Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Divergent reduction chemistry of NHC-aluminium(III) hydrides†
Fáinché
Murphy
 ,
Alan R.
Kennedy
,
Alan R.
Kennedy
 and
Catherine E.
Weetman
and
Catherine E.
Weetman
 *
*
Department of Pure and Applied Chemistry, University of Strathclyde, 295 Cathedral Street, Glasgow, G1 1XL, UK. E-mail: Catherine.Weetman@strath.ac.uk
First published on 26th March 2025
Abstract
Understanding and controlling facile reduction chemistry is a key challenge in molecular main group chemistry. Herein, we report the divergent reduction chemistry of aluminium(III) hydrides supported by N-heterocyclic carbenes (NHCs). Choice of reducing agent and NHC ligand are key, with Al(II) dialanes, Al(II) cations, asymmetric Al(II) dialanes and ligand exchange reactions all identified via NMR and single crystal X-ray diffraction.
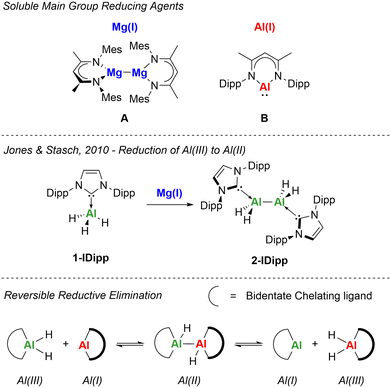
Low-oxidation state aluminium chemistry has rapidly evolved in recent years.1,2 Aluminium(I) and aluminium(II) compounds have been established since the 1990s,3–5 and have shown their transition metal like character through their high reactivity and ability to cleave strong bonds.6–8 Isolation of the first aluminium–aluminium double bond (dialumene)9 in 2017 and anionic aluminium nucleophiles (aluminyls)10 in 2018, has reignited interest in this field and has led to a range of new reactivity and bonding motifs. Access to reduced aluminium species has largely relied upon reductive dehalogenation of Al(III) halides with alkali metals. Stoichiometric reductions with aluminium halides results in formation of the Al(II) singly bonded species, known as dialanes.4 Whereas reactions with excess alkali metal reducing agents yield Al(I) monomers,5 Al(I) double bonds,9 Al(I) masked species11,12 or anionic Al(I)10,13 where there are no formal Al–Al bonds. Alternative routes to Al(I) or Al(II) species have focussed on the use of Al(III) hydrides and their reactivity with soluble β-diketiminate based reducing agents: Mg(I) (A) and Al(I) (B) (Fig. 1).11,14–16 In 2010, Jones, Stasch and co-workers reported the use of their Mg(I) reducing agent (A) to access an IDipp (IDipp = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene) stabilised dialane (2-IDipp) from the reduction of the IDipp-stabilised aluminium(III) trihydride (1-IDipp) (Fig. 1).14 Switching reducing agents to Roesky's Al(I) (B) and reacting with Al(III) dihydrides also results in formation of Al(II) dialanes, however this formation was found to be reversible.15 In a similar vein, Cowley and co-workers reported the isolation of a N,P-coordinated Al(II) dihydride which was also found to undergo reversible reductive elimination.17 This observed equilibria with the Al(II) species provided a new insight into factors that control reductive elimination and has led to transient formation of new Al(I) monomers, which can also dimerise to form new dialumenes depending on the sterics of the supporting ligand (Fig. 1).11
Recently our group examined the role of N-heterocyclic carbenes (NHCs) in the stabilisation of aluminium(III) trihydrides.18 Owing to their easily tuneable steric and electronic features, we reported the largest (1-IPr*) (IPr* = 1,3-bis(2,6-bis(diphenylmethyl)-4-methylphenyl)imidazo-2-ylidene) and smallest (1-ICy) (ICy = 1,3-bis(cyclohexyl)imidazol-2-ylidene) NHC aluminium(III) trihydrides to date, based on their percent buried volume (%Vbur) (Fig. 2). Consequently, we were interested in investigating the reduction chemistry of these NHC-alanes (1) towards various reducing agents to access Al(II) dialanes and target new Al(I) species.
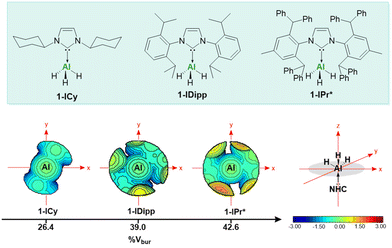 | ||
| Fig. 2 Topographic steric maps of NHC ligands in 1-ICy, 1-IDipp and 1-IPr*. The iso-contour curves of the steric maps are in Å. | ||
As mentioned previously, Jones, Stasch and co-workers reported the use of the Mg(I) dimer (compound A) with 1-IDipp, to yield the dialane complex 2-IDipp.14 Repetition of this reaction, with the larger (1-IPr*) and smaller (1-ICy) NHC-alanes also resulted in the formation of Al(II) dialanes in moderate to good yields (Scheme 1). NMR spectroscopy revealed marginal shifts in both 1H and 13C{1H} NMR spectra for 1vs.2. In both cases, 1H-DOSY NMR studies indicated the retention of a dimeric species in solution based on the estimated molecular weight (see ESI for details†). IR spectroscopy provided further confirmation, as a new sharp band was observed in each case at 1733 cm−1 (2-IPr*) and 1734 cm−1 (2-ICy) for the Al–H stretch. Unfortunately, numerous attempts to grow single crystals of 2 for single crystal X-ray diffraction (SC-XRD) analysis were unsuccessful, however, connectivity data was obtained for 2-IPr* which unequivocally highlights the dimeric nature of these reduced complexes (Fig. S38†). Additionally, an IPr* C–C coupled product was identified via SC-XRD where the carbene carbon C(1) has inserted into the C(6)–H bond to create a new 5-membered ring (Fig. S39†).
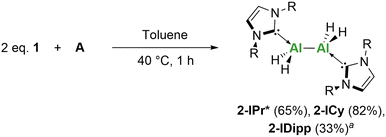 | ||
| Scheme 1 Synthesis of Al(II) dialanes using A. Yield (%). aPreviously reported.14 | ||
Alkali metal reducing agents have been widely used throughout organometallic chemistry to access compounds in their lower oxidation states. Combination of 1-IPr* with lighter alkali metal reducing agents (Li, NaH, 5% Na/NaCl and LiAlH4) resulted in no reaction (see ESI for details†). Use of the heavier alkali metal graphites (KC8, RbC8 and CsC8) with 1 resulted in the isolation of free NHC from the reaction mixtures, despite various attempts in varying the conditions (i.e., solvent, time, number of equivalents). However, in the case of 1-IPr* and KC8, additional minor species were identified in the 1H NMR spectrum. Fractional crystallisation allowed for the identification of one of the minor species, compound 3 (Fig. 3). Compound 3, now an N-heterocyclic aminal containing species, crystallises in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . Loss of the carbene character is confirmed upon comparison of bond lengths and angles around the central N(1)–C(1)–N(2) unit to the previously reported free carbene (IPr*) structure [average N–C(1) 1.469 Å (3) vs. 1.373 Å (IPr*) and N(1)–C(1)–N(2) 105.14° (3) vs. 101.2(2)° (IPr*)].19,20 Formation of N-heterocyclic aminal species from NHC-alanes has previously been reported by our group, which also supports the likely involvement of an Al–H species in the formation of 3.21 In addition to aminal formation, C–C coupling at the C(3) position with the C(Ph)2 carbon has also occurred (C(3)–C(4) 1.548(4) Å). This results in the formation of a second five-membered ring along with saturation of the backbone. Again the saturation of the backbone is confirmed on comparison to the free carbene [C(2)–C(3) 1.527(4) Å (3) vs. 1.344(4) Å (IPr*)] Whilst the mechanism of formation of 3 is unclear, C–H activation of CH-isopropyl or mesityl-CH3 groups have previously been reported for numerous low-oxidation state main group species.22–27 It is important to note here that under analogous conditions reaction of the free carbene IPr* and KC8 does not lead to the formation of 3. Therefore, this strongly suggests the involvement of the parent alumylene or another highly reactive aluminium hydride based intermediate.
. Loss of the carbene character is confirmed upon comparison of bond lengths and angles around the central N(1)–C(1)–N(2) unit to the previously reported free carbene (IPr*) structure [average N–C(1) 1.469 Å (3) vs. 1.373 Å (IPr*) and N(1)–C(1)–N(2) 105.14° (3) vs. 101.2(2)° (IPr*)].19,20 Formation of N-heterocyclic aminal species from NHC-alanes has previously been reported by our group, which also supports the likely involvement of an Al–H species in the formation of 3.21 In addition to aminal formation, C–C coupling at the C(3) position with the C(Ph)2 carbon has also occurred (C(3)–C(4) 1.548(4) Å). This results in the formation of a second five-membered ring along with saturation of the backbone. Again the saturation of the backbone is confirmed on comparison to the free carbene [C(2)–C(3) 1.527(4) Å (3) vs. 1.344(4) Å (IPr*)] Whilst the mechanism of formation of 3 is unclear, C–H activation of CH-isopropyl or mesityl-CH3 groups have previously been reported for numerous low-oxidation state main group species.22–27 It is important to note here that under analogous conditions reaction of the free carbene IPr* and KC8 does not lead to the formation of 3. Therefore, this strongly suggests the involvement of the parent alumylene or another highly reactive aluminium hydride based intermediate.
In further efforts to observe and isolate reactive low-oxidation state aluminium hydride species, we turned our attention to Roesky's β-diketiminate stabilised aluminium(I) complex (B). As a one-centre/two-electron reductant it can be utilised as a controlled and selective reducing agent, as well as a tool for new aluminium-element bond formations. In a similar vein, the tetrameric Al(I) system, [Cp*Al]4 has also been used for new aluminium–element bond formation28–31 as well as an Al(I) transfer reagent,32 as it can dissociate into its monomeric isomer at elevated temperatures.
Reactions of 1 with [Cp*Al]4 resulted in no reaction, even after prolonged heating. However, the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
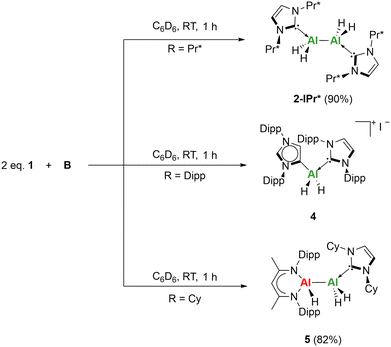
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reaction of 1-IPr* and B in C6D6 at room temperature resulted in the formation of compound 2-IPr* in an improved yield of 90% (Scheme 2). Interestingly, the 2
1 reaction of 1-IPr* and B in C6D6 at room temperature resulted in the formation of compound 2-IPr* in an improved yield of 90% (Scheme 2). Interestingly, the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reaction of 1-IDipp with B in C6D6 at room temperature did not result in the formation of 2-IDipp. Analysis by 1H NMR showed a characteristic 1H singlet at δ6.26 ppm for abnormal carbene formation, this correlates to δ123.9 ppm in the 13C{1H} spectrum by [1H, 13C] HSQC and corresponds to the C(5) position (see ESI for details†). Two 2H septets at δ3.35 and 2.56 ppm (JHH = 6.8 and 6.9 Hz) are now present corresponding to the new diisopropylphenyl environments of the abnormal NHC, alongside the 4H multiplet at δ2.73 ppm for the diisopropylphenyl environments of IDipp. Crystals suitable for SC-XRD analysis were grown from a concentrated toluene solution at −25 °C and matched that of the previously reported [(a-IDipp)AlH2(IDipp)]+[I−] (a-IDipp = 1,3-bis(2,6-diisopropylphenyl)imidazol-4-ylidene) by Stasch and co-workers.33 In their case the normal/abnormal aluminium hydride salt was formed through the reaction of IDippAlH2I with an additional equivalent of IDipp. It is thought that the iodine present in 4, is from the synthesis of B. Despite using crystalline material, compound 4 is preferentially formed even across multiple synthetic batches of B. Lastly, a 2
1 reaction of 1-IDipp with B in C6D6 at room temperature did not result in the formation of 2-IDipp. Analysis by 1H NMR showed a characteristic 1H singlet at δ6.26 ppm for abnormal carbene formation, this correlates to δ123.9 ppm in the 13C{1H} spectrum by [1H, 13C] HSQC and corresponds to the C(5) position (see ESI for details†). Two 2H septets at δ3.35 and 2.56 ppm (JHH = 6.8 and 6.9 Hz) are now present corresponding to the new diisopropylphenyl environments of the abnormal NHC, alongside the 4H multiplet at δ2.73 ppm for the diisopropylphenyl environments of IDipp. Crystals suitable for SC-XRD analysis were grown from a concentrated toluene solution at −25 °C and matched that of the previously reported [(a-IDipp)AlH2(IDipp)]+[I−] (a-IDipp = 1,3-bis(2,6-diisopropylphenyl)imidazol-4-ylidene) by Stasch and co-workers.33 In their case the normal/abnormal aluminium hydride salt was formed through the reaction of IDippAlH2I with an additional equivalent of IDipp. It is thought that the iodine present in 4, is from the synthesis of B. Despite using crystalline material, compound 4 is preferentially formed even across multiple synthetic batches of B. Lastly, a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reaction of 1-ICy with B in C6D6 at room temperature was monitored by 1H NMR spectroscopy. Complete consumption of B was observed after 1 h, with the formation of one equivalent of a new compound and one equivalent of unreacted starting compound 1-ICy. Recrystallisation via slow evaporation of a concentrated benzene solution gave a yellow crystalline product in 82% yield, that was characterised by multinuclear NMR, IR and UV-vis analysis. SC-XRD data obtained revealed the new compound to be the asymmetric comproportionation product (5) formed by the oxidative addition of B to 1-ICy (Scheme 2).
1 reaction of 1-ICy with B in C6D6 at room temperature was monitored by 1H NMR spectroscopy. Complete consumption of B was observed after 1 h, with the formation of one equivalent of a new compound and one equivalent of unreacted starting compound 1-ICy. Recrystallisation via slow evaporation of a concentrated benzene solution gave a yellow crystalline product in 82% yield, that was characterised by multinuclear NMR, IR and UV-vis analysis. SC-XRD data obtained revealed the new compound to be the asymmetric comproportionation product (5) formed by the oxidative addition of B to 1-ICy (Scheme 2).
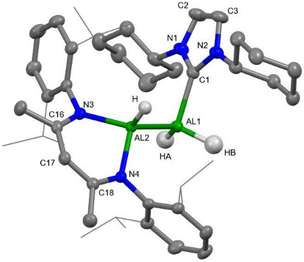
The molecular structure of 5 is depicted in Fig. 4 and is consistent with spectroscopic data. 5 crystallises in the monoclinic space group Cc. The asymmetric nature is evident from the molecular structure, with different ligand environments around the Al(II)–Al(II) bond. One end of the Al(II)–Al(II) unit is ligated by a molecule of ICy and the other by the β-diketiminate ligand. Both Al atoms are tetra-coordinated and adopt a distorted tetrahedral geometry with the coordination of one hydride (H) to Al(2) and two hydrides (HA and HB) to Al(1). Terminal hydrides were located from the electron difference map and refined freely. The Al(1)–Al(2) bond length of 2.5860(7) Å is slightly shorter in comparison to those previously reported for Al(II)–Al(II) bonds (average. 2.62 Å).4 While the asymmetric environment around the Al–Al bond is unique for aluminium(II) hydrides, a similar halide structural motif has been reported previously by Roesky and co-workers in 2017 where the supporting ligands were β-diketiminate and cAAC (cAAC = cyclic alkyl amino carbene).34
In contrast to other β-diketiminate Al(II) complexes where reversible reductive elimination was observed,15 compound 5 is remarkably stable. No equilibrium or exchange processes were observed in solution at room temperature or at elevated temperatures. Additionally, reactivity of 5 with excess 1-ICy resulted in no observed reaction, even after prolonged heating.
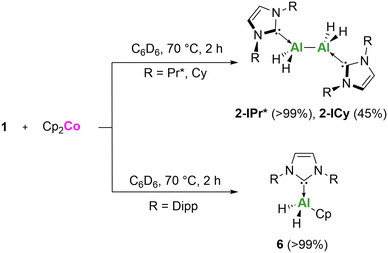
Given the observed divergent reactivity with A and B, we examined the reactivity of 1 towards the milder, one electron reducing agent Cobaltocene (Cp2Co), which is commercially available. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reaction of 1-ICy and 1-IPr* with Cp2Co produced compound 2 (Scheme 3). However, the 1
1 reaction of 1-ICy and 1-IPr* with Cp2Co produced compound 2 (Scheme 3). However, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reaction with 1-IDipp resulted in an alternate ligand exchange reaction producing compound 6. The identity of compound 6, was confirmed by SC-XRD with the data matching that of the previously reported Cp-substituted dihydride complexes (NHCAlH2Cp) reported by Radius and co-workers.35 Whilst the compounds are the same the synthetic routes are very different, with the previous report isolating 6via the salt metathesis reaction of NHCAlH2I and NaCp.
1 reaction with 1-IDipp resulted in an alternate ligand exchange reaction producing compound 6. The identity of compound 6, was confirmed by SC-XRD with the data matching that of the previously reported Cp-substituted dihydride complexes (NHCAlH2Cp) reported by Radius and co-workers.35 Whilst the compounds are the same the synthetic routes are very different, with the previous report isolating 6via the salt metathesis reaction of NHCAlH2I and NaCp.
Conclusions
In conclusion, we have investigated the reduction chemistry of three different NHC-alanes (1) towards new lower-oxidation state complexes. Whilst dimeric NHC-dialanes (2), with aluminium in the +2 oxidation state were prevalent with the soluble main group reducing agent Mg(I) (A), alternate complexes were isolated when changing reducing agents. Reaction with alkali metal reducing agents results in the isolation of free NHC in the majority of cases, with an intriguing C–C coupled species in the case of 1-IPr* indicating a likely Al(I) intermediate. Use of Roesky's Al(I) (B) also results in the formation of different Al(II) complexes, with a dialane (2-IPr*), cationic abnormal NHC complex (4) and asymmetric comproportionation product (5) all observed. These results highlight the versatility of NHC ligands in main group chemistry, and particularly their influence on reaction outcome with aluminium.Author contributions
FM: investigation, formal analysis, writing – original draft. ARK: crystallography, validation, data curation. CEW: writing, visualisation, supervision, funding acquisition, conceptualisation.Data availability
Data that supports the findings of this study are available from the University of Strathclyde Knowledge Base Pure data repository (DOI: https://10.15129/565e5634-b811-4663-8d28-666384dc2130).Conflicts of interest
There are no conflicts to declare.References
- K. Hobson, C. J. Carmalt and C. Bakewell, Chem. Sci., 2020, 11, 6942–6956 RSC.
- J. Hicks, P. Vasko, J. M. Goicoechea and S. Aldridge, Angew. Chem., Int. Ed., 2021, 60, 1702–1713 CrossRef CAS PubMed.
- C. Dohmeier, C. Robl, M. Tacke and H. Schnöckel, Angew. Chem., Int. Ed. Engl., 1991, 30, 564–565 CrossRef.
- P. Bag, C. Weetman and S. Inoue, Angew. Chem., Int. Ed., 2018, 57, 14394–14413 CrossRef CAS PubMed.
- C. Cui, H. W. Roesky, H.-G. Schmidt, M. Noltemeyer, H. Hao and F. Cimpoesu, Angew. Chem., Int. Ed., 2000, 39, 4274–4276 CrossRef CAS PubMed.
- P. P. Power, Nature, 2010, 463, 171–177 CrossRef CAS PubMed.
- C. Weetman and S. Inoue, ChemCatChem, 2018, 10, 4213–4228 CrossRef CAS.
- T. Chu and G. I. Nikonov, Chem. Rev., 2018, 118, 3608–3680 CrossRef CAS PubMed.
- P. Bag, A. Porzelt, P. J. Altmann and S. Inoue, J. Am. Chem. Soc., 2017, 139, 14384–14387 CrossRef CAS PubMed.
- J. Hicks, P. Vasko, J. M. Goicoechea and S. Aldridge, Nature, 2018, 557, 92–95 CrossRef CAS PubMed.
- C. Bakewell, K. Hobson and C. J. Carmalt, Angew. Chem., Int. Ed., 2022, 61, e202205901 CrossRef CAS PubMed.
- J. D. Queen and P. P. Power, Chem. Commun., 2023, 59, 43–46 RSC.
- M. P. Coles and M. J. Evans, Chem. Commun., 2023, 59, 503–519 RSC.
- S. J. Bonyhady, D. Collis, G. Frenking, N. Holzmann, C. Jones and A. Stasch, Nat. Chem., 2010, 2, 865–869 CrossRef CAS PubMed.
- T. Chu, I. Korobkov and G. I. Nikonov, J. Am. Chem. Soc., 2014, 136, 9195–9202 CrossRef CAS PubMed.
- X. Wang, R. F. Ligorio, F. Rüttger, D. M. J. Krengel, N. Graw, R. Herbst-Irmer, A. Krawczuk and D. Stalke, Dalton Trans., 2024, 53, 15441–15450 RSC.
- R. L. Falconer, G. S. Nichol, I. V. Smolyar, S. L. Cockroft and M. J. Cowley, Angew. Chem., Int. Ed., 2021, 60, 2047–2052 CrossRef CAS PubMed.
- F. Murphy, A. R. Kennedy and C. E. Weetman, Inorganics, 2023, 11, 13 CrossRef CAS.
- X. Xu, C. Gourlaouen, B. Jacques and S. Dagorne, Organometallics, 2023, 42, 2813–2825 CrossRef CAS.
- J. Balogh, A. M. Z. Slawin and S. P. Nolan, Organometallics, 2012, 31, 3259–3263 CrossRef CAS.
- T. Wilde, F. Murphy, C. R. T. Smylie, A. R. Kennedy and C. E. Weetman, Chem. – Asian J., 2024, 19, e202301058 CAS.
- D. P. Curran, A. Boussonnière, S. J. Geib and E. Lacôte, Angew. Chem., Int. Ed., 2012, 51, 1602–1605 CAS.
- D. Dhara, A. Jayaraman, M. Härterich, R. D. Dewhurst and H. Braunschweig, Chem. Sci., 2022, 13, 5631–5638 CAS.
- C. N. de Bruin-Dickason, A. J. Boutland, D. Dange, G. B. Deacon and C. Jones, Dalton Trans., 2018, 47, 9512–9520 CAS.
- E. W. Y. Wong, D. Dange, L. Fohlmeister, T. J. Haddlington and C. Jones, Aust. J. Chem., 2013, 66, 1144–1154 CAS.
- T. J. Haddlington and C. Jones, Chem. Commun., 2014, 50, 2321–2323 Search PubMed.
- M. D. Anker, M. Lein and M. P. Coles, Chem. Sci., 2019, 10, 1212–1218 RSC.
- F. Dankert and E. Hevia, Chem. – Eur. J., 2024, 30, e202304336 CrossRef CAS PubMed.
- S. G. Minasian, J. L. Krinsky, V. A. Williams and J. Arnold, J. Am. Chem. Soc., 2008, 130, 10086–10087 CrossRef CAS PubMed.
- M. T. Gamer, P. W. Roesky, S. N. Konchenko, P. Nava and R. Ahlrichs, Angew. Chem., Int. Ed., 2006, 45, 4447–4451 CrossRef CAS PubMed.
- J. D. Gorden, C. L. B. Macdonald and A. H. Cowley, Chem. Commun., 2001, 75–76 RSC.
- D. Sarkar, P. Vasko, A. F. Roper, A. E. Crumpton, M. M. D. Roy, L. P. Griffin, C. Bogle and S. Aldridge, J. Am. Chem. Soc., 2024, 146, 11792–11800 CAS.
- M. Trose, S. Burnett, S. J. Bonyhady, C. Jones, D. B. Cordes, A. M. Z. Slawin and A. Stasch, Dalton Trans., 2018, 47, 10281–10287 RSC.
- B. Li, S. Kundu, H. Zhu, H. Keil, R. Herbst-Irmer, D. Stalke, G. Frenking, D. M. Andrada and H. W. Roesky, Chem. Commun., 2017, 53, 2543–2546 RSC.
- L. Werner, S. Mann and U. Radius, Eur. J. Inorg. Chem., 2023, 26, e202300398 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General synthetic experimental details, NMR spectra and X-ray Crystallography. CCDC 2403858 and 2403860. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5dt00379b |
| This journal is © The Royal Society of Chemistry 2025 |