Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Deconvoluting capping ligand influence on photophysical properties in tetrathiafulvalene-based diradicaloids†
Nathan E.
Lopez‡
,
Lauren E.
McNamara‡
 ,
Sophie W.
Anferov
and
John S.
Anderson
,
Sophie W.
Anferov
and
John S.
Anderson
 *
*
Department of Chemistry, University of Chicago, Chicago, Illinois 60637, USA. E-mail: jsanderson@uchicago.edu
First published on 12th December 2024
Abstract
Tetrathiafulvalene-2,3,6,7-tetrathiolate (TTFtt) complexes are synthetically tunable and emit brightly in the near-infrared II region (NIR II, 1000–1700 nm). Their emission/absorption energies respond to the identity of the capping ligands on the metal center, but a detailed understanding of how ligand bonding interactions dictate photophysical properties is key to predictive design optimization. Here we assess the relative influence of ligand pi (π) backbonding versus sigma (σ) donation in these complexes across a new series of olefin- and phosphite-capped complexes. Increasing the backbonding character of peripheral ligands results in a hypsochromic shift in the absorption maxima, while stronger σ donation results in a bathochromic shift.
Introduction
The high depth penetration in tissue and improved spatial and temporal resolution of NIR II emitters make them promising for use in a wide variety of medical applications, including image-assisted surgeries and in vitro monitoring of disease progression.1–8 Developing dyes with tunable emission may further enable multicolor imaging and enhanced contrast.9–12 Beyond biomedical applications, NIR II dyes have attracted attention for use in OLEDs and information storage, with modular photophysical properties also proving advantageous in these areas.13–16 Despite these attributes, current leading NIR II dyes require elaborate scaffolds which are difficult to systematically modify.1–3,17–22 Our laboratory has recently reported a compact, modular TTFtt scaffold that absorbs and emits in the NIR II region.23,24 These molecules are also diradicals which further broadens their potential applications in fields such as quantum information science.25–29 Systematic changes to the electron-donating or -withdrawing character of capping triarylphosphine ligands results in predictive changes in photophysical properties; absorption and emission shift hypsochromically with increasing Hammett parameter.23,24 Diradical character is also responsive to the capping metal/ligand combination.25 While these examples demonstrate the tunability of this system, the explored span of capping ligand types is comparatively small and there is no clarity on the key interactions that determine shifts in absorption/emission. Such a detailed understanding is critical for optimal tuning of this emissive diradicaloid scaffold.Herein, a new series of Pt-capped TTFtt complexes has been synthesized and characterized to elucidate the relative influence of ligand σ-donation and π-accepting character on photophysical properties. Three new olefin- and three new triarylphosphite-capped PtTTFtt complexes were synthesized to probe a wider array of ligand properties (Fig. 1A). Natural bonding orbital (NBO) calculations provide insight into the relative π acidity (πb) and σ-donation of these ligands, which is then correlated with observed photophysical properties.30
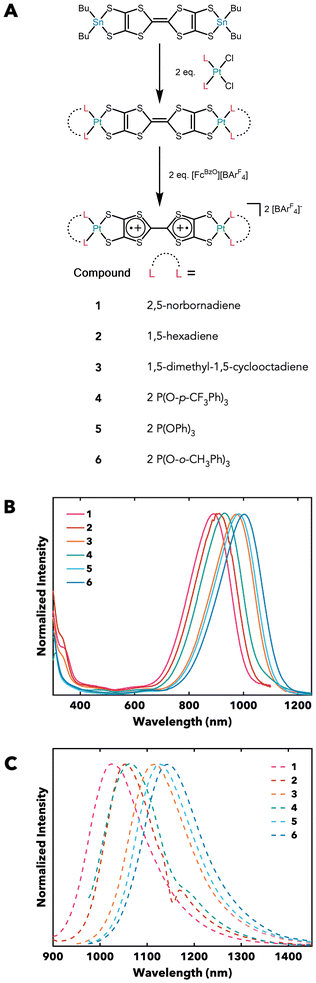 | ||
| Fig. 1 (A) Synthesis of new Pt-capped TTFtt analogs. See ESI† for further details and procedures. BArF4− = tetrakis[3,5-bis(trifluoromethyl)phenyl]borate. (B) Absorption of new analogs in dichloromethane (DCM) at 298 K. (C) Photoluminescence (PL) of new analogs in CH2Cl2 (2, 4) and CD2Cl2 (1, 3, 5, 6) at 298 K. | ||
Results and discussion
Synthesis and characterization of PtTTFtt compounds 1–6
Six new Pt-capped TTFtt analogs were synthesized according to previously established procedures (Fig. 1A, 1–6).23,31 Crystallographic characterization reveals no π stacking in the solid state; the dicationic fragments are encapsulated by BArF4− anions. The central C–C distances in the TTF core of these molecules have previously been shown to correlate with diradical character.25 This distance ranges from 1.38 to 1.44 Å across this series (4 is omitted due to poor bond resolution), suggesting moderate diradical character.24,25 Evans method analysis shows magnetic moments ranging from 0.79–2.05μB, further supporting population of a low-lying triplet state (Fig. S14–S19†). We note that while no π stacking is observed in the solid state, TTF fragments are also known to π stack in solution. This may explain the generally lower magnetic moments in solution than the value expected for a triplet (2.83μB). Room temperature CW EPR spectra display a single isotropic feature attributed to an organic radical at g ≈ 2 that also supports a thermally populated triplet state (Fig. S33–38†).The UV-vis-NIR spectra of these new compounds in DCM show hypsochromically-shifted absorption maxima compared to previously reported analogs (890–1005 nm, Fig. 1B).23,24,31 Compounds 1–6 also emit brightly in DCM, with maxima ranging from 1030–1143 nm (Fig. 1C). The photoluminescence (PL) spectra of compounds 1, 3, 5, and 6 are convoluted by the IR absorption of DCM (which onsets at ∼1150 nm), so for subsequent analysis, the maxima obtained from the PL spectra of these compounds in CD2Cl2 are used. We note what appears to be an anomalously bathochromic shift in the absorption and emission spectra of 3, particularly when compared with the other olefin complexes in this series. However, and as shown below, this is rationalized by the substantially better donor properties of 1,5-dimethyl-1,5-cyclooctadiene.
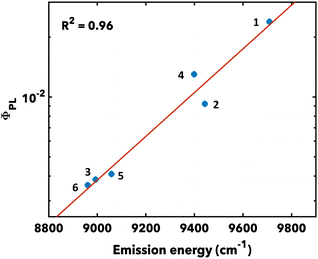
Photoluminescent quantum yields (PLQYs, ϕPL) range from 0.36–2.39% (Fig. 2).24 This trend generally follows the predictions of the energy gap law with larger PLQYs exhibited by higher energy-emitting examples. This series also displays high molar extinction coefficients (ε) from ∼40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 90
000 to 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 and correspondingly high brightness values (εϕPL) from ∼20
000 M−1 cm−1 and correspondingly high brightness values (εϕPL) from ∼20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 70
000 to 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1; these brightness values are substantially higher than commercially available NIR II dyes like IR-26.34 Overall, we note that the ϕPL in these complexes are among the brightest reported values for NIR II-emitting complexes.32,33
000 M−1 cm−1; these brightness values are substantially higher than commercially available NIR II dyes like IR-26.34 Overall, we note that the ϕPL in these complexes are among the brightest reported values for NIR II-emitting complexes.32,33
Natural bonding orbital (NBO) analysis
After photophysically characterizing complexes 1–6, we then turned to deconvoluting what ligand parameters most accurately predicted these observed photophysical trends. We initially performed a second-order perturbative energy analysis on the precursor LPtCl2 or L2PtCl2 fragments to investigate the strength of π-backbonding and σ-donation equitably (Table 1, Table S7,† and Fig. 3).30 The strengths of π-backbonding interactions are ∼28 kcal mol−1 for triarylphosphines, ∼40 kcal mol−1 for triarylphosphites, and ∼100 kcal mol−1 for olefins respectively. As expected, this analysis reveals that phosphites are stronger π-acids than previously examined phosphine ligands. More interestingly, phosphites are worse π-acids but comparable σ-donors to olefins. The strength of σ-donation from olefin C–C π bonds or P lone pairs to Pt–Cl antibonding orbitals spans a similar range of 540–620 kcal mol−1 for olefins and phosphites. The strength of these interactions is rationalized through the orbital overlap between donor orbitals (Pt 5d, P lone pair, or olefin C–C π) and accepting orbitals (Pt–Cl σ*, P–O σ*, or C–C π*). Examples of these interactions are depicted in Fig. 3. While the σ interaction appears to have comparable overlap between the olefins and phosphites, the C–C π* orbitals point more directly at the Pt 5d orbitals than the phosphite's P–O σ*, yielding a much stronger computed backbonding energy. Most importantly, the ability to quantify the relative ligand interactions across this series of complexes on one energetic scale enables us to analyze what ligand features correlate most closely with the observed photophysical properties.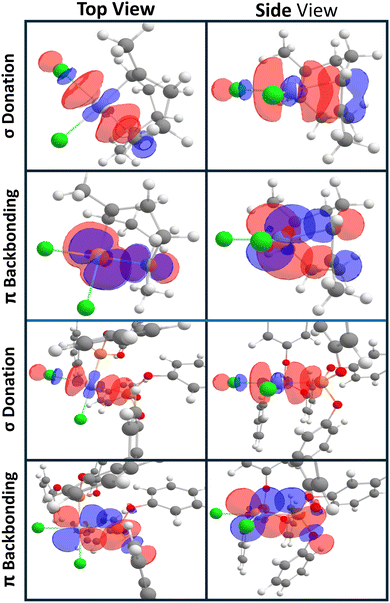 | ||
| Fig. 3 Top and side view visualization of σ and πb interaction of the chloride analogs of 3 (above blue line) and 5 (below blue line). | ||
| 1 | 2 | 3 | 4 | 5 | 6 | dppe23 | P(p-OMePh)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
PPh3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35 |
P(p-BrPh)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
|
|---|---|---|---|---|---|---|---|---|---|---|
| dppe = [{(dppe)Pt}2TTFtt][BAr4F]2, P(p-OMePh)3 = [{(P(p-OMePh)3)2Pt}2TTFtt][BAr4F]2, PPh3 = [{(PPh3)2Pt}2TTFtt][BAr4F]2, P(p-BrPh)3 = [{(P(p-BrPh)3)2Pt}2TTFtt][BAr4F]2 in CH2Cl2.a Value taken from fluorescence spectrum taken in CD2Cl2 | ||||||||||
| πb (kcal mol−1) | 109 | 100 | 96 | 37 | 40 | 37 | 33 | 27 | 28 | 28 |
| σ (kcal mol−1) | 580 | 559 | 551 | 587 | 556 | 617 | — | — | — | — |
| λ Abs (nm) | 890 | 914 | 975 | 931 | 984 | 1005 | 1044 | 1130 | 1100 | 1059 |
| E Abs (eV) | 1.394 | 1.357 | 1.272 | 1.332 | 1.261 | 1.234 | 1.188 | 1.098 | 1.128 | 1.171 |
| λ Em (nm) | 1030a | 1059 | 1115a | 1064 | 1127a | 1143a | 1202 | 1280 | 1266 | 1198 |
| EEm(eV) | 1.204 | 1.171 | 1.113 | 1.165 | 1.101 | 1.085 | 1.032 | 0.969 | 0.980 | 1.036 |
| ε (M−1 cm−1) | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 520 |
68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080 080 |
87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350 |
52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 366 366 |
63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 716 716 |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 808 808 |
107![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 868 868 |
| ϕ PL (%) | 2.39 | 0.92 | 0.38 | 1.30 | 0.41 | 0.36 | 0.136 | 0.041 | 0.07 | 0.171 |
| εϕ PL (M−1 cm−1) | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 553 553 |
62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634 634 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 288 288 |
56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 355 355 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 470 470 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 937 937 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 880 880 |
3887 | 7490 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 828 828 |
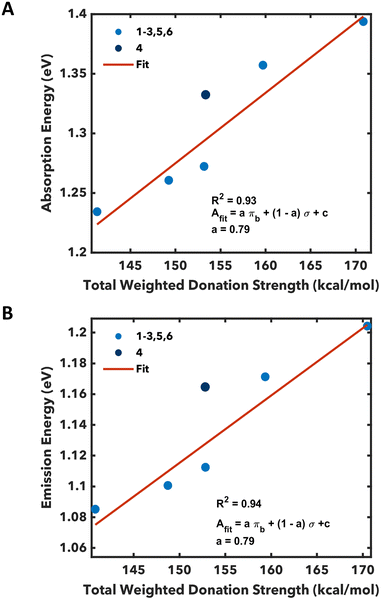
Regressions of the absorption and emission energy against ligand parameters were performed to understand what factors held the greatest predictive value. Both the phosphite and olefin complexes form their own independent sets when only π-backbonding interactions are considered, suggesting that some σ-effects must be considered (Fig. S48†). A weighted average of backbonding and σ-donation was used to address this possibility: Afit = aπb + (1 − a)σ + c (a is a weight ranging from 0 to 1 and A is the predicted absorption or emission energy). This analysis results in a satisfactory trend with absorption when the weighted average was comprised of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 π-backbonding
1 π-backbonding![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) σ-donation (79 and 21% respectively) with R2 = 0.93 (Fig. 4A). Similarly, an analysis for the emission maxima of these compounds results in a clear trend with an identical weight (a = 0.79 and R2 = 0.94, Fig. 4B). Finally, we note some anomalies in these analyses. Namely, while complex 4 possesses similar donation strength to the other phosphites, its absorption and emission are much more hypsochromically shifted. From a statistical analysis, the absorption/emission of 4 is >1 standard deviation from the mean, so it was removed from the dataset in all analyses. We suspect this anomalous behavior for 4 arises from solution-phase fluorophilic interactions with the BArF4− anions as similar outlier behavior has been observed in CF3 and F derivatives of other Pt-capped TTFtt complexes.24
σ-donation (79 and 21% respectively) with R2 = 0.93 (Fig. 4A). Similarly, an analysis for the emission maxima of these compounds results in a clear trend with an identical weight (a = 0.79 and R2 = 0.94, Fig. 4B). Finally, we note some anomalies in these analyses. Namely, while complex 4 possesses similar donation strength to the other phosphites, its absorption and emission are much more hypsochromically shifted. From a statistical analysis, the absorption/emission of 4 is >1 standard deviation from the mean, so it was removed from the dataset in all analyses. We suspect this anomalous behavior for 4 arises from solution-phase fluorophilic interactions with the BArF4− anions as similar outlier behavior has been observed in CF3 and F derivatives of other Pt-capped TTFtt complexes.24
While backbonding alone is insufficient in predicting photophysical properties of Pt-capped TTFtt complexes it still constitutes the majority of the predictive power in this model. We extended this analysis to include some previously reported phosphines (Table 1, Fig. S48 and 49†). We qualitatively observe that the bathochromically shifted features in phosphine-capped PtTTFtt compounds can be attributed to comparatively low backbonding character. Our observations here support previous time-dependent density functional theory (TD-DFT) analyses with phosphine-capped PtTTFtt complexes where increased donation strength of the phosphines results in a better energetic overlap between the Pt-ligand fragment and TTF core. This better overlap results in increased delocalization and bathochromically-shifted absorption and emission maxima.24 Similarly, an increase in π-backbonding from Pt to the capping ligands destabilizes this fragment relative to the TTF core, resulting in a poorer energetic overlap, less delocalization, and a hypsochromic shift. Meanwhile, σ-donation has a smaller but still significant effect.
Conclusions
Six new Pt-capped TTFtt compounds that absorb and emit in the NIR II region have been synthesized and characterized. The π-backbonding and σ-donation of the capping ligands in these complexes have been computed with second-order perturbative analysis within an NBO method. Trends between π-backbonding and σ-donation inform that the absorption and emission maxima is dominated by the strength of the backbonding interaction between Pt and its capping ligand. However, comparing these new complexes with previously reported phosphine analogs shows that backbonding alone is an insufficient predictor, and consideration of σ-donation is required. We find that the optimal weighting is ∼80%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20% πb/σ for the phosphite and olefin analogs. These results quantify an intuitive and synthetically facile method of tuning the photophysical properties of Pt-capped TTFtt complexes through modulation of the π-accepting character of the capping ligands.
20% πb/σ for the phosphite and olefin analogs. These results quantify an intuitive and synthetically facile method of tuning the photophysical properties of Pt-capped TTFtt complexes through modulation of the π-accepting character of the capping ligands.
Experimental
General considerations
All syntheses were performed under dry N2 in an MBraun UNIlab glovebox. Midwest Microlabs conducted all elemental analyses (C, H, N). All solvents were dried and N2-purged on a Pure Process Technology solvent system, filtered through activated alumina, and stored over 4 Å molecular sieves. TTFtt(SnBu2)2,31 [FcBzO][BArF4],31 P(O-o-CH3Ph)3,36 Pt{P(O-p-CF3Ph)3}2Cl2,37 Pt{P(O-o-CH3Ph)3}2Cl2,37 Pt(NBD)Cl2,38 Pt(HEX)Cl2,38 and Pt(Me2COD)Cl238 were prepared according to literature procedures (NBD = 2,5-norbornadiene, HEX = 1,5-hexadiene, and Me2COD = 1,5-dimethyl-1,5-cyclooctadiene). All other chemicals and reagents were purchased from commercial sources and used as received.Synthesis
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.55 (bs, [BArF4]−), 7.72 (bs, [BArF4]−). We note due to the air-sensitive nature of this compound, we did not run elemental analysis. See NMR and crystal structure for purity and composition/connectivity, respectively.
CH), 7.55 (bs, [BArF4]−), 7.72 (bs, [BArF4]−). We note due to the air-sensitive nature of this compound, we did not run elemental analysis. See NMR and crystal structure for purity and composition/connectivity, respectively.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (Z)), 5.07 (bd, HEX
CH2 (Z)), 5.07 (bd, HEX ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (E)), 5.93 (bm, HEX
CH2 (E)), 5.93 (bm, HEX ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.57 (s, [BArF4]−), 7.73 (s, [BArF4]−). Anal. calcd for 2, C82H44B2F48Pt2S8: C 37.74%, H 1.70%, N 0%; found: C 37.73%, H 1.87%, N none.
CH), 7.57 (s, [BArF4]−), 7.73 (s, [BArF4]−). Anal. calcd for 2, C82H44B2F48Pt2S8: C 37.74%, H 1.70%, N 0%; found: C 37.73%, H 1.87%, N none.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.56 (s, [BArF4]−), 7.72 (s, [BArF4]−). Anal. calcd for 3, C90H56B2F48Pt2S8: C 39.77%, H 2.08%, N 0%; found: C 40.10%, H 2.07%, N none.
CH), 7.56 (s, [BArF4]−), 7.72 (s, [BArF4]−). Anal. calcd for 3, C90H56B2F48Pt2S8: C 39.77%, H 2.08%, N 0%; found: C 40.10%, H 2.07%, N none.
Author contributions
Conceptualization: N. E. L., L. E. M., and J. S. A.; investigation and formal analysis: N. E. L., L. E. M., and S. W. A.; visualization: N. E. L., L. E. M., S. W. A. and J. S. A.; supervision: J. S. A.; all authors contributed to the writing of this work.Data availability
Crystallographic data for 1–6 has been deposited at the CCDC under 2377387, 2377388, 2377289, 2377390, 2377391, 2377392† and can be obtained from https://www.ccdc.cam.ac.uk/structures/.Conflicts of interest
J. S. A. and L. E. M. have a patent filed on this work.Acknowledgements
This work was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Grant DE-SC0019215, and the Army Research Office under Grant W911NF-23-1-0233. This work was done, in part, at the Chicago MRSEC, which is funded by the NSF through Grant DMR-1420709. Parts of this work were performed at the Soft Matter Characterization Facility at the Pritzker School of Molecular Engineering at the University of Chicago. We are grateful for the computational resources were supplied by the UChicago Computing Center. N.J., J.S.S., P.M.C., and C–Y. L. are thanked for helpful discussions.References
- G. Hong, A. L. Antaris and H. Dai, Nat. Biomed. Eng., 2017, 1, 1–22 CrossRef.
- A. Matsui, E. Tanaka, H. S. Choi, J. H. Winer, V. Kianzad, S. Gioux, R. G. Laurence and J. V. Frangioni, Surgery, 2010, 148, 87–95 CrossRef PubMed.
- B. Li, M. Zhao, L. Feng, C. Dou, S. Ding, G. Zhou, L. Lu, H. Zhang, F. Chen, X. Li, G. Li, S. Zhao, C. Jiang, Y. Wang, D. Zhao, Y. Cheng and F. Zhang, Nat. Commun., 2020, 11, 3102 CrossRef CAS PubMed.
- Y. Chen, Y. Yang and F. Zhang, Nat. Protoc., 2024, 1–22 Search PubMed.
- L.-Y. Huang, S. Zhu, R. Cui and M. Zhang, Anal. Chem., 2020, 92, 535–542 CrossRef CAS PubMed.
- Y. Song, G. Yu, B. Xie, K. Zhang and F. Huang, Appl. Phys. Lett., 2020, 117, 093302 CrossRef CAS.
- J.-J. Wu, X.-D. Wang and L.-S. Liao, ACS Photonics, 2019, 6, 2590–2599 CrossRef CAS.
- C.-L. Ho, H. Li and W.-Y. Wong, J. Organomet. Chem., 2014, 751, 261–285 CrossRef CAS.
- Y. Yang, Y. Chen, P. Pei, Y. Fan, S. Wang, H. Zhang, D. Zhao, B.-Z. Qian and F. Zhang, Nat. Nanotechnol., 2023, 18, 1195–1204 CrossRef CAS PubMed.
- D. Xu, J. Ge, Y. An, S. Bai, Z. Wang, S. Wu, Q. Dai, Z. Lu and G. Liu, Small, 2023, 19, 2300859 CrossRef CAS PubMed.
- J. Xu, N. Zhu, Y. Du, T. Han, X. Zheng, J. Li and S. Zhu, Nat. Commun., 2024, 15, 2845 CrossRef CAS PubMed.
- S. Zhu, S. Herraiz, J. Yue, M. Zhang, H. Wan, Q. Yang, Z. Ma, Y. Wang, J. He, A. L. Antaris, Y. Zhong, S. Diao, Y. Feng, Y. Zhou, K. Yu, G. Hong, Y. Liang, A. J. Hsueh and H. Dai, Adv. Mater., 2018, 30, 1705799 CrossRef PubMed.
- B. Wang, Z. Chen, X. Li, J. Zhou and Q. Zeng, J. Alloys Compd., 2020, 812, 152119 CrossRef CAS.
- H. Zhang, Y. Fan, P. Pei, C. Sun, L. Lu and F. Zhang, Angew. Chem., Int. Ed., 2019, 58, 10153–10157 CrossRef CAS PubMed.
- J.-L. He, F.-C. Kong, B. Sun, X.-J. Wang, Q.-S. Tian, J. Fan and L.-S. Liao, Chem. Eng. J., 2021, 424, 130470 CrossRef CAS.
- Y. Xiao, H. Wang, Z. Xie, M. Shen, R. Huang, Y. Miao, G. Liu, T. Yu and W. Huang, Chem. Sci., 2022, 13, 8906–8923 RSC.
- S. He, S. He, J. Song, J. Song, J. Qu, J. Qu, Z. Cheng and Z. Cheng, Chem. Soc. Rev., 2018, 47, 4258–4278 RSC.
- A. L. Antaris, H. Chen, S. Diao, Z. Ma, Z. Zhang, S. Zhu, J. Wang, A. X. Lozano, Q. Fan, L. Chew, M. Zhu, K. Cheng, X. Hong, H. Dai and Z. Cheng, Nat. Commun., 2017, 8, 15269 CrossRef CAS PubMed.
- X. Zeng, Y. Xiao, J. Lin, S. Li, H. Zhou, J. Nong, G. Xu, H. Wang, F. Xu, J. Wu, Z. Deng and X. Hong, Adv. Healthcare Mater., 2018, 7, 1800589 CrossRef PubMed.
- C. Qu, Y. Xiao, H. Zhou, B. Ding, A. Li, J. Lin, X. Zeng, H. Chen, K. Qian, X. Zhang, W. Fang, J. Wu, Z. Deng, Z. Cheng and X. Hong, Adv. Opt. Mater., 2019, 7, 1900229 CrossRef PubMed.
- J. Ding, M. Zhang, Y. Gao, C. Lu, M. Zhang and F. Li, J. Phys. Chem. Lett., 2023, 14, 8244–8250 CrossRef CAS PubMed.
- L. Wang, N. Li, W. Wang, A. Mei, J. Shao, W. Wang and X. Dong, ACS Nano, 2024, 18, 4683–4703 CrossRef CAS PubMed.
- L. E. McNamara, J.-N. Boyn, C. Melnychuk, S. W. Anferov, D. A. Mazziotti, R. D. Schaller and J. S. Anderson, J. Am. Chem. Soc., 2022, 144, 16447–16455 CrossRef CAS PubMed.
- L. E. McNamara, J.-N. Boyn, S. W. Anferov, A. S. Filatov, M. W. Maloney, D. A. Mazziotti, R. D. Schaller and J. S. Anderson, J. Am. Chem. Soc., 2024, 146, 17285–17295 CrossRef CAS PubMed.
- J.-N. Boyn, L. E. McNamara, J. S. Anderson and D. A. Mazziotti, J. Phys. Chem. A, 2022, 126, 3329–3337 CrossRef CAS PubMed.
- J. H. Cole and L. C. L. Hollenberg, Nanotechnology, 2009, 20, 495401 CrossRef PubMed.
- L. T. Hall, J. H. Cole, C. D. Hill and L. C. L. Hollenberg, Phys. Rev. Lett., 2009, 103, 220802 CrossRef CAS PubMed.
- L. T. Hall, C. D. Hill, J. H. Cole and L. C. L. Hollenberg, Phys. Rev. B:Condens. Matter Mater. Phys., 2010, 82, 045208 CrossRef.
- J. Köhler, Phys. Rep., 1999, 310, 261–339 CrossRef.
- A. Comas-Vives and J. N. Harvey, Eur. J. Inorg. Chem., 2011, 2011, 5025–5035 CrossRef CAS.
- J. Xie, J.-N. Boyn, A. S. Filatov, A. J. McNeece, D. A. Mazziotti and J. S. Anderson, Chem. Sci., 2020, 11, 1066–1078 RSC.
- R. Englman and J. Jortner, Mol. Phys., 1970, 18, 145–164 CrossRef CAS.
- Q. Yang, H. Ma, Y. Liang and H. Dai, Acc. Mater. Res., 2021, 2, 170–183 CrossRef CAS.
- S. Hatami, C. Würth, M. Kaiser, S. Leubner, S. Gabriel, L. Bahrig, V. Lesnyak, J. Pauli, N. Gaponik, A. Eychmüller and U. Resch-Genger, Nanoscale, 2014, 7, 133–143 RSC.
- L. E. McNamara, C. Melnychuk, J.-N. Boyn, S. W. Anferov, D. A. Mazziotti, R. D. Schaller and J. S. Anderson, Chem, 2024, 10, 2266–2282 Search PubMed.
- Y. D. Bidal, C. A. Urbina-Blanco, A. Poater, D. B. Cordes, A. M. Z. Slawin, L. Cavallo and C. S. J. Cazin, Dalton Trans., 2019, 48, 11326–11337 RSC.
- A. L. Fuller, F. R. Knight, A. M. Z. Slawin and J. D. Woollins, Eur. J. Inorg. Chem., 2010, 2010, 4034–4043 CrossRef.
- A. Lüning, M. Neugebauer, V. Lingen, A. Krest, K. Stirnat, G. B. Deacon, P. R. Drago, I. Ott, J. Schur, I. Pantenburg, G. Meyer and A. Klein, Eur. J. Inorg. Chem., 2015, 2015, 226–239 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2377387–2377392. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt03305a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |