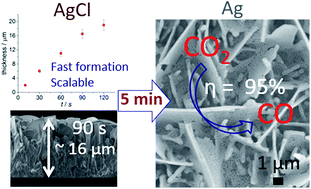
Rapid and scalable production of electrocatalysts with high conversion efficiency and product selectivity are essential for practical application of electrochemical CO2 reduction. Here we report highly efficient and selective free-standing silver (Ag) nanosheet-based electrocatalysts that were produced in less than 10 min via an electrochemical oxidative–reductive approach. The hierarchical structures of Ag nanosheets provide an enhanced surface area and favourable gas transport/diffusion. The interconnected nanosize Ag (∼45 ± 10 nm) sheets produce among the best performance for aqueous CO2 to CO reduction for this class of materials. The conversion selectivity was approximately 95% at an overpotential as low as 0.29 V. This rational experimental design strategy may inspire efforts towards developing rapidly synthesized and efficient CO2 reduction electrocatalysts.