Open Access Article
Open Access ArticleSynthesis of axially chiral diaryl ethers via NHC-catalyzed atroposelective esterification†‡
Yingtao
Wu
a,
Xin
Guan
a,
Huaqiu
Zhao
a,
Mingrui
Li
a,
Tianlong
Liang
a,
Jiaqiong
Sun
b,
Guangfan
Zheng
 *a and
Qian
Zhang
*a and
Qian
Zhang
 *ac
*ac
aKey Laboratory of Functional Organic Molecule Design & Synthesis of Jilin Province, Department of Chemistry, Northeast Normal University, Changchun, Jilin 130024, China. E-mail: zhenggf265@nenu.edu.cn; zhangq651@nenu.edu.cn
bSchool of Environment, Northeast Normal University, Changchun 130117, China
cState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
First published on 24th February 2024
Abstract
Axially chiral diaryl ethers bearing two potential axes find unique applications in bioactive molecules and catalysis. However, only very few catalytic methods have been developed to construct structurally diverse diaryl ethers. We herein describe an NHC-catalyzed atroposelective esterification of prochiral dialdehydes, leading to the construction of enantioenriched axially chiral diaryl ethers. Mechanistic studies indicate that the matched kinetic resolutions play an essential role in the challenging chiral induction of flexible dual-axial chirality by removing minor enantiomers via over-functionalization. This protocol features mild conditions, excellent enantioselectivity, broad substrate scope, and applicability to late-stage functionalization, and provides a modular platform for the synthesis of axially chiral diaryl ethers and their derivatives.
Introduction
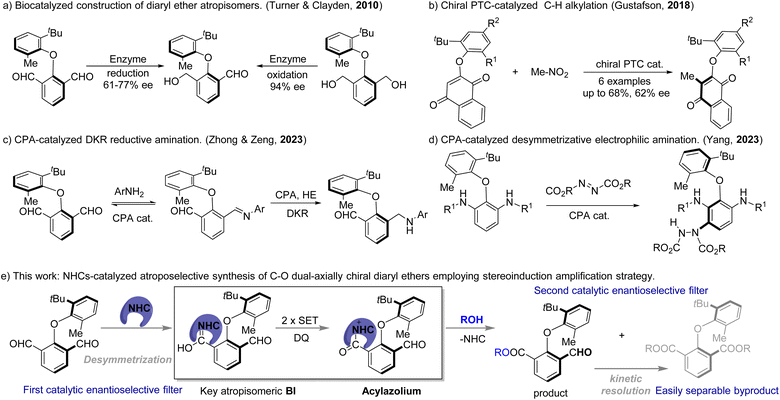
Atropisomerically enriched chiral frameworks find widespread applications in materials science,1 bioactive molecules,2 and asymmetric catalysis.3 Accordingly, significant progress has been achieved in the catalytic construction of axially chiral scaffolds, including biaryl atropisomers,4 axially chiral styrenes,5 amines,6 amides,7 boranes,8etc. As distinctive atropisomers, axially chiral diaryl ethers bearing two potential axes find unique applications in bioactive molecules.9 However, construction of C–O axially chiral diaryl ethers has received limited attention from organic chemists, probably owing to the challenges in more flexible dual-axial chirality control. In 1998, Fuji and coworkers10a discovered the first atropisomerism in diaryl ethers.10 In 2008, the Clayden group11 pioneered the first enantioselective synthesis of an axially chiral diaryl ether, that with sole dual-axial chirality. Developing a catalytic methodology for the asymmetric construction of diaryl ether-type atropisomers is highly desirable but more challenging. In this regard, Turner, Clayden, and co-workers12 developed an unprecedented biocatalyzed enantioselective construction of diaryl ether atropisomers via desymmetrizative oxidation/reduction of diols/dialdehydes (Scheme 1a). Even with the conceptual breakthrough, only one example of axially chiral diaryl ether was obtained. The Gustafson group13 developed the first organocatalyzed chiral induction through enantioselective C–H alkylation, although yields and enantioselectivities were not high (Scheme 1b). Very recently, the Zeng and Zhong14 group developed elegant chiral phosphoric acid (CPA) catalyzed asymmetric reductive amination of axially prochiral diaryl ethers (Scheme 1c) via dynamic kinetic resolution (DKR). Later, Yang's15 group developed CPA-catalyzed electrophilic remote amination of axially prochiral diaryl ethers, leading to highly enantioselective construction of diaryl ether-type atropisomers (Scheme 1d). Despite such significant progress, the development of a novel catalytic methodology for direct access to diaryl ether-type atropisomers is still an emerging area and in great demand.On the other hand, N-heterocyclic carbene catalysts (NHCs) exhibit unique reactivity in activating the carbonyl group.16 NHC-catalyzed transformations provide attractive alternatives for constructing axially chiral compounds17via desymmetrization,18 or (dynamic) kinetic resolution.19 NHC-catalyzed desymmetrization of axially prochiral dialdehydes18g provides chiral-NHC-bounded atropisomeric Breslow Intermediates (BIs) as critical intermediates, leading to the direct access to highly atropisomerically enriched aldehydes via two-step single electron oxidation and nucleophilic coupling. At the same time, the second enantiodifferentiation step for aldehydes could amplify the stereoinduction ability of the chiral catalyst, which might provide opportunities for challenging chiral induction of flexible dual-axial chirality, in analogy to the Horeau principle.20 As part of our continued interests in NHC-catalyzed transformations21 and asymmetric catalysis,18g,22 we now report an asymmetric esterification approach to axially chiral diaryl ethers by chiral NHC-catalyzed desymmetrization of dicarbaldehydes23 with alcohols/phenols23a–c,24 (Scheme 1e). The over-esterification could act as an additional stereocontrolling filter to improve the enantioselectivity, leading to excellent chiral induction for C–O axially chiral diaryl ethers.
Results and discussion
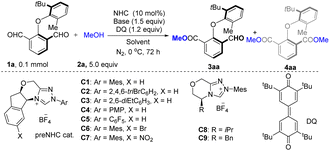
To probe the feasibility of our designed reaction, we commenced our investigation employing dicarbaldehydes (1a) and MeOH (2a) as the reactants in the model esterification reaction. Encouragingly, in the initial study, treatment of 1a and 2a in DCM employing C1 as the catalyst, DQ (1.2 equiv.) as the oxidant, and Cs2CO3 (1.5 equiv.) as the base at 0 °C under a N2 atmosphere for 72 h yielded the desired esterification product 3aa in 91% yield and 90% ee, along with a 5% diester byproduct 4aa (Table 1, entry 1). Next, a series of NHCs were screened (entries 2–9), and C1 was proven to be the best choice for this atroposelective esterification. Switching the Mes group of C1 to 2,4,6-triBrC6H2 (entry 2) or C6F5 (entry 5) had nearly no reactivity. The screening of various solvents indicated that DCM was the best solvent for this esterification (entries 10–16). Most of the solvents, such as DCE, MTBE, EtOAc, and toluene, gave acceptable yields and enantioselectivities. THF exhibited excellent chiral induction, delivering 3aa in 68% yield and 98% ee; however, diester 4aa was identified with a 19% yield because of high activity.| Entry | NHC cat. | Solvent | Base | 3aa (%) | 4aa (%) |
|---|---|---|---|---|---|
| Yield ee | |||||
| a Conditions: 1a (0.1 mmol), 2a (5.0 equiv.), NHC (10 mol%), base (1.5 equiv.) and DQ (1.2 equiv.), solvent (1.0 mL), 0 oC, N2 atmosphere, 72 h. Yields were determined by 1H NMR spectroscopic analysis of the crude reaction mixture employing CH2Br2 as the internal standard; ee was determined by chiral-phase HPLC analysis. b C1 (15 mol%). | |||||
| 1 | C1 | DCM | Cs2CO3 | 91 90 | 5 |
| 2 | C2 | DCM | Cs2CO3 | 7 35 | Trace |
| 3 | C3 | DCM | Cs2CO3 | 73 86 | 2 |
| 4 | C4 | DCM | Cs2CO3 | 84–67 | 11 |
| 5 | C5 | DCM | Cs2CO3 | 9–40 | Trace |
| 6 | C6 | DCM | Cs2CO3 | 74 91 | 2 |
| 7 | C7 | DCM | Cs2CO3 | 40 87 | Trace |
| 8 | C8 | DCM | Cs2CO3 | 59–16 | 31 |
| 9 | C9 | DCM | Cs2CO3 | 60 41 | 35 |
| 10 | C1 | CHCl3 | Cs2CO3 | 83 75 | 6 |
| 11 | C1 | DCE | Cs2CO3 | 86 91 | 10 |
| 12 | C1 | THF | Cs2CO3 | 68 98 | 19 |
| 13 | C1 | MTBE | Cs2CO3 | 87 91 | 8 |
| 14 | C1 | EtOAc | Cs2CO3 | 81 90 | 12 |
| 15 | C1 | MeCN | Cs2CO3 | 82 80 | 16 |
| 16 | C1 | Toluene | Cs2CO3 | 79 93 | 11 |
| 17 | C1 | DCM | Cs 2 CO 3 | 89 (88) 94 | 11 |
| 18b | C1 | DCM | K2CO3 | 80 89 | 11 |
| 19b | C1 | DCM | K3PO4 | 90 90 | 10 |
| 20b | C1 | DCM | DBU | 80 91 | 9 |
| 21b | C1 | DCM | DMAP | 82 89 | 8 |
Switching the catalyst loading to 15 mol% caused an increased yield of diester 4aa (11%), and 3aa was isolated in 88% yield and 94% ee (entry 17). This result indicates that the formation of byproduct 4aa could impact the enantioselectivity of 3aa. Unfortunately, the screening of various bases failed to afford improved results (entries 18–21); thus, entry 17 was identified as the standard conditions for the variation of the substrate.
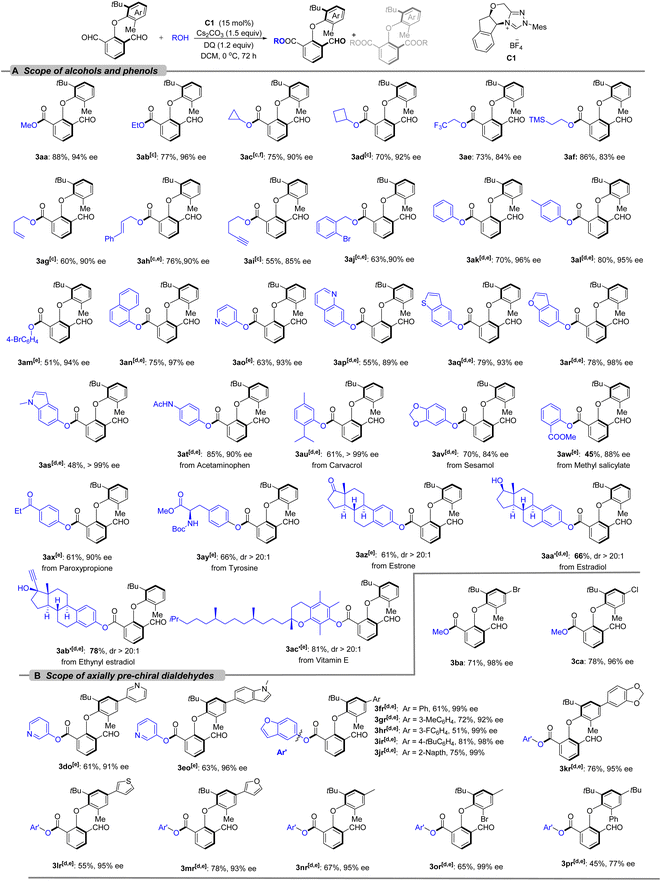
With the optimized conditions in hand, the scope and limitation of the atroposelective esterification system were examined (Scheme 2). First, the substrate scope of the alcohols was evaluated by the coupling with 2-(2-(tert-butyl)-6-methylphenoxy)isophthalaldehyde (1a) (Scheme 2A). A series of alcohols bearing primary alkyl (3aa, 3ab), secondary alkyl (3ac, 3ad), strained rings (3ac, 3ad), trifluoromethyl (3ae), TMS (3af), terminal (3ag) and internal (3ah) alkenyl, alkynyl (3ai), and benzyl groups (3aj) were well tolerated, delivering diaryl ether-type atropisomers in 55–88% yield and 83–96% ee. The absolute stereochemistry of 3aa was determined as the S configuration by HPLC compared with the known compounds14 (for details see the ESI†). Phenol was also a suitable substrate, generating 3ak–3am with up to 96% ee. Fused ring (3an), pyridine (3ao), isoquinoline (3ap), benzothiophene (3aq) benzofuran (3ar), and indole (3as) substituted phenols were all well tolerated, offering desired products with high enantioselectivity (in most cases >90% ee) and moderate yield (48–79%). Mild conditions and broad functional group tolerance encouraged us to carry out late-stage functionalization of natural products and bioactive compounds. Natural products including carvacrol (3au) and sesamol (3av), bioactive molecules such as methyl salicylate (3aw), paroxypropione (3ax), tyrosine (3ay), estrone (3az), estradiol (3aa′), and ethynyl estradiol (3ab′), and drugs such as acetaminophen (3at) and vitamin E (3ac′) were well tolerated in this system and delivered the axially chiral diaryl ethers in acceptable yields with good to excellent stereoselectivities (84–99% ee, or 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Non-cyclic secondary alcohols such as iPrOH (41%, 60% ee) and Ph2CHOH (70%, 74% ee) were tested; however, they gave decreased enantioselectivity. For tertiary alcohols (tBuOH), the esterification did not work at all. Other S-centered and N-centered nucleophiles were employed but failed to give satisfactory results (Scheme S1†).
1 dr). Non-cyclic secondary alcohols such as iPrOH (41%, 60% ee) and Ph2CHOH (70%, 74% ee) were tested; however, they gave decreased enantioselectivity. For tertiary alcohols (tBuOH), the esterification did not work at all. Other S-centered and N-centered nucleophiles were employed but failed to give satisfactory results (Scheme S1†).
Then, the scope of axially prochiral dialdehydes was explored (Scheme 2B). Dicarbaldehydes bearing halogens (3ba, 3ca), pyridinyl (3do), indolyl (3eo), substituted aryl (3fr–3ir, 3kr), naphthyl (3jr), thienyl (3lr), and furyl (3mr) on the aromatic ring could deliver the desired products with excellent enantioselectivity (in all cases >90% ee). Dicarbaldehydes with electron-donating methyl groups were well tolerated, giving the desired products 3nr and 3or with excellent ee. When the blocking group (methyl) of dicarbaldehyde was switched to bromine, nearly no reduction of ee was detected (3nrvs3or); however, when changing to the phenyl group, a drop in ee value to 77% was observed.
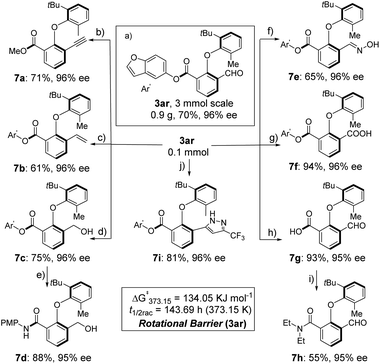
We conducted large-scale synthesis and follow-up transformations to further evaluate the synthetic value of the atroposelective esterification system. We successfully achieved gram-scale synthesis, obtaining compound 3ar with 70% yield and 96% ee (Scheme 3a). The racemization barrier of 3ar was up to ΔG≠rac = 134.05 kJ mol−1, corresponding to a half-life of 143.7 hours at 100 °C (i-PrOH). This relatively high racemization barrier of axially chiral diaryl ethers allows for excellent chiral retention in further transformations and thus might serve as a modular platform for diaryl ether-type atropisomers. Treatment of 3ar with P-(1-diazo-2-oxopropyl)-dimethyl ester produces transesterified alkyne 7a in 71% yield and 96% ee (Scheme 3b). Wittig reaction of 3ar could generate olefin-substituted diaryl ether-type atropisomers (Scheme 3c, 61%, 96% ee). 3ar could be reduced to 7c with 75% yield and 96% ee employing NaBH4 in THF/MeOH (Scheme 3d). Further treatment of 7c with 4-methoxy aniline in PhMe employing LiHMDS as the base results in an axially chiral amide (Scheme 3e). Condensation of the aldehyde group with NH2OH generated an oxime in 65% yield and 96% ee (Scheme 3f). Oxidation of the aldehyde group or hydrolysis of ester groups could yield chiral carboxylic acid 7f and 7g in good yields (Scheme 3g and h). Further condensation of 7g with diethylamine delivered amide 7h in 55% yield and 95% ee (Scheme 3i). Furthermore, cyclization of 3ar with TsNHNH2 and 2-bromo-3,3,3-trifluoropropene delivered pyrazole substituted diaryl ether 7i (Scheme 3j). No detectable enantioselectivity erosion was observed in all cases.
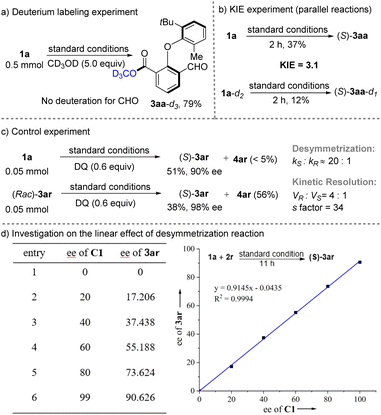
A series of mechanistic investigations were then conducted to probe the catalytic cycle and enantio-determining step of this esterification reaction (Scheme 4). Isotope exchange experiments resulted in no deuterium incorporation at the aldehyde group of 3aa-d3; reversible formation of the Breslow intermediate could be excluded (Scheme 4a). A parallel KIE experiment employing 1a and 1a-d2 in the coupling with MeOH gives KIE = 3.1 (Scheme 4b), indicating that BI formation might be involved in the rate-determining step. While optimizing the conditions, we found that the formation of diester byproducts could increase the ee value of the main product. We carried out a control experiment, and the results are shown in Scheme 4c. When the esterification was carried out with 60 mol% DQ, S-3ar (51%, 90% ee) was obtained with <5% 4ar; compared with 3ar (98% ee) under standard conditions, an additional stereocontrolling filter might exist beyond the desymmetrization. Furthermore, racemic 3ar could undergo efficient kinetic resolution, delivering S-3ar (38%, 98% ee) along with 4ar (56%). The minor enantiomer obtained after desymmetrization reacted fast in an over-functionalization (VR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Vs ≈ 4
Vs ≈ 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Removing minor enantiomers by the formation of diester byproducts is critical in improving the enantioselectivity (90% ee to 98% ee). On investigating the linear effect of desymmetrization of aldehydes, a linear correlation between the ee value of C1 and product 3ar was observed (Scheme 4d). The absence of a nonlinear effect indicated that a single NHC is presumably involved in the stereo-determining step.
1). Removing minor enantiomers by the formation of diester byproducts is critical in improving the enantioselectivity (90% ee to 98% ee). On investigating the linear effect of desymmetrization of aldehydes, a linear correlation between the ee value of C1 and product 3ar was observed (Scheme 4d). The absence of a nonlinear effect indicated that a single NHC is presumably involved in the stereo-determining step.
 | ||
| Scheme 4 Mechanistic studies and proposed mechanism. s = ln[(1 − Conv.)(1 − ees)]/ln[(1 − Conv.)(1 + ees)]. | ||
Conclusions
In summary, we have developed NHC-catalyzed facile and robust desymmetrization of readily accessible dialdehydes, leading to direct access to axially chiral diaryl ether derivatives. Mechanistic studies indicate that esterification proceeds via irreversible rate- and enantio-determination activation of the aldehyde followed by oxidative esterification. The matched kinetic resolutions play a critical role in enhancing the enantioselectivity by sacrificing minor enantiomers. The synthetic value of the esterification was further highlighted by the late-stage functionalization of natural products, bioactive molecules, and medicines (10 examples, up to 99% ee or >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr), and scale-up synthesis. This protocol features excellent chiral induction, mild conditions, good functional group tolerance, and broad substrate scope. The NHC-catalyzed desymmetrizative functionalization of axially prochiral diaryl ethers may provide modularized platforms for synthesizing challenging diaryl ether-type atropisomers and their derivatives.
1 dr), and scale-up synthesis. This protocol features excellent chiral induction, mild conditions, good functional group tolerance, and broad substrate scope. The NHC-catalyzed desymmetrizative functionalization of axially prochiral diaryl ethers may provide modularized platforms for synthesizing challenging diaryl ether-type atropisomers and their derivatives.
Data availability
Detailed synthetic procedures and complete characterization data for all new compounds can be found in the ESI.‡Author contributions
G. Z. and Q. Z. conceived the concept and directed the investigations. Y. W. conducted the majority of the experimental work. X. G., H. Z., M. L., and T. L. contributed to the preparation of substrates. J. S., G. Z., and Q. Z. wrote the manuscript with input from all the authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the NSFC (22193012, 22201033, 22001157, and 21831002), Jilin Educational Committee (JJKH20231295KJ, JJKH20231302KJ), Natural Science Foundation of Jilin Province (20230101047JC, YDZJ202201ZYTS338), and the Fundamental Research Funds for the Central Universities (2412022ZD012, 2412022QD016, 2412021QD007) for the generous financial support.Notes and references
- Selected reviews see: (a) L. Pu, Chem. Rev., 1998, 98, 2405–2494 CrossRef CAS PubMed; (b) G. A. Hembury, V. V. Borovkov and Y. Inoue, Chem. Rev., 2008, 108, 1–73 CrossRef CAS PubMed.
- (a) J. E. Smyth, N. M. Butler and P. A. Keller, Nat. Prod. Rep., 2015, 32, 1562–1583 RSC; (b) J. Clayden, W. J. Moran, P. J. Edwards and S. R. LaPlante, Angew. Chem., Int. Ed., 2009, 48, 6398–6401 CrossRef CAS PubMed.
- (a) Y. Chen, S. Yekta and A. K. Yudin, Chem. Rev., 2003, 103, 3155–3212 CrossRef CAS PubMed; (b) T. Akiyama, Chem. Rev., 2007, 107, 5744–5758 CrossRef CAS PubMed; (c) W. Tang and X. Zhang, Chem. Rev., 2003, 103, 3029–3070 CrossRef CAS PubMed; (d) D. Parmar, E. Sugiono, S. Raja and M. Rueping, Chem. Rev., 2014, 114, 9047–9153 CrossRef CAS PubMed.
- (a) J. K. Cheng, S. H. Xiang, S. Li, L. Ye and B. Tan, Chem. Rev., 2021, 121, 4805–4902 CrossRef CAS PubMed; (b) J. A. Carmona, C. Rodríguez-Franco, R. Fernández, V. Hornillos and J. M. Las-saletta, Chem. Soc. Rev., 2021, 50, 2968–2983 RSC; (c) J. K. Cheng, S. H. Xiang and B. Tan, Acc. Chem. Res., 2022, 55, 2920–2937 CrossRef CAS PubMed; (d) H. H. Zhang and F. Shi, Acc. Chem. Res., 2022, 55, 2562–2580 CrossRef CAS PubMed; (e) W. Qin, Y. Liu and H. Yan, Acc. Chem. Res., 2022, 55, 2780–2795 CrossRef CAS PubMed.
- (a) J. Feng, B. Li, Y. He and Z. Gu, Angew. Chem., Int. Ed., 2016, 55, 2186–2190 CrossRef CAS PubMed; (b) S. C. Zheng, S. Wu, Q. Zhou, L. W. Chung, L. Ye and B. Tan, Nat. Commun., 2017, 8, 15238 CrossRef PubMed; (c) P. Hu, L. Hu, X. X. Li, M. Pan, G. Lu and X. Li, Angew. Chem., Int. Ed., 2023, 63, e202312923 CrossRef PubMed.
- (a) H.-Y. Bai, F.-X. Tan, T.-Q. Liu, G.-D. Zhu, J.-M. Tian, T.-M. Ding, Z.-M. Chen and S.-Y. Zhang, Nat. Commun., 2019, 10, 3063 CrossRef PubMed; (b) J. Frey, A. Malekafzali, I. Delso, S. Choppin, F. Colobert and J. Wencel-Delord, Angew. Chem., Int. Ed., 2020, 59, 8844–8848 CrossRef CAS PubMed.
- (a) S.-L. Li, C. Yang, Q. Wu, H.-L. Zheng, X. Li and J.-P. Cheng, J. Am. Chem. Soc., 2018, 140, 12836–12843 CrossRef CAS PubMed; (b) H. Li, X. Yan, J. Zhang, W. Guo, J. Jiang and J. Wang, Angew. Chem., Int. Ed., 2019, 58, 6732–6736 CrossRef CAS PubMed; (c) Q.-J. Yao, P.-P. Xie, Y.-J. Wu, Y.-L. Feng, M.-Y. Teng, X. Hong and B.-F. Shi, J. Am. Chem. Soc., 2020, 142, 18266–18276 CrossRef CAS PubMed.
- (a) J. Yang, J.-W. Zhang, W. Bao, S.-Q. Qiu, S. Li, S.-H. Xiang, J. Song, J. Zhang and B. Tan, J. Am. Chem. Soc., 2021, 143, 12924–12929 CrossRef CAS PubMed; (b) K. Yang, Y. Mao, J. Xu, H. Wang, Y. He, W. Li and Q. Song, J. Am. Chem. Soc., 2021, 143, 10048–10053 CrossRef CAS PubMed.
- (a) K. C. Nicolaou, C. N. C. Boddy, S. Bräse and N. Winssinger, Angew. Chem., Int. Ed., 1999, 38, 2096–2152 CrossRef; (b) K. C. Nicolaou and C. N. C. Boddy, J. Am. Chem. Soc., 2002, 124, 10451–10455 CrossRef CAS PubMed.
- (a) K. Fuji, T. Oka, T. Kawabata and T. Kinoshita, Tetrahedron, 1998, 39, 1373–1376 CrossRef CAS; (b) M. S. Betson, J. Clayden, C. P. Worrall and S. Peace, Angew. Chem., Int. Ed., 2006, 45, 5803–5807 CrossRef CAS PubMed.
- J. Clayden, C. P. Worrall, W. J. Moran and M. Helliwell, Angew. Chem., Int. Ed., 2008, 47, 3234–3237 CrossRef CAS PubMed.
- B. Yuan, A. Page, C. P. Worrall, F. Escalettes, S. C. Willies, J. J. W. McDouall, N. J. Turner and J. Clayden, Angew. Chem., Int. Ed., 2010, 49, 7010–7013 CrossRef CAS PubMed.
- A. N. Dinh, R. R. Noorbehesht, S. T. Toenjes, A. C. Jackson, M. A. Saputra, S. M. Maddox and J. L. Gustafson, Synlett, 2018, 29, 2155–2160 CrossRef CAS PubMed.
- L. Dai, Y. Liu, Q. Xu, M. Wang, Q. Zhu, P. Yu, X. Zeng and G. Zhong, Angew. Chem., Int. Ed., 2023, 62, e202216534 CrossRef CAS PubMed.
- H. Bao, Y. Chen and X. Yang, Angew. Chem., Int. Ed., 2023, 62, e202300481 CrossRef CAS PubMed.
- (a) X. Bugaut and F. Glorius, Chem. Soc. Rev., 2012, 41, 3511–3522 RSC; (b) M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485–496 CrossRef CAS PubMed; (c) R. S. Menon, A. T. Biju and V. Nair, Chem. Soc. Rev., 2015, 44, 5040–5052 RSC; (d) K. J. R. Murauski, A. A. Jaworski and K. A. Scheidt, Chem. Soc. Rev., 2018, 47, 1773–1782 RSC; (e) P. Bellotti, M. Koy, M. N. Hopkinson and F. Glorius, Nat. Rev. Chem, 2021, 5, 711 CrossRef CAS PubMed; (f) Y. Sumoda and H. Ohmiya, Chem. Soc. Rev., 2021, 50, 6320–6332 RSC; (g) K. Liu, M. Schwenzer and A. Studer, ACS Catal., 2022, 12, 11984–11999 CrossRef CAS.
- J. Wang, C. Zhao and J. Wang, ACS Catal., 2021, 11, 12520–12531 CrossRef CAS.
- (a) S. Lu, S. B. Poh, Z. Rong and Y. Zhao, Org. Lett., 2019, 21, 6169–6172 CrossRef CAS PubMed; (b) G. Yang, D. Guo, D. Meng and J. Wang, Nat. Commun., 2019, 10, 3062 CrossRef PubMed; (c) S. Zhuo, T. Zhu, L. Zhou, C. Mou, H. Chai, Y. Lu, L. Pan, Z. Jin and Y. Chi, Angew. Chem., Int. Ed., 2019, 58, 1784–1788 CrossRef CAS PubMed; (d) S. Barik, S. Shee, S. Das, R. G. Gonnade, G. Jindal, S. Mukherjee and A. T. Biju, Angew. Chem., Int. Ed., 2021, 60, 12264–12268 CrossRef CAS PubMed; (e) J. Jin, X. Huang, J. Xu, T. Li, X. Peng, X. Zhu, J. Zhang, Z. Jin and Y. Chi, Org. Lett., 2021, 23, 3991–3996 CrossRef CAS PubMed; (f) X. Yang, L. Wei, Y. Wu, L. Zhou, X. Zhang and Y. Chi, Angew. Chem., Int. Ed., 2023, 62, e202211977 CrossRef CAS PubMed; (g) Y. Wu, M. Li, J. Sun, G. Zheng and Q. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202117340 CrossRef CAS PubMed; (h) W. Xiao, L. Lu, H. Jiang, X. He, J. Liu and W. Zhao, Chin. J. Org. Chem., 2022, 42, 2504–2514 CrossRef.
- (a) S. Lu, S. B. Poh and Y. Zhao, Angew. Chem., Int. Ed., 2014, 53, 11041–11045 CrossRef CAS PubMed; (b) D. Guo, Q. Peng, B. Zhang and J. Wang, Org. Lett., 2021, 23, 7765–7770 CrossRef CAS PubMed; (c) S. Lu, J. Y. Ong, H. Yang, S. B. Poh, X. Liew, C. S. D. Seow, M. W. Wong and Y. Zhao, J. Am. Chem. Soc., 2019, 141, 17062–17067 CrossRef CAS PubMed; (d) C. Zhao, D. Guo, K. Munkerup, K. Huang, F. Li and J. Wang, Nat. Commun., 2018, 9, 611 CrossRef PubMed; (e) K. Xu, W. Li, S. Zhu and T. Zhu, Angew. Chem., Int. Ed., 2019, 58, 17625–17630 CrossRef CAS PubMed; (f) C. Zhang, Y. Gao, H. Wang, B. Zhou and S. Ye, Angew. Chem., Int. Ed., 2021, 60, 13918–13922 CrossRef CAS PubMed; (g) T. Li, C. Mou, P. Qi, X. Peng, S. Jiang, G. Hao, W. Xue, S. Yang, L. Hao, Y. R. Chi and Z. Jin, Angew. Chem., Int. Ed., 2021, 60, 9362–9367 CrossRef CAS PubMed; (h) Y. Lv, G. Luo, Q. Liu, Z. Jin, X. Zhang and Y. Chi, Nat. Commun., 2022, 13, 36 CrossRef CAS PubMed; (i) B. Mondal, H. Chen, R. Maiti, H. Wang, H. Cai, C. Mou, L. Hao, H. Chai and Y. Chi, Org. Lett., 2023, 25, 8252–8257 CrossRef CAS PubMed; (j) S. Zhang, X. Wang, L. Han, J. Li, Z. Liang, D. Wei and D. Du, Angew. Chem., Int. Ed., 2022, 61, e202212005 CrossRef CAS PubMed; (k) J. Yan, R. Maiti, S. Ren, W. Tian, T. Li, J. Xu, B. Mondal, Z. Jin and Y. Chi, Nat. Commun., 2022, 13, 84 CrossRef CAS PubMed; (l) S. Zhang, S. Liu, X. Wang, S. Wang, H. Yang, L. Li, B. Yang, M. W. Wong, Y. Zhao and S. Lu, ACS Catal., 2023, 13, 2565–2575 CrossRef CAS; (m) K. Balanna, S. Barik, S. Barik, S. Shee, N. Manoj, R. G. Gonnade and A. T. Biju, ACS Catal., 2023, 13, 8752–8759 CrossRef CAS.
- (a) J. P. Vigneron, M. Dhaenens and A. Horeau, Tetrahedron, 1973, 29, 1055–1059 CrossRef CAS; (b) A. M. Harned, Tetrahedron, 2018, 74, 3797–3841 CrossRef CAS; (c) M. Burns, S. Essafi, J. R. Bame, M. P. Webster, S. Balieu, J. W. Dale, C. P. Butts, J. N. Harvey and V. K. Aggarwal, Nature, 2014, 513, 183–188 CrossRef CAS PubMed; (d) J. Merad, P. Borkar, F. Caijo, J. M. Pons, J. L. Parrain, O. Chuzel and C. Bressy, Angew. Chem., Int. Ed., 2017, 56, 16052–16056 CrossRef CAS PubMed.
- (a) L. Wang, R. Ma, J. Sun, G. Zheng and Q. Zhang, Chem. Sci., 2022, 13, 3169–3175 RSC; (b) L. Wang, J. Sun, J. Xia, M. Li, L. Zhang, R. Ma, G. Zheng and Q. Zhang, Sci. China: Chem., 2022, 65, 1938–1944 CrossRef CAS; (c) L. Wang, J. Sun, J. Xia, R. Ma, G. Zheng and Q. Zhang, Org. Chem. Front., 2023, 10, 1047–1055 RSC; (d) J. Sun, L. Wang, G. Zheng and Q. Zhang, Org. Chem. Front., 2023, 10, 4488–4515 RSC.
- (a) T. Liang, Y. Wu, J. Sun, M. Li, H. Zhao, J. Zhang, G. Zheng and Q. Zhang, Chin. J. Chem., 2023, 41, 3253–3260 CrossRef CAS; (b) G. Zhang, Y. Liang, T. Qin, T. Xiong, S. Liu, W. Guan and Q. Zhang, CCS Chem., 2020, 2, 1737–1745 Search PubMed; (c) G. Zhang ang and Q. Zhang, Chem Catal., 2023, 3, 100526 CrossRef; (d) J. Xia, Y. Guo, Z. Lv, J. Sun, G. Zheng and Q. Zhang, Molecules, 2024, 29, 790 CrossRef PubMed.
- (a) S. Staniland, B. Yuan, N. Gimnez-Agull, T. Marcelli, S. C. Willies, D. M. Grain-ger, N. J. Turner and J. Clayden, Chem. – Eur. J., 2014, 20, 13084–13088 CrossRef CAS PubMed; (b) H. Jiang, X.-K. He, X. Jiang, W. Zhao, L.-Q. Lu, Y. Cheng and W.-J. Xiao, J. Am. Chem. Soc., 2023, 145, 6944–6952 CrossRef CAS PubMed; (c) J. Liu, M. Zhou, R. Deng, P. Zheng and Y.-R. Chi, Nat. Commun., 2022, 13, 4793 CrossRef CAS PubMed; (d) M. Zhou, J. Liu, R. Deng, Q. Wang, S. Wu, P. Zheng and Y.-R. Chi, ACS Catal., 2022, 12, 7781–7788 CrossRef CAS; (e) H. Liu, P. He, X. Liao, Y. Zhou, X. Chen, W. Ou, Z. Wu, C. Luo, L. Yang and J. Xu, ACS Catal., 2022, 12, 9864–9871 CrossRef CAS; (f) X. Lv, J. Xu, C. Sun, F. Su, Y. Cai, Z. Jin and Y.-R. Chi, ACS Catal., 2022, 12, 2706–2713 CrossRef CAS; (g) S. Shee, S. S. Ranganathappa, M. S. Gadhave, R. Gogoi and A. T. Biju, Angew. Chem., Int. Ed., 2023, 62, e202311709 CrossRef CAS PubMed; (h) B. Zhou, X. Li, C. Zhang, Z. Wang and S. Ye, Angew. Chem., Int. Ed., 2023, e202314228, DOI:10.1002/anie.202314228; (i) Y. Wu, X. Guan, H. Zhao, M. Li, T. Liang, J. Sun, G. Zheng and Q. Zhang, ChemRxiv, 2023, preprint, DOI:10.26434/chemrxiv-2023-n9skl.
- (a) J. Guin, S.-D. Sarkar, S. Grimme and A. Studer, Angew. Chem., Int. Ed., 2008, 47, 8727–8730 CrossRef CAS PubMed; (b) S.-D. Sarkar, S. Grimme and A. Studer, J. Am. Chem. Soc., 2010, 132, 1190–1191 CrossRef PubMed; (c) W. Harnying, P. Sudkaow, A. Biswas and A. Berkessel, Angew. Chem., Int. Ed., 2021, 60, 19631–19636 CrossRef CAS PubMed.
Footnotes |
| † Dedicated to Professor Dennis Curran on the occasion of his 70th birthday. |
| ‡ Electronic supplementary information (ESI) available: See DOI: https://doi.org/10.1039/d3sc06444a |
| This journal is © The Royal Society of Chemistry 2024 |