Rebamipide nanocrystal with improved physicomechanical properties and its assessment through bio-mimicking 3D intestinal permeability model†
Md
Samim Sardar
a,
Kardile Punam
Kashinath
a,
Mamta
Kumari
a,
Sunil Kumar
Sah
a,
Kamare
Alam
b,
Ujjwal
Gupta
a,
Velayutham
Ravichandiran
*c,
Subhadeep
Roy
 *b and
Santanu
Kaity
*b and
Santanu
Kaity
 *a
*a
aDepartment of Pharmaceutics, National Institute of Pharmaceutical Education and Research (NIPER), Kolkata, West Bengal 700054, India. E-mail: skaity13@gmail.com
bDepartment of Pharmacology and Toxicology, National Institute of Pharmaceutical Education and Research, Kolkata, West Bengal 700054, India. E-mail: subhadeeproy.good@gmail.com; subhadeep@niperkolkata.ac.in
cDepartment of Natural Products, National Institute of Pharmaceutical Education and Research, Kolkata, West Bengal 700054, India
First published on 26th September 2024
Abstract
This study investigated the formulation and characterization of rebamipide nanocrystals (REB-NCs) to enhance the solubility and permeability of rebamipide, an anti-ulcer medication known for its low aqueous solubility and permeability, classified as BCS class IV. Employing high-pressure homogenization and wet milling techniques, we successfully achieved nanonization of rebamipide, resulting in stable nanosuspensions that were subsequently freeze-dried to produce REB-NCs with an average particle size of 223 nm. Comprehensive characterization techniques, including Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), X-ray diffraction (XRD), and differential scanning calorimetry (DSC) confirmed the crystalline nature of the nanocrystals and their compatibility with the selected excipients. The saturation solubility study revealed a remarkable three-fold enhancement in PBS pH 7.4 compared to rebamipide API, indicating the effectiveness of the nanocrystal formulation in improving drug solubility. Furthermore, 3D in-vitro permeability assessments conducted on Caco-2 cell monolayers demonstrated an noticeable increase in the permeability of REB-NCs relative to the pure active pharmaceutical ingredient (API), highlighting the promise of this formulation to enhance drug absorption. The dissolution profile of the nanocrystal tablets exhibited immediate release characteristics, significantly outperforming conventional formulations in terms of the dissolution rate. This research underscores the potential of nanomilling as a scalable, environment-friendly, and less toxic approach to significantly enhance the bioavailability of rebamipide. By addressing the challenges associated with the solubility and permeability of poorly water-soluble drugs, our outcome offers insightful information into developing efficient nanomedicine strategies for enhancing therapeutic outcomes.
1. Introduction
The quinolone derivative, [2-(4-chlorobenzoylamino)-3-[2(1H)-quinolinone-4-yl] propanoic acid], rebamipide (REB) is utilized to treat acute gastritis, chronic gastritis, and gastric mucosal lesions.1,2 It is also used in conditions like dry eye.3 The potent antiulcer drug, REB, works primarily by enhancing endogenous prostaglandin synthesis, scavenging free radicals generated from oxygen, and reducing inflammation and neutrophil activity. According to available research, REB is safe and effective against NSAID-induced GI injuries caused by different types of NSAIDs.4 Oral formulations of REB are eagerly awaited as potential solutions for gastrointestinal damage caused by NSAIDs. However, the effectiveness of REB is limited owing to its low solubility and permeability, resulting in a bioavailability of less than 10% in humans. Hence, it is classified as a biopharmaceutical classification system IV (BCS IV). Previous investigations, including solubility tests, have indicated that REB is nearly insoluble in polar and nonpolar solvents.5 Modification of such molecules into nanocrystals could be an effective strategy for the enhancement of solubility and permeability. Nanocrystals are pure drug crystals with diameters within the nanometer range. These crystals are stabilized or enclosed by a thin layer of inactive excipients. Nanocrystals consist only of drugs and do not contain carrier materials.6 In the pharmaceutical sector, nanocrystals are often defined as having sizes ranging from a few nanometers to 1000 nm. Consequently, researchers have turned to innovative formulation techniques, such as producing nanocrystals of ultrafine drug particles stabilized by excipients that retain a high loading capacity.7 Compared to other nanoparticles, such as polymeric nanoparticles, the high drug loading of nanocrystals (NCs) guarantees the efficient transport of drugs to cells or tissues and maintains a powerful therapeutic concentration to provide the desired pharmacological effects.8 Previous studies have explored various strategies for enhancing bioavailability of REB, including the development of nano-based formulations. REB nanocrystal based solid oral formulation significantly improved bioavailability compared to conventional formulations.9 Additionally, REB nanocrystals incorporated into hydrogels enhanced therapeutic effects in a hamster model of oral mucositis.10 However, these studies focused on specific applications and did not extensively investigate the physicochemical properties and stability of the REB nanocrystals. Despite these advancements, the literature lacks a comprehensive exploration of REB nanocrystals, particularly concerning the techniques for their preparation, characterization, and resultant in vitro and in vivo performance. Specifically, while high-pressure homogenization has been successfully employed in nanoparticle fabrication for various compounds, systematic studies dedicated to optimizing these methods for REB remain scarce. Additionally, the way that formulation components affect the stability and bioavailability of REB nanocrystals has not been thoroughly investigated. As such, there is a critical need to bridge these research gaps by enhancing the solubility and permeability of rebamipide through nanocrystal formulations and establishing a clear correlation between physicochemical properties and therapeutic outcomes.Microfluidization or milling was used to prepare the nanocrystal suspensions. The suspension was lyophilized to obtain a dried powder for tablet formulation development after mixing with other suitable excipients. The lyophilized powders were characterized using various techniques. An in vitro study was performed on Caco-2 cells single layers to access the permeability of the prepared REB-NCs. This study not only explores the synergistic effects of these methods on particle size reduction and solubility enhancement but also introduces a novel stabilization strategy using biocompatible surfactants to maintain the integrity of the nanocrystals. Furthermore, the comprehensive evaluation of the permeability profiles of nanocrystals through advanced in vitro models presents a significant advancement over traditional formulations, which often overlooks the impact of particle morphology on drug absorption. Moreover, our work includes a comparative analysis of the developed nanocrystal formulation against marketed formulations, thereby establishing the superiority of our approach in bioavailability and therapeutic outcomes. By addressing the limitations of previous studies and focusing on the specific challenges associated with rebamipide, our study contributes valuable knowledge to the field of nanomedicine. This also provides a feasible approach for improving the clinical outcomes of similar BCS class of therapeutic agents.
2. Materials and methods
2.1 Materials and reagents
The materials utilized in this study include various chemical reagents and laboratory equipment. Rebamipide was sourced from Dr Reddy's Laboratories, Hyderabad, India, having a molecular mass of 370.786 g mol−1 and purity of >99%. Crystal growth inhibitors included D-mannitol (purity: 99%, molecular weight: 182.17 g mol−1, Batch No: 9149161) and lactose monohydrate (purity: 98–99%, molecular weight: 360.31 g mol−1, Batch No: 5546975), both obtained from Sisco Research Laboratories Pvt. Ltd (SRL), Taloja, Maharashtra. Tween 80, a surfactant with a molecular mass of 1310 g mol−1 (Lot No: BCBW3163), was procured from Sigma-Aldrich Unilab, MO, USA. Solvents and buffers included acetonitrile and methanol (both gradient HPLC grade, CAS Nos: 75-05-8 and 67-57-1, respectively, from Advent Chembio), triethylamine (CAS No: 121-44-8), orthophosphoric acid (CAS No: 7664-58-2), sodium dihydrogen phosphate (CAS No: 7558-79-4), ammonium acetate (CAS No: 631-61-8), sodium hydroxide (CAS No: 1310-73-2), HPLC water (CAS No: 7732-18-5), disodium hydrogen phosphate (CAS No: 7558-79-4), from Merck Life Science, acetic acid (CAS No: 64-19-7, from Avera Synthesis), and sodium acetate (CAS No: 127-09-3, from Avera Synthesis). Cell culture media and supplements included DMEM (Catalog No: 11965-092), RPMI 1640 (Catalog No: 1875093), fetal bovine serum (Catalog No: 10082147), Anti–Anti (Catalog No: 15240096), and Geltrex (Catalog No: A1413302), all sourced from Gibco. The dyes and antibodies used were live/dead™ Viability/Cytotoxicity kit (Catalog No. L3224, Invitrogen), goat anti-mouse IgG2a Alexa Fluor™ 594 (Catalog No: S-21135), and Alexa Fluor™ 488 phalloidin (Catalog No. A12379). Other reagents included PBS buffer (Catalog No. 10010023, Gibco), Hank's balanced salt solution (HBSS) (Catalog No: 14025092, Gibco), Dulbecco's phosphate-buffered saline (Catalog No: TCL021, Gibco), HEPES buffer (Catalog No. TCL021, Himedia), and lucifer yellow (Catalog No. L453), dextran (40000) (Catalog No. D1845), and dimethyl sulfoxide (DMSO) (Catalog No: D12345). The laboratory equipment used was Corning Costar 6.5 mm/3 μm/12 inserts/24 healthy plates (Catalog No. 3415). All reagents were used as received with further purification where applicable, and Milli-Q grade water was utilized for all the study.2.2 Methods
2.3 Physicochemical characterization of particles
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 ratio. It was then carefully placed on a glass slide and allowed to dry under normal circumstances to prevent particle movement. Glass slide holds the mica and the sample. The data were shown using topographic and phase imaging techniques. AFM generates the topographic image by measuring the force between the tip and the surface. Meanwhile, phase imaging involves mapping the phase delay between the periodic signal that drives the cantilever and the cantilever's oscillations. Afterwards, the Igor Pro 6.38B01 software was used to flatten all the photos. Identical software was used for both section analysis and particle size determination.19
100 ratio. It was then carefully placed on a glass slide and allowed to dry under normal circumstances to prevent particle movement. Glass slide holds the mica and the sample. The data were shown using topographic and phase imaging techniques. AFM generates the topographic image by measuring the force between the tip and the surface. Meanwhile, phase imaging involves mapping the phase delay between the periodic signal that drives the cantilever and the cantilever's oscillations. Afterwards, the Igor Pro 6.38B01 software was used to flatten all the photos. Identical software was used for both section analysis and particle size determination.19
2.4. In vitro studies
The primary human intestinal cell lines Caco-2, HT-29, and HCT-15 cell lines were procured from the National Center for Cell Science, Pune, India. Caco-2 (passage 18) and HT-29 (passage 12) cells were cultured in DMEM. HCT-15 (passage number 8) were cultured in RPMI media with 10% fetal bovine serum and 1% antibiotic–antimycotic at 37° in a 5% CO2/95% air humidified incubator (Thermo HERA cell, USA).242.5. Intestinal permeability assessment
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per insert, with 3 μm pore size and 0.33 cm2 surface area). The media was changed every three days for 21 days. Caco-2 monolayer integrity was measured by analyzing transepithelial electrical resistance (TEER) and the permeability of zero permeable markers.
000 cells per insert, with 3 μm pore size and 0.33 cm2 surface area). The media was changed every three days for 21 days. Caco-2 monolayer integrity was measured by analyzing transepithelial electrical resistance (TEER) and the permeability of zero permeable markers.
 | (1) |
The percentage of LY passage was determined viaeqn (2):
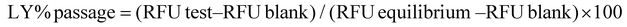 | (2) |
where RFU represents relative fluorescent units.
Similarly, the transport of standard drugs metoprolol (2.99 μM), Acyclovir (2.66 μM), prazosin (10 μM), digoxin (10 μM), and verapamil (10 μM) across the Caco-2 monolayer was evaluated in both the apical to basal and basal to apical direction. The apical and basal compartments were used to collect samples at 15, 30, 60, 90, 120, 150, and 180 minutes. The samples were subsequently substituted with an equivalent volume of the new assay media. The quantity of drug present in the samples was quantified via HPLC.30
The apparent permeability (Papp) of each model was calculated using the eqn (3):31
| Papp = (Vr × dQ/dt) × (1/A × C0) | (3) |
where Vr is the volume of the receiver compartment, dQ/dt is the rate of permeation (in millimoles per second), A is the membrane's surface area (cm2), and C0 is the initial drug concentration (μM). The efflux ratio or transport ratio was determined by calculating the ratio of Papp in the basal to apical direction versus Papp in the apical to basal direction, as shown in the following equation:32
| Efflux ratio (ER) = Papp(B to A)/Papp(A to B). | (4) |
The mobile phase consists of 10 mM phosphate buffer (pH 4.5) and acetonitrile to analyze rebamipide and its formulations. For REB, a gradient mode was used at a 1 mL min−1 flow rate with 10 μL of sample injection through a C18 column. The effluent was detected using an Agilent 1260 variable-wavelength diode-array detector. Rebamipide was measured at 228 °C with a typical retention time of 6.6 min.33 The gradient elution procedure for rebamipide is presented in Table 1.
| Run time (min) | Mobile phase-A: phosphate buffer pH 4.5 (%v/v) | Mobile phase-B: acetonitrile (%v/v) |
|---|---|---|
| 0 | 80 | 20 |
| 1 | 80 | 20 |
| 3 | 70 | 30 |
| 4.8 | 60 | 40 |
| 7 | 80 | 20 |
2.6. Manufacturing of final dosage form
| σ = 2F/πTD | (5) |
The tablet tensile strength (σ) was measured in megapascals (MPa), tablet breaking force (F) was measured in newtons (N), and tablet diameter (D) and thickness (H) were measured in millimeters (mm). The highest compaction pressure was 200 MPa. A PXRD study was conducted to examine the stress-induced polymorphic transitions. The calculated tensile strength data were plotted against compaction forces. In the compressibility study, the porosity of the compacts was measured. Porosity was determined by utilizing the density of the tablet (ρtablet) and the powder's actual density (ρtrue) using eqn (6).35
| Porosity = 1 − (ρtablet/ρtrue) | (6) |
Tablet density and powder actual density were measured, and porosity was calculated from this data. The actual density of powders was measured using a helium pycnometer (AccuPyc II 1340, Micrometric, USA). Then, for the compressibility study, porosity vs. different compaction forces of compacts were plotted. Compactibility was observed by plotting the graph tensile strength of compacts vs. porosity.36,37
3. Results and discussion
3.1 Nanocrystal by high-pressure homogenization
REB and other excipients were dispersed in Milli-Q water and sonicated for 15 min. Process optimization was performed by changing the pressure to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 psi, 15
000 psi, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 psi, and 20
000 psi, and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 psi and changing the number of cycles to 5, 10, 15, and 20 cycles. Each sample was evaluated using particle size analysis (ZS Xplorer software, Malvern Zeta Sizer). From the particle size estimation, it was found that the size of the F5 formulation was the minimum (345 nm). The particle size data for the other batches are presented in Table 2. Each microfluidised suspension was lyophilized (OPTICS Technology, India) for further use. Hence, the process parameters, such as a pressure of 15
000 psi and changing the number of cycles to 5, 10, 15, and 20 cycles. Each sample was evaluated using particle size analysis (ZS Xplorer software, Malvern Zeta Sizer). From the particle size estimation, it was found that the size of the F5 formulation was the minimum (345 nm). The particle size data for the other batches are presented in Table 2. Each microfluidised suspension was lyophilized (OPTICS Technology, India) for further use. Hence, the process parameters, such as a pressure of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 psi and 15 cycles, were considered optimum for high-pressure homogenization.
000 psi and 15 cycles, were considered optimum for high-pressure homogenization.
| Formulation number | Pressure (Psi) | Number of cycles | Particle size (nm) (with lactose) | Particle size (nm) (with mannitol) |
|---|---|---|---|---|
| Pure API particle size: 2920 nm. | ||||
| F1 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
10 | 509.6 | — |
| F2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
15 | 473.5 | — |
| F3 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
20 | 445.5 | — |
| F4 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
10 | 580.6 | — |
| F5 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 000 000
|
15 | 356.9 | — |
| F6 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
20 | 604.6 | — |
| F7 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
10 | 536.9 | — |
| F8 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
15 | 567.4 | — |
| F9 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
20 | 506.4 | — |
| F10 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
10 | — | 458.3 |
| F11 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
15 | — | 471.5 |
| F12 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
20 | — | 519.0 |
| F13 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
10 | — | 575.4 |
| F14 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 000 000
|
15 | — | 359.5 |
| F15 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
20 | — | 467.0 |
| F16 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
10 | — | 487.8 |
| F17 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
15 | — | 508.6 |
| F18 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
20 | — | 494.2 |
Process optimization showed that the optimized processing conditions reduced the particle size to a similar extent for lactose and mannitol (356.9 nm and 359.5 nm, respectively). Furthermore, we varied the amount of lactose and mannitol at optimized pressures and cycles to identify whether the excipient level affects particle size during processing through microfluidization. The observations are presented in Table 3. For both lactose and mannitol, we observed a larger particle size when the amount was increased (7.5 g) or decreased (2.5 g) than the central point (5.0 g).
| Formulation | Rebamipide (g) | Lactose (g) | Mannitol (g) | Tween 80 (1%) | Distilled water (mL) | Particle size (nm) |
|---|---|---|---|---|---|---|
| F19 | 1.0 | 2.5 | — | 1 | 25 | 586.8 |
| F5 | 1.0 | 5.0 | — | 1 | 25 | 356.9 |
| F20 | 1.0 | 7.5 | — | 1 | 25 | 654.7 |
| F21 | 1.0 | — | 2.5 | 1 | 25 | 436.9 |
| F14 | 1.0 | — | 5 | 1 | 25 | 359.5 |
| F22 | 1.0 | — | 7.5 | 1 | 25 | 690.5 |
3.2 Nanocrystal by media milling
Since we could not reduce the particle size beyond a certain level by microfluidization, and for both lactose and mannitol, the reduced particle size was almost identical, we explored another method, media milling by ball mail, in this study. The REB and other excipients were dispersed in Milli-Q water and sonicated for 15 min. Process optimization was performed by changing the milling duration from 30 to 60 and 90 min. The process optimization of each sample was performed by particle size analysis using the method mentioned earlier. Each microfluidised suspension was lyophilized for further use. Here, we observed that formulation B2 and B3 with mannitol resulted in a particle size of 223.5 nm and 222.7 nm, respectively (Table 4). Since the particle size did not differ significantly between 60 and 90 min ball-milled samples, we considered the milling condition of 60 min at a frequency of 30 s−1 as optimized to obtain the lowest possible particle size. However, using the ball-milling technique, we could not achieve significant size reduction in the case of a drug-excipient mixture with lactose monohydrate.| Formulation number | Time (min) | Frequency (per s) | Particle size (nm) (with lactose) | Particle size (nm) (with mannitol) |
|---|---|---|---|---|
| B1 | 30 | 30 | — | 230.1 |
| B2 | 60 | 30 | — | 223.5 |
| B3 | 90 | 30 | — | 222.7 |
| B4 | 30 | 30 | 762.6 | — |
| B5 | 60 | 30 | 762.4 | — |
| B6 | 90 | 30 | 854.6 | — |
Furthermore, composition optimization (Table 5) was performed using varying amounts of mannitol and lactose. In the case of lactose, no notable differences were observed in particle size among the batches. In the case of mannitol-containing batches, the batch executed with 5 g of mannitol showed the lowest particle size among all optimization trial batches.
| Formulation | Rebamipide (g) | Mannitol (g) | Lactose (g) | Tween 80 (% w/v) | Distilled water (mL) | Particle size (nm) |
|---|---|---|---|---|---|---|
| B7 | 1.0 | 2.5 | — | 1 | 25 | 277.3 |
| B2 | 1.0 | 5 | — | 1 | 25 | 223.5 |
| B8 | 1.0 | 7.5 | — | 1 | 25 | 371.4 |
| B9 | 1.0 | — | 2.5 | 1 | 25 | 758.0 |
| B5 | 1.0 | — | 5 | 1 | 25 | 762.4 |
| B10 | 1.0 | — | 7.5 | 1 | 25 | 767.0 |
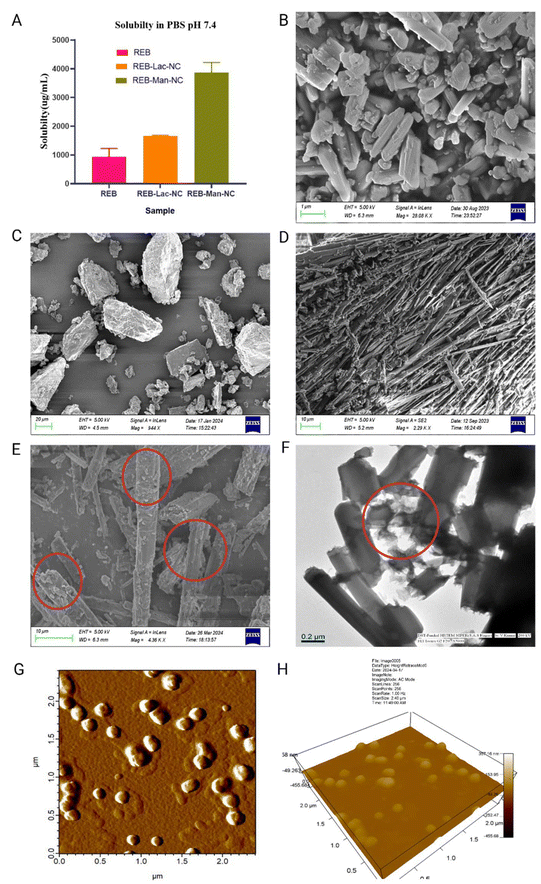
The composition was finalized based on the saturation solubility study of batches with minimum particle size, lactose monohydrate (356.9 nm), and mannitol (223.5 nm).
The energy input in ball milling can be relatively high, leading to a technique that reduces the particle to a significant mechanical stress on the particles.38 The liquid reduces the friction between the grinding media and particles, thereby minimizing the heat generated. Excessive heat can lead to the aggregation of particles. Liquid-assisted grinding (LAG) helps prevent particle aggregation, a common issue in high-energy processes such as microfluidization. Without proper control, microfluidization can sometimes lead to the re-agglomeration of particles. In LAG, the grinding energy is distributed more uniformly in the presence of the liquid, which can result in a finer and more consistent particle size reduction. In microfluidization, the energy distribution is less uniform, leading to a broader particle size distribution. LAG allows for more precise control over grinding parameters (e.g., liquid type, amount, and grinding time), which can be optimized to achieve smaller particle sizes.39 This could be why the base technique reduced the particle size to a lesser extent than microfluidization.
3.3 Physicochemical characterization of prepared nanocrystal powder
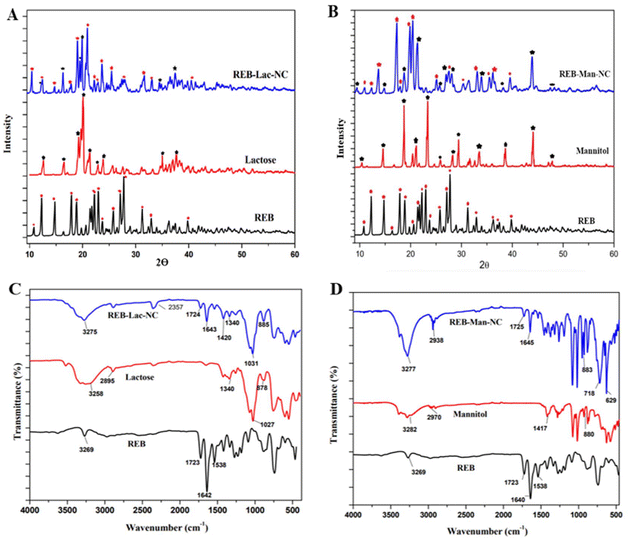
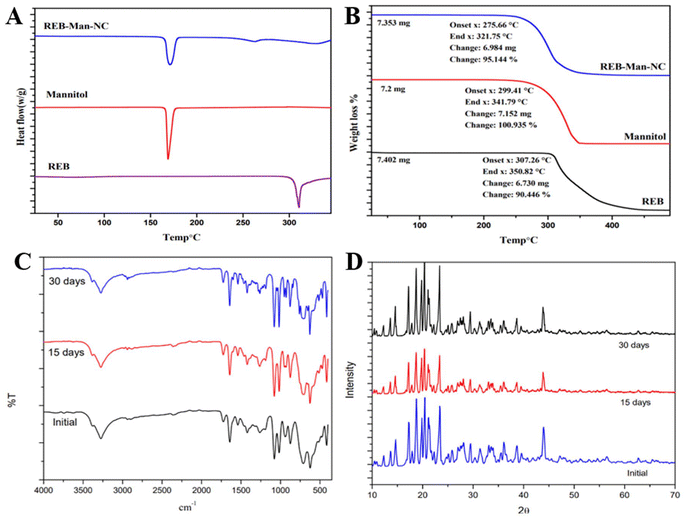
Clear and distinct peaks at specific 2θ values were observed in the diffractograms of rebamipide, confirming its crystalline nature. The diffractograms of the formulations showed that the peak positions of pure rebamipide and lyophilized nanocrystals were similar, indicating no significant differences, indicating that the lyophilized nanosuspension was in the same crystalline state. However, some new peaks were identified owing to the high-energy input during microfluidization. This aligns with research on rebamipide formulations, in which an attempt was made to improve their bioavailability by preparing lipid nanoemulsions or solid lipid nanoparticles.40,41
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester group), and 1642 cm−1 (CO–NH group) were consistent with the literature values for rebamipide.43 Similar retention of the characteristic peaks has been reported in studies involving other drugs, where nanonization did not affect their chemical structure.44 This consistency across different studies underscores the compatibility of rebamipide with the excipients used, as no new peaks or shifts were observed, indicating no chemical interaction between the drug and excipients. Tables 6 and 7 show the major band assignments for nanocrystals with lactose and mannitol, respectively. These results are aligned with previous data.41
O ester group), and 1642 cm−1 (CO–NH group) were consistent with the literature values for rebamipide.43 Similar retention of the characteristic peaks has been reported in studies involving other drugs, where nanonization did not affect their chemical structure.44 This consistency across different studies underscores the compatibility of rebamipide with the excipients used, as no new peaks or shifts were observed, indicating no chemical interaction between the drug and excipients. Tables 6 and 7 show the major band assignments for nanocrystals with lactose and mannitol, respectively. These results are aligned with previous data.41
| Peaks (API) | Functional group | Peaks (REB-Lac-NC) | Functional group |
|---|---|---|---|
| 3269 cm−1 | Aliphatic C–H stretching | 3275 cm−1 | Aliphatic C–H stretching |
| 1723 cm−1 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (ester) O (ester) |
1724 cm−1 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (ester) O (ester) |
| 1640 cm−1 | CO–NH | 1643 cm−1 | CO–NH |
| 1538 cm−1 | N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
885 cm−1 | ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–H CH–H |
| Peaks (API) | Functional group | Peaks (REB-Man-NC) | Functional group |
|---|---|---|---|
| 3269 cm−1 | Aliphatic C–H stretching | 3277 cm−1 | Aliphatic C–H stretching |
| 1723 cm−1 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (ester) O (ester) |
2938 cm−1 | CHO |
| 1640 cm−1 | CO–NH | 1725 cm−1 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (ester) O (ester) |
| 1538 cm−1 | N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
1645 cm−1 | CO–NH |
| 1337 cm−1 | R–NO2 | 883 cm−1 | ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–H CH–H |
As the goal of the study was to improve the physicochemical and mechanical properties of REB for improving its solubility, permeability, and development of suitable dosage form, and as we observed that the optimized premix with mannitol (B2) showed the most considerable solubility improvement, we selected the REB-mannitol nanocrystal (REB-Man-NC) for further characterization and formulation development.
3.4 In vitro studies
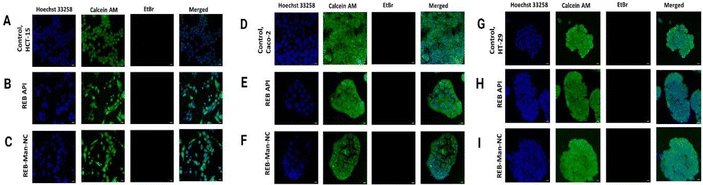
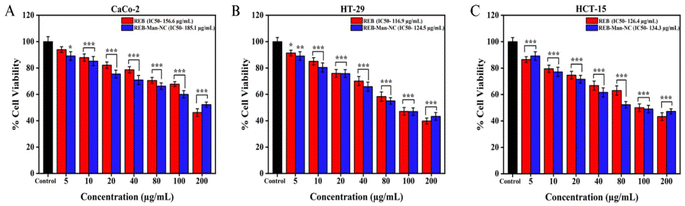 | ||
| Fig. 5 Effect on the cell viability after 24 h treatment with various concentrations of REB and REB-Man-NC on (A) Caco-2, (B) HT-29, and (C) HCT-15 cells. | ||
| S. no. | Formulation | Caco-2 (IC50) | HT-29 (IC50) | HCT-15 (IC50) |
|---|---|---|---|---|
| 1. | REB | 156.6 μg mL−1 | 116.9 μg mL−1 | 126.4 μg mL−1 |
| 2. | REB-Man-NC | 185.1 μg mL−1 | 124.5 μg mL−1 | 134.3 μg mL−1 |
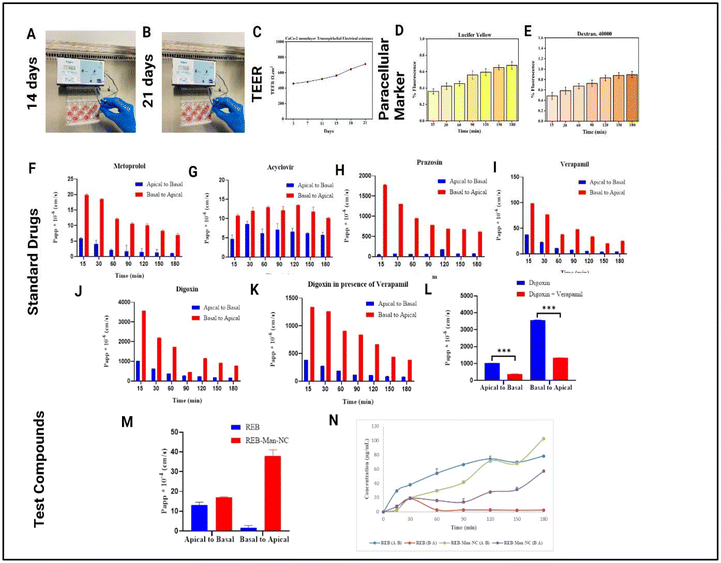
3.4.3.1 TEER measurement. The integrity of the Caco-2 monolayer was evaluated by TEER measurements for 21 days. Caco-2 cells showed a TEER value of 458 ± 12.56 Ωcm2 on day 3 and 710 ± 13.25 Ωcm2 on day 21. Caco-2 cells showed a gradual increase in TEER values after day 15 compared with the initial days, as shown in Fig. 7(A, B and C). Higher TEER values suggested the formation of a tight junction between the cells, further confirming monolayer formation. Studies have revealed that TEER values of 500–1000 Ωcm2 are indicative of fully formed tight junctions in differentiated Caco-2 monolayers.57,58
3.4.3.2 Permeability of paracellular markers.
3.4.3.2.1 Lucifer yellow. LY shows the potential to cross the intestinal epithelial barrier through three major paracellular pathways: pore, leak, and unrestricted pathways. In this research, the Caco-2 monolayer was grown for 21 days to establish a differentiated and functional intestinal barrier. The monolayer integrity was evaluated by measuring the LY passage. The result showed that the transport of LY through the Caco-2 monolayer was 0.67 ± 0.04% after 180 minutes (Fig. 7D), indicating the formation of a tight junction between Caco-2 cells. The low permeability of LY across Caco-2 cells is a strong indicator of the development of tight junctions between cells. Tight junctions serve as a seal between Caco-2 cells, establishing a robust barrier that significantly influences the paracellular permeability of LY.59,60
3.4.3.2.2 Dextran-40000. The permeability of dextran-40000 was evaluated after 21 days using a fluorescence plate reader in the Caco-2 monolayer. The results suggested that the permeability of dextran-40000 after 180 min was 0.89 ± 0.05% (Fig. 7E), indicating almost negligible permeability of dextran-40000 in the Caco-2 monolayer. Studies have shown that a Caco-2 monolayer with well-developed tight junctions displayed low paracellular permeability of dextran-40000.61,62
3.4.3.3 In vitro permeability assessment. The European Medicines Agency (EMA) and the Food and Drug Administration (FDA) emphasize the Caco-2 permeability assay as the “gold standard” for evaluating intestinal permeability under laboratory conditions.63 Standardization of the Caco-2 monolayer comprises a permeability study for standard compounds having high, moderate, and low permeability that range in human intestinal absorption ability.64 Caco-2 cells demonstrate elevated expression of P-glycoprotein (P-gp) transporters, which depends on the duration of culture, passage number, and culture conditions.65,66 A previous study reported higher expression of P-gp after 17 days of monolayer development.67 Therefore, standardization should include P-gp efflux substrates. In this study, five standard drugs with different BCS characteristics (metoprolol, prazosin, Acyclovir, digoxin, and verapamil) were used in the Caco-2 monolayer.68 To assess active transport mediated by P-gp transporters, 10 μM digoxin or 10 μM digoxin with 10 μM verapamil as an inhibitor was utilized. For the BCRP transport studies, 10 μM prazosin was used. In this study, experimental permeability data for the standard drugs (metoprolol, prazosin, Acyclovir, digoxin, and verapamil) and the test compound (rebamipide and its formulation) across the Caco-2 monolayer were evaluated using apparent permeability (Papp). The result showed that metoprolol (2.99 μM dose) was highly permeable with a value of 5.94 × 10−6 in the apical-to-basal transport and 19.9 × 10−6 in basal-to-apical transport, which declined slowly over a period of time, as shown in Fig. 7(F). Similar observations have been previously reported.69,70 The apparent permeability of Acyclovir (2.66 μM dose) was found to be 4.69 × 10−6 cm s−1 in the apical-to-basal direction and 10.8 × 10−6 cm s−1 in the basal-to-apical direction, which indicates less permeability than metoprolol. This suggests higher permeability of Acyclovir with a higher efflux ratio than that reported by Shah et al., with a Papp value of 0.352 × 10−6 cm s−1 at a dose of 2.5 mM.71 The apparent permeability of prazosin (10 μM dose) was 57.7 × 10−6 cm per in−1 the apical-to-basal transport and 1781 × 10−6 cm s−1 in the basal-to-apical direction. Prazosin showed the highest permeability as compared to the values reported by Kumar et al., 3.7 × 10−6 cm s−1 in the apical-to-basal transport and 38.2 × 10−6 cm s−1 in the basal-to-apical transport at a dose of 500 μM with a three times higher efflux ratio.72 Of all the standard drugs utilized in this study and one that was reported, digoxin exhibited the highest permeability, as shown in Fig. 7(J). The apparent permeability of digoxin (10 μM dose) was found to be 1019 × 10−6 cm s−1 in the apical-to-basal transport and 3569 × 10−6 cm s−1 in the basal-to-apical transport, as compared to the reported digoxin value of 1.45 × 10−6 cm s−1 in the apical-to-basal transport and 23.27 × 10−6 cm s−1 in the basal-to-apical transport at a dose of 1 μM.73 The apparent permeability of verapamil (10 μM dose) was found to be 37.4 × 10−6 cm s−1 in the apical-to-basal transport and 98.4 × 10−6 cm s−1 in the basal-to-apical transport as compared to the reported verapamil value, done by Jarc et al., 32.1 × 10−6 cm s−1 in the apical-to-basal transport and 28.6 × 10−6 cm s−1 in the basal-to-apical transport at a dose of 70 μM.74 When digoxin was administered with verapamil, a potent P-gp inhibitor, the Papp value of the drug decreased by one-third, as shown in Fig. 7(K and L), indicating that the P-gp transporter is responsible for drug efflux.
Similarly, the apparent permeabilities of the test compounds (rebamipide and its formulation) were calculated. The Papp values of rebamipide and its formulations at different time points are shown in Table S2 (ESI).† Enhanced permeability of rebamipide was obtained in our Caco-2 monolayer model at a dose of 10 μM, with values of 13.02 × 10−4 cm s−1 in the apical-to-basal transport and 1.66 × 10−4 cm s−1 in the basal-to-apical transport, in contrast to the published values of 1.51 × 10−5 cmin−1 the apical-to-basal transport and 2.50 × 10−5 cm s−1 in the basal-to-apical transport.75 The REB-Man-NC showed enhanced permeation with an eighteen-fold higher efflux ratio than the REB API over a 3 h period, as shown in Fig. 7(M) and Table 9. The increased permeability of REB-Man-NC may have been due to the presence of Tween-80 and mannitol in the formulation. The permeability of the majority of the low-permeability drugs was considerably enhanced by tween-80.76 As such, the permeability of mannitol is low, but the addition of other substances, such as interleukin-6, enhances its permeation.77 Thus, the permeability of the formulation was increased by the addition of mannitol and tween-80. In addition, the decreased particle size and increased solubility increase the concentration gradient across the membrane, thereby improving permeability.78
| System | Papp × 10−4 (cm s−1) | Efflux ratio | |
|---|---|---|---|
| Apical to basal | Basal to apical | ||
| REB | 13.02 | 1.66 | 0.12 |
| REB-Man-NC | 17.12 | 38.04 | 2.22 |
The result showed the higher permeation of both the test drugs (i.e., rebamipide) and standard drugs (i.e., metoprolol, prazosin, Acyclovir, digoxin, and verapamil). The Papp values for metoprolol, prazosin, Acyclovir, digoxin, and verapamil at different time points are listed in Table S1 (ESI).†
3.5 Tabletability, compressibility, and compactibility study
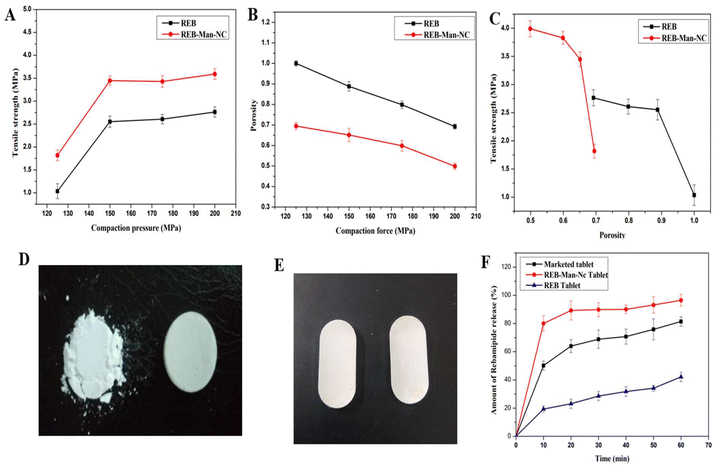
Tabletability is the correlation between the tensile strength of a tablet and applied compression pressure. Tabletability, compressibility, and compactibility profiles were plotted to assess the tableting performance of REB and REB-Man-NC; these profiles are given in Fig. 8(A), (B), and (C), respectively. To create these plots, the physical dimensions and breaking force of each compact were measured at each pressure point as the compression pressure increased from 125 to 200 MPa, as shown in Fig. 8(A). The porosity of the powder compressibility profile was a function of the applied pressure. Under a given stress, more compressible materials have a higher solid fraction and lower final porosity, which is a prerequisite for a good tabletability profile. As shown in Fig. 8(B), REB-Man-NC also showed a good compressibility and tabletability profile. The tensile strength of the tablets is shown as a function of porosity in the compactibility profile. Therefore, powdered materials are considered to be more compact if they produce stronger compacts at a given porosity.As shown in Fig. 8(C), REB-Man-NC has a lower bed porosity than pure REB, indicating the superior compressibility and compactable characteristics of nanocrystals. Finally, a tabletability profile was created by our analysis, and we noticed an upward trend in tensile strength as compression pressure increased, reaching a plateau at higher pressures. The data suggest that a compression force within the 175–200 MPa range was optimal for achieving the maximum tensile strength in the compact. Further increase in the compression force did not contribute to an enhancement in the tensile strength of the tablet. Typically, the strength of a tablet increases with increasing compression pressure, eventually stabilizing the compacted mass. However, an inverse relationship was observed when tablet strength diminished with increasing pressure. This phenomenon is indicative of over-compaction, which can lead to tablet flaws.79 Upon examining the tabletability graph, it was discernible that the tablet's tensile strength increased with increasing compression pressure, but reached a saturation point at higher pressures. The tensile strength apex of the tablet was attained using a compression force of 200 MPa. From these plots, we can observe that the nanocrystals have better tabletability characteristics than the pure REB. Nanocrystal powders have far better tableting behavior than pure REB API powders, which showed very weak compaction properties. Without other excipients, complete tablets can be formed with the premix over the compaction pressure range. Furthermore, after 24 h of rest, there was no sign of delamination or capping of tablets.
3.6 Preparation of tablet formulation
Tablets containing the lyophilized nanocrystals were developed by mixing the lyophilized REB-Man-NC with other extra-granular materials. The exact final composition and batch size are presented in Table 10. The images of the compressed tablets are presented in Fig. 8(E). The tablets were compressed by direct compression method. Excipients like MCC 102 (diluent), magnesium stearate (lubricant), and polyplasdone XL (super disintegrant) were used for tablet manufacturing. The details of the tablet unit composition are presented in Table 10. These excipients have excellent flowability and compressibility, making them suitable for direct compression. A mix of lyophilized nanocrystals with the tablet excipients was subjected to direct compression by a rotary compression machine using a caplet shape “D” type 19 mm × 8 mm punch by direct compression method. Weight variation was found to be 700 ± 2.07 mg. The hardness range of the tablet was found to be 12.2 ± 2.7 kp. The friability of the tablet was found to be 0.144% w/w. The drug content uniformity was analyzed by UV-visible spectroscopy. The % drug content of the tablets was observed to be 97.52 ± 2.03%. The results were within the range, indicating uniformity of drug content. The tablet shows an average disintegration time of 11 minutes 42 s.| Sl no. | Components | Mg/unit | Gram/batch (50 units) |
|---|---|---|---|
| Intra-granular | |||
| 1 | REB-Man-NC premix | 600.00 | 30.00 |
| Extra-granular | |||
| 2 | Microcrystalline cellulose 102 | 64.00 | 3.20 |
| 3 | Crospovidone XL | 30.00 | 1.50 |
| 4 | Magnesium stearate | 6.00 | 0.30 |
| Total weight of a tablet | 700.00 | 35.000 | |
3.7 In vitro dissolution study
The dissolution tests for the manufactured tablets were conducted in triplicate using a USP type II (paddle) apparatus. The tests utilized 900 mL of phosphate buffer at pH 7.4 as the dissolution medium, maintained at a temperature of 37 ± 0.5 °C. The paddle speed was set to 100 RPM for the REB-Man-NC tablet dissolution study. The dissolution characteristics of the REB-Man-NC powder, in-house tablet formulation with REB-Man-NC, and marketed formulation are presented in Fig. 8(F). The dissolution velocity of the REB-Man-NC tablet was faster than that of the marketed 100 mg tablet formulation. Nearly 76% of the REB was dissolved in just ten minutes from the nanocrystal tablet. This was better than the marketed tablet, from which near about 50% of REB was dissolved under the same conditions. The API showed only 20% dissolution after ten minutes. After 15 min, the REB-Man-NC tablet showed nearly 81% drug release, which is close to official drug release criteria for immediate release tablets (should release ≥85% drug in 15 min). The faster dissolution in the REB-Man-NC tablet formulation may be attributed to its extreme size reduction to the nanoscale level and its association with highly water-soluble mannitol. From this study, it can be concluded that REB-Man-NC tablets have the potential to show more significant dissolution and intestinal permeability improvement.4. Conclusion
In conclusion, this study successfully demonstrated that nanocrystalline rebamipide (REB) significantly enhances its solubility and permeability, addressing the critical limitations posed by its low bioavailability as a BCS class IV drug. By applying high-pressure homogenization and wet milling techniques, REB was converted into nanocrystals with an average particle size of 223 nm, leading to an impressive three-fold increase in its solubility in phosphate buffer at pH 7.4. Comprehensive characterization methods, including FT-IR, DSC, and PXRD, confirmed the crystalline stability and compatibility of the nanocrystals with the excipients. In vitro studies utilizing Caco-2 monolayers have validated an eighteen-fold enhancement in permeability compared to pure REB. Simultaneously, the dissolution profiles of the nanocrystal tablets indicated close to immediate drug release characteristics, further underscoring their therapeutic potential. These findings establish nano-milling as an effective and scalable platform technology to increase the rate of dissolution and solubility of drugs that are poorly soluble in water, such as rebamipide. Our research not only provides a promising approach for improving oral bioavailability but also contributes new insights into the formulation strategies necessary for maximizing the clinical efficacy of this important therapeutic agent.Author contributions
S. K. and S. R. conceptualized the study and designed the studies and methodologies. V. R. helped with funding and manuscript review. M. S. S., K. P. K., S. K. S., and U. G. performed manufacturing and material characterization. M. K., K. A., S. K. S., and U. G. performed permeation-related experiments. M. S. S., K. P. K., S. K. S., and M. K. drafted the manuscript. All authors have reviewed the final version of the manuscript and approved its submission.Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
S. K. would like to thank the Department of Pharmaceutics, National Institute of Pharmaceutical Education and Research, Kolkata, for supporting this work. S. R. acknowledges the Department of Pharmacology and Toxicology, National Institute of Pharmaceutical Education and Research, Kolkata, for supporting this work. The authors are thankful to the Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India for their generous funding support to the institute. We acknowledge the analytical support from Dr. Rekha Bhar (Anton Paar). The authors also acknowledge instrumental support from Shiv Nadar University, IoE, Department of Chemistry for AFM study. All graphics were developed using a licensed version of BioRender.References
- B. B. Huang, G. F. Li, J. H. Luo, L. Duan, K. Nobuaki and Y. Akira, World J. Gastroenterol., 2008, 14, 4928–4937 CrossRef PubMed.
- T. Arakawa, K. Higuchi, Y. Fujiwara, T. Watanabe, K. Tominaga, E. Sasaki, N. Oshitani, T. Yoshikawa and A. S. Tarnawski, Dig. Dis Sci., 2005, 50, S3–S11 CrossRef PubMed.
- S. Shrivastava, P. Patkar, R. Ramakrishnan, M. Kanhere and Z. Riaz, Oman J. Ophthalmol., 2018, 11, 207 CrossRef.
- S. Zhang, Q. Qing, Y. Bai, H. Mao, W. Zhu, Q. Chen, Y. Zhang and Y. Chen, Dig. Dis Sci., 2013, 58, 1991–2000 CrossRef.
- T. W. Wong, J. Pharm. Pharmacol., 2011, 63, 1497–1512 CrossRef.
- J. U. Junghanns and R. H. Muller, Int. J. Nanomed., 2008, 3, 295 Search PubMed.
- Y. Lu, Y. Chen, R. A. Gemeinhart, W. Wu and T. Li, Nanomedicine, 2015, 10, 2537–2552 CrossRef CAS.
- P. Couvreur, P. Tulkenst, M. Roland, A. Trouet and P. Speiser, FEBS Lett., 1977, 84, 323–326 CrossRef CAS.
- Y. Guo, Y. Wang and L. Xu, Asian J. Pharm. Sci., 2015, 10, 223–229 CrossRef.
- N. Nagai, R. Seiriki, S. Deguchi, H. Otake, N. Hiramatsu, H. Sasaki and N. Yamamoto, Pharmaceutics, 2020, 12, 532 CrossRef PubMed.
- P. S. Rathod, M. R. Narkhede and S. L. Dongare, Curr. Nanomed., 2023, 14, 4–12 CrossRef.
- Y. Li, L. Deng, T. Dai, Y. Li, J. Chen, W. Liu and C. Liu, Food Control, 2022, 137, 108794 CrossRef.
- R. Wahyuni, H. Lucida, G. Revilla, F. Ismed and E. Zaini, Trop. J. Nat. Prod. Res., 2024, 8, 5801 Search PubMed.
- S. K. Misra and K. Pathak, Industrial Applications of Nanocrystals, 2022, pp. 153–178 Search PubMed.
- X. Shang, M. Fu, Z. Cai, L. Wei, S. Ge, M. Ge and Y. Wang, J. Mol. Struct., 2023, 1288, 135786 CrossRef.
- S. Goel, V. Agarwal and M. Sachdeva, Curr. Nanosci., 2021, 18, 291–303 CrossRef.
- Z. Huang, S. Staufenbiel and R. Bodmeier, Pharm. Res., 2022, 39, 949–961 CrossRef PubMed.
- Y. Ogawa and J. L. Putaux, Cellulose, 2019, 26, 17–34 CrossRef.
- V. Binyukov, E. Mil, L. Matienko, A. Albantova and A. Goloshchapov, Micro, 2023, 3, 382–390 CrossRef.
- D. Gol, S. Thakkar and M. Misra, Drug Dev. Ind. Pharm., 2018, 44, 1458–1466 CrossRef PubMed.
- M. E. Melian, A. Paredes, B. Munguía, M. Colobbio, J. C. Ramos, R. Teixeira, E. Manta, S. Palma, R. Faccio and L. Domínguez, AAPS PharmSciTech, 2020, 21, 1–15 CrossRef PubMed.
- A. Hivechi, S. H. Bahrami, R. A. Siegel, A. Siehr, A. Sahoo, P. B. Milan, M. T. Joghataei, M. Amoupour and S. Simorgh, Mater. Sci. Eng., C, 2021, 121, 111855 CrossRef PubMed.
- N. Sreeharsha, N. R. Naveen, P. Anitha, P. S. Goudanavar, S. Ramkanth, S. Fattepur, M. Telsang, M. Habeebuddin and M. K. Answer, Pharmaceuticals, 2022, 15, 311 CrossRef PubMed.
- K. Alam, L. Nair, S. Mukherjee, K. Kaur, M. Singh, S. Kaity, V. Ravichandiran, S. Banerjee and S. Roy, Bio-Des. Manuf., 2024, 7, 320–357 CrossRef.
- K. Alajmi, M. Hartford, N. S. Roy, A. Bhattacharya, S. Kaity, B. L. Cavanagh, S. Roy and K. Kaur, J. Mater. Chem. B, 2024, 12, 9268–9282 RSC.
- S. Roy, A. Sharma and S. Ghosh, Biochem. Biophys. Res. Commun., 2023, 643, 39–47 CrossRef.
- D. Gartzke and G. Fricker, J. Pharm. Sci., 2014, 103, 1298–1304 CrossRef.
- A. Weller, M. B. Hansen, R. Marie, A. C. Hundahl, C. Hempel, P. J. Kempen, H. L. Frandsen, L. Parhamifar, J. B. Larsen and T. L. Andresen, Front. Bioeng. Biotechnol., 2022, 10 DOI:10.3389/fbioe.2022.965200.
- I. Hubatsch, E. G. E. Ragnarsson and P. Artursson, Nat. Protoc., 2007, 2, 2111–2119 CrossRef PubMed.
- T. P. Stockdale, V. L. Challinor, R. P. Lehmann, J. J. De Voss and J. T. Blanchfield, ACS Omega, 2019, 4, 7658–7666 CrossRef.
- N. Li, Z. Sui, Y. Liu, D. Wang, G. Ge and L. Yang, RSC Adv., 2018, 8, 34514–34524 RSC.
- D. A. Volpe, P. J. Faustino, A. B. Ciavarella, E. B. Asafu-Adjaye, C. D. Ellison, L. X. Yu and A. S. Hussain, Clin. Res. Regul. Aff., 2007, 24, 39–47 CrossRef.
- M. K. Jeoung, C. S. Kim, N. H. Kim, J. T. Hong, Y. Chung, Y. Park, K. S. Kim and D. Moon, J. Liq. Chromatogr. Relat. Technol., 2004, 27, 1925–1935 CrossRef.
- C. C. Sun, J. Adhes. Sci. Technol., 2011, 25, 483–499 CrossRef.
- K. S. Khomane, P. K. More, G. Raghavendra and A. K. Bansal, Mol. Pharm., 2013, 10, 631–639 CrossRef.
- F. Haruna, Y. E. Apeji, C. Oparaeche, A. R. Oyi and M. Gamlen, Future J. Pharm. Sci., 2020, 6, 1–9 CrossRef.
- L. Mareczek, C. Riehl, M. Harms and S. Reichl, Pharm. Res., 2024, 41, 185–197 CrossRef.
- C. C. Piras, S. Fernández-Prieto and W. M. De Borggraeve, Nanoscale Adv., 2019, 1, 937–947 RSC.
- Z. H. Loh, A. K. Samanta and P. W. Sia Heng, Asian J. Pharm. Sci., 2015, 10, 255–274 CrossRef.
- A. Narala, S. Guda and K. Veerabrahma, AAPS PharmSciTech, 2019, 20, 1–9 CrossRef.
- N. G. Mirmeera and K. Kannan, Int. J. Appl. Pharm., 2022, 14, 143–150 Search PubMed.
- Methods of nanonization of drugs for enhancing their dissolution, https://www.researchgate.net/publication/312068815_Methods_of_nanonization_of_drugs_for_enhancing_their_dissolution, (accessed 14 September 2024).
- Rebamipide|C19H15ClN2O4|CID 5042 - PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Rebamipide, (accessed 9 June 2024).
- How to Properly Compare Spectra, and Determining Alkane Chain Length From Infrared Spectra, https://www.spectroscopyonline.com/view/how-properly-compare-spectra-and-determining-alkane-chain-length-infrared-spectra, (accessed 14 September 2024).
- J. Hu, Y. Dong, W. K. Ng and G. Pastorin, Adv. Powder Technol., 2018, 29, 957–963 CrossRef.
- M. Chen, W. Zhang, H. Wu, C. Guang and W. Mu, Appl. Microbiol. Biotechnol., 2020, 104, 6941–6951 CrossRef PubMed.
- M. Saffari, A. Ebrahimi and T. Langrish, Eur. J. Pharm. Sci., 2016, 83, 52–61 CrossRef PubMed.
- K. Akhtar, S. A. Khan, S. B. Khan and A. M. Asiri, Handbook of Materials Characterization, 2018, pp. 113–145 Search PubMed.
- J. Liu, Z. Xiong, X. Geng, M. Cui and M. B. Adinortey, BioMed Res. Int., 2020, 1, 7196782 Search PubMed.
- C. V. Abiaziem, A. B. Williams, A. I. Inegbenebor, C. T. Onwordi, C. O. Ehi-Eromosele and L. F. Petrik, Rasayan J. Chem., 2020, 13, 177–187 CrossRef.
- N. G. Mirmeera and K. Kannan, Int. J. Appl. Pharm., 2022, 14, 143–150 Search PubMed.
- A. Narala, S. Guda and K. Veerabrahma, AAPS PharmSciTech, 2019, 20, 1–9 CrossRef.
- S. H. Jeon and Y. T. Sohn, Arch. Pharmacal Res., 2016, 39, 508–515 CrossRef.
- X. Guo, Y. Wu and X. Xie, Sci. Rep., 2017, 7, 1–12 CrossRef.
- H. G. T. Nguyen, B. Toman, R. D. van Zee, C. Prinz, M. Thommes, R. Ahmad, D. Kiska, J. Salinger, I. M. Walton, K. S. Walton, D. P. Broom, M. J. Benham, H. Ansari, R. Pini, C. Petit, J. Adolphs, A. Schreiber, T. Shigeoka, Y. Konishi, K. Nakai, M. Henninger, T. Petrzik, C. Kececi, V. Martis, T. Paschke, E. Mangano and S. Brandani, Adsorption, 2023, 29, 113–124 CrossRef.
- S. Mourdikoudis, R. M. Pallares and N. T. K. Thanh, Nanoscale, 2018, 10, 12871–12934 RSC.
- I. Ogulur, Y. Pat, T. Aydin, D. Yazici, B. Rückert, Y. Peng, J. Kim, U. Radzikowska, P. Westermann, M. Sokolowska, R. Dhir, M. Akdis, K. Nadeau and C. A. Akdis, J. Allergy Clin. Immunol., 2023, 151, 469–484 CrossRef PubMed.
- I. Lozoya-Agullo, F. Araújo, I. González-Álvarez, M. Merino-Sanjuán, M. González-Álvarez, M. Bermejo and B. Sarmento, Mol. Pharm., 2017, 14, 1264–1270 CrossRef.
- P. Lian, S. Braber, S. Varasteh, H. J. Wichers and G. Folkerts, Sci. Rep., 2021, 11, 13186 CrossRef.
- P. Hoffmann, M. Burmester, M. Langeheine, R. Brehm, M. T. Empl, B. Seeger and G. Breves, PLoS One, 2021, 16, e0257824 CrossRef.
- S. K. Sah, K. Alam, M. Kumari, M. R. Malootty, S. Nath, V. Ravichandiran, S. Roy and S. Kaity, Eur. J. Pharm. Biopharm., 2024, 114480 CrossRef CAS.
- C. Li, X. Bai, X. Liu, Y. Zhang, L. Liu, L. Zhang, F. Xu, Y. Yang and M. Liu, Front. Microbiol., 2021, 12, 634185 CrossRef PubMed.
- M. Kus, I. Ibragimow and H. Piotrowska-Kempisty, Pharmaceutics, 2023, 15, 2523 CrossRef.
- International Council For Harmonisation Of Technical Requirements For Pharmaceuticals For Human Use Ich Harmonised Guideline Biopharmaceutics Classification System-Based Biowaivers M9 Final version, 2019.
- Y. Shirasaka, M. Kawasaki, T. Sakane, H. Omatsu, Y. Moriya, T. Nakamura, T. Sakaeda, K. Okumura, P. Langguth and S. Yamashita, Drug Metab. Pharmacokinet., 2006, 21, 414–423 CrossRef CAS PubMed.
- S. M. D. K. Ganga Senarathna and A. Crowe, Pharmazie, 2015, 70, 798–803 Search PubMed.
- P. Anderle, E. Niederer, W. Rubas, C. Hilgendorf, H. Spahn-Langguth, H. Wunderli-Allenspach, H. P. Merkle and P. Langguth, J. Pharm. Sci., 1998, 87, 757–762 CrossRef CAS.
- Step, Committee for Medicinal Products for Human Use ICH M9 guideline on biopharmaceutics classification system-based biowaivers ICH M9 on biopharmaceutics classification system-based biowaivers, 2020.
- T. Jarc, M. Novak, N. Hevir, T. L. Rižner, M. E. Kreft and K. Kristan, J. Pharm. Pharmacol., 2019, 71, 1231–1242 CrossRef CAS.
- T. Incecayir, Y. Tsume and G. L. Amidon, Mol. Pharm., 2013, 10, 958–966 CrossRef CAS.
- P. Shah, V. Jogani, P. Mishra, A. K. Mishra, T. Bagchi and A. Misra, Drug Dev. Ind. Pharm., 2008, 34, 279–288 CrossRef CAS PubMed.
- L. Kumar, C. L. Meena, Y. B. Pawar, B. Wahlang, K. Tikoo, R. Jain and A. K. Bansal, AAPS PharmSciTech, 2013, 14, 141–150 CrossRef CAS.
- Y. Cai, C. Xu, P. Chen, J. Hu, R. Hu, M. Huang and H. Bi, J. Pharmacol. Toxicol. Methods, 2014, 70, 175–181 CrossRef CAS.
- T. Jarc, M. Novak, N. Hevir, T. L. Rižner, M. E. Kreft and K. Kristan, J. Pharm. Pharmacol., 2019, 71, 1231–1242 CrossRef CAS.
- M. Miyake and D. Nakai, Xenobiotica, 2017, 47, 821–824 CrossRef CAS.
- B. D. Rege, X. Yu. Lawrence, A. S. Hussain and J. E. Polli, J. Pharm. Sci., 2001, 90, 1776–1786 CrossRef CAS PubMed.
- M. Miyake and D. Nakai, Xenobiotica, 2017, 47, 821–824 CrossRef CAS PubMed.
- S. Rao, Y. Song, F. Peddie and A. M. Evans, Int. J. Nanomed., 2011, 6, 1245 CAS.
- 〈1062〉 Tablet Compression Characterization, https://doi.usp.org/USPNF/USPNF_M99395_02_01.html, (accessed 17 May 2024).
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr03137g |
| This journal is © The Royal Society of Chemistry 2024 |