Open Access Article
Open Access ArticleMechanochemical transformation of tetraaryl[3]cumulenes to benzofulvenes via electrophilic iodocyclization†
Fumitoshi
Yagishita
 *ab,
Shoma
Mukai
a,
Sota
Abe
a,
Shoko
Ueta
a,
Yasushi
Yoshida
*ab,
Shoma
Mukai
a,
Sota
Abe
a,
Shoko
Ueta
a,
Yasushi
Yoshida
 c,
Yukihiro
Arakawa
c,
Yukihiro
Arakawa
 a,
Keiji
Minagawa
a and
Yasushi
Imada
a,
Keiji
Minagawa
a and
Yasushi
Imada
 a
a
aDepartment of Applied Chemistry, Tokushima University, 2-1 Minamijosanjima, Tokushima 770-8506, Japan. E-mail: yagishitaf@tokushima-u.ac.jp
bInstitute of Post-LED Photonics, Tokushima University, 2-1 Minamijosanjima, Tokushima 770-8506, Japan
cDepartment of Applied Chemistry and Biotechnology, Graduate School of Engineering, Chiba University, 1-33 Yayoi-cho, Inage-ku, Chiba 263-8522, Japan
First published on 10th June 2024
Abstract
We demonstrate solvent-free mechanochemical iodocyclization of tetraaryl[3]cumulenes using N-iodosuccinimides as the first example of the molecular transformation of cumulenes based on mechanochemistry. This mechanochemical reaction provides the corresponding benzofulvenes in good yields, overcoming the limitation of conventional methods using organic solvents.
Molecular transformation based on mechanochemistry has drawn considerable attention in organic synthesis owing to some advantages such as shorter reaction time and reduction or elimination of solvent use.1 Therefore, many efforts have been devoted to developing mechanochemical synthesis including oxidation,2 reduction,3 condensation,4 metalation,5 and transition metal-catalysed cross-coupling reactions.6 In addition, the mechanochemistry-based synthetic methods overcome the solubility issues in organic synthesis and enable the molecular transformation of insoluble organic compounds under milling conditions.7
Cumulenes, which have cumulative C–C double bonds, are recognized as an important class of sp-hybridized carbons that have unique structural properties and reactivities leading to a wide variety of hydrocarbon structures through dimerization,8 trimerization,9 intermolecular cycloaddition,10 and related cyclization reactions.11 Although the mechanochemical synthesis of odd-numbered tetraaryl[n]cumulenes has been reported,12 the molecular transformation of cumulenes based on mechanochemistry remains unexplored.
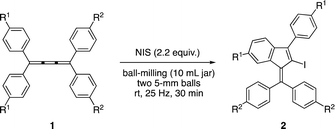
Recently, we reported the iodocyclization reactions of tetraaryl[3]cumulenes affording benzofulvene and tetraaryl[5]cumulene affording fulvene scaffolds in solution, respectively.13 For example, the electrophilic iodocyclization reaction of tetraaryl[3]cumulenes 1 using N-iodosuccinimide (NIS) proceeded smoothly via an iodonium intermediate in nitromethane at ambient temperature and gave the corresponding benzofulvenes 2 in good-to-high yields (Scheme 1a).13a We also reported that the other common organic solvents were found to be ineffective for this reaction. Due to the potential applications of benzofulvene as a versatile building block for functional materials,14 it is important to develop environmentally friendly synthetic methods. Here we demonstrate the solvent-free iodocyclization reaction of tetraaryl[3]cumulenes 1 leading to benzofulvenes 2 under milling conditions (Scheme 1b).
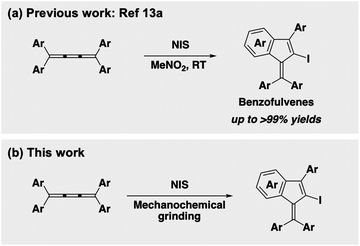 | ||
| Scheme 1 Construction of a benzofulvene scaffold by the iodocyclization reactions of tetraaryl[3]cumulenes. | ||
Initially, we aimed to optimize the reaction conditions for the iodocyclization reaction of tetraaryl[3]cumulenes 1 using tetraphenyl[3]cumulene (1a) as a model substrate. The results are summarized in Table 1.
| Entry | Number of balls | ν (Hz) | Time (min) | X (equiv.) | Yieldb (%) |
|---|---|---|---|---|---|
| a Conditions: 1a (0.3 mmol) and NIS (X equiv.) in a stainless-steel ball-milling jar (10 mL). b The yields were determined by 1H NMR spectroscopy using 1,3,5-trimethoxybenzene as an internal standard. c The values in parentheses are the diameters of balls. d ZrO2 jar and balls were used instead of stainless-steel jar and balls. e 5 mL stainless-steel jar was used instead of 10 mL stainless-steel jar. | |||||
| 1 | 2 (15 mm)c | 25 | 30 | 1.2 | 41 |
| 2 | 2 (15 mm)c | 25 | 30 | 1.2 | 22 |
| 3 | 1 (15 mm)c | 25 | 30 | 1.2 | 19 |
| 4 | 3 (15 mm)c | 25 | 30 | 1.2 | 21 |
| 5 | 4 (15 mm)c | 25 | 30 | 1.2 | 12 |
| 6 | 6 (15 mm)c | 25 | 30 | 1.2 | 14 |
| 7 | 2 (15 mm)c | 25 | 30 | 1.2 | 14 |
| 8 | 2 (15 mm)c | 20 | 30 | 1.2 | 35 |
| 9 | 2 (15 mm)c | 30 | 30 | 1.2 | 37 |
| 10 | 2 (15 mm)c | 25 | 60 | 1.2 | 32 |
| 11 | 2 (15 mm)c | 25 | 90 | 1.2 | 25 |
| 12 | 2 (15 mm)c | 25 | 30 | 2.2 | 71 |
| 13 | 2 (15 mm)c | 25 | 30 | 3.3 | 62 |
| 14d | 2 (15 mm)c | 25 | 30 | 2.2 | 63 |
| 16e | 2 (10 mm)c | 25 | 30 | 2.2 | 53 |
When 0.3 mmol of 1a and 1.2 equiv. of NIS were placed in a 10 mL stainless-steel jar equipped with two stainless-steel balls of 15 mm diameter and agitated at 25 Hz in a vibratory ball-mill for 30 minutes, the objective benzofulvene 2a was obtained in 41% yield (entry 1). The use of iodine instead of NIS as the iodonium cation source decreased the yield of 2a (entry 2). The other combinations of the number of balls and their diameter also resulted in lower yields (entry 1 vs. entries 3–7). The reactions at the milling frequencies of 20 or 30 Hz led to low yields (entries 8 and 9). The longer reaction time slightly decreased the yields of 2a (entries 10 and 11). Notably, increasing the amount of NIS improved the reaction and the benzofulvene 2a was obtained in up to 71% yields (entries 12 and 13). The use of a ZrO2 jar and balls resulted in lower yield than the use of a stainless-steel jar and balls (entry 14). We also conducted the reaction in a 5 mL jar equipped with two stainless-steel balls of 10 mm diameter. However, the use of a 5 mL jar was ineffective for this reaction (entry 15). Because the use of 2.2 equiv. of NIS led to the highest yield, we found that the reaction using 2.2 equiv. of NIS in a 10 mL stainless-steel jar equipped with two stainless-steel balls of 15 mm diameter at a milling frequency of 25 Hz for 30 minutes provides the optimum conditions for this reaction (entry 12).
Next, we investigated the substrate scope using various tetraaryl[3]cumulenes 1 under the optimized reaction conditions (Table 2).
| Entry | Substrate | R1 | R2 | Product | Yieldb (%) |
|---|---|---|---|---|---|
| a Conditions: 1 (0.3 mmol) and NIS (0.66 mmol) in a stainless-steel ball-milling jar (10 mL) equipped with two stainless-steel balls of 15 mm diameter. b Isolated yields. | |||||
| 1 | 1a | H | H | 2a | 69 |
| 2 | 1b | Me | Me | 2b | 91 |
| 3 | 1c | OMe | OMe | 2c | 14 |
| 4 | 1d | F | F | 2d | 0 |
| 5 | 1e | Cl | Cl | 2e | Trace |
| 6 | 1f | OMe | H | 2f | 61 |
| 7 | 1g | OMe | Me | 2g | 90 |
| 8 | 1h | OMe | Cl | 2h | 82 |
The reaction using tetrakis(p-methylphenyl)[3]cumulene (1b) proceeded smoothly under milling conditions; the corresponding benzofulvene 2b was obtained in 91% yield. On the other hand, mechanochemical iodocyclization of 1c gave the benzofulvene 2c in low yield owing to the formation of unidentified oligomer products under milling conditions. Similar unidentified oligomer formation was observed in the absence of NIS under milling conditions. Because the formation of oligomer products was observed after the mechanochemical reaction of 1c even in the absence of NIS, we consider that the oligomerization of 1c preferentially occurs rather than intermolecular reaction under milling conditions. Halogen substituents such as F (1d) and Cl (1e) at aryl rings were ineffective for electrophilic iodocyclization owing to the unfavorable nucleophilic attack of the electron-deficient aryl ring on iodonium intermediates. It was found that the regioselective iodocyclization of unsymmetrical tetraaryl[3]cumulenes 1f–h occurred from the electron-rich aromatic ring to the iodonium cation, affording the corresponding benzofulvenes 2f–h as the sole product in good yield, respectively. The structures of 2f and 2g were determined by 1H NMR according to our previous report.13a The structure of 2h was determined by NMR spectroscopy, HRMS, and single-crystal crystallographic analysis. X-ray analysis of 2h unequivocally revealed that the iodocyclization occurred at the p-methoxyphenyl ring, resulting in the formation of a methoxy-substituted benzofulvene scaffold (Fig. S1†).
Recently, Ito and co-workers have developed a high-temperature ball-milling technique for the palladium-catalysed Suzuki–Miyaura cross-coupling reaction of insoluble aryl halides with aryl boronic acids.7b Their accomplishments encouraged us to demonstrate the iodocyclization reaction of tetraaryl[3]cumulenes under high-temperature milling conditions. In this study, we demonstrated the iodocyclization reactions of 1a and 1e using a temperature-controllable heating gun with a preset temperature of 245 °C under milling conditions. After heating for 30 minutes, the internal temperature reached 144 °C, which was confirmed with an infrared radiation thermometer. Notably, the reaction of 1a proceeded smoothly even in the presence of 1.2 equiv. of NIS and provided the benzofulvene 2a in 90% yield (Scheme 2). Unfortunately, the high-temperature ball-milling technique did not improve the iodocyclization of tetrakis(p-chlorophenyl)[3]cumulene (1e) and the reaction afforded a trace amount of complex mixture.
We previously reported the utility of 1-(diphenylmethylene)-3-phenyl-2-iodo-1H-indene (2a) as a coupling partner in the common transition metal-catalysed cross-coupling reactions in solution, leading to tetrafunctionalized benzofulvenes.13a From the viewpoint of green chemistry, we conducted the mechanochemical Suzuki–Miyaura cross-coupling reaction of 2a with p-methoxyphenylboronic acid under liquid-assisted griding (LAG) conditions. The reaction proceeded smoothly at high temperature conditions and provided the corresponding tetraarylated benzofulvene 3 in 98% yield (Scheme 3).
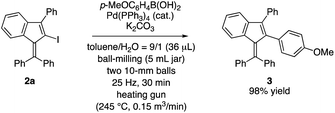 | ||
| Scheme 3 Mechanochemical Suzuki–Miyaura cross-coupling reaction of benzofulvene 2a with p-methoxyphenylboronic acid at high temperature. | ||
Conclusions
In conclusion, we have demonstrated the solvent-free mechanochemical iodocyclization of seven tetraaryl[3]cumulenes. Although the reactions of 1d and 1e having four electron deficient aryl rings did not proceed owing to their low nucleophilicities, the reactions of other tetraaryl[3]cumulenes gave the corresponding benzofulvenes 2 in up to 91% yield even under milling conditions. This environmentally friendly approach enabled the molecular transformation of tetraaryl[3]cumulenes to benzofulvenes without using nitromethane, which has explosive properties. The high-temperature ball-milling technique was also found to promote the iodocyclization reaction of 1a, providing benzofulvene 2a in 90% yield even in the presence of 1.2 equiv. of NIS. In addition, the LAG mechanochemical Suzuki–Miyaura cross-coupling reaction of 2a provided the highly functionalized benzofulvene scaffold in 98% yield.Author contributions
Fumitoshi Yagishita: conceptualization, methodology, writing – original draft; Shoma Mukai: investigation; Sota Abe: investigation; Shoko Ueta: investigation; Yasushi Yoshida: investigation; Yukihiro Arakawa: investigation; Keiji Minagawa: writing – review & editing; Yasushi Imada: supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Notes and references
- G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7668 RSC.
- (a) T. Szuppa, A. Stolle, B. Ondruschka and W. Hopfe, ChemSusChem, 2010, 3, 1181 CrossRef CAS PubMed; (b) O. Y. Posudievsky, O. A. Khazieieva, V. G. Koshechko and V. D. Pokhodenko, J. Mater. Chem., 2012, 22, 12465 RSC; (c) S. Dabral, H. Wotruba, J. G. Hernández and C. Bolm, ACS Sustainable Chem. Eng., 2018, 6, 3242 CrossRef CAS.
- (a) J. Mack, D. Fulmer, S. Stofel and N. Santos, Green Chem., 2007, 9, 1041 RSC; (b) A. Y. Li, A. Segalla, C.-J. Li and A. Moores, ACS Sustainable Chem. Eng., 2017, 5, 11752 CrossRef CAS; (c) Y. Gao, K. Kubota and H. Ito, Angew. Chem., Int. Ed., 2023, 62, e202217723 CrossRef CAS PubMed.
- (a) Z. Zhang, Y.-W. Dong, G.-W. Wang and K. Komatsu, Chem. Lett., 2004, 33, 168 CrossRef CAS; (b) B. Rodríguez, A. Bruckmann and C. Bolm, Chem. - Eur. J., 2007, 13, 4710 CrossRef PubMed; (c) M. Banerjee, A. Chatterjee, V. Kumar, Z. T. Bhutia, D. K. Khandare, M. S. Majik and B. G. Roy, RSC Adv., 2014, 4, 39606 RSC.
- (a) P. A. Julien, K. Užarević, A. D. Katsenis, S. A. J. Kimber, T. Wang, O. K. Farha, Y. Zhang, J. Casaban, L. S. Germann, M. Etter, R. E. Dinnebier, S. L. James, I. Halasz and T. Friščić, J. Am. Chem. Soc., 2016, 138, 2929 CrossRef CAS PubMed; (b) K. Ralphs, C. Zhang and S. L. James, Green Chem., 2017, 19, 102 RSC; (c) N. Pétry, T. Vanderbeeken, A. Malher, Y. Bringer, P. Retailleau, X. Bantreil and F. Lamaty, Chem. Commun., 2019, 55, 9495 RSC; (d) P. Milbeo, F. Quintin, L. Moulat, C. Didierjean, J. Martinez, X. Bantreil, M. Calmés and F. Lamaty, Tetrahedron Lett., 2021, 63, 152706 CrossRef CAS; (e) V. K. Singh, A. Chamberlain-Clay, H. C. Ong, F. León, G. Hum, M. Y. Par, P. Daley-Dee and F. García, ACS Sustainable Chem. Eng., 2021, 9, 1152 CrossRef CAS.
- (a) Q. Cao, J. L. Howard, E. Wheatley and D. L. Browne, Angew. Chem., Int. Ed., 2018, 57, 11339 CrossRef CAS PubMed; (b) K. Kubota, T. Seo, K. Koide, Y. Hasegawa and H. Ito, Nat. Commun., 2019, 10, 111 CrossRef PubMed; (c) T. Seo, T. Ishiyama, K. Kubota and H. Ito, Chem. Sci., 2019, 10, 8202 RSC; (d) C. Zhu, S.-C. Lee, H. Chen, H. Yue and M. Rueping, Angew. Chem., Int. Ed., 2022, 61, e202204212 CrossRef CAS PubMed; (e) T. Seo, K. Kubota and H. Ito, J. Am. Chem. Soc., 2023, 145, 6823 CrossRef CAS PubMed.
- (a) G.-W. Wang, K. Komatsu, Y. Murata and M. Shiro, Nature, 1997, 387, 583 CrossRef CAS; (b) T. Seo, N. Toyoshima, K. Kubota and H. Ito, J. Am. Chem. Soc., 2021, 143, 6165 CrossRef CAS PubMed.
- (a) H. D. Hartzler, J. Am. Chem. Soc., 1966, 88, 3155 CrossRef CAS; (b) F. W. Nader, C.-D. Wacker, H. Irngartinger, U. Huber-Patz, R. Jahn and H. Rodewald, Angew. Chem. Int. Ed. Engl., 1985, 24, 852 CrossRef; (c) M. Iyoda, M. Oda, Y. Kai, N. Kanehisa and N. Kasai, Chem. Lett., 1990, 19, 2149 CrossRef.
- (a) W. R. Dolbier Jr and S.-H. Dai, J. Am. Chem. Soc., 1970, 92, 1774 CrossRef; (b) M. Iyoda, S. Tanaka, H. Otani, M. Nose and M. Oda, J. Am. Chem. Soc., 1988, 110, 8494 CrossRef CAS; (c) N. Islam, T. Ooi, T. Iwasawa, M. Nishiuchi and Y. Kawamura, Chem. Commun., 2009, 574 RSC.
- (a) T. Asakawa, M. Iinuma, T. Furuta, S. Fujii, T. Kan and K. Tanaka, Chem. Lett., 2006, 35, 512 CrossRef CAS; (b) J. A. Januszewski, F. Hampel, C. Neiss, A. Görling and R. R. Tykwinski, Angew. Chem., Int. Ed., 2014, 53, 3743 CrossRef CAS PubMed; (c) P. Gawel, C. Dengiz, A. D. Finke, N. Trapp, C. Boudon, J.-P. Gisselbrecht and F. Diederich, Angew. Chem., Int. Ed., 2014, 53, 4341 CrossRef CAS PubMed.
- (a) B. Alcaide, P. Almendros, S. Cembllín, T. Fernández and C. Martínez del Campo, Chem. - Eur. J., 2016, 22, 11667 CrossRef CAS PubMed; (b) A. Konishi, S. Satake and M. Yasuda, Chem. Lett., 2020, 49, 589 CrossRef CAS; (c) M. A. Johnson, J. A. Meckes, M. U. Bühringer, Z. Zhou, Y. Kuwatani, M. J. Ferguson, Z. Wei, M. Iyoda, M. A. Petrukhina and R. R. Tykwinski, J. Phys. Org. Chem., 2023, 36, e4454 CrossRef CAS; (d) A. Konishi, S. Imai, S. Satake, K. Chiba and M. Yasuda, Chem. - Eur. J., 2023, 29, e202301255 CrossRef CAS PubMed.
- K. Ardila-Fierro, C. Bolm and J. G. Hernández, Angew. Chem., Int. Ed., 2019, 58, 12945 CrossRef CAS PubMed.
- (a) F. Yagishita, K. Hoshi, Y. Yoshida, S. Ueta, K. Minagawa, Y. Imada and K. Kawamura, Eur. J. Org Chem., 2021, 235 CrossRef CAS; (b) K. Hoshi, M. Yasuda, T. Nakamura, Y. Yoshida, S. Ueta, K. Minagawa, Y. Kawamura, Y. Imada and F. Yagishita, Org. Biomol. Chem., 2021, 19, 7594 RSC.
- (a) T. Nakano, K. Takewaki, T. Yade and Y. Okamoto, J. Am. Chem. Soc., 2001, 123, 9182 CrossRef CAS PubMed; (b) S. Chen, Q. Li, S. Sun, Y. Ding and A. Hu, Macromolecules, 2017, 50, 534 CrossRef CAS; (c) J. Terasawa, Y. Shibata, Y. Kimura and K. Tanaka, Chem. - Asian J., 2018, 13, 505 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures and NMR spectra. CCDC 2340176. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4mr00022f |
| This journal is © The Royal Society of Chemistry 2024 |