Identifying the nanostructure of residual Li in high-Ni cathodes for lithium-ion batteries†
Wooyoung
Jin‡
a,
Yujin
Kim‡
b,
Haeseong
Jang‡
cd,
Yehyeon
Gu
b,
Namhyung
Kim
 e,
Hyomyung
Lee
b,
Junhyeok
Kim
b,
Sinho
Choi
a,
Kyu-Nam
Jung
f,
Ki-Hun
Nam
g,
Jaephil
Cho
e,
Hyomyung
Lee
b,
Junhyeok
Kim
b,
Sinho
Choi
a,
Kyu-Nam
Jung
f,
Ki-Hun
Nam
g,
Jaephil
Cho
 *bh and
Hyungyeon
Cha
*bh and
Hyungyeon
Cha
 *a
*a
aUlsan Advanced Energy Technology R&D Center, Korea Institute of Energy Research (KIER), 25 Techno Saneop-ro 55 Beon-gil, Nam-gu, Ulsan 44776, Republic of Korea. E-mail: hcha@kier.re.kr
bDepartment of Energy Engineering, School of Energy and Chemical Engineering, Ulsan National Institute of Science and Technology (UNIST), 50, UNIST-gil, Ulsan 44919, Republic of Korea. E-mail: jpcho@singml.com
cDepartment of Advanced Materials Engineering, Chung-Ang University, 4726, Seodong-daero, Daedeok-myeon, Anseong, Gyeonggi-do 17546, Republic of Korea
dDepartment of Smart City, Chung-Ang University, Seoul 06974, Republic of Korea
eDepartment of Materials System Engineering, Pukyong National University, 45 Yongso-ro, Nam-gu, Busan, 48513, Republic of Korea
fRenewable Energy Institute, Korea Institute of Energy Research (KIER), 152 Gajeong-ro, Yuseong-gu, Daejeon 34129, Republic of Korea
gBattery Research Division, Korea Electrotechnology Research Institute (KERI), 12, Jeongiui-gil, Seongsan-gu, Changwon-si, Gyeongsangnam-do 51543, Republic of Korea
hSMLab, 27, Gacheongongdan 1-gil, Samnam-eup, Ulju-gun, Ulsan 44953, Republic of Korea
First published on 12th December 2024
Abstract
With the increasing demand for higher energy density in lithium-ion batteries (LIBs), designing high-Ni cathodes with maximized Ni content is becoming essential. This pursuit leads to an increase in surface residual Li compounds (Li2CO3 and LiOH), triggering notorious issues such as severe side reactions, gas evolution, and the necessity for additional manufacturing processes. However, an understanding of the residual Li chemistry is still lacking. In this study, the presence of residual Li compounds in both the primary and secondary particle levels in conventional polycrystalline high-Ni cathodes was investigated. Residual Li compounds exist in a crystalline phase not only on the surfaces of secondary particles, but also within the intergranular pores between the primary particles. The identification of residual Li implies that designing and controlling the intergranular pores in high-Ni cathodes is necessary. Finally, post-treatment strategies aimed at controlling residual Li compounds in high-Ni cathodes are proposed and the characteristics of each strategy are described.
Introduction
In the global energy field, a shift from internal combustion engines to electrified systems is occurring, promoting environmentally friendly and sustainable transportation. By 2022, worldwide electric vehicle (EV) sales exceeded 10 million, with projections indicating an estimated 45 million sales by 2030, representing approximately 35% of the total automobile sales for that year.1 The increasing demands for EVs have led to wide consumer acceptance, making driving range improvement a crucial factor for their widespread adoption. Ni-rich layered oxides (LiNixCoyMnzO2 and LiNixCoyAlzO2, x + y + z = 1) have been considered as the most feasible materials for improving the energy density and enhancing driving mileage.2,3Over the last decades, increasing the Ni content has been regarded in both academia and industry as feasible method of improving the cell energy density, as doing so can increase the specific capacity by 10–30% compared to when conventional LiNi0.6Co0.2Mn0.2O2 is used.4 However, deleterious phenomena such as anisotropic volume changes with micro-crack formation5,6 and irreversible phase transformation7 accelerate the capacity fading of Ni-rich cathodes. In addition to structural problems, interfacial issues derived from residual Li compounds are exacerbated, severely deteriorating the battery performance.8 Increasing the Ni content enhances charge transferability, rapidly increasing the formation of residual Li compounds on the surface layer. These compounds, such as LiOH, LiHCO3, and Li2CO3, form through reactions with the ambient atmosphere when unstable Li2O is generated on the surface during synthesis9,10 or through the spontaneous reduction of unstable trivalent Ni ions to divalent Ni ions.11
The interfacial residual Li layer possesses insulating properties, which can cause reaction heterogeneity by inhibiting the electronic and ionic connections between the particles.10,12 Additionally, as residual Li accumulates, the formation of transition metal compounds leads to the loss of redox centers.13 Furthermore, excessive accumulation causes imbalances within the electrode. From a cell perspective, the side reactions between Li2CO3 and LiPF6 cause gas evolution and HF formation, which cause battery safety concerns.14,15 In addition, alkaline environment induced by LiOH triggers polyvinylidene fluoride binder dehydrofluorination,16 resulting in slurry gelation,17 which adversely affects industrial manufacturing processes. Hence, residual Li compounds critically impact the overall battery system. To mitigate the negative effects of residual Li compounds, various methods have been employed, including commonly used washing processes,18 surface stabilization with gas-phase reaction,17 and surface reconstruction with coating materials.2,19,20 However, compared to studies on mitigating residual Li compounds, in-depth research on the characteristics of residual Li is lacking. To the best of our knowledge, the direct observation of residual Li in high-Ni cathodes has rarely been discussed.
In this study, we comprehensively analyzed the residual Li in polycrystalline LiNi0.8Co0.1Mn0.1O2 by investigating the presence of residual Li compounds at the nanoscale and examining the particle interfaces in detail before and after their removal. We discovered that residual Li compounds were present on the surface layers of the secondary particles and in the intergranular pores between the primary particles within the secondary particles. Specifically, Li2CO3 was observed in a crystalline state within these intergranular pores. Building on this understanding, we devised several strategies for effectively removing residual Li from high Ni-rich cathodes. We also identified various solutions, from standard washing to advanced treatments such as surface reconstruction and grain boundary minimization, and assessed their characteristics and limitations. Our research contributes to the fundamental understanding of residual Li compounds and offers insights that will facilitate the design of advanced high-Ni cathodes for use in practical applications.
Experimental details
Material preparation
Spherical Ni0.8Co0.1Mn0.1(OH)2 was synthesized via conventional co-precipitation. Nickel(II) sulfate hexahydrate (NiSO4·6H2O), cobalt(II) sulfate heptahydrate (CoSO4·7H2O), and manganese(II) sulfate (MnSO4·4H2O) were used as transition metal sources in a molar ratio of 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, at a concentration of 2 M. Ammonia (NH4OH) and sodium hydroxide (NaOH) solutions were prepared at 0.4 M and 4 M, respectively. The transition metal solution and the solution containing NaOH (300 mL h−1) and NH4OH (30 mL h−1) were pumped into a 5 L reaction under a N2 atmosphere at 50 °C. The pH was set to 11.3, and the reaction proceeded for 19 h. The final precursor powder was obtained through washing, filtering, and drying at 120 °C for 12 h. The dried precursor was mixed with lithium hydroxide (LiOH·H2O) in a molar ratio of 1
0.1, at a concentration of 2 M. Ammonia (NH4OH) and sodium hydroxide (NaOH) solutions were prepared at 0.4 M and 4 M, respectively. The transition metal solution and the solution containing NaOH (300 mL h−1) and NH4OH (30 mL h−1) were pumped into a 5 L reaction under a N2 atmosphere at 50 °C. The pH was set to 11.3, and the reaction proceeded for 19 h. The final precursor powder was obtained through washing, filtering, and drying at 120 °C for 12 h. The dried precursor was mixed with lithium hydroxide (LiOH·H2O) in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.01 and calcined under an O2 atmosphere at 820 °C for 10 h, designated as bare NCM. To produce the washed NCM, NCM powder was stirred in deionized water at a mass ratio of 1
1.01 and calcined under an O2 atmosphere at 820 °C for 10 h, designated as bare NCM. To produce the washed NCM, NCM powder was stirred in deionized water at a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 5 min, followed by drying at 120 °C in a vacuum oven for 6 h. Synthesize LiNi0.88Co0.1Al0.02O2, conventional co-precipitation process was carried out using nickel(II) sulfate hexahydrate (NiSO4·6H2O), cobalt(II) sulfate heptahydrate (CoSO4·7H2O), and manganese(II) sulfate (MnSO4·H2O) with a molar ratio of 0.88
1 for 5 min, followed by drying at 120 °C in a vacuum oven for 6 h. Synthesize LiNi0.88Co0.1Al0.02O2, conventional co-precipitation process was carried out using nickel(II) sulfate hexahydrate (NiSO4·6H2O), cobalt(II) sulfate heptahydrate (CoSO4·7H2O), and manganese(II) sulfate (MnSO4·H2O) with a molar ratio of 0.88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02. Prepared Ni0.88Co0.1Al0.02(OH)2 precursor and LiOH·H2O was mixed with a molar ratio of 1
0.02. Prepared Ni0.88Co0.1Al0.02(OH)2 precursor and LiOH·H2O was mixed with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.01 and annealed at 780 °C for 18 h in oxygen blowing condition. Artificial graphite (BTR) was prepared as active material for the anode.
1.01 and annealed at 780 °C for 18 h in oxygen blowing condition. Artificial graphite (BTR) was prepared as active material for the anode.
Characterization
Residual Li compounds were quantified using a potentiometric titrator (888 Titrando, Metrohm). First, 5 g of NCM powder was stirred in 50 mL of deionized water for 15 min. The filtered solution was titrated with 0.1 M hydrochloric acid. X-ray diffraction (XRD) patterns were obtained by using a Rigaku D/MAX 2500V/PC diffractometer with Cu Kα radiation. Morphological observations of the powder and electrodes were conducted by performing SEM (Verios 460, FEI). Cross-sectioned electrodes were prepared using an Ar-ion milling system (Model 1040 Nanomill, Fischione). Structural and chemical analyses of the NCM powders were performed with a high-resolution transmission electron microscope (HR-TEM, ARM300, JEOL), operating at 300 kV. Li K-edge spectra were collected by conducting EELS (Quantum 965, Gatan). The HR-TEM and EELS samples were prepared using a focused ion beam (FIB, Helios 450 HP, FEI). The chemical compositions of the NCM powders were analyzed by performing ICP-OES (OPTIMA 8300, PerkinElmer). The particle size distribution information was collected using the Fraunhofer approximation with a laser diffraction particle size analyzer (CILAS 1090). The BET surface area was measured using a N2 adsorption–desorption analyzer (TriStar II 3020, Micromeritics). The pore size distribution was estimated from adsorption data using the Barrett–Joyner–Halenda model. Before measurement, the samples were dried at 120 °C for 2 h.Electrochemical characterization
The NCM powders were mixed with carbon black and poly(vinylidene fluoride) binder in a weight ratio of 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 in N-methyl-2-pyrrolidinone (NMP). This slurry was coated onto an Al current collector, with a loading level of ∼11 mg cm−2 and a loading density of 3.1 g cm−3. Next, 2032R coin-type half-cells were assembled in an Ar-filled glove box, using Li metal as a counter electrode. The electrolyte for the half-cell was 1 M LiPF6 in ethylene carbonate, ethyl methyl carbonate, and diethyl carbonate in a volume ratio of 30
3 in N-methyl-2-pyrrolidinone (NMP). This slurry was coated onto an Al current collector, with a loading level of ∼11 mg cm−2 and a loading density of 3.1 g cm−3. Next, 2032R coin-type half-cells were assembled in an Ar-filled glove box, using Li metal as a counter electrode. The electrolyte for the half-cell was 1 M LiPF6 in ethylene carbonate, ethyl methyl carbonate, and diethyl carbonate in a volume ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (Panax Etec). A polypropylene/polyethylene/polypropylene separator was used (Celgard 2320). All cathode tests were conducted using a constant current (CC)–constant voltage (CV) mode within a voltage range of 3.0–4.3 V. For the formation cycles, two cycles were repeated with a 0.1C rate. Subsequent electrochemical cycling tests were conducted at a charge rate of 0.5C and discharge rate of 1C. For GITT analysis, a constant current of 0.1C was applied for 10 min, followed by a rest period of 10 s. In terms of full-cell design, the N/P ratio was 1.15. A graphite anode was additionally fabricated as a counter electrode with Super P, and carboxymethyl cellulose, and styrene butadiene rubber in a weight ratio of 96
30 (Panax Etec). A polypropylene/polyethylene/polypropylene separator was used (Celgard 2320). All cathode tests were conducted using a constant current (CC)–constant voltage (CV) mode within a voltage range of 3.0–4.3 V. For the formation cycles, two cycles were repeated with a 0.1C rate. Subsequent electrochemical cycling tests were conducted at a charge rate of 0.5C and discharge rate of 1C. For GITT analysis, a constant current of 0.1C was applied for 10 min, followed by a rest period of 10 s. In terms of full-cell design, the N/P ratio was 1.15. A graphite anode was additionally fabricated as a counter electrode with Super P, and carboxymethyl cellulose, and styrene butadiene rubber in a weight ratio of 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5. The loading level was 6.4 mg cm−2 and loading density was 1.4 g cm−3. The full cells were assembled in a pouch type configuration and tested in the voltage range of 3.0–4.2 V. Then, measurements of 1.3 M LiPF6 in ethylene, ethyl methyl carbonate, and diethyl carbonate in a volume ratio of 30
1.5. The loading level was 6.4 mg cm−2 and loading density was 1.4 g cm−3. The full cells were assembled in a pouch type configuration and tested in the voltage range of 3.0–4.2 V. Then, measurements of 1.3 M LiPF6 in ethylene, ethyl methyl carbonate, and diethyl carbonate in a volume ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 with 10% fluoroethylene carbonate, 0.5% vinylene carbonate, and 0.2% lithium tetrafluoroborate (Panax Etec). EIS (VSP-300, BioLogic) was performed in a frequency range of 1000 kHz to 10 mHz with an amplitude of 10 mV in a fully charged cell.
20 with 10% fluoroethylene carbonate, 0.5% vinylene carbonate, and 0.2% lithium tetrafluoroborate (Panax Etec). EIS (VSP-300, BioLogic) was performed in a frequency range of 1000 kHz to 10 mHz with an amplitude of 10 mV in a fully charged cell.
Results and discussion
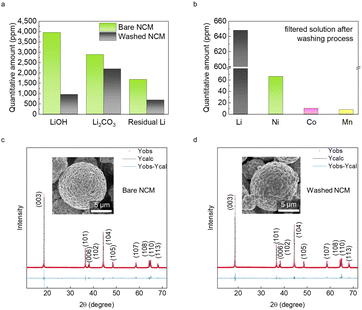
A pristine bi-modal LiNi0.8Co0.1Mn0.1O2 (NCM) cathode material was successfully synthesized using a conventional co-precipitation technique, yielding ∼300 g powder with a distribution of large particle size of ∼10 μm and small particle size of ∼1 μm. The scanning electron microscopy (SEM) images in Fig. S1† show the bare NCM. To investigate the quantitative and qualitative properties of the residual Li compounds, we added a washing process, a technique commonly used to eliminate residual Li compounds. To obtain washed NCM, bare NCM powder was stirred with deionized water in a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 5 min, followed by 6 h of vacuum drying at 120 °C. The amounts of the residual Li compounds, especially LiOH and Li2CO3, were measured using the titration method. The total amount of residual Li was calculated using the following equation:
1 for 5 min, followed by 6 h of vacuum drying at 120 °C. The amounts of the residual Li compounds, especially LiOH and Li2CO3, were measured using the titration method. The total amount of residual Li was calculated using the following equation:| Total residual Li (ppm) = (2 × atomic weight of Li)/(molecular weight of Li2CO3) × Li2CO3 (ppm) + (atomic weight of Li)/(molecular weight of LiOH) × LiOH (ppm). |
Fig. 1a shows a considerable reduction in the residual Li content of washed NCM compared with that of bare NCM. The decrease in LiOH is much greater than that in Li2CO3 because of the higher solubility of LiOH. To eliminate the effect of drying step after washing, we also measured the residual Li content of dried NCM. Although there has been tiny change of the compositions, we speculate that drying process does not significantly change the total amount of residual Li contents (Table S1†). The reduction in residual Li content in the powder was also confirmed by inductively coupled plasma optical emission spectrometry (ICP-OES) (Table S2†). Interestingly, the filtered solution after washing contained transition metals originating from the Ni-rich cathode particles. Because of the Ni-rich phase of NCM, Ni leaching is dominant (66 ppm), whereas Co (10 ppm) and Mn (8 ppm) leaching is negligible (Fig. 1b). Close observation of the particle surface to investigate the morphological changes revealed no noticeable changes in morphology upon washing (insets in Fig. 1c and d). However, this unnecessary leaching of transition metals could give rise to structure transformation of NCM. Hence, we conducted Rietveld refinement of X-ray diffraction analysis with bare and washed NCM. An α-NaFeO2 structure with an R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group is clearly observed in both bare NCM and washed NCM (Fig. 1c and d). However, the degree of cation mixing in washed NCM (3.7%) is twice as high as that in NCM (1.9%), indicating reduced divalent Ni ions migrating to Li slabs after washing (Table S3†). These results suggest that the localized nanostructure at the surface collapses, whereas the bulk structure is well-preserved after washing.
m space group is clearly observed in both bare NCM and washed NCM (Fig. 1c and d). However, the degree of cation mixing in washed NCM (3.7%) is twice as high as that in NCM (1.9%), indicating reduced divalent Ni ions migrating to Li slabs after washing (Table S3†). These results suggest that the localized nanostructure at the surface collapses, whereas the bulk structure is well-preserved after washing.
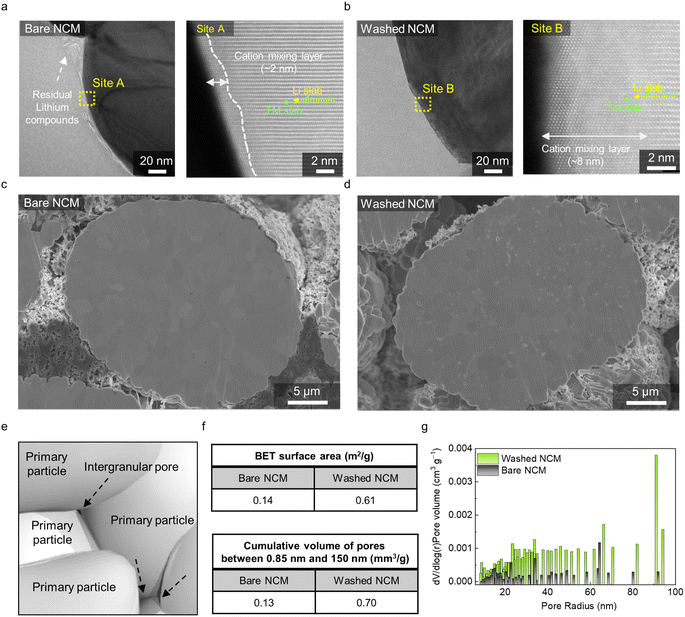
To investigate the nanostructural changes depending on the washing process, we performed high-resolution transmission electron microscopy (HR-TEM). Bare and washed NCM particles were thinned through using a focused ion beam. Low-magnification images are provided in Fig. S2.† In the high-magnification images of the bare NCM, nanoscale residual Li compounds are observed at the surface (Fig. 2a). Beneath the residual Li layer, a very thin cation mixing layer of ∼2 nm and a well-ordered layered structure are observed in the particles, demonstrating the conventional structure of the high-Ni cathode material.21 In contrast, the washed NCM (Fig. 2b) exhibits a clean surface without any byproducts. This finding suggests that washing effectively dissolves residual Li compounds. However, residual Li compounds increase the pH of the washing solution to >11, easily dissolving the transition metal ions. Subsequently, divalent Ni ions migrate into the Li slabs, forming a rock-salt phase. Therefore, Fig. 2b and S2† shows a severe structural change with an ∼8–11 nm cation mixing layer, consistent with the Rietveld results.
To obtain a better understanding of the washing effect at the secondary particle level, we closely examined the morphological characteristics of bare and washed NCM by performing SEM. Both the cross-sectioned electrodes were prepared (Fig. S3†) for the analyses. The SEM image of bare NCM shows a densely packed interior structure without noticeable pores (Fig. 2c). In contrast, the washed NCM particles show clear pore structures between the primary particles (Fig. 2d). Considering that washing effectively eliminated the residual Li compounds, we speculate that the exposed pores originated from sites with the initial residual Li compounds. A conventional high-Ni cathode particle has a secondary particle structure, which inevitably includes intergranular pores between the primary particles, as shown in Fig. 2e. Thus, residual Li compounds are generated on the surfaces of the secondary particles, and intergranular pores are produced inside the secondary particles. Thus, controlling the intergranular pores is as important as controlling the particle surfaces. Because of the exposed intergranular pore after washing, the Brunauer–Emmett–Teller (BET) surface area of the washed NCM powder is considerably increased compared to that of the bare NCM powder; specifically, the surface area is 0.61 m2 g−1 in the former case, representing a four-fold increase compared to that in the latter case (Fig. 2f). Because the radii of most of the intergranular pore are on the order of tens of nanometers, we also calculated the cumulative pore volumes between 0.85 and 150 nm. The results showed trends similar to that of the overall surface area results (Fig. 2f and g).
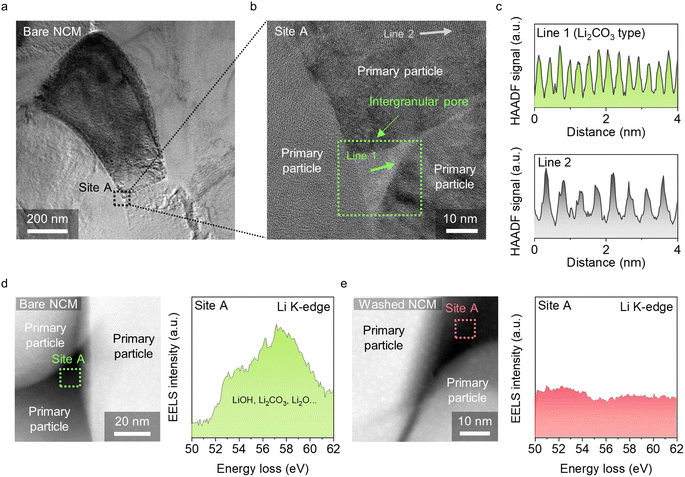
The results reveal that the intergranular pores are predominantly filled with residual Li compounds, which can easily be removed using a simple washing process. To characterize the properties of the residual Li compounds in the intergranular pores, we performed HR-TEM analysis of the bare and washed NCM particles. Bare NCM particles were prepared (Fig. 3a), and intergranular pores were carefully observed at site A. The HR-TEM image of site A shows intergranular pores comprising residual Li compounds with a clear crystalline structure between the three primary particles (Fig. 3b). The high-angle annular dark-field (HAADF) signal collected from line 1 at site A indicate that the residual Li compound may be crystalline Li2CO3,22 whereas the HAADF signal from line 2 indicates the conventional d-space of the Ni-rich layered structure (Fig. 3c).23
This assignment was further supported by electron energy loss spectroscopy (EELS) measurements. EELS is a powerful tool for investigating Li-based compounds because of the high ionization cross-section of the shallow Li K-edge. Therefore, it has been employed to investigate Li-comprised solid electrolyte interphase in Li-ion batteries. EELS was performed on the intergranular pore region in bare NCM (Fig. 3d) and washed NCM (Fig. 3e). We observed a broad Li K-edge peak at approximately 50–60 eV, which could be assigned to a combination of a series of Li compounds such as LiOH, Li2CO3, and Li2O.24,25 Each compound has a distinct electronic environment surrounding the central Li atoms; thus, the merged spectrum appears to exhibit a broad peak. In contrast to the bare NCM particles, the washed NCM particles do not show any evidence of Li in the intergranular pores (Fig. 3e).
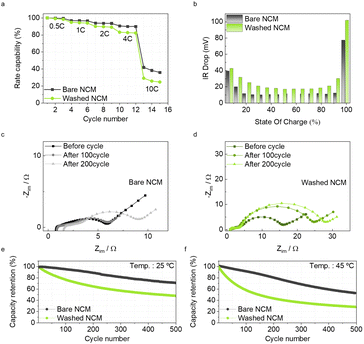
Although washing effectively reduces residual Li compounds, it is accompanied by transition metal dissolution, eventually leading to structural changes. Hence, we evaluated the electrochemical performance of the bare and washed NCM cathodes. The electrochemical behaviors of the bare and washed NCM cathodes were assessed using coin-type 2032R half-cells with cathode loadings of 11–12 mg cm−2 and an electrode density around 3.1 g cm−3, within a voltage window of 3.0–4.3 V. The initial discharge capacities of bare and washed NCM are similar (203 mA h g−1 and 202 mA h g−1, respectively), at a 0.1C rate at 25 °C, as depicted in Fig. S4.† However, as the cycle number increases, the washed NCM cathode exhibits severe polarization during 50 cycles (Fig. S5a and b†). The derivative capacity plots (dQ/dV) are shown in Fig. S5b and c.† During the 50 cycles, the peak position in the anodic reaction shifts to higher voltages, whereas the peak position in cathodic reaction shifts to lower voltages in both the bare and washed NCM cases. However, the washed NCM shows shifts more remarkable than those of the bare NCM. These observations suggest that increased polarization with lack of phase transition in washed NCM eventually leads to capacity fading.
Furthermore, pouch-type full-cells paired with traditional graphite anodes were fabricated to assess their long-term cycling performance and rate capability. After the formation cycles at a rate of 0.1C, the discharge rate capability was measured from 0.5C to 10C, as shown in Fig. 4a. The bare NCM has a better rate capability than the washed NCM, demonstrating a larger difference at a high rate. The galvanostatic intermittent titration technique analysis results in Fig. 4b reveal that the overall IR drop across all state-of-charge (SOC) levels for washed NCM is much higher than that for bare NCM, implying a higher ionic resistance of washed NCM, which contributes to poor rate capability. This finding is further corroborated by the Nyquist plots obtained by performing electrochemical impedance spectroscopy (EIS) analysis, enabling the investigation of the interfacial and charge-transfer resistances for each sample. Immediately after the formation cycles, the interfacial resistance of the washed NCM is notably higher, and its charge-transfer resistance increases with cycling, unlike that of bare NCM, which remains stable for up to 100 cycles (Fig. 4c and d). We speculate that the initial structural change induced by transition metal dissolution during washing increased the charge transfer resistance, which accelerated multiple degradations of the high-Ni cathode. In addition, washing affected the long-term cycle performance at both room temperature and a high temperature of 45 °C. When the bare and washed NCM full cells underwent 500 cycles at a 1C rate, the washed NCM full cells showed inferior cycle stability despite containing fewer residual Li compounds (Fig. 4e and f). These results imply that eliminating residual Li compounds without damaging the particle structure is important for high-Ni cathodes.
Based on our results, we propose three methods of improving the integrity of high-Ni cathode materials with reduced residual Li (Fig. 5). The first involves washing the powder with a transition metal containing solvent to maintain a layered structure incorporating a specific transition-metal-rich surface. A recently published paper demonstrated the feasibility of this approach in practical applications, providing superior cycle life of up to 6000 cycles.19 We also verified the Co-ion dissolved washing effect. Simple washing followed by annealing considerably reduces the residual Li compounds while offering excellent uniformity and structural stability (Fig. S6†). Thus, the washed sample demonstrates better cycle performance. However, this wet process is accompanied by additional drying and solvent recovery steps, which are time- and energy-consuming and further cause re-agglomeration of the powder. Alternatively, a dry coating approach can eliminate residual Li compounds.26,27 Numerous studies have reported effective coating precursors that react with residual Li compounds to form artificial layers in advance. However, this method still has limitations in terms of its ability to eliminate residual Li compounds from the intergranular pores of the polycrystalline Ni-rich particles. Therefore, we suggest one-body structured single-crystalline Ni-rich particles as a promising method for controlling residual Li compounds. Although the amount of residual Li can vary depending on the synthesis process, the selection of such particles requires only the removal of residual Li at the particle surface because they inherently lack intergranular pores. Fig. S7† demonstrates the superior performance of single-crystalline NCM with very low residual Li compound and moisture contents. Despite the obstacles to commercializing high-Ni cathodes, a one-body structured single-crystalline material design that minimizes grain boundaries is ideal for controlling the residual Li.
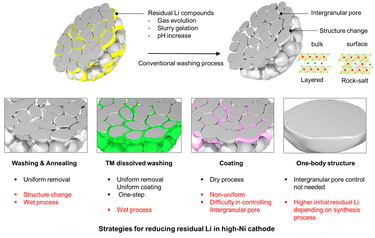 | ||
| Fig. 5 Illustration of promising strategies for reducing residual Li compounds in high-Ni cathode materials. | ||
Conclusions
We investigated residual Li compounds in high-Ni cathode materials to characterize their nanoscale structures and distributions. Residual Li compounds appeared in the both surface and intergranular pores between the primary particles at the nanoscale, as evidenced by the HR-TEM and EELS spectra. Furthermore, BET results demonstrated considerable residual Li compounds in the intergranular pores compared to that on the surface, emphasizing the importance of controlling the inner region. Although brief washing easily removes residual Li compounds, it dissolves transition metals from the cathode lattice, leading to structural degradation and poor electrochemical performance. Therefore, we propose a washing process integrating a reactant, dry coating, and morphology turning into a one-body single-crystalline structure for precise control of residual Li compounds. We identified the presence and characteristics of residual Li in high-Ni cathodes, focusing on engineering methodologies to mitigate them, ultimately anticipating the creation of novel material design strategies to promote further practical utilization of LIBs. This work demonstrates the importance of considering the complex chemistry of residual Li when developing strategies to control the residual Li in high-Ni cathodes.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Wooyoung Jin: conceptualization, methodology, investigation, formal analysis, data curation, validation, writing – original draft, writing – review & editing, Yujin Kim: methodology, investigation, formal analysis, data curation, writing – original draft, writing – review & editing, Haeseong Jang: formal analysis, data curation, writing – original draft, writing – review & editing, Yehyeon Gu: conceptualization, methodology, investigation, Namhyung Kim: investigation, Hyomyung Lee: formal analysis, Junhyeok Kim: formal analysis, Sinho Choi: visualization, formal analysis, Kyu-Nam Jung: data curation, funding acquisition, Ki-Hun Nam: data curation, Jaephil Cho: conceptualization, supervision, visualization, validation, resources, project administration, methodology, investigation, formal analysis, writing – original draft, writing– review & editing, Hyungyeon Cha: conceptualization, supervision, visualization, validation, resources, project administration, methodology, investigation, formal analysis, writing – original draft, writing– review & editing, funding acquisition.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This research was supported by a National Research Council of Science & Technology (NST) grant by the Korea Government (MSIT) (No. GTL24011-000) and National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST) (2022M3J1A1085412).References
- IEA, Global EV Outlook 2023, IEA, Paris, 2023, https://www.iea.org/reports/global-ev-outlook-2023, licence: CC BY 4.0 Search PubMed.
- J. Kim, H. Lee, H. Cha, M. Yoon, M. Park and J. Cho, Adv. Energy Mater., 2018, 8, 1702028 CrossRef.
- B. Namkoong, N. Y. Park, G. T. Park, J. Y. Shin, T. Beierling, C. S. Yoon and Y. K. Sun, Adv. Energy Mater., 2022, 12, 2200615 CrossRef CAS.
- H. Cao, Y. Zhang, J. Zhang and B. Xia, Solid State Ionics, 2005, 176, 1207–1211 CrossRef CAS.
- A. O. Kondrakov, A. Schmidt, J. Xu, H. Geßwein, R. Mönig, P. Hartmann, H. Sommer, T. Brezesinski and J. r. Janek, J. Phys. Chem. C, 2017, 121, 3286–3294 CrossRef CAS.
- G. W. Nam, N.-Y. Park, K.-J. Park, J. Yang, J. Liu, C. S. Yoon and Y.-K. Sun, ACS Energy Lett., 2019, 4, 2995–3001 CrossRef CAS.
- F. Wu, N. Liu, L. Chen, N. Li, J. Dong, Y. Lu, G. Tan, M. Xu, D. Cao and Y. Liu, J. Energy Chem., 2021, 62, 351–358 CrossRef CAS.
- Y. Kim, H. Park, J. H. Warner and A. Manthiram, ACS Energy Lett., 2021, 6, 941–948 CrossRef CAS.
- K. Min, K. Park, S. Y. Park, S.-W. Seo, B. Choi and E. Cho, Sci. Rep., 2017, 7, 7151 CrossRef PubMed.
- D.-H. Cho, C.-H. Jo, W. Cho, Y.-J. Kim, H. Yashiro, Y.-K. Sun and S.-T. Myung, J. Electrochem. Soc., 2014, 161, A920 CrossRef CAS.
- H. Liu, Z. Zhang, Z. Gong and Y. Yang, Electrochem. Solid-State Lett., 2004, 7, A190 CrossRef CAS.
- A. Grenier, H. Liu, K. M. Wiaderek, Z. W. Lebens-Higgins, O. J. Borkiewicz, L. F. Piper, P. J. Chupas and K. W. Chapman, Chem. Mater., 2017, 29, 7345–7352 CrossRef CAS.
- R. Jung, R. Morasch, P. Karayaylali, K. Phillips, F. Maglia, C. Stinner, Y. Shao-Horn and H. A. Gasteiger, J. Electrochem. Soc., 2018, 165, A132 CrossRef CAS.
- S. E. Renfrew and B. D. McCloskey, J. Am. Chem. Soc., 2017, 139, 17853–17860 CrossRef CAS.
- C.-H. Jo, D.-H. Cho, H.-J. Noh, H. Yashiro, Y.-K. Sun and S. T. Myung, Nano Res., 2015, 8, 1464–1479 CrossRef CAS.
- G. Ross, J. Watts, M. Hill and P. Morrissey, Polymer, 2000, 41, 1685–1696 CrossRef CAS.
- W. M. Seong, K. H. Cho, J. W. Park, H. Park, D. Eum, M. H. Lee, I. s. S. Kim, J. Lim and K. Kang, Angew. Chem., Int. Ed., 2020, 59, 18662–18669 CrossRef CAS.
- X. Xiong, Z. Wang, P. Yue, H. Guo, F. Wu, J. Wang and X. Li, J. Power Sources, 2013, 222, 318–325 CrossRef CAS.
- H.-H. Ryu, H.-W. Lim, S. G. Lee and Y.-K. Sun, Nat. Energy, 2024, 9, 47–56 CrossRef CAS.
- X. Li, C. Zhao, J. He, Y. Li, Y. Wang, L. Liu, J. Huang, C. Li, D. Wang and J. Duan, Electrochim. Acta, 2022, 406, 139879 CrossRef CAS.
- H. Cha, J. Kim, H. Lee, N. Kim, J. Hwang, J. Sung, M. Yoon, K. Kim and J. Cho, Adv. Mater., 2020, 32, 2003040 CrossRef CAS.
- R. Wang, X. Yu, J. Bai, H. Li, X. Huang, L. Chen and X. Yang, J. Power Sources, 2012, 218, 113–118 CrossRef CAS.
- U.-H. Kim, G.-T. Park, P. Conlin, N. Ashburn, K. Cho, Y.-S. Yu, D. A. Shapiro, F. Maglia, S.-J. Kim, P. Lamp, C. S. Yoon and Y.-K. Sun, Energy Environ. Sci., 2021, 14, 1573–1583 RSC.
- M. Boniface, L. Quazuguel, J. Danet, D. Guyomard, P. Moreau and P. Bayle-Guillemaud, Nano Lett., 2016, 16, 7381–7388 CrossRef CAS.
- F. Wang, J. Graetz, M. S. Moreno, C. Ma, L. Wu, V. Volkov and Y. Zhu, ACS Nano, 2011, 5, 1190–1197 CrossRef CAS PubMed.
- J. Kim, H. Cha, H. Lee, P. Oh and J. Cho, Batteries Supercaps, 2020, 3, 309–322 CrossRef CAS.
- J. Kim, H. Ma, H. Cha, H. Lee, J. Sung, M. Seo, P. Oh, M. Park and J. Cho, Energy Environ. Sci., 2018, 11, 1449–1459 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta07384c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |