Donor–acceptor type triphenylamine-based porous aromatic frameworks (TPA-PAFs) for photosynthesis of benzimidazoles †
Xinmeng
Xu
a,
He
Wang
a,
Zhenwei
Zhang
b,
Jiali
Li
b,
Xiaoming
Liu
 b,
Xin
Tao
b,
Xin
Tao
 *a and
Guangshan
Zhu
a
*a and
Guangshan
Zhu
a
aKey Laboratory of Polyoxometalate and Reticular Material Chemistry of Ministry of Education, College of Chemistry, Northeast Normal University, Changchun 130024, P. R. China. E-mail: taox091@nenu.edu.cn
bCollege of Chemistry, Jilin University, Changchun 130012, P. R. China
First published on 8th May 2024
Abstract
The development of efficient and recyclable photocatalysts for organic synthesis is of great interest. This study presents the synthesis of triphenylamine-based porous aromatic frameworks (TPA-PAFs) in an alternating donor–acceptor (D–A) manner. The light absorption range and the optical band gaps of TPA-PAFs are effectively tuned by changing the electron acceptor units, which further determine their photocatalytic properties. As a result, TPA-PAFs exhibit excellent catalytic performance for the photosynthesis of benzimidazoles in high yields (up to 99%), broad substrate scope (18 examples), and good recyclability (up to 10 cycles). This work provides a feasible approach toward the facile design and synthesis of efficient and stable PAF-based photocatalysts, which further broadens the application of PAFs catalytic materials in photocatalytic organic synthesis.
Introduction
Visible-light-driven photocatalytic reactions have received widespread attention for their advantages of high efficiency, ease of operation, and mild reaction conditions.1 Photocatalysts are indispensable to realize the conversion of solar energy into chemicals. Most organic molecules are unable to effectively absorb light at visible wavelengths, while suitable photocatalysts can transport electrons and provide active sites thus effectively achieving catalytic transformations.2 Recently, various classes of photocatalysts have been developed, including semiconductor materials (g-C3N4,3 TiO2,4,5etc.), organometallic complexes (Ru(bpy)3Cl2, bpy = bipyridine,6 Ir(ppy)3, ppy = 2-phenylpyridine,7etc.), molecular organic dyes (fluorescein,8etc) and polymetallic oxides.9 However, most of these photocatalysts suffer from inherent problems such as poor stability, low recyclability and reusability, and high cost, resulting in limitations for practical applications. Therefore, the development of low-cost, catalytically efficient, easily separable, and metal-free heterogeneous catalysts is a feasible way to solve this problem.Porous aromatic frameworks (PAFs) with highly stable skeleton structures, high specific surface areas, easy modification and functionalization have emerged as a promising class of porous organic materials in recent years.10 Recently, PAFs have been extensively applied in various fields such as gas adsorption and separation,11–13 catalysis,14 and trace ion extraction.15,16 In addition, the higher surface area, extended π-conjugated backbone, and lamellar stacking structure of PAFs provide optimal channels for carrier migration and charge separation.17 Meanwhile, the ability to tune the photoenergy band gap, redox potential, and light absorption range through careful framework design makes PAFs an ideal platform for visible-light-driven catalytic organic transformations.18,19 Benzimidazoles and their derivatives have attracted considerable attentions due to their biological activities such as antifungal, anticancer, antiviral, analgesic, anti-tuberculosis and anti-arrhythmic.20–22 Moreover, benzimidazole compounds can be used as engineering materials and are widely used in flame retardant materials,23 solar cells,24 corrosion inhibitors25 and other industries. In view of their immense application prospects, the synthesis of benzimidazoles and their derivatives is of great importance. Currently, there are several synthetic methods that require harsh reaction conditions such as strong acids and high temperatures relying on condensation reactions between o-phenylenediamines and carboxylic acid derived compounds, most of which are not conducive to large-scale production and environmental protection. In contrast, the synthesis of benzimidazoles and their derivatives achieved under visible-light-driven conditions is a green and easy-to-operate synthetic strategy.26 For example, the Pan group constructed two porous HCPs with the s-tetrazine unit, and the suitable energy band structure and pore size enabled efficient photocatalytic synthesis of benzimidazole in ethanol in 99% yield.27 The Huo group designed and synthesized a novel photocatalyst (BTT-TPA-COF) by a strategy that efficiently regulates the energy band structure and photoelectric properties and achieved the synthesis of benzimidazole in 97% yield.28 In addition, the Liu group synthesized three CMPs based on benzotrithiophene units and tuned the linking units to precisely regulate the “bandgap engineering”, which realized the efficient photocatalytic synthesis of benzimidazole and its derivatives under an oxygen atmosphere.29 It is well known that the construction of alternating donor (D)–acceptor (A) type conjugated systems is a very effective method to enhance the separation of photogenerated carriers. By adjusting the structures and ratios of different types of D and A fragments, precise control of the energy band structure and photoelectric properties of D–A type materials can be achieved, which may realize the tailored photocatalysts to meet the requirements for specific catalytic reactions. Therefore, the regulation of energy levels, band gaps and photoelectric properties in PAFs by changing the D–A structure can effectively improve the photocatalytic efficiency.30,31
Triphenylamine (TPA) serves as an electron-rich photocatalytic active unit, possessing unique photophysical properties and excellent hole transport. It finds wide applications as a donor in the field of photoelectric chemistry.32,33 In addition, anthraquinone (AQ) exhibits strong electron-deficient characteristics and unique redox activity,34 making it suitable as an acceptor in D–A type PAFs. Constructing triphenylamine-based PAFs in combination with different acceptors as linkers and studying their structure–property correlations would be significant for the design and synthesis of organo-polymeric photocatalysts. Taking these factors into account, two D–A type PAFs containing triphenylamine structural units were synthesized as metal-free heterogeneous photocatalysts (PAF-380 and PAF-381) in this study, and the modulation of the bandgap and energy levels was achieved by varying the connected acceptor units. The incorporation of the D–A system effectively enhances the charge separation, broadens the light absorption range, and reduces the energy bandgap. Under the irradiation of 460 nm blue light, PAF-380 showed excellent catalytic activity for the synthesis of benzimidazoles with good substrate compatibility. Importantly, the structure remains unchanged after ten catalytic cycles with a slight loss of catalytic activity. The energy band analysis and photoelectrochemical experiments reveal the mechanism behind the photocatalytic reaction.
Results and discussion
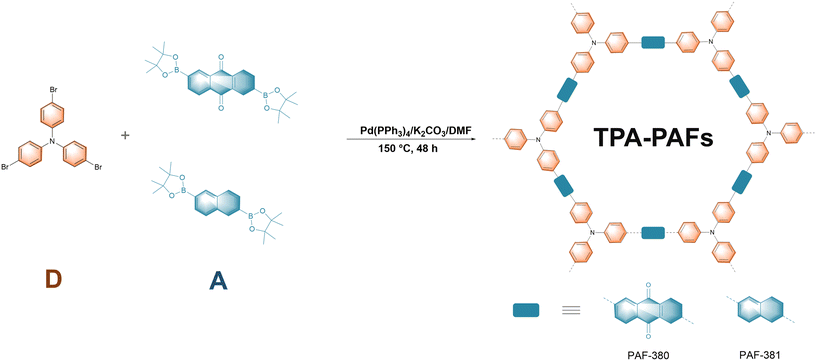
As shown in Scheme 1, TPA-PAFs with different electron-acceptor linkers are synthesized in high yields (95% and 91%) via palladium-catalysed Suzuki–Miyaura cross-coupling reaction of 2,6-bis(pinacolatoboryl)anthraquinone or 2,6-bis(pinacolatoboryl)naphthalene with tris(4-bromophenyl)amine named as PAF-380 and PAF-381, respectively. The obtained TPA-PAFs are fluffy solid powders that are insoluble in common organic solvents and are stable in dilute acid–base solutions. The TPA-PAFs are characterized by Fourier transform infrared (FT-IR) spectroscopy. The FT-IR spectra of the synthesized TPA-PAFs in comparison with the corresponding monomers are depicted in Fig. 1a and Fig. S1.† The stretching vibration of the aliphatic C–H bonds (2973 cm−1 and 2992 cm−1) of the boronic ester groups in the monomers almost disappeared in PAF-380 and PAF-381, which indicate that the polymerization reactions are successful (Fig. 1a and Fig. S1†). Meanwhile, a typical signal of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in the anthraquinone moiety is observed at 1674 cm−1 in PAF-380. The peak at 1596 cm−1 in the PAF-381 could be attributed to the stretching vibration of the aryl ring skeleton. These results indicate that the anthraquinone and naphthalene structural units are successfully introduced into the conjugated skeleton of PAF-380 and PAF-381. The characteristic signals observed for PAF-380 are consistent with PTPA-AQ reported by Samanta et al.35 The solid-state 13C cross-polarized magic angle spinning (CP/MAS) nuclear magnetic resonance shows the signals at 146 ppm for aromatic carbon attached to the nitrogen atom in both TPA-PAFs (Fig. 1b). The signal at 182 ppm in PAF-380 could be assigned to the carbonyl group in the anthraquinone unit. The aromatic carbon atoms of PAF-381 are located at 113–142 ppm (Fig. S2†). These results suggest that the TPA-PAFs are successfully synthesized. The elemental composition and chemical bonding of the synthesized TPA-PAFs are also characterized by X-ray photoelectron spectroscopy (XPS). In the C 1s XPS spectrum of PAF-380, four peaks with binding energies of 284.85, 284.11, and 285.42 eV are attributed to C–N, C
O group in the anthraquinone moiety is observed at 1674 cm−1 in PAF-380. The peak at 1596 cm−1 in the PAF-381 could be attributed to the stretching vibration of the aryl ring skeleton. These results indicate that the anthraquinone and naphthalene structural units are successfully introduced into the conjugated skeleton of PAF-380 and PAF-381. The characteristic signals observed for PAF-380 are consistent with PTPA-AQ reported by Samanta et al.35 The solid-state 13C cross-polarized magic angle spinning (CP/MAS) nuclear magnetic resonance shows the signals at 146 ppm for aromatic carbon attached to the nitrogen atom in both TPA-PAFs (Fig. 1b). The signal at 182 ppm in PAF-380 could be assigned to the carbonyl group in the anthraquinone unit. The aromatic carbon atoms of PAF-381 are located at 113–142 ppm (Fig. S2†). These results suggest that the TPA-PAFs are successfully synthesized. The elemental composition and chemical bonding of the synthesized TPA-PAFs are also characterized by X-ray photoelectron spectroscopy (XPS). In the C 1s XPS spectrum of PAF-380, four peaks with binding energies of 284.85, 284.11, and 285.42 eV are attributed to C–N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and C–C, respectively. In addition, the characteristic signal of C
C, and C–C, respectively. In addition, the characteristic signal of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 286.42 eV (Fig. 1c) is observed as well. The single peak at 399.92 eV in N 1s XPS spectra of PAF-380 indicates that all nitrogen atoms exist in the form of C–N bond (Fig. S3b†). The characteristic signal of C
O at 286.42 eV (Fig. 1c) is observed as well. The single peak at 399.92 eV in N 1s XPS spectra of PAF-380 indicates that all nitrogen atoms exist in the form of C–N bond (Fig. S3b†). The characteristic signal of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 531.34 eV and a peak at 532.68 eV also appeared in O 1s XPS spectrum of PAF-380 (Fig. S3c†), which may be caused by the adsorption of oxygen and water from the surface of PAF-380.36,37 The nitrogen adsorption isotherms of the two TPA-PAFs are measured at 77 K (0–1 bar) (Fig. 1d). The Brunauer–Emmett–Teller (BET) surface area of PAF-380 and PAF-381 are 356 m2 g−1, and 189 m2 g−1, respectively. The PAF-380 pore size distribution calculated using the nonlocal density-functional theory (NL-DFT) model is at 1.9 nm and 4.8 nm, and the PAF-381 pore size distribution is mainly concentrated at 1.9 nm. In contrast, PAF-380 with a higher surface area and abundant mesopores could provide suitable transport channels for the reactants and the products in the photocatalytic process. The powder X-ray diffraction (PXRD) curves indicate that PAF-380 and PAF-381 are amorphous (Fig. S5†). Thermogravimetric analysis (TGA) under a nitrogen atmosphere shows less than 5% mass loss before 300 °C for TPA-PAFs (Fig. S6†). The skeleton starts to collapse with increasing temperature; both TPA-PAFs still maintain 80% of their mass at 670 °C, indicating their excellent thermal stability. The scanning electron microscopy (SEM) images showed a micrometre-scale morphological structure for TPA-PAFs. PAF-380 shows an irregular morphology and PAF-381 shows an aggregated spherical particle morphology (Fig. 2a and b). The high-resolution transmission electron microscopy (TEM) images show that PAF-380 does not have a long-range ordered crystalline structure, which is consistent with the PXRD results (Fig. 2c). The elemental mapping of energy dispersive X-ray spectra collected in the TEM analyses clearly showed that C, N and O elements are uniformly distributed on the skeleton of PAF-380 (Fig. 2d–f).
O at 531.34 eV and a peak at 532.68 eV also appeared in O 1s XPS spectrum of PAF-380 (Fig. S3c†), which may be caused by the adsorption of oxygen and water from the surface of PAF-380.36,37 The nitrogen adsorption isotherms of the two TPA-PAFs are measured at 77 K (0–1 bar) (Fig. 1d). The Brunauer–Emmett–Teller (BET) surface area of PAF-380 and PAF-381 are 356 m2 g−1, and 189 m2 g−1, respectively. The PAF-380 pore size distribution calculated using the nonlocal density-functional theory (NL-DFT) model is at 1.9 nm and 4.8 nm, and the PAF-381 pore size distribution is mainly concentrated at 1.9 nm. In contrast, PAF-380 with a higher surface area and abundant mesopores could provide suitable transport channels for the reactants and the products in the photocatalytic process. The powder X-ray diffraction (PXRD) curves indicate that PAF-380 and PAF-381 are amorphous (Fig. S5†). Thermogravimetric analysis (TGA) under a nitrogen atmosphere shows less than 5% mass loss before 300 °C for TPA-PAFs (Fig. S6†). The skeleton starts to collapse with increasing temperature; both TPA-PAFs still maintain 80% of their mass at 670 °C, indicating their excellent thermal stability. The scanning electron microscopy (SEM) images showed a micrometre-scale morphological structure for TPA-PAFs. PAF-380 shows an irregular morphology and PAF-381 shows an aggregated spherical particle morphology (Fig. 2a and b). The high-resolution transmission electron microscopy (TEM) images show that PAF-380 does not have a long-range ordered crystalline structure, which is consistent with the PXRD results (Fig. 2c). The elemental mapping of energy dispersive X-ray spectra collected in the TEM analyses clearly showed that C, N and O elements are uniformly distributed on the skeleton of PAF-380 (Fig. 2d–f).
 | ||
| Fig. 2 SEM images of PAF-380 (a) and PAF-381 (b). (c) TEM image of PAF-380. TEM elemental mapping images of C (d), O (e), and N (f) for PAF-380. | ||
The light absorption range of TPA-PAFs is accurately detected by UV-visible diffuse reflectance spectroscopy (Fig. 3a). A wide range of light absorption by PAF-380, up to about 600 nm, with high intensity in both the UV and visible regions is observed. In contrast, the maximum absorption range of PAF-381 is up to about 450 nm. This also corresponds to the colour difference between PAF-380 (dark red) and PAF-381 (yellow green). The optical band gaps (Eg) of the TPA-PAFs calculated based on Tauc plots are 1.94 eV and 2.72 eV, respectively (Fig. 3b). To evaluate the energy levels of the TPA-PAFs, electrochemical Mott–Schottky analysis is performed (Fig. 3c), which reveals that the conduction band (CB) edges of PAF-380 and PAF-381 are −0.93 V and −1.17 V, respectively. The valence band (VB) values of PAF-380 and PAF-381 were calculated to be 1.01 eV, and 1.56 eV by combining the optical bandgap and CB values (Fig. 3d). These results indicate that connecting different acceptor units can effectively modulate the energy band structure of TPA-PAFs. It is well known that the separation efficiency and transport rate of photogenerated electrons and holes are crucial for photocatalytic efficiency. Therefore, we further investigate the photoelectric properties of TPA-PAFs by transient photocurrent response testing and electrochemical impedance spectroscopy (EIS) (Fig. 3e and f). Under visible light irradiation, both TPA-PAFs show obvious intermittent on–off irradiation response. PAF-380 exhibits a higher intensity of photocurrent, compared with PAF-381, suggesting that electrons and holes could be separated and transferred rapidly to the surface of the catalyst. In addition, both TPA-PAFs exhibit similar impedances, where PAF-380 has a smaller resistance value than PAF-381. These results imply that the introduction of different acceptors could affect their photogenerated charge separation and transfer efficiency.
The unique features such as excellent stability, porous nature, and a wide range of light absorption of TPA-PAFs make them potential candidates as photocatalysts for the synthesis of organic compounds, such as benzimidazoles and their derivatives. To evaluate the photocatalytic activity of TPA-PAFs, we selected the condensation cyclization reaction between 1,2-phenylenediamine (1a) and benzaldehyde (2a) as a model reaction to optimize the photocatalytic reaction conditions (Table 1). Under the irradiation of a 460 nm blue LED light (30 W), this reaction proceeds well under an air atmosphere using PAF-380 as the photocatalyst in a protic solvent (e.g. ethanol) giving product 3a in 94% yield after 2 h (Table 1, entry 1). Under similar conditions, much lower yields ranging from 5% to 23% in aprotic solvents such as N,N-dimethylformamide (DMF), tetrahydrofuran (THF), toluene, CH3CN, acetone and CH2Cl2 are obtained for the PAF-380 catalysed photosynthesis of compound 3a (Table 1, entries 2–7). This is probably because ethanol may affect the production of photo-induced electrons and holes, which could further facilitate the electron transfer process and stabilize the reaction products.38 In contrast, PAF-381 shows lower photocatalytic activities than PAF-380 for this reaction giving compound 3a in 0–79% yields (Table 1, entries 8–14). This is because the introduction of AQ acceptor to TPA-PAFs could increase its photogenerated charge separation efficiency, which further determines its excellent photocatalytic performance. It is also found that the catalyst dosage is another crucial factor for their photocatalytic performance. It is worth noting that increasing the amount of PAF-380 does not positively affect the catalytic efficiency (Table 1, entries 15–18). The optimal photocatalyst dosage is found to be 2 mg (5.3 mol%). This phenomenon could be attributed to the impeded diffusion caused by the high concentration of the solid catalyst, which results in an unsatisfactory light absorption efficiency.
| Entry | Catalyst | (mg, mol%)b | Solvent | Yieldc (%) |
|---|---|---|---|---|
| a o-Phenylenediamine (0.2 mmol), benzaldehyde (0.2 mmol), solvent (3.0 mL), 460 nm blue LED light (30 W, 0.11 W cm−2), 298 K, 2 h. b The molar ratio of repeating units of the catalysts versus substrates. c Isolated yields. d Reaction time: 3 h. e Under irradiation of 460 nm blue LED light (24 W, 0.08 W cm−2). rt: room temperature. | ||||
| 1 | PAF-380 | 2 mg, 5.3 mol% | Ethanol | 94% |
| 2 | PAF-380 | 2 mg, 5.3 mol% | DMF | 23% |
| 3 | PAF-380 | 2 mg, 5.3 mol% | THF | 5% |
| 4 | PAF-380 | 2 mg, 5.3 mol% | Toluene | 20% |
| 5 | PAF-380 | 2 mg, 5.3 mol% | CH3CN | 22% |
| 6 | PAF-380 | 2 mg, 5.3 mol% | Acetone | 18% |
| 7 | PAF-380 | 2 mg, 5.3 mol% | CH2Cl2 | 15% |
| 8 | PAF-381 | 2 mg, 6.8 mol% | Ethanol | 79% |
| 9 | PAF-381 | 2 mg, 6.8 mol% | DMF | 13% |
| 10 | PAF-381 | 2 mg, 6.8 mol% | THF | Trace |
| 11 | PAF-381 | 2 mg, 6.8 mol% | Toluene | Trace |
| 12 | PAF-381 | 2 mg, 6.8 mol% | CH3CN | 11% |
| 13 | PAF-381 | 2 mg, 6.8 mol% | Acetone | 9% |
| 14 | PAF-381 | 2 mg, 6.8 mol% | CH2Cl2 | 7% |
| 15 | PAF-380 | 1 mg, 2.6 mol% | Ethanol | 87% |
| 16 | PAF-380 | 3 mg, 7.9 mol% | Ethanol | 93% |
| 17 | PAF-380 | 4 mg, 10.6 mol% | Ethanol | 90% |
| 18 | PAF-380 | 5 mg, 13.2 mol% | Ethanol | 89% |
| 19d | PAF-380 | 2 mg, 5.3 mol% | Ethanol | 99% |
| 20e | PAF-380 | 2 mg, 5.3 mol% | Ethanol | 80% |
Furthermore, the reaction yield is increased by extending the reaction time to 3 h under similar reaction conditions, which resulted in 99% yield (Table 1, entry 19). In addition, photocatalytic experiments under the irradiation of a low wattage 460 nm blue LED lamp (24 W, 0.08 W cm−2) only afford 80% yield (Table 1, entry 20). Based on the above experimental results, 2 mg (5.3 mol%) catalyst dosage, 30 W blue LED light as the light source, 3 h reaction time, under an air atmosphere, at room temperature would be the optimal reaction conditions for the PAF-380 catalysed photosynthesis of benzimidazole and its derivatives.
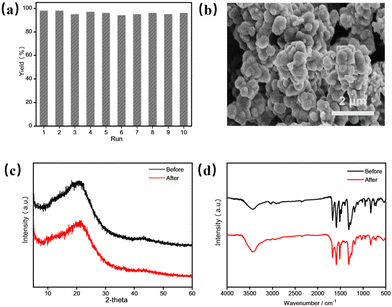
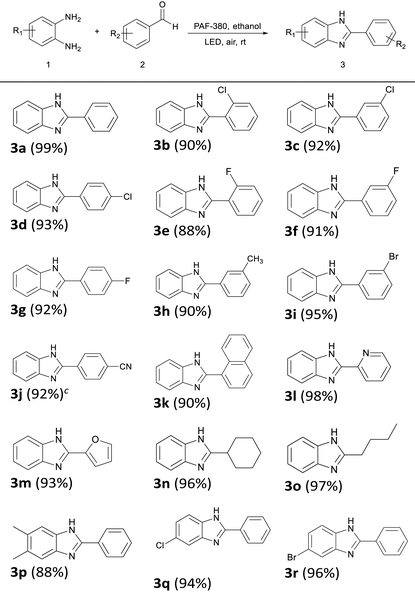
With the optimized reaction conditions in hand, we further explored the compatibility of aldehyde and amine substrates to PAF-380 catalysed photosynthesis of benzimidazole derivatives (Table 2). A series of benzaldehyde derivatives with electron-withdrawing and electron-donating substituents are employed as reaction substrates, which are successfully converted to the corresponding target products in high yields under the optimized reaction conditions using PAF-380 as the photocatalyst (Table 2, 3b–3j). For the photosynthesis of the cyano-substituted product 3j, a longer reaction time (7 h) is required. The yields of the products with electron-withdrawing groups (–F, –Cl, –Br) were found to be higher than those with electron-donating groups (–CH3). When the same substituent attaches to different positions of the phenyl group of the benzaldehyde, higher yield is found for the para-substituted benzaldehydes rather than the ortho- and meta-substitutions, probably due to the steric hindrance effect. Moreover, a high yield of 90% was achieved using 1-naphthaldehyde as the substrate (Table 2, 3k). It is noteworthy that PAF-380 also exhibits excellent catalytic performance for aliphatic aldehydes, with yields exceeding 95% for target products 3n and 3o. In addition, this catalytic system is equally applicable for heteroatom-containing aromatic aldehydes such as furfuraldehyde or picolinaldehyde, which afford corresponding products (Table 2, 3l–3m) in yields up to 98%. Furthermore, we also test the suitability of substituted 1,2-benzenediamine substrates (Table 2, 3p–3r). High yields (94–96%) of substituted benzimidazoles are achieved when 4-chloro or -bromo substituted 1,2-benzenediamine are used as the substrate. A moderate yield (88%) is obtained in the case of 4,5-dimethyl-substituted 1,2-benzenediamine. The above experimental results indicate that PAF-380 shows great substrate compatibility as a photocatalyst for the efficient synthesis of benzimidazole and its derivatives. In parallel, the substrate scope of PAF-381 catalysed photosynthesis of benzimidazole derivatives was also tested (Table S1†), which showed lower yields in comparison with those obtained from the similar conditions under PAF-380 catalysis. Moreover, recyclability is an essential parameter for heterogeneous catalysts. Recycling experiments were conducted to test the recyclability of PAF-380 employing the model reaction of 1,2-benzenediamine with benzaldehyde (Fig. 4a). It is found that PAF-380 can be recycled from the photocatalytic system by simple filtration, washing with ethanol and drying in vacuo, and subsequently reused for the next catalytic cycle. After ten cycles there is no obvious loss of photocatalytic activity observed for the PAF-380 catalyst. The recycled PAF-380 material maintains its original aggregation morphology and its chemical structure, indicating that PAF-380 has excellent stability under the conditions applied in the entire photocatalysis and recycling process (Fig. 4b–d). However, the surface area of the recycled PAF-380 decreased (Fig. S9,† SABET = 121 m2 g−1), probably due to the presence of residual organic molecules in its pores. Finally, we explore the applicability of this photocatalytic system for gram-scale synthesis of benzimidazole using 1,2-phenylenediamine and benzaldehyde as reactants (Scheme S1†). A 94% yield (912 mg) of the target product is isolated, indicating that PAF-380 is indeed an efficient and stable photocatalyst that is potentially applicable for large-scale synthesis of benzimidazole and its derivatives.
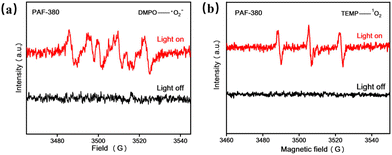
To gain a mechanistic insight into this photocatalytic reaction system, a series of probe experiments were carried out (Table 3). Lower yields of 25% and 14% are obtained in a dark environment or without the addition of the photocatalyst, respectively (Table 3, entries 2 and 3), indicating that blue light and PAF-380 photocatalyst are both necessary for this catalytic system. There was no production of the target product under a N2 atmosphere (Table 3, entry 4), demonstrating that oxygen may be essential for the photocatalytic reaction. The reaction yield is obviously inhibited by the addition of CuCl2 and KI as e− scavenger and h+ scavenger, respectively (Table 3, entries 5 and 6), verifying that electrons and holes are both involved in this photocatalytic reaction process. Furthermore, benzoquinone (BQ) was added to the reaction system as a scavenger of O2˙− to verify the reactive oxygen species (Table 3, entry 7), and a slight decrease in yield (72%) is observed. However, an apparent low yield of 15% was obtained when NaN3 was added to capture 1O2 (Table 3, entry 8), indicating that 1O2 plays a dominant role in this photocatalytic reaction system. In addition, 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) and 2,2,6,6-tetramethylpiperidine (TEMP) are used as spin trapping agents in the electron spin resonance (ESR) experiments to detect the existence of O2˙− and 1O2 in PAF-380 photocatalytic system, respectively. As shown in Fig. 5, a typical DMPO-O2˙− signal pattern and a typical TEMPO-1O2 signal pattern are both observed, which confirms that the two reactive oxygen species O2˙− and 1O2 are both produced in the PAF-380 photocatalytic synthesis of benzimidazole. It should be noted that a small amount of 2-methylbenzimidazole was detected in the catalytic reaction mixture by gas chromatography-mass spectrometry (GC-MS) (Fig. S10†). This indicates that 2-methylbenzimidazole was formed by the reaction of o-phenylenediamine with a small amount of acetaldehyde generated from the oxidation of ethanol.
| Entry | Scavenger | Time (h) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: o-phenylenediamine (0.2 mmol), benzaldehyde (0.2 mmol), PAF-380 (2 mg, 5.3 mol%), ethanol (3.0 mL), 460 nm blue LED light with 30 W (0.11 W cm−2). b Isolated yields. c In darkness. d Without catalyst. e Under N2 atmosphere. rt: room temperature. | |||
| 1 | — | 2 | 94% |
| 2c | — | 2 | 25% |
| 3d | — | 2 | 14% |
| 4e | — | 2 | Trace |
| 5 | CuCl2 | 2 | 34% |
| 6 | KI | 2 | 28% |
| 7 | BQ | 2 | 72% |
| 8 | NaN3 | 2 | 15% |
Based on the above experimental results and the conclusions reported in related literature,27 a possible reaction mechanism for the photosynthesis of benzimidazoles catalysed by PAF-380 is proposed (Fig. 6). Initially, a condensation reaction between 1,2-benzenediamine and benzaldehyde could occur rapidly to generate 2-(benzylideneamino)aniline. Under the irradiation of blue light, the photogenerated h+ could oxidize the in situ generated 2-(benzylideneamino)aniline to give a nitrogen centered radical cation intermediate I, which could be deprotonated to afford a neutral nitrogen centered radical intermediate II. Reduction by photogenerated e− and ring-closure gives an anionic intermediate III that could be easily protonated to generate intermediate IV. Oxidation by reactive oxygen species e.g. O2˙− and 1O2, and elimination of H2O2 (Fig. S11†) finally give target product 3a.
 | ||
| Fig. 6 Proposed mechanism for the photosynthesis of benzimidazole in the presence of PAF-380 under visible-light irradiation. | ||
Conclusions
In conclusion, two triphenylamine-based D–A type TPA-PAFs have been synthesized from the Suzuki–Miyaura cross-coupling reaction. The surface area, light absorption range and optical band gap were tuned by changing the acceptor units, leading to the enhancement of photocatalytic activity. Compared to PAF-381, the stronger push–pull effect between the electron-donating triphenylamine and more electron-accepting anthraquinone groups in PAF-380 favours the separation of the charge carriers, thus exhibiting excellent catalytic activity for the photosynthesis of benzimidazoles. Moreover, PAF-380 exhibited excellent recyclability, stability, and substrate compatibility. Overall, this work provides a feasible strategy for constructing PAF-based catalytic materials, which further broadens the application of PAF materials in photocatalysis.Author contributions
Xinmeng Xu and He Wang: conceptualization, methodology, formal analysis, investigation, resources, data curation, writing – original draft, and writing – reviewing and editing; Zhenwei Zhang and Jiali Li: formal analysis, investigation, resources, and data curation; Xiaoming Liu and Guangshan Zhu: visualization, project administration, and supervision; Xin Tao: validation, resources, writing – original draft, writing – reviewing and editing, visualization, project administration, supervision, and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Natural Science Foundation of China (Grant No. 52173195) is gratefully acknowledged.References
- K. Luo, Y. Chen, W. Yang, J. Zhu and L. Wu, Org. Lett., 2016, 18, 452–455 CrossRef CAS PubMed.
- Y. Zhi, S. Ma, H. Xia, Y. Zhang, Z. Shi, Y. Mu and X. Liu, Appl. Catal., B, 2019, 244, 36–44 CrossRef CAS.
- J. Jiang, Z. Xiong, H. Wang, G. Liao, S. Bai, J. Zou, P. Wu, P. Zhang and X. Li, J. Mater. Sci. Technol., 2022, 118, 15–24 CrossRef CAS.
- Q. Guo, Z. Ma, C. Zhou, Z. Ren and X. Yang, Chem. Rev., 2019, 119, 11020–11041 CrossRef CAS PubMed.
- F. Kayaci, S. Vempati, C. O. Akgun, I. Donmez, N. Biyikli and T. Uyar, Nanoscale, 2014, 6, 5735–5745 RSC.
- L. Wang, H. Zhang, Z. Zhang, J. Zhang, Y. He, Q. Li, J. Bao, M. Fang and Y. Wu, Chem. – Asian J., 2023, 18, e202300297 CrossRef CAS PubMed.
- A. K. Clarke, A. P. Parkin, R. J. K. Taylor, W. P. Unsworth and J. A. Rossi-Ashton, ACS Catal., 2020, 10, 5814–5820 CrossRef CAS PubMed.
- Z. Li, H. Song, R. Guo, M. Zuo, C. Hou, S. Sun, X. He, Z. Sun and W. Chu, Green Chem., 2019, 21, 3602–3605 RSC.
- S. Zhao and X. Zhao, J. Catal., 2018, 366, 98–106 CrossRef CAS.
- Y. Tian and G. Zhu, Chem. Rev., 2020, 120, 8934–8986 CrossRef CAS PubMed.
- P. Zhang, X. Zou, J. Song, Y. Tian, Y. Zhu, G. Yu, Y. Yuan and G. Zhu, Adv. Mater., 2020, 32, 1907449 CrossRef PubMed.
- S. Zhang, J. Li, J. Liu, S. Jiang, X. Chen, H. Ren, T. X. Liu, X. Zou and G. Zhu, J. Membr. Sci., 2021, 632, 119372 CrossRef CAS.
- W. Wang, Y. Yuan, F. Sun and G. Zhu, Chin. Chem. Lett., 2014, 25, 1407–1410 CrossRef CAS.
- Y. Yang, Y. Yang, T. Wang, Y. Tian, X. Jing and G. Zhu, Microporous Mesoporous Mater., 2020, 306, 110393 CrossRef CAS.
- B. Li, Y. Zhang, D. Ma, Z. Shi and S. Ma, Nat. Commun., 2014, 5, 5537 CrossRef CAS PubMed.
- B. Aguila, Q. Sun, H. Cassady, C. W. Abney, B. Li and S. Ma, ACS Appl. Mater. Interfaces, 2019, 11, 30919–30926 CrossRef CAS PubMed.
- J. Jiang, R. Luo, X. Zhou, Y. Chen and H. Ji, Adv. Synth. Catal., 2018, 360, 4402–4411 CrossRef CAS.
- N. Huber and K. A. I. Zhang, Eur. Polym. J., 2020, 140, 110060 CrossRef CAS.
- J. Wang, P. Zhang, C. Wang, X. Zheng, Y. Zhao, L. Li and S. Miao, Mater. Des., 2020, 186, 108371–108377 CrossRef CAS.
- S. K. Bagaria, N. Jangir and D. K. Jangid, Sustainable Chem. Pharm., 2023, 31, 100932 CrossRef CAS.
- K. Chakrabarti, M. Maji and S. Kundu, Green Chem., 2019, 21, 1999–2004 RSC.
- K. Tateyama, K. Wada, H. Miura, S. Hosokawa, R. Abe and M. Inoue, Catal. Sci. Technol., 2016, 6, 1677–1684 RSC.
- T. Liu, Y. Wang, J. Yu and Z. Hu, Composites, Part A, 2022, 163, 107253 CrossRef CAS.
- J. Wang, J. Zhu, C. Li, Y. Lin, Y. Yang, Z. Ma and Y. Lu, Adv. Funct. Mater., 2023, 33, 2304449 CrossRef CAS.
- J. A. Calderón, F. A. Vásquez and J. A. Carreño, Mater. Chem. Phys., 2017, 185, 218–226 CrossRef.
- Z. Li, H. Song, R. Guo, M. Zuo, C. Hou, S. Sun, X. He, Z. Sun and W. Chu, Green Chem., 2019, 21, 3602–3605 RSC.
- W.-K. An, S.-J. Zheng, H.-Z. Zhang, T.-T. Shang, H.-R. Wang, X.-J. Xu, Q. Jin, Y. Qin, Y. Ren, S. Jiang, C.-L. Xu, M.-S. Hou and Z. Pan, Green Chem., 2021, 23, 1292–1299 RSC.
- B. Luo, Y. Chen, Y. Zhang and J. Huo, J. Catal., 2021, 402, 52–60 CrossRef CAS.
- S. Han, Z. Li, Y. Zhi, H. Xia, X. Chen and X. Liu, J. Mater. Chem. A, 2021, 9, 3333–3340 RSC.
- S. Ghosh, D. Sarkar, S. Bastia and Y. S. Chaudhary, Nanoscale, 2023, 15, 10939–10974 RSC.
- L. Cao, C. Wang, H. Wang, X. Xu, X. Tao, H. Tan and G. Zhu, Angew. Chem., Int. Ed., 2024, e202402095 CAS.
- W. Huang, T. Jia, G. Zhou, S. Chen, Q. Hou, S. Luo, G. Shi and B. Xu, Electrochim. Acta, 2018, 283, 1284–1290 CrossRef CAS.
- Y. Wang, G. Ji, W. Ye, F. Zhang, Y. Wang, Y. Zhao and Z. Liu, ACS Sustainable Chem. Eng., 2022, 10, 9460–9468 CrossRef CAS.
- F. Yu, Z. Zhu, C. Li, W. Li, R. Liang, S. Yu, Z. Xu, F. Song, Q. Ren and Z. Zhang, Appl. Catal., B, 2022, 314, 121467 CrossRef CAS.
- S. Sau and S. K. Samanta, Chem. Commun., 2023, 59, 635–638 RSC.
- C. Dong, J. Chu, L. Cao, F. Cui, S. Liang, X. Zhang, X. Tao, H. Wang and G. Zhu, CCS Chem., 2023, 5, 607–615 CrossRef CAS.
- X. Dong, Y. Wang, F. Huang and X. Lang, Chem. Eng. J., 2023, 469, 143934 CrossRef CAS.
- C. Wang and D. Astruc, Chem. Soc. Rev., 2014, 43, 7188–7216 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr00779d |
| This journal is © The Royal Society of Chemistry 2024 |