Quorum sensing inhibiting dihydropyrrol-2-ones embedded polymer/graphene oxide nanocomposite waterborne antimicrobial coatings†
Renxun
Chen
 *a,
Namrata
Maslekar
b,
Sudip
Chakraborty
a,
Le N. M.
Dinh
b,
Yin
Yao
c,
Per B.
Zetterlund
*a,
Namrata
Maslekar
b,
Sudip
Chakraborty
a,
Le N. M.
Dinh
b,
Yin
Yao
c,
Per B.
Zetterlund
 b,
Naresh
Kumar
b,
Naresh
Kumar
 a and
Vipul
Agarwal
a and
Vipul
Agarwal
 *b
*b
aSchool of Chemistry, University of New South Wales (UNSW) Sydney, Sydney 2052, Australia
bCluster for Advanced Macromolecular Design (CAMD), School of Chemical Engineering, University of New South Wales, Sydney, NSW 2052, Australia. E-mail: r.chen@unsw.edu.au; agarwalvipul84@gmail.com
cMark Wainwright Analytical Centre, University of New South Wales, Sydney, NSW 2052, Australia
First published on 25th July 2024
Abstract
With increasing antibiotic resistance and hospital acquired microbial infections, there has been a growing interest to explore alternate antimicrobial approaches. This is particularly challenging when aiming to protect surfaces over a large area to avoid contact mediated infection transmission. Quorum sensing (QS) inhibition has emerged as an alternate antimicrobial approach overcoming evolutionary stress driven resistance observed in antibiotic treatment. However, specific surface orientation requirements and limited work on delivery of small molecule QS inhibiting compounds have limited their widespread applicability certainly when it comes to coating large surfaces. Here, we report antimicrobial nanocomposite coatings overcoming the dependence on molecular orientation of QS inhibiting dihydropyrrol-2-ones (DHP) analogues and release small molecule analogues. In a systematic study, we developed poly(styrene-stat-n-butyl acrylate)/graphene oxide (GO)/DHP analogue nanocomposite antimicrobial coatings that can be easily applied to surfaces of any length scale and studied their efficacy against Staphylococcus aureus. The polymer nanocomposite was designed to undergo coating formation at ambient temperature. The antimicrobial coatings exhibited DHP dose dependent antimicrobial response both in the supernatant growth media with a ∼7-log10 reduction in cell growth and virtually a complete inhibition in cell adhesion on the surface in the best coating compared to controls. When compared, DHP-Br coatings outperformed other DHP analogues (–F and –Ph) both in limiting the cell growth in the media and cellular adhesion on the coating surface. This is the first example of nanocomposite coatings comprising QS inhibiting compounds, and their exceptional performance is expected to pave the way for further research in the field.
Introduction
The global increase in infections is becoming a critical threat to human health driven by the emergence of evolutionary drug resistance in most common bacteria towards existing antibiotics.1 One of the biggest contributors to increasing infections is nosocomial (hospital-acquired) infections caused by the increasing number of clinical surgical procedures including implants.2 Implants have transformed human life by significantly improving both quality of life and life expectancy. However, implants are also highly prone to microbial infection, which remains one of the major causes of implant failure.3 It is now widely accepted that bacteria survive in sterile clinical environments by adhering to solid surfaces including implants, surgical equipment, surgical tables, monitoring/anaesthesia equipment, and drapes.2 In the absence of effective infection control, performing clinical procedures is anticipated to become impossible.Once a bacterial infection has been contracted, antibiotics remain the first and most effective line of treatment. Although alternatives such as antimicrobial organic and inorganic compounds, peptides, peptide mimics, peptoids and polymers have shown promise,1,4–11 however, their clinical translation has been limited. Therefore, common consensus has been emerging to avoid exposure to microbes, a lesson learned from the recent Covid-19 infection. One approach to counter surface contact-mediated infection exposure is to use antimicrobial coatings as a first line of defence. Furthermore, deeper mechanistic understanding of antibiotic resistance has revealed that an alternate strategy to avoid resistance could be to develop small molecules to specifically target less evolutionary stressing strategies such as innate cellular peptides, which are necessary for intercellular communications (quorum sensing, QS) in Gram-positive bacteria.12,13 QS signalling is also crucial for bacterial cell growth, adhesion, and biofilm formation, and therefore targeting QS is proposed to be a non-growth inhibitory mechanism avoiding evolutionary survival pressure on both Gram-positive and Gram-negative bacteria.14–17
To target QS, synthetic derivatives of lactone-based molecules that mimic the N-acyl homoserine lactones have gathered attention.18–21 These synthetic mimics have been shown to interact with a specific saturable AgrC receptor on the cytoplasmic membrane on Gram-positive Staphylococcus aureus thus interfering with its QS system along with dissipating both the membrane potential and the pH gradient of cells.22,23 Furthermore, these synthetic compounds also inhibit biofilm formation and the expression of the virulence gene lasB in the case of Gram-negative Pseudomonas aeruginosa,20,24,25 therefore being effective against a wide class of bacteria. One class of QS inhibitors is dihydropyrrol-2-ones (DHP) analogues, which have been shown to prevent adhesion and colonisation of different bacterial species specifically when bound to substrates.26 DHP analogues are believed to be less likely to induce antibacterial resistance with a broad spectrum antibacterial activity and low to no cytotoxicity towards eukaryotic cells.19 Previous studies have highlighted that specific surface orientation of bound DHP analogues is more important than overall concentration towards their antibacterial efficacy.26 Despite the promising results, one of the challenges potentially limiting large-scale clinical application of DHP analogues is the need for specific molecular orientation of surface immobilized DHPs or potential release from loaded substrates. Therefore, coating strategies overcoming the dependence on molecular orientation of DHP analogues could open the avenue for commercial translation of these compounds.
In the case of surface induced infections such as in hospitals or highly populated communal environments such as shopping centres and public transport, antimicrobial coatings that can be easily applied to a large area while adhering to different substrates are desired. Antimicrobial coatings developed to date operate under three main strategies – releasing antimicrobial agents, contact-killing, and bacterial cell repelling/anti-adhesion.27 Based on decades of research, it is now widely accepted that the next generation of antimicrobial coatings should encompass more than one of these main strategies.2 To this end, different polymer coatings have been explored mainly based on hydrophilic polymers with most effective dual surface and biocidal releasing coatings made using layer-by-layer approaches.28–32 However, layer-by-layer approaches can be cumbersome to apply over large areas and mainly rely on the chemical compatibility between the polymer used and the biocidal agent.2
There are other desired features warranted from next generation coatings, including (i) fast acting with short incubation time requirement avoiding interim transmission of the bacteria, (ii) sustained release of loaded antimicrobial agents within the therapeutic window allowing killing of both adhered and adjacent planktonic (suspended) bacteria but low enough to limit cytotoxicity toward eukaryotes, (iii) release poorly charged (i.e., with no or only few polar groups compared to its size) or biocidal small molecules, (iv) long-term stability (retain coating properties over time including substrate adhesion), (v) easily scalable, and (vi) mass producible at low cost.
To this end, we have developed next generation antibacterial coatings encompassing most of the above-mentioned features. We used poly(styrene-stat-n-butyl acrylate) (P(St-stat-nBA)) as a polymer matrix, graphene oxide (GO) as a hydrophilic antibacterial agent, and dihydropyrrol-2-ones (DHP) analogues as QS inhibitor small molecules. The polymer/GO nanocomposite dispersions were prepared using different emulsion-based polymerisation methods and the obtained latexes were supplemented with different DHP analogues. The developed water-based (emulsion) coatings can be easily applied over large areas by simple dip or spray coating methods. The polymer matrix was designed to undergo coating formation at ambient temperature and adhere to a range of substrates making them highly attractive for commercial translation overcoming the limitations with existing coatings. We also hypothesise that (i) plastic coatings can be used to deliver small molecules, and (ii) using this simple approach we can overcome the orientation dependent antibacterial efficacy of DHPs. This is to the best of our knowledge the first systematic study exploring the potential of vinyl polymer composite antimicrobial coatings with an ability to deliver relatively hydrophobic DHPs. We envisage that this study will potentially establish traditional vinyl polymer (P(St-stat-nBA))-based hydrophobic paints commonly used in commercial and clinical buildings as antimicrobial coatings.
Materials and methods
Styrene (St, Sigma Aldrich, 99%) and n-butyl acrylate (nBA, Sigma Aldrich, 99%) monomers were purified by passing through a column of activated basic aluminium oxide (Ajax) to remove the inhibitors. The aqueous dispersion of graphene oxide (GO, Graphenea, 4 mg mL−1, ∼600 nm by DLS33) was used as received. The as-purchased initiator azobisisobutyronitrile (AIBN, Sigma Aldrich) dissolved in acetone was purified by recrystallisation in water. Hexadecane (HD, Sigma Aldrich, 99%), potassium persulfate (KPS, Sigma Aldrich, 99%), and sodium dodecyl sulfate (SDS, Sigma Aldrich, ≥99%) were used as received. Pure antimicrobial compound stock solutions were prepared by dissolving 20 mg of a compound in 200 μL of ethanol overnight under contract stirring. All experiments were carried out in Milli-Q water.Coating preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 wt% of St and nBA (0.525 g each, 7 wt% rel. to water), HD (0.0525 g, 5 wt% rel. to monomer), AIBN (0.05 g, 0.25 M rel. to HD and monomer), SDS (11.525 mg, 1 wt% rel. to organic phase), and antimicrobial compounds (0.1, 1, 5 and 10 wt% rel. to monomer). Next, the sonicated GO dispersion was added to the organic phase and magnetically stirred at room temperature for 15 min. Subsequently, this aqueous and organic phase mixture was ultrasonicated on ice at 70% amplitude for 10 min to prepare a miniemulsion. The obtained miniemulsion was degassed for 20 min under nitrogen on ice and allowed to polymerize at 70 °C for 24 h under constant stirring.
50 wt% of St and nBA (0.525 g each, 7 wt% rel. to water), HD (0.0525 g, 5 wt% rel. to monomer), AIBN (0.05 g, 0.25 M rel. to HD and monomer), SDS (11.525 mg, 1 wt% rel. to organic phase), and antimicrobial compounds (0.1, 1, 5 and 10 wt% rel. to monomer). Next, the sonicated GO dispersion was added to the organic phase and magnetically stirred at room temperature for 15 min. Subsequently, this aqueous and organic phase mixture was ultrasonicated on ice at 70% amplitude for 10 min to prepare a miniemulsion. The obtained miniemulsion was degassed for 20 min under nitrogen on ice and allowed to polymerize at 70 °C for 24 h under constant stirring.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 wt% of St and nBA (10 wt% rel. to the aqueous phase) were mixed with KPS (0.25 M rel. to organic phase), and water (15 g) in a 25 mL glass vial using magnetic stirring for 30 min. The mixture was then degassed for 20 min under nitrogen and polymerized at 70 °C for 6 h under magnetic stirring.
50 wt% of St and nBA (10 wt% rel. to the aqueous phase) were mixed with KPS (0.25 M rel. to organic phase), and water (15 g) in a 25 mL glass vial using magnetic stirring for 30 min. The mixture was then degassed for 20 min under nitrogen and polymerized at 70 °C for 6 h under magnetic stirring.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (mg
20 (mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μL) ratio by constant magnetic stirring at 500 rpm overnight at room temperature. Next, 0.5, 1, 5, and 10 wt% (rel. to monomer) of the diluted DHP-X compound mixture was added to 500 μL of the neat soap-free, physical mixing, and miniemulsion nanocomposite latexes each. The P(St-stat-nBA)/GO/DHP-X latexes were then subjected to magnetic stirring for 15 min at 500 rpm to obtain the corresponding nanocomposite latexes.
μL) ratio by constant magnetic stirring at 500 rpm overnight at room temperature. Next, 0.5, 1, 5, and 10 wt% (rel. to monomer) of the diluted DHP-X compound mixture was added to 500 μL of the neat soap-free, physical mixing, and miniemulsion nanocomposite latexes each. The P(St-stat-nBA)/GO/DHP-X latexes were then subjected to magnetic stirring for 15 min at 500 rpm to obtain the corresponding nanocomposite latexes.
In vitro antibacterial study
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 repeatedly) to obtain different concentrations of cells, which were then plated on nutrient agar plates and incubated overnight at 37 °C. Plates were subsequently taken out and cell colonies were counted using a colony counter.
10 repeatedly) to obtain different concentrations of cells, which were then plated on nutrient agar plates and incubated overnight at 37 °C. Plates were subsequently taken out and cell colonies were counted using a colony counter.
Results and discussion
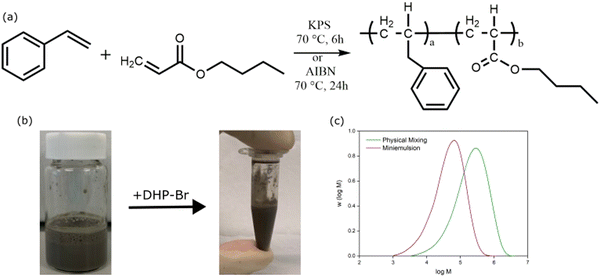
Nanocomposite latexes were prepared using miniemulsion polymerisation and ‘physical mixing’. Miniemulsion polymerisation is a one-pot synthesis strategy to prepare polymer/filler nanocomposite colloids (Scheme 1). In miniemulsion polymerisation, polymerisation proceeds through droplet nucleation of submicron-sized monomer droplets decorated with filler particles dispersed in a continuous aqueous phase to form filler particles decorated polymer particles in the case of nanocomposites.37,38 In ‘physical mixing’, a latex comprising polymer particles dispersed in a continuous aqueous phase is synthesised using soap-free emulsion polymerisation, which is then mixed with an aqueous dispersion of filler particles to obtain a colloidally stable nanocomposite.33 In physical mixing, typically polymer particles are not decorated with filler particles instead both polymer and filler particles are colloidally suspended in a continuous aqueous phase. The nanocomposite coatings prepared using these two methods exhibit significantly different surface and mechanical properties due to the difference in the way filler is distributed or organised around or at the interface of polymer particles in the latex as previously demonstrated by us.33,35,39 Therefore, we aimed to compare the antimicrobial performance of coatings prepared using the two preparation methods (miniemulsion versus physical mixing).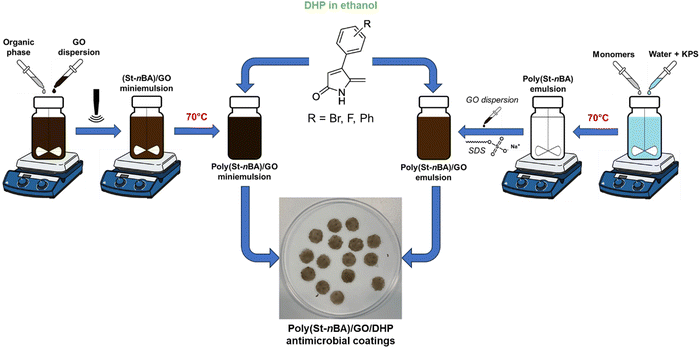 | ||
| Scheme 1 Schematic showing the two methods used to prepare P(St-stat-nBA)/GO/DHP nanocomposite coatings. | ||
Based on our previous work, we used a statistical copolymer of styrene and n-butyl acrylate (P(St-stat-nBA)) with an innate ability to undergo coating formation at ambient temperature.36,40 The weight ratio of St and nBA was kept at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which yields a copolymer with a theoretical glass transition temperature (Tg) of ∼3 °C (based on the Fox equation).41 Miniemulsion polymerisation was conducted at 70 °C using AIBN as initiator, 5 wt% GO (relative to monomer) and 1 wt% SDS (relative to organic phase) for 24 h. GO was selected for its intrinsic hydrophilic and antimicrobial properties.42–44 Soap-free emulsion polymerisation was conducted using KPS as initiator at 60 °C for 24 h, and the obtained latex was then mixed with 5 wt% GO (relative to monomer) and 1 wt% SDS (relative to organic phase). We observed no coagulation or phase separation in latexes obtained from both miniemulsion (Fig. 1b) and soap-free emulsion polymerisations. The monomer conversion was ∼95% in miniemulsion polymerisation and ∼97% in soap-free emulsion polymerisation, and the polymer particle sizes obtained from the two synthesis methods were in the typical range reported previously (Table S1, ESI†).34,45
1, which yields a copolymer with a theoretical glass transition temperature (Tg) of ∼3 °C (based on the Fox equation).41 Miniemulsion polymerisation was conducted at 70 °C using AIBN as initiator, 5 wt% GO (relative to monomer) and 1 wt% SDS (relative to organic phase) for 24 h. GO was selected for its intrinsic hydrophilic and antimicrobial properties.42–44 Soap-free emulsion polymerisation was conducted using KPS as initiator at 60 °C for 24 h, and the obtained latex was then mixed with 5 wt% GO (relative to monomer) and 1 wt% SDS (relative to organic phase). We observed no coagulation or phase separation in latexes obtained from both miniemulsion (Fig. 1b) and soap-free emulsion polymerisations. The monomer conversion was ∼95% in miniemulsion polymerisation and ∼97% in soap-free emulsion polymerisation, and the polymer particle sizes obtained from the two synthesis methods were in the typical range reported previously (Table S1, ESI†).34,45
The molecular weight was Mn = ∼2.4 × 104 g mol−1 for miniemulsion polymerisation and Mn = ∼10.3 × 105 g mol−1 for the polymer synthesised using soap-free emulsion polymerisation (Fig. 1c), both of which were within the typical ranges reported for similar systems.46–50 Next, we added dihydropyrrol-2-ones (DHP) analogues to the latexes prepared using the two polymerisation methods. Nanocomposite latexes were dropcasted on glass coverslips and allowed to dry at ambient temperature overnight to obtain uniform coatings. Neat polymer, neat polymer loaded with DHP analogues, and neat polymer loaded with GO with no DHP analogue coatings were used as controls.
We hypothesised that P(St-stat-nBA)/GO/DHP nanocomposite coatings would exhibit very high antimicrobial activity due to the combination of different mechanisms: (i) physical damage compromising the membrane integrity of microbial cells due to sharp edges of GO sheets on the coating surface,42,51 (ii) QS inhibition due to DHP analogues,20,26,52,53 and (iii) oxidative stress induced by GO via superoxide anion-independent oxidation.43,44,51
Subsequently, we systematically investigated the antimicrobial activity of the coatings using a Gram-positive Staphylococcus aureus SA38 bacterial strain. First, we compared the effect of nanocomposite synthesis strategy on antimicrobial performance of coatings. To this end, we compared nanocomposite coatings prepared using the ‘physical mixing’ and miniemulsion polymerisation. The antimicrobial activity was investigated in terms of reduction in bacterial cell growth (a) in the supernatant surrounding different coating, and (b) by surface adhesion on different coatings.
Antimicrobial activity in the supernatant
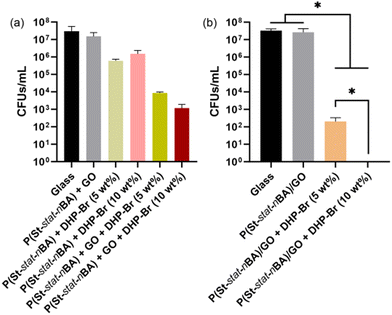
The bacterial cell growth in the supernatant data is shown in Fig. 2. In the case of physical mixing coatings, we observed a reduction in bacterial cell growth in the supernatant in all coatings compared to uncoated glass control, albeit to different extents, indicative of antimicrobial activity of the coatings. For example, a marginal reduction in cell growth was observed in polymer + GO control coatings, which did not reach significance, indicating that the inclusion of GO is not inducing any prominent antimicrobial response (Fig. 2a). A marginal reduction in cell growth was also observed for polymer + DHP-Br coatings at both 5 wt% and 10 wt% DHP-Br loading (relative to the polymer) compared to glass and polymer + GO control coatings (p < 0.05). However, in the case of polymer + GO + DHP-Br coatings, a considerable reduction in bacterial cell growth was observed compared to all different controls (uncoated glass, polymer + GO, polymer + DHP-Br) coatings (Fig. 2a). Between polymer + GO + DHP-Br coatings, we observed some reduction in the microbial cell growth, however, the difference between 5 wt% and 10 wt% DHP-Br containing coatings did not reach significance (p > 0.05). The observed antimicrobial response in the supernatant is supported by the release of DHP-Br from the respective coatings. The DHP-Br release was higher in polymer + GO + DHP-Br coatings (∼14–20 μg) compared to polymer + DHP-Br coatings (∼11–13 μg) after 24 h incubation. Furthermore, a higher amount of the active compound (DHP-Br) was released from coatings containing 10 wt% DHP-Br than 5 wt% DHP-Br (for both polymer + DHP-Br control and polymer + GO + DHP-Br coatings) after 24 h incubation (Fig. S1, ESI†).In the case of miniemulsion coatings, we observed no noticeable difference in bacterial cell growth in the supernatant between uncoated glass control and polymer/GO control coatings (Fig. 2b). Polymer + DHP-Br control coatings were not included in this experiment due to their similarity to the physical mixing control coating. In miniemulsion coatings, the inclusion of DHP-Br in polymer/GO coatings revealed significant reduction in cell growth in the supernatant in a dose dependent manner (polymer/GO + DHP-Br (10 wt%) < polymer/GO + DHP-Br (5 wt%)) (p < 0.05). The reduction in cell growth was ∼7-log10 in polymer/GO + DHP-Br (10 wt%) coatings compared to ∼5-log10 in polymer/GO + DHP-Br (5 wt%) coatings which estimate to be >99.9999% reduction. Surprisingly, we observed no measurable viable cell population in the supernatant in polymer/GO + DHP-Br (10 wt%) coatings. This significantly superior antimicrobial performance of 10 wt% DHP-Br coating (∼7-log10 reduction) can potentially be attributed to the presence of greater amount of DHP on the surface of these coatings which can result in higher release of DHP-Br (∼15 μg compared to ∼8 μg from 5 wt% DHP-Br coatings) in 24 h (Fig. S2, ESI†).
Comparatively, miniemulsion polymer/GO + DHP-Br coatings exhibited a significantly greater reduction in cell growth in the supernatant compared to physical mixing polymer + GO + DHP-Br coatings at both 5 wt% and 10 wt% (DHP-Br) concentrations, respectively. Considering the physical mixing and miniemulsion cell growth in the supernatant data, we hypothesise a potential combined effect of DHP-Br and GO in polymer + GO + DHP-Br coatings which was greater than control coatings comprising GO or DHP-Br alone. It is postulated that (i) π–π and hydrophobic–hydrophobic interactions between the aromatic groups and (ii) H-bonding interactions between GO and DHP-Br would lead to the presence of a greater amount of DHP-Br on the film surface. This GO mediated higher amount of DHP-Br on the coating surface would lead to exposure of microbial cells to antimicrobial DHP-Br. To explain the observed difference in miniemulsion and physical mixing coatings, based on our previous work, we know that there is higher amount of GO present on the miniemulsion coating surface compared to physical mixing coatings. Therefore, based on our hypothesis of considerable interactions between DHP-Br and GO sheets, we hypothesise that there would be a higher amount of DHP-Br on miniemulsion coating surface compared to physical mixing coatings (EDS analysis – Fig. S3 and S4, ESI†). It is assumed that in the case of polymer + DHP-Br coatings, DHP-Br would be embedded within the bulk with very limited presence on the film surface to induce any meaningful antimicrobial response. Furthermore, the negligible antimicrobial response of polymer + GO control coatings regardless of the synthesis strategy can be attributed to the lack of exposed GO sheets on the coating surface to induce any physical damage mediated cell death.
Antimicrobial activity on the coating surface
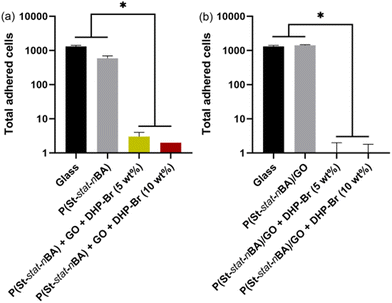
Instead of growing in the supernatant, bacterial cells can grow by adhering to a coating surface. We used SEM to quantify the number of adhered cells on different coatings after one day of growth (Fig. 3 and 4). Notably, DHP-Br is surface active and has been shown to function by inhibiting surface adhesion of bacterial cells. As evident from the SEM images, in the case of physical mixing coatings, a significantly higher number of surface adhered cells were observed on the uncoated glass and polymer + GO control coatings compared to the polymer + GO + DHP-Br coatings. Quantitative analysis of SEM images revealed an almost linear reduction in log(adhered cell number) going from uncoated glass to polymer + GO to increasing concentration of DHP in polymer + DHP-Br coatings (p < 0.05) (Fig. 3 and 5a). At 5 wt% and 10 wt% (DHP-Br) concentrations (in polymer + DHP-Br coatings), no noticeable difference in surface adhered cells was observed indicating that 5 wt% DHP-Br was sufficient to almost inhibit bacterial cell adhesion completely. Furthermore, the retention of round cellular morphology in GO containing coatings confirmed that there was no GO sheet mediated physical damage to adhered cells. In the case of miniemulsion films, we again observed no inhibitory effect on neat glass and polymer/GO coatings (Fig. 4 and 5b). It is only after the inclusion of DHP-Br that we observed an almost complete inhibition of surface adhesion of bacterial cells (p < 0.05) (Fig. 4 and 5b). Based on the physical mixing and miniemulsion data, we attributed the observed significant inhibition response on nanocomposite (polymer + GO + DHP-Br) coatings to the presence of DHP-Br on coating surfaces, probably mediated by the inter-molecular interactions between GO and DHP-Br. Considering all the antimicrobial data, it can be concluded that miniemulsion coatings exhibited a significantly stronger antimicrobial response compared to their physical mixing coating counterparts. Furthermore, it can be deduced that incorporation of DHP-Br within hydrophobic nanocomposite coatings can overcome the need for specific-orientation of DHP-Br on the surface to be effective against Gram-positive S. aureus cells as reported previously.26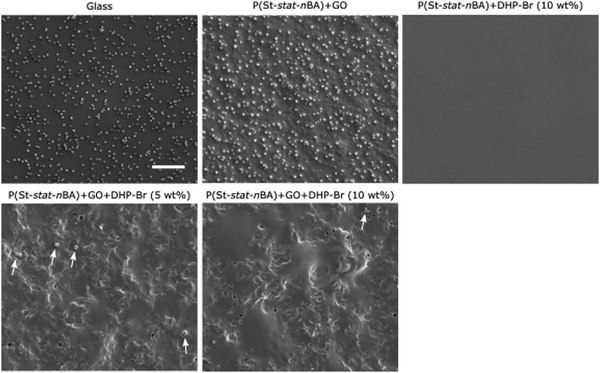 | ||
| Fig. 3 SEM images showing bacterial cell adhesion exhibiting characteristic spherical morphology on physical mixing coatings. White arrows indicate surface adhered cells. Scale bar = 10 μm. | ||
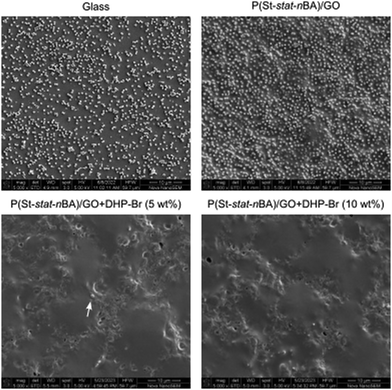 | ||
| Fig. 4 SEM images showing bacterial cell adhesion exhibiting characteristic spherical morphology on miniemulsion coatings. White arrow indicates surface adhered cells. Scale bar = 10 μm. | ||
Furthermore, difference in the release of DHP-Br from different coatings could be reasoned behind the observed differences in the cell growth in the supernatant. To this end, we conducted release of DHP-Br from different coatings.
Antimicrobial performance of DHP-Br analogue coatings
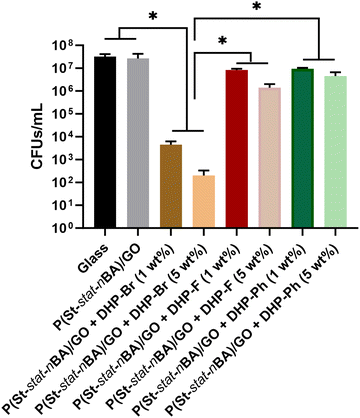
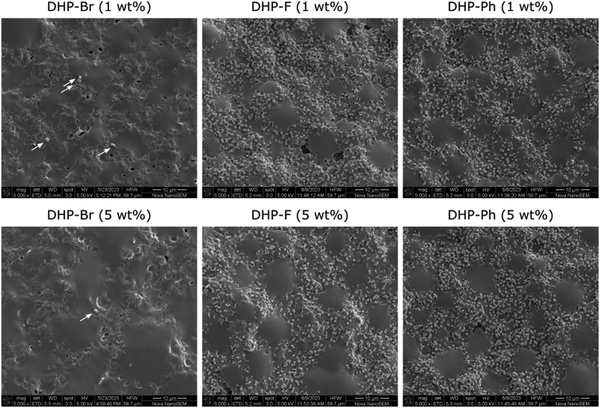
Next, we investigated the influence of the –Br group on DHP antimicrobial activity by substituting it with other electronegative functional groups (–F and –Ph). These DHP (–F and –Ph) analogues in isolation (as a neat compound) have been previously shown by us to demonstrate antimicrobial activity against different bacterial strains including S. aureus and P. aeruginosa.14,19,24,26 However, no considerable difference in antimicrobial activity was observed between halogenated DHP (–F, –Cl, –Br) analogues, expect for –Cl which exhibited lower activity than other compounds (–F and –Br).19 Coatings comprising DHP analogues (–F and –Ph) were developed in the same way as polymer/DHP-Br coatings and their antimicrobial activity was investigated against S. aureus in terms of both growth in the supernatant and by surface adhesion. In the supernatant, compared to DHP-Br coatings, both –F and –Ph analogues exhibited significantly lower antimicrobial activity (p < 0.05), i.e. a greater number of viable cells were observed on both (–F and –Ph) coatings after 24 h of incubation (Fig. 6). Between –F and –Ph coatings, –F coatings exhibited considerably better antimicrobial performance (i.e. reduction in the number of viable cells) than –Ph coatings, albeit only at 5 wt% loading. At 1 wt% loading, no noticeable difference in the number of viable cells was observed between –F and –Ph analogue coatings. Furthermore, compared to controls (untreated and polymer/GO coating), no significant reduction in number of viable cells in the supernatant (p > 0.05) was observed at both 1 wt% and 5 wt% loading (Fig. 6). Despite the evidence of some antimicrobial activity in terms of viable cells in the supernatant, the reduction in cell numbers is not commercially or clinically relevant regardless of the two analogue (–F and –Ph) loadings. Overall, supernatant data revealed the importance of functional groups on the antimicrobial performance of DHP compounds in nanocomposite coatings.In the case of bacterial cell growth by surface adhesion, we observed no noticeable difference in the number of adhered cells between –F and –Ph coatings. Out of the three DHP compounds investigated, –Br coatings were significantly more effective inhibiting cellular adhesion showing an almost negligible number of adhered cells (Fig. 7).
Conclusions
The aim of the present work was to develop nanocomposite strategy to produce antimicrobial coatings embedded with QS inhibiting small molecules (DHP analogues). P(St-stat-nBA)/GO/DHP analogue nanocomposite coatings were prepared using two methods, miniemulsion and physical mixing (polymer synthesised using soap-free emulsion polymerisation). P(St-stat-nBA) was designed to undergo coating formation at ambient temperature on virtually any substrate. The antimicrobial performance was investigated against Staphylococcus aureus in terms of their growth in the growth media and surface adhesion in response to their incubation with coatings. A dose dependent antimicrobial response was observed on coatings prepared using both miniemulsion and physical mixing. When compared the two methods, miniemulsion coatings significantly outperformed physical mixing coatings in terms of cellular growth in the supernatant with the highest growth reduction of ∼7-log10 on miniemulsion DHP-Br (10 wt%) coatings. However, coatings prepared using both methods exhibited similar almost complete inhibition of cellular adhesion on the coating surface. When compared, DHP-Br coatings significantly outperformed DHP-F and DHP-Ph analogues regardless of the concentration in both the supernatant and surface adhesion. Based on the obtained data it was concluded that inclusion of DHP analogues overcame the previously reported specific conformational orientation requirement of DHP analogues to be effective antimicrobial compounds. Furthermore, observed cellular growth inhibition in the supernatant could have been caused by the release of these analogues from the coatings. The simplicity of the developed nanocomposites and their ease of applicability on different substrates make them highly attractive for commercial application and inspire further research on QS inhibitor-based antimicrobial coatings.Data availability
Data for this article are available at UNSW public repository at https://doi.org/10.26190/unsworks/30295.Conflicts of interest
No conflict to declare.Acknowledgements
V. A. acknowledges the National Health and Medical Research Council (NHMRC), Australia, for an Early Career Fellowship (GNT1139060) and UNSW Safety Net Fellowship. The authors acknowledge the facilities and the scientific and technical assistance of Microscopy Australia at the Electron Microscope Unit (EMU) within the Mark Wainwright Analytical Centre (MWAC) at UNSW Sydney.References
- R. Namivandi-Zangeneh, E. H. H. Wong and C. Boyer, ACS Infect. Dis., 2021, 7, 215–253 CrossRef CAS PubMed
.
- M. Cloutier, D. Mantovani and F. Rosei, Trends Biotechnol., 2015, 33, 637–652 CrossRef CAS PubMed
.
- J. M. Schierholz and J. Beuth, J. Hosp. Infect., 2001, 49, 87–93 CrossRef CAS PubMed
.
- A. Bian, Y. Sun, J. Guan, L. Xie, H. Yang, P. Han, H. Lin, H. Qiao, X. Zhang and Y. Huang, J. Ind. Eng. Chem., 2024, 135, 94–109 CrossRef CAS
.
- F. Jia, D. Xu, Y. Sun, W. Jiang, H. Yang, A. Bian, Y. Liu, K. Liu, S. Zhang, Y. Wang, H. Qiao, H. Lin, J. Lan and Y. Huang, Ceram. Int., 2023, 49, 35703–35721 CrossRef CAS
.
- B. Wang, J. Lan, H. Qiao, L. Xie, H. Yang, H. Lin, X. Li and Y. Huang, Colloids Surf., B, 2023, 224, 113188 CrossRef CAS PubMed
.
- H. X. Luong, T. T. Thanh and T. H. Tran, Life Sci., 2020, 260, 118407 CrossRef CAS PubMed
.
- L.-j Zhang and R. L. Gallo, Curr. Biol., 2016, 26, R14–R19 CrossRef CAS PubMed
.
- M. Haktaniyan and M. Bradley, Chem. Soc. Rev., 2022, 51, 8584–8611 RSC
.
- F. A. García, T. F. Fuentes, I. P. Alonso, R. A. Bosch, A. E. Brunetti and N. P. Lopes, J. Nat. Prod., 2024, 87, 600–616 CrossRef PubMed
.
- P. Chen, T. Ye, C. Li, P. Praveen, Z. Hu, W. Li and C. Shang, Nat. Prod. Rep., 2024, 41, 331–346 RSC
.
- S. P. Diggle, A. Gardner, S. A. West and A. S. Griffin, Philos. Trans. R. Soc., B, 2007, 362, 1241–1249 CrossRef CAS PubMed
.
- Ö. Acet, D. Erdönmez, B. Ö. Acet and M. Odabaşı, Arch. Microbiol., 2021, 203, 3739–3749 CrossRef PubMed
.
- C. Grandclément, M. Tannières, S. Moréra, Y. Dessaux and D. Faure, FEMS Microbiol. Rev., 2015, 40, 86–116 CrossRef PubMed
.
- S. P. Diggle, S. A. Crusz and M. Cámara, Curr. Biol., 2007, 17, R907–R910 CrossRef CAS PubMed
.
- B. Neil, G. L. Cheney, J. A. Rosenzweig, J. Sha and A. K. Chopra, Appl. Microbiol. Biotechnol., 2024, 108, 205 CrossRef CAS PubMed
.
- Y. Qu, Y. Zou, G. Wang, Y. Zhang and Q. Yu, ACS Appl. Mater. Interfaces, 2024, 16, 13353–13383 CrossRef CAS PubMed
.
- C. E. McInnis and H. E. Blackwell, Bioorg. Med. Chem., 2011, 19, 4820–4828 CrossRef CAS PubMed
.
- T. Das, S. Sabir, R. Chen, J. Farrell, F. H. Kriel, G. S. Whiteley, T. O. Glasbey, J. Manos, M. D. P. Willcox and N. Kumar, Molecules, 2022, 27, 1169 CrossRef CAS PubMed
.
- C. M. Al-Matarneh, A. Nicolescu, I. C. Marinas, M. C. Chifiriuc, S. Shova, M. Silion and M. Pinteala, Future Med. Chem., 2023, 15, 1369–1391 CrossRef CAS PubMed
.
- W. R. J. D. Galloway, J. T. Hodgkinson, S. D. Bowden, M. Welch and D. R. Spring, Chem. Rev., 2011, 111, 28–67 CrossRef CAS PubMed
.
- T. J. Polaske, C. G. Gahan, K. E. Nyffeler, D. M. Lynn and H. E. Blackwell, Cell Chem. Biol., 2022, 29(605–614), e604 Search PubMed
.
- E. J. Murray, R. C. Crowley, A. Truman, S. R. Clarke, J. A. Cottam, G. P. Jadhav, V. R. Steele, P. O’Shea, C. Lindholm, A. Cockayne, S. R. Chhabra, W. C. Chan and P. Williams, J. Med. Chem., 2014, 57, 2813–2819 CrossRef CAS PubMed
.
- S. Sabir, T. T. Yu, R. Kuppusamy, B. Almohaywi, G. Iskander, T. Das, M. D. P. Willcox, D. S. Black and N. Kumar, Antibiotics, 2021, 10, 321 CrossRef CAS PubMed
.
- J. Liu, Q.-X. Chen, W.-F. Wu, D. Wang, S.-Y. Zhao, J.-H. Li, Y.-Q. Chang, S.-G. Zeng, J.-Y. Hu, Y.-J. Li, J.-X. Du, S.-M. Jiao, H.-C. Xiao, Q. Zhang, J. Xu, J.-F. Zhao, H.-B. Zhou, Y.-H. Wang, J. Zou and P.-H. Sun, Eur. J. Med. Chem., 2024, 263, 115972 CrossRef CAS PubMed
.
- A. Taunk, R. Chen, G. Iskander, K. K. K. Ho, B. Almohaywi, D. S. Black, M. D. P. Willcox and N. Kumar, Molecules, 2019, 24, 2676 CrossRef CAS PubMed
.
- M. Salwiczek, Y. Qu, J. Gardiner, R. A. Strugnell, T. Lithgow, K. M. McLean and H. Thissen, Trends Biotechnol., 2014, 32, 82–90 CrossRef CAS PubMed
.
- A. C. Pinho and A. P. Piedade, Polymers, 2020, 12, 2469 CrossRef CAS PubMed
.
- Y.-T. Hung, L. A. McLandsborough, J. M. Goddard and L. J. Bastarrachea, LWT, 2018, 97, 546–554 CrossRef CAS
.
- A. Jain, L. S. Duvvuri, S. Farah, N. Beyth, A. J. Domb and W. Khan, Adv. Healthcare Mater., 2014, 3, 1969–1985 CrossRef CAS PubMed
.
- S. Gupta, Y. M. Puttaiahgowda, A. Nagaraja and M. D. Jalageri, Polym. Adv. Technol., 2021, 32, 4642–4662 CrossRef CAS
.
- C.-G. Wang, N. E. B. Surat'man, J. J. Q. Mah, C. Qu and Z. Li, J. Mater. Chem. B, 2022, 10, 9349–9368 RSC
.
- Y. Fadil, L. N. M. Dinh, M. O. Y. Yap, R. P. Kuchel, Y. Yao, T. Omura, U. A. Aregueta-Robles, N. Song, S. Huang, F. Jasinski, S. C. Thickett, H. Minami, V. Agarwal and P. B. Zetterlund, ACS Appl. Mater. Interfaces, 2019, 11, 48450–48458 CrossRef CAS PubMed
.
- N. Maslekar, R. A. Mat Noor, R. P. Kuchel, Y. Yao, P. B. Zetterlund and V. Agarwal, Nanoscale Adv., 2020, 2, 4702–4712 RSC
.
- B. N. Tran, S. C. Thickett, V. Agarwal and P. B. Zetterlund, ACS Appl. Polym. Mater., 2021, 3, 5145–5154 CrossRef CAS
.
- V. Agarwal, Y. Fadil, A. Wan, N. Maslekar, B. N. Tran, R. A. Mat Noor, S. Bhattacharyya, J. Biazik, S. Lim and P. B. Zetterlund, ACS Appl. Mater. Interfaces, 2021, 13, 18338–18347 CrossRef CAS PubMed
.
- J. M. Asua, Prog. Polym. Sci., 2002, 27, 1283–1346 CrossRef CAS
.
- Y. Fadil, S. C. Thickett, V. Agarwal and P. B. Zetterlund, Prog. Polym. Sci., 2022, 125, 101476 CrossRef CAS
.
- L. N. M. Dinh, B. N. Tran, V. Agarwal and P. B. Zetterlund, ACS Appl. Polym. Mater., 2022, 4, 1867–1877 CrossRef CAS
.
- N. Maslekar, Y. Fadil, P. B. Zetterlund and V. Agarwal, ACS Appl. Nano Mater., 2023, 6, 5177–5186 CrossRef CAS
.
- T. G. Fox, Bull. Am. Phys. Soc., 1956, 1, 123 CAS
.
- F. Perreault, A. F. de Faria, S. Nejati and M. Elimelech, ACS Nano, 2015, 9, 7226–7236 CrossRef CAS PubMed
.
- V. Agarwal and P. B. Zetterlund, Chem. Eng. J., 2021, 405, 127018 CrossRef CAS
.
- A. Radhi, D. Mohamad, F. S. Abdul Rahman, A. M. Abdullah and H. Hasan, J. Mater. Res. Technol., 2021, 11, 1290–1307 CrossRef CAS
.
- M. G. Saborio, N. Maslekar, Y. Yao, P. B. Zetterlund and V. Agarwal, ACS Appl. Nano Mater., 2023, 6, 2413–2420 CrossRef CAS
.
- B. N. Tran, S. Bhattacharyya, Y. Yao, V. Agarwal and P. B. Zetterlund, ACS Appl. Nano Mater., 2021, 4, 12461–12471 CrossRef CAS
.
- M. G. Saborio, K. Privat, B. N. Tran, P. B. Zetterlund, V. Agarwal and F. Estrany, ACS Appl. Nano Mater., 2022, 5, 3686–3700 CrossRef CAS
.
- L. N. M. Dinh, L. N. Ramana, R. P. Kuchel, V. Agarwal and P. B. Zetterlund, Polym. Chem., 2020, 11, 5790–5799 RSC
.
- F. Gao, X. Wu, D. Wu, J. Yu, J. Yao, Q. Qi, Z. Cao, Q. Cui and Y. Mi, Colloids Surf., A, 2020, 587, 124363 CrossRef CAS
.
- S. M. J. Merritt, A. M. Wemyss, S. Farris, S. Patole, G. Patias, D. M. Haddleton, B. Shollock and C. Wan, ACS Appl. Polym. Mater., 2020, 2, 725–731 CrossRef CAS
.
- S. Gungordu Er, M. Edirisinghe and T. A. Tabish, Adv. Healthcare Mater., 2023, 12, 2201523 CrossRef CAS PubMed
.
- K. K. K. Ho, N. Cole, R. Chen, M. D. P. Willcox, S. A. Rice and N. Kumar, Antimicrob. Agents Chemother., 2012, 56, 1138–1141 CrossRef CAS PubMed
.
- S. Sabir, D. Suresh, S. Subramoni, T. Das, M. Bhadbhade, D. S. Black, S. A. Rice and N. Kumar, Bioorg. Med. Chem., 2021, 31, 115967 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb01026d |
| This journal is © The Royal Society of Chemistry 2024 |