Open Access Article
Open Access ArticleBioactive metal sulfide nanomaterials as photo-enhanced chemodynamic nanoreactors for tumor therapy
Houjuan Zhu a,
Chui Yu Chana,
Jerry Zhi Xiong Henga,
Karen Yuanting Tanga,
Casandra Hui Teng Chai
a,
Chui Yu Chana,
Jerry Zhi Xiong Henga,
Karen Yuanting Tanga,
Casandra Hui Teng Chai a,
Hui Ling Tanb,
Xian Jun Loh
a,
Hui Ling Tanb,
Xian Jun Loh *a,
Enyi Ye
*a,
Enyi Ye *a and
Zibiao Li
*a and
Zibiao Li *ab
*ab
aInstitute of Materials Research and Engineering, Agency for Science, Technology and Research (A*STAR), 2 Fusionopolis Way, 138634, Singapore. E-mail: lohxj@imre.a-star.edu.sg; yeey@imre.a-star.edu.sg; lizb@imre.a-star.edu.sg
bInstitute of Sustainability for Chemicals, Energy and Environment (ISCE2) A*STAR (Agency for Science, Technology and Research), Singapore 138634, Singapore
First published on 10th April 2025
Abstract
Metal sulfide nanomaterials (MeSNs) are highly promising for biomedical applications due to their low toxicity, good dispersibility, high stability, adjustable particle sizes, and good biocompatibility. Their unique chemical and light-conversion properties also enable them to function as photothermal or photodynamic agents, enhancing chemodynamic therapy (CDT) of tumors. This makes MeSNs valuable as photo-enhanced CDT nanoagents, advancing precision and multi-modal tumor treatment. This review examines recent advancements in MeSNs for photo-enhanced chemodynamic tumor ablation, comparing their effectiveness in CDT. It highlights the roles of photothermal, photodynamic, and photocatalytic effects in enhancing treatment efficacy. MeSN-based nanoreactors are categorized by composition into iron sulfide, copper sulfide, other unary, and multi-MeSNs for their applications in tumor therapy. Additionally, this review discusses challenges, limitations, and future biomedical applications of MeSNs, offering insights into their potential for next-generation cancer treatments.
1. Introduction
Tumors pose a significant and growing threat to human health, claiming millions of lives annually with an alarming upward trend in incidence and mortality rates. Traditional treatment methods such as surgery, chemotherapy (CHT), precision oncology,1–3 and radiotherapy (RT) have long been the cornerstone of cancer management.4–9 However, their efficacy is often undermined by severe side effects and complications, which limit their widespread application and impact patients' quality of life. In response to these challenges, a variety of nanomedicine-based innovative tumor treatment modalities have emerged in recent years.10,11 These include chemodynamic therapy (CDT),12 which exploits tumor microenvironment (TME)-specific catalytic reactions to produce toxic reactive oxygen species (ROS); photodynamic therapy (PDT),13–15 which uses light-activated agents to induce cellular damage; photothermal therapy (PTT),16,17 which relies on localized heat generation to ablate tumors; sonodynamic therapy (SDT),18 which employs ultrasound to activate therapeutic agents; and starvation therapy (ST) that disrupts the nutrient supply essential for tumor growth.19–21 Collectively, these advanced approaches represent a paradigm shift in oncology, offering targeted and less invasive alternatives to conventional treatments.Chemodynamic therapy (CDT) has recently emerged as a groundbreaking cancer treatment strategy, garnering attention for its innovative mechanism and significant potential advantages.22 It leverages the unique TME to catalyse reactions that selectively destroy cancer cells. Specifically, CDT utilizes iron or other transition metal ions (such as Cu, Co, Mn, etc.) to convert excess hydrogen peroxide (H2O2) in tumors into highly cytotoxic hydroxyl radicals (˙OH) through Fenton/Fenton-like reactions under acidic conditions.23 These ˙OH radicals induce oxidative stress, which disrupts critical cellular processes like DNA replication and protein function, ultimately triggering cancer cell apoptosis.24,25 CDT offers several notable benefits over traditional therapies. It is a non-invasive treatment modality with high tumor selectivity and minimal side effects. The tumor-specific selectivity is rooted in the acidic nature of the TME and the elevated H2O2 levels within cancer tissues.26 In contrast, noncancerous regions, which typically exhibit a neutral or alkaline pH and lower H2O2 concentrations, are less affected because Fenton/Fenton-like reactions are challenging to initiate under these conditions. This precise targeting minimizes off-target toxicity, addressing a major limitation of many conventional cancer therapies. Moreover, unlike PDT, CDT does not rely on external factors such as oxygen availability or laser irradiation.27–29 This independence makes it particularly advantageous for treating hypoxic tumors, which are resistant to oxygen-dependent treatments. These unique properties make CDT a promising alternative for more targeted cancer treatment.
Despite its potential, several challenges hinder the clinical application of CDT: (1) low endogenous H2O2 levels limit the Fenton/Fenton-like reactions for effective ROS production in many tumors;15,30–32 (2) mild tumor acidity (pH 5.6–6.8) decreases the catalytic efficiency of Fenton/Fenton-like reactions;33 (3) high glutathione (GSH) concentration enhances ˙OH scavenging and reduces oxidative stress, thereby diminishing the therapeutic efficacy of CDT.34 To overcome these limitations, researchers have explored various strategies to improve CDT's effectiveness. One of the most promising approaches is combining CDT with other therapeutic modalities to create synergistic effects. In particular, phototherapy including PTT and PDT complements CDT by enhancing ROS production and reaction kinetics.15,27,30 The localized heat from PTT accelerates the reaction rate of Fenton/Fenton-like processes, boosting ˙OH production. PDT generates additional ROS, further amplifying oxidative stress within the tumor. By integrating CDT with phototherapy, the nanocatalytic efficiency of Fenton/Fenton-like reactions can be significantly enhanced.35,36 This synergistic approach improves the overall therapeutic impact by generating higher ROS concentrations and accelerating reaction kinetics. While CDT and its combination strategies show great promise, continued innovation is necessary to address current limitations. Enhancing the catalytic efficiency of CDT agents, increasing endogenous H2O2 availability, and reducing tumor GSH levels are critical research directions. With ongoing advancements in nanomaterial engineering and therapeutic integration, CDT has the potential to become a cornerstone of next-generation cancer treatment, offering targeted and minimally invasive options for improved patient outcomes.
In line with this principle of Fenton/Fenton-like reaction, most CDT nanoagents are designed as metal-containing bioactive nanomaterials, including metal–organic frameworks,37–40 metal sulfides,28,41–44 metal carbides,36 and metal oxides.45–47 These nanomaterials play a crucial role in catalysing ROS production, thereby enhancing the therapeutic impact of CDT. Recently, these metal-based nanoagents have attracted significant attention in biomedical research due to their ability to amplify the efficacy of various therapeutic approaches. Among the diverse types of CDT nanoagents, MeSNs stand out as particularly promising for biomedical applications.48–51 Their exceptional physical properties – such as low toxicity, excellent dispersibility in biological environments, high physiological stability, and tunable particle size-make them highly biocompatible and effective in clinical settings. Beyond their favorable physical attributes, MeSNs exhibit a range of notable chemical characteristics that are instrumental in therapeutic applications. For instance, their Fenton-like catalytic activity enables efficient ROS generation, which is critical for inducing oxidative damage in tumor cells. Additionally, MeSNs possess unique light-conversion properties, allowing them to serve as both photothermal and/or photodynamic agents for enhanced chemodynamic cancer therapies. In summary, the combination of their distinctive physical and chemical properties makes MeSNs an invaluable tool as photo-enhanced CDT nanoagents in the field of tumor treatment, where they contribute to significant advancements in precision and multi-modal therapeutic approaches.
To date, although a growing number of research studies have explored the use of MeSNs for tumor therapy,19,52–54 and a growing body of reviews have systematically summarized their applications in therapy, imaging, and drug delivery, a comprehensive and detailed overview specifically addressing the application of MeSNs in CDT for tumors is still notably absent. This review aims to address this gap by providing an in-depth examination of recent advances in the use of MeSNs for photo-enhanced chemodynamic-induced tumor ablation (Fig. 1). It summarizes and compares various types of MeSNs employed in photo-enhanced CDT of tumors (Table 1). According to relevant references, the photothermal, photodynamic, and photocatalytic effects of MeSNs are individually analysed, highlighting their role in improving CDT efficacy against tumors. These mechanisms are further clarified in terms of their respective contributions, including heat-accelerated ROS generation rates and external energy-driven increases in ROS concentrations. Furthermore, based on their synthetic composition, photo-enhanced chemodynamic nanoreactors are categorized into iron sulfide nanomaterials, copper sulfide nanomaterials, other unary MeSNs, and binary MeSNs. The application of each category in photo-enhanced CDT for cancer is discussed and emphasized in detail. Finally, this review not only addresses the current limitations and potential challenges of MeSNs as CDT nanoagents but also explores their future prospects and broader applications in other biomedical fields.
| Nanomaterials | Composition | Size (nm) | PCE (%) | Irradiation conditions | Cell line/animal model | Treatment/imaging | Ref. |
|---|---|---|---|---|---|---|---|
| FeSx-based nanomaterials | PEG-FeS2 NCs | 180–200 | 28.6 | 808 nm | 4T1 cells | PTT-CDT/MRI | 26 |
| 1.5 W cm−2, 0.33 W cm−2 | 4T1 tumor-bearing mice | ||||||
| Sa@FeS | NA | 29.6 | 1064 nm | 4T1 cells | CDT | 65 | |
| 1.5 W cm−2 | 4T1 tumor-bearing mice | ||||||
| Carbon@FeS2 | ∼125 | 27.2 | 808 nm | HeLa cells | PTT-CDT/US-PAI-MRI | 66 | |
| 1.5 W cm−2 | HeLa tumor-bearing mice | ||||||
| FeS-PEG-CAI NPs | ∼20 | 56.51 | 1064 nm | 4T1 cells | PTT-CDT-GT/PAI-MRI-US | 67 | |
| 1.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| BSO-FeS2 NPs | ∼7.27 | 49.5 | 808 nm | 4T1 cells | PTT-PDT-CDT/PAI | 68 | |
| 1.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| FeS2@SRF@BSA NPs | ∼100 | NA | 808 nm | 4T1 cells | PTT-PDT-CDT/FLI | 69 | |
| 1.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| FeS-GOx | ∼12 | NA | 808 nm | 4T1 cells | PTT-CDT/US-PAI-MRI | 70 | |
| NDs | 1.8 W cm−2, 1.6 W cm−2 | 4T1 tumor-bearing mice | |||||
| BSA-FeS2 NPs | 163.0–212.4 | 29.7 | 1064 nm | 4T1 cells | PTT-CDT-ICD | 72 | |
| 1.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| PVP-Fe3S4 NSs | ∼120 | 64.3 | 915 nm | HeLa, SKO-V3 cells | PTT-CDT/MRI | 73 | |
| 15–25 | 0.5 W cm−2 | HeLa tumor-bearing mice | |||||
| PEG-Fe3S4 NPs | ∼20 | 45 | 1064 nm | 4T1, SH-SY5Y cells | PTT-CDT/PAI | 74 | |
| 1.5 W cm−2 | SH-SY5Y tumor-bearing mice | ||||||
| CuSx-based nanomaterials | PEG-Cu2−xS NDs | 2–5 | 30.8 | 1064 nm | 4T1 cells | PTT-CDT/PAI | 79 |
| 1.5 W cm−2 | 4T1 tumor-bearing mice | ||||||
| AIBA@CuS-FA NPs | ∼13 | 47.5 | 1064/808 nm | KB cells | PTT-PDT-CDT | 80 | |
| 0.5 W cm−2 | KB tumor-bearing mice | ||||||
| GOx@CuS nanocomposites | ∼8 | NA | 808 nm | B16F10, MDA-MB-231 cells | PTT-PDT-CDT-ST | 81 | |
| 1.0 W cm−2 | B16F10 tumor-bearing mice | ||||||
| Cu-MOF | ∼85 | 45.7 | 808 nm | L929, HeLa, CT26 cells | PTT-CDT | 89 | |
| 1.0 W cm−2 | CT26 tumor-bearing mice | ||||||
| PVP-Cu9S8 NPs | ∼36.3 | NA | 1064 nm | 4T1 cells | PTT-CDT/PAI | 82 | |
| 0.2 W cm−2 | 4T1 tumor-bearing mice | ||||||
| CuS@COFs-BSA-FA/DOX | ∼300 | 21.5 | 808 nm | 4T1 cells | PTT-CDT-CHT | 90 | |
| 1.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| Cu9S8 NPs | ∼18.05 | NA | 808 nm | 4T1 cells | PTT-CDT/PAI | 91 | |
| 1.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| CCM-Lap-CuS NPs | ∼3.3 | NA | 1064 nm | 4T1, RAW264.7 cells | PTT-CDT/FLI-PAI | 92 | |
| 1.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| hollow mesoporous CuS NPs | ∼130 | 22.5 | 808 nm | LO-2, MCF-7, A549, MDA-MB-231 cells | PTT-CDT | 93 | |
| 1.0 W cm−2 | MCF-7 tumor-bearing mice | ||||||
| HCuS NPs-HCQ-PEG | ∼170 by DLS | 46 | 1060 nm | 4T1 cells | PTT-CDT/PAI | 94 | |
| 1.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| D-HCuS@HA | ∼177.9 by DLS | NA | 808 nm | 4T1 cells | PTT-CDT-CHT/FLI | 95 | |
| 2.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| CuS-ICG@PDA-FA NPs | ∼210.5 | 61.2 | 808 nm | 4T1 cells | PTT-PDT-CDT/FLI | 96 | |
| 0.8 W cm−2 | 4T1 tumor-bearing mice | ||||||
| DOX@H-Cu9S8-PEG NPs | ∼100 | 40.9 | 1064 nm | HUVEC, CT26 cells | PTT-CDT-CHT | 97 | |
| 1.0 W cm−2 | CT26 tumor-bearing mice | ||||||
| HM-CuS-HA(GOx)-Lf NPs | ∼50 | NA | 808 nm | C6 cells | PTT-CDT-ST-CHT/CT-MRI | 99 | |
| 1.0 W cm−2 | C6 tumor-bearing mice | ||||||
| DOX/CuS@Cu-MOF/PEG nanocomposites | Lengths: ∼200; widths: ∼40 | 39.6 | 1064 nm | 4T1 cells | PTT-CDT-CHT/PAI | 100 | |
| 1.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| HP-PCN@CuS nanoplatform | 5–20 | 42.3 | 808 nm | 4T1, HepG2, Caco-2, L02 cells | PTT-PDT-CDT | 101 | |
| 1.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| SiO2@Cu7S4 NCs | 5–8 | 30.3 | 808 nm | HeLa cells | PTT-PDT-CDT | 102 | |
| 1.3 W cm−2 | HeLa tumor-bearing mice | ||||||
| PS@Cu9S8 nanocatalysts | ∼156.1 by DLS | 42.34 | 1064 nm | 4T1 cells | PTT-CDT | 103 | |
| 1.5 W cm−2 | 4T1 tumor-bearing mice | ||||||
| Cu9S5 NSs | 2–3 | 58.96 | 808 nm | 3T3, 4T1 cells | PTT-CDT | 104 | |
| 1.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| 1064 nm | |||||||
| 1.0 W cm−2 | |||||||
| CuS@G5-PEG NPs | ∼25 | NA | 808 nm | 4T1 cells | PTT-CDT | 105 | |
| 1.2 W cm−2 | 4T1 tumor-bearing mice | ||||||
| Alg-CuS/BSA NPs | ∼22 | NA | 808 nm | 4T1 cells | PTT-PDT-CDT | 106 | |
| 1.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| CuS NPs | ∼196 by DLS | NA | 808 nm | CT26 cells | PTT-CDT-CHT | 107 | |
| 0.5 W cm−2 | CT26 tumor-bearing mice | ||||||
| CuS-GOx NPs | ∼8 | NA | 850 nm | MDA-MB-231, A375, HEK-293 cells | PTT-CDT-ST | 108 | |
| 1.5 W cm−2 | A375 tumor-bearing mice | ||||||
| Fuc-CuS NPs-DOX | 10–20 | 39.77 | 808 nm | B16F10 cells | PTT-CDT/CT | 110 | |
| 1.0 W cm−2 | B16F10 tumor-bearing mice | ||||||
| Hollow CuS NPs | NA | 30 | 808 nm | A549 cells | PTT-CDT | 111 | |
| 0.5 W cm−2 | A549 tumor-bearing mice | ||||||
| Liposome-Cu-GOx nanocarriers | ∼56.46 by DLS | 33.8 | 1064 nm | CT26 cells | PTT-CDT | 112 | |
| 1.0 W cm−2 | CT26 tumor-bearing mice | ||||||
| AG@Cu9S8@dOMV | ∼161 | 44.35 | 1064 nm | GL261 cells | PTT-CDT-ST-CHT/FLI | 113 | |
| 1.0 W cm−2 | GL261 tumor-bearing mice | ||||||
| CoSx-based nanomaterials | CoS2 NCs | ∼19.79 | 60.4 | 808 nm | 4T1 cells | PTT-CDT/PAI | 115 |
| 1.0 W cm−2, 0.75 W cm−2 | 4T1 tumor-bearing mice | ||||||
| Co2.19S4 NDs | 3–5 | 52 | 880 nm | 4T1, 3T3 cells | PTT-CDT-ST | 116 | |
| 1.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| BSA-CoSx QDs | ∼5.8 | 40.5 | 808 nm | A431, 4T1 cells | PTT-CDT | 117 | |
| 0.8 W cm−2 | 4T1 tumor-bearing mice | ||||||
| ZIF@Co3S4-ICG | ∼254 | 40.5 | 808 nm | 4T1 cells | PTT-PDT-CDT | 118 | |
| 1.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| GOx@PCoS | ∼180 | 45.06 | 808 nm | 4T1 cells | PTT-PDT-CDT/MRI | 119 | |
| 1.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| Other unitary-metal sulfide-based nanomaterials | MnS@PDA | ∼100 | 24.48 | 808 nm | LLC, HUVEC, 3T3 cells | PTT-CDT/MRI-FLI | 124 |
| 1.5 W cm−2 | LLC tumor-bearing mice | ||||||
| Bi2S3@Bi@PDA-HA/Art NRs | ∼120; ∼30 | 23.35 | 808 nm | 4T1 cells | PTT-PDT-CDT | 125 | |
| 1.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| PEG-Ag2S-Ag | ∼17 | 56.8 | 808 nm | L929, HeLa cells | PTT-CDT/FLI | 128 | |
| 2.0 W cm−2, | HeLa tumor-bearing mice | ||||||
| 1.0 W cm−2 | |||||||
| Multi-metal sulfide-based nanomaterials | Cu2ZnSnS4@BSA | ∼12.6 by DLS | 31.6 | 808 nm | HepG-2 cells | PTT-CDT/MRI-PAI | 151 |
| 1.0 W cm−2 | H22 tumor-bearing mice | ||||||
| Ag2S-Cu(II)-Cu2−xSe NPs | 34.2 | 61.2 | 808 nm | L929, MRC-5, HeLa cells | PTT-CDT | 127 | |
| 1.0 W cm−2 | |||||||
| ZnxMn1−xS@PDA | ∼280 | 32.79 | 808 nm | HUVECs, L02, L929, CT26, HeLa, 4T1 cells | PTT-CDT | 129 | |
| 2.0 W cm−2, | 4T1 tumor-bearing mice | ||||||
| 1.0 W cm−2 | |||||||
| TPZ@Cu-SnS2−x/PLL | ∼300 | 32.8 | 808 nm | L929, 4T1 cells | PTT-CDT-CHT/CT-PAI | 130 | |
| 0.8 W cm−2 | 4T1 tumor-bearing mice | ||||||
| PEG-Cu5FeS4 NPs | ∼22 | 45.9 | 1064 nm | 4T1 cells | PTT-CDT/MRI | 131 | |
| 1.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| Mn doped Cu7S4 NPs | ∼90 | 40.3; 33.4 | 808 nm | BV-2, PC3, HeLa cells | PTT-CDT-CHT | 132 | |
| 1.5 W cm−2 | HeLa tumor-bearing mice | ||||||
| 1064 nm | |||||||
| 1.5 W cm−2 | |||||||
| BSA-Ag:CuS NPs | ∼67 | 37.8 | 1064 nm | 4T1 cells | PTT-CDT | 133 | |
| 1.25 W cm−2 | 4T1 tumor-bearing mice | ||||||
| Co3−xCuxS4 NPs | ∼250 | 46.7 | 808 nm | L929, 4T1 cells | PTT-CDT | 134 | |
| 1.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| BiNS-Fe@Fe-PVP | 48.6 | 37.9 | 808 nm | HepG-2, Eca109 cells | PTT-PDT-CDT/CT-MRI-PAI | 135 | |
| 1.31 W cm−2 | HepG-2 tumor-bearing mice | ||||||
| Mn-CoS@carbon-BSA-DOX | ∼50 | 66.3 | 808 nm | HepG-2 cells | PTT-CDT/MRI-CT | 136 | |
| 0.75 W cm−2 | H22 tumor-bearing mice | ||||||
| CoSnS2 NCs | ∼320 | 47.23 | 808 nm | L02, HUVECs, HC11, 4T1 cells | PTT-CDT | 137 | |
| 1.5 W cm−2 | 4T1 tumor-bearing mice | ||||||
| PEG-MoS2/Co3S4 NFs | 100–150 | 39.8 | 1064 nm | HepG-2, NCM 460 cells | PTT-PDT-CDT/MRI | 138 | |
| 0.8 W cm−2 | 4T1 tumor-bearing mice | ||||||
| PEG-FeS2/CoS2NSs | ∼80 | 50.5 | 1064 nm | HepG-2 cells | PTT-PDT-CDT/MRI-CT | 139 | |
| 0.8 W cm−2 | HepG-2 tumor-bearing mice | ||||||
| PVP-NiS2/FeS2 NPs | NA | 36.74 | 808 nm | 4T1 cells | PTT-PDT-CDT/MRI-PAI | 140 | |
| 1.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| PEG-Cu2MoS4 @GOx | ∼142.5 by DLS | 63.3 | 1064 nm | L929, HeLa cells | PTT-PDT-CDT-ST | 141 | |
| 0.48 W cm−2 | U14 tumor-bearing mice | ||||||
| PEG-CuxMnySz | ∼140 | 56.7 | 1064 nm | 4T1 cells | PTT-CDT/MRI-PAI | 143 | |
| 1.0 W cm−2 | 4T1 tumor-bearing mice | ||||||
| PEG-CuMo2S3 | ∼230 | 38.2 | 1064 nm | B16-F10 cells | PTT-PDT-CDT | 145 | |
| NPs | 0.68 W cm−2 | ||||||
| BSA-CuFeS2 NPs | ∼4.9 | 38.8 | 808 nm | 4T1 cells | PTT-CDT/MRI-FLI | 148 | |
| 1.5 W cm−2 | 4T1 tumor-bearing mice | ||||||
| CuS-Fe@polymer | ∼100 | NA | 808 nm | HeLa, NIH3T3 cells | PTT-CDT | 149 | |
| 0.5 W cm−2, 0.75 W cm−2 | 4T1 tumor-bearing mice | ||||||
| Bi2S3@Fe/Mn-ZIF-8/MTX | ∼259 by DLS | 54 | 808 nm | HepG2 cells | PTT-CDT-CHT/MRI-CT | 152 | |
| 0.75 W cm−2 | HepG2 tumor-bearing mice | ||||||
| DOX@Fe(III)@WS2-PVP | ∼108 | 39.1 | 808 nm | HT29 cells | PTT-CDT-CHT | 153 | |
| 1.0 W cm−2 | HT29 tumor-bearing mice | ||||||
| PEI-CuMnS | ∼20 | 67.8 | 808 nm | 4T1 cells | PTT-PDT-CDT | 154 | |
| NPs | 1.0 W cm−2 | 4T1 tumor-bearing mice | |||||
2. Overview of MeSN-based photo-enhanced chemodynamic nanoreactors
2.1 Synthesis methods, in vivo metabolic pathways, and long-term toxicity of MeSNs
Metal sulfide nanomaterials (MeSNs) have garnered significant attention due to their diverse applications, particularly in biomedical fields. Various synthesis methods, including co-precipitation, hydrothermal synthesis, and sol–gel processes, enable precise control over particle size and morphology-critical factors for optimizing their properties. Recently, environmentally friendly green synthesis approaches using plant extracts or microbial systems have gained interest as sustainable alternatives that minimize the use of toxic chemicals. Furthermore, surface functionalization techniques such as PEGylation or ligand attachment enhance the biocompatibility and targeting capabilities of MeSNs, thereby improving therapeutic efficacy while reducing potential side effects.Also, MeSNs after administration undergo complex in vivo metabolic processes that influence their biodistribution, metabolism, and biodegradation. Factors such as particle size, surface charge, and functionalization impact their distribution across tissues and organs, with the liver, spleen, and kidneys being primary accumulation sites. These organs play essential roles in nanoparticle metabolism and excretion, making it crucial to understand these pathways to predict the long-term biological behavior of MeSNs. Additionally, certain MeSNs can undergo biodegradation, releasing metal ions that may interact with biological systems, requiring further investigation into their therapeutic potential and associated toxicity.
Assessing the long-term toxicity of MeSNs is vital to ensure their safety for biomedical use. Toxicological evaluations, including acute and chronic toxicity studies, examine potential impacts on organ function, histopathology, and overall physiological health. One major concern is the release of metal ions, which may induce cytotoxic effects if present at harmful concentrations. Besides, MeSNs can provoke immune responses, potentially leading to chronic inflammation or other adverse effects. Comprehensive research on these aspects is necessary to mitigate risks and optimize the biocompatibility of MeSNs.
A thorough understanding of the synthesis methods, in vivo metabolic pathways, and long-term toxicity of MeSNs is essential for their safe and effective biomedical applications. Continued research in these areas will contribute to the advancement of targeted therapies, ultimately improving patient outcomes in cancer treatment and beyond.
2.2 Influence of metal elements on MeSN properties
Metal sulfide nanomaterials (MeSNs) have been widely reported in biomedical applications due to their highly tunable physicochemical performances, which are primarily dictated by the type of incorporated metal cation. Factors such as the metal's electronic configuration, oxidation state, coordination environment, and ionic radius play a crucial role in shaping the nanoparticles' morphology, crystallinity, surface charge, optical characteristics, biodegradability, and biocompatibility. These structural and chemical variations directly impact their suitability for diverse biomedical applications, ranging from therapeutics and imaging to biosensing and drug delivery. This section explores the distinctive properties of unitary MeSNs, including CuSx, FeSx, CoSx, AgSx, MnSx, MoSx, NiSx, and BiSx, with a focus on their biomedical relevance. A detailed comparative summary of these materials is provided in Table 2, highlighting their key physicochemical attributes to show the potential applications.| MeSNs | Properties |
|---|---|
| FeSx | Magnetic properties, Fenton catalytic effect |
| CuSx | Fenton-like catalytic, localized surface plasmon resonance (LSPR) |
| CoSx | Fenton-like catalytic, electrocatalytic performance, magnetic properties |
| AgSx | NIR-II fluorescence, localized surface plasmon resonance (LSPR), antibacterial properties |
| MnSx | T1-weighted MRI contrast ability, modulating oxidative stress, redox-mediated catalytic activity |
| MoSx | Photothermal, photodynamic and photocatalytic properties, redox-mediated catalytic activity |
| NiSx | Electrocatalytic activity, magnetically responsive properties |
| BiSx | Strong X-ray attenuation, photothermal properties |
Beyond unitary MeSNs, multi-metal sulfide nanomaterials (MMeSNs), such as CuFeS2, CoSnS2, Cu2MoS4, CuMnS, and Cu2ZnSnS4, have been engineered to harness the synergistic advantages of multiple metal cations within a single nanostructure. By integrating different metal ions, these heterostructured materials exhibit enhanced photothermal conversion efficiency, improved catalytic activity, superior magnetic responsiveness, and increased structural stability, broadening their functional versatility in biomedical applications. MMeSNs facilitate multi-modal therapeutic strategies, including simultaneous bioimaging, targeted drug delivery, and combination therapies, such as PTT, PDT, and CDT. Also, their improved electronic and optical properties, attributed to bandgap modulation and electron transfer effects, position them as highly promising candidates for applications in photoacoustic imaging (PAI), fluorescence-guided diagnostics, and TME modulation. However, despite these advantages, the complexity of MMeSN synthesis, which often requires precise control over stoichiometry, crystal phase, and heterojunction formation, poses significant challenges. Furthermore, the potential toxicity and bioaccumulation risks associated with the release of multiple metal ions necessitate systematic cytotoxicity evaluations, in vivo biodistribution studies, and long-term biocompatibility assessments to guarantee their clinical safety.
The biomedical performance of MeSNs is highly dependent on the intrinsic chemical and physical characteristics of the incorporated metal ions. Each metal confers specific functional attributes, such as localized surface plasmon resonance (LSPR) in CuSx and AgSx, magnetic properties in FeSx and CoSx, redox-mediated catalytic activity in MoSx and MnSx, and high X-ray attenuation in BiSx, all of which can be strategically leveraged for targeted therapies, advanced bioimaging techniques, and theranostic applications. However, critical variations in toxicity, ion release kinetics, and biodegradability necessitate careful material optimization, surface engineering, and systematic biocompatibility assessments. While MMeSNs present a promising avenue for next-generation biomedical applications, further mechanistic studies are essential to fully elucidate their biological interactions, metabolic pathways, and long-term toxicity profiles. Advancements in their nanomaterial synthesis, functionalization strategies, and controlled drug delivery systems will be pivotal in accelerating their clinical translation and regulatory approval for safe and effective biomedical applications.
2.3 Mechanism of photo-enhanced chemodynamic effects
In light of the above summary, it is evident that while MeSN-based CDT offers substantial advantages over conventional cancer treatments, such as high specificity, minimal invasiveness, and the ability to exploit the unique TME,55 it remains limited in its clinical applications due to several inherent challenges. These challenges include suboptimal catalytic activity under physiological conditions,30,56 insufficient production of ROS required for effective tumor destruction,57,58 and the presence of intracellular antioxidants like GSH, which can neutralize ROS and mitigate the therapeutic efficacy of CDT.43,59 To address these limitations and maximize the therapeutic potential of MeSN-based CDT, researchers have developed an array of innovative strategies aimed at overcoming these barriers.60–63 These strategies are largely focused on three interconnected objectives:(1) Designing highly efficient MeSN-based CDT agents: recent advances in nanotechnology have enabled the creation of MeSNs with superior catalytic properties. By engineering nanomaterials with enhanced surface area, higher reactivity, and optimized compositions, these CDT agents demonstrate improved efficiency in catalysing reactions that produce ROS, even under the relatively mild conditions of the TME.
(2) Optimizing environmental conditions: tumors exhibit distinct microenvironmental characteristics, such as hypoxia, acidity (low pH), and high levels of reducing agents like GSH. These unique properties can be exploited to improve CDT performance. By developing MeSNs that respond to acidic pH or modulating environmental factors such as temperature, researchers aim to amplify the catalytic reactions and boost the production of ROS directly within the tumor.
(3) Amplifying oxidative stress in tumors: enhancing oxidative stress within tumors is critical for effective CDT. Strategies to increase ROS levels include elevating the availability of H2O2, a key substrate for Fenton and Fenton-like reactions, or depleting GSH, which acts as an antioxidant and scavenges ROS. By tipping the redox balance in favor of oxidative damage, these approaches improve the overall efficacy of CDT.
Among these strategies, light activation has emerged as a highly promising technique due to its ability to achieve precise, localized treatment while minimizing off-target effects. Light activation leverages the unique properties of specific wavelengths, particularly within the near infrared (NIR)-I biological window (650–950 nm), which allows for deep tissue penetration and low absorption by healthy tissues. This makes it an ideal candidate for enhancing the performance of MeSN-based CDT. Light activation enhances the catalytic efficiency of MeSNs by triggering or amplifying chemical reactions that generate ROS. This process is particularly advantageous because it allows for spatiotemporal control over the activation of MeSNs, ensuring that therapeutic effects are confined to the tumor site. Furthermore, by reducing the required dosage or the need for extreme conditions, light activation minimizes potential side effects and improves the overall safety profile of the therapy. Upon exposure to light, MeSNs absorb energy and utilize it to catalyse the production of ROS, which are crucial for inducing oxidative stress and destroying cancer cells. This enhancement in ROS production occurs through two main mechanisms: (1) photothermal effects: light absorption by MeSNs generates heat, which accelerates Fenton/Fenton-like reactions. These reactions involve the reduction of transition-metal ions from a higher valence state to a lower one, facilitating the rapid decomposition of H2O2 into highly reactive ˙OH. Additionally, the heat generated during the photothermal process promotes the conversion of intracellular GSH into its oxidized form (GSSG). This depletes the tumor's antioxidant defences, further increasing oxidative stress. To better understand these processes, Fig. 2A depicts photothermal-triggered electron transfer and thermal activation of the Fenton catalytic cycle. These visualizations can help clarify the interplay between thermal energy, redox activity, and ROS production at the nanoscale. Moreover, integrating theoretical approaches – such as density functional theory (DFT) calculations or molecular dynamics simulations – could provide valuable insights into activation energy barriers, electron density redistribution, and transition-state dynamics under photothermal stimulation. These combined experimental and computational strategies will be instrumental in advancing the mechanistic understanding of light-enhanced CDT and in guiding the rational design of next-generation nanocatalysts for synergistic cancer therapy; (2) photodynamic and photocatalytic effects: in addition to the photothermal effects, light-activated MeSNs can act as photosensitizers to generate singlet oxygen (1O2) through energy transfer to molecular oxygen. Simultaneously, they can function as photocatalysts, producing superoxide ions (O2˙−) and ˙OH from water via photochemical reactions. These additional ROS species further amplify the oxidative damage inflicted on cancer cells.
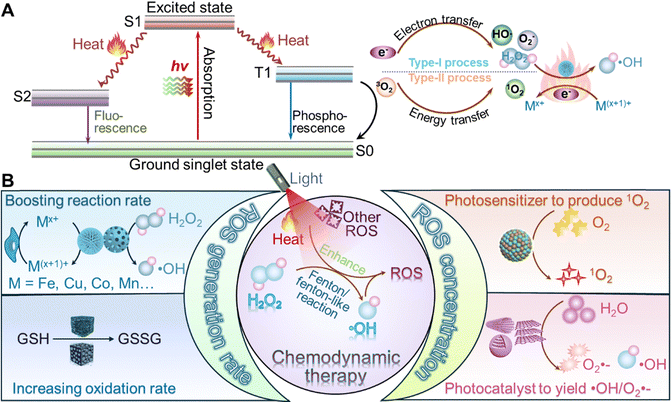 | ||
| Fig. 2 (A) The mechanistic diagrams for the photothermal-triggered electron transfer pathways, (B) Illustration representing the main mechanism for photo-enhanced CDT from MeSN nanoreactors. | ||
As depicted in Fig. 2B, the combined effects of heat and light activation significantly enhance the therapeutic outcomes of MeSN-based CDT. Heat generated by light activation not only accelerates the catalytic conversion of H2O2 into ROS but also disrupts the tumor's redox balance by depleting GSH. At the same time, light-driven photodynamic and photocatalytic processes contribute to the production of various ROS species, including 1O2, O2˙−, and ˙OH, further intensifying oxidative stress within cancer cells. Collectively, these mechanisms demonstrate the immense potential of light activation as a versatile and effective strategy for improving the performance of MeSN-based CDT. By integrating light-responsive properties into CDT agents, researchers aim to develop more efficient, targeted, and safe cancer therapies that address the current limitations of conventional treatment modalities.
3. MeSN-based photo-enhanced chemodynamic nNanoreactor for tumor therapy
3.1 Iron sulfide-based nanoreactor
Iron sulfide-based nanomaterials are emerging as a unique class of agents with significant potential for tumor therapy.36,46,64,65 These materials release Fe2+, which catalyses the conversion of H2O2 into highly ˙OH through the Fenton reaction. The accumulation of ˙OH induces oxidative stress within tumor cells, ultimately leading to cell death, a process known as CDT. Additionally, iron sulfides exhibit strong NIR absorption, enabling them to generate localized heat for PTT and produce photoinduced electron–hole pairs for PDT. These properties make iron sulfides well-suited for combination therapies, offering a powerful approach to tumor treatment.Iron, as one of the most abundant elements on Earth and in the human body, makes iron sulfides cost-effective, safe, and biocompatible. The Fe2+ released by these nanomaterials can be excreted from the body, reducing the risk of organ retention and minimizing concerns about the accumulation of nanoparticles. These favorable properties make iron sulfides an excellent candidate for tumor therapy. One of the most commonly used iron sulfides for tumor treatment is iron(II) disulfide (FeS2), also known as pyrite. For example, Bu's group developed pyrite-based PEG (FeS2-PEG) nanocubes for synergistic PTT and CDT.31 Both in vitro and in vivo studies demonstrated that the localized heat generated from PTT enhanced the CDT effects, leading to reduced tumor size. Similarly, hollow carbon-coated FeS2 (HPFe2@C) nanocatalysts were designed to improve CDT against tumors under light irradiation.66 The carbon coating helped convert NIR light into heat, enhancing the PTT effect. The hollow porous carbon structure enhanced the conversion of NIR light into localized heat, boosting the PTT effect. Furthermore, the incorporation of glucose oxidase (GOx) within the nanocatalyst facilitated in situ conversion of glucose into H2O2, providing more ˙OH for effective CDT. The reduced glucose in tumor cells also triggered a starvation effect. To target cancer cells specifically, folic acid (FA) was added to the surface of the nanocatalyst.
Recently, Wang and colleagues used carbonic anhydrase inhibitor (CAI) to modify ferrous sulfide nanoparticles (FeS-PEG-CAI NPs) for disrupting the metabolic balance within tumors and enhancing tumor elimination by inducing acidosis (Fig. 3A).67 The FeS-PEG-CAI NPs were stable under normal physiological conditions and exhibited excellent photothermal properties under 1064 nm-irradiation (Fig. 3B). Under acidic conditions, however, the FeS-PEG-CAI NPs broke down into functional components, including CAI, Fe2+, and H2S. This degradation triggered the Fenton reaction, where Fe2+ catalysed the production of ˙OH as the pH decreased, enhancing the CDT effect through PTT (Fig. 3C). In vivo, the therapeutic potential of the FeS-PEG-CAI NPs was tested in mice. After nanoparticle injection for 8 h, the mice were exposed to laser irradiation. Infrared thermal images showed that the NPs generated localized tumor heat, raising the temperature by 11.27 °C, higher than that in the FeS-PEG group without CAI and acidosis (Fig. 3D). Tumor volume measurements revealed that the FeS-PEG-CAI NPs caused the greatest tumor inhibition, demonstrating the synergistic effect of PTT-enhanced CDT (Fig. 3E and F). These findings highlight the potential of FeS-PEG-CAI NPs combined with light-induced PTT for effective tumor therapy.
 | ||
| Fig. 3 (A) Graphical representation of FeS-PEG-CAI NPs for the photo-enhanced tumor therapeutic mechanism. (B) Temperature variation in FeS-PEG-CAI NP dispersions at different concentrations irradiated by a 1064 nm laser. (C) Electron spin resonance (ESR) spectra of FeS-PEG-CAI NPs across varied pH and temperature conditions. (D) Infrared thermal imaging of tumor areas with laser irradiation. (E) Tumor volume progression in 4T1 tumor-bearing mice over 14 days. Reprinted with permission from ref. 67. Copyright 2022, Elsevier. | ||
FeS2 has a small band gap of 0.96 eV, allowing it to act as a photosensitizer for PDT when activated by an 808-nm laser. This makes FeS2-based nanomaterials ideal for combined PTT, PDT, and CDT, as they can produce both O2˙− and ˙OH to destroy tumor cells.68 To enhance ROS accumulation, FeS2 NPs can be modified with L-buthionine–sulfoximine (BSO), which blocks GSH synthesis, increasing the effectiveness of the combined therapy. FeS2-based nanomaterials can also be loaded with chemotherapeutic drugs to improve their therapeutic effectiveness. For instance, sorafenib (SRF) inhibits tumor cell growth and blood vessel formation, boosting ROS accumulation.69 Paclitaxel (PTX) can also be loaded into these nanomaterials to trigger tumor cell apoptosis. When PTX-loaded nanoreactors (FeS-GOx@PTX) are combined with cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) checkpoint blockade, they promote the infiltration of cytotoxic T lymphocytes (CTLs) into distant tumors, enhancing PTT/CDT/immunotherapy for tumor elimination.70 Another approach incorporates immunotherapy by loading stimulator of interferon genes (STING) agonists into the nanoreactor. For example, a nanoreactor with the STING agonist cGAMP, FeS2-BSA, and bromelain can work together under 1064-nm laser irradiation, enabling PTT-induced CDT and immunotherapy to effectively kill tumor cells.71 Fe3S4 has emerged as a promising iron sulfide for tumor therapy, offering several advantages over FeS2. Unlike FeS2, Fe3S4 is ferrimagnetic, enabling its use as a magnetic resonance imaging (MRI) contrast agent and for magnetic hyperthermia treatment.72 Additionally, Fe3S4 can be transformed into smaller nanoparticles and Fe ions, which are more easily excreted from the body, making it a safer option for long-term therapy. For instance, Guan and colleagues utilized Fe3S4 tetragonal nanosheets for MRI-guided PTT and CDT, achieving complete excretion of the materials from the body.73 Similarly, Dong's group developed GOx and PEG-modified Fe3S4 nanoplates (Fe3S4-PEG-GOD),74 which achieved over 80% shrinkage of both tumors under NIR-II irradiation. These nanocatalysts also provided excellent PAI, further enhancing their therapeutic potential.
3.2 Copper sulfide-based nanoreactors
Copper sulfide-based (Cu2−xS, 0 ≤ x ≤ 1) nanocatalysts have emerged as promising tools for antitumor therapy and imaging due to their affordability, abundance,75 simple synthesis, low toxicity, biodegradability, and scalability.76–78 They feature unique properties, such as LSPR,79 strong absorption in the NIR region, and high photothermal conversion efficiency (PCE).80 Additionally, copper ion d–d orbital transitions ensure stable photothermal performance across various sizes, shapes, and environments.81These properties make Cu2−xS nanocatalysts highly effective in multiple therapeutic applications.83–87 They serve as contrast agents for PAI and as photothermal agents in PTT. Their ability to generate ROS upon light activation also makes them valuable for PDT.88 Furthermore, their catalytic versatility extends to Fenton-like reactions,80 where they outperform iron-based catalysts across different pH levels. Specifically, Cu2−xS reacts with H2O2 in the TME to produce cytotoxic ˙OH, enabling CDT.79 The superior reactivity of Cu+ with H2O2, compared to Fe2+, and the synergistic heat generation during PTT further enhance CDT efficacy. For example, copper-based metal–organic framework nanoparticles (HKUST-1) were converted into NIR-activated copper sulfide using an H2S-triggered reaction to improve colon cancer treatment through PTT, CDT, and gas therapy (GT).89 These combined effects make Cu2−xS nanocatalysts powerful candidates for multimodal therapies, including PTT, PDT, CDT, and their combinations. Recent advancements in Cu2−xS nanocatalysts have focused on improving treatment outcomes through integrated therapeutic strategies. For instance, although CuS nanoparticles (NPs) have inherent photothermal properties, An et al.82 developed a Cu9S8 NPs platform that operates at room temperature under low-power, short-duration NIR-II laser irradiation, enhancing immunotherapy via plasmon-induced CDT in a photo-Fenton process. As shown in Fig. 4A, self-doped Cu9S8 NPs enter the bloodstream and act as efficient Fenton-like agents with strong NIR-II absorption. Mild NIR-II laser irradiation promotes Cu(II)/Cu(I) conversion, boosting ROS generation in the TME, leading to oxidative stress, immunogenic cell death (ICD), and enhanced antitumor immunotherapy. The ESR spectrum in Fig. 4B confirms significantly increased ˙OH generation under NIR-II laser exposure, indicating enhanced ˙OH levels through plasmonic effects. Under 1064 nm laser exposure, Cu9S8 NPs generate a photocurrent that stops when the laser is turned off (Fig. 4C), while CuS NPs show no photocurrent, suggesting that Cu9S8 NPs rapidly undergo LSPR dephasing, releasing electrons and holes. Fig. 4D and E demonstrate that this synergistic approach significantly ablated primary tumors and inhibited the growth of distant tumors compared to the control group. The enhanced efficacy of anti-PD-L1 therapy via NIR-II laser-assisted photo-Fenton-like reactions was further confirmed by a reduction in tumor size for both primary and distant tumors (Fig. 4F), highlighting the significantly enhanced chemodynamic efficiency of Cu9S8 NPs with NIR-II irradiation.
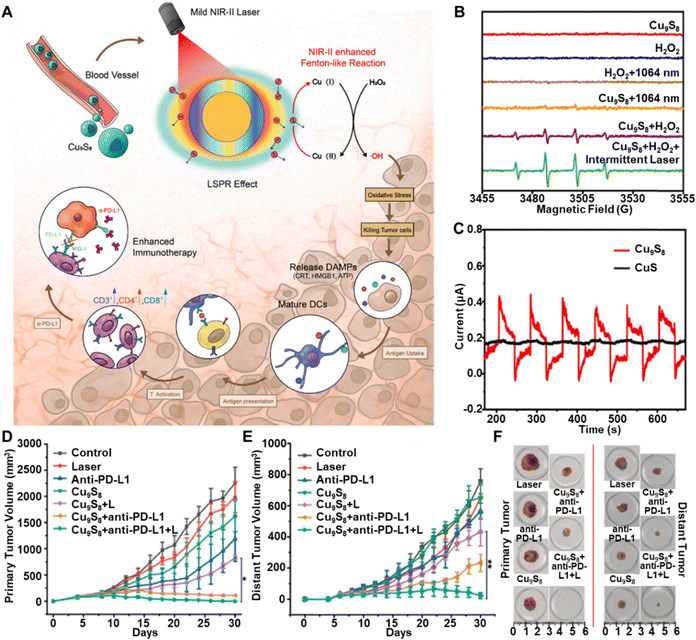 | ||
| Fig. 4 (A) Diagram showing the mechanism of NIR-II laser-triggered photo-Fenton-like reactions to enhance immunotherapy. (B) The ESR spectrum for different experimental groups. (C) The photocurrent behaviours of Cu9S8 nanoparticles and CuS after undergoing six cycles of laser exposure. (D) and (E) The monitoring the primary and distant tumor volume following various treatments. (F) Representative images of tumors following different treatments. Reprinted with permission from ref. 82. Copyright 2022, Elsevier. | ||
Several strategies have been developed for photo-enhanced CDT of targeted and effective anti-tumor therapy. Folic acid (FA), known to target tumors by binding to the folate receptor (FR) overexpressed on many cancer cells, is commonly used in these approaches. Based on this insight, Sun and colleagues functionalized CuS NPs with a FA ligand to selectively target folate receptors on tumor cells.80 The resulting hybrid system, AIBA@CuS-FA, combines fluorescence imaging-guided PTT, oxygen-independent PDT-like therapy, and CDT, effectively killing cancer cells under low-power NIR-II laser irradiation. Similarly, CuS-based nanostructures functionalized with BSA-FA were developed as a TME-responsive platform for targeted PTT, CDT, and CHT.90 This platform targets folate receptors on tumor cells, improving drug uptake, stability, and biocompatibility. It integrates CuS NPs with COFs for PTT and CDT, while DOX is loaded into COF mesopores for CHT. Upon NIR irradiation and in the acidic TME, DOX is released, and H2O2 levels rise, enhancing CDT. In a unique approach, Liu et al.91 developed a one-pot method to create LDH-CuS nanocomposites (NCs) that localize in cancer cell lysosomes. Under a NIR laser, these NCs increase temperature and release copper ions, generating ROS to enhance CDT. They also promote electron transfer, preventing hole recombination and increasing 1O2 for PDT. The ROS induce lysosomal lipid peroxidation, leading to cell death via lysosomal membrane permeabilization. Additionally, a biomimetic nanozyme, CD47@CCM-Lap-CuS NPs, was engineered for combined CDT and PTT in breast cancer treatment.92 This nanozyme supplies H2O2 in situ and targets breast cancer cells via CD47 overexpression, enhancing targeted therapy efficacy.
Increasing the surface area of copper sulfide-based nanoparticles significantly enhances their catalytic and therapeutic performance. By providing more active sites for H2O2 reactions, these nanoparticles boost PTT combined with CDT and support other synergistic treatments. Hollow nanostructures, with their high surface area, have been shown to improve these properties.93–96 For example, Wang et al. developed hollow Cu9S8 NPs with 1.7 times the surface area of solid counterparts,91 improving photothermal properties and chemotherapeutic efficiency. Liu et al. coated hollow Cu9S8 NPs with PEG and loaded them with DOX, creating DOX@H-Cu9S8/PEG nanocomposites that efficiently combine PTT, CHT, and CDT with high drug-loading capacity and NIR-triggered drug release.97 Building on this, PEGylated pH-responsive peptides (PEG-pHLIP) and lauric acid (LA)-modified hollow CuS nanoparticles (HCuS NPs) were developed for a smart drug delivery system,98 encapsulating the stress granule (SG) inhibitor ISRIB to enhance PTT and CDT, effectively inhibiting primary tumors and preventing metastasis. Hu et al. applied hollow CuS nanoparticles in a photo-responsive drug delivery platform, D-HCuSHA, combining hyaluronic acid (HA) and losartan for synergistic anti-tumor therapy.95 Upon laser irradiation, DDTC in CuS reacts with Cu2+ to form Cu(DDTC)2, inducing apoptosis and ICD, while losartan enhances T cell infiltration in the TME. For glioblastoma multiforme (GBM), hollow mesoporous copper sulfide nanoparticles (CTHG-Lf NPs) were modified with lactoferrin (Lf), GOx, and HA to improve blood–brain barrier (BBB) permeability and target GBM cells.99 This modification prevents drug leakage, enabling targeted release and supporting CHT, CDT, PTT, and ST for enhanced treatment.
Porous hybrid platforms, with their high surface areas, are highly effective for enhancing catalytic and therapeutic performance in CDT. For instance, Geng et al. developed a copper-based metal–organic framework (Cu-MOF) that incorporates CuS nanodots (NDs) on both the surface and within the pores. This design increases surface area and enables the integration of PTT, CDT, and CHT.100 The addition of PEG improves dispersibility, while the platform's high DOX loading capacity and responsive drug release in acidic TME further enhance therapeutic outcomes. Similarly, a hybrid nanoplatform combining hierarchically porous porphyrinic coordination networks (HP-PCN) with CuS NPs was developed to enable robust ROS generation for PDT, in addition to CDT and PTT.101 In another innovative design, Sun et al. created SiO2@Cu7S4 nanotubes with dual valency, improving ROS generation and accelerating both CDT and PDT under 808-nm laser irradiation.102 Additionally, flower-like PS@Cu9S8 nanocatalysts were fabricated using biomineralization strategies, providing a high surface area for enhanced CDT and serving as laser-cavity mirrors for NIR-II light, resulting in high PCE.103 In vivo studies confirmed strong therapeutic efficacy with minimal toxicity. By integrating PTT, CDT, PDT, and CHT, these multimodal platforms enhance antitumor efficacy while minimizing side effects.
Driven by the outstanding performance of nanosheets, ultrathin Cu9S5 nanosheets (NSs) with sulfur vacancies were designed through a one-pot method, enabling multimodal therapies,104 including PTT, CDT, PDT, and GSH depletion under NIR laser irradiation, as shown in Fig. 5A. Photothermal performance tests demonstrated a positive correlation between Cu9S5 NS concentration and temperature increase under 1064-nm laser exposure (Fig. 5B). With increasing concentration of Cu9S5 NSs, the maximum temperatures were found to increase to 65.9 °C, indicating the good photothermal effect. Additionally, 1O2 production from Cu9S5 NSs was assessed through measuring absorption at 380 nm of 9,10-anthracenediylbis(methylene) dimalonic acid (ABDA) and H2O2 under 1064-nm laser irradiation, confirming efficient 1O2 production for PDT (Fig. 5C). Also, ˙OH generation from Cu9S5 NS catalysed H2O2 was assessed using terephthalic acid (TAOH). Laser irradiation led to a notable increase in fluorescence intensity at 425 nm, indicating photothermal enhanced CDT (Fig. 5D). In vivo antitumor efficacy was evaluated in a breast cancer mouse model. Tumor growth inhibition was most significant under NIR II laser irradiation (Fig. 5E), highlighting the ability of NIR-II laser irradiation to induce rapid temperature increases, 1O2 and ˙OH production, triggering apoptosis through PTT, PDT, and CDT.
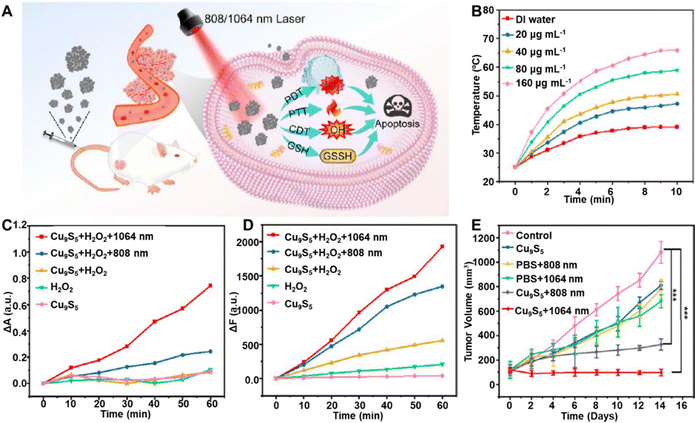 | ||
| Fig. 5 (A) Diagrammatic representation of Cu9S5 NSs for PTT/PDT/CDT under NIR-I and NIR-II irradiation. (B) Temperature evolution of Cu9S5 NSs with varying concentrations under a 1064-nm laser. (C) Absorption drop at 380 nm in ABDA solution mediated by Cu9S5 NSs and H2O2. (D) Reduction of the fluorescence intensity at 425 nm in the TAOH solution facilitated by Cu9S5 NSs and H2O2. (F) Tumor volume trends under different treatment conditions. Reprinted with permission from ref. 104. Copyright 2024, American Chemical Society. | ||
A critical limitation of CDT is the insufficient concentration of H2O2 in tumors, which restricts its ability to eliminate cancer cells resistant to oxidative stress. To address this challenge, researchers have developed strategies for in situ H2O2 generation, primarily using GOx. GOx catalyses the oxidation of glucose into gluconic acid and H2O2, simultaneously depleting glucose to starve tumor cells and sustaining ˙OH production to enhance CDT efficacy. In a paradigm, Kong et al.105 developed a biomimetic enzyme system, CuS/G5-GOx, designed for pH-responsive CDT and enhanced PTT. A Generation 5 poly(amidoamine) (G5) dendrimer protected GOx until tumor-specific release, effectively preventing breast cancer recurrence and metastasis under laser irradiation. Similarly, Singh et al.81 synthesized GOx-coated CuS nanocomposites (GOx@CuS) integrating PTT, PDT, ST, and chloride-accelerated CDT under NIR irradiation. The presence of chloride ions enhanced Cu-based CDT (Cl-Cu CDT), boosting ROS production. Within glucose- and chloride-rich tumors, GOx@CuS formed a nano-cascade system that regenerated H2O2, achieving effective tumor eradication. Another promising approach involves mesoporous Cu2−xS NPs (HMCu2−xS) functionalized with GOx and oxygen-loaded perfluoropentane (PFP) (PO@HMCG).88 Upon NIR irradiation, PFP transitioned to gas, releasing oxygen to sustain ROS generation, which demonstrated strong antitumor effects without toxicity in vivo.
A delivery patch offers a promising site-specific approach for cancer treatment.106 For instance, a thermosensitive hydrogel co-loaded with CuS NPs and camptothecin (CPT) can induce apoptosis while activating nicotinamide adenine dinucleotide phosphate oxidase (NOX) to generate H2O2. The heat generated by CuS NPs enhances CDT, while CPT further amplifies CDT efficacy by blocking DNA synthesis.107 To endow the benefits of the patch to photothermal enhanced CDT combining with GOx, researchers have developed various patches, including microneedles (MNs), hydrogels, and membranes, designed to penetrate the skin's outer layer for efficient GOx and CuS NP delivery.108 For instance, a dissolvable MN loaded with GOx-CuS NCs utilizes tumor glucose to generate H2O2, synergizing ST, PTT, and CDT for melanoma treatment.109 An innovative bubble pump microneedle system (BPMN-CuS/DOX) was developed for transdermal cancer therapy, integrating CDT, PTT, and CHT.110 Encapsulated NaHCO3 generates CO2 in the acidic TME, enhancing drug penetration and controlled DOX release. Additionally, fucoidan coating improves biocompatibility and immune regulation, while CuS NPs facilitate PTT and CDT. DOX further increases H2O2 levels, amplifying CDT efficacy.
Another strategy for developing nanoplatforms for anti-tumor treatment involves using a polymeric matrix to encapsulate CuS NPs and other active agents, enabling controlled release in response to PTT-generated heat. For example, Ning and coworkers co-loaded hollow CuS NPs and β-lapachone (Lap) into agarose hydrogels, facilitating a self-sustaining H2O2 supply to enhance CDT efficacy.111 Various researchers have employed this approach to integrate PTT with enhanced CDT by ensuring a self-sustaining supply of H2O2. For instance, Wu et al.112 designed a thermos-responsive liposome shell loaded with CuS NPs and GOx, enabling controlled H2O2 production. More recently, He et al. developed AG@Cu9S8@dOMV NPs,113 which were camouflaged with lipopolysaccharide-free bacterial outer membrane vesicles (dOMV) to improve BBB penetration and GBM targeting. These nanoparticles, loaded with AQ4N and GOx, consumed glucose and oxygen, inducing ST, generating H2O2 for CDT, and activating AQ4N under hypoxic conditions. Upon NIR-II irradiation, Cu9S8 enhanced CDT efficacy and drug release. In vivo studies demonstrated significant GBM inhibition without increasing heat shock protein (HSP) expression.
In conclusion, CuS NPs show great potential for cancer treatment by integrating PTT, CDT, and PDT. To enhance their efficacy, CuS NPs can be loaded with anticancer drugs such as DOX for CHT, as well as GOx, which not only facilitates the in situ generation of H2O2 to enhance CDT but also achieves ST via glucose consumption. Furthermore, CuS NPs can be tailored for specific tumor targeting by adding ligands such as FA. Studies have demonstrated that CuS nanoplatforms effectively inhibit primary tumors and help prevent metastasis with minimal toxicity.
3.3 Cobalt sulfide-based nanoreactors
Beyond the commonly used transition metal ions in CDT, cobalt has emerged as a promising candidate, particularly in the form of cobalt complexes. Recent studies have highlighted the potential of cobalt-based nanomaterials in enhancing ROS generation, thereby improving tumor targeting and therapeutic efficacy.114 As an exemplary work, monodispersed CoS2 nanoclusters were developed using the La Mer scheme to achieve a synergistic PTT/CDT approach.115 These nanoclusters exhibited excellent PCE and catalytic activity for Fenton-like reactions, significantly enhancing cancer cell eradication in both in vitro and in vivo models while maintaining biodegradability and low toxicity. A key feature of Wang's work was the defect-engineered, loosely stacked CoS2 nanocrystals, which increased catalytic sites for converting endogenous H2O2 into cytotoxic ˙OH while enabling oxidation into smaller, excretable species via the reticuloendothelial system. Building on this, Zhao et al. synthesized two-dimensional cobalt chalcogenide nanodots (Co2.19S2 NDs) through a one-step solvothermal method, demonstrating potent ST properties.116 With strong optical absorption, efficient photothermal conversion, and rapid degradation into Co2+ and S2−, these NDs synergistically enhanced CDT and PTT. Their study demonstrated effective tumor suppression through ST and PTT-enhanced CDT, achieving significant antitumor efficacy.Leveraging the exceptional Fenton-like performance of CoS nanomaterials, Zhu et al. developed sulfur-deficient biodegradable cobalt sulfide quantum dots (CoSx QDs) to enhance cancer therapy.117 These QDs induce cancer cell death through four key mechanisms (Fig. 6A): (1) Fenton-like reaction, (2) GSH depletion, (3) PTT, and (4) PTT-enhanced Fenton-like reaction. The defect-engineered CoSx QDs catalyse endogenous H2O2 into ROS, causing oxidative damage while oxidizing GSH to GSSG, further amplifying ROS production by disrupting cellular antioxidant defences. Their strong photothermal properties enable direct cancer cell ablation, with heat further accelerating the Fenton-like reaction and ROS generation. The photothermal effect of CoSx QDs was concentration-dependent, with a significant temperature increase observed within five minutes (Fig. 6B). ESR spectra confirmed enhanced Fenton-like activity at higher temperatures (pH 6.5, 40 °C), indicating increased ROS production and accelerated CDT (Fig. 6C). In vivo, tumor temperatures in mice injected with CoSx QDs rapidly rose to 52 °C, demonstrating effective hyperthermia-induced tumor inhibition (Fig. 6D). While CoSx QDs exhibited strong ˙OH generation in the TME, the Fenton-like reaction alone was insufficient for complete tumor eradication (Fig. 6E and F). However, this demonstrated that their biodegradable CoSx QDs significantly enhanced CDT and PTT efficacy, leading to complete tumor suppression under NIR irradiation.
 | ||
| Fig. 6 (A) Graphical representation of biodegradable CoSx QDs for photothermal and heat-enhanced CDT of tumors. (B) Relevant photothermal heating profiles of CoSx QDs at varying concentrations under 808-nm laser irradiation. (C) ESR spectra of 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) capturing ˙OH under diverse conditions. (D) IR images of tumor-bearing mice following laser irradiation after intravenous administration of saline or CoSx QDs. (E) Tumor growth progression in various mouse groups. (F) Digital images of surgically removed tumors. Reprinted with permission from ref. 117. Copyright 2022, American Chemical Society. | ||
Given the limitations of single-modality therapies, researchers have increasingly adopted multi-modal approaches to improve tumor ablation. For instance, Jiang et al. developed a trimodal PDT/PTT/CDT strategy, effectively enhancing therapeutic efficacy through a synergistic “kill three birds with one stone” approach.118 They incorporated the photosensitizer indocyanine green (ICG) into hollow Co3S4 NPs, which degrade in the acidic TME to release Co2+, triggering a localized Fenton-like reaction and generating cytotoxic ˙OH for CDT. The Co3S4-ICG nanocomplex exhibited superior therapeutic potential, efficiently producing 1O2 for PDT and achieving a high PCE (40.5%), enabling simultaneous PDT and PTT under laser irradiation, ultimately leading to effective tumor ablation. Further advancing this multi-modal paradigm, GOx-encapsulated mesoporous hollow Co9S8 nanoreactors were designed to coat with polyphenol block copolymers (POEGMA-b-PDOPA) to enhance tumor ablation.119 Their GOx@PCoS nanoreactors, exhibiting superior PCE (45.06%), facilitated potent phototherapy and oxygen generation under NIR laser irradiation. To maximize therapeutic efficacy, they developed a multifunctional cascade nanoreactor integrating CDT, GSH depletion, PDT, ST, and PTT. These studies underscore the potential of multi-modal strategies in improving antitumor efficacy and advancing cancer treatment.
3.4 Other unitary metal sulfide-based nanoreactors
Beyond copper, cobalt, and iron sulfide-based materials, other binary metal sulfides have also gained attention as promising candidates for photo-enhanced CDT nanotheranostics in tumor treatment. These nanomaterials leverage the unique properties of transition metal sulfides, such as their excellent PCE, redox activity, and ability to catalyse the decomposition of H2O2 into ROS. When exposed to NIR light, these nanomaterials generate localized heat, enhancing their CDT efficiency by accelerating Fenton-like reactions and promoting oxidative stress within tumor cells. Additionally, their tunable band structures enable synergistic interactions with PDT and PTT, further amplifying their antitumor effects. Recent studies have demonstrated that binary metal sulfides, including manganese sulfide (MnS), molybdenum oxo-sulfide (MoxS2−x),35,120,121 bismuth sulfide (Bi2S3), and silver sulfide (Ag2S), exhibit significant potential in tumor theranostics, offering multimodal imaging capabilities, controlled drug release, and targeted therapeutic effects.To further enhance anticancer efficacy, doping with manganese (Mn) has been explored to boost free electron flow and activate both radical and non-radical pathways for catalytic degradation. The release of Mn2+ also enables MRI and Mn-based CDT.58,122,123 Within this context, one notable example is MnS nanocomposites. Ma et al. demonstrated the effectiveness of a PDA-coated MnS nanocluster as both a contrast agent for MRI and a nanoplatform for synergistic ferroptosis-photothermal therapy (Fig. 7A).124 The MnS@PDA nanoplatform was created by coating spherical MnS with PDA, enhancing the interaction between the pH-sensitive MnS and water molecules through hydrogen bonding. This interaction improved tumor MRI efficiency, with the spin–lattice relaxation time increasing from 5.76 mM−1 s−1 for unmodified MnS to 19.33 mM−1 s−1 for the nanocomposite at pH 5.5. MnS@PDA also exhibited notable photothermal properties, with temperature rising in proportion to nanocomposite concentration (Fig. 7B). The combination of PTT and MnS-mediated effects significantly enhanced antitumor activity. In the acidic TME, MnS releases hydrogen sulfide (H2S), sensitizing tumor cells to PTT by inhibiting mitochondrial respiration and ATP production, which reduces heat shock protein levels. Additionally, released Mn2+ exhibits potent peroxide (POD) and glutathione oxidase (GSHox) activities, converting abundant H2O2 into highly reactive ˙OH, depleting GSH, and inducing synergistic ferroptosis and apoptosis through catalytic cell death, further amplified by photothermal effects (Fig. 7C). In vivo studies confirmed the remarkable antitumor efficacy of MnS@PDA, achieving complete tumor volume inhibition under NIR irradiation (Fig. 7D).
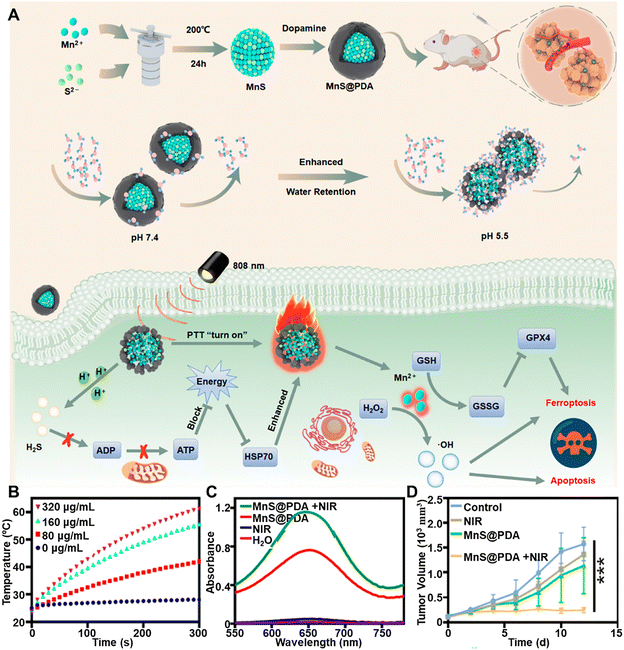 | ||
| Fig. 7 (A) Diagram depicting the MnS@PDA synthesis process, the role of the PDA shell in modulating water molecule interactions across different pH levels, and the underlying mechanisms by which MnS@PDA enhances PTT and ferroptosis-based tumor treatment. (B) Temperature variations of MnS@PDA at varying concentrations under photothermal irradiation. (C) UV-vis absorption spectra of TMB in the presence of H2O2, comparing conditions with and without NIR irradiation. (D) Tumor growth trends following different treatment regimens. Reprinted with permission from ref. 124. Copyright 2024, American Chemical Society. | ||
Except for the aforementioned MeSNs, a NIR-responsive Bi2S3@Bi Z-scheme heterostructure was developed for synergistic PTT, CDT, and PDT in hypoxic tumors.125 Hydrazine post-treatment of solvothermally synthesized Bi2S3 nanorods induced the formation of a Bi-layer, enhancing both PTT and PDT efficacy. Additionally, coating with PDA/ammonium bicarbonate (PDA/ABC) and HA enabled targeted artesunate (Art) delivery. Art-mediated transferrin degradation elevated intracellular Fe2+ levels, promoting ROS generation for efficient CDT. Furthermore, Ag2S NPs have emerged as an attractive therapeutic platform.126,127 Bio-responsive Janus nanoparticles (JNPs) based on Ag2S were designed for precise NIR-II bioimaging and tumor therapy.128 The asymmetric Ag2S-sulfur heterostructure, coated with polyethylene glycol (PEG), facilitated dual-mode treatment. Ag2S JNPs exhibited strong PTT capability, while silver (Ag) catalyzed the decomposition of H2O2 into highly reactive ˙OH, enhancing CDT. The Janus structure further amplified CDT effects, achieving a high PCE of 56.8%. These JNPs demonstrated potent antitumor efficacy, resulting in a tumor inhibition rate of 96.2% through PTT-enhanced CDT.
3.5 Binary metal sulfide-based nanoreactors
Building on the use of MeSNs in photo-enhanced Fenton/Fenton-like tumor therapy, binary structures have been developed to enhance PCE, ROS generation, and advanced imaging capabilities compared to individual metal sulfides. These complex formations offer improved stability, biocompatibility, and multifunctionality by integrating multiple therapeutic modalities such as PTT and CDT.129,130 This subsection highlights the advantages of binary and ternary metal sulfides over their individual counterparts, particularly in terms of high PCE, enhanced ROS generation, advanced imaging capabilities, and synergistic therapeutic effects. These benefits underscore their potential as superior alternatives for targeted tumor therapy.Several strategies have been employed to improve their NIR absorption capabilities. A prominent approach is leveraging the LSPR effect, which enhances the photothermal properties of ternary Cu5FeS4 NPs.131 This strategy enables Cu5FeS4 NPs to achieve a PCE of 45.9%, substantially surpassing unitary counterparts such as CuFeS2 (36.6%) and FeS2 (24.4%). The improvement arises from modulating the LSPR characteristic peak shift toward longer wavelengths by increasing Cu/Fe ratios. Doping metals into CuS NPs with inherent photothermal properties has been shown to enhance chemodynamic antitumor efficacy. In one design,132 Mn-doped Cu7S4 nanospheres (Cu(Mn)S) exhibited high PCE (40.3% NIR-I, 33.4% NIR-II), enabling PTT, CDT, and CHT for effectively inhibiting tumor growth in vivo. Through a similar approach, doping Ag into CuS (BSA-Ag:CuS NPs) achieves a 37.8% of PCE for enhanced PTT and CDT of halted tumors.133
Other binary systems have demonstrated improved photothermal properties through compositional, surface, and morphological modifications. For instance, Cu integration in Co3−xCuxS4 nanoparticles enhances NIR absorption without compromising their crystalline structure.134 Similarly, surface decoration of Fe on BiNS in BiNS-Fe@Fe enhances the SPR effect and introduces deep-level defects, generating additional electrons to improve optical absorption and photothermal efficiency.135 Hollow core–shell structures, such as Mn–CoS@C and CoSnS2, utilize multiple reflections of incident light within the hollow structure to achieve notable improvements in PCE and stability.136,137 The advantages of binary metal sulfides over unitary counterparts are exemplified by MoS2/Co3S4@PEG nanoflowers, which exhibit a PCE of 39.4% under a 1064 nm laser compared to 33.2% for MoS2@PEG.138 Similarly, PEGylated Co3−xCuxS4 achieves a PCE of 46.7% under an 808-nm laser, far exceeding the 33.1% observed for Co3S4.134 These examples underscore the robust PTT functionalities of binary metal sulfide-based nanoreactors.
In Fenton-like reactions, binary metal sulfides leverage multivalent redox cycles to enhance catalytic efficiency. Elements such as Cu, Mo, Co, and Sn can transition between multiple oxidation states, continuously regenerating active catalytic species and sustaining high levels of ROS production.141–146 For instance, the Cu+/Cu2+ and Mo4+/Mo6+ redox pairs in materials like Mo–Cu9S5 enable dynamic cycling, which boosts Cu+ generation and enhances Fenton-like reactions.142 The photothermal effect in PTT further accelerates these reactions, as elevated temperatures increase reaction rates and improve ROS generation, as demonstrated in studies on Cu5FeS4 and CuS/Gd2O3, which highlight the temperature-dependent efficiency of Fenton-like reactions.131,147 To overcome limitations in Fenton-like reactions, innovative strategies have been developed. These include the design of pH-independent catalysts like CuFeS2 for synergistic CDT-PDT tumor therapy148 and the use of prodrug systems, such as CuS–Fe@polymer, to ensure a sustained release of Fe2+ for prolonged Fenton activity.149 Another effective approach is GSH depletion in cancer cells, which prevents the scavenging of generated ˙OH. For example, Co3−xCuxS4 and BSA-(Bi3+/Cu2+)-DATS nanoreactors combine GSH depletion with redox cycling to maximize ROS generation and Fenton-like reaction efficiency.134,150
By integrating these strategies, binary metal sulfides significantly improve the catalytic performance and amplify therapeutic effects in photo-enhanced Fenton/Fenton-like tumor therapy, establishing them as highly effective alternatives to individual metal sulfides.
 | ||
| Fig. 8 (A) Illustrative depiction of the synthesis and structural design of ZMS@PDA hollow nanospheres for enhanced synergistic PTT/PDT of cancer. (B) Heat variation in ZMS@PDA suspensions under NIR laser stimulation. (C) MB decomposition following exposure to varying concentrations of H2O2. (D) Thermal infrared visualization across different treatment groups. (E) Normalized tumor growth trajectories. (F) Images of excised tumors from various treatment groups. Reprinted with permission from ref. 129. Copyright 2021, American Chemical Society. | ||
Similar synergistic effects of PTT and CDT have been observed in other studies, including those involving MoS2/FeGA,144 BSA-CuFeS2,148 and Cu2ZnSnS4@BSA (CZTS@BSA),151 which also achieved enhanced tumor elimination and growth inhibition through combined therapeutic modalities.
Nanoreactors capable of attenuating X-rays can also serve as efficient CT imaging agents. Studies involving Bi2S3@Fe/Mn-ZIF-8, BSA-CuS/Gd2O3 NPs, BiNS-Fe@Fe and Mn–CoS@C highlight trimodal imaging capabilities, including CT imaging.135,136,147,152 For example, the bismuth in BiNS-Fe@Fe, a high atomic number element, enables superior X-ray attenuation for CT imaging, while magnetic Fe ions contribute to MRI, and robust NIR absorption facilitates PAI. Similarly, Lv et al. demonstrated the efficacy of their bimetallic chalcogenides as CT contrast agents, offering integrated MRI/CT/photothermal imaging functionality.
To improve tumor-specific therapeutic efficacy while minimizing adverse effects, Zhu et al. developed a poly(L-lysine) (PLL)-encapsulated, tirapazamine (TPZ)-loaded, Cu-doped SnS2 nanosphere (TPZ@Cu-SnS2−x/PLL) for enhanced tumor-specific therapy with minimal side effects (Fig. 9A).130 Copper doping and sulfur vacancies were introduced into Cu–SnS2−x nanospheres using a sacrificial template method, improving their photothermal and catalytic properties. This modification resulted in a temperature increase of up to 27.5 °C and a PCE of 32.8% (Fig. 9B). The Cu doping enhanced NIR absorption and carrier separation, optimizing the material for PTT. The hollow nanostructure provided high stability and a drug loading capacity of 93% for TPZ, with PLL enabling pH- and temperature-responsive release. TPZ release was significantly enhanced in the acidic TME and elevated temperatures from PTT, improving therapeutic efficacy. This intelligent nanoplatform combines PTT and CHT for synergistic tumor treatment with minimal off-target effects. In vitro, Cu–SnS2−x/PLL nanocomposites exhibited excellent catalytic activity, generating ˙OH from H2O2 due to Cu ions (Fig. 9C). The presence of GSH further enhanced the catalytic activity by converting Cu2+ to Cu+, promoting ROS generation. Sulfur vacancies also facilitated charge separation, increasing PDT efficacy by preventing carrier recombination. Further studies confirmed enhanced cytotoxicity under NIR irradiation, leading to significant apoptosis in cancer cells. In vivo, nanocomposite accumulation in tumor tissues was monitored via dual-modality imaging: NIR absorption enabled PAI, and Sn's high X-ray attenuation allowed CT imaging for precise, real-time therapy monitoring. The combined effects of ROS generation, GSH depletion, and controlled drug release induced oxidative stress, mitochondrial damage, and apoptosis without systemic toxicity (Fig. 9D). The strategic incorporation of copper ions, sulfur vacancies, and multimodal imaging guidance enhanced therapeutic outcomes, integrating PDT, PTT, CDT, and CHT into a unified tumor treatment platform.
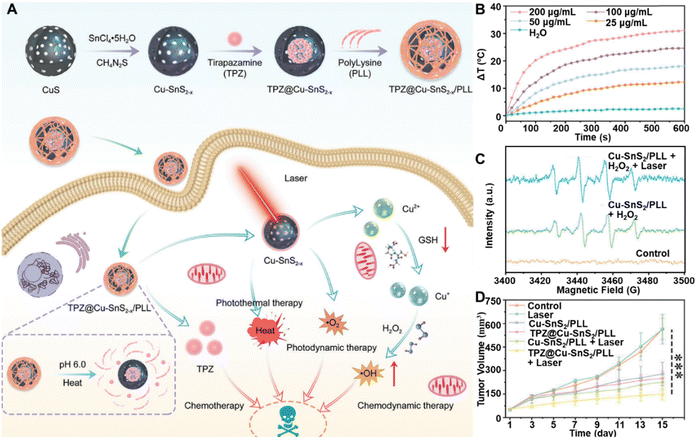 | ||
| Fig. 9 (A) Schematic representation of the synthesis process of TPZ@Cu–SnS2−x/PLL nanocomposites and their underlying mechanism in facilitating synergistic cancer therapy. (B) Laser-induced temperature changes in TPZ@Cu-SnS2−x/PLL at varying concentrations. (C) ESR analysis of ˙OH production following different treatments. (D) Tumor volumes in mice subjected to various treatments. Reprinted with permission from ref. 130. Copyright 2024, Wiley. | ||
In a similar vein, Wu et al. developed biodegradable doxorubicin-loaded Fe(III) species-WS2-polyvinylpyrrolidone (DOX@Fe(III)@WS2-PVP) nanocapsules for combined PTT/CDT/CHT.153 Other studies have also successfully combined multiple therapies to create multifunctional nanotheranostic platforms. Examples include Cu2MoS4/GOx for CDT/PTT/ST/immunotherapy,141 and pH-responsive supramolecular hydrogels encapsulating CuMnS2 and TiO2−x@Cu,S-MONs@GOx for PTT/CDT/GT/ST.154,155
In summary, the application of binary and ternary MeSNs in photo-enhanced Fenton/Fenton-like tumor therapy has led to significant advancements in PCE, ROS generation, and imaging capabilities. These multifunctional nanostructures offer improved stability, biocompatibility, and therapeutic efficacy. Key strategies, such as LSPR effects, compositional modifications, and core–shell configurations, have been instrumental in enhancing these properties. By integrating multiple therapeutic modalities, including PTT and CDT, these nanoplatforms achieve synergistic effects that minimize invasiveness and side effects while maximizing antitumor efficacy. The inclusion of advanced imaging capabilities ensures precise tumor localization and monitoring, supporting effective diagnosis and treatment planning. These innovations underscore the potential of metal sulfide nanoreactors as transformative tools for targeted tumor therapy.
4. Conclusions and future prospects
As a catalysis-driven approach toward cancer treatment, CDT has attracted considerable attention for its ability to selectively target tumors by exploiting their unique microenvironment. Particularly promising are CDT-based combinatorial systems that incorporate photo-activation, which have emerged as highly encouraging strategies for cancer theranostics. These systems synergistically combine the advantages of CDT with photo-responsive modalities, such as PTT or PDT, to achieve precise tumor targeting and enhanced therapeutic efficacy. Photo-activation not only amplifies ROS production from nanoagents, but also presents spatiotemporal control over treatment, minimized off-target effects and improved safety profiles. The integration of photo-activated systems with CDT enhances the versatility of cancer theranostics and has great potential for achieving robust therapeutic outcomes, offering a pathway to more effective and personalized cancer treatments.In this review, we have provided an overview of the typical Fenton MeSNs and their photo-enhanced catalytic mechanisms, highlighting their ability to improve the efficiency of CDT through synergistic photoactivation. Additionally, we have summarized the recent advancements in the design and application of MeSNs for photo-enhanced CDT, emphasizing their potential to revolutionize cancer treatment by combining precise tumor targeting with robust therapeutic outcomes. Despite these promising developments, research in this field is still in its early stages, and many challenges need to be addressed before MeSN-based therapies can be used in clinical settings. Future efforts should focus on improving the biocompatibility and stability of MeSNs, enhancing their ability to target tumors specifically, and ensuring their safety and effectiveness through thorough preclinical and clinical studies. Collaboration across different fields will be crucial to overcome these challenges and unlock the full potential of MeSNs in advanced cancer treatment.
To achieve a meaningful clinical impact, several key issues and challenges for MeSNs still need to be addressed as follows. (1) Limited efficacy: CDT is often limited by the TME. The amount of H2O2 and acidity in cancer cells is usually too low to trigger the Fenton/Fenton-like reaction, and high levels of GSH can reduce ROS production. While many MeSNs have been created to address these issues, more advanced and versatile systems are needed to overcome these TME challenges and improve CDT effectiveness. (2) Unclear mechanism: Although the Fenton/Fenton-like reaction for ROS production has been well studied, the precise mechanisms and molecular damage caused by ROS are not fully understood. This gap hinders efforts to improve catalytic efficiency. Gaining a deeper understanding of the CDT process in vivo is essential for advancing cancer treatment. (3) Insufficient stability: Many MeSNs in this field suffer from low stability and high aggregation. Improving their stability for prolonged circulation in the body is crucial for advancing the field. Modifying MeSNs with functional polymers like PEG or using stable organic carriers such as liposomes, dendrimers, and micelles can help achieve this. (4) Inadequate biosafety: The biosafety of MeSNs is crucial for their clinical use. While their biocompatibility has been studied in animal models during treatment, the long-term toxicity is still unclear and needs more research. Biodegradation is important for MeSNs because it allows the body to eliminate them. Therefore, thorough studies on the absorption, distribution, metabolism, and excretion (ADME) of MeSNs are necessary to ensure their safety and future use.
Overall, photo-activated CDT represents a promising approach to improving cancer treatment. It has attracted significant research interest and led to rapid advancements in the field. While various MeSNs have shown excellent photo-enhanced chemodynamic anti-tumor in animal models, more work is needed at both the basic and clinical levels to make these therapies viable for widespread clinical use. Overcoming the remaining challenges will likely accelerate the growth and development of this field.
Author contributions
Conceptualization and supervision: H. J. Investigation, resources, and visualization: H. J. Writing original draft: H. J., C. Y., J. Z., T. Y., C. C. H. and T. H. Review and editing: X. J., E. Y. and Z. B.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare no conflicts to declare.Acknowledgements
The authors would like to acknowledge the A*STAR Science and Engineering Research Council (SERC) Central Research Fund (Use-inspired Basic Research) for support of this project.Notes and references
- R. El Khaled El Faraj, S. Chakraborty, M. Zhou, M. Sobol, D. Thiele, L. M. Shatford-Adams, M. Correa Cassal, A.-K. Kaster, S. Dietrich, P. A. Levkin and A. A. Popova, Adv. Healthcare Mater., 2025, 14, 2401820 CrossRef CAS PubMed.
- B. Cuglievan and V. Subbiah, Nat. Rev. Clin. Oncol., 2025, 22, 155–156 Search PubMed.
- D. Niraula, K. C. Cuneo, I. D. Dinov, B. D. Gonzalez, J. B. Jamaluddin, J. J. Jin, Y. Luo, M. M. Matuszak, R. K. Ten Haken, A. K. Bryant, T. J. Dilling, M. P. Dykstra, J. M. Frakes, C. L. Liveringhouse, S. R. Miller, M. N. Mills, R. F. Palm, S. N. Regan, A. Rishi, J. F. Torres-Roca, H.-H. M. Yu and I. El Naqa, Nat. Commun., 2025, 16, 1138 CrossRef CAS PubMed.
- A. S. Cleary, T. L. Leonard, S. A. Gestl and E. J. Gunther, Nature, 2014, 508, 113–117 Search PubMed.
- J. L. Counihan, E. A. Grossman and D. K. Nomura, Chem. Rev., 2018, 118, 6893–6923 CrossRef CAS PubMed.
- P. Anand, A. B. Kunnumakara, C. Sundaram, K. B. Harikumar, S. T. Tharakan, O. S. Lai, B. Sung and B. B. Aggarwal, Pharm. Res., 2008, 25, 2097–2116 Search PubMed.
- J. Shi, P. W. Kantoff, R. Wooster and O. C. Farokhzad, Nat. Rev. Cancer, 2017, 17, 20–37 Search PubMed.
- R. S. Riley, C. H. June, R. Langer and M. J. Mitchell, Nat. Rev. Drug Discovery, 2019, 18, 175–196 CrossRef CAS PubMed.
- J. Zhou, L. Rao, G. Yu, T. R. Cook, X. Chen and F. Huang, Chem. Soc. Rev., 2021, 50, 2839–2891 RSC.
- B. Geng, J. Hu, X. He, Z. Zhang, J. Cai, D. Pan and L. Shen, Adv. Mater., 2024, 36, 2313670 Search PubMed.
- B. Geng, J. Hu, Y. Li, S. Feng, D. Pan, L. Feng and L. Shen, Nat. Commun., 2022, 13, 5735 CrossRef CAS PubMed.
- Z. Tang, Y. Liu, M. He and W. Bu, Angew. Chem., Int. Ed., 2019, 58, 946–956 CrossRef CAS PubMed.
- H. Zhu, W. Zan, W. Chen, W. Jiang, X. Ding, B. L. Li, Y. Mu, L. Wang, S. Garaj and D. T. Leong, Adv. Mater., 2022, 34, 2200004 CrossRef CAS PubMed.
- H. Zhu, J. Li, X. Qi, P. Chen and K. Pu, Nano Lett., 2018, 18, 586–594 CrossRef CAS PubMed.
- H. Zhu, Y. Fang, Q. Miao, X. Qi, D. Ding, P. Chen and K. Pu, ACS Nano, 2017, 11, 8998–9009 CrossRef CAS PubMed.
- H. Zhu, B. Li, C. Yu Chan, B. Low Qian Ling, J. Tor, X. Yi Oh, W. Jiang, E. Ye, Z. Li and X. Jun Loh, Adv. Drug Delivery Rev., 2023, 192, 114644 Search PubMed.
- H. Zhu, J. Zheng, X. Y. Oh, C. Y. Chan, B. Q. L. Low, J. Q. Tor, W. Jiang, E. Ye, X. J. Loh and Z. Li, ACS Nano, 2023, 17, 7953–7978 CrossRef CAS PubMed.
- X. Pan, H. Wang, S. Wang, X. Sun, L. Wang, W. Wang, H. Shen and H. Liu, Sci. China Life Sci., 2018, 61, 415–426 Search PubMed.
- Y. Zhuang, S. Han, Y. Fang, H. Huang and J. Wu, Coord. Chem. Rev., 2022, 455, 214360 CrossRef CAS.
- X. Li, J. F. Lovell, J. Yoon and X. Chen, Nat. Rev. Clin. Oncol., 2020, 17, 657–674 CrossRef PubMed.
- J. D. Martin, H. Cabral, T. Stylianopoulos and R. K. Jain, Nat. Rev. Clin. Oncol., 2020, 17, 251–266 CrossRef PubMed.
- L. Zhang, C.-X. Li, S.-S. Wan and X.-Z. Zhang, Adv. Healthcare Mater., 2022, 11, 2101971 CrossRef CAS PubMed.
- B. Kwon, E. Han, W. Yang, W. Cho, W. Yoo, J. Hwang, B.-M. Kwon and D. Lee, ACS Appl. Mater. Interfaces, 2016, 8, 5887–5897 CrossRef CAS PubMed.
- E. Hwang and H. S. Jung, Chem. Commun., 2020, 56, 8332–8341 Search PubMed.
- Q. Sun, Z. Wang, B. Liu, F. He, S. Gai, P. Yang, D. Yang, C. Li and J. Lin, Coord. Chem. Rev., 2022, 451, 214267 Search PubMed.
- P. Manivasagan, A. Joe, H.-W. Han, T. Thambi, M. Selvaraj, K. Chidambaram, J. Kim and E.-S. Jang, Mater. Today Bio, 2022, 13, 100197 CrossRef CAS PubMed.
- X. Wang, X. Zhong, Z. Liu and L. Cheng, Nano Today, 2020, 35, 100946 CrossRef CAS.
- T. He, X. Qin, C. Jiang, D. Jiang, S. Lei, J. Lin, W.-G. Zhu, J. Qu and P. Huang, Theranostics, 2020, 10, 2453 CrossRef CAS PubMed.
- C. Zhang, D. Li, P. Pei, W. Wang, B. Chen, Z. Chu, Z. Zha, X. Yang, J. Wang and H. Qian, Biomaterials, 2020, 237, 119835 Search PubMed.
- L.-S. Lin, T. Huang, J. Song, X.-Y. Ou, Z. Wang, H. Deng, R. Tian, Y. Liu, J.-F. Wang, Y. Liu, G. Yu, Z. Zhou, S. Wang, G. Niu, H.-H. Yang and X. Chen, J. Am. Chem. Soc., 2019, 141, 9937–9945 CrossRef CAS PubMed.
- Z. Tang, H. Zhang, Y. Liu, D. Ni, H. Zhang, J. Zhang, Z. Yao, M. He, J. Shi and W. Bu, Adv. Mater., 2017, 29, 1701683 CrossRef PubMed.
- X. Wang, X. Zhong, H. Lei, Y. Geng, Q. Zhao, F. Gong, Z. Yang, Z. Dong, Z. Liu and L. Cheng, Chem. Mater., 2019, 31, 6174–6186 CrossRef CAS.
- B. Ma, S. Wang, F. Liu, S. Zhang, J. Duan, Z. Li, Y. Kong, Y. Sang, H. Liu and W. Bu, J. Am. Chem. Soc., 2018, 141, 849–857 CrossRef PubMed.
- F. Gong, N. Yang, X. Wang, Q. Zhao, Q. Chen, Z. Liu and L. Cheng, Nano Today, 2020, 32, 100851 CrossRef CAS.
- F. Jiang, B. Ding, S. Liang, Y. Zhao, Z. Cheng, B. Xing and J. Lin, Biomaterials, 2021, 268, 120545 CrossRef CAS PubMed.
- Y. Liu, W. Zhen, Y. Wang, J. Liu, L. Jin, T. Zhang, S. Zhang, Y. Zhao, S. Song and C. Li, Angew. Chem., Int. Ed., 2019, 131, 2429–2434 CrossRef.
- X. Tan, D. Liao, C. Rao, L. Zhou, Z. Tan, Y. Pan, A. Singh, A. Kumar, J. Liu and B. Li, J. Solid State Chem., 2022, 314, 123352 CrossRef CAS.
- Y. Pan, Z. Luo, X. Wang, Q. Chen, J. Chen, Y. Guan, D. Liu, H. Xu and J. Liu, Dalton Trans., 2020, 49, 5291–5301 RSC.
- W. Zhu, J. Zhao, Q. Chen and Z. Liu, Coord. Chem. Rev., 2019, 398, 113009 Search PubMed.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232–1268 Search PubMed.
- H. Yu, Y. Yang, T. Jiang, X. Zhang, Y. Zhao, G. Pang, Y. Feng, S. Zhang, F. Wang and Y. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 27536–27547 Search PubMed.
- X. Wang, J. Wang, J. Pan, F. Zhao, D. Kan, R. Cheng, X. Zhang and S.-K. Sun, ACS Appl. Mater. Interfaces, 2019, 11, 33650–33658 CrossRef CAS PubMed.
- L. H. Fu, Y. R. Hu, C. Qi, T. He, S. Jiang, C. Jiang, J. He, J. Qu, J. Lin and P. Huang, ACS Nano, 2019, 13, 13985–13994 Search PubMed.
- C. Xu, J. Li, C. Ou, J. Yang, S. Fu, W. Hu, L. Wang, Z. Wang, L. Hai, L. Deng and D. He, Chem. Eng. J., 2024, 499, 156170 CrossRef CAS.
- C. Yao, W. Wang, P. Wang, M. Zhao, X. Li and F. Zhang, Adv. Mater., 2018, 30, 1704833 CrossRef PubMed.
- M. Saeed, W. Ren and A. Wu, Biomater. Sci., 2018, 6, 708–725 RSC.
- L. S. Lin, J. Song, L. Song, K. Ke, Y. Liu, Z. Zhou, Z. Shen, J. Li, Z. Yang and W. Tang, Angew. Chem., Int. Ed., 2018, 6, 708–725 Search PubMed.
- S. Y. H. Wu, K. C. Yang, C. L. Tseng, J. C. Chen and F. H. Lin, J. Nanopart. Res., 2011, 13, 1139–1149 CrossRef CAS.
- Y. Chen, G. Zhao, S. Wang, Y. He, S. Han, C. Du, S. Li, Z. Fan, C. Wang and J. Wang, Biomater. Sci., 2019, 7, 3450–3459 RSC.
- Y. Cheng, Y. Chang, Y. Feng, H. Jian, Z. Tang and H. Zhang, Angew. Chem., Int. Ed., 2018, 57, 246–251 Search PubMed.
- R. Yang, R. Li, L. Zhang, Z. Xu, Y. Kang and P. Xue, J. Mater. Chem. B, 2020, 8, 7766–7776 Search PubMed.
- W. Fei, Y. Zhang, Y. Ye, C. Li, Y. Yao and Zhang, J. Mater. Chem. B, 2020, 8, 10461–10473 Search PubMed.
- W. Fei, X. Zhang, X. Fan, Y. Ye, M. Zhao, C. Zheng, Y. Li and X. Zheng, J. Nanobiotechnol., 2021, 19, 93 CrossRef CAS PubMed.
- H. Ranji-Burachaloo, P. A. Gurr, D. E. Dunstan and G. G. Qiao, ACS Nano, 2018, 12, 11819–11837 CrossRef CAS PubMed.
- Z. Tang, Y. Liu, M. He and W. Bu, Angew. Chem., 2019, 131, 958–968 CrossRef.
- L. Yang, J. Wang, S. Yang, Q. Lu, P. Li and N. Li, Theranostics, 2019, 9, 3992–4005 CrossRef CAS PubMed.
- G. K. Balendiran, R. Dabur and D. Fraser, Cell Biochem. Funct., 2004, 22, 343–352 CrossRef CAS PubMed.
- L. S. Lin, J. Song, L. Song, K. Ke, Y. Liu, Z. Zhou, Z. Shen, J. Li, Z. Yang, W. Tang, G. Niu, H. H. Yang and X. Chen, Angew. Chem., Int. Ed., 2018, 57, 4902–4906 CrossRef CAS PubMed.
- J. Lu, Y. Yang, Q. Xu, Y. Lin, S. Feng, Y. Mao, D. Wang, S. Wang and Q. Zhao, Coord. Chem. Rev., 2023, 474, 214861 CrossRef CAS.
- S. Shen, Y. Chao, Z. Dong, G. Wang, X. Yi, G. Song, K. Yang, Z. Liu and L. Cheng, Adv. Funct. Mater., 2017, 27, 1700250 CrossRef.
- W. Yin, L. Yan, J. Yu, G. Tian, L. Zhou, X. Zheng, X. Zhang, Y. Yong, J. Li and Z. Gu, ACS Nano, 2014, 8, 6922–6933 CrossRef CAS PubMed.
- S. Q. Li, L. Sun, M. Hou, Q. Chen, R. Yang, L. Zhang, Z. Xu, Y. Kang and P. Xue, ACS Appl. Mater. Interfaces, 2018, 11, 417–429 CrossRef PubMed.
- Q. Z. Lu, L. F.-y. Huang, R. Cao, L. Zhang, G.-h. Tan, N. He, J. Huang, G. Wang and Z. Zhang, Sci. Rep., 2017, 7, 41571 CrossRef PubMed.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang and S. Perrett, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed.
- W. Wang, J. Song, W. Yu, M. Chen, G. Li, J. Chen, L. Chen, L. Yu and Y. Chen, Adv. Funct. Mater., 2024, 34, 2400929 Search PubMed.
- F. Wu, Q. Zhang, M. Zhang, B. Sun, Z. She, M. Ge, T. Lu, X. Chu, Y. Wang, J. Wang, N. Zhou and A. Li, ACS Appl. Mater. Interfaces, 2020, 12, 10142–10155 Search PubMed.
- J. Wang, Z. Sun, S. Wang, C. Zhao, J. Xu, S. Gao, M. Yang, F. Sheng, S. Gao and Y. Hou, J. Am. Chem. Soc., 2022, 144, 19884–19895 CrossRef CAS PubMed.
- S. Xiao, Y. Lu, M. Feng, M. Dong, Z. Cao, X. Zhang, Y. Chen and J. Liu, Chem. Eng. J., 2020, 396, 125294 CrossRef CAS.
- M. Feng, M. Li, R. Dai, S. Xiao, J. Tang, X. Zhang, B. Chen and J. Liu, Biomater. Sci., 2021, 10, 258–269 RSC.
- H. Ren, J. Yong, Q. Yang, Z. Yang, Z. Liu, Y. Xu, H. Wang, X. Jiang, W. Miao and X. Li, Acta Pharm. Sin. B, 2021, 11, 3244–3261 Search PubMed.
- M. Zhan, X. Yu, W. Zhao, Y. Peng, S. Peng, J. Li and L. Lu, J. Nanobiotechnol., 2022, 20, 23 Search PubMed.
- Y. M. Feng, Y. Lu, Y. Yang, M. Zhang, Y. J. Xu, H. L. Gao, L. Dong, W. P. Xu and S. H. Yu, Sci. Rep., 2013, 3, 2994 Search PubMed.
- G. Guan, X. Wang, B. Li, W. Zhang, Z. Cui, X. Lu, R. Zou and J. Hu, Nanoscale, 2018, 10, 17902–17911 Search PubMed.
- X. Liu, W. Feng, H. Xiang, B. Liu, M. Ye, M. Wei, R. Dong, Y. Chen and K. Dong, Chem. Eng. J., 2021, 411, 128364 Search PubMed.
- M. B. Gawande, A. Goswami, F. X. Felpin, T. Asefa, X. Huang, R. Silva, X. Zou, R. Zboril and R. S. Varma, Chem. Rev., 2016, 116, 3722–3811 Search PubMed.
- Y. Li, W. Lu, Q. Huang, M. Huang, C. Li and W. Chen, Nanomedicine, 2010, 5, 1161–1171 CrossRef CAS PubMed.
- M. Wang, H. Zhu, Y. Xue, Y. Duan, H. Tian, Q. Liu, Y. Zhang, Z. Li, X. J. Loh, E. Ye, G. Yin, X. Wang, X. Ding and D. T. Leong, Bioact. Mater., 2024, 42, 628–643 CAS.
- H. Sun, Y. Zhang, S. Chen, R. Wang, Q. Chen, J. Li, Y. Luo, X. Wang and H. Chen, ACS Appl. Mater. Interfaces, 2020, 12, 30145–30154 Search PubMed.
- R. Hu, Y. Fang, M. Huo, H. Yao, C. Wang, Y. Chen and R. Wu, Biomaterials, 2019, 206, 101–114 CrossRef CAS PubMed.
- Y. Sun, H. Shi, X. Cheng, L. Wu, Y. Wang, Z. Zhou, J. He, H. Y. Chen and D. Ye, CCS Chem., 2020, 3, 1336–1349 CrossRef.
- P. Singh, B. Youden, Y. Yang, Y. Chen, A. Carrier, S. Cui, K. Oakes, M. Servos, R. Jiang and X. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 41464–41472 Search PubMed.
- L. An, C. Wang, Q. Tian, C. Tao, F. Xue, S. Yang, X. Zhou, X. Chen and G. Huang, Nano Today, 2022, 43, 101397 CrossRef CAS.
- P. M. Zhang, X. Liu, Q. Luo, Q. Wang, L. Zhao, G. Deng, R. Ge, L. Zhang, J. Hu and J. Lu, Chem. Eng. J., 2020, 389, 124450 CrossRef.
- J. Yao, F. Yang, F. Zheng, C. Yao, J. Xing, X. Xu and A. Wu, ACS Appl. Mater. Interfaces, 2021, 13, 54770-–54782 CrossRef CAS PubMed.
- K. Du, S. Zhao, J. Feng, X. Gao, K. Liu, X. Wang, M. Zhang, Y. Li, Y. Lu and H. Zhang, J. Mater. Chem. B, 2021, 9, 7216–7228 Search PubMed.
- Y. Chen, P. Liu, C. Zhou, T. Zhang, T. Zhou, D. Men, G. Jiang and L. Hang, Acta Biomater., 2023, 158(1), 649–659 CrossRef CAS PubMed.
- L. S. Li, P. W. Chen, X. J. Zhao, D. Cheng, B. B. Liu, X. J. Tang, W. Q. Zhu, X. Yang and M. X. Zhao, J. Colloid Interface Sci., 2025, 680, 202–214 CrossRef CAS PubMed.
- S. Wang, A. Riedinger, H. Li, C. Fu, H. Liu, L. Li, T. Liu, L. Tan, M. J. Barthel, G. Pugliese, F. De Donato, M. Scotto D’Abbusco, X. Meng, F. Manna, H. Meng and L. T. Pellegrino, ACS Nano, 2015, 9, 1788–1800 CrossRef CAS PubMed.
- Y. Li, J. Zhou, L. Wang and Z. Xie, ACS Appl. Mater. Interfaces, 2020, 12, 30213–30220 CrossRef CAS PubMed.
- S. Wang, Y. Pang, S. Hu, J. Lv, Y. Lin and M. Li, Chem. Eng. J., 2023, 451, 138864 CrossRef CAS.
- Y. Wang, L. An, J. Lin, Q. Tian and S. Yang, Chem. Eng. J., 2020, 385, 123925 CrossRef CAS.
- Z. Zhan, W. Zeng, J. Liu, L. Zhang, Y. Cao, P. Li, H. Ran and Z. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 24071–24083 Search PubMed.
- P. An, S. Yin, Y. Qiang, F. Shui, Q. Zhang, C. Zhao, H. Zhou and F. Yu, New J. Chem., 2023, 47, 16494–16504 RSC.
- Z. Wei, W. Si, M. Huang, M. Lu, W. Wang, C. Liang, X. Dong and Y. Cai, Adv. Healthcare Mater., 2024, 13, 2402367 CrossRef CAS PubMed.
- H. Hu, W. Zhang, L. Lei, F. Tong, H. Zhang, Y. Zhang, W. Yang, Y. Tang, R. Lin and X. Xia, Chin. Chem. Lett., 2024, 35, 108765 CrossRef CAS.
- Y. Yang, W. Zheng, J. Zhang, J. Guo, Q. Liu, H. Wang, F. Xu and Z. Bao, ACS Appl. Bio Mater., 2025, 8, 676–687 CrossRef CAS PubMed.
- X. Liu, P. Geng, N. Yu, Z. Xie, Y. Feng, Q. Jiang, M. Li, Y. Song, W. Lian and Z. Chen, J. Colloid Interface Sci., 2022, 615, 38–49 Search PubMed.
- F. Tong, Y. Hu, Y. Xu, Y. Zhou, R. Xie, T. Lei, Y. Du, W. Yang, S. He, Y. Huang, T. Gong and H. Gao, Acta Pharm. Sin. B, 2023, 13, 3471–3488 CrossRef CAS PubMed.
- Y. Cao, L. Jin, S. Zhang, Z. Lv, N. Yin, H. Zhang, T. Zhang, Y. Wang, Y. Chen, X. Liu and G. Zhao, Eur. J. Pharm. Sci., 2023, 180, 106319 CrossRef CAS PubMed.
- P. Geng, N. Yu, D. K. Macharia, R. Meng, P. Qiu, C. Tao, M. Li, H. Zhang, Z. Chen and W. Lian, Chem. Eng. J., 2022, 441, 135964 CrossRef CAS.
- G. Wei, X. Lian, X. Qin, Y. Zhao, L. Cai, Q. Chen, J.-J. Zou and J. Tian, Mater. Des., 2022, 224, 111302 CrossRef CAS.
- M. Sun, D. Yang, W. Fanqi, Z. Wang, H. Ji, Z. Liu, S. Gai, F. Zhang and P. Yang, J. Mater. Chem. B, 2020, 8, 5707–5721 RSC.
- Q. Zou, H. Pan, X. Zhang and and C. Zhang, J. Mater. Chem. B, 2023, 11, 4740–4751 RSC.
- T. Xu, S. Liu, Z. Wei, W. Zhang, G.-Y. Zhang, T. Liu, L. Dai, P. Yin and Y. Song, ACS Appl. Mater. Interfaces, 2024, 16, 14489–14502 CrossRef CAS PubMed.
- L. Kong, F. Yuan, P. Huang, L. Yan, Z. Cai, T. Lawson, W. Wu, S. Chou and Y. Liu, Small, 2020, 16, 2004161 CrossRef CAS PubMed.
- B. Colak, M. C. Cihan and Y. N. Ertas, ACS Appl. Nano Mater., 2022, 6, 16076–16085 CrossRef.
- W. Tang, X. Li, Z. Liu, L. Meng, D. Zhu and Q. Huang, Front. Bioeng. Biotechnol., 2022, 10, 1003777 CrossRef PubMed.
- P. Singh, Y. Chen, B. Youden, D. Oakley, A. Carrier, K. Oakes, M. Servos, R. Jiang and X. Zhang, Int. J. Pharm., 2024, 652, 123814 Search PubMed.
- M. Moetamani-Ahmadi, A. Mahmoud Ahmadzadeh, M. Alaei, N. Zafari, Z. Negahbanzaferanloo, A. M. Pourbagher-Shahri, F. Forouzanfar, H. Fiuji, H. Mahaki, M. Khazaei, I. S. Gataa, G. A. Ferns, G. J. Peters, J. Batra, A. K.-y. Lam, E. Giovannetti, H. TanzadehPanah and A. Avan, Int. J. Pharm., 2024, 652, 123839 Search PubMed.
- J. Tao, B. Wang, Y. Dong, X. Chen, S. Li, T. Jiang and X. Zhao, ACS Appl. Mater. Interfaces, 2023, 15, 40267–40279 CrossRef CAS PubMed.
- S. Ning, J. Mo, R. Huang, B. Liu, B. Fu, S. Ding, H. Yang, Y. Cui and L. Yao, Front. Bioeng. Biotechnol., 2023, 11, 1191014 Search PubMed.
- S. Wu, C. Liu, W. Li, C. Zhang, D. Chen, C. Xu, L. Su and X. Wang, J. Mater. Chem. B, 2023, 11, 2455–2465 Search PubMed.
- Y. He, Y. Pan, X. Zhao, L. Ye, L. Liu, W. Wang, M. Li, D. Chen, Y. Cai and X. Mou, Chem. Eng. J., 2023, 471, 144410 Search PubMed.
- H. Tian, H. Zhu, Y. Xue, M. Wang, K. Xing, Z. Li, X. J. Loh, E. Ye, X. Ding, B. L. Li, X. Yin and D. T. Leong, Nanoscale Horiz., 2024, 9, 1190–1199 Search PubMed.
- X. Wang, X. Zhong, Z. Zha, G. He, Z. Miao, H. Lei, Q. Luo, R. Zhang, Z. Liu and L. Cheng, Applied Materials Today, 2020, 18, 100464 CrossRef.
- L. Zhao, Q. Yang, W. Guo, F. Zhang, K. Yu, C. Yang and F. Qu, J. Colloid Interface Sci., 2021, 600, 390–402 CrossRef CAS PubMed.
- H. Zhu, S. Huang, M. Ding, Z. Li, J. Li, S. Wang and D. T. Leong, ACS Appl. Mater. Interfaces, 2022, 14, 25183–25196 CrossRef CAS PubMed.
- Y. Jiang, Y. Lu, L. Lei, S. Zhou, L. Yang, X. Yang, Z. Xu, J. Liu and Y. Liu, Mater. Sci. Eng., C, 2021, 130, 112465 CrossRef CAS PubMed.
- X. Li, M. He, Q. Zhou, D. Dutta, N. Lu, B. Li and Z. Ge, ACS Appl. Mater. Interfaces, 2022, 14, 50601–50615 CrossRef CAS PubMed.
- G. Pidamaimaiti, X. Huang, K. Pang, Z. Su and F. Wang, New J. Chem., 2021, 45, 10296–10302 RSC.
- W. B. Dirersa, G. Getachew, A. Wibrianto, A. S. Rasal, V. S. Gurav, M. Z. Fahmi and J. Y. Chang, J. Colloid Interface Sci., 2023, 647, 528–545 CrossRef CAS PubMed.
- Z. Mo, M. Qiu, K. Zhao, H. Hu, Q. Xu, J. Cao, Y. Luo, L. Liu, Z. Xu, C. Yi, Z. Xiong, G. Liao and S. Yang, J. Colloid Interface Sci., 2022, 611, 193–204 CrossRef CAS PubMed.
- S. Liang, G. Liao, W. Zhu and L. Zhang, Biomater. Res., 2022, 26, 32 CrossRef CAS PubMed.
- G. Ma, X. Zhang, K. Zhao, S. Zhang, K. Ren, M. Mu, C. Wang, X. Wang, H. Liu, J. Dong and X. Sun, ACS Nano, 2024, 18, 3369–3381 CrossRef CAS PubMed.
- J. Lv, X. Wang, X. Zhang, R. Xu, S. Hu, S. Wang and M. Li, Asian J. Pharm. Sci., 2023, 18, 100798 Search PubMed.
- Y. Lu, Y. Wu, Z. Tang, Y. Hou, M. Cui, S. Huang, B. Long, Z. Yu, M. Z. Iqbal and X. Kong, Sensors, 2023, 23, 8930 CrossRef CAS PubMed.
- T. Han, D. Cui, M. Wu, Q. Sun, Y. Chen, N. Niu and L. Chen, Microchem. J., 2024, 206, 111466 CrossRef CAS.
- J. Bao, R. Liu, Z. Yu, Z. Cheng and B. Chang, Adv. Funct. Mater., 2024, 34, 2316646 CrossRef CAS.
- J. Ruan, H. Liu, B. Chen, F. Wang, W. Wang, Z. Zha, H. Qian, Z. Miao, J. Sun, T. Tian, Y. He and H. Wang, ACS Nano, 2021, 15, 11428–11440 CrossRef CAS PubMed.
- Y. Zhu, R. Zhao, L. Feng, W. Wang, Y. Xie, H. Ding, B. Liu, S. Dong, P. Yang and J. Lin, Small Methods, 2024, 8, 2400125 CrossRef CAS PubMed.
- Z. Wang, Y. Wang, H. Guo, N. Yu, Q. Ren, Q. Jiang, J. Xia, C. Peng, H. Zhang and Z. Chen, J. Colloid Interface Sci., 2021, 592, 116–126 CrossRef CAS PubMed.
- M. Wang, Q. Huang, R. Ma, S. Wang, X. Li, Y. Hu, S. Zhu, M. Zhang and Q. Huang, Colloids Surf., B, 2024, 234, 113689 CrossRef CAS PubMed.
- Z. Qin, M. Qiu, Q. Zhang, S. Yang, G. Liao, Z. Xiong and Z. Xu, J. Mater. Chem. B, 2021, 9, 8882–8896 RSC.
- Y. Jiang, H. Lu, X. Yuan, Y. Zhang, L. Lei, Y. Li, W. Sun, J. Liu, D. Scherman and Y. Liu, J. Mater. Chem. B, 2022, 10, 8082–8093 RSC.
- S. Ma, J. Xie, L. Wang, Z. Zhou, X. Luo, J. Yan and G. Ran, ACS Appl. Mater. Interfaces, 2021, 13, 10728–10740 CrossRef CAS PubMed.
- K. Lv, L. Wang, Y. Ma, F. Zhang, W. Guo, K. Yu, F. Qu and H. Lin, Biomater. Adv., 2022, 136, 212778 CrossRef CAS PubMed.
- X. Zhu, Z. Chu, B. Chen, Q. Jin, X. Ma, J. Yang, Y. Jiang, W. Wang, Z. Zha and and H. Qian, Mater. Chem. Front., 2022, 6, 1522–1532 RSC.
- K. Kang, L. Wang, K. Yu, Y. Ma, F. Qu and and H. Lin, Biomater. Adv., 2023, 144, 213168 CrossRef CAS PubMed.
- L. Wang, K. Kang, H. Hou, Y. Ma, K. Yu, F. Qu and and H. Lin, J. Colloid Interface Sci., 2022, 625, 145–157 CrossRef CAS PubMed.
- S. Xu, S. Zhou, L. Xie, W. Dou, R. Zhang, B. Zhao, Y. Xu, X. Fu and M. Yuan, Chem. Eng. J., 2023, 460, 141639 CrossRef CAS.
- M. Chang, M. Wang, M. Wang, M. Shu, B. Ding, C. Li, M. Pang, S. Cui, Z. Hou and J. Lin, Adv, Mater, 2019, 31, e1905271 CrossRef PubMed.
- Z. Zhou, Z. Gao, W. Chen, X. Wang, Z. Chen, Z. Zheng, Q. Chen, M. Tan, D. Liu, Y. Zhang and Z. Hou, Acta Biomater., 2022, 151, 600–612 CrossRef CAS PubMed.
- Y. Zhu, Y. Pan, Z. Guo, D. Jin, W. Wang, M. Liu, M. Zong, X. Zheng, Y. Wu, L. Wang, C. Tian, J. Cheng and Y. Liu, Adv. Healthcare Mater., 2023, 12, 2202198 CrossRef CAS PubMed.
- Z. Liu, N. Zeng, J. Yu, C. Huang and and Q. Huang, Front. Bioeng. Biotechnol., 2022, 10, 998571 CrossRef PubMed.
- Q. Fang and X. Yin, J. Nanopart. Res., 2023, 25, 106 CrossRef CAS.
- X. M. Sun, L. Wang, Y. Zhuo, S. Xu, H. Liu, X. Jiang, Z. Lu, X. Wang, Y. Wang, G. Yue, B. Feng, H. Rao and D. Wu, Small, 2024, 20, 2309593 CrossRef PubMed.
- M. Luo, H. Yukawa, K. Sato, M. Tozawa, M. Tokunaga, T. Kameyama, T. Torimoto and Y. Baba, ACS Appl. Mater. Interfaces, 2022, 14, 34365–34376 CrossRef CAS PubMed.
- Q. Chen, Y. Luo, W. Du, Z. Liu, S. Zhang, J. Yang, H. Yao, T. Liu, M. Ma and H. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 18133–18144 CrossRef CAS PubMed.
- X. Nie, L. Xia, H. L. Wang, G. Chen, B. Wu, T. Y. Zeng, C. Y. Hong, L. H. Wang and Y. Z. You, ACS Appl. Mater. Interfaces, 2019, 11, 31735–31742 CrossRef CAS PubMed.
- Z. W. Tao, Z. Tuo, F. Wu, K. Mu, C. Xu, Y. Shi, Z. Sun, Y. Wang, Y. Li, Z. Zhong, L. Zhou, J. Wang, J. Liu, Y. Du and S. Zhang, Regener. Biomater., 2022, 9, rbac045 CrossRef PubMed.
- L. Tan, J. Wan, W. Guo, C. Ou, T. Liu, C. Fu, Q. Zhang, X. Ren, X. J. Liang, J. Ren, L. Li and X. Meng, Biomaterials, 2018, 159, 108–118 CrossRef CAS PubMed.
- P. Dash, N. Nataraj, P. K. Panda, C. L. Tseng, Y. C. Lin, R. Sakthivel and R. J. Chung, ACS Appl. Mater. Interfaces, 2024, 17, 222–234 CrossRef PubMed.
- C. Wu, S. Wang, J. Zhao, Y. Liu, Y. Zheng, Y. Luo, C. Ye, M. Huang and H. Chen, Adv. Funct. Mater., 2019, 29, 1901722 CrossRef.
- A. Dong, S. Huang, Z. Qian, S. Xu, W. Yuan and B. Wang, J. Mater. Chem. B, 2023, 11, 10883–10895 RSC.
- Y. Luo, L. Zhang, S. Wang, Y. Wang, J. Hua, C. Wen, S. Zhao and H. Liang, ACS Appl. Mater. Interfaces, 2023, 15, 38309–38322 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |