Bright and persistent green emitting MgGa2O4:Mn2+ for phosphor converted white light emitting diodes†
Reshmi T.
Parayil
ab,
Santosh K.
Gupta
 *ab,
Malini
Abraham
cd,
Deepak
Tyagi
e,
Subrata
Das
*ab,
Malini
Abraham
cd,
Deepak
Tyagi
e,
Subrata
Das
 cd,
Mohit
Tyagi
bf,
N. S.
Rawat
bg and
Manoj
Mohapatra
ab
cd,
Mohit
Tyagi
bf,
N. S.
Rawat
bg and
Manoj
Mohapatra
ab
aRadiochemistry Division, Bhabha Atomic Research Centre, Trombay, Mumbai-400085, India. E-mail: santoshg@barc.gov.in; santufrnd@gmail.com
bHomi Bhabha National Institute, Anushaktinagar, Mumbai – 400094, India
cMaterials Science and Technology Division, CSIR-National Institute for Interdisciplinary Science and Technology, Thiruvananthapuram, Kerala 695019, India
dAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad-201002, India
eChemistry Division, Bhabha Atomic Research Centre, Trombay, Mumbai-400085, India
fTechnical Physics Division, Bhabha Atomic Research Centre, Trombay, Mumbai-400085, India
gRadiation Physics and Advisory Division, Bhabha Atomic Research Centre, Mumbai-400085, India
First published on 18th November 2024
Abstract
Narrow band green emitting phosphors have gained widespread attention due to their application in white light emitting diode (wLED) backlight displays. Commercial backlight displays have a broad band green phosphor which limits their performance. In this work, bright, narrow and thermally stable green emitting MgGa2O4:Mn2+ (MGO-Mn) has been synthesized. Time-resolved emission spectroscopy suggested that Mn2+ ions are distributed at both Mg2+ and Ga3+ sites of the MGO spinel, which resulted in a high internal quantum efficiency of 63%. The colour purity of MGO-Mn (76.4%) superseded that of the commercial green phosphor β-SiAlON:Eu2+ (59.12%). Doping-induced creation of oxygen vacancies endows MGO-Mn with excellent persistent luminescence with a time duration of more than 900 s upon 4 min charging with 270 nm UV light and persistent radioluminescence of more than 6000 s when charged with X-rays for 1 min. Finally, tunable white LEDs (cool and neutral white LEDs) are fabricated by combining the RGB mixture of the green phosphor with commercial red and blue phosphors along with a 280 nm UV LED chip. This work also showcases the importance of different annealing atmospheres in the photoluminescence and persistent luminescence of the MGO-Mn phosphor.
1. Introduction
Presently, there is high demand for white light emitting diodes (wLEDs) in backlight display devices, which are the fourth-generation lighting sources having the benefits of low power consumption, long lifetime, high brightness, low heat emission and environmental friendliness.1,2 The commercial wLED consists of a cerium(III) doped yttrium aluminium garnet phosphor coated on a blue LED chip.3 Many research studies are going on for the development of a green emitting phosphor with better emission properties. Researchers have reported a good number of green emitting phosphors that find application in areas like white light emitting diodes, backlight displays, optical temperature sensors, anticounterfeiting applications, persistent emission etc.4–10 The most reported green phosphors are rare earth based phosphors which possess many disadvantages such as safety related issues in mining and the high cost of rare earth ions.11 The reported rare earth based green phosphors include Li2Ca2Si2O7:Eu2+,12 Ba2CaZn2Si6O17:Tb3+,13 Ca6La4(SiO4)2(PO4)4O2:Eu2+,14 NaBaB9O15:Eu2+,15 RbLi(Li3SiO4)2:Eu2+,16 CaAl4O7:Ce3+,Tb3+,17 Ca2YHf2Al3O12:Ce3+,Tb3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 and NaSrLa(MoO4)O3:Er3+.19
18 and NaSrLa(MoO4)O3:Er3+.19
The commercially used green phosphor β-SiAlON:Eu2+ emits at 527 nm with an FWHM of 50 nm.20 Another important green phosphor is CsPbBr3 quantum dots (QDs) with an FWHM value of 20 nm and a high quantum efficiency.21 Even though QDs have superior emission properties, they cannot withstand the junction temperature of the LEDs and also undergo degradation when exposed to a humid environment.22 Another important disadvantage is the toxicity associated with Pb, which leads to the search for lanthanide activated green phosphors with narrow band emission. These include RbNa(Li3SiO4)2:Eu2+ (FWHM ∼ 41 nm),16 Ba[Li2(Al2Si2)N6]:Eu2+ (FWHM ∼ 57 nm),23 SrGa2S4:Eu2+ (FWHM ∼ 47 nm),24 and Ba2LiSi7AlN12:Eu2+ (FWHM ∼ 61 nm),25 which have higher green emission efficiency due to the allowed transition of Eu2+(4f–5d). But they have poor thermal stability and high fabrication costs arising from the use of rare earth ions, which paved the urgent need for cheap and thermally stable green emitting phosphors. Transition metal activated (Mn2+) phosphors have been investigated intensely to circumvent the above-mentioned issues. These mainly include Sr2MgAl22O36:Mn2+ (FWHM ∼ 26 nm),26 MgAl2O4:Mn2+ (FWHM ∼ 35 nm),6 BaZnAl10O17:Mn2+ (FWHM ∼ 31 nm)27 and Zn2SiO4:Mn2+ (FWHM ∼ 42 nm).28
Another important application of phosphors is in the area of persistent luminescence (PersL) in which the luminescence lasts for a longer duration even after the stoppage of the excitation source which can be UV, visible, IR or high energy X-ray radiation.29,30 PersL can be used in many areas such as display screens, afterglow toys, bioimaging, information storage, anticounterfeiting etc.31,32 Hu et al.33 reported colour tunable persistent luminescence in oxyfluoride glass which lasted for an hour. Due to the change in the coordination environment of Mn2+, the persistent colour evolves from red to yellow and then to green. Ma et al.34 reported the X-ray excited PersL in MgF2:Mn2+ wherein Ca2+ incorporation leads to tunability.
Lanthanide doped phosphors have attracted great attention due to their high color purity and stable narrow band emission, but their high cost and low abundance can limit the scalability of high quality phosphors for different applications. Another important aspect is the tunability in the emission which can be achieved in a transition metal ion doped phosphor and this flexibility is limited in lanthanide-based phosphors. In terms of thermal stability, transition metal ions can maintain the luminescence properties at elevated temperatures compared to lanthanides.
The manganese ion is an ideal luminescent centre that can replace the costly rare-earth ions and can be used as a rare earth free and cost-effective dopant for phosphor applications. Depending upon the crystal field strength of the host matrices, Mn2+ can exhibit emission in the green and red regions. In a tetrahedral (weak field) environment, Mn2+ exhibits green emission, whereas in an octahedral (strong field) environment, it exhibits red emission. So choosing a host material can control the emission of Mn2+ ions.35,36 Developing a Mn doped material for wLEDs and persistent light emission is a growing field.
In this work we have employed solvent free solid-state synthesis for the preparation of Mn-doped MgGa2O4 (MGO-Mn) under different annealing atmospheres. The spinel which is annealed under a reducing atmosphere has shown superior emission properties that can be excited by using UV, visible and X-ray radiation with an internal quantum yield of 63%. In addition to that, the material also exhibits longer UV and X-ray activated persistent luminescence. Finally, the synthesized green phosphor is mixed with commercial red and blue phosphors in the appropriate composition to produce white light.
2. Experimental
2.1. Synthesis and LED fabrication
The precursors used for the synthesis are MgO (SPEX pure), Ga2O3 and MnCO3. The precursors were weighed according to their stoichiometry and ground in a mortar and pestle for 15 min, followed by heating in a muffle furnace at 1350 °C for 5 h under an air atmosphere and then final grinding. This is represented as MGO-Mn(A). Another sample was prepared under an inert atmosphere, which is represented as MGO-Mn(I). The air annealed samples were again annealed under a reducing atmosphere at 1350 °C for 10 h. This sample is represented as MGO-Mn(R). The doping percentage of Mn was 2% in all the above samples.To assemble a phosphor LED device (pc-LED), the mixture of the as-obtained optimized green phosphor, the BAM:Eu2+ blue phosphor, and the Y2O3:Eu3+ red phosphor (at different ratios) was combined with a 280 nm UV LED chip. Initially, the phosphor mixture was excellently mixed with epoxy resin to obtain phosphor paste. Then the subsequent mixture was coated onto the surface of the 280 nm UV chip to obtain a pc-LED device. Finally, the device features of the obtained pc-LED device were verified using a CCD spectrophotometer, including electroluminescence spectra, chromatic colour coordinates (CIE), colour rendering (CRI), and colour temperature (CCT).
2.2. Instrumentation
X-ray diffraction was performed to confirm the phase purity of the materials on a benchtop Proto X-ray diffractometer with CuKα as the monochromatic source. FTIR measurements were carried out on an Alpha Bruker system in ATR mode.The Raman spectral measurements have been carried out on a micro Raman spectrometer. The FESEM and EDS mapping of the samples were carried out in a Field emission scanning electron microscope (Carl Zeiss Make, GEMINISEM300 Model). All the photoluminescence measurements were done on a fluorescence spectrometer (FLS 1000, Edinburgh make) with a 450 W Xenon lamp as the excitation source. Emission photographs were captured under a 265 nm UV lamp using a Nikon camera. The quantum yield measurements were carried out on an integrating sphere with BaSO4 as the reference. Low-temperature photoluminescence measurements were carried out using a cryostat assembly with the fluorescence spectrometer and liquid nitrogen as the coolant. For decay measurements, a 150 W microsecond flash lamp was used as the excitation source with variable frequencies from 0.1 to 100 Hz. Persistent luminescence measurements were carried out using a PTI QM-400 spectrofluorometer equipped with a 450 W Xenon lamp. Thermoluminescence measurements were performed using a Riso TL/OSL-DA-15 reader system and the signals were recorded at a heating rate of 2 K/s. XPS measurements were carried out on a SPECS instrument with a PHOBIOS 100/150 delay line detector. EPR measurements were performed on a Bruker X-band EPR spectrometer. X-ray excited radioluminescence (RL) measurements were carried out using a customized X-ray generator with a W tube operating at 100 kV and 4 mA. RL emission was recorded with the help of an optical fiber-based Avantes spectrometer. The thermal stability and electroluminescence properties of the obtained samples were determined using a high-sensitivity CCD spectrophotometer (Maya 2000 Pro) by utilizing a 280 nm LED chip. The CIE colour coordinates have been plotted by incorporating the emission values in CIE 1931.3. Results and discussion
3.1. Phase, vibrational spectroscopy and morphology structural analysis
X-ray powder diffraction has been used to evaluate the phase purity and crystal structure of the synthesized material. The respective XRD patterns are displayed in Fig. 1a. The XRD pattern of the synthesized material matches well with the standard pattern of MgGa2O4 suggesting that the Mn2+ ion is doped into the host lattice with a spinel crystal structure. No other impurity phases are found in the XRD pattern. This observation is consistent with the fact that the ionic radius of the dopant Mn2+ is 0.066 nm, which is close to its substitute Mg2+/Ga3+ (0.057 nm/0.047 nm) in tetrahedral coordination, whereas that of Mn2+ is 0.067 nm, which is close to its substitute Mg2+/Ga3+ (0.072 nm/0.055 nm) in octahedral coordination. The space group corresponding to the crystal structure is Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. In this structure, Mg occupies the tetrahedral site (MgO4), whereas Ga occupies the octahedral site (GaO6). Although the crystal structure is spinel, some of the Mg ions can occupy the octahedral site and the same number of Ga can occupy the tetrahedral site, thus forming a partial inverse spinel structure. This creates anti-site defects in the crystal structure and the inversion can reach up to 44% depending upon the synthesis methods.37
m. In this structure, Mg occupies the tetrahedral site (MgO4), whereas Ga occupies the octahedral site (GaO6). Although the crystal structure is spinel, some of the Mg ions can occupy the octahedral site and the same number of Ga can occupy the tetrahedral site, thus forming a partial inverse spinel structure. This creates anti-site defects in the crystal structure and the inversion can reach up to 44% depending upon the synthesis methods.37
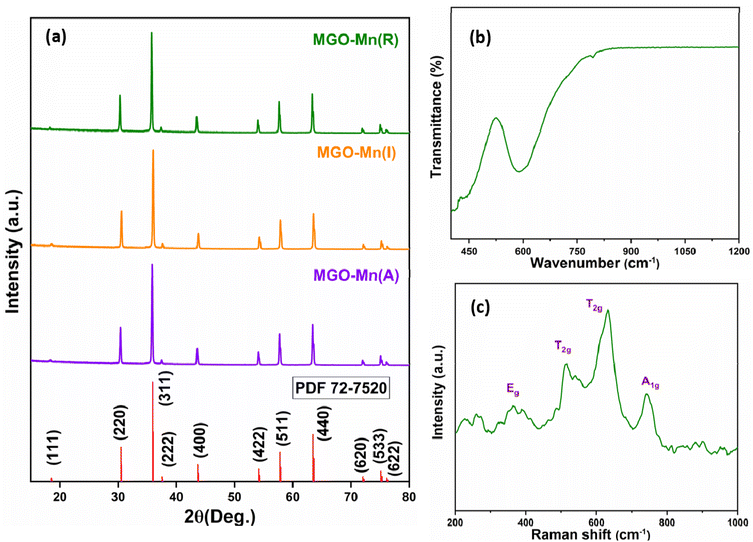 | ||
| Fig. 1 (a) Powder XRD patterns of MGO-Mn(A), MGO-Mn(I) and MGO-Mn(R), (b) FTIR spectrum and (c) Raman spectrum of MGO-Mn(R). | ||
The FTIR spectrum of MGO-Mn(R) is shown in Fig. 1b with two bands at 450 cm−1 and 605 cm−1, which correspond to the stretching modes of Ga–O and Mg–O bonds respectively.38,39 The shoulder peak around 800 cm−1 corresponds to the Ga–O vibration in the GaO4 unit.40 The FTIR spectra of MGO-Mn(A) and MGO-Mn(I) are shown in Fig. S1†, which have similar bands to those of MGO-Mn(R) except the shoulder peak at 800 cm−1. The shoulder peak corresponds to the inversion of the Ga3+ cation, which has also been reported by Naka et al.41 in CoGa2O4, which confirms that partial inversion is observed in MgGa2O4.
The Raman spectrum of MGO-Mn(R) is presented in Fig. 1c. From group theory analysis, the total number of vibrational modes are A1g + Eg + 3T2g + T1g + 4T1u + 2A2u + 2Eu + 2T2u. Among these only five modes, (A1g + Eg + 3T2g) are Raman active. The asymmetric peaks are split into doublets which indicate that the spinel has a partially inverted structure. The peak at 741 cm−1 corresponds to the A1g mode, the peaks at 633 and 517 cm−1 correspond to the T2g mode and the peak at 365 cm−1 corresponds to the Eg mode.34,35
The FESEM image of the MGO-Mn(R) sample shown in Fig. S2a† depicts irregular shapes with sub-micron size. The high temperature heating has resulted in an appreciable extent of aggregation as well. The EDS mapping of the same is shown in Fig. S2b–S2f†, which confirms the uniform distribution of elements Mg, Ga, O and Mn in the synthesized phosphor.
3.2. X-ray photoelectron spectroscopy (XPS) and electron spin resonance (ESR) spectroscopy
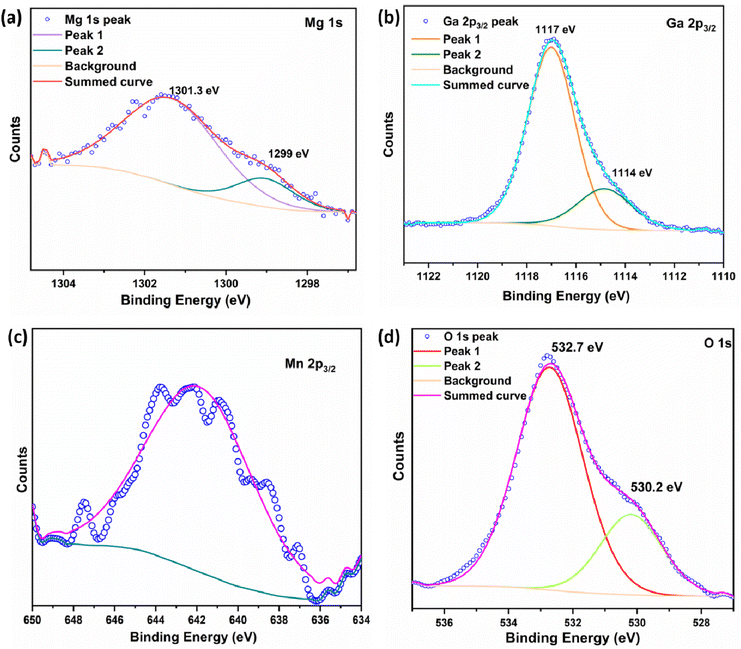
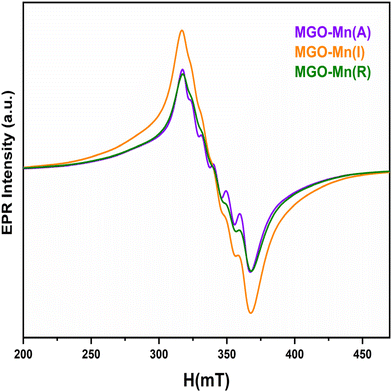
The XPS high resolution spectra of Mg, Ga, Mn and O are shown in Fig. 2. The high-resolution spectra of Mg 1s and Ga 2p3/2 are shown in Fig. 2a and b, which are asymmetric in nature and the curve can be fitted with two peaks, which confirms that Mg and Ga occupy both octahedral and tetrahedral sites. The peak values of Mg 1s are 1301.3 eV and 1299 eV, which correspond to Mg2+ at octahedral and tetrahedral sites respectively.42 The fitted peak values for Ga 2p3/2 are 1117 eV and 1114 eV, which correspond to Ga3+ at octahedral and tetrahedral sites respectively.43,44 The high-resolution spectrum of Mn 2p3/2 is shown in Fig. 2c with a binding energy value of 641.8 eV.45 The O 1s spectrum is shown in Fig. 2d, which has an asymmetric peak and can be fitted to two peaks with binding energy values of 530.2 and 532.7 eV, which correspond to lattice oxygen and an oxygen vacancy, respectively.46,4755Mn is the major isotope of Mn with 100% natural abundance and nuclear spin I = 5/2. Thus, it is expected to give six hyperfine lines due to hyperfine interactions with an electron field. Mn in both +2 and +4 states is EPR active with d5 and d3 configurations respectively. Mn2+ is expected to show an EPR spectrum close to g = 2.0 due to the half-filled configuration, whereas the Mn4+ system with the d3 configuration is expected to show EPR spectra at lower g values than the divalent species. Fig. 3 shows the EPR spectra of the MGO-Mn(A), MGO-Mn(I) and MGO-Mn(R) samples which showed the six hyperfine lines of Mn2+. The MGO-Mn(A) and MGO-Mn(R) phosphors have the same spectral features and the same g values (1.99) as those of MGO-Mn(I). However, the MGO-Mn(I) sample has an unresolved spectrum due to spin exchange broadening and also has higher absorption intensity when compared with MGO-Mn(A) and MGO-Mn(R). This is primarily because of the higher Mn2+ concentration which leads to the large spin broadening (50.8 mT).483.3. Time resolved photoluminescence
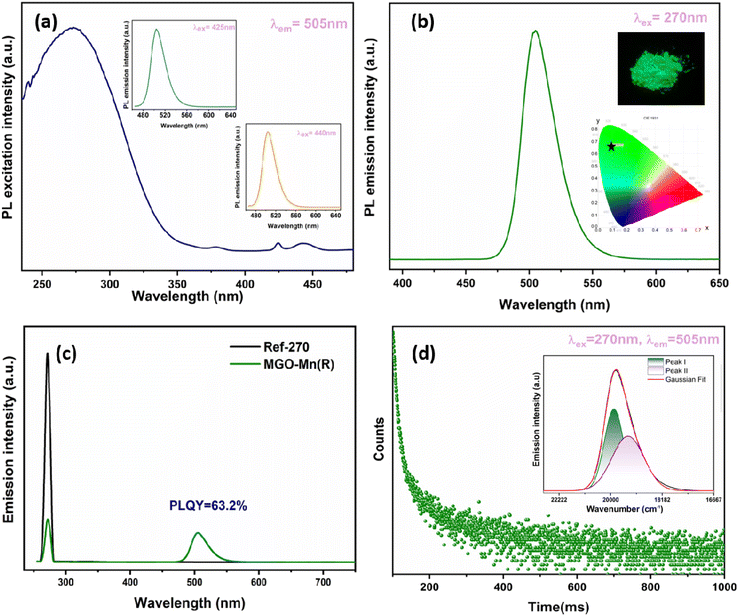
Fig. 4a presents the excitation spectrum of MGO-Mn(R) with an emission wavelength of 505 nm. A broad band can be observed in the region from UV to near UV, which comprises host absorption and Mn2+ → O2− charge transfer. Along with that, three other weak absorption peaks can be seen in the blue region at 380, 425 and 440 nm, which correspond to the forbidden d–d transitions 4A1 → 4T2 (4D), 4A1 → 4T1 (4G) and 4A1 → 4T2 (4G) of Mn2+ ions respectively.49,50 The emission spectra of MGO-Mn(R) with excitation wavelengths of 425 nm and 440 nm are shown in the inset of excitation spectrum and the emission spectrum with an excitation wavelength of 270 nm are shown in Fig. 4b; all the emission spectra consist of a broad band in the green region with a peak maximum at 505 nm, which is due to the 4T1(4G) → 6A1(6S) d–d transition. The corresponding CIE diagram is shown in the inset with CIE coordinates as (0.085, 0.654). The inset presents the digital image of this sample captured using a Nikon camera under 265 nm UV lamp excitation, which shows bright green colour that can be seen by the naked eye. The FWHM of MGO-Mn(R) is calculated to be 31 nm which is less than that of the commercial green phosphor β-SiAlON:Eu2+ (54 nm). This suggests that the MGO-Mn(R) green phosphor can be potentially used in backlight displays. Along with that, the phosphors can be efficiently excited by blue light from 425 or 440 nm. The green emission observed here reveals that Mn2+ occupies the low crystal field (tetrahedral coordination).51 The emission spectra of MGO-Mn(A) and MGO-Mn(I) are shown in Fig. S3.†To further evaluate the applicability, the internal quantum efficiency (IQE) and external quantum efficiency (EQE) have been calculated by using the integrating sphere method and the spectrum is shown in Fig. 4c. The internal quantum efficiency can be calculated by using the equation:52
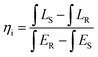 | (1) |
The IQE value is obtained as 63.2%. The IQE values for MGO-Mn(A) and MGO-Mn(I) are shown in Fig. S4.†
The external quantum efficiency (EQE) can be calculated by using the equation:
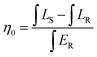 | (2) |
The EQE value is obtained as 50%. The green emission at 505 nm is expected to originate from Mn2+ occupying the tetrahedral site. In the crystal structure of MGO, as already discussed, both Mg2+ and Ga3+ have tetrahedral coordination due to the partial inversion, which is confirmed from the FTIR, Raman and XPS studies. In order to determine the site occupancy of the Mn2+ ions, the decay profile was recorded with an excitation of 270 nm and the emission was monitored at 505 nm, which is shown in Fig. 4d. The decay profile follows a bi-exponential decay and can be fitted in the following equation:1
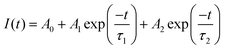 | (3) |
The lifetime values obtained after fitting are 5.6 and 58.3 ms with a relative percentage of 69 and 31, respectively, which confirms that the Mn2+ ion occupies two different sites. It can be proposed that the short lifetime value corresponds to Mn2+ occupying the Ga3+ site whereas the long lifetime corresponds to Mn2+ at the Mg2+ site. So, the broad emission in the green region can be fitted to two peaks by Gaussian fitting with peak values of 503 and 516 nm.53,54 We speculate that they correspond to Mn2+ occupying the [MgO4] and [GaO4] sites, respectively. The substitution of Mn2+ at Ga3+ can create charge compensating defects such as oxygen vacancy related defects around Mn2+, which is why the peak at 516 nm is broader.
The colour purity of the green phosphor can be calculated by using the following equation:
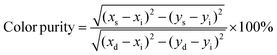 | (4) |
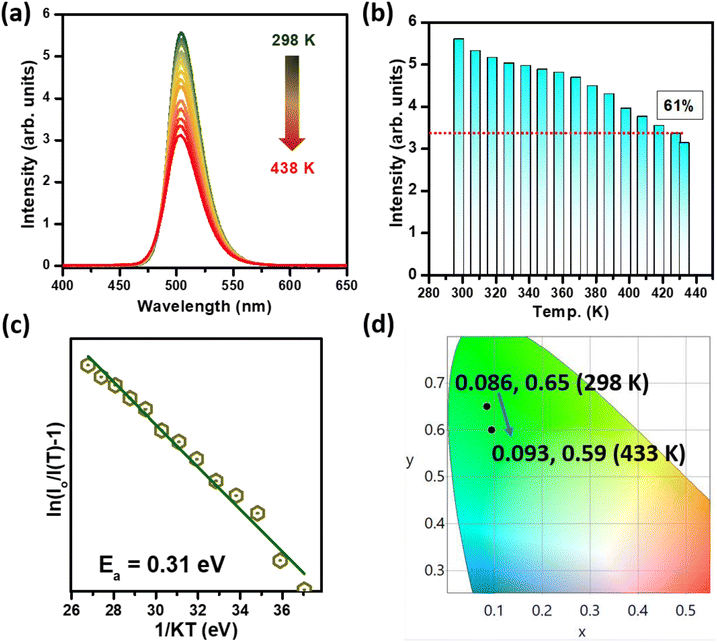
Fig. 5a shows the excitation spectra of MGO-Mn(R) in the temperature range of 80–280 K with an emission wavelength of 505 nm. The same spectral features are observed in the low temperature region with only variation in their intensity. Fig. 5b shows the emission spectra with an excitation wavelength of 270 nm. With an increase in temperature from 80 to 280 K, the emission intensity reduces monotonically, although the emission peak positions barely shift in this temperature range. Fig. 5c shows the corresponding contour plot of the emission profile, and the intensity variation with temperature can be seen from the contour plots. Fig. 5d represents the plot of integral intensity and FWHM as a function of temperature. With an increase in temperature the emission intensity gets reduced whereas the FWHM gets enhanced. When the temperature increases the thermally activated luminous centre more strongly interacts with the thermally activated phonon, which increases the population density of the phonon and thus the electron–phonon interaction becomes more prominent, which results in the enhancement of the FWHM value.55 Some recently reported Mn2+ and Eu2+ activated green phosphors with their emission wavelengths and FWHM values are compared in Table 1.
| Green phosphor | Emission wavelength (nm) | FWHM (nm) | Ref. |
|---|---|---|---|
| Sr2MgAl22O36:Mn2+ | 518 | 26 | 26 |
| MgAl2O4:Mn2+ | 535 | 35 | 6 |
| SrAl2Si2O8:Mn2+ | 517 | 2 | 56 |
| NaGa8Al3O17:Mn2+ | 510 | 33 | 57 |
| NaGa11O17:Mn2+ | 505 | 29 | 57 |
| ZnB2O4:Mn2+ | 541 | 41 | 58 |
| Zn2GeO4:Mn2+ | 534 | 49.5 | 35 |
| NaAl11O17:Mn2+ | 508 | 33 | 59 |
| Sr2ZnGe2O7:Mn2+ | 535 | 48 | 60 |
| LaMgAl11O19:Mn2+ | 517 | 24 | 61 |
| Na2MgAl10O17:Mn2+ | 513 | 27 | 62 |
| BaZnAl10O17:Mn2+ | 516 | 31 | 27 |
| Rb3Na(Li3SiO4)4:Eu2+ | 527 | 42 | 52 |
| RbLi(Li3SiO4)2:Eu2+ | 530 | 53 | 16 |
| Ba2LiSi7AlN12:Eu2+ | 515 | 61 | 25 |
| (Ba,Sr)SiO4:Eu2+ | 530 | 72 | 63 |
| Ba[Li2(Al2Si2)N6]:Eu2+ | 532 | 57 | 23 |
| MgGa2O4:Mn2+(R) | 505 | 31 | This work |
3.4. Persistent luminescence and thermoluminescence
The PersL decay curve is shown in Fig. 6a with an excitation wavelength of 270 nm and an emission wavelength of 505 nm. The decay curve was acquired by illuminating the MGO-Mn(R) sample with 270 nm UV light for 4 min. Afterglow of more than 15 min is observed, which demonstrates that the material is a good persistent luminescent phosphor. In order to correlate the persistence properties, thermoluminescence (TL) was carried out by illuminating the phosphor materials with UV light for 4 min followed by recording the spectrum (Fig. 6b). The trap depth value can be calculated by using the equation:| E = Tm/500 | (5) |
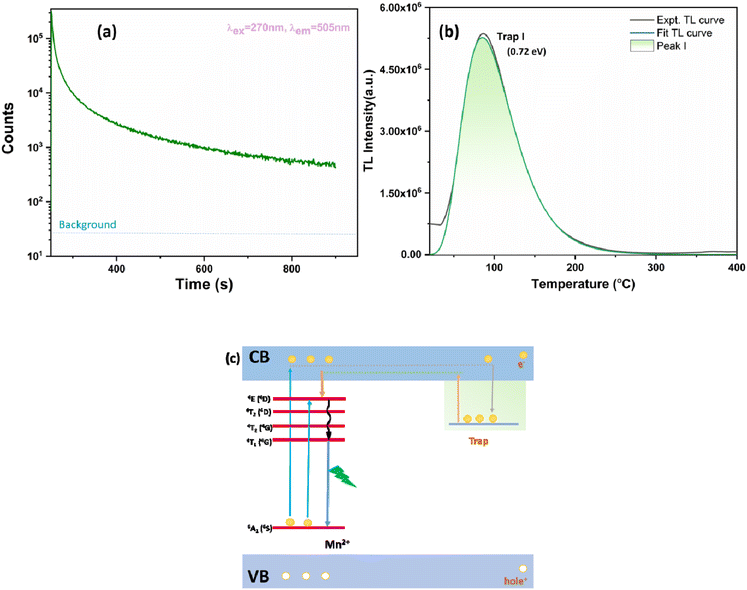 | ||
| Fig. 6 (a) Persistent decay plot, (b) thermoluminescence spectrum of MGO-Mn(R), and (c) schematics for the persistence mechanism. | ||
The radioluminescence spectrum of MGO-Mn(R) is presented in Fig. 7a, in which the spectral features are the same as those with the UV excitation, which suggests that the origin of emission is from Mn2+ ions. Fig. 7b represents the radioluminescence spectra as a function of X-ray voltage starting from 20 kV to 100 kV and a constant current of 4 mA. By increasing the X-ray voltage, the spectral features do not change but the RL intensity increases monotonically, as shown in Fig. 7c. Another important feature observed is the X-ray excited persistent luminescence of the material which lasts for around 100 min, as presented in Fig. 7d.
3.5. Temperature-dependent photoluminescence
Thermal stability is recognized as a crucial parameter for any phosphor material to be used in devices. Fig. 8a summarizes the emission spectra of the MGO-Mn(R) green phosphor recorded at various temperatures ranging from 298 to 438 K. It is realized that the integrated emission intensity of the measured system reduced gradually with mounting temperatures. However, even at around 423 K, the MGO-Mn(R) green phosphor preserves nearly 61% of its room temperature emission intensity (Fig. 8b). With the help of the Arrhenius plot (Fig. 8c), reported elsewhere,64,65 the activation energy for the present sample was estimated to be 0.31 eV. The CIE color coordinates were also measured for the emission spectra recorded at various temperatures ranging from 298 K to 438 K. The results presented in Fig. 8d clearly showed negligible variations in the measured coordinates at terminal temperatures, indicating the device potential of the present green phosphor.3.6. Device performance
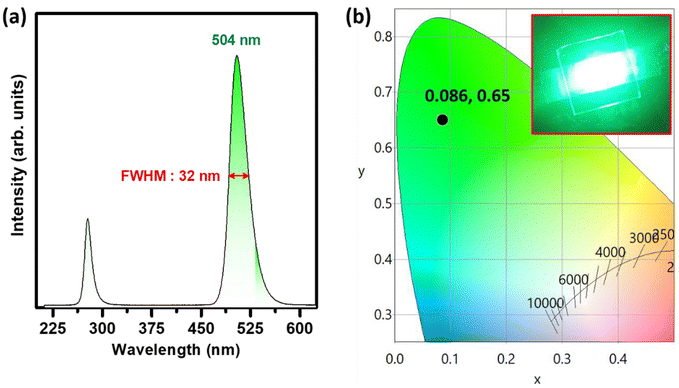
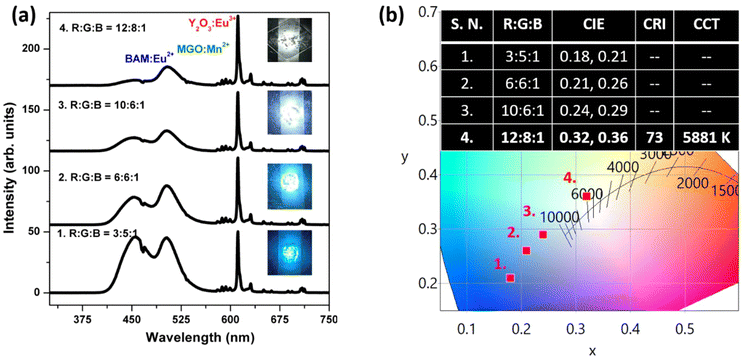
In order to check the credibility of the optimized green phosphor in white LED fabrication, a green pc-LED has been fabricated by mixing this green phosphor along with resin followed by coating the phosphor + resin mixture on a 280 nm UV LED source with an operating current of 3 mA. The resulting electroluminescence spectrum, shown in Fig. 9a, demonstrates a narrow band green emission at 504 nm with an FWHM of ∼32 nm along with the LED spectral band situated at 280 nm. The resulting CIE coordinates can be seen from Fig. 9b, which are located in the green region, and the direct digital image of the illuminated pc-LED source is also depicted in the inset of the same figure. Such narrow band emission and appreciable thermal stability at elevated operating temperatures make the system suitable for white LED applications.Finally, numerous white LEDs were made up using a mixture of commercial Y2O3:Eu3+, an optimized green-emitting MGO-Mn(R) phosphor, and the commercial BAM:Eu2+ blue phosphor in various ratios. The different batches of powder mixtures were then mixed with resin and individually coated on identical 280 nm UV LED chips. Fig. 10a represents the EL spectra of the fabricated white LEDs with the various RGB mixing ratios along with the digital images of the respective illuminated devices in the insets. As seen in the CIE diagram depicted in Fig. 10b, by adjusting the RGB ratio at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the white light emission of the fabricated white LEDs shifted from cool white light to pure white light with a CCT of 5881 K and a CRI of 73, as observed from the CIE coordinates depicted in the inset of Fig. 10b.
1, the white light emission of the fabricated white LEDs shifted from cool white light to pure white light with a CCT of 5881 K and a CRI of 73, as observed from the CIE coordinates depicted in the inset of Fig. 10b.
Table 2 represents the reported works on Mn2+ doped MgGa2O4, which are compared with our work.
| Work | Ref. |
|---|---|
| This work only reported excitation and emission spectra | 66 |
| This work has shown the stabilization of both Mn2+ and Mn4+ in MgGa2O4 and explored it for thermometry applications | 67 |
| This work is purely material synthesis work where authors have shown the effect of different kinds of fluxes on the PL of MgGa2O4:Mn2+ phosphors | 68 |
| This work reported the comparison between MgGa2O4:Mn2+ and ZnGa2O4:Mn2+ nanophosphors synthesized by the glycothermal method | 69 |
| This work is purely material synthesis work where authors have used the spray pyrolysis method, prevented surface residual non-radiative defect sites and produced highly luminescent and spherical phosphors | 70 |
| This is an upconversion paper where authors have reported the visible upconversion in Yb3+, Mn2+ co-doped MgGa2O4 phosphors | 71 |
| We have reported bright green emission from MgGa2O4:Mn2+ with an IQE of 63% and EQE of 50% with a colour purity of 76.4%, which superseded that of the commercial green phosphor β-SiAlON:Eu2+(59.12%) and carried out thermal stability measurements, thermoluminescence, afterglow under UV and X-ray and finally LED fabrication | This work |
4. Conclusion
Here in this work, we have synthesized bright, pure and persistent deep green emitting MGO-Mn phosphors using the solid-state method. The synthesized phosphor was characterized by using XRD, FTIR, EDS and Raman spectroscopy. We could achieve an IQE of more than 63% and EQE of 50% in the sample annealed under a reduced atmosphere. The same sample was utilized in designing a pc-LED which showed deep green emission with a colour purity of 76.4%, which is superior to the commercial green phosphor. The optimum green phosphor can retain 61% at 423 K relative to the PL intensity observed at room temperature and the activation energy is estimated to be 0.31 eV, suggesting good thermal stability. In addition to this, the phosphor can be excited by using UV, visible and X-ray radiation, depicting efficient photo and radioluminescence. Interestingly both UV activation and X-ray activation show persistent luminescence properties driven by the presence of oxygen vacancy-related shallow traps. For evaluating the real-time device performance of the MGO-Mn phosphor, a prototype white-LED was fabricated by combining a 280 nm UV chip and a mixture of tri-color emitting phosphors, and by tuning the RGB phosphor composition, we could achieve both cool and pure white LEDs.Data availability
The data are available from the corresponding author on reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge Dr Apurav Guleria, BARC Mumbai, for helping with Raman measurements and Dr Jithendra Bahadur BARC Mumbai for FESEM and EDS measurements.References
- R. T. Parayil, S. K. Gupta, M. Abraham, S. Das, S. S. Pitale, K. Sudarshan and M. Mohapatra, Ultra-bright and thermally stable deep red emitting doped yttrium zirconate nanoparticles for tunable white LEDs and indoor plant growth, Mater. Adv., 2023, 4(22), 5594–5604 RSC.
- T. P. R. Parayil, G. D. Patra, B. Modak, K. Sudarshan, M. Sonawane, S. Sen and S. K. Gupta, Uranium-Sensitized Luminescence Enhancement in Li2B4O7:U6+,Eu3+ and Potential Application towards Color-Tunable Phosphors and Actinide Sensor, ACS Appl. Opt. Mater., 2023, 1(1), 179–192 CrossRef.
- T. Hasegawa, M. Iwaki, R. Tanaka, S.-W. Kim, S. Yin and K. Toda, Phase stabilization of red-emitting olivine-type NaMgPO4:Eu2+ phosphors via molten-phase quenching, Inorg. Chem. Front., 2020, 7(21), 4040–4051 RSC.
- X. Zhang, J. Zhang, R. Wang and M. Gong, Photo-Physical Behaviors of Efficient Green Phosphor Ba2MgSi2O7:Eu2+ and Its Application in Light-Emitting Diodes, J. Am. Ceram. Soc., 2010, 93(5), 1368–1371 CrossRef CAS.
- J. H. Chung and J. H. Ryu, Photoluminescence and LED application of β-SiAlON:Eu2+ green phosphor, Ceram. Int., 2012, 38(6), 4601–4606 CrossRef CAS.
- E. H. Song, Y. Y. Zhou, Y. Wei, X. X. Han, Z. R. Tao, R. L. Qiu, Z. G. Xia and Q. Y. Zhang, A thermally stable narrow-band green-emitting phosphor MgAl2O4:Mn2+ for wide color gamut backlight display application, J. Mater. Chem. C, 2019, 7(27), 8192–8198 RSC.
- Q. Dong, J. Yang, J. Cui, F. Xu, F. Yang, J. Peng, F. Du, X. Ye and S. Yang, A narrow-band ultra-bright green phosphor for LED-based applications, Dalton Trans., 2020, 49(6), 1935–1946 RSC.
- N. Latha, D. R. Lavanya, G. P. Darshan, B. R. Radha Krushna, H. B. Premkumar, H. C. Prameela and H. Nagabhushana, Green emanating BiOCl:Tb3+ phosphors for strategic development of dermatoglyphics and anti-counterfeiting applications, Inorg. Chem. Commun., 2022, 138, 109266 CrossRef CAS.
- Y. Hua and J. S. Yu, Strong Green Emission of Erbium(III)-Activated La2MgTiO6 Phosphors for Solid-State Lighting and Optical Temperature Sensors, ACS Sustainable Chem. Eng., 2021, 9(14), 5105–5115 CrossRef CAS.
- S. Wang, X. Liu, B. Qu, Z. Song, Z. Wang, S. Zhang, F. Wang, W.-T. Geng and Q. Liu, Green persistent luminescence and the electronic structure of β-Sialon:Eu2, J. Mater. Chem. C, 2019, 7(40), 12544–12551 RSC.
- B. Srivastava, S. Gupta, Y. Li and Y. Mao, Bright Persistent Green Emitting Water-Dispersible Zn2GeO4:Mn Nanorods, Dalton Trans., 2020, 49, 7328–7340 RSC.
- J. S. Kim, H. J. Song, H.-S. Roh, D. K. Yim, J. H. Noh and K. S. Hong, Luminescent characteristics of green emitting Li2Ca2Si2O7:Eu2+ phosphor, Mater. Lett., 2012, 79, 112–115 CrossRef CAS.
- G. Annadurai, M. Jayachandiran, S. M. M. Kennedy and V. Sivakumar, Synthesis and photoluminescence properties of Ba2CaZn2Si6O17:Tb3+ green phosphor, Mater. Sci. Eng., B, 2016, 208, 47–52 CrossRef CAS.
- Y. Xia, Y.-G. Liu, Z. Huang, M. Fang, M. S. Molokeev and L. Mei, Ca6La4(SiO4)2(PO4)4O2:Eu2+: a novel apatite green-emitting phosphor for near-ultraviolet excited w-LEDs, J. Mater. Chem. C, 2016, 4(21), 4675–4683 RSC.
- Y. Zhuo, S. Hariyani, J. Zhong and J. Brgoch, Creating a Green-Emitting Phosphor through Selective Rare-Earth Site Preference in NaBaB9O15:Eu2+, Chem. Mater., 2021, 33(9), 3304–3311 CrossRef CAS.
- M. Zhao, H. Liao, L. Ning, Q. Zhang, Q. Liu and Z. Xia, Next-Generation Narrow-Band Green-Emitting RbLi(Li3SiO4)2:Eu2+ Phosphor for Backlight Display Application, Adv. Mater., 2018, 30(38), 1802489 CrossRef PubMed.
- D. Jia, J. Zhu, B. Wu and S. E, Luminescence and energy transfer in CaAl4O7:Tb3+, Ce3+, J. Lumin., 2001, 93(2), 107–114 CrossRef CAS.
- S. Wang, B. Devakumar, Q. Sun, J. Liang, L. Sun and X. Huang, Highly efficient near-UV-excitable Ca2YHf2Al3O12:Ce3+,Tb3+ green-emitting garnet phosphors with potential application in high color rendering warm-white LEDs, J. Mater. Chem. C, 2020, 8(13), 4408–4420 RSC.
- J. Xue, H. M. Noh, S. H. Park, B. R. Lee, J. H. Kim and J. H. Jeong, NUV light induced visible emission in Er3+-activated NaSrLa(MoO4)O3 phosphors for green LEDs and thermometer, J. Am. Ceram. Soc., 2020, 103(2), 1174–1186 CrossRef CAS.
- Y. Li, Q. Yang, Z. Wang, G. Wang, B. Zhang, Q. Zhang and D. Yang, Rapid fabrication of SnO2 nanoparticle photocatalyst: computational understanding and photocatalytic degradation of organic dye, Inorg. Chem. Front., 2018, 5(12), 3005–3014 RSC.
- C. Li, Z. Zang, W. Chen, Z. Hu, X. Tang, W. Hu, K. Sun, X. Liu and W. Chen, Highly pure green light emission of perovskite CsPbBr3 quantum dots and their application for green light-emitting diodes, Opt. Express, 2016, 24(13), 15071–15078 CrossRef CAS PubMed.
- X. Shen, C. Sun, X. Bai, X. Zhang, Y. Wang, Y. Wang, H. Song and W. W. Yu, Efficient and Stable CsPb(Br/I)3@Anthracene Composites for White Light-Emitting Devices, ACS Appl. Mater. Interfaces, 2018, 10(19), 16768–16775 CrossRef CAS PubMed.
- P. Strobel, S. Schmiechen, M. Siegert, A. Tücks, P. J. Schmidt and W. Schnick, Narrow-Band Green Emitting Nitridolithoalumosilicate Ba[Li2(Al2Si2)N6]:Eu2+ with Framework Topology whj for LED/LCD-Backlighting Applications, Chem. Mater., 2015, 27(17), 6109–6115 CrossRef CAS.
- Y. Ito, T. Hori, T. Kusunoki, H. Nomura and H. Kondo, A phosphor sheet and a backlight system providing wider color gamut for LCDs, J. Soc. Inf. Disp., 2014, 22(8), 419–428 CrossRef CAS.
- T. Takeda, N. Hirosaki, S. Funahshi and R.-J. Xie, Narrow-Band Green-Emitting Phosphor Ba2LiSi7AlN12:Eu2+ with High Thermal Stability Discovered by a Single Particle Diagnosis Approach, Chem. Mater., 2015, 27(17), 5892–5898 CrossRef CAS.
- Y. Zhu, Y. Liang, S. Liu, H. Li and J. Chen, Narrow-Band Green-Emitting Sr2MgAl22O36:Mn2+ Phosphors with Superior Thermal Stability and Wide Color Gamut for Backlighting Display Applications, Adv. Opt. Mater., 2019, 7(6), 1801419 CrossRef.
- H. Li, Y. Liang, S. Liu, W. Zhang, Y. Bi, Y. Gong and W. Lei, Highly Efficient Green-Emitting Phosphor BaZnAl10O17:Mn2+ with Ultra-Narrow Band and Extremely Low Thermal Quenching for Wide Color Gamut LCD Backlights, Adv. Opt. Mater., 2021, 9(24), 2100799 CrossRef CAS.
- C. Bertail, S. Maron, V. Buissette, T. Le Mercier, T. Gacoin and J.-P. Boilot, Structural and Photoluminescent Properties of Zn2SiO4:Mn2+ Nanoparticles Prepared by a Protected Annealing Process, Chem. Mater., 2011, 23(11), 2961–2967 CrossRef CAS.
- Y. Hu, Y. Yang, X. Zhang, X. Wang, X. Li, Y. Li, T. Li and H. Zhang, X-ray-Excited Super-Long Green Persistent Luminescence from Tb3+ Monodoped β-NaYF4, J. Phys. Chem. C, 2020, 124(45), 24940–24948 CrossRef CAS.
- S. Vaidyanathan, Recent progress on lanthanide-based long persistent phosphors: an overview, J. Mater. Chem. C, 2023, 11(26), 8649–8687 RSC.
- A. Balhara, S. K. Gupta, M. Abraham, B. Modak, S. Das, C. Nayak, H. V. Annadata and M. Tyagi, Trap esngineering through chemical doping for ultralong X-ray persistent luminescence and anti-thermal quenching in Zn2GeO4, J. Mater. Chem. C, 2024, 12(5), 1728–1745 RSC.
- S. K. Gupta, B. Modak, M. Abraham, S. Das, R. Gupta, K. G. Girija, M. Mohapatra and K. Sudarshan, Defect induced tunable light emitting diodes of compositionally modulated zinc gallium germanium oxides, Chem. Eng. J., 2023, 474, 145595 CrossRef CAS.
- T. Hu, H. Lin, J. Xu, B. Wang, J. Wang and Y. Wang, Color-tunable persistent luminescence in oxyfluoride glass and glass ceramic containing Mn2+:α-Zn2SiO4 nanocrystals, J. Mater. Chem. C, 2017, 5(6), 1479–1487 RSC.
- M. Ma, L. Li, C. Cai, Y. Han and Y. Yang, X-ray excited (Mg,Ca)F2:Mn2+ for persistent luminescence modulation, J. Lumin., 2022, 252, 119376 CrossRef CAS.
- J. Xue, F. Li, F. Liu, H. M. Noh, B. R. Lee, B. C. Choi, S. H. Park, J. H. Jeong and P. Du, Designing ultra-highly efficient Mn2+-activated Zn2GeO4 green-emitting persistent phosphors toward versatile applications, Mater. Today Chem., 2022, 23, 100693 CrossRef CAS.
- R. T. Parayil, S. K. Gupta and M. Mohapatra, A review on defect engineered NIR persistent luminescence through transition metal ion (Cr, Mn, Fe and Ni) doping: Wider perspective covering synthesis, characterization, fundamentals and applications, Coord. Chem. Rev., 2025, 522, 216200 CrossRef CAS.
- B. Jiang, F. Chi, X. Wei, Y. Chen and M. Yin, A self-activated MgGa2O4 for persistent luminescence phosphor, J. Appl. Phys., 2018, 124(6), 063101 CrossRef.
- A. Mondal and J. Manam, Structural and Luminescent Properties of Si4+ Co-Doped MgGa2O4:Cr3+ Near Infra-Red Long Lasting Phosphor, ECS J. Solid State Sci. Technol., 2017, 6(7), R88 CrossRef CAS.
- A. Mondal, S. Das and J. Manam, Hydrothermal synthesis, structural and luminescent properties of a Cr3+ doped MgGa2O4 near-infrared long lasting nanophospor, RSC Adv., 2016, 6(86), 82484–82495 RSC.
- K. Girija, S. Thirumalairajan, V. R. Mastelaro and D. Mangalaraj, Photocatalytic degradation of organic pollutants by shape selective synthesis of β-Ga2O3 microspheres constituted by nanospheres for environmental remediation, J. Mater. Chem. A, 2015, 3(6), 2617–2627 RSC.
- T. Naka, T. Nakane, S. Ishii, M. Nakayama, A. Ohmura, F. Ishikawa, A. de Visser, H. Abe and T. Uchikoshi, Cluster glass transition and relaxation in the random spinel CoGa2O4, Phys. Rev. B: Condens. Matter Mater. Phys., 2021, 103(22), 224408 CrossRef CAS.
- P. Liu, Y. Zhang, B. Li, L. Han and Y. Xu, Trap depth engineering in MgGa2O4: Bi3+ for muticolor dynamic anti-counterfeiting, encryption and optical temperature sensing applications, Chem. Eng. J., 2022, 437, 135389 CrossRef CAS.
- N. Li, J. Liu, X. Duan, F. Yu and H. Jiang, Synthesis and microstructure of Co/Ni: MgGa2O4 nanoparticles, J. Nanopart. Res., 2017, 19(8), 285 CrossRef.
- R. T. Parayil, S. K. Gupta, M. Pal, A. Dutta, D. Tyagi, K. Sudarshan and M. Mohapatra, ZnGa2−xAlxO4 (x = 0 ≤ 2) spinel for persistent light emission and HER/OER bi-functional catalysis, RSC Adv., 2023, 13(44), 31101–31111 RSC.
- C. Yu, L. Zhang, J. Shi, J. Zhao, J. Gao and D. Yan, A Simple Template-Free Strategy to Synthesize Nanoporous Manganese and Nickel Oxides with Narrow Pore Size Distribution, and Their Electrochemical Properties, Adv. Funct. Mater., 2008, 18(10), 1544–1554 CrossRef CAS.
- R. T. Parayil, S. K. Gupta, K. Garg, S. Jangra, S. Samanta, K. Sudarshan, M. Mohapatra and T. C. Nagaiah, Enhanced electrocatalytic performance of bismuth-doped zinc stannate towards OER and HER through oxygen vacancies: p-block metal ion doping empowering d-block, Sustainable Energy Fuels, 2024, 8(14), 3136–3144 RSC.
- R. T. Parayil, S. K. Gupta, K. Garg, S. Mehta, K. Sudarshan, M. Mohapatra and T. C. Nagaiah, Improved catalytic activity on transitioning from inverse to normal spinel in Zn2−xGa2xSn1−xO4: a robust bifunctional OER and HER electrocatalyst, Sustainable Energy Fuels, 2024, 8(10), 2144–2152 RSC.
- N. Singh, V. Singh, G. Sivaramaiah, J. L. Rao, P. K. Singh, M. S. Pathak, S. J. Dhoble and M. Mohapatra, EPR and optical properties of Eu2+ and Mn2+ co-doped MgSrAl10O17 blue–green light emitting powder phosphors, J. Lumin., 2016, 178, 479–486 CrossRef CAS.
- Q. Zhou, L. Dolgov, A. M. Srivastava, L. Zhou, Z. Wang, J. Shi, M. D. Dramićanin, M. G. Brik and M. Wu, Mn2+ and Mn4+ red phosphors: synthesis, luminescence and applications in WLEDs. A review, J. Mater. Chem. C, 2018, 6(11), 2652–2671 RSC.
- Y. Li, S. Qi, P. Li and Z. Wang, Research progress of Mn doped phosphors, RSC Adv., 2017, 7(61), 38318–38334 RSC.
- Y. Feng, R. Liu, L. Zhang, Z. Li, Y. Su and Y. Lv, Raspberry-Like Mesoporous Zn1.07Ga2.34Si0.98O6.56:Cr0.01 Nanocarriers for Enhanced Near-Infrared Afterglow Imaging and Combined Cancer Chemotherapy, ACS Appl. Mater. Interfaces, 2019, 11(48), 44978–44988 CrossRef CAS PubMed.
- M. Liao, Q. Wang, Q. Lin, M. Xiong, X. Zhang, H. Dong, Z. Lin, M. Wen, D. Zhu, Z. Mu and F. Wu, Na Replaces Rb towards High-Performance Narrow-Band Green Phosphors for Backlight Display Applications, Adv. Opt. Mater., 2021, 9(17), 2100465 CrossRef CAS.
- Y. Pan, Y. Tang, X. Yin, M. Qiang, X. Yao and D. Zhang, ZnAl2O4:Mn2+ transparent phosphor ceramic with narrow-band green emission by spark plasma sintering, J. Lumin., 2024, 265, 120198 CrossRef CAS.
- C. Wang, X. Wang, Y. Zhou, S. Zhang, C. Li, D. Hu, L. Xu and H. Jiao, An Ultra-Broadband Near-Infrared Cr3+-Activated Gallogermanate Mg3Ga2GeO8 Phosphor as Light Sources for Food Analysis, ACS Appl. Electron. Mater., 2019, 1(6), 1046–1053 CrossRef CAS.
- Y. Yan, H. Dai, J. Li, C. Wang, W. Zhang and G. Zhu, Narrow-band green-emitting Cs3ZnCl5: Mn2+ phosphors with abnormal thermal quenching, J. Lumin., 2023, 258, 119821 CrossRef CAS.
- B. Wang, Y. Kong, Z. Chen, X. Li, S. Wang and Q. Zeng, Thermal stability and photoluminescence of Mn2+ activated green-emitting feldspar phosphor SrAl2Si2O8: Mn2+ for wide gamut w-LED backlight, Opt. Mater., 2020, 99, 109535 CrossRef CAS.
- G. Lu, Y. Wang, K. Ma, X. Chen, W. Geng, T. Liu, S. Xu, J. Zhang and B. Chen, A high-efficiency near-ultraviolet excited Mn2+ doped narrow-band emission phosphor via a strong charge transfer for wide color gamut WLED, Ceram. Int., 2024, 50(9, Part B), 16190–16200 CrossRef CAS.
- H. Chen and Y. Wang, Photoluminescence and cathodoluminescence properties of novel rare-earth free narrow-band bright green-emitting ZnB2O4:Mn2+ phosphor for LEDs and FEDs, Chem. Eng. J., 2019, 361, 314–321 CrossRef CAS.
- R. Cao, D. Peng, H. Xu, S. Jiang, Z. Luo, H. Ao and P. Liu, Synthesis and luminescence properties of NaAl11O17:Mn2+ green phosphor for white LEDs, J. Lumin., 2016, 178, 388–391 CrossRef CAS.
- Y. Fang, Y. Li, C. Wang, W. Yin, T. Shi, G. Zhang, G. Zhao, X. Zhou, J. Yang, D. Wu, L. Dong and J. Hou, Intense green light emission in Sr2ZnGe2O7:Mn2+ phosphors by the design of high symmetry melilite structure, Luminescence, 2024, 39(1), e4555 CrossRef CAS PubMed.
- Z. Wu, C. Li, F. Zhang, S. Huang, F. Wang, X. Wang and H. Jiao, High-performance ultra-narrow-band green-emitting phosphor LaMgAl11O19:Mn2+ for wide color-gamut WLED backlight displays, J. Mater. Chem. C, 2022, 10(19), 7443–7448 RSC.
- X. Wu, Y. Liang, Y. Xue, H. Li, Y. Dou, W. Zhang, Q. Wang and C. Han, Synthesis and luminescence properties of a novel narrow band green-emitting Na2MgAl10O17:Mn2+ phosphor for backlight display, J. Alloys Compd., 2024, 990, 174397 CrossRef CAS.
- K. A. Denault, J. Brgoch, M. W. Gaultois, A. Mikhailovsky, R. Petry, H. Winkler, S. P. DenBaars and R. Seshadri, Consequences of Optimal Bond Valence on Structural Rigidity and Improved Luminescence Properties in SrxBa2−xSiO4:Eu2+ Orthosilicate Phosphors, Chem. Mater., 2014, 26(7), 2275–2282 CrossRef CAS.
- M. Abraham, A. K. Kunti, K. K. Thejas, N. Amador-Mendez, N. Gogneau, K. G. Nishanth, M. Tchernycheva and S. Das, The elevated colour rendering of white-LEDs by microwave-synthesized red-emitting (Li, Mg)3RbGe8O18:Mn4+ nanophosphors, Dalton Trans., 2021, 50(8), 3044–3059 RSC.
- M. Abraham, K. K. Thejas, A. K. Kunti, N. Amador-Mendez, R. Hernandez, J. Duras, K. G. Nishanth, S. K. Sahoo, M. Tchernycheva and S. Das, Strategically Developed Strong Red-Emitting Oxyfluoride Nanophosphors for Next-Generation Lighting Applications, Adv. Opt. Mater., 2024, 2401356 CrossRef CAS.
- G. K. B. Costa, S. S. Pedro, I. C. S. Carvalho and L. P. Sosman, Preparation, structure analysis and photoluminescence properties of MgGa2O4:Mn2+, Opt. Mater., 2009, 31(11), 1620–1627 CrossRef CAS.
- B. Zhu, L. Wang, Q. Shi, H. Guo, J. Qiao, C. E. Cui and P. Huang, MgGa2O4:Mn2+, Mn4+: A dual-emitting phosphors with unique optical temperature sensing, J. Alloys Compd., 2023, 948, 169717 CrossRef CAS.
- W. Ahn, M. Im and Y. J. Kim, Effects of flux on the luminescence of MgGa2O4:Mn2+ phosphors, Mater. Res. Bull., 2017, 96, 254–257 CrossRef CAS.
- M. Takesada, M. Osada and T. Isobe, Glycothermal Synthesis and Photoluminescence of MgGa2O4:Mn2+ Nanophosphors: Comparison to ZnGa2O4:Mn2+ Nanophosphors, J. Electrochem. Soc., 2009, 156(5), J97 CrossRef CAS.
- S. Choi, K. Kim, Y.-M. Moon, B.-Y. Park and H.-K. Jung, Rapid synthesis of spherical-shaped green-emitting MgGa2O4:Mn2+ phosphor via spray pyrolysis, Mater. Res. Bull., 2010, 45(8), 979–981 CrossRef CAS.
- E. H. Song, J. L. Wang, D. C. Yu, S. Ye and Q. Y. Zhang, Anomalous tunable visible to near infrared emission in the Mn2+-doped spinel MgGa2O4 and room-temperature upconversion in the Mn2+ and Yb3+-codoped spinel, J. Mater. Chem. C, 2014, 2(41), 8811–8816 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4dt02960g |
| This journal is © The Royal Society of Chemistry 2025 |