Alessandra Lattanzi, Claudia De Fusco, Alessio Russo, Albert Poater and Luigi Cavallo
Chem. Commun., 2012,48, 1650-1652
DOI:
10.1039/C2CC17488J,
Communication
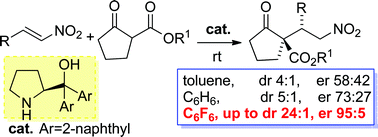
A dramatic enhancement of the diastereo- and enantioselectivity in the nitro-Michael addition reaction organocatalysed by a commercially available α,α-L-diaryl prolinol was disclosed when performing the reaction in unconventional