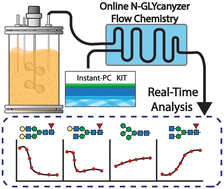
With the transition toward continuous bioprocessing, process analytical technology (PAT) is becoming necessary for rapid and reliable in-process monitoring during biotherapeutics manufacturing. Bioprocess 4.0 is looking to build end-to-end bioprocesses that include PAT-enabled real-time process control. This is especially important for drug product quality attributes that can change during bioprocessing, such as protein N-glycosylation, a critical quality attribute for most monoclonal antibody (mAb) therapeutics. Glycosylation of mAbs is known to influence their efficacy as therapeutics and is regulated for a majority of mAb products on the market today. Currently, there is no method to truly measure N-glycosylation using on-line PAT, hence making it impractical to design upstream process control strategies. We recently described the N-GLYcanyzer workflow: an integrated PAT unit that measures mAb N-glycosylation within 3 hours of automated sampling from a bioreactor. Here, we integrated Agilent's Instant Procainamide (InstantPC) based chemistry workflow into the N-GLYcanyzer PAT unit to allow for nearly 10× faster near real-time analysis of mAb glycoforms. Our methodology is explained in detail to allow for replication of the PAT workflow as well as present a case study demonstrating the use of this PAT to autonomously monitor a mammalian cell perfusion process at the bench scale to gain increased knowledge of mAb glycosylation dynamics during continuous biologics manufacturing using Chinese hamster ovary (CHO) cells.