Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mechanochemical indium(0)-mediated Barbier allylation of carbonyl compounds: unexpected immiscible water additive effect for hydrophobic reagents†
Nuri
Kim‡
a,
Eun Sul
Go‡
a and
Jeung Gon
Kim
 *ab
*ab
aDepartment of Chemistry, Research Institute of Physics and Chemistry, Jeonbuk National University, Jeonju, 54896, Republic of Korea. E-mail: jeunggonkim@jbnu.ac.kr
bDepartment of JBNU-KIST Industry-Academia Convergence Research, Jeonbuk National University, Jeonju, 54896, Republic of Korea
First published on 22nd February 2024
Abstract
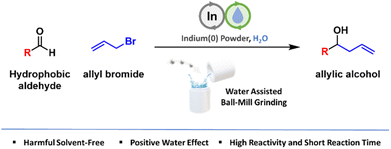
Indium-mediated Barbier allylation exhibited a positive effect with the addition of water under mechanochemical ball-milling conditions. A small amount of water as an additive selectively boosted the allylation of solid-state hydrophobic aldehydes despite their immiscibility. The broad scope and scalability of this method are also demonstrated herein.
Barbier allylation represents a highly efficient and mild method for forming C–C bonds.1 This popular method combines allyl halides with zero-valent metals to generate metal allyl intermediates. These intermediates then readily react with a wide array of carbonyl compounds, including aldehydes, ketones, imines, and more, producing homoallylic alcohols and amines that expand the possibilities in chemical synthesis. Many metals demonstrate remarkable reactivity and group 13 indium creates its own distinct domain.2 Indium allylation shows exceptional functional group tolerance, enabling broad scope and facile operation under ambient conditions. Additionally, its employment in green chemistry practices, utilizing environmentally benign or less toxic indium metal and solvents such as water, has garnered unique attention.
One of the most notable features of indium-mediated reactions is the use of water as a solvent.3 Water is highly desirable for chemical transformations due to its environmentally benign nature, and organoindium reagents have shown excellent stability and reactivity under aqueous conditions. However, the poor water solubility of many organic materials has limited the use of aqueous conditions. This issue becomes more pronounced when handling solid reagents.
To address this problem, we highlight the use of mechanochemistry. Mechanical energy, supplied by methods such as ball-milling or twin-screws, can promote chemical transformations with minimal or no solvent use.4 Recently, there has been significant progress in mechanochemistry, with successes in enhancing chemical reactivity, improving green metrics, and discovering new chemistry, establishing it as a vital approach for scale-up and commercialization.5 We have achieved success with the mechanochemical Barbier allylation reaction using indium metal. While water as a solvent is incompatible with organic solid reagents, it enhances the reaction under solvent-free mechanochemical conditions, outperforming the more sluggish neat reactions (Scheme 1).
Among many mechanochemical reactions with zero-valent metals,6 a closely related study on mechanochemical ball-milling, zinc-mediated Barbier allylation, was reported by Browne and coworkers.7 They demonstrated that a series of aldehydes and ketones underwent high-yielding allylations using zinc flakes and allyl bromide. The reaction proceeded well with just zinc, aldehydes, and allyl bromide; however, the addition of a liquid additive, DMSO (1.5 equiv.), improved the reaction to a quantitative level. Notably, the study did not explore the use of water as an additive, probably due to the corrosiveness of zinc in water. Suzuki and coworkers also explored bismuth-mediated allylation using a solvent-free ball-milling approach.8 While bismuth is recognized for its ability to promote organic transformations in aqueous media, including allylation reactions,9 their study did not investigate the use of water or any other liquid additives.
In this work, we commenced with solid 4-phenyl-benzaldehyde (1a), allyl bromide (1.5 equiv.), and indium powder (325 mesh, 2.0 equiv.) as outlined in Table 1. The reaction mixture was placed in a 10 mL Teflon jar along with three 7 mm stainless steel (SS) balls. A vibratory motion was applied at a frequency of 30 Hz using a Retsch MM400 mixer mill for 30 minutes. The resulting mixture was analyzed using proton nuclear magnetic resonance spectroscopy (1H NMR) against a CH2Br2 standard. Although all aldehydes had disappeared, only 33% of the intended homoallylic alcohol product, 2a, was detected (entry 1), with the remaining 67% comprising side products that are insoluble in the organic phase.
| Entry | Alternations to the standard conditions | Time (min) | Aldehyde conv.a (%) | Product yielda (%) |
|---|---|---|---|---|
| a Based on 1H NMR analysis of the crude reaction mixture using CH2Br2 as the internal standard. | ||||
| 1 | None | 30 | >99 | 33 |
| 2 | H2O (40 μL) | 30 | >99 | 98 |
| 3 | Methanol (40 μL) | 30 | >99 | 86 |
| 4 | 1-Hexanol (40 μL) | 30 | 99 | 80 |
| 5 | DMSO (40 μL) | 30 | >99 | 89 |
| 6 | THF (40 μL) | 30 | >99 | 55 |
| 7 | DMF (40 μL) | 30 | >99 | 72 |
| 8 | Allyl-Br 1.1 equiv., H2O (40 μL) | 30 | 98 | 37 |
| 9 | In 1.1 equiv., H2O (40 μL) | 30 | 98 | 67 |
| 10 | SS 10 mL jar instead of Teflon, H2O (40 μL) | 30 | >99 | 98 |
| 11 | Zirconia jar (10 mL) and balls (8 mm × 3), H2O (40 μL) | 30 | >99 | 98 |
| 12 | H2O (40 μL) | 5 | >99 | 98 |
| 13 | 20 Hz, H2O (40 μL) | 30 | >99 | 48 |
| 14 | H2O 1 mL as solvent in 4 mL vial with magnetic stirring | 30 | 63 | 51 |
The next set of experiments investigated liquid-assisted grinding (LAG) (entries 2–7).10 The addition of a small volume of liquid to the overall mass of reactants, generally between 0.1 and 1.0 μL mg−1, facilitates reagent mixing or creates a more favorable reaction environment, thus leading to higher efficiency and selectivity. A series of liquids (40 μL) demonstrated a positive effect, increasing the yield of product 2a. Notably, water as an additive provided the best allylation selectivity with a 98% yield (entry 2). For many solid-state ball-milling processes, the addition of a suitable solvent can reduce crystallinity, leading to better dispersion. In this case, poorly miscible water facilitated allylation over other side reactions. Initially, we hypothesized in situ acetal formation upon water treatment. However, infrared (IR) spectroscopy of the post-ball-milling mixture of 1a and water did not indicate any conversion from aldehyde with no changes in the IR spectra corresponding to carbonyls (1693 cm−1) except for a broad water signal at above 3000 cm−1 (see Fig. S1†). When organic transformation is accelerated in a water medium, the hydrophobic effect and hydrogen bonding are generally proposed mechanisms.11 The hydrophobic effect is relevant when organic substrates are immersed in a large quantity of water, which does not align with the conditions of LAG. Moreover, IR spectra of the 1a and water mixture did not exhibit any carbonyl group shift, ruling out hydrogen bonding activation. Therefore, the specific role of water in enhancing the reaction remains elusive. Other protic solvents, such as methanol (entry 3) and 1-hexanol (entry 4), also improved the selectivity for 2a. A series of polar aprotic solvents, including dimethyl sulfoxide (entry 5), tetrahydrofuran (entry 6), and dimethylformamide (entry 7), were examined. While reports of zinc-mediated mechanochemical allylation suggest that coordinating liquids in LAG might facilitate better conversion by aiding zinc disassembly, our findings show that while protic solvents noted improvement, it was less significant than that with protic ones.
The amounts of reagents were scrutinized. Using less of both allyl bromide (1.1 equiv., entry 8) and indium (1.1 equiv., entry 9) resulted in a loss of reactivity to 37% and 67% respectively. Employing a harder SS jar did not hamper the efficiency (entry 10). Another widely used setup, employing a zirconia jar (10 mL) with three zirconia balls (8 mm each), also achieved quantitative conversion to product 2b (entry 11). While the indium reaction showed a strong deviation in aldehyde conversions and allylation products, aldehyde remained intact (5% conv.). The reaction time could be shortened to 5 minutes, achieving full conversion to homoallylic alcohol 2a (entry 12). However, lowering mixing frequency to 20 Hz resulted in poor allylation selectivity (48%, entry 13). A conventional reaction involving an excess of water (1 mL) also produced 2a without mechanical treatment, but the efficiency was considerably lower at 51% (entry 14).
Next, we scrutinized the scope of aldehydes (Scheme 2). A brief reaction time of 5 minutes proved effective for most substrates, regardless of their physical state. Water-assisted grinding maintained high efficiency, exemplified by liquid benzaldehyde achieving a 95% yield (2b). The anticipated electronic effect was observed; an electron-withdrawing para-chloride group enhanced the electrophilic efficiency of the carbonyl unit, yielding nearly quantitative results (95%, 2c). Conversely, an electron-donating para-methoxy group decreased the allylation efficiency (75%, 2d). Notably, benzaldehyde with a nitro substituent failed to undergo allylation (2e). 4-Nitro-benzaldehyde disappeared, but no product soluble in organic solvent was isolated. The previous report on aqueous indium allylation also observed no selectivity towards allylation.12 Other hydrophobic solid aldehydes, such as 1-pyrenyl and 9-anthracene aldehydes (melting points of 123 °C and 103 °C, respectively), underwent indium allylation almost quantitatively, with yields of 90% (2f) and 96% (2g).
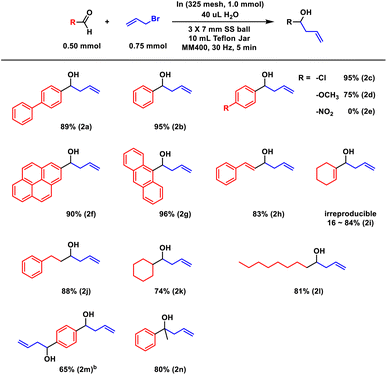 | ||
| Scheme 2 Scope of carbonyl compounds. aAll reactions were performed twice, and average values are reported. b0.25 mmol substrate with 0.75 mmol allyl bromide and 1.0 mmol indium. | ||
A variety of aliphatic aldehydes also maintained high efficiency. However, enone-type substrates yielded mixed results. Cinnamaldehyde cleanly converted to homoallylic alcohol (83%, 2h), while 1-cyclohexenyl aldehyde exhibited irreproducible yields (2i). No conjugate products were formed other than 2h and 2i. Saturated alkyl aldehydes, both linear and cyclic, showed similar reactivities (2j–l). Remarkably, terephthalaldehyde underwent predominant double allylation, with no mono-allylation product detected in the crude NMR analysis (2m). Lastly, when we applied the representative ketone, acetophenone, under identical conditions, a commendable yield of 80% was achieved (2n).
A gram-scale allylation was initiated using aldehyde 1a (1 g), placed in a 25 mL Teflon container along with three 7 mm stainless steel (SS) balls. The initial attempt, involving transfer conditions of 30 Hz for 5 minutes, did not achieve full conversion, resulting in 59% conversion of 1a and a 57% yield of the product 2b. Adjusting the procedure to include six cycles of 5-minute milling with 5-minute breaks in between led to complete conversion and a 90% isolation yield (Scheme 3).
Other structurally related reactions, such as propargylation and crotylation, were explored, as depicted in Schemes 4 and 5. Metal allylation can proceed via two distinct reaction pathways: SN2 and SN2′.13 In propargylation reactions, the SN2 pathway predominated, predominantly yielding propargyl alcohols across various aldehydes. The standard aldehyde, 4-phenyl-benzaldehyde, displayed a somewhat slower rate, leading to an incomplete reaction and a reduced yield of 69%. The ratio of propargyl to allenyl alcohol was observed to be 4.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (3a
1 (3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a). Other aldehydes, such as 4-chloro-benzaldehyde and hydrocinnamaldehyde, achieved better efficiency, with yields of 81% (3b + 4b) and 80% (3c + 4c), respectively, and demonstrated similar selectivity (3b
4a). Other aldehydes, such as 4-chloro-benzaldehyde and hydrocinnamaldehyde, achieved better efficiency, with yields of 81% (3b + 4b) and 80% (3c + 4c), respectively, and demonstrated similar selectivity (3b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4b = 4.2
4b = 4.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3c
1, 3c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4c = 4.1
4c = 4.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).10 In contrast, crotylation reactions exclusively yielded SN2′ products, showing high reactivity under similar conditions. However, these reactions did not exhibit meaningful stereoselection between the syn (5) and anti (5′) products, aligning with the results from previous aqueous indium allylation studies.14 In all cases, the products showed a near equimolecular ratio of syn and anti structures.3a
1).10 In contrast, crotylation reactions exclusively yielded SN2′ products, showing high reactivity under similar conditions. However, these reactions did not exhibit meaningful stereoselection between the syn (5) and anti (5′) products, aligning with the results from previous aqueous indium allylation studies.14 In all cases, the products showed a near equimolecular ratio of syn and anti structures.3a
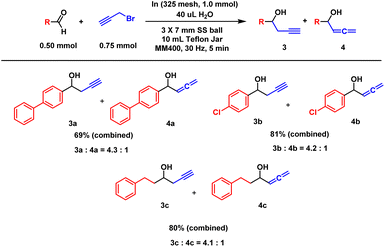 | ||
| Scheme 4 Mechanochemical indium propargylation. aAll reactions were performed twice, and average values are reported. | ||
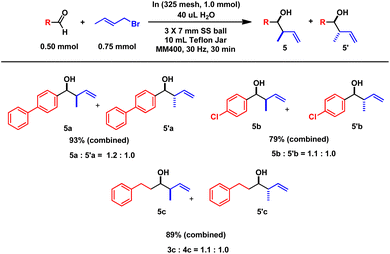 | ||
| Scheme 5 Mechanochemical indium crotylation. aAll reactions were performed twice, and average values are reported. | ||
Conclusions
This communication highlights another achievement in solvent-free mechanochemical reactions: indium-mediated Barbier allylations. Whereas previous aquatic reactions were limited in their scope of aldehydes in the case of hydrophobicity, the newly disclosed solvent-free ball-milling allylation has removed its barrier and enhanced green metrics. The surprising effectiveness of the immiscible water additive not only enhanced indium allylations but also conveyed a significant message: sometimes, a poor solvent can be a beneficial choice of additive in mechanochemistry.Author contributions
Conceptualization, funding acquisition, supervision, and writing: JGK; investigation, data curation, formal analysis, and validation: NK and ESG.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was supported by the National Research Foundation of Korea (NRF-2019R1A2C1087769).References
- (a) C.-J. Li, Tetrahedron, 1996, 52, 5643–5668 CrossRef CAS; (b) T. Hiyama, M. Sawahata and M. Obayashi, Chem. Lett., 1983, 1237–1238 CrossRef CAS; (c) T. Imamoto, T. Kusumoto, Y. Tawarayama, Y. Sugiura, T. Mita, Y. Hatanaka and M. Yokoyama, J. Org. Chem., 1984, 49, 3904–3912 CrossRef CAS; (d) C.-J. Li and W.-C. Zhang, J. Am. Chem. Soc., 1998, 120, 9102–9103 CrossRef CAS; (e) B. Sain, D. Prajapati and J. S. Sandhu, Tetrahedron Lett., 1992, 33, 4795–4798 CrossRef CAS; (f) M. Wada and K. Akina, Tetrahedron Lett., 1985, 26, 4211–4212 CrossRef CAS; (g) J. H. Dam, P. Fristrup and R. Madsen, J. Org. Chem., 2008, 73, 3228–3235 CrossRef CAS PubMed.
- (a) Z.-L. Shen, S.-Y. Wang, Y.-K. Chok, Y.-H. Xu and T.-P. Loh, Chem. Rev., 2013, 113, 271–401 CrossRef CAS PubMed; (b) P. H. Li, Bull. Korean Chem. Soc., 2007, 28, 17–28 CrossRef; (c) S. Araki, H. Ito and Y. Butsugan, J. Org. Chem., 1988, 53, 1831–1833 CrossRef CAS; (d) C.-J. Li and T.-H. Chan, Tetrahedron Lett., 1991, 32, 7018–7020 Search PubMed.
- (a) M. B. Issac and T.-H. Chan, Tetrahedron Lett., 1995, 36, 8957–8960 CrossRef; (b) S. Nakamura, Y. Hara, T. Furukawa and T. Hirashita, RSC Adv., 2017, 7, 15582–15585 RSC; (c) X.-H. Yi, Y. Meng and C.-J. Li, Tetrahedron Lett., 1997, 38, 4731–4734 CrossRef CAS; (d) T.-H. Chan and Y. Yang, J. Am. Chem. Soc., 1999, 121, 3228–3229 CrossRef CAS; (e) T.-P. Loh and X.-R. Li, Eur. J. Org Chem., 1999, 1999, 1893–1899 CrossRef; (f) C.-J. Li and T.-H. Chan, Tetrahedron, 1999, 55, 11149–11176 CrossRef CAS.
- (a) S. J. James and T. Friščić, Chem. Soc. Rev., 2013, 42, 7494–7496 RSC; (b) J.-L. Do and T. Friščić, ACS Cent. Sci., 2017, 3, 13–19 CrossRef CAS PubMed; (c) J. L. Howard, Q. Cao and D. L. Browne, Chem. Sci., 2018, 9, 3080–3094 RSC; (d) T. Friščić, C. Mottillo and H. M. Titi, Angew. Chem., Int. Ed., 2020, 59, 1018–1029 CrossRef PubMed.
- (a) J. Anderson and J. Mack, Green Chem., 2018, 20, 1435–1443 RSC; (b) J. F. Reynes, V. Isoni and F. García, Angew. Chem., Int. Ed., 2023, 62, e202300819 CrossRef CAS PubMed.
- A. C. Jones, J. A. Leitch, S. E. Raby-Buck and D. L. Browne, Nat. Synth., 2022, 1, 763–775 CrossRef.
- J. Yin, R. T. Stark, I. A. Fallis and D. L. Browne, J. Org. Chem., 2020, 85, 2347–2354 CrossRef CAS PubMed.
- S. Wada, N. Hayashi and H. Suzuki, Org. Biomol. Chem., 2003, 1, 2160–2163 RSC.
- K. Smith, S. Lock, G. A. El-Hiti, M. Wada and N. Miyoshi, Org. Biomol. Chem., 2004, 2, 935–938 RSC.
- (a) P. Ying, J. Yu and W. Su, Adv. Synth. Catal., 2021, 363, 1246–1271 CrossRef CAS; (b) J. L. Howard, Y. Saratov, L. Resusseau, C. Schotten and D. L. Browne, Green Chem., 2017, 19, 2798–2802 RSC.
- (a) R. Breslow, Acc. Chem. Res., 1991, 24, 159–164 CrossRef CAS; (b) M. Cortes-Clerget, J. Yu, J. R. A. Kincaid, P. Walde, F. Gallou and B. H. Lipshutz, Chem. Sci., 2021, 12, 4237–4266 RSC.
- V. J. Bryan, PhD Thesis, McGill University, 1999.
- S. Dutta, T. Bhattacharya, D. B. Werz and D. Maiti, Chem, 2021, 7, 555–605 CAS.
- M. B. Issac and T.-H. Chan, J. Chem. Soc., Chem. Commun., 1995, 1003–1004 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4mr00005f |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |