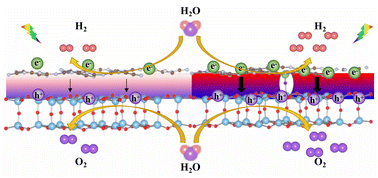
The high recombination rate of photocarriers inhibits the photocatalytic efficiency of heterostructures due to the lack of a driving force for electron–hole separation. Herein, through Li–F co-doping, there was a large amount of interfacial charge transfer at the interface of the Li@g-C3N4/F@TiO2-B(001) heterostructure because of the noticeable Fermi level difference between the separated components. The built-in electric field induced by the significant interfacial charge transfer provided a strong driving force for the separation of photoinduced electron–hole pairs. Under the synergistic effect of the built-in electric field and band bending, the photoinduced electrons accumulated on the side of Li@g-C3N4, while the photoinduced holes were retained on the side of F@TiO2-B(001), indicating that the hydrogen evolution reaction (HER) takes place at the Li@g-C3N4 surface while the oxygen evolution reaction (OER) occurs at the F@TiO2-B(001) surface. Thermodynamic analysis of the HER proved that the Li@g-C3N4/F@TiO2-B(001) heterostructure as a HER photocatalyst had the lowest overpotential and strongest photocatalytic capacity compared with the pristine g-C3N4 and undoped g-C3N4/TiO2-B(001) heterostructure. The Li–F co-doping enhanced the interfacial interactions of the Li@g-C3N4/F@TiO2-B(001) heterostructure, promoted the separation of electron–hole pairs, and improved the photocatalytic performance. We believe that metal–nonmetal co-doping is a good strategy for promoting the separation of photoinduced carriers, which can also be used in other heterostructures to improve the photocatalytic capacity.