CuPW12O40 | Ethanol–water mixture | Wheat stalk lignin | Diethyl maleate | 110 |
| [BSmIm]CuPW12O40 | Ethanol | Raw lignocellulosic biomass | Diethyl maleate | 111 |
| [PyBS]5PV2Mo10O40 | H2O | Cellobiose/cellulose | Formic acid and levulinic acid | 81 |
| [MIMPS]3HPMo11VO40, [PyPS]3HPMo11VO40, [TEAPS]3HPMo11VO40, [BMIm]3HPMo11VO40 | H2O | Cellulose | Formic acid | 79 |
Since lignin is resistant to conversion due to its complex structure and stable linkages, acid-catalyzed lignin degradation to phenolic monomers has gained great interest due to its potential for producing biofuels and chemicals.112 Back in 2016, Welton et al. used acidic H5PV2Mo10O40 as a catalyst coupled with protic IL [HC4Im][HSO4] as the solvent, together with molecular oxygen and hydrogen peroxide to depolymerize lignin samples extracted from pine and willow.106 Besides phenols and functionalized aromatics, vanillin and syringaldehyde were the main products extracted from the IL. This system succeeds in the synergistic properties of ILs to depolymerize lignin and the remarkable properties of the POM to oxidize the lignin fragments into useful platform chemicals. Exchanging the protons with imidazolium cations produced [HC4Im]5PV2Mo10O40 and [HC2Im]5PV2Mo10O40 hybrids, which also showed high efficiency in the oxidation of lignin fragments into platform compounds in aqueous acidic ILs solutions.80 Lignin conversion could reach to 76% with 77% selectivity of ketone products (acetovanillone, 3-methoxyphenol, and 4-methylcatechol). With [HC4Im]3PMo12O40 as a catalyst produced phenolic products (mainly m(p)-cresol, veratrole, vanillin). It was found the POM–IL type and the IL acidity had a big influence on the reaction process. The lignin molecule not only had intermolecular hydrogen bonds and π–π stacking with the imidazole ring, but also formed stronger hydrogen bonds with POM anions, thus making the lignin structure more flexible and easier to depolymerize. Later, a series of POM–ILs were reported for the selective cleavage of the β-O-4 linkage to value-added aromatic chemicals under mild conditions. Lv and Yang et al. reacted H5PV2Mo10O40 with 1-methyl-3-(4-sulfobutyl)-1H-imidazolium (C4C1Im–SO3) zwitterions to form the HPMoV–C4C1Im–SO3H POM–IL assembly,107 which could also selectively and effectively convert various β-O-4 lignin models into aromatic acids and phenols under mild homogeneous conditions. It could be precipitated by the addition of ethyl acetate to the homogeneous post-reaction solution, thus could be reused for five runs. Similarly, IL [C2OHC1Im]Br (1-(hydroxyethyl)-3-methylimidazole bromide) was assembled with H5PVnMo12−nO40 (n = 1–3) to form POM–ILs with different V molar ratios.108 Among them, [C2COOHC1Im]4PVMo11O40 could oxidize glucose and its upstream biomass derivatives to glycolic acid under O2 with 88% conversion and 61% selectivity, which is even applicable to the oxidation of raw lignocellulose.
Deep eutectic solvent cation betaine was combined with Dawson-type H6V2Mo18O62 to form BetH5V2Mo18O62 material, and it could be used as a trinitarian catalyst for one-pot lignocellulose transformation.109 The incorporation of Bet+ cation with POM anion not only increases the activity due to the strong hydrogen bonding interactions between the Bet+ cation and substrate 2-phenoxy-1-phenylethanol but also forms a micro-environment that concentrates solid lignocellulose to overcome mass-transferring barriers. Furthermore, the Bet+ endowed H6V2Mo18O62 with temperature responsibility, ensuring the recyclability of BetH5V2Mo18O62.
Besides acidic HPMoV, neutral vanadium pentoxide stabilized by IL is also efficient for biomass oxidation. Hou et al. used imidazolium IL [BIm][Pic] to stabilize the vanadium oxocluster catalysts, and the catalyst showed superior activity for one-step selective oxidation to cleave the β-O-4 linkage of lignin model compounds to phenols, eaters, and acids.64 It was found the presence of mixed-valence state of V4+ and V5+ in V oxocluster catalysts was crucial for enhancing the catalytic activity, and the oligomeric states of V species could be tuned by adjusting the molar ratio of IL [Bim][Pic] to V. Especially, 1-butylimidazolium also played a facilitating role in the cleavage of C–O bonds by the action of the protonated IL cations. Notably, the catalyst can be recycled with the catalytic activity did not decrease significantly even after five cycles (Fig. 5). This catalytic system demonstrates a new approach for the selective aerobic oxidation of β-O-4 lignin without any co-catalysts.
 | ||
| Fig. 5 IL-stabilized vanadium oxocluster catalysts for selective oxidative cleavage of β-O-4 lignin model compounds. Adapted from ref. 64 with permission from American Chemical Society, copyright 2023. | ||
Meanwhile, POM–IL [BSmIm]CuPW12O40 catalytic systems have been developed for the production of diethyl maleate in Li's group.110,111 Under optimized conditions, 94% wheat stalk lignin was oxidized with 73% diethyl maleate selectivity over [BSmIm]CuPW12O40via oxidative ring cleavage of lignin, followed by in situ esterification in one pot.110 A series of phenol, guaiacol, syringol, and their derivatives could be oxidatively converted into diethylmaleate. Later, to broaden the substrate scope, a one-step selective conversion of raw lignocellulosic biomass directly to diethyl maleate over the [BSmIm]CuPW12O40 catalyst was proposed.111 All major fractions of lignocellulose could be converted simultaneously into diethyl maleate with high selectivity. It was found that the relatively low incorporation of the Cu2+ sites in [BSmIm]CuPW12O40 (in comparison with that of CuHPW12O40) gives vacant orbitals for molecular oxygen, which is responsible for the excellent catalytic activity. Additionally, the [BSmim]CuPW12O40 could be easily recovered from the mixture after the reaction by simple temperature control.
One-pot transformation of cellulose directly into formic acid (FA) and levulinic acid (LA) is possible via vanadium-containing POM–IL catalysts.113 A relatively high selectivity of LA (46.3%) and FA (26.1%) together with 100% cellobiose conversion could be obtained by using [PyBS]5PV2Mo10O40 as a catalyst.81 It could be easily recycled four times without a significant loss of activity. Meanwhile, a similar POM–IL [MIMPS]3HPVMo11O40 was applied in the conversion of cellulose into FA with over 50% FA yield and a high FA concentration of ∼10% in aqueous solution.79 Notably, these POM–ILs acted as bifunctional catalysts, with –SO3H functionalized cations catalyzing cellulose hydrolysis to glucose and POM anions catalyzing glucose oxidation to FA.
Overall, acidic IL–MOC materials are efficient for biomass oxidation, representing an innovative approach with significant implications for sustainable energy and chemical production. The synergistic effect between the IL cation and MOC anion facilitates the controlled oxidation of biomass components, such as lignocellulosic materials, under mild conditions to value-added products without any additives.
Recently, Hou's group developed novel carboxylic acid-functionalized imidazolium IL-stabilized Ti oxoclusters.65 These oxoclusters, well stabilized in ILs and highly dispersed in methanol, showed excellent desulfurization efficiency under mild conditions. Complete removal of sulfur compounds from model oil was achieved using the Ti oxo-HSO4 catalyst, with methanol identified as the most effective extractant. It was indicated that Ti–OOH species were indeed the active species and responsible for the sulfur removal on the Ti oxocluster catalysts. Moreover, the HSO4− anion could activate the Ti–OOH species by forming hydrogen bonds, facilitating the electrophilic oxygen transfer in the ODS reaction. This strategy provides an alternative and green route for constructing stable MOC catalytic systems (Scheme 5).
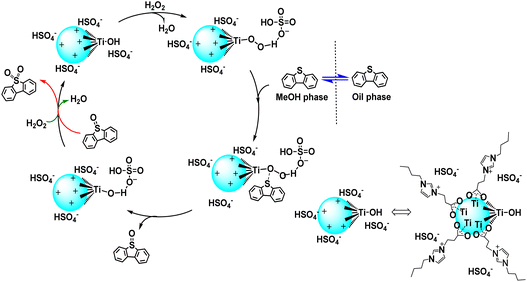 | ||
| Scheme 5 Proposed mechanism for the extraction and catalytic oxidative desulfurization (ECODS) system catalyzed by Ti oxocluster catalyst. Adapted from ref. 65 with permission from American Chemical Society, copyright 2022. | ||
Several review papers focusing on ODS reactions using POM–ILs as catalysts have been published, including works by Yu et al.,116 Zhao et al.,117 Ong et al.,118 and Anbia et al.119 Specifically, the reported examples until March 2021 are detailedly summarized by Anbia et al.119 Beyond the commonly studied tungstenphosphoric or phosphomolybdenum-based POM–(P)ILs,120,121 tungstovanadate-based POM–IL [C2(MIm)2]2VW12O40 (MIm = 1-methylimidazolium) was also investigated as a catalyst in ODS with 100% sulfur removal efficiency and sulfone product selectivity in 20 min, with a high turnover frequency (TOF) value (53.2 h−1).122
In addition, the heterogeneous carboxyl-functionalized bilayer POM–IL catalysts were also developed, demonstrating superior activity for ODS (Scheme 6).123 The optimized catalyst, SiO2 (50 nm)–BiIL–PW (Keggin-type), exhibits good amphiphilicity for both sulphur-containing compounds and hydrogen peroxide, thereby providing a quasi-homogeneous microenvironment for the heterogeneous catalysis. Complete sulfur removal can all be achieved for four model fuels free of extractant. It was demonstrated that POM and carrier have significant effects on the catalytic activities. Notably, no decrease in catalytic efficiency was observed even after the eight cycles in scale-up experiments. These results make the catalyst promising for industrial application.
 | ||
| Scheme 6 The diagram of ODS catalyzed by SiO2–BiIL–CO2H–POM. Adapted from ref. 123 with permission from Elsevier, copyright 2023. | ||
Besides, SiO2, graphene oxide (GO) was also used as a supported material for POM–IL in ODS reactions.124,125 One example involves the synthesis of POM–ILs with monomer, dimer, and poly IL, and then further loaded on the surface of GO.124 The influences of IL type and carrier on ODS activity were thoroughly investigated. The other example is the covalent immobilization of POM–IL on the amine-modified magnetic GO (APTES-mGO).125 The employed ILs were imidazolium cation-based, with varying alkyl chain lengths (Cn[MIm]Cl, n = 4, 8, 12, and 16). Among these, [C12MIm]5PV2Mo10/APTES-mGO, with a C12 alkyl length chain, showed the highest sulfur removal percentage due to its desirable amphiphilicity, which facilitated a stable Pickering emulsion system, enhancing the contact area between phases and overcoming mass transfer resistance. The emulsion breakage and the catalyst recycling can be achieved using a permanent magnet. Notably, the designed catalyst could be recovered 8 times without significant loss of its catalytic activity.
Phosphovanadomolybdate (V-POM) in other compositions (H7[P2Mo17VO62], H8[P2Mo16V2O62] and H9[P2Mo15V3O62]) were combined with amphiphilic PIL (Scheme 7).21 These composites served as efficient heterogeneous catalysts for the direct hydroxylation of benzene to phenol with H2O2 in the liquid phase. The synergistic catalytic effect between the V-POM anion and carboxylic acid-functionalized PIL cation framework contributed to the high catalytic performance. Meanwhile, H2PMo11VO402− anion-based imidazolium IL was also employed as a catalyst for the hydroxylation of benzene by H2O2 without using a solvent.134 The homogeneous emulsion formed by the catalyst and substrates during the reaction facilitated the catalytic process. After the reaction, the catalyst could self-precipitate from the reaction media and proved to be recyclable. Moreover, mesoporous MCM-41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 135 and functionalized chloromethylated polystyrene136 were employed as supported materials for V-POMs to enhance the recyclability of the active species.
135 and functionalized chloromethylated polystyrene136 were employed as supported materials for V-POMs to enhance the recyclability of the active species.
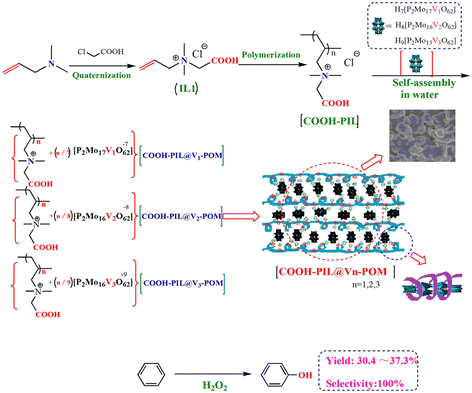 | ||
| Scheme 7 Direct hydroxylation of benzene to phenol with H2O2 catalyzed by three porous functionalized PIL/phosphovanadomolybdate ionic composites. Adapted from ref. 21 with permission from Wiley-VCH Verlag GmbH, Weinheim, copyright 2020. | ||
In addition to V-POMs, vanadium oxocluster itself is also effective for hydroxylation of benzene. For example, VxOy supported on PIL (VxOy/PIL) demonstrated much higher catalytic performance than the analogous catalyst VxOy/P, prepared with only the polymer support (P), without the IL. The superior catalyst performance of VxOy/PIL might be assigned to a higher specific surface area, stronger absorbent ability, and more active sites than the smooth and bulky VxOy/P.137 Furthermore, the hydrophobic side chain of the IL in the catalyst was beneficial for the access of benzene and the removal of the generated phenol from the surface of the catalyst, which also played an important role in improving the catalytic performance. In addition, the catalyst is highly stable and recycled at least three times without a significant decline in the phenol yield.
5.2 The acid catalysis
Meanwhile, POM–IL immobilized on MOF was prepared and applied for the esterification of acidic vegetable oil.95 MOFs (UiO-66-2COOH) were functionalized with Keggin-type POM acids first and then further incorporated with sulfonated acidic ILs. The resulting ILs/POM/UiO-66-2COOH solid catalyst combines Brønsted and Lewis acid sites, exhibiting a high surface area, increased acidity, and exceptional catalytic activities with long-term stability and reusability for one-pot biodiesel production via esterification of the low-cost acidic oils. Therefore, this solid catalyst has potential for practical application in the catalytic biodiesel production, and would provide a guide for selecting suitable POM–ILs for the preparation of solid acid catalysts.
| Catalysts | Amount/g | T/°C | t | Conv./% | Yield/% | Reuse cycles | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Cat. | Sub. | Sol. | |||||||
| Substrate: polyethylene terephthalate (PET), product: bis(hydroxyethyl) terephthalate (BHET), solvent: ethylene glycol. | |||||||||
| Na12[WZn3(H2O)2(ZnW9O34)2] | 0.025 | 5.0 | 20.0 | 190 | 40 min | 100 | 84 | Four | 138 |
| [PyPs]6[WZn3(H2O)2(ZnW9O34)2] | 0.04 | 5.0 | 20.0 | 195 | 0.5 h | 100 | 85 | Five | 139 |
| [BMIm]4[Ti(H2O)TiMo11O39] | 0.21 | 5.0 | 30.0 | 190 | 5 h | 100 | 85 | Six | 83 |
Polyethylene terephthalate (PET) is widely used in daily life, particularly in food packaging. To decrease environmental pollution and resource wastage issues, PET degradation is of great significance. Compared with the physical and hydrolysis methods, PET alcoholysis is a more favored degradation approach. Lu et al. found that transition-metal-substituted POMs Na12[WZnM2(H2O)2(ZnW9O34)2] (M = Zn2+, Mn2+, Co2+, Cu2+, Ni2+) can efficiently catalyze PET alcoholysis,138 with superior activity than transition single-substituted catalyst K6SiW11ZnO39(H2O) due to their specific sandwich structure, increased active sites, and outstanding coordination ability with PET carbonyl oxygen. Then they synthesized a series of POM–ILs with different anion–cation molar ratios to investigate the possible impact of the layer spacing of the catalyst on PET degradation since polymer degradation simultaneously requires the active site of the catalyst to match multiple reaction sites in the polymer long chain (Scheme 8).139 It was found that the adjustable layer spacing (1.00–1.63 nm) in these POM–ILs, achieved by incorporating [WZn3(H2O)2(ZnW9O34)2]12 anion with organic cations such as pyridinium (Py), 3-(pyridine-1-ium-1-yl)propane-1-sulfonate (PyPs), 1-(3-sulfonic group) trimethylamine (TEAPs), and 1-methyl-3-(3-sulfopropyl)imidazolium (MIMPs), was a crucial parameter. When the catalyst layer spacing was adjusted to coincide with the spacing of 1.32 nm between the carbonyl active sites on both sides of the PET benzene ring, the active sites of the catalyst (Zn atom in the POM and N atom in the pyridine ring) corresponded exactly to the reactive sites in PET's long chain. This enabled the shearing of the carbonyl functional group at a fixed point on the nanoscale, promoting fast and complete reaction. Thus the transformation of POM into POM–IL not only enhances thermal stability but also introduces anionic and cationic synergistic catalysis. Under optimal conditions, the proposed catalytic strategy achieved complete PET degradation in just 30 minutes, with a remarkable 85% yield of bis(hydroxyethyl) terephthalate (BHET). Impressively, the BHET yield remained consistently above 85% even after five cycles, highlighting the sustainability and robustness of the approach. This innovative strategy of precisely modulating the spacing of the catalytic active site represents a significant advancement in the field of PET alcoholysis, which also provides a new approach for designing functional catalytic materials tailored for depolymerizing waste plastics into high value-added chemicals.
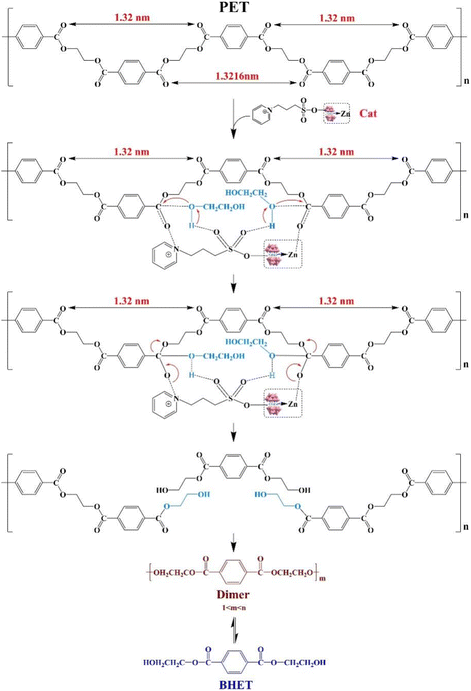 | ||
| Scheme 8 The schematic illustration of the possible mechanism of PET alcoholysis with POM–IL. Adapted from ref. 139 with permission from Wiley-VCH Verlag GmbH, Weinheim, copyright 2023. | ||
Furthermore, titanium-centered POM–ILs, denoted as [BMIm]4–6[M(H2O)TiMo11O39] (M = Cu2+, Fe2+, Pb2+, Ti4+, and Zn2+), were evaluated in the alcoholysis of bottle-grade PET, using waste mineral water bottles as samples.83 Among the catalysts, [BMIm]4[Ti(H2O)TiMo11O39] exhibited optimal catalytic efficacy, achieving a 100% alcoholysis rate and a 85% BHET yield with a catalyst dosage of 0.6% at 190 °C for 5 hours. Impressively, even after six cycles, the alcoholysis rate remained as high as 94% with 76% BHET yield. These findings highlight the potential of the prepared POM–IL catalysts in catalyzing the degradation of bottle-grade PET, offering a promising avenue for sustainable waste management. Meanwhile, this POM–IL [BMIm]4[Ti(H2O)TiMo11O39] behaved as a phase transfer catalyst for the transesterification reaction between dimethyl carbonate and phenol to synthesize diphenyl carbonate.84 Under optimized conditions, the phenol conversion reached 46%, and the overall selectivity of mono- and di-phenyl carbonate products was 99%. Moreover, the catalyst could be reused four to five times due to its characteristic of a “homogeneous phase at high temperature and separation at low temperature”.
5.3 The CO2 activation and utilization
The utilization of CO2, a greenhouse gas and also a valuable C1 feedstock, reduces reliance on traditional fossil fuel-based resources, fosters more sustainable pathways, and contributes to the concept of a circular economy by closing the carbon loop.The POM–IL catalytic systems demonstrate the ability to activate CO2 through cooperative interactions between the POM and IL components. The POM acts as a redox-active entity, facilitating electron transfer during the activation of CO2, while the IL provides a stabilizing medium for the POM and influences the solubility and reactivity of CO2. The catalytic transformations of CO2 using POM–IL catalytic systems have been summarized previously,140,141 which demonstrates the advantages of POM-based ILs in the emerging field of CO2 utilization, e.g. CO2 capture, the cycloaddition of CO2 to epoxides, and reduction of CO2. Here, we mainly focus on new catalytic designing and new catalytic effects in CO2 utilization by POM–IL catalytic systems in recent three years.
5.4 The catalytic carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbonylations form the basis of many types of reactions, e.g. hydroformylation, hydrocarboxylation/hydroesterification, aminocarbonylation, and N-formylation of CO2, etc.Catalytic hydroformylation is a pivotal industrial process in which alkenes react with carbon monoxide and hydrogen, typically in the presence of a transition metal catalyst like rhodium, to yield aldehydes. The POM–IL–Rh SAC (MTOA)5[SiW11O39Rh] (MOTA = methyltrioctylammonium cation) is active for the hydroformylation of alkenes to produce aldehydes at an ultralow loading of Rh (ca. 3 ppm) (Fig. 6), displaying exceptional catalytic activity and long storage stability.100 For styrene hydroformylation, both the conversion and the aldehyde yield can reach almost 99%, and a TOF as high as 9000 h−1 was obtained without using any phosphine ligand. This IL catalyst, miscible with n-hexane at reaction temperatures, contributed to the exceptionally high activity, while the bulk organic cation not only prevented active Rh species from leaching during reactions but also stabilized Rh1 species from reducing into Rh0 species.
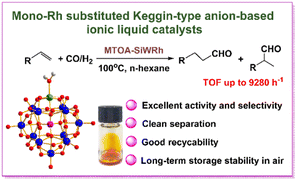 | ||
| Fig. 6 Ionic liquid-immobilized rhodium single-atom catalyst for hydroformylation. For the ball-and-stick structure of the [SiW11O39Rh(H2O)]5− framework: Si, W, O, Rh and H atoms are shown in pink, blue, red, green and gray spheres, respectively (inset). Adapted from ref. 100 with permission from Wiley-VCH Verlag GmbH, Weinheim, copyright 2022. | ||
Catalytic hydrocarboxylation is a transformative process wherein alkynes or alkenes undergo carboxylation in the presence of carbon monoxide and water, typically facilitated by transition metal catalysts like nickel or palladium. The Keggin-type POM–IL–Pd SACs [(n-C4H9)4N]6[SiW11O39Pd] and [(n-C4H9)4N]5[PW11O39Pd], with Pd atoms incorporated into the POM structure, exhibited high efficiency in the olefin hydrocarboxylation reaction under two-phase conditions, achieving up to 95% conversion of styrene and 95% carboxylic acid yield at 110 °C.88 The tetrabutylammonium cation played a crucial role in stabilizing the Pd catalyst, allowing for easy storage and consecutive catalytic recycling. Mechanistic studies revealed a reaction pathway proceeding through the “Pd–H” pathway, with the Pd SAC system exhibiting superior performance and stability even without phosphine ligands. This catalytic system offers advantages such as broad substrate suitability and easy quantitative recovery, with ongoing efforts to further enhance its efficiency.
The catalytic N-formylation of CO2 involves the incorporation of CO2 into organic substrates to form N-formyl compounds. The POM–IL–Ru SAC ([TOMA6SiW11O39Ru(dmso)], TOMA = methyltrioctylammonium) with the single-atom Ru, confirmed to be anchored into the framework of the POM, demonstrated high efficiency in the N-formylation of amines with CO2 and H2 in toluene-IL biphasic media (Fig. 7).89 The binary IL system, involving the POM-based IL and [BMIm]OAc, exhibited a sponge-like structure, ensuring efficient contact between catalytic sites and substrates. Experimental results and the DFT calculations indicated a pathway that involves formate intermediates, and the process is a three-component (morpholine, Ru–POM–IL, and BMImOAc) synergistic process, which significantly reduces the reaction energy barrier and enhances the conversion and yield, showcasing the catalytic system's advantages in terms of high efficiency, robustness, and recyclability in N-formylation reactions.
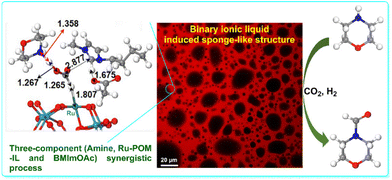 | ||
| Fig. 7 Ruthenium single-atom anchored in POM–ILs for N-formylation of amines with CO2 and H2. Adapted from ref. 89 with permission from American Chemical Society, copyright 2023. | ||
Additionally, Hou's group developed new-type organic ammonium cation-stabilized Nb oxoclusters, which can efficiently catalyze the reductive amination of CO2 with amines using PhSiH3 as a reducing agent.142 The reaction proceeds smoothly under ambient conditions and affords formamides with excellent selectivity. Notably, the Nb oxocluster catalysts showed good recyclability in consecutive recycles and wide substrate suitability. Mechanistic studies suggest that the N-formylation reaction proceeds via a silyl formate reaction path. The organic methyltrioctylammonium cation could not only prevent Nb oxoclusters from aggregation, but also provide the suitable hydrophobic environment to accelerate the accessibility of organic reactants to the catalytic active centers. As a result, the POM–IL catalyzed CO2 activation showcases the potential for developing sustainable and environmentally friendly processes for utilizing CO2 as a carbon source, contributing to the field of green chemistry and carbon capture and utilization.
5.5 Catalytic hydrogenation/hydrogenolysis
Catalytic hydrogenation and hydrogenolysis are chemical processes used to modify or transform organic compounds by introducing hydrogen atoms into the molecular structure. POMs doped with Pd,143 Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 144–147 and Ru148,149 nanoparticles (NPs) are reported to be efficient hydrogenation/hydrogenolysis catalysts. Han et al. designed a multifunctional catalyst denoted as Pd NP/POM–IL composite supported on SiO2 (Pd/H2[POM–IL]@SiO2) for the reductive hydrolysis of C–O bonds in diaryl ethers in water, yielding selective ketone products in high yields (>80%) (Fig. 8).150 The alcohol byproducts, which are common products from this kind of reaction, and complicate product purification are deliberately excluded. The catalytic reaction cannot proceed in the absence of either a catalyst or H2 (in 3 MPa N2). Without the incorporation of Pd NPs, the POM–IL@SiO2 alone could not catalyze the reaction either. So the unique performance of the catalyst results from the excellent cooperation of Pd NPs, POM, and imidazolium cation, with Pd and POM co-catalyze the reaction and imidazolium cation enhances the accumulation of the reactants due to its hydrophobic nature. The catalyst exhibited efficacy not only for diaryl ethers but also for alkyl aryl ethers, facilitating their transformation into the corresponding ketones. Notably, the catalyst demonstrated robustness and stability throughout the reaction even after five successive reuses. This study introduces a promising avenue for the valorization of lignin derivatives, including authentic lignin from raw biomass, into valuable chemical products in a sustainable, efficient, and highly selective manner.
144–147 and Ru148,149 nanoparticles (NPs) are reported to be efficient hydrogenation/hydrogenolysis catalysts. Han et al. designed a multifunctional catalyst denoted as Pd NP/POM–IL composite supported on SiO2 (Pd/H2[POM–IL]@SiO2) for the reductive hydrolysis of C–O bonds in diaryl ethers in water, yielding selective ketone products in high yields (>80%) (Fig. 8).150 The alcohol byproducts, which are common products from this kind of reaction, and complicate product purification are deliberately excluded. The catalytic reaction cannot proceed in the absence of either a catalyst or H2 (in 3 MPa N2). Without the incorporation of Pd NPs, the POM–IL@SiO2 alone could not catalyze the reaction either. So the unique performance of the catalyst results from the excellent cooperation of Pd NPs, POM, and imidazolium cation, with Pd and POM co-catalyze the reaction and imidazolium cation enhances the accumulation of the reactants due to its hydrophobic nature. The catalyst exhibited efficacy not only for diaryl ethers but also for alkyl aryl ethers, facilitating their transformation into the corresponding ketones. Notably, the catalyst demonstrated robustness and stability throughout the reaction even after five successive reuses. This study introduces a promising avenue for the valorization of lignin derivatives, including authentic lignin from raw biomass, into valuable chemical products in a sustainable, efficient, and highly selective manner.
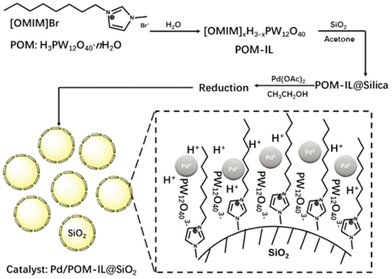 | ||
| Fig. 8 Schematic illustration of the process for the synthesis of multifunctional catalyst Pd/POM–IL@SiO2 (Pd/Hx[OMIM]3−xPW12O40@SiO2, x = 1, 2, 3) and its structure. Adapted from ref. 150 with permission from Royal Society of Chemistry, copyright 2018. | ||
In quinoline hydrogenation, our recent investigation demonstrates that the POM anion-stabilizing Pt nanocatalysts can greatly facilitate to forming oxygen vacancies adjacent to Pt0 species on POM catalysts.147 The oxygen vacancy concentration of the POM catalyst exhibited a decisive role in the catalytic hydrogenation of quinoline in the aqueous media. Further studies indicated H2O actually participated in the hydrogenation and acted as an indirect hydrogen source. Especially, H2O was adsorbed at oxygen vacancies and underwent homolytic cleavage into OH− and H− species, and the forming interfacial platinum–hydroxyl species can efficiently promote the heterolytic H2 dissociation for hydrogenation. It was noteworthy that organic cations exert a remarkable impact on oxygen vacancy density, which implies that the catalytic performance enables it to be tuned by the cation structure.
5.6 The application of IL–MOCs in electrochemistry
Electrochemistry is the study of chemical processes that cause electrons to move, it is becoming increasingly important due to its environmental friendliness and profound influence on fields such as materials science, environmental monitoring, and sustainable energy solutions. The utilization of either IL151 or POM152 in electrochemistry has gained significant attention due to their unique properties, including high ionic conductivity and reversible redox activity. The hybrid IL–MOCs also serve as promising catalysts and electroactive materials with higher conductivity and better thermostability, capitalizing on the inherent advantages of ILs and MOCs.153 The study of the [S2W18O62]4− POM reduction in 12 different ILs with a range of different anions and cations revealed strong solvation and ion-pairing effects between the IL cation and the negatively charged POM reduction products.154 Both the IL and the POM structures can influence the kinetics and thermodynamics of the electron transfer processes, and this should be considered when employing these promising materials for relevant electrochemical applications.POM–ILs based on the phosphomolybate anion [PMo12O40]3− and organic imidazolium, pyridinium, and phosphorous cations were prepared and used as electrochromic materials with deep eutectic solvents as electrolytes to design efficient electrochromic devices.155 Additionally, Mo-based electrodes derived from POM–IL, denoted as N/P-MoO2@carbon, were designed and exhibited advanced electrochemical performance.156 The contents of C, N, P, and the pore geometry of N/P-MoO2@carbon networks can be easily tailored by adjusting the precursor's cyano group position, resulting in enhanced lithium storage performance. Moreover, composite catalysts composed of Pd, POM–IL, and N,P-codoped coal-based carbon fibers have been developed, exhibiting superior activity for formic acid electrooxidation compared to non-enhanced Pd/N, P–C composites.157 This enhanced performance is attributed to the synergistic effects of the POM anion and the longer alkyl chains in the imidazole IL, leading to smaller catalyst particles and increased active sites being exposed, thus better electrochemical performance.
Furthermore, a Co-based CoW12–IL–graphene oxide system was reported as an efficient electrocatalyst for water oxidation in neutral pH conditions (Scheme 9).158 This new hybrid material was prepared by covalent attachment of IL species to graphene oxide and then electrostatic interaction of supported imidazolium cations with Keggin type [CoW12O40]6− polyanions, it is the strong electrostatic interaction between imidazolium cations and CoW12 species prevents the leaching of catalytically active species into the solution. It exhibited remarkable stability which demonstrated the efficiency and stability of the oxygen evolution reaction process in neutral pH.
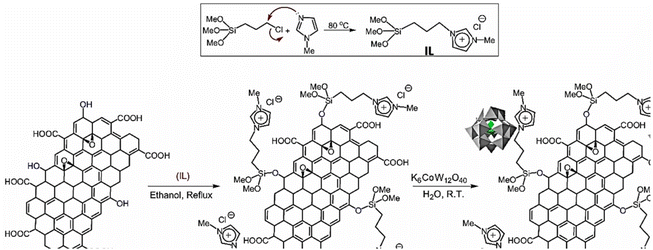 | ||
| Scheme 9 Schematic exhibition of the preparation of CoW12–IL–graphene oxide hybrid nanomaterial. Adapted from ref. 158 with permission from Elsevier, copyright 2022. | ||
Overall, the tunable nature of IL–MOCs allows for precise control over the electrochemical properties, making them suitable for applications such as energy storage devices, sensors, and electrocatalysis. The synergistic effects between the IL environment and the MOCs enhance the stability and conductivity of the electroactive materials, paving the way for advancements in electrochemical technologies with improved efficiency and performance.
6. Conclusion
To gain deep insight into the development of new IL–MOC systems in catalytic application, it's crucial to consider specific aspects such as ease of synthesis, catalytic efficiency, stability, environmental impact, etc. The IL–MOC hybrids are easy to prepare via anion exchange or simple mixing, offering advantages over traditional ILs or MOCs. Ongoing research focuses on enhancing their structural diversity and functional properties to broaden their applicability across various fields.ILs can not only stabilize but also regulate MOC structure, reactivity, and catalytic performance. By interacting with MOCs, ILs can alter MOCs’ coordination environment, structural arrangement and even oxygen vacancy density. This interaction may lead to changes in the size, shape, and surface properties of MOCs. Additionally, ILs can affect the redox behavior of MOCs by donating or accepting electrons, and they can also modify the acidity, basicity, hydrophilicity or hydrophobicity. Meanwhile, it has been proved that the composition and morphology of MOCs are quite significant factors affecting the catalytic efficiency. Thus ILs can ultimately influence MOCs’ catalytic activity and selectivity. Furthermore, ILs provide a unique solvent environment that surrounds MOCs, influencing their solubility, stability, and accessibility to reactants. The solvation effects of ILs can enhance the dispersion of MOCs, increase their surface area, and facilitate the diffusion of reactant molecules to active sites.
IL–MOCs offer higher catalytic efficiency with increased reaction rates, higher selectivity, and overall improved performance in catalytic processes. They also exhibit improved stability due to the high thermal stability and non-volatility characteristics of ILs, allowing for easy separation and better recyclability. Additionally, the versatility and flexibility of IL–MOCs, stemming from ILs’ tunability, make them applicable for various types of catalytic reactions, while their green solvent properties reduce environmental impact by simplifying reaction conditions, minimizing waste generation, and lowering overall costs. Moreover, ILs are non-volatile, which means they are less likely to evaporate than traditional solvents. This makes them safer and more attractive to use than traditional solvents, particularly in industrial applications where solvent loss is a concern.
However, there are still some challenges to overcome, such as the cost and availability of ILs, as they are currently more expensive than traditional solvents. Further research is needed to develop new ILs with the desired properties. Additionally, there is still a need for a better understanding of the underlying chemistry of IL–MOCs. Especially, understanding the role of IL in stabilizing/regulating MOCs will foster new discoveries of catalytic reactions that are accelerated by IL–MOC catalysts. This will be essential for developing new and improved IL–MOCs for a wider range of applications. Despite these challenges, IL–MOCs are a promising new class of catalysts with a wide range of potential applications.
In summary, IL–MOCs are a fascinating class of materials with promising catalytic applications. The unique combination of MOCs and ILs brings forth several noteworthy advantages in various chemical transformations. As researchers continue to explore the potential of IL–MOCs, we can anticipate even more remarkable applications in the near future. For example, new IL–MOC combinations could be explored to expand the catalytic scope and improve efficiency. This involves synthesizing and characterizing a diverse range of IL–MOCs to identify promising candidates for various chemical transformations. Meanwhile, detailed mechanistic studies are essential to understand the reaction pathways and active sites involved in IL–MOC catalysis. Techniques such as spectroscopy, microscopy, and computational modeling can provide valuable insights into the fundamental processes occurring at the molecular level, aiding in catalyst design and optimization. Moreover, the stability and recyclability of IL–MOC catalysts could be further enhanced for practical applications and sustainability. Beyond catalytic oxidation reactions, IL–MOCs already offered exciting opportunities for emerging applications such as biomass conversion, CO2 utilization, and renewable energy storage. Future studies should explore these novel application areas and assess the feasibility and potential impact of IL–MOC catalysis. Although the field of IL–MOC hybrids is still young and there are still some challenges to overcome, the utilization of IL–MOCs is preferable from the viewpoint of both industry and society as they align more closely with the principles of green chemistry.
Abbreviations
| IL | Ionic liquid |
| MOC | Metal oxocluster |
| IL–MOCs | IL-stabilized metal oxoclusters |
| PIL | Polymeric ionic liquid |
| POMs | Polyoxometalates |
| POM–ILs | POM-based ILs |
| HPAs | Heteropoly acids |
| PEG | Polyethylene glycol |
| [Hbet][NTf2] | Protonated betaine bis(trifluoromethylsulfonyl)imide |
| EMIm | 1-Ethyl-3-methylimidazolium |
| BMIm | 1-Butyl-3-methylimidazolium |
| Gr | Graphene |
| HDIm | Protic N-dodecylimidazolium |
| MNP | Magnetic nanoparticles |
| BPy | N-Butylpyridinium |
| P4,4,4,n | Quaternary phosphonium cation |
| DMIm | 1-Dodecyl-3-methylimidazolium |
| TTA | Tetradecyl trimethyl ammonium |
| TBA | Tetrabutylammonium |
| NH4–Nb | Ammonium peroxoniobate |
| Pic | Picolinate ions |
| Bim | 1-Butylimidazolium |
| MIMPS | Methylimidazolium propyl sulfobetaine |
| Py | Pyridinium |
| PyPS | 3-(Pyridine-1-ium-1-yl) propane-1-sulfonate |
| TEAPS | 1-(3-Sulfonic group) trimethylamine |
| Dopy | N-n-Dodecylpyridinium |
| Im-PEG-Im | PEG-functionalized alkylimidazolium |
| C12MIm | 1-Dodecyl-3-methylimidazolium |
| CTA | Cetyltrimethyl-ammonium |
| ODA | Octadecylmethylammonium |
| Co4PW–PDDVAC | A porous POM-based composite |
| t Bu-Hptz | 2-tert-Butyl-5-(2-pyridyl)tetrazole |
| TOMA | Methyltrioctylammonium |
| PS–IL–PW | [α-PW12O40]3− Immobilized on IL-modified polystyrene resin beads |
| CMC | Carboxymethyl cellulose |
| AIL | Acidic IL |
| SACs | Single-atom catalysts |
| POM-SACs | POM-supported SACs |
| MOFs | Metal–organic frameworks |
| DEDSA | Diethyldisulphoammonium |
| BSmIm | –SO3H-functionalized imidazolium cation |
| [C2OHC1Im] | 1-(Hydroxyethyl)-3-methylimidazole |
| POMs-ILs@MOFs | MOF encapsulated POMs-based ILs |
| TON | Turnover number |
| TOF | Turnover frequency |
| HPMoV | H5PMo10V2O40 |
| FA | Formic acid |
| LA | Levulinic acid |
| ODS | Oxidative desulfurization |
| PET | Polyethylene terephthalate |
| BHET | Bis(hydroxyethyl) terephthalate |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the support from the National Key Research and Development Program of China (2022YFA1504903), State Key Laboratory of Petroleum Molecular & Process Engineering and the National Natural Science Foundation of China (21978095). Y. Qiao would like to thank the financial support by the Max Planck Society.References
- S. S. Wang and G. Y. Yang, Chem. Rev., 2015, 115, 4893–4962 CrossRef CAS PubMed
.
-
U. Schubert, in Macromolecules Containing Metal and Metal-Like Elements, ed. A. S. Abd-El-Aziz, C. E. Carraher Jr., C. U. Pittman Jr. and M. Zeldin, John Wiley & Sons, 2005, ch. 2, vol. 7, pp. 55–71 Search PubMed
.
- M. Carraro and S. Gross, Materials, 2014, 7, 3956–3989 CrossRef PubMed
.
- C. Wang, J. H. Yan, S. X. Chen and Y. Liu, ChemPlusChem, 2023, 88, e202200462 CrossRef CAS PubMed
.
- F. de Azambuja, J. Moons and T. N. Parac-Vogt, Acc. Chem. Res., 2021, 54, 1673–1684 CrossRef PubMed
.
- D. E. Salazar Marcano, N. D. Savić, K. Declerck, S. A. M. Abdelhameed and T. N. Parac-Vogt, Chem. Soc. Rev., 2024, 53, 84–136 RSC
.
- J. W. Zhong, J. Pérez-Ramírez and N. Yan, Green Chem., 2021, 23, 18–36 RSC
.
- D. M. Cheng, K. Li, H. Y. Zang and J. J. Chen, Energy Environ. Mater., 2023, 6, e12341 CrossRef CAS
.
- A. Misra, I. F. Castillo, D. P. Müller, C. González, S. Eyssautier-Chuine, A. Ziegler, J. M. de la Fuente, S. G. Mitchell and C. Streb, Angew. Chem., Int. Ed., 2018, 57, 14926–14931 CrossRef CAS PubMed
.
- I. Majeed, Z. Ahmad, N. AlMasoud, T. S. Alomar, S. Hussain, H. M. Asif, F. Mansoor, Z. Nazar and Z. M. El-Bahy, Polyhedron, 2023, 243, 116577 CrossRef CAS
.
- C. L. Hill, J. Mol. Catal. A: Chem., 2007, 262, 2–6 CrossRef CAS
.
- A. Patel, N. Narkhede, S. Singh and S. Pathan, Catal. Rev., 2016, 58, 337–370 CrossRef CAS
.
- Q. D. Liu and X. Wang, Matter, 2020, 2, 816–841 CrossRef
.
- B. Smarsly and H. Kaper, Angew. Chem., Int. Ed., 2005, 44, 3809–3811 CrossRef CAS PubMed
.
- Y. X. Qiao and Z. S. Hou, Curr. Org. Chem., 2009, 13, 1347–1365 CrossRef CAS
.
- S. Ivanova, ISRN Chem. Eng., 2014, 2014, 963792 Search PubMed
.
- Y. Martinetto, B. Pégot, C. Roch-Marchal, B. Cottyn-Boitte and S. Floquet, Eur. J. Inorg. Chem., 2020, 228–247 CrossRef CAS
.
- E. Ahmed and M. Ruck, Angew. Chem., Int. Ed., 2012, 51, 308–309 CrossRef CAS PubMed
.
- C. B. Hong, T. Wang and H. C. Liu, Inorg. Chem., 2023, 62, 4054–4065 CrossRef CAS PubMed
.
- A. B. Bourlinos, K. Raman, R. Herrera, Q. Zhang, L. A. Archer and E. P. Giannelis, J. Am. Chem. Soc., 2004, 126, 15358–15359 CrossRef CAS PubMed
.
- X. Z. Li, H. Y. Xue, Q. Lin and A. M. Yu, Appl. Organomet. Chem., 2020, 34, e5606 CrossRef CAS
.
- S. X. Mao, Q. H. Zhou, H. L. Guo, M. Du, W. S. Zhu, H. M. Li, J. Y. Pang, D. B. Dang and Y. Bai, Fuel, 2023, 333, 126392 CrossRef CAS
.
- H. X. Chen, S. T. Hou, H. Y. Cui, C. Wang, M. Zhang, H. P. Li, H. Xu, J. Q. Wu and W. S. Zhu, J. Mol. Liq., 2023, 379, 121650 CrossRef CAS
.
- S. Y. Zhang, Q. Zhuang, M. Zhang, H. Wang, Z. M. Gao, J. K. Sun and J. Y. Yuan, Chem. Soc. Rev., 2020, 49, 1726–1755 RSC
.
- P. Nockemann, B. Thijs, S. Pittois, J. Thoen, C. Glorieux, K. Van Hecke, L. Van Meervelt, B. Kirchner and K. Binnemans, J. Phys. Chem. B, 2006, 110, 20978–20992 CrossRef CAS PubMed
.
- P. Nockemann, B. Thijs, K. V. Hecke, L. V. Meervelt and K. Binnemans, Cryst. Growth Des., 2008, 8, 1353–1363 CrossRef CAS
.
- P. G. Rickert, M. R. Antonio, M. A. Firestone, K.-A. Kubatko, T. Szreder, J. F. Wishart and M. L. Dietz, Dalton Trans., 2007, 5, 529–531 RSC
.
- P. G. Rickert, M. R. Antonio, M. A. Firestone, K.-A. Kubatko, T. Szreder, J. F. Wishart and M. L. Dietz, J. Phys. Chem. B, 2007, 111, 4685–4692 CrossRef CAS PubMed
.
- S. W. Lin, W. L. Chen, Z. M. Zhang, W. L. Liu and E. B. Wang, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2008, E64, m954 CrossRef PubMed
.
- S. W. Lin, W. L. Liu, Y. G. Li, Q. Wu, E. B. Wang and Z. M. Zhang, Dalton Trans., 2010, 39, 1740–1744 RSC
.
- S. L. Linguito, X. Zhang, M. Padmanabhan, A. V. Biradar, T. T. Xu, T. J. Emge, T. Asefa and J. Li, New J. Chem., 2013, 37, 2894–2901 RSC
.
- T. L. Greaves and C. J. Drummond, Chem. Soc. Rev., 2008, 37, 1709–1726 RSC
.
- H. W. Yang, L. J. Bai, D. L. Wei, L. X. Yang, W. X. Wang, H. Chen, Y. Z. Niu and Z. X. Xue, Chem. Eng. J., 2019, 358, 850–859 CrossRef CAS
.
- Z. K. Guan, M. M. Wang, G. X. Sun, X. Xue, Y. F. Chen, Y. Zhang, T. Zhao, H. F. Shi, C. Feng, Y. Pan and Y. Q. Liu, Fuel, 2024, 357, 129887 CrossRef CAS
.
- Y. T. Gao, M. Choudhari, G. K. Such and C. Ritchie, Chem. Sci., 2022, 13, 2510–2527 RSC
.
- Z. H. Ma, J. H. Zhang, H. Q. Zhan, M. Xu, H. W. Yang, L. X. Yang, L. J. Bai, H. Chen, D. L. Wei and W. X. Wang, Process Saf. Environ. Prot., 2020, 140, 26–33 CrossRef CAS
.
- A. Misra, K. Kozma, C. Streb and M. Nyman, Angew. Chem., Int. Ed., 2020, 59, 596–612 CrossRef CAS PubMed
.
- T. V. Marinho, E. Schreiber, R. E. Garwick, W. W. Brennessel and E. M. Matson, Inorg. Chem., 2023, 62, 15616–15626 CrossRef CAS PubMed
.
- P. F. Ji, Y. Song, T. Drake, S. S. Veroneau, Z. K. Lin, X. D. Pan and W. B. Lin, J. Am. Chem. Soc., 2018, 140, 433–440 CrossRef CAS PubMed
.
- U. Schubert, Coord. Chem. Rev., 2017, 350, 61–67 CrossRef CAS
.
- A. Banerjee, R. Theron and R. W. J. Scott, ChemSusChem, 2012, 5, 109–116 CrossRef CAS PubMed
.
-
K. Kumari, P. Singh and G. K. Mehrotra, in Advanced Energy Materials, ed. A. Tiwari and S. Valyukh, John Wiley & Sons, 2014, pp. 529–553 Search PubMed
.
- S. P. Ding, Y. L. Guo, M. J. Hülsey, B. Zhang, H. Asakura, L. M. Liu, Y. Han, M. Gao, J.-y. Hasegawa, B. T. Qiao, T. Zhang and N. Yan, Chem, 2019, 5, 3207–3219 CAS
.
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772–3789 CrossRef CAS PubMed
.
- R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792–793 CrossRef PubMed
.
- J. Dupont and J. D. Scholten, Chem. Soc. Rev., 2010, 39, 1780–1804 RSC
.
- Y. X. Qiao, Z. S. Hou, H. Li, Y. Hu, B. Feng, X. R. Wang, L. Hua and Q. F. Huang, Green Chem., 2009, 11, 1955–1960 RSC
.
- Y. X. Qiao, H. Li, L. Hua, L. Orzechowski, K. Yan, B. Feng, Z. Pan, N. Theyssen, W. Leitner and Z. S. Hou, ChemPlusChem, 2012, 77, 1128–1138 CrossRef CAS
.
- J. Z. Chen, L. Hua, C. Chen, L. Guo, R. Zhang, A. J. Chen, Y. H. Xiu, X. R. Liu and Z. S. Hou, ChemPlusChem, 2015, 80, 1029–1037 CrossRef CAS PubMed
.
- J. Z. Chen, C. Chen, R. Zhang, L. Guo, L. Hua, A. J. Chen, Y. H. Xiu, X. R. Liu and Z. S. Hou, RSC Adv., 2015, 5, 25904–25910 RSC
.
- D. Liu, J. Z. Gui, F. Lu, Z. L. Sun and Y.-K. Park, Catal. Lett., 2012, 142, 1330–1335 CrossRef CAS
.
- Y. Martinetto, B. Pégot, C. Roch-Marchal, M. Haouas, B. Cottyn-Boitte, F. Camerel, J. Jeftic, D. Morineau, E. Magnier and S. Floquet, New J. Chem., 2021, 45, 9751–9755 RSC
.
- Y. Martinetto, S. Basset, B. Pégot, C. Roch-Marchal, F. Camerel, J. Jeftic, B. Cottyn-Boitte, E. Magnier and S. Floquet, Molecules, 2021, 26, 496 CrossRef CAS PubMed
.
- A. de Zordo-Banliat, K. Grollier, N. Vanthuyne, S. Floquet, T. Billard, G. Dagousset, B. Pégot and E. Magnier, Angew. Chem., Int. Ed., 2023, 62, e202300951 CrossRef CAS PubMed
.
- C. Chen, X. G. Zhao, J. Z. Chen, L. Hua, R. Zhang, L. Guo, B. N. Song, H. M. Gan and Z. S. Hou, ChemCatChem, 2014, 6, 3231–3238 CrossRef CAS
.
- C. Chen, H. Y. Yuan, H. F. Wang, Y. F. Yao, W. B. Ma, J. Z. Chen and Z. S. Hou, ACS Catal., 2016, 6, 3354–3364 CrossRef CAS
.
- W. B. Ma, H. Y. Yuan, H. F. Wang, Q. Q. Zhou, K. Kong, D. F. Li, Y. F. Yao and Z. S. Hou, ACS Catal., 2018, 8, 4645–4659 CrossRef CAS
.
- Q. Q. Zhou, M. Ye, W. B. Ma, D. F. Li, B. J. Ding, M. Y. Chen, Y. F. Yao, X. Q. Gong and Z. S. Hou, Chem. – Eur. J., 2019, 25, 4206–4217 CrossRef CAS PubMed
.
- Q. Q. Zhou, B. B. Xu, X. Tang, S. Dai, B. J. Ding, D. F. Li, A. N. Zheng, T. Zhang, Y. F. Yao, X. Q. Gong and Z. S. Hou, Langmuir, 2021, 37, 8190–8203 CrossRef CAS PubMed
.
- B. J. Ding, R. Zhang, Q. Q. Zhou, W. B. Ma, A. N. Zheng, D. F. Li, Y. F. Yao and Z. S. Hou, Mol. Catal., 2021, 500, 111342 CrossRef CAS
.
- W. B. Ma, C. Chen, K. Kong, Q. F. Dong, K. Li, M. M. Yuan, D. F. Li and Z. S. Hou, Chem. – Eur. J., 2017, 23, 7287–7296 CrossRef CAS PubMed
.
- W. B. Ma, Y. X. Qiao, N. Theyssen, Q. Q. Zhou, D. F. Li, B. J. Ding, D. Q. Wang and Z. S. Hou, Catal. Sci. Technol., 2019, 9, 1621–1630 RSC
.
- Q. Q. Zhou, R. Zhang, D. F. Li, B. J. Ding, A. N. Zheng, Y. F. Yao, X. Q. Gong and Z. S. Hou, Catal. Sci. Technol., 2020, 10, 7601–7612 RSC
.
- Y. J. Wang, B. J. Ding, J. J. He, Z. J. Ding and Z. S. Hou, Energy Fuels, 2023, 37, 5429–5440 CrossRef CAS
.
- T. Zhang, X. J. Chen, B. J. Ding, Z. J. Ding, Y. J. Wang, H. W. Qiu, Y. J. Jiang, S. Dai and Z. S. Hou, Energy Fuels, 2022, 36, 1402–1416 CrossRef CAS
.
- Y. Leng, J. Wang, D. R. Zhu, X. Q. Ren, H. Q. Ge and L. Shen, Angew. Chem., Int. Ed., 2009, 48, 168–171 CrossRef CAS PubMed
.
- L. L. Liu, C. C. Chen, X. F. Hu, T. Mohamood, W. H. Ma, J. Lin and J. C. Zhao, New J. Chem., 2008, 32, 283–289 RSC
.
- S. S. Wang, W. Liu, Q. X. Wan and Y. Liu, Green Chem., 2009, 11, 1589–1594 RSC
.
- N. Kashyap, S. Das and R. Borah, Polyhedron, 2021, 196, 114993 CrossRef CAS
.
- D. Liang, N. Zhu, H. L. Zhu, Z. Y. Hu, D. L. Cao and N. Sang, Catal. Commun., 2016, 79, 49–52 CrossRef CAS
.
- P. B. Hao, M. J. Zhang, W. Zhang, Z. Y. Tang, N. Luo, R. Tan and D. H. Yin, Catal. Sci. Technol., 2018, 8, 4463–4473 RSC
.
- H. Li, Y. X. Qiao, L. Hua, Z. S. Hou, B. Feng, Z. Y. Pan, Y. Hu, X. R. Wang, X. G. Zhao and Y. Yu, ChemCatChem, 2010, 2, 1165–1170 CrossRef CAS
.
- H. Li, Z. S. Hou, Y. X. Qiao, B. Feng, Y. Hu, X. R. Wang and X. G. Zhao, Catal. Commun., 2010, 11, 470–475 CrossRef CAS
.
- B. S. Chhikara, R. Chandra and V. Tandon, J. Catal., 2005, 230, 436–439 CrossRef CAS
.
- X. X. Han, K. Ouyang, C. H. Xiong, X. J. Tang, Q. Chen, K. W. Wang, L. L. Liu, C.-T. Hung and S.-B. Liu, Appl. Catal., A, 2017, 543, 115–124 CrossRef CAS
.
- L. Hua, Y. X. Qiao, H. Li, B. Feng, Z. Y. Pan, Y. Y. Yu, W. W. Zhu and Z. S. Hou, Sci. China: Chem., 2011, 54, 769–773 CrossRef CAS
.
- L. Hua, Y. X. Qiao, Y. Y. Yu, W. W. Zhu, T. Cao, Y. Shi, H. Li, B. Feng and Z. S. Hou, New J. Chem., 2011, 35, 1836–1841 RSC
.
- L. Jing, J. Shi, F. M. Zhang, Y. J. Zhong and W. D. Zhu, Ind. Eng. Chem. Res., 2013, 52, 10095–10104 CrossRef CAS
.
- J. L. Xu, H. Y. Zhang, Y. F. Zhao, Z. Z. Yang, B. Yu, H. J. Xu and Z. M. Liu, Green Chem., 2014, 16, 4931–4935 RSC
.
- J. W. Zhang, X. Y. Zhu, X. X. Xu, Q. Q. Sun, L. G. Wei, K. L. Li, S. R. Zhai and Q. D. An, J. Environ. Chem. Eng., 2022, 10, 108260 CrossRef CAS
.
- K. X. Li, L. L. Bai, P. N. Amaniampong, X. L. Jia, J.-M. Lee and Y. H. Yang, ChemSusChem, 2014, 7, 2670–2677 CrossRef CAS PubMed
.
- S. Xue, G. J. Chen, Z. Y. Long, Y. Zhou and J. Wang, RSC Adv., 2015, 5, 19306–19314 RSC
.
- Z. B. Liao, Y. L. Duan, L. Y. Guo, R. R. Zheng, L. Y. Wang, Y. M. Chen, L. N. Zhang and X. Qian, New J. Chem., 2023, 47, 4337–4345 RSC
.
- Z. Z. Jiang, H. Y. Wang, L. N. Shan, R. R. Zheng, X. D. Zhao, Z. B. Liao and L. Y. Guo, Catal. Lett., 2023, 153, 1308–1318 CrossRef CAS
.
- J. X. Yang, N. Chu and X. W. Chen, Molecules, 2023, 28, 3307 CrossRef CAS PubMed
.
- T. M. A. Bakker, S. Mathew and J. N. H. Reek, Sustainable Energy Fuels, 2019, 3, 96–100 RSC
.
- M. S. Nunes, A. C. Gomes, P. Neves, R. F. Mendes, F. A. A. Paz, A. D. Lopes, M. Pillinger, I. S. Gonçalves and A. A. Valente, Catal. Today, 2023, 423, 114273 CrossRef CAS
.
- Y. Ma, Y. J. Jiang, X. J. Wei, Q. P. Peng, S. Dai and Z. S. Hou, ACS Sustainable Chem. Eng., 2022, 10, 15389–15401 CrossRef CAS
.
- Y. Ma, C. Chen, Y. J. Jiang, X. J. Wei, Y. Liu, H. Y. Liao, H. F. Wang, S. Dai, P. F. An and Z. S. Hou, ACS Catal., 2023, 13, 10295–10308 CrossRef CAS
.
- X. J. Lang, Z. Li and C. G. Xia, Synth. Commun., 2008, 38, 1610–1616 CrossRef CAS
.
- S. Abednatanzi, K. Leus, P. G. Derakhshandeh, F. Nahra, K. De Keukeleere, K. Van Hecke, I. Van Driessche, A. Abbasi, S. P. Nolan and P. V. Der Voort, Catal. Sci. Technol., 2017, 7, 1478–1487 RSC
.
- L. Hua, J. Z. Chen, C. Chen, W. W. Zhu, Y. Y. Yu, R. Zhang, L. Guo, B. N. Song, H. M. Gan and Z. S. Hou, New J. Chem., 2014, 38, 3953–3959 RSC
.
- S. W. Li, R. J. Lou, R. N. Biboum, B. Lepoittevin, G. J. Zhang, A. Dolbecq, P. Mialane and B. Keita, Eur. J. Inorg. Chem., 2013, 1882–1889 CrossRef
.
- R. J. Liu, S. W. Li, G. J. Zhang, A. Dolbecq, P. Mialane and B. Keita, J. Cluster Sci., 2014, 25, 711–740 CrossRef CAS
.
- W. L. Xie and F. Wan, Chem. Eng. J., 2019, 365, 40–50 CrossRef CAS
.
- M. M. Jin, Q. T. Niu, G. D. Liu, Z. G. Lv, C. D. Si and H. Y. Guo, J. Mater. Sci., 2020, 55, 8199–8210 CrossRef CAS
.
- H. Y. Zhao, Y. Z. Li, J. W. Zhao, L. Wang and G. Y. Yang, Coord. Chem. Rev., 2021, 443, 213966 CrossRef CAS
.
- Y. Y. Li, X. F. Wu, Q. Y. Wu, H. Ding and W. F. Yan, Ind. Eng. Chem. Res., 2014, 53, 12920–12926 CrossRef CAS
.
- R. J. Liu and C. Streb, Adv. Energy Mater., 2021, 11, 2101120 CrossRef CAS
.
- X. J. Wei, Y. J. Jiang, Y. Ma, J. Fang, Q. B. Peng, W. Xu, H. Y. Liao, F. X. Zhang, S. Dai and Z. S. Hou, Chem. – Eur. J., 2022, 28, e202200374 CrossRef CAS PubMed
.
- G. D. Yadav, R. K. Mewada, D. P. Wagh and H. G. Manyar, Catal. Sci. Technol., 2022, 12, 7245–7269 RSC
.
- J. Liu, Y. Liu, Y. Liu, Y. Wang, F. Q. Wu, Z. Zhou and Z. B. Zhang, ACS Sustainable Chem. Eng., 2023, 11, 12934–12945 CrossRef CAS
.
- N. Sun, X. Y. Jiang, M. L. Maxim, A. Metlen and R. D. Rogers, ChemSusChem, 2011, 4, 65–73 CrossRef CAS PubMed
.
- H. Y. Deng, W. B. B. Xu, D. Zhang, X. Y. Li and J. Y. Shi, Polymers, 2023, 15, 2401 CrossRef CAS PubMed
.
- J. A. Abia and R. Ozer, BioResources, 2013, 8, 2924–2933 CrossRef
.
- G. F. De Gregorio, R. Prado, C. Vriamont, X. Erdocia, J. Labidi, J. P. Hallett and T. Welton, ACS Sustainable Chem. Eng., 2016, 4, 6031–6036 CrossRef
.
- X. Xin, Z. Li, M. Z. Chi, M. Zhang, Y. Y. Dong, H. J. Lv and G. Y. Yang, Green Chem., 2023, 25, 2815–2824 RSC
.
- K. X. Li, L. Z. Huang, Y. Zhang, Z. T. Zhu, J. Zhao, G. J. Xia and Y. G. Min, Ind. Eng. Chem. Res., 2023, 62, 18337–18349 CrossRef CAS
.
- Z. H. Li, Y. M. Li, Y. N. Chen, Q. W. Wang, M. Jadoon, X. H. Yi, X. Z. Duan and X. H. Wang, ACS Catal., 2022, 12, 9213–9225 CrossRef CAS
.
- Z. P. Cai, J. X. Long, Y. W. Li, L. Ye, B. L. Yin, L. J. France, J. C. Dong, L. R. Zheng, H. Y. He, S. J. Liu, S. C. E. Tsang and X. H. Li, Chem, 2019, 5, 2365–2377 CAS
.
- Z. P. Cai, R. J. Chen, H. Zhang, F. K. Li, J. X. Long, L. L. Jiang and X. H. Li, Green Chem., 2021, 23, 10116–10122 RSC
.
- Z. Wu, L. Hu, Y. T. Jiang, X. Y. Wang, J. X. Xu, Q. F. Wang and S. F. Jiang, Biomass Convers. Biorefin., 2023, 13, 519–539 CrossRef
.
- Y. C. Hou, M. G. Niu and W. Z. Wu, Ind. Eng. Chem. Res., 2020, 59, 16899–16910 CrossRef CAS
.
- F. Boshagh, M. Rahmani, K. Rostami and M. Yousefifar, Energy Fuels, 2022, 36, 98–132 CrossRef CAS
.
- M. Haghighi and S. Gooneh-Farahani, Environ. Sci. Pollut. Res., 2020, 27, 39923–39945 CrossRef CAS PubMed
.
- F. Liu, J. Yu, A. B. Qazi, L. Zhang and X. K. Liu, Environ. Sci. Technol., 2021, 55, 1419–1435 CrossRef CAS PubMed
.
- J. R. Li, Z. Yang, S. W. Li, Q. P. Jin and J. S. Zhao, J. Ind. Eng. Chem., 2020, 82, 1–16 CrossRef
.
- X. B. Lim and W.-J. Ong, Nanoscale Horiz., 2021, 6, 588–633 RSC
.
- M. Ahmadian and M. Anbia, Energy Fuels, 2021, 35, 10347–10373 CrossRef CAS
.
- H. Liu, H. Xu, M. Hua, L. Chen, Y. Wei, C. Wang, P. Wu, F. Zhu, X. Chu, H. Li and W. Zhu, Fuel, 2020, 260, 116200 CrossRef CAS
.
- J. Wang, B. Yang, X. L. Peng, Y. C. Ding, S. S. Yu, F. Q. Zhang, L. F. Zhang, H. D. Wu and J. Guo, Chem. Eng. J., 2022, 429, 132446 CrossRef CAS
.
- X. X. Xing, H. L. Guo, T. M. He, X. An, H. P. Li, W. S. Zhu, H. M. Li, J. Y. Pang, D. B. Dang and Y. Bai, ACS Sustainable Chem. Eng., 2022, 10, 11533–11543 CrossRef CAS
.
- Y. Z. Wang, Z. Z. Li, Z. W. Liu and X. Y. Shi, J. Mol. Liq., 2023, 373, 121245 CrossRef CAS
.
- Y. Gao, L. Cheng, R. Gao, G. Hu and J. Zhao, J. Hazard. Mater., 2021, 401, 123267 CrossRef CAS PubMed
.
- F. Mohammadi-Nejati and S. Shahhosseini, Fuel Process. Technol., 2023, 252, 107980 CrossRef CAS
.
- H. Ge, Y. Leng, F. Zhang, C. Zhou and J. Wang, Catal. Lett., 2008, 124, 250–255 CrossRef CAS
.
- Y. Leng, J. Wang, D. Zhu, L. Shen, P. Zhao and M. Zhang, Chem. Eng. J., 2011, 173, 620–626 CrossRef CAS
.
- P. Zhao, J. Wang, G. Chen, Y. Zhou and J. Huang, Catal. Sci. Technol., 2013, 3, 1394–1404 RSC
.
- P. Zhao, Y. Leng and J. Wang, Chem. Eng. J., 2012, 204–206, 72–78 CrossRef CAS
.
- P. Zhao, Y. Zhou, Y. Liu and J. Wang, Chin. J. Catal., 2013, 34, 2118–2124 CrossRef CAS
.
- Z. Long, Y. Zhou, W. Ge, G. Chen, J. Xie, Q. Wang and J. Wang, ChemPlusChem, 2014, 79, 1590–1596 CrossRef CAS
.
- X. Cai, Q. Wang, Y. Liu, J. Xie, Z. Long, Y. Zhou and J. Wang, ACS Sustainable Chem. Eng., 2016, 4, 4986–4996 CrossRef CAS
.
- Z. Long, Y. Zhang, G. Chen, J. Shang, Y. Zhou, J. Wang and L. Sun, ACS Sustainable Chem. Eng., 2019, 7, 4230–4238 CrossRef CAS
.
- C. Yuan, X. Gao, Z. Pan, X. Li and Z. Tan, Catal. Commun., 2015, 58, 215–218 CrossRef CAS
.
- M. Taheri, M. Ghiaci, A. Moheb and A. Shchukarev, Appl. Organomet. Chem., 2019, 33, e5012 CrossRef
.
- H. Wang, L. Fang, Y. Yang, L. Zhang and Y. Wang, Catal. Sci. Technol., 2016, 6, 8005–8015 RSC
.
- H. Han, T. Jiang, T. Wu, D. Yang and B. Han, ChemCatChem, 2015, 7, 3526–3532 CrossRef CAS
.
- P. T. Fang, B. Liu, J. L. Xu, Q. Zhou, S. J. Zhang, J. Y. Ma and X. M. Lu, Polym. Degrad. Stab., 2018, 156, 22–31 CrossRef CAS
.
- P. T. Fang, X. Zheng, R. Q. Zhang, J. L. Xu, D. X. Yan, Q. Zhou, J. Y. Xin, C. Y. Shi, S. Q. Xia and X. M. Lu, ChemCatChem, 2023, 15, e202200712 CrossRef CAS
.
- M. Y. Wang, R. Ma and L. N. He, Sci. China: Chem., 2016, 59, 507–516 CrossRef CAS
.
-
M. Y. Wang and X. B. Ma, in Encyclopedia of Ionic Liquids, ed. S. J. Zhang, Springer, Singapore, 2019. DOI:10.1007/978-981-10-6739-6_35-2
.
- Y. Ma, X. J. Wei, J. Fang, Q. B. Peng, W. Xu and Z. S. Hou, Green Chem. Eng., 2022, 3, 146–156 CrossRef
.
- A. Patel, J. Patel and S. Pathan, Inorg. Chem., 2023, 62, 6970–6980 CrossRef CAS PubMed
.
- M. A. Alotaibi, E. F. Kozhevnikova and I. V. Kozhevnikov, Chem. Commun., 2012, 48, 7194–7196 RSC
.
- B. Zhang, G. Sun, S. P. Ding, H. Asakura, J. Zhang, P. Sautet and N. Yan, J. Am. Chem. Soc., 2019, 141, 8185–8197 CrossRef CAS PubMed
.
- S. J. Li, Y. B. Ma, Y. Zhao, R. J. Lou, Y. P. Zhao, X. S. Dai, N. N. Ma, C. Streb and X. N. Chen, Angew. Chem., Int. Ed., 2023, 62, e202314999 CrossRef CAS PubMed
.
- X. J. Wei, Y. J. Jiang, Y. Ma, H. Y. Liao, S. Dai, P. F. An, Z. Q. Wang, X. Q. Gong and Z. S. Hou, ACS Catal., 2024, 14, 5344–5355 CrossRef CAS
.
- A. Modvig, C. Kumpidet, A. Riisager and J. Albert, Materials, 2019, 12, 2175 CrossRef CAS PubMed
.
- Q. B. Peng, X. G. Zhao, M. Y. Chen, J. J. Wang, K. Cui, X. J. Wei and Z. S. Hou, Mol. Catal., 2022, 517, 112049 CrossRef CAS
.
- Z. R. Zhang, M. Y. Liu, J. L. Song, H. Z. Liu, Z. B. Xie, S. S. Liu, Q. L. Meng, P. Zhang and B. X. Han, Green Chem., 2018, 20, 4865–4869 RSC
.
- M. Armand, F. Endres, D. R. MacFarlane, H. Ohno and B. Scrosati, Nat. Mater., 2009, 8, 621–629 CrossRef CAS PubMed
.
- T. Ueda, ChemElectroChem, 2018, 5, 823–838 CrossRef CAS
.
- Z. Q. Wang, L. M. Ai, X. S. Lv, Z. J. Wang, F. W. He and Q. Y. Wu, Int. J. Electrochem. Sci., 2020, 15, 223–230 CrossRef CAS
.
- J. I. Phillips, S. Azuma, J. Lee, T. Ueda and D. S. Silvester, Aust. J. Chem., 2022, 75, 865–876 CrossRef CAS
.
- H. Cruz, N. Gomes, F. Mirante, S. S. Balula, L. C. Branco and S. Gago, ChemistrySelect, 2020, 5, 12266–12271 CrossRef CAS
.
- G. J. Chen, L. Zhang, Y. D. Zhang, K. Liu, Z. Y. Long and Y. Wang, J. Mater. Chem. A, 2019, 7, 7194–7201 RSC
.
- M. R. Lou, R. Y. Wang, L. L. Yang, D. Z. Jia, Z. P. Sun, L. X. Wang, Y. Guo, X. C. Wang, J. Zhang and H. L. Shi, Appl. Surf. Sci., 2020, 516, 146137 CrossRef CAS
.
- S. Shahsavarifar, M. Masteri-Farahani and M. R. Ganjali, Colloids Surf., A, 2022, 632, 127812 CrossRef CAS
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |