Open Access Article
Open Access ArticleProton-conducting copper-based MOFs for fuel cells
Byong June
Kim
 a,
Sun Ho
Park
a,
Sun Ho
Park
 a,
Mariana L.
Díaz-Ramírez
a,
Mariana L.
Díaz-Ramírez
 ab and
Nak Cheon
Jeong
ab and
Nak Cheon
Jeong
 *ab
*ab
aDepartment of Physics & Chemistry, DGIST, Daegu 42988, Korea. E-mail: nc@dgist.ac.kr
bCenter for Basic Science, DGIST, Daegu 42988, Korea
First published on 27th January 2025
Abstract
Metal–organic frameworks (MOFs) are emerging as promising alternatives for proton-conductive materials due to their high porosity, large surface area, stability, and relatively low cost. Among these, copper-based MOFs (Cu-MOFs) stand out with unique advantages, including open metal sites, variable valence states, and strongly electrophilic Cu centers. In this review, we discuss recent advances and developments in the use of Cu-MOFs as proton-conductive materials, with a particular focus on their application as proton exchange membranes (PEMs). We introduce the most common strategies employed to date and review the key features that have contributed to the construction of efficient proton transport pathways in Cu-MOFs. Additionally, we review PEMs fabricated via direct thin-film deposition or as mixed-matrix membranes (MMMs) incorporating Cu-MOF fillers. Finally, we address the challenges that must be overcome in the coming years to develop more robust Cu-MOFs and to create more efficient thin films and Cu-MOF-based MMMs.
1. Introduction
Fuel cells have garnered attention as advantageous energy conversion technologies due to their ability to convert chemical energy into electrical energy efficiently. Compared to combustion engines, fuel cells produce lower or even zero emissions of regulated pollutants. Consequently, they are considered for applications across diverse fields, including transportation,1,2 distributed energy generation systems,3–5 and portable electronic devices.6,7 Among the fuel cell technologies currently developing, those utilizing solid-phase electrolyte membranes show the most promise. Proton-exchange membrane fuel cells (PEMFCs), which use H2, methane, or methanol as fuel, are particularly prevalent due to their production of water vapor as the only byproduct. Direct methanol fuel cells (DMFCs), a subset of PEMFCs specifically using methanol as fuel, are especially suited for portable devices.8,9The core of these fuel cell technologies is the proton-exchange membrane (PEM), placed between two porous carbon-based electrodes that contain metal nanoparticles, such as platinum, which serve as catalysts for electrochemical reactions (see Fig. 1(a)). The primary function of the PEM is to facilitate proton transport from the anode to the cathode while electrons travel through an external circuit to the cathode.10,11
Two crucial properties of the PEM are paramount: (i) its ability to transport protonic species such as H+, H3O+, and NH4+, which dictates proton conductivity (σ), depending largely on the concentration and mobility of proton carriers, and (ii) the activation energy (Ea) required for proton transport, which depends on the transport pathway. Depending on the magnitude of the activation energy, the proton transport mechanism can be classified into two categories: (i) the vehicle model, in which proton carriers move like vehicular entities with higher activation energy (>0.5 eV), and (ii) the Grotthuss model, characterized by lower activation energy (≤0.5 eV), where protons hop through hydrogen bonds (H-bonds).12–14 The Grotthuss transport, generally conducted by H-bonds between the water molecules, is preferable since the low activation energy correlates with efficient proton conduction at low temperatures. Therefore, incremental water content in the PEM under low humidity conditions is one of the primary considerations for enhancing the performance of proton conductivity by forming H-bonds.
Among commercially available PEM materials, Nafion—a copolymer comprised of a hydrophobic backbone of perfluoroethylene with randomly distributed hydrophilic sulfonic acid groups—exhibits high proton conductivity (0.1 S cm−1) at low temperatures.15 Recently, several polymers, such as perfluorosulfonic acid (PFSA),16 sulfonated polyphenylene (SPP),17 and poly(2,3,5,6-tetrafluorostyrene-4-phosphonic acid),18 with improved proton conductivity, mechanical strength, and temperature resistance have newly been developed. However, Nafion-based PEMs suffer from limitations such as excessive swelling and water loss at high temperatures, high production costs, and low durability, which have hindered their broader application in PEMFCs.19,20 As a result, substantial research efforts have been directed toward exploring novel proton-conductive materials with superior proton transport capabilities.
Crystalline porous materials have attracted significant interest as promising candidates for proton-conducting applications. Metal–organic frameworks (MOFs), composed of metal ions or metal-oxo clusters coordinated with organic linkers, have emerged as particularly noteworthy materials for PEMs due to their ability to be precisely engineered to meet specific criteria for pore size and the chemical environment by selecting suitable metal ions21,22 and organic linkers with appropriate lengths, bulkiness,23 and functional groups.24,25 Their substantial void spaces facilitate the incorporation of proton-conducting media such as water,26 non-volatile acids,27 and protic organic molecules or ions,28,29 thereby enhancing conductivity. The high crystallinity of MOFs, characterized by long-range order, theoretically offers uninterrupted pathways for proton transport, which is crucial for understanding and modeling proton conduction mechanisms within porous materials.30,31 Historically, after first reported by Kanda et al. in 1979,32 the MOF proton conductivity has begun to be intensively studied with copper dithiooxamidate.33 Then, the research has been broadly expanded to other MOFs comprised of various metal ions, such as zinc,34 chromium,35 cerium,36 and iron,37 giving rise to much progress on the topic. Nevertheless, we focus this review on the copper-based metal–organic frameworks (Cu-MOFs) due to Cu's natural abundance (60 ppm in Earth's crust),38 its cost-efficiency, and Cu-MOFs’ specific physicochemical features such as redox activity, high specific surface area, and, in certain instances, the potential presence of open metal sites.39–42 These characteristics, combined with their structural diversity, have positioned Cu-MOFs as promising materials to use as PEMs in fuel cells.
Pioneering studies on Cu-containing proton-conductive coordination polymers, assembled with disubstituted dithiooxamide linkers, demonstrated proton conductivities ranging from 10−6 to 10−4 S cm−1 at 27 °C under water vapor-saturated air conditions.43–45 In the study, it was postulated that an H-bonds network between free water molecules, nitrogen/sulfur atoms, and –OH groups within the MOF framework facilitated efficient proton conduction. Although these initial studies did not provide a comprehensive understanding of the proton transport mechanism, significant advancements have been made in the field of Cu-MOFs as proton conductors: diverse functionalities have been incorporated to enhance proton transport capacity, highly stable frameworks have been engineered, and insights into conduction mechanisms have been partially elucidated.
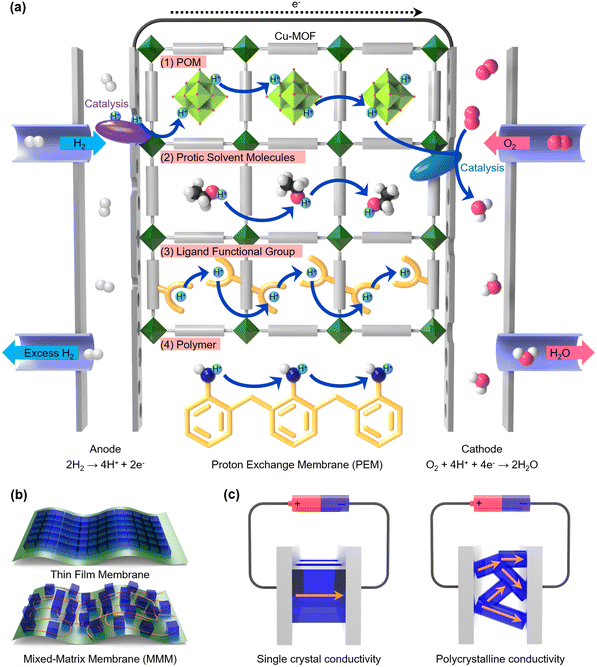
This highlight aims to delineate the progress in leveraging Cu-MOFs as proton conductors for fuel cells, structured into two main sections. The first section focuses on single-crystalline and poly-crystalline Cu-MOFs and their efficacy as proton-conducting materials. This section is subdivided into four subsections based on the modifications made to enhance hydrophilicity and facilitate proton transport: (1) the incorporation of polyoxometalates (POMs) into the pores, (2) the inclusion of H-bond-forming guest molecules such as protic solvent molecules, (3) functionalization of organic linkers capable of forming H-bonds or containing acid groups, and (4) incorporating polymers with acidic functional groups, such as sulfonate poly(ether ether ketone) (see Fig. 1(a)).
While MOFs have shown potential as proton exchange membranes, their micron-scale sizes and brittleness present challenges for their industrial application. The second main section introduces the MOF fabrication methods with two strategies to overcome these challenges and thereby enhance the properties of fuel cell membranes (see Fig. 1(b)). (1) The first strategy involves growing Cu-MOFs as thin films on a membrane, which arranges proton conduction pathways and reduces grain boundaries—key obstacles to proton mobility. (2) The second strategy involves fabricating composite materials with proton-conductive polymers as mixed-matrix membranes (MMMs), providing proton transport pathways between MOF crystals through the polymer matrix. Each method relates to two different approaches for measuring proton conductivity: single-crystalline conductivity and polycrystalline conductivity (see Fig. 1(c)). Single-crystalline conductivity provides an intrinsic property measured with an individual crystal, reflecting its inherent ability to conduct protons. By contrast, polycrystalline conductivity is measured after packing the crystals into a pellet, which differs from single-crystalline conductivity. Thus, distinguishing the conductivity based on the measurement method is crucial for evaluating their suitability as fuel cell membranes. The final section offers an overview of the findings and presents ideas and recommendations for advancing the development and understanding of these promising materials.
2. Proton conductivity in single- and poly-crystalline copper MOF
2.1. Polyoxometalate-incorporated MOFs
Polyoxometalates (POMs) are self-assembled polyatomic clusters containing transition metal oxo-anions with well-defined connectivity. Due to their oxygen-enriched surfaces, POMs can serve as relay centers in proton conduction, and their strong Brønsted acidity allows them to provide abundant protons readily.46–48 However, the water solubility and structural instability of POMs hinder their direct use in proton-exchange membrane fuel cells (PEMFCs). Therefore, strategies that exploit their advantageous properties while improving their stability are required, such as immobilizing POMs in porous materials or constructing more stable frameworks using them.One approach to enhance the stability of POMs is to use organic linkers that covalently or coordinatively bond with MOFs, serving as connecting agents instead of metal clusters. For example, using a Cu(II)-Schiff-base cation and a 1D chain [Cu(DMF)4(SiW12O40)]n2n− to form a 3-dimensional network with hydrophilic cavities (see Fig. 2(a)).49 This material exhibited moderate conductivity, reaching 5.94 × 10−4 S cm−1 at 100 °C under 98% RH, primarily due to constrained intergrain proton transfer. A previous study which exploits different strategies reported a Cu-MOF with polyoxomolybdate connecting nodes (Cu3Mo5P2), where water molecules coordinated to the Cu center, forming a one-dimensional channel with a conductivity of 2.2 × 10−5 S cm−1 at 28 °C under 98% RH.50 However, the conductivity decreased above 42 °C as water molecules were dislocated from the 1-D channel. Later, Keggin anions [PM12O40]3− (M = Mo or W) were employed as bidentate linkers to assemble Cu-MOFs based on 2,2′-bipyridyl-3,3′-dicarboxylic acid (H2bpdc), specifically [H{Cu(Hbpdc)(H2O)2}2(PM12O40)·nH2O]n, as reported by Duan and his group.51 The resulting one-dimensional hydrophilic channels, lined with water molecules H-bonded to oxygen atoms of the polyanion or the H2bpdc linkers, provided suitable pathways for proton conduction (Fig. 2(b)). The maximum conductivity for these materials, measured at 100 °C under 98% RH, was 1.25 × 10−3 S cm−1 for [H{Cu(Hbpdc)(H2O)2}2(PMo12O40)·nH2O]n and 1.56 × 10−3 S cm−1 for [H{Cu(Hbpdc)(H2O)2}2(PW12O40)·nH2O]n, although the loss of water molecules above 100 °C caused a drop in conductivity of up to six orders of magnitude at 150 °C.
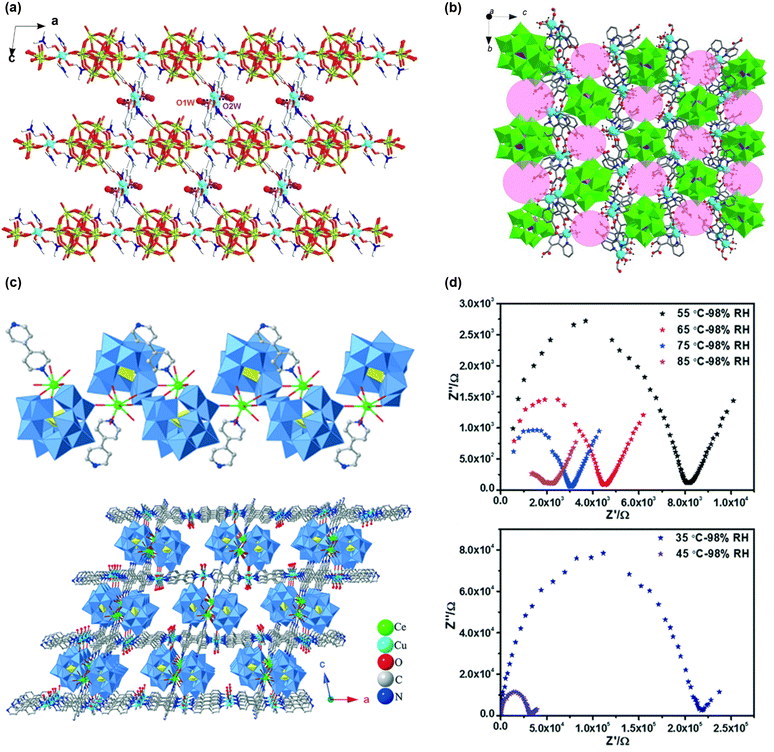 | ||
| Fig. 2 (a) View of the 2D organic–inorganic composite layer with hydrophilic cavities from the “ABAB” packing arrangement of the metal-Schiff-base cations and 1D organic–inorganic anionic chains along the ac plane. Cu, O, Si, N and W are represented as cyanine, red, green, blue and yellow, respectively. Reproduced with permission from ref. 49, Copyright 2014, Wiley, VCH. (b) View of the 3D [{Cu(Hbpdc)(H2O)2}2{PM12O40}]n− unit with 1D the hydrophilic channels (pink circles) along the a axis. Reproduced with permission from ref. 51, Copyright 2013, Wiley, VCH. (c) The structure of the 1D chain in 1; and the view of the 3D framework structure in 1. Polyhedral codes: WO6, blue; PO4, yellow. (d) Nyquist plots for 1 at different temperatures at 98% RH; reproduced with permission from ref. 52, Copyright 2021, Royal Society of Chemistry. | ||
POMs as connecting agents for copper–pyridine complexes have also been investigated. Zhang and coworkers developed two POM-based frameworks: H2[Cu2OL3(H2O)2][Ce(L)(H2O)3(PW11O39)]·17H2O and H4[CuL3]2[Ln(H2O)3(PW11O39)]2·28H2O (L = 4,4′-bipyridine).52 These frameworks connect [Ln(PW11O39)]4− with copper–bipyridine complexes, forming a 3D structure by stacking 2D layers (Fig. 2(c)). The proton conductivity increased from 3.255 × 10−6 S cm−1 to 3.175 × 10−4 S cm−1 as temperatures increased (Fig. 2(d)), with proton transport activation energy calculated as 1.445 eV at lower temperatures and 0.456 eV at higher temperatures than 55 °C. Both regions exhibited higher activation energy than 0.4 eV, indicating a vehicle-type proton transport mechanism, as the authors claimed. PXRD patterns demonstrated that the framework retained its structure even after exposure to humid conditions, supporting the notion that incorporating POM enhances stability.
Incorporating POMs as guest entities into MOF pores is another viable strategy to improve stability. Wei and colleagues reported the preparation of a proton-conducting MOF, [{Cu4(dpdo)12}{H(H2O)27(CH3CN)12}{PW12O40}3]n (with dpdo = 4,4′-bipyridine-N,N′-dioxide), where [PW12O40]3− was hosted in the MOF cavities along with a large number of water molecules.53 The material achieved a maximum conductivity of 1.25 × 10−4 S cm−1 at 100 °C under 98% RH, with an endothermic dissociation process of H+(H2O)27 clusters yielding an activation energy of 0.82 eV. Lun and coworkers synthesized four polynuclear coordination polymers with POM coordination into the pores, one of which was a copper-based MOF with trinuclear Cu3(OH) subunits (Fig. 3(a) and (b)).54 This Cu-MOF exhibited the highest proton conductivity with the value of 3.05 × 10−5 S cm−1 at 25 °C and 98% RH, with high thermal (retaining its structure up to 300 °C) and hydrolytic stability. By the way, increasing the temperature to 85 °C resulted in an increased proton conductivity with the value of 2.57 × 10−4 S cm−1, and the subsequent incorporation of Nafion into the Cu-MOF under the same conditions further increased the conductivity up to 1.28 × 10−2 S cm−1, implying that the synergetic interaction between MOFs and Nafion allows the formation of reconfigurable hydrogen bonds, which enhance proton transfer.
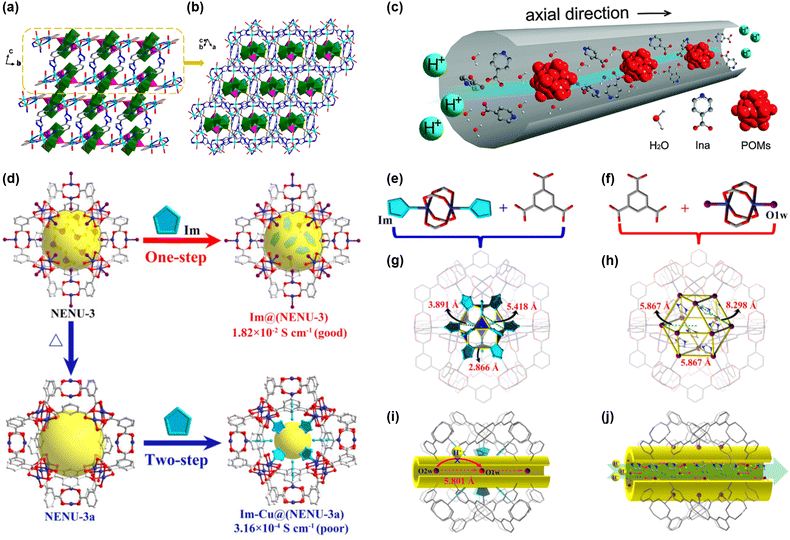 | ||
| Fig. 3 Summary of the structure of NENU-530: (a) ball-and-stick representation of the packing arrangement of staggered 2D sheet-like structure in NENU-530. (b) The 3D framework of NENU-530. Reproduced with permission from ref. 54, Copyright 2016, Wiley, VCH. (c) Schematic of the proton-conducting pathway constructed by POMs, Ina and water molecules arranged alternately in the nano-channels. Reproduced with permission from ref. 55, Copyright 2014, Royal Society of Chemistry. (d) Comparison of the one- and two-step synthesis of the proton conductors Im@(NENU-3) and Im-Cu@(NENU-3a), and their corresponding proton conductivities. BTC ligand and the paddle-wheel Cu2 units in Im-Cu@(NENU-3b) (e) and NENU-3 (f). The cuboctahedral cage B in Im-Cu@(NENU-3b) (g) and NENU-3 (h) are fabricated by the BTC ligands and the Cu2 units, and with the different pore spaces and window sizes with much more free imidazole molecules inside the pore spaces of NENU-3. (i) Speculative pathway of the proton conduction for Im-Cu@(NENU-3a) based on single crystal X-ray data showing the actual positions of the absorbed water molecules within the structure. (j) Schematic view of the possible proton-conductive pathways in Im@(NENU-3). Water molecules are shown in violet or red. Red arc arrows show the protons hop along hydrogen-bonding networks. Red dashed arrows represent transport of protons through self-diffusion of protonated water. (Guest molecules and H atoms are omitted for clarity.) Reproduced with permission from ref. 56, Copyright 2017, American Chemical Society. | ||
First envisioned as heterogeneous catalysts for hydrolysis of esters, Liu and colleagues evaluated NENU-3 (HKUST-1 loaded with phosphotungstic acid) as a proton-conducting material.55 The introduction of H3PW12O40 improved the retention of water molecules in the pores of HKUST-1, even at 90 °C. Consequently, proton transfer was facilitated, and the activation energy was reduced to 0.41 eV in NENU-3 (vs. 0.69 eV in HKUST-1), and conductivity increased to 4.76 × 10−5 S cm−1 at 90 °C under 70% RH (refer to the fact that the conductivity of pristine HKUST-1 was 1.08 × 10−8 S cm−1 under same conditions). Inspired by these results, authors sought to further improve the conductivity of NENU-3 by exchanging Cu-coordinated water molecules with isonicotinic acid (Ina), which could act both as a proton donor and H-bonding acceptor, as pictured in Fig. 3(c). Proton conductivity in NENU-3-Ina reached 1.81 × 10−3 S cm−1 at 90 °C under 70% RH, and its Ea was 0.36 eV. Later, when instead of Ina, imidazole (Im) was loaded in NENU-3 to give Im@NENU-3, conductivity improved by an order of magnitude 1.82 × 10−2 S cm−1 at 90 °C under 70% RH, which could be maintained up to 12 h, as investigated by Ye and coauthors.56 Notably, they found that the strategy followed for loading the Im had a huge effect on the conductivity of the resulting material, as schematized in Fig. 3(d). When Im was loaded in a pristine NENU-3, with water molecules coordinated to the Cu center to obtain Im@NENU-3, it resulted in a high concentration of free imidazole molecules in the pores. Thus, the proton conductivity was two orders of magnitude higher than that of Im-loaded activated NENU-3 (Im-Cu@NENU-3a), where Im was coordinated to Cu centers and in a lesser amount than Im@NENU-3. This result manifested that forming an extended H-bond network (promoted by a high concentration of free imidazole and water molecules, as illustrated in Fig. 3(e)–(j)) was indispensable to reach good proton conductivities. Although the conductivity of Im@NENU-3 was higher than that of previously reported NENU-3-Ina, the activation energy of Im@NENU-3 was slightly higher (0.57 eV).
The inclusion of POMs in Cu-MOFs has resulted in moderate enhancement of proton conductivity, as summarized in Table 1. No significant differences in structure and proton-conducting properties were observed between these compounds, implying that metal in the POM is irrelevant to its proton-conduction pathway. However, its moisture retention ability is enhanced by its high hydrophilicity and water capture ability, and it conducts enhanced proton conductivity in relatively low humid conditions and high temperatures. Notably, both compounds retained water molecules up to 100 °C; however, activation energy was exceptionally high (1.02 eV) among the POM-Cu-MOFs reviewed. The insertion of POMs in Cu3(BTC)2 (BTC = benzentricarboxylate; also referred to as HKUST-1) to produce NENU-3 improved its proton conductivity; however, the best values were obtained for NENU-3 loaded with additional H-bond-forming entities like isonicotinic acid (Ina) or proton carriers like imidazole (Im). Of the eight materials, only Im@NENU-3 was evaluated for long-term proton conductivity (12 hours), successfully demonstrating its structural stability by using PXRD measurements.
| Material | σ (S cm−1) | E a (eV) | Temperature (°C) | % RH | Ref. |
|---|---|---|---|---|---|
| a L = N,N′-bis[(2-hydroxy-3-methoxyphenyl)methylidene]hydrazine hydrate. | |||||
| [{Cu3(L)2(H2O)4}{Cu(dmf)4(SiW12O40)}·9H2O]na | 5.94 × 10−4 | 0.32 | 100 | 98 | 49 |
| Cu3Mo5P2 | 2.2 × 10−5 | 0.23 | 28 | 98 | 50 |
| [H{Cu(Hbpdc)(H2O)2}2(PMo12O40)·nH2O]n | 1.25 × 10−3 | 1.02 | 100 | 98 | 51 |
| [H{Cu(Hbpdc)(H2O)2}2(PW12O40)·nH2O]n | 1.56 × 10−3 | 1.02 | 100 | 98 | 51 |
| [{Cu4(dpdo)12}{H(H2O)27(CH3CN)12}(PW12O40)3]n | 1.25 × 10−4 | 0.82 | 100 | 98 | 53 |
| NENU-3 | 4.76 × 10−5 | 0.41 | 90 | 70 | 55 |
| NENU-3-Ina | 1.81 × 10−3 | 0.36 | 90 | 70 | 55 |
| HKUST-1 | 1.08 × 10−8 | 0.69 | 90 | 70 | 55 |
| Im@NENU-3 | 1.82 × 10−2 | 0.57 | 90 | 70 | 56 |
2.2. Incorporating protic guest molecules
Encapsulation of guest molecules was one of the strategies explored to study and enhance proton conductivity in MOFs. Our group reported a conceptual example that proton conductivity could be tuned by changing the coordinating solvent molecules at Cu(II) centers in HKUST-1.57 While protic solvent molecules residing in the pores of HKUST-1 typically form a simple H-bond network without proton conduction, coordinating water molecules at Cu centers trigger forming the H-bond network to transfer protons and thereby transform it into a conductive one. Although water is generally a poor proton donor, its acidity increases when coordinated to a transition metal center, enabling it to donate protons to pore-filling protic solvent molecules such as methanol (MeOH), as shown in Fig. 4(a) and (b). Consequently, the conductivity of H2O-loaded HKUST-1 (H2O-HK) reached 1.5 × 10−5 S cm−1 at room temperature under a MeOH atmosphere. Moreover, the acidity of the coordinated molecules plays a decisive role in proton conduction: low-acidic solvent molecules (MeOH or EtOH) do not provide sufficient protons to impact conductivity significantly (Fig. 4(c)).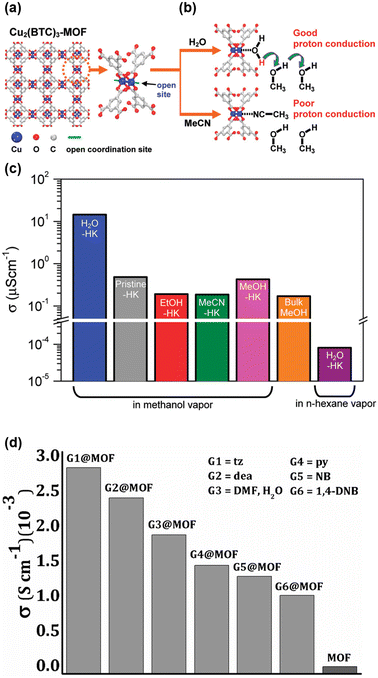 | ||
| Fig. 4 (a) Illustrations of the HKUST-1 structure in a two-dimensional view along the (100) direction. (b) HKUST-1 nodes with the Cu-paddlewheel environment and qualitative representations of proton transfer from Cu(iI) centers coordinated with water and ethanol. (c) Proton conductivities of H2O-HK, pristine-HK, EtOH-HK, MeCN-HK, MeOH-HK, and bulk MeOH under a MeOH or n-hexane atmosphere as indicated. Reproduced with permission from ref. 57, Copyright 2012, American Chemical Society. (d) Bar diagram representation of the proton conductivities of the activated complex [1] and its guest-incorporated adducts. Reproduced with permission from ref. 58, Copyright 2016, Wiley, VCH. | ||
Building on this concept, Khatua and coworkers explored the effect of various guest molecules in the pores of MOFs. They employed a Cu(I)-MOF with a V-shaped flexible terpyridine-based linker [{(C44H48O9Cu2I2)n}⊃(DMF)(MeCN)] and loaded different molecules such as H2O, MeOH, dimethyl sulfoxide (DMSO), N,N′-dimethylformamide (DMF), diethylamine (DEA), nitrobenzene (NB), 1,4-dinitrobenzene (DNB), pyridine (py), and 1H-1,2,4-triazole (tz).58 Under anhydrous conditions, the conductivity was negligible without guest molecules. However, conductivity improved significantly under humid conditions (95% RH), exceeding 10−3 S cm−1 for several combinations, including [{(C44H48O9Cu2I2)n}⊃μ(H2O)(DMF)], [{(C44H48O9Cu2I2)n}⊃NB], [{(C44H48O9Cu2I2)n}⊃DNB], [{(C44H48O9Cu2I2)n}⊃py], and [{(C44H48O9Cu2I2)n}⊃tz]. The highest values were observed for [{(C44H48O9Cu2I2)n}⊃(H2O)(DMF)] (1.89 × 10−3 S cm−1 at 65 °C) and [{(C44H48O9Cu2I2)n}⊃tz] (2.89 × 10−3 S cm−1 at 80 °C) (Fig. 4(d)).
The correlation between proton conductivity and the species of solvent molecules highlights the importance of H-bonds, particularly involving water molecules, in achieving high conductivity. Zhang and colleagues proposed that a MOF with linkers containing a high proportion of oxygen (O) and nitrogen (N) atoms could retain adsorbed water molecules even at reduced RH, thereby maintaining good conductivity.59 The authors prepared an ionic Cu-based MOF using 4-carboxypyrazole (Cpz) as a linker, specifically NH4[Cu3(OH)(C4H2N2O2)3] (hereafter CuCpz), an anionic framework built from Cu3(μ3-OH) bridges connected by Cpz linkers (Fig. 5(a)–(c)). This framework could absorb up to 385 water molecules per unit cell at 25 °C due to the presence of hydrophilic atoms (Cu(II), O, and N) and counter cations (NH4+). Remarkably, during desorption, water contents remained at 385 water molecules per unit cell until the relative humidity dropped to 40%. The conductivity of CuCpz reached 2.45 × 10−3 S cm−1 at 100% RH, and it only decreased to 1.26 × 10−3 S cm−1 when RH was lowered to 43%. Notably, to achieve 1.26 × 10−3 S cm−1 at 43% RH, CuCpz needed to be saturated with water before reducing the RH. When the RH was directly set to 40% from a dry state, conductivity was significantly lower at only 1 × 10−6 S cm−1, indicating that the abundance of hydrophilic atoms created a robust H-bond network that helped retain water molecules as humidity levels decreased (Fig. 5(d)).
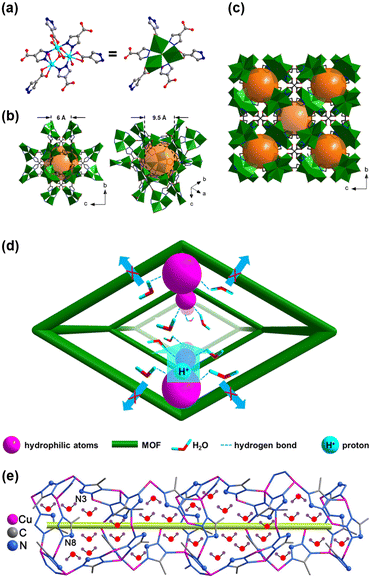 | ||
| Fig. 5 Schematic diagrams of CuCpz. (a) Cu3(μ3-OH) clusters in CuCpz; (b) accessible window apertures of the cage structure; and (c) unit cell. All NH4+ ions in the pore and the hydrogen atoms of the ligands are omitted for clarification. The Cu, O, N, and C are shown in cyan, red, blue, and gray, respectively. (d) Proposed holding effect scheme of the hydrophilic atoms in the CuCPz pores for enhancing the proton conductivity at reduced RH. Reproduced with permission from ref. 59, Copyright 2022, American Chemical Society. (e) Tunnel in the 3D anionic [Cu3(mtz)4]− framework of 1 with schematic guest water molecules. Red and purple balls present O and H atoms, respectively. The green, yellow bar highlights the proton pathway. Reproduced with permission from ref. 64, Copyright 2021, American Chemical Society. | ||
Due to the small size of guest molecules relative to the large pore space, substantial disorder among the filling molecules was observed, making it challenging to establish clear correlations between conductivity and interactions among guest molecules, the host framework, and proton carriers. To improve the molecular ordering within the framework, the addition of functional groups to the linker that can form strong H-bonds with water molecules in the pores is a viable approach. For example, Gil-Hernández and colleagues utilized a mesoxalate linker to build two anionic Cu-MOFs [(H3O){Cu7(Hmesox)5(H2O)7}·9H2O]n and [(NH4)0.6(H3O)0.4{Cu7(Hmesox)5(H2O)7}·11H2O]n (mesox = mesoxalate), which contains carboxylate and hydroxyl groups to stabilize water molecules.60 In these frameworks, conductivity arose from cations (H3O+ and NH4+) located in the pore space alongside crystallized water molecules. Despite the presence of different cations, the conductivity did not significantly differ between these materials: both showed nearly identical proton conduction behavior, reaching maximum conductivity at 23 °C under 100% RH (6.5 × 10−5 S cm−1) and decreasing by three orders of magnitude after heating to 85 °C, in which conditions water loss occurred, disrupting the H-bond network.
In a subsequent study, Gil-Hernández and coworkers investigated the impact of different cations on the proton-conducting properties of three new mesoxalate-based Cu-MOFs: (H3O)[Cu9(Hmesox)6(H2O)6Cl]·8H2O, (NH2Me2)0.4(H3O)0.6[Cu9(Hmesox)6(H2O)6Cl]·8H2O, and (enH2)0.25(enH)1.5[Cu6(Hmesox)3(mesox)(H2O)6Cl0.5]Cl0.5·5.25H2O (NH2Me2 = dimethylammonium, enH2, enH = ethylenediammonium).61 Unlike their previously reported materials, the conductivity of all three new MOFs increased as the temperature increased up to 80 °C, showing the highest conductivity (1.87 × 10−3 S cm−1) at 80 °C under 95% RH for (enH2)0.25(enH)1.5[Cu6(Hmesox)3(mesox)(H2O)6Cl0.5]Cl0.5·5.25H2O. This improvement in conductivity was attributed to three main factors: (1) a greater number of coordinated water molecules compared to the other two compounds; (2) stronger interactions between crystallized water molecules and the framework, which assists in retaining water molecules at higher temperatures; and (3) in (enH2)0.25(enH)1.5[Cu6(Hmesox)3(mesox)(H2O)6Cl0.5]Cl0.5·5.25H2O, the mesoxalate was fully deprotonated, supplying an extra proton carrier to maintain charge neutrality and thereby, resulting in proton carriers three times more than in (H3O)[Cu9(Hmesox)6(H2O)6Cl]·8H2O and (NH2Me2)0.4(H3O)0.6[Cu9(Hmesox)6(H2O)6Cl]·8H2O. These findings highlight the importance of both the number and nature of proton carriers in determining conductivity.
Improving the concentration and ordering proton carriers further enhances conductivity.66,67 One effective approach involves incorporating biomolecules, such as amino acids, peptides, or polysaccharides, into MOFs. Grancha and coworkers reported an MOF, [Ca(II)Cu(II)6{(S,S)-alamox}3(OH)2(H2O)]·32H2O, using an oxamidato linker {(S,S)-alamox} driven from natural amino acids.62 This material featured honeycomb-like hexagonal channels filled with H-bonded water molecules forming a 1D ribbon array. The highly ordered water molecules enabled modest conductivity (8.6 × 10−4 S cm−1 at 80 °C under 95% RH). Based on the crystal structure and theoretical calculations, the authors proposed a Grotthuss-type proton-hopping mechanism involving the cleavage and formation of covalent O–H bonds, followed by a reorganization of solvent molecules within the pores.
Another example comes from Li and colleagues, who synthesized a 2D homochiral MOF based on L-hydroxyproline: [Cu2(Htzehp)2(4,4′-bipy)]·3H2O (Htzehp = N-[2-(1H-tetrazol-5-yl)ethyl]-L-hydroxyproline).63 This MOF achieved anisotropic conductivity of 1.39 × 10−4 S cm−1 at 30 °C under 95% RH along the [100] direction, compared to the conductivity two orders of magnitude lower in the [010] direction under the same conditions. Activation energies along the [100] and [010] directions were 0.48 eV and 0.56 eV, respectively, suggesting different proton transport mechanisms: a Grotthuss-type mechanism in the [100] direction, while a vehicle mechanism in the [010] direction.
Tetrazoles, known for forming H-bonds due to their nitrogen-rich structure, also demonstrate potential as MOF linkers. Lu and colleagues synthesized an ultra-stable NaCu3(mtz)4 (mtz = 5-methyltetrazolate), which exhibited good thermal and chemical stability.64 The material demonstrated outstanding proton conductivity (1.33 × 10−2 S cm−1) at 70 °C under 100% RH, with an Ea of 0.34 eV, indicative of Grotthuss-type proton conduction (Fig. 5(e)).
Introducing ion pairs within MOF cavities to form clathrates has also proven effective. You and coauthors demonstrated the enhanced proton conductivity of benchmark HKUST-1 by incorporating NH4Br (NH4Br@HKUST-1).65 Proton conductivity significantly increased under high relative humidity (99% RH) up to 8.99 × 10−4 S cm−1, while pristine HKUST-1 alone remained low in conductivity (10−8 S cm−1). Though NH4Br@HKUST-1 displayed a higher activation energy than HKUST-1 (1.42 eV vs. 0.69 eV), the primary transport mechanism remained the vehicle type in both materials. These results demonstrate that the insertion of additional guest molecules capable of improving proton transport pathways can yield high conductivity (∼10−3 S cm−1) in some cases, as seen in [{(C44H48O9Cu2I2)n}⊃(H2O)(DMF)] and [{(C44H48O9Cu2I2)n}⊃tz] (Table 2).
| Material | σ (S cm−1) | E a (eV) | Temperature (°C) | % RH | Ref. |
|---|---|---|---|---|---|
| H2O-HK | 1.5 × 10−5 | NR | 25 | — | 57 |
| MeCN-HK | 2.0 × 10−7 | NR | 25 | — | 57 |
| EtOH-HK | 2.0 × 10−7 | NR | 25 | — | 57 |
| [{(C44H48O9Cu2I2)n}⊃deal] | 3.77 × 10−7 | 0.79 | 50 | 0 | 58 |
| [{(C44H48O9Cu2I2)n}⊃(H2O)(DMF)] | 1.89 × 10−3 | 1.10 | 65 | 95 | 58 |
| [{(C44H48O9Cu2I2)n}⊃tz] | 2.89 × 10−3 | 1.55 | 80 | 95 | 58 |
| CuCpz | 1.80 × 10−2 | 0.33 | 80 | 100 | 59 |
| [(NH4)0.6(H3O)0.4{Cu7(Hmesox)5(H2O)7}·11H2O]n | 6.5 × 10−5 | NR | 23 | 100 | 60 |
| [(H3O){Cu7(Hmesox)5(H2O)7}·9H2O]n | 6.5 × 10−5 | NR | 23 | 100 | 60 |
| (H3O)[Cu9(Hmesox)6(H2O)6Cl]·8H2O | 1.16 × 10−4 | 0.43 to 0.5 | 80 | 95 | 61 |
| (NH2Me2)0.4(H3O)0.6[Cu9(Hmesox)6(H2O)6Cl]·8H2O | 1.85 × 10−4 | 0.43 to 0.5 | 80 | 95 | 61 |
| (enH2)0.25(enH)1.5[Cu6(Hmesox)3(mesox)(H2O)6Cl0.5]Cl0.5·5.25H2O | 1.87 × 10−3 | 0.43 to 0.5 | 80 | 95 | 61 |
| [CaIICuII6{(S,S)-alamox}3(OH)2(H2O)]·32H2O | 8.6 × 10−4 | 0.34 | 80 | 95 | 62 |
| [Cu2(Htzehp)2(4,4′-bipy)]·3H2O | 1.39 × 10−4 | 0.48 | 30 | 95 | 63 |
| NaCu3(mtz)4 | 1.33 × 10−2 | 0.34 | 70 | 100 | 64 |
| NH4Br@HKUST-1 | 8.99 × 10−4 | 0.68 | 25 | 99 | 65 |
| HKUST-1 | 1.04 × 10−8 | 1.42 | 25 | 99 | 65 |
2.3. Ligand functionalization
Developing proton-conducting materials that perform well under low humidity conditions is essential to ensure reliable PEM performance across diverse applications. This aim can be achieved by creating H-bonds pathways using fewer guest molecules through ligand functionalization. This section discusses two primary strategies: exploiting ligands with (1) H-bonding capabilities and (2) highly acidic properties.The first strategy, which uses H-bond-forming ligands, typically employs pyridine or pyrazole-based ligands that contain nitrogen atoms for forming H-bonding. Ruan and coauthors reported [Cu(atz)2(H2O)2]·H2O (Hatz = 5-aminotetrazole) MOFs, where the atz ligand forms a two-dimensional framework by connecting two Cu(II) ions (Fig. 6(a)).68 The atz ligand can act as both a proton donor and acceptor, allowing water molecules to create H-bond pathways that serve as proton conduction channels (Fig. 6(b)). Similarly, Tayade and colleagues designed MOFs with bipyridine glycoluril, with N–H bonds acting as H-bond donors and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds acting as H-bond acceptors.69 This MOF forms H-bonds when water molecules coordinate with the MOF, creating aligned H-bond networks that act as proton conduction pathways (Fig. 6(c)).
O bonds acting as H-bond acceptors.69 This MOF forms H-bonds when water molecules coordinate with the MOF, creating aligned H-bond networks that act as proton conduction pathways (Fig. 6(c)).
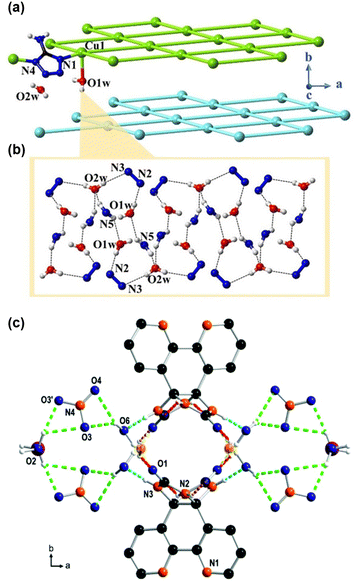 | ||
| Fig. 6 (a) The stacking sql networks for complex 1, among that atz− ligands can be considered as sticks. (b) The coordinated water molecules O1w and free water molecules O2w are linked into 1D hydrogen transport channel by hydrogen bonds with atz− ligands. Reproduced with permission from ref. 68, Copyright 2021, Wiley, VCH. (c) Ligand NH⋯O (red) ligand donor–acceptor bonds are responsible for the formation of channels along [001]. In addition ligand NH⋯O water (bluish), water OH⋯O nitrate (green) and water OH⋯O ligand (yellow) hydrogen donor–acceptor bonds are observed (color code: N = orange, O = blue and Cu = purple). Reproduced with permission from ref. 69, Copyright 2017, Royal Society of Chemistry. | ||
Multifunctional organic linkers, where highly electronegative atoms such as nitrogen, oxygen, or sulfur are rich, can provide a suitable pore environment for good proton transport. For example, Li's group developed a 2D layered Cu-MOF, [Cu(p-IPhHIDC)]n, using an imidazole-based linker (p-IPhH3IDC = 2-(p-N-imidazol-1-yl)phenyl-1H-imidazole-4,5-dicarboxylic acid).70 Notably, some carboxylic acid groups protonated within the framework resulted in a proton conductivity of 1.51 × 10−3 S cm−1 at 100 °C under 98% RH due to H-bond networks with interlayer water molecules. In contrast, a similar Cu-MOF ([Cu4(HDMPhIDC)4(H2O)4]n), where H3DMPhIDC is 2-(3,4-dimethyl)phenyl-4,5-imidazole dicarboxylic acid, showed a conductivity of 2.58 × 10−5 S cm−1 at the same temperature and humidity.71 Although both [Cu(p-IPhHIDC)]n and [Cu4(HDMPhIDC)4(H2O)4]n contained uncoordinated carboxylic acid groups, the higher nitrogen content in [Cu(p-IPhHIDC)]n provided more proton hopping sites for higher proton conductivity. This result suggests that the number of N-atoms plays a crucial role in conductivity, even though differences in the overall structure are not considered.
Continuing with this approach, Li's group further studied a thiourea-based metal-mixed Cu(I/II)-MOF, featuring a complex H-bond system formed by interactions among O-, N-atoms from the linker, free water, methanol, and coordinated DMF molecules: [{Cu(I)3Cu(II)3L3(DMF)2(CH3OH)(H2O)}·3CH3OH]n, where L represents [3-(4-methyl-benzoyl)-thioureido]-acetic acid.72 This MOF, noted for its high thermal and chemical stability, exhibited a conductivity of 3.78 × 10−4 S cm−1 at 98% RH and 100 °C, which was one order of magnitude higher than the conductivity of the H-bonded organic framework formed solely by the linker, L. The improved conductivity was attributed to higher proton mobility facilitated by free solvent molecules, particularly water, within the channels of the Cu(I/II)-MOF.
The second strategy involves incorporating highly acidic functional groups, like sulfonic acid, into MOFs. Organic polymers containing sulfonic acid, such as Nafion, are known for their high proton conductivities (∼10−1 S cm−1) due to the strong Brønsted acidity of sulfonic acids and their ability to form extended H-bond networks.73–75 Inspired by such polymers, Cu-MOFs with sulfonic acid or sulfonate groups on their framework linkers have been extensively studied. For instance, disodium-2,2′-disulfonate-4,4′′-oxydibenzoic acid (Na2H2DSOA) formed a porous 3-dimensional MOF, Cu-DSOA, based on a tetrameric copper-cluster framework with H3O+ ions as charge-balancing cations (Fig. 7(a) and (b)).76 Proton conductivity was relatively low (10−6 S cm−1) at room temperature, even under high relative humidity (98% RH), but increased significantly, reaching 1.9 × 10−3 S cm−1 at 85 °C under the same humidity. The relatively high activation energy (1.04 eV) indicates that proton transfer likely occurs through the vehicle mechanism, suggesting that the long-range directional mobility of H3O+ ions improves as the temperature increases, and this behavior, along with the higher dielectric constant of water molecules at elevated temperatures, contributes to enhanced conductivity. Another example is Cu4(L)2(OH)2(DMF)2, where L represents 5-sulfoisophthalate.38 This MOF features a tetranuclear Cu cluster and irregular 1D channels lined with abundant H-bonds between sulfonate/carboxylate groups and DMF molecules (Fig. 7(c)–(f)).77 The highest conductivity recorded in this MOF was 7.4 × 10−4 S cm−1 at 95 °C under 95% RH.
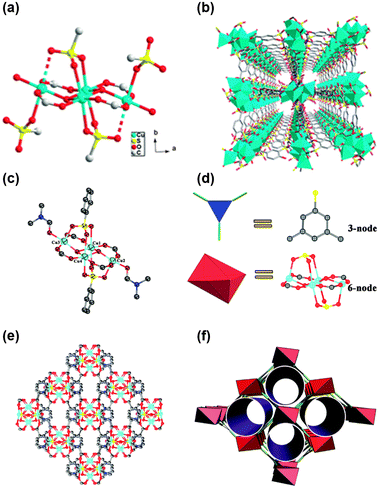 | ||
| Fig. 7 (a) A view of a tetrameric copper cluster. (b) Central projection of Cu-DSOA viewed down the c-axis. The solvent water molecules and hydrogen atoms are omitted for clarity. Reproduced with permission from ref. 76, Copyright 2013, Royal Society of Chemistry. (c) The asymmetric unit of 1; (d) the three-dimensional structure viewed along the b-axis; (e) ball-and-stick and polyhedral representations of the 3-connected L ligand and 6-connected [Cu4(OH)2(CO2)4(SO3)2] cluster, respectively; (f) the (3,6)-connected 3D non-interpenetrating network of 1. Reproduced with permission from ref. 77, Copyright 2015, Royal Society of Chemistry. | ||
Combining these two strategies by mixing different ligands—one containing H-bond-forming groups (like pyridine, pyrazole, or amine) and the other containing sulfonate groups—can also improve conductivity. For instance, the mixed-linker Cu-MOF [{Cu2(sba)2(bpg)2(H2O)3}·5H2O]n, where sba is 4-sulfobenzoate, and bpg is bipyridine glycoluril, exhibited one-dimensional chains of water molecules filled in hydrophilic channels (Fig. 8(a)).78 The maximum conductivity, 0.94 × 10−2 S cm−1 at 80 °C under 95% RH, was attributed to the presence of extensive networks of H-bonded water molecules interacting with the uncoordinated oxygens of sulfonate, carboxylate, and glycoluril groups in the wall of MOF channels.
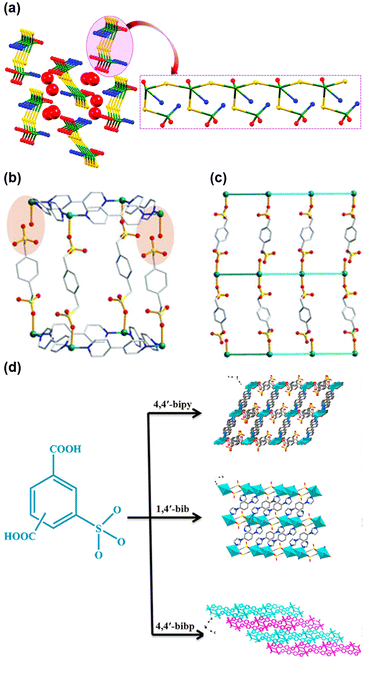 | ||
| Fig. 8 (a) Showing the node-and-linker-type representation of 1D chain in 1; the copper nodes are represented in green, sba linkers in yellow, BPG ligands in blue and water molecules are in red colour. Reproduced with permission from ref. 78, Copyright 2019, Royal Society of Chemistry. Crystallographic view of TMOF-2 (Cu, teal; C, gray; S, yellow; O, red; N, blue): (b) one single primitive-cubic net with the missing metal–ligand connectivities highlighted; (c) a single primitive extended framework viewed along the a axis in which 4,4′-bipy is simplified as a rod for clarity; reproduced with permission from ref. 79, Copyright 2017, Royal Society of Chemistry. (d) Three new Cu(II) coordination polymers, [Cu5(μ3-OH)4(STP)2(4,4′-bipy)2(H2O)2]·4H2O (CTGU-20), [Cu2(μ2-OH)(SIP)(1,4′-bib)2] (CTGU-21) and [Cu2(SIP)(4,4′-bibp)2(HCOO)]·3H2O (CTGU-22) and based on two less-developed isomeric sulfo-functionalized benzene dicarboxylic acid linkers were synthesized and characterized. Reproduced with permission from ref. 80, Copyright 2021, Elsevier. | ||
Although organosulfonates are known to create relatively fragile frameworks due to their weak coordination with metal cations, the incorporation of sulfonate groups into MOFs has been explored to improve conductivity. Notably, Zhang and coauthors reported the doubly interpenetrated and permanently porous TMOF-2, constructed using copper nitrate, 1,4-benzenedimethanesulfonate (1,4-BDMS), and 4,4′-bipyridine (4,4′-bipy).79 The TMOF-2 structure showed dangling sulfonate groups and open Cu(II) sites, forming a network of H-bonded water molecules upon adsorption and thereby creating effective pathways for proton transport (Fig. 8(b) and (c)). As a result, TMOF-2 achieved a conductivity of 1.23 × 10−4 S cm−1 with an Ea of 0.37 eV, consistent with a proton-hopping (Grotthuss) mechanism.
Moi and coauthors further explored using a mixture of two different ligands: 1,2,4-triazol-4-amine (T4A) as an H-bond-forming component and 1,5-naphthalenedisulfonic acid as an acidic group. Incorporating amine and sulfonates into a MOF created an appropriate distance between these functional groups, resulting in a high proton conductivity of 0.53 × 10−3 S cm−1 at 80 °C and 98% RH.81 Additionally, a number of oxygen atoms from sulfonate groups available to form H-bonds and guest water molecules influenced conductivity, as exemplified by Cu-MOFs such as CTGU-20, CTGU-21, and CTGU-22, reported by Yu and colleagues.80 While all three frameworks are of difference in structure as depicted in Fig. 8(d), CTGU-20 displayed the highest conductivity (2.86 × 10−4 S cm−1 under 98% RH), seemingly due to its completely uncoordinated sulfonate groups, which were closely spaced and allowed efficient water molecule retention.
Phosphonates have also been studied for their potential as building blocks for proton-conducting MOFs due to their uncoordinated acidic hydroxyl groups, which can form H-bond networks with guest water molecules. For instance, BAM-3, [Cu(H2PhDPA)(dpe)2(H2O)2·2H2O]n (with H2PhDPA = phenylene diphosphonate and dpe = 1,2-di(4-pyridyl)ethylene), reported by Rautenberg and coworkers in 2022, demonstrated modest conductivity of 1.4 × 10−5 S cm−1 at 50 °C under 98% RH, although it was suffered from dehydration and a phase transition above 50 °C, which negatively affected its performance.82
CuCpz and NaCu3(mtz)4, anionic MOFs based on N-rich linkers (Cpz = 4-carboxypyrazole and mtz = 5-methyltetrazolate, respectively) exhibited the best proton conductivities (>10−2 S cm−1). CuCpz, which displayed the highest conductivity at 80 °C under 100% RH among the materials discussed in this section, maintained conductivity for up to 120 hours without notable structural damage, even as relative humidity was lowered from 100% RH to 43% RH. This stability contrasts with many other materials, which typically experience an abrupt decrease in conductivity when humidity levels drop. In the case of NaCu3(mtz)4, its proton conductivity was elevated and remained stable for up to 8 hours, although it required high humidity to achieve these results. Despite numerous uncoordinated nitrogen atoms available to form H-bonds and facilitate efficient proton hopping, the loss of water molecules at temperatures above 70 °C limited its working conditions.
Among the sulfonic acid/sulfonate group-containing Cu-MOFs reviewed (summarized in Table 3), two frameworks showed high proton conductivity (>10−3 S cm−1): the mixed-linker [{Cu2(sba)2(bpg)2(H2O)3}·5H2O]n (sba = 4-sulfobenzoate, bpg = bipyridine glycoluril) and tetranuclear copper-cluster Cu-DSOA (DSOA = 2,2′-disulfonate-4,4′′-oxydibenzoic acid). Both frameworks contained a high number of water molecules per asymmetric unit, indicating strong water dependence for proton conductivity. Furthermore, high proton conductivity was maintained for over 12 hours in the mixed-linker MOF under high temperatures and humidity conditions, with its structural stability confirmed by PXRD. Conversely, Cu-DSOA partially lost crystallinity after standard impedance measurements, revealing less structural stability.
| Material | σ (S cm−1) | E a (eV) | Temperature (°C) | % RH | Ref. |
|---|---|---|---|---|---|
| a L = 5-sulfoisophthalate. | |||||
| Cu-DSOA | 1.9 × 10−3 | 1.04 | 85 | 98 | 76 |
| Cu4(L)2(OH)2(DMF)2a | 7.4 × 10−4 | 1.32 | 95 | 95 | 77 |
| [{Cu2(sba)2(bpg)2(H2O)3}·5H2O]n | 0.94 × 10−2 | 0.64 | 80 | 95 | 78 |
| TMOF-2 | 1.23 × 10−4 | 0.37 | 90 | 98 | 79 |
| CTGU-20 | 2.86 × 10−4 | NR | 50 | 98 | 80 |
| CTGU-21 | 1.58 × 10−5 | NR | 50 | 98 | 80 |
| CTGU-22 | 4.58 × 10−5 | NR | 50 | 98 | 80 |
2.4. Composites with acid-functionalized polymers
While highly ordered structures provide a deep understanding of the underlying mechanisms of proton conduction, they have limitations in the performance of proton conductivity when incorporated into practical applications. Grain boundaries in MOF microcrystallites and MOF-polymer mixed matrices can disrupt proton conduction channels, thus diminishing the material's potential conductivity. By contrast, the incorporation of a polymer with acidic groups can help bridge these grain boundaries and enhance proton conductivity.Mahimai and colleagues explored Cu-MOF-polymer composites using 2,6-naphthalene dicarboxylic acid as an organic linker.83 They used polystyrene as the composite material but added a sulfonate group to the polymer to increase acidity and, thereby, proton conductivity (Fig. 9(a)). This composite material achieved a maximum conductivity of 1.65 × 10−2 S cm−1. Additionally, they further enhanced proton conductivity to 3.1 × 10−2 S cm−1 by casting the MOF with additional ligands, specifically 1,2-dimethyl-3-propylimidazolium iodide. This study demonstrates the importance of selecting appropriate composite materials to improve proton conductivity.
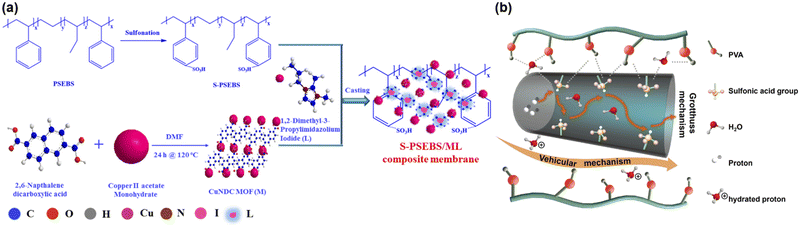 | ||
| Fig. 9 (a) Schematic representation for preparation of S-PSEBS/ML composite membrane. Reproduced with permission from ref. 83, Copyright 2022, American Chemical Society. (b) The possible proton transport pathways of CL-PVA/Cu-MOF-15. Reproduced with permission from ref. 84, Copyright 2023, Elsevier. | ||
Another strategy involves incorporating the polymer directly into the MOF pores to enable direct interactions between the MOF's organic linkers and the polymer. Li and coauthors synthesized a Cu-MOF with a sulfonate-imidazolium ion pair inside the MOF pores using 1-(1-ethyl-3-imidazolio)-propane-3-sulfonate (MIMS).84 In addition, they incorporated poly(vinyl alcohol) (PVA), which contains hydroxyl groups that can facilitate proton conduction (Fig. 9(b)). This strategy achieved a maximum conductivity of 2.3 × 10−3 S cm−1, nearly double that of the commercial Nafion-115 (1.25 × 10−3 S cm−1). Furthermore, the activation energy for proton transport was reduced from 0.96 eV to 0.42 eV, indicating a combined Grotthuss and vehicle mechanism for proton transport.
Additionally, disrupting the long-range order without fully transforming the material into an amorphous phase can enhance proton conductivity. Disordered structures, such as metal–organic gel (MOG) materials, present fascinating alternatives due to their numerous defective sites and solvent-absorbing networks, which can potentially improve conductivity.85,86 Tang and colleagues investigated this approach by reducing the crystallinity of HKUST-1 and, thus, converting it into a MOG-type HKUST-1, which resulted in a mesoporous, low-crystallinity network with deficient N2 adsorption capacity.87 At 80 °C under 75% RH, the resulting proton conductivities were 7.2 × 10−5 and 1.15 × 10−3 S cm−1 for HKUST-1 and MOG-HKUST-1, respectively, showing that a less ordered three-dimensional network provided an easier proton transfer pathway. However, MOG-type HKUST-1 was unstable and lost its conductivity after only three days.
3. MOF fabrications
In the studies, the conductivity of Cu-MOFs was primarily assessed in the form of compacted pellets of microcrystalline material. However, this method does not adequately represent the actual working conditions of proton-exchange membrane fuel cells (PEMFCs). It restricts a proper evaluation of the performance of Cu-MOFs as components of PEMs. Typically obtained as microcrystalline powders, Cu-MOFs face challenges such as limited proton transfer over long distances due to grain boundaries, and their brittle nature prevents them from forming films independently, which limits their application as solid-state electrolytes in fuel cells.To be effectively integrated into PEMs, Cu-MOFs should be able to produce a uniform and durable film or be well-dispersed in a membrane. Different strategies have been developed to address this challenge, such as depositing Cu-MOFs as thin films on a conductive substrate or integrating Cu-MOFs into membranes to create mixed-matrix membranes. Relevant studies concerning these strategies are discussed in the following sections.
3.1. Thin films
In 2014, Qi and coauthors achieved the in situ growth of a micron-thick (∼0.15 μm) dense HKUST-1 film on a copper foil under mild conditions.88 The resulting film exhibited a conductivity of 0.69 × 10−3 S cm−1 after exposal for three days at 98% RH, which was an order of magnitude higher than the microcrystalline HKUST-1 reported by Jeong in 2012.57 However, the activation energy for this HKUST-1 microfilm was not reported.Given the promising proton conduction performance of NENU-3, a derivative of HKUST-1 encapsulating the polyoxometalate [PW12O40]3−, several studies have explored the electrochemical thin film fabrication of this material with controlled crystallite size and thickness.89,90 Zhang and collaborators investigated the proton conductivity of a 12-μm-thick NENU-3 film, finding that its conductivity was negligible as-synthesized but increased substantially to 2.9 × 10−5 S cm−1 at 40 °C under 97% RH after humidification for 3 days. For comparison, an HKUST-1 film grown using the same methodology exhibited a maximum conductivity of 1.8 × 10−6 S cm−1 at 35 °C under 97% RH. The activation energy for the NENU-3 film was estimated to be 0.38 eV, significantly lower than the activation energy of 0.61 eV for the pristine HKUST-1, suggesting that the POM clusters are critical proton carriers whose solvation depends on temperature.
Smart materials responding to external stimuli such as light are highly desirable for various applications, including remote control of fundamental material properties.91 Two notable studies focused on controlling the reversible conductivity of composite materials through light irradiation. The first involved azobenzene derivatives that can switch trans-to-cis isomerization under UV light and cis-to-trans back-isomerization with thermal treatment. In 2018, Müller and coauthors grew a film of Cu2(F2AzoBDC)2(dabco) (F2AzoBDC = (E)-2-((2,6-difluorophenyl)diazenyl)terephthalate, dabco = 1,4-diazabicyclo[2.2.2]octane) on a quartz substrate, subsequently loading it with 1,4-butanediol or 1,2,3-triazole (Fig. 10(a)).92 When the film was virtually empty, it exhibited negligible proton conduction in either the cis- or trans-form at 25 °C. However, when loaded with guest molecules, such as 1,4-butanediol or 1,2,3-triazole, conductivity was much enhanced in the trans-form, reaching up to 1.24 × 10−4 S cm−1 for 1,2,3-triazole@Cu2(F2AzoBDC)2(dabco). Conductivity could be switched between 7.9 × 10−5 S cm−1 (cis) and 1.24 × 10−4 S cm−1 (trans) by irradiating green light (530 nm) and violet (400 nm), respectively (Fig. 10(b)).
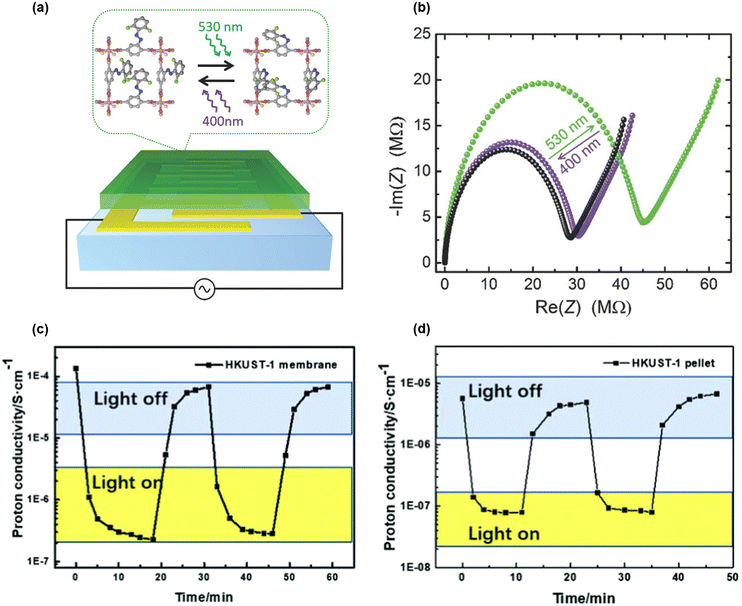 | ||
| Fig. 10 (a) Schematization of surface mounted Cu2(F2AzoBDC)2(dabco) film: Cu2(F2AzoBDC)2(dabco) is the green layer on the interdigitated gold electrodes (yellow layer) on quartz substrate (light blue layer). Cu2(F2AzoBDC)2(dabco) structure viewed along the [001] direction. Black lines show the connection to the electric circuit and the impedance spectrometer. Representation of photoswitching between cis- and trans-fluorinated azobenzene side groups in Cu2(F2AzoBDC)2(dabco) when irradiated by green light (530 nm from trans to cis) and with violet light (400 nm from cis to trans). (b) Nyquist plot of the impedance of triazole@Cu2(F2AzoBDC)2(dabco). The black data is measured in the pristine sample (trans), green after irradiation with green light (cis), and violet after irradiation with violet light (trans). Reproduced with permission from ref. 92, Copyright 2018, Wiley, VCH. (c) and (d) Proton conductivity changes of the HKUST-1 (c) membrane and (d) pellet with and without light at 55 °C under 95% RH. Reproduced with permission from ref. 93, Copyright 2021, Royal Society of Chemistry. | ||
In another study, Wang and coauthors prepared an HKUST-1 membrane using a solid confined conversion process.93 Due to HKUST-1's intrinsic photothermal properties, the authors evaluated the membrane's conductivity in the dark at 55 °C under 95% RH (1.35 × 10−4 S cm−1), which significantly dropped to 2.27 × 10−7 S cm−1 under a Xe lamp at 0.5 sun intensity. Remarkably, conductivity could be restored to its initial value by returning the membrane to darkness (Fig. 10(c)). This behavior was attributed to the loss of pore-filling water molecules due to local heating effects from the photothermal properties of HKUST-1 when exposed to light. Furthermore, the membrane showed better proton conductivity than pelletized microcrystalline HKUST-1 under similar conditions (Fig. 10(d)).
Pelletized powder materials generally exhibit lower conductivity compared to the thin films reported in these studies, as summarized in Table 4. Among them, the HKUST-1 microfilm reported by Qi and colleagues achieved the highest conductivity (10−4 S cm−1). This comparison highlights the significant influence of the deposition method on the films’ conductivities. For example, the 0.15 μm-thick HKUST-1 thin film produced by using a mother solution deposition technique demonstrated ∼383 times higher conductivity compared to a (10 times thicker) film synthesized via a solid confined conversion process and showed 5 times higher conductivity than one (0.12 μm thick) created using an electrochemical method. However, to achieve the maximum conductivity of 10−4 S cm−1, the HKUST-1 microfilm required three days of exposure to humid conditions (98% RH at 25 °C) to ensure adequate water retention within its pores. Furthermore, although these thin films are characterized as dense, individual crystallite grains are still distinguishable, suggesting that grain boundaries may substantially hinder proton conductivity. Therefore, developing more uniform Cu-MOF thin films is essential to enhance the conductivity further.
3.2. Mixed-matrix membranes
Among Cu-based MOFs, HKUST-1 is considered a benchmark material for studying proton conductivity due to its paddlewheel-like dimeric Cu units linked by trimesic acid (benzene-1,3,5-tricarboxylate) anions. Also, the ease of its synthesis on a gram scale from inexpensive chemicals has made it the most studied Cu-MOF as a filler for mixed-matrix membranes. In an early study, Kim and colleagues incorporated various amounts of HKUST-1 into Nafion to enhance its conductivity.94 The membranes were prepared using a solution casting method, and acids were fed into them by soaking them in a 50 wt% H3PO4 solution. While water uptake by the membranes decreased with increasing HKUST-1 content, the maximum acid uptake occurred with the 2.5 wt% content of HKUST-1. The acid-doped 2.5 wt% HKUST-1/Nafion membrane exhibited the highest conductivity, 1.8 × 10−2 S cm−1 at 25 °C under 100% RH.More recently, Li and collaborators investigated the proton-conducting properties of two Cu12S6-cluster-based MOFs incorporated into Nafion.95 The respective conductivities were found to be 3.63 × 10−5 and 2.75 × 10−5 S cm−1 for [Cu12(MES)6(H2O)3]n and ([Cu12(MPS)6(H2O)4]·6H2O)n (H2MES = 2-mercaptoethanesulfonate acid, H2MPS = 2-mercaptopropanesulfonate acid) at 60 °C under 98% RH, with activation energies of 0.12 eV and 0.22 eV, respectively. The differences in proton conductivities were attributed to structural variations between the MOFs: [Cu12(MES)6(H2O)3]n contained one additional partially coordinated μ2-SO3 group per asymmetric unit, making it more acidic than [Cu12(MPS)6(H2O)4]. Moreover, although [Cu12(MES)6(H2O)3]n had fewer water molecules per copper unit, these molecules were firmly coordinated to the Cu centers, forming a more ordered H-bond network, thereby facilitating easier proton transport.
Nafion is known for its high methanol permeability, which can adversely affect DMFC performance.96,97 Alternative sulfonated polymers, such as SPEEK, have been investigated to mitigate methanol crossover. However, even SPEEK-based composites have exhibited low stability and conductivity at elevated temperatures.98,99 Consequently, HKUST-1 has also been studied as a filler in SPEEK-based MMMs. In a 2018 study, Niluroutu and coworkers dispersed HKUST-1 varying its amount (1, 2, 3, and 5 wt%) into SPEEK to form composite membranes, each approximately 170 μm thick, denoted as SP/CT-MOF-n, where n represents the Cu-MOF contents (wt%) in the membrane.100 Proton incorporation was achieved by loading H2SO4 groups into the prepared membranes. The composite with 3 wt% HKUST-1 (SP/CT-MOF-3) exhibited the highest conductivity (4.5 × 10−2 S cm−1) at 70 °C under 98% RH and the lowest activation energy (0.08 eV) among the prepared membranes. The carboxylate groups in HKUST-1 contributed to better connectivity for the ionic channels of SPEEK by forming H-bonds with the sulfonic groups of SPEEK, as illustrated in Fig. 11(a). The inclusion of 3 wt% HKUST-1 reduced methanol permeability to nearly half (4.26 × 10−7 cm2 s−1) compared to pristine SPEEK (7.95 × 10−7 cm2 s−1) due to the strong interaction of methanol molecules with HKUST-1, while the sulfonic groups in the SPEEK facilitated hydronium ion transport. The electrochemical selectivity, defined as the ratio between conductivity and methanol permeability, was estimated for the prepared composite materials, with SP/CT-MOF-3 showing the highest value (∼1.1 × 105 S cm−3 s).
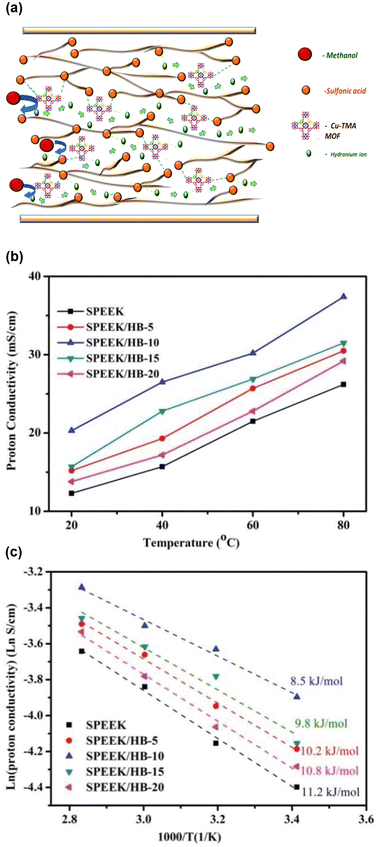 | ||
| Fig. 11 (a) Illustration of HKUST-1 in sPEEK matrix, SP/CT-MOF-3, depicting hydrogen bond network, proton transport pathways and low methanol permeability. Reproduced with permission from ref. 100, Copyright 2018, Royal Society of Chemistry. (b) Proton conductivity and (c) Arrhenius plots of pristine SPEEK and HKUST-1/BPO4 in sPEEK matrix. Reproduced with permission from ref. 101, Copyright 2021, Wiley VCH. | ||
Further advancing this approach, Hu and collaborators prepared a hybrid material by combining HKUST-1 and boron phosphate (BPO4), an inorganic compound known for its ability to retain water molecules up to 300 °C owing to the strong H-bonds formed between water molecules and boron or phosphorus.101 The resulting HKUST-1/BPO4 hybrid materials (HB) were dispersed in SPEEK membranes for DMFC applications. The amount of HKUST-1 in the SPEEK was kept constant, while the BPO4 content 5, 10, 15, 20 wt% relative to HKUST-1, named as HB-5, HB-10, HB-15, HB-20, respectively. Increasing BPO4 content improved methanol permeability, oxidative stability, and mechanical strength while reducing area by polymeric swelling compared to pristine SPEEK. All composite membranes showed higher conductivity than pristine SPEEK, with the highest conductivity (3.74 × 10−2 S cm−1) at 80 °C under 100% RH and the lowest activation energy (0.09 eV) reported for the sample containing 10 wt% BPO4. Further increased BPO4 content made the proton transport path more tortuous, resulting in lower conductivity and higher activation energy, as depicted in Fig. 11(b) and (c).
Biopolymer-based PEMs have gained attention for their high availability, non-toxicity, and abundance of active functional groups.102–104 Chitosan, in particular, has been highlighted for DMFC applications due to its rigid structure, crystallinity, and hydrophilicity, attributed to its amine, hydroxyl, and ether functional groups.105,106 Divya and coauthors fabricated sulfonated chitosan membranes incorporating HKUST-1 as a filler in varying amounts (0.25, 0.5, or 1 wt% relative to chitosan).107 The 0.5 wt% HKUST-1 composite, designated as S-Chitosan-0.5, showed the best conductivity (6.19 × 10−3 S cm−1) at 80 °C, lower methanol permeability (3.01 × 10−8 cm2 s−1), and higher membrane selectivity (1.78 × 105 S cm−3 s). Although the maximum conductivity achieved in this study was lower than that reported for SP/CT-MOF-3, the membrane selectivity of S-chitosan-0.5 was higher.
Poly(vinylpyrrolidone) (PVP) and poly(vinylidene fluoride) (PVDF) blends have been explored as alternatives for high-temperature PEMFCs.108–110 Moi and coworkers prepared PVP/PVDF membranes incorporating different amounts of Cu-SAT, [{Cu2(μ2-OH)2(NDS)(T4A)2}·2DMF]n (H2NDS = 1,5-naphthalenedisulfonic acid, T4A = 1,2,4-triazol-4-amine), a MOF containing sulfonate and amine groups.111 They found that the maximum amount of Cu-SAT effectively integrated into the membrane was 60 wt% (designated as MMM-4). Among the prepared membranes, MMM-4 demonstrated a 52% increase in conductivity (8 × 10−4 S cm−1) at 80 °C under 98% RH and a 20% reduction in activation energy (0.187 eV) compared to the pristine Cu-SAT. They found that the time-dependent conductivity of MMM-4 remained nearly unchanged even for five days, indicating its potential for practical applications. In a subsequent study conducted in 2022, Bao and collaborators developed PVP/PVDF composite membranes with an increased loading of up to 50 wt% of [Cu(BTTA)H2O]n·6nH2O (BTTA = 2,5-bis(1,2,4-triazol-1-yl)terephthalate).112 The membrane containing 50 wt% MOF (Cu-MOF@PP-50) exhibited the highest conductivity and lowest activation energy among the prepared membranes, achieving 4.36 × 10−4 S cm−1 at 80 °C under 98% RH with an activation energy of 0.09 eV. This result represents a two-order magnitude increase in conductivity compared to pristine [Cu(BTTA)H2O]n·6nH2O and an 11-fold increase compared to the bare PVP/PVDF membrane. While PVP/PVDF composite membranes have been investigated as candidates for high-temperature PEMFCs (intended to operate between 120 °C and 200 °C), they have only been tested up to 80 °C. Therefore, evaluating these materials at higher temperatures is crucial to verify their suitability for such applications. Moreover, compared to MMMs made from other polymers, the proton conductivity of PVP/PVDF composite membranes is at least one order of magnitude lower, even with a lower MOF content (vice versa, a higher polymer content). Given their high chemical stability, proton activation via soaking in oxo-acid solutions could enhance performance, but this or other enhancement strategies remain unexplored.
Among the MMMs discussed, those based on Nafion and SPEEK with HKUST-1 fillers exhibited the highest conductivity (∼10−2 S cm−1), as summarized in Table 5. However, only the SPEEK-based MMMs were tested at high temperatures (SP/CT-MOF-3 and SPEEK/HB-10, at 70 °C and 80 °C, respectively). Notably, SP/CT-MOF-3 evaluated under open circuit voltage conditions (where methanol crossover was maximized) for 50 hours showed better operational stability than pristine SPEEK. In the case of SPEEK/HB-10, its stability was evaluated and then found to be 17% higher than that of pristine SPEEK (up to 332 minutes). Furthermore, its proton conductivity was maintained for over 72 hours.
| Material | Polymer | σ (S cm−1) | E a (eV) | Temperature (°C) | % RH | Ref. |
|---|---|---|---|---|---|---|
| 2.5 wt% HKUST-1/Nafion | Nafion | 1.8 × 10−2 | NR | 25 | 100 | 94 |
| [Cu12(MES)6(H2O)3]n/Nafion | Nafion | 3.63 × 10−5 | 0.12 | 60 | 98 | 95 |
| [{Cu12(MPS)6(H2O)4}·6H2O]n/Nafion | Nafion | 2.75 × 10−5 | 0.22 | 60 | 98 | 95 |
| SP/CT-MOF-3 | SPEEK | 4.5 × 10−2 | 0.08 | 70 | 98 | 100 |
| SPEEK/HB-10 | SPEEK | 3.74 × 10−2 | 0.09 | 80 | 100 | 101 |
| S-Chitosan-0.5 | Sulfonated chitosan | 6.19 × 10−3 | NA | 80 | NA | 107 |
| MMM-4 | PVP/PVDF | 8.0 × 10−4 | 0.19 | 80 | 98 | 111 |
| Cu-MOF@PP-50 | PVP/PVDF | 4.36 × 10−4 | 0.09 | 80 | 98 | 112 |
The MOF-filled MMMs showed modest conductivity levels but consistently exhibited low activation energies (0.22 eV), comparable to those of pristine SPEEK at 90% RH. These findings underscore the potential of Cu-MOFs for MMMs, presenting a promising opportunity to create composite materials with high proton conductivity.
4. Conclusions
This highlight introduces the proton conductivity of Cu-MOFs in different forms: single crystal, microcrystalline powders, thin films, and fillers in mixed-matrix membranes. For single-crystalline and microcrystalline Cu-MOFs, we highlighted diverse approaches explored by various research groups to design or enhance pathways for water-assisted proton transport within Cu-MOF channels. The predominant strategies involved utilizing linkers rich in hydrophilic atoms, such as oxygen, nitrogen, or sulfur; incorporating polyoxometalates (POMs) as integral building units or guests within the pores; and introducing guest molecules capable of forming H-bonds. The use of N-rich linkers, in particular, has led to highly proton-conductive materials, underlining the significance of stable, uninterrupted H-bond networks between the framework and guest molecules to support efficient proton hopping.Combining multiple strategies to improve proton conductivity, such as adding additional proton carriers (like imidazole) to an already POM-loaded MOF, resulted in a six-order magnitude increase in proton conductivity, even at relatively low humidity levels, making this approach particularly attractive for further exploration. We also interpret the role of guest molecules and proton carriers in different Cu-MOFs, emphasizing the importance of well-ordered structures to elucidate the relationships between structure and proton transport performance in Cu-MOFs.
Regarding Cu-MOF thin films, most research has focused on synthesizing HKUST-1 thin films, resulting in only moderate proton conductivities, mainly because HKUST-1 inherently exhibits low conductivity. Although further efforts should be made to explore other Cu-MOFs with high proton conductivity in their microcrystalline forms, developing optimized deposition techniques for each Cu-MOF is essential to enhance their performance as thin films.
Cu-MOFs in MMMs represent a relatively underexplored area, offering opportunities for significant advancements. So far, the most effective polymers for these composites have been Nafion and SPEEK, though they come with known limitations. Most studies have concentrated on proton conductivity and activation energy, often neglecting other essential evaluations. Comprehensive assessments are necessary, including not only long-term conductivity but also thermal, chemical, and mechanical stability (especially in response to swelling cycles). Research on optimizing the balance between membrane thickness, conductivity, and fuel crossover will pave the way for more efficient and robust proton-exchange membranes.
The future of Cu-MOFs in fuel cells is promising, particularly as advancements continue to refine their integration into PEMs and explore new methods to enhance their conductivity, stability, and overall performance. By leveraging their unique properties, Cu-MOFs could play a crucial role in developing next-generation energy conversion technologies.
Author contributions
B. J. K., S. H. P. and M. L. D.-R. wrote the original draft. B. J. K. visualized the work. M. L. D.-R. conceptualized the study. N. C. J. supervised all the papers.Data availability
No primary research results, software or code have been included, and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Ministry of Science and ICT (MSIT) of Korea under the auspices of the Basic Science Research Program sponsored by the National Research Foundation (RS-2023-NR077060) and by the DGIST R&D Program (25-HRHR+-01).Notes and references
- S. Chakraborty, D. Elangovan, K. Palaniswamy, A. Fly, D. Ravi, D. A. S. Seelan and T. K. R. Rajagopal, Energies, 2022, 15, 9520 CrossRef CAS.
- H. S. Das, C. W. Tan and A. H. M. Yatim, Renewable Sustainable Energy Rev., 2017, 76, 268–291 CrossRef.
- L. Venturelli, P. E. Santangelo and P. Tartarini, Appl. Therm. Eng., 2009, 29, 3469–3475 CrossRef CAS.
- F.-C. Wang, P.-C. Kuo and H.-J. Chen, Int. J. Hydrogen Energy, 2013, 38, 5845–5856 CrossRef CAS.
- K. Nikiforow, P. Koski, H. Karimäki, J. Ihonen and V. Alopaeus, Int. J. Hydrogen Energy, 2016, 41, 14952–14970 CrossRef CAS.
- I. Gatto, A. Saccà, R. Pedicini, E. Passalacqua and A. Carbone, Int. J. Hydrogen Energy, 2021, 46, 27687–27699 CrossRef CAS.
- Y. Shen, X. Yan, L. An, S. Shen, L. An and J. Zhang, Appl. Energy, 2022, 313, 118781 CrossRef CAS.
- S. Sun, M. Zhao, Q. Wang, S. Xue, Q. Huang, N. Yu and Y. Wu, Small, 2023, 2205835 CrossRef CAS PubMed.
- S. Zou, Y. Li, H. Jin, F. Ning, P. Xu, Q. Wen, S. Pan, X. Dan, W. Li and X. Zhou, Adv. Energy Mater., 2021, 12, 2103178 CrossRef.
- M. W. Ellis, M. R. Von Spakovsky and D. J. Nelson, Proc. IEEE, 2001, 89, 1808–1818 CrossRef CAS.
- J. Lindorfer, D. C. Rosenfeld and H. Böhm, Fuel Cells: Energy Conversion Technology, in Future Energy, ed. T. M. Letcher, Elsevier, Amsterdam, 3rd edn, 2020, ch. 23, pp. 495–517 Search PubMed.
- P. Colomban and A. Novak, Proton conductors: classification and conductivity, in Proton conductors: Solids, membranes and gels – materials and devices, ed. P. Colomban, Cambridge University Press, Cambridge, 1992, ch. 23, pp. 38–60 Search PubMed.
- J. A. Elliott and S. J. Paddison, Phys. Chem. Chem. Phys., 2007, 9, 2602–2618 RSC.
- G. A. Ludueña, T. D. Kühne and D. Sebastiani, Chem. Mater., 2011, 23, 1424–1429 CrossRef.
- L. Y. Zhu, Y. C. Li, J. Liu, J. He, L. Y. Wang and J. D. Lei, Pet. Sci., 2022, 19, 1371–1381 CrossRef CAS.
- Y. Oshiba, J. Tomatsu and T. Yamaguchi, J. Power Sources, 2018, 394, 67–73 CrossRef CAS.
- Z. Long and K. Miyatake, iScience, 2021, 24, 102962 CrossRef CAS PubMed.
- V. Atanasov, A. Oleynikov, J. Xia, S. Lyonnard and J. Kerres, J. Power Sources, 2017, 343, 364–372 CrossRef CAS.
- N. Sulaiman, M. A. Hannan, A. Mohamed, E. H. Majlan and W. R. Wan Daud, Renewable Sustainable Energy Rev., 2015, 52, 802–814 CrossRef.
- P. Zegers, J. Power Sources, 2006, 154, 497–502 CrossRef CAS.
- N. E. Wong, P. Ramaswamy, A. S. Lee, B. S. Gelfand, K. J. Bladek, J. M. Taylor, D. M. Spasyuk and G. K. H. Shimizu, J. Am. Chem. Soc., 2017, 139, 14676–14683 CrossRef CAS PubMed.
- Z. Y. Zhang, G. X. Qin, X. M. Li, H. L. Dong, S. Wan, Y. H. Ni, J. Liu, Z. Q. Chen and Z. Su, Inorg. Chem., 2022, 61, 15166–15174 CrossRef CAS PubMed.
- S. Mukhopadhyay, J. Debgupta, C. Singh, R. Sarkar, O. Basu and S. K. Das, ACS Appl. Mater. Interfaces, 2019, 11, 13423–13432 CrossRef CAS PubMed.
- M. K. Sarango-Ramirez, J. Park, J. Kim, Y. Yoshida, D. W. Lim and H. Kitagawa, Angew. Chem., Int. Ed., 2021, 60, 20173–20177 CrossRef CAS PubMed.
- A. Shigematsu, T. Yamada and H. Kitagawa, J. Am. Chem. Soc., 2011, 133, 2034–2036 CrossRef CAS PubMed.
- A. Mallick, T. Kundu and R. Banerjee, Chem. Commun., 2012, 48, 8829–8831 RSC.
- V. G. Ponomareva, K. A. Kovalenko, A. P. Chupakhin, D. N. Dybtsev, E. S. Shutova and V. P. Fedin, J. Am. Chem. Soc., 2012, 134, 15640–15643 CrossRef CAS PubMed.
- F. M. Zhang, L. Z. Dong, J. S. Qin, W. Guan, J. Liu, S. L. Li, M. Lu, Y. Q. Lan, Z. M. Su and H. C. Zhou, J. Am. Chem. Soc., 2017, 139, 6183–6189 CrossRef CAS PubMed.
- M. Lupa, P. Kozyra and D. Matoga, ACS Appl. Energy Mater., 2023, 6, 9118–9123 CrossRef CAS.
- S. Pili, S. P. Argent, C. G. Morris, P. Rought, V. Garcia-Sakai, I. P. Silverwood, T. L. Easun, M. Li, M. R. Warren, C. A. Murray, C. C. Tang, S. Yang and M. Schroder, J. Am. Chem. Soc., 2016, 138, 6352–6355 CrossRef CAS PubMed.
- J. Chen, Q. Mei, Y. Chen, C. Marsh, B. An, X. Han, I. P. Silverwood, M. Li, Y. Cheng, M. He, X. Chen, W. Li, M. Kippax-Jones, D. Crawshaw, M. D. Frogley, S. J. Day, V. Garcia-Sakai, P. Manuel, A. J. Ramirez-Cuesta, S. Yang and M. Schroder, J. Am. Chem. Soc., 2022, 144, 11969–11974 CrossRef CAS PubMed.
- S. Kanda, K. Yamashita and K. Ohkawa, Bull. Chem. Soc. Jpn., 2006, 52, 3296–3301 CrossRef.
- Y. Nagao, T. Kubo, K. Nakasuji, R. Ikeda, T. Kojima and H. Kitagawa, Synth. Met., 2005, 154, 89–92 CrossRef CAS.
- J. Y. Choi, M. Stodolka, N. Kim, H. T. B. Pham, B. Check and J. Park, Chem, 2023, 9, 143–153 CAS.
- M.-Y. Zhang, X.-N. Qin, C.-X. Zhang and Q.-L. Wang, ACS Appl. Nano Mater., 2024, 7, 7063–7071 CrossRef CAS.
- H. Huang, Z. Zhong, J. Li and H. Li, ACS Appl. Energy Mater., 2024, 7, 10804–10814 CrossRef CAS.
- H. Bunzen, A. Javed, D. Klawinski, A. Lamp, M. Grzywa, A. Kalytta-Mewes, M. Tiemann, H.-A. K. von Nidda, T. Wagner and D. Volkmer, ACS Appl. Nano Mater., 2019, 2, 291–298 CrossRef CAS.
- W. M. Haynes, CRC Handbook of Chemistry and Physics, CRC Press, Boca Raton, FL, 2016–2017 Search PubMed.
- Y. Chen, Y. Zhang, Q. Huang, X. Lin, A. Zeb, Y. Wu, Z. Xu and X. Xu, ACS Appl. Energy Mater., 2022, 5, 7842–7873 CrossRef CAS.
- C. Kong, G. Jiang, Y. Sheng, Y. Liu, F. Gao, F. Liu and X. Duan, Chem. Eng. J., 2023, 460, 141803 CrossRef CAS.
- R. Singh, G. Singh, N. George, G. Singh, S. Gupta, H. Singh, G. Kaur and J. Singh, Catalysts, 2023, 13, 130 CrossRef CAS.
- J. E. Cun, X. Fan, Q. Pan, W. Gao, K. Luo, B. He and Y. Pu, Adv. Colloid Interface Sci., 2022, 305, 102686 CrossRef CAS PubMed.
- S. Kanda, K. Yamashita and K. Ohkawa, Bull. Chem. Soc. Jpn., 1979, 52, 3296–3301 CrossRef CAS.
- Y. Nagao, M. Fujishima, R. Ikeda, S. Kanda and H. Kitagawa, Synth. Met., 2003, 133–134, 431–432 CrossRef CAS.
- Y. Nagao, T. Kubo, K. Nakasuji, R. Ikeda, T. Kojima and H. Kitagawa, Synth. Met., 2005, 154, 89–92 CrossRef CAS.
- Y. Zhou, J. Yang, H. Su, J. Zeng, S. P. Jiang and W. A. Goddard, J. Am. Chem. Soc., 2014, 136, 4954–4964 CrossRef CAS PubMed.
- W. J. Liu, L. Z. Dong, R. H. Li, Y. J. Chen, S. N. Sun, S. L. Li and Y. Q. Lan, ACS Appl. Mater. Interfaces, 2019, 11, 7030–7036 CrossRef CAS PubMed.
- N. Ogiwara, T. Iwano, T. Ito and S. Uchida, Coord. Chem. Rev., 2022, 462, 214524 CrossRef CAS.
- M. L. Wei, J. J. Sun and X. Y. Duan, Eur. J. Inorg. Chem., 2013, 345–351 Search PubMed.
- C. Dey, T. Kundu and R. Banerjee, Chem. Commun., 2012, 48, 266–268 RSC.
- M. Wei, X. Wang and X. Duan, Chem. – Eur. J., 2013, 19, 1607–1616 CrossRef CAS PubMed.
- R.-T. Zhang, H.-P. Xiao, Z. Li, M. Wang, Y.-F. Xie, Y.-D. Ye, X.-X. Li and S.-T. Zheng, CrystEngComm, 2021, 23, 2973–2981 RSC.
- M. Wei, L. Chen and X. Duan, J. Coord. Chem., 2014, 67, 2809–2819 CrossRef CAS.
- H.-J. Lun, Z.-M. Zhang, Y.-H. Sun, M.-M. Wang, J.-J. Cai, X.-Y. Liang, Y.-M. Li and Y. Bai, Inorg. Chem., 2023, 62, 17093–17101 CrossRef CAS PubMed.
- Y. Liu, X. Yang, J. Miao, Q. Tang, S. Liu, Z. Shi and S. Liu, Chem. Commun., 2014, 50, 10023–10026 RSC.
- Y. Ye, W. Guo, L. Wang, Z. Li, Z. Song, J. Chen, Z. Zhang, S. Xiang and B. Chen, J. Am. Chem. Soc., 2017, 139, 15604–15607 CrossRef CAS PubMed.
- N. C. Jeong, B. Samanta, C. Y. Lee, O. K. Farha and J. T. Hupp, J. Am. Chem. Soc., 2012, 134, 51–54 CrossRef CAS PubMed.
- S. Khatua, A. Kumar Bar and S. Konar, Chem. – Eur. J., 2016, 22, 16277–16285 CrossRef CAS PubMed.
- Z. Zhang, Q. Han, S. Zhang, X. Guo, H. Huang, F. Yang and C. Zhong, ACS Sustainable Chem. Eng., 2022, 10, 11867–11874 CrossRef CAS.
- B. Gil-Hernández, S. Savvin, G. Makhloufi, P. Núñez, C. Janiak and J. Sanchiz, Inorg. Chem., 2015, 54, 1597–1605 CrossRef PubMed.
- B. Gil-Hernández, S. Millan, I. Gruber, M. Quirós, D. Marrero-López, C. Janiak and J. Sanchiz, Inorg. Chem., 2022, 61, 11651–11666 CrossRef PubMed.
- T. Grancha, J. Ferrando-Soria, J. Cano, P. Amorós, B. Seoane, J. Gascon, M. Bazaga-García, E. R. Losilla, A. Cabeza, D. Armentano and E. Pardo, Chem. Mater., 2016, 28, 4608–4615 CrossRef CAS.
- R. Li, S. H. Wang, X. X. Chen, J. Lu, Z. H. Fu, Y. Li, G. Xu, F. K. Zheng and G. C. Guo, Chem. Mater., 2017, 29, 2321–2331 CrossRef CAS.
- Y. B. Lu, Y. Q. Liao, L. Dong, S. D. Zhu, H. R. Wen, J. Huang, X. X. Dai, P. Lian, X. M. Jiang, R. Li and Y. R. Xie, Chem. Mater., 2021, 33, 7858–7868 CrossRef CAS.
- Y. W. You, C. Xue, Z. F. Tian, S. X. Liu and X. M. Ren, Dalton Trans., 2016, 45, 7893–7899 RSC.
- J. M. Taylor, R. K. Mah, I. L. Moudrakovski, C. I. Ratcliffe, R. Vaidhyanathan and G. K. Shimizu, J. Am. Chem. Soc., 2010, 132, 14055–14057 CrossRef CAS PubMed.
- Q. Tang, Y. Liu, S. Liu, D. He, J. Miao, X. Wang, G. Yang, Z. Shi and Z. Zheng, J. Am. Chem. Soc., 2014, 136, 12444–12449 CrossRef CAS PubMed.
- Z.-Y. Ruan, Y.-B. Liang, S.-L. Tan, Y.-X. Tang, W.-Q. Lin, J.-Z. Wu and Y.-C. Ou, Chem. – Asian J., 2021, 16, 931–936 CrossRef CAS PubMed.
- S. B. Tayade, V. M. Dhavale, A. S. Kumbhar, S. Kurungot, P. Lönnecke, E. Hey-Hawkins and B. Pujari, Dalton Trans., 2017, 46, 6968–6974 RSC.
- Z. Sun, S. Yu, L. Zhao, J. Wang, Z. Li and G. Li, Chem. – Eur. J., 2018, 24, 10829–10839 CrossRef CAS PubMed.
- W. Chen, C. Yang, S. Yu, Z. Li and G. Li, Polyhedron, 2019, 158, 377–385 CrossRef CAS.
- Z.-B. Sun, Y.-L. Li, Z.-H. Zhang, Z.-F. Li, B. Xiao and G. Li, New J. Chem., 2019, 43, 10637–10644 RSC.
- M. E. Scofield, H. Liu and S. S. Wong, Chem. Soc. Rev., 2015, 44, 5836–5860 RSC.
- J. P. Guthrie, Can. J. Chem., 1978, 56, 2342–2354 CrossRef CAS.
- H. Dong, H. Du, S. R. Wickramasinghe and X. Qian, J. Phys. Chem. B, 2009, 113, 14094–14101 CrossRef CAS PubMed.
- X. Y. Dong, R. Wang, J. B. Li, S. Q. Zang, H. W. Hou and T. C. W. Mak, Chem. Commun., 2013, 49, 10590–10592 RSC.
- X. Meng, S. Y. Song, X. Z. Song, M. Zhu, S. N. Zhao, L. L. Wu and H. J. Zhang, Chem. Commun., 2015, 51, 8150–8152 RSC.
- S. B. Tayade, R. Illathvalappil, V. Lapalikar, D. Markad, S. Kurungot, B. Pujari and A. S. Kumbhar, Dalton Trans., 2019, 48, 11034–11044 RSC.
- G. Zhang and H. Fei, Chem. Commun., 2017, 53, 4156–4159 RSC.
- H. G. Yu, B. Li, S. Liu, C. Jiang, Y. S. Li, Y. P. Wu, J. Zhao and D. S. Li, J. Solid State Chem., 2021, 294, 121860 CrossRef CAS.
- R. Moi, A. Ghorai, S. Banerjee and K. Biradha, Cryst. Growth Des., 2020, 20, 5557–5563 CrossRef CAS.
- M. Rautenberg, B. Bhattacharya, C. Das and F. Emmerling, Inorg. Chem., 2022, 61, 10801–10809 CrossRef CAS PubMed.
- B. M. Mahimai, G. Sivasubramanian, S. Moorthy and P. Deivanayagam, Ind. Eng. Chem. Res., 2022, 61, 8081–8090 CrossRef CAS.
- Z. Li, Y. Zhou, J. Hu, C. Shi, S. Liu, Y. Ge, T. Zhou and Y. Ye, Chem. Eng. J., 2024, 480, 148146 CrossRef CAS.
- D. Gao, J. Tang, F. Zhang, C. Wen, L. Feng, C. Wan, F. Qu and X. Liang, J. Colloid Interface Sci., 2023, 650, 19–27 CrossRef CAS PubMed.
- S. Ma, J. Xu, S. Sohrabi and J. Zhang, J. Mater. Chem. A, 2023, 11, 11572–11606 RSC.
- H. Tang, X. Lv, J. Du, Y. Liu, J. Liu, L. Guo, X. Zheng, H. Hao and Z. Liu, Appl. Organomet. Chem., 2022, 36, e6777 CrossRef CAS.
- Y. Qi, S. Lin, C. Chen, Y. Liu, Z. Qiao, X. Kuang, Q. Su and H. Y. Chao, J. Mater. Chem. A, 2014, 2, 8849–8853 RSC.
- L. Yang, H. Naruke and T. Yamase, Inorg. Chem. Commun., 2003, 6, 1020–1024 CrossRef CAS.
- C. Y. Sun, S. X. Liu, D. D. Liang, K. Z. Shao, Y. H. Ren and Z. M. Su, J. Am. Chem. Soc., 2009, 131, 1883–1888 CrossRef CAS PubMed.
- C. L. Jones, A. J. Tansell and T. L. Easun, J. Mater. Chem. A, 2016, 4, 6714–6723 RSC.
- K. Muller, J. Helfferich, F. Zhao, R. Verma, A. B. Kanj, V. Meded, D. Bleger, W. Wenzel and L. Heinke, Adv. Mater., 2018, 30, 1706551 CrossRef PubMed.
- S. Wang, P. Li, S. Fan, Z. Fang, X. Wang, Z. Li and X. Peng, Dalton Trans., 2021, 50, 2731–2735 RSC.
- H. J. Kim, K. Talukdar and S. J. Choi, J. Nanopart. Res., 2016, 18, 47 CrossRef.
- J. M. Li, T. Y. Xu, Y. L. Zhao, X. L. Hu and K. H. He, Dalton Trans., 2021, 50, 7484–7495 RSC.
- A. V. da Rosa and J. C. Ordóñez, in Fundamentals of Renewable Energy Processes, ed. A. V. da Rosa and J. C. Ordóñez, Academic Press, Oxford, 4th edn, 2022, pp. 317–417 Search PubMed.
- F. Samimi and M. R Rahimpour, in Methanol, ed. A. Basile and F. Dalena, Elsevier, 2018, pp. 381–397 Search PubMed.
- G. Chen, L. Ge, J. H. Lee, Z. Zhu and H. Wang, Matter, 2022, 5, 2031–2053 CrossRef CAS.
- A. Al-Ahmed, A. S. Sultan and S. M. J. Zaidi, in Ion Exchange Technology I: Theory and Materials, ed. Inamuddin and M. Luqman, Springer, Netherlands, Dordrecht, 2012, ch. 12, pp. 437–451 Search PubMed.
- N. Niluroutu, K. Pichaimuthu, S. Sarmah, P. Dhanasekaran, A. Shukla, S. M. Unni and S. D. Bhat, New J. Chem., 2018, 42, 16758–16765 RSC.
- F. Hu, F. Zhong, J. Wang, T. Qu, J. Ni, G. Zheng, C. Gong and H. Liu, Macromol. Mater. Eng., 2021, 306, 2100053 CrossRef CAS.
- S. Li, G. Cai, S. Wu, A. Raut, W. Borges, P. R. Sharma, S. K. Sharma, B. S. Hsiao and M. Rafailovich, Int. J. Mol. Sci., 2022, 23, 15245 CrossRef CAS PubMed.
- M. T. Musa, N. Shaari, S. K. Kamarudin and W. Y. Wong, Int. J. Energy Res., 2022, 46, 16178–16207 CrossRef CAS.
- N. Shaari and S. K. Kamarudin, Polym. Test., 2020, 81, 106183 CrossRef CAS.
- M. Purwanto, L. Atmaja, M. A. Mohamed, M. T. Salleh, J. Jaafar, A. F. Ismail, M. Santoso and N. Widiastuti, RSC Adv., 2016, 6, 2314–2322 RSC.
- K. N. Lupatini, J. V. Schaffer, B. Machado, E. S. Silva, L. S. N. Ellendersen, G. I. B. Muniz, R. J. Ferracin and H. J. Alves, J. Polym. Environ., 2018, 26, 2964–2972 CrossRef CAS.
- K. Divya, M. S. Sri Abirami Saraswathi, A. Nagendran and D. Rana, J. Appl. Polym. Sci., 2022, 139, e52829 CrossRef CAS.
- Z. Guo, X. Xu, Y. Xiang, S. Lu and S. P. Jiang, J. Mater. Chem. A, 2015, 3, 148–155 RSC.
- T. Wen, Z. Shao, H. Wang, Y. Zhao, Y. Cui and H. Hou, Inorg. Chem., 2021, 60, 18889–18898 CrossRef CAS PubMed.
- L. Sun, Q. Gu, H. Wang, J. Yu and X. Zhou, RSC Adv., 2021, 11, 29527–29536 RSC.
- R. Moi, A. Ghorai, S. Banerjee and K. Biradha, Cryst. Growth Des., 2020, 20, 5557–5563 CrossRef CAS.
- Y. L. Bao, J. Y. Zheng, H. P. Zheng, G. D. Qi, J. R. An, Y. P. Wu, Y. L. Liu, W. W. Dong, J. Zhao and D. S. Li, J. Solid State Chem., 2022, 310, 123070 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |