Recent advances and perspectives of 1D/2D carbon materials for high-performance flexible zinc ion batteries
Qingqing
Zheng
 a,
Zewei
Hu
a,
Liyang
Liu
a,
Haiying
Lu
a,
Xin
Wang
a,
Yongpeng
Lei
a,
Zewei
Hu
a,
Liyang
Liu
a,
Haiying
Lu
a,
Xin
Wang
a,
Yongpeng
Lei
 a,
Chao
Han
b and
Weijie
Li
a,
Chao
Han
b and
Weijie
Li
 *a
*a
aState Key Laboratory for Powder Metallurgy, Central South University, Changsha, 410083, China. E-mail: li-306@csu.edu.cn
bSchool of Materials Sciences and Engineering, Central South University, Changsha, 410083, China
First published on 12th July 2024
Abstract
Flexible zinc ion batteries (FZIBs) have garnered significant attention owing to their cost-effectiveness, environmental friendliness, excellent flexibility and advanced security. Nevertheless, the electrochemical performance of FZIBs, such as energy density and cycling life, has yet to be improved compared to that of conventional rigid zinc-ion batteries (ZIBs). Due to the excellent electrical conductivity and mechanical properties exhibited by advanced one or two dimensional (1D/2D) carbon materials, they are increasingly recognized to play a key role in constructing flexible electrodes and improving the capacity, energy density/power density of FZIBs. However, no review comprehensively summarizes the functions and advances of 1D/2D carbon materials in FZIBs to date. In this review, a comprehensive overview of the development background of 1D/2D carbon materials (carbon nanotubes, graphene, MXenes and carbon fiber), highlighting their great advantages and functions of applications in FZIBs is given. Detailed summaries of recent advancements and the current challenges of 1D/2D carbon materials for high-performance FZIBs along with promising strategies are provided. First, the essential requirements and challenges of FZIBs and the fundamental aspects of 1D/2D carbon materials, including the development background and the unique advantages of these 1D/2D carbon materials applied in FZIBs are summarized. Then, the latest developments of these 1D/2D materials in FZIBs, which could function as active materials, conductive networks, current collectors or Zn hosts in FZIBs are discussed. In addition, the application of 1D/2D carbon materials in separators and gel electrolytes is specially emphasized. Finally, the development prospect of 1D/2D carbon materials used in FZIBs is briefly discussed.
1. Introduction
With the continuous progress of technology, the application of flexible electronics is becoming increasingly widespread. Examples include implantable electronic skins in medicine, wearable health monitoring systems, smart clothing, and flexible displays.1–4 However, traditional energy storage devices, such as supercapacitors, are too large, heavy, and expensive to be compatible with flexible electronic devices.5,6 Therefore, developing small, lightweight, low-cost, and fast-charging flexible energy storage devices is an important challenge for technological innovation in flexible electronics. Various studies have been conducted on flexible batteries, which could be an integral part of flexible electronic products.7,8As a resource-rich metal in the world, zinc has attracted a lot of attention due to its cheap price, non-toxic and environmentally friendly nature, low redox potential (−0.76 V versus the standard hydrogen electrode (SHE)), and high theoretical capacity (820 mA h g−1 or 5855 mA h cm−3).9–12 Moreover, compared with traditional liquid electrolytes based on organic solvents, aqueous electrolytes display higher biocompatibility and environmental friendliness, and avoid safety concerns.13,14 Thus, ZIBs are suitable for developing flexible batteries for flexible electronic products. However, the employment of aqueous electrolytes is not suitable in flexible batteries due to the potential liquid leakage under deformation.15 In addition, aqueous electrolytes tend to induce the growth of zinc dendrites and the formation of soluble ZnO2 on the zinc anode, which poison the cathode, resulting in rapid capacity attenuation.16–18 Polymer/hydrogel electrolytes are becoming crucial in addressing specific challenges in flexible battery technology due to their relatively low water content. Furthermore, the formation of a solid–solid interface between the electrodes and polymer/hydrogel electrolytes, rather than a solid–liquid interface, significantly enhances the stability of the battery system.19–21 This alteration mitigates the risks associated with dendrite formation and the degradation of cathode materials. However, polymer/hydrogel electrolytes usually possess low ionic conductivity and high interfacial resistance. Therefore, the batteries consisting of polymer/hydrogel electrolytes exhibit poor rate performance and high resistance.22–24 In addition, conventional metal oxide cathodes suffer from inherently low conductivity, which hinders rapid electron transfer. Their mechanical instability also renders them unable to cope with repeated volume changes during the intercalation/deintercalation cycles.13,25 Moreover, when these cathode materials are directly exposed to mild acid electrolytes, it typically leads to the dissolution of active materials, which accelerates the capacity loss.26 Simultaneously, flexible zinc anodes require robust mechanical stability to ensure the preservation of electrochemical performance and structural integrity during deformation such as bending or stretching. However, flexible zinc anodes are often susceptible to mechanical damage or fatigue.27,28 This problem could cause short circuits of batteries. Collectively, these challenges in both cathode and anode materials contribute to suboptimal zinc storage outcomes, manifesting in lower energy density and a restricted cycling life.
To tackle the mentioned challenges and pursue for the high-performance and excellent mechanical properties of FZIBs, it requires the utilization of flexible battery components (e.g., cathodes, anodes and electrolytes).29,30 Over the past decade, 1D/2D carbon materials have increasingly been applied in FZIBs owing to their superior electrical conductivity, mechanical properties, and adjustable porous structures.31,32 These 1D materials include carbon nanotubes (CNTs), carbon fibers (CFs), and 2D materials include graphene and MXenes.33,34 For example, Zhi et al.35 explained the design principles and device performance of flexible fiber-based batteries. Hu et al.36 introduced the design mechanisms of carbon nanotubes (CNTs), carbon fibers (CFs), and other carbon materials in flexible batteries based on thin films and fibers that can be bent. Moreover, previous reviews on FZIBs have primarily focused on gel electrolytes. For instance, He and colleagues reviewed recent advancements in polymer electrolytes, particularly focusing on the synthesis and characterization of gel electrolytes. Their work aimed to provide insights from laboratory research to commercialization.37 Similarly, Li's team systematically discussed and evaluated the preparation methods of functional gel electrolytes for FZIBs from a fabrication perspective.38 Zhang and colleagues introduced the latest research progress and rational design strategies of flexible quasi-solid-state ZIBs from the perspectives of mechanisms, design principles and applications.39 However, until now, no review has comprehensively reported the progress of application of 1D/2D carbon materials in the cathodes, anodes, gel electrolytes and separators of FZIBs. With the attention to flexible electronics increasing, there is an urgent need for a systematic and timely review of the recent advancements in the use of 1D/2D carbon materials in FZIBs to provide potential directions for the next generation of FZIBs.
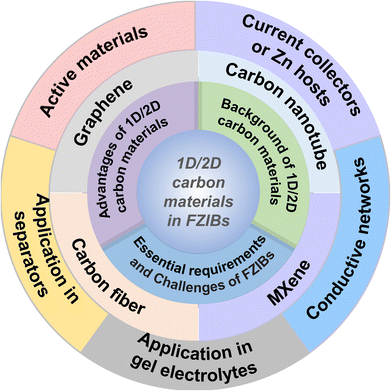
In this review, we aim to figure out the development background of 1D/2D carbon materials, highlighting their great advantages and functions of applications in FZIBs. Detailed summaries of recent advancements and the current challenges of 1D/2D carbon materials for high-performance FZIBs along with promising strategies are provided. As depicted in Fig. 1, this review begins by introducing the essential requirements and challenges of FZIBs. Currently summarizing the fundamental aspects of 1D/2D carbon materials (carbon nanotubes, graphene, MXenes and carbon fiber), including the development background and the unique advantages of these 1D/2D carbon materials applied in FZIBs. Then, the latest developments of these 1D/2D materials in FZIBs, which could function as active materials, conductive networks, current collectors or Zn hosts in FZIBs are discussed. In addition, the application of 1D/2D carbon materials in separators and gel electrolytes is specially emphasized. Finally, the development prospect of 1D/2D carbon materials used in FZIBs is briefly discussed. We hope to provide valuable suggestions for the research and development of 1D/2D carbon material applying in FZIBs through this review.
2. Fundamentals of 1D/2D carbon materials in FZIBs
2.1. Essential requirements and challenges of FZIBs
FZIBs represent an emerging field of energy storage technologies, particularly for applications requiring flexibility, such as wearable electronics, flexible displays, and medical implants.40 FZIBs offer a viable alternative to traditional LIBs due to the plentiful supply of zinc in the world, low cost, and safety.41 Nonetheless, their progress also faces several challenges and complexities in the configuration and materials used.Firstly, there are some essential requirements for the configuration of FZIBs, as summarized in Fig. 2a. (1) Basic configuration: similar to traditional ZIBs, the basic configuration of FZIBs comprises cells assembled with Zn foil anodes and cathodes that include binders, conductive additives, and pure active materials on metal current collectors. During the discharge process, anodic zinc releases electrons and generates Zn2+ ions, which are subsequently dissolved by a Zn2+-containing electrolyte. Currently, the Zn2+ ions present in the electrolyte traverse the separator and insert into the cathode. The flow of electrons from the anode to the cathode through an external circuit provides the power necessary to operate electrical devices.42,43 However, the restricted flexibility of zinc metal is inadequate for wearable or flexible applications. Additionally, zinc metal is susceptible to the shape memory effect, preventing it from enduring continuous bending. In order to achieve a truly flexible zinc anode, two common methods have been employed. The first of these involves the application of coating pastes, comprising Zn powders, conductive and binding additives on flexible and conductive current collectors. Another approach is to directly electrodeposit zinc onto flexible substrates. A comparable methodology for the fabrication of flexible cathodes is to deposit active cathode materials upon flexible substrates. This approach is also applicable to the coating of pastes on flexible substrates, which is effective for powder cathode materials. In addition, inspired by the requirement for leak-proof designs, gel electrolytes are favoured for constructing ZIBs in flexible configurations.44 (2) Flexible device design: the design of the device plays a crucial role in ensuring that the batteries meet the essential requirements for performance and flexibility. Several key aspects of flexible device design include the development of fiber battery design and sandwich structure battery design. Fiber ZIBs exhibit linear shapes and omni-directional flexibility, which can liberate ZIBs from rigid constraints and bestow upon them greater shape versatilities and design freedoms. The sandwich structure adheres to the conventional principles of battery design. The fabrication of sandwiched ZIBs typically involves numerous steps, including the preparation of flexible electrodes, the sandwiching of the electrolyte and separator between two thin-film electrodes, and subsequent packaging processes.44,45 (3) Withstand deformation: FZIBs must withstand deformation while maintaining their electrochemical performance. Therefore, the transition from traditional rigid and brittle materials to flexible alternatives is essential for every component of ZIBs.46–49
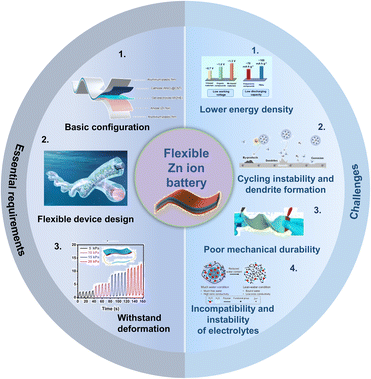 | ||
| Fig. 2 Essential requirements and challenges of FZIBs. Basic configuration image: reproduced with permission from ref. 50. Copyright 2023, American Chemical Society. Flexible device design image: reproduced with permission from ref. 28. Copyright 2024, Wiley. Withstand deformation image: reproduced with permission from ref. 28 Copyright 2024, Wiley. Lower energy density image: reproduced with permission from ref. 25. Copyright 2022, Wiley. Cycling instability and dendrite formation image: reproduced with permission from ref. 21. Copyright 2023, Wiley. Poor mechanical durability image: reproduced with permission from ref. 43. Copyright 2019, Elsevier. Incompatibility and instability of electrolytes image: reproduced with permission from ref. 24. Copyright 2023, Springer Nature. | ||
Currently, the challenges of FZIBs can be summarized as follows: (1) lower energy density: while ZIBs offer many advantages, their energy density typically lags behind that of LIBs, which is a significant challenge for many applications where both high energy density and flexibility are required. (2) Cycling instability and dendrite formation: repeated charge and discharge cycles can lead to the formation of Zn dendrites. These dendrites can pierce the separator, causing short circuits and battery failure.50 (3) Poor mechanical durability: the repeated bending and flexing in applications can strain the mechanical integrity of battery components, leading to potential failures in the electrical connections and separator breakdown.41,51 (4) Incompatibility and instability of electrolytes: aqueous electrolytes in ZIBs can be prone to water splitting at high voltages, limiting their voltage window and overall performance, while gel electrolytes could expand the voltage range but may introduce other issues of instability or incompatibility.52
Addressing these challenges requires innovative approaches in materials science and engineering. For instance, exploring advanced 1D/2D carbon materials for electrodes and separators could enhance the mechanical and electrochemical properties of FZIBs. Additionally, developing novel gel electrolytes with improved stability and compatibility will be crucial for the future success of FZIBs.
2.2. Background of 1D/2D carbon materials
1D/2D carbon materials represent an intriguing and advanced frontier in materials science, due to their distinctive structural, electrical, mechanical, and thermal properties. These materials have gained substantial interest for a broad range of applications including electronics, energy storage, generation, sensors, and composite materials.53 The following are the background and basic properties of 1D/2D carbon materials:2.3. Advantages of 1D/2D carbon materials in FZIBs
To address the mentioned challenges of FZIBs, the rational design and construction of carbon-based hybrid electrodes is considered as an effective approach benefiting from multiple exceptional properties of 1D/2D carbon materials. These materials offer numerous advantages (Fig. 3a):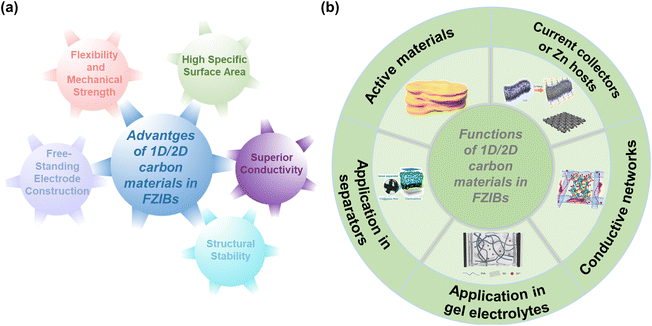 | ||
| Fig. 3 An overview of (a) the advantages and (b) functions of 1D/2D carbon materials in FZIBs. Active materials image: reproduced with permission from ref. 29. Copyright 2020, Wiley. Conductive networks image: reproduced with permission from ref. 34. Copyright 2022, Elsevier. Current collectors or Zn hosts image: reproduced with permission from ref. 33. Copyright 2019, Wiley. Application in gel electrolytes image: reproduced with permission from ref. 31. Copyright 2021, AAAS. Application in separators image: reproduced with permission from ref. 30. Copyright 2020, Wiley. | ||
Due to the above advantages, 1D/2D carbon materials are excellent candidates for various components in FZIBs, which could be utilized as active materials, conductive networks, current collectors or Zn hosts in FZIBs. Especially, 1D/2D carbon materials are also widely applied in modified separators and gel electrolytes in FZIBs (Fig. 3b). Table 1 summarizes the functions of 1D/2D carbon materials in FZIBs. The details of the functions of 1D/2D carbon materials in FZIBs will be stated in the following text.
| Function | Cathode | Anode | Electrolyte | Current density | Cycling number | Capacity retention (%) | Flexibility | Ref. |
|---|---|---|---|---|---|---|---|---|
| Active materials | ZnMn2O4 | NHVO@Ti3C2Tx | 3 m Zn (CF3SO3)2 | 2 A g−1 | 6000 | 92.1 | Folded | 69 |
| V2CTX MXene | Flexible Zn | PAMHS | 0.5 A g−1 | 120 | Twisted | 70 | ||
| Conductive networks | α-MnO2@CNT | Zn@CNT | 2 M ZnSO4/0.2 MnSO4/PVA | 32.5C | 1000 | 100 | Bent | 71 |
| ZMO/CNT | Zn foil | 1.0 M ZnSO4/0.1 M MnSO4 | 3 A g−1 | 2000 | 97.01 | Bent and twisted | 34 | |
| CaVO/CNTs | Zn foil | 3 M Zn (CF3SO3)2 | 10 A g−1 | 3000 | 90.7 | Bent | 72 | |
| CVO/RCNTs | Zn foil | 2 M Zn (CF3SO3)2/PVA | 5 A g−1 | 1400 | 61.5 | Bent | 73 | |
| KVO/SWCNT | Zn foil | 4 M Zn (CF3SO3)2 | 5 A g−1 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
91 | Bent | 74 | |
| MnO2/CNT/PAA | Zn foil | 2 M ZnSO4/0.1 M MnSO4 | 1.5 A g−1 | 1000 | 82 | Bent | 75 | |
| PDA/CNT/MnO2 | Zn foil | 3.3 M ZnSO4 | 0.2 A g−1 | 500 | Bent | 76 | ||
| MnO2 | Zn power/CNTs | 2 M ZnSO4/0.1 M MnSO4 | 3 A g−1 | 2000 | 91.1 | Bent | 77 | |
| CNT/MnO2 | ZCN | 2 M ZnSO4 + 0.2 M MnSO4 | 3 A g−1 | 1000 | 75.3 | Bent | 78 | |
| MnO2/rGO | Zn foil | 2 M ZnSO4/0.1 M MnSO4 | 6 A g−1 | 500 | 79 | Bent | 79 | |
| MnO2/rGO | Zn foil | 2 M ZnSO4/0.1 M MnSO4 | 2 A g−1 | 2000 | 99.87 | Bent and folded | 80 | |
| RGO/NVO | Zn foil | 1 MZnSO4/1 M Na2SO4 | 1 A g−1 | 2000 | 94 | Bent | 81 | |
| MnO2/EG | Zn/EG | 2 M ZnSO4/0.1 M MnSO4 | 1C | 480 | Bent | 82 | ||
| Ti3C2Tx@MnO2 micro flowers | Zn foil | 2.0 M ZnSO4/0.1 M MnSO4 | 0.5 A g−1 | 2000 | 90.6 | Bent | 83 | |
| CC@MnO2@MXene | Zn foil | PVA/ZnSO4/MnSO4 | 1 A g−1 | 800 | 51.4 | Bent | 84 | |
| VO2/MXene | Zn foil | PVA/Zn (CF3SO3)2 | 5 A g−1 | 2500 | 72.1 | Bent | 85 | |
| ZMO@Ti3C2Tx | Zn foil | Gelatin-based | 1 A g−1 | 5000 | 92.4 | Flat, bent, and twisted | 86 | |
| Current collectors or Zn hosts | Co3O4 NSs@CNTF | Zn NSs@CNTF | 2 M ZnSO4/0.0005 M CoSO4 | 5 A g−1 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
97.27 | Bent and twisted | 87 |
| CNT/MnO2 foams | Zn foil | 2 M ZnSO4/0.005 M MnSO4 | 10 mA cm−2 | 2500 | 83.5 | Folded | 88 | |
| MnO2@CNT | Zn wire | 2 M ZnTFSI/0.2 M MnCl2/PVA | 2 A g−1 | 100 | 98 | Bent and twisted | 89 | |
| CNT–MnO2@PEDOT | Zn/CNT | PVA/LiCl/ZnCl2/MnSO4 | 20 mA cm−2 | 1000 | 88.7 | Bent and twisted | 33 | |
| FSM@FGF | Zn foil | 2 M ZnSO4 | 1 A g−1 | 300 | 82.7 | Folded | 90 | |
| ZOV array | Zn array | Fumed silica/ZnSO4 | 20C | 2000 | 89 | Bent and twisted | 91 | |
| MnO2@N-VG@CC | Zn@N-VG@CC | PVA/Zn (CF3SO3)2 | 2 A g−1 | 300 | 80 | Bent and twisted | 92 | |
| PANI/SWCNTs | Zn/RGO–SWCNTs | PVA/Zn (CF3SO3)2 | 1 A g−1 | 1000 | 97.3 | Flat, bent, and twisted | 93 | |
| N-CNSs@MnO2 | N-CNSs@Zn | PVA | 8 A g−1 | 500 | 76.5 | Bent and twisted | 94 | |
| Al2O3@VSe2NSs@N-CNFs | Zn NSs@CNT | CMC/ZnSO4 | 1 A g−1 | 2500 | 86.2 | Bent | 95 | |
| V2O5-CFC | Zn foil | 2 M ZnSO4/0.1 M MnSO4 | 0.5 A g−1 | 1000 | Bent | 96 | ||
| V2O3@CNFs | Zn@CC | 2 M Zn (CF3SO3)2 | 2 A g−1 | 1000 | Bent and twisted | 97 | ||
| MnO2@CC | Zn foil | PAM/ZnSO4/MnSO4 | 0.1 A g−1 | 300 | Bent | 98 | ||
| MnO2 | CC@MnO2-UTF@Zn | 2 M ZnSO4/0.2 M MnSO4 | 1 A g−1 | 300 | 81 | Flat and bent | 99 | |
| VS2/CC | Zn/CC | PVA/Zn (CH3COO)2/Mn (CH3COO)2· | Flat, bent, and twisted | 100 | ||||
| Application in gel electrolytes | MnO2 | Zn foam | PVA/GO | 0.3 A−1 | 1000 | 98 | Flat, bent, and twisted | 31 |
| V2O5 | Zn foil | V2O5/GO/PVA | 1 A g−1 | 1000 | 96 | Bent and twisted | 101 | |
| V2O5 | Zn foil | PVA/Zn (CF3SO3)2/TiO2 | 0.2 A g−1 | 100 | 99.8 | Flat and bent | 102 | |
| Application in separators | V2O5 | Zn foil | 2 M ZnSO4 | 5 A g−1 | 1000 | 75 | Flat and bent | 30 |
| V2O5 | Zn foil | 2 M Zn (CF3SO3)2 | 1 A g−1 | 1000 | 78.7 | Bent | 103 |
3. 1D/2D carbon materials for active materials
1D/2D carbon materials could provide numerous extraordinary properties, which contribute to them being attractive candidates as active materials for FZIBs. Firstly, 1D/2D carbon materials have a high specific surface area (SSA) and abundant pore structure, which can provide more embedded zinc sites, with a higher embedded zinc capacity. Secondly, 1D/2D carbon materials with excellent ionic conductivity facilitate fast ionic transport and reaction kinetics to improve the charge/discharge rate performance of batteries.42 Additionally, 1D/2D carbon materials also possess reliable chemical and cycling stability, which can reduce the capacity degradation and cycling life loss of the batteries. There have been several studies focusing on the application of 1D/2D carbon materials as active materials for FZIBs. For example, 1D/2D carbon materials such as MXenes, which have been adopted as active materials for FZIBs. These studies have proved that 1D/2D carbon materials could exhibit well-embedded zinc properties, high specific capacity and excellent cycling stability in FZIBs.Modeled on the method of inhibiting dendrite growth in a lithium-ion battery system, the construction of rocking-chair type zinc-free metal anode materials will be an effective way to solve the growth of zinc dendrites.14 MXene is a class of 2D layered transition metal carbides or nitrides, with interlayer spaces capable of accommodating and storing zinc ions, which makes it an ideal zinc ion storage material. As active materials for zinc storage, MXenes offer high capacity, rapid ion transport, and excellent mechanical properties, among other advantages. Yuan et al. synthesized (NH4)2V10O25·8H2O@Ti3C2Tx (NHVO@Ti3C2Tx) films by vacuum filtration of 1D ultrathin NHVO nanobelts and layered Ti3C2Tx nanosheets. The composite film exhibited a low working potential of 0.59 V and could be utilized as an anode for “rocking-chair” type ZIBs. More importantly, 2D Ti3C2Tx not only provided a flexible substrate for NHVO, which could be bent and folded, with an excellent mechanical performance, but also stabilized the structural changes of NHVO nanobelts during charge and discharge. In the meantime, the utilization of MXenes as active materials yielded a specific capacity. Consequently, the NHVO@Ti3C2Tx thin film electrode delivered a remarkable capacity of 514.7 mA h g−1 over 6000 cycles, retaining a capacity of up to 84.2%. The “rocking-chair” Zn-ion full battery constructed by pairing the NHVO@Ti3C2Tx thin film anode and the ZnMn2O4 cathode exhibited a maximum specific capacity of 131.7 mA h g−1 and a maximum energy density of 97.1 W h kg−1, which is better than those of the previously reported “rocking-chair” Zn-ion battery.69 In addition, Li.et al. presented an aqueous zinc hybrid-ion battery (ZHIB) featuring a remarkable capacity enhancement over 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, utilizing a 2D layered V2CTX MXene cathode. Here, “TX” denoted the surface functional groups such as –F, –OH, and = O, which significantly differed from those in previously reported ZHIBs. The delamination of V2CTX MXene enabled a continuous exposure of active sites, and the phase transition product in conjunction with V2CTX MXene contributed continuously to capacity, which accounted for the unusual capacity enhancement.70
000 cycles, utilizing a 2D layered V2CTX MXene cathode. Here, “TX” denoted the surface functional groups such as –F, –OH, and = O, which significantly differed from those in previously reported ZHIBs. The delamination of V2CTX MXene enabled a continuous exposure of active sites, and the phase transition product in conjunction with V2CTX MXene contributed continuously to capacity, which accounted for the unusual capacity enhancement.70
While MXenes are promising in these diverse applications, several challenges need addressing to fully exploit their potential: (1) MXenes are susceptible to oxidation, which can degrade their properties. Enhancing their stability under environmental conditions is crucial for practical applications. (2) Methods for the synthesis and processing of MXenes need improvement to ensure that they can be produced in large quantities without losing quality. (3) Concerns regarding the toxicity of MXenes and their precursors, particularly those involving hazardous chemicals like hydrofluoric acid, need to be addressed. Developing safer synthesis methods and handling protocols is essential. Addressing these challenges will be key to advancing the use of MXenes as active materials across various domains. With ongoing research and development, MXenes hold the potential to revolutionize multiple industries by providing high-performance alternatives to traditional materials.
4. 1D/2D carbon materials for conductive networks
The combination of 1D/2D carbon materials with superior electrical conductivity and electrode materials can significantly enhance the conductivity and flexibility of electrodes, thereby improving their zinc storage performance. For details, 1D carbon materials, such as carbon nanotubes, exhibit elongated and thin structures resembling rolled-up single-layer carbon atoms, facilitating the formation of continuous conductive pathways. 2D carbon materials, like graphene, consist of a single-layer planar lattice of carbon atoms with a smooth and extensive surface area, promoting efficient electron transport and conductivity. These structural features enable electrons to move freely along extended 1D or 2D structures, creating longer electron transport paths and ultimately forming superior conductive networks.424.1. Carbon nanotubes as conductive networks
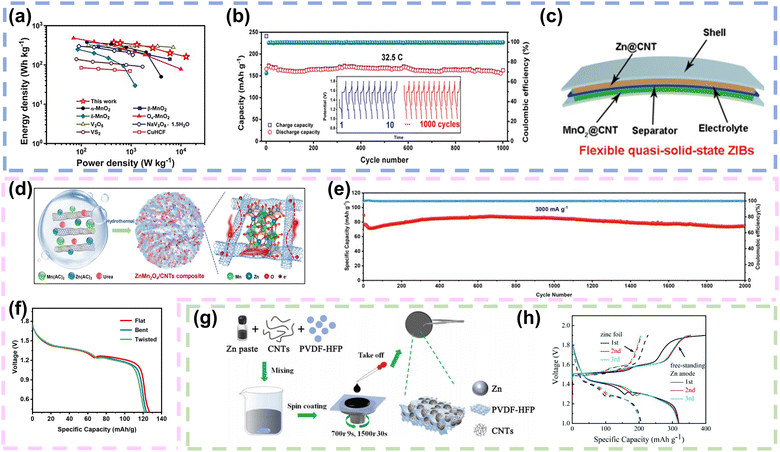 | ||
| Fig. 4 Carbon nanotubes as conductive networks in FZIBs. (a) Ragone plot illustrating the energy and power densities in comparison with that of other previously reported cathode materials in aqueous ZIBs. (b) The cycling performance and galvanostatic charge/discharge curve of α-MnO2@CNTs at 32.5C. (c) The fabricated flexible quasi-solid-state ZIBs. Reproduced with permission from ref. 71. Copyright 2020, Elsevier. (d) Scheme illustrating the fabrication of ZMO/CNTs. (e) Cycling performance of the ZMO/CNT electrode at 3000 mA g−1. (f) Discharging curves at 100 mA g−1 under different conditions of FZIBs. Reproduced with permission from ref. 34. Copyright 2022, Elsevier. (g) The fabrication process of the free-standing Zn anode. (h) Discharge/charge curves of ZIBs with the freestanding Zn anode and zinc foil at 0.3 A g−1. Reproduced with permission from ref. 77. Copyright 2021, the Royal Society of Chemistry. | ||
Furthermore, CNTs provide mechanical strength to the cathode structure. Their high tensile strength and flexibility contribute to maintaining the integrity of the electrode even under mechanical stress, which is particularly important for flexible or wearable applications. As depicted in Fig. 4d, Gao et al. synthesized ZMO/CNT cathodes by anchoring ZnMn2O4 particles onto CNTs through a one-step hydrothermal method. Due to robust interfacial interactions (Mn–O–C bond) enhanced both the electron and ion transport pathways, the ZMO/CNT cathode demonstrated high capacity and remarkable cycling life, with a capacity retention of 97.01% even after 2000 cycles (Fig. 4e). In addition, the FZIB was assembled with the ZMO/CNT as the cathode and zinc foil as the anode, which was enough for it to be bent or twisted and the electrochemical impedance in the bent or twisted states was similar to that in the flat state (Fig. 4f).34 Parallelly, CaVO microflowers were successfully synthesized using a hydrothermal method. Subsequently, CaVO/CNT films were effectively prepared using spray printing technology. The CaVO microflowers were enveloped by a network of CNTs, expediting electron transport and enhancing electrical conductivity. Furthermore, the fabricated CaVO/CNT film demonstrated remarkable flexibility, and its electrochemical performance remained stable under different bending conditions.72
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, the freestanding zinc thin film electrode exhibited a specific capacity of 318.5 mA h g −1 at a current density of 0.3 A g−1 (Fig. 4h).77 In a similar manner, nanocellulose was employed as a film-forming agent, and dispersed Zn microspheres along with CNTs were assembled into free-standing ZCN thin film electrodes through a straightforward vacuum filtration process. In this study, Zn powders structured with microspheres of several μm in diameter provided numerous advantages as active materials. Among these, including plentiful electroactive sites and 3D diffusion pathways contributed to the reversible plating/stripping of Zn2+ ions and ultimately enhanced the cycling life and performance stability of ZIBs.78
8, the freestanding zinc thin film electrode exhibited a specific capacity of 318.5 mA h g −1 at a current density of 0.3 A g−1 (Fig. 4h).77 In a similar manner, nanocellulose was employed as a film-forming agent, and dispersed Zn microspheres along with CNTs were assembled into free-standing ZCN thin film electrodes through a straightforward vacuum filtration process. In this study, Zn powders structured with microspheres of several μm in diameter provided numerous advantages as active materials. Among these, including plentiful electroactive sites and 3D diffusion pathways contributed to the reversible plating/stripping of Zn2+ ions and ultimately enhanced the cycling life and performance stability of ZIBs.78
By introducing CNTs with various nanostructures and excellent properties, the issues associated with cathodes and anodes in FZIBs can be effectively addressed. When conductive CNTs are combined with low conductivity cathode materials, the resulting hybrid electrodes are expected to exhibit excellent zinc storage performance.42 While CNTs offer significant advantages as conductive networks in both cathodes and anodes of FZIBs, their integration into battery systems also presents challenges. (1) Dispersion and homogeneity: achieving a uniform dispersion of CNTs within electrode materials is challenging due to their tendency to agglomerate. Proper dispersion techniques are essential to ensure that CNTs are evenly distributed, maximizing their beneficial effects on conductivity and mechanical properties. (2) Cost and scalability: the production of CNTs can be costly, and scaling these processes while maintaining quality and performance poses significant challenges. Reducing the cost of CNT production is crucial for their widespread adoption in commercial FZIB applications.
CNTs offer several compelling advantages for both cathodes and anodes in FZIBs, enhancing ionic conductivity, structural integrity and mechanical durability. However, addressing challenges related to dispersion, cost, and material compatibility is crucial for realizing the full potential of CNTs in this promising area of energy storage technology. Ongoing research and development are expected to continue improving the integration of CNTs in FZIBs, paving the way for more reliable, safe, and efficient flexible batteries.
4.2. Graphene as a conductive network
Owing to the advantages of high intrinsic strength, great transmittance, superior thermal conductivity and high electron mobility, graphene is considered as an advantageous conductive network for enhancing the electrical conductivity of FZIBs. The exceptional electrical conductivity facilitates rapid and efficient electron movement, which is critical for high-performance batteries. Its superior thermal conductivity helps manage heat dissipation, enhancing the safety and longevity of devices. Additionally, the high intrinsic strength of graphene contributes to the mechanical stability of the conductive network, ensuring durability during repeated charge–discharge cycles. Additionally, graphene nanosheets can be assembled with active materials into conductive and flexible composite films, preventing the self-stacking of graphene nanosheets and the agglomeration of active materials. Most importantly, graphene nanosheets can be easily assembled into 1D fibres and 2D films. What's more, its high electrical conductivity enables rapid and efficient charge transport within the FZIBs, thereby reducing internal resistance and enhancing the power density of FZIBs.105 For example, Huang and colleagues deposited MnO2 nanosheets and reduced graphene oxide (rGO) sheets onto carbon cloth to fabricate a binder-free MnO2/rGO hybrid cathode by vacuum filtration (Fig. 5a). Due to the uniform deposition of MnO2/rGO on carbon cloth without a binder, the electrode's internal resistance was reduced. Consequently, in comparison to the traditional MnO2 cathode, the hybrid cathode exhibited higher capacity (Fig. 5b), excellent rate performance (Fig. 5c), and stable cycling performance. When the Zn//MnO2/rGO battery was assembled, the highly flexible battery maintained a capacity retention rate of 90% after 500 cycles under conditions of bending at an angle close to 180°.79 More importantly, the nanostructures are currently a research hotspot for further realizing high-energy/power density, long life, light weight, and superior mechanical properties under various deformation conditions. In 2018, Wang et al. constructed MnO2/rGO lightweight thin film electrodes, which employed 1D ultralong MnO2 nanowires with a high aspect ratio and 2D rGO nanosheets. The unique 1D/2D structure enabled the thin film electrode to have excellent mechanical deformation performance, and it could be folded into shapes such as paper airplanes (Fig. 5d). Impressively, the free-standing hybrid nanoarchitecture could reduce the transfer path of electrons, which allowed the electrode to exhibit an energy/power density of 436 W h kg−1/9.2 kW kg−1(Fig. 5e).80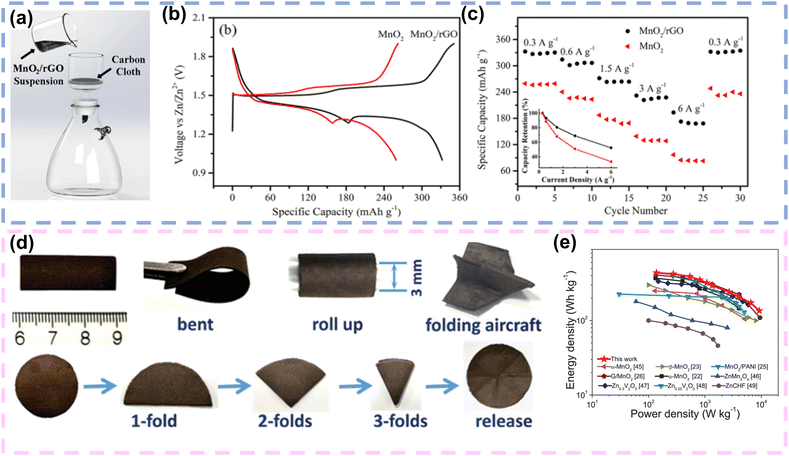 | ||
| Fig. 5 Graphene as a conductive network in FZIBs. (a) Schematic illustration of the vacuum filtration on carbon cloth to prepare MnO2/rGO electrodes. (b) Comparison of the charging and discharging profiles of the samples at a current density of 0.3 A g−1. (c) Specific capacities (normalized over the mass of MnO2) of the samples at different current densities. Reproduced with permission from ref. 79. Copyright 2018, Springer nature. (d) The digital photos of the MnO2/rGO nanocomposite membrane demonstrating flexibility under bent, rolled, and folded conditions. (e) Ragone plot of our cells compared with previously reported ZIBs. Reproduced with permission from ref. 80. Copyright 2020, Wiley. | ||
Similarly, Wan and colleagues fabricated free-standing RGO/NVO films using high aspect ratio NVO nanobelts and RGO through vacuum filtration. Benefiting from the multilayer connection between the RGO sheet and the NVO nanobelt, the RGO/NVO hybrid film not only exhibited excellent mechanical performance, but also much improved electrical conductivity. The manufactured flexible soft-packaged ZIBs were able to operate stably under different conditions and showed no significant capacity decay after 100 cycles.81 Significantly, Dong's group generated transparent graphene nanosheets with a thickness of 14 μm, which were prepared by simple electrochemical exfoliation. The flexible hybrid films were prepared by vacuum filtration of electrochemically exfoliated graphene (EG) nanosheets and ultrathin MnO2 nanosheets. The availability of EG provided free-standing MnO2/EG films with superior flexibility. In addition, the highly flexible EG nanosheets could also serve as a conductive substrate for electrodepositing Zn, which was obtained as a Zn/EG film electrode instead of rigid Zn foil. Owing to the significant effect of EG on slowing down the dissolution of Mn and regulating the volume change during charge/discharge cycles, the MnO2/EG//Zn/EG full battery assembled with MnO2/EG as the cathode and Zn/EG as the anode exhibited a high specific capacity of 300 mA h g−1 at 0.2C. A critical feature of the soft-packaged MnO2/EG//Zn/EG battery was its outstanding flexibility, allowing the battery to maintain the brightness of a small bulb even when bent and deformed. This further validated the importance of EG nanosheets in flexible and high-performance ZIBs.82
In particular, mass loading refers to the amount of active material per unit area in the cathode, which directly influences the energy density and overall performance of batteries. According to recent studies, He's team successfully synthesized a sodium and copper co-intercalated boron titanate manganese oxide (NCMO) cathode. When using TIMCAL graphite & carbon as a conductive material, it exhibited highly reversible Mn deposition and dissolution processes under high-quality loading, attributed not only to the dual-ion co-intercalation increasing the manganese conversion on the cathode surface but also to the stability of the host material during charge/discharge cycles.106 Moreover, in high-loading cathode ZIBs, the use of 1D/2D carbon materials can create a conductive network that improves electron transport pathways and mechanical stability. This network allows for a higher amount of active material to be effectively utilized, thereby increasing the overall capacity and energy density of FZIBs. The high specific surface area and excellent electrical conductivity of these 1D/2D carbon materials facilitate the uniform distribution and robust connection of active materials, thus supporting higher mass loadings without compromising the battery's performance. This network allows for a higher amount of active material to be effectively utilized, thereby increasing the overall capacity and energy density of FZIBs. Furthermore, the structural integrity provided by 1D/2D carbon materials can prevent the detachment of active materials during cycling, enhancing the longevity and cycling stability of FZIBs.
Although graphene offers numerous benefits, its large-scale production remains cost-intensive and technically challenging. Reducing the cost of graphene production through improved synthesis methods is crucial for its broader adoption in commercial applications. Moreover, achieving effective integration of graphene with existing battery materials requires advanced material engineering techniques. What's more, while graphene's general properties are well understood, optimizing these properties for specific battery configurations and applications remains an area of active research. In conclusion, graphene as a conductive network in FZIBs represents a significant advancement in battery technology, particularly for applications requiring high flexibility, durability, and performance. Its ability to enhance both the cathode and anode properties addresses several limitations of traditional battery materials, positioning graphene as a key material in the future advancement of energy storage devices. Continuing advancements in graphene processing and integration will likely unlock further potential of this versatile material in flexible battery applications.
4.3. MXenes as conductive networks
Benefiting from their abundant functional groups, high conductivity, superior electrochemically active surface area, hydrophilicity and excellent mechanical properties, MXenes are considered as an appropriate 2D template or conductive network to accommodate for carrying high capacity active materials.107,108 First of all, MXenes typically demonstrate high electrical conductivity, often ranging from 10^4 to 10^5 S m−1. This high conductivity facilitates efficient electron transport, reducing internal resistance in batteries and improving their power density. Secondly, MXenes possess a large SSA, and this high SSA enhances electrode–electrolyte contact, promoting ion transport and electrochemical reactions, thereby enhancing battery performance. What's more, MXenes demonstrate good chemical stability, maintaining robust performance in various electrochemical environments. For instance, Shi et al. synthesized 3D Ti3C2Tx@MnO2 microflower electrodes with a gas-phase spray drying approach. The etching Ti3C2Tx nanosheets were uniformly dispersed in Mn (NO3)2 salt solvent to form a mixed solution, which was introduced into a spray atomizer to generate aerosol droplets. These droplets were subsequently transferred into a high-temperature reactor under the action of a carrier gas. Benefiting from the solvent evaporation and capillary forces generated by the Ti3C2Tx nanosheets in the high temperature reactor, 3D Ti3C2Tx@MnO2 microflower structures were successfully constructed (Fig. 6a). The Ti3C2Tx nanosheets were encapsulated with MnO2 nanoparticles. Notably, the (002) characteristic peak of Ti3C2Tx-Mxene and the typical characteristic peak of γ-MnO2 were clearly shown in the XRD pattern of 3D Ti3C2Tx@MnO2 microflowers, consisting of 1 × 1 (≈2.3 × 2.3 Å, pyrolusite) and 1 × 2 (≈2.3 × 4.6 Å, ramsdellite) randomly oriented tunnels composed of γ-MnO2, which facilitated Zn2+ storage. When employed as a cathode, the 3D Ti3C2Tx@MnO2 microflower structure demonstrated commendable reversible capacity and rate performance. Impressively, it showcased a remarkable capacity retention of 90.6% even after undergoing an extensive 2000 cycles. Pairing the 3D Ti3C2Tx@MnO2 microflower cathode with a Zn nanosheet anode electrodeposited on carbon cloth, the FZIB was assembled with gel electrolyte (Fig. 6b). Fig. 6c illustrated the impressive power delivery of the FZIB after full charging. A single device was capable of powering both an electric clock and a light-emitting diode (LED). Even during dynamic bending, the FZIB maintained its performance, with the LED indicator continuing to emit a bright red light. Impressively, the FZIB exhibited similar specific capacity and cycling performance under different bending conditions (Fig. 6d).83 Similarly, Qi et al. utilized carbon cloth with remarkable flexibility and conductivity as the substrate. They employed an electrodeposition process to grow MnO2 nanosheets directly onto its surface. To further improve the conductivity, MXene coating was added on the surface of the MnO2 nanosheets to synthesize a binder-free CC@MnO2@MXene electrode. The electrode exhibited excellent electrochemical performance owing to the presence of MXenes, which improved the conductivity of the electrode and facilitated the co-intercalation mechanism of Zn2+ and H+ (Fig. 6e). Fig. 6f displayed the CV curves of the quasi-solid CC@MnO2@MXene//Zn flexible battery in both normal and bent states. The curves exhibited two reduction peaks at approximately 1.19 and 1.3 V, along with a broad oxidation peak at around 1.7 V, demonstrating that bending did not affect the electrochemical performance of CC@MnO2@MXene.84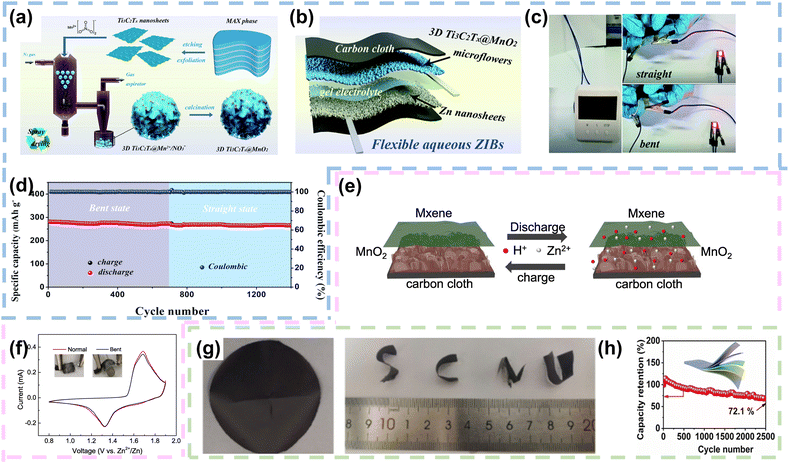 | ||
| Fig. 6 MXenes as conductive networks in FZIBs. (a) Schematic depiction of the synthesis process of 3D Ti3C2Tx@MnO2 microflowers. (b) Fabrication procedure for the FZIB. (c) Real-time image demonstrating the FZIB supplying power under both straight and bent conditions. (d) Cycling performance in the straight and bent states at a current density of 100 mA g−1. Reproduced with permission from ref. 83. Copyright 2020, the Royal Society of Chemistry. (e) Schematic representation of intercalation and deintercalation mechanisms of the CC@MnO2@MXene electrode. (f) CV curves under normal and bending conditions. Reproduced with permission from ref. 84 Copyright 2022, Elsevier. (g) Optical images of the VO2/MXene hybrid films. (h) Cycling performance of the VO2/MXene hybrid films at a current density of 5 A g−1. Reproduced with permission from ref. 85. Copyright 2021, Wiley. | ||
Furthermore, considering the inherent excellent conductivity and mechanical properties of 2D MXenes, Shi et al. fabricated VO2/MXene hybrid films through a simple vacuum filtration process. Within the hybrid film, VO2 nanoparticles were embedded within MXene sheets, establishing a 3D layered conductive network. Optical images revealed that the VO2/MXene hybrid film exhibited high flexibility and remained free of cracks under various bending and folding states (Fig. 6g). The specific capacity of the VO2/MXene hybrid film reached up to 228.5 mA h g−1, with an energy density of up to 197.2 W h kg−1. To demonstrate the application of VO2/MXene hybrid films in flexible energy storage devices, the researchers designed a quasi-solid-state FZIB with the hybrid film as the cathode material. Moreover, the flexible battery exhibited remarkable mechanical flexibility and exceptional Zn2+ storage capacity, maintaining a capacity retention of 72.1% after 2500 cycles at 5 A g−1(Fig. 6h).85 In conclusion, the ability of MXenes to form continuous conductive pathways enhances electron transport efficiency in batteries, leading to improved electrochemical performance. Their high surface area provides ample active sites for electrochemical reactions, increasing the capacity and rate performance of energy storage devices. Additionally, MXenes possess good mechanical flexibility and chemical stability, which are crucial for maintaining the structural integrity and longevity of electrodes during cycling. Furthermore, MXenes can be functionalized to tailor their surface chemistry, allowing for the optimization of their electrochemical properties to meet specific application needs. The combination of these properties positions MXenes as a promising material for advanced conductive networks, contributing to the development of high-performance and durable energy storage systems.
5. 1D/2D carbon materials for current collectors or Zn hosts
Traditional metal current collectors, owing to their high mass, are unsuitable for achieving light weight and flexibility. To address this issue, researchers focused on 1D/2D materials, leveraging their inherent structural advantages to develop lightweight and flexible current collectors. Beyond their role as current collectors, 1D/2D carbon materials also function effectively as Zn hosts for electrochemical deposition of zinc; this approach replaces the use of heavy and rigid zinc foil, which improves energy density and enables high mechanical flexibility of FZIBs.109,1105.1. CNTs as current collectors or Zn hosts
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles through the implementation of an electrolyte dynamics engineering strategy, which involved the addition of trace amounts of Co2+ cations to a 2 M ZnSO4 aqueous electrolyte to prevent the dissolution of the Co3O4 NSs@CNTF cathode (Fig. 7b and c). Moreover, the electrochemical performance of the assembled fiber shaped ZIB remained stable even after 2000 bending cycles at 120 angle, demonstrating remarkable flexibility and durability. Finally, the fiber shaped ZIBs were integrated into a sweater and connected in series to power a smart watch with high safety, indicating significant potential for applications in flexible and wearable electronics.87 In addition, to accommodate the volume change during the additional MnO2 redox deposition and dissolution, Li and co-workers electrodeposited MnO2 nanoflakes on CNT films to obtain a binder-free CNT@MnO2 film, where MnO2 nanoflakes were uniformly dispersed on the CNT surface (Fig. 7d), since the 3D porous structure of the CNT could provide a robust scaffold for MnO2 deposition and dissolution and electrolyte reservation. As shown in Fig. 7e, the pouch-type Zn–MnO2 battery exhibited good cycling durability with about 83.5% capacity retention after 2500 cycles.88 Similarly, Ma and colleagues firstly fabricated CNT fibers with diameters of approximately 80–100 μm by using Fe films as catalysts in a chemical vapor deposition (CVD) process, where arrays of CNTs were grown directly on silicon wafers. Subsequently, MnO2@CNT fiber electrodes were achieved in an aqueous Mn (NO3)2 solution through a chronoamperometric electrochemical deposition process, resulting in MnO2 nanosheets densely wound and grew vertically on the surface of the CNT fibers. The Zn//MnO2 cable-like micro battery assembled with MnO2@CNT fibers as the cathode, zinc wire as the anode and ZnCl2 gel polymer as the electrolyte achieved a specific capacity of 290 mA h g−1 at 0.1 A g−1. In addition, the Zn//MnO2 cable-like microcell exhibited superior flexibility, which was almost identical to the flat galvanostatic charge/discharge curve after 100 bending cycles.89
000 cycles through the implementation of an electrolyte dynamics engineering strategy, which involved the addition of trace amounts of Co2+ cations to a 2 M ZnSO4 aqueous electrolyte to prevent the dissolution of the Co3O4 NSs@CNTF cathode (Fig. 7b and c). Moreover, the electrochemical performance of the assembled fiber shaped ZIB remained stable even after 2000 bending cycles at 120 angle, demonstrating remarkable flexibility and durability. Finally, the fiber shaped ZIBs were integrated into a sweater and connected in series to power a smart watch with high safety, indicating significant potential for applications in flexible and wearable electronics.87 In addition, to accommodate the volume change during the additional MnO2 redox deposition and dissolution, Li and co-workers electrodeposited MnO2 nanoflakes on CNT films to obtain a binder-free CNT@MnO2 film, where MnO2 nanoflakes were uniformly dispersed on the CNT surface (Fig. 7d), since the 3D porous structure of the CNT could provide a robust scaffold for MnO2 deposition and dissolution and electrolyte reservation. As shown in Fig. 7e, the pouch-type Zn–MnO2 battery exhibited good cycling durability with about 83.5% capacity retention after 2500 cycles.88 Similarly, Ma and colleagues firstly fabricated CNT fibers with diameters of approximately 80–100 μm by using Fe films as catalysts in a chemical vapor deposition (CVD) process, where arrays of CNTs were grown directly on silicon wafers. Subsequently, MnO2@CNT fiber electrodes were achieved in an aqueous Mn (NO3)2 solution through a chronoamperometric electrochemical deposition process, resulting in MnO2 nanosheets densely wound and grew vertically on the surface of the CNT fibers. The Zn//MnO2 cable-like micro battery assembled with MnO2@CNT fibers as the cathode, zinc wire as the anode and ZnCl2 gel polymer as the electrolyte achieved a specific capacity of 290 mA h g−1 at 0.1 A g−1. In addition, the Zn//MnO2 cable-like microcell exhibited superior flexibility, which was almost identical to the flat galvanostatic charge/discharge curve after 100 bending cycles.89
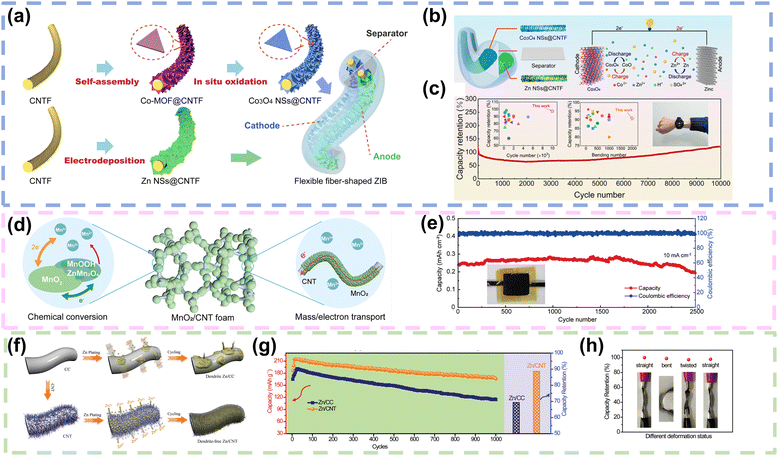 | ||
| Fig. 7 CNTs as current collectors or Zn hosts in FZIBs. (a) Schematic depiction of the preparation process of fiber shaped Co3O4 NSs@CNTF and Zn NSs@CNTF electrodes. (b) Schematic diagram of the mechanism of the aqueous ZIB employing Co3O4 NSs@CNTF as the cathode and zinc as the anode. (c) Long-term cycling performance of a fiber-shaped ZIB at a current density of 5 A g−1; comparison of long-term cycling performances for the fiber-shaped ZIB in this work and a previous FZIB and an image of flexible fiber shaped ZIBs integrated into a sweater to charge a smart watch. Reproduced with permission from ref. 87. Copyright 2021, American Chemical Society. (d) Diagram illustrating the MnO2/CNT foam cathode with a reversible chemical conversion and a 3D hierarchical structure for mass and charge transport. (e) Cycling performance of the assembled Zn–MnO2 battery that was tested at 10 mA cm−2. Reproduced with permission from ref. 88. Copyright 2021, Wiley. (f) The schematic illustrations of Zn deposition on CC and CNT electrodes. (g) Cycling performance measured at 20 mA cm−2 of the Zn//MnO2 batteries with Zn/CC and Zn/CNT anodes tested in aqueous electrolyte with a coin cell. (h) Capacity retention of the quasi-solid-state Zn//MnO2 battery with a Zn/CNT anode under different deformation states. Reproduced with permission from ref. 33. Copyright 2019, Wiley. | ||
In a word, while CNTs have shown great potential in the realm of flexible energy storage devices, the development of CNT-based current collectors is still an evolving field with many challenges to overcome. One major obstacle is the inherently low electrical conductivity of individual CNTs when assembled into macroscopic structures, which limits their performance as current collectors. Furthermore, the mechanical properties of CNTs can degrade over long-term cycling or under repeated mechanical deformation, affecting the durability and reliability of the devices. Advancements in material engineering, surface chemistry, and fabrication techniques are essential to fully realize the potential of CNTs in creating high-performance, durable, and flexible energy storage devices. Future research should focus on addressing these challenges to enable the widespread adoption of CNT-based current collectors in commercial applications.
The application of CNTs as host materials for zinc anodes in FZIBs not only enhances the electrochemical performance and mechanical flexibility of the batteries but also significantly extends their lifespan, indicating broad potential for future applications. Future research should concentrate on optimizing the structure and fabrication processes of carbon nanotubes to further improve the performance and commercial viability of FZIBs.
5.2. Graphene as a current collector or Zn host
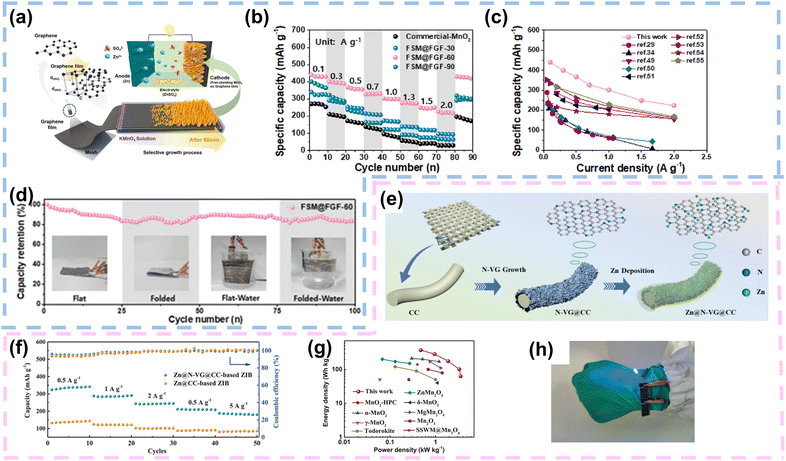 | ||
| Fig. 8 Graphene as a current collector or Zn host in FZIBs. (a) Schematic illustration depicting the selective growth process of free-standing MnO2 on a flexible graphene film for use as a binder-free cathode for FZIBs. (b) Comparative rate performances of commercial MnO2, FSM@FGF-30, FSM@FGF-60, and FSM@FGF-90 at potential and current densities of 1.0–1.9 V and 0.1–2.0 A g−1, respectively. (c) Comparative specific capacity, including those of previously reported ZIB materials. (d) Cycling performance of FSM@FGF for up to 100 cycles at a current density of 0.5 A g−1. Reproduced with permission from ref. 90 Copyright 2022, Elsevier. (e) Schematic illustrations of preparing N-VG@CC and the Zn@N-VG@CC electrode. (f) Rate performance of the coin ZIBs based on Zn@CC and Zn@N-VG@CC anodes. (g) Ragone plot of the quasi-solid-state ZIB compared with other reported energy storage devices. (h) Flexible demonstration of two quasi-solid-state ZIBs connected in series. Reproduced with permission from ref. 92 Copyright 2021, Wiley. | ||
Graphene-based current collectors have been successfully employed in various cathode materials, including transition metal oxides, vanadium-based materials, and others. The use of graphene has resulted in batteries with higher energy densities, improved cycling stability, and enhanced rate performance compared to conventional current collectors. Future research in this area should focus on optimizing the synthesis and processing methods of graphene to further improve its electrical conductivity, mechanical properties, and compatibility with different cathode materials. Additionally, exploring new graphene-based composite materials and hybrid structures could lead to further advancements in battery performance and applications. In conclusion, graphene-based current collectors hold great promise for improving the electrochemical performance and mechanical flexibility of cathodes in various battery applications, including portable electronics, electric vehicles, and energy storage systems. Continued research and development are expected to accelerate the commercialization of graphene-based battery technologies in the near future.
In conclusion, graphene as a host material for zinc in ZIBs holds great promise for advancing the development of high-performance batteries with enhanced energy density, cycling stability, and safety. Continued research and innovation in this area is anticipated to hasten the adoption of graphene-based materials in next-generation energy storage technologies.
5.3. Carbon fibers as current collectors or Zn hosts
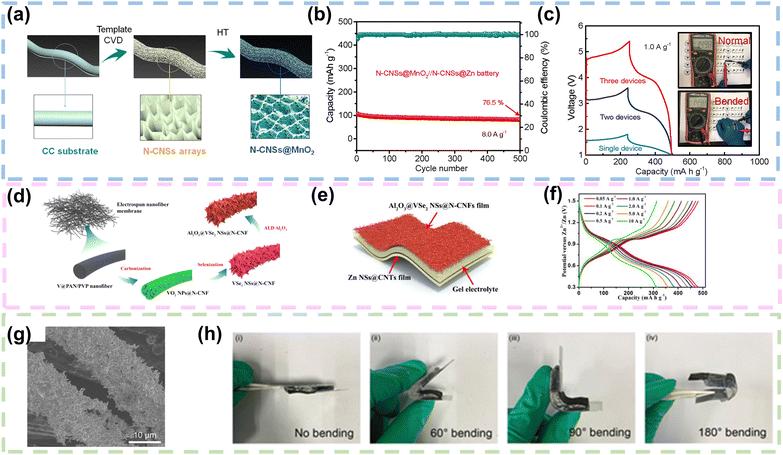 | ||
| Fig. 9 CFs as current collectors or Zn hosts in FZIBs. (a) Schematic illustration preparation process of a flexible N-CNSs@MnO2 cathode. (b) Cycling performance of the quasi-solid-state N-CNSs@MnO2//N-CNSs@Zn battery at 8.0 A g−1. (c) Galvanostatic charge/discharge curves obtained at a current density of 1.0 A g−1 for a single quasi-solid-state device and tandem devices consisting of two and three batteries units connected in series. Reproduced with permission from ref. 94 Copyright 2020, Elsevier. (d) Schematic illustration of the preparation process for the Al2O3@VSe2 NSs@N-CNF film. (e) Schematic diagram illustration of the flexible QAZIB. (f) Galvanostatic charge/discharge curves of the flexible QAZIB at different current densities. Reproduced with permission from ref. 95 Copyright 2021, Elsevier. (g) SEM images of the Zn nanowires/CC anode. (h) Optical images of the self-healing flexible Zn-ion battery after bending to different angles. Reproduced with permission from ref. 100 Copyright 2021, Elsevier. | ||
Moreover, V2O3 nanoparticles (NPs) were embedded into carbon nanofibers by electrospinning to achieve V2O3@Carbon Nanofiber (V2O3@CNF) flexible binder-free ZIB cathode materials. The V2O3@CNF electrodes could withstand bending or twisting and exhibit excellent mechanical performance. After carbonization of the prepared V2O3@CNF cathode material, the pyridine and pyrrole N not only provided electrons to the π bond in the CNFs but also led to a number of structural defects, thus providing a large quantity of active sites. The V2O3@CNFs provided a superior specific capacity of 220 mA h g−1, with a current density of 50 mA g−1.97 Moreover, Cao's group developed a MnO2/CC hybrid electrode by modifying carbon cloth with zeolitic imidazolate frame-work-67 (ZIF-67). The carbon cloth substrate was enveloped by intricately arranged ultra-thin MnO2 nanosheets, forming a distinctive hollow polyhedral structure. This MnO2/CC electrode demonstrated a synergistic Zn2+ and H+ co-intercalation mechanism, attributed to its exceptional reversibility. The substantial surface area of the MnO2-loaded hollow polyhedron significantly enhanced electrolyte infiltration, mitigated volume expansion challenges, and concurrently promoted the conductivity of the underlying CC.98
The use of CFs as cathode current collectors in FZIBs presents a promising approach for improving the performance and durability of these energy storage devices. The integration of advanced materials and innovative fabrication techniques can lead to significant enhancements in electrochemical properties, mechanical flexibility, and overall battery efficiency, making CFs a valuable component in the development of next-generation flexible energy storage solutions.
The scalability and versatility of CF-based zinc anode current collectors open new avenues for the advancement of next-generation flexible, stretchable, and customizable energy storage solutions. This approach not only enhances the performance and durability of ZIBs but also broadens their application potential in wearable electronics and other flexible devices.
6. Application of 1D/2D carbon materials in gel electrolytes
For the gel electrolyte of FZIBs, 1D/2D carbon materials can also be added as fillers into the gel electrolyte. Firstly, their large SSA and porous structure facilitate improved electrolyte–electrode interactions, enhancing ion transport kinetics and overall battery performance.114–116 Secondly, the presence of functional groups on the surface of these carbon fillers can promote better adhesion between the electrolyte and the electrode interfaces, thereby enhancing the mechanical stability of the battery. Moreover, the addition of 1D/2D carbon materials can effectively inhibit the growth of dendrites during cycling, thus improving the long-term cycling stability and safety of FZIBs.28 Overall, the utilization of these carbon fillers holds great promise for optimizing the gel electrolyte formulation and advancing the practical applications of FZIBs.117Typically, 2D functional fillers provide great SSA, enabling increased number of active interfaces with polymers in composite polymer electrolytes (CPEs). GO, the most typical 2D filler, has been widely adopted in CPEs due to its numerous oxygen-containing functional groups. Specifically, GO possesses a long-range ordered monoatomic layer structure that is expected to create fast ion transport channels at the polymer–GO interface. Xiao et al. introduced GO as a filler into the ZnSO4/MnSO4-PVA-based gel electrolyte. As depicted in Fig. 10a, there were three main steps in the preparation of the GO-containing polymer hydrogel electrolyte (GPHE): (i) mixing PVA and GO, (ii) freeze/thaw processing, and (iii) ZnSO4/MnSO4 solution soaking. The molecular chain of PVA provided the framework for stiffness maintenance, while ions were transported within the ZnSO4/MnSO4 solution. In addition, a detailed finite element analysis (FEA) of the random distribution of GO in the GPHE was conducted using COMSOL Multiphysics, based on Monte Carlo simulations. At high molecular weights, ion transport was primarily achieved through microscopic processes that were independent of the polymer length and viscosity (Fig. 10b). Moreover, the hydrogen bond formed between GO and the PVA polymer affected GO dispersion, enhancing ion mobility and forming a layered structure that bound molecularly to the PVA polymer. The resulting composite polymer electrolyte containing 0.32 wt% GO demonstrated favorable performance with an ionic conductivity of 21 mS cm−1 (Fig. 10c). A rechargeable solid-state zinc-ion fiber battery was developed, maintaining 98.0% capacity after over 1000 charging and discharging cycles (Fig. 10d). To demonstrate practical applications, the solid-state Zn/MnO2 fiber battery successfully powered a textile-based active node (TBAN) for continuous monitoring of various physiological signals and wireless charging (Fig. 10e and f). The lightweight, low-cost, and high-performance Zn/MnO2 fiber battery showed potential for mass production, highlighting its capability to power e-textiles for personalized healthcare.31 Chen and co-workers fabricated V2O5/GO/PVA gel electrolytes with 5 wt% GO, which exhibited about a two orders of magnitude enhancement in mechanical performance compared with pure PVA gel electrolytes.101 As mentioned above, 1D/2D carbon materials as fillers typically possess abundant functional groups. These groups can significantly enhance interactions with polymers and zinc salts, maximizing the anchoring of anions and reducing the affinity between zinc ions and polymers. This accelerates zinc ion transport and improves the compatibility at the filler–polymer interface.
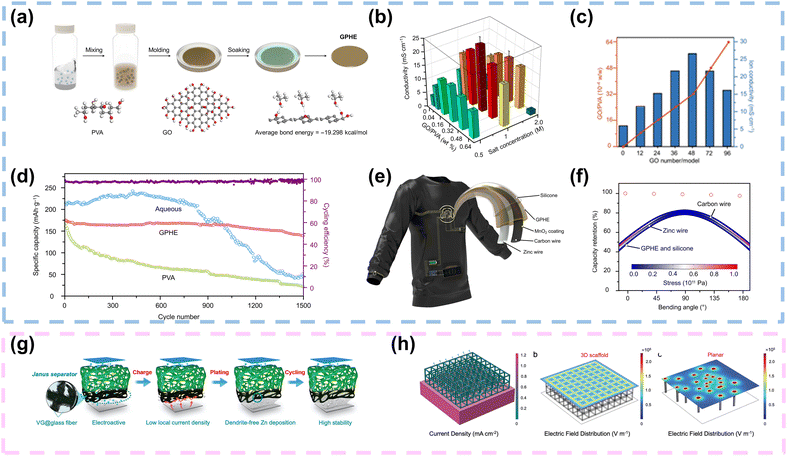 | ||
| Fig. 10 Application of 1D/2D carbon materials in gel electrolytes and separators. (a) Schematic illustration of the preparation process of GPHE. (b) Measurement of ion conductivity of GPHEs with different PVA–GO mass ratios and soaking concentrations. (c) Calculation results of ion conductivity of GPHEs with different GO masses. (d) Cycling performance of coin cells with aqueous electrolyte, PVA electrolyte, and GPHE at a current density of 0.3 A g−1. (e) Illustration and design of a flexible ZIB TBAN. (f) PHE-based ZIB fiber maintaining stable capacity output under different bending angles. The inset image shows bending stress and deformation of ZIB fibers. Reproduced with permission from ref. 31 Copyright 2021, AAAS. (g) A Janus separator employing a VG carpet directly grown on one side to reduce local current density and homogenize ion distribution. (h) Current distribution in the 3D scaffold. Electric field distribution of the Janus separator structure and pristine separator structure. Reproduced with permission from ref. 30 Copyright 2020, Wiley. | ||
7. Application of 1D/2D carbon materials in separators
Separators play a crucial role in battery systems by preventing direct contact between cathodes and anodes, thereby mitigating the risk of short circuits. Separator engineering, which includes modification and functionalization, has emerged as an innovative and effective strategy for developing high-performance energy storage devices.118,119Typically, GF separators dominate in aqueous ZIBs due to their hydrophilic nature, high ionic conductivity, and porous structure. In situ modification of GF separators with conductive carbon materials, particularly graphene, presents a promising strategy to enhance electrochemical performance of ZIBs. Li et al. employed plasma-enhanced chemical vapor deposition (PECVD) to grow 3D VG carpets on one side of GF separators, creating Janus separators with an ample porous structure and large SSA to ensure uniform electric field distribution and reduced local current density. Additionally, the introduction of oxygen and nitrogen functional groups enhanced zincophilicity, regulating homogeneous Zn2+ fluxes and stabilizing Zn anodes (Fig. 10g). A simplified 3D model was constructed using the Janus separator structure, and as shown in Fig. 10h, the introduction of a 3D conductive VG scaffold significantly increased the surface area and effectively reduced the current density, outperforming 2D planar graphene. According to the 3D model, reducing the current density delayed the initiation of dendritic growth. The 3D VG scaffold provided a uniform electric field, facilitating the uniform deposition of Zn onto the VG framework. In contrast, the absence of a 3D scaffold in planar configurations easily triggered single nucleation points, leading to an uneven electric field which promoted the formation of Zn dendrites. Consequently, these robust Janus separators facilitated stable cycling of Zn//Zn symmetrical batteries for 300 hours at 0.5 mA cm−2 for 0.5 mA h cm−2. Furthermore, Zn//V2O5 batteries achieved a high energy density of 182 W h kg−1 and withstood deformation without significant electrochemical decay.30 Additionally, to achieve low-cost separators, Wu and colleagues proposed a functional cellulose nanofiber/graphene oxide (CG) separator. This separator featured abundant pores ranging from 10 to 50 nm, a smooth surface, and ample zincophilic oxygen-containing functional groups. These properties facilitated strong interactions with zinc, enabling uniform distribution of Zn2+ ions and promoting dendrite-free Zn anodes.103
While advancements have been notable, carbon separator research remains at an early stage. Presently, the prevalent GF separators suffer from fragility and large pores, prone to issues like zinc dendrite growth and short circuits.120 Additionally, the separator/electrolyte and separator/electrode interface design has been overlooked, crucial for constructing FZIBs. To address this, an integrated system amalgamating all components into a single monolith could effectively stabilize interfaces and structures, ensuring resilience against deformations.
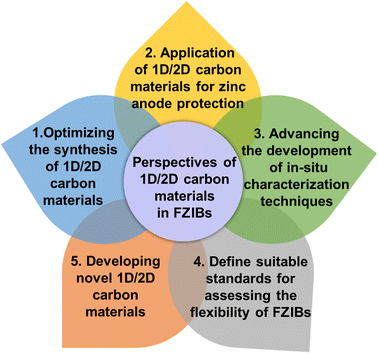
8. Conclusions and perspectives
This review provides a comprehensive summary of recent research advancements in 1D/2D carbon materials (carbon nanotubes, graphene, MXenes, and carbon fibers) for FZIBs. It mainly introduces the significant functions of these 1D/2D materials as active materials, conductive networks, current collectors or Zn hosts in enhancing the performance of FZIBs. In the meantime, 1D/2D carbon materials are also widely applied in modified separators and gel electrolytes in FZIBs. In future commercial developments, 1D/2D carbon materials also demonstrate immense potential. Firstly, they can significantly enhance the energy density and cycling life of FZIBs, thereby meeting the market demand for high-performance batteries. Secondly, due to their superior mechanical flexibility, these materials can be used to develop various wearable devices and flexible electronic products, presenting new opportunities for the consumer electronics market. Despite the extensive progress in the research of 1D/2D carbon materials in FZIBs, there are still many unresolved issues which are discussed as follows (Fig. 11):(1) Optimizing the synthesis of 1D/2D carbon materials. Precise synthesis methods allow for the creation of 1D/2D carbon materials with tailored structures and surface chemical properties to fulfill specific functional or component requirements. Although existing 1D/2D carbon materials generally meet the demands of high-performance energy storage devices, the unique structural and surface characteristics of 1D/2D carbon materials, such as high conductivity and hydrophilicity, assume crucial significance when considering the distinctions between FZIBs and other energy storage systems. In this context, precise modulation of the hydrophilic and hydrophobic nature of 1D/2D carbon materials becomes particularly pertinent.
(2) Application of 1D/2D carbon materials for zinc anode protection. Addressing the challenges posed by poor electrochemical and cycling stability of zinc metal anodes has driven researchers to predominantly focus on structural design of the zinc material. This involves modifying the zinc surface through the incorporation of supplementary protective layers, alongside optimizing the electrolyte composition. Within the context of 1D/2D carbon materials, which serve as both the core material and protective layer, the compatibility and intimate contact at the interfaces, namely the 1D/2D carbon material/zinc and/or 1D/2D carbon material/separators, emerge as critical factors for sustaining a stable microstructure and uniform zinc deposition. In essence, ensuring compatibility and close interaction at these interfaces is pivotal for stabilizing the microstructure and promoting homogeneous zinc deposition.
(3) Advancing the development of in situ characterization techniques. Currently, research on the energy storage mechanisms of zinc heavily relies on non-in situ characterization methods, which fall short in accurately revealing the dynamic changes in chemical composition, morphological evolution, oxidation states, and other factors of active materials under real experimental conditions. As a consequence, our understanding of the energy storage mechanisms of active materials and the continuous structural evolution of metal oxides during charge and discharge processes remains incomplete. Furthermore, a more profound investigation is required to discern the impact of introducing 1D/2D carbon materials on energy storage mechanisms. Moreover, a more detailed exploration of the interfacial interactions between 1D/2D carbon materials and metal oxides, as well as their effects on electrochemical performance, is necessary. To tackle these challenges, employing advanced in situ visualization and spectroscopic characterization techniques such as synchrotron X-ray absorption spectroscopy (XAS), transmission X-ray microscopy (TXM), X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), and atomic force microscopy (AFM), plays an indispensable role in resolving these issues and gaining a deeper understanding of the role of 1D/2D carbon materials. Additionally, to achieve mass production of 1D/2D carbon materials, it is crucial to specifically consider costs. Some studies may opt for expensive raw materials, and complex testing techniques also constitute a significant portion of research expenses. Therefore, finding a relative balance between cost and outcomes could accelerate the process of commercial application.
(4) Define suitable standards for assessing the flexibility of FZIBs. Presently, the evaluation of conventional ZIBs typically encompasses parameters such as specific capacity, rate performance, cycling stability, energy density, and power density. Within this context, the discharge depth and current density significantly influence the cycling life of ZIBs. Furthermore, the validation of their exceptional flexibility often involves tests such as bending, twisting, and stretching in FZIBs. However, there currently exists a divergence in experimental methodologies and geometric parameters. This disparity hampers the progression of FZIBs. Consequently, it becomes essential to formulate a set of suitable standards to facilitate the smooth advancement and interdisciplinary application of FZIBs.
(5) Developing novel 1D/2D carbon materials. Although existing 1D/2D carbon materials have been widely used in many fields and have demonstrated great performance, the exploration of novel 1D/2D carbon materials is still an ongoing and evolving process. In addition, by focusing on green synthesis methods and utilizing renewable resources, researchers can minimize the environmental impact of producing 1D/2D carbon materials. Furthermore, it is essential to consider the end-of-life disposal and recyclability of 1D/2D carbon materials. Designing materials that are easily recyclable or biodegradable will ensure that their use does not lead to long-term environmental pollution. This approach aligns with the principles of a circular economy, promoting the reuse and recycling of materials to reduce waste and resource consumption. Integrating these considerations into the development of novel 1D/2D carbon materials will not only enhance their commercial viability but also support broader environmental and societal goals. By prioritizing safety and environmental impact alongside performance and cost, the next generation of 1D/2D carbon materials can meet the demands of both industry and the planet, paving the way for a sustainable energy future.
In conclusion, advanced 1D/2D carbon materials have made significant progress in enhancing the electrochemical performance of FZIBs. However, there are still challenges and limitations that require further research and resolution. The path ahead remains filled with challenges, necessitating ongoing efforts and exploration.
Data availability
This is a review paper. Data availability is not applicable to this article as no new data were created or analyzed in this study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for financial support from the National Natural Science Foundation of China (Youth Program, No. 52204378 and No.22309209) and the Natural Science Foundation of Hunan province in China (Grant No. 2023JJ40709).References
- H. Huang, L. Han, J. Li, X. Fu, Y. Wang, Z. Yang, X. Xu, L. Pan and M. Xu, J. Mater. Chem. A, 2020, 8, 10291–10300 RSC.
- A. Sumboja, J. Liu, W. G. Zheng, Y. Zong, H. Zhang and Z. Liu, Chem. Soc. Rev., 2018, 47, 5919–5945 RSC.
- C. Yoo, S. Lee, Y. Song, W. Chang, M. K. Park, Y. Ko and J. Cho, Carbon Energy, 2023, 5, e335 CrossRef CAS.
- Y. Gao, L. Yu, J. C. Yeo and C. T. Lim, Adv. Mater., 2020, 32, e1902133 CrossRef PubMed.
- H. R. Lim, H. S. Kim, R. Qazi, Y. T. Kwon, J. W. Jeong and W. H. Yeo, Adv. Mater., 2020, 32, e1901924 CrossRef PubMed.
- W. Heng, S. Solomon and W. Gao, Adv. Mater., 2022, 34, e2107902 CrossRef PubMed.
- J. He, N. Wang, Z. Cui, H. Du, L. Fu, C. Huang, Z. Yang, X. Shen, Y. Yi, Z. Tu and Y. Li, Nat. Commun., 2017, 8, 1172 CrossRef PubMed.
- Y. M. J. H. Zhou, F. Wu, Y. T Hao, W. W. Ma, L. Li, M. Xie and R. J. Chen, Angew. Chem., Int. Ed., 2023, 62, e202304454 CrossRef PubMed.
- Y. Du, Y. Li, B. B. Xu, T. X. Liu, X. Liu, F. Ma, X. Gu and C. Lai, Small, 2022, 18, 2104640 CrossRef CAS PubMed.
- H. Li, L. Ma, C. Han, Z. Wang, Z. Liu, Z. Tang and C. Zhi, Nano Energy, 2019, 62, 550–587 CrossRef CAS.
- X. Wang, L. Liu, Z. Hu, C. Peng, C. Han and W. Li, Adv. Energy Mater., 2023, 13, 2302927 CrossRef CAS.
- H. Lu, L. Liu, J. Zhang and J. Xu, J. Colloid Interface Sci., 2022, 617, 422–429 CrossRef CAS PubMed.
- Z. W. Hu, L. Y. Liu, X. Wang, Q. Q. Zheng, C. Han and a. W. J. Li, Adv. Funct. Mater., 2024, 2313823 CrossRef CAS.
- H. Wang, K. Wang, E. Jing, M. Wei, J. Xiong, D. Zhong, Y. Zuo, B. Liang and P. Pei, Energy Storage Mater., 2024, 103451 CrossRef.
- J. Zhu, Z. Tie, S. Bi and Z. Niu, Angew Chem. Int. Ed. Engl., 2024, e202403712 CAS.
- L. Liu, H. Lu, C. Han, X. Chen, S. Liu, J. Zhang, X. Chen, X. Wang, R. Wang, J. Xu, H. K. Liu, S. X. Dou and W. Li, ACS Nano, 2023, 17, 23065–23078 CrossRef CAS PubMed.
- H. Yu, D. Chen, Q. Li, C. Yan, Z. Jiang, L. Zhou, W. Wei, J. Ma, X. Ji, Y. Chen and L. Chen, Adv. Energy Mater., 2023, 13, 2300550 CrossRef CAS.
- L. Y. Liu, X. Y. Wang, Z. W. Hu, X. Wang, Q. Q. Zheng, C. Han, J. T. Xu, X. Xu, H. K. Liu, S. X. Dou and W. J. Li, Angew. Chem., Int. Ed., 2024, e202405209 CAS.
- Y. Song, W. Li, K. Zhang, C. Han and A. Pan, Adv. Energy Mater., 2024, 14, 2303352 CrossRef CAS.
- K. R. Ryan, M. P. Down, N. J. Hurst, E. M. Keefe and C. E. Banks, eScience, 2022, 2, 365–381 CrossRef.
- X. Meng, S. Zhou, J. Li, Y. Chen, S. Lin, C. Han and A. Pan, Adv. Funct. Mater., 2023, 34, 2309350 CrossRef.
- J. Wu, Y. Wang, D. Deng, Y. Bai, M. Liu, X. Zhao, X. Xiong and Y. Lei, J. Mater. Chem. A, 2022, 10, 19304–19319 RSC.
- Y. Gao, C. Wang, H. Wang, C. Feng, H. Pan, Z. Zhang, J. He and Q. Wang, Chem. Eng. J., 2023, 474, 145446 CrossRef CAS.
- Y. Wang, Q. Li, H. Hong, S. Yang, R. Zhang, X. Wang, X. Jin, B. Xiong, S. Bai and C. Zhi, Nat. Commun., 2023, 14, 3890 CrossRef CAS PubMed.
- P. Ruan, S. Liang, B. Lu, H. J. Fan and J. Zhou, Angew. Chem., Int. Ed., 2022, 61, e202200598 CrossRef CAS PubMed.
- B. Huang, Z. Sun and G. Sun, eScience, 2022, 2, 243–277 CrossRef.
- W. Li, C. Han, Q. Gu, S. L. Chou, J. Z. Wang, H. K. Liu and S. X. Dou, Adv. Energy Mater., 2020, 10, 2303352 Search PubMed.
- K. Zhu, J. Luo, D. Zhang, N. Wang, S. Pan, S. Zhou, Z. Zhang, G. Guo, P. Yang, Y. Fan, S. Hou, Z. Shao, S. Liu, L. Lin, P. Xue, G. Hong, Y. Yang and Y. Yao, Adv. Mater., 2024, e2311082 CrossRef PubMed.
- H. Liu, Z. Xin, B. Cao, B. Zhang, H. J. Fan and S. Guo, Advanced Science, 2023, 11, 2305806 CrossRef PubMed.
- C. Li, Z. Sun, T. Yang, L. Yu, N. Wei, Z. Tian, J. Cai, J. Lv, Y. Shao, M. H. Rummeli, J. Sun and Z. Liu, Adv. Mater., 2020, 32, e2003425 CrossRef PubMed.
- X. Xiao, X. Xiao, Y. H. Zhou, X. Zhao, G. R. Chen, Z. X. Liu, Z. H. Wang, C. Y. Lu, M. L. Hu, A. Nashalian, S. Shen, K. D. Xie, W. W. Yang, Y. J. Gong, W. B. Ding, P. Servati, C. Han, S. X. Dou, W. J. Li and J. Chen, Sci. Adv., 2021, eabl3742 CrossRef CAS PubMed.
- W. Li, C. Han, K. Zhang, S. Chou and S. Dou, J. Mater. Chem. A, 2021, 9, 6671–6693 RSC.
- Y. Zeng, X. Zhang, R. Qin, X. Liu, P. Fang, D. Zheng, Y. Tong and X. Lu, Adv. Mater., 2019, 31, 1903675 CrossRef PubMed.
- F. Gao, B. Mei, X. Xu, J. Ren, D. Zhao, Z. Zhang, Z. Wang, Y. Wu, X. Liu and Y. Zhang, Chem. Eng. J., 2022, 448, 137742 CrossRef CAS.
- F. Mo, G. Liang, Z. Huang, H. Li, D. Wang and C. Zhi, Adv. Mater., 2020, 32, e1902151 CrossRef PubMed.
- K. K. Fu, J. Cheng, T. Li and L. Hu, ACS Energy Lett., 2016, 1, 1065–1079 CrossRef CAS.
- H. Dong, J. Li, J. Guo, F. Lai, F. Zhao, Y. Jiao, D. J. L. Brett, T. Liu, G. He and I. P. Parkin, Adv. Mater., 2021, 33, e2007548 CrossRef PubMed.
- X. Li, D. Wang and F. Ran, Energy Storage Mater., 2023, 56, 351–393 CrossRef.
- W. H. Wang, C. W. Li, S. Z. Liu, J. C. Zhang, D. J. Zhang, J. M. Du, Q. C. Zhang and Y. G. Yao, Adv. Energy Mater., 2023, 2300250 CrossRef CAS.
- D. Wang, C. Han, F. Mo, Q. Yang, Y. Zhao, Q. Li, G. Liang, B. Dong and C. Zhi, Energy Storage Mater., 2020, 28, 264–292 CrossRef.
- C. Tian, J. Wang, R. Sun, T. Ali, H. Wang, B. B. Xie, Y. Zhong and Y. Hu, Angew. Chem., Int. Ed., 2023, 62, e202310970 CrossRef CAS PubMed.
- L. Wu and Y. Dong, Energy Storage Mater., 2021, 41, 715–737 CrossRef.
- Z. Liu, D. Wang, Z. Tang, G. Liang, Q. Yang, H. Li, L. Ma, F. Mo and C. Zhi, Energy Storage Mater., 2019, 23, 636–645 CrossRef.
- P. Yu, Y. Zeng, H. Zhang, M. Yu, Y. Tong and X. Lu, Small, 2019, 15, 1804760 CrossRef PubMed.
- X. Zhong, Z. Zheng, J. Xu, X. Xiao, C. Sun, M. Zhang, J. Ma, B. Xu, K. Yu, X. Zhang, H. M. Cheng and G. Zhou, Adv. Mater., 2023, 35, e2209980 CrossRef PubMed.
- L. Zhang, L. Chen, X. Zhou and Z. Liu, Adv. Energy Mater., 2015, 5, 550–587 Search PubMed.
- Y. Li, Z. Wang, Y. Cai, M. E. Pam, Y. Yang, D. Zhang, Y. Wang and S. Huang, Energy Environ. Mater., 2022, 5, 823–851 CrossRef CAS.
- X. Wang, C. Han, S. X. Dou and W. J. Li, Nano Mater. Sci., 2022 DOI:10.1016/j.nanoms.2022.10.004.
- C. Han, H. Li, A. Pan, S. Dou, Y. Liu, J. Liu and W. Li, Chem. Eng. J., 2024, 481, 148073 CrossRef CAS.
- Q. He, Z. Chang, Y. Zhong, S. Chai, C. Fu, S. Liang, G. Fang and A. Pan, ACS Energy Lett., 2023, 8, 5253–5263 CrossRef CAS.
- S. Liu, W. Liu, D. Ba, Y. Zhao, Y. Ye, Y. Li and J. Liu, Adv. Mater., 2023, 35, e2110423 CrossRef PubMed.
- S. Huang, L. Hou, T. Li, Y. Jiao and P. Wu, Adv. Mater., 2022, 34, e2110140 CrossRef PubMed.
- S. Li, Y. Liu, X. Zhao, Q. Shen, W. Zhao, Q. Tan, N. Zhang, P. Li, L. Jiao and X. Qu, Adv. Mater., 2021, 33, 2007480 CrossRef CAS PubMed.
- K. Shirvanimoghaddam, I. Ozen, P. Wu, S. Jafarzadeh, R. Yadav and M. Naebe, Small Struct., 2024, 2300539 CrossRef CAS.
- F.-Z. Zhang, X.-B. Liu, C.-M. Yang, G.-D. Chen, Y. Meng, H.-B. Zhou and S.-H. Zhang, Mater. Today, 2024, 74, 203–234 CrossRef.
- J. A. Naveed, H. J. Yang, J. Yang, Y. N. Nuli and J. L. Wang, Angew. Chem. Int. Ed., 2019, 58, 2760–2764 CrossRef PubMed.
- J. Zhou, M. Xie, F. Wu, Y. Mei, Y. Hao, L. Li and R. Chen, Adv. Mater., 2022, 34, e2106897 CrossRef PubMed.
- S. Abdolhosseinzadeh, X. Jiang, H. Zhang, J. Qiu and C. Zhang, Mater. Today, 2021, 48, 214–240 CrossRef CAS.
- N. Zhang, S. Huang, Z. Yuan, J. Zhu, Z. Zhao and Z. Niu, Angew. Chem., Int. Ed., 2021, 60, 2861–2865 CrossRef CAS PubMed.
- Y. Liu, Y. Jiang, Z. Hu, J. Peng, W. Lai, D. Wu, S. Zuo, J. Zhang, B. Chen, Z. Dai, Y. Yang, Y. Huang, W. Zhang, W. Zhao, W. Zhang, L. Wang and S. Chou, Adv. Funct. Mater., 2020, 31, 2008033 CrossRef.
- C. Wan, J. Huang, K. Chen, C. Jiang, Q. Wu, P. Huang, Q. Xu, S. Qin and H. Xie, Energy Storage Mater., 2024, 69, 103384 CrossRef.
- X. Chang, X. Cheng, H. Zhang, W. Li, L. He, X. Yin, X. Liu, J. Yu, Y. T. Liu and B. Ding, Adv. Funct. Mater., 2023, 33, 2215168 CrossRef CAS.
- G. Ge, Y. Wu, E. van der Heide, Z. Chen, J. Zhu and X. Zhuang, J. Energy Storage, 2024, 90, 111900 CrossRef.
- S. Zhai, N. Wang, X. Tan, K. Jiang, Z. Quan, Y. Li and Z. Li, Adv. Funct. Mater., 2021, 31, 2008894 CrossRef CAS.
- S. Pandiaraj, S. Aftab, G. Koyyada, F. Kabir, H. H. Hegazy and J. H. Kim, Mater. Today Energy, 2024, 101629 CrossRef CAS.
- X. Guan, Z. Li, X. Geng, Z. Lei, A. Karakoti, T. Wu, P. Kumar, J. Yi and A. Vinu, Small, 2023, 19, e2207181 CrossRef PubMed.
- B. Sun, N. Wang, X. Xie, L. Zhong, L. He, S. Komarneni and W. Hu, J. Mater. Sci. Technol., 2025, 209, 251–261 CrossRef.
- D. Yuan, Y. Dou, Z. Wu, Y. Tian, K. H. Ye, Z. Lin, S. X. Dou and S. Zhang, Chem. Rev., 2022, 122, 957–999 CrossRef CAS PubMed.
- X. Wang, Y. Wang, Y. Jiang, X. Li, Y. Liu, H. Xiao, Y. Ma, Y. y. Huang and G. Yuan, Adv. Funct. Mater., 2021, 31, 2103210 CrossRef CAS.
- X. Li, M. Li, Q. Yang, H. Li, H. Xu, Z. Chai, K. Chen, Z. Liu, Z. Tang, L. Ma, Z. Huang, B. Dong, X. Yin, Q. Huang and C. Zhi, ACS Nano, 2020, 14, 541–551 CrossRef CAS PubMed.
- S. Bi, Y. Wu, A. Cao, J. Tian, S. Zhang and Z. Niu, Mater. Today Energy, 2020, 18, 100548 CrossRef CAS.
- Y. Du, X. Wang, Y. Zhang, H. Zhang, J. Man, K. Liu and J. Sun, Chem. Eng. J., 2022, 434, 134642 CrossRef CAS.
- J. Song, W. Wang, Y. Fang, S. Wang, D. He, R. Zhao and W. Xue, Appl. Surf. Sci., 2022, 578, 152053 CrossRef CAS.
- F. Wan, S. Huang, H. Cao and Z. Niu, ACS Nano, 2020, 14, 6752–6760 CrossRef CAS.
- J. Zhang, Y. Huang, Z. Li, C. Gao, S. Jin, S. Zhang, X. Wang and H. Zhou, Nanotechnology, 2020, 31, 375401 CrossRef CAS.
- X. Yue, H. Liu and P. Liu, Chem. Commun., 2019, 55, 1647–1650 RSC.
- C. Gao, J. Wang, Y. Huang, Z. Li, J. Zhang, H. Kuang, S. Chen, Z. Nie, S. Huang, W. Li, Y. Li, S. Jin, Y. Pan, T. Long, J. Luo, H. Zhou and X. Wang, Nanoscale, 2021, 13, 10100–10107 RSC.
- A. Wang, W. Zhou, M. Chen, A. Huang, Q. Tian, X. Xu and J. Chen, J. Colloid Interface Sci., 2021, 594, 389–397 CrossRef CAS PubMed.
- Y. Huang, J. Liu, Q. Huang, Z. Zheng, P. Hiralal, F. Zheng, D. Ozgit, S. Su, S. Chen, P.-H. Tan, S. Zhang and H. Zhou, npj Flex. Electron., 2018, 2, 21 CrossRef.
- J. Wang, J.-G. Wang, H. Liu, Z. You, Z. Li, F. Kang and B. Wei, Adv. Funct. Mater., 2021, 31, 2007397 CrossRef CAS.
- F. Wan, X. Wang, S. Bi, Z. Niu and J. Chen, Sci. China Chem., 2019, 62, 609–615 CrossRef CAS.
- F. Shi, C. Mang, H. Liu and Y. Dong, New J. Chem., 2020, 44, 653–657 RSC.
- M. Shi, B. Wang, C. Chen, J. Lang, C. Yan and X. Yan, J. Mater. Chem. A, 2020, 8, 24635–24644 RSC.
- M. Qi, F. Li, Z. Zhang, Q. Lai, Y. Liu, J. Gu and L. Wang, J. Colloid Interface Sci., 2022, 615, 151–162 CrossRef CAS PubMed.
- Z. Shi, Q. Ru, Z. Pan, M. Zheng, F. Chi-Chun Ling and L. Wei, Chemelectrochem, 2021, 8, 1091–1097 CrossRef CAS.
- M. Shi, B. Wang, Y. Shen, J. Jiang, W. Zhu, Y. Su, M. Narayanasamy, S. Angaiah, C. Yan and Q. Peng, Chem. Eng. J., 2020, 399, 135386 Search PubMed.
- Y. Lu, H. Zhang, H. Liu, Z. Nie, F. Xu, Y. Zhao, J. Zhu and W. Huang, Nano Lett., 2021, 21, 9651–9660 CrossRef CAS.
- X. Shen, X. Wang, Y. Zhou, Y. Shi, L. Zhao, H. Jin, J. Di and Q. Li, Adv. Funct. Mater., 2021, 31, 2101579 CrossRef CAS.
- K. Wang, X. Zhang, J. Hang, X. Zhang, X. Sun, C. Li, W. Liu, Q. Li and Y. Ma, ACS Appl. Mater. Interfaces, 2018, 10, 24573–24582 CrossRef CAS PubMed.
- Y. G. Lee, J. Lee and G. H. An, Chem. Eng. J., 2021, 414, 128916 CrossRef CAS.
- D. Chao, C. R. Zhu, M. Song, P. Liang, X. Zhang, N. H. Tiep, H. Zhao, J. Wang, R. Wang, H. Zhang and H. J. Fan, Adv. Mater., 2018, 30, e1803181 CrossRef PubMed.
- Q. Cao, H. Gao, Y. Gao, J. Yang, C. Li, J. Pu, J. Du, J. Yang, D. Cai, Z. Pan, C. Guan and W. Huang, Adv. Funct. Mater., 2021, 31, 2103922 CrossRef CAS.
- M. Yao, Z. Yuan, S. Li, T. He, R. Wang, M. Yuan and Z. Niu, Adv. Mater., 2021, 33, e2008140 CrossRef PubMed.
- Y. Zhang, S. Deng, Y. Li, B. Liu, G. Pan, Q. Liu, X. Wang, X. Xia and J. Tu, Energy Storage Mater., 2020, 29, 52–59 CrossRef.
- J. Yang, H. Yang, C. Ye, T. Li, G. Chen and Y. Qiu, Energy Storage Mater., 2022, 46, 472–481 CrossRef.
- N. Xu, C. Yan, W. He, L. Xu, Z. Jiang, A. Zheng, H. Wu, M. Chen and G. Diao, J. Power Sources, 2022, 533, 231358 CrossRef CAS.
- X. Liu, Z. Wang, Y. Niu, C. Liu, H. Chen, X. Ren, Z. Liu, W.-M. Lau and D. Zhou, ACS Appl. Energy Mater., 2022, 5, 3525–3535 CrossRef CAS.
- F. Wu, X. Gao, X. Xu, Y. Jiang, X. Gao, R. Yin, W. Shi, W. Liu, G. Lu and X. Cao, Chemsuschem, 2020, 13, 1537–1545 CrossRef CAS PubMed.
- Y. Chen, J. Li, S. Zhang, J. Cui and M. Shao, Adv. Mater. Interfaces, 2020, 7, 2196–7350 Search PubMed.
- J. Liu, J. Long, Z. Shen, X. Jin, T. Han, T. Si and H. Zhang, Adv. Sci., 2021, 8, 2004689 CrossRef CAS PubMed.
- R. G. Lv, H. Y. Wu, Z. H. Jiang, A. Y. Zheng, H. Y. Yu and M. Chen, Electrochim. Acta, 2022, 140998 CrossRef CAS.
- C. K. Liu, Y. Tian, Y. L. An, Q. L. Yang, S. L. Xiong, J. K. Feng and Y. T. Qian, Chem. Eng. J., 2022, 430, 132748 CrossRef CAS.
- J. Cao, D. Zhang, C. Gu, X. Wang, S. Wang, X. Zhang, J. Qin and Z. S. Wu, Adv. Energy Mater., 2021, 11, 2101299 CrossRef CAS.
- X. Xia, J. Yang, Y. Liu, J. Zhang, J. Shang, B. Liu, S. Li and W. Li, Adv. Sci., 2022, e2204875 Search PubMed.
- X. Gao, H. Wu, C. Su, C. Lu, Y. Dai, S. Zhao, X. Hu, F. Zhao, W. Zhang, I. P. Parkin, C. J. Carmalt and G. He, Energy Environ. Sci., 2023, 16, 1364–1383 RSC.
- X. Gao, C. Shen, H. Dong, Y. Dai, P. Jiang, I. P. Parkin, H. Zhang, C. J. Carmalt and G. He, Energy Environ. Sci., 2024, 17, 2287–2297 RSC.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- M. Naguib, V. N. Mochalin, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2014, 26, 992–1005 CrossRef CAS PubMed.
- J. Zheng, J. A. Lochala, A. Kwok, Z. D. Deng and J. Xiao, Adv. Sci., 2017, 4, 1700032 CrossRef PubMed.
- J. Shin, J. Lee, Y. Park and J. W. Choi, Chem. Sci., 2020, 11, 2028–2044 RSC.
- S. Yang, Y. Cheng, X. Xiao and H. Pang, Chem. Eng. J., 2020, 384, 123294 CrossRef CAS.
- J. Xu, Z. Dong, K. Huang, L. Wang, Z. Wei, L. Yu and X. Wu, Scr. Mater., 2022, 209, 114368 CrossRef CAS.
- Y. Zhuang, Y. Xie, B. Fei, D. Cai, Y. Wang, Q. Chen and H. Zhan, J. Mater. Chem. A, 2021, 9, 21313–21322 RSC.
- L. Chen, T. Xiao, J. L. Yang, Y. Liu, J. Xian, K. Liu, Y. Zhao, H. J. Fan and P. Yang, Angew. Chem., Int. Ed., 2024, e202400230 CAS.
- Q. Liu, Z. Yu, Q. Zhuang, J. K. Kim, F. Kang and B. Zhang, Adv. Mater., 2023, 35, e2300498 CrossRef PubMed.
- Y. Zhang, L. Zhao, Y. Liang, X. Wang and Y. Yao, eScience, 2022, 2, 110–115 CrossRef.
- H. Xia, G. Xu, X. Cao, C. Miao, H. Zhang, P. Chen, Y. Zhou, W. Zhang and Z. Sun, Adv. Mater., 2023, 35, e2301996 CrossRef PubMed.
- W. Zhou, M. Yang, M. Chen, G. Zhang, X. Han, J. Chen, D. Ma and P. Zhang, Adv. Funct. Mater., 2024, 2315444 CrossRef CAS.
- F. Shen, H. Du, H. Qin, Z. Wei, W. Kuang, N. Hu, W. Lv, Z. Yi, D. Huang, Z. Chen and H. He, Small, 2024, 20, e2305119 CrossRef PubMed.
- L. Wu, Y. Zhang, P. Shang, Y. Dong and Z.-S. Wu, J. Mater. Chem. A, 2021, 9, 27408–27414 RSC.
| This journal is © The Royal Society of Chemistry 2024 |