The synergistic effect of MOF and biomass achieving dual breakthroughs in material structure and performance
Ran
Li
,
Xian
Wang†
,
Chengwei
Lin
,
Xinxiang
Zhang
 * and
Zhanhui
Yuan
* and
Zhanhui
Yuan
 *
*
College of Materials Engineering, Fujian Agriculture and Forestry University, Fuzhou 350002, P. R. China. E-mail: xxzhang0106@163.com; zhanhuiyuan@fafu.edu.cn
First published on 3rd December 2024
Abstract
In order to cope with the environmental crisis and the depletion of non-renewable resources, the combination of metal–organic framework (MOF) and biomass materials has become an important breakthrough in sustainable materials science. With its unique pore structure and adjustable performance, MOF significantly improves the thermal stability and mechanical strength of biomass materials, and broadens its application in energy, material manufacturing and other fields. MOF–biomass composites show excellent properties in carbon capture, water treatment and biomedicine, which is of great significance to environmental protection and green chemical industry. This paper summarizes the latest research progress of MOF–biomass composites, including their application in wastewater treatment, air purification, food packaging and other fields, and discusses the green synthesis method, biological interface integration strategy and its potential in biocatalysis, sensing and medical fields. The development and application of MOF–biomass composites provide a new way to solve environmental problems and promote sustainable development. Furthermore, we critically analyze the key challenges and future directions. Our sincere hope is that this review will stimulate greater research interest in applications of biomass/MOF composites, and offer valuable insights for their design and utilization strategies.
1. Introduction
In the midst of growing environmental concerns and the depletion of non-renewable energy sources, there is an urgent global need for sustainable materials. Biomass, as a renewable and biodegradable resource, is at the vanguard of this quest, presenting a multitude of applications spanning from energy generation to material fabrication.1 Biomass encompasses a wide range of materials, including lignocellulosic biomass (such as wood, agricultural residues, and energy crops), algae, and waste materials from the forestry and agricultural sectors. Each type of biomass possesses unique properties and applications, from energy generation to material fabrication. Biomass, as a renewable and environmentally friendly material, is characterized by low cost and abundant reserves, reducing reliance on fossil fuels, decreasing greenhouse gas emissions, and being more environmentally friendly. However, the inherent limitations of biomass, such as its modest thermal stability and inadequate mechanical resilience, often restrict its broader utility. MOFs are a class of sophisticated porous materials constituted by metal ions or clusters interconnected with organic linkers. MOFs' distinct characteristics, namely their expansive surface area, adaptable pore dimensions, and modularity in synthesis,2 position them as prime enhancers for biomass composites, overcoming the inherent drawbacks of biomass.3,4MOF and biomass materials occupy distinct yet synergistic niches within the realm of materials science. Biomass materials inherently possess high porosity and large surface areas, characteristics that are also found in MOFs. The combination of the two can enhance these properties, representing one aspect of their synergistic effects. Furthermore, biomass materials contain various functional groups that can be modified and utilized, with MOFs capable of binding to specific functional groups, leading to a more uniform distribution of MOFs. Additionally, biomass materials exhibit excellent biodegradability and biocompatibility, while MOFs can be designed with specific functions. The integration of these materials allows for the creation of environmentally friendly functional materials. The fusion of these two realms through MOF–biomass composites introduces a potent strategy to elevate the performance and utility of biomass materials across a spectrum of applications. These are also the places where biomass carriers are more attractive than other carriers.
The combination of MOFs with biomass not only retains the advantages of each but also enhances overall performance through synergistic effects. In particular, they can facilitate the creation of superior adsorbents for carbon capture and sequestration, a pivotal strategy in combating climate change.5–8 Moreover, these composite materials act as catalysts in the transformation of biomass into high-value chemicals, thereby harnessing renewable resources for a more sustainable chemical industry.9–11 The integration of MOF within biomaterials for drug delivery systems and tissue engineering not only pushes the boundaries of biomedicine but also underscores the potential for producing more robust, chemically stable, and multifunctional biomaterials.12 The unique properties of MOF–biomass composites extend their potential to fields such as flexible electronics, biosensing, and environmental remediation. These materials exhibit high conductivity, mechanical flexibility, and biocompatibility, making them ideal for the development of next-generation electronic devices that are both technologically advanced and environmentally responsible. Additionally, their high adsorption capacity and selectivity for pollutants make them effective solutions for water purification and air pollution control. This reciprocal relationship sees MOFs enhancing the physical properties of biomass, while biomass serves as a sustainable and economical feedstock for MOF production.
In essence, the merging of MOFs and biomass materials paves the way for the creation of eco-friendly and biocompatible materials that hold promise in biomedicine, energy production, and environmental remediation.6,13,14 We will discuss the green synthesis methods, biological interface integration strategies, and potential applications in biocatalysis, sensing, and medical fields. By critically analyzing the key challenges and future directions, we hope to stimulate greater research interest and offer valuable insights for the design and utilization of MOF–biomass composites. These materials hold the potential to address pressing environmental issues and promote sustainable development, making them a transformative force for the 21st century (Fig. 1).
 | ||
| Fig. 1 Timeline of MOF–biomass composite materials. (Reproduced with permission from ref. 15–30, copyright 2015 American Chemical Society, copyright 2016 Wiley, copyright 2017 American Chemical Society, copyright 2018 Elsevier, copyright 2019 American Chemical Society, copyright 2019 Elsevier, copyright 2020 Elsevier, copyright 2020 Wiley, copyright 2021 Elsevier, copyright 2021 Wiley, copyright 2022 Elsevier, copyright 2023 Elsevier, copyright 2023 Elsevier, copyright 2023 American Chemical Society, copyright 2023 Elsevier, copyright 2024 Springer Nature, copyright 2024 Nature.) | ||
2. Properties and synthesis of MOFs with biomaterial matrices
2.1 Key factors influencing MOF stability
MOFs have emerged as a class of materials with exceptional potential for various applications, especially when integrated with biomass materials.6 Their stability, a critical aspect for practical implementation, is influenced by several key factors, including the choice of metal ions, organic linkers, and the synthesis conditions.31–33 For example, the use of zirconium or titanium ions can lead to highly robust MOFs, such as UiO-66 and MIL-125, which are known for their excellent chemical and thermal stability.3 The choice of organic linkers can affect the pore size and functionality of the framework. For instance, the use of bipyridine-based linkers can introduce redox-active sites within the MOF structure, which can be beneficial for catalytic applications.34One of the key factors that influence the stability of MOFs under operational conditions is the strength of the coordination bonds between the metal ions and the organic ligands. MOFs with strong coordination bonds are more stable and less likely to undergo degradation or structural collapse. The choice of metal ions and organic ligands can also affect the stability of MOFs.35 Walton proposed several guidelines that may be useful in the selection of further chemically stable MOFs based on this criterion. The Irving–Williams series describes the relative stability of first row transition series bivalent metal ions (Mn < Fe < Co < Ni < Cu > Zn) with a wide variety of ligands and can therefore be used as a guiding tool in the synthesis of stable structures.36 Xiong et al.37 also revealed that MOFs based on Cu2+ are more stable than their Co2+ and Zn2+ analogues, and that the N-oxide ligand endows the MOFs with a higher affinity for CO2. In addition, the use of functionalized organic ligands, such as those containing carboxylic, amino, or hydroxyl groups, can enhance the stability of MOFs by increasing the number of coordination sites and the strength of the interactions between the ligands and the metal ions.35
In addition to stability, the properties of MOFs, such as pore size, surface area, and chemical functionality, can be tuned by varying the metal ions and organic ligands used in their synthesis.15,38–40 This makes MOFs highly versatile and adaptable to a wide range of applications. For example, MOFs with large pore sizes and high surface areas are ideal for use in gas storage and separation,41,42 while those with specific chemical functionalities are useful for catalysis and drug delivery.43
2.2 Synthesis techniques for MOF–biomass composites
To harness the synergy of MOFs with biomass, innovative synthesis methods are employed:Solvothermal/hydrothermal synthesis is the most widely used method for the synthesis of MOFs. This method involves the reaction of metal ions and organic ligands in a solvent, such as water,16 ethanol, and DMF, at elevated temperatures and pressures, as a result of high yield and uniform particle size distribution. The use of biomaterials, such as cellulose and chitosan, as templates or supports for the synthesis of MOFs can significantly improve the mechanical strength and stability of the resulting MOF–biomass composites. These composites exhibit high surface area and tunable porosity, making them ideal for applications in pollution control, such as air and water purification. They also show promise in energy storage, with potential use in the development of advanced battery materials and supercapacitors due to their excellent electrochemical properties. Furthermore, the controlled morphology and high surface area enhance catalytic efficiency, making these composites suitable for various chemical reactions.
There are two strategies for solvothermal synthesis. The first strategy is to synthesize the MOF separately and then mix it with the biomass matrix. As shown in Fig. 2a, MIL-101 was solvothermally synthesized after mixing Fe3+ and terephthalic acid, and then a mixed solution of MIL-101 and chitosan was used to form MIL-101/CS sponge.44 The advantage is that the synthesis conditions of the MOF, such as temperature, time and solvent type, can be accurately controlled, so as to obtain a MOF with a specific structure and properties. In addition, this method can further improve the performance of MOFs through post-synthesis modification, such as constructing bifunctional MOF catalysts through post-synthesis modification of organic ligands and unsaturated metal sites.48 This method can accurately control the properties of MOFs, but it may increase the synthesis steps and costs.
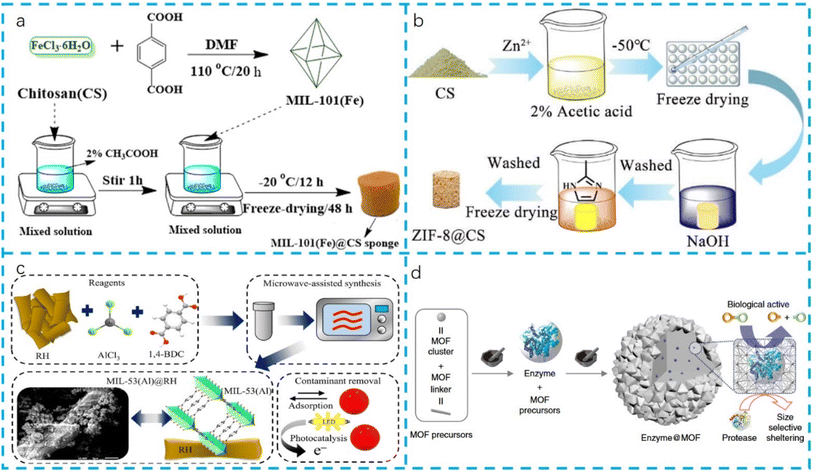 | ||
| Fig. 2 Preparation of MOF–biomass composite materials via various methods: (a) solvothermal (reproduced from ref. 44 with permission from Elsevier, copyright 2020); (b) in situ growth (reproduced from ref. 45 with permission from RSC, copyright 2020); (c) microwave-assisted synthesis (reproduced from ref. 46 with permission from Elsevier, copyright 2023); (d) mechanochemical process (reproduced from ref. 47 with permission from Nature, copyright 2019). | ||
The second strategy is to directly mix the reactant with the biomass matrix in the process of synthesizing the MOF. The advantage of this method is that it can simplify the synthesis process, reduce the steps and possibly introduce new physical and chemical properties into the MOF. For example, a solution containing metal ions (Co(NO3)2, NiCl2, and terephthalic acid) is prepared with a specific molar ratio. This solution is then mixed with a CMC aerogel in a Teflon-lined stainless steel autoclave and heated at 120 °C for 14 hours to allow for the in situ growth of the MOF on the CMC aerogel. The resulting precursor is then washed with DMF and methanol and freeze-dried to obtain the final MOF–CMC aerogel composite.49 This method can also take advantage of the renewability and environmental friendliness of biomass matrices, providing a new way for the sustainable development of MOFs, but it may be difficult to accurately control the properties of MOFs, because the complexity of biomass matrices may affect the crystal structure and properties of MOFs. Niu et al.50 reported the synthesis of a chitosan-supported MOF composite using the solvothermal method. The resulting Zn-MOF/chitosan composite had high adsorption capacity for chromium(VI) and methyl orange through electrostatic attraction and non-electrostatic action respectively. According to Nasser Abdelhamid's research,51 the 3D printed Cellulose-ZIF8 (CelloZIF-8) composite material begins with the formulation of a thixotropic ink using SiO2-based materials and TOCNF (Tall Oil Carboxylate Nanofibers). This ink is then used to 3D print the desired structures using a 3D printer. After printing, the structures are subjected to a hydrothermal treatment to induce the in situ growth of ZIF-8 crystals on the 3D printed framework. The materials are then tested for their adsorption and catalytic properties, showing high efficiency in dye removal and CO2 adsorption. The process is binder-free and does not require additional chemical reagents, making it cost-effective and environmentally friendly.
In situ growth of MOFs in biomass composites is an innovative strategy combining MOFs with biomass materials, which are usually used to prepare continuous MOF films. It ensures strong interface adhesion between the MOF and the biomass substrate, leading to better mechanical stability and durability of the composite materials. This is crucial for applications where the material's integrity under stress is essential. The method allows for customizable properties by adjusting the growth conditions, such as temperature and pH. This flexibility enables the fine-tuning of the composite's properties to meet specific application requirements. The versatility of in situ growth means it can be applied to a wide range of biomass substrates, including cellulose fibers and polymer matrices, expanding the scope of potential applications in biomedical, sensors and food packaging. In situ growth of MOFs can effectively utilize the natural advantages of biomass, such as renewability, biocompatibility and environmental friendliness, and at the same time endow materials with new functional characteristics. By growing MOFs on the surface or inside of biomass, composite materials with excellent mechanical strength, stability and biocompatibility can be prepared, which is of great significance for developing new environmental protection materials and sustainable development materials. As shown in Fig. 2b, the ZIF-8@CS composite sponge is prepared using a modified in situ method.45 The process involves dissolving chitosan and Zn(CH3COOH)2·6H2O in acetic acid solution, followed by vacuum freeze-drying to obtain the primary Zn2+@CS sponges. These sponges are then immersed in a solution containing 2-methylimidazole for the in situ growth of ZIF-8 crystals. After washing and another round of vacuum freeze-drying, the white ZIF-8@CS sponges are obtained. Table 1 shows more MOF–biomass composites prepared by the in situ growth method.
| Adsorbent | Preparation route | MOF types | Metal core | Linker | Pollutant | Adsorption capacity (mg g−1) | pH | Reusability | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| ZIF-8@chitosan/PVA nanofiber | Rest for 24 hours; electrospinning | ZIF-8 | Zn | 2-Methylimidazole | Malachite green | 1000 | 6 | 5, 90% | Langmuir isotherm model | 52 |
| Zn-MOF/chitosan (ZnBDC/CSC) composite | Solvothermal method | Zn-MOF | Zn | 1,4-Benzenedicarboxylic acid | Cr(VI)) methyl orange (MO) | 225 | 5 | 5, 84% | Langmuir isotherm model | 50 |
| 202 | ||||||||||
| Nano-MIL-101(Fe)@chitosan (CS) hybrid sponge | Solvothermal method | MIL-101 | Fe | Terephthalic acid | Acid red 94 | 4518 | — | 3, 87% | Langmuir isotherm | 44 |
| Chitosan/UiO-66 composite porous monolith | Solvothermal method; ice-templating | UiO-66 | Zr | Benzene-1,4-dicarboxylate | Congo red (CR) | 246.21 | 7 | 4, 90% | Langmuir and Freundlich models | 53 |
| ZIF-67/BC/CH aerogel | In situ growth | ZIF-67 | Co | 2-Methylimidazole | Cu2+ | 200.6 | 6 | 5, 72% | Pseudo-second-order kinetic model | 18 |
| Cr6+ | 152.1 | 5, 81% | ||||||||
| ZIF-67 MOF@aminated chitosan composite bead | Solvothermal method | ZIF-67 | Co | 2-Methylimidazole | Cr(VI) | 119.05 | 2 | 7, 60% | Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich (D–R) | 54 |
| Chitosan-ZIF-8 composite beads | Room temperature growth | ZIF-8 | Zn | 2-Methylimidazole | Cu(II) | 165.7 | 5 | 5 | Langmuir and Freundlich isotherm models | 55 |
| Pb(II) | 131.4 | |||||||||
| Chitosan-g-PNVCL/ZIF-8 composite nanofibers | Stirring for 6 hours | ZIF-8 | Zn | 2-Methylimidazole | Cr(VI) | 269.2 | 3 | 3, a slight decrease | Redlich-Peterson isotherm model | 56 |
| As(V) | 258.5 | |||||||||
| Phenol | 152.3 | |||||||||
| MIL-100(Fe)-nano-modified chitosan | Solvothermal method | MIL-100(Fe) | Fe | 1,3,5-Benzenetricarboxylic acid | Sb(III) | 52.91 | 11 | Freundlich model | 57 | |
| Chitosan-GO/ZIF (GCZ8A) foam | Reaction at 25 °C for 4 hours | ZIF-8-Ag | Zn | 2-Methylimidazole | Uranium(VI) | 361.01 | 7 | 5, 33.7% | Freundlich model | 58 |
| MOF-808/chitosan | Solvothermal process | MOF-808 | Zr | 1,3,5-Benzenetricarboxylic acid | Cr(VI) | 320 | 5 | 6 | Langmuir isotherm model | 59 |
| MIL-53(Fe)/chitosan composite hydrogel spheres | Solvothermal method | MIL-53(Fe) | Fe | Terephthalate | CR | 590.8 | Neutral | 3, 85% | Pseudo-first-order model; Langmuir model | 60 |
| UiO-66/CB | Stirring for 2 hours | UiO-66 | Zr | BDC | As(III) | 88.6 | 7 | 5, 86.9% | Freundlich model | 61 |
| UiO-66-NH2-modified chitosan composite | Solvothermal method | UiO-66-NH2 | Zr | 2-Aminoterephthalic acid | Hg(II) | 896.8 | 6 | 5 | Langmuir isotherm model | 62 |
| TIF-A1/chitosan composite beads | Secondary growth method | TIF-A1 | Zn | Adenine isonicotinic acid | Pb(II) | 397.3 | 5, 99% | Langmuir model | 63 | |
| ZIF8–ZnO–cotton fiber | Solvothermal reaction | ZIF-8 | Zn | 2-Methylimidazole | As(V) | 52.5 | 7 | — | 64 | |
| BC@ZIF-8 composite aerogel | In-situ growth | ZIF-8 | Zn | 2-Methylimidazole | Pb2+ and Cd2+ | 390 and 220 | 5.5 ± 0.5 | Pseudo-second-order kinetic model | 22 | |
| ZIF-8@CNF@cellulose foam | Solvothermal method | ZIF-8 | Zn | 2-Methylimidazole | Rhodamine B | 24.6 | 23 | |||
| Cr(VI) | 35.6 | |||||||||
| DMF | 45.2 g g−1 | |||||||||
| UiO-66-NH2 grafted onto cellulose fibers | One-pot synthesis | UiO-66-NH2 | Zr | 2-Amino-1,4,7-triazacyclononane | Cr(VI) | 78.2% | 65 | |||
| MO | 84.5% | |||||||||
| UiO-66 and UiO-66-NH2@cellulose aerogels | In situ growth | UiO-66 and UiO-66-NH2 | Zr | 2-Aminoterephthalic acid | Pb2+ | 89.4 | 5 | Pseudo-second-order kinetic model | 66 | |
| Cu2+ | 31.23 | |||||||||
| NH2-MIL-53/WC hybrid membrane | Self-sacrificial template strategy | Al | 2-Aminoterephthalic acid | Pb2+ | 223.4 | 6 | Langmuir isotherm model | 67 | ||
| HKUST-1/cellulose/chitosan composite aerogel | In situ growth method | HKUST-1 | Zn | 2,3,5,6-Tetrafluorophenyl | Methylene blue (MB) | 526.3 | 7.5 | Desorption with HCl solution; 90% | The Freundlich model | 68 |
| HKUST-1/PANNs/RC composite aerogel | In-situ growth and solvothermal methods | HKUST-1 | Cu | 1,3,5-Benzenetricarboxylate | MB | 522.01 | 5, 92% | 69 | ||
| Cu-BTC@cellulose acetate membrane | In-situ immobilization | Cu | BTC | Dimethoate | 321.9 | 5, 77.5% | Langmuir isotherm model | 70 | ||
| MIL-53@PC | Sonicate | MIL-53 | Fe | 2-Hydroxybenzaldehyde | MO | 936.5 | 2 | Pseudo-first-order (PFO) and pseudo-second-order (PSO) models | 71 | |
| 3D printed cellulose-ZIF8 | In-situ growth | ZIF-8 | Zn | Imidazolate | Cu2+ | 101.2 | 51 | |||
| Co2+ | 33.5 | |||||||||
| Co-MOF-cellulose based composite | In situ growth method | ZIF-8 and ZIF-67 | Zn and Co | 2-Methylimidazole | Cr(VI) | 145 | Neutral | 5, 90% | Langmuir and pseudo-second-order model | 72 |
| Fluorescent bacterial cellulose@Zr-MOF | Solvothermal method | Zr-MOF | Zr | 1,3,5-Tri(4-carboxyphenyl)benzene | Cr(VI) | 90 | 3 to 9 | 73 | ||
| Fe/ZIF-8@cellulose bioadsorbent | In-situ growth | ZIF-8 | Fe Zn | 2-Methylimidazole | Tetracycline | 1359.2 | 5, 63.5% | Langmuir isotherm model | 74 | |
| MIL-100(Fe)/cellulose | Stirred at ambient temperature for 24 h | MIL-100(Fe) | Fe | Benzene-1,3,5-tricarboxylic acid | MB | 384.615 | 6.5 | 5, without significant loss | Langmuir model | 75 |
| MIL-88(Fe)cellulose-based filter | Solvothermal method | MIL-88(Fe) | Fe | Terephthalic acid | MB | 602 | Neutral | 5, 82.29% | Langmuir isotherm | 76 |
| Ni/Co-MOF-CMC aerogel | Solvothermal reaction | Ni Co | Terephthalic acid | Cu2+ | 233.99 | 2 to 5 | Langmuir isotherm model | 49 | ||
| Cellulose cotton-based UiO-66 MOF | Solvothermal process | UiO-66 | Zr | p-Phthalic acid | Rhodamine B | 68.5 | 10 | Langmuir model | 77 | |
| Pb(II) | 65 | |||||||||
| SA/CMC/Cu-BDC composite beads | Solvothermal method | Cu | Terephthalic acid | Tetracycline | 180.5 | 7 | 3 | Langmuir isotherm model | 78 | |
| UiO-66-NH2 incorporated PVA/cellulose nanofiber composite aerogel | Solvothermal reaction | UiO-66-NH2 | Zr | 2-Aminoterephthalic acid | Formaldehyde | Decreases from 12.85 to 11.12 mg g−1 | 5 | 79 | ||
| TMPA@MOF-801 aerogel | Solvothermal process | MOF-801 | Zr | Fumarate | Cr(VI) | 350.64 | 2 | 6, 88% | Pseudo-second-order model and Langmuir model | 80 |
| MIL@TH@FP composite | — | MIL-125-NH2 | Ti | Hg2+ | 182.8 | 6.8 | 5 | Langmuir and pseudo-second-order models | 81 | |
| Cr3+ | 109.7 | |||||||||
| Cu2+ | 134.1 | |||||||||
| EC/ZIF-67 and EC/MIL-88A nanocomposites | Green synthesis routes in water at room temperature and atmospheric pressure | ZIF-67 | Zr | 2-Methyl imidazole | CR | 99.80% | 7 | 5 | Freundlich model | 82 |
| MIL-88A | Fe | Fumaric acid | 90.90% | |||||||
| SCC-CuMOF | Solvothermal method | CuMOF | Cu | Trimesic acid | Pb2+ | 531.38 | 5 | Langmuir model | 83 | |
| Aminated ZIF-8 composite cellulose aerogel | Stirring at room temperature | ZIF-8 | Zn | 2-Methylimidazole | Mo(VI) | 872.39 | 3 | 5, 80% | Langmuir and pseudo-second-order model | 84 |
| Ni-MOF-74 lignin-based nanofiber materials | Solvothermal reaction | Ni-MOF-74 | Ni | 2,5-Dihydroxyterephthalic acid | Chlorobenzene | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 490 490 |
Langmuir model | 85 | ||
| NH2-UIO@L | Solvothermal method | UIO | Zr | 2-Amino terephthalic acid | MO | 214.13 | 3–10 | 6, 97.66% | Dubinin–Radushkevich and pseudo-second-order models | 86 |
| MB | 243.31 | 11–12 | 6, 99.15% | Temkin and pseudo-second-order models | ||||||
| NH2-MIL@L | One-step hydrothermal method | MIL | Fe | Phenolated lignin | MO | 195.31 | 6, 91.2% | Temkin model and pseudo-second-order kinetics | 87 | |
| MB | 481.34 | 6, 93.4% | Dubinin–Radushkevich model and pseudo-second-order kinetics | |||||||
| UIO-g-NL | Solvothermal reaction; Schiff base reaction | UIO-66-NH2 | Zr | Aminated lignin | MO | 961.54 | 6, >90% | Pseudo-second-order kinetic and Freundlich isotherm models | 88 | |
| MB | 1120.7 | Selective adsorption of both dyes (MO and MB) is pH-dependent | ||||||||
| PdNPs/UiO-66-NH2/LP | Solvothermal method | UiO-66-NH2 | Zr | 2-Aminoterephthalic acid | Cr6+ | Efficient removal of Cr6+ in 10 min | 4, >50% | First-order kinetics | 89 |
Microwave-assisted synthesis is a rapid and efficient method for the synthesis of MOFs,90–92 offering significant advantages of energy efficiency and uniform heating over traditional methods. This method involves the use of microwave radiation to heat the reaction mixture, resulting in faster reaction rates and shorter synthesis times. The ability to reduce reaction times makes microwave-assisted synthesis particularly suitable for large-scale production, highlighting its advantage in terms of rapid synthesis. The use of microwave-assisted synthesis for the synthesis of MOFs with biomaterial matrices can improve the homogeneity and dispersion of the MOFs in the biomaterial matrices.46 Kevin et al.46 reported the design of MOF@Biomass layered hybrids through in situ growth from rice husk (RH) and microwave-assisted synthesized MIL-53(Al) particles that enable the reduction of reaction times, as shown in Fig. 2c. Couzon et al.93 discussed the development of a new synthesis method for MOF–textile composites using microwave irradiation. This method allows for the direct integration of MOFs onto textiles, which could enhance the protective capabilities of the material against chemical and nuclear hazards.
A mechanochemical approach provides a rapid and efficient route to MOFs directly from a metal oxide and without bulk solvent,94,95 which not only speeds up the process but also minimizes the use of organic solvents and strong acids, aligning with environmentally friendly practices. In the pharmaceutical industry, the improved enzyme stability and activity make these composites suitable for controlled drug delivery systems and topical medications, potentially improving the efficacy and safety of such treatments. Noorian96 reported a novel approach to encapsulating bioactive molecules within MOFs using a simple mechanochemical method. The focus is on developing controlled topical drug delivery systems, which could potentially improve the efficacy and safety of topical medications. By encapsulating enzymes within MOFs using this method, their stability and activity are enhanced, even under harsh conditions. This is particularly important for applications where the enzymes need to maintain their functionality in challenging environments. Fig. 2d provides the preparation process of encapsulating enzymes within MOFs using a ball milling strategy. The method is rapid, green, and minimizes the use of organic solvents and strong acids. Enzymes such as β-glucosidase, invertase, β-galactosidase, and catalase are encapsulated in ZIF-8, UiO-66-NH2, or Zn-MOF-74. The process is initiated by adding MOF precursors and the lyophilized enzyme into a zirconia grinding jar and grinding at a frequency of 8 Hz for 5 minutes. The resulting powder is then collected, washed, and stored for further use. The synthesis time is optimized to 5 minutes for industrial applicability. The amount of enzyme loading is controlled, and the encapsulation efficiency and bioactivity are evaluated. The method is shown to be general and can be applied to various enzymes and MOF, demonstrating improved enzyme stability and activity even under harsh conditions. Additionally, the encapsulation of biomolecules within MOFs can improve the sensitivity and selectivity of biosensors for detecting various analytes, making them more reliable and efficient.
Electrospinning is a versatile and scalable method for the synthesis of nanofibers and nanofiber-based composites. This method involves the use of an electric field to draw a polymer solution or melt into a thin fiber. This makes it an ideal method for industrial applications where mass production is required. The resulting nanofiber structure also improves the mechanical properties of the composites, making them suitable for structural applications that demand strength and durability. It can incorporate various functional materials into the nanofibers to enhance the multifunctionality of the composites, allowing them to perform multiple roles within a single material system. The use of electrospinning for the synthesis of MOF–biomass composites can improve the mechanical properties and functionality of the resulting composites.17 Laurila et al.97 reported the in situ crystal growth of HKUST-1 on electrospun cellulose nanofibers. And Li98 showed another in situ growth of HKUST-1 on a composite material consisting of electrospun polyacrylonitrile nanofibers and regenerated cellulose aerogel. In contrast, Bahmani et al.99 discussed the synthesis and characterization of electrospun polyacrylonitrile/cellulose acetate/MIL-125/TiO2 composite nanofibers. These nanofibers are designed to function as efficient photocatalysts and as a system for delivering anticancer drugs.
The synthesis of MOF–biomass composites employs diverse techniques to capitalize on the unique properties of both components, yielding materials with enhanced performance. Methods such as solvothermal/hydrothermal reactions, microwave-assisted synthesis, electrospinning, and mechanochemical approaches facilitate the creation of composites with improved stability, adsorption capabilities, and functional properties. These composites show promise in areas like pollution control, protective gear enhancement, and drug delivery systems, showcasing the potential of MOF–biomass integration across multiple industries.
2.3 Properties of MOFs with different metal ions and organic linkers
MOF–biomass composites represent an innovative class of hybrid materials that integrate the unique characteristics of MOFs with the natural abundance and functionality of biomass derivatives. These composites exhibit a suite of properties that transcend those of their individual components, offering enhanced performance and versatility across various applications.18,67,100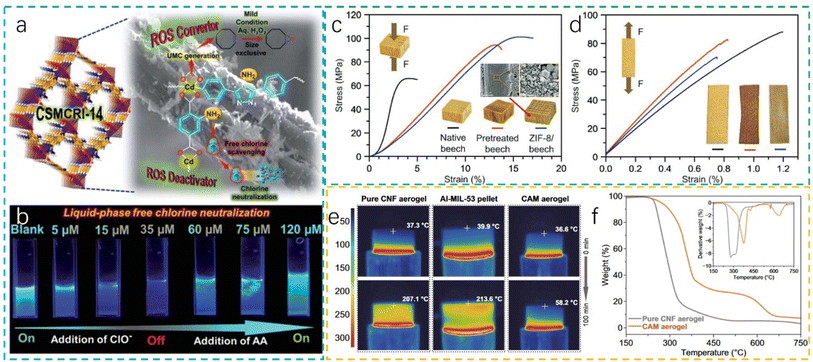 | ||
| Fig. 3 (a) Graphical representation of the applications carried out using CSMCRI-14 and (b) stepwise liquid-phase free chlorine neutralization via addition of AA in the aqueous phase dispersion of CSMCRI-14 (reproduced from ref. 15 with permission from RSC, copyright 2022); (c and d) compressive and tensile stress–strain curves of native beech, pretreated beech and ZIF-8/beech composite (reproduced from ref. 19 with permission from Wiley, copyright 2020); (e) infrared side-view images of the pure CNF aerogel, the pure Al-MIL-53 pellet, and the CAM aerogel, with the temperature of the top surfaces and (f) TGA analysis curves of the pure CNF and CAM aerogels under air flow (reproduced from ref. 13 with permission from Springer, copyright 2020). | ||
In conclusion, MOF–biomass composites, with their multifaceted properties and application versatility, stand at the forefront of material innovation. As research progresses, these composites are poised to revolutionize fields ranging from sustainable energy storage to precision medicine, underscoring the importance of continuous exploration and optimization of these remarkable hybrid materials.
2.4 Interaction of MOFs with biomass-derived materials and biological molecules
The realm of MOFs in conjunction with biomass-derived materials and biological molecules has sparked significant interest due to the unique synergies that emerge from these interactions. These associations not only underscore the versatility of MOFs in interfacing with natural systems but also pave the way for novel applications in biotechnology, medical science, and environmental remediation.124,125 By delving into the intricate mechanisms governing these interactions, we unravel the potential for designing advanced materials tailored for specific functions within biological contexts.The pore size of MOFs is an important factor affecting their interaction with biomolecules. By adjusting the pore size of MOFs, the selective adsorption of biomolecules with specific size can be realized.127 In addition, the functionalization of MOF surface is also a key factor to regulate their interaction with biomolecules. By introducing specific functional groups (as shown in Fig. 4),62 the interaction between MOFs and biomolecules can be enhanced, thus improving the adsorption efficiency and selectivity.129,130 Some interesting phenomena have been found in the study of the interaction between MOFs and biomolecules. For example, by using specific organic ligands and metal sources, MOFs with specific pore structure and surface functionalization can be synthesized, and these MOFs can effectively adsorb and separate organic dyes44,131,132 and heavy metal ions.107,111,113 In addition, some studies show that MOFs can also be used as drug carriers, and effective drug loading and controlled release can be achieved by adjusting the structure and functionalization of MOFs.133,134
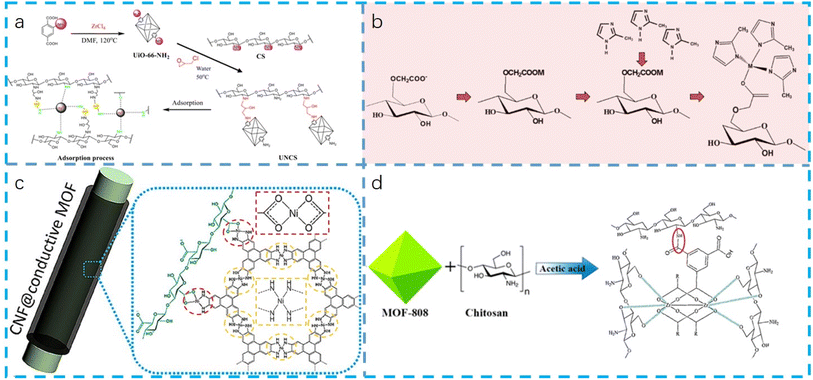 | ||
| Fig. 4 The interaction between MOFs and biomolecules. (a) Possible prepared processes and adsorption mechanism of UNCS (Reproduced with permission from ref. 62, Copyright 2023 Elsevier); (b) schematic of the growth mechanism of ZIF-8 and ZIF-67 MOF on CM cotton fabric (Reproduced with permission from ref. 128, Copyright 2021 Elsevier); (c) possible interactions between MOF and CNF in CNF@c-MOF hybrid nanofibers (Reproduced with permission from ref. 20, Copyright 2019 American Chemical Society); (d) possible interactions between MOF and chitosan in the composite (Reproduced with permission from ref. 59, Copyright 2022 Elsevier). | ||
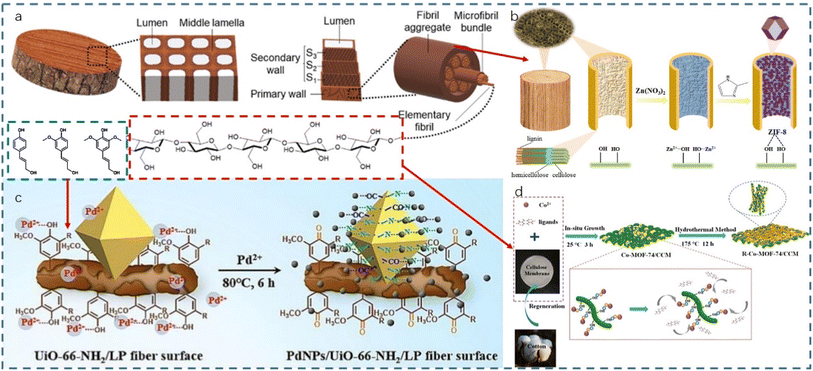 | ||
| Fig. 5 Chemical structure of wood (a) (reproduced from ref. 138 with permission from RSC, copyright 2022); schematic of the preparation of ZIF-8/wood composites (b) (reproduced from ref. 139 with permission from Elsevier, copyright 2021); binding mechanism of UiO-66NH2 and PdNPs with lignin (c) (reproduced from ref. 89 with permission from Elsevier, copyright 2024); in situ growth of Co-MOF-74 on cellulose (d) (reproduced from ref. 135 with permission from Elsevier, copyright 2021). | ||
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, which serve as active centers for CO2 fixation into cyclic carbonates and Knoevenagel condensation reactions. The bio-MOF(Cu)/GO demonstrates exceptional performance in CO2 conversion under mild conditions, achieving over 99.9% conversion with a turnover number (TON) of 525 in a solvent-free reaction at 1 bar CO2 pressure.
O groups, which serve as active centers for CO2 fixation into cyclic carbonates and Knoevenagel condensation reactions. The bio-MOF(Cu)/GO demonstrates exceptional performance in CO2 conversion under mild conditions, achieving over 99.9% conversion with a turnover number (TON) of 525 in a solvent-free reaction at 1 bar CO2 pressure.
 | ||
| Fig. 6 (A) Schematic diagram of the fabrication of CA/PM/EUG. (Reproduced from ref. 142 with permission from Elsevier, copyright 2024.) (B) Schematic illustration of the preparation of FeTPt@CCM and its capability of synergistic chemotherapy, PDT, and ferroptosis therapy. (a) The design of the multi-bioinspired “Trojan Horse” drug vehicle. (b) The preparation processes of FeTPt through microfluidics, and the generation of FeTPt@CCM through CCM coating. (Reproduced from ref. 143 with permission from Wiley, copyright 2023.) | ||
The interface between MOF and biomass-derived materials and biological molecules presents a fertile ground for innovation, pushing the boundaries of materials science towards more sustainable, biocompatible, and intelligent solutions. As our understanding of these interactions deepens and new synthesis strategies emerge, we edge closer to a future where MOF-based materials play a pivotal role in energy, catalysis, advancing healthcare, environmental protection, and beyond. The intricate dance between MOF and biological entities underscores the potential for transformative technologies that harmoniously integrate synthetic and natural systems.
3. Applications of MOFs in biomass materials
The synergy of MOFs with biomass aims to create a new generation of materials with enhanced properties for a multitude of applications, including but not limited to, catalysis,11,100,149 adsorption,19,21,118 energy storage,20,32,150 and environmental remediation.120 This comprehensive exploration delves into the intricate world of MOF–biomass composites, illuminating their potential, advantages, challenges, and the scientific advancements driving this exciting frontier.3.1 Enhancing biomass properties with MOFs
3.2 Biomedical applications
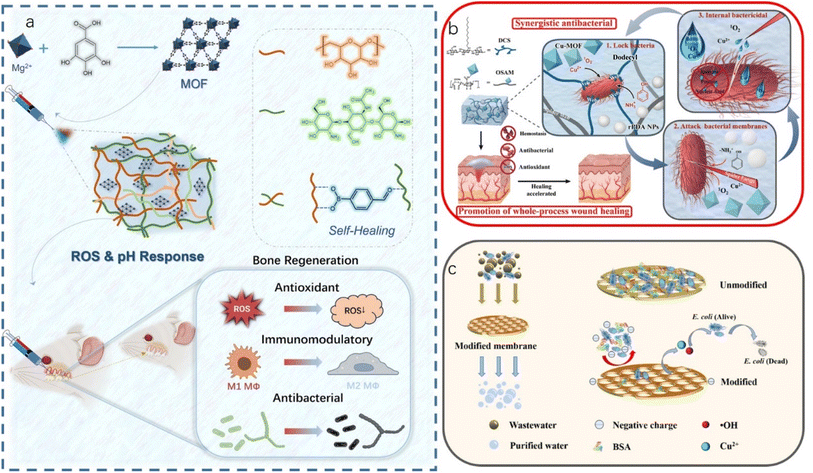 | ||
| Fig. 7 (a) The schematic illustration of the preparation, application and bone regeneration promotion mechanism of CSBDX@MOF (reproduced from ref. 16 with permission from Springer Nature, copyright 2024); (b) bio-inspired synergistic antibacterial hydrogel for synergistic antibacterial and whole-process promotion of wound healing (reproduced from ref. 164 with permission from Elsevier, copyright 2023) and (c) the antifouling mechanism (reproduced from ref. 165 with permission from Elsevier, copyright 2023). | ||
3.3 Environmental remediation
The high porosity and extensive surface area characteristic of MOFs' network structures are pivotal for adsorption processes. They provide a vast surface for the adsorption of various pollutants, encompassing heavy metals(Cr6+,50,54,59 Cu2+,18,49,55 Pb2+,22,63,67,83 As5+,56,64 As3+,61 Hg2+,62,81 Sb3+,57 U6+,58 Co2+ (ref. 51)), dyes (malachite green,52 congo red,45,53,60,82 rhodamine B,23,77 methyl orange,65,71,86,87 methylene blue,75,76,88 acid red44), and organic compounds (dimethoate,70 tetracycline,74,78 formaldehyde,79 chlorobenzene85). The remarkable surface area of MOF//////// facilitates a higher number of binding sites, thereby augmenting total adsorption capacity and efficiency. However, most MOF is in powder form, and it will be difficult to recover if it is directly used for water pollution treatment.
Conversely, biomass, sourced naturally from plant materials, agricultural residues, or microorganisms, significantly contributes to the enhanced stability and biocompatibility of these composites.175 Biomass materials are typically replete with functional groups such as hydroxyls, carboxyls, and amines, which can engage in interactions with both the MOF and the pollutants present in water. This interaction not only bolsters the composite's stability but also facilitates the selective adsorption of specific contaminants. For example, researchers report the development of a novel ZIF-8/wood composite for the removal of water pollutants, specifically focusing on the in situ growth of ZIF-8 within the aligned channels of natural balsa wood.176 This method creates a hierarchical structure with a high surface area, which facilitates efficient and rapid water filtration. The composite displays an excellent affinity and compatibility between the ZIF-8 coating and the wood's lumen, allowing for high-speed water transport. Tested with a 7.9 wt% loading of ZIF-8, a three-layered ZIF-8/wood filter (ZW-2) effectively treated 800 mL of a 10 mg L−1 Cu2+ solution.
Furthermore, incorporating biomass into MOF composites mitigates potential toxicity concerns associated with pristine MOF.152 Certain MOF may harbor heavy metals or other potentially hazardous constituents that could leach out during usage, posing threats to human health and the environment. By amalgamating MOF with biomass, the leaching of these toxic elements can be substantially reduced, rendering the composites safer for environmental applications.
Beyond their high adsorption capacity and enhanced stability, MOF–biomass composites exhibit commendable reusability – as shown in Table 1 – a critical attribute for practical water treatment technologies. Post-contaminant adsorption, these composites can often be regenerated and reused multiple times without a significant decline in performance, curtailing the costs and waste associated with water purification processes.
In summary, the synergistic integration of MOF and biomass within composites furnishes a sustainable and efficacious solution for water purification, tackling the challenge of removing a diverse array of contaminants while maintaining high efficiency, stability, and safety. This innovative approach holds immense promise for the future of water treatment technologies, particularly in addressing global concerns regarding water scarcity and pollution.
3.4 Energy storage and conversion
 | ||
| Fig. 8 (a) Schematic illustration for the preparation of the ZIF-8@BC/ANF composite membrane and its critical role as an LIB separator; (b) microstructure design and strengthening mechanism of the ZIF-8@BC/30%ANF composite separator (reproduced from ref. 186 with permission from Elsevier, copyright 2022). (c) Schematic diagram of the preparation process of the LC@UiO-66-NH2 separator; (d) the Zn deposition process with the LC@UiO-66-NH2 separator; (e) cycling performance of Zn//AC ZHSs with different separators at 1 A g−1 (reproduced from ref. 187 with permission from Wiley, copyright 2023). | ||
The MOF exhibits good application prospects in lithium batteries because the efficient ionic sieve can realize selective passage of Li+ and prevent the LiPSs from shuttling to the anode.188–190 The battery separator can be obtained by loading the MOF to the biomass membrane.191 Yan's research explores the use of monodispersed metal–organic framework (MOF)-modified nanofibers as versatile building blocks for constructing separators and composite polymer electrolytes in safe lithium–sulfur batteries.24 The study demonstrates that these nanofibers, derived from bacterial cellulose (BC), exhibit excellent thermal stability and mechanical properties, effectively adsorb lithium polysulfides (LiPSs), and enhance lithium-ion (Li+) transfer and uniform deposition. The resulting BCSZ membrane separator maintains a stable potential for 2500 hours and significantly improves the rate performance and capacity retention of lithium–sulfur batteries. When used as fillers in composite polymer electrolytes, the ionic conductivity and Li+ transference number are enhanced, leading to a stable quasi-solid-state lithium symmetric battery for 3000 hours and a low capacity decay rate of 0.038% per cycle over 800 cycles. This approach shows promising potential for achieving high-performance and safe lithium–sulfur batteries. Ma et al.192 also presented a novel method for preparing multifunctional and flexible composite aerogels by in situ growth of MOF nanoparticles on bacterial cellulose. The composite aerogels, BC@ZIF-8 and BC@UiO-66, exhibit excellent performance in heavy metal ion adsorption and as intermediate layers in high-performance lithium–sulfur batteries, demonstrating low capacity decay rates and effective suppression of polysulfide shuttling.
The electrolyte is designed to address the challenges of uneven magnesium deposition and dendrite formation, which are common issues in LMBs.193 Wang et al.194 presented a study on the preparation and application of a novel 3D-on-1D MOF@cellulose acetate (HCA) gel polymer electrolyte (GPE) in lithium–magnesium hybrid batteries (LMBs). The MOF@HCA GPE is synthesized using a metal ion pre-anchored strategy, which ensures uniform distribution of Mg2+ ions and improves ionic conductivity. MOFs are capable of adsorbing anions, which helps to inhibit their transportation, thus promoting a more homogeneous ion flux and preventing the formation of Mg dendrites. The study demonstrates that this electrolyte significantly enhances the cycling stability and efficiency of LMBs, with a capacity retention of 96% after 600 cycles at 30 °C. The research confirms that the MOF@HCA GPE offers a promising strategy for the design of functional bio-based GPEs and improves the performance of LMBs.
3.5 Agriculture
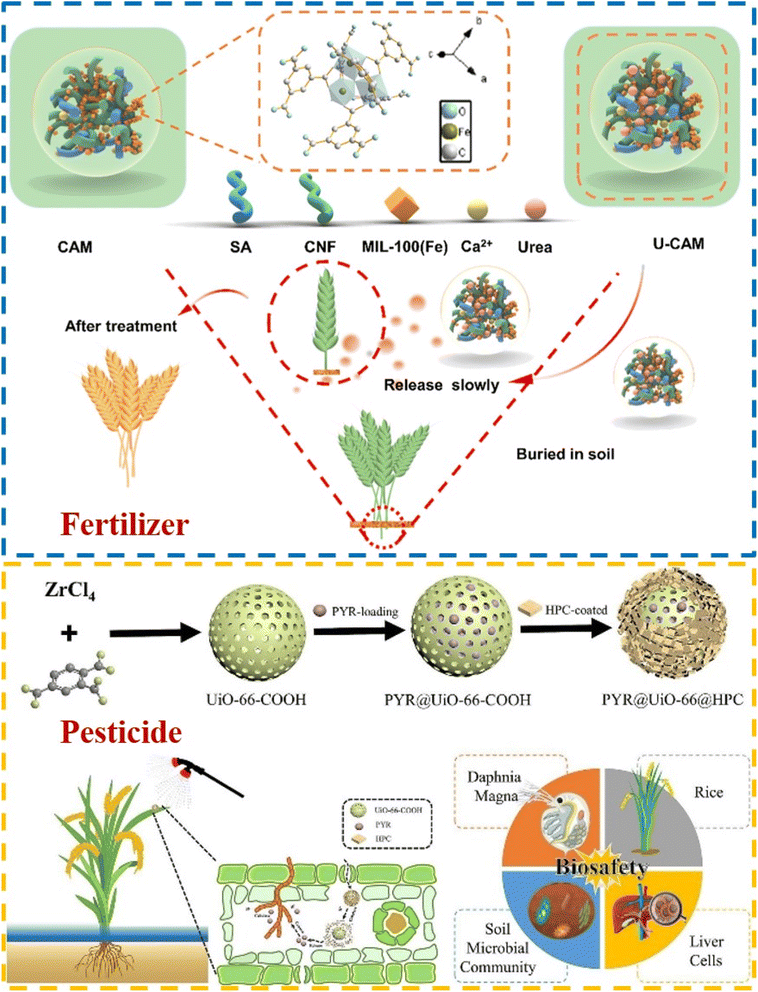 | ||
| Fig. 9 Fertilizer: the simplified scheme of the preparation of the U-CAM and its application in the slow release of the urea (reproduced from ref. 207 with permission from Elsevier, copyright 2023); pesticide: schematic diagram for the formation of PYR@UiO-66@HPC and mechanism of PYR release triggered by plant infestation by pathogenic bacteria (reproduced from ref. 209 with permission from Elsevier, copyright 2021). | ||
3.6 Food industry
 | ||
| Fig. 10 (a) Schematic description of the preparation of HKUST-1@CMCS, the regeneration of HKUST-1@CMCS, phosphate-stimulated drug release without Cu(II) ion residues, and antimicrobial activities and food preservation of dimethyl fumarate-loaded HKUST-1@CMCS film (reproduced from ref. 219 with permission from Elsevier, copyright 2020). (b) A novel cellulose nanocrystal/MOF composite (MC) developed as a stabilizer for alkenyl succinic anhydride (ASA) pickering emulsions for advanced food packaging applications (reproduced from ref. 220 with permission from Elsevier, copyright 2024). | ||
3.7 Electronics and sensors
One of the key attributes that make MOF–biomass composites particularly appealing for flexible electronics is their high conductivity.222 Although MOF themselves may not inherently possess high electrical conductivity, the addition of conductive fillers or the modification of the organic linkers can significantly enhance this property.223–225 When combined with conductive biomass derivatives, such as carbon nanotubes or graphene extracted from plant fibers, the composites exhibit remarkable electrical conductivity, enabling the efficient flow of current even when the material is subjected to deformation. Mechanical flexibility is another critical factor for flexible electronics, and MOF–biomass composites excel in this domain as well. The porous nature of MOFs allows for the accommodation of strain without compromising structural integrity, while the inherent flexibility of biomass components, especially those derived from plant sources, contributes to the overall pliability of the composite.226,227 Wang et al.228 presented a study on the in situ growth of electrically conductive MOFs (EC-MOF) on a wood cellulose scaffold to create flexible, robust, and hydrophobic membranes for use in wearable supercapacitors, as shown in Fig. 11a–c. The research, involves incorporating EC-MOF (NiCAT or CuCAT) onto a porous TEMPO-oxidized wood (TOW) scaffold using electrostatic forces, followed by room-temperature densification to produce ultrathin membranes. These membranes exhibit exceptional mechanical properties, high electrical conductivity, and hydrophobicity, with the potential for improved electrochemical performance over traditional EC-MOF powder electrodes.
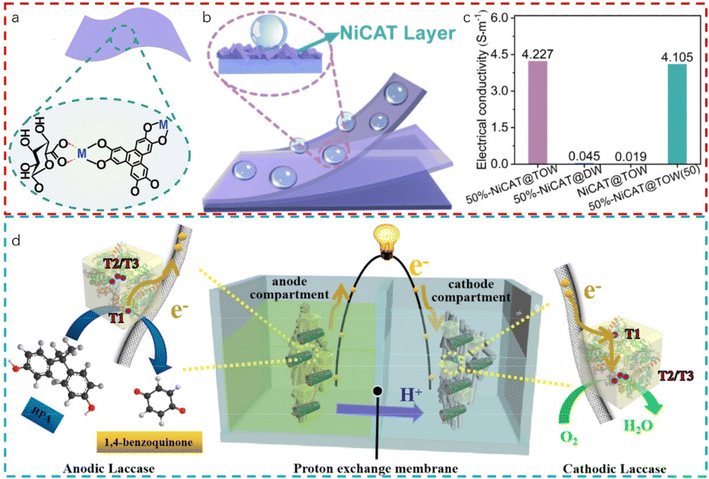 | ||
| Fig. 11 (a) Schematic illustration of 50%-NiCAT@TOW membrane; (b) schematic diagram of the hydrophobic mechanism of the 50%-NiCAT@TOW membrane; (c) comparison of the electrical conductivity results of 50%-NiCAT@TOW, 50%-NiCAT@DW, NiCAT@TOW membrane and 50%-NiCAT@TOW membrane after 50 times bending with an angle of ∼120 °C (reproduced from ref. 228 with permission from Elsevier, copyright 2024). (d) Schematic of the single-LAC biofuel cell with BPA as fuel and O2 as the final electron acceptor (reproduced from ref. 229 with permission from Elsevier, copyright 2020). | ||
Biocompatibility is yet another distinguishing feature of MOF–biomass composites, which is particularly advantageous for applications where direct contact with human skin or biological tissues is involved, such as in biomedical sensors and implants. Researchers have developed conductive metal–organic framework (c-MOF) nanolayers on cellulose nanofibers (CNFs) to create high-performance flexible supercapacitors.20 The authors successfully fabricated CNF@c-MOF hybrid nanofibers through interfacial synthesis, resulting in freestanding nanopapers with high conductivity, hierarchical porosity, mechanical strength, and flexibility. These properties facilitate efficient charge transfer and electrolyte transport, leading to excellent electrochemical performance. The CNF@c-MOF nanopapers exhibit significantly higher conductivity and capacitance compared to pure c-MOF powders and CNF-c-MOF papers, attributed to the continuous c-MOF nanolayers on CNFs. The assembled symmetric supercapacitor based on CNF@Ni-HITP nanopapers demonstrates high stability, mechanical flexibility, and superior electrochemical performance, making it suitable for flexible electronic devices. Moreover, the biodegradability of biomass components in these composites offers an eco-friendly edge, aligning with the growing global push towards sustainable manufacturing practices. This is particularly important in the context of electronic waste management, where the disposal of traditional electronic devices poses significant environmental challenges.230
In summary, the integration of MOFs with biomass materials in composites has opened up new avenues for the development of flexible electronics. These composites, characterized by high conductivity, mechanical flexibility, and biocompatibility, hold great promise for the creation of next-generation electronic devices that are not only technologically advanced but also environmentally responsible. As research progresses, we can expect to see a wider array of applications for MOF–biomass composites in fields ranging from consumer electronics to biomedicine, reflecting their status as a transformative material for the 21st century.
The integration of MOFs with biomass materials presents a fertile ground for innovation, pushing the boundaries of what is achievable with sustainable materials. As research continues to unravel the complexities of MOF–biomass interactions and refine synthesis techniques, the potential for developing materials with unparalleled performance, sustainability, and functionality becomes increasingly tangible. From catalysis to environmental remediation, the future of MOF–biomass composites promises a transformative impact across multiple industries. As we embark on this journey, addressing the existing challenges and capitalizing on the unique attributes of MOF will be pivotal in realizing a sustainable materials future.
4. Challenges and future perspectives
While MOF–biomass composites have shown significant potential in various applications, there are still several challenges that need to be addressed for their widespread adoption and commercialization. In this section, we will discuss the main challenges and outline the future perspectives for MOF–biomass composites' research.4.1 Challenges
(1) Scalability and cost-effectiveness: current synthesis methods for MOF–biomass composites often involve complex and time-consuming processes that limit their scalability and economic viability. Developing efficient and cost-effective synthesis routes is crucial for the commercialization of these materials. Currently, the synthesis of MOF–biomass composites using hydrothermal or solvothermal methods is often time-consuming, with processes lasting from several hours to multiple days. This extended duration raises the cost of synthesis. Additionally, the substantial use of solvents in these methods exerts environmental pressure due to the potential for solvent waste and its associated environmental impact.(2) Stability and durability: MOF–biomass composites may face issues related to stability and durability under various environmental conditions, especially in harsh or dynamic environments. Ensuring the long-term stability and durability of these materials is essential for their practical applications.
(3) Toxicity and biocompatibility: although MOF–biomass composites generally exhibit good biocompatibility, concerns regarding their toxicity, especially for biomedical applications, must be thoroughly investigated. Comprehensive toxicity assessments are required to ensure their safe use in various applications.
(4) Mechanical properties: the mechanical properties of MOF–biomass composites can be limited due to the brittle nature of MOFs. Improving the mechanical properties, such as flexibility and toughness, is necessary for applications requiring robust materials.
(5) Functionalization and tunability: while MOF–biomass composites can be functionalized for specific applications, achieving precise control over their properties remains challenging. Developing methods for fine-tuning the properties of these materials is critical for their optimal performance in various applications.
4.2 Future perspectives
(1) Advanced synthesis techniques: developing innovative synthesis techniques that enable the large-scale production of MOF–biomass composites while maintaining their properties is a key area for future research. This includes exploring one-pot synthesis methods, continuous flow processes, and green chemistry approaches. For instance, Li et al.26 prepared scalable multifunctional MOF–textiles with excellent ultraviolet (UV) resistance, organic contamination degradation, antibacterial activity, self-cleaning, antifouling, and oil-water separation performances.(2) Enhanced stability and durability: research efforts should focus on improving the stability and durability of MOF–biomass composites under various environmental conditions. This can be achieved by modifying the MOF structure, optimizing the composite composition, and incorporating protective coatings. This can involve the introduction of more robust metal nodes or the use of stronger organic linkers, which can significantly bolster the composite's resilience. The ratio of biomass to MOF components should be fine-tuned to achieve a balance between biodegradability and long-term performance. The incorporation of additives or cross-linking agents can also reinforce the composite, making it more resistant to environmental stressors such as moisture, temperature fluctuations, and chemical exposure.80 The application of protective coatings can provide an additional layer of defense against environmental factors that might compromise the integrity of the composite. These coatings can be designed to be resistant to UV radiation, water, and microbial attack, thereby extending the service life of the MOF–biomass composites.
(3) Comprehensive toxicity assessments: conducting thorough toxicity assessments, including long-term studies and in vivo evaluations, is essential to ensure the safe use of MOF–biomass composites in various applications, particularly in biomedical and food-related areas. Long-term studies provide insights into the chronic effects of these composites, which are crucial for understanding their potential impacts over extended periods of exposure. In vivo evaluations, on the other hand, offer a direct assessment of the composites' toxicity within living organisms, allowing researchers to observe biological responses and potential adverse effects in real-time. These assessments are vital for ensuring that MOF–biomass composites do not pose a threat to human health or the environment.162 By conducting these thorough evaluations, researchers can develop guidelines for safe usage and establish a foundation for the responsible integration of MOF–biomass composites into various applications, thereby supporting both innovation and safety in materials science.
(4) Tunable and functional materials: advancing the functionalization and tunability of MOF–biomass composites through the use of smart materials, responsive linkers, and stimuli-responsive properties will enable their customization for specific applications. Smart materials, known for their ability to mimic biological behavior and respond to external stimuli such as temperature, humidity, and pressure, can be integrated into MOF–biomass composites to create intelligent, responsive materials that adapt to their environment. This integration allows for the development of composites that can change their properties in response to specific triggers, enhancing their versatility and efficiency in targeted applications. The incorporation of stimuli-responsive properties into MOF–biomass composites enables them to exhibit changes in their structure or chemical composition when exposed to specific stimuli. This can lead to the development of materials that are self-healing, self-cleaning, or that can actively respond to pollutants in the environment, making them highly customizable for a wide range of applications.16
(5) Sustainable development: focusing on the sustainable production and disposal of MOF–biomass composites is essential for minimizing their environmental impact. This includes utilizing renewable resources, developing biodegradable composites, and implementing circular economy principles. Harnessing renewable resources like agricultural byproducts and plant residues for MOF–biomass composites not only cuts reliance on fossil fuels but also sustains agricultural and forest ecosystems. These biomass-derived materials are a greener, cost-effective substitute for petroleum-based polymers in materials science. The creation of biodegradable MOF–biomass composites is pivotal for curbing environmental pollution. Unlike synthetic materials that linger and pollute, biomass materials can be broken down by natural microorganisms, turning into harmless natural byproducts. This makes them ideal for disposable items and packaging, reducing plastic dependence and pollution.218 Incorporating circular economy principles into MOF–biomass composites' lifecycle is crucial for sustainability. This includes recycling bio-based components, a key aspect of a circular economy. Innovative recycling technologies and the use of novel biocomposites can decrease waste and environmental impact, aligning biocomposite development with a circular model for eco-friendly production.
5. Conclusion
In conclusion, the integration of MOF with biomass materials offers a promising approach for the development of novel composites with enhanced properties and functionalities. The tunable properties of MOFs allow for precise control over the modification effects, while the sustainable nature of biomass materials provides cost-effective and renewable sources for the production of these composites. However, several challenges remain to be addressed for the widespread application of MOF–biomass composites, including optimizing the synthesis conditions, improving the compatibility between MOF and biomass matrixes, and developing green and sustainable synthesis methods.Future research should focus on addressing these challenges and exploring the potential applications of MOF–biomass composites in emerging fields such as energy, catalysis, and biomedicine. The development of novel MOF structures and compositions, as well as hierarchical and composite MOF structures, will further enhance the performance of these materials and expand their applications. Ultimately, the integration of MOF with biomass materials has the potential to revolutionize various industries and contribute to the development of a more sustainable and environmentally friendly future.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the Natural Science Foundation of Fujian Province (2021J01107) and China Postdoctoral Science Foundation (2020M682603).References
- A. Saravanakumar, P. Vijayakumar, A. T. Hoang, E. E. Kwon and W.-H. Chen, Bioresour. Technol., 2023, 370, 128562 CrossRef CAS PubMed.
- Z. Cheng, X. Li, L. Zhang, Z. Yuan, H. Zheng, H. Guo, X. Zhao, J. Chen, Y. Pan, X. Chen, Y. Feng, Z. Luo, G. Tang, X. Jin and J. He, Chem. Eng. J., 2023, 475, 145912 CrossRef CAS.
- Y. Lu, C. Liu, C. Mei, J. Sun, J. Lee, Q. Wu, M. A. Hubbe and M.-C. Li, Coord. Chem. Rev., 2022, 461, 214496 CrossRef CAS.
- H. N. Abdelhamid and A. P. Mathew, Coord. Chem. Rev., 2022, 451, 214263 CrossRef CAS.
- V. Atiroğlu, A. Atiroğlu and M. Özacar, Food Chem., 2021, 349, 129127 CrossRef PubMed.
- Y. H. Cheung, K. Ma, M. C. Wasson, X. Wang, K. B. Idrees, T. Islamoglu, J. Mahle, G. W. Peterson, J. H. Xin and O. K. Farha, Angew. Chem., Int. Ed., 2022, 61, e202202207 CrossRef CAS PubMed.
- G. W. Peterson, D. T. Lee, H. F. Barton, T. H. Epps and G. N. Parsons, Nat. Rev. Mater., 2021, 6, 605–621 CrossRef CAS.
- H. Kim, S. Yang, S. R. Rao, S. Narayanan, E. A. Kapustin, H. Furukawa, A. S. Umans, O. M. Yaghi and E. N. Wang, Science, 2017, 356, 430–434 CrossRef CAS PubMed.
- S. Wang, C. Wang and Q. Zhou, ACS Appl. Mater. Interfaces, 2021, 13, 29949–29959 CrossRef CAS PubMed.
- J. Su, X. Yang, H. Shi, S. Yao and M. Zhou, J. Colloid Interface Sci., 2024, 669, 336–348 CrossRef CAS PubMed.
- V. V. Karve, D. T. Sun, O. Trukhina, S. Yang, E. Oveisi, J. Luterbacher and W. L. Queen, Green Chem., 2020, 22, 368–378 RSC.
- S. Javanbakht, M. Pooresmaeil, H. Hashemi and H. Namazi, Int. J. Biol. Macromol., 2018, 119, 588–596 CrossRef CAS PubMed.
- S. Zhou, V. Apostolopoulou-Kalkavoura, M. V. Tavares Da Costa, L. Bergström, M. Strømme and C. Xu, Nano-Micro Lett., 2020, 12, 9 CrossRef PubMed.
- G. W. Peterson, D. T. Lee, H. F. Barton, T. H. Epps and G. N. Parsons, Nat. Rev. Mater., 2021, 6, 605–621 CrossRef CAS.
- R. Goswami, B. D. Bankar, S. Rajput, N. Seal, R. S. Pillai, A. V. Biradar and S. Neogi, J. Mater. Chem. A, 2022, 10, 4316–4332 RSC.
- Q. Luo, Y. Yang, C. Ho, Z. Li, W. Chiu, A. Li, Y. Dai, W. Li and X. Zhang, J. Nanobiotechnol., 2024, 22, 287 CrossRef CAS PubMed.
- W. Lu, C. Duan, Y. Zhang, K. Gao, L. Dai, M. Shen, W. Wang, J. Wang and Y. Ni, Carbohydr. Polym., 2021, 258, 117676 CrossRef CAS PubMed.
- D. Li, X. Tian, Z. Wang, Z. Guan, X. Li, H. Qiao, H. Ke, L. Luo and Q. Wei, Chem. Eng. J., 2020, 383, 123127 CrossRef CAS.
- K. Tu, B. Puértolas, M. Adobes-Vidal, Y. Wang, J. Sun, J. Traber, I. Burgert, J. Pérez-Ramírez and T. Keplinger, Adv. Sci., 2020, 7, 1902897 CrossRef CAS PubMed.
- S. Zhou, X. Kong, B. Zheng, F. Huo, M. Strømme and C. Xu, ACS Nano, 2019, 13, 9578–9586 CrossRef CAS PubMed.
- T. Saeed, A. Naeem, I. U. Din, M. Farooq, I. W. Khan, M. Hamayun and T. Malik, J. Hazard. Mater., 2022, 427, 127902 CrossRef CAS PubMed.
- N. Wang, X.-K. Ouyang, L.-Y. Yang and A. M. Omer, ACS Sustainable Chem. Eng., 2017, 5, 10447–10458 CrossRef CAS.
- S. Ma, M. Zhang, J. Nie, J. Tan, S. Song and Y. Luo, Carbohydr. Polym., 2019, 208, 328–335 CrossRef CAS PubMed.
- K. Yan, C. Shen, H. Wang, F. Tao, C. Zhou, C. Dong, G. Zhang, X. Chen, L. Zhang, Y. Luo and X. Xu, ACS Appl. Mater. Interfaces, 2023, 15, 29094–29101 CrossRef CAS PubMed.
- S. Feng, Q. Tang, Z. Xu, K. Huang, H. Li and Z. Zou, Food Hydrocolloids, 2023, 135, 108193 CrossRef CAS.
- W. Li, Z. Yu, Y. Zhang, C. Lv, X. He, S. Wang, Z. Wang, B. He, S. Yuan, J. Xin, Y. Liu, T. Zhou, Z. Li, S. C. Tan and L. Wei, Nat. Commun., 2024, 15, 5297 CrossRef CAS PubMed.
- Y. Song, H. Li, T. Shan, P. Yang, S. Li, Z. Liu, C. Liu and C. Shen, Carbohydr. Polym., 2023, 302, 120377 CrossRef CAS PubMed.
- L. E. Lange and S. K. Obendorf, ACS Appl. Mater. Interfaces, 2015, 7, 3974–3980 CrossRef CAS PubMed.
- H. Zhu, X. Yang, E. D. Cranston and S. Zhu, Adv. Mater., 2016, 28, 7652–7657 CrossRef CAS PubMed.
- J. Ko, S. K. Kim, Y. Yoon, K. H. Cho, W. Song, T.-H. Kim, S. Myung, S. S. Lee, Y. K. Hwang, S.-W. Kim and K.-S. An, Mater. Today Energy, 2018, 9, 11–18 CrossRef.
- Z. Kang, L. Fan and D. Sun, J. Mater. Chem. A, 2017, 5, 10073–10091 RSC.
- K. Tu, Y. Ding and T. Keplinger, Carbohydr. Polym., 2022, 291, 119539 CrossRef CAS PubMed.
- F. S. Arghavan, T. J. Al-Musawi, G. A. Rumman, R. Pelalak, A. Khataee and N. Nasseh, J. Environ. Chem. Eng., 2021, 9, 105619 CrossRef CAS.
- R. Fang, A. Dhakshinamoorthy, Y. Li and H. Garcia, Chem. Soc. Rev., 2020, 49, 3638–3687 RSC.
- A. Herbst and C. Janiak, Crystengcomm, 2017, 19, 4092–4117 RSC.
- N. C. Burtch, H. Jasuja and K. S. Walton, Chem. Rev., 2014, 114, 10575–10612 CrossRef CAS PubMed.
- Y. Xiong, Y. Fan, D. Damasceno Borges, C. Chen, Z. Wei, H. Wang, M. Pan, J. Jiang, G. Maurin and C. Su, Chem.–A Euro. J., 2016, 22, 16147–16156 CrossRef CAS PubMed.
- Z. Kang, L. Fan and D. Sun, J. Mater. Chem. A, 2017, 5, 10073–10091 RSC.
- X.-F. Zhang, Z. Wang, M. Ding, Y. Feng and J. Yao, J. Mater. Chem. A, 2021, 9, 23353–23363 RSC.
- G. Khandelwal, N. P. Maria Joseph Raj and S.-J. Kim, J. Mater. Chem. A, 2020, 8, 17817–17825 RSC.
- D. E. Jaramillo, D. A. Reed, H. Z. H. Jiang, J. Oktawiec, M. W. Mara, A. C. Forse, D. J. Lussier, R. A. Murphy, M. Cunningham, V. Colombo, D. K. Shuh, J. A. Reimer and J. R. Long, Nat. Mater., 2020, 19, 517–521 CrossRef CAS PubMed.
- H. Li, L. Li, R.-B. Lin, W. Zhou, Z. Zhang, S. Xiang and B. Chen, EnergyChem, 2019, 1, 100006 CrossRef PubMed.
- M. Wu and Y. Yang, Adv. Mater., 2017, 29, 1606134 CrossRef PubMed.
- Y. Wang, K. Wang, J. Lin, L. Xiao and X. Wang, Int. J. Biol. Macromol., 2020, 165, 2684–2692 CrossRef CAS PubMed.
- L. Liu, Y. Ma, W. Yang, C. Chen, M. Li, D. Lin and Q. Pan, New J. Chem., 2020, 44, 15459–15466 RSC.
- K. J. Fernández-Andrade, A. A. Fernández-Andrade, L. Á. Zambrano-Intriago, L. E. Arteaga-Perez, S. Alejandro-Martin, R. J. Baquerizo-Crespo, R. Luque and J. M. Rodríguez-Díaz, Chemosphere, 2023, 314, 137664 CrossRef PubMed.
- T.-H. Wei, S.-H. Wu, Y.-D. Huang, W.-S. Lo, B. P. Williams, S.-Y. Chen, H.-C. Yang, Y.-S. Hsu, Z.-Y. Lin, X.-H. Chen, P.-E. Kuo, L.-Y. Chou, C.-K. Tsung and F.-K. Shieh, Nat. Commun., 2019, 10, 5002 CrossRef PubMed.
- B. Li, Y. Zhang, D. Ma, L. Li, G. Li, G. Li, Z. Shi and S. Feng, Chem. Commun., 2012, 48, 6151 RSC.
- Q. Kong, H. Zhang, P. Wang, Y. Lan, W. Ma and X. Shi, Int. J. Biol. Macromol., 2023, 244, 125169 CrossRef CAS PubMed.
- C. Niu, N. Zhang, C. Hu, C. Zhang, H. Zhang and Y. Xing, Carbohydr. Polym., 2021, 258, 117644 CrossRef CAS PubMed.
- H. Nasser Abdelhamid, S. Sultan and A. P. Mathew, Chem. Eng. J., 2023, 468, 143567 CrossRef CAS.
- N. M. Mahmoodi, M. Oveisi, A. Taghizadeh and M. Taghizadeh, Carbohydr. Polym., 2020, 227, 115364 CrossRef CAS PubMed.
- L. Wen, X. Chen, C. Chen, R. Yang, M. Gong, Y. Zhang and Q. Fu, Arabian J. Chem., 2020, 13, 5669–5678 CrossRef CAS.
- A. M. Omer, E. M. Abd El-Monaem, M. M. Abd El-Latif, G. M. El-Subruiti and A. S. Eltaweil, Carbohydr. Polym., 2021, 265, 118084 CrossRef CAS PubMed.
- C. Wang, Q. Sun, L. Zhang, T. Su and Y. Yang, J. Environ. Chem. Eng., 2022, 10, 107911 CrossRef CAS.
- E. Bahmani, S. Koushkbaghi, M. Darabi, A. ZabihiSahebi, A. Askari and M. Irani, Carbohydr. Polym., 2019, 224, 115148 CrossRef CAS PubMed.
- N. Xiong, P. Wan, G. Zhu, F. Xie, S. Xu, C. Zhu and A. S. Hursthouse, Sep. Purif. Technol., 2020, 236, 116266 CrossRef CAS.
- X. Guo, H. Yang, Q. Liu, J. Liu, R. Chen, H. Zhang, J. Yu, M. Zhang, R. Li and J. Wang, Chem. Eng. J., 2020, 382, 122850 CrossRef CAS.
- F. M. Valadi, S. Shahsavari, E. Akbarzadeh and M. R. Gholami, Carbohydr. Polym., 2022, 288, 119383 CrossRef CAS PubMed.
- Y. Jin, Y. Li, Q. Du, B. Chen, K. Chen, Y. Zhang, M. Wang, Y. Sun, S. Zhao, Z. Jing, J. Wang, X. Pi and Y. Wang, Microporous Mesoporous Mater., 2023, 348, 112404 CrossRef CAS.
- Y. Wei, R. Zou, Y. Xia, Z. Wang, W. Yang, J. Luo and C. Liu, Mater. Today Chem., 2022, 26, 101091 CrossRef CAS.
- X. Yan and H. Ge, Int. J. Biol. Macromol., 2023, 232, 123329 CrossRef CAS PubMed.
- Y. Ma, D. You, Y. Fang, J. Luo, Q. Pan, Y. Liu, F. Wang and W. Yang, Sep. Purif. Technol., 2022, 294, 121223 CrossRef CAS.
- M. Schelling, M. Kim, E. Otal, M. Aguirre and J. P. Hinestroza, Cellulose, 2020, 27, 6399–6410 CrossRef CAS.
- T. Hashem, A. H. Ibrahim, C. Wöll and M. H. Alkordi, ACS Appl. Nano Mater., 2019, 2, 5804–5808 CrossRef CAS.
- C. Lei, J. Gao, W. Ren, Y. Xie, S. Y. H. Abdalkarim, S. Wang, Q. Ni and J. Yao, Carbohydr. Polym., 2019, 205, 35–41 CrossRef CAS PubMed.
- Y. Gu, Y. Wang, H. Li, W. Qin, H. Zhang, G. Wang, Y. Zhang and H. Zhao, Chem. Eng. J., 2020, 387, 124141 CrossRef CAS.
- Q. Liu, H. Yu, F. Zeng, X. Li, J. Sun, C. Li, H. Lin and Z. Su, Carbohydr. Polym., 2021, 255, 117402 CrossRef CAS PubMed.
- X. Li, L. Wang, S. Li, S. Yu, Z. Liu, Q. Liu and X. Dong, Int. J. Biol. Macromol., 2024, 274, 133381 CrossRef CAS PubMed.
- R. M. Abdelhameed, H. Abdel-Gawad and H. E. Emam, J. Environ. Chem. Eng., 2021, 9, 105121 CrossRef CAS.
- Z. Yuan, F. Li, X. Zhang, M.-C. Li, Y. Chen, C. F. D. Hoop, J. Qi and X. Huang, Environ. Res., 2024, 248, 118263 CrossRef CAS PubMed.
- Y. Lin, Q. Wang, Y. Huang, J. Du, Y. Cheng, J. Lu, Y. Tao and H. Wang, Int. J. Biol. Macromol., 2023, 247, 125559 CrossRef CAS PubMed.
- S. Jiang, Z. Yan, Y. Deng, W. Deng, H. Xiao and W. Wu, Int. J. Biol. Macromol., 2024, 262, 129854 CrossRef CAS PubMed.
- A. Liu, J. Liu, S. He, J. Zhang and W. Shao, Int. J. Biol. Macromol., 2023, 225, 40–50 CrossRef CAS PubMed.
- S. Abbasi, Sci. Rep., 2024, 14, 14497 CrossRef CAS PubMed.
- S. V. Kamath, J. R. Attokkaran, A. S. Maraddi, A. Samage, G. B. D'Souza, H. Yoon and S. K. Nataraj, Chem. Eng. J., 2024, 479, 147805 CrossRef CAS.
- H. Yang, P. Zhang, Q. Zheng, M. U. Hameed and S. Raza, Int. J. Biol. Macromol., 2023, 253, 126986 CrossRef CAS PubMed.
- A. Ehsani, H. Aghdasinia, M. E. Farshchi, S. Rostamnia and A. Khataee, Surf. Interfaces, 2023, 36, 102506 CrossRef CAS.
- W. Qu, Z. Wang, M. Qin, X. Yang, F. Zhang, Z. Wang, D. Ji and D. Yu, Sep. Purif. Technol., 2023, 325, 124673 CrossRef CAS.
- J. Zhao, J. He, L. Liu, S. Shi, H. Guo, L. Xie, X. Chai, K. Xu, G. Du and L. Zhang, Sep. Purif. Technol., 2023, 327, 124942 CrossRef CAS.
- A. M. Munshi, N. A. Alamrani, H. Alessa, M. Aljohani, S. F. Ibarhiam, F. A. Saad, S. D. Al-Qahtani and N. M. El-Metwaly, Cellulose, 2023, 30, 7235–7250 CrossRef CAS.
- F. Aghaei, S. Tangestaninejad, M. Bahadori, M. Moghadam, V. Mirkhani, I. Mohammadpoor−Baltork, M. Khalaji and V. Asadi, J. Colloid Interface Sci., 2023, 648, 78–89 CrossRef CAS PubMed.
- H. Zhao, J. Sun, Y. Du, M. Zhang, Z. Yang, J. Su, X. Peng, X. Liu, G. Sun and Y. Cui, J. Solid State Chem., 2023, 322, 123928 CrossRef CAS.
- Z. Qiu, F. Gao, Y. Zhang, J. Li, Y. You, X. Lv and J. Dang, Sep. Purif. Technol., 2024, 338, 126478 CrossRef CAS.
- Y. Hu, Y. Zhou, Z. Yang, F. Ren, Z. Chen, Y. Fu, R. Dong, L. Wang, Q. Zhou and H. Qin, J. Mater. Sci., 2022, 57, 1536–1544 CrossRef CAS.
- C. Wang, C. Xing, X. Feng, S. Shang, H. Liu, Z. Song and H. Zhang, Int. J. Biol. Macromol., 2023, 250, 126092 CrossRef CAS PubMed.
- C. Wang, X. Feng, Y. Tian, X. Huang, S. Shang, H. Liu, Z. Song and H. Zhang, Environ. Res., 2024, 251, 118651 CrossRef CAS PubMed.
- C. Wang, X. Feng, S. Shang, H. Liu, Z. Song and H. Zhang, Bioresour. Technol., 2023, 388, 129781 CrossRef CAS PubMed.
- L. Zhang, Y. Huang, J. Zhang, E. Zhu, J. Ma and Z. Wang, Int. J. Biol. Macromol., 2024, 255, 128187 CrossRef CAS PubMed.
- H.-T. T. Nguyen, K.-N. T. Tran, L. Van Tan, V. A. Tran, V.-D. Doan, T. Lee and T. D. Nguyen, Mater. Chem. Phys., 2021, 272, 125040 CrossRef CAS.
- Z. Zhao, H. Li, K. Zhao, L. Wang and X. Gao, Chem. Eng. J., 2022, 428, 131006 CrossRef CAS.
- R. Vakili, S. Xu, N. Al-Janabi, P. Gorgojo, S. M. Holmes and X. Fan, Microporous Mesoporous Mater., 2018, 260, 45–53 CrossRef CAS.
- N. Couzon, M. Ferreira, S. Duval, A. El-Achari, C. Campagne, T. Loiseau and C. Volkringer, ACS Appl. Mater. Interfaces, 2022, 14, 21497–21508 CrossRef CAS PubMed.
- G. Kaur, A. Anthwal, P. Kandwal and D. Sud, Inorg. Chim. Acta, 2023, 545, 121248 CrossRef CAS.
- P. A. Julien, K. Užarević, A. D. Katsenis, S. A. J. Kimber, T. Wang, O. K. Farha, Y. Zhang, J. Casaban, L. S. Germann, M. Etter, R. E. Dinnebier, S. L. James, I. Halasz and T. Friščić, J. Am. Chem. Soc., 2016, 138, 2929–2932 CrossRef CAS PubMed.
- S. A. Noorian, N. Hemmatinejad and J. A. R. Navarro, Microporous Mesoporous Mater., 2020, 302, 110199 CrossRef CAS.
- E. Laurila, J. Thunberg, S. P. Argent, N. R. Champness, S. Zacharias, G. Westman and L. Öhrström, Adv. Eng. Mater., 2015, 17, 1282–1286 CrossRef CAS.
- X. Li, L. Wang, S. Li, S. Yu, Z. Liu, Q. Liu and X. Dong, Int. J. Biol. Macromol., 2024, 274, 133381 CrossRef CAS PubMed.
- E. Bahmani, H. Seyyed Zonouzi, S. Koushkbaghi, F. Keyvani Hafshejani, A. Fassadi Chimeh and M. Irani, Cellulose, 2020, 27, 10029–10045 CrossRef CAS.
- R. M. Abdelhameed, M. El-Shahat and H. E. Emam, Carbohydr. Polym., 2020, 247, 116695 CrossRef CAS PubMed.
- M. Yousefian and Z. Rafiee, Carbohydr. Polym., 2020, 228, 115393 CrossRef PubMed.
- X. Shen, C. Zhang, B. Han and F. Wang, Chem. Soc. Rev., 2022, 51, 1608–1628 RSC.
- Y. Wang, Z. Tang, M. Chen, J. Zhang, J. Shi, C. Wang, Z. Yang and J. Wang, Energy Convers. Manage., 2020, 222, 113227 CrossRef CAS.
- H. Hamedi, S. Javanbakht and R. Mohammadi, J. Ind. Eng. Chem., 2024, 133, 454–463 CrossRef CAS.
- Y. Hassanpouraghdam, M. Pooresmaeil and H. Namazi, Int. J. Biol. Macromol., 2022, 221, 256–267 CrossRef CAS PubMed.
- M. Kim, L. K. Njaramba, Y. Yoon, M. Jang and C. M. Park, Carbohydr. Polym., 2024, 324, 121436 CrossRef CAS PubMed.
- S. Hu, T. Huang, N. Zhang, Y. Lei and Y. Wang, Carbohydr. Polym., 2022, 277, 118809 CrossRef CAS PubMed.
- Y. Zeng, J. Yuan, Z. Ran, X. Zhan, X. Li, H. Ye, J. Dong, G. Cao, Z. Pan, Y. Bao, J. Tang, X. Liu and Y. He, Int. J. Biol. Macromol., 2024, 263, 130368 CrossRef CAS PubMed.
- Z. Ansari-Asl, Z. Shahvali, R. Sacourbaravi, E. Hoveizi and E. Darabpour, Microporous Mesoporous Mater., 2022, 336, 111866 CrossRef CAS.
- R. Li, D. Huang, L. Lei, S. Chen, Y. Chen, G. Wang, L. Du, W. Zhou, J. Tao and H. Chen, J. Mater. Chem. A, 2023, 11, 2595–2617 RSC.
- P. Shi, X. Hu and M. Duan, J. Cleaner Prod., 2021, 290, 125794 CrossRef CAS.
- H. M. Nassef, G. A. A. M. Al-Hazmi, A. A. Alayyafi, M. G. El-Desouky and A. A. El-Bindary, J. Mol. Liq., 2024, 394, 123741 CrossRef CAS.
- N. A. Al-Dhabi, G. A. Esmail and M. Valan Arasu, J. Cleaner Prod., 2020, 277, 124252 CrossRef CAS.
- E. Y. Kayhan, A. Yildirim, M. B. Kocer, A. Uysal and M. Yilmaz, Int. J. Biol. Macromol., 2023, 240, 124426 CrossRef CAS PubMed.
- H. Djahaniani, N. Ghavidel and H. Kazemian, Int. J. Biol. Macromol., 2023, 242, 124627 CrossRef CAS PubMed.
- Z. Hu, S. Li, S. Wang, B. Zhang and Q. Huang, Food Chem., 2021, 338, 127839 CrossRef CAS PubMed.
- H. Wang, X. Qian, X. An and Z. Chang, Int. J. Biol. Macromol., 2024, 270, 132151 CrossRef CAS PubMed.
- Z. Huang, C. Xiong, L. Ying, W. Wang, S. Wang, J. Ding and J. Lu, Chem. Eng. J., 2022, 449, 137722 CrossRef CAS.
- A. K. Mohamed and M. E. Mahmoud, Carbohydr. Polym., 2020, 245, 116438 CrossRef CAS PubMed.
- S. Kumar, S. Jain, M. Nehra, N. Dilbaghi, G. Marrazza and K.-H. Kim, Coord. Chem. Rev., 2020, 420, 213407 CrossRef CAS.
- Z. Wang, T. Liu, M. Asif, Y. Yu, W. Wang, H. Wang, F. Xiao and H. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 27936–27946 CrossRef CAS PubMed.
- H. M. Nassef, G. A. A. M. Al-Hazmi, A. A. Alayyafi, M. G. El-Desouky and A. A. El-Bindary, J. Mol. Liq., 2024, 394, 123741 CrossRef CAS.
- Q. Wei, S. Xue, W. Wu, S. Liu, S. Li, C. Zhang and S. Jiang, Chem. Rec., 2023, 23, e202200263 CrossRef CAS PubMed.
- J. Liu, J. Liang, J. Xue and K. Liang, Small, 2021, 17, 2100300 CrossRef CAS PubMed.
- C. Doonan, R. Riccò, K. Liang, D. Bradshaw and P. Falcaro, Acc. Chem. Res., 2017, 50, 1423–1432 CrossRef CAS PubMed.
- Y. Pan, H. Li, J. Farmakes, F. Xiao, B. Chen, S. Ma and Z. Yang, J. Am. Chem. Soc., 2018, 140, 16032–16036 CrossRef CAS PubMed.
- R. Anderson, J. Rodgers, E. Argueta, A. Biong and D. A. Gómez-Gualdrón, Chem. Mater., 2018, 30, 6325–6337 CrossRef CAS.
- H. S. Jhinjer, A. Singh, S. Bhattacharya, M. Jassal and A. K. Agrawal, J. Hazard. Mater., 2021, 411, 125056 CrossRef CAS PubMed.
- Y. Li, R. Wang, X. Liu, K. Li and Q. Xu, Nanotechnology, 2023, 34, 202002 CrossRef CAS PubMed.
- P. Pachfule, Y. Chen, J. Jiang and R. Banerjee, J. Mater. Chem., 2011, 21, 17737 RSC.
- C. Wang, C. Xing, X. Feng, S. Shang, H. Liu, Z. Song and H. Zhang, Int. J. Biol. Macromol., 2023, 250, 126092 CrossRef CAS PubMed.
- C. Wang, X. Feng, Y. Tian, X. Huang, S. Shang, H. Liu, Z. Song and H. Zhang, Environ. Res., 2024, 251, 118651 CrossRef CAS PubMed.
- P.-H. Tong, L. Zhu, Y. Zang, J. Li, X.-P. He and T. D. James, Chem. Commun., 2021, 57, 12098–12110 RSC.
- S. Tarasi, A. Ramazani, A. Morsali, M.-L. Hu, S. Ghafghazi, R. Tarasi and Y. Ahmadi, Inorg. Chem., 2022, 61, 13125–13132 CrossRef CAS PubMed.
- C. Hou, L. Fu, Y. Wang, W. Chen, F. Chen, S. Zhang and J. Wang, Carbohydr. Polym., 2021, 273, 118548 CrossRef CAS PubMed.
- J. Zhou, M. Song, X. Hu, W. Zhang and Z. Deng, Crit. Rev. Environ. Sci. Technol., 2023, 53, 1586–1612 CrossRef CAS.
- Y. Zhang, S. Ni, X. Wang, W. Zhang, L. Lagerquist, M. Qin, S. Willför, C. Xu and P. Fatehi, Chem. Eng. J., 2019, 372, 82–91 CrossRef CAS.
- A. Spieß, J. Wiebe, E. Iwaschko, D. Woschko and C. Janiak, Mol. Syst. Des. Eng., 2022, 7, 1682–1696 RSC.
- X.-F. Zhang, Z. Wang, L. Song and J. Yao, Sep. Purif. Technol., 2021, 266, 118527 CrossRef CAS.
- J. Su, H. Xia, F. Ge, X. Yang, M. Zhou and J. Jiang, Fuel Process. Technol., 2023, 247, 107764 CrossRef CAS.
- S. Perveen, R. Zhai, X. Chen, T. Kanwal, M. R. Shah, M. Lu, B. Ding and M. Jin, Int. J. Biol. Macromol., 2024, 274, 133339 CrossRef CAS PubMed.
- H. Pang, Y. Wu, Q. Tao, Y. Xiao, W. Ji, L. Li and H. Wang, Int. J. Biol. Macromol., 2024, 263, 130523 CrossRef CAS PubMed.
- Q. Zhang, G. Kuang, H. Wang, Y. Zhao, J. Wei and L. Shang, Adv. Sci., 2023, 10, 2303818 CrossRef CAS PubMed.
- D. Lv, W. He, W. Liu, Y. Cheng, Y. Cui, X. Zhou, Y. Xue, S. Yu, N. Zhang, H. Meng, Y. Guan, J.-H. Sun and X.-M. Shi, Biomacromolecules, 2024, 25(7), 4449–4468 CrossRef CAS PubMed.
- L. Chen, W. Huang, M. Hao, F. Yang, H. Shen, S. Yu and L. Wang, Int. J. Biol. Macromol., 2023, 242, 124881 CrossRef CAS PubMed.
- M. Pooresmaeil, Y. Hassanpouraghdam and H. Namazi, Eur. Polym. J., 2023, 184, 111802 CrossRef CAS.
- R. Abazari, N. Ghorbani, J. Shariati, R. S. Varma and J. Qian, Inorg. Chem., 2024, 63, 12667–12680 CrossRef CAS PubMed.
- M. Chen, R. Dong, J. Zhang, H. Tang, Q. Li, H. Shao and X. Jiang, ACS Appl. Mater. Interfaces, 2021, 13, 18554–18562 CrossRef CAS PubMed.
- Y. Pan, H. Li, M. Lenertz, Y. Han, A. Ugrinov, D. Kilin, B. Chen and Z. Yang, Green Chem., 2021, 23, 4466–4476 RSC.
- C. Xu, X. Kong, S. Zhou, B. Zheng, F. Huo and M. Strømme, J. Mater. Chem. A, 2018, 6, 24050–24057 RSC.
- K. Li, J. Yang and J. Gu, Acc. Chem. Res., 2022, 55, 2235–2247 CrossRef CAS PubMed.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469–472 CrossRef CAS PubMed.
- W. Zou, H. Li, M. Liu and Y. Lv, Appl. Catal., A, 2023, 656, 119120 CrossRef CAS.
- W. Gao, Y. Li, X. Zhang, M. Qiao, Y. Ji, J. Zheng, L. Gao, S. Yuan and H. Huang, Nano Lett., 2024, 24, 3404–3412 CrossRef CAS PubMed.
- H. Nabipour, F. Aliakbari, K. Volkening, M. J. Strong and S. Rohani, Int. J. Biol. Macromol., 2024, 259, 128875 CrossRef CAS PubMed.
- Y. Sun, S. Ding, X. Zhao, D. Sun, Y. Yang, M. Chen, C. Zhu, B. Jiang, Q. Gu, H. Liu and M. Zhang, Adv. Mater., 2024, 2401094 CrossRef CAS PubMed.
- X. Zeng, P. Li, S. Yan and B.-F. Liu, Chem. Eng. J., 2023, 452, 139517 CrossRef CAS.
- X. Huang and C. S. Brazel, J. Controlled Release, 2001, 73, 121–136 CrossRef CAS PubMed.
- Q. Sun, H. Bi, Z. Wang, C. Li, X. Wang, J. Xu, H. Zhu, R. Zhao, F. He, S. Gai and P. Yang, Biomaterials, 2019, 223, 119473 CrossRef CAS PubMed.
- F. Xiong, Z. Qin, H. Chen, Q. Lan, Z. Wang, N. Lan, Y. Yang, L. Zheng, J. Zhao and D. Kai, J. Nanobiotechnol., 2020, 18, 139 CrossRef CAS PubMed.
- X. Xu, Y. Chen, Y. Zhang, Y. Yao and P. Ji, J. Mater. Chem. B, 2020, 8, 9129–9138 RSC.
- Y. Xu, S. Wang, J. Xiong, P. Zheng, H. Zhang, S. Chen, Q. Ma, J. Shen, T. Velkov, C. Dai and H. Jiang, Adv. Healthcare Mater., 2024, 13, 2303839 CrossRef CAS PubMed.
- Y. Wang, W. Qi, Z. Mao, J. Wang, R. C. Zhao and H. Chen, Mater. Today Nano, 2023, 23, 100363 CrossRef CAS.
- H. Zheng, M. Zhu, D. Wang, Y. Zhou, X. Sun, S. Jiang, M. Li, C. Xiao, D. Zhang and L. Zhang, Sep. Purif. Technol., 2023, 306, 122599 CrossRef CAS.
- Y. Wang, W. Qi, Z. Mao, J. Wang, R. C. Zhao and H. Chen, Mater. Today Nano, 2023, 23, 100363 CrossRef CAS.
- M. Shyngys, J. Ren, X. Liang, J. Miao, A. Blocki and S. Beyer, Front. Bioeng. Biotechnol., 2021, 9, 603608 CrossRef PubMed.
- C. Liu, X. Xu, W. Cui and H. Zhang, Eng. Regener., 2021, 2, 105–108 Search PubMed.
- M. D. Telgerd, M. Sadeghinia, G. Birhanu, M. P. Daryasari, A. Zandi-Karimi, A. Sadeghinia, H. Akbarijavar, M. H. Karami and E. Seyedjafari, J. Biomed. Mater. Res., Part A, 2019, 107, 1841–1848 CrossRef CAS PubMed.
- M. Barjasteh, S. M. Dehnavi, S. Ahmadi Seyedkhani, S. Y. Rahnamaee and M. Golizadeh, Surf. Interfaces, 2023, 36, 102631 CrossRef CAS.
- W. Zhu, M. Han, D. Kim, Y. Zhang, G. Kwon, J. You, C. Jia and J. Kim, Environ. Res., 2022, 205, 112417 CrossRef CAS PubMed.
- Q. Miao, L. Jiang, J. Yang, T. Hu, S. Shan, H. Su and F. Wu, J. Water Process Eng., 2022, 50, 103348 CrossRef.
- M. Khajavian, E. Salehi and V. Vatanpour, Carbohydr. Polym., 2020, 247, 116693 CrossRef CAS PubMed.
- M. Jamali and A. Akbari, J. Environ. Chem. Eng., 2021, 9, 105175 CrossRef CAS.
- J. Jiang, Y. Shi, M. Wu, M. Rezakazemi, T. M. Aminabhavi, R. Huang, C. Jia and S. Ge, Chem. Eng. J., 2024, 494, 152932 CrossRef CAS.
- X.-F. Zhang, Z. Wang, L. Song and J. Yao, Sep. Purif. Technol., 2021, 266, 118527 CrossRef CAS.
- Z. Wang, F. Yin, X.-F. Zhang, T. Zheng and J. Yao, Sep. Purif. Technol., 2022, 293, 121095 CrossRef CAS.
- S. Ma, M. Zhang, J. Nie, J. Tan, B. Yang and S. Song, Carbohydr. Polym., 2019, 203, 415–422 CrossRef CAS PubMed.
- S. Ma, M. Zhang, J. Nie, B. Yang, S. Song and P. Lu, Cellulose, 2018, 25, 5999–6010 CrossRef CAS.
- Z. Chen, X. He, J. Ge, G. Fan, L. Zhang, A. M. Parvez and G. Wang, Ind. Crops Prod., 2022, 186, 115269 CrossRef CAS.
- Q. Huang, C. Zhao and X. Li, Cellulose, 2021, 28, 3097–3112 CrossRef CAS.
- Y. Ma, Y. Zhu, Y. Lei, Y. Yang, L. Li, Q. Zhu and W. Zhang, J. Alloys Compd., 2023, 946, 169365 CrossRef CAS.
- R. Du, Y. Wu, Y. Yang, T. Zhai, T. Zhou, Q. Shang, L. Zhu, C. Shang and Z. Guo, Adv. Energy Mater., 2021, 11, 2100154 CrossRef CAS.
- L. Du, B. Zhang, W. Deng, Y. Cheng, L. Xu and L. Mai, Adv. Energy Mater., 2022, 12, 2200501 CrossRef CAS.
- Z. Zhang, M. Liu, C. Li, W. Wenzel and L. Heinke, Small, 2022, 18, 2200602 CrossRef CAS PubMed.
- S. Zhang, J. Luo, F. Zhang, M. Du, H. Hui, F. Zhao, X. He and Z. Sun, J. Membr. Sci., 2022, 652, 120461 CrossRef CAS.
- H. Ma, J. Yu, M. Chen, X. Han, J. Chen, B. Liu and S. Shi, Adv. Funct. Mater., 2023, 33, 2307384 CrossRef CAS.
- H. Zhu, S. Dong, J. Xiong, P. Wan, X. Jin, S. Lu, Y. Zhang and H. Fan, J. Colloid Interface Sci., 2023, 641, 942–949 CrossRef CAS PubMed.
- Y. Yang, S. Ma, M. Xia, Y. Guo, Y. Zhang, L. Liu, C. Zhou, G. Chen, X. Wang, Q. Wu, L. Yang and Z. Hu, Mater. Today Phys., 2023, 35, 101112 CrossRef CAS.
- R. Razaq, M. M. U. Din, D. R. Småbråten, V. Eyupoglu, S. Janakiram, T. O. Sunde, N. Allahgoli, D. Rettenwander and L. Deng, Adv. Energy Mater., 2024, 14, 2302897 CrossRef CAS.
- L. Zhou, H. Pan, G. Yin, Y. Xiang, P. Tan, X. Li, Y. Jiang, M. Xu and X. Zhang, Adv. Funct. Mater., 2024, 34, 2314246 CrossRef CAS.
- X. Ma, Y. Lou, X.-B. Chen, Z. Shi and Y. Xu, Chem. Eng. J., 2019, 356, 227–235 CrossRef CAS.
- R. Davidson, A. Verma, D. Santos, F. Hao, C. Fincher, S. Xiang, J. Van Buskirk, K. Xie, M. Pharr, P. P. Mukherjee and S. Banerjee, ACS Energy Lett., 2019, 4, 375–376 CrossRef CAS.
- Y. Wang, Y. Huang and Y. Fu, Carbohydr. Polym., 2023, 314, 120919 CrossRef CAS PubMed.
- T. Zhao, H. Wu, X. Wen, J. Zhang, H. Tang, Y. Deng, S. Liao and X. Tian, Coord. Chem. Rev., 2022, 468, 214642 CrossRef CAS.
- M. Mao, X. Wu, Y. Hu, Q. Yuan, Y.-B. He and F. Kang, J. Energy Chem., 2021, 52, 277–283 CrossRef CAS.
- X. Pu, B. Jiang, X. Wang, W. Liu, L. Dong, F. Kang and C. Xu, Nano-Micro Lett., 2020, 12, 152 CrossRef CAS PubMed.
- C. Yin, C. Pan, X. Liao, Y. Pan and L. Yuan, ACS Appl. Mater. Interfaces, 2021, 13, 35837–35847 CrossRef CAS PubMed.
- Z. Cao, H. Zhang, B. Song, D. Xiong, S. Tao, W. Deng, J. Hu, H. Hou, G. Zou and X. Ji, Adv. Funct. Mater., 2023, 33, 2300339 CrossRef CAS.
- Y. Wang, Y. Liu, H. Wang, S. Dou, W. Gan, L. Ci, Y. Huang and Q. Yuan, J. Mater. Chem. A, 2022, 10, 4366–4375 RSC.
- X. Liu, F. Yang, W. Xu, Y. Zeng, J. He and X. Lu, Adv. Sci., 2020, 7, 2002173 CrossRef CAS PubMed.
- X. Zeng, J. Zhao, Z. Wan, W. Jiang, M. Ling, L. Yan and C. Liang, J. Phys. Chem. Lett., 2021, 12, 9055–9059 CrossRef CAS PubMed.
- Z. Wang, J. Hu, L. Han, Z. Wang, H. Wang, Q. Zhao, J. Liu and F. Pan, Nano Energy, 2019, 56, 92–99 CrossRef CAS.
- H. Yang, Z. Chang, Y. Qiao, H. Deng, X. Mu, P. He and H. Zhou, Angew. Chem., Int. Ed., 2020, 59, 9377–9381 CrossRef CAS PubMed.
- L. Cao, D. Li, T. Deng, Q. Li and C. Wang, Angew. Chem., Int. Ed., 2020, 59, 19292–19296 CrossRef CAS PubMed.
- C.-Y. Liu, Y.-D. Wang, H. Liu, Q. Chen, X. Jiang, H. Jia and J.-P. Lang, Composites, Part B, 2024, 272, 111227 CrossRef CAS.
- Y. Wang, H. Shaghaleh, Y. A. Hamoud, S. Zhang, P. Li, X. Xu and H. Liu, Int. J. Biol. Macromol., 2021, 187, 262–271 CrossRef CAS PubMed.
- X. Lin, L. Guo, H. Shaghaleh, Y. A. Hamoud, X. Xu and H. Liu, Int. J. Biol. Macromol., 2021, 191, 483–491 CrossRef CAS PubMed.
- Y. Ma, M. Yu, Y. Wang, S. Pan, X. Sun, R. Zhao, Z. Sun, R. Gao, X. Guo, Y. Xu and X. Wu, Chem. Eng. J., 2023, 462, 142190 CrossRef CAS.
- S. Pezhhanfar, M. A. Farajzadeh, S. A. Hosseini-Yazdi and M. R. A. Mogaddam, J. Food Compos. Anal., 2023, 123, 105578 CrossRef CAS.
- W. Meng, Z. Tian, P. Yao, X. Fang, T. Wu, J. Cheng and A. Zou, Colloids Surf., A, 2020, 604, 125266 CrossRef CAS.
- Y. Shan, C. Xu, H. Zhang, H. Chen, M. Bilal, S. Niu, L. Cao and Q. Huang, Nanomaterials, 2020, 10, 2000 CrossRef CAS PubMed.
- S. Song, M. Wan, W. Feng, Y. Tian, X. Jiang, Y. Luo and J. Shen, Langmuir, 2022, 38, 10867–10874 CrossRef CAS PubMed.
- P. Ananthi, K. Hemkumar and A. Pius, ACS Food Sci. Technol., 2024, 4, 1462–1471 CrossRef CAS.
- D.-Y. Zhang, J.-X. Yang, E.-J. Liu, R.-Z. Hu, X.-H. Yao, T. Chen, W.-G. Zhao, L. Liu and Y.-J. Fu, Int. J. Biol. Macromol., 2022, 211, 470–480 CrossRef CAS PubMed.
- J. Wang, S. Zhou, F. Lu, S. Wang and Q. Deng, Food Chem., 2024, 451, 139451 CrossRef CAS PubMed.
- S. Ribes, A. Fuentes, P. Talens and J. M. Barat, Food Chem., 2018, 239, 704–711 CrossRef CAS PubMed.
- A. Khan, Z. Riahi, J. T. Kim and J.-W. Rhim, Food Chem., 2024, 455, 139911 CrossRef CAS PubMed.
- G. Huang, Y. Li, Z. Qin, Q. Liang, C. Xu and B. Lin, Carbohydr. Polym., 2020, 233, 115848 CrossRef CAS PubMed.
- Z. Rui, D. Yu and F. Zhang, Ind. Crops Prod., 2024, 207, 117771 CrossRef CAS.
- M. Tavassoli, A. Khezerlou, M. A. Sani, M. Hashemi, S. Firoozy, A. Ehsani, F. Khodaiyan, S. Adibi, S. M. A. Noori and D. J. McClements, Int. J. Biol. Macromol., 2024, 259, 129182 CrossRef CAS PubMed.
- S. Behboudikhiavi, G. Chanteux, B. Babu, S. Faniel, F. Marlec, K. Robert, D. Magnin, F. Lucaccioni, J. O. Omale, P. Apostol, L. Piraux, C. Lethien and A. Vlad, Small, 2024, 2401509 CrossRef CAS PubMed.
- K. Yuan, K. Tao, T. Song, Y. Zhang, T. Zhang, F. Wang, S. Duan, Z. Chen, L. Li, X. Zhang, D. Zhong, Z. Tang, T.-B. Lu and W. Hu, J. Am. Chem. Soc., 2024, 146, 6893–6904 CrossRef CAS PubMed.
- Q. Zhang, S. Jiang, T. Lv, Y. Peng and H. Pang, Adv. Mater., 2023, 35, 2305532 CrossRef CAS PubMed.
- C. Huang, W. Sun, Y. Jin, Q. Guo, D. Mücke, X. Chu, Z. Liao, N. Chandrasekhar, X. Huang, Y. Lu, G. Chen, M. Wang, J. Liu, G. Zhang, M. Yu, H. Qi, U. Kaiser, G. Xu, X. Feng and R. Dong, Angew. Chem., Int. Ed., 2024, 63, e202313591 CrossRef CAS PubMed.
- C. Yan, J. Wei, J. Guan, Z. Shao and S. Lv, Carbon, 2023, 213, 118187 CrossRef CAS.
- J. Qiao, Q. Song, X. Zhang, S. Zhao, J. Liu, G. Nyström and Z. Zeng, Adv. Sci., 2024, 11, 2400403 CrossRef CAS PubMed.
- Z. Wang, B. Sun, J. Liao, S. Cao, L. Li, Q. Wang and C. Guo, Int. J. Biol. Macromol., 2024, 255, 127989 CrossRef CAS PubMed.
- X. Li, D. Li, Y. Zhang, P. Lv, Q. Feng and Q. Wei, Nano Energy, 2020, 68, 104308 CrossRef CAS.
- F. O. Ongondo, I. D. Williams and T. J. Cherrett, Waste Manage., 2011, 31, 714–730 CrossRef CAS PubMed.
- M. Miao, L. Mu, S. Cao, Y. Yang and X. Feng, Carbohydr. Polym., 2022, 291, 119587 CrossRef CAS PubMed.
- Q. Lu, T. Su, Z. Shang, D. Jin, Y. Shu, Q. Xu and X. Hu, Biosens. Bioelectron., 2021, 184, 113229 CrossRef CAS PubMed.
- Y. Shu, T. Su, Q. Lu, Z. Shang, Q. Xu and X. Hu, Anal. Chem., 2021, 93, 16222–16230 CrossRef CAS PubMed.
- A. Afzalinia and M. Mirzaee, ACS Appl. Mater. Interfaces, 2020, 12, 16076–16087 CrossRef CAS PubMed.
- X. Li, X. Li, D. Li, M. Zhao, H. Wu, B. Shen, P. Liu and S. Ding, Biosens. Bioelectron., 2020, 168, 112554 CrossRef CAS PubMed.
- L. Huang, W. Huang, R. Shen and Q. Shuai, Food Chem., 2020, 330, 127212 CrossRef CAS PubMed.
Footnote |
| † Xian Wang: Joint first author. |
| This journal is © The Royal Society of Chemistry 2025 |
