Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Earth-abundant transition metal complexes in light-emitting electrochemical cells: successes, challenges and perspectives
Ginevra
Giobbio
 *a,
Rubén D.
Costa
*a,
Rubén D.
Costa
 *b and
Sylvain
Gaillard
*b and
Sylvain
Gaillard
 *a
*a
aNormandy University, ENSICAEN, UNICAEN, CNRS, LCMT, 6 Bd du Maréchal Juin, 14050 Caen, France. E-mail: sylvain.gaillard@ensicaen.fr
bTechnical University of Munich, Campus Straubing for Biotechnology and Sustainability, Chair of Biogenic Functional Materials, 22 Schulgasse, 94315 Straubing, Germany. E-mail: ruben.costa@tum.de
First published on 13th January 2025
Abstract
Light-emitting electrochemical cells (LECs) are an attractive technology in the field of solid state light devices (SSLDs) as their simple architectures allow the preparation of cost-effective lighting devices. Consequently, low-cost and sustainable emitters are highly desirable. Transition metal complexes are attractive in this field as they have been proved to possess compatible optoelectronic properties. Nowadays, the best emitters are based on platinum and iridium class metals, which is a limitation for industrial production. Due to this concern, researchers have turned their attention to Earth-abundant metal complexes. However, the abundance of these metals should not blind us to a consideration of their cost. Herein, the photophysical properties of the most interesting Earth-abundant metal complexes and their performance in LECs are put into context with respect to their real cost based on their metal precursors, revealing some surprises.
Introduction
Sustainable lighting technologies are a key research area, as 15% of global electricity consumption is addressed to lighting.1 Nowadays, highly inefficient traditional incandescent bulbs and fluorescent lamps are being replaced step by step by less energy-demanding solid-state lighting devices (SSLDs).2 This is crucial for environmental concerns as population growth and the ever-increasing need for illumination and screen systems will result in a continuous increase in energy demand. Furthermore, electricity generation still relies on fossil fuels, and the use of high-efficiency lighting technologies could directly contribute to a cut in greenhouse gas emission in good agreement with the goals of the Paris Agreement.3The first reported SSLD was a light emitting diode (LED) in 19274 and since 1968, the technology has become a commercial reality and is widespread in the lighting market. In parallel, the organic light emitting diodes (OLEDs) reported first by Pope, Kallmann and Magnate,5 have emerged at this time to overcome to the color tunability issue of LEDs and offered an alternative thin-film multilayer-based device. Nevertheless, color availability is not the sole issue to solve in SSLDs, where the cost and availability of raw materials are critical concerns.6 Based on this point, the components of inorganic semiconductors to elaborate LEDs using low-abundant and expensive elements is a limitation for the global lighting sector. The next big share of cost in the lighting sector is device fabrication costs. Indeed, the semiconductor technology of LEDs requires materials characterized by high crystallinity,7 while high-performance OLEDs are fabricated by step-by-step vacuum deposition of the different layers.8 This vacuum deposition needs special equipment and inert atmosphere conditions, which increase the cost of production. Alternatively, OLEDs can be elaborated by wet deposition methods, but their performance and up-scalability are still a concern.9 Alternatively, light-emitting electrochemical cells (LECs) were proposed by Pei in 1995, combining a wet deposition process and a simpler device architecture with (virtually) just one layer.10 Such a difference in device architecture is related to the different working principles of OLEDs and LECs. Briefly, in an OLED the applied voltage causes the injection of holes and electrons from the electrodes to the active layer, forming excitons. Thus, to ensure high mobility and efficient recombination, a multilayer architecture is required. Conversely, LECs are based on the migration of ionic species under bias, due to the strong interfacial electric field of the electrodes. More detailed aspects of the working principles of OLEDs11 and LECs12 have been thoroughly reviewed in the literature.
In brief, after this historical and societal introduction, LECs may offer an opportunity for the inexpensive and easy large-scale production of lighting devices.13 Nevertheless, this technology has not reached the maturity of the LEDs and OLEDs that are currently commercially available in, for example, for smartphones, TVs, and lighting applications.14 Indeed, LECs are usually less efficient, bright and stable than OLEDs, and the development of emitters that address these issues is still a current challenge.
In this context, the emitters need to possess several specific properties, such as the ability to reach very high internal quantum efficiencies and electrochemical stability. Here, ionic transition metal complexes (iTMCs) show great potential thanks to their efficient phosphorescence or thermally activated delayed fluorescence (TADF).11,15 Phosphorescent emitters were based on rare and expensive metals such as Ir, Pd or Pt due to their high spin–orbit coupling (SOC). However, their low abundance on Earth does not align well with the principles of low manufacturing cost and sustainability.16 Therefore, research groups focused on exploiting the photophysical properties of ITMCs based on more abundant metals.17 Nevertheless, the metal precursors for the synthesis of abundant transition metal complexes are not always inexpensive and our discussion will pay particular attention to this point. It will start with the most relevant emitters that have already been applied in LECs. Then, it will pursue potential future new members according to their photophysical properties or the recent developments in new LEC architecture strategies that might allow their use. For convenience, Table 1 summarizes the transition metals that are discussed, with their abundance in the Earth's crust and the prices of the commercially available precursors used for synthesis of the emitters.
| Metal | Abundance17 (ppm) | Precursor in synthesis | Pricea (euro per mol) |
|---|---|---|---|
| a Calculated from the best price found in the Merck catalogue.18 | |||
| Ir | 0.000037 | IrCl 3 ·xH 2 O |
58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 000 000
|
| Ti | 4136 | Ti(OtBu)4 | 1700 |
| Ti(OiPr)4 | 50 | ||
| Cp2TiCl2 | 855 | ||
| Mn | 774 | MnBr2 | 854 |
| MnCl2 | 38 | ||
| Cr | 135 | CrCl3 | 30 |
| CrCl2 | 3000 | ||
| Zr | 132 | Zr(Bn)4 | 107![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| ZrCl4 | 377 | ||
| Zn | 72 | Zn(OAc)2·2H2O | 27 |
| Zn(NO3)2·6H2O | 26 | ||
| ZnEt2 | 506 | ||
| Cu | 27 | [Cu(CH3CN)4][PF6] | 5000 |
| [Cu(CH3CN)4][BF4] | 5713 | ||
| Cu2O | 1322 | ||
| CuCl | 506 | ||
| CuBr | 334 | ||
| CuI | 117 | ||
| Cu(0) powder | 863 | ||
| W | 1 | WCl6 | 3640 |
| WO2Cl2 | 4300 | ||
| Ag | 0.055 | AgPF6 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
| AgBF4 | 4653 | ||
| Ag2O | 4282 | ||
| AgClO4 | 1991 | ||
Discussion
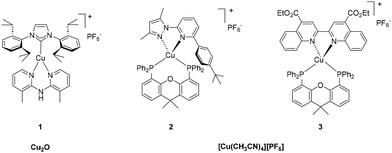
So far, the most studied abundant transition metal for the synthesis of emitters in LECs is copper. As established since the pioneering works of McMillin and co-workers, the luminescent properties of copper(I) complexes are based on metal-to-ligand charge transfer (MLCT) transitions that allow easy modulation of the emission properties by ligand design.19 Furthermore, they have been demonstrated to be efficient TADF emitters and, in turn, perfect candidates for sustainable lighting technologies.20 Even if Cu(I) complexes offer a wide opportunity for the modulation of emission color, their excited-state dynamics still makes them challenging.21 Indeed, pseudo-tetrahedral Cu(I) complexes are prone to flattening distortion in the excited state due to the formal +II oxidation state of the metal centre. In this scenario, the Cu(II) metal centre normally adopts a square-planar geometry, which leads to non-radiative decay pathways. This drawback can be tackled by ligand design to limit or fully avoid this flattening of the complex.22 Nevertheless, this molecular design focusing on geometric conservation has sometimes limited the fine-tuning of color emission. Indeed, deep-blue23,24 or far-red25,26 emissive mononuclear Cu(I) complexes have only recently been obtained. As a consequence, white-emitting LECs based on Cu(I) complexes as emitters are still rare in the literature.27To illustrate this statement, Cu(I) complexes bearing NHC dipyridylamine ligands have been designed to avoid the tetrahedral distortion, and the versatility of the N^N ligand driving the emission of the complexes led to a strongly blue-emissive compound (1, λmax = 497 nm, PLQY = 0.86) allowing the first example of a high-energy emissive Cu(I)-based LEC (22.2 cd m−2 for t1/2 = 16.5 min) (Fig. 1).23,28 Another representative blue-emitting copper complex was reported by Costa et al. based on a tetragonal Cu(I) complex bearing pyrazol-pyridine as N^N ligand (2) (Fig. 1). Here, the remarkable photophysical properties (λmax = 470 nm, PLQY = 0.42) and performance in devices (205 cd m−2, t1/2 = 1.5 min, and EQE = 0.11%) were obtained from the electron-donating effect of the N^N ligand and the sterical hindrance generated by both N^N and P^P ligands.24
With respect to low-energy emitting LECs, red-emissive Cu(I) complexes present a challenge, as the low-energy excited state is often affected by vibrational relaxation, in competition with emissive radiative decay pathways. Therefore, the design of Cu(I) complexes with high PLQY and deep-red emission is still a challenge.29,30 In this context, an interesting strategy was to lower the energy of the π* orbitals of the ligand. Following this approach, in 2019, Fresta et al. reported a series of tetrahedral Cu(I) complexes bearing a biquinoline ligand possessing π-extended and electron-poor heteroaromatic rings compared to conventional bipyridine-type ligands, leading to significant red-shifted emission for the brightest complex (3, λmax = 675 nm, PLQY = 0.056) (Fig. 1).25 The resulting red-emitting LEC exhibited is the actual benchmark with an irradiance of 129.8 μW cm−2 and a power efficiency of 0.19 lm W−1.25
White-emitting LECs are scarce in the literature as they generally follow a host–guest approach that requires efficient red emitters. In this context, the best performing white-emissive devices have been obtained by blending blue- and red-emissive Cu(I) complexes in around a 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio in the active layer. Such prepared devices led to a luminance of 30 cd m−2, a lifetime of 2 h, an EQE of 0.3%, and chromaticity with colour coordinates (x/y) of 0.30/0.35 according to the Commission internationale de l’éclairage (CIE).24
2 molar ratio in the active layer. Such prepared devices led to a luminance of 30 cd m−2, a lifetime of 2 h, an EQE of 0.3%, and chromaticity with colour coordinates (x/y) of 0.30/0.35 according to the Commission internationale de l’éclairage (CIE).24
From the synthetical point of view, the aforementioned tetrahedral heteroleptic [Cu(N^N)(P^P)][X] complexes have been prepared following standard procedures using a [Cu(CH3CN)4][X] copper precursor with various counterions (Fig. 1). Even if the abundance in the Earth's crust of copper is almost one million times higher than that of iridium, the prices between the metal precursors does not follow this trend. Indeed, if the commercial price of the most common iridium and copper sources are compared, only a factor of around 12 exists between them (58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 € per mol for IrCl3·xH2O vs. 5000 € per mol for [Cu(CH3CN)4][PF6], Table 1). Notably, the [Cu(CH3CN)4][X] copper precursor can be prepared from less expensive Cu2O (1322 € per mol, Table 1), but the reaction requires an inert atmosphere to avoid oxidation of the air-sensitive product.31 Additionally, the price of the ligands also has to be considered. Indeed, diphosphines (DPEPhos and Xantphos) are often used as ligands for the copper emitters. However, the cost of such diphosphine derivatives can be high (DPEPhos: 2870 € per mol or Xantphos: 12
000 € per mol for IrCl3·xH2O vs. 5000 € per mol for [Cu(CH3CN)4][PF6], Table 1). Notably, the [Cu(CH3CN)4][X] copper precursor can be prepared from less expensive Cu2O (1322 € per mol, Table 1), but the reaction requires an inert atmosphere to avoid oxidation of the air-sensitive product.31 Additionally, the price of the ligands also has to be considered. Indeed, diphosphines (DPEPhos and Xantphos) are often used as ligands for the copper emitters. However, the cost of such diphosphine derivatives can be high (DPEPhos: 2870 € per mol or Xantphos: 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 930 € per mol) compared to the heteroaromatic derivatives used for the iridium emitters (2-phenylpyridine: 756 € per mol).18
930 € per mol) compared to the heteroaromatic derivatives used for the iridium emitters (2-phenylpyridine: 756 € per mol).18
Moving back to trigonal Cu(I) complexes bearing NHC ligands and bis-pyridyl derivatives, three different strategies can be adopted to achieve the [Cu(NHC)X] intermediate. First, the imidazolium pro-ligand can be deprotonated in the presence of a base, providing the free NHC that binds Cu(I) halide.32 This procedure involves inexpensive Cu(I) sources, such as metal halides (CuCl = 506 € per mol; CuBr = 334 € per mol; CuI = 117 € per mol, Table 1). However, the procedure has to be run under strictly anhydrous and deoxygenated conditions due to the generation of free carbene in the reaction mixture and because CuX (X = Cl, Br and I) are air-sensitive. Finally, the procedure with Cu2O appears to be a good compromise in terms of cost (1322 € per mol, Table 1) and reaction conditions as the reagents are less sensitive and the procedure does not require the use of a strong base.32a,33
However, up to now Cu(I)-based LECs have suffered from shorter device lifetimes compared to Ir(III)-based LECs. Therefore, optimization of performance often requires joint efforts in terms of device architecture and the molecular design of more stable copper emitters. As a result, some research groups have turned their attention to other Earth-abundant transition metals.
Moving down the 11th column, growing attention has been dedicated to Ag(I) complexes as emitters in SSLD devices. As the 4d orbitals of the Ag(I) metal centre are typically lower in energy than those of the ligand, the emission in Ag(I) complexes is generally attributed to ligand-centred (LC) transitions with minimal metal contribution.34,35 For this reason, the metal centre in tetrahedral Ag(I) complexes avoids the flattening issue related to copper(I) complexes. In addition, the higher ligand field splitting of the Ag(I) metal centre allows the easy design of high-energy emissive complexes that show higher stability against oxidation processes.34,36 Interestingly, a wide range of photophysical properties, including TADF,37 phosphorescence,38 dual emission,39 and aggregation induced dual phosphorescence40 have recently been reported.
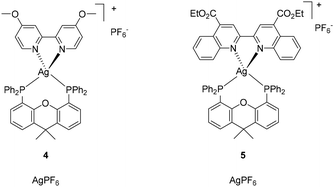
Nevertheless, the implementation of Ag(I)-based emitters in LECs is still rare in the literature and just a few examples have been reported so far.35,39–42 The first reported emissive Ag(I)-based LECs showed remarkable brightness of 395 cd m−2.41 The actual benchmark for a white-emitting Ag(I)-based LEC was achieved with [Ag(N^N)(P^P)][PF6] (4) complexes exhibiting a luminance of 35 cd m−2 and a lifetime for the device of about 80 h, which is 40 times greater than the best stability of a white-emitting Cu(I)-based LEC at this time (Fig. 2).42 Notably, these Ag(I) complexes presented a very broad emission band, compromising the monochromatic preparation of the LEC. However, the possibility of effective color-tuning for Ag(I) complexes and related devices was recently demonstrated using [Ag(N^N)(P^P)][PF6], where N^N is a biquinoline derivative and P^P is Xantphos (5) (Fig. 2). The resulting Ag(I) complex showed bright-red luminescence and, when implemented in multi-layered LECs, led to an irradiance of ∼40 μW cm−2, EQE of 0.006%, and stability of 5 h.39 Considering the cost of the synthesis of such complexes, the procedure mainly involved a very light- and moisture-sensitive AgPF6 precursor (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 € per mol, Table 1). For this reason, a procedure that could start from less expensive AgBF4 (4653 € per mol) or even better the less sensitive and cheapest Ag2O (4282 € per mol) would be of interest (Table 1).
300 € per mol, Table 1). For this reason, a procedure that could start from less expensive AgBF4 (4653 € per mol) or even better the less sensitive and cheapest Ag2O (4282 € per mol) would be of interest (Table 1).
Similar to Cu(I) and Ag(I), Zn(II) complexes have a d10 electronic configuration. However, due to the higher ionization potential of the metal centre, MLCT transitions are often disfavoured. Indeed, the lowest-lying states commonly originate from LC and LLCT excitation processes, which result in fluorescence emission.43 However, re-investigation of the photophysical properties of Zn(II) complexes proved that the SOC of the Zn2+ ion is weak but sometimes enough to allow a more suitable TADF process.44
Finally, phosphorescent Zn(II) complexes have also been known in the literature since 1971.45 In detail, Zn(II)-porphyrins exhibited deep-red emission that inspired their use as emitters in LECs.46–48 The most remarkable result was the NIR(II)-emissive LEC (λEL = 900 nm) reported by Wang and co-workers, which performed with an irradiance of 36 μW cm−2 and an EQE of 0.028% (6) (Fig. 3).48 Considering the cost of such an emitter, the most attractive aspects are the zinc abundance of 72 ppm and the low cost of the metal precursor Zn(OAc)2·2H2O (27 € per mol), which is almost 200 times less expensive than [Cu(CH3CN)4][PF6] (5000 € per mol) (Table 1). However, modulation of the photophysical properties of Zn(II) complexes may require huge synthetic efforts. Indeed, the porphyrins in the complex display a sophisticated ligand design that might increase the total cost of the emitter. Notably, if a brief survey of the OLED field is undertaken, some other metal precursors, such as Zn(NO3)2·6H2O (26 € per mol) or ZnEt2 (506 € per mol), have also been described for the synthesis of emitters (Table 1).49
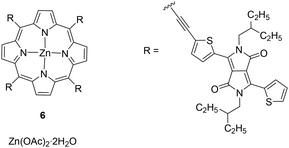 | ||
| Fig. 3 Zn(II)-based porphyrin complex reported by Wang and co-workers.48 | ||
Among many different valence states available for the manganese ion, Mn2+ possesses a d5 electronic configuration and shows metal centered (MC) radiative transitions that are strongly affected by the crystal field strength.50 Consequently, the geometry of Mn(II) complexes can be tetrahedral or octahedral, depending on the nature of the ligands. The tetrahedral coordination requires a weaker field strength and these Mn(II) complexes are often characterized by green emission.50,51 Conversely, octahedral Mn(II) complexes display a longer lifetime with an emission wavelength spanning from orange to NIR.50,52 Additionally, the resulting emission from Mn(II) complexes is particular as it results from a spin flip (SF) transition. For Mn(II) complexes, this SF proceeds by energy transfer from the initial excitation of the ligand or the counterion.50,51 For such a scenario, the energy level of the ligand or counter anion excited state has to be higher by 2.88 eV than the energy level of the last populated d orbital of the Mn(II) metal centre.51
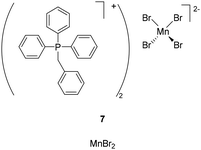
As a consequence, some Mn(II) complexes have been applied in the field of OLEDs,53 but just one example of an Mn(II)-based LEC has been reported using tetrahedral [Ph3PBn][MnBr4] (7), showing remarkable photophysical properties in powder form (λmax = 512 nm, PLQY = 1) (Fig. 4). The resulting host–guest-structured device exhibited white emission with a luminance of 46 cd m−2 and an EQE of 0.045%.54 Even if the performances are still modest, this new incoming type of Mn(II) might offer new opportunities to provide very low-cost LECs, as manganese is almost 30 times more abundant (774 ppm) than copper (27 ppm). This fact is also reflected by the low price of the Mn(II) precursors (MnBr2 = 854 € per mol and MnCl2 = 38 € per mol, Table 1).
At this stage, blue emitters are missing, but new development of this family might be attractive as the synthesis of both tetrahedral and octahedral Mn(II) complexes follows straightforward pathways which do not require an inert atmosphere.
Notably, 7 was also applied in OLEDs and, following a host–guest approach, the best Mn(II)-based devices showed intense, narrow emission with brightness of 5231 cd m−2 and EQE of 11.42% (Fig. 4).55 This example offers the prospect of the re-exploration of metal-based emitters used in OLED for LEC devices and vice versa. So, this overlap in the molecular design of the Mn(II) luminescent complexes used both in OLEDs and in LECs is forcing us to discuss whether the other elements that have been more extensively studied in OLED devices could inspire the development of new candidates in LEC technology. Additionally, the reader should be warned that neutral complexes might be implemented in LEC devices thanks to ionic polymers being used as hosts.56 Of course, metals previously mentioned in the first part of our discussion could be cited again and the reader can refer to some reviews to gain an overview of this field.15,57 From our point of view, it is more interesting to open the discussion to other metals.
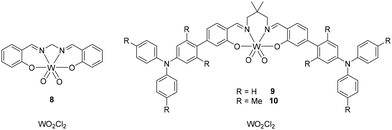
Ironically, tungsten used in an incandescent bulb in its metal(0) state can provide complexes that present interesting properties as emissive dopants in LECs. Indeed, tungsten (1 ppm in the Earth's crust, Table 1) possesses a large SOC, which should ease the intersystem crossing (ISC) between the singlet and the triplet excited states, leading to efficient phosphorescent emitters.58 A representative example in OLEDs was W(VI) complexes bearing Schiff bases.59 These complexes have attracted growing interest, as they are also TADF emitters.58–60 Additionally, their d0 configuration results in ILCT and LMCT characters, implying that simple modification of the Schiff base ligands could allow tuning of the emission color.61
In 2017, Che's group reported complex 8 that poorly emits at 600 nm (PLQY of 0.028 in dichloromethane solution) (Fig. 5). However, when used as a dopant (5 wt%) in 1,3-bis(N-carbazolyl)benzene (mCP) thin-film the PLQY increased to 0.22 and the OLED device featured an EQE of 4.79% with luminance of 40 cd m−2.58 Afterwards, the ligand design achieved for complexes 9 and 10 led to a further improvement in device performance (Fig. 5).59 Considering the cost of the synthesis, these complexes were prepared starting from WO2Cl2, which has similar cost to [Cu(CH3CN)4][PF6] (4300 € per mol vs. 5000 € per mol, Table 1). As the price of this W(VI) source is comparable to Cu(I), attention has to be paid to the price of the ligands for the development of interesting emitters for LECs.
Moving to the fourth group, luminescent Zr(IV) complexes have attracted the attention of researchers due to their electron-deficient d0 configuration. Indeed, the emission of Zr(IV) complexes is based on ligand-to-metal charge transfer (LMCT).62 Therefore, the design of highly electron-donating ligands is a valuable strategy for modulation of wavelength, lifetimes and PLQYs.62 Recently, TADF emission from Zr(IV) complexes 11 was also proven by Milsmann and co-workers (Fig. 6).62 As mentioned above, such an emission mechanism is highly desirable for application in SSLDs and it increased the interest in Zr(IV)-based emitters. Thus, it is worth noting that zirconium is the fourth-most Earth-abundant transition metal (132 ppm) (Table 1). To the best of our knowledge, just one example of OLEDs using Zr(IV)-based emitters has been reported in the literature.63 In detail, Zr(IV) tetra(8-hydroxyquinoline) was used in an OLED device showing an EQE of 1.1%. Interestingly, complex 12 has been prepared starting from inexpensive ZrCl4 (377 € per mol, Table 1) (Fig. 6). However, complex 11 was prepared from an expensive commercially available Zr(Bn)4 (107![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 € per mol, Table 1) (Fig. 6).62 However, Zr(Bn)4 can be prepared by starting from ZrCl4 in the presence of Grignard's reagent BnMgCl in cryogenic conditions followed by multi-step purification that sometimes requires very low-temperature conditions.64 Therefore, the development of Zr(IV)-based emitters could be an option with respect to the cost of the metal source for cost-effective devices.
000 € per mol, Table 1) (Fig. 6).62 However, Zr(Bn)4 can be prepared by starting from ZrCl4 in the presence of Grignard's reagent BnMgCl in cryogenic conditions followed by multi-step purification that sometimes requires very low-temperature conditions.64 Therefore, the development of Zr(IV)-based emitters could be an option with respect to the cost of the metal source for cost-effective devices.
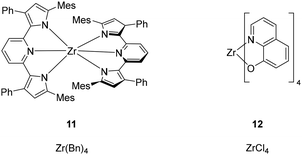 | ||
| Fig. 6 Representative Zr(IV)-based complexes. Left: TADF emitter reported by Milsmann; right: Zr(IV)-based emitter implemented in an OLED. | ||
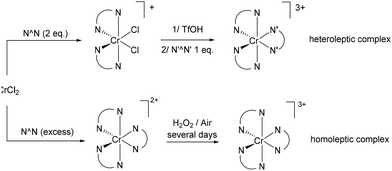
Finally, among Earth-abundant transition metal complexes not applied in LECs or OLEDs, some elements deserve a few words. First, the photophysical properties of Cr(III) complexes have still not gained attention for either OLEDs or LECs, due to its d3 electronic configuration. In addition, the photophysical behavior of Cr(III) complexes is influenced primarily by d–d transitions involving SP processes.65 Thus, Cr(III) complexes are generally characterized by low PLQY, long lifetime (μs–ms range), and narrow far-red emission.65,66 Nevertheless, ligand design to create strong-field ligands could efficiently modulate the d-orbital splitting and consequently increase the PLQY, preventing non-radiative decay.67 Interestingly, the modulation of the emission wavelength appears to be governed by the nephelauxetic effect of the ligand set and even if theoretical prediction is still difficult to perform, the design of new complexes might open the door to new emitters.66,68,69 Thus, the most convenient procedure for the synthesis of Cr(III) complexes involves an oxidation step of the metal centre and the formation of a bis-diimine complex with the general formula of [Cr(N^N)2(CF3SO3)2][CF3SO3], where the two labile triflate ligands can be displaced by different N′^N′ ligands, leading to the formation of a heteroleptic complex (Scheme 1).70 With respect to cost concerns, the abundance of chromium in the Earth's crust is 135 ppm and the moderate costs of the Cr(II) precursors (CrCl2: 3000 € per mol, Table 1) make this class of complexes potentially interesting.
Finally, luminescent Ti(IV) complexes are still scarcely reported, but are of growing interest. As with Zr(IV) complexes, the metal centre is characterized by a d0 electronic configuration, which favours LMCT and ILCT transitions.71 Even if these emission mechanisms are convenient, as they allow modulation of the photophysical properties through straightforward ligand design, the instability of Ti(IV) complexes in solution and their sensitivity to pH prevent their widespread implementation in many different fields.72,73 However, titanium is the second-most Earth-abundant transition metal and the synthetic procedures already reported involve inexpensive Ti(IV) precursors (Ti(OtBu)4 = 1700 € per mol; Ti(OiPr)4 = 50 € per mol, Table 1).71–73 Additionally, the incorporation of another metal into some bis-alkynyl titanocene(IV) complexes (13–15) reported by Wagenknecht and co-workers showed enhanced stability and increased lifetime in the μs range (Fig. 7).74 Additionally, the metal precursor (CpTiCl2), is air-stable and commercially available at low cost (855 € per mol, Table 1).
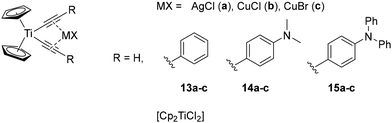 | ||
| Fig. 7 Titanocene(IV) complexes reported by Wagenknecht and co-workers.74 | ||
Conclusions and outlook
Even if Ir(III)-based emitters are the most efficient and best models to develop the best performing LECs, the cost of these complexes confronts the technology with issues of sustainability and supply risk. For these reasons, the door has been opened to the development of emitters based on Earth-abundant transition metals, with Cu(I) complexes as frontrunners. Nevertheless, LECs prepared with such emitters suffer from lifetime issues and the provision of inexpensive and stable emitters for high-performing LECs remains a challenge.For the future development of new emitters in LECs, organometallic chemists have to bear in mind that the complexes should be either phosphorescent or TADF emitters, based on Earth-abundant metals, considering the price of both the precursor and the coordinated ligands.
Considering these key properties, copper chemistry should be dedicated to the synthesis of more stable emitters, as rainbow colors are now accessible and they have proved to be highly interesting TADF emitters. Recently reported Ag(I) complexes appeared to provide very stable LECs, but efforts should be dedicated to color tuning and enhanced PLQY.
A few new approaches using complexes of Zn(II) and Mn(II) as emitters have recently been reported and could lead to promising LECs. However, these examples are still too rare to provide any template for molecular design.
Finally, the recent design architecture of some LEC devices allowed the use of neutral emitters that have been applied in OLED technology. This new opportunity might lead to investigation of all the above-mentioned neutral metal complexes but not only these. Indeed, W(VI) and Zr(IV) complexes that have been applied to OLEDs may now find new interest in LECs as well as the other Earth-abundant elements that have not been explored in SSLDs.
Data availability
All data are reported in the literature from references that are cited in the bibliography section of our article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the “Ministère de L'Enseignement Supérieur et de la Recherche”, CNRS (Centre National de la Recherche Scientifique) and LABEX SynOrg (ANR-11-LABX-0029). S. G. acknowledges the “Région Normandie” (G. G.), the Graduate School of Research XL-Chem (ANR-18-EURE-0020 XL-Chem) for funding.References
- United Nation Climate Change , https://unfccc.int/ (accessed November 2024).
- Lighting – IEA , https://www.iea.org/energy-system/buildings/lighting (accessed November 2024).
- European Council – Council of the European Union , https://www.consilium.europa.eu/en/policies/climate-change/paris-agreement/(accessed November 2024).
- O. V. Lossev, London Edinburgh Philos. Mag. J. Sci., 1928, 6, 1024–1044 CrossRef CAS.
- M. Pope, H. P. Kallmann and P. Magnate, J. Chem. Phys., 1963, 38, 2042–2043 CrossRef CAS.
- C. C. Pavel, A. Marmier, E. Tzimas, T. Schleicher, D. Schlüler, M. Buchert and D. Blagoeva, Phys. Status Solidi A, 2016, 213, 2937–2946 CrossRef CAS.
- C. J. Humphreys, MRS Bull., 2008, 33, 459–470 CrossRef.
- J. H. Kwon, S. Yoo, R. Lampande and S. Kim, Vacuum Deposition, Springer Japan, Tokyo, 2019 Search PubMed.
- X.-Y. Zeng, Y.-Q. Tang, X.-Y. Cai, J.-X. Tang and Y.-Q. Li, Mater. Chem. Front., 2023, 7, 1166–1196 RSC.
- Q. Pei, G. Yu, C. Zhang, Y. Yang and A. J. Heeger, Science, 1995, 269, 1086–1088 CrossRef CAS.
- (a) H. Yersin, A. F. Rausch, R. Czerwieniec, T. Hofbeck and T. Fischer, Coord. Chem. Rev., 2011, 255, 2622–2652 CrossRef CAS; (b) D. Volz, J. Photonics Energy, 2016, 6, 020901 CrossRef.
- Light-Emitting Electrochemical Cells, ed. R. D. Costa, Springer International Publishing, Cham, 2017 Search PubMed.
- Q. Pei and R. D. Costa, Adv. Funct. Mater., 2020, 30, 2002879 CrossRef CAS.
- G. Hong, X. Gan, C. Leonhardt, Z. Zhang, J. Seibert, J. M. Busch and S. Bräse, Adv. Mater., 2021, 33, 2005630 CrossRef CAS.
- G. U. Mahoro, J. Fernandez-Cestau, J.-L. Renaud, P. B. Coto, R. D. Costa and S. Gaillard, Adv. Opt. Mater., 2020, 8, 2000260 CrossRef CAS.
- R. Czerwieniec, M. J. Leitl, H. H. H. Homeier and H. Yersin, Coord. Chem. Rev., 2016, 325, 2–28 CrossRef CAS.
- CRC Handbook of Chemistry and Physics, ed. W. M. Haynes, CRC Press/Taylor and Francis, Boca Raton, FL, 95th edn, Internet Version 2015, accessed December 2014 Search PubMed.
- Merck-France , https://www.sigmaaldrich.com/FR/en, (accessed November 2024).
- D. R. McMillin, M. T. Buckner and B. T. Ahn, Inorg. Chem., 1977, 16, 943–945 CrossRef CAS.
- C. E. Housecroft and E. C. Constable, J. Mater. Chem. C, 2022, 10, 4456–4482 RSC.
- M. Iwamura, S. Takeuchi and T. Tahara, Acc. Chem. Res., 2015, 48, 782–791 CrossRef CAS PubMed.
- D. R. McMillin, J. R. Kirchhoff and K. V. Goodwin, Coord. Chem. Rev., 1985, 64, 83–92 CrossRef CAS.
- M. Elie, F. Sguerra, F. Di Meo, M. D. Weber, R. Marion, A. Grimault, J.-F. Lohier, A. Stallivieri, A. Brosseau, R. B. Pansu, J.-L. Renaud, M. Linares, M. Hamel, R. D. Costa and S. Gaillard, ACS Appl. Mater. Interfaces, 2016, 8, 14678–14691 CrossRef CAS.
- L. M. Cavinato, S. Wölfl, A. Pöthig, E. Fresta, C. Garino, J. Fernandez-Cestau, C. Barolo and R. D. Costa, Adv. Mater., 2022, 34, 2109228 CrossRef CAS.
- E. Fresta, M. D. Weber, J. Fernandez-Cestau and R. D. Costa, Adv. Opt. Mater., 2019, 7, 1900830 CrossRef CAS.
- E. Fresta, G. U. Mahoro, L. M. Cavinato, J.-F. Lohier, J.-L. Renaud, S. Gaillard and R. D. Costa, Adv. Opt. Mater., 2022, 10, 2101999 CrossRef CAS.
- E. Fresta and R. D. Costa, Adv. Funct. Mater., 2020, 30, 1908176 CrossRef CAS.
- M. D. Weber, E. Fresta, M. Elie, M. E. Miehlich, J.-L. Renaud, K. Meyer, S. Gaillard and R. D. Costa, Adv. Funct. Mater., 2018, 28, 1707423 CrossRef.
- A. Jouaiti, L. Ballerini, H.-L. Shen, R. Viel, F. Polo, N. Kyritsakas, S. Haacke, Y.-T. Huang, C.-W. Lu, C. Gourlaouen, H.-C. Su and M. Mauro, Angew. Chem., Int. Ed., 2023, 62, e202305569 CrossRef CAS.
- B. Pashaei, S. Karimi, H. Shahroosvand and M. Pilkington, Adv. Funct. Mater., 2020, 30, 1908103 CrossRef CAS.
- K. A. McNitt, K. Parimal, A. I. Share, A. C. Fahrenbach, E. H. Witlicki, M. Pink, D. K. Bediako, C. L. Plaisier, N. Le, L. P. Heeringa, D. A. Vander Griend and A. H. Flood, J. Am. Chem. Soc., 2009, 131, 1305–1313 CrossRef CAS.
- (a) O. Santoro, A. Collado, A. M. Z. Slawin, S. P. Nolan and C. S. J. Cazin, Chem. Commun., 2013, 49, 10483 RSC; (b) N. P. Mankad, T. G. Gray, D. S. Laitar and J. P. Sadighi, Organometallics, 2004, 23, 1191 CrossRef CAS; (c) C. Michon, A. Ellern and R. J. Angelici, Inorg. Chim. Acta, 2006, 359, 4549 CrossRef CAS; (d) N. Schneider, V. César, S. Bellemin-Laponnaz and L. H. Gade, J. Organomet. Chem., 2005, 690, 5556 CrossRef CAS.
- C. A. Citadelle, E. L. Nouy, F. Bisaro, A. M. Z. Slawin and C. S. J. Cazin, Dalton Trans., 2010, 39, 4489 RSC.
- M. Z. Shafikov, A. F. Suleymanova, R. Czerwieniec and H. Yersin, Inorg. Chem., 2017, 56, 13274–13285 CrossRef CAS PubMed.
- G. Giobbio, L. Greffier, S. Lipinski, A. Montrieul, J.-F. Lohier, M. Linares, R. D. Costa and S. Gaillard, Dalton Trans., 2024, 53, 18607–18615 RSC.
- C.-W. Hsu, C.-C. Lin, M.-W. Chung, Y. Chi, G.-H. Lee, P.-T. Chou, C.-H. Chang and P.-Y. Chen, J. Am. Chem. Soc., 2011, 133, 12085–12099 CrossRef CAS PubMed.
- A. V. Artem'ev, M. Z. Shafikov, A. Schinabeck, O. V. Antonova, A. S. Berezin, I. Y. Bagryanskaya, P. E. Plusnin and H. Yersin, Inorg. Chem. Front., 2019, 6, 3168 RSC.
- M. Z. Shafikov, R. Czerwieniec and H. Yersin, Dalton Trans., 2019, 48, 2802 RSC.
- S. Lipinski, L. M. Cavinato, T. Pickl, G. Biffi, A. Pöthig, P. B. Coto, J. Fernández-Cestau and R. D. Costa, Adv. Opt. Mater., 2023, 11, 2203145 CrossRef CAS.
- G. Giobbio, P. B. Coto, J.-F. Lohier, J.-L. Renaud, S. Gaillard and R. D. Costa, Dalton Trans., 2024, 53, 12307–12315 RSC.
- O. Moudam, A. C. Tsipis, S. Kommanaboyina, P. N. Horton and S. J. Coles, RSC Adv., 2015, 5, 95047–95053 RSC.
- E. Fresta, J. M. Carbonell-Vilar, J. Yu, D. Armentano, J. Cano, M. Viciano-Chumillas and R. D. Costa, Adv. Funct. Mater., 2019, 29, 1901797 CrossRef.
- J. Tang, H.-Y. Yin and J.-L. Zhang, Inorg. Organomet. Transition Met. Complexes Biol. Mol. Living Cells, 2017, 1–53 Search PubMed.
- (a) Y. Sakai, Y. Sagara, H. Nomura, N. Nakamura, Y. Suzuki, H. Miyazaki and C. Adachi, Chem. Commun., 2015, 51, 3181–3184 RSC; (b) N. Lüdtke, J. Kuhnt, T. Heil, A. Steffen and C. M. Marian, ChemPhotoChem, 2023, 7, e202200142 CrossRef; (c) M. Mitra, O. Mrózek, M. Putscher, J. Guhl, B. Hupp, A. Belyaev, C. l. M. Marian and A. Steffen, Angew. Chem., Int. Ed., 2024, 63, e202316300 CrossRef CAS.
- I. Chan, W. van Dorp, T. Schaafsma and J. van der Waals, Mol. Phys., 1971, 22, 753–760 CrossRef CAS.
- K. T. Weber, K. Karikis, M. D. Weber, P. D. Coto, A. Cherisiadis, D. Charitaki, G. Charalambidis, P. Angaridis, A. G. Coutsolelos and R. D. Costa, Dalton Trans., 2016, 45, 13284–13288 RSC.
- M. D. Weber, J. E. Wittmann, A. Burger, O. B. Malcıoğlu, J. Segarra-Martí, A. Hirsch, P. B. Coto, M. Bockstedte and R. D. Costa, Adv. Funct. Mater., 2016, 26, 6737–6750 CrossRef CAS.
- M. Mone, S. Tang, P. Murto, B. A. Abdulahi, C. Larsen, J. Wang, W. Mammo, L. Edman and E. Wang, Chem. Mater., 2019, 31, 9721–9728 CrossRef CAS.
- T. J. Rashamuse, R. L. Mohlala, E. M. Coyanis and N. P. Magwa, Molecules, 2023, 28, 5272 CrossRef CAS.
- P. Tao, S.-J. Liu and W.-Y. Wong, Adv. Opt. Mater., 2020, 8, 2000985 CrossRef CAS.
- J. Chen, Q. Zhang, F. Zheng, Z. Liu, S. Wang, A. Wu and G. Guo, Dalton Trans., 2015, 44, 3289 RSC.
- Y. Tanabe and S. Sugano, J. Phys. Soc. Jpn., 1954, 9, 766 CrossRef CAS.
- (a) Y. Qin, P. Tao, L. Gao, P. She, S. Liu, X. Li, F. Li, H. Wang, Q. Zhao, Y. Miao and W. Huang, Adv. Opt. Mater., 2019, 7, 1801160 CrossRef; (b) A. Jana, V. G. Sreea, Q. Ba, S. C. Choc, S. U. Lee, S. Cho, Y. Jo, A. Meena, H. Kim and H. Im, J. Mater. Chem. C, 2021, 9, 11314–11323 RSC.
- B. Adranno, S. Tang, V. Paterlini, V. Smetana, O. Renier, G. Bousrez, L. Edman and A.-V. Mudrig, Adv. Photonics Res., 2023, 4, 2200351 CrossRef CAS.
- V. G. Sree, A. Jana, S. C. Cho, S. U. Lee, S. Cho, J. I. Sohn and H. Im, J. Chem. Eng., 2023, 474, 145936 CrossRef.
- (a) I.-S. Shin, H.-C. Lim, J.-W. Oh, J.-K. Lee, T.-H. Kim and H. Kim, Electrochem. Commun., 2011, 13, 64–67 CrossRef CAS; (b) E. Fresta and R. D. Costa, J. Mater. Chem. C, 2017, 5, 5643–5675 RSC; (c) C. Zhang, R. Liu, D. Zhang and L. Duan, Adv. Funct. Mater., 2020, 30, 1907156 CrossRef CAS; (d) K. Yasuji, T. Sakanoue, F. Yonekawa and K. Kanemoto, Nat. Commun., 2023, 14, 992 CrossRef CAS.
- C. Bizzarri, E. Spuling, D. M. Knoll, D. Volz and S. Bräse, Coord. Chem. Rev., 2018, 373, 49–82 CrossRef CAS.
- K.-T. Yeung, W.-P. To, C. Sun, G. Cheng, C. Ma, G. S. Ming Tong, C. Yang and C.-M. Che, Angew. Chem., Int. Ed., 2017, 56, 133–137 CrossRef CAS PubMed.
- K.-T. Chan, T.-L. Lam, D. Yu, L. Du, D. L. Phillips, C.-L. Kwong, G. S. M. Tong, G. Cheng and C.-M. Che, Angew. Chem., Int. Ed., 2019, 58, 14896–14900 CrossRef CAS PubMed.
- V. Ferraro, C. Bizzarri and S. Bräse, Adv. Sci., 2024, 11, 2404866 CrossRef CAS.
- C. Boulechfar, H. Ferkous, A. Delimi, A. Djedouani, A. Kahlouche, A. Boublia, A. S. Darwish, T. Lemaoui, R. Verma and Y. Benguerba, Inorg. Chem. Commun., 2023, 150, 110451 CrossRef CAS.
- Y. Zhang, T. S. Lee, J. M. Favale, D. C. Leary, J. L. Petersen, G. D. Scholes, F. N. Castellano and C. Milsmann, Nat. Chem., 2020, 12, 345–352 CrossRef CAS PubMed.
- S. Garon, E. K. C. Lau, S.-L. Chew, S. T. Lee and M. E. Thompson, J. Soc. Info. Disp., 2005, 13, 405–409 CrossRef CAS.
- K. J. Covert, A.-R. Mayol and P. T. Wolczanski, Inorg. Chim. Acta, 1997, 263, 263–278 CrossRef CAS.
- W. R. Kitzmann and K. Heinze, Angew. Chem., Int. Ed., 2023, 62, e202213207 CrossRef CAS PubMed.
- F. Reichenauer, C. Wang, C. Förster, P. Boden, N. Ugur, R. Báez-Cruz, J. Kalmbach, L. M. Carrella, E. Rentschler, C. Ramanan, G. Niedner-Schatteburg, M. Gerhards, U. Resch-Genger and K. Heinze, J. Am. Chem. Soc., 2021, 143, 11843–11855 CrossRef CAS PubMed.
- S. Otto, M. Dorn, C. Förster, M. Bauer, M. Seitz and K. Heinze, Coord. Chem. Rev., 2018, 359, 102–111 CrossRef CAS.
- N. Sinha, P. Yaltseva and O. S. Wenger, Angew. Chem., Int. Ed., 2023, 62, e202303864 CrossRef CAS.
- A. Benchohra, J. Chong, C. M. Cruz, C. Besnard, L. Guénée, A. Rosspeintner and C. Piguet, Inorg. Chem., 2024, 63, 3617–3629 CrossRef CAS PubMed.
- J.-R. Jiménez, B. Doistau, M. Poncet and C. Piguet, Coord. Chem. Rev., 2021, 434, 213750 CrossRef.
- G. Khalil, C. Orvain, L. Fang, L. Barloy, A. Chaumont, C. Gaiddon, M. Henry, N. Kyritsakas and P. Mobian, Dalton Trans., 2016, 45, 19072 RSC.
- A. Erdoğmuş, M. Dormuş, A. L. Uğur, O. Avciata, U. Avciata and T. Nyokong, Synth. Met., 2010, 160, 1868–1876 CrossRef.
- Y. Arslanoğlu, E. Hayran and E. Hamuryudan, Dyes Pigm., 2013, 97, 340–346 CrossRef.
- M. Barker, T. J. Whittemore, H. C. London, J. M. Sledesky, E. A. Harris, T. M. Smith Pellizzeri, C. D. McMillen and P. S. Wagenknecht, Inorg. Chem., 2023, 62, 17870–17882 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |