Open Access Article
Open Access ArticleDo Arctic local sources of pollution influence the exposure of ringed seals (Pusa hispida) analyzed in contaminant monitoring programs?†
Houde M. *a,
Facciola N.a,
Routti H.
*a,
Facciola N.a,
Routti H. b,
Vorkamp K.
b,
Vorkamp K. c,
Søndergaard J.
c,
Søndergaard J. d and
Muir D. C. G.
d and
Muir D. C. G. e
e
aAquatic Contaminants Research Division, Environment and Climate Change Canada, Montreal, QC, Canada. E-mail: magali.houde@ec.gc.ca
bNorwegian Polar Institute, Fram Centre, Tromsø, Norway
cDepartment of Environmental Science, Aarhus University, Roskilde, Denmark
dDepartment of Ecoscience, Aarhus University, Roskilde, Denmark
eAquatic Contaminants Research Division, Environment and Climate Change Canada, Burlington, ON, Canada
First published on 28th April 2025
Abstract
The monitoring of contaminants in Arctic wildlife is mainly designed for long-range transported chemicals and supports international initiatives to mitigate global threats from chemical pollution. As human activities increase in the Arctic, the question of potential impacts of local pollution on wildlife exposure has emerged. In this study, we examined the current monitoring practices and the state of knowledge of pollutants in Arctic ringed seals (Pusa hispida) from Canada, Greenland, Norway, Russia, and Alaska. We complemented this synthesis with a case study of ringed seal exposure from different locations in Canada (2008–2022) to test the potential influence of local pollution sources. Multivariate constrained ordination was applied to examine the effects of ringed seals biological metrics (age, sex, length, and stable isotopes as dietary proxies), settlement size, and potential local contaminant sources (infrastructures, industries) on contaminant concentrations [polychlorinated biphenyls (PCBs), organochlorine pesticides (OCPs), per- and polyfluoroalkyl substances (PFAS), polybrominated diphenyl ethers (PBDEs), trace elements] in seals. Results indicated that biological data, location, local source variables and sampling year explained 8.4% (PBDEs), 31.8% (OCPs), 41.0% (trace elements), 45.1% (PFAS), and 65.8% (PCBs) of the contaminant variation in seals through time. Variation partitioning showed that, out of these percentages, local source variables uniquely explained less than 4% of variation for PBDEs, OCPs, trace elements and PFAS compared to 17.5% for PCBs. Results suggest an effect of local contaminant sources that is tightly linked to study site characteristics as well as seal biology. Local sources should be identified and assessed at sampling sites close to communities to ensure efficient contaminant monitoring and effectiveness of mitigation measures.
Environmental significanceThe monitoring of contaminants in Arctic wildlife is important to assess the long-range transport of chemicals and support international initiatives mitigating pollution. Local sources of pollution may increase with climate change and socioeconomic development of the Arctic and potentially influence levels of contaminants found in wildlife. This study examined the state of knowledge of pollutants in ringed seals (Pusa hispida) across the Arctic, identified examples of ringed seal exposure to local sources of pollution, and presents a preliminary analysis of the influences of local pollution in ringed seals across Canada. Results suggest an effect of local contaminant sources that is tightly linked to study sites and seal biology. Recommendations are given to better assess the potential influence of local sources in wildlife contaminant monitoring are suggested. |
Introduction
Pollution is a global issue impacting ecosystems worldwide, including remote polar regions. The Arctic Monitoring and Assessment Programme (AMAP) was established in the early 1990s to monitor and assess environmental contaminants and their effects in Arctic regions.1 For decades, AMAP has investigated levels and trends of pollutants in various environmental media and human samples and their effects on ecosystems and human health and presented these findings in scientific assessment reports and summaries for policymakers.2,3 The long-term monitoring of contaminants in Arctic wildlife has two main purposes. The first is to assess pollutant concentrations in the Arctic environment, including their change over time, which is important information for chemicals management. This includes international initiatives to mitigate global threats from contaminants such as the United Nations Stockholm Convention on Persistent Organic Pollutants (POPs). Thus, an important aspect of the Arctic monitoring programs has been to document long-range environmental transport to remote regions, as one of the screening criteria of the Stockholm Convention, as well as the effectiveness of international regulations. The second major purpose of the monitoring programs is to generate information on the potential exposure of Arctic Indigenous and local populations, who rely on local fish and wildlife in their traditional diet. It has been documented repeatedly that human populations in the Arctic are highly exposed to POPs and mercury because of the bioaccumulative characteristics of these chemicals and their biomagnification through the food web.3The pollutants monitored in Arctic biota include chemicals of the original dirty dozen POPs (e.g., polychlorinated biphenyls [PCBs] and organochlorine pesticides such as dichlorodiphenyltrichloroethane [DDT]), and new POPs such as some per- and polyfluoroalkyl substances (PFAS) (specifically, perfluorooctane sulfonic acid [PFOS], perfluorooctanoic acid [PFOA], perfluorohexane sulfonic acid [PFHxS]). Monitoring programs have also included various chemicals of emerging Arctic concern (CEACs), a term that summarizes compounds of concern in the Arctic that are not covered by the Stockholm Convention, such as non-regulated PFAS, emerging brominated flame retardants (EBFRs) and current-use pesticides.4 The monitoring efforts also include mercury and trace elements in biota and thus support the United Nations' Minamata Convention on Mercury.
The Arctic is not only affected by long-range transported pollution; a wide range of pollutants may also be emitted locally, for example, from community solid waste sites, airports, sewage outflows, shipping, tourism, and resource extraction (i.e., mining, oil and gas exploration).5,6 These studies have shown that polycyclic aromatic hydrocarbons (PAHs) and other CEACs are emitted from a variety of local sources in addition to POPs from former uses. Legacy POPs, such as PCBs, have been found in high concentrations at former military installations, as well as in buildings and electrical installations in Arctic settlements.7,8 The Arctic is experiencing fast socioeconomic development and industrialization, which will likely increase the number of local pollution sources and the variety of chemicals emitted.5,9,10
The ringed seal (Pusa hispida) is a key species for monitoring spatial and temporal trends of contaminants in the Arctic.11–17 This long-lived species has an important role in marine food webs, is distributed across the Arctic and is of great cultural, economic, and nutritional importance for Arctic Indigenous Peoples in Canada and Greenland. Collaborations with northern and Indigenous communities, including hunters, have enabled the long-term collection of ringed seal samples at multiple locations within the Arctic.18 The ringed seal is currently listed as a species of least concern by the International Union for Conservation of Nature in Greenland,19 as vulnerable in Norway,20 as special concern in Canada,21 as threatened in the United States,22 and is not listed in Russia.21 Highly dependent on sea ice, ringed seals are impacted by climate-related change,23,24 food web dynamics and disease spread.21,22
The objectives of this study were to (1) examine the current monitoring practices and the state of knowledge of pollutants in Arctic ringed seals from Canada, Greenland, Norway, Russia, and the United States (Alaska), (2) identify examples of local/regional source pollution in ringed seal contaminant levels, in the Arctic and beyond, (3) conduct a preliminary analysis of the influence from local Arctic contaminant sources using monitoring data in ringed seal from the Canadian Arctic, and (4) provide recommendations to better assess the potential influence of local sources in wildlife contaminant monitoring.
Monitoring contaminants in Arctic ringed seals
Contaminants in ringed seals are currently studied in monitoring programs in Canada, Greenland and Norway, although the frequency varies. Long-term temporal trends have focused on POPs, additional unregulated PFAS, and mercury (Hg), but ringed seals from different regions have also been studied for the presence of additional trace elements and CEACs. In this study, we gathered information from all studies of contaminants in ringed seals by searching ‘Web of Science’, ‘Scopus’ and ‘Google Scholar’ with the following keywords: ringed seals, Pusa hispida/Phoca hispida, pinnipeds, contaminants, pollution, monitoring, Arctic, north, temporal contaminant trends. Manual checks of the results lead to a few additions of studies that were known to the authors and not identified in the literature search, for example referring to one-off screening studies outside the regular time trend monitoring.In Canada, the Northern Contaminants Program was created in the 1990s to monitor pollutants in multiple Arctic wildlife species following concerns of northerners about the high levels of contaminants found in their traditional foods. Results have been published over time in a series of annual Synopses of Research Reports (2005–2017) and Canadian Arctic Contaminant Assessment Reports (1997–2017).25 Contaminant monitoring in ringed seal has thus been on-going for more than 30 years across the Canadian Arctic in collaboration with Indigenous partners. The current monitoring sites include Sachs Harbour (Beaufort Sea), Resolute Bay (High Arctic), Arviat (Western Hudson Bay) and Nain (Labrador) (Fig. 1). Through this program, temporal and spatial trends of multiple contaminants e.g., PCBs, polybrominated diphenyl ethers (PBDEs), OCPs, PFAS, short-chain chlorinated paraffins (SCCPs), and trace elements, including mercury, have been reported (see Table 1 for review). Multiple articles have quantified these POPs and others (e.g., polychlorinated dibenzodioxins/dibenzofurans, PCDD/Fs) in ringed seals from different sites through time.26–37 Results for legacy POPs show that PCBs and OCPs are declining in concentrations,38 but trends were generally found to be region and tissue-specific, while the feeding ecology of the seals, inferred from stable isotopes and fatty acids, was found to be closely linked to bioaccumulation of contaminants.39,40 Moreover, CEACs such as substituted diphenylamine antioxidants and benzotriazole UV stabilizers,41 EBFRs,42 and plastics43 have also been studied in ringed seals in the Canadian Arctic.
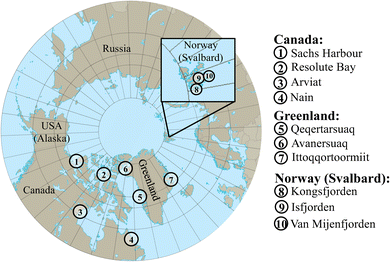 | ||
| Fig. 1 Sampling locations for ringed seal long-term contaminant monitoring in Canada, Greenland (part of Kingdom of Denmark), and Norway (Svalbard). | ||
| Region/site | Chemicalsa | Year of collection | Tissue | Reference |
|---|---|---|---|---|
| a PCB: polychlorinated biphenyls; DDT: dichlorodiphenyltrichloroethane; DDE: dichlorodiphenyldichloroethylene; HCH: hexachlorocyclohexane; HCB: hexachlorobenzene; HCBD: hexachlorobutadiene; CHL: chlordanes; PCDD/F: polychlorinated dibenzodioxins/dibenzofurans; HBCD: hexabromocyclododecane; TBBPA: tetrabromobisphenol A; PCN: polychlorinated naphthalenes; EBFR: emerging brominated flame retardants; PBDE: polybrominated diphenyl ethers; OH-PCB/PBDE: hydroxylated metabolites of PCBs and PBDEs, respectively; MeSO2-PBDE: methylsufonyl metabolite of PBDEs; SCCP: short-chain chlorinated paraffins; MCCP: medium-chain chlorinated paraffins; UV: ultra-violet; PFCA: perfluoroalkyl carboxylic acids; PFSA: perfluoroalkyl sulfonic acids; PFOS: perfluorooctane sulfonic acid; PFOA: perfluorooctanoic acid; PFNA: perfluorononanoic acid; FASA: perfluoroalkane sulfonamide; PFHxS: perfluorohexane sulfonic acid; F53: polyfluorinated ether sulfonate; F53B: chlorinated polyfluorinated ether sulfonate; FTOH: fluorotelomer alcohols; FTA: fluorotelomer saturated acid; FTUA: fluorotelomer unsaturated acid; FTCA: fluorotelomer carboxylic acids; FOSA: heptadecafluorooctane sulfonamide; PFOSA: perfluorooctane sulfonamide; Al: aluminium; Ag: silver; As: arsenic, Ba: barium; Be: beryllium; Cd: cadmium; Co: cobalt; Cr: chromium; Cu: copper; Hg: mercury; Ni: nickel; Mn: manganese; Mo: molybdenum; Pb: lead; Sr: strontium; Sn: tin; Sb: antimony; Se: selenium; V: vanadium; Zn: zinc. | ||||
| Canada | ||||
| Holman (now Ulukhaktok) | PCB, DDT | 1972, 1981 | Blubber | 26 |
| Resolute Bay, Grise Fiord, Admiralty Inlet | PCB, DDT, CHL, HCH, HCB | 1972, 1975, 1976, 1983, 1984 | Blubber, liver | 44 |
| Belcher Islands | PCB, DDT, CHL, HCH, dieldrin | 1989 | Blubber, kidney, liver, muscle | 27 |
| Sachs Harbour, Ulukhaktok, Paulatuk, Shingle Point, Salluit, Wakeham Bay, George River, Inukjuak, Resolute Bay, Sanikiluaq, Eureka | Total Hg, Cd, Pb, Se, Cu, Zn | 1989–1994 | Liver, kidney, muscle | 45 |
| Belcher Islands (Sanikiluaq) | PCB, DDT, CHL, HCH, dieldrin, mirex | 1991 | Blubber | 28 |
| Qikiqtarjuaq, Cambridge Bay, Coral Harbour, Kangiqsujuaq Kangiqsualujjuaq, Inukjuak, Pangnirtung, Rankin Inlet, Resolute Bay, Sachs Harbour, Talurjuaq, Tuktoyaktuk | PCB, DDT, HCH, CHL, HCB, toxaphene, dieldrin | 1983–1989 | Blubber | 46 |
| Ulukhaktok, Grise Fiord | PFCA, PFSA, FOSA | 1998, 2001 | Liver | 47 |
| Ulukhaktok | PCB, PCDD/F | 1981, 1991, 1996, 2000 | Blubber | 29 |
| Quaqtaq, Salluit, Kangirsuk | Toxaphene | 1998 | Blubber | 48 |
| Qikiqtarjuaq | PCB, DDT, CHL, HCH, HCB, dieldrin, mirex | 2001 | Blubber, liver, muscle | 30 |
| Resolute Bay, Arviat | PFCA, PFSA, 8:2/10:2 FTCA/FTUCA | 1972, 1992, 1993, 1998, 2000, 2004, 2005 | Liver | 49 |
| Inukjuak, Pangnirtung, Grise Fiord, Gjoa Haven, Pond Inlet, Arctic Bay, Sachs Harbor, Qikiqtarjuaq, Nain, Arviat, Resolute Bay | PFCA, PFSA, PFOSA, 8:2/10:2 FTUCA, 8:2 FTA | 2002–2005 | Liver | 50 |
| Sachs Harbour | PFCA, PFSA, 8:2/10:2 FTA, 8:2/10:2 FTUA, 7:3 FTA/FTUA | 2004 | Blubber, liver, blood | 31 |
| Ulukhaktok, Co-op Site, Woodward Point, Kuuk River | Hg | 14th century–2003 | Canine teeth | 51 |
| Ulukhaktok | HCH | 1978–2006 | Blubber | 32 |
| Ulukhaktok | Total Hg | 1973–2007 | Muscle | 33 |
| Ulukhaktok | PCB, DDT, CHL, HCH, HCB, dieldrin, mirex, toxaphene | 1993–2008 | Blubber | 52 |
| Beaufort Sea, Lancaster Sound, East Baffin, Hudson Bay | Hg | 1999–2009 | Liver, muscle | 53 |
| Beaufort Sea, Lancaster Sound, East Baffin, Hudson Bay | PCB, DDT, HCB, CHL, HCB, HCH, toxaphene, PBDE, HBCD, PFCA, PFSA, PFOSA | 1972–2010 | Blubber, liver | 54 |
| Ulukhaktok | PCB, DDT, CHL, HCB, heptachlor epoxide, dieldrin, mirex, endrin | 1972–2010 | Blubber | 34 |
| Saglek Fjord | PCB, DDT, HCH, CHL, HCB, dieldrin | 2008–2011 | Blubber | 35 |
| Nachvak Fjord, Saglek Fjord, Okak Bay, Anaktalak Bay | PCB | 2008 | Blubber | 36 |
| Sachs Harbour, Ulukhaktok, Arviat, Inukjuaq, Grise Fiord, Arctic Bay, Resolute Bay, Pangnirtung, Gjoa Haven, Pond Inlet, Nachvak Fjord, Saglek Fjord, Okak Bay, Anaktalak Bay | Total Hg, Cd | 2007–2011 | Liver, muscle | 37 |
| Arviat, Inukjuaq, Resolute Bay, Grise Fiord, Arctic Bay, Sachs Harbour, Pangnirtung, Ungava, Qikiqtarjuaq, Nain, Okak, Saglek, Gjoa Haven | PBDE, EBFR, dechlorane plus | 1998–2013 | Blubber | 42 |
| Sachs Harbour, Ulukhaktok, Arviat, Kangiqsualujjuaq, Quaqtaq, Inukjuaq, Grise Fiord, Arctic Bay, Resolute Bay, Pangnirtung, Qikiqtarjuaq, Gjoa Haven, Makkovik, Nain | PCB, DDT, HCB, HCH, CHL, toxaphene | 1972–2016 | Blubber | 38 |
| Resolute Bay, Arviat, Sachs Harbour, Lake Melville | Substituted diphenylamine antioxidants, benzotriazole UV stabilizers | 2016–2017 | Liver | 41 |
| Ulukhaktok | PBDE | 1981–2015 | Blubber | 55 |
| 22 sites from Beaufort Sea, Central Arctic, Baffin Island, Hudson Bay, Ungava, Nunatsiavut | Total Hg, Se | 1972–2017 | Liver, muscle | 39 |
| Arviat | Total Hg | 2003–2015 | Muscle | 56 |
| Arviat | SCCP | 2017 | Blubber | 57 |
| Arviat, Nain, Resolute Bay, Sachs Harbour | SCCP | 2016 | Blubber | 58 |
| Arviat | PFOS, PFNA | 1992–2020 | Liver | 59 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Greenland | ||||
| Greenland | Total Hg | 1984–1987 | Liver, kidney, muscle | 60 |
| Greenland | Pb, Cd, Hg, Se | 1978–1991 | Liver, kidney, muscle | 61 |
| Avanersuaq, Danmarkshavn, Ittoqqortoormiit, Nanortalik, Upernavik, Uummannaq, Kong Oscars Fjord | Hg, Cd, Se | 1978–1986 | Liver, kidney, muscle | 62 |
| Ittoqqortoormiit, Avanersuaq, Qeqertarsuaq, Nanortalik | PCB, DDT, HCH, HCB, trans-nonachlor, toxaphene | 1994 | Blubber | 63 |
| Ittoqqortoormiit, Qeqertarsuaq, Avanersuq | PCB, DDT, CHL, HCH, HCB, Cd, Hg, Se | 1999–2000 | Blubber, liver, kidney, muscle | 64 |
| Ittoqqortoormiit | PCB, DDT, PBDE, CHL, HCH, HCB, toxaphene | 2001 | Blubber | 65 |
| Ittoqqortoormiit, Qeqertarsuaq | PFCA, PFOS, PFHxS, PFOSA | 1982–2003 | Liver | 66 |
| Ittoqqortoormiit, Qeqertarsuaq, Avanersuq | PFOA, PFOS, PFHxS, PFOSA | 1998–2002 | Liver | 67 |
| Ittoqqortoormiit | PCDD/PCDF, coplanar PCB | 1986–2003 | Blubber | 68 |
| Ittoqqortoormiit | PCB, PBDE | 1986–2004 | Blubber | 69 |
| Ittoqqortoormiit, Qeqertarsuaq | HBCD, TBBPA, methyl-TBBPA | 2002 | Blubber, liver | 70 |
| Ittoqqortoormiit, Qeqertarsuaq | HCH | 1986–2006 | Blubber | 71 |
| Qeqertarsuaq | PCB, DDT, PBDE, CHL, HCH, HCB, toxaphene | 1982–2006 | Blubber | 72 |
| Ittoqqortoormiit | PCB, PBDE, HBCD | 1986–2008 | Blubber | 17 |
| Ittoqqortoormiit, Qeqertarsuaq, Avanersuq | Hg | 1984–2010 | Liver | 73 |
| Ittoqqortoormiit | PCN | 1986, 2000, 2006 | Blubber | 74 |
| Qaanaaq | PFCA, PFSA | 1984, 1998, 2006 | Liver | 75 |
| Ittoqqortoormiit | HBCD | 1986–2010 | Blubber | 76 |
| Ittoqqortoormiit, Qeqertarsuaq | PFCA, PFOS, PFHxS, PFOSA | 1982–2010 | Liver | 77 |
| Qeqertarsuaq | PCB-52, PCB-153, p,p′-DDE, HCB, HCH | 1994–2010 | Blubber | 23 |
| Ittoqqortoormiit, Qeqertarsuaq | Toxaphene | 1986–2012 | Blubber | 78 |
| Ittoqqortoormiit, Qeqertarsuaq | PCB, BDE-47, HBCD, EBFR, dechlorane plus | 2012 | Blubber | 79 |
| Ittoqqortoormiit | PFCA, PFSA, F-53B, FOSA | 2011, 2012 | Liver | 80 |
| Ittoqqortoormiit, Qeqertarsuaq | Endosulfan, SCCP, octachlorostyrene | 1986–2014 | Blubber | 81 |
| Ittoqqortoormiit, Qeqertarsuaq | HCBD | 2014 | Blubber | 82 |
| Ittoqqortoormiit, Qeqertarsuaq | PCB, DDT, HCB, α-HCH | 1986–2016 | Blubber | 83 |
| Qeqertarsuaq, Ittoqqortoormiit, Qaanaaq | SCCP | 2016 | Blubber | 58 |
| Avanersuaq/Qaanaaq | Hg, Se, Cd | 2018 | Liver | 84 |
| Qaanaaq, Qeqertarsuaq | Hg, Se, Cd | 2008 | Liver | 85 |
| Ittoqqortoormiit | Non-target screening | 2018 | Blubber, liver | 86 |
| Ittoqqortoormiit | PFOS, PFNA | 1986–2021 | Liver | 59 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Norway | ||||
| Spitsbergen, Svalbard | PCB, DDT, HCH, CHL, PCDD/F, HCB, dieldrin | 1986 | Blubber | 87 |
| Tempelfjorden and Kongsfjorden, Svalbard | PCB, DDE | 1990 | Blubber, kidney, liver | 88 |
| Northern coast of Norway | PCB, DDT, CHL, HCB, HCH, Hg, Cd | 1987–1994 | Blubber | 89 |
| Svalbard | Cd, Hg, Zn, Se | 1978–1986 | Liver, kidney, muscle | 62 |
| Kongsfjorden, Svalbard | PCB, toxaphene | 1996 | Blubber | 90 |
| Kongsfjorden, Svalbard | Toxaphene | 1992 | Blubber | 91 |
| East of Svalbard (northern ice area) | PCB, DDT, HCH, CHL, HCB | 1995 | Blubber | 92 |
| Kongsfjorden, Svalbard | PCB, DDE | 1992 | Blubber | 93 |
| Kongsfjorden, Svalbard | PCB, DDT, CHL, HCH, HCB, Cd | 1994, 1996 | Blood | 94 |
| Kongsfjorden, Svalbard | Hg, Se, Cd, Pb | 1996 | Liver, kidney, muscle | 95 |
| Barents Sea | PCB, p,p′-DDE, HCH, CHL, HCB | 1995 | Blubber | 96 |
| Kongsfjorden, Svalbard | PCB, DDT | 1996, 1998 | Liver | 97 |
| Kongsfjorden, Svalbard | PCB, PBDE, HCB, CHL, DDE, toxaphene | 1996, 2004 | Blubber | 98 |
| Tempelfjorden and Van Mijenfjorden, Svalbard | PCB, OH-PCB, MeSO2-PCB | 2007 | Liver, plasma | 99 |
| Tempelfjorden and Van Mijenfjorden, Svalbard | p,p′-DDE, MeSO2-DDE, CHL, HCB, toxaphene | 2007 | Liver, plasma | 100 |
| Tempelfjorden and Van Mijenfjorden, Svalbard | PBDE, OH-PBDE | 2007 | Liver, plasma | 101 |
| Tempelfjorden, Svalbard | EBFR, PBDE | 2007 | Liver | 102 |
| Kongsfjorden, Svalbard | PFCA, PFSA, FTOH, 6:2 FTS, FTCA, FTUCA, EBFR, SCCP, MCCP | 2010 | Plasma | 103 |
| Kongsfjorden, Svalbard | OPE | 2010 | Blubber | 104 |
| Kongsfjorden, Tempelfjorden, Billefjorden, Van Mijenfjorden, Svalbard | PFCA, PFSA, FASA | 1990–2010 | Plasma | 105 |
| Tempelfjorden, Svalbard | PFOS, PFOA, F53, F53B | 2007 | Liver | 106 |
| Tempelfjorden, Yoldiabukta, Svalbard | EBFR | 2014 | Blubber | 107 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| United States (Alaska) | ||||
| Barrow (now Utquigvik) and Nome | PCB, DDT, HCB, γ-HCH, CHL, heptachlor epoxide, dieldrin | 1988–1989 | Liver, kidney, blubber | 108 |
| Bering Sea | PCB, DDT, CHL | 1989–1995 | Blubber | 109 |
| Barrow, Nome | PCB, DDT, HCB, γ-HCH, CHL, dieldrin, heptachlor epoxide, 35 elements | 1988, 1991 | Liver, kidney, blubber | 110 |
| Barrow, Nome | 36 elements, methylmercury | 1988–1989 | Liver, kidney | 111 |
| Barrow, Nome | DDT, PCB, HCB, HCH, CHL, mirex, toxaphene, dieldrin, As, Cd, Cu, Hg, Se, Ag, V, Zn | 1988–1993 | Liver | 112 |
| Barrow, Nome | PCB, DDT, HCB, HCH, CHL, toxaphene, heptachlor epoxide, dieldrin, 37 elements | 1988–1993 | Liver, kidney, blubber | 113 |
| Barrow | PCB, DDT, HCH, CHL, HCB | 1996 | Blubber | 114 |
| Barrow, Nuiqsut, Point Lay | HCB, CHL, DDT, HCH, PCB | 1997–1999 | Blubber | 115 |
| Point Hope, Shishmaref, Little Diomede, Hooper Bay | PFCA, PFSA, PFOSA | 2003–2007 | Liver | 116 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Russia | ||||
| Dikson | DDT, PCB, CHL, HCH, HCB | 1995 | Blubber | 117 |
| Sorocskaya Guba Bay, Lake Lagoda, Lake Saimaa | HCB, PCB, DDT, HCH | 1988–1995 | Blubber | 118 |
| Dvina Bay, Onega Bay | HCH, CHL, DDT, HCB, PCB | 1998, 2001 | Blubber | 119 |
| Lavrentiya | PCB, PCDD/F | 2002 | Blubber | 120 |
| Lavrentiya Bay | HCH, DDT, PBDE, CHL, HCB, PCB, toxaphene, Hg, Pb, Cd | 2000, 2002 | Blubber, kidney, liver, muscle | 121 |
| Kalgalaksha Bay, Vaygach Island, Dikson Island, Vankarem | DDT, PCB, PBDE, PCDD/F, HCH, CHL, HCB, mirex, methoxychlor, endrin, toxaphene | 2001–2005 | Blubber | 122 |
| Enmelen, Sireniki | DDT, HCH, HCB, CHL, PCB, mirex | 2016 | Blubber, muscle | 123 |
| Enmelen, Sireniki | Pb, As, Cd, Hg, Cu, Zn, Ni, Cr, Al, Mn, Ba, Sr, Co, V, Be, Mo, Sn, Sb | 2016 | Blubber, muscle | 124 |
In Greenland, the AMAP Core Programme was formally established in 1994, following earlier sample collections of ringed seals that were also included in retrospective analyses.69 The program is ongoing and includes sampling of ringed seals approximately every two years at three sites, Qeqertarsuaq (Disko Island, central West Greenland), Ittoqqortoormiit (central East Greenland), and Avanersuaq/Qaanaaq (Northwest Greenland) (Fig. 1 and Table 1). The samples are collected by local hunters.125 Ringed seals from Qaanaaq are only analyzed for trace elements (mercury, cadmium, selenium), while specimens from the other locations are analyzed for lipophilic POPs (e.g., PCBs; OCPs including DDTs, hexachlorobenzene [HCB], hexachlorocyclohexanes [HCHs], chlordanes [CHLs] and toxaphene; PBDEs; hexabromocyclododecane [HBCD]) in blubber as well as PFAS in liver. Regular screening of CEACs (e.g., EBFRs, PFAS, phthalates, octachlorostyrene) and chemicals later regulated as POPs (e.g., hexachlorobutadiene, SCCPs) in ringed seals has been included in the program.70,79–82,126,127 Suspect and non-target screening has also recently been conducted to identify CEACs in ringed seal blubber and liver samples.86 Retrospective studies that were not continued in the regular monitoring program included PCDDs/PCDFs, coplanar PCBs and endosulfan.68,81 POP concentrations have been found to be generally higher in the Eastern region than in West Greenland.69,72,77 Temporal trends of chemicals have been reported in several publications (Table 1). The results show that POP concentrations have generally been decreasing in ringed seals from Greenland,17,83 whereas PFAS trends have been more variable.59,77
In Norway (Svalbard), PCBs, OCPs including DDTs, CHLs, HCB, toxaphene, and PBDEs have been monitored in ringed seals with relatively low frequency since the 1990s103,128 (Fig. 1 and Table 1). Emerging brominated flame retardants and organophosphate esters (OPEs),102,104,107 SCCPs and medium-chain chlorinated paraffins (MCCPs), PCB/PBDE metabolites,100,101 toxaphene,91,100 PFAS,105,106 and trace elements62,95 have been reported in seals collected at different sites (see ESI Fig. S1† for specific sampling locations).103 Time trends for PFAS were reported for the period of 1990 to 2010,105 while trends for POPs (CHLs; dichlorodiphenyldichloroethylene [DDE]; PCB-153; HCHs; HCB) have been reported under the environmental monitoring program for Svalbard and Jan Mayen (MOSJ).128 Ringed seals from this Arctic region have been used as a reference population for studying biotransformation and effects of contaminants in highly exposed Baltic ringed seals.129–136
In the United States (Alaska) and Russia, concentrations of contaminants in ringed seals have been reported, however, there are currently no known long-term contaminant monitoring programs in ringed seals in these regions, although a national Russian Arctic contaminants program has been proposed.137 Various studies have reported levels of PCBs, DDTs, CHLs, HCHs, HCB, PBDEs, PCDD/Fs, and trace elements in ringed seal tissues collected across different regions of the Russian Arctic.117,118,120–124 Information for ringed seals from Alaska are mainly from the 1980s and 1990s and include assessments of PCBs, DDTs, CHLs, HCHs, dieldrin and multiple trace elements (e.g., Hg and methylmercury; see Table 1).108–113,115,138 Perfluoroalkyl carboxylic/sulfonic acids (PFCAs, PFSAs) and perfluorooctane sulfonamide (PFOSA) were also reported in seal liver collected in Alaska between 2003 and 2007.116
The circumpolar assessment of contaminants in ringed seals has enabled comparisons between regions and over time.10,11,13,15,29 The long-term trends established by these Arctic monitoring programs are among the longest recorded for wildlife globally. These monitoring programs have also provided a platform for assessing time trends of new POPs and CEACs in seals. For instance, temporal analyses indicated that PBDEs increased significantly from 1998 to 2008 in ringed seals from East Baffin and decreased in recent years, which fluctuated slightly over time at several other locations.42 Increasing trends of perfluorononanoic acid (PFNA) were also reported in ringed seals from East Greenland and Western Hudson Bay, whereas PFOS trends were highly variable.59
The time series of contaminants in ringed seals from the Canadian Arctic and Greenland have reached a time span that provides sufficient power for statistical analyses, for example studies on the influence of climate change on contaminant levels in ringed seals.38,39,77,83 Seawater temperature, sea ice extent, and the Arctic Oscillation Index were found to be associated with POP concentrations in ringed seal in East and West Greenland.83 Contaminant concentrations (including PCB and Hg) were also found to be correlated with environmental factors (air temperature, precipitation, sea ice coverage, oscillation indices) in seals in the Canadian Arctic.38,39 These influences were still minor compared to the overall decrease of most POPs due to declining primary and secondary emissions5 but might gain importance with the changing climate.
These long-term monitoring programs have mainly been designed to detect chemicals that are transported from industrialized mid-latitude locations to the Arctic via air, oceans, and rivers representing long-range environmental transport, together with the purpose of monitoring human exposure. As more information emerges on local pollution sources in the Arctic, such as local infrastructures and industrial activities,6 and projected increases in human activity are foreseen in a warming Arctic,10,139 local influences on wildlife exposure need to be considered. Furthermore, screening efforts for CEACs are increasing and include current-used chemicals for which emission sources are more difficult to identify. Local sources of CEACs in the Arctic might be associated with ongoing use (e.g., in consumer products, building materials, vehicles), rendering them difficult to control. This raises the question of the potential influence of chemicals emitted from Arctic local sources on ringed seal exposure and consequences for interpretation of findings from ongoing spatial and temporal trend monitoring studies.
Examples of known local or regional sources of contamination in seals
Contaminants in ringed seals have also been analyzed outside the Arctic where the environment is impacted from both local sources and long-range transported pollutants. One example is the Baltic Sea where ringed seal tissues have been analyzed to study time trends of contaminants and their effects.133 This semi-enclosed brackish water environment receives pollution from inland activities located in the vast catchment area of nine heavily populated countries, including municipal, agricultural, and industrial sources.140–142 In addition, ship traffic, fisheries and tourism may lead to direct emissions to the sea that also receives contaminants from the deposition of airborne pollutants. Given these aggregated pollution sources, the Baltic Sea is less suitable than the Arctic to reflect the long-range environmental transport of chemicals that is assessed for the Stockholm Convention. Consequently, the research and monitoring of contaminants in ringed seals of the Baltic Sea have not distinguished between different types of sources. Starting from the late 1960s, high levels of legacy contaminants have been reported in ringed seals from the Baltic Sea143,144 and the contaminant exposure has been associated with effects on reproduction, immune, and endocrine functions as well as population declines.129,133,134,136,145–148 The Baltic Marine Environment Protection Commission, formerly Helsinki Commission (HELCOM), was established in 1974 to protect the marine environment of the Baltic Sea from all sources of pollution. Time trends indicated that some chemical groups such as PBDEs, PCBs, DDTs, and PCDD/Fs have declined since the 1970s in ringed seal tissues, whereas other chemicals (brominated dioxins and furans) remained stable.97,149Ringed seals potentially exposed to locally emitted contaminants were studied at Saglek Bay (Nunatsiavut, northern Labrador, Canada), where a former military radar station with PCB contamination was located. Although PCB contaminated soils were removed in the 1990s and early 2000, the adjacent bay had become contaminated with PCBs, leading to elevated PCB levels in sediments, invertebrates, fish, seabirds, and ringed seals.6,7 Unique PCB profiles (high proportions of highly chlorinated congeners) and high PCB concentrations were found in ringed seal tissues compared to other Canadian locations.35 Moreover, stable isotopes of nitrogen and carbon, fatty acid, and satellite telemetry data indicated that the home range/habitat use of seals and time spent in the inlets were important determinants of their PCB concentrations, indicating local contamination.36,150
Local sources of pollution, along with long-range pollution, were suggested to contribute to plasma PFAS concentrations and blubber PBDE concentrations in another ice-associated seal species, the Weddell seal (Leptonychotes weddellii) of the Antarctic.151,152 These seals were sampled close to the permanent research stations of the United States, New Zealand, and Italy. The local source hypothesis was supported by geographical differences in PFAS levels in seawater indicating potential point sources of contamination153 as well as PCB, PBDE, and trace element concentrations reported at high levels in wastewater sludge, sediment, invertebrates, and/or fish around the American station.154–156 These studies also reported a rapid decrease in concentrations with increasing distance from the point source. Together these examples suggest that local sources might influence the levels and patterns of contaminants in ringed seals and related species, but the information is limited to a handful of studies with only one on Arctic seal populations.
Case study: the influence of local anthropogenic sources on contaminant concentrations in ringed seals analyzed under the Canadian monitoring program
To increase our understanding of the potential influence of local anthropogenic sources on the ringed seal contaminant exposure in the Arctic, we conducted an exploratory pilot study based on data from the Canadian Northern Contaminants Program. Canadian ringed seal samples are typically collected near communities where local pollution sources might exist. Specific anthropogenic local pollution sources have not been formally studied in the four communities with ongoing ringed seal monitoring across the Canadian Arctic (i.e., Sachs Harbour, Northwest Territories; Resolute Bay and Arviat, Nunavut; Nain, Labrador). For this case study, we incorporated information on human population sizes, community infrastructures, mines, airports, military sites, and industries that have been identified as potential local sources of pollution in other studies6,157,158 (Tables 2 and S1†). However, limited data existed on contaminant emissions for locations included in those studies. A distance-based redundancy analysis (db-RDA) with forward selection of variables and variation partitioning was performed to analyze this environmental dataset. Constrained ordination is a multivariate regression that helps visualize the relationship between response variables (herein the suite of contaminants in seal tissues) and a set of explanatory variables (herein the potential local source pollution, seal biological data, and study site location). In turn, variation partitioning teases apart the unique versus combined effect of explanatory variable bundles represented via Venn diagrams. Several studies have applied redundancy analyses to link contaminant profiles to environmental variables (e.g., water chemistry, habitat characteristics, biological factors) in fish, invertebrates, and marine mammals.159–161 Here, we build on this previous experience by testing the additional influence of local point source contamination in Arctic wildlife.| Level | Variable | Units/scale | Reference |
|---|---|---|---|
| Observation-level | Age | Years | Ref. 39 and Houde et al. unpublished |
| Sex | Categorical (male/female) | ||
| Size (maximum girth) | cm | ||
| Carbon isotope (δ13C) | ‰ | ||
| Nitrogen isotope (δ15N) | ‰ | ||
| Sampling year | Year | ||
| Community-level | Sampling location | Categorical (Sachs Harbour, Resolute Bay, Arviat, Nain) | Ref. 39 and Houde et al. unpublished |
| Human population | Human population size in each settlement | NASA Socioeconomic Data and Applications Center (SEDAC) population estimator162 | |
| Mines | Distance to closest active mine, km | Minerals and mining database (Natural Resource Canada; https://natural-resources.canada.ca/our-natural-resources/minerals-mining/10858) | |
| Power source | MW h per year | Remote communities energy database (Natural Resource Canada; https://open.canada.ca/data/en/dataset/0e76433c-7aeb-46dc-a019-11db10ee28dd) | |
| Airports | Number of flights per year | Flightradar24 https://www.flightradar24.com | |
| Military site | Distance to nearest Distant Early Warning (DEW) line site, km | GoogleEarth for distance measurements | |
| Solid waste | Distance from landfill to shoreline, km | GoogleEarth for distance measurements | |
| Wastewater | m3 per day | Nunavut, Labrador, and Northwest Territories water board issue permits |
A total of five db-RDAs were realized, with each focusing on a different contaminant class as the response matrix: trace elements (liver), PCB homolog groups (blubber), individual PBDEs (blubber), OCPs (blubber), and PFAS (liver) measured in ringed seals from the four Canadian locations between 2008 and 2022 (Fig. 1). Specific chemicals included in each contaminant class are detailed in Table S2.† For this study, airports, wastewater facilities, solid waste disposal sites, mines, and military sites identified in and around communities where seal sampling occurred were considered as local source variables. Source of energy and human population size through time were also considered as local source variables. The information was collected from available public sources (Tables 2 and S1†). For most of the local source variables, obtaining specific information (e.g., volume of solid waste produced each year) and quantifying certain variables (e.g., emission from airports) proved challenging due to the lack of available data. In cases where data were only available for a single point in time (e.g., power use, flow of wastewater), this static value was used for all years for that community. Due to this limitation, we acknowledge that these variables will not fully capture temporal variability of contaminants emitted from point sources. By their nature, the local source variables may correlate strongly with the location effect. Biological characteristics of the ringed seals such as the age of the animal, sex, and maximum length were included in the analyses as they are known to influence contaminant variation in ringed seals.38,42 Carbon (δ13C) and nitrogen (δ15N) bulk stable isotopes were also included as these variables are important indicators of trophic level.24 Detailed methods for obtaining each biological variable are provided in Houde et al.38 Lastly, location (community) and sampling years were also included as explanatory variables.
All statistical analyses were conducted in R version 4.3.1. Prior to performing the db-RDAs, contaminant data were transformed using the decostand function from the vegan package in R163 to select the best transformation among chord, Hellinger, and log-chord. The transformation that provided the highest R2 value was retained. Multicollinearity among explanatory variables was assessed using Variance Inflation Factor (VIF). The human population size variable exhibited high collinearity and was removed to ensure better model interpretation for all db-RDAs. Using the ordistep function, a forward selection approach using bundles (local source bundle: mine, power source, airports, military sites (Distant Early Warning [DEW] line sites), wastewater, solid waste; biological variable bundle: seal age, sex, size, δ13C, δ15N) was then conducted to ensure the inclusion of local source variables in the model while also reducing redundancy. The forward selected variables, along with sampling year and location, were incorporated into the db-RDA models using the capscale function in the vegan package with Euclidean distance.163 To identify the relative contribution of local source contamination on the observed contaminant variation, variance partitioning using all the explanatory variables was run with the varpart function. This approach allowed for a direct comparison between Venn diagrams and ensured that all predefined variable bundles (e.g., local source, biological, location and sampling year) had representation in the diagrams. All local source contamination variables were considered community-level variables as they were spatially structured factors tied to community-based characteristics. This contrasts with observation-level variables that were specific to individual seals.
Case study results and discussion
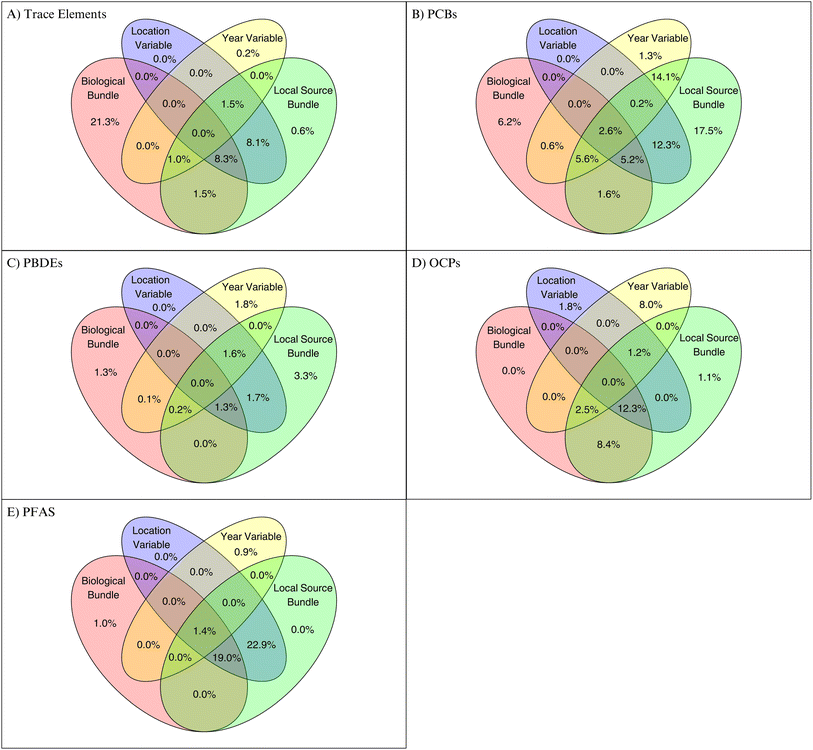
The forward selected db-RDA results showed that seal biological data, community location, local source variables and sampling year explained a considerable portion of the ringed seal contaminant variation. These models explained 41.0% of the variation in trace element concentrations in ringed seals (R2 adjusted for number of variables and sample size), 45.1% for PFAS levels, 8.4% for PBDEs, 31.8% for OCPs, and 65.8% for PCBs. Results of forward selected variables included in each db-RDA can be found in Table S3.† The variation partitioning (Fig. 2) showed that the local source variables uniquely explained none of the variation in PFAS concentrations, 0.6% in trace elements, 3.3% in PBDEs, 1.1% in OCPs, and 17.5% in PCBs. The partition of variation uniquely explained by location variables was consistently 0% for all contaminant groups other than OCPs (1.8%) (Fig. 2). It is challenging to separate the effect of community-level source variables from the geographical position of the community and, as expected, the contribution of the location variable overlapped with local contaminant source variables (0–22.9% co-explained). Generally, the biological variable bundle and the sampling year represented large unique contributions to the explained variation for all contaminant groups. These results are expected as biological variables are known to be closely related to contaminant concentrations in seals via diet differences, age, sex, and body condition of the animal,13 and sampling year captures temporal changes in ringed seal contaminant levels likely related to changes in emissions (e.g., as a consequence of chemical regulation) and environmental changes (e.g., ice cover, oscillation patterns).38 Nonetheless, it is interesting to note that a large part of the location, local source, and biological effects could not be distinguished as 1.3–19.0% of the variation in seal contamination was co-explained by all three bundles. While the local source bundles did not show strong unique contributions on their own, their combined influence with the other variables is evident.For the trace element db-RDA, distance from the mine and distance to military sites (DEW line site) showed negative relationships with copper and manganese, suggesting that the closer these infrastructures/sites are, the higher the copper and manganese concentrations in liver of ringed seals (Fig. 3A). Distance to military sites (DEW line sites) showed a positive relationship with cadmium suggesting that the further the military site, the higher the cadmium concentrations. While these relationships are unusual, a combination of factors not assessed in this study, including regional land cover, geochemical composition and permafrost thaw could potentially explain these findings.164,165 As seen in Fig. 2A and 3A, biological variables (e.g. seal size) also play a substantial role in explaining trace metal variation. For PCBs, the db-RDA indicated numerous relationships between the PCB homolog groups and local source variables (Fig. 3B). Community power consumption showed positive relationships with the octa, hepta, and nona PCB homolog groups. The tetra PCB homolog group showed a positive relationship with distance to mine. Out of all db-RDA models, PCBs showed the highest explained variance and a large shared and unique contribution of the bundle of the local sources in the variance partitioning. PCBs were widely used in infrastructure such as electrical transformers, capacitors, and building materials prior to their ban and these results might be a reflection of PCB from older infrastructures although actual measurements are limited. PCBs were detected in soils near the Resolute Bay airport and studies of DEW line sites have shown evidence of the spread of PCBs from the radar stations to the surrounding region.166–168
Fire training sites, airports, landfills, wastewater discharges, and consumer goods have been identified as typical local sources of PFAS in the Arctic (reviewed by Lohmann et al.59). Our results for PFAS indicated that individual seals analyzed through the monitoring program were grouped strongly by location indicating geographic influence on contamination patterns (Fig. 3E). However, variance partitioning revealed that location itself did not explain unique variation rather it was shared with the biological and local source bundles (Fig. 2E). These results suggest that while geographical differences influence PFAS variation, likely related to source regions and long-range transported patterns (e.g., sources in Asia affecting the western and High Arctic vs. North America and Europe affecting the eastern part of Canada). Besides geographical differences related to long-range transport, previous studies have documented PFAS contamination near Arctic airports (Resolute Bay, Canada, and Svalbard, Norway) further supporting the role of local source influences.168,169
Although the variation explained for PBDEs was small, some weak relationships could be observed. Power source showed positive relationships with PBDE-47, -99, -100, -153, and -209, suggesting that an increase in annual power usage could have influenced concentrations of these congeners in ringed seal blubber (Fig. 3C). Even though the production and use of PBDEs have been phased out since the mid-2000s in North America and Europe, older products and infrastructure may still contain PBDEs from before the bans.170 For OCPs, the db-RDA showed a positive relationship between α-chlordane and distance to mine and a negative relationship between number of flights and γ-chlordane (Fig. 3D). In the variance partitioning, the local source bundle co-explains the majority of the OCP variance with the biological bundle and location variable, suggesting that a combination of geographical factors and seal characteristics are influencing the relationships seen in the db-RDA. As agricultural activities are limited or non-existent in the Canadian Arctic, the unique local influence for OCPs is likely small.
The persistence of the regulated contaminants studied here, along with the potential influence of historical contamination and secondary emissions, makes it difficult to pinpoint with certainty if relationships observed through the db-RDA analyses are solely due to local source influences. Our results indicate that it is likely a combination of factors that influence POP concentrations in ringed seals. Conducting the same analyses with non-regulated chemicals of current concern (e.g. plastic related compounds, EBFRs, emerging PFAS) would be interesting to do, however, long-term trends for these compounds are generally not available and their levels in seals are usually lower than legacy contaminants. Overall, results from this pilot study suggest that human-associated Arctic point sources may contribute to some degree to the contaminant exposure of ringed seals, but that this influence is likely small and difficult to distinguish from biological and temporal variables and geographical differences due to long-range transport.
Challenges in estimating the contribution of local sources to contaminants in ringed seals
Specific source contributions may be difficult to assess for contaminants in high trophic level marine species due to their typically large-scale spatial movements and their foraging ecology across different food webs.171,172 Some ringed seals from different parts of the Arctic have been reported to travel over large distances (>1000 km) at least seasonally or during certain life stages.173–175Ringed seal samples intended for contaminant monitoring are often collected at significant distances from hamlets to avoid the influence of local sources. For example, none of the ringed seals from Svalbard were collected close to human settlements. Although local pollutant sources have been described for Svalbard,8,169,176 their contribution to pollutant exposure in the environment remains at a small spatial scale (<20 km)177 and thus their influence on pollutant exposure for species that move over large spatial scales, like ringed seals, is difficult to assess. Spatial differences may also be related to local oceanographic conditions as suggested for differences in PFAS concentrations observed in ringed seals sampled in different fjords from the western coast of Svalbard.105 In Greenland, the monitoring sites are located far (>200 km) from active and major legacy mine sites178 and other industrial point sources. Local settlements adjacent to the monitoring sites are comprised of small communities of <1000 residents. Therefore, the influence of local point sources is considered low but cannot be ruled out and should be assessed on a compound-specific basis. Previous studies from Greenland showed indications of influences of community size on levels of PCBs and PBDEs in shorthorn sculpin (Myoxocephalus scorpius), whereas no difference was seen for OCPs in the same samples.179 Potential local contaminant sources at the Greenland monitoring sites include sewage and open waste burning from waste dumps in the settlements, release of effluents and spills from harbors as well as shipping (including cargo ships, cruise ships, and fishing vessels), but no detailed information is available for a quantitative assessment. In Canada, local inputs of PCBs from DEW line sites were reported for soil,166,167 and human activities in and around Iqaluit, Nunavut were found to be a source of PCBs, Hg, PAHs, and PFAS in sediment from Frobisher Bay.180 PCB and DDT levels in seawater collected around Cambridge Bay, Nunavut also suggested local inputs from the town and military site.181,182 Chemical concentrations found in water and sediment may distinctly represent local source signals as compared to pinnipeds, that accumulate contaminants over a long-life span and integrate exposure from different sources including different prey species.
Recommendations to better assess the potential influence of local sources in wildlife contaminant monitoring
Long-term monitoring of contaminants in marine mammals such as ringed seals has important applications in the protection of Arctic ecosystems and human health as it integrates temporal changes in water quality and ecosystem state, provides information on contaminant exposure from traditional foods to local population, identifies emerging issues, and supports the evaluation of the effectiveness of mitigation of recognized pollutants. The question whether the measurements are influenced by emissions from local sources is important regarding the assessment of long-range environmental transport under the Stockholm Convention. Furthermore, a general understanding of contaminant transport and accumulation in Arctic ecosystems is needed for efficient regulatory action.One key aspect in understanding the potential influence of local sources in remote regions is the knowledge of the history of the site/community and its surroundings, as compiled in part for this study. Past and present potential local/regional sources of contamination should be identified. These can include industrial facilities (e.g., mines), military sites, transportation (e.g., airport, port), power/heating systems, wastewater treatment, waste disposal/landfills, livestock, and urban runoff. Knowledge is needed of which substances are actually emitted from these sources. Secondary sources of contamination in the area should also be noted. For example, permafrost thawing and glacier melting sites can potentially act as secondary sources for certain contaminants.183–186 Maps indicating the location of pollution sources should be created with this information and, ultimately, modeling and machine learning can be used to track sources of pollution.187,188 Integration of hydrological and air modeling could also help predict the dispersion patterns of contaminants around local sources and help identify sites for sample collection.
Ringed seals or other marine mammals may not be the perfect indicator species for monitoring local contamination sources. However, if the primary purpose of the monitoring campaign is human contaminant exposure, local sources should be considered as a representation of the real-life situation, including all possible dietary exposure pathways. It will also be relevant to be able to identify pollution source mitigation measures as different environmental management agencies will likely be responsible for actions at local, regional and international levels.
Although POPs, such as PCBs and OCPs, have been banned for up to several decades, their monitoring is still crucial, for several reasons. The effectiveness of regulations should be ensured, as reflected in decreasing concentrations in Arctic ringed seals. Furthermore, despite decreasing concentrations, some POPs, such as PCBs, are still at levels in wildlife and humans that cause health concerns and require attention.189 Other compounds, such as long-chain PFCAs are increasing in some Arctic wildlife populations and cause co-exposure situations that are far from understood.59 Climate change affects the environmental distribution of POPs and/or ecosystem structures, which is captured in an integrated way in the contaminant time series.190 In addition to POP and heavy metal monitoring, ringed seals also have been important species for screening studies and should be considered for further surveillance of CEACs.4
Higher resolution analyses of local sources might identify specific compounds that are relevant to monitor locally, in connection with specific sources, such as wastewater emissions or landfill leachates. In addition to collecting samples from wildlife for contaminant monitoring, abiotic (air, water, sediment) and other biotic (invertebrates, fish) samples around the northern communities and from the species habitat and food web should be collected. However, these collections are not always simple due to the remote locations and harsh environmental conditions. This highlights the importance of community engagement and collaboration with Indigenous Arctic Peoples.18,191 Local communities have information about potential sources of contamination as well as valuable insights and knowledge about local environmental issues, and know when and where to collect samples. Local knowledge can also help inform the movement of animals and support interpretation of contaminant data. When resources and expertise are available, satellite tracking devices and photography (e.g. camera trap sampling stations) can also be used to investigate the movement ecology of the studied wildlife and links to potential contaminant exposure.192 Other techniques such as compound specific isotopic composition and chemical fingerprinting can also be of interest to help identify specific sources of contamination.
Finally, the monitoring of contaminants in Arctic wildlife requires balancing the ongoing measurements at the same sites over time with adjustment based on the emergence of new contaminants and evolving local conditions. Contaminant monitoring should also consider any changes in the species that are being harvested by local people, such that contaminant data are most relevant for communities.
Conclusion
Arctic monitoring programs including ringed seals (and/or other marine mammals) have been designed to monitor concentrations of long-range transported chemicals over time and generate information on exposure of local human populations via their traditional diet. As Arctic data are often used to assess long-range environmental transport, it is important to be able to relate measurements in the Arctic to local or distant sources. Knowledge of sources is also important to enable mitigation actions via the competent authorities at the local, regional or international level. This study of contaminants in ringed seals from the Arctic highlights the valuable knowledge obtained from long-term monitoring of contaminants. Our preliminary analysis using monitoring data from the Canadian Arctic detected limited effects of local sources of contamination accumulation in seals. However, it should be noted that our analysis was biased towards chemicals which are banned globally and no longer included in products of current use. As climate change continues and as Arctic communities continue to change in size, infrastructure, and tourist and industrial activity, among other factors, the relative importance of local versus distant contaminant sources affecting wildlife and country foods may change in the future. The assessment of local emissions of contaminants within the Arctic becomes therefore more critical and study designs should consider these developments.Data availability
The list of potential local sources of contamination can be found in the ESI.† Contaminants data from Norway are available on the Environmental monitoring of Svalbard and Jan Mayen website (https://mosj.no/en/indikator/influence/pollution/pollutants-in-ringed-seals/). Metadata from Canada can be found on the Polar Data Catalogue (https://www.polardata.ca/) and the Environment and Climate Change open government portal (https://open.canada.ca/en). Greenland biota contaminant data can be accessed at the International Council for the Exploration of the Sea (ICES) Data Centre via the DOME database (https://dome.ices.dk/). Some temporal trend information can be obtained from the Arctic Monitoring Assessment Programme (AMAP) assessment data portal (https://www.amap.no/ahat). Additional data can be available on request.Author contributions
M. Houde – conceptualization, investigation, writing – original draft, writing – review & editing, project administration. N. Facciola – data curation, methodology, formal analysis, writing – original draft, writing – review & editing. H. Routti – data curation, writing – original draft, writing – review & editing. K. Vorkamp – investigation, data curation, writing – original draft, writing – review & editing. J. Søndergaard – data curation, writing – review & editing, validation, project administration. D. C. G. Muir – writing – review & editing, validation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The monitoring of contaminants in Arctic ringed seal could not be done through time without the collaboration of northern and Indigenous communities and hunters. We are very grateful for their knowledge and expertise that are the basis of this long-term work. In Canada, the Nunatsiavut Government and the Hunters and Trappers organizations of Sachs Harbour, Arviat and Resolute are acknowledged for their co-leadership in ringed seal contaminants monitoring. Zofia Taranu from Environment and Climate Change Canada is thanked for the help with statistics and her review of the manuscript as well as Cynthia de Wit (Stockholm University) and Melissa McKinney (McGill University) for their input on the text.References
- L.-O. Reiersen, K. Vorkamp and R. Kallenborn, The role of the Arctic Monitoring and Assessment Programme (AMAP) in reducing pollution of the Arctic and around the globe, Environ. Sci. Ecotechnol., 2024, 17, 100302 CrossRef CAS PubMed.
- AMAP, AMAP Assessment Report: Arctic Pollution Issues, Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway, 1998 Search PubMed.
- AMAP, AMAP Assessment 2020: POPs and Chemicals of Emerging Arctic Concern: Influence of Climate Change, Arctic Monitoring and Assessment Programme (AMAP), Tromsø, Norway, 2021 Search PubMed.
- AMAP, AMAP Assessment 2016: Chemicals of Emerging Arctic Concern, Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway, 2017 Search PubMed.
- H. Hung, C. Halsall, H. Ball, T. Bidleman, J. Dachs, A. De Silva, M. Hermanson, R. Kallenborn, D. Muir, R. Sühring, X. Wang and S. Wilson, Climate change influence on the levels and trends of persistent organic pollutants (POPs) and chemicals of emerging Arctic concern (CEACs) in the Arctic physical environment – A review, Environ. Sci.: Processes Impacts, 2022, 24, 1577–1615 RSC.
- R. Kallenborn, G. W. Gabrielsen, K. Vorkamp, L.-O. Reiersen, A. Evenset, K. B. Pedersen, S. Corsolini, N. Ademollo, H. Langberg, W. F. Hartz, F. von Hippel, D. C. G. Muir, C. de Wit, M. J. Gunnarsdottir, P. E. Jensen, M. Kirkelund, G. Breedveld, E. Barbaro and S. Bengtson Nash, Industrial and public infrastructure as local Arctic pollutant sources of organic contaminants in the Arctic, Environ. Sci.: Adv., 2025 Search PubMed , In Preparation.
- Z. A. Kuzyk, J. P. Stow, N. M. Burgess, S. M. Solomon and K. J. Reimer, PCBs in sediments and the coastal food web near a local contaminant source in Saglek Bay, Labrador, Sci. Total Environ., 2005, 351–352, 264–284 CrossRef CAS PubMed.
- M. Jartun, R. T. Ottesen, T. Volden and Q. Lundkvist, Local sources of polychlorinated biphenyls (PCB) in Russian and Norwegian settlements on Spitsbergen Island, Norway, J. Toxicol. Environ. Health, Part A, 2009, 72, 284–294 CrossRef CAS PubMed.
- J. Schmale, S. R. Arnold, K. S. Law, T. Thorp, S. Anenberg, W. R. Simpson, J. Mao and K. A. Pratt, Local Arctic air pollution: A neglected but serious problem, Earths Future, 2018, 6, 1385–1412 CrossRef.
- D. C. G. Muir, M. J. Gunnarsdóttir, K. Koziol, F. A. von Heppel, D. Szumińska, N. Ademollo, S. Corsolini, A. De Silva, G. W. Gabrielsen, R. Kallenborn, Ż. Polkowska, E. Kruemmel and K. Vorkamp, Local versus long range transport of POPs in the Arctic: Future developments related to climate change, Environ. Sci.: Adv., 2024, 4, 355–408 Search PubMed.
- B. M. Braune, P. M. Outridge, A. T. Fisk, D. C. G. Muir, P. A. Helm, K. Hobbs, P. F. Hoekstra, Z. A. Kuzyk, M. Kwan, R. J. Letcher, W. L. Lockhart, R. J. Norstrom, G. A. Stern and I. Stirling, Persistent organic pollutants and mercury in marine biota of the Canadian Arctic: An overview of spatial and temporal trends, Sci. Total Environ., 2005, 351–352, 4–56 CrossRef CAS PubMed.
- D. Muir, R. Bossi, P. Carlsson, M. Evans, A. De Silva, C. Halsall, C. Rauert, D. Herzke, H. Hung, R. Letcher, F. Rigét and A. Roos, Levels and trends of poly- and perfluoroalkyl substances in the Arctic environment – An update, Emerging Contam., 2019, 5, 240–271 CrossRef.
- D. Muir, F. Riget, M. Cleemann, J. Skaare, L. Kleivane, H. Nakata, R. Dietz, T. Severinsen and S. Tanabe, Circumpolar trends of PCBs and organochlorine pesticides in the Arctic marine environment inferred from levels in ringed seals, Environ. Sci. Technol., 2000, 34, 2431–2438 CrossRef CAS.
- D. Muir, B. Braune, B. De March, R. Norstrom, R. Wagemann, L. Lockhart, B. Hargrave, D. Bright, R. Addison, J. Payne and K. Reimer, Spatial and temporal trends and effects of contaminants in the Canadian Arctic marine ecosystem: A review, Sci. Total Environ., 1999, 230, 83–144 CrossRef CAS PubMed.
- K. Vorkamp and D. C. G. Muir, in Implications and Consequences of Anthropogenic Pollution in Polar Environments, ed. R. Kallenborn, Springer Berlin Heidelberg, Berlin, Heidelberg, 2016, pp. 229–251 Search PubMed.
- F. Rigét, A. Bignert, B. Braune, M. Dam, R. Dietz, M. Evans, N. Green, H. Gunnlaugsdóttir, K. S. Hoydal, J. Kucklick, R. Letcher, D. Muir, S. Schuur, C. Sonne, G. Stern, G. Tomy, K. Vorkamp and S. Wilson, Temporal trends of persistent organic pollutants in Arctic marine and freshwater biota, Sci. Total Environ., 2019, 649, 99–110 CrossRef PubMed.
- K. Vorkamp, F. F. Rigét, R. Bossi and R. Dietz, Temporal trend of hexabromocyclododecane, polybrominated diphenyl ethers and polychlorinated biphenyls in ringed seals from East Greenland, Environ. Sci. Technol., 2011, 45, 1243–1249 CrossRef CAS PubMed.
- M. Houde, E. M. Krümmel, T. Mustonen, J. Brammer, T. M. Brown, J. Chételat, P. E. Dahl, R. Dietz, M. Evans, M. Gamberg, M.-J. Gauthier, J. Gérin-Lajoie, A. L. Hauptmann, J. P. Heath, D. A. Henri, J. Kirk, B. Laird, M. Lemire, A. E. Lennert, R. J. Letcher, S. Lord, L. Loseto, G. A. MacMillan, S. Mikaelsson, E. A. Mutter, T. O'Hara, S. Ostertag, M. Robards, V. Shadrin, M. Smith, R. Stimmelmayr, E. Sudlovenick, H. Swanson, P. J. Thomas, V. K. Walker and A. Whiting, Contributions and perspectives of Indigenous Peoples to the study of mercury in the Arctic, Sci. Total Environ., 2022, 841, 156566 CrossRef CAS PubMed.
- L. Lowry, Pusa Hispida, Ringed Seal, The IUCN Red List of Threatened Species 2016, e.T41672A45231341, 2016, p. 15, DOI:10.2305/IUCN.UK.2016-1.RLTS.T41672A45231341.en.
- Norwegian Biodiversity Information Centre, Department for Nature Management, Oslo, Norway, https://www.biodiversity.no/.
- COSEWIC, Committee on the Status of Endangered Wildlife in Canada (COSEWIC) assessment and status report on the Ringed Seal Pusa hispida in Canada, Ottawa, Canada, 2019, pp. xii + 82, https://www.canada.ca/en/environment-climate-change/services/species-risk-public-registry.html Search PubMed.
- Ringed seal, Five-Year Status Review: Summary and Evaluation, National Marine Fisheries Service and Alaska Fisheries Science Center, United States, 2024, p. 241.
- F. Rigét, K. Vorkamp, K. A. Hobson, D. C. G. Muir and R. Dietz, Temporal trends of selected POPs and the potential influence of climate variability in a Greenland ringed seal population, Environ. Sci.: Processes Impacts, 2013, 15, 1706 RSC.
- K. Borgå, M. A. McKinney, H. Routti, K. J. Fernie, J. Giebichenstein, I. Hallanger and D. C. G. Muir, The influence of global climate change on accumulation and toxicity of persistent organic pollutants and chemicals of emerging concern in Arctic food webs, Environ. Sci.: Processes Impacts, 2022, 24, 1544–1576 RSC.
- Government of Canada, Canadian Arctic Contaminants Assessment Report Series, 2019, https://science.gc.ca/site/science/en/northern-contaminants-program/publications/canadian-arctic-contaminants-assessment-report-series, Accessed December 2024.
- R. F. Addison, M. E. Zinck and T. G. Smith, PCBs have declined more than the DDT group residues in Arctic ringed seals (Phoca hispida) between 1972 and 1981, Environ. Sci. Technol., 1986, 20, 253–256 CrossRef CAS PubMed.
- M. Cameron and I. M. Weis, Organochlorine contaminants in the country food diet of the Belcher Island Inuit, Northwest Territories, Canada, Arctic, 1993, 46, 42–48 CrossRef.
- M. E. Cameron, T. L. Metcalfe, C. D. Metcalfe and C. R. Macdonald, Persistent organochlorine compounds in the blubber of ringed seals (Phoca hispida) from the Belcher Islands, Northwest Territories, Canada, Mar. Environ. Res., 1997, 43, 99–116 CrossRef CAS.
- R. F. Addison, M. G. Ikonomou, M. P. Fernandez and T. G. Smith, PCDD/F and PCB concentrations in Arctic ringed seals (Phoca hispida) have not changed between 1981 and 2000, Sci. Total Environ., 2005, 351–352, 301–311 CrossRef CAS PubMed.
- M. L. Mallory, B. M. Braune, M. Wayland and K. G. Drouillard, Persistent organic pollutants in marine birds, arctic hare and ringed seals near Qikiqtarjuaq, Nunavut, Canada, Mar. Pollut. Bull., 2005, 50, 95–102 CrossRef CAS PubMed.
- C. R. Powley, S. W. George, M. H. Russell, R. A. Hoke and R. C. Buck, Polyfluorinated chemicals in a spatially and temporally integrated food web in the Western Arctic, Chemosphere, 2008, 70, 664–672 CrossRef CAS PubMed.
- R. F. Addison, D. C. G. Muir, M. G. Ikonomou, L. Harwood and T. G. Smith, Hexachlorocyclohexanes (HCH) in ringed seal (Phoca hispida) from Ulukhaktok (Holman), NT: Trends from 1978 to 2006, Sci. Total Environ., 2009, 407, 5139–5146 CrossRef CAS PubMed.
- A. Gaden, S. H. Ferguson, L. Harwood, H. Melling and G. A. Stern, Mercury trends in ringed seals (Phoca hispida) from the Western Canadian Arctic since 1973: Associations with length of ice-free season, Environ. Sci. Technol., 2009, 43, 3646–3651 CrossRef CAS PubMed.
- R. F. Addison, D. C. Muir, M. G. Ikonomou, L. Harwood, T. G. Smith and J. Alikamik, Temporal trends in “legacy” organochlorine contaminants in blubber of ringed seals (Phoca hispida) from Ulukhaktok, NT, Canada between 1972 and 2010, Sci. Total Environ., 2014, 466–467, 564–576 CrossRef CAS PubMed.
- T. M. Brown, A. T. Fisk, C. C. Helbing and K. J. Reimer, Polychlorinated biphenyl profiles in ringed seals (Pusa Hispida) reveal historical contamination by a military radar station in Labrador, Canada, Environ. Toxicol. Chem., 2014, 33, 592–601 CrossRef CAS PubMed.
- T. M. Brown, S. J. Iverson, A. T. Fisk, R. W. Macdonald, C. C. Helbing and K. J. Reimer, Local contamination, and not feeding preferences, explains elevated PCB concentrations in Labrador ringed seals (Pusa hispida), Sci. Total Environ., 2015, 515–516, 188–197 CrossRef CAS PubMed.
- T. M. Brown, A. T. Fisk, X. Wang, S. H. Ferguson, B. G. Young, K. J. Reimer and D. C. G. Muir, Mercury and cadmium in ringed seals in the Canadian Arctic: Influence of location and diet, Sci. Total Environ., 2016, 545–546, 503–511 CrossRef CAS PubMed.
- M. Houde, X. Wang, T.-L. L. Colson, P. Gagnon, S. H. Ferguson, M. G. Ikonomou, C. Dubetz, R. F. Addison and D. C. G. Muir, Trends of persistent organic pollutants in ringed seals (Phoca hispida) from the Canadian Arctic, Sci. Total Environ., 2019, 665, 1135–1146 CrossRef CAS PubMed.
- M. Houde, Z. E. Taranu, X. Wang, B. Young, P. Gagnon, S. H. Ferguson, M. Kwan and D. C. G. Muir, Mercury in ringed seals (Pusa hispida) from the Canadian Arctic in relation to time and climate parameters, Environ. Toxicol. Chem., 2020, 39, 2462–2474 CrossRef CAS PubMed.
- N. Facciola, M. Houde, D. C. G. Muir, S. H. Ferguson and M. A. McKinney, Feeding and contaminant patterns of sub-arctic and arctic ringed seals: Potential insight into climate change-contaminant interactions, Environ. Pollut., 2022, 313, 120108 CrossRef CAS PubMed.
- Z. Lu, A. O. De Silva, J. F. Provencher, M. L. Mallory, J. L. Kirk, M. Houde, C. Stewart, B. M. Braune, S. Avery-Gomm and D. C. G. Muir, Occurrence of substituted diphenylamine antioxidants and benzotriazole UV stabilizers in Arctic seabirds and seals, Sci. Total Environ., 2019, 663, 950–957 CrossRef CAS PubMed.
- M. Houde, X. Wang, S. H. Ferguson, P. Gagnon, T. M. Brown, S. Tanabe, T. Kunito, M. Kwan and D. C. G. Muir, Spatial and temporal trends of alternative flame retardants and polybrominated diphenyl ethers in ringed seals (Phoca hispida) across the Canadian Arctic, Environ. Pollut., 2017, 223, 266–276 CrossRef CAS PubMed.
- A. M. Jardine, J. F. Provencher, S. J. Insley, L. Tauzer, W. D. Halliday, M. P. T. Bourdages, M. Houde, D. Muir and J. C. Vermaire, No accumulation of microplastics detected in western Canadian ringed seals (Pusa hispida), Mar. Pollut. Bull., 2023, 188, 114692 CrossRef CAS PubMed.
- D. C. G. Muir, R. J. Norstrom and M. Simon, Organochlorine contaminants in arctic marine food chains: accumulation of specific polychlorinated biphenyls and chlordane-related compounds, Environ. Sci. Technol., 1988, 22, 1071–1079 CrossRef CAS PubMed.
- R. Wagemann, S. Innes and P. R. Richard, Overview and regional and temporal differences of heavy metals in Arctic whales and ringed seals in the Canadian Arctic, Sci. Total Environ., 1996, 186, 41–66 CrossRef CAS PubMed.
- I. M. Weis and D. C. G. Muir, Geographical variation of persistent organochlorine concentrations in blubber of ringed seal (Phoca hispida) from the Canadian Arctic: Univariate and multivariate approaches, Environ. Pollut., 1997, 96, 321–333 CrossRef CAS PubMed.
- J. W. Martin, M. M. Smithwick, B. M. Braune, P. F. Hoekstra, D. C. G. Muir and S. A. Mabury, Identification of long-chain perfluorinated Acids in biota from the Canadian Arctic, Environ. Sci. Technol., 2004, 38, 373–380 CrossRef CAS PubMed.
- B. Gouteux, M. Lebeuf, M. O. Hammill, D. C. G. Muir and J.-P. Gagné, Comparison of toxaphene congeners levels in five seal species from Eastern Canada: What is the importance of biological factors?, Environ. Sci. Technol., 2005, 39, 1448–1454 Search PubMed.
- C. M. Butt, D. C. G. Muir, I. Stirling, M. Kwan and S. A. Mabury, Rapid response of Arctic ringed seals to changes in perfluoroalkyl production, Environ. Sci. Technol., 2007, 41, 42–49 CrossRef CAS PubMed.
- C. M. Butt, S. A. Mabury, M. Kwan, X. Wang and D. C. G. Muir, Spatial trends of perfluoroalkyl compounds in ringed seals (Phoca hispida) from the Canadian Arctic, Environ. Toxicol. Chem., 2008, 27, 542–553 Search PubMed.
- P. M. Outridge, K. A. Hobson and J. Savelle, Long-term changes of mercury levels in ringed seal (Phoca hispida) from Amundsen Gulf, and beluga (Delphinapterus leucas) from the Beaufort Sea, western Canadian Arctic, Sci. Total Environ., 2009, 407, 6044–6051 Search PubMed.
- A. Gaden, S. H. Ferguson, L. Harwood, H. Melling, J. Alikamik and G. A. Stern, Western Canadian Arctic ringed seal organic contaminant trends in relation to sea ice break-up, Environ. Sci. Technol., 2012, 46, 4427–4433 Search PubMed.
- CACAR – Canadian Arctic Contaminants Assessment Report III – 2012: Mercury in Canada's North/Northern Contaminants Program, ed. J. Chételat and B. M. Braune, Aboriginal Affairs and Northern Development Canada, R74-2/1-2013E-PDF, Canada, 2012, vol. xxiii, p. 276 Search PubMed.
- D. Muir, P. Kurt-Karakus, and J. Stow., CACAR – Canadian Arctic Contaminants Assessment Report on Persistent Organic Pollutants, Aboriginal Affairs and Northern Development Canada, Canada, 2013, pp. 273–422 Search PubMed.
- R. F. Addison, D. C. G. Muir, M. G. Ikonomou, C. Dubetz, T. G. Smith and J. Alikamik, Temporal trends in polybrominated diphenylethers (PBDEs) in blubber of ringed seals (Pusa hispida) from Ulukhaktok, NT, Canada between 1981 and 2015, Arch. Environ. Contam. Toxicol., 2020, 79, 167–176 CrossRef CAS PubMed.
- D. J. Yurkowski, E. S. Richardson, N. J. Lunn, D. C. G. Muir, A. C. Johnson, A. E. Derocher, A. D. Ehrman, M. Houde, B. G. Young, C. D. Debets, L. Sciullo, G. W. Thiemann and S. H. Ferguson, Contrasting temporal patterns of mercury, niche dynamics, and body fat indices of polar bears and ringed seals in a melting icescape, Environ. Sci. Technol., 2020, 54, 2780–2789 Search PubMed.
- N. Facciola, S. Pedro, M. Houde, A. T. Fisk, S. H. Ferguson, H. Steer, D. C. G. Muir and M. A. McKinney, Measurable levels of short-chain chlorinated paraffins in Western Hudson Bay fishes but limited biomagnification from fish to ringed seals, Environ. Toxicol. Chem., 2021, 40, 2990–2999 CrossRef CAS PubMed.
- K. Vorkamp, R. Young, M. Houde, H. Routti and F. Rigét, Geographical trends of short-chain chlorinated paraffins (SCCPs) in ringed seals from the Arctic, Scientific Report from DCE – Danish Centre for Environment and Energy, 2023, https://dce.au.dk/fileadmin/dce.au.dk/Udgivelser/Videnskabelige_rapporter_500-599/SR582.pdf Search PubMed.
- R. Lohmann, K. Abass, E. C. Bonefeld-Jørgensen, R. Bossi, R. Dietz, S. Ferguson, K. J. Fernie, P. Grandjean, D. Herzke, M. Houde, M. Lemire, R. J. Letcher, D. Muir, A. O. De Silva, S. K. Ostertag, A. A. Rand, J. Søndergaard, C. Sonne, E. M. Sunderland, K. Vorkamp, S. Wilson and P. Weihe, Cross-cutting studies of per- and polyfluorinated alkyl substances (PFAS) in Arctic wildlife and humans, Sci. Total Environ., 2024, 176274 Search PubMed.
- R. Dietz, C. O. Nielsen, M. M. Hansen and C. T. Hansen, Organic mercury in Greenland birds and mammals, Sci. Total Environ., 1990, 95, 41–51 Search PubMed.
- R. Dietz, F. Riget and P. Johansen, Lead, cadmium, mercury and selenium in Greenland marine animals, Sci. Total Environ., 1996, 186, 67–93 CrossRef CAS PubMed.
- R. Dietz, P. Paludan-Müller, C. T. Agger and C. O. Nielsen, Cadmium, mercury, zinc and selenium in ringed seals (Phoca hispida) from Greenland and Svalbard, NAMMCO Sci. Publ., 1998, 1, 242 Search PubMed.
- M. Cleemann, F. Riget, G. Paulsen, J. De Boer and R. Dietz, Organochlorines in Greenland ringed seals (Phoca hispida), Sci. Total Environ., 2000, 245, 103–116 Search PubMed.
- AMAP, AMAP Greenland and the Faroe Islands 1997-2001, The Environment of Greenland, ed F. Riget, J. Christensen and P. Johansen, Danish Cooperation for Environment in the Arctic Ministry of Environment, 2003, vol. 2, p. 193 Search PubMed.
- K. Vorkamp, J. Christensen and F. Riget, Polybrominated diphenyl ethers and organochlorine compounds in biota from the marine environment of East Greenland, Sci. Total Environ., 2004, 331, 143–155 CrossRef CAS PubMed.
- R. Bossi, F. F. Riget and R. Dietz, Temporal and spatial trends of perfluorinated compounds in ringed seal (Phoca hispida) from Greenland, Environ. Sci. Technol., 2005, 39, 7416–7422 CrossRef CAS PubMed.
- R. Bossi, F. F. Riget, R. Dietz, C. Sonne, P. Fauser, M. Dam and K. Vorkamp, Preliminary screening of perfluorooctane sulfonate (PFOS) and other fluorochemicals in fish, birds and marine mammals from Greenland and the Faroe Islands, Environ. Pollut., 2005, 136, 323–329 Search PubMed.
- F. Riget, J. Vikelsøe and R. Dietz, Levels and temporal trends of PCDD/PCDFs and non-ortho PCBs in ringed seals from East Greenland, Mar. Pollut. Bull., 2005, 50, 1523–1529 Search PubMed.
- F. Rigét, K. Vorkamp, R. Dietz and S. C. Rastogi, Temporal trend studies on polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in ringed seals from East Greenland, J. Environ. Monit., 2006, 8, 1000–1005 Search PubMed.
- M. Frederiksen, K. Vorkamp, R. Bossi, F. Rigét, M. Dam and B. Svensmark, Method development for simultaneous analysis of HBCD, TBBPA, and dimethyl-TBBPA in marine biota from Greenland and the Faroe Islands, Int. J. Environ. Anal. Chem., 2007, 87, 1095–1109 Search PubMed.
- F. Rigét, K. Vorkamp, R. Dietz and D. C. G. Muir, Levels and temporal trends of HCH isomers in ringed seals from West and East Greenland, J. Environ. Monit., 2008, 10, 935 RSC.
- K. Vorkamp, F. F. Rigét, M. Glasius, D. C. G. Muir and R. Dietz, Levels and trends of persistent organic pollutants in ringed seals (Phoca hispida) from Central West Greenland, with particular focus on polybrominated diphenyl ethers (PBDEs), Environ. Int., 2008, 34, 499–508 Search PubMed.
- F. Rigét, R. Dietz and K. A. Hobson, Temporal trends of mercury in Greenland ringed seal populations in a warming climate, J. Environ. Monit., 2012, 14, 3249 RSC.
- A. Rotander, B. van Bavel, F. Rigét, G. A. Auðunsson, A. Polder, G. W. Gabrielsen, G. Víkingsson, B. Mikkelsen and M. Dam, Polychlorinated naphthalenes (PCNs) in sub-Arctic and Arctic marine mammals, 1986–2009, Environ. Pollut., 2012, 164, 118–124 Search PubMed.
- A. Rotander, A. Kärrman, B. V. Bavel, A. Polder, F. Rigét, G. A. Auðunsson, G. Víkingsson, G. W. Gabrielsen, D. Bloch and M. Dam, Increasing levels of long-chain perfluorocarboxylic acids (PFCAs) in Arctic and North Atlantic marine mammals, 1984–2009, Chemosphere, 2012, 86, 278–285 Search PubMed.
- K. Vorkamp, K. Bester and F. F. Rigét, Species-specific time trends and enantiomer fractions of hexabromocyclododecane (HBCD) in biota from East Greenland, Environ. Sci. Technol., 2012, 46, 10549–10555 CrossRef CAS PubMed.
- F. Rigét, R. Bossi, C. Sonne, K. Vorkamp and R. Dietz, Trends of perfluorochemicals in Greenland ringed seals and polar bears: Indications of shifts to decreasing trends, Chemosphere, 2013, 93, 1607–1614 CrossRef PubMed.
- K. Vorkamp, F. F. Rigét and R. Dietz, Toxaphene in the aquatic environment of Greenland, Environ. Pollut., 2015, 200, 140–148 CrossRef CAS PubMed.
- K. Vorkamp, R. Bossi, F. F. Rigét, H. Skov, C. Sonne and R. Dietz, Novel brominated flame retardants and dechlorane plus in Greenland air and biota, Environ. Pollut., 2015, 196, 284–291 CrossRef CAS PubMed.
- W. A. Gebbink, R. Bossi, F. F. Rigét, A. Rosing-Asvid, C. Sonne and R. Dietz, Observation of emerging per- and polyfluoroalkyl substances (PFASs) in Greenland marine mammals, Chemosphere, 2016, 144, 2384–2391 Search PubMed.
- K. Vorkamp, F. F. Rigét, R. Bossi, C. Sonne and R. Dietz, Endosulfan, short-chain chlorinated Paraffins (SCCPs) and octachlorostyrene in wildlife from Greenland: Levels, trends and methodological challenges, Arch. Environ. Contam. Toxicol., 2017, 73, 542–551 CrossRef CAS PubMed.
- J. E. Balmer, H. Hung, K. Vorkamp, R. J. Letcher and D. C. G. Muir, Hexachlorobutadiene (HCBD) contamination in the Arctic environment: A review, Emerging Contam., 2019, 5, 116–122 Search PubMed.
- F. Rigét, K. Vorkamp, I. Eulaers and R. Dietz, Influence of climate and biological variables on temporal trends of persistent organic pollutants in Arctic char and ringed seals from Greenland, Environ. Sci.: Processes Impacts, 2020, 22, 993–1005 RSC.
- J. O. Boyi, C. Sonne, R. Dietz, F. Rigét, U. Siebert and K. Lehnert, Gene expression and trace elements in Greenlandic ringed seals (Pusa hispida), Environ. Res., 2024, 244, 117839 CrossRef CAS PubMed.
- E. U. Andersen-Ranberg, P. S. Leifsson, F. F. Rigét, J. Søndergaard, S. Andersen, A. K. O. Alstrup, R. Dietz and C. Sonne, Element concentrations and histopathology of liver and kidney in West Greenland ringed seals (Pusa hispida), Animals, 2024, 14, 1739 Search PubMed.
- L. Zhu, R. Bossi, P. N. Carvalho, F. F. Rigét, J. H. Christensen, P. Weihe, E. C. Bonefeld-Jørgensen and K. Vorkamp, Suspect and non-target screening of chemicals of emerging Arctic concern in biota, air and human serum, Environ. Pollut., 2024, 360, 124605 Search PubMed.
- M. Oehme, P. Fürst, C. Krüger and H. A. Meemken, Presence of polychlorinated dibenzo-p-dioxins, dibenzofurans and pesticides in Arctic seal from Spitzbergen, Chemosphere, 1988, 17, 1291–1300 Search PubMed.
- F. F. Daelemans, F. Mehlum, C. Lydersen and P. J. C. Schepens, Mono-ortho and non-ortho substituted PCBs in Arctic ringed seal (Phoca hispida) from the Svalbard area: Analysis and determination of their toxic threat, Chemosphere, 1993, 27, 429–437 Search PubMed.
- J. U. Skaare, Environmental pollutants in marine mammals from the Norwegian coast and Arctic, Sci. Total Environ., 1996, 186, 25–27 CrossRef CAS PubMed.
- J. Wolkers, I. C. Burkow, C. Lydersen, S. Dahle, M. Monshouwer and R. F. Witkamp, Congener specific PCB and polychlorinated camphene (toxaphene) levels in Svalbard ringed seals (Phoca hispida) in relation to sex, age, condition and cytochrome P450 enzyme activity, Sci. Total Environ., 1998, 216, 1–11 CrossRef CAS PubMed.
- S. Føreid, T. Rundberget, T. Severinsen and J. U. Skaare, Determination of toxaphenes in fish and marine mammals, Chemosphere, 2000, 41, 521–528 CrossRef PubMed.
- L. Kleivane, T. Severinsen and J. U. Skaare, Biological transport and mammal to mammal transfer of organochlorines in Arctic fauna, Mar. Environ. Res., 2000, 49, 343–357 CrossRef CAS PubMed.
- T. Severinsen, J. U. Skaare and C. Lydersen, Spatial distribution of persistent organochlorines in ringed seal (Phoca hispida) blubber, Mar. Environ. Res., 2000, 49, 291–302 CrossRef CAS PubMed.
- K. Bang, B. M. Jenssen, C. Lydersen and J. U. Skaare, Organochlorine burdens in blood of ringed and bearded seals from north-western Svalbard, Chemosphere, 2001, 44, 193–203 CrossRef CAS PubMed.
- M. L. Fant, M. Nyman, E. Helle and E. Rudbäck, Mercury, cadmium, lead and selenium in ringed seals (Phoca hispida) from the Baltic Sea and from Svalbard, Environ. Pollut., 2001, 111, 493–501 CrossRef CAS PubMed.
- H. Hop, K. Borgå, G. W. Gabrielsen, L. Kleivane and J. U. Skaare, Food web magnification of persistent organic pollutants in poikilotherms and homeotherms from the Barents Sea, Environ. Sci. Technol., 2002, 36, 2589–2597 CrossRef CAS PubMed.
- M. Nyman, J. Koistinen, M. L. Fant, T. Vartiainen and E. Helle, Current levels of DDT, PCB and trace elements in the Baltic ringed seals (Phoca hispida baltica) and grey seals (Halichoerus grypus), Environ. Pollut., 2002, 119, 399–412 CrossRef CAS PubMed.
- H. Wolkers, B. A. Krafft, B. van Bavel, L. B. Helgason, C. Lydersen and K. M. Kovacs, Biomarker responses and decreasing contaminant levels in ringed seals (Pusa hispida) from Svalbard, Norway, J. Toxicol. Environ. Health, Part A, 2008, 71, 1009–1018 CrossRef CAS PubMed.
- H. Routti, R. J. Letcher, A. Arukwe, B. Van Bavel, N. G. Yoccoz, S. Chu and G. W. Gabrielsen, Biotransformation of PCBs in relation to phase I and II xenobiotic-metabolizing enzyme activities in ringed seals (Phoca hispida) from Svalbard and the Baltic Sea, Environ. Sci. Technol., 2008, 42, 8952–8958 CrossRef CAS PubMed.
- H. Routti, B. van Bavel, R. J. Letcher, A. Arukwe, S. Chu and G. W. Gabrielsen, Concentrations, patterns and metabolites of organochlorine pesticides in relation to xenobiotic phase I and II enzyme activities in ringed seals (Phoca hispida) from Svalbard and the Baltic Sea, Environ. Pollut., 2009, 157, 2428–2434 CrossRef CAS PubMed.
- H. Routti, R. J. Letcher, S. Chu, B. van Bavel and G. W. Gabrielsen, Polybrominated diphenyl ethers and their hydroxylated analogues in ringed seals (Phoca hispida) from Svalbard and the Baltic Sea, Environ. Sci. Technol., 2009, 43, 3494–3499 CrossRef CAS PubMed.
- K. Sagerup, D. Herzke, M. Harju, A. Evenset, G. N. Christensen, E. Fuglei, J. Aars, H. Strøm and G. W. Gabrielsen, New Brominated Flame Retardants in Arctic Biota, 2010, SPFO-rapport 1070/2010 TA-2630/2010, Norway, p. 32 Search PubMed.
- Norwegian Climate and Pollution Agency, Perfluorinated Alkylated Substances (PFAS), Brominated Flame Retardants (BFR) and Chlorinated Paraffins (CP) in the Norwegian Environment Screening, NILU, Tromsø, 2013, p. 110 Search PubMed.
- I. G. Hallanger, K. Sagerup, A. Evenset, K. M. Kovacs, P. Leonards, E. Fuglei, H. Routti, J. Aars, H. Strøm, C. Lydersen and G. W. Gabrielsen, Organophosphorous flame retardants in biota from Svalbard, Norway, Mar. Pollut. Bull., 2015, 101, 442–447 CrossRef CAS PubMed.
- H. Routti, G. W. Gabrielsen, D. Herzke, K. M. Kovacs and C. Lydersen, Spatial and temporal trends in perfluoroalkyl substances (PFASs) in ringed seals (Pusa hispida) from Svalbard, Environ. Pollut., 2016, 214, 230–238 CrossRef CAS PubMed.
- M. Schlabach, G. W. Gabrielsen, D. Herzke, L. Hanssen, H. Routti and A. Borgen, Screening of PFAS and Dechlorane Compounds in Selected Arctic Top Predators, NILU report 40/2017, Norway, 2017, p. 34 Search PubMed.
- A. Lippold, M. Harju, J. Aars, P. Blévin, J. Bytingsvik, G. W. Gabrielsen, K. M. Kovacs, J. L. Lyche, C. Lydersen, A. H. Rikardsen and H. Routti, Occurrence of emerging brominated flame retardants and organophosphate esters in marine wildlife from the Norwegian Arctic, Environ. Pollut., 2022, 315, 120395 CrossRef CAS PubMed.
- M. M. Schantz, B. J. Koster, S. A. Wise and P. R. Becker, Determination of PCBs and chlorinated hydrocarbons in marine mammal tissues, Sci. Total Environ., 1993, 139–140, 323–345 CrossRef CAS PubMed.
- M. M. Krahn, P. R. Becker, K. L. Tilbury and J. E. Stein, Organochlorine contaminants in blubber of four seal species: Integrating biomonitoring and specimen banking, Chemosphere, 1997, 34, 2109–2121 CrossRef CAS PubMed.
- P. R. Becker, S. A. Wise, M. M. Schantz, B. J. Koster and R. Zeisler, Alaska Marine Mammal Tissue Archival Project: Sample Inventory and Results of Analyses of Selected Samples for Organic Compounds and Trace Elements, U.S. Department of Commerce, 1992, p. 144 Search PubMed.
- R. Zeisler, R. Demiralp, B. J. Koster, P. R. Becker, M. Burow, P. Ostapczuk and S. A. Wise, Determination of inorganic constituents in marine mammal tissues, Sci. Total Environ., 1993, 139–140, 365–386 CrossRef CAS PubMed.
- P. R. Becker, E. A. Mackey, M. M. Schantz, R. Demiralp, R. R. Greenberg, B. J. Koster, S. A. Wise and D. C. G. Muir, Concentrations of Chlorinated Hydrocarbons, Heavy Metals and Other Elements in Tissues Banked by the Alaska Marine Mammal Tissue Archival Project, National Institute of Standards and Technology, Gaithersburg, United States, 1995, p. 126 Search PubMed.
- P. R. Becker, E. A. Mackey, R. Demiralp, M. M. Schar, B. J. Kostel and S. A. Wise, Concentrations of chlorinated hydrocarbons and trace elements in marine mammal tissues archived in the U.S. National Biomonitoring Specimen Bank, Chemosphere, 1997, 34, 2067–2098 CrossRef CAS PubMed.
- J. R. Kucklick, W. D. J. Struntz, P. R. Becker, G. W. York, T. M. O'Hara and J. E. Bohonowych, Persistent organochlorine pollutants in ringed seals and polar bears collected from northern Alaska, Sci. Total Environ., 2002, 287, 45–59 CrossRef CAS PubMed.
- P. F. Hoekstra, T. M. O'Hara, S. M. Backus, C. Hanns and D. C. G. Muir, Concentrations of persistent organochlorine contaminants in bowhead whale tissues and other biota from northern Alaska: Implications for human exposure from a subsistence diet, Environ. Res., 2005, 98, 329–340 CrossRef CAS PubMed.
- L. T. Quakenbush and J. J. Citta, Perfluorinated contaminants in ringed, bearded, spotted, and ribbon seals from the Alaskan Bering and Chukchi Seas, Mar. Pollut. Bull., 2008, 56, 1809–1814 CrossRef CAS PubMed.
- H. Nakata, S. Tanabe, R. Tatsukawa, Y. Koyama, N. Miyazaki, S. Belikov and A. Boltunov, Persistent organochlorine contaminants in ringed seals (Phoca hispida) from the Kara Sea, Russian Arctic, Environ. Toxicol. Chem., 1998, 17, 1745–1755 CrossRef CAS.
- A. Kostamo, N. Medvedev, J. Pellinen, H. Hyvärinen and J. V. K. Kukkonen, Analysis of organochlorine compounds and extractable organic halogen in three subspecies of ringed seal from Northeast Europe, Environ. Toxicol. Chem., 2000, 19, 848–854 CrossRef CAS.
- D. Muir, T. Savinova, V. Savinov, L. Alexeeva, V. Potelov and V. Svetochev, Bioaccumulation of PCBs and chlorinated pesticides in seals, fishes and invertebrates from the White Sea, Russia, Sci. Total Environ., 2003, 306, 111–131 CrossRef CAS PubMed.
- Z. Amirova, E. Kruglov, S. Vlasov, E. Loshkina and R. Khalilov, PCBs and PCDD/Fs distribution in tissues and organs of marine animals in Russian Arctic, POPs in marine mammals: levels and effects, Organohalogen Compd., 2004, 66, 1506–1514 CAS.
- AMAP, Persistent Toxic Substances, Food Security and Indigenous Peoples of the Russian North. Final Report, Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway, 2004, p. 192 Search PubMed.
- V. Savinov, D. C. G. Muir, V. Svetochev, O. Svetocheva, S. Belikov, A. Boltunov, L. Alekseeva, L.-O. Reiersen and T. Savinova, Persistent organic pollutants in ringed seals from the Russian Arctic, Sci. Total Environ., 2011, 409, 2734–2745 CrossRef CAS PubMed.
- A. A. Dudarev, V. S. Chupakhin, S. V. Vlasov and S. Yamin-Pasternak, Traditional Diet and Environmental Contaminants in Coastal Chukotka II: Legacy POPs, Int. J. Environ. Res. Public Health, 2019, 16, 695 CrossRef CAS PubMed.
- A. A. Dudarev, V. Chupakhin, S. Vlasov and S. Yamin-Pasternak, Traditional Diet and Environmental Contaminants in Coastal Chukotka III: Metals, Int. J. Environ. Res. Public Health, 2019, 16, 699 CrossRef CAS PubMed.
- F. Rigét, K. Vorkamp, R. Bossi, C. Sonne, R. J. Letcher and R. Dietz, Twenty years of monitoring of persistent organic pollutants in Greenland biota. A review, Environ. Pollut., 2016, 217, 114–123 CrossRef PubMed.
- K. Vorkamp, M. Dam, F. Rigét, P. Fauser, R. Bossi and A. B. Hansen, Screening of «new» contaminants in the marine environment of Greenland and the Faroe Islands, NERI Technical Report no. 525, 2004. https://www2.dmu.dk/1_viden/2_publikationer/3_fagrapporter/rapporter/fr525.pdf Search PubMed.
- J. E. Balmer, A. D. Morris, H. Hung, L. Jantunen, K. Vorkamp, F. Rigét, M. Evans, M. Houde and D. C. G. Muir, Levels and trends of current-use pesticides (CUPs) in the Arctic: An updated review, 2010–2018, Emerging Contam., 2019, 5, 70–88 CrossRef.
- Norwegian Polar Institute, MOSJ, Environmental monitoring program for Svalbard and Jan Maye, https://mosj.no/en/indikator/influence/pollution/pollutants-in-ringed-seals/, accessed December 10, 2024.
- M. G. Castelli, M. Rusten, A. Goksøyr and H. Routti, mRNA expression of genes regulating lipid metabolism in ringed seals (Pusa hispida) from differently polluted areas, Aquat. Toxicol., 2014, 146, 239–246 CrossRef CAS PubMed.
- M. Kanerva, H. Routti, Y. Tamuz, M. Nyman and M. Nikinmaa, Antioxidative defense and oxidative stress in ringed seals (Pusa hispida) from differently polluted areas, Aquat. Toxicol., 2012, 114–115, 67–72 CrossRef CAS PubMed.
- M. Nyman, H. Raunio and O. Pelkonen, Expression and inducibility of members in the cytochrome P4501 (CYP1) family in ringed and grey seals from polluted and less polluted waters, Environ. Toxicol. Pharmacol., 2000, 8, 217–225 CrossRef CAS PubMed.
- M. Nyman, H. Raunio, P. Taavitsainen and O. Pelkonen, Characterization of xenobiotic-metabolizing cytochrome P450 (CYP) forms in ringed and grey seals from the Baltic Sea and reference sites, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2001, 128, 99–1112 CAS.
- M. Nyman, M. Bergknut, M. L. Fant, H. Raunio, M. Jestoi, C. Bengs, A. Murk, J. Koistinen, C. Bäckman, O. Pelkonen, M. Tysklind, T. Hirvi and E. Helle, Contaminant exposure and effects in Baltic ringed and grey seals as assessed by biomarkers, Mar. Environ. Res., 2003, 55, 73–99 CrossRef CAS PubMed.
- H. Routti, A. Arukwe, B. M. Jenssen, R. J. Letcher, M. Nyman, C. Bäckman and G. W. Gabrielsen, Comparative endocrine disruptive effects of contaminants in ringed seals (Phoca hispida) from Svalbard and the Baltic Sea, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2010, 152, 306–312 Search PubMed.
- H. Routti, R. J. Letcher, A. Arukwe, B. van Bavel, N. G. Yoccoz, S. Chu and G. W. Gabrielsen, Biotransformation of PCBs in relation to phase I and II xenobiotic-metabolizing enzyme activities in ringed seals (Phoca hispida) from Svalbard and the Baltic Sea, Environ. Sci. Technol., 2008, 42, 8952–8958 CrossRef CAS PubMed.
- G. M. Troisi, S. J. Barton, O. Liori and M. Nyman, Polychlorinated biphenyls (PCBs) and sex hormone concentrations in ringed and grey seals: A possible link to endocrine disruption?, Arch. Environ. Contam. Toxicol., 2020, 78, 513–524 CrossRef CAS PubMed.
- A. A. Dudarev and J. O. Odland, Forty-Year biomonitoring of environmental contaminants in Russian Arctic: Progress, gaps and perspectives, Int. J. Environ. Res. Public Health, 2022, 19, 11951 CrossRef PubMed.
- P. R. Becker, Concentration of chlorinated hydrocarbons and heavy metals in Alaska Arctic marine mammals, Mar. Pollut. Bull., 2000, 40, 819–829 CrossRef CAS.
- T. Heleniak, The future of the Arctic populations, Polar Geogr., 2021, 44, 136–152 CrossRef.
- HELCOM, Hazardous Substances in the Baltic Sea an Integrated Thematic Assessment of Hazardous Substances in the Baltic Sea, Baltic Sea Environment Proceedings No. 120B, Helsinki Commission, Baltic Marine Environment Protection Commission, Finland, 2010, p. 119 Search PubMed.
- M. McLachlan and F. Undeman, Dioxins and PCBs in the Baltic Sea, Helcom Baltic Sea Environment Proceedings No. 171, Helsinki, Finland, 2020, p. 24 Search PubMed.
- HELCOM, PCBs, Dioxins and Furans. HELCOM Core Indicator Report, ISSN 2343-2543, 2018, p. 20 Search PubMed.
- M. Olsson, B. Karlsson and E. Ahnland, Diseases and environmental contaminants in seals from the Baltic and the Swedish west coast, Sci. Total Environ., 1994, 154, 217–227 CrossRef CAS PubMed.
- K. Haraguchi, M. Athanasiadou, Å. Bergman, L. Hovander and S. Jensen, CB and PCB methyl sulfones in selected groups of seals from Swedish waters, Ambio, 1992, 21, 546549 Search PubMed.
- E. Helle, M. Olsson and S. Jensen, DDT and PCB levels and reproduction in ringed seal from the Bothnian Bay, Ambio, 1976, 5, 188–189 CAS.
- E. Helle, Lowered reproductive capacity in female ringed seals (Pusa hispida) in the Bothnian Bay, northern Baltic Sea, with special reference to uterine occlusions, Ann. Zool. Fenn., 1980, 17, 147–158 Search PubMed.
- C. Sonne, U. Siebert, K. Gonnsen, J.-P. Desforges, I. Eulaers, S. Persson, A. Roos, B.-M. Bäcklin, K. Kauhala, M. Tange Olsen, K. C. Harding, G. Treu, A. Galatius, E. Andersen-Ranberg, S. Gross, J. Lakemeyer, K. Lehnert, S. S. Lam, W. Peng and R. Dietz, Health effects from contaminant exposure in Baltic Sea birds and marine mammals: A review, Environ. Int., 2020, 139, 105725 CrossRef CAS PubMed.
- S. Jensen, A. G. Johnels, M. Olsson and G. Otterlind, DDT and PCB in marine animals from Swedish waters, Nature, 1969, 224, 247–250 CrossRef CAS PubMed.
- F. Bjurlid, A. Roos, I. Ericson Jogsten and J. Hagberg, Temporal trends of PBDD/Fs, PCDD/Fs, PBDEs and PCBs in ringed seals from the Baltic Sea (Pusa hispida botnica) between 1974 and 2015, Sci. Total Environ., 2018, 616–617, 1374–1383 CrossRef CAS PubMed.
- T. M. Brown, S. Luque, B. Sjare, A. T. Fisk, C. C. Helbing and K. J. Reimer, Satellite telemetry informs PCB source apportionment in a mobile, high trophic level marine mammal: The ringed seal (Pusa hispida), Environ. Sci. Technol., 2014, 48, 13110–13119 CrossRef CAS PubMed.
- S. J. Trumble, E. M. Robinson, S. R. Noren, S. Usenko, J. Davis and S. B. Kanatous, Assessment of legacy and emerging persistent organic pollutants in Weddell seal tissue (Leptonychotes weddellii) near McMurdo Sound, Antarctica, Sci. Total Environ., 2012, 439, 275–283 CrossRef CAS PubMed.
- H. Routti, B. A. Krafft, D. Herzke, R. Eisert and O. Oftedal, Perfluoroalkyl substances detected in the world's southernmost marine mammal, the Weddell seal (Leptonychotes weddellii), Environ. Pollut., 2015, 197, 62–67 CrossRef CAS PubMed.
- M. Cai, H. Yang, Z. Xie, Z. Zhao, F. Wang, Z. Lu, R. Sturm and R. Ebinghaus, Per- and polyfluoroalkyl substances in snow, lake, surface runoff water and coastal seawater in Fildes Peninsula, King George Island, Antarctica, J. Hazard. Mater., 2012, 209–210, 335–342 CrossRef CAS PubMed.
- M. C. Kennicutt, S. J. McDonald, J. L. Sericano, P. Boothe, J. Oliver, S. Safe, B. J. Presley, H. Liu, D. Wolfe, T. L. Wade, A. Crockett and D. Bockus, Human contamination of the marine environment- Arthur Harbor and McMurdo Sound, Antarctica, Environ. Sci. Technol., 1995, 295, 1279–1287 CrossRef PubMed.
- A. Negri, K. Burns, S. Boyle, D. Brinkman and N. Webster, Contamination in sediments, bivalves and sponges of McMurdo Sound, Antarctica, Environ. Pollut., 2006, 143, 456–467 CrossRef CAS PubMed.
- R. C. Hale, S. L. Kim, E. Harvey, M. J. La Guardia, T. M. Mainor, E. O. Bush and E. M. Jacobs, Antarctic research bases: Local sources of polybrominated diphenyl ether (PBDE) flame retardants, Environ. Sci. Technol., 2008, 42, 1452–1457 CrossRef CAS PubMed.
- M. J. Gunnarsdottir, Ongoing legacy contamination from a military radar station in Iceland: a case study, Environ. Sci.: Adv., 2024, 3, 972–982 CAS.
- P. E. Jensen, S. Gewurtz, M. Hanson, P. Rossi, M. Velmitskaya, I. B. Overjordet, H. O. Andradottir, A. Dotson, R. Mortensen, K. Hoydal, L. T. Hansen, H. Kvitsand, D. Borato, A. Richard and R. Jamieson, The importance of wastewater as source of POPs and CEACs in the Arctic environment, Environ. Sci.: Adv., 2025 Search PubMed , Submitted.
- J. Chételat, M. Amyot and E. Garcia, Habitat-specific bioaccumulation of methylmercury in invertebrates of small mid-latitude lakes in North America, Environ. Pollut., 2011, 159, 10–17 CrossRef PubMed.
- C. Andvik, E. Jourdain, A. Borgen, J. L. Lyche, R. Karoliussen, T. Haug and K. Borgå, Intercorrelations of chlorinated paraffins, dechloranes, and legacy persistent organic pollutants in 10 species of marine mammals from Norway, in light of dietary niche, Environ. Sci. Technol., 2024, 58, 14797–14811 CrossRef CAS PubMed.
- J. O. Bustnes, K. Borgå, T. Dempster, E. Lie, T. Nygård and I. Uglem, Latitudinal distribution of persistent organic pollutants in pelagic and demersal marine fish on the Norwegian coast, Environ. Sci. Technol., 2012, 46, 7836–7843 CrossRef CAS PubMed.
- NASA Socioeconomic Data and Applications Center (SEDAC) population estimator, Center for International Earth Science Information Network, 2018, https://www.earthdata.nasa.gov/data/tools/sedac-population-estimator.
- J. Oksanen, G. Simpson, F. Blanchet, R. Kindt, P. Legendre, P. Minchin, R. O'Hara, P. Solymos, M. Stevens, E. Szoecs, H. Wagner, M. Barbour, M. Bedward, B. Bolker, D. Borcard, G. Carvalho, M. Chirico, M. De Caceres, S. Durand, H. Evangelista, R. FitzJohn, M. Friendly, B. Furneaux, G. Hannigan, M. Hill, L. Lahti, D. McGlinn, M. Ouellette, E. Ribeiro Cunha, T. Smith, A. Stier, C. Ter Braak, J. Weedon, Package ‘vegan’, Community Ecology Package, 2024, version 2.6-8, URL https://github.com/vegandevs/vegan.
- C. R. Perryman, J. Wirsing, K. A. Bennett, O. Brennick, A. L. Perry, N. Williamson and J. G. Ernakovich, Heavy metals in the Arctic: Distribution and enrichment of five metals in Alaskan soils, PLoS One, 2020, 15, e0233297 CrossRef CAS PubMed.
- S. V. Loiko, O. S. Pokrovsky, T. V. Raudina, A. Lim, L. G. Kolesnichenko, L. S. Shirokova, S. N. Vorobyev and S. N. Kirpotin, Abrupt permafrost collapse enhances organic carbon, CO2, nutrient and metal release into surface waters, Chem. Geol., 2017, 471, 153–165 CrossRef CAS.
- D. A. Bright, W. T. Dushenko, S. L. Grundy and K. J. Reimer, Evidence for short-range transport of polychlorinated biphenyls in the Canadian Arctic using congener signatures of PCBs in soils, Sci. Total Environ., 1995, 160–161, 251–263 CrossRef.
- J. P. Stow, J. Sova and K. J. Reimer, The relative influence of distant and local (DEW-line) PCB sources in the Canadian Arctic, Sci. Total Environ., 2005, 342, 107–118 CrossRef CAS PubMed.
- A. Cabrerizo, D. C. G. Muir, A. O. De Silva, X. Wang, S. F. Lamoureux and M. J. Lafrenière, Legacy and emerging persistent organic pollutants (POPs) in terrestrial compartments in the High Arctic: Sorption and secondary sources, Environ. Sci. Technol., 2018, 52, 14187–14197 CrossRef CAS PubMed.
- J. S. Skaar, E. M. Ræder, J. L. Lyche, L. Ahrens and R. Kallenborn, Elucidation of contamination sources for poly- and perfluoroalkyl substances (PFASs) on Svalbard (Norwegian Arctic), Environ. Sci. Pollut. Res., 2019, 26, 7356–7363 CrossRef CAS PubMed.
- G. Abbasi, L. Li and K. Breivik, Global historical stocks and emissions of PBDEs, Environ. Sci. Technol., 2019, 53, 6330–6340 CrossRef CAS PubMed.
- T. R. Ross, G. W. Thiemann, B. G. Young and S. H. Ferguson, Complementary diet analyses reveal intraspecific and temporal variation in ringed seal (Pusa hispida) foraging in the Canadian high arctic, Polar Biol., 2022, 45, 465–480 CrossRef.
- S. J. Insley, L. M. Tauzer, W. D. Halliday, J. Illasiak, R. Green, A. Kudlak and J. Kuptana, Ringed seal diet and body condition in the Amundsen Gulf region, Eastern Beaufort Sea, Arctic, 2021, 74, 127–138 CrossRef.
- W. R. Ogloff, S. H. Ferguson, A. T. Fisk, M. Marcoux, N. E. Hussey, A. Jaworenko and D. J. Yurkowski, Long-distance movements and associated diving behaviour of ringed seals (Pusa hispida) in the eastern Canadian Arctic, Arctic Sci., 2021, 7, 494–511 CrossRef.
- L. A. Harwood, T. G. Smith, J. C. Auld, H. Melling and D. J. Yurkowski, Seasonal movements and diving of ringed seals, Pusa hispida, in the Western Canadian Arctic, 1999–2001 and 2010–11, Arctic, 2015, 68, 193 CrossRef.
- C. Freitas, K. M. Kovacs, R. A. Ims, M. A. Fedak and C. Lydersen, Ringed seal post-moulting movement tactics and habitat selection, Oecologia, 2008, 155, 193–204 CrossRef PubMed.
- M. E. Granberg, A. Ask and G. W. Gabrielsen, Local Contamination in Svalbard Overview and Suggestions for Remediation Actions, Brief Report no. 044, Norwegian Polar Institute, Tromsø, Norway, 2017, p. 48 Search PubMed.
- A. M. Ali, H. A. Langberg, S. E. Hale, R. Kallenborn, W. F. Hartz, Å.-K. Mortensen, T. M. Ciesielski, C. A. McDonough, B. M. Jenssen and G. D. Breedveld, The fate of poly- and perfluoroalkyl substances in a marine food web influenced by land-based sources in the Norwegian Arctic, Environ. Sci.: Processes Impacts, 2021, 23, 588–604 RSC.
- J. Søndergaard and A. Mosbech, Mining pollution in Greenland - the lesson learned: A review of 50 years of environmental studies and monitoring, Sci. Total Environ., 2022, 812, 152373 CrossRef PubMed.
- M. Glasius, J. H. Christensen, J. Platz and K. Vorkamp, Halogenated organic contaminants in marine fish and mussels from southern Greenland - pilot study on relations to trophic levels and local sources, J. Environ. Monit., 2005, 7, 127–131 RSC.
- M. C. Bartley, T. Tremblay, A. O. De Silva, C. Michelle Kamula, S. Ciastek and Z. Z. A. Kuzyk, Sedimentary records of contaminant inputs in Frobisher Bay, Nunavut, Environ. Sci. Ecotechnol., 2024, 18, 100313 CrossRef CAS PubMed.
- D. A. Bright, W. T. Dushenko, S. L. Grundy and K. J. Reimer, Effects of local and distant contaminant sources: polychlorinated biphenyls and other organochlorines in bottom-dwelling animals from an Arctic estuary, Sci. Total Environ., 1995, 160–161, 265–283 CrossRef CAS PubMed.
- R. Lohmann, B. Vrana, D. Muir, F. Smedes, J. Sobotka, E. Y. Zeng, L.-J. Bao, I. J. Allan, P. Astrahan, R. O. Barra, T. Bidleman, E. Dykyi, N. Estoppey, G. Fillmann, N. Greenwood, P. A. Helm, L. Jantunen, S. Kaserzon, J. V. Macías, K. A. Maruya, F. Molina, B. Newman, R. M. Prats, M. Tsapakis, M. Tysklind, B. L. Van Drooge, C. J. Veal and C. S. Wong, Passive-sampler-derived PCB and OCP concentrations in the waters of the world - First results from the AQUA-GAPS/MONET network, Environ. Sci. Technol., 2023, 57, 9342–9352 CrossRef CAS PubMed.
- M. Langer, T. S. Von Deimling, S. Westermann, R. Rolph, R. Rutte, S. Antonova, V. Rachold, M. Schultz, A. Oehme and G. Grosse, Thawing permafrost poses environmental threat to thousands of sites with legacy industrial contamination, Nat. Commun., 2023, 14, 1721 CrossRef CAS PubMed.
- K. R. Miner, J. D'Andrilli, R. Mackelprang, A. Edwards, M. J. Malaska, M. P. Waldrop and C. E. Miller, Emergent biogeochemical risks from Arctic permafrost degradation, Nat. Clim. Change, 2021, 11, 809–819 CrossRef CAS.
- K. Christensen, Thawing permafrost releases industrial contaminants into Arctic communities, Environ. Health Perspect., 2024, 132, 032001 CrossRef PubMed.
- F. Pawlak, K. Koziol and Z. Polkowska, Chemical hazard in glacial melt? The glacial system as a secondary source of POPs (in the Northern Hemisphere). A systematic review, Sci. Total Environ., 2021, 778, 145244 CrossRef CAS PubMed.
- Y. Zhuang, X. Liu, Z. Yuan, H. Sheng and J. Gao, A process-based model to track water pollutant generation at high resolution and its pathway to discharge, Water Resour. Res., 2023, 59, e2023WR034738 CrossRef.
- J. A. Charbonnet, A. E. Rodowa, N. T. Joseph, J. L. Guelfo, J. A. Field, G. D. Jones, C. P. Higgins, D. E. Helbling and E. F. Houtz, Environmental source tracking of per- and polyfluoroalkyl substances within a forensic context: Current and future techniques, Environ. Sci. Technol., 2021, 55, 7237–7245 CrossRef CAS PubMed.
- AMAP, AMAP Assessment 2021: Human Health in the Arctic, Arctic Monitoring and Assessment Programme (AMAP), Tromsø, Norway, 2021, p. 240 Search PubMed.
- K. Vorkamp, P. Carlsson, S. Corsolini, C. A. de Wit, R. Dietz, M. O. Gribble, M. Houde, V. Kalia, R. J. Letcher, A. Morris, F. F. Rigét, H. Routti and D. C. G. Muir, Influences of climate change on long-term time series of persistent organic pollutants (POPs) in Arctic and Antarctic biota, Environ. Sci.: Processes Impacts, 2022, 24, 1643–1660 RSC.
- R. E. Kwiatkowski, Indigenous community based participatory research and health impact assessment: A Canadian example, Environ. Impact Assess. Rev., 2011, 31, 445–450 CrossRef.
- A. Langwieder, A. Coxon, N. Louttit, S. Varty, F. Boulanger, S. Diamond, J. Lameboy, A. Jolly, G. Natawapineskum, D. Okimaw and M. M. Humphries, Community-led non-invasive polar bear monitoring in the Eeyou Marine Region of James Bay, Canada: insights on distribution and body condition during the ice-free season, FACETS, 2023, 8, 1–12 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00418c |
| This journal is © The Royal Society of Chemistry 2025 |