Open Access Article
Open Access ArticleSolution-processed structural colors and their applications
Wei-Jie
Feng
 a,
Jennie
Paik
a and
L. Jay
Guo
a,
Jennie
Paik
a and
L. Jay
Guo
 *ab
*ab
aMacromolecular Science and Engineering, The University of Michigan, Ann Arbor 48109, USA. E-mail: guo@umich.edu
bDepartment of Electrical Engineering and Computer Science, The University of Michigan, Ann Arbor 48109, USA
First published on 13th September 2024
Abstract
High-quality and brilliant structural colors have been successfully produced using vacuum-based deposition and patterning technology in recent decades. Nevertheless, the major obstacles of high production costs and limited scalability impede the commercialization of these vibrant color products. Solution-processed structural colors, on the other hand, are renowned for their cost-effectiveness, scalability, and versatility. In this review, we provide an overview of prevalent solution-based techniques for structural color synthesis, along with their potential applications. Emphasizing the versatility of solution-processed structural colors, we discuss their capabilities in both color tuning and new ways of modifying refractive indices of dielectrics.
1. Introduction
The realm of color constitutes a captivating facet of our visual encounters, intriguing both scientists and artists throughout history. The multitude of colors permeating our surroundings arises from the absorption or reflection of light by pigments, dyes, or minerals. This phenomenon is entirely contingent on the inherent optical characteristics of the materials involved. Conversely, an alternative avenue for color generation lies in the properties and arrangement of the structure of, rather than the specific, constituent materials. Visible colors that are produced through the interaction between light and micro- or nano-structures (e.g. thin film interference, particle resonance) are commonly referred to as structural colors. Structural colors are prevalent in the natural world,1,2 where they serve pivotal roles in diverse biological functions such as mate selection, camouflage, and communication. Notable instances include the iridescent hues found in butterfly wings,3,4 peacock feathers,5 beetle shells,6 and bird feathers7 (Fig. 1). These colors emerge through the interference of light waves with nanostructures inherent in these materials. In contrast to conventional organic dyes, structural colors offer several advantages.8 | ||
| Fig. 1 Examples of structural color in nature. (a) Morpho butterfly with brilliant blue wings, (b) colorful peacock feather, (c) blue feather barb of a male plum-throated coting. | ||
Firstly, they exhibit resistance to fading over time, as their coloration is not produced by chemical substances that could be susceptible to environmental factors like light, heat, or moisture. Secondly, they have the capacity to generate a broader spectrum of colors, deriving their hues from the properties of nanostructures rather than the material composition. Thirdly, and important to this review, their production is feasible using environmentally friendly materials and processes, eliminating dependence on toxic chemicals or heavy metals.
In recent decades, fueled by inspiration drawn from nature, researchers have crafted a myriad of artificial designs for structural colors. These include photonic crystals,9–14 metamaterials,15–17 and plasmonics,18–23 amongst others, each showcasing a spectrum of color effects characterized by remarkable vibrancy. From the understanding of the inherent physical principles, a multitude of fabrication technologies have emerged to bring these designs to fruition. Techniques such as physical vapor deposition (PVD),24,25 atomic layer deposition (ALD),26 sputtering,27,28 photolithography,29–32 nanoimprinting,33,34 self-assembly35–39 have been developed to realize these intricate designs. Readers can refer to other reviews that cover these technologies and basic principles of colors with respect to human perception.40 While each method boasts unique strengths and weaknesses, a significant drawback is the reliance on high vacuum conditions41–44 for film deposition or intricate, multi-step patterning processes21,32,34,45,46 used by many of these techniques. This limitation significantly hampers their potential applications for large-scale and cost-effective production. In contrast solution-processed deposition methods are immensely desirable for their inherent simplicity, cost-effectiveness, and scalability, thereby rendering large-scale production of structural color coatings significantly more achievable. Moreover, the incorporation of solutions or suspensions introduces substantial versatility in manipulating material composition and morphology. In this comprehensive review, our emphasis will be on the diverse strategies developed to date for solution-based structural color fabrication. We will undertake a thorough comparison of their merits and limitations, accompanied by illustrative examples spanning a wide array of applications. While many photonic structures can indeed be fabricated using traditional vacuum-based processes, we will explore alternative routes through solution processes, where they offer the potential to reduce costs and unlock new possibilities.
The structure of the paper will unfold as follows: Section 2 will present an extensive review of the most widely adopted solution-based methods for structural color fabrication, delving into their underlying chemistry. In Section 3, our focus will shift to one of the most distinctive aspects of solution-processed structural color: tunability. Many examples will be provided to showcase how structural color derived from solutions responds to various external stimuli (such as electricity, temperature, solvent, etc.) through spatial tuning, along with their potential applications. We will also explore the feasibility of creating artificial optical materials through refractive index (RI) tuning and elucidate how these novel optical properties contribute to unique color perceptions. Section 4 will offer a comprehensive summary of solution-processed structural color and provide insights into the present challenges as well as future research directions.
2. Solution-based fabrication strategies
2.1. Structural color from self-assembly
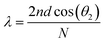 , where n is the average refractive index, d is the PC period, θi is the incident angle, and N is an integer that characterizes the order of the bands.
, where n is the average refractive index, d is the PC period, θi is the incident angle, and N is an integer that characterizes the order of the bands.
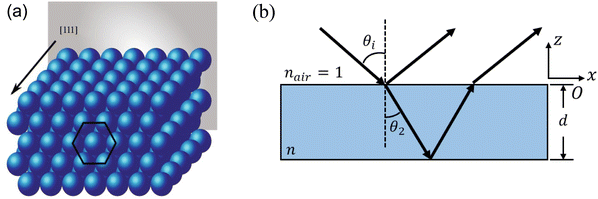 | ||
Fig. 2 Schematic illustration of (a) an opal structure [Reproduced from ref. 47, with permission from Springer Publishing, Copyright 2022] and (b) Bragg's law where n is the average refractive index, d is the PC period, θi is the incident angle, θ2 is the angle of refraction (where sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θi = n θi = n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ2) and N is an integer that characterizes the order of the bands. θ2) and N is an integer that characterizes the order of the bands. | ||
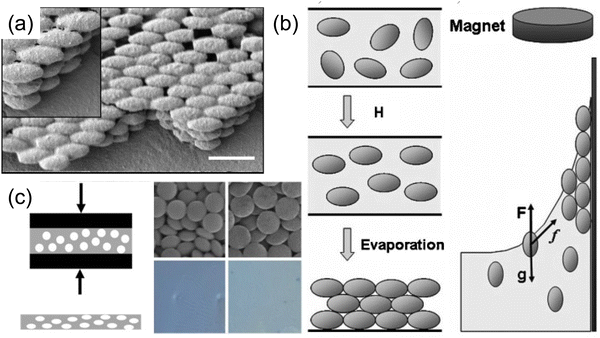
With a careful tuning of the colloidal particle size and the material composition, self-assembled PCs with various colors can be made. Typical fabrication utilizes a convective assembly method, where the capillary force pushes the mono-dispersed colloidal particle to self-pack into a single-crystal film at the front of an evaporating solution. An early example of silica colloidal particles with sizes from 260–400 nm produces colors in blue, yellow and red (Fig. 3a and b).52 Other methods, including external field (i.e. electric field, gravity, etc.) induced PhC self-assembly,53,54 spin-coating,55,56 electrospinning,57etc. were later on developed based on the initial ideal of convective assembly, i.e. balancing the rate of colloidal particle self-arrangement and the rate of solvent evaporation becomes the key to a highly ordered photonic structure. In addition to the opal structure, much stronger photonic behavior can be realized if a higher dielectric contrast within the system can be obtained, e.g. through use of titanium oxide58 to achieve higher reflectance. By switching to a higher refractive index nanoparticle made of synthetic melanin, Xiao et al.59 has created a self-assembled PhC with a broader stopband. A higher color purity was also obtained with the absorbing nature of melanin where the reflective background is greatly suppressed (Fig. 3c and d). However, the high index PhC structure poses a challenge in the synthesis of high index colloidal particles, namely in size, uniformity, suspension stability, etc. Hence, structural modification is employed to enable a universal method of getting high index material into the PhC structure. The opal structure is treated as a template where the air voids in the initial self-assembled opal structure are replaced with a higher refractive index material. The original silica or polymer template is either chemically etched away or thermally decomposed. The resulting inverse opal structure (Fig. 3e) now contains more spherical air void space with higher contrast to backbone material. In an early demonstration with TiO2 backbone,60 the PBG widened and gave a stronger iridescent color appearance. Other metal oxide61–63 (e.g. SnO2, ZrO2, etc.) were also demonstrated to give vivid color across the entire visible spectrum.
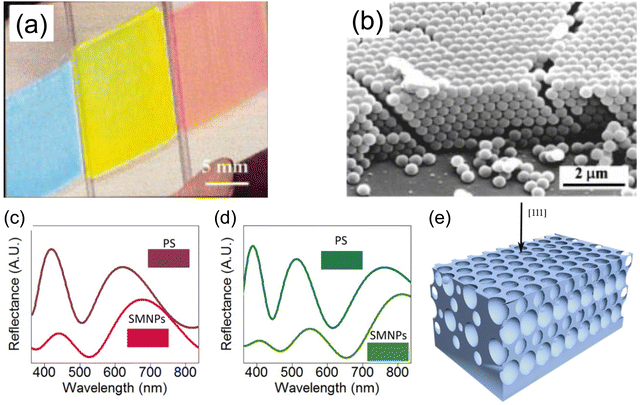 | ||
| Fig. 3 (a) Structural color of SiO2 colloidal particle self-assembled photonic crystal. (b) SEM of photonic crystal showing the arrangement of SiO2 particles. [Reproduced from ref. 52, with permission from American Chemical Society, Copyright 1999]. (c) and (d) Reflection spectra showing the suppression of background reflection with light absorbing synthetic melanin nanoparticles (SMNP). [Reproduced from ref. 59, with permission from American Chemical Society, Copyright 2015]. (e) Schematic illustration of an inverse-opal structure. [Reproduced from ref. 60, with permission from American Association for the Advancement of Science, Copyright 1998]. | ||
While highly ordered close-packed colloidal particles gives crystalline structure (Fig. 4a), it makes the stopband center wavelength angle dependent as light shines upon different crystal planes producing varied optical phases; thus, iridescent color is always present. Though appealing, consistent color perception is desired in many applications, which cannot be satisfied by the self-assembly accompanied by the long-range order. Besides, the spontaneous nature of self-assembly cannot ensure a perfect crystalline structure throughout the macroscopic film, tiny assembly defects could deteriorate the local color appearance and challenge the large-scale color uniformity. Hence, another colloidal self-assembly structure featuring short range order but long-range disorder64 (Fig. 4b), in the form of amorphous photonic crystal or photonic glass (PhG), opens another path for non-iridescent structural color generation. With the lack of long-range ordering, PhG-based structural color appears to be more diffusive and non-iridescent. In contrast to balancing the solvent evaporation rate and colloidal self-assembly rate, locking/disturbing/freezing the assembly process of colloidal particles ensures a long-range disorder in PhG. One commonly used method utilizes a glass phase transition of the colloidal system. As shown in Fig. 4c and d, a polymeric gel particle underwent a phase transition, forming a non-closed-packed state as the gel suspension concentration increased. Distinctive color from blue to green to red can be produced65,66 with various average inter-particle distances from differences in particle surface coverage. Another fabrication method adopts the nature of spinodal phase separation. This strategy has been widely adopted by nature where bird feather barbs show a spinodal deposition phase of the keratin structure, leading to a brilliant blue color.7,67,68 A template-based synthesis using the feather barb with sol–gel chemistry leads to an inorganic replica of similar structural color69,70 (Fig. 5a–c). To the best of our knowledge, no direct bottom-up self-assembled PhG structural color has been made with spinodal decomposition. However, a dewetting process during thermal anneal can produce a similar pattern but in 2D. With the dewetted pattern as a litho-free mask, followed by a metal-assisted chemical etch process, a colored silicon substrate can be made71 (Fig. 5d and e). Other methods including spray coating72 or rapid thermal evaporation also work well by having these colloidal particles lose their mobility before entering the thermodynamic equilibrium state.
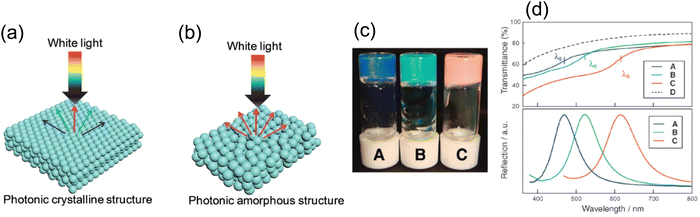 | ||
| Fig. 4 Schematic illustration of (a) photonic crystal (PhC) and (b) photonic glass (PhG) showing specular and diffusive reflection. [Reproduced from ref. 64, with permission from Royal Society of Chemistry, Copyright 2019]. (c) Glassy colloidal arrays in liquid crystal showing structural color and (d) their corresponding reflection spectra. [Reproduced from ref. 65, with permission from American Chemical Society, Copyright 2009; Reproduced from ref. 66, with permission from Royal Society of Chemistry, Copyright 2009]. | ||
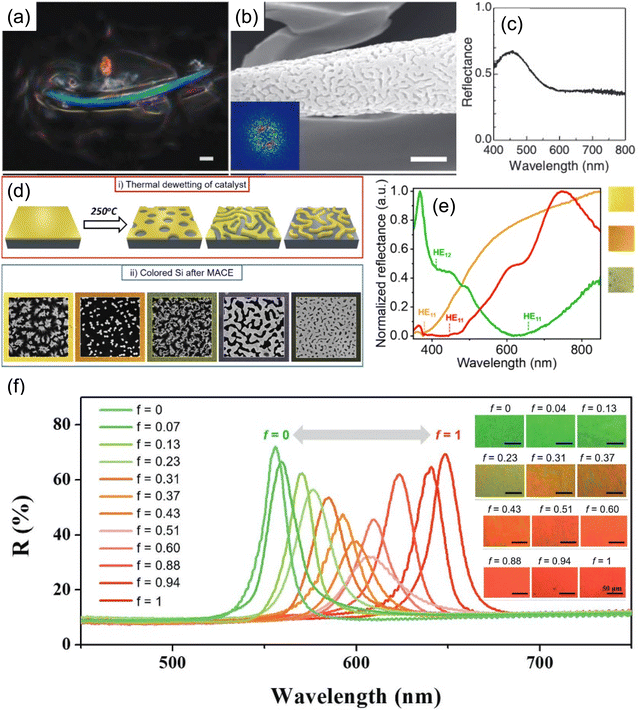 | ||
| Fig. 5 (a) Spinodal phase of SiO2 structural color using beetle elytral surface as template and its (b) SEM image and (c) reflection spectrum. [Reproduced from ref. 66,70, with permission from Royal Society of Chemistry, Copyright 2009 and 2013]. (d) Schematic illustration of thermal dewetting followed by metal-assisted chemical etching (MACE) for color Si fabrication. (e) Optical images and reflection spectra of color Si. [Reproduced from ref. 71, with permission from American Chemical Society, Copyright 2023]. (f) Color transition from green to red by changing the ratio of two nanocolloids with different sizes. The reflection spectra showing the transition from specular reflection to diffusive reflection. [Reproduced from ref. 73, with permission from American Chemical Society, Copyright 2019]. | ||
In addition to PhC and PhG, a transition in the assembly ordering can be realized with the co-assembly of colloidal particles of various size.74,75 By varying the fill fraction of the bi-disperse particles (one small and one large in size),73 the assembly structure gradually changes from a highly ordered PhC to disordered PhG (Fig. 5f), thus changing from specular color appearance to a more diffusive one. The reflection peak wavelength is therefore a superposition of the diffraction wavelengths for crystalline structures composed of only small or large particles.
 | ||
| Fig. 6 (a) SEM image of stretched PVA photonic crystal. [Reproduced from ref. 76, with permission from American Chemical Society, Copyright 2009]. (b) Schematic illustration of magnetic field induced γ-Fe2O3–SiO2 particles elongation. [Reproduced from ref. 54, with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Copyright 2009]. (c) Synthesis of heat compressed discoidal particles and their self-assembled structural color. [Reproduced from ref. 77, with permission from American Chemical Society, Copyright 2022]. | ||
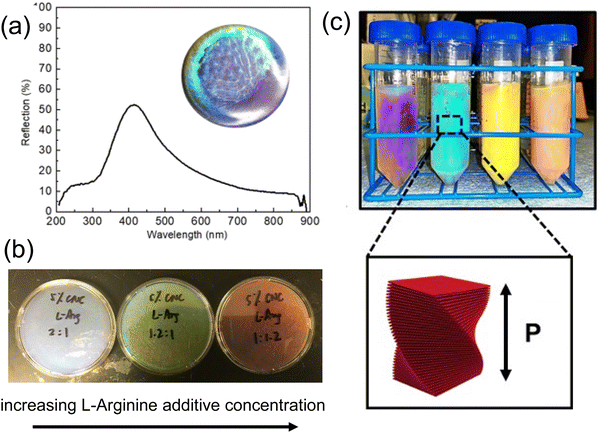
While the assembly of high-aspect ratio particles either leads to a disordered structure or nematic-like liquid crystal phase (not capable of forming a well-defined stopband), a slight twist at the particle level makes a different story. Many plants and fruits78,79 take advantage of the twist in their cell walls (Fig. 7a), where cellulose fiber aligns and twists into a chiral nematic phase (Fig. 7b), leaving a well-defined pitch in between. These helical structures serve as chiral Bragg-reflectors, creating stopbands for light with certain circular polarization. This leads to stunning structural colors. This masterpiece has been replicated by artificially synthesizing a high aspect ratio (∼15–20) cellulose nanocrystal (CNC). CNCs can be derived directly from paper pulp through sulfuric acid hydrolysis, where the amorphous region of the cellulose fibers is decomposed while the crystalline region is preserved.80Fig. 7c gives an overview of the self-assembly process of CNC.81–89 A typical CNC could have a rod-like shape with a diameter of 10–20 nm and length of 150–200 nm. The chiral natural of the glucose unit within the cellulose backbone gives a twist to the CNC surface and enables the self-assembly into the chiral nematic phase. The stopband wavelength is determined by the pitch within the assembled helix, where the resonance wavelength λ is a function of pitch length p, the film refractive index n and the angle of incidence θ according to the de Vries formula90λ = np![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (θ). The chiral nature of the film also imposes selectivity in light polarization upon reflection, giving around 50% reflection within the photonic stopband (Fig. 8a). With the addition of small molecular additives,87,91–96 a spectrum of the colors from violet to red can be produced continuously by varying the CNC-additive ratio (Fig. 8b, e.g. adding L-arginine). Continuous roll-to-roll fabrication of the CNC film under controlled solvent evaporation give brilliant structural color pigments for future scalable production.97
(θ). The chiral nature of the film also imposes selectivity in light polarization upon reflection, giving around 50% reflection within the photonic stopband (Fig. 8a). With the addition of small molecular additives,87,91–96 a spectrum of the colors from violet to red can be produced continuously by varying the CNC-additive ratio (Fig. 8b, e.g. adding L-arginine). Continuous roll-to-roll fabrication of the CNC film under controlled solvent evaporation give brilliant structural color pigments for future scalable production.97
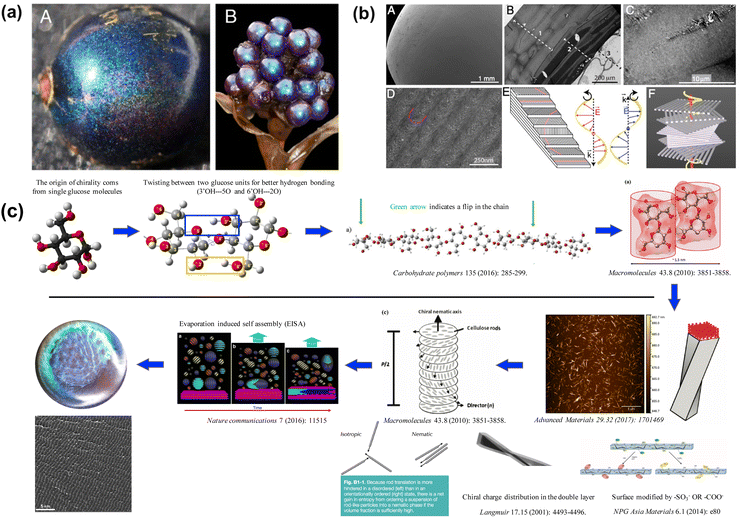 | ||
| Fig. 7 (a) Photo of Pollia condensate pericarp. (b) Microscope image of the helicoidal cell walls. [Reproduced from ref. 78, with permission from National Academy of Science, Copyright 2012; Reproduced from ref. 79, with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Copyright 2013]. (c) Schematic illustration of evaporation-induced self-assembly of CNC. The chiral nature of glucose is passed all the way up to the super-molecular structure. | ||
Most polymer self-assembly falls in the category of block copolymers (BCPs), where the polymer backbone is made of two or more units with distinctive chemical properties. Phase separation between these distinctive backbone segments enables various ordered structures from lamellae, to cylinders, to close-packed micelles106 (Fig. 9a and b) and more depending on the relative ratio of the segments. Though the assembly morphology as well as the BCP formulation has been widely investigated, it's quite challenging for these BCP assembled domains to reach the size capable of producing structural colors. The major difficulty lies in synthesizing sufficiently large molecular weight polymers as well as achieving large refractive index contrast between the domains. A commonly used strategy to bring structural color to BCP assembly is by expanding the domain size with external stimuli, which at the same time modulates the refractive index of the domain. Hence, functional chemical groups responsive to external stimuli are frequently incorporated into the BCP backbone. One popular candidate is 2-vinyl pyridine, where a reversible quaternization can be made with various stimuli. In an early attempt, the polystyrene-block-poly(2-vinyl pyridine) (PS-b-P2VP) self-assembly lamellae, the P2VP domain was swelled (and the domain refractive index was lowered) in reduced salt concentrations to give a photonic stopband near the infrared (Fig. 9c).107 Further modulation of the PS-P2VP system also involved stimuli as pH,108 solvents,109,110 and temperature,111 among others. Other BCP assembly phases including gyroid112,113 and micelles114 are also made possible with delicate polymer design and concentration control. Following the idea of template synthesis, a degradable BCP template was used to achieve large index contrast between the distinctive domains. With the exposure to UV light, the PMMA domain undergoes photo-degradation, which can be removed in acetic acid in a polystyrene-block-poly(methyl methacrylate) (PS-b-PMMA) assembled gyroid structure.113 A replacement is then made with the infiltration of high refractive index oxide (e.g. TiO2) precursor under sol–gel process to give a more brilliant blue color (Fig. 9d).
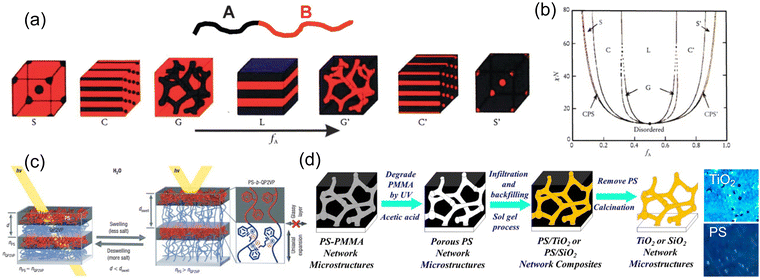 | ||
| Fig. 9 (a) Various self-assembly phases of BCP and (b) phase diagram. [Reproduced from ref. 106, with permission from Royal Society of Chemistry, Copyright 2012]. (c) Photo-responsive PS-P2VP BCP showing reversible color changes. [Reproduced from ref. 107, with permission from Nature Publishing Group, Copyright 2007]. (d) TiO2 gyroid structural color made out of BCP self-assembly template. [Reproduced from ref. 113, with permission from American Chemical Society, Copyright 2018]. | ||
An alternative strategy for fabricating larger BCP self-assembly domains is via brush block copolymers (BBCPs) where various distinctive polymer side chains are grafted to a linear polymer backbone. The increasing degree of polymerization (DP) as well as the longer side chain significantly expand the self-assembly domain size, and no external stimuli is needed to further push the domain scale into visible wavelength. Grafting through ring opening metathesis polymerization (ROMP) is a common strategy for BBCP synthesis115 (Fig. 10a). A widely studied ω-norbornenyl based backbone is typically adopted due to its easy accessibility and structural rigidity. Macromonomer (MM) moieties like polystyrene, polyhydroxystyrene,116 poly(methyl methacrylate)117,118etc. are frequently used as sides chains to enable BBCP structural colors. Yu et al. developed a graft through method of BBCP synthesis of ω-norbornenyl polystyrene (NPSt) and ω-norbornenyl poly(4-tert-butoxystyrene) (NPtBOS) via ROMP.119 Over 10 BBCPs with various molecular weights (MWs) have been synthesized with lamellae domain sizes ranging from 40 nm to 200 nm. These self-assembled structures are much larger compared to a high-DP linear BCP and can produce structural coloration from blue to pink (Fig. 10b). Note that the alkyl spacer unit in the MMs are the key to enable a high MW synthesis, where the bulky side chain steric hinderance is being mitigated. However, these flexible alkyl chains could reduce backbone rigidity and lead to an insufficient increase in domain sizing.118 One method of bypassing the incorporation of the flexible spacer while maintaining a sufficient domain size is by introducing a semicrystalline BBCP with poly(ethylene oxide) (PEO). A hierarchical structure of both lamellae and crystalized PEO domains (Fig. 10c) is formed under annealing.120 In addition, a large refractive index change from 1.46 to 1.64 has been observed upon PEO domain crystallization hence giving a more brilliant color appearance. Shape-memory characteristics are also observed due to well-defined PEO phase transition temperature. Another strategy to compromise the steric hinderance-backbone rigidity dilemma is a dendritic design of MM side chain with BBCP. An AB3-type MM with dodecyl and fluorobenzyl units radiates from the center benzyl ring into three branches,121 forming a wedge-like shape with increased steric hinderance but less flexibility. Thus, these wedge-like units give enough MM accessibility during ROMP for high MW BBCP synthesis while keeping the backbone rigidity. The bulk BBCP is then extruded into filament for 3D printing into various geometrically different structural colored objects (Fig. 10d). A spin-off company named “Cypris” is now demonstrating even more promising BBCP-based structural color paints (Fig. 10e) for industrial scale production.122 Astonishing rainbow color from blue to red shows up only after several minutes of paint drying.
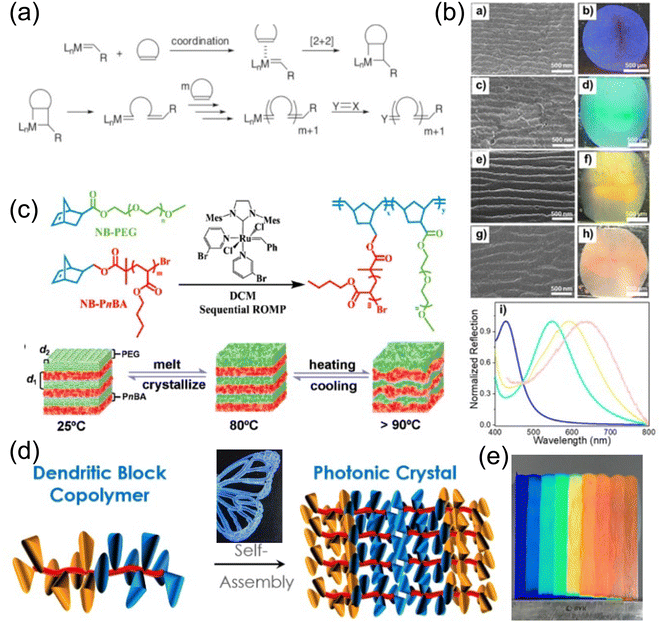 | ||
| Fig. 10 (a) Illustration of ring opening metathesis polymerization (ROMP) process. [Reproduced from ref. 115, with permission from Elsevier Publishing Group, Copyright 2007]. (b) Self-assembly of BBCP with NPSt and NPtBOS. [Reproduced from ref. 118, with permission from American Chemical Society, Copyright 2019]. (c) A hierarchical lamellar BBCP with crystalizing PEO for structural memory photonic crystal. [Reproduced from ref. 120, with permission from American Chemical Society, Copyright 2020]. (d) Synthetic strategy of AB3-type BBCP for 3D structural color printing. [Reproduced from ref. 121, with permission from American Chemical Society, Copyright 2017]. (e) Rainbow structural color produced by Cypris Materials Inc. [Reproduced from ref. 122, with permission from Cypris Materials Inc, Copyright 2023]. | ||
In summary, the self-assembly of nanocolloids and polymers presents a straightforward approach to producing large-scale structural color. This process relies on the intermolecular forces between particles and thus can be easily scale up for bulk production. In addition, minimal external energy is usually required for the assembly process compared to traditional vacuum-based deposition methods. As a result, significant energy savings are achieved. However, structural defects from the self-assembly process poses a big challenge in the way of commercializing self-assembled structural color, where large scale color uniformity can hardly be guaranteed. Therefore, grinding self-assembled structural color into micro-pigments provides a potential method for its real-world application.
2.2. Multilayer coating
As demonstrated in the previous section, photonic crystals synthesized through nanoparticles or block-copolymer self-assembly gives various colors; however, their color properties are largely compromised when ground into micro/nano-flakes for general coating applications. Moreover, these photonic crystals usually take several to hundreds of micron thicknesses (i.e. CNC or HPC films) to achieve high color brilliance, which largely limits their application in thin film coatings. Multilayer pearlescent pigments, on the other hand, utilize light interference to give brilliant color with sub-micrometer film thickness. Typical thin-film coating methods (Fig. 11)123 from solution phase includes dip-coating,124,125 spin-coating,126 and spray coating.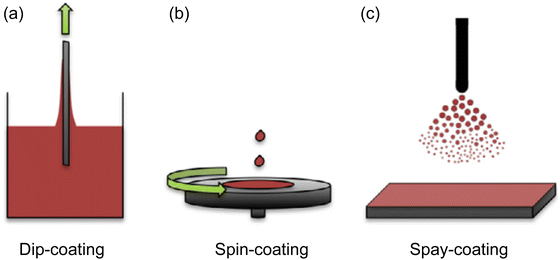 | ||
| Fig. 11 Schematic illustration of three commonly used thin film coating method (a) dip-coating, (b) spin-coating and (c) spray coating. [Reproduced from ref. 123, with permission from Taylor and Francis Publishing Group, Copyright 2013]. | ||
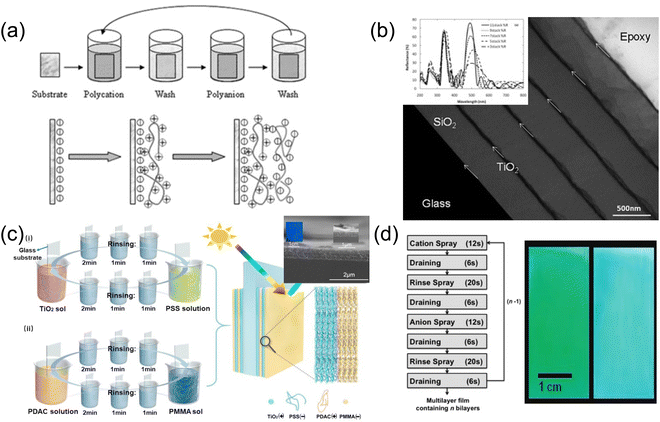 | ||
| Fig. 12 (a) Schematic illustration of a LbL process where alternative coatings of polycation and polyanion are implemented. [Reproduced from ref. 127, with permission from American Chemical Society, Copyright 2020]. (b) SEM and reflection spectra of PAH-SiO2 or TiO2-PVS multilayers. [Reproduced from ref. 129, with permission from Royal Society of Chemistry, Copyright 2009]. (c) Hydrophobic Bragg reflector made of PMMA particles with LbL coating. [Reproduced from ref. 130, with permission from Elsevier Publishing Group, Copyright 2021]. (d) Spray coating LbL TiO2/SiO2 Bragg stacks featuring large area uniform structural colors. [Reproduced from ref. 131, with permission from American Chemical Society, Copyright 2011]. | ||
To create a reflection stopband centered at λ0, a quarter-wave Bragg stack with alternating high index (nH) and low index (nL) satisfying nHdH = nLdL = λ0 is anticipated, where dH and = dL represent the thicknesses of the high index and low index layers respectively. Inorganic nanoparticles like SiO2 and TiO2 are commonly introduced to increase the layer index contrast,129 as demonstrated in Fig. 12b. A bilayer structure with a cation–anion pair (e.g. PAH-SiO2 or TiO2-PVS) is first formed by two-step LbL coating, featuring a thickness of a few nanometers (mainly determined by the size of nanoparticles). The resulting bilayer coating process is then repeated multiple times to achieve desired thickness. With proper tuning of the LbL cycles and further calcination (to remove the organic component), a SiO2/TiO2 Bragg stack is formed with a stopband centered in a visible wavelength. As the removal of the polyelectrolyte leads to a collapse of the nanoparticle assembly, the index of the layer could be fine-tuned according to the particle size and the choice of the polyelectrolyte, allowing customization of the stack properties. Though the charged polyelectrolyte typically results in a hydrophilic coated surface, the rich solution chemistry enables hydrophobic polymer incorporation into the polyelectrolyte framework and thus expands the potential of LbL coating. In a recent example, Yu et al.130 fabricated a hydrophobic structural color coating by introducing hydrophobic PMMA particles (prepared with negative charge) into PDAC layer (Fig. 12c). The as prepared structural colored TiO2/PMMA stack thus becomes capable of changing its color appearance upon organic vapor uptake.
Compared to dip-coating LbL, which takes a very long time to complete the entire stack considering the thickness of a single bi-layer coating, spray coating provides a more efficient strategy for LbL stack fabrication. As shown in Fig. 12d,131 a spray-coated TiO2/SiO2 LbL film was fabricated via multiple cycles of cation spray-draining-rinse spray-draining-anion spray-draining-rinse spray-draining. Compared to a similar dip-coated structure, the spray coating method significantly reduces a bilayer coating time from 36 min to 90 s while preserving color uniformity and smooth interface between layers. An iridescent 11-stack green-cyan structural color film (Fig. 12d right) with high reflectivity over 90% was demonstrated after calcining the LbL film under 550 °C.
Spin coating provides an effective way of rapid solvent removal through centrifugal force, leaving a thin layer of material on surface. The thickness of the spin-coated film can be tuned by solute concentration, solvent evaporation rate and spin rate. Silvia et al. has demonstrated a 1-D Bragg reflector132 with alternative SiO2/TiO2 layers directly out of SiO2 and TiO2 nanoparticle solutions (Fig. 13a). Methanol was chosen as a cosolvent with water for the nanoparticle solutions to prevent coagulation and enhance the solvent volatility during spin-coating. The as-spun film thickness ranges from 40–200 nm in accordance with nanoparticle solution concentration from 1–6 wt%. Subsequent layers can be coated continuously without further stabilization. The entire Bragg stack can therefore be built within a few minutes followed by annealing to stabilize the layers mechanically. Infiltration of polymer materials such as polydimethylsiloxane (PDMS) into the Bragg stack further enables the making of a flexible structural color133 (Fig. 13b). In addition to inorganic Bragg reflectors, polymeric materials134 are excellent thin film candidates for direct spin-coating. Several examples using high index polymers like hyperbranched polyvinyl sulfide,135 poly(N-vinylcarbazole),136etc. have been used in a full-polymer Bragg reflector through spin coating and feature brilliant structural colors.
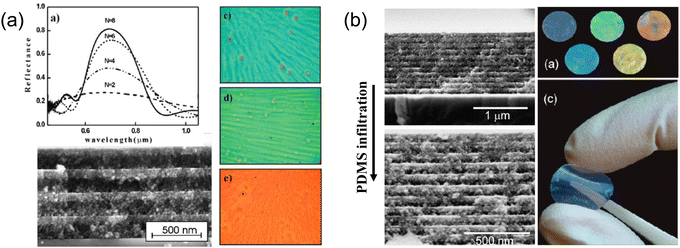 | ||
| Fig. 13 (a) Optical and SEM image of spin-on alternative SiO2/TiO2 layers of Bragg stacks and their reflection spectra. [Reproduced from ref. 132, with permission from American Chemical Society, Copyright 2008]. (b) PDMS infiltrated inorganic Bragg reflector featuring flexible structural colors. [Reproduced from ref. 133, with permission from American Chemical Society, Copyright 2010]. | ||
For dielectric layer coatings, metal alkoxides are the most common oxide precursors, where they can be easily dissolved in various types of solvents and are ready to use. When water is introduced, metal alkoxides undergo hydrolysis to form metal hydroxides, which then condense into metal oxides. Thus, the sol–gel process provides a universal method for film formation from a variety of dielectrics. The coating conditions determine the sol–gel kinetics and thus the film properties including thickness and density, and composition can be changed accordingly. One strategy of depositing SiO2/TiO2 multilayer Bragg reflector utilizes an electro-assisted sol–gel process (Fig. 14a), where a bias is applied to a conductive substrate (cathode) to reduce water to OH− and create a basic local environment.137,138 The sol–gel condensation reaction kinetics are then boosted, triggering deposition of the oxide material onto the substrate. The deposited layer thickness is determined by the applied bias and deposition time. The as-deposited film, after being withdrawn from the solution and dried, is ready for the deposition of the next layer.139 The multilayer film shows fairly smooth interfaces as well as a decent stop-band. Similarly, spin-coating of titanium alkoxide is adopted in structural colored photovoltaic cell fabrication to balance the color performance and PV cell efficiency.140 Rapid spinning of the titanium alkoxide solution triggers the TiO2 condensation as solvent evaporates. The as-spun film thickness is thus determined by the spin-rate as well as the evaporation rate.
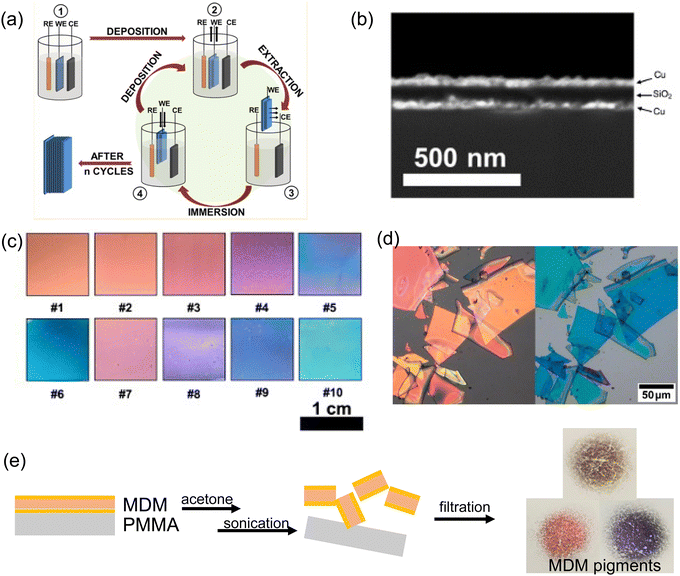 | ||
| Fig. 14 (a) Schematic illustration of electro-assisted sol–gel process. [Reproduced from ref. 137, with permission from American Chemical Society, Copyright 2017]. (b) Cross-section SEM showing F–P type (Cu/SiO2/Cu) structural color and (c) their brilliant color appearances.141 (d) Cracked TiO2/SiO2 Bragg reflector flakes due to internal sol–gel process stress.142 (e) MDM structural color pigments lifted off by sonication. | ||
However, due to the internal stress buildup inside the sol–gel film with increased thickness, overall stack thickness is usually limited to a few micrometers to avoid cracking or delamination. Therefore, though sol–gel based thin film coating is very effective with oxide films tens to hundreds of nanometers thick, it is not suitable for complicated photonic structure fabrication. However, high-performance structural colors can be produced with structures simpler and thinner than photonic crystals or Bragg reflectors. The Fabry–Perot (F–P) cavity structure has less than four layers and has been explored as a popular candidate. A lossy cavity is required to absorb the resonant light energy and leaves a resonance dip in the reflection spectrum, giving subtractive colors like cyan, magenta, and yellow. For example, we have demonstrated a tri-layer metal-dielectric-metal (MDM)-type F–P cavity fabricated using a full solution process. The fabrication method combines electroless deposition of metal layer (Cu) with a sol–gel coated SiO2 or TiO2 deposited through dip-coating.141 Various iridescent colors from orange to cyan have been produced with a total stack thickness under 200 nm (Fig. 14b and c). Hence, dip-coating of the sol–gel film provides a decent amount of control in film thicknesses by varying the precursor solution concentration or the withdrawal rate. Notably, such full solution-processed MDM method ensures solution compatibility across all dielectric and metal layers without compromising layer quality. The coated film shows a low surface roughness of 1.8 nm across a centimeter scale sample surface. Further, we have demonstrated that a modified F-P layer structure with the sequence of high-RI/low-RI/Absorber (HLA) could also produce brilliant and vivid structural color with proper balance between the radiative and absorptive decay rate of the F–P cavity.143 With a similar sol–gel process, TiO2/SiO2/Si or ZrO2/SiO2/Si stacks can be dip-coated from metal alkoxide solution. These HLA structural colors could easily span the entire CMY space by varying the dielectric layer (i.e. high RI or low RI) thickness.
However, the fragmentation method efficiency and yield are insufficient for large-scale production. A more practical way of structural color pigment fabrication involves an additive method to coat flake substrate bottom-up. Mica, a smooth sheet layered silicate, is widely adopted as a perfect flake substrate. Over the past decades, various metal oxides, including Fe2O3, TiO2, Pb(OH)2, PbCO3, BiOCl, (Sn,Sb)O2, etc. have all been demonstrated as good candidates for pearlescent mica pigment synthesis.144,145 Deposition of a metal salt precursor is trigged under carefully chosen conditions to selectively deposit onto the activated mica surface and avoid bulk precipitation. The filtered pigments are then calcinated under high temperature for dehydration and crystalized into metal oxides.
Solution-processed multilayer coating offers a simple and intuitive layer by layer method for multilayer film color production. While it mimics the thin film deposition commonly takes place in vacuum chambers, it allows the quick fabrication of multilayer structure under ambient condition. With careful engineering controls, large scale uniformly colored film can be fabricated continuously with multiple coatings. Hence the cost is greatly reduced by getting rid of the harsh fabrication environment and the cost in tool maintenance. One challenge still presents in the complexity of chemistry involved in solution-processed multilayer coating. In addition to the internal stress caused by different material lattice matching (i.e. usually a concern in vacuum-based deposition), chemical compatibility between multilayer coatings as well as their compatibility with substrate is of great concern during fabrication. Reliable and compatible chemical coating recipes are highly desirable in future research and development.
2.3. Structural color produced from electrochemical methods
Electrochemical methods are another way to shape the structure of thin films. It is well-known that anodization of aluminum surfaces leads to the formation of cylindrical holes with honeycomb structures.146 The aluminum surface is oxidized in acidic aqueous solution under high voltage and the radial current distribution leads to a cylindrical hole throughout the oxidized layer. This technique has been widely adopted in modern aluminum surface coloration where dye pigments are encapsulated inside the anodized aluminum oxide (AAO) holes. Since the hole size is directly proportional to the current density across the surface, Liu et al. has designed a stack of AAO layers with different refractive indexes,147 mimicking a 1D Bragg-type reflector. A pulsed anodization of aluminum is carried out. By varying the current passed through the aluminum surface with different pulse duration, AAO layers with various porosity and thickness are formed (Fig. 15a). The refractive index of each porous layer can be approximated with a linear combination of the RI from Al2O3 and air by the fill fraction. The resulting multilayer stack shows brilliant color in both transmission and reflection due to the formation of a high contrast, wide stopband within the visible range. Furthermore, a highly ordered porous AAO film can be obtained with a novel self-ordering electrolyte, namely etidronic acid.148 The aluminum surface is anodized under a self-ordering voltage (210–270 V for etidronic acid) followed by selective dissolution of aluminum oxide in CrO3/H3PO4 solution. In contrast to the AAO Bragg stack, the structural color produced with self-ordering anodization comes from a 2D photonic crystal. Large hole sizes from 500–800 nm can be obtained, giving rainbow color from red to violet (Fig. 15b).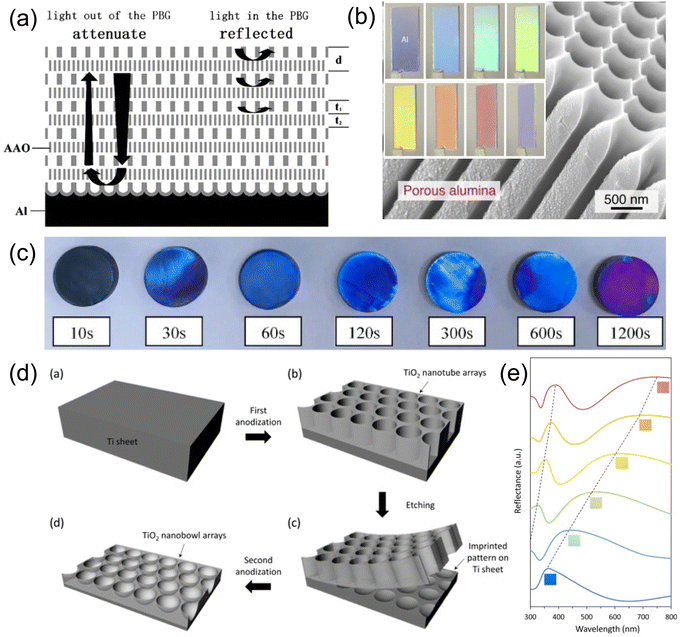 | ||
| Fig. 15 (a) Schematic illustration of porous AAO Bragg stack with pulsed anodization of aluminum. [Reproduced from ref. 147, with permission from Elsevier Publishing Group, Copyright 2011]. (b) Structural colored AAO photonic crystal from self-ordered anodization process. [Reproduced from ref. 148, with permission from Elsevier Publishing Group, Copyright 2015]. (c) Gradual change of color in anodized titanium film over various anodization times. [Reproduced from ref. 149, with permission from Elsevier Publishing Group, Copyright 2023]. (d) A schematic diagram demonstrating the fabrication of TiO2 nanobowl array on titanium film and (e) their corresponding refraction spectra. [Reproduced from ref. 150, with permission from American Chemical Society, Copyright 2016]. | ||
Other metals like platinum, titanium, silicon etc. can be anodized as well. Due to the high refractive index of TiO2, structural colors from anodized TiO2 on Ti can be readily produced. Fig. 15c demonstrates structural color produced via Ti anodization under different anodization times.149 The SEM images revealed changes to the TiO2 surface morphology where indentation and protrusions inside the lamellar structure are critical for color production. More ordered TiO2 arrays (Fig. 15d) were grown directly on top of the Ti sheet through two-step anodization.150 A potential of 30–75 V was applied in the first anodization step, producing a rough and irregular TiO2 honeycomb structure. The TiO2 layer was then removed with a sticky tape, leaving an array of concave cavities on the Ti sheet surface. A second anodization step was then carried out, forming hierarchical TiO2 bowl arrays. A rainbow color can be visualized by increasing the air cavity size inside the bowl array. The reflection spectrum features two broad stop-bands (Fig. 15e) where two peaks correspond to the first and second order diffraction from the periodic TiO2 bowl surface respectively.
Electrophoretic deposition (EPD), on the other hand, utilizes electrostatic force to move charged particles towards the electrode surface for deposition. Negatively charged SiO2 nanoparticles pre-synthesized by sol–gel chemistry are forced to deposit onto an ITO glass anode under applied voltage.151 A well-ordered PhC was formed with close-packed SiO2 particles under low EPD voltage (∼5 V, 25 min), while an amorphous PhG was obtained once the EPD voltage increased to 90 V (1 min). The difference in the resulting structural color could be clearly seen by the change in color iridescence and angle-resolved spectra. Compared to the convective deposition method of PhC or PhG described in Sections 2.1.1 and 2.1.2, EPD uses a much shorter deposition time to deposit conformal and uniform structural colors across curved surfaces (Fig. 16a). Another example152 uses EPD of pre-synthesized polystyrene (PS) onto carbon fiber to give core–shell type structural color fibers with colors in red, green and blue (Fig. 16b). The flexibility and relative high throughput of the EPD structural color fiber shows promising applications in developing dye-free fabrics or textiles.
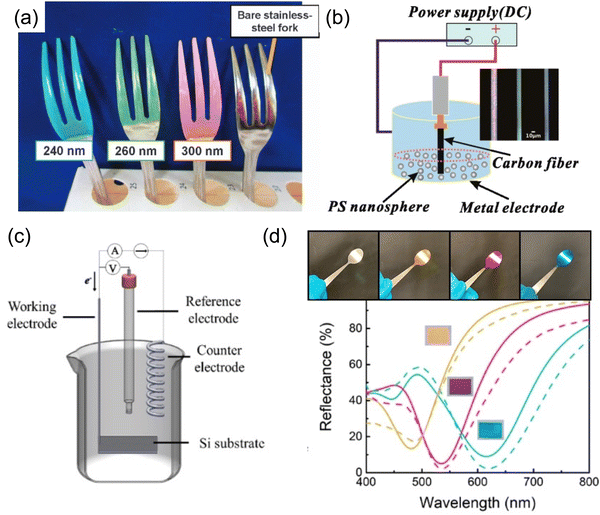 | ||
| Fig. 16 (a) Structural colored forks coated with electrophoretic deposition of SiO2 nanoparticle on curved surfaces. [Reproduced from ref. 151, with permission from Nature Publishing Group, Copyright 2017]. (b) Photonic crystal of PS particles deposited by EPD on carbon fibers. [Reproduced from ref. 152, with permission from American Chemical Society, Copyright 2013]. (c) A schematic illustration of an electrochemical deposition setup. (d) Electrodeposited Au/Cu2O/Au structural color on curved stainless steel spoon surface and their corresponding reflection spectra. [Reproduced from ref. 153, with permission from American Chemical Society, Copyright 2019]. | ||
In contrast to the process of anodization or EPD, where a large voltage is required to induce electrochemical responses, electrochemical deposition uses a very small voltage to trigger a chemical reaction at the electrode–electrolyte interface for layer deposition. Electrodeposition of F–P cavity-type structural color (Fig. 16c) has been demonstrated with an Au/Cu2O/Au trilayer stack on a heavily n-doped Si substrate.153 As a reduction potential was applied to the cathode, the positively charged metal ion (i.e. Au(III)) is reduced into its metallic state on the Si substrate. Similarly, Cu2O is reduced from a copper citrate complex by carefully controlling the solution pH and applied potential to prevent over reduction into metallic copper. The top Au layer is carefully deposited with a solution pH ∼10–11 compatible with the Cu2O layer while applying a relative low reduction potential to avoid aggressive hydrogen evolution, which can peel off the underlying layers. Shiny yellow, magenta, and cyan colors are produced (Fig. 16d) conformally on both flat Si substrates and curved stainless steel spoon surfaces, demonstrating promise in coating objects with complex geometries.
In brief, electrochemical methods provide new pathways toward structural color fabrication. Chemical reactions are triggered at the electrode–electrolyte interface with the flow of electrons gauged through the voltage applied. With the ability of both 2D growth thin film growth and 3D sculpting of the surface, electrochemical methods unlock the various possible photonic structures. Moreover, with careful control on the surface current distribution, the film thickness can be tuned to achieve either uniform or gradient color appearance. Thus allowing special visual effect to be produced with one deposition. However, the limitation with electrochemical methods is quite obvious where a conductive substrate is always required. The process window of electrochemical methods is another concerned due to side reaction and layer compatibility with in the electrolyte.
2.4. Structural color from particles
Though nanoparticle solutions alone show fascinating transmissive colors, immobilization of these nanoparticles further expands their use in plasmonic structural colors. Over the past five years, it has been shown that the incorporation of disordered nanoparticles into various photonic structures can lead to very different colors from when a continuous metal layer is used. The optical property of the nanoparticle layer evolves with changes to layer morphology, giving distinctive color appearances. As shown in Fig. 17b, a discontinuous metal layer (i.e. metal nanoparticles) gives rise to primary reflective peaks in a F–P type structure,163–166 polarizonic colors (named after electronic oscillation at optical frequency) on metal substrate,167–169 gap-plasmon-resonance color with ultrathin dielectric layer170,171etc. These initial attempts of nanoparticle deposition require quite special deposition techniques including arc plasma deposition, cluster beam deposition, e-beam evaporation, etc., making them far from practical for mass production. However, immobilizing solution-based nanoparticles is a promising pathway for cost-effective plasmonic structural color fabrication.
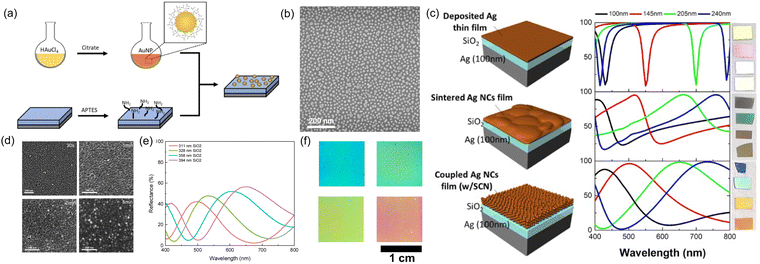 | ||
| Fig. 17 (a) Schematic illustration of gold nanoparticle synthesis and immobilization/deposition on substrate. (b) SEM image of gold nanoparticles deposited on surface. (c) Schematic configurations, absorption and reflection of various Ag/SiO2/Ag structural color. [Reproduced from ref. 172, with permission from American Chemical Society, Copyright 2019]. (d) SEM images showing the evolution of electroless deposited copper on substrate surface. (e) Reflection spectra and (f) color appearance of Cu nanoparticle/SiO2/Cu structural color. [Reproduced from ref. 141, with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Copyright 2013]. | ||
One straightforward solution deposition method utilizes the electrostatic attraction of charges between nanoparticle surface and substrate.173,174 As shown in Fig. 17a, gold nanoparticles of desired size capped with negatively-charged citrate ligands were pre-synthesized in aqueous solution. On the other hand, positive charges were functionalized onto a silica-based substrate through silanization with (3-aminopropyl)triethoxysilane (APTES). A gold nanoparticle monolayer was adsorbed onto the silica surface after immersing the substrate into the nanoparticle solution. The nanoparticle surface density can be well controlled (Fig. 17b) by immersion time, ionic strength and nanoparticle solution concentration. In another example, oleylamine-capped silver nanocubes were spin coated onto an APTES-treated SiO2/Ag substrate.172 Vivid colors were produced with a wider absorption stopband (Fig. 17c) than a sintered or smooth thin silver film. Another method of nanoparticle immobilization is via direct growth of nanoparticles on substrate surface. A seed layer is first required as a nucleation and growth center. Electroless deposition of metal provides a perfect method to implement this process. Fig. 17d records a time-evolution of copper electroless plating in both surface morphology and color appearance.141 The top copper layer first forms discrete particles (30 s), then grows into islands (1 min), and finally overlaps with each other (2 min). A particulate morphology is always observed even with extending deposition time (≥5 min) due to the repetitive growth pattern, i.e. particle-island-film. Hence, the copper film reflectivity increases with deposition time while always featuring low reflectivity in lower wavelengths compared to a vacuum-deposited copper film. With short electroless deposition times of copper on top of SiO2/Cu surface (i.e. F–P type cavity), a series of additive colors with reflective peaks can be clearly observed (Fig. 17e and f). As in the case of gold nanoparticles, the addition of chloroauric acid (HAuCl4) and hydroxylamine (NH2OH) facilitates the growth of the seed layer into larger nanoparticles. Note that the pre-deposited seed layer catalyzes the sluggish reduction reaction kinetics between HAuCl4 and NH2OH, thus enabling in situ growth of gold nano-domains on the surface.175–178
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dpi. Further engineering of the particle refractive index, size and interparticle distance allows for separation and tuning of the multipole contribution in dielectric particle scatting (in this case, the electric dipole ED and electric quadrupole EQ), giving a rarely observed red color. Disordered ZnO particle aggregates can be obtained via dip-coating, where the surface coverage and therefore interparticle spacing can be well controlled.182 Several potential applications of NAIR have been demonstrated in the field of colored photovoltaic cells,35 automobile paints,183 anticounterfeiting184etc. As a final note for this section, Mie resonance induced color is only observable for particles of relatively high refractive index. Low index particles, e.g. SiO2, only produce broad resonance that is hardly visible. Zhou et al. reported that, after coating with a thin metal cap, the single particle resonance becomes highly pronounced and the resulting color strongly correlates with the particle size.185
000 dpi. Further engineering of the particle refractive index, size and interparticle distance allows for separation and tuning of the multipole contribution in dielectric particle scatting (in this case, the electric dipole ED and electric quadrupole EQ), giving a rarely observed red color. Disordered ZnO particle aggregates can be obtained via dip-coating, where the surface coverage and therefore interparticle spacing can be well controlled.182 Several potential applications of NAIR have been demonstrated in the field of colored photovoltaic cells,35 automobile paints,183 anticounterfeiting184etc. As a final note for this section, Mie resonance induced color is only observable for particles of relatively high refractive index. Low index particles, e.g. SiO2, only produce broad resonance that is hardly visible. Zhou et al. reported that, after coating with a thin metal cap, the single particle resonance becomes highly pronounced and the resulting color strongly correlates with the particle size.185
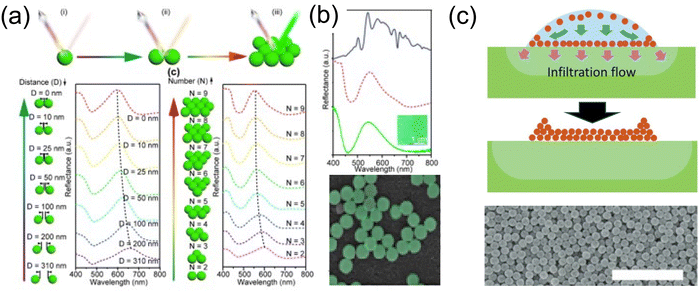 | ||
| Fig. 18 (a) Schematic illustration of NAIR and their reflection spectra with various configurations. (b) Simulated reflection spectrum of aggregates constructed of nine 310 nm dielectric spheres (red dotted line), experimental reflection (green solid line), and dark-field reflection (blue solid line) spectrum of aggregates constructed of 310 nm ZnS spheres (green solid line). The inset is the digital image of this aggregate and their false colored SEM image. [Reproduced from ref. 180, with permission from American Chemical Society, Copyright 2020]. (c) Schematic illustration of infiltration-driven nonequilibrium colloidal assembly and the resulted SEM. Scale bar: 2 μm. [Reproduced from ref. 181, with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Copyright 2018]. | ||
With confined space, nano-/micro-particle-derived structural colors distinguish themselves from self-assembled structural colors, where colors are produced within one single particle or particle cluster instead of bulk assemblies. Hence, particle-derived colors never suffer from assembly uniformity or defects. Besides, resonance inside particles could be added to other structural color platforms to produce richer color appearance. However, note the scattering nature of these colored-particles would likely lead to a more diffusive color appearance, special treatment or preparation methods are needed to produce bright reflective color.
3. Tunability of solution-based structural color
3.1. Responsive structural color via spatial tuning
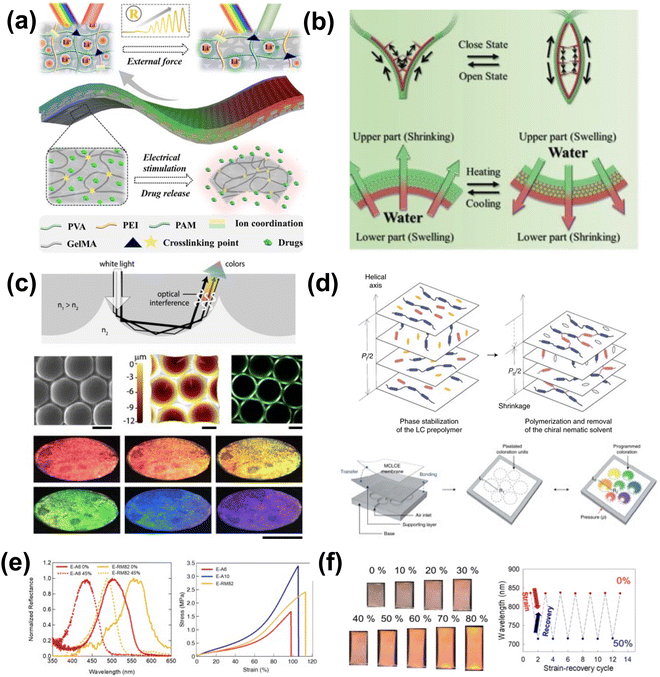 | ||
| Fig. 19 (a) Schematic diagram of the inverse opal scaffold-based structural color ionic hydrogel patch encapsulating drugs where drug release is triggered by electrical stimulation. [Reproduced from ref. 186, with permission from American Chemical Society, Copyright 2023]. (b) Schemes of the bilayer structural color hydrogel with multienvironment survivability. [Reproduced from ref. 187, with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Copyright 2019]. (c) Microwells and domes with structural coloration appearance from total internal reflection. [Reproduced from ref. 188, with permission from American Chemical Society, Copyright 2020]. (d) Programmable structural coloration of membranes based on Poisson effect. Schematic diagram showing the pixelated structural coloration platform, consisting of a base with air channels, a supporting layer and a structural color membrane. [Reproduced from ref. 189, with permission from Nature Publishing Group, Copyright 2022]. (e) Reflectance spectra (left) of crosslinked LC elastomers under tensile strain of 0% (solid line) and 45% (dashed line). Stress–strain curves (right) of the chiral nematic liquid crystal elastomers. (f) Photographs of the chiral nematic liquid crystal elastomer under applied tensile strains of 0–80% and mechano-optical stability of the chiral nematic liquid crystal elastomer film during compressive/tensile strain cycles from 0% to 50%. [Reproduced from ref. 190,191, with permission from Multidisciplinary Digital Publishing Institute, Copyright 2021]. | ||
Strain can also be used to modulate the spacing of structurally colored liquid crystals (LCs). Though liquid crystal-based structural color is difficult to fabricate into free-standing materials, several efforts have been made to enable sufficient robustness for use in mechanoresponsive sensing, such as supporting the LC films on a more robust polymer, incorporating them directly into the polymer backbone, or encapsulation by a more robust polymer. Kim et al. developed a system for colorimetric pressure readout utilizing a crosslinked copolymer elastomer incorporating a chiral nematic LC189 (Fig. 19d). The LC was allowed to self-assemble and stabilize, and the resulting film was mounted to a PDMS film that was then mounted to an air inlet. Under applied air pressure and therefore plane stress, the LC's pitch changed, thus changing the observed color. Similarly, Ku et al. improved the response, thermal, and environmental stability of LC-incorporated polymer networks by introducing noncovalent intermolecular crosslinking via a cyanobiphenyl derivative capable of π–π and dipole–dipole interactions.190,191 The resulting material exhibited fast mechanoresponsive optical behavior and recovery under applied and released tensile strain (Fig. 19e). Thus, the unique spatial properties of LC structural color can be modulated through deformation to create mechanoresponsive structural color (Fig. 19f), provided the LC is supported in a sufficiently robust material.
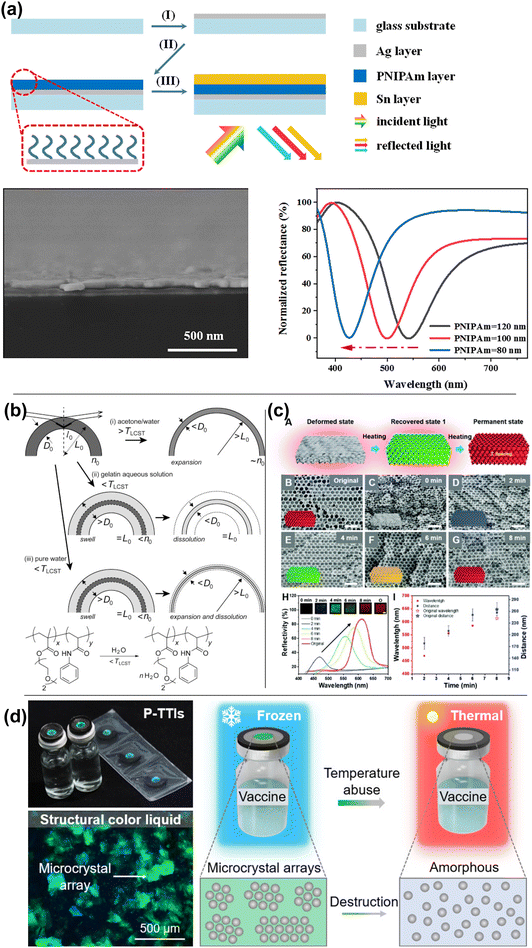 | ||
| Fig. 20 (a) Schematic diagram of the preparation process, SEM image and reflection spectra of the proposed asymmetric F–P-type cavity for subtractive structural colors. [Reproduced from ref. 192, with permission from American Chemical Society, Copyright 2022]. (b) Schematic illustration of change in diameter 2L, thickness of the shell D, refractive index n, and the structural color of SCBs composed of poly(diethylene glycol monomethyl ether methacrylate)-co-poly(N-phenylacrylamide) (PDegMA–PNPAM) random copolymer under several stimuli. [Reproduced from ref. 193, with permission from American Chemical Society, Copyright 2018]. (c) Color tuning of the shape memory polymer-based PhC film through heat. SEM image showing the morphology change during deformation/recovery cycle along with the corresponding reflection spectra. [Reproduced from ref. 194, with permission from Royal Society of Chemistry, Copyright 2020]. (d) Illustration of thermal-triggered structural color destruction based on triggering agent-diffusion-induced irreversible disassembly of liquid colloidal PCs for indicating the time–temperature history of the vaccine. [Reproduced from ref. 195, with permission from American Chemical Society, Copyright 2023]. | ||
Thermal transitions can be combined with other unique material properties, such as freezing point or shape memory, to trigger structural color changes. Using a thermally-triggered shape memory transition, Wang et al. created laser-writable photonic crystal paper using an inverse opal structured shape memory polymer.194 The paper is held in its compressed, colorless state due to shape memory until it is written on using an NIR laser, upon which the shape memory is released and the photonic crystal is allowed to expand to its native state (Fig. 20c), which is red. The written color is controlled by the duration of the writing, with shorter periods resulting in blue-shifted colors. While heat can be used to recover color in thermoresponsive materials, it can be used to destroy color in others. To tackle the problem of time-temperature history indication during vaccine cold storage, Huang et al. developed a system where photonic microcrystal arrays wrapped in glycerol are exposed to a triggering agent consisting of a frozen aqueous glycol solution.195 The glycerol-wrapped photonic crystals are sensitive to glycol concentration, which is controlled by the triggering agent's melting temperature (Fig. 20d). Upon melting of the triggering agent, the glycol mixes irreversibly with the glycerol-wrapped particles, thereby destroying them and their color. Thermal transitions can be applied to structurally colored materials in unique ways outside of using LCST polymer materials.
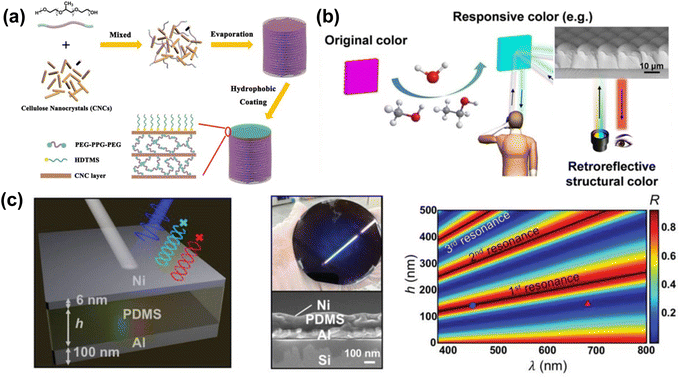 | ||
| Fig. 21 (a) Schematic illustration for the preparation of hydrophobic CNC nanocomposite films with structural color. [Reproduced from ref. 196, with permission from American Chemical Society, Copyright 2020]. (b) The color of the egg-waffle film remains angle-independent when the retroreflective structural color film responds to varied humidity and organic vapors (methanol and ethanol). [Reproduced from ref. 197, with permission from Elsevier Publishing Group, Copyright 2021]. (c) Ni/PMDS/Al structural color demonstrating a solvent-responsive color change due to the volume change of the PDMS layer. [Reproduced from ref. 198, with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Copyright 2019]. | ||
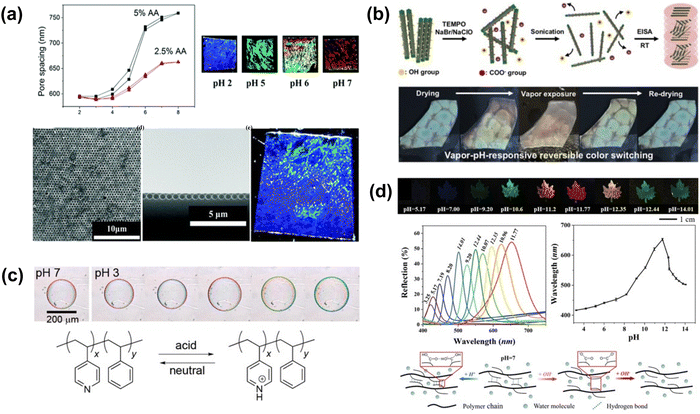 | ||
| Fig. 22 (a) pH-dependent color change of the 2D inverse opal hydrogel via pH modulated pore spacing. [Reproduced from ref. 199, with permission from Royal Society of Chemistry, Copyright 2014]. (b) Schematic of the fabrication of oxidized CNC (OxCNC) films by evaporation-induced self-assembly. PEG/OxCNC composite film undergoes reversible color change upon vapor exposure. [Reproduced from ref. 200, with permission from Elsevier Publishing Group, Copyright 2019]. (c) Schematic illustration showing the mechanism of pH responsiveness for the films assembled by the copolymer nanoparticles. [Reproduced from ref. 202, with permission from American Chemical Society, Copyright 2016]. (d) Color and reflection spectra of the leaf pattern in response to solution with different pH value. [Reproduced from ref. 201, with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Copyright 2019]. | ||
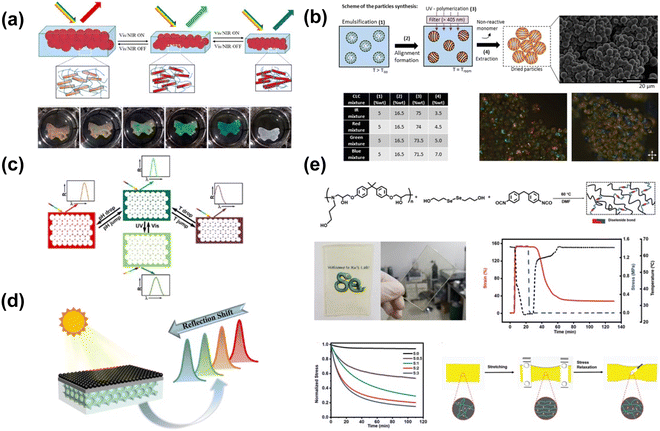 | ||
| Fig. 23 (a) Scheme of the photonic hydrogel responsive to the NIR light with color and volume variation. [Reproduced from ref. 203, with permission from American Chemical Society, Copyright 2021]. (b) Scheme, SEM and polarized optical microscopy (POM) images of cholesteric liquid crystalline (CLC) polymer particles synthesis with various CLC monomer mixing formula. [Reproduced from ref. 205, with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Copyright 2020]. (c) Model of environmental stimuli (temperature, pH or light) induced longitudinal swelling of the inverse opal hydrogel sensor. [Reproduced from ref. 206, with permission from Elsevier Publishing Group, Copyright 2018]. (d) Power-free and self-cleaning solar light detector based on the temperature-sensitive structural color and photothermal effect. [204]. (e) Diselenide-containing shape memory material and the controlled stress relaxation. [Reproduced from ref. 207, with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Copyright 2020]. | ||
On a molecular scale, dyes or chemical moieties that change shape under irradiation can be used to induce spatial changes in light-responsive structural color. The cis–trans isomerization of azobenzene is triggered upon changing between 365 and 450 nm light. Though the trans-isomer is 3.5 Å larger than the cis-isomer, cis-azobenzene occupies a larger volume as the phenyl rings are not coplanar.208 Belmonte et al. incorporated an azobenzene dye into particles of crosslinked chiral nematic cholesteric liquid crystal (CLC) copolymer205 (Fig. 23b). The particles contain cholesteric layers arranged in a parallel, asymmetric configuration; interaction of light with these layers yields visible color. Cis–trans isomerization causes spatial changes in the CLC helical direction; elongation long the helical axis causes the color to red-shift. These particles exhibit spot- and arc-like reflective color from red to blue, and are independently light- and temperature responsive. Similarly, Zhao et al. imbued UV response to a temperature- and pH-responsive inverse opal structural color hydrogel by incorporating UV-responsive spiropyran into the copolymer.206 Exposure to UV light causes the hydrogel's reflection peak to broaden and red-shift due to spatial changes affecting the hydrogel's refractive index caused by the spiropyran's response to UV light (Fig. 23c). In its native form, spiropyrans consist of heterocyclic functional groups in orthogonal planes. Under UV exposure, one of the heterocycles breaks, thereby changing the volume occupied by the molecule. One unique strategy to create light-responsive structural color films uses diselenide metathesis and shape memory properties. By applying strain, Liu et al. induced birefringent transmissive structural color in a film cast from a diselenide bond-containing shape memory polymer207 (Fig. 23e). The films show a strong yellow color under polarized light. The diselenide bond is broken under visible light irradiation, thus releasing strain and eliminating the color. By masking certain areas of the film, it is possible to create an image, thus showing potential applications in counterfeit protection. Light-responsive chemical moieties are a powerful tool that can induce spatial changes in a bulk material, thereby allowing for multimodally responsive structural color.
Electrochromic volume changes in polymers are often realized through a combination of responses, e.g. thermal or swelling responses. Park et al. used water splitting under 4V bias to impart deswelling in an inverse opal photonic gel fabricated from a charged polymer on a transparent electrode.209 Under deswelling, the color changed from red to green as the photonic crystal structure was compressed (Fig. 24a). Froyen et al. employed an electrothermal effect to create electrically-responsive structural color,210 using a thermally conductive silver nanowire substrate to heat a cholesteric liquid crystal (CLC) ink deposited on top (Fig. 24b). Increasing the applied voltage increases the heat and blue-shifts the observed color, going from colorless to red to green to blue as the liquid crystal transition is triggered. By using voltage to trigger volume change through thermal or swelling mechanisms, significant color change can be observed in electroresponsive structural color materials.
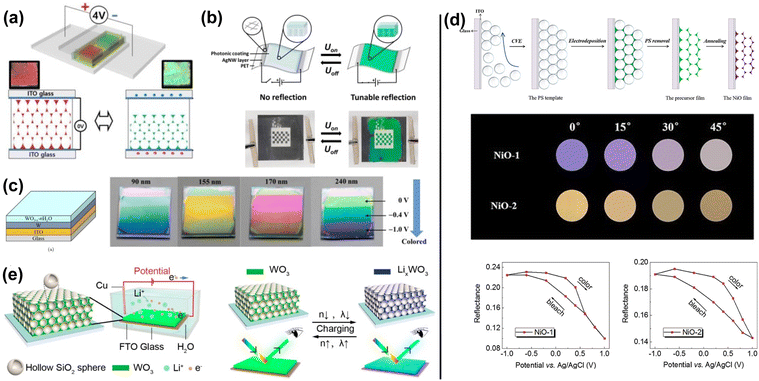 | ||
| Fig. 24 (a) Schematic illustration of the longitudinal deswelling of inverse opal photonic gel with voltage bias from the fully swollen state. [Reproduced from ref. 209, with permission from Elsevier Publishing Group, Copyright 2020]. (b) Schematic representation of the electrochromic structural colored foil. A flexible, transparent silver nanowire/poly(ethylene terephthalate) (AgNW/PET) heater induces reflection band shifting under electrical stimulation. [Reproduced from ref. 210, with permission from American Chemical Society, Copyright 2022]. (c) Different color states (applied voltages of 0 V, −0.4 V and −1 V respectively) of four electrochromic films with various tungsten oxide thicknesses containing the colors of blue, yellow, pink and green. [Reproduced from ref. 211, with permission from World Scientific, Copyright 2021]. (d) Schematic diagram of the preparation of inverse opal NiO films. Reflectance of photonic band gap peaks at different potentials of two different NiO films is shown. [Reproduced from ref. 212, with permission from American Physical Society, Copyright 2021]. (e) Schematic demonstration of WO3 PhC under electrochromic on–off states. [Reproduced from ref. 213, with permission from American Chemical Society, Copyright 2023]. | ||
3.2. Artificial optical properties via refractive index tuning
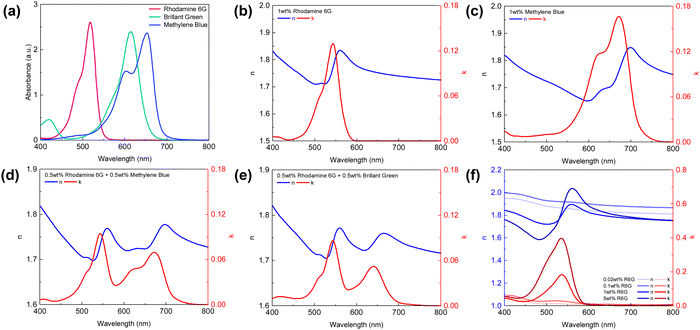
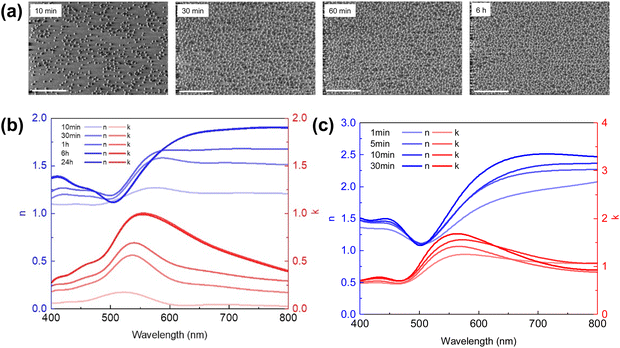
We chose three dye molecules—rhodamine 6G (R6G), brilliant green (BG), and methylene blue (MB)—due to their good solubility in both water and ethanol as well as their compatibility with the dielectric precursors tetraethyl orthosilicate (TEOS) and titanium(IV) tetraisopropoxide (TTIP). As shown in Fig. 25a, the three dyes show a narrow absorption peak with ∼100–150 nm bandwidth at three different wavelengths. Hence, local modification of the refractive index becomes possible with a dye-doped dielectric layer (dD). The dD layer can be deposited using a typical dip-coating method. Herein, we chose TiO2 as the matrix material since it has a different real part of the refractive index than that of the dye.227Fig. 25b gives the experimentally determined refractive index of the coated dD layer from 1 wt% dye dissolved in the TiO2 precursor solution. A quick comparison between the dyes’ absorption spectra peaks and the imaginary part k of the coated dielectric reveals that the absorptive features of dyes have been integrated into the dielectric refractive index (Fig. 25b and c). The real part of the refractive index n also shows a distinctive anomalous dispersion feature due to the Kramers–Kronig relation228 (K–K relation) between n and k. Further combination of the dye molecules leads to more complex refractive index behavior (Fig. 25d and e), where the refractive index starts to oscillate across the spectrum due to the absorption of dyes at different wavelengths. The magnitude of the imaginary part of the RI can also be tuned by the dye concentration in the precursor solution. A continuous change of the imaginary refractive index has been shown in Fig. 25d with increasing R6G concentration.
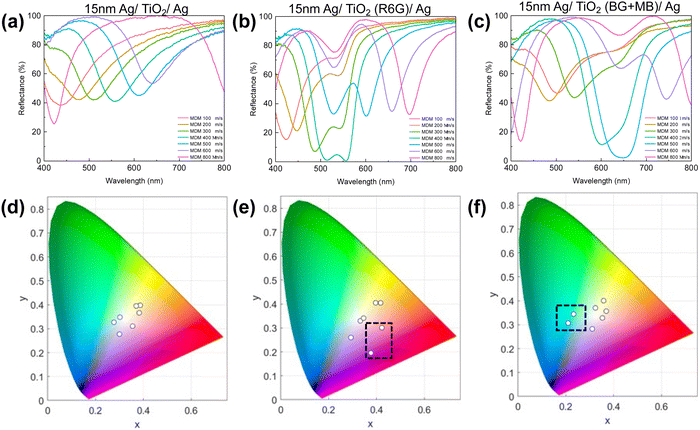
TiO2 precursor solution with 1 wt% R6G or MB was dip-coated onto a silver layer coated substrate followed by another 15 nm silver layer deposited on top of the TiO2 layer, resulting in a typical metal–dD–metal (M–dD–M) structure. Silver is chosen as both the top and bottom metal layer to maximize the lightness due to its low optical loss, and absorption in the structure is primarily governed by the dyes doped in the dielectric layer. Compared to undoped dip-coated TiO2 samples, the samples doped with either R6G or BG/MB show much lower reflectivity when cavity resonance wavelength matches the dye-absorbing wavelength (Fig. 26a–c), giving a high contrast in the reflection spectra. The off-resonance wavelengths are only slightly affected as the dielectric layer is almost lossless in those wavelength regions. Hence, the overall reduction of lightness is mitigated compared to the un-doped samples because the absorptive property is only introduced near the resonance wavelength. Moreover, as the dyes boost their absorption efficiency inside the resonance cavity, the color chromaticity has been greatly improved as well (Fig. 26d–f). We can therefore expect that, with minimum amount of dye molecules inside the cavity, such an approach could benefit the coloring industry with its the environmental impact by reducing the use of dyes.229
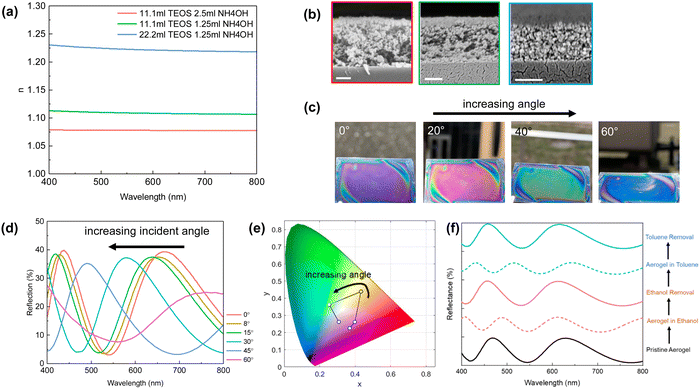
For the first time, we introduce SiO2 aerogel as an ultra-low refractive index dielectric to the F–P cavity and demonstrate its super-iridescent color performance. The preparation of SiO2 aerogel follows the logic of sol–gel chemistry232–235 and uses a weak base (i.e. ammonia) to catalyze the condensation reactions. The TEOS molecules form small SiO2 nanoparticles, and further undergo agglomeration once the nanoparticles reach a critical size. As more SiO2 nanoparticles join, a SiO2 framework forms and transforms the precursor solution into a porous gel. For the air void to form without collapsing the framework,236 we incorporated a volatile solvent (e.g. hexane) to enhance the rate of solvent removal during spin-coating. SiO2 aerogels with different refractive indexes are prepared with different TEOS concentrations. The resulting refractive index varies from 1.07 to 1.23 (Fig. 27a) with the increasing amount of TEOS being added. The low refractive index variation can be attributed to the porosity difference within the sample (Fig. 27b). The obtained tri-layer MDM structural color consists of 15nm Al/SiO2 aerogel/Si and shows a very iridescent color (Fig. 27c) upon angle variation. Reflection spectra have been measured from oblique incident angles from 0° to 60° with 15° interval. As shown in Fig. 27d, significant blue shifts in the resonance wavelength were observed under increased viewing angle from normal. A change in the visual appearance is more drastic with the lowest refractive index (n = 1.07) aerogel, where color travels almost a closed trajectory on the CIE color space (Fig. 27e) upon angle variation. Such color iridescence is staggering where all three secondary colors (cyan, magenta, yellow) are reached within 60° angle variation. The possibility of substituting the air void with other medium like ethanol or toluene has also been explored where a high-order resonance can be obtained along with a dramatic color change (Fig. 27f). The entire process is fully reversible with the removal of solvent and thus provides new insight into designing solvent/vapor-based colorimetric sensors.
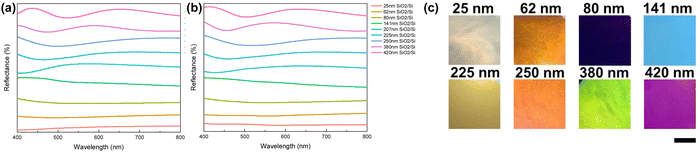
A three-layer particle/dielectric/metal structure with various dielectric layer thicknesses were fabricated from dip-coating of SiO2 on Si substrate followed by gold nanoparticle immobilization. Prominent reflection peaks appear (Fig. 29a) when the SiO2 layer is thick (>100 nm), while interestingly, a broadband absorber is seen when the layer is thin (<80 nm), matching with the transfer-matrix method (TMM) simulation (Fig. 29b) using the effective refractive index of the gold nanoparticle layer. Various colors are therefore produced (Fig. 29c). Mao et al.163 has provided a detailed explanation of such spectrum behavior where the disordered nanoparticles serve as a broadband absorber and the absorptive decay rate is equally partitioned into each wavelength.237 No resonance mode is built up when the dielectric layer is thin (i.e. broadband absorber), while in a thicker dielectric layer, a mismatch shows up between the absorption decay rates and the radiation decay rates at the resonance wavelength, leading to a strong reflection peak. The non-continuous particulate metal layer thus enables new ways of tuning the optical property of broadband lossy dielectric across the visible spectrum.
4. Summary and future outlook
4.1. Merits of solution-processed structural color
Overall, solution-processed structural color offers a cost-effective, scalable, and flexible approach to generating color through nanoscale manipulation of light. The simplicity and cost-effectiveness of solution processing methods, such as self-assembly, dip-coating, spray coating, electrochemical and electrostatic-based deposition, enable large-scale production, making it economically viable for applications like displays and sensors. Many of these approaches also allow for easy integration with flexible substrates, facilitating the development of bendable and stretchable devices. Additionally, the potential for energy efficiency makes solution-processed structural color versatile in design and environmentally friendly. Table 1 presents a comparison of commonly employed methods for structural color coating. Each method offers a niche application regarding material choice, achievable film thickness and application scenarios.| Methods | Material choice | Unit film thickness | Advantage | Disadvantage |
|---|---|---|---|---|
| Self-assembly | Polymers, colloids | >103 nm | Facile, scalable | Low-throughput, non-uniform |
| Layer-by-layer coating (LbL) | Polyelectrolytes | ∼100 nm | Conformal, precise thickness control | Very low-throughput |
| Dip coating | Polymers, particles, dielectrics | 101–102 nm | Facile, scalable, no pre-syntheis | Edge effect, non-conformal |
| Spin coating | Polymers, particles, dielectrics | 101–102 nm | Facile, rapid | Edge effect, non-conformal, non-scalable |
| Spray coating | Particles, polymers | >102 nm | Facile, rapid, scalable, conformal | Nozzle clogging |
| Electroless deposition (ELD) | Metals | >100 nm | Facile, rapid, scalable, conformal | Require fresh solution |
| Electrodeposition (ED) | Metals, dielectrics | >100 nm | Facile, rapid, scalable, conformal | Require conductive substrate, secondary reaction possible |
| Electrophoretic deposition (EPD) | Charged particles | Depend on particle size | Facile, conformal | Require conductive substrate large voltage and pre-synthesis |
Moreover, solution-processed structural colors stand out for their exceptional tunability in responding to external stimuli and altering optical properties, which would be challenging to achieve through vacuum-deposited materials. A range of solution-processable materials is employed to tune the size, refractive index, and spacing in producing the structural colors. Polymers undergoing volume changes in response to strain, temperature, or solvent swelling, at the bulk and macromolecular scale, can be integrated into photonic crystals or Fabry–Perot cavities, altering their spacing and resulting color. At the molecular and atomic scale, polymers and sol–gel oxides responsive to light, electricity, or pH can modulate their volume or refractive index, affecting the optical path length within incorporated photonic structures. Solution methods also facilitate the effortless mixing and formulation of material components, offering versatility in creating and modulating effective refractive index properties. The artificial synthesis of optical materials, in turn, imparts distinctive color perceptions, thereby enhancing the appeal for decorations, sensing, and anti-counterfeiting, amongst other applications.
4.2. Future perspectives
Solution processes leverage solution chemistry to enable versatile and facile material and photonic structure design. Compared to mature vacuum-based deposition technology, solution-processed methods for fabricating structural color still encounter significant challenges before achieving commercial viability: (1) color uniformity over large area. Solution process, being a bottom-up fabrication method, faces limitations in achieving uniformity across the entire film. Molecules or nano-/micro-particles cannot perceive the global environment outside their entirety. This can result in non-uniformity or defects at the macro-scale, such as edge effects. Rigorous engineering control is essential to maintain a consistent micro-environment across the substrate. (2) Encapsulation. Structural color films produced through solution processing tend to be less compact and more fragile, making them prone to scratching and environmental damage. While their susceptibility to environmental changes makes them suitable for sensor applications, it also compromises color stability when consistent color perception is required. Therefore, applying a protective layer atop the colored film becomes crucial to prevent external molecule infiltration and mitigate scratching. Common strategies involve additional coating layers of materials like acrylic polymer or hard dielectrics such as ZrO2 onto the colored structure. (3) Compatibility. Ensuring compatibility between each layer coating in terms of structure and recipe is paramount in solution-processed structures. Materials with different properties, such as metals and dielectrics, must not only adhere closely to each other without inducing excessive internal stress but also remain intact under various fabrication conditions. Further research efforts are necessary to develop reliable and compatible coating recipes for future advancements in this field. Hence, we emphasize the significance of future interdisciplinary research, with ample opportunity for collaboration between the chemistry, nanofabrication, materials science, and photonics communities.Engineering novel optical materials through solution-based synthesis remains an emerging area of research. Similar to doping dye into dielectrics, active components like fluorescent molecules can also be doped into the dielectric and further incorporated into resonant cavities. Potential applications in the field of emissive devices, sensors and even lasers could be possible. On the other hand, solution-synthesized particles can have various shapes such as nanorods,157,158,238,239 nanodisks161,240,241 and even nanopolyhedrons242,243 and components with absorption/resonance wavelength varying across the visible spectrum. A careful study and control on nanoparticle geometry, arrangements, order could further revolutionize the field of optical metasurface or metamaterial fabrication, where large scale production and cost-effective devices could be made possible.
Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Conflicts of interest
Certain aspects of the content presented in the manuscript is the subject of a US provisional patent application.Acknowledgements
The authors would like to acknowledge the support by the National Science Foundation (PFI- 2213684).References
- J. Sun, B. Bhushan and J. Tong, Structural coloration in nature, RSC Adv., 2013, 3, 14862–14889 RSC.
- S. Kinoshita, Structural Colors in the Realm of Nature, 2008, DOI:10.1142/9789812709752_fmatter.
- G. S. Smith, Structural color of Morpho butterflies, Am. J. Phys., 2009, 77, 1010–1019 CrossRef.
- S. Kinoshita, S. Yoshioka, Y. Fujii and N. Okamoto, Photophysics of Structural Color in the Morpho Butterflies, Forma, 2002, 17, 103–121 Search PubMed.
- J. Zi, et al., Coloration strategies in peacock feathers, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 12576 CrossRef CAS PubMed.
- A. E. Luna, D. C. Skigin, M. E. Inchaussandague and A. R. Alsina, Structural color in beetles of South America, in The Nature of Light: Light in Nature III, vol. 778205, 2010, DOI:10.1117/12.858695.
- H. Yin, et al., Amorphous diamond-structured photonic crystal in the feather barbs of the scarlet macaw, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 10798–10801 CrossRef CAS.
- B. Datta, E. F. Spero, F. J. Martin-Martinez and C. Ortiz, Socially-Directed Development of Materials for Structural Color, Adv. Mater., 2022, 34, 2100939 CrossRef CAS PubMed.
- Y. Liu, et al., Structural color three-dimensional printing by shrinking photonic crystals, Nat. Commun., 2019, 10, 4340 CrossRef.
- X. Su, H. Xia, S. Zhang, B. Tang and S. Wu, Vivid structural colors with low angle dependence from long-range ordered photonic crystal films, Nanoscale, 2016, 9, 3002–3009 RSC.
- Y. Zhang, et al., Using Cuttlefish Ink as an Additive to Produce-Non-iridescent Structural Colors of High Color Visibility, Adv. Mater., 2015, 27, 4719–4724 CrossRef CAS PubMed.
- V. L. Colvin, From Opals to Optics: Colloidal Photonic Crystals, MRS Bull., 2001, 26, 637–641 CrossRef CAS.
- D. Yan, et al., Flexible construction of cellulose photonic crystal optical sensing nano-materials detecting organic solvents, Analyst, 2018, 144, 1892–1897 RSC.
- H. K. Raut, et al., Hierarchical Colorful Structures by Three-Dimensional Printing of Inverse Opals, Nano Lett., 2021, 21, 8602–8608 CrossRef CAS PubMed.
- H. Galinski, et al., Scalable, ultra-resistant structural colors based on network metamaterials, Light: Sci. Appl., 2017, 6, e16233 CrossRef CAS PubMed.
- L. Feng, P. Huo, Y. Liang and T. Xu, Photonic Metamaterial Absorbers: Morphology Engineering and Interdisciplinary Applications, Adv. Mater., 2020, 32, e1903787 CrossRef.
- F. Cheng, et al., Aluminum plasmonic metamaterials for structural color printing, Opt. Express, 2015, 23, 14552–14560 CrossRef CAS PubMed.
- M. Song, et al., Colors with plasmonic nanostructures: A full-spectrum review, Appl. Phys. Rev., 2019, 6, 041308 CAS.
- J. Xue, et al., Scalable, full-colour and controllable chromotropic plasmonic printing, Nat. Commun., 2015, 6, 8906 CrossRef CAS.
- H. Wang, et al., Full Color Generation Using Silver Tandem Nanodisks, ACS Nano, 2017, 11, 4419–4427 CrossRef CAS PubMed.
- T. Xu, Y.-K. Wu, X. Luo and L. J. Guo, Plasmonic nanoresonators for high-resolution colour filtering and spectral imaging, Nat. Commun., 2010, 1, 59 CrossRef PubMed.
- A. E. Schlather, P. Gieri, M. Robinson, S. A. Centeno and A. Manjavacas, Nineteenth-century nanotechnology: The plasmonic properties of daguerreotypes, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 13791–13798 CrossRef CAS.
- A. Kristensen, et al., Plasmonic colour generation, Nat. Rev. Mater., 2016, 2, 16088 CrossRef.
- C. Yang, et al., Compact Multilayer Film Structures for Ultrabroadband, Omnidirectional, and Efficient Absorption, ACS Photonics, 2016, 3, 590–596 CrossRef CAS.
- K.-T. Lee, S. Y. Han, Z. Li, H. W. Baac and H. J. Park, Flexible High-Color-Purity Structural Color Filters Based on a Higher-Order Optical Resonance Suppression, Sci. Rep., 2019, 9, 14917 CrossRef PubMed.
- B. A. Rorem, et al., Integrating Structural Colors with Additive Manufacturing Using Atomic Layer Deposition, ACS Appl. Mater. Interfaces, 2022, 14, 31099–31108 CrossRef CAS PubMed.
- X. Yuan, et al., Properties and application of multi-functional and structurally colored textile prepared by magnetron sputtering, J. Ind. Text., 2022, 51, 1295–1311 CrossRef CAS.
- M. Huang, et al., Structural coloration and its application to textiles: a review, J. Text. Inst., 2020, 111, 756–764 CrossRef CAS.
- F. Cheng, J. Gao, T. S. Luk and X. Yang, Structural color printing based on plasmonic metasurfaces of perfect light absorption, Sci. Rep., 2015, 5, 11045 CrossRef CAS PubMed.
- H. Wang, et al., Full Color and Grayscale Painting with 3D Printed Low-Index Nanopillars, Nano Lett., 2021, 21, 4721–4729 CrossRef CAS.
- S. Sun, et al., All-Dielectric Full-Color Printing with TiO2 Metasurfaces, ACS Nano, 2017, 11, 4445–4452 CrossRef CAS PubMed.
- Y. Shen, et al., Structural Colors from Fano Resonances, ACS Photonics, 2015, 2, 27–32 CrossRef CAS.
- C. Zhang, et al., Printed photonic elements: nanoimprinting and beyond, J. Mater. Chem. C, 2016, 4, 5133–5153 RSC.
- A. F. Kaplan, T. Xu and L. J. Guo, High efficiency resonance-based spectrum filters with tunable transmission bandwidth fabricated using nanoimprint lithography, Appl. Phys. Lett., 2011, 99, 143111 CrossRef.
- Z. Li, et al., High-Efficiency, Mass-Producible, and Colored Solar Photovoltaics Enabled by Self-Assembled Photonic Glass, ACS Nano, 2022, 16, 11473–11482 CrossRef CAS.
- A. L. Liberman-Martin, C. K. Chu and R. H. Grubbs, Application of Bottlebrush Block Copolymers as Photonic Crystals, Macromol. Rapid Commun., 2017, 38, 1700058 CrossRef.
- D.-P. Song, T. H. Zhao, G. Guidetti, S. Vignolini and R. M. Parker, Hierarchical Photonic Pigments via the Confined Self-Assembly of Bottlebrush Block Copolymers, ACS Nano, 2019, 13, 1764–1771 CAS.
- K. P. Velikov, A. Moroz and A. V. Blaaderen, Photonic crystals of core-shell colloidal particles, Appl. Phys. Lett., 2002, 80, 49–51 CrossRef CAS.
- E. S. A. Goerlitzer, R. N. K. Taylor and N. Vogel, Bioinspired Photonic Pigments from Colloidal Self-Assembly, Adv. Mater., 2018, 30, 1706654 CrossRef PubMed.
- D. Wang, et al., Structural color generation: from layered thin films to optical metasurfaces, Nanophotonics, 2023, 12, 1019–1081 CrossRef CAS.
- K. Mao, et al., Angle Insensitive Color Filters in Transmission Covering the Visible Region, Sci. Rep., 2016, 6, 19289 CrossRef CAS PubMed.
- C. Ji, et al., Decorative near-infrared transmission filters featuring high-efficiency and angular-insensitivity employing 1D photonic crystals, Nano Res., 2019, 12, 543–548 CrossRef CAS.
- Z. Yang, C. Ji, Q. Cui and L. J. Guo, High-Purity Hybrid Structural Colors by Enhancing Optical Absorption of Organic Dyes in Resonant Cavity, Adv. Opt. Mater., 2020, 8, 2000317 CrossRef CAS.
- C. Yang, et al., Compact Multilayer Film Structure for Angle Insensitive Color Filtering, Sci. Rep., 2015, 5, 9285 CrossRef CAS.
- N. S. King, et al., Fano Resonant Aluminum Nanoclusters for Plasmonic Colorimetric Sensing, ACS Nano, 2015, 9, 10628 CrossRef CAS PubMed.
- A. E. Goodling, et al., Colouration by total internal reflection and interference at microscale concave interfaces, Nature, 2019, 566, 523–527 CrossRef CAS PubMed.
- S. D. Abdurakhmonov, M. S. Ashurov, S. O. Klimonsky and N. V. Tcherniega, Numerical Simulation of Optical Properties of Photonic Crystals with Inverse Opal Structure, Bull. Lebedev Phys. Inst., 2022, 49, 137–144 CrossRef.
- C. D. Dushkin, K. Nagayama, T. Miwa and P. A. Kralchevsky, Colored multilayers from transparent submicrometer spheres, Langmuir, 1993, 9, 3695–3701 CrossRef CAS.
- S. John, Strong localization of photons in certain disordered dielectric superlattices, Phys. Rev. Lett., 1987, 58, 2486–2489 CrossRef CAS PubMed.
- E. Yablonovitch, Inhibited Spontaneous Emission in Solid-State Physics and Electronics, Phys. Rev. Lett., 1987, 58, 2059–2062 CrossRef CAS.
- S. A. Asher, J. Holtz, L. Liu and Z. Wu, Self-Assembly Motif for Creating Submicron Periodic Materials. Polymerized Crystalline Colloidal Arrays, J. Am. Chem. Soc., 1994, 116, 4997–4998 CrossRef CAS.
- P. Jiang, J. F. Bertone, K. S. Hwang and V. L. Colvin, Single-Crystal Colloidal Multilayers of Controlled Thickness, Chem. Mater., 1999, 11, 2132–2140 CrossRef CAS.
- B. Frka-Petesic, G. Guidetti, G. Kamita and S. Vignolini, Controlling the Photonic Properties of Cholesteric Cellulose Nanocrystal Films with Magnets, Adv. Mater., 2017, 29, 1701469 CrossRef PubMed.
- T. Ding, K. Song, K. Clays and C. Tung, Fabrication of 3D Photonic Crystals of Ellipsoids: Convective Self-Assembly in Magnetic Field, Adv. Mater., 2009, 21, 1936–1940 CrossRef CAS.
- H. Wang, K. P. Yan, J. Xie and M. Duan, Fabrication of ZnO colloidal photonic crystal by spin-coating method, Mater. Sci. Semicond. Process., 2008, 11, 44–47 CrossRef CAS.
- R. Pozas, A. Mihi, M. Ocaña and H. Míguez, Building Nanocrystalline Planar Defects within Self-Assembled Photonic Crystals by Spin-Coating, Adv. Mater., 2006, 18, 1183–1187 CrossRef CAS.
- W. Yuan, N. Zhou, L. Shi and K.-Q. Zhang, Structural Coloration of Colloidal Fiber by Photonic Band Gap and Resonant Mie Scattering, ACS Appl. Mater. Interfaces, 2015, 7, 14064–14071 CrossRef CAS PubMed.
- H. Nakamura, M. Ishii, A. Tsukigase, M. Harada and H. Nakano, Close-Packed Colloidal Crystalline Arrays Composed of Silica Spheres Coated with Titania, Langmuir, 2006, 22, 1268–1272 CrossRef CAS PubMed.
- M. Xiao, et al., Bio-Inspired Structural Colors Produced via Self-Assembly of Synthetic Melanin Nanoparticles, ACS Nano, 2015, 9, 5454–5460 CrossRef CAS PubMed.
- J. E. G. J. Wijnhoven and W. L. Vos, Preparation of Photonic Crystals Made of Air Spheres in Titania, Science, 1998, 281, 802–804 CrossRef CAS PubMed.
- G. I. N. Waterhouse, W.-T. Chen, A. Chan and D. Sun-Waterhouse, Achieving Color and Function with Structure: Optical and Catalytic Support Properties of ZrO2 Inverse Opal Thin Films, ACS Omega, 2018, 3, 9658–9674 CrossRef CAS PubMed.
- J. E. S. Hoeven, A. V. van der, Shneidman, N. J. Nicolas and J. Aizenberg, Evaporation-Induced Self-Assembly of Metal Oxide Inverse Opals: From Synthesis to Applications, Acc. Chem. Res., 2022, 55, 1809–1820 CrossRef PubMed.
- F. Liu, B. Shan, S. Zhang and B. Tang, SnO2 Inverse Opal Composite Film with Low-Angle-Dependent Structural Color and Enhanced Mechanical Strength, Langmuir, 2018, 34, 3918–3924 CrossRef CAS PubMed.
- P. Liu, et al., Self-assembled colloidal arrays for structural color, Nanoscale Adv., 2019, 1, 1672–1685 RSC.
- Y. Takeoka, M. Honda, T. Seki, M. Ishii and H. Nakamura, Structural Colored Liquid Membrane without Angle Dependence, ACS Appl. Mater. Interfaces, 2009, 1, 982–986 CrossRef CAS PubMed.
- K. Ueno, A. Inaba, Y. Sano, M. Kondoh and M. Watanabe, A soft glassy colloidal array in ionic liquid, which exhibits homogeneous, non-brilliant and angle-independent structural colours, Chem. Commun., 2009, 3603–3605 RSC.
- R. O. Prum, R. H. Torres, S. Williamson and J. Dyck, Coherent light scattering by blue feather barbs, Nature, 1998, 396, 28–29 CrossRef CAS.
- R. O. Prum, E. R. Dufresne, T. Quinn and K. Waters, Development of colour-producing -keratin nanostructures in avian feather barbs, J. R. Soc., Interface, 2009, 6, S253–S265 CrossRef CAS PubMed.
- L. Shi, et al., Macroporous oxide structures with short-range order and bright structural coloration: a replication from parrot feather barbs, J. Mater. Chem., 2009, 20, 90–93 RSC.
- Y. Zhang, B. Dong, X. Liu and J. Zi, Replication of spinodally decomposed structures with structural coloration from scales of the longhorn beetle Sphingnotus mirabilis, Bioinspiration Biomimetics, 2013, 8, 045003 CrossRef CAS PubMed.
- A. Farhadi, T. Bartschmid and G. R. Bourret, Dewetting-Assisted Patterning: A Lithography-Free Route to Synthesize Black and Colored Silicon, ACS Appl. Mater. Interfaces, 2023, 15, 44087–44096 CrossRef CAS PubMed.
- P. Liu, et al., Bio-inspired robust non-iridescent structural color with self-adhesive amorphous colloidal particle arrays, Nanoscale, 2017, 10, 3673–3679 RSC.
- G. H. Lee, J. B. Kim, T. M. Choi, J. M. Lee and S. Kim, Structural Coloration with Nonclose-Packed Array of Bidisperse Colloidal Particles, Small, 2019, 15, e1804548 CrossRef PubMed.
- T. Liu, B. VanSaders, S. C. Glotzer and M. J. Solomon, Effect of Defective Microstructure and Film Thickness on the Reflective Structural Color of Self-Assembled Colloidal Crystals, ACS Appl. Mater. Interfaces, 2020, 12, 9842–9850 CrossRef CAS PubMed.
- T. Liu, B. VanSaders, J. T. Keating, S. C. Glotzer and M. J. Solomon, Effect of Particles of Irregular Size on the Microstructure and Structural Color of Self-Assembled Colloidal Crystals, Langmuir, 2021, 37, 13300–13308 CrossRef CAS PubMed.
- P. P. Lele and E. M. Furst, Assemble-and-Stretch Method for Creating Two- and Three-Dimensional Structures of Anisotropic Particles, Langmuir, 2009, 25, 8875–8878 CrossRef CAS PubMed.
- T. Liu, T. Liu, F. Gao, S. C. Glotzer and M. J. Solomon, Structural Color Spectral Response of Dense Structures of Discoidal Particles Generated by Evaporative Assembly, J. Phys. Chem. B, 2022, 126, 1315–1324 CrossRef CAS PubMed.
- S. Vignolini, et al., Pointillist structural color in Pollia fruit, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 15712–15715 CrossRef CAS PubMed.
- S. N. Fernandes, et al., Structural Color and Iridescence in Transparent Sheared Cellulosic Films, Macromol. Chem. Phys., 2013, 214, 25–32 CrossRef CAS.
- B. G. Rånby, A. Banderet and L. G. Sillén, Aqueous Colloidal Solutions of Cellulose Micelles, Acta Chem. Scand., 1949, 3, 649–650 CrossRef.
- K. Conley, L. Godbout, M. T. Whitehead and T. G. van de Ven, Origin of the twist of cellulosic materials, Carbohydr. Polym., 2016, 135, 285–299 CrossRef CAS PubMed.
- J. Pan, W. Hamad and S. K. Straus, Parameters Affecting the Chiral Nematic Phase of Nanocrystalline Cellulose Films, Macromolecules, 2010, 43, 3851–3858 CrossRef CAS.
- J. P. F. Lagerwall, et al., Cellulose nanocrystal-based materials: from liquid crystal self-assembly and glass formation to multifunctional thin films, NPG Asia Mater., 2014, 6, e80 CrossRef CAS.
- B. Frka-Petesic, G. Guidetti, G. Kamita and S. Vignolini, Controlling the Photonic Properties of Cholesteric Cellulose Nanocrystal Films with Magnets, Adv. Mater., 2017, 29, 1701469 CrossRef PubMed.
- J. Araki and S. Kuga, Effect of Trace Electrolyte on Liquid Crystal Type of Cellulose Microcrystals, Langmuir, 2001, 17, 4493–4496 CrossRef CAS.
- P.-X. Wang, W. Y. Hamad and M. J. MacLachlan, Structure and transformation of tactoids in cellulose nanocrystal suspensions, Nat. Commun., 2016, 7, 11515 CrossRef PubMed.
- X. Mu and D. G. Gray, Formation of Chiral Nematic Films from Cellulose Nanocrystal Suspensions Is a Two-Stage Process, Langmuir, 2014, 30, 9256–9260 CrossRef CAS PubMed.
- A. G. Dumanli, et al., Controlled, Bio-inspired Self-Assembly of Cellulose-Based Chiral Reflectors, Adv. Opt. Mater., 2014, 2, 646–650 CrossRef CAS PubMed.
- T. G. Parton, et al., Chiral self-assembly of cellulose nanocrystals is driven by crystallite bundles, Nat. Commun., 2022, 13, 2657 CrossRef CAS PubMed.
- G. Kamita, et al., Biocompatible and Sustainable Optical Strain Sensors for Large-Area Applications, Adv. Opt. Mater., 2016, 4, 1950–1954 CrossRef CAS.
- C. E. Boott, A. Tran, W. Y. Hamad and M. J. MacLachlan, Cellulose Nanocrystal Elastomers with Reversible Visible Color, Angew. Chem., Int. Ed., 2020, 59, 226–231 CrossRef CAS PubMed.
- K. Adstedt, et al., Chiral Cellulose Nanocrystals with Intercalated Amorphous Polysaccharides for Controlled Iridescence and Enhanced Mechanics, Adv. Funct. Mater., 2020, 30, 2003597, DOI:10.1002/adfm.202003597.
- J. Wang, Q. Cheng, L. Lin and L. Jiang, Synergistic Toughening of Bioinspired Poly(vinyl alcohol)–Clay–Nanofibrillar Cellulose Artificial Nacre, ACS Nano, 2014, 8, 2739–2745 CrossRef CAS PubMed.
- S. Beck, J. Bouchard and R. Berry, Controlling the Reflection Wavelength of Iridescent Solid Films of Nanocrystalline Cellulose, Biomacromolecules, 2011, 12, 167–172 CrossRef CAS PubMed.
- K. E. Shopsowitz, W. Y. Hamad and M. J. MacLachlan, Flexible and Iridescent Chiral Nematic Mesoporous Organosilica Films, J. Am. Chem. Soc., 2012, 134, 867–870 CrossRef CAS PubMed.
- K. E. Shopsowitz, H. Qi, W. Y. Hamad and M. J. MacLachlan, Free-standing mesoporous silica films with tunable chiral nematic structures, Nature, 2010, 468, 422–425 CrossRef CAS PubMed.
- B. E. Droguet, et al., Large-scale fabrication of structurally coloured cellulose nanocrystal films and effect pigments, Nat. Mater., 2022, 21, 352–358 CrossRef CAS PubMed.
- T. Heinze, Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials, Adv. Polym. Sci., 2015, 1–52, DOI:10.1007/12_2015_319.
- M. H. Godinho, D. G. Gray and P. Pieranski, Revisiting (hydroxypropyl) cellulose (HPC)/water liquid crystalline system, Liq. Cryst., 2017, 1–13, DOI:10.1080/02678292.2017.1325018.
- R. S. Werbowyj and D. G. Gray, Liquid Crystalline Structure In Aqueous Hydroxypropyl Cellulose Solutions, Mol. Cryst. Liq. Cryst., 1976, 34, 97–103 CrossRef CAS.
- H. Shimokawa, K. Hayata, M. Fukawa and S. Furumi, Fabrication of Reflective Color Films from Cellulose Derivatives, J. Photopolym. Sci. Technol., 2020, 33, 467–471 CrossRef CAS.
- R. S. Werbowyj and D. G. Gray, Optical properties of hydroxypropyl cellulose liquid crystals. I. Cholesteric pitch and polymer concentration, Macromolecules, 1984, 17, 1512–1520 CrossRef CAS.
- C. H. Barty-King, et al., Mechanochromic, Structurally Colored, and Edible Hydrogels Prepared from Hydroxypropyl Cellulose and Gelatin, Adv. Mater., 2021, 33, 2102112, DOI:10.1002/adma.202102112.
- H.-L. Liang, et al., Roll-to-roll fabrication of touch-responsive cellulose photonic laminates, Nat. Commun., 2018, 9, 4632 CrossRef PubMed.
- Y.-S. Yang, Y. Zhou, F. B. Y. Chiang and Y. Long, Temperature-responsive hydroxypropylcellulose based thermochromic material and its smart window application, RSC Adv., 2016, 6, 61449–61453 RSC.
- Y. Mai and A. Eisenberg, Self-assembly of block copolymers, Chem. Soc. Rev., 2012, 41, 5969–5985 RSC.
- Y. Kang, J. J. Walish, T. Gorishnyy and E. L. Thomas, Broad-wavelength-range chemically tunable block-copolymer photonic gels, Nat. Mater., 2007, 6, 957–960 CrossRef CAS PubMed.
- Y.-B. Baek, S.-H. Choi and D.-M. Shin, Tunable Photonic Band Gap of PS-b-P2VP Lamellar Film Using Metal Ions and pH Gradation, J. Nanosci. Nanotechnol., 2015, 15, 1624–1627 CrossRef CAS PubMed.
- H. S. Lim, J.-H. Lee, J. J. Walish and E. L. Thomas, Dynamic Swelling of Tunable Full-Color Block Copolymer Photonic Gels via Counterion Exchange, ACS Nano, 2012, 6, 8933–8939 CrossRef CAS PubMed.
- Y. Ahn, E. Kim, J. Hyon, C. Kang and Y. Kang, Photonic Crystals: Photoresponsive Block Copolymer Photonic Gels with Widely Tunable Photosensitivity by Counter-Ions, Adv. Mater., 2012, 24, OP89 CAS.
- J. J. Walish, Y. Fan, A. Centrone and E. L. Thomas, Controlling Thermochromism in a Photonic Block Copolymer Gel, Macromol. Rapid Commun., 2012, 33, 1504–1509 CrossRef CAS PubMed.
- E.-L. Lin, W.-L. Hsu and Y.-W. Chiang, Trapping Structural Coloration by a Bioinspired Gyroid Microstructure in Solid State, ACS Nano, 2017, 12, 485–493 CrossRef PubMed.
- C.-S. Wu, et al., Flexible or Robust Amorphous Photonic Crystals from Network-Forming Block Copolymers for Sensing Solvent Vapors, Anal. Chem., 2018, 90, 4847–4855 CrossRef CAS PubMed.
- M. Poutanen, et al., Block Copolymer Micelles for Photonic Fluids and Crystals, ACS Nano, 2018, 12, 3149–3158 CrossRef CAS PubMed.
- D. E. Fogg and H. M. Foucault, Comprehensive Organometallic Chemistry III, Transit. Met. Catal. Polym. Synth., 2007, 623–652, DOI:10.1016/b0-08-045047-4/00163-1.
- Y.-G. Yu, et al., Hydrogen Bonding-Mediated Phase Transition of Polystyrene and Polyhydroxystyrene Bottlebrush Block Copolymers with Polyethylene Glycol, Macromolecules, 2019, 52, 4349–4358 CrossRef CAS.
- H.-B. Seo, Y.-G. Yu, C.-G. Chae, M.-J. Kim and J.-S. Lee, Synthesis of ultrahigh molecular weight bottlebrush block copolymers of ω-end-norbornyl polystyrene and polymethacrylate macromonomers, Polymer, 2019, 177, 241–249 CrossRef CAS.
- M.-J. Kim, et al., Norbornenyl Macromonomers: In Situ Synthesis by End-Capping of Living Anionic Polymers Using a Norbornenyl-Functionalized α Phenyl Acrylate and Their Ring-Opening Metathesis Polymerization, Macromolecules, 2019, 52, 103–112 CrossRef CAS.
- Y.-G. Yu, et al., Precise Synthesis of Bottlebrush Block Copolymers from ω End-Norbornyl Polystyrene and Poly(4-tert-butoxystyrene) via Living Anionic Polymerization and Ring-Opening Metathesis Polymerization, Macromolecules, 2018, 51, 447–455 CrossRef CAS.
- T. Guo, et al., Structure Memory Photonic Crystals Prepared by Hierarchical Self-Assembly of Semicrystalline Bottlebrush Block Copolymers, Macromolecules, 2020, 53, 3602–3610 CrossRef CAS.
- B. M. Boyle, T. A. French, R. M. Pearson, B. G. McCarthy and G. M. Miyake, Structural Color for Additive Manufacturing: 3D-Printed Photonic Crystals from Block Copolymers, ACS Nano, 2017, 11, 3052–3058 CrossRef CAS PubMed.
- Cypris offers structural coloration with help from the EPA, BASF & better ventures. Focus Pigment, 7 ( 2023).
- B. Faure, et al., Dispersion and surface functionalization of oxide nanoparticles for transparent photocatalytic and UV-protecting coatings and sunscreens, Sci. Technol. Adv. Mater., 2013, 14, 023001 CrossRef CAS PubMed.
- L. Landau and B. Levich Dynamics of Curved Fronts, Part II Collect Pap Dragging Liq Mov Plate 141–153 ( 1988) DOI:10.1016/b978-0-08-092523-3.50016-2.
- M. Faustini, B. Louis, P. A. Albouy, M. Kuemmel and D. Grosso, Preparation of Sol−Gel Films by Dip-Coating in Extreme Conditions, J. Phys. Chem. C, 2010, 114, 7637–7645 CrossRef CAS.
- M. Pichumani, P. Bagheri, K. M. Poduska, W. González-Viñas and A. Yethiraj, Dynamics, crystallization and structures in colloid spin coating, Soft Matter, 2013, 9, 3220 RSC.
- E. Guzmán, R. G. Rubio and F. Ortega, A closer physico-chemical look to the Layer-by-Layer electrostatic self-assembly of polyelectrolyte multilayers, Adv. Colloid Interface Sci., 2020, 282, 102197 CrossRef PubMed.
- W. Chen and T. J. McCarthy, Layer-by-Layer Deposition: A Tool for Polymer Surface Modification, Macromolecules, 1997, 30, 78–86 CrossRef CAS.
- P. Kurt, D. Banerjee, R. E. Cohen and M. F. Rubner, Structural color via layer-by-layer deposition: layered nanoparticle arrays with near-UV and visible reflectivity bands, J. Mater. Chem., 2009, 19, 8920–8927 RSC.
- X. Yu, W. Ma and S. Zhang, Hydrophobic polymer-incorporated hybrid 1D photonic crystals with brilliant structural colors via aqueous-based layer-by-layer dip-coating, Dyes Pigm., 2021, 186, 108961 CrossRef CAS.
- G. M. Nogueira, D. Banerjee, R. E. Cohen and M. F. Rubner, Spray-Layer-by-Layer Assembly Can More Rapidly Produce Optical-Quality Multistack Heterostructures, Langmuir, 2011, 27, 7860–7867 CrossRef CAS PubMed.
- S. Colodrero, M. Ocaña and H. Míguez, Nanoparticle-Based One-Dimensional Photonic Crystals, Langmuir, 2008, 24, 4430–4434 CrossRef CAS PubMed.
- M. E. Calvo and H. Miguez, Flexible, Adhesive, and Biocompatible Bragg Mirrors Based on Polydimethylsiloxane Infiltrated Nanoparticle Multilayers, Chem. Mater., 2010, 22, 3909–3915 CrossRef CAS.
- P. Lova, et al., Engineering the Emission of Broadband 2D Perovskites by Polymer Distributed Bragg Reflectors, ACS Photonics, 2018, 5, 867–874 CrossRef CAS.
- S. Gazzo, et al., High refractive index hyperbranched polyvinylsulfides for planar one-dimensional all-polymer photonic crystals, J. Polym. Sci., Part B: Polym. Phys., 2016, 54, 73–80 CrossRef CAS.
- G. Manfredi, C. Mayrhofer, G. Kothleitner, R. Schennach and D. Comoretto, Cellulose ternary photonic crystal created by solution processing, Cellulose, 2016, 23, 2853–2862 CrossRef CAS.
- G. Giordano, N. Vilà, E. Aubert, J. Ghanbaja and A. Walcarius, Multi-layered, vertically-aligned and functionalized mesoporous silica films generated by sequential electrochemically assisted self-assembly, Electrochim. Acta, 2017, 237, 227–236 CrossRef CAS.
- G. Giordano, C. Durante, A. Gennaro and M. Guglielmi, Multilayer Deposition of Silica Sol–Gel Films by Electrochemical Assisted Techniques, J. Phys. Chem. C, 2016, 120, 28820–28824 CrossRef CAS.
- G. Giordano, et al., SiO2–TiO2 multilayer via electrochemical deposition: characterization of reflection and refractive index, J. Sol-Gel Sci. Technol., 2019, 89, 196–204 CrossRef CAS.
- W. Zhang, et al., Highly Efficient Perovskite Solar Cells with Tunable Structural Color, Nano Lett., 2015, 15, 1698–1702 CrossRef CAS PubMed.
- W. Feng, C. Ji and L. J. Guo, Primary and Secondary Reflective Color Realized by Full-Solution-Processed Multi-Layer Structures, Adv. Opt. Mater., 2023, 11, 2300456 CrossRef CAS.
- T. Yasuda, K. Nishikawa and S. Furukawa, Structural colors from TiO2/SiO2 multilayer flakes prepared by sol–gel process, Dyes Pigm., 2012, 92, 1122–1125 CrossRef CAS.
- W.-J. Feng, Y. Cheng and L. J. Guo, Temporal Coupled Mode Analysis of Chromaticity in Trilayer Subtractive Structural Colors, ACS Photonics, 2023, 10, 2784–2792 CrossRef CAS.
- G. Pfaff and J. Weitzel, Color. Plast., 2003, 226–241, DOI:10.1002/0471721581.ch15.
- F. J. Maile, G. Pfaff and P. Reynders, Effect pigments—past, present and future, Prog Org Coat, 2005, 54, 150–163 CrossRef CAS.
- F. Keller, M. S. Hunter and D. L. Robinson, Structural Features of Oxide Coatings on Aluminum, J. Electrochem. Soc., 1953, 100, 411–419 CrossRef CAS.
- Y. Liu, Y. Chang, Z. Ling, X. Hu and Y. Li, Structural coloring of aluminum, Electrochem. Commun., 2011, 13, 1336–1339 CrossRef CAS.
- T. Kikuchi, O. Nishinaga, S. Natsui and R. O. Suzuki, Fabrication of Self-Ordered Porous Alumina via Etidronic Acid Anodizing and Structural Color Generation from Submicrometer-Scale Dimple Array, Electrochim. Acta, 2015, 156, 235–243 CrossRef CAS.
- O. Yilmaz, M. F. Ebeoglugil, R. Dalmis and T. Dikici, Effect of anodizing time on the structural color and photocatalytic properties of the TiO2 films formed by electrochemical method, Mater. Sci. Semicond. Process., 2023, 167, 107768 CrossRef CAS.
- H. N. Umh, S. Yu, Y. H. Kim, S. Y. Lee and J. Yi, Tuning the Structural Color of a 2D Photonic Crystal Using a Bowl-like Nanostructure, ACS Appl. Mater. Interfaces, 2016, 8, 15802–15808 CrossRef CAS PubMed.
- K. Katagiri, et al., Structural color coating films composed of an amorphous array of colloidal particles via electrophoretic deposition, NPG Asia Mater., 2017, 9, e355–e355 CrossRef CAS.
- N. Zhou, A. Zhang, L. Shi and K.-Q. Zhang, Fabrication of Structurally-Colored Fibers with Axial Core–Shell Structure via Electrophoretic Deposition and Their Optical Properties, ACS Macro Lett., 2013, 2, 116–120 CrossRef CAS PubMed.
- C. Ji, S. Acharya, K. Yamada, S. Maldonado and L. J. Guo, Electrodeposition of Large Area, Angle-Insensitive Multilayered Structural Colors, ACS Appl. Mater. Interfaces, 2019, 11, 29065–29071 CrossRef CAS PubMed.
- N. G. Bastus, F. Merkoçi, J. Piella and V. Puntes, Synthesis of Highly Monodisperse Citrate-Stabilized Silver Nanoparticles of up to 200 nm: Kinetic Control and Catalytic Properties, Chem Mater., 2014, 26, 2836–2846 CrossRef CAS.
- P. Zhao, N. Li and D. Astruc, State of the art in gold nanoparticle synthesis, Coord. Chem. Rev., 2013, 257, 638–665 CrossRef CAS.
- E. S. Kooij, E. A. M. Brouwer, H. Wormeester and B. Poelsema, Ionic Strength Mediated Self-Organization of Gold Nanocrystals: An AFM Study, Langmuir, 2002, 18, 7677–7682 CrossRef CAS.
- L. Scarabelli, A. Sánchez-Iglesias, J. Pérez-Juste and L. M. Liz-Marzán, A “Tips and Tricks” Practical Guide to the Synthesis of Gold Nanorods, J. Phys. Chem. Lett., 2015, 6, 4270 CrossRef CAS PubMed.
- C. J. Murphy, et al., Gold nanorod crystal growth: From seed-mediated synthesis to nanoscale sculpting, Curr. Opin. Colloid Interface Sci., 2011, 16, 128–134 CrossRef CAS.
- X. Ye, et al., Seeded Growth of Monodisperse Gold Nanorods Using Bromide-Free Surfactant Mixtures, Nano Lett., 2013, 13, 2163–2171 CrossRef CAS PubMed.
- J. Pérez-Juste, I. Pastoriza-Santos, L. M. Liz-Marzán and P. Mulvaney, Gold nanorods: Synthesis, characterization and applications, Coord. Chem. Rev., 2005, 249, 1870–1901 CrossRef.
- S. Chen, Z. Fan and D. L. Carroll, Silver Nanodisks: Synthesis, Characterization, and Self-Assembly, J. Phys. Chem. B, 2002, 106, 10777–10781 CrossRef CAS.
- S. Zhou, et al., Facile Synthesis of Silver Nanocubes with Sharp Corners and Edges in an Aqueous Solution, ACS Nano, 2016, 10, 9861–9870 CrossRef CAS PubMed.
- P. Mao, et al., Manipulating disordered plasmonic systems by external cavity with transition from broadband absorption to reconfigurable reflection, Nat. Commun., 2020, 11, 1538 CrossRef CAS PubMed.
- P. Mao, et al., Disorder-Induced Material-Insensitive Optical Response in Plasmonic Nanostructures: Vibrant Structural Colors from Noble Metals, Adv. Mater., 2021, 33, 2007623 CrossRef CAS PubMed.
- J. Kim, H. Oh, M. Seo and M. Lee, Generation of Reflection Colors from Metal–Insulator–Metal Cavity Structure Enabled by Thickness-Dependent Refractive Indices of Metal Thin Film, ACS Photonics, 2019, 6, 2342–2349 CrossRef CAS.
- Y. H. Kim, et al., Reflection color tuning of a metal–insulator–metal cavity structure using arc plasma deposition of gold nanoparticles, Appl. Surf. Sci., 2021, 562, 150140 CrossRef CAS.
- M. A. Rahman, S. M. K. Vivek, S. H. Kim and J. Y. Byun, Polarizonic-interference colouration of stainless steel surfaces by Au-Al2O3 nanocomposite thin film coating, Appl. Surf. Sci., 2020, 505, 144428 CrossRef CAS.
- M. Abdelaziz, S. Homaeigohar, M. K. Hedayati, M. A. Assad and M. Elbahri, Solar Aluminum Kitchen Foils with Omnidirectional Vivid Polarizonic Colors, Adv. Opt. Mater., 2019, 7, 1900737 CrossRef.
- M. A. Assad, S. Homaeigohar and M. Elbahri, Reflective Coloration from Structural Plasmonic to Disordered Polarizonic, Adv. Photonics Res., 2021, 2100009, DOI:10.1002/adpr.202100009.
- D. Franklin, et al., Self-assembled plasmonics for angle-independent structural color displays with actively addressed black states, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 13350–13358 CrossRef CAS PubMed.
- P. Cencillo-Abad, D. Franklin, P. Mastranzo-Ortega, J. Sanchez-Mondragon and D. Chanda, Ultralight plasmonic structural color paint, Sci. Adv., 2023, 9, eadf7207 CrossRef CAS PubMed.
- S.-J. Kim, H.-K. Choi, H. Lee and S.-H. Hong, Solution-Processable Nanocrystal-Based Broadband Fabry–Perot Absorber for Reflective Vivid Color Generation, ACS Appl. Mater. Interfaces, 2019, 11, 7280–7287 CrossRef CAS PubMed.
- C. Gao, et al., Adsorption of Silver Nanoparticles on Modified Surfaces, Key Eng. Mater., 2015, 645–646, 75–79 Search PubMed.
- X. Rao, et al., High density gold nanoparticles immobilized on surface via plasma deposited APTES film for decomposing organic compounds in microchannels, Appl. Surf. Sci., 2018, 439, 272–281 CrossRef CAS.
- K. R. Brown and M. J. Natan, Hydroxylamine Seeding of Colloidal Au Nanoparticles in Solution and on Surfaces, Langmuir, 1998, 14, 726–728 CrossRef CAS.
- K. R. Brown, L. A. Lyon, A. P. Fox, B. D. Reiss and M. J. Natan, Hydroxylamine Seeding of Colloidal Au Nanoparticles. 3. Controlled Formation of Conductive Au Films, Chem. Mater., 2000, 12, 314–323 CrossRef CAS.
- S. M. Tabakman, Z. Chen, H. S. Casalongue, H. Wang and H. Dai, A New Approach to Solution-Phase Gold Seeding for SERS Substrates, Small, 2011, 7, 499–505 CrossRef CAS PubMed.
- K. R. Brown, D. G. Walter and M. J. Natan, Seeding of Colloidal Au Nanoparticle Solutions. 2. Improved Control of Particle Size and Shape, Chem. Mater., 2000, 12, 306–313 CrossRef CAS.
- H. Hulst Christoffel, Light Scattering by Small Particles. (Courier Corporation, 1981).
- Y. Wu, J. Ren, S. Zhang and S. Wu, Nanosphere-Aggregation-Induced Reflection and Its Application in Large-Area and High-Precision Panchromatic Inkjet Printing, ACS Appl. Mater. Interfaces, 2020, 12, 10867–10874 CrossRef CAS PubMed.
- L. Bai, et al., Large-Scale Noniridescent Structural Color Printing Enabled by Infiltration-Driven Nonequilibrium Colloidal Assembly, Adv. Mater., 2018, 30, 1705667 CrossRef PubMed.
- J. Ren, et al., Mie resonant structural colors based on ZnO spheres and their application in multi-color Pattern: Especially realization of red color, Chem. Eng. J., 2023, 474, 145530 CrossRef CAS.
- J. Ren, Y. Wu, Y. Han, S. Zhang and S. Wu, Noniridescent and Robust Structural-Colored Coating for Automotives Based on the Mie Scattering of ZnO Spheres, Ind. Eng. Chem. Res., 2022, 61, 18772–18779 CrossRef CAS.
- Y. Wu, et al., Polarization-Dependent Structural Colors in ZnS Nanosphere-Based Photonic Crystals for Anticounterfeiting Applications, ACS Appl. Nano Mater., 2022, 5, 423–429 CrossRef CAS.
- J. Zhou, et al., Visualizing Mie Resonances in Low-Index Dielectric Nanoparticles, Phys. Rev. Lett., 2018, 120, 253902 CrossRef CAS PubMed.
- Y. Wang, L. Sun, G. Chen, H. Chen and Y. Zhao, Structural Color Ionic Hydrogel Patches for Wound Management, ACS Nano, 2023, 17, 1437–1447 CrossRef CAS PubMed.
- Z. Zhang, et al., Bioinspired Bilayer Structural Color Hydrogel Actuator with Multienvironment Responsiveness and Survivability, Small Methods, 2019, 3, 1900519 CrossRef CAS.
- A. E. Goodling, S. Nagelberg, M. Kolle and L. D. Zarzar, Tunable and Responsive Structural Color from Polymeric Microstructured Surfaces Enabled by Interference of Totally Internally Reflected Light, ACS Mater. Lett., 2020, 2, 754–763 CrossRef CAS.
- S.-U. Kim, et al., Broadband and pixelated camouflage in inflating chiral nematic liquid crystalline elastomers, Nat. Mater., 2022, 21, 41–46 CrossRef CAS PubMed.
- K. Ku, et al., Effect of Crosslinkers on Optical and Mechanical Behavior of Chiral Nematic Liquid Crystal Elastomers, Molecules, 2021, 26, 6193 CrossRef CAS PubMed.
- K. Ku, et al., Environmentally Stable Chiral-Nematic Liquid-Crystal Elastomers with Mechano-Optical Properties, Appl. Sci., 2021, 11, 5037 CrossRef CAS.
- C. Liu, et al., Dynamic Color Display with Viewing-Angle Tolerance Based on the Responsive Asymmetric Fabry–Perot Cavity, ACS Appl. Mater. Interfaces, 2022, 14, 7200–7207 CrossRef CAS PubMed.
- K. Higashiguchi, N. Morita and K. Matsuda, Structural Colored Balloon Composed of Temperature-Responsive Polymers Showing LCST Behavior, Langmuir, 2018, 34, 12853–12860 CrossRef CAS PubMed.
- Y. Wang, Q. Zhao and X. Du, Inkless multi-color writing and copying of laser-programmable photonic crystals, Mater. Horiz., 2020, 7, 1341–1347 RSC.
- C. Huang, Y. Shang, J. Hua, Y. Yin and X. Du, Self-Destructive Structural Color Liquids for Time–Temperature Indicating, ACS Nano, 2023, 17, 10269–10279 CrossRef CAS PubMed.
- C. Sun, et al., Bioinspired Hydrophobic Cellulose Nanocrystal Composite Films as Organic-Solvent-Responsive Structural-Color Rewritable Papers, ACS Appl. Mater. Interfaces, 2020, 12, 26455–26463 CrossRef CAS PubMed.
- C. Ji, J. Zeng, S. Qin, M. Chen and L. Wu, Angle-independent responsive organogel retroreflective structural color film for colorimetric sensing of humidity and organic vapors, Chin. Chem. Lett., 2021, 32, 3584–3590 CrossRef CAS.
- S. D. Rezaei, et al., Tunable, Cost-Effective, and Scalable Structural Colors for Sensing and Consumer Products, Adv. Opt. Mater., 2019, 7, 1900735 CrossRef.
- F. Xue, et al., Two-dimensional inverse opal hydrogel for pH sensing, Analyst, 2014, 139, 6192–6196 RSC.
- H. Yang, et al., Color-spectrum-broadened ductile cellulose films for vapor-pH-responsive colorimetric sensors, J. Ind. Eng. Chem., 2019, 80, 590–596 CrossRef CAS.
- J. Liao, et al., Multiresponsive Elastic Colloidal Crystals for Reversible Structural Color Patterns, Adv. Funct. Mater., 2019, 29, 1902954 CrossRef.
- K. Higashiguchi, J. Imai and K. Matsuda, Structural Colored Balloons Responsive to pH Change, Langmuir, 2016, 32, 4945–4951 CrossRef CAS PubMed.
- Y. Xia, et al., Near-Infrared Light Induced Dynamic Structural Color Change of Amorphous Photonic Hydrogel, ACS Appl. Polym. Mater., 2021, 3, 757–764 CrossRef CAS.
- M. Xiong, et al., Power-Free and Self-Cleaning Solar Light Detector Based on the Temperature-Sensitive Structural Color and Photothermal Effect, ACS Appl. Mater. Interfaces, 2021, 13, 33566–33573 CrossRef CAS PubMed.
- A. Belmonte, et al., Dual Light and Temperature Responsive Micrometer-Sized Structural Color Actuators, Small, 2020, 16, e1905219 CrossRef PubMed.
- W. Zhao, et al., Visual multi-triggered sensor based on inverse opal hydrogel, Colloids Surf., A, 2018, 554, 93–99 CrossRef CAS.
- C. Liu, Z. Fan, Y. Tan, F. Fan and H. Xu, Tunable Structural Color Patterns Based on the Visible-Light-Responsive Dynamic Diselenide Metathesis, Adv. Mater., 2020, 32, 1907569 CrossRef CAS PubMed.
- O. N. Oliveira, M. Raposo and A. Dhanabalan, Handbook of Surfaces and Interfaces of Materials, 1–63, ( 2001), DOI:10.1016/b978-012513910-6/50047-5.
- J. Park, S. Yoon, N. Heo and W. Lee, Electrochromic inverse opal photonic gel containing charged hydrogel in aqueous media for full color reflective display, J. Ind. Eng. Chem., 2020, 88, 117–126 CrossRef CAS.
- A. A. F. Froyen, et al., Ink-Deposited Transparent Electrochromic Structural Colored Foils, ACS Appl. Mater. Interfaces, 2022, 14, 39375–39383 CrossRef CAS PubMed.
- S. Zhang, et al., Reflective Structural Color Tunability of Inorganic Electrochromic Devices by Interferometric Modulation, Int. J. Nanosci., 2021, 20, 2150054 CrossRef CAS.
- H.-Y. Qu, et al., Multicolored absorbing nickel oxide films based on anodic electrochromism and structural coloration, J. Appl. Phys., 2021, 129, 123105 CrossRef CAS.
- X. Ran, J. Ren, S. Zhang, Y. Wu and S. Wu, Multicolor Electrochromic Display and Patterned Device Based on Hollow-SiO2-Supported WO3 Photonic Crystals, ACS Appl. Mater. Interfaces, 2023, 15, 41763–41771 CrossRef CAS PubMed.
- M. Seo, et al., Printing of Highly Vivid Structural Colors on Metal Substrates with a Metal-Dielectric Double Layer, Adv. Opt. Mater., 2019, 7, 1900196 CrossRef.
- M. A. Rahman, D. K. Kim, J.-K. Lee and J. Y. Byun, To realize a variety of structural color adjustments via lossy-dielectric-based Fabry–Perot cavity structure, Nanophotonics, 2022, 11, 4855–4868 CrossRef CAS.
- S. Rossi and M. P. Jonsson, Highly reflective optical nanocavities for structural coloration by combining broadband absorber and FabryProt effects, J. Optom., 2021, 23, 015001 Search PubMed.
- M. A. Kats, R. Blanchard, P. Genevet and F. Capasso, Nanometre optical coatings based on strong interference effects in highly absorbing media, Nat. Mater., 2013, 12, 20–24 CrossRef CAS PubMed.
- S. Ayas, et al., Colorimetric detection of ultrathin dielectrics on strong interference coatings, Opt. Lett., 2018, 43, 1379–1382 CrossRef CAS PubMed.
- O. Hemmatyar, S. Abdollahramezani, Y. Kiarashinejad, M. Zandehshahvar and A. Adibi, Full color generation with Fano-type resonant HfO2 nanopillars designed by a deep-learning approach, Nanoscale, 2019, 11, 21266–21274 RSC.
- A. S. Rana, M. Zubair, M. S. Anwar, M. Saleem and M. Q. Mehmood, Engineering the absorption spectra of thin film multilayer absorbers for enhanced color purity in CMY color filters, Opt. Mater. Express, 2020, 10, 268 CrossRef CAS.
- D. Kim, et al., Manipulation of resonance orders and absorbing materials for structural colors in transmission with improved color purity, Opt. Express, 2022, 30, 11740 CrossRef CAS PubMed.
- C. Yang, et al., Tunable, omnidirectional structural color on reflection based on metal-SiOx-metal structure, Appl. Phys. Lett., 2016, 109, 241104 CrossRef.
- C.-S. Park, V. R. Shrestha, S.-S. Lee, E.-S. Kim and D.-Y. Choi, Omnidirectional color filters capitalizing on a nano-resonator of Ag-TiO2-Ag integrated with a phase compensating dielectric overlay, Sci. Rep., 2015, 5, 8467 CrossRef CAS PubMed.
- K. Lee, S. Seo and L. J. Guo, High-Color-Purity Subtractive Color Filters with a Wide Viewing Angle Based on Plasmonic Perfect Absorbers, Adv. Opt. Mater., 2015, 3, 347–352 CrossRef CAS.
- Z. Yang, C. Ji, D. Liu and L. J. Guo, Enhancing the Purity of Reflective Structural Colors with Ultrathin Bilayer Media as Effective Ideal Absorbers, Adv. Opt. Mater., 2019, 7, 1900739 CrossRef CAS.
- H. Pan, et al., Wide gamut, angle-insensitive structural colors based on deep-subwavelength bilayer media, Nanophotonics, 2020, 9, 3385–3392 CrossRef.
- C. J. Huang, K. C. Liao and Y. K. Su, Structure Property of Titanium Dioxide Thin Films in Sintered Temperature by the Sol-Gel Method, Key Eng. Mater., 2008, 368–372, 1465–1467 CAS.
- V. Lucarini, K.-E. Peiponen, J. J. Saarinen and E. M. Vartiainen, Kramers-Kronig Relations in Optical Materials Research, Springer Ser. Opt. Sci., 2005, 110, 1, DOI:10.1007/b138913.
- E. Rio and F. Boulogne, Withdrawing a solid from a bath: How much liquid is coated?, Adv. Colloid Interface Sci., 2017, 247, 100–114 CrossRef CAS PubMed.
- Q. Yang and L. R. Zhao, Characterization of nano-layered multilayer coatings using modified Bragg law, Mater. Charact., 2008, 59, 1285–1291 CrossRef CAS.
- M. Born et al. , Principles of Optics, ( 1999), DOI:10.1017/cbo9781139644181.
- B. T. Mekonnen, et al., Preparation of aerogel and its application progress in coatings: a mini overview, J. Leather Sci. Eng., 2021, 3, 25 CrossRef CAS.
- H.-S. Yang, S.-Y. Choi, S.-H. Hyun, H.-H. Park and J.-K. Hong, Ambient-dried low dielectric SiO2 aerogel thin film, J. Non-Cryst. Solids, 1997, 221, 151–156 CrossRef CAS.
- P. Lin, M. Mah and J. J. Talghader Synthesis and Characterizations of a Very Low Index Silica Aerogel Optical Thin Film, Opt Interf Coatings Conf Oic 2022 WD.5 ( 2022) DOI:10.1364/oic.2022.wd.5.
- S. H. Hyun, J. J. Kim and H. H. Park, Synthesis and Characterization of Low-Dielectric Silica Aerogel Films, J. Am. Ceram. Soc., 2000, 83, 533–540 CrossRef CAS.
- S. S. Prakash, C. J. Brinker, A. J. Hurd and S. M. Rao, Silica aerogel films prepared at ambient pressure by using surface derivatization to induce reversible drying shrinkage, Nature, 1995, 374, 439–443 CrossRef CAS.
- C. Liu, et al., Enhanced energy storage in chaotic optical resonators, Nat. Photonics, 2013, 7, 473–478 CrossRef CAS.
- Y. Y. Yu, S. S. Chang, C. L. Lee and C. C. Wang, Gold Nanorods: Electrochemical Synthesis and Optical Properties, J. Phys. Chem. B, 1997, 101, 6661–6664 CrossRef CAS.
- Y. Niidome, H. Takahashi, S. Urakawa, K. Nishioka and S. Yamada, Immobilization of Gold Nanorods on the Glass Substrate by the Electrostatic Interactions for Localized Plasmon Sensing, Chem. Lett., 2004, 33, 454–455 CrossRef CAS.
- C. Kuttner, et al., Seeded Growth Synthesis of Gold Nanotriangles: Size Control, SAXS Analysis, and SERS Performance, ACS Appl. Mater. Interfaces, 2018, 10, 11152–11163 CrossRef CAS PubMed.
- Y.-H. Chang, W.-H. Hsu, S.-L. Wu and Y.-C. Ding, The synthesis of a gold nanodisk–molecular layer–gold film vertical structure: a molecular layer as the spacer for SERS hot spot investigations, Mater. Chem. Front., 2016, 1, 922–927 RSC.
- B. Saute and R. Narayanan, Solution-based direct readout surface enhanced Raman spectroscopic (SERS) detection of ultra-low levels of thiram with dogbone shaped gold nanoparticles, Analyst, 2010, 136, 527 RSC.
- Y. Sun and C. An, Shaped gold and silver nanoparticles, Front. Mater. Sci., 2010, 5, 1–24 Search PubMed.
| This journal is © the Partner Organisations 2024 |