Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Techno-economic analysis of indirect carbonation processes for carbon sequestration using mining waste†
Katherine Vaz Gomes *a,
Caleb M. Woodallb,
Hélène Pilorgéa,
Peter Psarrasa and
Jennifer Wilcoxa
*a,
Caleb M. Woodallb,
Hélène Pilorgéa,
Peter Psarrasa and
Jennifer Wilcoxa
aDepartment of Chemical and Biomolecular Engineering, University of Pennsylvania, Philadelphia, PA, USA
bIndependent Consultant, Washington, DC, USA
First published on 5th February 2025
Abstract
Carbon mineralization offers the potential to durably store gigatonne-scale CO2 emissions, with mining waste representing an especially promising feedstock due to its relatively small particle size, global availability, and opportunities for decarbonizing the mining sector. Despite significant research into the scale and potential of this technology, there remains a lack of techno-economic analyses (TEAs) that comprehensively capture the full-process costs of indirect carbonation using a pH-swing approach. This approach enables both CO2 storage in carbonates, potentially usable to decarbonize concrete, and the extraction of critical minerals, incorporating the costs and revenues of coupling these processes. To address this gap, we developed a Class IV TEA tailored to estimate the costs and life cycle assessment (LCA) of combining critical mineral extraction and carbon mineralization in mining wastes. The model evaluates scenarios for various waste types (i.e.., legacy asbestos waste, aggregate quarry tailings, platinum group metal tailings) under different extraction conditions (acid type, temperature, strength) and carbonation parameters. Additionally, sensitivity analyses explore the effects of reactor design, internal acid–base recycling, and other factors on process costs and carbon efficiency. Our findings show carbon efficiencies of up to 95%, depending on process design. Acid–base recycling is critical for cost-effective and carbon-negative operations: without recycling, process costs exceed $3000 per tCO2 and yield a carbon efficiency of −280%, while internal acid regeneration reduces costs to $500–800 per tCO2 with carbon efficiencies ranging from 41–72%. Process costs vary by waste type and process conditions, ranging from $800–1800 per tCO2 (assuming 10% reagent makeup), with the carbonate precipitation step contributing 34–78% of total costs. The TEA highlights that acid–base recycling is essential for scaling the pH-swing process on mine tailings and should be a research priority to enable gigatonne-scale CO2 storage by mid-century. Additionally, selectively recovering critical minerals in wastes where magnesium and calcium are not exclusively leached could significantly offset capital costs.
1 Introduction
The ratification of the Paris Agreement by 188 countries, aiming to limit climate change to below two degrees Celsius,1 indicates that cumulative greenhouse gas emissions should stay under 1000 billion tonnes (Gt) relative to preindustrial levels.2 The urgent need to address climate change and reduce greenhouse gas emissions has spurred significant interest in carbon capture and storage (CCS) and carbon dioxide removal (CDR) technologies.3 Among the emerging solutions, the concept of storing carbon through reacting with alkaline cations extracted from mine tailings has gained considerable attention due to its potential for additional environmental co-benefits4 This innovative approach not only provides a durable storage solution for CO2 but also offers the opportunity to neutralize and utilize mine tailings, thereby addressing environmental concerns associated with mining activities.5Carbon mineralization is a promising strategy for achieving gigatonne-scale CO2 storage while addressing industrial and mining waste challenges. This process aligns with the principles of a circular economy, which emphasizes the reuse of materials and reduction of waste. Globally, it is estimated that alkaline industrial wastes, including mine tailings, could store up to 4.02 Gt of CO2 per year, highlighting the immense potential of this approach to contribute to carbon management goals.6 Beyond storage, the integration of mineralization with critical mineral recovery offers an opportunity to produce low-carbon metals for use in renewable energy infrastructure and battery technologies, further contributing to decarbonization efforts and closing material loops in the transition to a sustainable economy.7
Despite its promise, several challenges and barriers remain. One critical challenge is understanding the factors that control the kinetics of cation lability during extraction processes, which directly impacts the leaching efficiency of magnesium and calcium from silicate minerals.8 Recent studies have highlighted the importance of mineral surface area and polymorphic variations in optimizing leaching conditions, but further research is needed to develop predictive models and scalable solutions.9 Another key barrier is the identification of reliable alkalinity sources. Geospatial analyses and studies on high-alkalinity mine tailings, such as serpentinite and platinum group metal (PGM) tailings, are critical for pinpointing suitable sites for mineralization.10–12 Without overcoming these barriers, the deployment of carbon mineralization at scale will remain constrained by resource availability and geochemical variability.
Opportunities, however, are abundant. Tailoring extraction and carbonation processes to specific types of mine tailings, such as those generated by PGM mining, could unlock vast new reservoirs for CO2 storage. Pilot-scale industrial ventures are already exploring these possibilities. For instance, Arca, in partnership with nickel mines such as Vale and Talon Metals, has initiated projects to integrate passive carbon drawdown in tailings pits.13 These initiatives not only reduce the environmental footprint of mining operations but also meet the growing demand for low-carbon critical minerals. Tesla's call for “environmentally-sensitive” nickel mining underscores this market demand, suggesting that coupling carbon mineralization with critical mineral extraction could play a pivotal role in ensuring the availability of sustainable materials for clean technologies.14
One potential method of employing mine tailings for carbon sequestration is indirect carbonation (IDC).15 IDC is a two-step engineered process which extracts cations from mineral phases and then precipitates carbonate minerals from the extracted material. The literature base includes a variety of extraction agents and carbonation methods for the mine tailings IDC process.16–19 A promising method of IDC for mine tailings is a pH swing regime in which acids leach cations from the minerals and then base is added to create an alkaline solution to precipitate calcium and magnesium carbonates.20
Despite the growing interest in CO2 storage in mine tailings, a crucial knowledge gap exists in terms of detailed cost models that can inform accurate estimates of project expenses and potential revenue. The lack of reliable economic projections hinders the ability of mine owners, CDR project developers, and policy makers to make well-informed decisions swiftly, as the race to mid-century climate goals intensifies. A summary of technoeconomic estimates for mineral carbonation from the academic literature over the past twenty years is provided in Table 1. Many of the older references in Table 1 may not provide reliable cost estimates for modern carbon mineralization enterprises involving mine tailings. However, they are included in the review to illustrate how cost estimates have shifted in order of magnitude over time. These references are not suitable for direct comparison with more recent studies and are included to give a historical overview of the evolution of techno-economic estimates.
| Article title | Authors (date) | CO2 source | Alkalinity source | Cost estimate |
|---|---|---|---|---|
| CO2 storage as carbonate minerals, report PH3/17 for IEA greenhouse Gas R&D programme | Newall (1999) | Coal fired power plant | Silicate mined for purpose/Mg-rich brine | $60–$100 per tCO2 |
| Carbonate chemistry for sequestering fossil carbon | Lackner (2002) | Coal fired power plant | Magnesium silicates mined for purpose | $70 per tCO2 |
| Energy and economic evaluation of ex situ aqueous mineral carbonation | O’Connor (2004) | Coal fired power plant | Silicate minerals mined for purpose | $50 per tCO2 |
| Mineral carbonation: energy costs of pretreatment options and insights gained from flow loop reaction studies | Penner (2004) | Coal fired power plant | Magnesium silicates mined for purpose | $69 per tCO2 |
| Aqueous mineral carbonation: mineral availability, pretreatment, reaction parametrics, and process studies | W. O'Connor, K. Rush, G. E. Gerdemann, L. R. Penner (2005) | Industrial point source | Silicate minerals mined for purpose | $54–$427 per tCO2 |
| Carbon dioxide capture and storage | IPCC (2005) | Industrial point source | Silicate minerals mined for purpose | $50–$100 per tCO2 |
| Ex situ aqueous mineral carbonation | S. Gerdemann, W. O'Conner, D. Dahlin, L. Penner, H. Rush (2007) | Coal fired power plant | Magnesium silicate minerals mined for purpose | $54–$69 per tCO2 |
| Cost evaluation of CO2 sequestration by aqueous mineral carbonation | W. Huijen, R. Comans, G. Witkamp (2007) | Industrial point source | Wollastonite minerals and steel slag | $106–140 per tCO2 |
| Economic Feasibility and sensitivity analysis of integrating industrial-scale mineral carbonation into mining operations | M. Hitch, G. Dipple (2012) | Industrial point source | Nickel tailings | $82.51 per tCO2 |
| Technical & economic evaluation of a mineral carbonation process using southern Quebec mining wastes for CO2 sequestration of raw flue gas with by-product recovery | L. Pasquier, G. Mercier, J. Blais, E. Cecchi, S. Kentish (2016) | Point source capture on cement kiln | Asbestos tailings | $146–$282 per tCO2 |
| Techno-economic and environmental evaluation of nano-calcium carbonate production using steel slag | J. Lee, K. Hwan Ryu, H. Yong Ha, K. Jung, J. H. Lee (2020) | Coal fired power plant | Steel slag | $483 per tCaCO3 |
| Techno-economic assessment of a carbon capture and utilization process for the production of plaster-like construction materials | J. Galvez-Martos, A. Elhoweris, A. Hakki, Y. Al-horr (2020) | Coal fired power plant | Desalination brine | $410 per tCO2 |
Cost estimates have generally increased over time (Table 1). One contributing factor may be early studies’ overly optimistic assumptions regarding the availability of low-cost, low-carbon electricity during the energy transition34 (Table 1). Most economic estimates in the literature are based on CO2 sourced from coal-fired power plants co-located with mineralization projects, which overlooks the higher costs associated with more dilute CO2 sources, such as those from natural gas plants or direct air capture.3 Furthermore, many estimates fail to account for the additional costs and revenues related to external factors, such as co-product generation, material handling and transportation, and the industrial application or disposal of carbonate products.35 (Table 2)
| Attribute | Low detail, low-cost estimates | High detail, high-cost estimates |
|---|---|---|
| Material costs | Absent costs for virgin mineral and industrial byproducts as raw material feedstocks | Market cost considerations for both virgin materials and industrial byproducts as raw material feedstocks |
| Energy availability | Best case scenario assumption for locally available low-cost renewable energy | Scaleup costs contextualized with power generation and distribution local to specific sites where possible |
| Waste heat | Waste heat assumed to meet thermal energy requirements, with no associated cost or carbon intensity | Detailed cost estimates for additional required heating including energy sourcing such as fossil fuel and renewable energy to provide high grade heat |
| Materials of construction | No specialty materials of construction for system components in estimate where corrosion resistance or reinforced materials may be required | Cost considerations for appropriate reinforced materials of construction where applicable |
| Waste and disposal costs | Absent OPEX costs for post-processing such as waste treatment, disposal, and transport | Waste stream treatment and disposal OPEX cost estimates |
To bridge this critical gap, this study presents a comprehensive techno-economic analysis (TEA) model specifically designed for the mineralization of industrial waste. This TEA model takes into account various engineering designs, variable feeds, and site-specific conditions to provide more accurate and detailed cost projections. By considering details such as gross and net CO2 storage costs, internal rate of return (IRR), capital expenditure (CAPEX), and operational expenditure (OPEX), this TEA model offers the necessary insights for economic evaluation and decision-making. The net storage cost is calculated using eqn (1), where process emissions comprise the emissions associated with the electricity, heat, and material transport, raw materials, and labour.
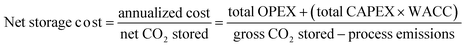 | (1) |
The distinguishing feature of this TEA model lies in its ability to capture a high level of detail both in terms of input parameters and output results. By allowing specific project details, such as engineering designs and variable feed characteristics, to be input, the model can generate customized cost estimates tailored to the unique requirements of each project. Moreover, the TEA model provides a comprehensive overview of costs associated with gross and net storage, enabling stakeholders to assess the economic viability of CO2 storage in mine tailings.
The outcomes of this study hold significant implications for various stakeholders involved in CCS and CDR initiatives. Mine owners can evaluate the economic feasibility of implementing CO2 storage in their tailings, potentially converting liabilities into assets. CDR project developers can utilize the TEA model to assess the financial viability of incorporating mine tailings as a storage medium in their carbon removal strategies. Policymakers can rely on the results to make informed decisions regarding the allocation of resources and the development of supportive regulations and incentives. This study addresses the pressing need for reliable economic projections in the field of CO2 storage in mine tailings. By introducing a detailed and customizable TEA model, this research contributes to the understanding of the economic potential of utilizing mine tailings for CO2 storage.
2 Methods and assumptions
The excel-based model includes major process steps that include choice of energy source, alkaline feed, and reagents. The model includes a main dashboard for the design and operating process conditions and a summary dashboard with the capital and operating costs of the process. The TEA model used in this work includes several features to allow a flexible yet precise method of comparing the economic impact of engineering decisions on the overall process design of indirect carbonation. The model uses established process design economic relationships.36–38 The major process units in the model are the following:The dashboard includes options for process conditions for each of the major steps included in Table 3. The Prefeed Block allows for input regarding particle size and desired grinding size of the initial feed, efficiency of any upfront iron removal by magnetic separations, and transport of the alkaline feed to the IDC site (based on distance and mode). The Extraction Block is sensitive to the temperature, pressure, medium (type, concentration and solid to liquid loading ratio), along with the extraction efficiencies and residence time. The options to define the extraction efficiency is especially useful for using the model to simulate the process economics based on bench-scale experimental data. In addition, the model presents an option in engineering valuable metal co-recovery: electroextraction or chemical precipitation by pH swing. The electroextraction module is built on proven economic algorithms for electrowinning, drawing from well-established literature, including publications from the American Electroplaters and Surface Finishers Society.39,40 The dashboard also provides space to define process conditions for the carbonation step, i.e., temperature, pressure, water addition, residence time, and reaction efficiency. Finally, the model includes a panel for the electrochemical regeneration of acids and bases within the process.40
| Process ID | Description |
|---|---|
| PreFeed | |
| 0.1 | Mining/aggregate |
| 0.2 | Front-end loader/hauling |
| 0.3 | Hopper/screening facility |
| 0.4 | Hydrocyclone |
| 0.5 | Wet milling |
| 0.6 | Magnesite separation |
| Extraction | |
| 1.1.1 | CSTR 1 |
| 1.1.2 | CSTR 2 |
| 1.2 | Effluent phase separation |
| 1.3 | Waste solid wash |
| 1.4 | Waste solid disposal |
| Chemical precipitation or electroextraction | |
| 2.1.1 | CSTR 1 |
| 2.1.2 | CSTR 2 |
| 2.2 | Hydrocyclone |
| 2.3.1 | CSTR 1 |
| 2.3.2 | CSTR 2 |
| Carbonation | |
| 3.1.1 | CSTR 1 |
| 3.1.2 | CSTR 2 |
| 3.2 | Hydrocyclone |
| 3.3 | Drying |
| Electrochemical recycling | |
| 4.1 | Chloralkali electroylsis |
| 4.2 | H2/Cl2 fuel cell |
Overlaying the settings for the process operating parameters, the model includes options for defined system-wide values. The Plant Economic Inputs panel allows for specific values to be set for discount rate, the weighted average cost of capital, chemical engineering plant cost index (CEPCI), project lifetime, and the operational time per year. The model also features a revenue panel where assigned values can be set for the potential sale of aggregate and valuable metals from the process, along with values from the 45Q tax credit and the voluntary carbon market.
In addition to the core IDC process, the TEA also includes a module dedicated to the separation of valuable metals that might be co-leached with magnesium and calcium during extraction. The model includes an internal revenue model which is sensitive to the sale of aggregate, the 45Q tax credit, the voluntary carbon market, and the production of valuable metals. The TEA model provides process emissions and cost estimates for a continuous process that is flexible to a variety of scales, unit operations, thermodynamics, and kinetics. It takes, as input, results derived from bench-scale experiments as well as baseline assumptions supported in the literature and yields results on the basis of NPV, capital and operating expenses, and cost per net tonne of CO2 stored. The model includes databases of the physical and chemical properties of a representative set of reagents, mineral feedstocks and their alkalinity content, heat and energy sources with their respective emissions factors and costs.3,41 The following analysis assumes critical mineral recovery efficiencies of up to 95%, aiming to demonstrate the impact of a highly efficient recovery step on the overall process economics. However, this assumption will require further validation at both lab and bench scales.
In particular, the model includes a database of potential alkaline feed materials ranging from industrial wastes to mined materials. The minerals included in the feeds database include periclase, brucite, forsterite, lizardite, chrysotile, lime, portlandite, wollastonite, larnite, anorthite, and diopside. The model includes composition and particle size information for tailings used in the parallel experimental workstream to this one, where tailings are tested in the CEC Lab. The database also includes published values on the MgO and CaO content of steel slag,42 blast furnace slag,43,44 basic oxygen furnace slag,43–46 electric arc furnace slag,43,47 fly ash,48 cement kiln dust,49 and air pollution control residue.50
The model considers site specific details which would be influential to the overall process economics of mineralization. Examples of factors specific to locations would be the price and carbon intensity of electricity, specific to the local grid of a certain zip code within the United States.51 The model considers the impact of specified Ca and Mg products from the following list: calcite (CaCO3), aragonite (CaCO3), vaterite (CaCO3), dolomite (CaMg(CO3)2), huntite (CaMg3(CO3)4), magnesite (MgCO3), nesquehonite (MgCO3·3H2O), hydromagnesite (Mg5(CO3)4(OH)2·4H2O), and artinite (Mg2(CO3)(OH)2·3H2O). Another example of a customizable feature is the choice of grass roots (cost for designing a process at a virgin location), total module (total cost for purchase and installation), and bare module reporting (cost of process without supporting infrastructure and interconnection).
In addition to engineering and economic parameters, the model incorporates provisions for plant-wide operational settings and accounts for the operational time per year, considering shutdown periods for maintenance, safety, and other reasons. It estimates labour expenses by reflecting how the entire process is uniquely represented in the analysis.38 The model considers labour wages and maximum work hours per week in its cost projections; while these details may seem minor, they are explicitly highlighted in many of the climate provisions within the Inflation Reduction Act.52 The model further considers parameters like the discount rate and weighted average capital cost (WACC).
Designed to interface with bench-scale experiments and data from the literature, the model utilizes kinetic expressions for the dissolution of industrial waste in the critical mineral extraction phase to calculate space time and determine reactor sizing for this part of the process. Similarly, it applies kinetic expressions based on reaction efficiency for carbonation to size and scale continuous stirred tank reactors (CSTRs) for the carbonation step. The model assumes first-order kinetics for both the leaching of materials from mining waste and the precipitation of carbonates from the leachate, with the kinetic constant calculated using residence time and extraction/carbonation efficiency. In addition to calculating the costs associated with the process, the model also estimates the carbon efficiency by calculating the ratio of carbon emitted via the mineralization process (e.g., from material transportation, reagents, electricity, heat) to the gross amount of carbon stored in the process.
3 Results
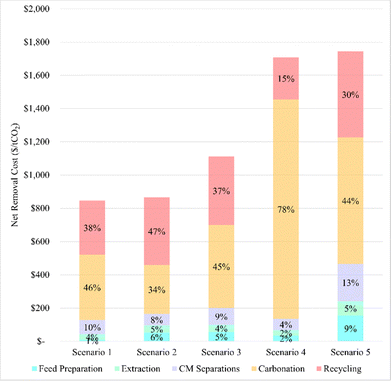
A major benefit of having an adaptable TEA for the indirect carbonation process is that different process designs can be compared on their potential to provide cost-effective, durable carbon storage, using mineral waste as a raw material. To highlight the major cost levers, 5 scenarios are compared (Table 4). All 5 scenarios model pH-Swing indirect carbonation with 6 M HCl coupled to critical mineral recovery. Across the five scenarios, there are 3 types of mineral feedstocks considered: legacy asbestos tailings, serpentinite tailings, and platinum group element tailings.| Parameter | Scenario 1 | Scenario 2 | Scenario 3 | Scenario 4 | Scenario 5 |
|---|---|---|---|---|---|
| Feedstock source | Legacy asbestos waste (CA) | Serpentinite tailings (PA-MD) | Serpentinite tailings (PA-MD) | Serpentinite tailings (PA-MD) | PGM Tailings (MT) |
| Acid type and ratio | 66 g L−1 6 M HCl | 57 g L−1 6 M HCl | 57 g L−1 6 M HCl | 57 g L−1 6 M HCl | 212 g L−1 6 M HCl |
| Extraction temperature (°C) | 50 | 50 | 50 | 50 | 60 |
| Ca & Mg extraction efficiency (%) | 70% | 70% | 70% | 70% | 85% |
| Critical mineral recovery (%) | 95% | 95% | 95% | 95% | 70% |
| Carbonation conditions | 60 °C, 10 bar, 1 h | 60 °C, 10 bar, 1 h | 150 °C, 10 bar, 3 h | 60 °C, 10 bar, 1 h | 60 °C, 10 bar, 1 h |
| Reaction efficiency (%) | 95% | 95% | 95% | 95% | 95% |
| Reactor configuration | 2 CSTRs for extraction, 6 for carbonation | 2 CSTRs for extraction, 6 for carbonation | 2 CSTRs for extraction, 6 for carbonation | 1 reactor for extraction, 1 reactor for carbonation | 2 CSTRs for extraction, 6 for carbonation |
| Challenges | Remote location and limited utilities | Higher carbonation temperature | Limited reactor volume | Higher acid concentration and temperature |
3.1 Process comparison
Scenario 1 highlights the costs associated with accessing legacy mining waste at a long-abandoned mine. This scenario focuses on tailings from the King City Asbestos Company in California, where the mine has been closed for several decades.53–55 It accounts for the challenges of operating an industrial plant in a location where utilities like electricity and water may not be readily available. The tailings, with a particle size of approximately 500 μm (based on their d50 value), require grinding in a ball mill to achieve a size of 75 μm to improve extraction kinetics. The extraction process employs 6 M HCl at a solid-to-liquid ratio of 56 g L−1 and a temperature of 50 °C, aiming to extract 70% of major cations (e.g., Mg, Ca, Na, K) and critical minerals (e.g., Ni, Co, Cr) based on the tailings' elemental composition. In practice, the leaching of elements from silicate minerals is often not uniform, meaning that applying a single extraction efficiency to all elements may not be realistic. However, for the sake of simplicity, this assumption is made here. The model, when provided with sufficient experimental data, can account for individual extraction efficiencies for each element, offering greater accuracy. The TEA examines a system processing 1 million tonnes of tailings annually, with the goal of sequestering a quarter-million tonnes of CO2 each year. The system assumes solar energy costs and carbon intensity ($5.56 per GJ and 7 kg CO2e per GJ).3 Scenario 1 further assumes that 95% of the critical minerals can be recovered from the acid leachate, with 75% of the Mg and Ca in the leachate advancing to carbonation. Carbonation occurs at 60 °C, 10 bar CO2, for 60 minutes, achieving a 95% reaction efficiency. The makeup streams of acid, base, and water are each 2% of the total volumetric requirement.Scenario 2 explores the use of serpentinite mine tailings from the Penn-MD Quarry as a raw material.56 Outside of the costs associated with accessing legacy waste, Scenario 1 shares identical processing conditions with Scenario 2.
Scenario 3 follows the same feed preparation, extraction, and critical mineral recovery conditions as Scenario 2 but introduces changes to the engineering parameters for carbonation. In this scenario, carbonation occurs at 150 °C and 10 bar CO2 for 3 hours, aiming to produce anhydrous magnesium carbonate, which may be more suitable as an industrial feedstock for specific applications than its hydrated counterparts.57–59
Scenario 4 examines the impact of reactor volume on net storage costs during both the extraction and carbonation steps. In the other scenarios, a series of Continuous Stirred-Tank Reactors (CSTRs) is used to minimize capital costs associated with large-volume reactors. The cost optimization algorithm determined that 2 CSTRs were ideal for extraction and 6 for carbonation in Scenario 2. However, in Scenario 4, both steps are limited to a single reactor each, to demonstrate how volume mitigation techniques in process design can influence net storage costs.
Scenario 5 provides a comparison to Scenario 2, utilizing a mineral feedstock with lower concentrations of Mg and Ca, necessitating higher temperatures and increased acid during extraction. This is consistent with previous literature, which indicates that Mg and Ca in complex tecto-silicate heavy mine tailings are typically less labile than in serpentine feedstocks. Scenario 5 assumes the alkalinity and elemental composition of the Stillwater tailings, as previously characterized in the literature.60 Unlike Scenario 2, Scenario 5 involves extraction at 60 °C with 212 g L−1 HCl, achieving 85% extraction of Mg and Ca but only 70% of the available critical minerals.
The analysis of feedstock selection reveals notable variations in net storage costs. In the baseline scenario, Scenario 2, the serpentinite feedstock results in a net storage cost of $870 per tonne of CO2. In contrast, using Stillwater tailings as a hypothetical feedstock leads to a significantly higher net storage cost of $1700 per tonne of CO2, while asbestos results in a lower net storage cost of $850 per tonne of CO2.
The disparity in costs can be attributed to the distinct chemical and mineralogical properties of the feedstocks. Stillwater, with a lower amount of magnesium and calcium compared to the serpentinite or asbestos waste minerals, possesses a reduced carbon storage potential on a mass basis. Additionally, Stillwater is bound in more complex tectosilicates, which necessitates more aggressive extraction conditions to render the Mg and Ca components labile. These factors, including the lower amount of magnesium and calcium and the intricate silicate structure, contribute to the increased costs associated with Stillwater relative to more labile phyllosilicate feedstocks.61
The absence of volume mitigation strategies results in a doubling of the net storage costs compared to the baseline scenario. This underscores the importance of employing volume-reduction engineering strategies in both extraction and carbonation processes. Techniques such as connecting reactors in series or optimizing the specific type of reactor, like a continuous stirred tank reactor (CSTR), can significantly enhance efficiency and minimize the reactor's overall volume requirements. Volume mitigation is crucial due to its impact on operational efficiency and capital costs. Specifically, the pH swing integrated IDC process, being capital intensive, contrasts with the operationally intensive processes, as detailed in the solid/liquid loading section.
The primary distinction between these scenarios lies in the reaction conditions provided during carbonation. This parameter has a considerable impact on the overall cost efficiency of the process. For magnesium-rich feedstocks, in particular, higher temperatures, pressures, and extended residence times may be necessary to prevent the formation of hydrated carbonates, which is important if these carbonates are to be used in the built environment (e.g. within concrete formulations).62–66 The carbonation conditions influence not only the size and morphology of the carbonates but also their properties, which can affect their compatibility with various industrial applications.60
3.2 Acid recycling
The dissolution of rocks in acid has been studied for centuries and remains a well-established concept. A wealth of research exists on optimizing rock dissolution for indirect carbonation, with studies examining variables such as acid concentration, temperature, and the use of mineral versus organic acids, among other engineering parameters.67–75 However, this body of work has often lacked a comprehensive economic analysis, leaving unclear which specific factors most influence the overall cost of the process. Scenario 2 requires 719![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 liters of 12 M HCl per year, 345
000 liters of 12 M HCl per year, 345![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of solid NaOH per year, and approximately 7.5 billion liters of water to sequester a gross total of 250
000 tonnes of solid NaOH per year, and approximately 7.5 billion liters of water to sequester a gross total of 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of CO2, without internal regeneration systems for acid, base, and water.
000 tonnes of CO2, without internal regeneration systems for acid, base, and water.
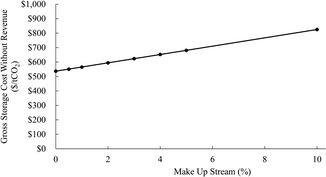
Exploring the acid extraction step in an economic analysis revealed the strain, in both cost and carbon, of constantly purchasing and neutralizing HCl (Fig. 2 and 3) 6 M HCl costs $300 per t and has an emissions factor of 0.89 CO2e per kg HCl.76,77 Without any internal acid recycling, the process costs over $3000 per tCO2 and emits more than two times the CO2 it sequesters.78–80 Viable options for the regeneration of HCl at scale include electrochemical methods, such as chlor-alkali fuel cells and bipolar membrane electrodialysis. The TEA model includes the cost of an electrolyzer and fuel cell for NaOH and HCl recycling, along with the operating costs associated with electrode degradation overtime and electricity necessary to run the cell. While this makes the process highly sensitive to the cost of electricity it enables a more realistic pathway to net negativity if the produced energy is sufficiently low carbon.
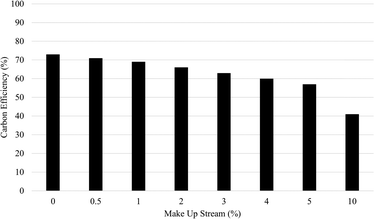 | ||
| Fig. 3 Impact of increasing acid and base make up streams on total carbon efficiency of IDC using chlor-alkali based electrolysis for recovery. | ||
Not only is regeneration a necessary part of the system, but the efficacy of the regeneration process has a significant impact on both cost and carbon efficiency. Comparing an ideal recycling case (one with no necessary internal make up stream to account for lost acid and base) to a case where to make up streams are 10% of the total acid and base requirement, the carbon efficiency of the system drops from 72% to 41%, with the net storage cost nearly tripling. This underlies the importance of maximizing internal management and recovery of acidic and basic reagents on process economic viability.
3.3 Solid to liquid ratio
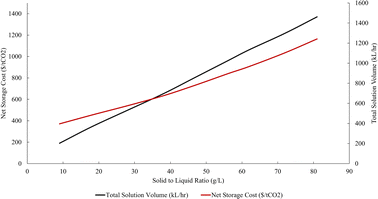
Along with the requirement for acid generation, the solid to liquid ratio used in the extraction step can significantly impact the overall economics. In Scenario 297![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t tailings per h are processed at 56 g tailings per L HCl. The solid to liquid ratio in the extraction step was modulated between 9 and 81 g L−1 to demonstrate the impact that the volume of the extraction step has on the capital cost of this part of the process. The TEA model includes an algorithm that optimizes the capital cost for the extraction step based on the cost of a series of CSTRs. For this analysis, the solid to liquid ratios 9–47 g L−1 were optimized to 1 CSTR, where 56–81 g L−1 were 2 CSTRs in series (Fig. 4). In addition, the TEA model includes the additional costs of corrosion resistant steel that is appropriate to handle concentrated acid at the industrial scale.
000 t tailings per h are processed at 56 g tailings per L HCl. The solid to liquid ratio in the extraction step was modulated between 9 and 81 g L−1 to demonstrate the impact that the volume of the extraction step has on the capital cost of this part of the process. The TEA model includes an algorithm that optimizes the capital cost for the extraction step based on the cost of a series of CSTRs. For this analysis, the solid to liquid ratios 9–47 g L−1 were optimized to 1 CSTR, where 56–81 g L−1 were 2 CSTRs in series (Fig. 4). In addition, the TEA model includes the additional costs of corrosion resistant steel that is appropriate to handle concentrated acid at the industrial scale.
The solid to liquid ratio used in extraction is directly connected to the capital cost of the unit operations used for extraction at scale. This is interesting because the economic implications of the solid to liquid ratio are not often discussed with the literature base. Specifically, many papers within the past ten years have used an excess of acid to increase the extraction kinetics:63,69–71 the gains in extraction efficiency are eclipsed by the steep increase in capital cost to accommodate these solid to liquid ratios. The installed capital cost increases linearly until the solid-to-liquid ratio reaches 54 g L−1, which aligns with the point at which the model indicates a shift from a single CSTR to two CSTRs in series for optimal cost-effective extraction.
As the solid-to-liquid ratio increases, there is a notable rise in total solution volume and, correspondingly, the net storage cost. For instance, the net storage cost per unit of total solution volume shows a discernible increase with higher solid-to-liquid ratios. Specifically, the configuration with a solid-to-liquid ratio of 62 g L−1 and a total solution volume of 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L h−1 incurs a net storage cost of $970 per tCO2. In comparison, the configuration with a solid-to-liquid ratio of 9 g L−1 and a total solution volume of 202
000 L h−1 incurs a net storage cost of $970 per tCO2. In comparison, the configuration with a solid-to-liquid ratio of 9 g L−1 and a total solution volume of 202![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670 L h−1 has a much lower net storage cost of, $400 per tCO2.
670 L h−1 has a much lower net storage cost of, $400 per tCO2.
3.4 Coupling to critical mineral recovery
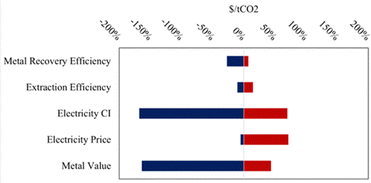
The high capital cost of pH-swing indirect carbonation may be offset by coupling the process to critical mineral recovery, which could provide additional value. The leading hydrometallurgical technique to separate metal phases from aqueous solutions is electroextraction. However, by using the pH-swing mechanism at the center of the process may be utilized to separate valuable metal phases before the carbonation step. As base is added to the extraction solution, the solubilities of potentially valuable metal hydroxides decrease causing them to precipitate out of solutions.81 Both electroextraction and hydroxide precipitation may prove to be scalable paths to scale critical mineral recovery coupled to indirect carbonation.The baseline, lower, and upper bounds for the variables of interest in the sensitivity analysis are shared below in Table 5. In this analysis, metal recovery efficiency denotes the quantity of valuable metals from the original tailings that can be recovered using either the pH-swing or electroextraction technologies The two tornado plots below show the impact of varying some of the engineering levers that impact cost on each of the critical mineral recovery processes, electroextraction ($660 per t net CO2) and chemical precipitation ($512 per t net CO2) respectively (Fig. 5 and 6). Both processes are highly sensitive to the carbon intensity (CI) of the electricity used, highlighting the need to decarbonize all sectors, including the power sector, while also reducing and removing CO2 emissions. In this analysis, the net storage cost is used as the parameter of interest to demonstrate the impact on both cost and carbon efficiency.
| Category | Low value | Base value | High value |
|---|---|---|---|
| Metal value ($ per t) | 100 | 500 | 2000 |
| Electricity price ($ per MW h) | 15 | 20 | 60 |
| Electricity carbon intensity (kgCO2 per GJ) | 3 | 7 | 49 |
| Extraction efficiency (%) | 50 | 70 | 95 |
| Metal recovery efficiency (%) | 80 | 95 | 99.9 |
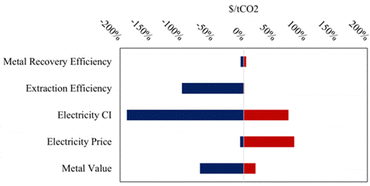 | ||
| Fig. 6 Sensitivity analysis on net storage cost coupled to electroextraction critical mineral recovery. | ||
The process that includes electroextraction (modelled as electrowinning) is far more sensitive to the separation efficiency of the critical minerals in question, likely due to the increased capital and operating costs associated with operating the electroextraction. The primary operating costs associated with the electroextraction process are the electricity needed to run the electrolytic cell and the replacement of electrodes that will degrade overtime.40 Similarly, the electroextraction process is more sensitive to the extraction efficiencies of the relevant minerals upstream of the critical minerals separations, when compared to the chemical precipitation process: this is likely because the chemical precipitation process can occur over multiple reactors each with a stepwise pH change, leading to more effective separations of minerals from solutions compared to electroextraction which uses a single unit to electroplate minerals from solutions.
4 Discussion
The integrated TEA model provides critical insights into the key cost drivers of pH-swing indirect carbonation, highlighting the significant roles of reactor design, internal recycling, and feedstock selection in achieving cost-effectiveness and carbon efficiency. In the baseline case, ultramafic tailings with 70% extraction efficiency and 95% carbonation efficiency achieved the lowest net storage costs. However, even these costs remain significantly higher than previously estimated costs for large-scale mineralization of mine tailings.21–23 This finding suggests that while mine tailings offer a promising pathway for widespread, durable CO2 storage, the associated costs may be substantially higher than previously assumed, even when coupled with a revenue source such as critical mineral recovery.Although critical mineral recovery provides some economic offset, its impact was modest in the scenario models. For instance, with mineral values set at $500 per t, the overall cost reductions were limited. Sensitivity analyses (Fig. 5 and 6) reveal that increasing mineral value to $1500 per t, still less than 25% of the market value of many critical minerals,82 could reduce net storage costs by as much as 66%. This underscores the importance of the condition and form of mineral outputs, as their value to downstream refineries significantly influences the overall economics of the process.
The analysis further emphasizes the critical role of efficient internal acid–base recycling, such as via chlor-alkali electrolysis, in achieving cost-effective and net-negative emissions. Given the reliance of this process on electrochemical technologies for reagent regeneration, the system is highly sensitive to the availability of low-cost, low-carbon electricity. This dependence is not unique to this process; many technologies vital to the energy transition will similarly require access to “green electrons” to maximize carbon abatement potential.83
Experimental parameters, such as the solid-to-liquid ratio during extraction, also play a pivotal role in scalability and costs. While higher solid concentrations generally reduce capital expenses, they require careful trade-offs with operational efficiency, as they increase reactor complexity. The findings suggest that slurry-based systems may provide more economical extraction pathways, balancing cost and carbon efficiency at scale. These insights can help guide bench-scale experiments toward factors most critical for delivering cost-effective mineralization at scale, such as optimizing the trade-offs between solid–liquid ratios and the strength and type of acid used.
The scale of the opportunity for carbon mineralization in mining tailings is immense, particularly in the United States, which produces approximately 9 billion tonnes of tailings annually. Specific case studies underscore the potential of leveraging these tailings for both CO2 sequestration and critical mineral recovery.4
The Sibanye-Stillwater platinum group metal (PGM) mine in Nye, Montana, generates roughly 1 million tonnes of tailings annually, excluding stockpiles from decades of legacy mining.60 Using approximately 900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of tailings per year, the TEA demonstrates a potential net storage cost range of $1330–2000 per tonne of CO2. This process could yield 78
000 tonnes of tailings per year, the TEA demonstrates a potential net storage cost range of $1330–2000 per tonne of CO2. This process could yield 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of carbonate minerals annually while recovering 220
000 tonnes of carbonate minerals annually while recovering 220![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of critical minerals, showcasing the dual benefit of carbon storage and resource extraction.
000 tonnes of critical minerals, showcasing the dual benefit of carbon storage and resource extraction.
Similarly, the Mt. Keith nickel mine in Australia, which produces 11 million tonnes of tailings per year, has an estimated CO2 sequestration capacity of 4 million tonnes per year.84 Indirect carbonation at this site could be achieved at a cost of $720–1070 per tonne of CO2, highlighting the economic feasibility of scaling up such processes in nickel mining operations.
Abandoned mine sites also present significant opportunities. For example, the King City Asbestos Company in California has extensive legacy tailings.85 A project utilizing just 4% of the tailings available at the site each year (about 815![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes) could sequester approximately 72
000 tonnes) could sequester approximately 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of CO2 annually—equivalent to the emissions of a commercial-scale glass manufacturing plant.
000 tonnes of CO2 annually—equivalent to the emissions of a commercial-scale glass manufacturing plant.
These examples highlight the scalability, economic potential, and dual environmental benefits of integrating carbon mineralization into mining operations, offering a pathway toward achieving significant CO2 sequestration while contributing to the critical mineral supply chain. The TEA presented in this article has the potential to identify the cost-range and major cost levers associated with commercial scale pH-swing indirect carbonation at a mine, given details on the availability of tailings, the location of the mine, and the geochemical composition of the tailings.
5 Conclusions
The costs predicted by the TEA in this analysis are notably higher than those reported in earlier literature. This discrepancy arises because the current analysis incorporates often-overlooked details, such as additional costs for waste disposal, reinforced materials of construction where applicable, and scale-up costs contextualized to local power generation and resource availability. While these more comprehensive cost estimates reveal that indirect carbonation at scale may be more expensive than previously anticipated, coupling the process with critical mineral recovery could offset these costs.This integration not only enhances economic viability but also supports broader sustainability goals, creating a pathway for carbon mineralization to simultaneously address climate change and resource efficiency challenges. Reagent recycling is absolutely essential for the success of indirect carbonation technologies, both in terms of cost-effectiveness and carbon efficiency. Dependence on virgin reagents is economically prohibitive and environmentally unsustainable.
The integrated TEA model offers a clear understanding of the most significant cost drivers in pH-swing indirect carbonation, revealing how reactor volume, internal recycling, and feedstock selection shape both the cost-effectiveness and carbon efficiency of industrial-scale systems. In the baseline case (Scenario 1), ultramafic tailings, with 70% extraction efficiency and 95% carbonation efficiency (at 60 °C and 10 bar CO2), delivered the lowest net storage costs. The pH-swing process is particularly sensitive to energy costs, recycling efficiency, and capital expenditures related to extraction and carbonation reactors. The TEA highlights that efficient internal acid–base recycling, potentially achieved through chlor-alkali electrolysis, is essential for a cost-effective system capable of achieving net negative emissions.
The TEA model also excels in identifying which bench-scale experimental parameters translate into critical cost levers at scale. For instance, the solid-to-liquid ratio in the extraction phase plays a pivotal role in reactor design and capital costs, with higher acid concentrations and volumes necessitating greater investment in corrosion-resistant equipment. The findings suggest that higher solid concentrations generally lower capital costs, indicating that slurry-based systems may be more economical for extraction than processes operating with excess acid. However, there is a balance to be struck between capital investment and operational efficiency—lower solid-to-liquid ratios reduce capital expenditure but may compromise extraction efficiency, whereas higher ratios increase complexity and reactor requirements.
5.1 Recommendations
Future efforts should focus on developing a predictive algorithm for the leaching kinetics of calcium, magnesium, and critical minerals from various sources of mine tailings. Such an algorithm could dramatically accelerate the scale-up of carbon mineralization technologies by eliminating the need for time-intensive extraction trials to estimate recoverable alkalinity and critical minerals. Additionally, integrating this algorithm into the TEA model would enable parallel cost assessments, providing a more robust framework for decision-making and improving the feasibility of large-scale implementation.The form and value of metal outputs should be carefully considered, as these factors will directly correlate to their potential worth to downstream refineries. Optimizing the solid-to-liquid ratio in extraction processes should also be a priority for future research, with an aim to develop slurry-based systems that reduce capital costs associated with extraction steps. This optimization must be paired with meticulous tracking of critical minerals that co-leach during the process to ensure their efficient recovery and accurate accounting.
In scenarios where calcium and magnesium are not selectively leached, critical minerals can be co-recovered alongside the carbon mineralization process. The TEA reveals that these valuable elements could help offset the substantial costs, particularly in feedstocks rich in critical minerals and rare earth elements. This opportunity is timely, given recent funding from the U.S. Department of Energy aimed at building a domestic supply chain for critical minerals from industrial waste streams.86
This research provides a valuable cost-projection framework for projects utilizing mine tailings for ex situ carbon mineralization. While the Class IV TEA does not offer detailed itemized costs, it identifies the key economic levers to consider when developing such processes. Additionally, the model offers insights into carbon efficiency, helping stakeholders balance emissions with higher energy demands.
This research presents the most comprehensive TEA for the carbonation and critical mineral recovery of mine tailings thus presented in the literature to date. This model can adapt to different mines: considering their mineralogy, location, scale, and other characteristic details. The model not only accounts for the equipment and operating needs at scale, but also considers external factors such as transportation, waste disposal, and reactor optimization. This holistic approach sets a new benchmark for evaluating the techno-economic feasibility of carbon mineralization, providing a scalable, adaptable framework that paves the way for transforming mine tailings into a cornerstone of sustainable carbon storage and critical mineral recovery.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.References
- United Nations Framework Convention on Climate Change, Paris Agreement, Presented at the Report of the Conference of the Parties to the United Nations Framework Convention on Climate Change (21st Session, 2015: Paris), 2015.
- M. Allen, D. Frame, C. Huntingford, C. Jones, J. Lowe, M. Meinshausen and N. Meinshausen, Nature, 2009, 458, 1163–1166 CrossRef CAS PubMed.
- C. on Developing a Research Agenda for Carbon Dioxide Removal and R. Sequestration, Negative Emissions Technologies and Reliable Sequestration: A Research Agenda, National Academies Press, Washington, DC, 2019 Search PubMed.
- D. Sandalow, R. Aines, J. Friedmann, P. Kelemen, C. McCormick, I. Power, B. Schmidt and S. Wilson, Carbon Mineralization Roadmap, Icef innovation roadmap project technical report, 2021 Search PubMed.
- P. Kinnunen, M. Karhu, E. Yli-Rantala, P. Kivikytö-Reponen and J. Mäkinen, Cleaner Eng. Technol., 2022, 8, 100499 CrossRef.
- S. Pan, Y. Chen, L. Fan, H. Kim, X. Gao, T. Ling, P. Chiang, S. Pei and G. Gu, Nat. Sustainability, 2020, 3, 399–405 CrossRef.
- V. Masindi, S. Foteinis, P. Renforth, J. Ndiritu, J. Maree, M. Tekere and E. Chatzisymeon, Ecol. Eng., 2022, 183, 106740 CrossRef.
- X. Lu, G. M. Dipple and C. C. Turvey, Int. J. Greenhouse Gas Control, 2025, 141, 104315 CrossRef CAS.
- X. Lu, K. J. Carroll, C. C. Turvey and G. M. Dipple, Appl. Geochem., 2022, 140, 105285 CrossRef CAS.
- M. Madankan and P. Renforth, Int. J. Greenhouse Gas Control, 2023, 130, 104010 CrossRef CAS.
- K. Steinthorsdottir, G. M. Dipple, J. A. Cutts, C. C. Turvey, D. Milidragovic and S. M. Peacock, J. Petrol., 2022, 63, egac100 CrossRef.
- C. M. Woodall, K. Vaz Gomes, A. Voigt, K. Sundmacher and J. Wilcox, RSC Sustainability, 2024, 2, 3320–3333 RSC.
- L.-P. Elufisan, Carbon minerisation: ARCA expands global footprint, Mining digital technical report, 2023 Search PubMed.
- Nickel-a mineral with a challenging role in clean tech, International energy forum technical report, 2024.
- C. Kunzler, N. Alves, E. Pereira, J. Nienczewski, R. Ligabue, S. Einloft and J. Dullius, Energy Procedia, 2011, 4, 1010–1017 CrossRef CAS.
- J. L. Hamilton, S. Wilson, B. Morgan, A. L. Harrison, C. C. Turvey, D. J. Paterson, G. M. Dipple and G. Southam, Econ. Geol., 2020, 115, 303–323 CrossRef.
- X. Lu, K. J. Carroll, C. C. Turvey and G. M. Dipple, Appl. Geochem., 2022, 140, 105285 CrossRef CAS.
- V. Prigiobbe and M. Mazzotti, Chem. Eng. Sci., 2011, 66, 6544–6554 CrossRef CAS.
- M. Hänchen, V. Prigiobbe, G. Storti, T. Seward and M. Mazzotti, Geochim. Cosmochim. Acta, 2006, 70, 4403–4416 CrossRef.
- A. Azdarpour, M. Asadullah, R. Junin, M. Manan, H. Hamidi and A. R. M. Daud, Energy Proc., 2014, 61, 2783–2786 CrossRef CAS.
- J.-L. Gálvez-Martos, A. Elhoweris, A. Hakki and Y. Al-horr, J. CO2 Util., 2020, 38, 59–67 CrossRef.
- J. Lee, K. H. Ryu, H. Y. Ha, K.-D. Jung and J. H. Lee, J. CO2 Util., 2020, 37, 113–121 CrossRef CAS.
- N. McQueen, P. Kelemen and G. Dipple, et al., Nat. Commun., 2020, 11, 3299 CrossRef CAS PubMed.
- L.-C. Pasquier, G. Mercier, J.-F. Blais, E. Cecchi and S. Kentish, Int. J. Greenhouse Gas Control, 2016, 50, 147–157 CrossRef CAS.
- M. Hitch and G. Dipple, Miner. Eng., 2012, 39, 268–275 CrossRef CAS.
- S. J. Gerdemann, W. K. O’Connor, D. C. Dahlin, L. R. Penner and H. Rush, Environ. Sci. Technol., 2007, 41, 2587–2593 CrossRef CAS PubMed.
- H. Herzog, K. Smekens, P. Dadhich, J. Dooley, Y. Fujii, O. Hohmeyer and K. Riahi, IPCC Special Report on Carbon Dioxide Capture and Storage, Cambridge University Press, Cambridge, UK, 2005, pp. 339–340 Search PubMed.
- L. R. Penner, W. K. O’Connor, D. C. Dahlin, S. J. Gerdemann and G. E. Rush, Mineral carbonation: energy costs of pretreatment options and insights gained from flow loop reaction studies, Report from the US Department of Energy Office of Scientific and Technical Information, 2024, https://www.osti.gov/biblio/899009 Search PubMed.
- W. O’Connor, D. Dahlin, G. Rush, S. Gerdemann and L. Penner, Greenhouse Gas Control Technologies 7, Elsevier Science Ltd, Oxford, 2005, pp. 2011–2015 Search PubMed.
- K. S. Lackner, Annu. Rev. Environ. Resources, 2002, 27, 193–232 Search PubMed.
- W. O’Connor, D. Dahlin, G. Rush, S. Gerdemann, L. Penner and D. Nilsen, Aqueous Mineral Carbonation: Mineral Availability, Pretreatment, Reaction Parametrics, and Process Studies, National Energy Technology Laboratory, Office of Fossil Energy, US DOE Technical Report DOE/ARC-TR-04-002, 2005.
- P. Newall, S. Clarke, H. Haywood, H. Scholes, N. Clarke, P. King and R. Barley, CO2 storage as carbonate minerals, IEA Greenhouse Gas RD Programme Report PH3/17, 1999.
- W. J. Huijgen, R. N. Comans and G.-J. Witkamp, Energy Convers. Manage., 2007, 48, 1923–1935 CrossRef CAS.
- R. Weron, Int. J. Forecasting, 2014, 30, 1030–1081 CrossRef.
- C. M. Woodall, N. McQueen, H. Pilorgé and J. Wilcox, Greenhouse Gases: Sci. Technol., 2019, 9, 1096–1113 CrossRef CAS.
- R. Turton, R. C. Bailie, W. B. Whiting and J. A. Shaeiwitz, Analysis, Synthesis, and Design of Chemical Processes, Pearson Education, 3rd edn, 2008 Search PubMed.
- W. D. Seider, D. R. Lewin, J. D. Seader, S. Widagdo, R. Gani and K. M. Ng, Product and Process Design Principles: Synthesis, Analysis and Evaluation, Wiley Global Education, 4th edn, 2016, p. 784 Search PubMed.
- D. W. Green and R. H. Perry, Perry's Chemical Engineers’ Handbook, McGraw-Hill, New York, Chicago, San Francisco, Lisbon, London, Madrid, Mexico City, Milan, New Delhi, San Juan, Seoul, Singapore, Sydney, Toronto, 8th edn, 2008 Search PubMed.
- Training Course: Electroplating and Surface Finishing, 2014 Search PubMed.
- C. Stinn and A. Allanore, Electrochem. Soc. Interface, 2020, 29, 44 CrossRef.
- L. J. Müller, A. Kätelhön, S. Bringezu, S. McCoy, S. Suh, R. Edwards, V. Sick, S. Kaiser, R. Cuéllar-Franca, A. El Khamlichi, J. H. Lee, N. von der Assen and A. Bardow, Energy Environ. Sci., 2020, 13, 2979–2992 RSC.
- N. McQueen, C. M. Woodall, P. Psarras and J. Wilcox, Carbon Capture and Storage, The Royal Society of Chemistry, 2019 Search PubMed.
- D. M. Proctor, K. A. Fehling, E. C. Shay, J. L. Wittenborn, J. J. Green, C. Avent, R. D. Bigham, M. Connolly, B. Lee, T. O. Shepker and M. A. Zak, Environ. Sci. Technol., 2000, 34, 1576–1582 CrossRef CAS.
- J. Liu, Q. Yu, Z. Zuo, F. Yang, Z. Han and Q. Qin, Cem. Concr. Compos., 2019, 95, 19–24 CrossRef CAS.
- E.-E. Chang, A.-C. Chiu, S.-Y. Pan, Y.-H. Chen, C.-S. Tan and P.-C. Chiang, Int. J. Greenhouse Gas Control, 2013, 12, 382–389 CrossRef CAS.
- R. Ragipani, S. Bhattacharya and S. K. Akkihebbal, Waste Manage., 2020, 117, 179–187 CrossRef CAS PubMed.
- D. Bonenfant, L. Kharoune, S. Sauvé, R. Hausler, P. Niquette, M. Mimeault and M. Kharoune, Ind. Eng. Chem. Res., 2008, 47, 7610–7616 CrossRef CAS.
- Z. Wei, B. Wang, G. Falzone, E. C. La Plante, M. U. Okoronkwo, Z. She, T. Oey, M. Balonis, N. Neithalath, L. Pilon and G. Sant, J. CO2 Util., 2018, 23, 117–127 CrossRef CAS.
- D. Huntzinger, J. Gierke, S. Kawatra, T. Eisele and L. Sutter, Environ. Sci. Technol., 2009, 43, 1986–1992 CrossRef CAS PubMed.
- A. Bogush, J. A. Stegemann, I. Wood and A. Roy, Waste Manage., 2015, 36, 119–129 CrossRef CAS PubMed.
- U.S. Environmental Protection Agency, Emissions & Generation Resource Integrated Database (eGRID), https://www.epa.gov/egrid, 2023, Accessed: 2023-09-10.
- U. S. Congress, H.R.5376 – Inflation Reduction Act, 2022, https://www.congress.gov/bill/117th-congress/house-bill/5376, Accessed: 2024-10-10.
- F. A. Mumpton and C. S. Thompson, Clays Clay Miner., 1975, 23, 131–143 CrossRef CAS.
- K. M. Finstad, R. D. Aines, G. M. Dipple, A.-M. Doucet, F. A. Jones, B. Ladd, M. M. Smith, C. Turvey and B. Schmidt, AGU Fall Meeting Abstracts, 2023, pp. V41B–012 Search PubMed.
- B. M. Schmidt, 2024, https://netl.doe.gov/sites/default/files/netl-file/24CM/24CM_CTS2_8_Schmidt.pdf#page=1.00, FWP-FEW0278.
- F. Goff, G. Guthrie, B. Lipin, M. Fite, S. Chipera, D. Counce, E. Kluk and H. Ziock, Albany Res. Center, 2000 Search PubMed , https://www.osti.gov/biblio/754045.
- J. Morrison, G. Jauffret, J. L. Galvez-Martos and F. P. Glasser, Cem. Concr. Res., 2016, 85, 183–191 CrossRef CAS.
- T. Matschei, B. Lothenbach and F. Glasser, Cem. Concr. Res., 2007, 37, 551–558 CrossRef CAS.
- F. Winnefeld, E. Epifania, F. Montagnaro and E. M. Gartner, Cem. Concr. Res., 2019, 126, 105912 CrossRef CAS.
- C. M. Woodall, X. Lu, G. Dipple and J. Wilcox, Minerals, 2021, 11, 844 CrossRef CAS.
- C. M. Woodall, A Multi-Faceted Approach to Carbon Mineralization Advancement, Worcester Polytechnic Institute, 2021, ch. 4, pp. 43–65 Search PubMed.
- E. J. Swanson, K. J. Fricker, M. Sun and A.-H. A. Park, Phys. Chem. Chem. Phys., 2014, 16, 23440–23450 RSC.
- R. Zevenhoven, M. Slotte, J. Åbacka and J. Highfield, Energy, 2016, 117, 604–611 CrossRef CAS.
- C. Jiménez-López, E. Caballero, F. Huertas and C. Romanek, Geochim. Cosmochim. Acta, 2001, 65, 3219–3231 CrossRef.
- H. S. Santos, H. Nguyen, F. Venâncio, D. Ramteke, R. Zevenhoven and P. Kinnunen, Inorg. Chem. Front., 2023, 10, 2507–2546, 10.1039/D2QI02482A.
- R. Zevenhoven, D. Legendre, A. Said and M. Järvinen, Energy, 2019, 175, 1121–1129 CrossRef CAS.
- S. Teir, H. Revitzer, S. Eloneva, C.-J. Fogelholm and R. Zevenhoven, Int. J. Miner. Process., 2007, 83, 36–46 CrossRef CAS.
- W. H. Huang and W. D. Keller, Am. Mineral., 1970, 55, 2076–2094 CAS.
- W. H. Huang and W. D. Keller, Am. Mineral., 1971, 56, 1082–1095 CAS.
- J. Drever and L. Stillings, Colloids Surf., A, 1997, 120, 167–181 CrossRef CAS.
- D. E. Lazo, L. G. Dyer and R. D. Alorro, Miner. Eng., 2017, 100, 115–123 CrossRef CAS.
- F. Crundwell, Hydrometallurgy, 2013, 139, 132–148 CrossRef CAS.
- G. E. Seil and Staff, J. Am. Ceram. Soc., 1943, 26, 218–236 CrossRef CAS.
- R. P. Multhauf and R. A. Gilbert, Alchemy, https://www.britannica.com/topic/alchemy, Accessed: 2024-09-11.
- S. Welch and W. Ullman, Geochim. Cosmochim. Acta, 1996, 60, 2939–2948 CrossRef CAS.
- C. McKinley and A. Ghahreman, Miner. Process. Extr. Metall., 2018, 127, 157–168 CAS.
- Euro Chlor, An Eco-profile and Environmental Product Declaration of the European Chlor-Alkali Industry, Euro chlor technical report, 2022.
- R. A. Pepper, S. J. Couperthwaite and G. J. Millar, Miner. Eng., 2016, 99, 8–18 CrossRef CAS.
- F. Crundwell, Hydrometallurgy, 2016, 161, 34–44 CrossRef CAS.
- S. Gudbrandsson, D. Wolff-Boenisch, S. R. Gislason and E. H. Oelkers, Geochim. Cosmochim. Acta, 2014, 139, 154–172 CrossRef CAS.
- Q. Zhao, C. Jun Liu, M. Fa Jiang, H. Saxén and R. Zevenhoven, Miner. Eng., 2015, 79, 116–124 CrossRef CAS.
- IEA, Global Critical Minerals Outlook 2024, International Energy Agency technical report, 2024.
- M. Wei, C. McMillan and S. de la Rue du Can, Curr. Systainably, 2019, 140–148 Search PubMed.
- S. Wilson, A. L. Harrison, G. M. Dipple, I. M. Power, S. L. Barker, K. Ulrich Mayer, S. J. Fallon, M. Raudsepp and G. Southam, Int. J. Greenhouse Gas Control, 2014, 25, 121–140 CrossRef CAS.
- W. S. Wise and W. P. Moller, Rocks Miner., 1995, 70, 30–35 CrossRef.
- FECM, Funding Notice: Regional Scale Collaboration to Facilitate a Domestic Critical Minerals Future: Carbon Ore, Rare Earth, and Critical Minerals (CORE-CM) Initiative, 2024, DEFOA-0003077.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ya00567h |
| This journal is © The Royal Society of Chemistry 2025 |