Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Topological variety and self-sorting in homo- and heteroleptic PdnL2n metallo-supramolecular assemblies
Laura
Neukirch
 and
Guido H.
Clever
and
Guido H.
Clever
 *
*
Department of Chemistry and Chemical Biology, TU Dortmund University, Otto-Hahn-Str. 6, 44227 Dortmund, Germany. E-mail: guido.clever@tu-dortmund.de
First published on 12th June 2025
Abstract
A plethora of nanoscale PdnL2n-type architectures has been synthesized through the coordination-driven self-assembly of Pd(II) ions and organic bis-monodentate bridging ligands. While initially, the focus was on homoleptic structures, comprising one type of ligand per assembly, the field has recently shifted towards reducing symmetry in heteroleptic multicomponent assemblies, containing two or more distinct ligands in defined positions. In parallel, the incorporation of functional moieties such as binding and catalytic sites, photoswitches and redox units has seen a steep development. While empirical data have been gathered on the relationship between the ligand structure and assembly outcome for a limited number of cases, confidently forecasting the result of reacting a given ligand with Pd(II) cations often still remains challenging and has been mastered only for the simplest systems. Additionally, new PdnL2n topologies – along with subtle factors driving their formation (such as counter anion or guest templation and solvation effects) – are discovered continuously. For designing metallosupramolecular assemblies for application, it is of pivotal importance to increase predictabilty and gain control over assembly topology, as the structure and properties are often closely connected. To raise awareness for the problem's complexity, we commence this review by exploring the surprising breadth of topological diversity among homoleptic PdnL2n (n = 2–8) architectures that has so far been found experimentally. We next discuss strategies for increasing the structural complexity even further through the non-statistical self-assembly of heteroleptic cages, the orientational self-sorting of asymmetric ligands, and chiral self-sorting effects. Special emphasis will be placed on factors governing the particular self-assembly outcome as well as on rationalization approaches based on computations or geometrical considerations.
1. Introduction
Since the beginning of the field of supramolecular chemistry, researchers have aspired to understand and mimic the high selectivity and specificity of structure formation, binding processes and catalysed reactions found in natural systems.1 A closer look into the nanosized cavities of enzymes reveals their often highly unsymmetric, multi-functionalized and dynamic nature.2 While imitating certain aspects of the structural and functional complexity of natural systems by means of classical covalent synthesis is possible in principle,3,4 such approaches usually involve resource-consuming multi-step organic conversions and tedious purification tasks. The concept of dynamic self-assembly represents an attractive alternative to this: the combination of suitable molecular building blocks with a pre-programmed connectivity allows for the formation of large and intricate supramolecular architectures, usually under thermodynamic or sometimes kinetic control.5 Supramolecular assembly approaches can be classified according to the interactions that drive recognition and binding between the different building blocks. Popular approaches are based on dynamic covalent reactions,6–9 hydrogen bonding,10–13 and metal coordination.14–22 In the latter case, metal ions are combined with organic building blocks equipped with a set of suitable donor groups, serving as ligands to bridge two or more metal nodes. Often, transition metal cations with defined coordination geometries are used to enhance predictability of the self-assembly outcome. This approach allows for the construction of extended structures such as coordination polymers or metal organic frameworks (MOFs) as well as discrete, soluble entities such as defined rings, helicates and cages. This review only covers the latter class of non-polymeric compounds. Pioneering examples of such supramolecular coordination architectures include Saalfrank's Mg(II)-based adamantoid assembly from 1988,23 Lehn's helicates,24 Raymond's Ga(III) catecholate tetrahedra,25 and Stang's Pt(II)-based rings and cages.26 As a very versatile metal node, Pd(II) was found to assemble with nitrogen-containing heterocyclic donors such as pyridines and isoquinolines to form defined supramolecular architectures. Aside from the fixed square-planar coordination geometry of d8-configured Pd(II) cations, their diamagnetism (beneficial for NMR analytics), suitable ligand exchange kinetics and the thermodynamic stability of their N-ligand complexes render them highly attractive for realizing a large variety of metal-mediated assemblies.16In the early 1990s Makoto Fujita pioneered the construction of two- and three-dimensional assemblies based on cis-protected Pd(II) cations and organic bridging panel-shaped ligands.20,27 In 1998, McMorran and Steel reported the first self-assembly of a helical Pd2L4 cage by employing a Pd(II) salt with a non-coordinating hexafluorophosphate counter anion and bridging bis-pyridyl ligands with a flexible backbone structure.28 Since these initial studies, interest in constructing and applying such Pd(II) assemblies blossomed.21,29–31 Incipiently, the focus of the field was mainly on reporting the successful design or serendipitous finding of new coordination assemblies, largely driven by their aesthetic appeal and the quest for unprecedented topologies. In the last decade, the field transitioned towards implementing functionality into coordination cages, thus bringing them closer to application. In this direction, strategies for modular multicomponent self-assembly started to be developed, allowing the arrangement of several different organic building blocks in close proximity without the need for extensive organic synthesis. Examples for functions that have been incorporated into PdnL2n assemblies include catalytic activity,32–37 light harvesting chromophores,38 fluorophores,39–42 (photo)-redox activity,43–45 photoswitches,46–50 and solubility-controlling groups for higher-order self-assembly.51–53 Furthermore, many assemblies possess a cavity in which guest molecules can be encapsulated, allowing them to serve as molecular reaction vessels32,54–56 and high-affinity receptors,57–59 among other functions.60 However, most architectures reported so far have high symmetry, sharply contrasting with the low symmetry environments found in natural host systems.61–63 Therefore, efforts have been devoted to lowering the symmetry of such artificial self-assemblies by the controlled use of (I) matching organic ligands to form heteroleptic cages,21,64 (II) asymmetric ligands,30 and (III) different metal-ions to form heteronuclear assemblies.65 Self-sorting has also been investigated in the context of organic cages.66
Despite vast strides being made in this field, many of the rules governing the formation of specific and sometimes surprising topologies – often closely connected to the functionality of the assemblies – still remain elusive. Thus, we begin this review by exploring the various topologies found for PdnL2n homoleptic assemblies, i.e. architectures constructed from only one kind of organic ligand (Scheme 1a). While self-assembly is an equilibrium reaction, for simplicity, we use simple arrows throughout the remaining review. Next, we set out to summarise strategies developed for achieving integrative self-sorting to defined heteroleptic assemblies (Scheme 1b). We will then discuss approaches for orientational self-sorting to form homoleptic cages based on asymmetric ligands (Scheme 1c). Lastly, we describe the formation of cages via chiral self-sorting phenomena (Scheme 1d).
While there are many reports on assemblies with cis- or trans-protected Pd(II) cations and assemblies with ligands of higher denticity15,21 as well as on heteronuclear cages,65 we limit our discussion here to architectures based on bis-monodentate organic ligands and unprotected Pd(II) cations. We start this review by highlighting the breadth of topological diversity among homoleptic PdnL2n (n = 2–8) architectures. We then discuss strategies for the non-statistical self-assembly of heteroleptic cages, followed by orientational self-sorting of asymmetric ligands and chiral self-sorting. Special emphasis will be placed on factors governing the particular self-assembly outcome and attempts to rationalize experimental results based on computations or geometrical considerations.
2. Homoleptic dinuclear assemblies (n = 2)
The most basic and best explored family member among PdnL2n cages consists of two Pd(II) ions bridged by four organic ligands. After the initial work on a quadruple stranded helicate Pd2L4 by McMorran and Steel,28 a multitude of cages and helicates has been reported, with many studies focusing on the creation of larger cavities67,68 for the encapsulation of big58,69,70 or multiple58,71,72 guests and/or towards the incorporation of functionalities in the ligand backbone.38–40,42–47,50,51 As the field of the so-called lantern-shaped Pd2L4 cages has been reviewed recently,29 we will only briefly discuss some key parameters governing their formation and highlight prominent examples. Some helicates will be tackled with special emphasis on the degree of helicity. Finally, we describe in more depth some recent examples for some rather sophisticated dinuclear topologies, including those that do not obey the principle of maximum site occupancy, i.e. assemblies of stoichiometry Pd2L2 and Pd2L3.2.1. Lantern-shaped cages and helicates
The geometry of rigid bispyridyl-type bridging ligands can be described through the binding angle α, defined as the angle between the two nitrogen donor vectors. For ligands with collinear vectors, so-called lantern-shaped cages are preferably formed (Fig. 1a). A prominent example for a lantern-shaped cage is Pd2(1.1a)4, (note the naming scheme used herein: ligand 1.1a reacts to form cage 1.2a), initially reported by Hooley and coworkers in 2010.73 Here, the π-surfaces of the ligands are oriented in a perpendicular fashion with respect to the longer axis of the cavity (the latter going through both Pd centres), allowing for inward-pointing functionalities to be placed in the backbones. Lusby and coworkers exploited the inward pointing α-protons of the coordinating pyridines for forming multiple hydrogen bonds to the oxygens of quinones, resulting in encapsulation with high association constants.74 Furthermore, the same group used 1.2b for efficiently catalysing cycloadditions to the thus activated substrates inside the cage cavity.33–35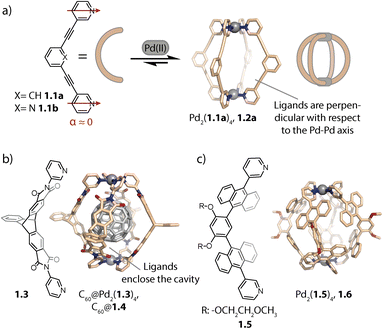 | ||
| Fig. 1 (a) Self-assembly of lantern-shaped cage 1.2a. (b) Capsule 1.4 based on a triptycene-derived ligand, encapsulating a C60 fullerene. (c) Capsule 1.6 based on ligands with anthracene panels. | ||
On the other hand, ligands with aromatic surfaces that partially enclose the cavity have been employed to assemble Pd2L4 capsules. For example, we designed curved ligand 1.3 based on triptycene58,75 (and ligands with a similarly shaped backbone76) aiming at a size match between the cavity of the corresponding capsule Pd2(1.3)4, 1.4 and fullerene C60 (Fig. 1b). This allowed selective binding of C60 and thereby its solubilization in polar organic solvents.
Yoshizawa and co-workers reported capsule Pd2(1.5)4, 1.6 based on ligand 1.5, whose methoxy ethoxy substituents endow the assembly with good solubility, even in water (Fig. 1c). The cavity of 1.6 is surrounded by an array of anthracene panels, creating a hydrophobic confinement. Owing to these two key properties, capsule 1.6 is capable of binding a range of neutral guests in aqueous solution.59 Recently, the same group used a similar capsule decorated with sugar substituents to induce chirality on an encapsulated fullerene.77 Our group, in collaboration with Gunnlaugsson and coworkers, has also achieved chirality transfer onto fullerenes (C60 and C70) with a readily accessible, relatively lightweight Pd2L4 cage with ligands constructed from Tröger's base.78
Certain factors such as the ligand geometry,38,79,80 steric congestion at the backbone81 or donor site,82 and attractive inter-ligand interactions83,84 can favour a helical twisting of the ligands around the Pd–Pd axis. For characterizing the degree of helicity, the azimuthal angle β can be used. It is defined as the dihedral angle between the two N–Pd coordination bonds of the same ligand (Fig. 2a).
 | ||
| Fig. 2 (a) Overview of azimuthal angles β of some Pd2L4 helicates formed with different bis-monodentate organic ligands (the β values were taken from the corresponding publication when available or were determined based on the X-ray crystal structure as described in ref. 96). (b) Structure of helicate 1.18a in comparison to isomeric mesocate 1.18b. (c) Structure of ligands 1.19 and mesocate 1.20. | ||
In their initial report, McMorran and Steel found one PF6− anion encapsulated in the cavity of helicate Pd2(1.7)4 which is based on flexible ligand 1.7 (Fig. 2a).28 This recently enticed the authors to study helicate formation in the presence of a variety of counter anions. Tetrahedral anions were found to be preferred over octahedral PF6− and amongst the anions tested, ClO4− showed the strongest binding, probably due to a perfect size match. On decreasing the size of the anions, the assembly shrinks along its Pd–Pd axis, concomitant with an increase in helicity, enabling an almost constant degree of cavity filling. The largest azimuthal angle of 85° was observed with iodine.85
Chand and coworkers reported in 2010 on helicate Pd2(1.8)4 consisting of piperazine-based ligand 1.8. The ligand adopts a flattened anti conformation upon self-assembly and the helicate is characterized by an azimuthal angle of around 45°.86 In 2012, the same group reported a helicate with a similar azimuthal angle by employing another flexible ligand 1.9 based on a central pyridine (not participating in metal coordination) that was equipped with amide linkers.87 The ligand can adopt different conformations depending on the orientation of the amide linkages relative to the backbone. It was found that the carbonyl bonds rotate outside of the helicate, presumably to avoid electronic repulsion with the central pyridine nitrogen and for enabling H-bonding between the NH groups and the encapsulated anion. In contrast, the same ligand with a benzene core formed, albeit under different conditions, a lantern-shaped cage, as reported by Puddephatt and coworkers in 2004. In this case, one of the two carbonyl groups of each ligand indeed points inside the cavity.88
In 2018, Gan and coworkers showed that ligand 1.10 assembles to form a mixture of D4-symmetric helicate and C4h-symmetric mesocate when combined with Pd(NO3)2. Selective formation of helicate or mesocate was favoured with a B12F122− guest or with tetrafluoroborate, respectively. Furthermore, addition of the chiral anion Δ-TRISPHAT led to an enrichment of one of the helical enantiomers, as the authors showed by CD spectroscopy.89
Additionally, in 2021, Chand and coworkers reported the anion-controlled self-assembly of helicate versus lantern-shaped cages: ligand 1.11 with urea linkers yields non-helical Pd2(1.11)4 in the presence of NO3− while a helicate with an angle of approximately 60° is formed with ClO4− anions.90
Sun and coworkers reported in 2015 on helicate Pd2(1.12)4 comprising anthracene-based ligands 1.12 equipped with benzimidazole donor moieties. Ligand twisting, resulting in a D4-symmetrical helicate with an azimuthal angle of around 75°, can be traced back to the steric repulsion between the anthracene backbones. The latter creates a hydrophobic cavity which encapsulates a nitrate anion that is stabilized by multiple H-bonds to the acidic imidazole protons. The authors showed that the binding affinity towards nitrate is significantly higher as compared to those of other anions tested.81
Crowley and coworkers reported helicate Pd2(1.14a)4 based on a small triazine-terminated ligand modified with benzyl groups. The helicate is further stabilized by π–π interactions and the azimuthal angle reaches around 90°.91 In a follow-up study, the group modified the triazine appended moiety as well as the spacer. For the latter, the helicates were shown to form reliably when the 1,3-phenylene moiety was substituted with a flexible propyl chain but not when substituted with a 1,4-phenylene group. Furthermore, the helical structure displayed tolerance across all tested exohedral triazine substituents, ranging from alkyl chains to electron-rich and -poor aromatic groups.92 In the context of biological activity, the Crowley and Giles group studied the stability of Pd2(1.14a–c)4 (Fig. 2a) and 1.2b (see Fig. 1a) against biological nucleophiles. Here, the helicates showed higher stability compared to lantern-shaped cage 1.2b, presumably due to the increased donor strength of the triazine and the sterically shielded Pd(II) centres. Furthermore, Pd2(1.14b)4 proved more stable compared to Pd2(1.14a)4 and Pd2(1.14c)4, originating most likely from the more restricted access to the coordination sites, owing to agglomeration of the alkyl chains.93
We have reported the self-assembly of helicates from various ligands possessing 8-isoquinoline donor moieties. The choice of this donor moiety leads to strongly convergent bonding vectors and hence, the ligands have to severely twist for bridging the two Pd(II) ions. The helical arrangement is further stabilized by π–π interactions between the ligands. Self-assembly of ligands 1.15 (ref. 79) and 1.16a, 1.16b (ref. 80 and 94) which are based on azulene and acridone backbones, respectively, led to helicates with azimuthal angles of around 140°. We also synthesized helicates based on well-known coal-tar dyes; in this case, piperazine linkers were used, allowing for conserving the absorption properties of the dyes.38 The corresponding ligands based on acridone, azulene, or coal-tar dyes equipped with 3-pyridyl donors instead of 8-isoquinolines assemble to form interlocked double cages95 or to lantern-shaped cages,38,79 respectively. This can be traced back to the nearly collinear binding vectors of the latter, showcasing how the topology of the dinuclear species in terms of its helical twist can be controlled with the ligand geometry.
In order to attain even greater azimuthal angles, we recently investigated the self-assembly of carbazole-based ligand 1.17 with an even more negative binding angle. Formation of helicate Pd2(1.17)4, 1.18a was observed with various different Pd(II) salts and the azimuthal angles ranged between 171 and 176° (Fig. 2b). In each case, one anion was found to be encapsulated within the cavity. Interestingly, with an increase in the size of anion, the Pd–Pd distance decreased, concomitant with a widening of the cage i.e. a change in the shape from prolate to oblate. While this might sound contradictory to the earlier report of McMorran and Steel,85 the structural adaptation serves the same purpose, that is to retain a favourable cavity filling. Helicate 1.18a exists in a counter anion- and solvent dependent equilibrium with the corresponding mesocate 1.18b. In the latter, the ligands adopt Cs symmetry, resulting in a significantly larger Pd–Pd distance (16.8 Å as compared to 9.3 Å) and an overall achiral structure.96
Crowley and coworkers modified ligand 1.1b by either exchanging the pyridine with quinoline or isoquinoline donors (1.19a and 1.19b)83 or by equipping the pyridines with amino groups at ortho- or meta-position to the nitrogens (1.19c and 1.19d).97 Ligands 1.19b and 1.19d form, similarly to parent ligand 1.1b, a lantern-shaped cage. However, self-assembly of 1.19a and 1.19c which possess modifications in closer proximity to the coordinating nitrogen resulted in the formation of a mesocate Pd2(1.19a)4, 1.20 and a helicate Pd2(1.19c)4 (Fig. 2c). For Pd2(1.19c)4, this was explained by hydrogen bonds between the amino groups in the twisted coordination environment.
2.2. Unusual dinuclear assemblies
Severin and coworkers observed that acridone-based helicate Pd2(1.16b)4, initially reported by us,80 interacts with a fifth ligand 1.16b, when Pd(II) and 1.16b are combined in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio. In order to get insight into the nature of this association, the authors added 1.16c which is a derivative of 1.16b, lacking the nitrogen donors. The authors observed the formation of a 1
5 ratio. In order to get insight into the nature of this association, the authors added 1.16c which is a derivative of 1.16b, lacking the nitrogen donors. The authors observed the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
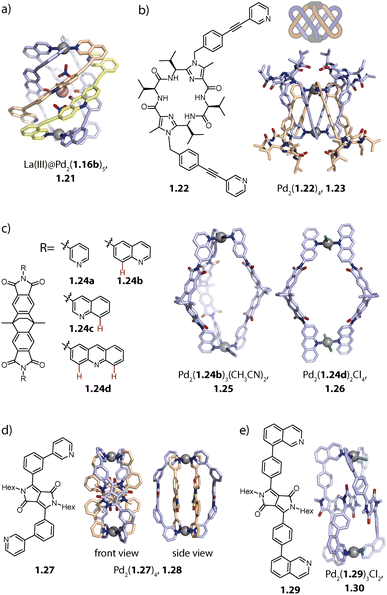
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex between 1.16c and helicate Pd2(1.16b)4. The association of compound 1.16c with Pd2(1.16b)4 is presumably driven by strong ligand-to-ligand π–π stacking interactions. For obtaining a discrete penta-stranded helicate Pd2L5, the authors exploited the multiple inward pointing carbonyl groups of the helicate: oxophilic, high-valency La(III) binds to the pocket of the helicate and stabilizes the arrangement of five ligands 1.16bvia coordination bonds. In La(III)@Pd2(1.16b)5, 1.21, three ligands coordinate to both Pd(II) centres while the two remaining ones coordinate via one donor group only, giving rise to an assembly of overall low symmetry (Fig. 3a).84
1 complex between 1.16c and helicate Pd2(1.16b)4. The association of compound 1.16c with Pd2(1.16b)4 is presumably driven by strong ligand-to-ligand π–π stacking interactions. For obtaining a discrete penta-stranded helicate Pd2L5, the authors exploited the multiple inward pointing carbonyl groups of the helicate: oxophilic, high-valency La(III) binds to the pocket of the helicate and stabilizes the arrangement of five ligands 1.16bvia coordination bonds. In La(III)@Pd2(1.16b)5, 1.21, three ligands coordinate to both Pd(II) centres while the two remaining ones coordinate via one donor group only, giving rise to an assembly of overall low symmetry (Fig. 3a).84
Our group obtained a complex, interlocked architecture Pd2(1.22)4, 1.23 with bis-pyridyl ligand 1.22 which is based on a peptidic macrocycle (Fig. 3b). The assembly is chiral and represents an entirely new catenated motif for Pd(II) architectures. The high ligand flexibility allows it to chelate to a single metal ion to give mononuclear complex Pd(1.22)2, possessing a butterfly structure. Self-assembly in acetonitrile, followed by the addition of chloride or triflimide salts, however, eventually led to the formation of a dinuclear complex. The Pd(II) centres in the C2-symmetric structure are chelated in a cis-configuration and the two Pd(1.22)2 moieties, also termed lemniscates, are held together by two mechanical bonds. A closer look at the X-ray structure reveals that this peculiar architecture is stabilized by (a) a counter anion that binds between the two Pd(II) centres, (b) inter-ligand π–π stacking interactions and (c) embedding of pyridine donor moieties in the macrocyclic backbone cavity of the adjacent ligand.98
Moreover, we showed that the introduction of steric congestion on the donor site can result in the formation of Pd2Lm, m < 4 species, thereby challenging the principle of maximum site occupancy. While pyridine-equipped ligand 1.24a based on a dibenzo-2.2.2-bicyclooctane backbone assembles to form capsule Pd2(1.24a)4, the same ligand with 6-quinoline donors 1.24b yields bowl Pd2(1.24b)3(CH3CN)2, 1.25 when Pd(II) and 1.24b are combined in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio (Fig. 3c). This outcome can be explained by steric repulsion: in capsule Pd2(1.24b)4, the distances between protons 8H (red in Fig. 3c) of neighbouring ligands are smaller than the sum of their van der Waals distances, leading to steric repulsion, while they have more space and thus surmount this distance in bowl 1.25.76 Interestingly, when ligand 1.24c, equipped with 3-quinoline donors is employed, capsule Pd2(1.24c)4 is formed as a thermodynamic product. For overcoming steric stress, the donor groups arrange in a propeller geometry, leading to an overall helical twist of the assembly. Acridine as a donor moiety introduces two hydrogen substituents that are oriented in a similar direction to the nitrogen donor vectors. Ligand 1.24d hence has a further increased steric hindrance compared to 1.24b and 1.24c, favouring the formation of ring Pd2(1.24d)2(CH3CN)4/(Cl)4, 1.26.82 This topological variance has consequences for the fullerene binding capabilities of the assemblies. While capsule Pd2(1.24a)4 is able to bind C60 but not C70, the open bowl 1.25 allows for binding of both. Helically twisted Pd2(1.24c)4 and ring 1.26, on the other hand, showed no or weak affinity towards fullerenes. Partial solvent exposure of the surface of C60 in bowl 1.25 was exploited for controlling the covalent functionalization of fullerenes with the bowl serving as a non-covalent protecting group.
3 ratio (Fig. 3c). This outcome can be explained by steric repulsion: in capsule Pd2(1.24b)4, the distances between protons 8H (red in Fig. 3c) of neighbouring ligands are smaller than the sum of their van der Waals distances, leading to steric repulsion, while they have more space and thus surmount this distance in bowl 1.25.76 Interestingly, when ligand 1.24c, equipped with 3-quinoline donors is employed, capsule Pd2(1.24c)4 is formed as a thermodynamic product. For overcoming steric stress, the donor groups arrange in a propeller geometry, leading to an overall helical twist of the assembly. Acridine as a donor moiety introduces two hydrogen substituents that are oriented in a similar direction to the nitrogen donor vectors. Ligand 1.24d hence has a further increased steric hindrance compared to 1.24b and 1.24c, favouring the formation of ring Pd2(1.24d)2(CH3CN)4/(Cl)4, 1.26.82 This topological variance has consequences for the fullerene binding capabilities of the assemblies. While capsule Pd2(1.24a)4 is able to bind C60 but not C70, the open bowl 1.25 allows for binding of both. Helically twisted Pd2(1.24c)4 and ring 1.26, on the other hand, showed no or weak affinity towards fullerenes. Partial solvent exposure of the surface of C60 in bowl 1.25 was exploited for controlling the covalent functionalization of fullerenes with the bowl serving as a non-covalent protecting group.
Formation of Pd2L3-type bowls along with Pd2L2-type rings was also observed for bis-picolyl ligands, i.e. when the steric congestion at the pyridine is increased through the introduction of methyl groups.99 Furthermore, coordination rings are also formed when doubly protected Pd(II) cations or a moiety such as PdCl2 is employed.100–102
Recently, we reported a self-penetrated homoleptic assembly based on ligand 1.27 which possesses two key attributes: (a) strongly convergent donor vectors and (b) diketopyrrolopyrrole (DPP) backbones, known for their high propensity to interact via π–π stacking. Doubly-bridged ravel Pd2(1.27)4, 1.28 is obtained as a racemic mixture, belonging to point group D2. The two inner ligands adopt an S-shaped conformation which allows them to cross the centre of the assembly. The other two ligands sit on the outer positions and are helically twisted (Fig. 3d). Overall, four DPP moieties are stacked whereby neighbouring backbones are rotated by roughly 90°. The assembly is reminiscent of heteroleptic trans-Pd2A2B2 cages,103 which will be described later. In ligand 1.29 the DPP backbone is equipped with sterically demanding isoquinoline donors which led to the formation of self-penetrated bowl Pd2(1.29)3, 1.30. In contrast to previously reported bowl structures, the central and one of the outer ligands adopt an S-shaped conformation. This allows three DPP moieties to stack and gives rise to a C2-symmetric assembly, not leaving any cavity for guest binding (Fig. 3e).104
In summary, here we showcased a series of experimentally observed topologies for assemblies possessing only two Pd(II) ions. The design principles for lantern-shaped cages and helicates are well established. For the former, collinear bis-monodentate ligands are utilized; helical twisting is driven by steric constraints, inter-ligand interactions, and/or strongly convergent donor vectors. We also touched upon examples where the steric demand of the donor groups resulted in the formation of more open bowl or ring structures. It is notoriously more difficult to design intricate self-penetrated or interwoven assemblies whose formation is often governed by close-ligand interactions, templation, solvent effects or a combination thereof.
3. Homoleptic multinuclear assemblies (n = 3–8)
Dinuclear cages (Chapter 2) and spheres PdnL2nn ≥ 12 have garnered great attention and for the latter, guidelines for their rational design have been described by Fujita and coworkers.105 Furthermore, most functional assemblies reported so far belong to either of these classes.64,105 Less attention has been paid to assemblies with intermediate sizes (n = 3–8), which will be described in this chapter. We discuss the assemblies in the order of increasing nuclearity and complexity, starting with three- to five-membered rings. Next, we will discuss pseudo-tetrahedral and hexanuclear species such as cubes, octahedra, and rings. Finally, we address more complex supramolecular coordination assemblies, such as interlocked cages. For clarity, different assemblies based on the same ligand are usually discussed together; hence, the order is not strictly followed.3.1. Coordination rings
For the nuclearities n = 3, 4, and 5, the assemblies of the highest symmetry are rings. Fujita and coworkers synthesized the first three-membered ring in 2006 which will be described later.106 The same group showed that ligand 2.1, possessing a binding angle of 63°, assembles to form the entropically favoured three-membered ring Pd3(2.1)6, 2.2 in acetonitrile, while a four-membered ring Pd4(2.1)8, 2.3 is formed in DMSO (Fig. 4a). Interestingly, X-ray crystal structure analysis revealed that the cavity of 2.3 in the solid state is occupied by an aggregate of DMSO and nitrate molecules. Hence, the solvent dependency might be explained by a templation effect. Self-assembly in the presence of other anions yields mixtures of tri- and tetranuclear species, supporting this conclusion.107 Previously, a four-membered ring Pd4L8 had only been reported by Chand, Fujita and coworkers in 2003.108 A similar solvent-dependent equilibrium between 3- and 4-membered rings was recently described in mechanistic detail by Li and coworkers.109 | ||
| Fig. 4 Self-assembly of homoleptic rings PdnL2n (n = 3–5). (a) Solvent-controlled self-assembly of three- and four-membered rings 2.2 and 2.3. (b) Self-assembly of three- and four-membered rings 2.5 and 2.6 controlled by the ligand length. (c) Three-membered ring 2.8 based on a rotationally flexible ferrocene-based ligand. (d) Control over the ring size achieved through ligand angle adjustment (all X-ray structures; for 2.10, a DFT model is shown). For Pd6L12 rings, see Fig. 5b and 8d (α refers to the binding angle of the free ligand and the values for ligands 2.1, 2.4, 2.9, 2.11, and 2.13 were taken from ref. 112). | ||
Previously, the same group also investigated the self-assembly of metalloligands 2.4a and 2.4b with binding angles of 60°, differing solely in their length and hence in their flexibility. While short ligand 2.4a cleanly assembles to form four-membered ring Pd4(2.4a)6, 2.5, ligand 2.4b forms trinuclear ring Pd3(2.4b)6, 2.6 (Fig. 4b). Hence, the increased flexibility of ligand 2.4b allows the formation of the entropically favoured smaller assembly.110
Crowley and coworkers studied the self-assembly of ferrocene-based ligand 2.7, which can, in contrast to the rather rigid ligands discussed beforehand, adopt a variety of different binding angles owing to its rotational flexibility (Fig. 4c). Interestingly, the three-membered ring Pd3(2.7)6, 2.8 was obtained as the sole species. Presumably, this is the smallest assembly accessible without significant ring strain; hence, the outcome can be rationalized based on entropic considerations.111
We recently studied the self-assembly of a series of ligands under systematic variation of the binding angle α under similar conditions (DMSO, [Pd(CH3CN)4](BF4)2) for deriving a relationship between the ligand bent angle and ring nuclearity (Fig. 4d). Ligand 2.9 with an angle of 28° assembles to form the three-membered ring Pd3(2.9)6, 2.10 and phenanthrene-based ligand 2.11 with an angle of 63° forms, as was shown previously,80 the four-membered ring Pd4(2.11)8, 2.12. The binding angle of fluorenone based ligand 2.13 is further increased to 84° which allowed us to cleanly assemble the first non-templated five-membered ring Pd5(2.13)10, 2.14. Self-assembly of a dibenzothiophene-based ligand with an intermediate binding angle of 78° leads to the formation of a mixture of four- and five-membered rings, showcasing that the ring size can be controlled by the bent angle when self-assembly conditions and ligand flexibility remain constant. It has to be noted that the self-assembly of the discussed ligands displayed solvent dependency and also tetra- and hexanuclear species, most probably octahedra, could be observed by mass spectrometric analysis. By treating the ligands as rigid triangles, a geometrical model was derived which reproduced the observed trends in a qualitative fashion. Major bottlenecks of such simple geometrical considerations, however, are omission of the ligand flexibility and solvent effects, hence serving only as a rule of thumb apporach.112
Crowley and coworkers synthesized a heterometallic Pd3(PtL2)6 ring by employing a ligand with a bidentate and two monodentate binding sites. In the first step, Pt-based metalloligand L2Pt was synthesized through coordination via the bidentate site. This was followed by self-assembly with Pd(II). When L was reacted with only Pd(II) cations, a mixture of different assemblies was obtained and Pd3(PdL2)6 was identified as the main component. The heterometallic ring was shown to incorporate planar aromatic guests within its clefts and was used for catalysing the [4 + 2] cycloaddition of anthracene with singlet oxygen.113
In 2014 and 2015, the groups of Mukherjee and Chand assembled a three-membered ring Pd3L6 from simple 1,3-diimidazole-benzene ligands.114,115 Chand showed that a similar ligand with benzimidazole donors also assembles to form a three-membered ring and that this assembly allows for gel formation owing to the additional π-surfaces.115
Furthermore, we equipped a photoswitchable dithienylethene (DTE) backbone with para-pyridine donors. Opening and closing of the DTE moiety upon irradiation with UV or visible light, respectively, is accompanied by a drastic change in the ligand bent angle. This allowed for reversibly switching between a mixture of rings (Pd3L6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd4L8 3
Pd4L8 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and a Pd24L48 sphere. While assembly in the presence of tetrafluoroborate anions afforded the aforementioned mixture of three- and four-membered rings, the trinuclear species was formed as the sole species with a Pd(II) nitrate salt. Presumably, this can be attributed to a templation effect.116
1) and a Pd24L48 sphere. While assembly in the presence of tetrafluoroborate anions afforded the aforementioned mixture of three- and four-membered rings, the trinuclear species was formed as the sole species with a Pd(II) nitrate salt. Presumably, this can be attributed to a templation effect.116
Templation of Pd3L6 by nitrate anions was also reported by Jung and coworkers. The authors showed that self-assembly of a very flexible bis-pyridyl ligand 2.15 with different Pd(II) precursors results in entropically favoured lemniscates Pd(2.15)2, 2.16. In contrast, when Pd(II) nitrate was used as the metal source, a clean three-membered ring Pd3(2.15)6, 2.17 was formed (Fig. 5a).117
In a similar fashion, Sun and coworkers showed nitrate templated formation of the three-membered ring Pd3(2.18)6, 2.19 (Fig. 5b). Surprisingly, rings of various nuclearities, ranging from n = 3–7 were assembled with ligand 2.18, strictly depending on the anion(s) present in solution. Akin to the three-membered ring, the six- and seven-membered rings Pd6(2.18)12, 2.20 and Pd7(2.18)14, 2.21 were obtained through direct assembly with the BF4−, or PF6−/OTf− salts, respectively (red arrows in Fig. 5b). In contrast, four-membered ring Pd4(2.18)8, 2.22 could only be accessed via ring-to-ring transformation by addition of HSO4− to preformed 2.20 or 2.21 (dark blue arrows) and five-membered ring Pd5(2.18)10, 2.23 by addition of Mo7O246− to any of the preformed rings (beige arrows). From the different transformation processes, the authors concluded that the templation effect for this system is decreased in the order Mo7O246− > NO3− > SO42− > BF4− > PF6− = OTf−. The high adaptivity was attributed to (a) the methylene group which enables rotation as well as bending and hence adoption of numerous binding angles and (b) the acidic CH protons which might contribute to specific anion binding.118
3.2. Tetrahedra
In contrast to para-pyridine-based ligands which often form three-membered and/or four-membered rings,107,110,112 small ligands equipped with meta-pyridine donors tend to assemble to form pseudo-tetrahedra as tetranuclear species.106,119,120 The first example of a tetrahedron Pd4L8 was reported by Fujita and coworkers in 2006. The authors showed that short ligand 2.24 forms a triangular species Pd3(2.24)6, 2.25 with triflate as the counter anion while a tetrahedron Pd4(2.24)8, 2.26 was obtained when a nitrate salt was employed (Fig. 6a). Note that Pd4L8 assemblies can only give rise to pseudo-tetrahedral structures in which two oppositely arranged edges are doubly bridged while the remaining four edges are bridged by a single ligand each. Hence, each vertex consists of a tetra-coordinated Pd(II) cation (whereas in a real tetrahedron, each vertex connects only three edges). Elongation of the ligand with one phenyl linker results in a dynamic equilibrium between both species: with nitrate, the triangular ring and the tetrahedron coexist in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 while the ring is favoured when a Pd(II) triflate salt is employed. With tosylate, the tetrahedral species is yielded as the sole assembly.106
1 while the ring is favoured when a Pd(II) triflate salt is employed. With tosylate, the tetrahedral species is yielded as the sole assembly.106
Mukherjee and coworkers studied the self-assembly of small bis-meta-pyridyl ligands of a similar geometry. Self-assembly of ligand 2.27 with a Pd(II) nitrate salt yielded, similar to 2.24, tetrahedral cage Pd4(2.27)8, 2.29 exclusively. In contrast, ligand 2.30 assembles to form triangular Pd3(2.30)6, 2.31 under the same conditions (Fig. 6b). The self-assembly of ligand 2.27 was furthermore studied with Pd(II) tetrafluoroborate salt in DMSO and ACN. While the tetrahedral species 2.29 was favoured in ACN with this counter anion as well, a mixture of ring 2.28 and tetrahedron 2.29 was obtained in DMSO. Self-assembly of ligand 2.30 yielded a dynamic mixture in both solvents with the triangular species 2.31 as the main component in ACN and tetrahedral 2.32 in DMSO. From the investigation of the equilibrium of both DMSO systems at various temperatures, the authors were able to conclude that the tetrahedra 2.29 and 2.32 are entropically favoured over the corresponding rings but are enthalpically disfavoured; hence, the equilibrium shifts towards the larger species at higher temperatures.120
Moreover, our lab investigated the self-assembly of fluorene-derived ligands 2.33.119,121 Considering the flat ligands, two binding situations, namely the concave and the convex mode, can be differentiated (Fig. 6c). A binding mode in which the two donor vectors are oriented in opposite directions would most probably not lead to a discrete assembly. Fluorenone-119 and N-methyl carbazole121 based ligands 2.33a and 2.33c assemble to form a mixture of the three-membered ring Pd3(2.33a/c)6, 2.34a/c and tetrahedral Pd4(2.33a/c)8, 2.35a/c in acetonitrile. In contrast, ligand 2.33b based on N-unsubstituted carbazole cleanly assembles to form the tetrahedral species 2.35b. Even though the ligands in the assembly are not flat, the two distinct binding modes are observed in the X-ray crystal structure of 2.35a (Fig. 6c, middle): the ligands sitting on the singly bridged edges adopt a rather convex binding mode, and hence the backbone functionality points outside, while the ligands occupying the doubly bridged edges adopt a concave binding mode. When fluorene-based ligand 2.33d with two hexyl chains is employed, octahedral Pd6(2.33d)12, 2.36d is obtained. All ligands adopt a convex binding mode, allowing for assembly to this higher nuclearity species. Occupation of double-bridged edges is not possible with 2.33d owing to steric hindrance; hence, formation of homoleptic rings and tetrahedra is impeded.119 It is worth noting that the backbone steric bulk does serve as a topology-controlling moiety here that does not compromise the size of the inner cavity. This contrasts with the first study on the backbone steric bulk-controlled formation of octahedra by Severin and coworkers where the overall size of the ligand backbone was increased so much that it reaches into the central cavity, as will be discussed later.122,123
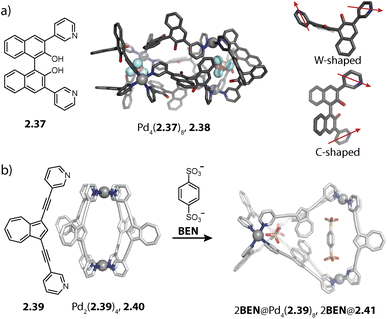
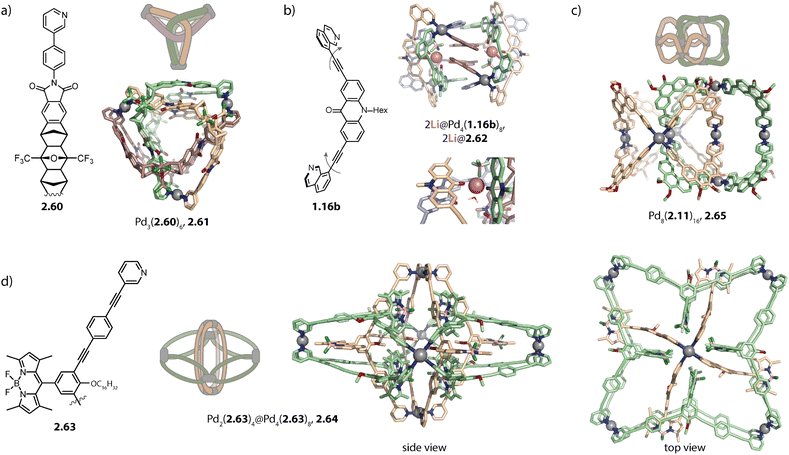
Lützen and coworkers applied chiral BINOL-based ligand 2.37 for assembling a tetranuclear species Pd4(2.37)8, 2.38 in which the Pd(II) ions are arranged in a tetrahedral fashion (Fig. 7a). In contrast to the aforementioned tetrahedra possessing singly and doubly bridged edges, all neighboring metal ions are doubly bridged in 2.38. In this distorted macrocyclic structure, the ligands adopt two distinct conformations: ligands bridging the long edges have a W-shaped conformation with rather divergent binding angles while ligands bridging the short edges possess a C-shaped conformation with a smaller binding angle. This arrangement results in three pockets: two smaller peripheral pockets, each encapsulating one tetrafluoroborate anion, and a central cavity. Self-assembly of 2.37 with other Pd(II) sources did not result in a defined species, showing that the tetrafluoroborate anions serve as the template for 2.38.124
We recently reported an unprecedented structural switch between a lantern-shaped cage Pd2(2.39)4, 2.40 and a distorted tetrahedron Pd4(2.39)8, 2.41 (Fig. 7b). Bis-pyridyl ligand 2.39 with an azulene backbone assembles with Pd(II) to a classical lantern-shaped cage 2.40, as can be expected based on the geometry of the ligand (Chapter 2.1). Conversion into 2.41 was achieved upon addition of 1,4-benzene-bissulfonate (BEN). While the conformation of the ligands sitting on the doubly bridged edges closely resembles the situation in the lantern-shaped cage, the pyridines of the ligands on the singly bridged edges are flipped by approximately 180°. The shorter Pd–Pd distances, connecting the nodes of the double-bridged edges, match the size of the BEN guest, allowing for close attractive electrostatic interactions. Hence, maximizing host–guest interactions is presumably the driving force for the observed structural switch, aided by an energetic contribution resulting from a circular arrangement of the dipolar backbones of the singly bridged edges. Intriguingly, the switch could be reversed by addition of a competitive host.79
3.3. Hexanuclear species
Rigid ligands with nearly rectangular geometry assemble to form cubes in which the Pd(II) ions are arranged in an octahedral fashion. This can be regarded as an extension of the above discussed series of rigid ligands to even larger binding angles. The first cube Pd6(2.41b)12, 2.43 was reported by Fujita and coworkers in 2009 and is assembled from dibenzofurane ligand 2.41b (Fig. 8a).125 Also, smaller ligand 2.41a and carbazole-based ligand 2.42 assemble to form analogous cubes, showcasing the reliability of the design approach based on the binding angle of rigid ligands. Reek and coworkers showed that cubes Pd6(2.41a)12 and Pd6(2.42)12 bind fullerene and that the binding affinity can be tuned by altering the electronic properties of the ligands.126Furthermore, the group of Lützen reported BINOL-based ligands which possess, in contrast to ligand 2.37 discussed above, para-pyridine donor groups and hence larger binding angles. The ligands self-assemble to form Pd6L12 cubes and Pd12L24 spheres.127
Mukherjee and coworkers reported the first octahedra based on bis-imidazole ligands 2.44 (Fig. 8b). Similar to the cube-like structure, the Pd(II) ions in Pd6(2.44)12, 2.45 are arranged in an octahedral fashion but due to the different ligand geometry, neighboring Pd(II) are bridged in a rather linear fashion.128
Severin and coworkers reported clathrochelate ligands 2.46 which possess a similar geometry compared to ligand 2.24 introduced by the group of Fujita. However, the increased thickness of the backbone allowed for topology control: while less bulky 2.46a assembles to form the entropically favoured tetrahedron Pd4(2.46a)8, ligands 2.46b–d with increased steric bulk only allow the formation of larger octahedra Pd6(2.46b–d)12, 2.47b–d (Fig. 8c). Presumably, the larger species is favoured here in order to avoid repulsive steric interactions between the ligands in the smaller assembly. Furthermore, the authors showed with the help of enforced disassembly experiments that the decreased thickness of ligand 2.46d as compared to 2.46c translates into an enhanced thermodynamic stability of 2.47d. In turn, narcissistic self-sorting was observed when ligand 2.46d was combined with ligand 2.24. In contrast, a combination of 2.46c, 2.24, and Pd(II) resulted in a library of mixed-ligand species.122,123
Recently, we reported the surprising conformational flexibility of short acridone-based ligand 1.16a. In solution, helically twisted Pd2(1.16a)4 is formed which is, depending on the solvent and counter anion, transformable into its mesocate isomer. Surprisingly, when we attempted to crystallize the mesocate, six-jagged ring Pd6(1.16a)12, 2.48 was obtained (Fig. 8d). Here, the ligands adopt an S-shaped conformation and pairs of ligands bridge neighbouring Pd(II) ions, resulting in an overall C6h-symmetric structure. Crystal packing analysis revealed that the rings stack cofacially to form columnar, hexagonal channels that are separated by triangular gaps. The templated six-membered ring 2.22 reported by Sun and coworkers as described earlier and this solid-state structure are the first reported Pd6L12 rings.94
3.4. Interlocked and other unusual motifs
Lantern-shaped coordination cages can undergo dimerization through catenation. Thereby, the upper part of the ligands of one cage penetrates through the windows of the other cage. The most frequently observed interlocked double cages possess a fourfold symmetry and an alternating stack of Pd(II) cations and counter anions which reduces the Coulomb repulsion between the closely positioned Pd(II) ions. We have reviewed interpenetrated double cages in depth in the past129 and here, we will focus on some factors that allow for controlling interpenetration and on a recently reported new motif.In 2008, Kuroda and coworkers reported Pd4L8 interlocked double cages for the first time. In this work, a combination of an organic ligand and Pd(NO3)2 in DMSO led to the formation of a monomeric cage Pd2L4 as a kinetic intermediate which was then converted into the quadruply interlocked double cage Pd4L8. Interestingly, when other Pd(II) sources were used for the self-assembly, the equilibrium was shifted towards the monomeric cage. Also, the addition of a naphthalene-monosulfonate guest led to a preference for the monomeric cage.130
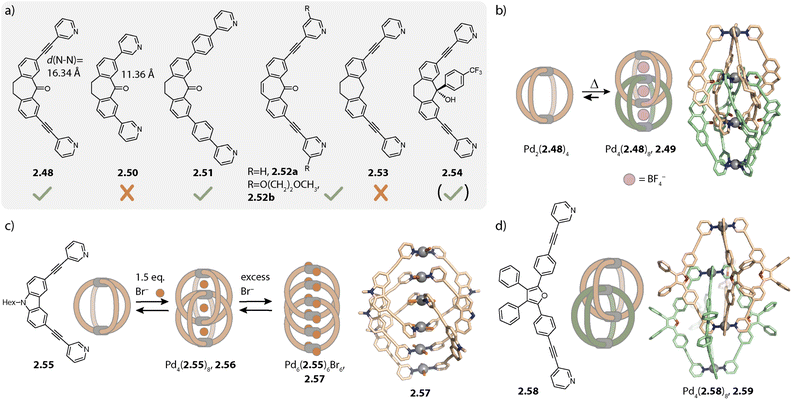
Later, we reported that dibenzosuberone-based ligand 2.48 assembles to form a similar interpenetrated cage Pd4(2.48)8, 2.49 with [Pd(CH3CN)4](BF4)2 in acetonitrile (Fig. 9b). Akin to the report of Kuroda and coworkers, the monomeric cage was observed to be a kinetic intermediate. In contrast to ligand 2.48 with alkyne spacers, double cage formation is not observed for ligand 2.50 with a shorter N⋯N distance (11.36 Å vs. 16.34 Å) under the same conditions. The pockets of a hypothetical double cage Pd4(2.50)8 would be too small for the incorporation of BF4− (or any other) anions, resulting in a preference for the monomeric cage Pd2(2.50)4.131
For further controlling double- vs. monomeric cage formation, we investigated how structural modifications of the ligand 2.48 affect its ability to dimerize. Elongation of the linker by exchanging the alkyne with a phenyl spacer (2.51) and desaturation of the ligand backbone (2.52a) as well as modifications of the pyridines of 2.52 with methoxy ethoxy chains (2.52b) did not impede interpenetration. In contrast, deoxygenation of the backbone (2.53) led to formation of the monomeric cage Pd2(2.53)4. Close examination of the X-ray crystal structure in combination with DFT calculations revealed that the double cage structure 2.49 seems to be further stabilized by interactions between the oxygens of the carbonyl groups and the closely packed inner Pd(pyridine)4 plane of the other cage unit.132 This interaction, however, was not necessarily required for the interlocked cage reported by the group of Kuroda130 and for double cages with other ligands based on phenothiazine133 or helicene,134 reported by us.
The propensity for intercalation is also altered when inward-pointing steric bulk is installed on the ligand backbone. Derivative 2.54 which possesses the same N⋯N distance and binding angle as compared to 2.48 forms lantern-shaped cage Pd2(2.54)4 under similar self-assembly conditions to those used for obtaining double cage 2.49. However, the addition of chloride to Pd2(2.54)4 again resulted in double cage formation. This can be rationalized based on steric considerations: due to the comparably large size of BF4− anions which would have to be encapsulated in the central pocket, the outer pockets of a potential cage BF4@Pd4(2.54)8 would be small, resulting in repulsive interactions between the attached aromatic groups. However, with chloride as the central anion, the outer pockets are enlarged, offering sufficient space for the bulky substituents.135 Yamashina, Toyota and coworkers showed, on the other hand, that exohedral steric bulk can also control dimerization.136
Similar chloride-induced interpenetration was observed with carbazole-based ligand 2.55 possessing shorter N⋯N distances as compared to 2.48. Only halides Cl− and Br−, but not BF4−, can be sandwiched between the Pd(py)4 planes, allowing for catenation of two cages to give Pd4(2.55)8, 2.56 (Fig. 9c). In this case, addition of stoichiometric amounts of halides led, however, to a mixture of the interlocked double cage with the monomeric cage and the free ligand. Addition of over-stoichiometric amounts of halides leads to further ligand release. This can be explained by competition between halides and organic ligands coordinating to Pd(II). Unexpectedly, triple catenated (PdBr2)6(2.55)6, 2.57, carrying no net charge, was crystallized. Here, the Pd(II) ions are arranged in a linear stack with noticeably shorter Pd⋯Pd distances as compared to the distances in the corresponding double cage (4.36 Å vs. 6.73 Å). The bromide ligands are arranged in a helical fashion and the close proximity of the ligand backbones suggests favourable π–π interactions to be the key driving force for the observed structure.137
Bloch and coworkers reported the halide-triggered assembly of double cages that bind bisulfates with high affinity.138 The group of Hiraoka described a system of transformable quadruply interpenetrated cages in which different halide-dependent states lead to different reactivities.139
Most often, the degree of catenation is related to the symmetry of either the assembly or the ligand employed. This holds for the described quadruply interlocked cages with C4 or D4 symmetry, for triply interlocked C3 symmetric cages of Fujita,140 and for quintuply interlocked double cages based on C5 symmetric ligands reported by Nitschke and coworkers.141 However, recently, we reported unprecedented triply interlocked double cage Pd4(2.58)8, 2.59 with long furane-based ligand 2.58 (Fig. 9d). Similar to what has been reported for quadruply interlocked double cages, monomeric cage Pd2(2.58)4 was formed as a kinetic product. Heating led to a mixture of the monomeric cage with quadruply and triply interlocked double cage 2.59. At room temperature, the latter was observed to be the thermodynamic product. Here, two opposing windows of a first cage are each penetrated by one bridging ligand of a second cage while a third window of the first cage is penetrated by two ligands of the second cage. This leaves one window of the first cage free. The peculiar structure is stabilized by π–π interactions between the electron rich furane backbones and the electron poor pyridines. A reference ligand with the same geometry as 2.58 but a silole backbone that possesses additional methyl substituents assembles to form a monomeric cage. Due to the methyl substituents, π–π interactions involving the backbones are hampered, which highlights the necessity of intense π–π interactions for the formation of an interlocked cage.142
Aside from a simple three-membered ring, a double-trefoil knot structure Pd3(2.60)6, 2.61 was observed for the stoichiometry Pd3L6 when long concave ligand 2.60 was employed (Fig. 10a). Similar to the ring, each Pd(II) pair is bridged by two organic ligands; however, the ligands are intertwined. This allows for close interactions between ligands 2.60 which possess multiple sites for π–π and H-bond interactions. Among the different possibilities for the arrangement of the ligands in 2.61, the peculiar structure was assigned employing a combination of NOESY NMR spectroscopy and molecular modelling, considering the symmetry of the ligands and the measured vs. calculated H⋯H distances. Double trefoil knot 2.61 consists of two chiral hemispheres with opposite configurations, forming an overall meso structure. The lack of preference for chiral guests of opposite configurations is thus in full accordance with the proposed structure.143
Severin and coworkers recently reported a structural switch from acridone based helicate Pd2(1.16b)4 to tetranuclear low symmetry assembly Pd4(1.16b)8, 2.62 upon addition of a Li salt. The structure consists of two Pd2(1.16b)3 distorted bowls that are bridged by two more ligands 1.16b which sit in the centre of the structure and are involved in π–π interactions (Fig. 10b). Two binding pockets are each occupied by one LiBF4 and a water molecule. The Li cation is stabilized by a tetrahedral environment of BF4−, water, and two carbonyl oxygens of the acridone ligands. The water molecule is furthermore engaged in H-bonds to two adjacent carbonyl oxygen atoms. Under strictly dry conditions, the peculiar, compact structure is not observed, underscoring the importance of the water molecules. Furthermore, the structural switch was selective for Li as it could not be induced by addition of other alkali metal salts.144 The same group incorporated lantern-shaped cages with polymerizable appendices as dynamic crosslinks in acrylamide hydrogels.145
Lützen and coworkers reported a cage-in-ring assembly Pd2(2.63)4@Pd4(2.63)8, 2.64, resembling a rotaxane (Fig. 10d). The shape of ligand 2.63 reminds to simple banana-shaped ligands known to assemble to form lantern-shaped cages Pd2L4. However, it stands out due to the length of its arms and possesses BODIPY and alkoxy substituents, having a propensity for engaging in π–π and van-der-Waals interactions, respectively. In the centrally positioned lantern-shaped cage Pd2(2.63)4, the ligands adopt a C-shaped conformation, leading to outward-pointing BODIPY-moieties and inward-pointing alkoxy chains. The ligands forming the four-membered ring Pd4(2.63)8 possess a W-shaped conformation in which the pyridine moieties are flipped by approximately 180°. Hence, the BODIPY substituents are endohedrally located allowing for π–π interaction with the backbones of Pd2(2.63)4. Additionally, the outer ring possesses clefts which enable the intercalation of the BODIPY moieties of Pd2(2.63)4.146
Catenation of PdnL2n architectures with n > 2 was so far only observed for four-membered rings 2.12 based on phenanthrene ligand 2.11. Octanuclear Pd8(2.11)16, 2.65 consists of two mechanically interlocked D4h-symmetric rings 2.12 that are rotationally displaced by 90° (Fig. 10c). The assembly displays D2d symmetry and can be described as a huge Hopf-link. Noteworthily, the interlocked motif was only observed in the presence of nitrate, either upon direct assembly with Pd(NO3)2 or upon addition of a nitrate salt to a preformed mixture of tri- and tetranuclear assemblies. Presumably, nitrate anions possess the ideal size for templating the dimerization. Furthermore, switching the solvent from acetonitrile to DMSO impeded interlocking, similar to the dimerization of most lantern-shaped cages. Furthermore, higher order assembly to vesicular structures was achieved by equipping ligand 2.11 with hexyloxy chains. Each modified 2.65 assembly carries 32 hexyloxy chains, promoting aggregation via hydrophobic interactions in acetonitrile.147
To conclude, assemblies of higher nuclearity can be accessed with ligands possessing large bent angles, with flexible ligands and a suited template, or through interlocking of lower nuclearity assemblies. Starting off with architectures of rather high symmetry, namely rings, tetrahedra, and octahedra, we presented how the assembly size can be rationalized with the bent angle of the underlying ligand. We have also discussed more sophisticated architectures such as a cage-in-ring assembly, a double trefoil knot, and interlocked rings, among others, found serendipitously. Here, solvent and templation effects as well as ligand flexibility challenge the rationalization and prediction of the outcome.
4. Integrative self-sorting
As we can see, the investigation of homoleptic cage assembly has already produced an astonishing variety of topologies and it has enabled the rationalization of some key design principles. However, the functionality of such cages is inevitably limited as they carry only one kind of organic ligand – the building block that usually imbues the assembly with its function. For overcoming this constraint, multiple kinds of ligands can be combined, giving rise to so-called heteroleptic assemblies.64,148 The synthesis of such multicomponent cages is, however, not trivial in terms of the self-assembly strategy: when ligands of too similar size and shape are combined, the formation of an entropically favoured statistical mixture (i.e. assemblies with different stoichiometries and stereo configurations) is usually observed.148,149 The drawback of working with such mixtures is that only a subset of assemblies then comes with the desired functionality, and observed properties may not be correlated with a particular structure, impeding the optimization of the most selective and capable derivatives. For limiting the number of possible stoichiometries and isomers, cis-protected Pd(II) sources have been successfully employed in the past. This has been combined with steric constraints by Fujita,150 with differently charged donors by Mukherjee and others,151,152 as well as with variation in the ligand denticity153 to achieve integrative self-sorting. Subsequently, strategies for the non-statistical self-assembly of bis-monodentate organic ligands carrying pyridine, isoquinoline, or imidazole donors with naked Pd(II) ions were developed by Crowley,154 Hooley,155 Severin,156 and us.80,99,121,157 We will first describe Pd2L4 heteroleptic cages as they represent the most basic and largest family and will subsequently address heteroleptic cages of higher nuclearity.4.1. Pd2A2B2 cages
Inspired by the work of Fujita and coworkers on heteroleptic assemblies based on cis-protected Pd(II),150 we exploited steric constraints at the donor sites for yielding Pd2A2B2-type cages with naked Pd(II) cations. For this, we coined the term “coordination sphere engineering” (CSE). Picolyl ligands o-3.1 and i-3.1 differ from the typical banana-shaped ligands discussed in Chapter 1 in the methyl-group adjacent to the nitrogen donor, either pointing outside (o-3.1) or inside (i-3.1) the tentative cavity of the respective assembly (Fig. 11a). Increased steric repulsion renders potential homoleptic species Pd2(o-3.1)4 or Pd2(i-3.1)4 energetically unfavourable; indeed, bowls or mixtures of bowls and rings are obtained when only one kind of ligand is employed. In contrast, the combination of both ligands o-3.1 and i-3.1 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio results in the formation of heteroleptic cage cis-Pd2(o-3.1)2(i-3.1)2, 3.3. Here, the repulsive steric interactions are decreased while the principle of maximum site occupancy is satisfied. DFT calculations and the X-ray crystal structure of a mononuclear Pd(picolyl)4 complex support that the cis-isomer possesses higher stability as compared to a tentative trans-isomer. The combination of acridone-based o-3.1 with phenothiazine-based i-3.2 led to a similar Pd2(o-3.1)2(i-3.2)2 cage, i.e. the platform tolerates incorporation of two different functional backbones. Surprisingly, the reverse combination of i-3.1 with o-3.2 did not allow for clean self-assembly to heteroleptic Pd2(i-3.1)2(o-3.2)2.99 This was ascribed to the high propensity of the phenothiazine-based ligand to form interlocked double cages.133 This study further revealed that this approach of sterically modifying the donor groups is not independent from the backbone geometries, in particular when the latter differs in the structure (with acridone being flat and phenothiazine being significantly bent along the tricyclic aromatic system) in that the degree of Pd(donor)4 helicity, as dictated by the methylated pyridines, will be mechanically linked to the backbone-based central geometry of such a cage.
1 ratio results in the formation of heteroleptic cage cis-Pd2(o-3.1)2(i-3.1)2, 3.3. Here, the repulsive steric interactions are decreased while the principle of maximum site occupancy is satisfied. DFT calculations and the X-ray crystal structure of a mononuclear Pd(picolyl)4 complex support that the cis-isomer possesses higher stability as compared to a tentative trans-isomer. The combination of acridone-based o-3.1 with phenothiazine-based i-3.2 led to a similar Pd2(o-3.1)2(i-3.2)2 cage, i.e. the platform tolerates incorporation of two different functional backbones. Surprisingly, the reverse combination of i-3.1 with o-3.2 did not allow for clean self-assembly to heteroleptic Pd2(i-3.1)2(o-3.2)2.99 This was ascribed to the high propensity of the phenothiazine-based ligand to form interlocked double cages.133 This study further revealed that this approach of sterically modifying the donor groups is not independent from the backbone geometries, in particular when the latter differs in the structure (with acridone being flat and phenothiazine being significantly bent along the tricyclic aromatic system) in that the degree of Pd(donor)4 helicity, as dictated by the methylated pyridines, will be mechanically linked to the backbone-based central geometry of such a cage.
Crowley and coworkers exploited attractive interactions around the coordination sites to steer integrative self-sorting. They designed ligands 3.4 and 3.5, differing solely in the presence or absence of amino groups on the pyridines. The combination of 3.4 with homoleptic cage Pd23.54 led to the formation of cage cis-Pd2(3.4)2(3.5)2, 3.6 for which the stereo configuration was assigned based on DFT computations (Fig. 11b). Preference for the cis-configuration was rationalized with the possibility for H-bond interactions between adjacent amino groups and additional H-bonds to the acidic α-protons of 3.5. In line with DFT computations that predict homoleptic Pd2(3.4)4 to be the most thermodynamically stable system, heteroleptic cis-Pd2(3.4)2(3.5)2 could not be accessed when the ligands, followed by the Pd(cations), or the preformed homoleptic cages were combined, i.e. it is a metastable, kinetic product.154
Aside from steric constraints, geometrical principles have also been exploited for the formation of heteroleptic assemblies. Examples of this include metallacycles with cis-protected Pd(II) or Pt(II) by Stang158 and Cu(II)-based coordination cages by Zhou,159 both exploiting different ligand bent angles, and Pd12A12B12 spheres by Fujita whereby A and B possess different lengths.160
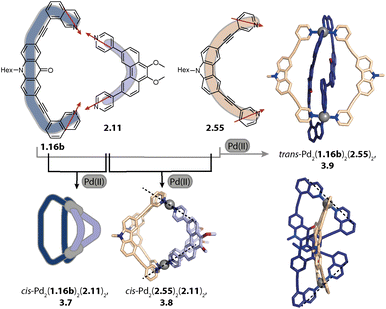
Concerning PdnL2n cages, we introduced in 2016 a strategy exploiting complementary ligand geometries for achieving control over self-sorting and coined it “shape complementary assembly” (SCA).80,103 Ligands with convergent (1.16b or 2.55) and divergent (2.11) binding vectors are combined to yield a single heteroleptic assembly in a non-statistical fashion (Fig. 12). Matching binding angles and suitable lengths allow for a square-planar coordination environment of the Pd(II) nodes, e.g. in cis-Pd2(1.16b)2(2.11)2, 3.7 and cis-Pd2(2.55)2(2.11)2, 3.8 without significant conformational strain. The severe twisting of the homoleptic helicate Pd2(1.16b)4 in combination with the high nuclearity of the homoleptic ring Pd4(2.11)8 drives the exergonic formation of 3.7, both from an enthalpic and an entropic point of view. Addition of ligand 1.16b to heteroleptic 3.8 results in transformation into 3.7 due to better shape complementarity of ligands 1.16b and 2.11. The complementarity of the binding angles leads to tilted Pd(donor)4 planes and therefore to a bent cavity shape. This translates into a higher guest binding affinity of 3.7 for bent as compared to linear disulfonate guests (opposite to a straight, homoleptic cage of comparable size). Unexpectedly, the combination of 1.16b and 2.55 also resulted in the formation of a defined species, trans-Pd2(1.16b)2(2.55)2, 3.9, in which ligands 1.16b adopt an S-shaped conformation and cross through the centre of ring Pd2(2.55)2 (Fig. 12; compare also to its homoleptic analogue in Fig. 3d). The SCA approach proved to be widely applicable and was exploited for the synthesis of amphiphilic cages,161 CPL-active cages,162,163 tetranuclear cages,164 and modular cage libraries for binding of phosphate esters.165
Recently, Jelfs Lewis and coworkers reported a joint theoretical and experimental approach aiming at predicting the integrative self-sorting of convergent and divergent ligands to give cis-Pd2A2B2 cages. From a theoretical perspective, the authors compared (a) the geometrical compatibility of ligand pairs and (b) the energy differences between homoleptic and heteroleptic species. The former yielded reasonable results in the sense that ligands for which integrative self-sorting was experimentally observed exhibited good geometrical compatibility. However, favourable geometric parameters did not ensure exclusive integrative self-sorting. Additionally, the relative energies of the overall assemblies were not congruent with the experimentally observed self-sorting outcome. These theoretical investigations were performed on single, static molecules, i.e. solvent and counter anion effects as well as entropic contributions were omitted in this simplified approach.166 In line with what was discussed for the topology control in homoleptic assemblies, these factors can be decisive for the assembly outcome, motivating to consider them in cage assembly rationalization or prediction schemes. Together with the Kast group, we recently showed that considering solvation effects gives valuable insight into homo-/heteroleptic assembly equilibria, while still being approximative and requiring further development.167
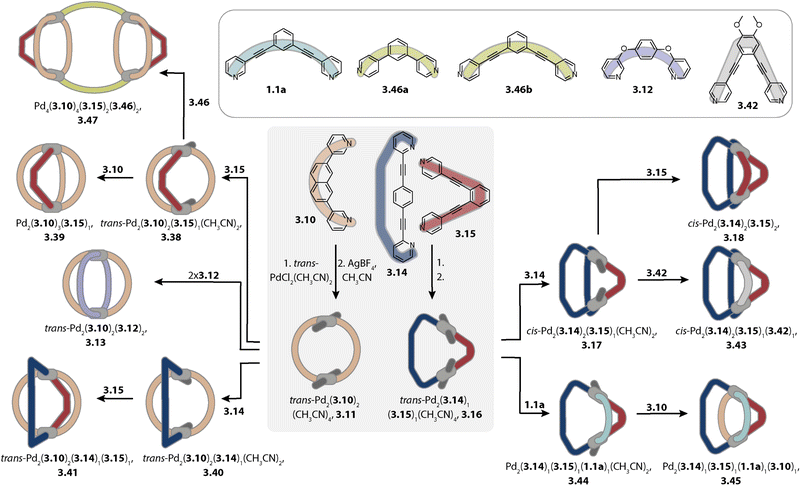
Hiraoka and coworkers formed cis- and trans-Pd2A2B2 cages in a stepwise fashion under kinetic control. In the first step, the authors synthesized ring Pd2(3.10)2Cl4 (Fig. 13). Chloride abstraction in the presence of acetonitrile allows for the transformation into ring Pd2(3.10)2(CH3CN)4, 3.11 with kinetically labile acetonitrile ligands sitting in trans-position to each other. Addition of two equivalents of a further distinguishable ligand 3.12 allowed for the formation of trans-Pd2(3.10)2(12)2, 3.13. In a similar vein, ring trans-Pd2(3.14)1(3.15)1(CH3CN)4, 3.16 with shape complementary ligands 3.14 and 3.15 was synthesized. In a stepwise fashion, the acetonitrile ligands were replaced by an additional ligand 3.14 to form heteroleptic bowl Pd2(3.14)2(3.15)1(CH3CN)2, 3.17 followed by incorporation of ligand 3.15, yielding cis-Pd2(3.14)2(3.15)2, 3.18 within 4 steps in total. The key to successful kinetic control was (a) weaker donor strength of acetonitrile ligands as compared to pyridine ligands, (b) choice of solvent and counter anions that do not promote rapid ligand scrambling, (c) kinetic stability of the cyclic intermediates, and (d) local reversibility of ligand–metal bond formation due to the presence of the solvent acetonitrile, allowing for error correction.168
4.2. Pd2A3B1 cages
Hooley and coworkers investigated in 2011 the effect of endohedral steric bulk on self-sorting. The authors synthesized derivatives of ligand 1.1a with substituents of increasing size on the central benzene ring (1.1c: amino group, 1.1d: trifluoroacetamide group, and 1.1e: N-phenyl urea, Fig. 14a). When ligand 1.1c was combined with 1.1a, a mixture of different species was obtained whose ratio could be varied by altering the ligand ratio. In contrast, the combination of 1.1a with 1.1d led to homoleptic 1.2a along with, for the first time, heteroleptic cage Pd2(1.1a)3(1.1d)1, 3.19. A further increase in steric bulk in 1.1e hampered the incorporation of the functionalized ligand into a lantern-shaped cage.155The first selective self-sorting to Pd2A3B1 cages was achieved by us in 2021 utilizing the CSE approach. Photoswitchable DTE-based ligand 3.20 was equipped with quinoline donor moieties. Similar to quinoline ligands described earlier (Chapter 2.1), ligand c-3.20 (the DTE photoswitch is in its closed form) assembles to form homoleptic bowl Pd2(c-3.20)3(solvent)2, c-3.21 since repulsive steric interactions at the coordination site prohibit coordination of a fourth quinoline ligand (Fig. 14b). The introduction of a ligand of the same length, equipped with sterically less demanding pyridine donor moieties (ligands 3.22a–3.23b) allowed for occupation of the fourth coordination sites. This strategy proved robust across different fourth ligands and hence allowed for the synthesis of a series of cages Pd2(c-3.20)3(3.22a–3.23b)1, 3.24a–3.25b. Depending on the linker (no linker in 3.22a and 3.23avs. a phenyl linker in 3.22b and 3.23b), the cages possess different cavity sizes which translates into size selective guest encapsulation.169
Moreover, we realized the installation of a fourth ligand on bowl-fullerene complex C70@1.25 in 2022. Again, coordination of a fourth ligand 1.24c with isoquinoline donor moieties is unfavoured due to steric repulsion at the coordination site. In contrast, when a similar ligand with naphthyridine donors 3.26 is employed, C70@Pd2(1.24c)3(3.26)1, C70@3.27 is obtained (Fig. 14c, left). In addition to the reduced steric bulk of the naphthyridine in comparison to isoquinoline, the nitrogen lone pairs of the former allow for attractive interactions with the hydrogens of the adjacent donor groups of 1.24c. Importantly, the CSE approach alone was not sufficient here for the clean formation of 3.27 in the absence of fullerene C70 as a template. Additionally, ligands 1.24c and 3.26 were combined in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with fullerene C60 as a template, whereby C60@trans-Pd2(1.24c)2(3.26)2, C60@3.28 was obtained (Fig. 14c, right). The combination of the CSE approach and templation by fullerene also allowed for the assembly of heteroleptic bowls: when acridine-equipped ligand 1.24d forming Pd2(1.24d)2(CH3CN)4 rings was combined with naphthyridine-equipped ligand 3.26 in the presence of C60 or C70, host–guest complexes C60/C70@trans-Pd2(1.24d)2(3.26)1(CH3CN)2 were selectively obtained. Here, naphthyridyl ligand 3.26 bridges one side of the ring Pd2(1.24d)2.157
1 ratio with fullerene C60 as a template, whereby C60@trans-Pd2(1.24c)2(3.26)2, C60@3.28 was obtained (Fig. 14c, right). The combination of the CSE approach and templation by fullerene also allowed for the assembly of heteroleptic bowls: when acridine-equipped ligand 1.24d forming Pd2(1.24d)2(CH3CN)4 rings was combined with naphthyridine-equipped ligand 3.26 in the presence of C60 or C70, host–guest complexes C60/C70@trans-Pd2(1.24d)2(3.26)1(CH3CN)2 were selectively obtained. Here, naphthyridyl ligand 3.26 bridges one side of the ring Pd2(1.24d)2.157
In 2024, Zhang and coworkers combined the CSE approach with the incorporation of endohedral steric bulk to form Pd2A3B1 cages with varying cavity volumes. Picolyl ligand 3.29 was combined with Pd(II) in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio for obtaining metastable bowl Pd2(3.29)3(CH3CN)2, 3.30 (Fig. 15, top). In the next step, pyridine ligands 1.1a, 1.1d, 1.1f, 1.1g, and 1.1h were added as ligand B to form cages Pd2(3.29)3B1, 3.31. While this stepwise synthesis allowed for obtaining the peculiar heteroleptic cages with sufficient selectivity, a one-pot reaction of the ligands with Pd(II) led to a mixture of different assemblies. For ligand B, possessing endohedral bulk (1.1d, 1.1f, 1.1g, and 1.1h), the heteroleptic cage 3.31 was, however, the main species. This showcases how the interplay of two steric control elements can steer integrative self-sorting. Noteworthily, the accessible cavity volume decreases depending on ligand B in the order 1.1a > 1.1f > 1.1g. This was shown to result in an alteration of the host–guest properties.170 Wang and coworkers also combined coordination sphere engineering and endohedral steric bulk for realizing Pd2A2B2 and Pd2A3B cages in a pathway-dependent approach.171
2 ratio for obtaining metastable bowl Pd2(3.29)3(CH3CN)2, 3.30 (Fig. 15, top). In the next step, pyridine ligands 1.1a, 1.1d, 1.1f, 1.1g, and 1.1h were added as ligand B to form cages Pd2(3.29)3B1, 3.31. While this stepwise synthesis allowed for obtaining the peculiar heteroleptic cages with sufficient selectivity, a one-pot reaction of the ligands with Pd(II) led to a mixture of different assemblies. For ligand B, possessing endohedral bulk (1.1d, 1.1f, 1.1g, and 1.1h), the heteroleptic cage 3.31 was, however, the main species. This showcases how the interplay of two steric control elements can steer integrative self-sorting. Noteworthily, the accessible cavity volume decreases depending on ligand B in the order 1.1a > 1.1f > 1.1g. This was shown to result in an alteration of the host–guest properties.170 Wang and coworkers also combined coordination sphere engineering and endohedral steric bulk for realizing Pd2A2B2 and Pd2A3B cages in a pathway-dependent approach.171
4.3. Pd2A2B1C1 cages
Zhang and coworkers reported in 2023 the first example of a heteroleptic Pd2A2B1C1-type cage incorporating three distinguishable ligands. Similar to their strategy for the synthesis of Pd2A3B1-type cages, the CSE approach was merged with the installation of endohedral steric bulk. The combination of picolyl ligand 3.29 with Pd(II) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio led to ring Pd2(3.29)2(CH3CN)4, 3.32 (Fig. 15, bottom). Next, one side of the ring was bridged by ligand 1.1d, acting as a ligand “B”. While ligand 1.1d is of similar shape to 1.1a it possesses endohedral steric bulk, hampering coordination of a second equivalent. Thus, a heteroleptic bowl trans-Pd2(3.29)2(1.1d)1(CH3CN)2, 3.33 is obtained. Noteworthily, bowl 3.33 can also be assembled directly from a mixture of ligands 3.29 and 1.1d with Pd(II), showing that its formation is pathway-independent. Lastly, a sterically less demanding ligand, such as unsubstituted 1.1a, can be implemented as ligand “C” to yield trans-Pd2(3.29)2(1.1d)1(1.1a)1, 3.35. The authors showed that different endohedral functionalities can be incorporated into the heteroleptic cage by changing the appended group in ligand B (1.1i: carboxylic acid and 1.1j: hydroxy group). Furthermore, the cavity can be enlarged by employing larger 3.34a as ligand C. On the other hand, a second endohedral moiety can be incorporated by functionalization of ligand C (3.34b).172
1 ratio led to ring Pd2(3.29)2(CH3CN)4, 3.32 (Fig. 15, bottom). Next, one side of the ring was bridged by ligand 1.1d, acting as a ligand “B”. While ligand 1.1d is of similar shape to 1.1a it possesses endohedral steric bulk, hampering coordination of a second equivalent. Thus, a heteroleptic bowl trans-Pd2(3.29)2(1.1d)1(CH3CN)2, 3.33 is obtained. Noteworthily, bowl 3.33 can also be assembled directly from a mixture of ligands 3.29 and 1.1d with Pd(II), showing that its formation is pathway-independent. Lastly, a sterically less demanding ligand, such as unsubstituted 1.1a, can be implemented as ligand “C” to yield trans-Pd2(3.29)2(1.1d)1(1.1a)1, 3.35. The authors showed that different endohedral functionalities can be incorporated into the heteroleptic cage by changing the appended group in ligand B (1.1i: carboxylic acid and 1.1j: hydroxy group). Furthermore, the cavity can be enlarged by employing larger 3.34a as ligand C. On the other hand, a second endohedral moiety can be incorporated by functionalization of ligand C (3.34b).172
In 2024, we combined the SCA approach with either inter-ligand interactions or with stoichiometry control to steer the selective formation of cis- or trans-Pd2A2B1C1 cages, respectively. In accordance with the SCA approach, ligand 1.24a assembles with ligands 2.33a or 2.33d to form cis-Pd2(1.24a)2(2.33)2 cages. While ligands 2.33a and 2.33d feature similar geometries, their electronic properties diverge: fluorene-based ligand 2.33d possesses two CH3 groups while fluorenone-based ligand 2.33a has a planar π-surface. CH3⋯π interactions between central parts of neighbouring ligands 2.33a and 2.33d foster selective formation of cis-Pd2(1.24a)2(2.33a)1(2.33d)1, 3.36 when the ligands are combined in a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with Pd(II) (Fig. 16a). For this, we coined the term “adjacent backbone interactions” (ABIs). Small ligands 2.33 also show shape complementarity to ligand 1.29 with strongly inward pointing donor vectors. Therefore, when 1.29, 2.33d, and 1.24a are combined with Pd(II) in a 1
1 with Pd(II) (Fig. 16a). For this, we coined the term “adjacent backbone interactions” (ABIs). Small ligands 2.33 also show shape complementarity to ligand 1.29 with strongly inward pointing donor vectors. Therefore, when 1.29, 2.33d, and 1.24a are combined with Pd(II) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio, trans-Pd2(1.24a)2(1.29)1(2.33d)1, 3.37 is obtained. That ligand 1.24a can adapt both, a conformation with rather convergent binding vectors, allowing for shape complementarity to ligands 2.33, and a conformation with nearly collinear binding vectors without considerable strain can be explained with its spring-like nature that enables adaptable lengths and binding angles.121,173
2 ratio, trans-Pd2(1.24a)2(1.29)1(2.33d)1, 3.37 is obtained. That ligand 1.24a can adapt both, a conformation with rather convergent binding vectors, allowing for shape complementarity to ligands 2.33, and a conformation with nearly collinear binding vectors without considerable strain can be explained with its spring-like nature that enables adaptable lengths and binding angles.121,173
4.4. Pd2A1B1C1D1 cages
Utilizing the pathway-dependent self-assembly approach described earlier, Hiraoka and coworkers formed Pd2A3B1, PdA2B1C1, as well as the first Pd2A1B1C1D1-type cage under kinetic control. Ring 3.11 was reacted with ligand 3.15 to yield intermediate bowl Pd2(3.10)2(3.15)1(CH3CN)2, 3.38 that was then converted into Pd2(3.10)3(3.15)1, 3.39 by the addition of a further equivalent of ligand 3.10 (Fig. 13). Reversing the last two steps did not lead to selective formation of 3.39 since homoleptic Pd2(3.10)4 can be formed. When intermediate bowl 3.38 was reacted with ligand 3.14, which is complementary in shape to ligand 3.15, cage trans-Pd2(3.10)2(3.14)1(3.15)1, 3.41 was obtained. 3.41 was also obtained when ligands 3.14 and 3.15 were added in the reverse order. Notably, this cage exhibited thermodynamic stability. This can be explained similar to the stability of cage 3.37: the ligands opposing each other either possess parallel binding vectors (ligand 3.10) or complementary geometries (3.14 and 3.15). In another vein, intermediate bowl 3.17 yielded cage cis-Pd2(3.14)2(3.15)1(3.42)1, 3.43 when ligand 3.42, only differing from ligand 3.15 by its exohedral substituents, was added. The culmination of the synthetic strategy represents the formation of a cage with four differentiable ligands: starting from ring 3.16, bowl intermediate Pd2(3.14)1(3.15)1(1.1a)1(CH3CN)2, 3.44 was obtained upon addition of ligand 1.1a. With ligand 3.10, bowl 3.44 was then converted into Pd2(3.14)1(3.15)1(1.1a)1(3.10)1, 3.45. Except for cage 3.41, heating of all assemblies discussed here resulted in a transformation into other, thermodynamically more stable compounds. In order to get insight into the factors influencing the respective cage stabilities, the authors determined the half-life of the cages and correlated them with different structural features. It was found that small deviations of the N⋯N distances of the ligands in the cages from their ideal distances are essential for high cage stability.168Finally, we published the first report on the thermodynamically controlled formation of Pd2A1B1C1D1-type cages in 2024. Based on our knowledge on the shape complementarity of larger ligands 1.24a (A) and 3.49 (B) with smaller ligands 2.33d (C) and 2.33a (D) as well as favourable inter-ligand interactions between the latter (ABI approach), we combined all four ligands with Pd(II) cations in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio. Intriguingly, out of 55 possible dinuclear species, one single assembly incorporating all four ligands was exclusively obtained, both in solution and the solid state. Considering the above-mentioned driving forces, two different isomers, namely Pd2(1.24a)1(3.49a)1(2.33d)1(2.33a)1, 3.51 (Pd2A1B1C1D1) and Pd2(1.24a)1(3.49a)1(2.33a)1(2.33d)1 (Pd2A1B1D1C1) would have been feasible whereby X-ray structure analysis revealed that only the former is formed (Fig. 16b). Concerning the observed stoichiometry, presumably, the incorporation of one equivalent of ligand 1.24a is favoured due to its ability to adapt its N⋯N distance to match the other ligands. Fluorenone-based ligand 3.49a could be replaced by carbazole-based ligand 3.49b, possessing a similar shape and flexibility. Furthermore, we showed that ligands 2.33b, 2.33c, and 2.33e, based on carbazole or fluorene, can likewise serve as ligand D since they also offer a π-surface for adjacent backbone interactions with ligand 2.33d. In contrast, dimethyl fluorene ligand 2.33d C proved to be vital for the selective outcome since substitution by ligands 2.33b, 2.33c, or 2.33e which offer only up to one CH3 group in the central backbone position resulted in the formation of mixtures of different species. Interestingly, when NDI-based ligand 3.53 was applied as ligand D, a swapped ligand arrangement was observed. In this case, ligand 2.33d offers its π-surface for CH⋯π-interactions with ligand 3.53. Probably, the alteration of the orientations of ligand C and D affects the shape complementarity with ligands A and B in a way that now favours the formation of pseudo-isomeric cage 3.52. Noteworthily, cage Pd2A1B1C1D1 could also be obtained in a stepwise fashion through several possible cage-to-cage transformation pathways, starting from the respective homoleptic cages, again supporting its characteristics as the final thermodynamic assembly product (Fig. 16c).121
2 ratio. Intriguingly, out of 55 possible dinuclear species, one single assembly incorporating all four ligands was exclusively obtained, both in solution and the solid state. Considering the above-mentioned driving forces, two different isomers, namely Pd2(1.24a)1(3.49a)1(2.33d)1(2.33a)1, 3.51 (Pd2A1B1C1D1) and Pd2(1.24a)1(3.49a)1(2.33a)1(2.33d)1 (Pd2A1B1D1C1) would have been feasible whereby X-ray structure analysis revealed that only the former is formed (Fig. 16b). Concerning the observed stoichiometry, presumably, the incorporation of one equivalent of ligand 1.24a is favoured due to its ability to adapt its N⋯N distance to match the other ligands. Fluorenone-based ligand 3.49a could be replaced by carbazole-based ligand 3.49b, possessing a similar shape and flexibility. Furthermore, we showed that ligands 2.33b, 2.33c, and 2.33e, based on carbazole or fluorene, can likewise serve as ligand D since they also offer a π-surface for adjacent backbone interactions with ligand 2.33d. In contrast, dimethyl fluorene ligand 2.33d C proved to be vital for the selective outcome since substitution by ligands 2.33b, 2.33c, or 2.33e which offer only up to one CH3 group in the central backbone position resulted in the formation of mixtures of different species. Interestingly, when NDI-based ligand 3.53 was applied as ligand D, a swapped ligand arrangement was observed. In this case, ligand 2.33d offers its π-surface for CH⋯π-interactions with ligand 3.53. Probably, the alteration of the orientations of ligand C and D affects the shape complementarity with ligands A and B in a way that now favours the formation of pseudo-isomeric cage 3.52. Noteworthily, cage Pd2A1B1C1D1 could also be obtained in a stepwise fashion through several possible cage-to-cage transformation pathways, starting from the respective homoleptic cages, again supporting its characteristics as the final thermodynamic assembly product (Fig. 16c).121
4.5. PdnAxBy assemblies with n > 2
Recently, we reported the first trinuclear heteroleptic assemblies Pd3A3B3. By applying the SCA approach, the long, convergent ligand 4.1 was combined with the small, divergent ligand 3.46a. This resulted in the formation of three-fold symmetric syn-cis-Pd3(3.46a)3(4.1)3, 4.3a assembly (Fig. 17). Here, cis refers to the ligand arrangement around the metal node and syn to the ligand orientation relative to the Pd3 plane, i.e. the assembly is overall bowl-shaped. Modification of the central benzene moiety (ligands 4.2b–e) allowed for obtaining endohedrally functionalized rings 4.3b–e. Noteworthily, when uncharged and small functionalities were incorporated, the self-assembly to Pd3A3B3-type rings was not disturbed. On the other hand, when the sterically more demanding tert-butyl ester modified ligand 4.2f was employed, a mixture of trinuclear and tetranuclear assemblies was obtained. A further increase in steric bulk (phenyl ester ligand 4.2g) led to the tetranuclear ring as the sole species. In a similar fashion, functionalization with a nitro group led to higher nuclearity ring Pd4(4.1)4(4.2h)4, 4.5h. Presumably, this can be traced back to reduced electrostatic repulsion between the nitro oxygens of ligands 4.2h when occupying neighboring edges in the larger assembly as compared to a tentative three-membered ring. Aside from the incorporation of steric bulk and charges, the formation of a Pd4A4B4-type assembly could also be steered by modification of the bent angle of the small ligand. When the central benzene moiety in 3.46a (α = 120°) was substituted by a thiophene or a selenophene moiety, the bent angle was increased to 149° (4.4a) and 151° (4.4b), respectively. In accordance with the trends observed for homoleptic assemblies (Fig. 4), this led to an increase in nuclearity, here to a Pd4A4B4-type ring. Interestingly, NMR analyses in combination with DFT computations suggest that for most of the four-membered rings investigated, more precisely the rings formed with 4.4a, 4.4b, and 4.2g, a saddle-shaped conformation is favoured over a bowl-shaped one.41In 2019, we reported the self-assembly of pill-shaped tetranuclear species by exploiting the charge separation strategy, widely utilized by Stang and coworkers,15 combined with our CSE approach. As discussed in the preceding section, the “free” coordination sites of quinoline-based Pd2A3-type bowls (such as 1.25, Fig. 18a) can be occupied by ligands lacking steric bulk close to the donor site. When instead of a convergent bis-pyridyl ligand a linear terephthalate 4.6 is employed, two bowls are bridged, giving rise to pill-shaped Pd4(1.7)6(4.6)2, 4.7. The negative charges of the terephthalates help compensate for the repulsion stemming from the close proximity of multiple Pd(II) cations. Additionally, the dimer-fullerene complexes 2C60@4.7 or 2C70@4.7 could also be accessed upon dimerization of preformed bowl-fullerene complexes C60@1.25 and C70@1.25.76
In another vein, Hiraoka and coworkers recently reported pill-shaped Pd4A4B2C2-type assemblies via dimerization of two Pd2A2B heteroleptic bowls under kinetic control. Starting from bowl 3.38, whose synthesis is described in Chapter 4.4, the authors added long or short bis-pyridyl ligands 3.46 with diverging binding angles. Similar to our approach, pill-shaped dimer Pd4(3.10)4(3.15)2(3.46)2, 3.47 was obtained, albeit here with three different bis-pyridyl ligands and higher positive charge (Fig. 13).168
In 2021, we reported a strategy for yielding heteroleptic tetrahedra by exploiting the role of backbone-centred steric bulk (as a special variant of the ABI approach). As described in Chapter 3.2, fluorene-derived ligands 2.33 can assemble to form rings, pseudo-tetrahedra, and octahedra, depending on the steric bulk installed at the backbone. While ligand 2.33a with low steric demand assembles to form a mixture of ring 2.34a and tetrahedron 2.35a, dihexyl-decorated ligand 2.33d forms entropically disfavoured homoleptic octahedron 2.36d (Fig. 18b). In the latter, the distance between the ligands is increased and they adopt a concave binding mode while the steric bulk is oriented to the exterior. Upon careful inspection of the ligand conformations and arrangement in tetrahedron 2.35a (Fig. 6), we suspected that the assembly could serve as a platform for combining sterically demanding ligands, sitting on the singly bridged edges, with ligands of low steric demand occupying the doubly bridged edges. Indeed, a combination of either ligands 2.33a and 2.33d with Pd(II) or combination of the preformed homoleptic assemblies 2.34a and 2.35a with 2.36d resulted in the selective formation of tetrahedron Pd4(2.33a)4(2.33d)4, 4.8 with the expected ligand arrangement (Fig. 18b).119
Yoshizawa, Chand, and coworkers reported the first and, until now, sole pentanuclear heteroleptic assembly in 2017 (elected as the molecule of the year in ACS Chemical & Engineering News). In the first step, the authors combined the rather flexible ligand 4.9 with linear ligand 4.10 and a cis-protected Pd(II) source, (tmeda)Pd(II). A trapezium-shaped complex Pd2(tmeda)2(4.9)1(4.10)1, 4.11 with an exterior angle β1 of 72° and an interior angle β2 of 104° was obtained, serving as a promising precursor to a higher order assembly (Fig. 18c).174 It was then anticipated that the combination of the same two ligands with unprotected Pd(II) should result in a circular arrangement of fused trapezium-shaped complexes 4.11. Comparing the angles observed in 4.11 with the angles in tentative triangular, square, and pentagonal arrangements of annelated trapezia suggested that, from a purely geometrical point of view, the pentagonal species should be favoured. Indeed, the combination of 4.9 and 4.10 with [Pd(CH3CN)4](BF4)2 in DMSO resulted in pentanuclear species Pd5(4.9)5(4.10)5, 4.12 which can be described as a truncated pentagram. Here, the interior angle reaches 108°, similar to the one of a regular pentagon. Noteworthily, ring 4.12 could also be accessed via different pathways, more precisely upon combination of the two homoleptic species or upon addition of one of the ligands to the homoleptic assembly of the respective other ligand, supporting that 4.12 is the thermodynamic product.175
In 2016, Mukherjee and coworkers reported a trigonal prismatic Pd6A6B6-type cage. First, the authors showed that urea-functionalized bis-pyridyl ligand 4.13 assembles with cis-protected (tmeda)Pd(II) to form a molecular triangle Pd3(tmeda)3(4.13)3. Aiming at a three-dimensional assembly, ligand 4.13 was combined with unprotected Pd(II) and short “clipping” ligand 4.14. In the resulting assembly Pd6(4.13)6(4.14)6, 4.15, in total six clipping ligands 4.14 bridge two triangles Pd3(4.13)3 (Fig. 18d). Urea-functionalized compounds are generally prone to self-association via hydrogen bonds which limits their capability of substrate recognition and hence their catalytic applicability. The large inter-ligand distances in the triangular prism prohibit such self-quenching interactions, rendering the assembly suitable for the recognition of nitro-olefins. Therefore, triangular prism 4.15 could be used as a heterogeneous catalyst for promoting Michael-additions and Diels–Alder reactions in water.176
In 2021, Severin and coworkers achieved selective assembly of Pd4A4B4-type tetrahedral, Pd6A6B6-type trigonal prismatic, and Pd8A8B8-type tetragonal prismatic architectures by variation of the binding angle of one of the employed ligands. Heteroleptic assembly formation was investigated with ligand 4.16, possessing collinear binding vectors. The ligand is predisposed for the formation of heteroleptic assemblies as its homoleptic cage Pd2(4.16)4, 4.17 is strained due its small Pd⋯Pd distance, amongst other factors (Fig. 19a). The combination of 4.16 with ligand 3.46a, possessing a bent angle of 120° led to selective formation of pseudo-tetrahedral Pd4(4.16)4(3.46a)4, 4.18a. In accordance with the binding modes observed in homoleptic tetrahedra, more open ligand 3.46a occupies the singly bridged edges while two ligands 4.16 form macrocyclic motif Pd2(4.16)2. Interestingly, a similar outcome was achieved when alkyne-spaced ligand 3.46b was employed; however, ligand 4.16 could not be replaced by its alkyne spaced analogue 1.1a. This apparent disparity can be explained with the higher strain of homoleptic 4.17 in comparison to the longer analogue 1.2a. The combination of wider ligand 4.4a, based on thiophene, with ligand 4.16 resulted in the formation of a trigonal prismatic cage Pd6(4.4a)6(4.16)6, 4.19. Similar to the assembly 4.15 reported by Mukherjee and coworkers, two counts of ligand 4.16 form macrocyclic moieties Pd2(4.16)2 that are bridged by ligands 4.4a. Next, the authors further increased the binding angle of the second ligand by applying linear ligands in which the para-pyridines were either bridged by a phenyl (4.20) or by a para-diethynylbenzene linker (4.22). From a purely geometrical point of view, the combination of a linear ligand with ligand 4.16 would lead to the formation of a tetragonal prismatic (cuboid) assembly. Indeed, the exclusive formation of Pd8(4.16)8(4.20)8, 4.21 was observed for short, and thus rigid, ligand 4.20. Conversely, the alkyne linkers endow ligand 4.22 with enough flexibility to allow for the formation of entropically favoured trigonal prism Pd6(4.16)6(4.22)6, 4.23 along with tetragonal prism Pd8(4.16)8(4.22)8, 4.24.156
Tetrahedral assemblies can be obtained upon the combination of 120° bent angle ligands 3.46 with ligand 4.16 (as just discussed, Fig. 19b, route I). Ligand 4.16 occupying the doubly-bridged edges can be replaced by ligand 4.25, possessing an angle of 60° (route II). Excitingly, the combination of the two phenyl-spaced ligands 3.46a and 4.26 led to novel species Pd6(3.46a)6(4.26)6, 4.28, a trigonal antiprismatic assembly (route III). Severin and coworkers accomplished this discovery by screening of a virtual combinatorial library. More precisely, the authors combined a variety of well-known ligands (1.1a, 1.8, 2.24, 2.55, bis-pyridyl analogue of 3.1, 3.46a) with substoichiometric amounts of a Pd(II) salt in order to favour formation of particularly stable assemblies. Examination of the X-ray crystal structure reveals that the peculiar ligand arrangement in 4.28 allows for a nearly ideal square planar coordination geometry of the Pd(II) nodes. For examining the generality of the assembly of hexanuclear heteroleptic cages, the authors employed ligands with similar angles albeit with different lengths and bulk. Hence, instead of ligand 4.26, clathrochelate ligand 2.46d was employed and ligand 3.46a was replaced by an analogue with similar clathrochelate moieties between the 1,3-benzene-core and the para-pyridines. Interestingly, this led to the formation of a hexanuclear prismatic architecture instead of an antiprismatic one as observed in 4.28.177 In another vein, the authors showed that ligand 1.1a assembles with ligand 4.25 to form dinuclear cage cis-Pd2(1.1a)2(4.25)2, 4.29. The tilted PdN4 planes point towards shape complementarity between the two ligands. This is achieved by the bending of the ligands owing to their flexible alkyne spacers. In order to further increase the complexity of the system, the authors aimed at incorporating all three kinds of ligands (i.e. with 0°, 60°, and 120° bent angles) within one assembly. For this, the self-assembly was examined with the various ligand combinations (eight combinations and different ratios). Solely the combination of ligands 4.16 and 4.25 with either 3.46a or 3.46b afforded assemblies comprising all three ligands (routes IVa and IVb). More specifically, tetrahedra Pd4(3.46a/b)4(4.16)x(4.25)4−x were obtained in which 120° ligand 3.46 occupies the singly bridged edges while ligands 4.16 and 4.25 are statistically distributed over the doubly bridged edges.208
Driven by the aim of preparing cages that carry multiple functionalities, vast progress has been achieved in the development of strategies for designing Pd(II)-based heteroleptic cages in the last decade. For favouring a heteroleptic coordination environment in dinuclear cages, the donor sites can be equipped with steric bulk or functionalities that allow for attractive secondary interactions (coordination sphere engineering, CSE). Alternatively, repulsive or attractive interactions are installed in more central ligand positions, as in the adjacent backbone interaction (ABI) approach. In other examples, cavity-filling steric bulk is employed to steer the self-sorting outcome. The necessity for such ancillary groups can be omitted when suitably shaped ligands are used in the shape complementary assembly (SCA) strategy. The combination of different strategies was shown to allow for accessing dinuclear cages with up to four differentiable ligands. Similar strategies have been employed for designing heteroleptic cages of higher nuclearity. In this case, exohedral steric bulk was also added to control selective self-sorting. For n = 6 and n = 8, some topologies were accessed that could never be observed when only one kind of ligand was employed, namely trigonal prismatic and antiprismatic as well as tetragonal prismatic assemblies. In contrast to dinuclear cages, defined heteroleptic assemblies of higher nuclearity which carry more than two kinds of ligands have so far rarely been reported and thus pose a challenge for future investigations.
5. Orientational self-sorting
An alternative approach for accessing PdnL2n assemblies of higher structural complexity is the use of ligands lacking the typical two-fold symmetry seen in all ligands discussed so far. On the one hand, this method can give rise to assemblies with Pd(II) ions sitting in differentiable coordination environments. On the other hand, it permits for the partitioning of functional groups within the cavity, holding promise for cages capable of binding low-symmetry guests. Most of the work reported so far focuses on the smallest family member, the Pd2L4 cage, where four isomers can be obtained depending on the relative arrangement (H: head and T: tail) amongst the ligands (Fig. 20, middle). Significant progress regarding the orientational self-sorting of asymmetric ligands was achieved in recent years by the groups of Lewis,179,187,189,193,197 Chand,191,192,194 and others180,181,186,188,196 from the experimental side and the group of Jelfs179,187,189 from the theoretical side.In 2020, Chand and coworkers showed that ligand 5.1, possessing an asymmetrical linker between the two pyridines, assembles to form a mixture of different isomers Pd2(5.1)4, 5.2 (Fig. 20, middle).178 Later, the ligand design was picked up by Lewis, Jelfs, and coworkers who substituted one of the donor moieties with sterically more demanding picolyl (5.1P) or quinolinyl (5.1Q) moieties. Interestingly, the picolyl-based ligand 5.1P shows a preference for the HTHT-5.2P isomer, while self-assembly of quinoline-based ligand 5.1Q leads to a higher fraction of HHTT-5.2Q isomer when DMSO is used as the solvent. Addition of acetonitrile led to an increase in the fraction of HTHT-5.2 for both ligands. Supported by DFT-computations, the authors suggested that the HTHT-5.2 isomer is favoured from a purely structural point of view while the HHTT-isomer offers a suitable pocket for binding of H-bond acceptor solvents such as DMSO. The latter effect is of particular importance for the ligand with quinoline donors due to the polarized quinoline proton. Pleasingly, this behaviour was also observed for the self-sorting of ligands 1.1Q and 1.1P: the former shows a preference for the HHTT-1.2Q isomer while the latter assembles to form HTHT-1.2P in acetonitrile and to form a mixture of the two isomers in DMSO. Ligands 5.3Q and 5.3P assemble to form three-membered rings Pd3(5.3)6, 5.4. Similar effects lead to a preference for all-HHTT-Pd3(5.3Q)6, 5.4Q in the case of 5.3Q in DMSO and for all-HTHT-Pd3(5.3P)6, 5.4P in the case of 5.3P in ACN.179
In 2023, Lusby, Crowley, and coworkers investigated the effect of attractive interactions within the coordination sphere on the orientational self-sorting. A selection of ligands, termed here R1-1.1-R2 (Fig. 21a), with variable hydrophilicity has been synthesized: ligands H-1.1-Alk3, H-1.1-Alk6, and H-1.1-Alk12 are equipped with a hydrophobic alkoxy chain on one of the pyridines and ligands EG-1.1-H, EG2-1.1-H, and EG4-1.1-H possess hydrophilic ethylene glycol chains of increasing length. Furthermore, ligands EG-1.1-Alk3, EG2-1.1-Alk6, and EG4-1.1-Alk12 have been synthesized which are functionalized with alkoxy chains on one ligand end and ethylene glycol chains on the other end that are of varying length. Intuitively, DFT computations supported that for the EG-1.1-Alk ligands, the HHHH-Pd2(1.1)4 isomer, in which hydrophobic and hydrophilic chains are segregated, should be favoured. A similar isomer was favoured for H-1.1-Alk ligands, while the HTHT-isomer was predicted to be the most stable for the EG-1.1-H-type ligands. The predicted energy differences between the isomers were, however, small. In accordance with this, the authors observed the formation of mixtures of isomers of Pd2(1.1)4 when the ligands were combined with Pd(II). Hence, dispersion interactions were not sufficient here for steering the self-sorting towards a single isomer. The authors also equipped one of the ligand ends with 2-amino pyridine. As discussed in Section 4.1, this strategy allowed for the formation of (kinetically stable) cis-Pd2A2B2 with the corresponding symmetrical ligands A and B. In a similar fashion, ligand 5.5a assembled to form HHTT-Pd2(5.5a)4, 5.6a (Fig. 21c). DFT computations supported that the orientational self-sorting was steered by H-bonding interactions between neighbouring amino groups and that the isomer was, similar to heteroleptic 3.6, a kinetic product. When the amino groups were positioned farther away from the nitrogen donor, more precisely in the meta-position for ligand 5.5b, a mixture of isomers was obtained, highlighting the importance of the intramolecular H-bonds for the selectivity.180
H-bonding interactions for driving orientational self-sorting were also exploited by Natarajan and coworkers. In 2019, the authors reported dinuclear cages Pd2(5.7)4, 5.8 formed by ligand 5.7 which is based on cholic acid and possesses two hydroxyl groups on its flanks (Fig. 21b). The latter allows for inter-ligand hydrogen bonds in the helically twisted HHHH-5.8 isomer. These interactions were vital for selectivity since removal of one or both hydroxy groups resulted in the formation of a mixture of isomers.181 Further recent reports by McTernan as well as Palma and coworkers on the stereoselective cage assembly from peptide-based bis-pyridyl ligands are noteworthy.182,183
Rissanen and coworkers reported in 2015 on the self-assembly of bile acid–based ligand 5.9a, having a multitude of chiral centres, to a single isomer of three-membered ring Pd3(5.9a)6, 5.10a (Fig. 21d). NMR experiments in combination with DFT computations and chloride-induced disassembly studies point towards an all-HHTT arrangement of the ligands in 5.10a.184
In a later study, the authors employed ligand 5.9b, which possesses, in contrast to ligand 5.9a a free carboxyl group. Assembly Pd3(5.9b)6, 5.10b could only be obtained through stepwise synthesis by first adding a Fe(III) or Cu(II) salt, followed by a transmetalation with Pd(II). Supported by ion mobility mass spectrometric experiments, the authors suppose that the ligands are arranged in an all-HHTT fashion, similarly to 5.10a. The unprotected carboxyl group renders 5.10b water soluble and amphiphilic. The authors also observed higher order self-assembly: addition of water to the cage (synthesized by transmetalation of the Cu(II) assembly) in DMSO led to aggregation and gel formation. Furthermore, investigation with transmission electron microscopy revealed evaporation-induced formation of hexagonal particles.185
Very recently, some of the authors incorporated a bile acid which is an epimer to 5.9a/b, into bis-pyridyl ligand 5.9c. Importantly, since the pyridine moiety is attached via the epimeric hydroxy group, ligand 5.9c has an altered binding angle. Assembly of 5.9c with Pd(II) led to a mixture of Pd3(5.9c)6, Pd4(5.9c)8, Pd5(5.9c)10, and Pd6(5.9c)12 species.186
Lewis and coworkers showed in 2020 that the asymmetry of ligand 5.11, in which the two ligand arms differ only in one alkyne spacer, is not sufficient for steering orientational self-sorting to one defined species (Fig. 22a). More precisely, the HHTT- and the HHHT-isomer were predicted to be relatively similar in energy. The bias for the HHTT-isomer could be sufficiently increased by endowing one end of the ligand with a picolyl donor moiety (5.12). Hence, orientational self-sorting was achieved here through a combination of steric and geometrical constraints (principle I). In another fashion, selectivity for the HHTT-isomer was achieved by increasing the geometrical constraints: in 5.13 and 5.14, the planes orthogonal to the donor vectors are parallel but have, however, a larger offset as compared to ligand 5.11 (principle II). In both cases, the HHTT-isomer was obtained within two hours at room temperature. Ligand 5.15 differs from ligand 5.13 in the alkyne spacer only, which increases the similarity between the two arms. Selective self-assembly to the HHTT-isomer was observed; however, the equilibration time was significantly longer as compared to the one for the self-assembly of ligands 5.13 and 5.14.187
Zhang, Wang and coworkers increased the asymmetry of ligand 5.11 by increasing the length of one linker (principle III). Self-assembly with phenyl spaced ligand 5.16 afforded HHTT-Pd2(5.16)4 as the main species along with traces of other species. A further increase in ligand dissymmetry by incorporating a phenyl–alkynyl-spaced linker in 5.17 afforded the same isomer HHTT-Pd2(5.17)4, 5.18 as the sole species. DFT computations support that by increasing the linker length, the energy difference between the HHTT- and the HHHT-isomer increases (5.11 < 5.16 < 5.17).188
In a combined theoretical and experimental study, Jelfs, Lewis, and Tarzia developed a workflow that aims at predicting the selective orientational self-sorting to HHTT-Pd2L4 cages with high throughput. For ranking the stability of the isomers of a virtual library of asymmetric ligands, three heuristics were considered: the energy difference ΔE between the two most stable isomers, as well as the sum of the deviations of the Pd(II)–nitrogen bonds from the average PdN4 plane D and the minimum square planar order parameter q (in an ideal square planar environment D = 0 and q = 1, Fig. 22b). From the isomers that were predicted to have parameters favouring the formation of a single HHTT-isomer (ΔE ≥ 6 kJ mol−1, D < 0.1 Å, q > 0.95), three were already shown to selectively form the HHTT-isomer (ligands 5.13, 5.14, and 5.15). For testing the fidelity of the approach, the authors experimentally investigated the self-assembly of five new ligands. While single isomers were obtained for ligands strictly obeying the set structural parameters, the approach was less reliable for small ΔE values. Hence, the parameters considered do not seem to describe all factors influencing the orientational self-sorting. Omitted effects include the templation by anions or solvents as well as ligand flexibility.189
In 2019, Ogata and Yuasa studied the self-assembly of ligand 5.19 which is equipped with a para-pyridine as well as an imidazole donor moiety. When 5.19 and the Pd(II) source are combined in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, a single isomer Pd2(5.19)4, 5.20 was obtained (Fig. 23a). NMR spectroscopic investigations in combination with DFT computations support that the HHTT-isomer is favoured. Steric repulsion between pyridine and imidazole protons caused a twisting of the assembly which led to the existence of two diastereomers if chiral ligand 5.19b was employed. The authors furthermore exploited the superior donor strength of imidazole: upon decreasing the 5.19: Pd(II) stoichiometry, open structure Pd1(5.19)4 was obtained, where the ligand only coordinates via the imidazole end. The structural conversion was accompanied by the release of an encapsulated anion.190
1 ratio, a single isomer Pd2(5.19)4, 5.20 was obtained (Fig. 23a). NMR spectroscopic investigations in combination with DFT computations support that the HHTT-isomer is favoured. Steric repulsion between pyridine and imidazole protons caused a twisting of the assembly which led to the existence of two diastereomers if chiral ligand 5.19b was employed. The authors furthermore exploited the superior donor strength of imidazole: upon decreasing the 5.19: Pd(II) stoichiometry, open structure Pd1(5.19)4 was obtained, where the ligand only coordinates via the imidazole end. The structural conversion was accompanied by the release of an encapsulated anion.190
The group of Chand employed ligand 5.21 with 4-aniline and meta-pyridine ends. The self-assembly was studied with various Pd(II) precursors using DMSO as the solvent. In each case, formation of a single isomer assigned to HHTT-Pd2(5.21)4, 5.22 was achieved, noteworthily with the aniline amino substituents acting as donors besides the pyridines (Fig. 23b). Furthermore, robust selectivity for this isomer was observed at different temperatures as well as at varying concentrations. The authors performed DFT computations and molecular dynamics (MD) studies which supported that the superior stability of the HHTT-isomer is driven on one hand by its least conformational strain and on the other hand by stabilization through electrostatic and van-der-Waals interactions.191
Additionally, the same group reported an asymmetric ligand with meta-pyridine and 3-aniline donor groups separated by an amide bond. This short ligand was shown to assemble to form a single isomer Pd3L6 which was, based on spectroscopic and computational investigations, assigned to the all-HHTT isomer.192
Lewis presented in 2021 the selective formation of HHTT-Pd2(5.23)4, 5.24 isomers with ligands 5.23, terminated with an isoquinoline and a triazole donor (Fig. 23c). The latter was introduced in the last step via a CuAAC click reaction, allowing for easy functionalization of the ligand. While the selectivity for the HHTT-isomer proved robust across a variety of different electronic situations for the triazole donor (ligands 5.23a–f), variation of the donor strength affected the self-assembly when ligand and Pd(II) were combined in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. For phthalimide-appended ligand 5.23e, a mononuclear Pd1(5.23e)4 complex was observed, similar to the work of Ogata and Yuasa.190 The ligand coordinates here solely via the isoquinoline donor moieties as the substituent in 5.23e (further) reduces the triazole donor strength. In contrast, when ligand 5.23a was employed, a mixture of free ligand, mono-, and dinuclear species was observed. Exploiting the isostructural nature of ligands 5.23, the author showed that statistical mixtures of homo- and heteroleptic cages were obtained when multiple ligands were combined.193
1 ratio. For phthalimide-appended ligand 5.23e, a mononuclear Pd1(5.23e)4 complex was observed, similar to the work of Ogata and Yuasa.190 The ligand coordinates here solely via the isoquinoline donor moieties as the substituent in 5.23e (further) reduces the triazole donor strength. In contrast, when ligand 5.23a was employed, a mixture of free ligand, mono-, and dinuclear species was observed. Exploiting the isostructural nature of ligands 5.23, the author showed that statistical mixtures of homo- and heteroleptic cages were obtained when multiple ligands were combined.193
Zhang and coworkers substituted the meta-pyridine in ligand 5.11 with a para-pyridine resulting in an increased binding angle of ligand 5.25 (Fig. 24a). In accordance with the trends observed for homoleptic cages (Chapter 2), this resulted in the formation of a species of higher nuclearity, here a three-membered ring. Noteworthily, geometric constraints prompted selective formation of all-HHTT-Pd3(5.25)6, isomer 5.26.188
Recently, Chand and coworkers reported the formation of a tetrahedral all-HHTT-Pd4(5.27)8, 5.28 by applying geometrically constrained ligand 5.27 with a bent angle of approximately 120° (Fig. 24b).194
In 2021, Severin and coworkers reported tetrahedral and octahedral assemblies with asymmetric ligands 5.29 and 5.31 that possess wide bent angles (Fig. 24c and d). The number of possible isomers reaches 35 in the case of the tetrahedral assembly and to 112 in the case of the octahedral one. Astonishingly, the authors observed the formation of single isomers all-HHTT-Pd4(5.29)8, 5.30 and all-HHTT-Pd6(5.31)12, 5.32 in which two different ligand ends are arranged in a cis-configuration on each Pd(II) centre.195 The preferred orientation of the ligands can be traced back to geometrical constraints, as was explored in a later study by the same authors on the basis of octahedral assemblies: ligand 5.31 can be characterized by the ratio of the two donor vectors a/b. Two ligands opposing each other in the assembly can either be oriented HH, TT, or HT. Only the latter case allows for a Pd–Pd–Pd angle of 90° required for an octahedron. Conceivable limitations of the so-called cis-rule are a small a/b ratio as well as ligand flexibility. Both aspects were altered in alkyne-spaced ligands 5.33 and 5.35. Ligand 5.33, possessing a large a/b ratio cleanly assembles, similarly to ligand 5.31, to form octahedron all-HHTT-Pd6(5.33)12, 5.34. When ligand 5.35 with a decreased asymmetry was employed, prolonged heating was required for the selective formation of all-HHTT-Pd6(5.35)12, 5.36. The approximate rectangular bent angle that allows for the formation of an octahedron is maintained in ligands 5.37 and 5.39, possessing triazole or imidazole donors, respectively. The former strictly follows the cis-rule, yielding all-HHTT-Pd6(5.37)12, 5.38. In contrast, a mixture of isomers Pd6(5.39)12, 5.40 was obtained with ligand 5.39, that differs merely in its electronic situation. The selectivity might be hampered by the formation of kinetically trapped intermediates due to the higher basicity of the imidazole donor moiety, as hypothesized by the authors.196
Only very recently, Chand and coworkers were able to combine the non-statistical self-assembly of heteroleptic cages with orientational self-sorting. For this, they employed two strategies: the SCA approach for forming cis-Pd2A2B2 cages as well as geometrical constraints for driving orientational self-sorting. For the former, the two ligands 5.27 and 5.41 were designed in a way that their donor vectors are convergent and divergent, respectively (for ligand 5.41 in both conformations, Fig. 25a). The geometric complementarity of 5.27 and 5.41 reduced the number of possible isomers from 31 to six. Furthermore, the structural constraints were expected to only allow a ligand arrangement in which the amido-pyridine ends of 5.41 oppose the pyridine ends of ligands 5.27 and the pyridine ends of 5.41 oppose the isoquinoline ends of 5.27. This leaves two possible isomers, namely HHH′H′-Pd2(5.27)2(5.41)2 and HTH′T′-Pd2(5.27)2(5.41)2. The authors found that HTH′T′-Pd2(5.27)2(5.41)2, 5.42 was formed selectively (Fig. 25b). The preference for the antiparallel arrangement of the ligands can be explained with increased steric strain when the two isoquinoline donors of 5.27 sit in cis-position to each other. Interestingly, substitution of the alkyne spacer in 5.27 by a phenyl spacer hampered self-sorting to a defined species. It was hypothesized that this is due to a length mismatch.194 Lewis and coworkers, on the other hand, achieved isomer-selective assembly of low-symmetry heteroleptic Pd2LA2LB2 cages through a combination of shape-complementary assembly and coordination sphere engineering.197
The repertoire of self-assemblies with asymmetric ligands has greatly expanded within recent years. In most cases, ligands with roughly parallel binding vectors have been employed for obtaining dinuclear Pd2L4 cages, which possess the smallest number of possible isomers. Additionally, all-HHTT isomers of higher nuclearity species have been reported, that are three-membered rings, tetrahedra, and octahedra. The development of strategies for orientational self-sorting was highly shaped by already existing ones for the non-statistical self-sorting of heteroleptic cages, that are strategies exploiting steric or geometric constraints or a combination thereof. This showcased that the design principles are transferrable; hence, it is expected that the two areas will mutually benefit from each other in the future. Furthermore, orientational self-sorting allows for differentiable coordination environments; however, the number of examples for species dealing with the consequences of this phenomenon remains scarce. Recently, strategies for integrative and orientational self-sorting have been transferred to Pt(II) cage chemistry by Preston, using a specific Pt(II) precursor that ensures sufficient kinetic accessibility of the thermodynamic products in the assembly reaction.198
6. Chiral cages
Chirality in metallo-supramolecular assemblies can be a consequence of the spatial arrangement of the components upon assembly formation or can stem from the inherent chirality of one or more components. The former, commonly referred to as a “soft” approach is promising for stimuli responsive systems due to its dynamicity. Chiral interaction partners, such as guests, can then transfer their chirality on the assembly via non-covalent interactions. The latter, “hard” approach can be advantageous for functional materials, e.g. for chiral sensing or asymmetric catalysis, due to its robustness.199 Employing chiral ligands as a racemic mixture endows the self-assembly process with a further layer of complexity: in the absence of self-sorting, a mixture of different diastereoisomers can be obtained. Narcissistic chiral self-sorting occurs under self-recognition, leading to a mixture of two assemblies bearing only ligands of the same handedness. In contrast, social chiral self-sorting is based on self-discrimination and may lead to overall achiral meso-compounds.The groups of Kubik and Lützen as well as our group used chiral, peptide-based ligands for assembling three-membered rings200 and doubly interlocked lemniscates.98 Furthermore, Lützen and coworkers reported homochiral tetrahedra, cubes, and dodecanuclear spheres based on BINOL ligands.124,127 Liu and coworkers utilized the BINOL-based cube for obtaining CPL-active host–guest complexes with BODIPY guests.201 The group of Zysman-Colman formed three- and four-membered rings for photophysical investigations based on paracyclophane202 and homochiral Ir(III)203 complexes. Furthermore, Rissanen and coworkers reported a series of assemblies with ligands based on bile acids,184,185 as described in Chapter 5. Aside from these architectures that were assembled from enantiomerically pure ligands, the chiral self-sorting of racemic mixtures of ligands gained particular attention and will be the focus of this chapter.
Lützen and coworkers reported in 2013 on the first system showing narcissistic chiral self-sorting in Pd2L4 cages. The authors studied the self-assembly of BINOL-based ligands M- or P-6.1 possessing axial chirality. When 6.1 is employed as a racemic mixture, six different isomers can potentially be obtained: two homochiral cages, two achiral, diastereomeric cages (meso-cis and meso-trans), and two cages carrying both enantiomers in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Self-assembly occurred under chiral self-recognition, leading to a narcissistic mixture of the two homochiral cages Pd2(M-6.1)4, MMMM-6.2 and Pd2(P-6.1)4, PPPP-6.2 (Fig. 26a).204
1 ratio. Self-assembly occurred under chiral self-recognition, leading to a narcissistic mixture of the two homochiral cages Pd2(M-6.1)4, MMMM-6.2 and Pd2(P-6.1)4, PPPP-6.2 (Fig. 26a).204
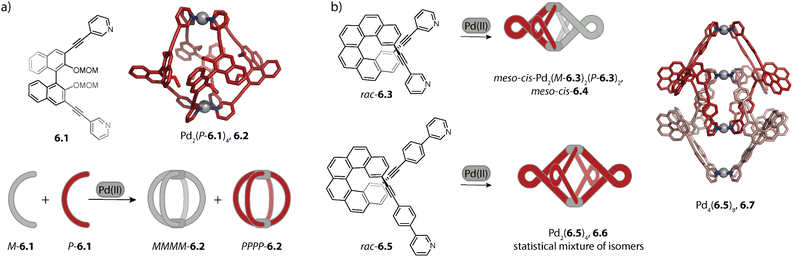 | ||
| Fig. 26 (a) Narcissistic chiral self-sorting to a pair of enantiomeric lantern-shaped cages 6.2. (b) Social chiral self-sorting to meso-cis-6.4 that is lost upon prolongation of the linker. | ||
Recently, we reported the narcissistic chiral self-sorting of Tröger-based ligands M-L and P-L into Pd2(M-L)4 and Pd2(P-L)4 capsules upon encapsulation of fullerene guests or upon crystallization, respectively.78
Social chiral self-sorting in Pd2L4 cages, on the other hand, was reported by us in 2019 for the first time. Ligands M- and P-6.3, based on a helicene backbone, assemble with Pd(II) to form dinuclear homochiral cages Pd2(M/P-6.3)4, MMMM/PPPP-6.4. When a racemic mixture of the ligand is employed, self-discrimination to meso-cis-Pd2(M-6.3)2(P-6.3)2 and meso-cis-6.4 is observed (Fig. 26b). Interestingly, a breakdown of chiral self-sorting was observed for ligands M- and P-6.5, differing only in the elongated linker between the helicene core and the pyridine donors. This was thought to originate from the increased distance between the chiral backbones in the assembly. The enantiopure monomeric cages Pd2(M/P-6.5)4 and MMMM/PPPP-6.6 were shown to discriminate between guests of opposite chirality. Furthermore, in line with the criteria for the formation of interlocked double cages (Chapter 2), elongated ligand 6.5 assembled in acetonitrile to form double cage Pd4(6.5)8, 6.7.134
Recently, the group of Natarajan observed social chiral self-sorting in Pd6L12 cubes. Ligands 6.8 possess axial chirality and a rectangular bent angle. Due to the increased number of components in hexanuclear in comparison to dinuclear cages, the number of possible isomers reaches 145. Excitingly, the authors observed the selective formation of a racemic pair of heterochiral cages Pd6(S-6.8)6(R-6.8)6, 6.9 (Fig. 27a).205
 | ||
| Fig. 27 (a) Social chiral self-sorting to a pair of cube enantiomers 6.9. (b) Solvent- and counter anion-dependent chiral self-sorting with a racemic mixture of ligands 6.10. | ||
The same group additionally explored the chiral self-sorting with an isomer of 6.8, ligand 6.10 possessing meta-pyridine donors. Intriguingly, when BArF− was employed as the counter anion which, owing to its large size, resides outside of the narrow cavity, no chiral self-sorting was observed (Fig. 27b). However, social chiral self-sorting to meso-cis-Pd2(S-6.10)2(R-6.10)2, meso-cis-6.11 was observed in the presence of NO3− in DMSO and narcissistic chiral self-sorting to the homochiral pair SSSS-6.11 and RRRR-6.11 with BF4−, PF6− and OTf−. Chiral self-sorting was furthermore shown to be solvent-dependent for NO3− or BF4− as counter anions: with NO3−, a solvent switch from DMSO to acetonitrile resulted in a mixture of meso-cis-6.11 and a pair of homochiral cages. Additionally, a mixture of different isomers was obtained for the self-assembly with BF4− in DMSO.206
We recently carried out a thorough investigation of the solvent dependency of chiral self-sorting in helical cages with ligands 6.12 and 6.14 that are based on axially chiral 1,1′-biazulene-2,2′-diamine, a structural motif reminiscent of widely used BINOL (with azulene being an isomer of the therein contained naphthalene). Short ligand 6.12 and long ligand 6.14 both assemble to form Pd2L4 cages; however, a striking difference in chiral self-sorting was observed. In DMSO and acetonitrile, racemic 6.12 selectively forms meso-trans-Pd2(S-6.12)2(R-6.12)2, meso-trans-6.13 while a mixture of isomers Pd2(6.14)4, 6.15 is obtained with racemic 6.14 under the same conditions (Fig. 28a). Close examination of the crystal structure of meso-trans-6.13 in combination with DFT computations including explicit solvation reveals that the meso-trans isomer is stabilized by individual solvent molecules that connect two adjacent ligands via hydrogen bonding. Accordingly, changing the solvent to nitromethane, a significantly weaker H-bond acceptor, resulted in a loss of chiral self-sorting (to be gained back by adding small amounts of DMSO). Such an H-bond tethering is not possible in 6.15 due to the larger distances between the amino groups in the ligand backbones.
Examples for the transfer of chirality from homochiral PdnL2n cages to encapsulated guests were reported, either giving rise to chiral guest discrimination134 or to induced guest chirality.77,78,201 Conversely, guest-to-host chirality transfer has been observed in terms of the stabilization of chirality in dynamically chiral hosts through binding of enantiopure guests.38,46,89
Very recently, Gan and coworkers reported a bidirectional transmission of chiral information between a guest and the exterior of a helical cage. The authors incorporated in helicates Pd2(6.16)4 another stereogenic centre: the 1,8-diazaanthracene segments can rotate relative to the ligand backbone, giving rise to different gauche conformations (Fig. 28b). Owing to steric constraints, helical and axial chirality are linked; more precisely, a PP-conformation of the ligand favours a positive gauche conformation (g+) while a MM-conformation favours g−. Achiral ligand 6.16a assembles to form meso-cis-Pd2(MM-6.16a)2(PP-6.16a)2 incorporating both gauche conformations. Encapsulation of L- or D-titrate guests induced a preference for one of the gauche conformations, meaning that the chirality is transmitted to the cage periphery. Homo-axial chirality could also be achieved through incorporation of point chirality in the appended segment of ligand 6.16b. Chiral helicates Pd2(MM-6.16b)4 and Pd2(PP-6.16b)4 were shown to discriminate between L- and D-tartrate guests.207
Natural or synthetic chiral backbones have been employed for bestowing coordination assemblies of various nuclearities with chirality. We paid particular attention to the chiral self-sorting of racemic mixtures of ligands to either meso-cages or a mixture of the two enantiopure cages. Prolongation of the linker can result in a loss of chiral self-sorting due to the increased distance between the ligand backbones. Furthermore, the solvent and the counter anions can have a pivotal effect on the outcome. A possible origin for the former is the specific interactions between solvent molecules and the ligands at the portals of the cage. We also touched upon a recent example in which the soft and hard chirality of helicates was exploited for chirality transmission from or to an encapsulated guest, respectively.
7. Conclusion
To conclude, we surveyed the topological diversity of di- to octanuclear assemblies that are accessible upon simple combination of bis-monodentate organic ligands and Pd(II) cations. The structure of the organic ligand, i.e. its geometry and steric demand, plays a key role in the self-assembly outcome. The stiffness of small, aromatic ligands allows them to be simplified as simple geometric units which facilitate structure prediction. In contrast, solvent and counter anions often exert a templation effect on self-assembly with highly flexible ligands, rendering their topologies notoriously more difficult to predict. Long organic ligands allow for mechanical interlocking which can be inhibited by equipping the ligands with endohedral steric bulk. Strategies for increasing structural complexity include the formation of heteroleptic cages with multiple organic building blocks as well as the usage of asymmetric ligands. For steering the self-sorting towards a defined species, the ligands have been endowed with geometric constraints, steric bulk has been installed at the backbone or at the donor moieties, and attractive as well as repulsive inter-ligand interactions have been exploited. Concerning dinuclear heteroleptic cages, the maximum level of self-sorting to provide Pd2ABCD cages with four differentiable ligands has been mastered very recently. Several larger heteroleptic architectures have been reported, most of them carrying two different kinds of ligands. Orientational self-sorting with asymmetric ligands flourished in the last couple of years and defined architectures with up to six Pd(II) nodes have been attained. We have also treated self-sorting effects with chiral ligands; chiral narcissistic and social self-sorting was shown to be highly dependent on solvent and counter anions.We anticipate that an enhanced understanding of the factors governing the formation of specific topologies as well as the reliability of strategies for selective self-sorting will allow the field to shift progressively towards more and more complex functional nanosystems. On one hand, the assemblies can be harnessed as platforms for combining multiple functional moieties in a combinatorial fashion. The overall assembly properties emerge then from the interplay of the various components, which holds promise for highly selective molecular receptors, catalysts, and drug delivery systems. Another field of application may be energy harvesting materials, i.e. in terms of gaining supramolecular control over the morphology of donor/acceptor-based charge separating layers in photovoltaic devices. While examples for functional cages incorporating two kinds of interoperating ligands have been reported (many by our lab), the newly developed strategies for the non-statistical combination of more than two kinds of ligands have not yet been exploited for applicable cages. On the other hand, as an additional layer of complexity, populations of coexisting assemblies have been reported by us very recently. In biological systems, numerous processes occur simultaneously, either in an orthogonal fashion or are closely interconnected. The dynamic nature of the coordination bond as well as the possibility of incorporating responsive moieties can be exploited for creating networks of communicating and coexisting assemblies. This offers great potential for the transmission and transduction of information or energy, which is of interest for the development of smart materials and of the field of systems chemistry.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
L. N. compiled the draft and created the figures. G. H. C. and L. N. both discussed and revised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financed by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under GRK2376, project number 331085229 (“Confinement-Controlled Chemistry”) and Germany's Excellence Strategy EXC2033, project number 390677874 (“RESOLV”).Notes and references
- J.-M. Lehn, Science, 1993, 260, 1762 CrossRef CAS.
- A. Stank, D. B. Kokh, J. C. Fuller and R. C. Wade, Acc. Chem. Res., 2016, 49, 809 CrossRef CAS.
- K. Selvakumar, L. Motiei and D. Margulies, J. Am. Chem. Soc., 2015, 137, 4892 CrossRef CAS PubMed.
- P. Parkinson, C. E. I. Knappke, N. Kamonsutthipaijit, K. Sirithip, J. D. Matichak, H. L. Anderson and L. M. Herz, J. Am. Chem. Soc., 2014, 136, 8217 CrossRef CAS PubMed.
- G. M. Whitesides and B. Grzybowski, Science, 2002, 295, 2418 CrossRef CAS PubMed.
- M. E. Briggs and A. I. Cooper, Chem. Mater., 2017, 29, 149 CrossRef CAS PubMed.
- F. Beuerle and B. Gole, Angew. Chem., Int. Ed., 2018, 57, 4850 CrossRef CAS PubMed.
- M. Mastalerz, Angew. Chem., Int. Ed., 2010, 49, 5042 CrossRef CAS.
- T. Kunde, T. Pausch and B. M. Schmidt, Eur. J. Org Chem., 2021, 2021, 5844 CrossRef CAS.
- L. R. MacGillivray and J. L. Atwood, Nature, 1997, 389, 469 CrossRef CAS.
- J. Rebek, Acc. Chem. Res., 2009, 42, 1660 CrossRef CAS PubMed.
- S. K. Yang and S. C. Zimmerman, Isr. J. Chem., 2013, 53, 511 CrossRef CAS.
- L. J. Prins, D. N. Reinhoudt and P. Timmerman, Angew. Chem., Int. Ed., 2001, 40, 2382 CrossRef CAS.
- M. M. J. Smulders, I. A. Riddell, C. Browne and J. R. Nitschke, Chem. Soc. Rev., 2012, 42, 1728 RSC.
- T. R. Cook and P. J. Stang, Chem. Rev., 2015, 115, 7001 CrossRef CAS.
- M. Han, D. M. Engelhard and G. H. Clever, Chem. Soc. Rev., 2014, 43, 1848 RSC.
- M. Pan, K. Wu, J.-H. Zhang and C.-Y. Su, Coord. Chem. Rev., 2019, 378, 333 CrossRef CAS.
- F. Würthner, C.-C. You and C. R. Saha-Möller, Chem. Soc. Rev., 2004, 33, 133 RSC.
- C. M. Hong, R. G. Bergman, K. N. Raymond and F. D. Toste, Acc. Chem. Res., 2018, 51, 2447 CrossRef CAS PubMed.
- M. Fujita, M. Tominaga, A. Hori and B. Therrien, Acc. Chem. Res., 2005, 38, 369 CrossRef CAS PubMed.
- D. Bardhan and D. K. Chand, Chem.–Eur. J., 2019, 25, 12241 CrossRef CAS.
- S. De, K. Mahata and M. Schmittel, Chem. Soc. Rev., 2010, 39, 1555 RSC.
- R. W. Saalfrank, A. Stark, K. Peters and H. G. von Schnering, Angew. Chem., Int. Ed., 1988, 27, 851 CrossRef.
- B. Hasenknopf, J.-M. Lehn, N. Boumediene, A. Dupont-Gervais, A. V. Dorsselaer, B. Kneisel and D. Fenske, J. Am. Chem. Soc., 1997, 119, 10956 CrossRef CAS.
- D. L. Caulder, R. E. Powers, T. N. Parac and K. N. Raymond, Angew. Chem., Int. Ed., 1998, 37, 1840 CrossRef CAS.
- P. J. Stang, Chem.–Eur. J., 1998, 4, 19 CrossRef CAS.
- M. Fujita, J. Yazaki and K. Ogura, J. Am. Chem. Soc., 1990, 112, 5645 CrossRef CAS.
- D. A. McMorran and P. J. Steel, Angew. Chem., Int. Ed., 1998, 37, 3295 CrossRef CAS.
- Z. T. Avery, J. L. Algar and D. Preston, Trends Chem., 2024, 6, 352 CrossRef.
- J. E. M. Lewis and J. D. Crowley, ChemPlusChem, 2020, 85, 815 CrossRef CAS.
- D. Bokotial, K. Acharyya, A. Chowdhury and P. S. Mukherjee, Angew. Chem., Int. Ed., 2024, 63, e202401136 CrossRef CAS PubMed.
- T. K. Piskorz, V. Martí-Centelles, R. L. Spicer, F. Duarte and P. J. Lusby, Chem. Sci., 2023, 14, 11300 RSC.
- V. Martí-Centelles, A. L. Lawrence and P. J. Lusby, J. Am. Chem. Soc., 2018, 140, jacs.7b12146 CrossRef PubMed.
- R. L. Spicer, A. D. Stergiou, T. A. Young, F. Duarte, M. D. Symes and P. J. Lusby, J. Am. Chem. Soc., 2020, 142, 2134 CrossRef CAS PubMed.
- T. A. Young, V. Martí-Centelles, J. Wang, P. J. Lusby and F. Duarte, J. Am. Chem. Soc., 2020, 142, 1300 CrossRef PubMed.
- S. Pradhan, A. Bhattacharyya and R. P. John, Tetrahedron Lett., 2016, 57, 1532 CrossRef CAS.
- M. Xu, B. Sun, D. A. Poole, E. O. Bobylev, X. Jing, J. Wu, C. He, C. Duan and J. N. H. Reek, Chem. Sci., 2023, 14, 11699 RSC.
- I. Regeni, B. Chen, M. Frank, A. Baksi, J. J. Holstein and G. H. Clever, Angew. Chem., Int. Ed., 2021, 60, 5673 CrossRef CAS PubMed.
- A. B. S. Elliott, J. E. M. Lewis, H. van der Salm, C. J. McAdam, J. D. Crowley and K. C. Gordon, Inorg. Chem., 2016, 55, 3440 CrossRef CAS.
- B. Aikman, R. Bonsignore, B. Woods, D. Doellerer, R. Scotti, C. Schmidt, A. A. Heidecker, A. Pöthig, E. J. Sayers, A. T. Jones and A. Casini, Dalton Trans., 2022, 51, 7476 RSC.
- K. E. Ebbert, E. Benchimol, A. Platzek, C. Drechsler, J. Openy, S. Hasegawa, J. J. Holstein and G. H. Clever, Angew. Chem., Int. Ed., 2024, 63, e202413323 CrossRef CAS PubMed.
- C. Li, B. Zhang, Y. Dong, Y. Li, P. Wang, Y. Yu, L. Cheng and L. Cao, Dalton Trans., 2020, 49, 8051 RSC.
- S. Ganta, J.-H. Borter, C. Drechsler, J. J. Holstein, D. Schwarzer and G. H. Clever, Org. Chem. Front., 2022, 9, 5485 RSC.
- V. Croué, S. Krykun, M. Allain, Y. Morille, F. Aubriet, V. Carré, Z. Voitenko, S. Goeb and M. Sallé, New J. Chem., 2017, 41, 3238 RSC.
- R. A. S. Vasdev, J. A. Findlay, A. L. Garden and J. D. Crowley, Chem. Commun., 2019, 55, 7506 RSC.
- R.-J. Li, J. J. Holstein, W. G. Hiller, J. Andréasson and G. H. Clever, J. Am. Chem. Soc., 2019, 141, 2097 CrossRef CAS PubMed.
- R. Li, M. Han, J. Tessarolo, J. J. Holstein, J. Lübben, B. Dittrich, C. Volkmann, M. Finze, C. Jenne and G. H. Clever, ChemPhotoChem, 2019, 3, 378 CrossRef CAS.
- S. Fu, Q. Luo, M. Zang, J. Tian, Z. Zhang, M. Zeng, Y. Ji, J. Xu and J. Liu, Mater. Chem. Front., 2019, 3, 1238 RSC.
- J. Zhu, X. Chen, X. Jin and Q. Wang, Chin. Chem. Lett., 2023, 34, 108002 CrossRef CAS.
- R. G. DiNardi, A. O. Douglas, R. Tian, J. R. Price, M. Tajik, W. A. Donald and J. E. Beves, Angew. Chem., Int. Ed., 2022, 61, e202205701 CrossRef CAS PubMed.
- S. Ganta, C. Drechsler, Y. Chen and G. H. Clever, Chem.–Eur. J., 2022, 28, e202104228 CrossRef CAS PubMed.
- S. Saha, Y.-T. Chen, S. Ganta, M. Gilles, B. Holzapfel, P. Lill, H. Rehage, C. Gatsogiannis and G. H. Clever, Chem.–Eur. J., 2022, 28, e202103406 CrossRef CAS PubMed.
- S. Saha, B. Holzapfel, Y.-T. Chen, K. Terlinden, P. Lill, C. Gatsogiannis, H. Rehage and G. H. Clever, J. Am. Chem. Soc., 2018, 140, 17384 CrossRef CAS PubMed.
- M. D. Ward, Chem. Commun., 2024, 60, 10464 RSC.
- Y. Xue, X. Hang, J. Ding, B. Li, R. Zhu, H. Pang and Q. Xu, Coord. Chem. Rev., 2020, 430, 213656 CrossRef.
- H. Takezawa and M. Fujita, Bull. Chem. Soc. Jpn., 2021, 94, 2351 CrossRef CAS.
- W.-L. Jiang, B. Huang, X.-L. Zhao, X. Shi and H.-B. Yang, Chem, 2023, 9, 2655 CAS.
- S. Hasegawa, A. Baksi, B. Chen and G. H. Clever, Org. Mater., 2022, 4, 222 CrossRef CAS.
- M. Yoshizawa and L. Catti, Acc. Chem. Res., 2019, 52, 2392 CrossRef CAS PubMed.
- S. Yadav, P. Kannan and G. Qiu, Org. Chem. Front., 2020, 7, 2842 RSC.
- D. Fujita, Y. Ueda, S. Sato, N. Mizuno, T. Kumasaka and M. Fujita, Nature, 2016, 540, 563 CrossRef CAS PubMed.
- D. Fujita, Y. Ueda, S. Sato, H. Yokoyama, N. Mizuno, T. Kumasaka and M. Fujita, Chem, 2016, 1, 91 CAS.
- R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Chem. Rev., 2011, 111, 6810 CrossRef CAS PubMed.
- S. Pullen, J. Tessarolo and G. H. Clever, Chem. Sci., 2021, 12, 7269 RSC.
- L. K. Moree, L. A. V. Faulkner and J. D. Crowley, Chem. Soc. Rev., 2024, 53, 25 RSC.
- K. Acharyya, S. Mukherjee and P. S. Mukherjee, J. Am. Chem. Soc., 2013, 135, 554 CrossRef CAS PubMed.
- D. Preston, K. M. Patil, A. T. O'Neil, R. A. S. Vasdev, J. A. Kitchen and P. E. Kruger, Inorg. Chem. Front., 2020, 7, 2990 RSC.
- S. M. Jansze, M. D. Wise, A. V. Vologzhanina, R. Scopelliti and K. Severin, Chem. Sci., 2017, 8, 1901 RSC.
- N. Kishi, Z. Li, K. Yoza, M. Akita and M. Yoshizawa, J. Am. Chem. Soc., 2011, 133, 11438 CrossRef CAS PubMed.
- M. Yamashina, T. Yuki, Y. Sei, M. Akita and M. Yoshizawa, Chem.–Eur. J., 2015, 21, 4200 CrossRef CAS PubMed.
- G. H. Clever, W. Kawamura, S. Tashiro, M. Shiro and M. Shionoya, Angew. Chem., Int. Ed., 2012, 51, 2606 CrossRef CAS PubMed.
- N. Kishi, Z. Li, Y. Sei, M. Akita, K. Yoza, J. S. Siegel and M. Yoshizawa, Chem.–Eur. J., 2013, 19, 6313 CrossRef CAS PubMed.
- P. Liao, B. W. Langloss, A. M. Johnson, E. R. Knudsen, F. S. Tham, R. R. Julian and R. J. Hooley, Chem. Commun., 2010, 46, 4932 RSC.
- D. P. August, G. S. Nichol and P. J. Lusby, Angew. Chem., Int. Ed., 2016, 55, 15022 CrossRef CAS PubMed.
- S. Hasegawa, S. L. Meichsner, J. J. Holstein, A. Baksi, M. Kasanmascheff and G. H. Clever, J. Am. Chem. Soc., 2021, 143, 9718 CrossRef CAS PubMed.
- B. Chen, J. J. Holstein, S. Horiuchi, W. G. Hiller and G. H. Clever, J. Am. Chem. Soc., 2019, 141, 8907 CrossRef CAS PubMed.
- H. Sasafuchi, M. Ueda, N. Kishida, T. Sawada, S. Suzuki, Y. Imai and M. Yoshizawa, Chem, 2024, 102332 Search PubMed.
- E. Benchimol, H. M. O'Connor, B. Schmidt, N. Bogo, J. J. Holstein, J. I. Lovitt, S. Shanmugaraju, C. J. Stein, T. Gunnlaugsson and G. H. Clever, Angew. Chem., Int. Ed., 2025, 64, e202421137 CrossRef CAS PubMed.
- A. Walther, I. Regeni, J. J. Holstein and G. H. Clever, J. Am. Chem. Soc., 2023, 145, 25365 CrossRef CAS PubMed.
- W. M. Bloch, Y. Abe, J. J. Holstein, C. M. Wandtke, B. Dittrich and G. H. Clever, J. Am. Chem. Soc., 2016, 138, 13750 CrossRef CAS PubMed.
- L.-P. Zhou and Q.-F. Sun, Chem. Commun., 2015, 51, 16767 RSC.
- B. Chen, S. Horiuchi, J. J. Holstein, J. Tessarolo and G. H. Clever, Chem.–Eur. J., 2019, 25, 14921 CrossRef CAS PubMed.
- R. A. S. Vasdev, L. F. Gaudin, D. Preston, J. P. Jogy, G. I. Giles and J. D. Crowley, Front. Chem., 2018, 6, 563 CrossRef CAS PubMed.
- S. Sudan, F. Fadaei-Tirani and K. Severin, Chem. Commun., 2023, 59, 8258 RSC.
- P. J. Steel and D. A. McMorran, Chem.–Asian J., 2019, 14, 1098 CrossRef CAS PubMed.
- H. S. Sahoo and D. K. Chand, Dalton Trans., 2010, 39, 7223 RSC.
- D. Tripathy, A. K. Pal, G. S. Hanan and D. K. Chand, Dalton Trans., 2012, 41, 11273 RSC.
- N. L. S. Yue, D. J. Eisler, M. C. Jennings and R. J. Puddephatt, Inorg. Chem., 2004, 43, 7671 CrossRef CAS PubMed.
- Q. Lin, L. Gao, B. Kauffmann, J. Zhang, C. Ma, D. Luo and Q. Gan, Chem. Commun., 2018, 54, 13447 RSC.
- M. Sarkar, H. Dasary and D. K. Chand, J. Organomet. Chem., 2021, 950, 121984 CrossRef CAS.
- J. D. Crowley and E. L. Gavey, Dalton Trans., 2010, 39, 4035 RSC.
- S. Ø. Scott, E. L. Gavey, S. J. Lind, K. C. Gordon and J. D. Crowley, Dalton Trans., 2011, 40, 12117 RSC.
- S. M. McNeill, D. Preston, J. E. M. Lewis, A. Robert, K. Knerr-Rupp, D. O. Graham, J. R. Wright, G. I. Giles and J. D. Crowley, Dalton Trans., 2015, 44, 11129 RSC.
- E. Benchimol, K. E. Ebbert, A. Walther, J. J. Holstein and G. H. Clever, Chem.–Eur. J., 2024, 30, e202401850 CrossRef CAS PubMed.
- S. Löffler, J. Lübben, L. Krause, D. Stalke, B. Dittrich and G. H. Clever, J. Am. Chem. Soc., 2015, 137, 1060 CrossRef PubMed.
- W. M. Bloch, S. Horiuchi, J. J. Holstein, C. Drechsler, A. Wuttke, W. Hiller, R. A. Mata and G. H. Clever, Chem. Sci., 2023, 14, 1524 RSC.
- D. Preston, S. M. McNeill, J. E. M. Lewis, G. I. Giles and J. D. Crowley, Dalton Trans., 2016, 45, 8050 RSC.
- T. R. Schulte, J. J. Holstein, L. Schneider, A. Adam, G. Haberhauer and G. H. Clever, Angew. Chem., Int. Ed., 2020, 59, 22489 CrossRef CAS PubMed.
- R. Zhu, W. M. Bloch, J. J. Holstein, S. Mandal, L. V. Schäfer and G. H. Clever, Chem.–Eur. J., 2018, 24, 12976 CrossRef CAS PubMed.
- F. A. Pereira, T. Fallows, M. Frank, A. Chen and G. H. Clever, Z. Anorg. Allg. Chem., 2013, 639, 1598 CrossRef CAS.
- Q. Zhang, L. He, J.-M. Liu, W. Wang, J. Zhang and C.-Y. Su, Dalton Trans., 2010, 39, 11171 RSC.
- A. Sautter, D. G. Schmid, G. Jung and F. Würthner, J. Am. Chem. Soc., 2001, 123, 5424 CrossRef CAS PubMed.
- W. M. Bloch, J. J. Holstein, W. Hiller and G. H. Clever, Angew. Chem., Int. Ed., 2017, 56, 8285 CrossRef CAS PubMed.
- I. Regeni, R. Chowdhury, K. Terlinden, S. Horiuchi, J. J. Holstein, S. Feldmann and G. H. Clever, Angew. Chem., Int. Ed., 2023, 62, e202308288 CrossRef CAS PubMed.
- K. Harris, D. Fujita and M. Fujita, Chem. Commun., 2013, 49, 6703–6712 RSC.
- D. K. Chand, K. Biradha, M. Kawano, S. Sakamoto, K. Yamaguchi and M. Fujita, Chem.–Asian J., 2006, 1, 82 CrossRef CAS PubMed.
- K. Suzuki, M. Kawano and M. Fujita, Angew. Chem., Int. Ed., 2007, 46, 2819 CrossRef CAS PubMed.
- D. K. Chand, M. Fujita, K. Biradha, S. Sakamoto and K. Yamaguchi, Dalton Trans., 2003, 2750 RSC.
- X.-C. Zhou, Y.-Z. Tan, Y.-Y. Ge, Y.-L. Lai, D. Luo, X.-P. Zhou and D. Li, Sci. China Chem., 2025, 1, 68 Search PubMed.
- Y.-Y. Ge, X.-C. Zhou, J. Zheng, J. Luo, Y.-L. Lai, J. Su, H.-J. Zhang, X.-P. Zhou and D. Li, Inorg. Chem., 2023, 62, 4048 CrossRef CAS PubMed.
- R. A. S. Vasdev, J. A. Findlay, D. R. Turner and J. D. Crowley, Chem.–Asian J., 2021, 16, 39 CrossRef CAS PubMed.
- L. Neukirch, M. D. Kulas, J. J. Holstein and G. H. Clever, Chem.–Eur. J., 2024, 30, e202400132 CrossRef CAS PubMed.
- D. Preston, J. J. Sutton, K. C. Gordon and J. D. Crowley, Angew. Chem., Int. Ed., 2018, 57, 8659 CrossRef CAS PubMed.
- D. Samanta and P. S. Mukherjee, Chem.–Eur. J., 2014, 20, 12483 CrossRef CAS PubMed.
- S. Ganta and D. K. Chand, Dalton Trans., 2015, 44, 15181 RSC.
- M. Han, Y. Luo, B. Damaschke, L. Gómez, X. Ribas, A. Jose, P. Peretzki, M. Seibt and G. H. Clever, Angew. Chem., Int. Ed., 2015, 55, 445 CrossRef PubMed.
- S. Lim, H. Moon, D. Kim and O.-S. Jung, Dalton Trans., 2021, 50, 14320 RSC.
- T. Zhang, L.-P. Zhou, X.-Q. Guo, L.-X. Cai and Q.-F. Sun, Nat. Commun., 2017, 8, ncomms15898 CrossRef PubMed.
- J. Tessarolo, H. Lee, E. Sakuda, K. Umakoshi and G. H. Clever, J. Am. Chem. Soc., 2021, 143, 6339 CrossRef CAS PubMed.
- A. Kumar, R. Banerjee, E. Zangrando and P. S. Mukherjee, Inorg. Chem., 2022, 61, 2368 CrossRef CAS PubMed.
- K. Wu, E. Benchimol, A. Baksi and G. H. Clever, Nat. Chem., 2024, 16, 584 CrossRef CAS PubMed.
- M. D. Wise, J. J. Holstein, P. Pattison, C. Besnard, E. Solari, R. Scopelliti, G. Bricogne and K. Severin, Chem. Sci., 2015, 6, 1004 RSC.
- S. M. Jansze, G. Cecot, M. D. Wise, K. O. Zhurov, T. K. Ronson, A. M. Castilla, A. Finelli, P. Pattison, E. Solari, R. Scopelliti, G. E. Zelinskii, A. V. Vologzhanina, Y. Z. Voloshin, J. R. Nitschke and K. Severin, J. Am. Chem. Soc., 2016, 138, 2046 CrossRef CAS PubMed.
- C. Klein, C. Gütz, M. Bogner, F. Topić, K. Rissanen and A. Lützen, Angew. Chem., Int. Ed., 2014, 53, 3739 CrossRef CAS PubMed.
- K. Suzuki, M. Tominaga, M. Kawano and M. Fujita, Chem. Commun., 2009, 1638 RSC.
- E. O. Bobylev, D. A. Poole, B. de Bruin and J. N. H. Reek, J. Am. Chem. Soc., 2022, 144, 15633 CrossRef CAS PubMed.
- C. Gütz, R. Hovorka, C. Klein, Q. Jiang, C. Bannwarth, M. Engeser, C. Schmuck, W. Assenmacher, W. Mader, F. Topić, K. Rissanen, S. Grimme and A. Lützen, Angew. Chem., Int. Ed., 2014, 53, 1693 CrossRef PubMed.
- B. Roy, R. Saha, A. K. Ghosh, Y. Patil and P. S. Mukherjee, Inorg. Chem., 2017, 56, 3579 CrossRef CAS PubMed.
- M. Frank, M. D. Johnstone and G. H. Clever, Chem.–Eur. J., 2016, 22, 14104 CrossRef CAS PubMed.
- M. Fukuda, R. Sekiya and R. Kuroda, Angew. Chem., Int. Ed., 2008, 47, 706 CrossRef CAS PubMed.
- S. Freye, J. Hey, A. Torras-Galán, D. Stalke, R. Herbst-Irmer, M. John and G. H. Clever, Angew. Chem., Int. Ed., 2012, 51, 2191 CrossRef CAS PubMed.
- T. R. Schulte, M. Krick, C. I. Asche, S. Freye and G. H. Clever, RSC Adv., 2014, 4, 29724 RSC.
- M. Frank, J. Hey, I. Balcioglu, Y. Chen, D. Stalke, T. Suenobu, S. Fukuzumi, H. Frauendorf and G. H. Clever, Angew. Chem., Int. Ed., 2013, 52, 10102 CrossRef CAS PubMed.
- T. R. Schulte, J. J. Holstein and G. H. Clever, Angew. Chem., Int. Ed., 2019, 58, 5562 CrossRef CAS PubMed.
- S. Freye, R. Michel, D. Stalke, M. Pawliczek, H. Frauendorf and G. H. Clever, J. Am. Chem. Soc., 2013, 135, 8476 CrossRef CAS PubMed.
- S. Watanabe, M. Yamashina, E. Tsurumaki and S. Toyota, ChemistryEurope, 2023, 1, e202300047 CrossRef.
- R. Zhu, J. Lübben, B. Dittrich and G. H. Clever, Angew. Chem., Int. Ed., 2015, 54, 2796 CrossRef CAS PubMed.
- J. I. Virtue, S. Tsoukatos, M. R. Johnston and W. M. Bloch, Chem. Sci., 2024, 15, 19119 RSC.
- T. Abe, Y. Zhang, K. Takeuchi and S. Hiraoka, Chem, 2025, 11, 102453 Search PubMed.
- Y. Yamauchi, M. Yoshizawa and M. Fujita, J. Am. Chem. Soc., 2008, 130, 5832 CrossRef CAS PubMed.
- T. K. Ronson, Y. Wang, K. Baldridge, J. S. Siegel and J. R. Nitschke, J. Am. Chem. Soc., 2020, 142, 10267 CrossRef CAS PubMed.
- P. Montes-Tolentino, A. S. Mikherdov, C. Drechsler, J. J. Holstein and G. H. Clever, Angew. Chem., Int. Ed., 2025, 64, e202423810 CrossRef CAS PubMed.
- D. M. Engelhard, S. Freye, K. Grohe, M. John and G. H. Clever, Angew. Chem., Int. Ed., 2012, 51, 4747 CrossRef CAS PubMed.
- S. Sudan, F. Fadaei-Tirani, R. Scopelliti, K. E. Ebbert, G. H. Clever and K. Severin, Angew. Chem., Int. Ed., 2022, 61, e202201823 CrossRef CAS PubMed.
- C. Hu, D. W. Chen, S. Sudan and K. Severin, Chem. Sci., 2025, 16, 5559 RSC.
- M. Käseborn, J. J. Holstein, G. H. Clever and A. Lützen, Angew. Chem., Int. Ed., 2018, 57, 12171 CrossRef PubMed.
- W. M. Bloch, J. J. Holstein, B. Dittrich, W. Hiller and G. H. Clever, Angew. Chem., Int. Ed., 2018, 57, 5534 CrossRef CAS PubMed.
- W. M. Bloch and G. H. Clever, Chem. Commun., 2017, 53, 8506 RSC.
- M. Frank, L. Krause, R. Herbst-Irmer, D. Stalke and G. H. Clever, Dalton Trans., 2014, 43, 4587 RSC.
- M. Yoshizawa, M. Nagao, K. Kumazawa and M. Fujita, J. Organomet. Chem., 2005, 690, 5383 CrossRef CAS.
- A. K. Bar, G. Mostafa and P. S. Mukherjee, Inorg. Chem., 2010, 49, 7647 CrossRef CAS PubMed.
- A. K. Bar, S. Raghothama, D. Moon and P. S. Mukherjee, Chem.–Eur. J., 2012, 18, 3199 CrossRef CAS PubMed.
- D. Samanta, S. Shanmugaraju, S. A. Joshi, Y. P. Patil, M. Nethaji and P. S. Mukherjee, Chem. Commun., 2012, 48, 2298 RSC.
- D. Preston, J. E. Barnsley, K. C. Gordon and J. D. Crowley, J. Am. Chem. Soc., 2016, 138, 10578 CrossRef CAS PubMed.
- A. M. Johnson and R. J. Hooley, Inorg. Chem., 2011, 50, 4671 CrossRef CAS PubMed.
- R. Li, F. Fadaei-Tirani, R. Scopelliti and K. Severin, Chem.–Eur. J., 2021, 27, 9439 CrossRef CAS PubMed.
- B. Chen, J. J. Holstein, A. Platzek, L. Schneider, K. Wu and G. H. Clever, Chem. Sci., 2022, 13, 1829 RSC.
- P. J. Stang and B. Olenyuk, Acc. Chem. Res., 1997, 30, 502 CrossRef CAS.
- J.-R. Li and H.-C. Zhou, Nat. Chem., 2010, 2, 893 CrossRef CAS PubMed.
- Q. Sun, S. Sato and M. Fujita, Angew. Chem., Int. Ed., 2014, 53, 13510 CrossRef CAS PubMed.
- S. Saha, B. Holzapfel, Y.-T. Chen, K. Terlinden, P. Lill, C. Gatsogiannis, H. Rehage and G. H. Clever, J. Am. Chem. Soc., 2018, 140, 17384 CrossRef CAS PubMed.
- K. Wu, J. Tessarolo, A. Baksi and G. H. Clever, Angew. Chem., Int. Ed., 2022, 61, e202205725 CrossRef CAS PubMed.
- J. Tessarolo, E. Benchimol, A. Jouaiti, M. W. Hosseini and G. H. Clever, Chem. Commun., 2023, 59, 3467 RSC.
- K. Wu, B. Zhang, C. Drechsler, J. J. Holstein and G. H. Clever, Angew. Chem., Int. Ed., 2021, 60, 6403 CrossRef CAS PubMed.
- A. Platzek, S. Juber, C. Yurtseven, S. Hasegawa, L. Schneider, C. Drechsler, K. E. Ebbert, R. Rudolf, Q. Yan, J. J. Holstein, L. V. Schäfer and G. H. Clever, Angew. Chem., Int. Ed., 2022, 61, e202209305 CrossRef CAS PubMed.
- A. Tarzia, W. Shan, V. Posligua, C. J. T. Cox, L. Male, B. D. Egleston, R. L. Greenaway, K. E. Jelfs and J. E. M. Lewis, Chem.–Eur. J., 2025, 31, e202403336 CrossRef CAS PubMed.
- K. E. Ebbert, F. Sendzik, L. Neukirch, L. Eberlein, A. Platzek, P. Kibies, S. M. Kast and G. H. Clever, Angew. Chem., Int. Ed., 2024, 64, e202416076 CrossRef PubMed.
- T. Abe, N. Sanada, K. Takeuchi, A. Okazawa and S. Hiraoka, J. Am. Chem. Soc., 2023, 145, 28061 CrossRef CAS PubMed.
- R.-J. Li, J. Tessarolo, H. Lee and G. H. Clever, J. Am. Chem. Soc., 2021, 143, 3865 CrossRef CAS PubMed.
- W.-T. Dai, T.-T. Liu, Q. Bai, Z. Zhang, P. Wang, W. Lu and Q. Zhang, Sci. China Chem., 2024, 67, 4110 CrossRef CAS.
- Z. Guo, H. Yu, J. Shi, N. Han, G. Wu, H. Zhang, B. Li and M. Wang, Angew. Chem., Int. Ed., 2025, 64, e202425369 CrossRef CAS PubMed.
- Y. Liu, S. Liao, W. Dai, Q. Bai, S. Lu, H. Wang, X. Li, Z. Zhang, P. Wang, W. Lu and Q. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202217215 CrossRef CAS PubMed.
- E. Benchimol, I. Regeni, B. Zhang, M. Kabiri, J. J. Holstein and G. H. Clever, J. Am. Chem. Soc., 2024, 146, 6905 CrossRef CAS PubMed.
- S. Prusty, S. Krishnaswamy, S. Bandi, B. Chandrika, J. Luo, J. S. McIndoe, G. S. Hanan and D. K. Chand, Chem.–Eur. J., 2015, 21, 15174 CrossRef CAS PubMed.
- S. Prusty, K. Yazaki, M. Yoshizawa and D. K. Chand, Chem.–Eur. J., 2017, 23, 12456 CrossRef CAS PubMed.
- P. Howlader, P. Das, E. Zangrando and P. S. Mukherjee, J. Am. Chem. Soc., 2016, 138, 1668 CrossRef CAS PubMed.
- S. Sudan, R.-J. Li, S. M. Jansze, A. Platzek, R. Rudolf, G. H. Clever, F. Fadaei-Tirani, R. Scopelliti and K. Severin, J. Am. Chem. Soc., 2021, 143, 1773 CrossRef CAS PubMed.
- S. Samantray, S. Krishnaswamy and D. K. Chand, Nat. Commun., 2020, 11, 880 CrossRef CAS PubMed.
- P. Molinska, A. Tarzia, L. Male, K. E. Jelfs and J. E. M. Lewis, Angew. Chem., Int. Ed., 2023, 62, e202315451 CrossRef CAS PubMed.
- R. A. S. Vasdev, D. Preston, C. A. Casey-Stevens, V. Martí-Centelles, P. J. Lusby, A. L. Garden and J. D. Crowley, Inorg. Chem., 2023, 62, 1833 CrossRef CAS PubMed.
- S. K. Sen and R. Natarajan, Inorg. Chem., 2019, 58, 7180 CrossRef CAS PubMed.
- B. E. Barber, E. M. G. Jamieson, L. E. M. White and C. T. McTernan, Chem, 2024, 10, 2792 CAS.
- D. F. Brightwell, K. Samanta, J. Muldoon, P. C. Fleming, Y. Ortin, L. Mardiana, P. G. Waddell, M. J. Hall, E. R. Clark, F. Fantuzzi and A. Palma, ChemistryEurope, 2025, 3, e202400050 CrossRef CAS.
- O. Jurček, P. Bonakdarzadeh, E. Kalenius, J. M. Linnanto, M. Groessl, R. Knochenmuss, J. A. Ihalainen and K. Rissanen, Angew. Chem., Int. Ed., 2015, 54, 15462 CrossRef PubMed.
- O. Jurček, Nonappa, E. Kalenius, P. Jurček, J. M. Linnanto, R. Puttreddy, H. Valkenier, N. Houbenov, M. Babiak, M. Peterek, A. P. Davis, R. Marek and K. Rissanen, Cell Rep. Phys. Sci., 2021, 2, 100303 CrossRef.
- O. Jurček, S. Chattopadhyay, E. Kalenius, J. M. Linnanto, A. Kiesilä, P. Jurček, P. Radiměřský and R. Marek, Angew. Chem., Int. Ed., 2024, 63, e202409134 CrossRef PubMed.
- J. E. M. Lewis, A. Tarzia, A. J. P. White and K. E. Jelfs, Chem. Sci., 2020, 11, 677 RSC.
- H. Yu, J. Li, C. Shan, T. Lu, X. Jiang, J. Shi, L. Wojtas, H. Zhang and M. Wang, Angew. Chem., Int. Ed., 2021, 60, 26523 CrossRef CAS PubMed.
- A. Tarzia, J. E. M. Lewis and K. E. Jelfs, Angew. Chem., Int. Ed., 2021, 60, 20879 CrossRef CAS PubMed.
- D. Ogata and J. Yuasa, Angew. Chem., Int. Ed., 2019, 58, 18424 CrossRef CAS PubMed.
- S. S. Mishra, S. V. K. Kompella, S. Krishnaswamy, S. Balasubramanian and D. K. Chand, Inorg. Chem., 2020, 59, 12884 CrossRef CAS PubMed.
- S. S. Mishra and D. K. Chand, Dalton Trans., 2022, 51, 11650 RSC.
- J. E. M. Lewis, Chem.–Eur. J., 2021, 27, 4454 CrossRef CAS PubMed.
- M. Parbin, V. Sivalingam and D. K. Chand, Angew. Chem., Int. Ed., 2024, 63, e202410219 CrossRef CAS PubMed.
- R.-J. Li, A. Marcus, F. Fadaei-Tirani and K. Severin, Chem. Commun., 2021, 57, 10023 RSC.
- J. de Montmollin, A. B. Solea, D. W. Chen, F. Fadaei-Tirani and K. Severin, Inorg. Chem., 2024, 63, 4583 CrossRef CAS PubMed.
- P. Molinska, L. Male and J. E. M. Lewis, ChemRxiv, 2024, DOI:10.26434/chemrxiv-2024-0thnh.
- Z. T. Avery, M. G. Gardiner and D. Preston, Angew. Chem., Int. Ed., 2025, 64, e202418079 CrossRef CAS PubMed.
- L.-J. Chen, H.-B. Yang and M. Shionoya, Chem. Soc. Rev., 2017, 46, 2555 RSC.
- L. M. Mesquita, J. Anhäuser, D. Bellaire, S. Becker, A. Lützen and S. Kubik, Org. Lett., 2019, 21, 6442 CrossRef CAS PubMed.
- X. Tang, H. Jiang, Y. Si, N. Rampal, W. Gong, C. Cheng, X. Kang, D. Fairen-Jimenez, Y. Cui and Y. Liu, Chem, 2021, 7, 2771 CAS.
- D. R. Martir, L. Delforce, D. B. Cordes, A. M. Z. Slawin, S. L. Warriner, D. Jacquemin and E. Zysman-Colman, Inorg. Chem. Front., 2020, 7, 232 RSC.
- D. R. Martir, D. Escudero, D. Jacquemin, D. B. Cordes, A. M. Z. Slawin, H. A. Fruchtl, S. L. Warriner and E. Z. Colman, Chem.–Eur. J., 2017, 23, 14358 CrossRef PubMed.
- C. Gütz, R. Hovorka, G. Schnakenburg and A. Lützen, Chem.–Eur. J., 2013, 19, 10890 CrossRef PubMed.
- S. Ghorai, S. Maji, B. Paul, K. Samanta, S. K. Sen and R. Natarajan, Chem.–Asian J., 2023, 18, e202201312 CrossRef CAS PubMed.
- S. Ghorai and R. Natarajan, Chem.–Eur. J., 2023, 29, e202203085 CrossRef CAS PubMed.
- C. Ma, Q. Cao, L. Yu, Z. Ma and Q. Gan, Angew. Chem., Int. Ed., 2024, 63, e202410731 CrossRef CAS PubMed.
- R.-J. Li, J. de Montmollin, F. Fadaei-Tirani, R. Scopelliti and K. Severin, Construction of Pd-based coordination cages with three geometrically distinct ligands, Dalton Trans., 2023, 52, 6451–6456 RSC.
| This journal is © The Royal Society of Chemistry 2025 |