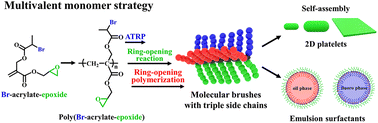
Heterografted molecular brushes (HMBs) bearing two or more types of side chains have attracted tremendous attention because of their asymmetric structures and multiple functionalities. However, the controlled synthesis of HMBs with three types of side chains remains a challenge. The structure–property–application correlations of HMBs with three types of side chains are still not clear. Herein, based on the multivalent monomer strategy, we report the synthesis of well-defined asymmetric HMBs comprising three different side chains by sequential reversible addition–fragmentation chain transfer (RAFT) polymerization, atom transfer radical polymerization (ATRP), thiol–epoxy coupling reaction, and ring-opening polymerization (ROP). PA-g-PAzo/PEG/PLA with a polyacrylate (PA) backbone, hydrophobic poly(azobenzene-methacrylate) (PAzo) brush, hydrophilic polyethylene glycol (PEG) brush, and hydrophobic polylactide (PLA) brush was synthesized and characterized. The self-assembly behavior of PA-g-PAzo/PEG/PLA with a short backbone and relatively strong intermolecular association in solutions was investigated. Well-defined platelets with tunable morphologies were constructed. Subsequently, PA-g-PFA/PEG/PLA with a fluorophilic poly(pentafluoropropyl acrylate) (PFA) brush, hydrophilic PEG brush, and lipophilic PLA brush was synthesized and further used as an efficient surfactant for the stabilization of different emulsions. Our study offers a platform for exploring the unique properties of asymmetric HMBs.