Open Access Article
Open Access ArticleTwo photon-responsive gold nanocapsules enable targeted photothermal hyperthermia of chemoresistant melanoma: injection-route-dependent efficacy and renal evidence of fragment clearance†
Paula Zamora-Pérez‡
a,
Qiutian She‡a,
Harrisson D. Santosb,
Jose J. Conesac,
M. Carmen Iglesias de la Cruzb,
Nuria Fernándezb,
Daniel Jaque b and
Pilar Rivera-Gil
b and
Pilar Rivera-Gil *a
*a
aIntegrative Biomedical Materials and Nanomedicine Lab, Department of Medicine and Life Sciences (MELIS), Pompeu Fabra University, PRBB, Carrer Doctor Aiguader 88, 08003 Barcelona, Spain. E-mail: pilar.rivera@upf.edu
bFluorescence Imaging Group, Departamento de Física de Materiales, Universidad Autónoma de Madrid, C/Francisco Tomás y Valiente 7, 28049 Madrid, Spain
cMistral Beamline, Experiment Division, ALBA Synchrotron (ALBA-CELLS), Barcelona, Spain
First published on 17th July 2025
Abstract
Melanoma is a highly aggressive skin cancer that often develops resistance to chemotherapy, underscoring the need for new treatment strategies. Here we evaluate plasmonic gold nanocapsules (AuNCs) as photoresponsive agents for two-photon luminescence-assisted photothermal therapy in chemoresistant melanoma models. The performance of the AuNCs was assessed in two-dimensional cell cultures, three-dimensional paclitaxel-resistant B16-F10 melanoma spheroids, and a subcutaneous melanoma mouse model under near-infrared excitation. In vitro, AuNCs alone exhibited no cytotoxicity, but under 830 nm two-photon excitation, they produced strong two-photon luminescence and thermal effects that increased with nanocapsule concentration and laser power. This led to transient oxidative stress, apoptosis induction, and effective melanoma cell ablation under optimal conditions (80 μg mL−1 AuNCs, 12 mW laser power). In vivo, the route of nanoparticle administration proved decisive. A single 4-min 806 nm irradiation after intratumoral injection uniformly heated the lesion (≈45–50 °C), yielded durable tumour eradication, and sequestered >99% of detected gold in the necrotic scab, with only trace renal clearance. In contrast, the same laser fluence after peritumoral injection generated a superficial hot rim, spared the tumour core, allowing eventual regrowth, and left ∼65% of the injected gold systemically redistributed, mainly in the spleen and liver. These findings highlight the potential of AuNCs as potent, image-guided photothermal agents for chemoresistant melanoma, offering targeted tumor destruction with limited systemic exposure. They reveal the injection route is a critical determinant of both therapeutic success and nanoparticle biodistribution.
Introduction
Cutaneous malignant melanoma accounts for ≤1% of skin cancers yet it is responsible for most related deaths. Globocan 2022 recorded 331![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 722 new cases and 58
722 new cases and 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 667 deaths worldwide.1 Independent burden of disease studies confirm the rising incidence while attributing a substantial proportion to ultraviolet radiation exposure.2–4 Surgical excision cures most stage I tumors, but once distant metastases develop, the 5-year survival rate falls below 15%.5 Immune-checkpoint inhibitors (ICIs) targeting CTLA-4, PD-1 and, more recently, the LAG-3 antibody relatlimab in combination with nivolumab, have revolutionized melanoma therapy.6,7 Furthermore, autologous tumor-infiltrating lymphocyte therapy has entered the clinic.8 Nevertheless, primary or acquired resistance occurs in ∼50% of patients and immune-related adverse events can be severe.9,10 For tumors harboring BRAF-V600 mutations, combined BRAF/MEK inhibition achieves high initial responses, but durable remissions remain uncommon and systemic toxicity limits chronic use.9 Chemotherapy and external-beam radiotherapy remain largely palliative because melanoma is intrinsically chemo- and radio-resistant. These limitations demand tumor-selective local treatments that eradicate residual disease while synergizing with systemic immunity.
667 deaths worldwide.1 Independent burden of disease studies confirm the rising incidence while attributing a substantial proportion to ultraviolet radiation exposure.2–4 Surgical excision cures most stage I tumors, but once distant metastases develop, the 5-year survival rate falls below 15%.5 Immune-checkpoint inhibitors (ICIs) targeting CTLA-4, PD-1 and, more recently, the LAG-3 antibody relatlimab in combination with nivolumab, have revolutionized melanoma therapy.6,7 Furthermore, autologous tumor-infiltrating lymphocyte therapy has entered the clinic.8 Nevertheless, primary or acquired resistance occurs in ∼50% of patients and immune-related adverse events can be severe.9,10 For tumors harboring BRAF-V600 mutations, combined BRAF/MEK inhibition achieves high initial responses, but durable remissions remain uncommon and systemic toxicity limits chronic use.9 Chemotherapy and external-beam radiotherapy remain largely palliative because melanoma is intrinsically chemo- and radio-resistant. These limitations demand tumor-selective local treatments that eradicate residual disease while synergizing with systemic immunity.
Photothermal therapy (PTT) offers an orthogonal physical modality that converts NIR light into cytotoxic heat localized to the tumor, while priming systemic antitumor immunity through immunogenic cell death. Hyperthermia kills cancer cells by raising the tumor temperature above a physiological level.11,12 Heat disrupts the endoplasmic reticulum and mitochondria homeostasis, drives reactive oxygen species (ROS) production and elicits ROS-driven apoptosis and ER stress-mediated cell death.13,14 Because melanomas already harbour elevated ROS due to melanin synthesis,15 an acute ROS surge is particularly cytotoxic. Local heat further boosts antitumour immunity by releasing tumor-associated antigens, promoting immunogenic cell death (ICD),16,17 improving blood flow and vascular permeability, and recruiting immune effector cells.18 Conventional “outside-in” heating with radiofrequency or microwave applicators produces centimeter-scale isotherms that can harm adjacent healthy tissue, limiting its clinical utility.19–22 Nanotechnology allows an “inside-out” approach: stimuli-responsive nanoparticles accumulate in the tumor and convert external physical energy (light, alternating magnetic fields, ultrasound or radiofrequency) into heat with sub-millimeter precision.20 Gold–silica nanoshells provided the first proof-of-concept for in vivo nPTT two decades ago23 and inspired a broad range of other photothermal nanomaterials such as semiconducting polymers,24 copper sulfide,25 FDA-listed Prussian-blue,26 and black-phosphorus quantum dots.27 Among the available nanoplatforms, gold nanoparticles,28,29 iron-oxide,30 or silica are commonly used in hyperthermia and stand out due to their biocompatibility and powerful photothermal performance.31–33
Regarding melanoma treatment, the most suitable approach for external small superficial lesions is NIR-induced NP-assisted PTT (nPTT),28,34,35 while deeper lesions may benefit from radiofrequency, magnetic or ultrasound heating.20 AuNPs are the best candidates for nPTT because of their facile and controlled synthesis, easy optical property tunability, targetability36 and biofunctionalization, loading capabilities, cellular uptake, and safe biological profile.37–42 At the nanoscale, collective surface-electron oscillations give rise to localized surface plasmon resonance (LSPR).43–45 Spectroscopy tracking of the LSPR predicts photothermal efficiency.46,47 Excitation in the “biological” NIR window (700–980 nm) maximizes tissue penetration, while two-photon excitation (2PE) of AuNPs additionally generates intrinsic luminescence for image-guided therapy.48–50 Besides, AuNPs can induce ROS-mediated cell death for photodynamic therapy (PDT).29,51–56 Singh et al. developed NIR-II-active, capsule-shaped, and rattle-like bimetallic nanoparticles, which exhibited excellent photothermal efficiency at low power density by using an NIR-II laser (1064 nm), affirming the photothermal efficacy of nanocapsules.31 Li et al. designed carbon–silica nanocapsules with gold nanoparticles inside the cavity. These multifunctional nanocapsules exhibiting excellent photothermal conversion and good biocompatibility achieved a synergistic effect between photothermal- and chemo-therapy.32 Grabowska-Jadach et al. reported plasmonic hollow gold nanoshells modified with aptamers selective towards nucelolin for the photothermal treatment of skin cancer. Their hollow gold nanoshells exhibited good water solubility, colloidal stability during modification and purification stages, as well as an LSPR band in the NIR region.33
Mechanistic studies show that plasmonic heat triggers membrane disruption, protein denaturation and oxidative stress57 and that sub-lethal hyperthermia promotes ICD, augmenting antigen presentation and synergizing with PD-1 blockade in melanoma models.58,59 Early clinical trials have confirmed the safety of nPTT in prostate (silica–gold nanoshells; NCT02680535).19 This trial demonstrates the clinical feasibility of intratumoral nanoparticle delivery and NIR laser parameters comparable to ours.
We previously engineered hollow ∼400 nm gold nanocapsules (AuNCs) by layer-by-layer deposition of polyelectrolytes and gold seed on silica templates followed by core dissolution and gold growth.29,60–62 The plasmonic surface exhibits broadband extinction across the first NIR window and converts 830 nm 2PE into heat with ∼37% efficiency, outperforming mass-matched nanorods or nanostars. Their internal cavity can host diagnostic or therapeutic cargoes, providing true theranostic capacity, while intrinsic two-photon luminescence (2PL) enables μm-scale tracking under clinically permissible NIR fluences. Importantly, their synthesis uses clinical-grade reagents and yields endotoxin-free dispersions, facilitating translation.
Here we exploit the dual imaging and heating functionality of AuNCs to eradicate multi-drug-resistant melanoma. In paclitaxel-resistant B16-F10 spheroids, a single 10 min 2PE pulse (830 nm, 12 mW) delivered with 80 μg mL−1 AuNCs generates abundant ROS, perforates the plasma membrane, and kills >90% of cells, whereas AuNCs or laser alone are innocuous.29 Cryo-soft X-ray tomography confirms AuNC fragmentation and μm-scale membrane pores, consistent with inside-out-hyperthermia. Translating these parameters to murine B16-F10 xenografts, a single 4-min 806 nm exposure following intratumoral (IT) AuNC delivery raises the entire lesion to a temperature of 45°–50 °C, achieves complete remission in all animals, and confines >99% of the injected gold within the necroric scab that is later shed. In contrast, peritumoral (PT) injection yields a superficial “hot rim”, leaves the core viable, allows regrowth, and redistributes ≈65% of the AuNCs to the liver and spleen. Thus, the injection route critically determines both therapeutic outcome and systemic nanoparticle burden.
Importantly, longitudinal studies are beginning to clarify the fate of residual gold and its immunological footprint. Dextran-coated 21 nm AuNPs persist in murine liver and spleen for at least two weeks yet cause no histopathological alterations or elevations in serum transaminases, creatinine or IL-6, arguing against chronic hepato- or nephron-toxicity.63 A comparative toxicology screen in C57BL/6 mice found that AuNPs transiently elevate IL-6 and TNF-α and expand memory CD8+ T-cells within 2 h, but all cytokine and activation markers return to baseline by 96 h, suggesting a self-limited innate response with potential adjuvant benefit.64 Particle engineering can further mitigate organ retention: glutathione-protected ultrasmall Au(25) nanoclusters achieve 36% renal clearance within 24 h and >90% overall elimination by 28 days, whereas albumin-coated analogues retain >95% of gold in mononuclear-phagocytic system organs and prolong cytokine activation.65 Notably, our IT protocol sequesters >99% of AuNCs in the desquamated eschar, virtually abolishing off-target accumulation. Collectively, these data support a broad therapeutic window and indicate that residual gold deposits are largely immunologically inert, or even mildly immunostimulatory, when particle size, surface chemistry and administration route are optimized.
AuNC-mediated nPTT therefore emerges as a single session, image-guided strategy for hyperthermia eradication of drug-resistant melanoma while minimizing systemic, off-target exposure. Given its capacity for cargo loading, the platform provides a rational foundation for combining local hyperthermia with ICIs or encapsulated chemotherapeutics, and the mechanistic insights obtained here will inform the broader clinical translation of IT nanomedicines.
Experimental
Synthesis and characterization of gold nanocapsules
Rhodamine B-loaded AuNCs (RhoB-AuNCs) were synthesized using the same LbL protocol described above, with the modification of employing a fluorescent PS template. The fluorescent PS spheres were prepared by radical polymerization. Briefly, 0.15 g of polyvinylpyrrolidone (PVP, MW 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kDa) and 0.128 g of 2,2′-azobis[2-(2-imidazolin-2-yl)propane]dihydrochloride (AIBA) were dissolved in 50 mL of argon-purged Milli-Q water. The solution was heated to 70 °C under reflux. After 5 g of styrene monomer was added, 15 mg of rhodamine B (Sigma-Aldrich) was introduced 5 minutes later, and the polymerization was allowed to proceed for 24 hours. The resulting fluorescent particles were collected by centrifugation at 9500 rpm for 30 minutes and washed three times with Milli-Q water. More details of the procedure are included in our previously published work.36
000 kDa) and 0.128 g of 2,2′-azobis[2-(2-imidazolin-2-yl)propane]dihydrochloride (AIBA) were dissolved in 50 mL of argon-purged Milli-Q water. The solution was heated to 70 °C under reflux. After 5 g of styrene monomer was added, 15 mg of rhodamine B (Sigma-Aldrich) was introduced 5 minutes later, and the polymerization was allowed to proceed for 24 hours. The resulting fluorescent particles were collected by centrifugation at 9500 rpm for 30 minutes and washed three times with Milli-Q water. More details of the procedure are included in our previously published work.36
The thermographic measurements of AuNCs in solution were performed under 806 nm excitation with a single-photon laser and images were recording with a FLIR thermocamera. For more details on the physicochemical properties of the NCs, please check our previous publication.29
| Laser | Purpose (figures) | λ (nm) | Regime | Beam/scan geometry | Average power at sample | Key dosimetry parameters* |
|---|---|---|---|---|---|---|
| Ti:Sa pulsed (Spectra-Physics MaiTai eHP DeepSee on Leica SP5): | 2 PE imaging/in vitro nPTT (Fig. 2 and 3 and Fig. S1C, S2, S5, S6, ESI†) | 830 | 80 fs, 80 MHz | Diffraction-limited focal spot, d = 0.264 μm (NA = 1.1 water). Raster-scanned 512 × 512 frame, 2 μs pixel dwell, 60 s total | 3–12 mW | Instantaneous focal irradiance (I, W cm−2) = (5.5–22) × 106 |
| Pulse energy = 38–150 pJ; peak power (Ppeak) = 0.47–1.9 kW | ||||||
| Time-averaged voxel irradiance (after duty-cycle ≈3.8 × 10−6) = 21–84 W cm−2 | ||||||
| Voxel dose over 60 s = 1–5 kJ cm−2 | ||||||
| Diode (Lumics LU0806T040) | Photothermal efficiency curves (Fig. 1D and Fig. S8 right, ESI†) | 806 | Continuous wave (CW) | 3 mm collimated beam on cuvette | 30 mW (1 W cm−2) | (1 W cm−2; 6 min) → 360 J cm−2 |
| CW diode (Lumics LU0806T040) | In vivo nPTT (Fig. 4 and Fig. S7, S8 left, S9, S10,ESI†) | 806 | CW | 15 mm defocused spot on tumour | 1.8 W (1 W cm−2) | (1 W cm−2; 4 min) → 240 J cm−2 |
830 nm (2PE, in vitro) and 806 nm (single-photon, AuNCs bulk and in vivo) lie on the same broadband LSPR shoulder of AuNCs, and no change in extinction is observed between 800 and 840 nm (Fig. 1C).
Photothermal efficiency
The photothermal efficiency (η) of AuNCs under laser irradiation at 806 nm was obtained by applying the Wang model. In this model a certain mass (M) of the solution containing the photothermal agents is excited with an infrared (806 nm) laser. The time evolution of the solution temperature is measured until a steady state is achieved and the temperature increment (ΔT) is determined. Then, the heating laser is tuned off and the thermal relaxation of the solution is recorded to determine its thermal relaxation time (τs). In a first order approximation the laser-to-heat conversion efficiency is given by:68
 | (1) |
Cell culture
For cellular studies, B16-F10 mouse melanoma adherent cells were cultured in high glucose (4500 mg L−1) Dulbecco's modified Eagle's medium (Gibco), supplemented with 1% penicillin–streptomycin (Hyclone) and 10% fetal bovine serum, FBS (Sigma Aldrich). Cells were cultured at 37 °C under a wet, modified atmosphere with 5% CO2. B16-F10 melanoma spheroids obtained from serum-free B16-F10 adherent cells were seeded at a concentration of 750 cells per well in low-attachment 96-well plates (Corning) and cultured for 5 days in a supplemented Mammocult Human Medium Kit (STEMCELL technologies).29 From the maximum projections, ROIs for each individual spheroid were performed.In vitro characterization: Cell viability and cellular sensitivity to paclitaxel
Paclitaxel (PTX) was dissolved in ethanol, before mixing it with the cell medium. We prepared a PTX working solution of 50 μM.(i) Cells were cultured as before. Trypan Blue staining was used to count (Countess™, C10228, Invitrogen™) the cells and to differentiate between live and dead cells in suspension. Live cells are impermeant to trypan blue and only enter the cell if the plasma membrane is damaged, thus being an indicator for cellular toxicity. Cells were treated for 24 h with PTX at different concentrations, 0.1–100 μM. As a control for viability, we used untreated cells. Then, we collected the cells by trypsinization of the adherent cells. We realized there were dead cells floating in the medium, therefore we also collected the medium from each well of the plate. Dead cells were stained with trypan blue (10 μL was added to 10 μL of cell medium). The viability was measured in % of the total amount of cells.
(ii) B16-F10 melanoma spheroids were incubated with PTX and AuNCs. Cell viability was measured using CytoCalcein violet 450 and propidium iodide, the total counts for each spheroid were obtained dividing the signal by the area of the spheroid. Mean ± SD were provided. ROScyt and apoptosis studies were performed in spheroids exposed to 30 μg mL−1 of AuNCs for 24 h, and treated with 6 μM CellROX deep red (Invitrogen) and with 10 μM apopxin (Abcam) for 1 h.
Meanwhile we sterilized an aliquot of the NC dispersion. The aliquot was centrifuged at 8500 rpm for 30 minutes. After centrifugation the supernatant was removed, the same amount of sterile Milli-Q water was added and a sterile new Eppendorf was used. The entire process was repeated 3 times. The cells were incubated with 80 μg mL−1 NCs for 24 h and the viability was measured with trypan blue as described before.
Imaging was performed with a Leica SP5 confocal microscope using a 40× water immersion objective. The images of the tumor spheres were acquired with an Andor Dragonfly 500 spinning disk confocal microscope using a 40× water immersion objective. A region of interest (ROI) for each spheroid was obtained in the maximum projection, and the cell viability was calculated considering the total count per spheroid of propidium iodide and CytoCalcein violet 450 signals divided by the area of the spheroid obtained with FIJI. ROS and apoptosis studies were performed using 3D melanoma spheroids grown for 5 days; the spheroids were exposed for 24 h to 30 μg mL−1 of AuNCs, treated with 6 μM CellROX green and/or CellROX deep red (Invitrogen) with 10 μM apopxin (Abcam), and incubated for 1 h.
In vitro nPTT
The treatment of chemoresistant B16 melanoma spheroids was performed with a titanium sapphire multiphoton laser coupled to a Leica SP5 confocal microscope (830 nm) (see the laser set up section above).The cryoSXT study was performed in B16 melanoma adherent cells seeded on Au-EM grids coated with holey carbon (R 2/2; Quantifoil) controlled so that 1–3 cells per grid square were obtained. Then, the cells were incubated for 24 h at 37 °C in a wet atmosphere and 5% CO2 before adding the AuNCs at a concentration of 30 μg mL−1. After that, the grids with control and AuNC-treated cells were incubated for 10 min with LysoTracker green (1000×), washed with PBS 1x and vitrified by plunge-freezing them using a Leica EM GP unit. The samples were maintained in liquid nitrogen until visualization. Samples were imaged in cryo-conditions first using an epifluorescence microscope (integrated in the X-ray microscope) and afterwards with a soft X-ray microscope (at the Mistral beamline, ALBA). Based on the FL signal, the cell candidates were selected. Then, 2D mosaics were acquired to assess sample quality and the best candidates were acquired at a photon energy of 520 eV from −65° to 65° at 1° intervals (exposure time: 1–3 seconds). The tilt series of images were normalized with the XMIPP 3 software package,70 aligned and reconstructed using IMOD.71 For a more detailed protocol read the work by Chiappi et al. 2016.72
Synchrotron cryo-soft X-ray microscopy
A CryoSXT study was performed in B16-F10 melanoma adherent cells seeded onto Au-EM grids coated with holey carbon (R 2/2; Quantifoil) so that 1–3 cells per grid square were obtained. The cells were then incubated for 24 h at 37 °C in a wet atmosphere and 5% CO2 before adding the AuNCs at a concentration of 30 μg mL−1. When necessary, the cells were irradiated with an 806 nm laser at 3 mW. Subsequently, the grids with control and AuNC-treated cells were incubated for 10 min with LysoTracker green (1000×), washed with PBS 1×, and vitrified by plunge-freezing using a Leica EM GP unit. The samples were maintained in liquid nitrogen until visualization. The samples were imaged under cryo-conditions first with an epifluorescence microscope (integrated in the X-ray microscope) and then with a transmission soft X-ray microscope (Mistral Beamline, ALBA synchrotron). Based on the FL signal, the cell candidates were selected. Next, 2D images mosaics were acquired to locate the areas for tilt series collection (−65° to 65° at 1° intervals and exposure time per image 1–3 s) at 520 eV photon energy. The tilt series were normalized with the XMIPP 3 software package,70 and aligned and reconstructed using IMOD.70,71 Five cells per condition at the minimum were considered for the analysis. A more detailed protocol is provided in ref. 72.In vivo nPTT studies
All the experiments were conducted in accordance with the European Union directives 63/2010UE and Spanish regulation RD 53/2013. The use of these animals was also approved by the Animal Ethics Committee of the Universidad Autónoma de Madrid. We used 6 to 12-week-old female C57BL/6 mice to induce melanoma, a well-established and widely used tumor model.73 Melanoma tumors were induced by subcutaneous inoculation of 4 million B16-F10 melanoma cells. The measurements of the tumor volume were obtained daily with a digital caliper. After 5–7 d of tumoral growth, the nPTT was performed by administration of AuNCs and SPE at 806 nm at 1 W cm−2 for 4 minutes.The thermal images were obtained with a FLIR thermocamera and the thermographs were obtained with the commercial software of the same brand (see the previous laser set up section). Temperature was monitored by infrared thermography at the skin surface.
For the ICP-MS study, after 21 days of nPTT the animals were euthanized, and the necropsy was performed for the obtention of the samples for ICP-MS. The digestion of the sample was performed with HNO3, H2O2, and HCl in a Teflon reactor, heated to 90 °C in a heating oven. The digestion solution was diluted with a solution containing HCl and thiourea. The final volume was calculated by weight and weight/volume ratio. Samples were digested with three blanks worked in parallel. The determination of Au was performed in a PerkinElmer Nexion 350d instrument under standard conditions. The calibration was performed with standards prepared from certified standard solutions traceable to NIST. The digested sample was analysed directly.
Results and discussion
Synthesis and physicochemical characterization of gold nanocapsules for photothermal therapy
Photothermal responsive hollow plasmonic gold nanocapsules (AuNCs) were engineered by templating Au seeds onto polystyrene spheres through layer-by-layer (LbL) assembly followed by a silica coating shell, core dissolution, in situ gold growth and silica shell removal.60,66 Transmission-electron micrographs (Fig. 1A and Fig. S1A, B, ESI†) show complete loss of the electron-lucent silica layer while preserving a dense monolayer of ∼10 nm Au nanoparticles, and the outer diameter of the hollow structures remains 400 ± 20 nm, matching the template size. The capsules display intense dark-field scattering with a narrow ensemble peak at 620 nm (Fig. 1B), indicative of good colloidal stability and size homogeneity. Their UV-vis-NIR extinction spectrum is dominated by a broad localised surface-plasmon resonance (LSPR) that extends well into the first biological window48,49 (700–950 nm) (Fig. 1C; cf., SectionS1 and Fig. S1A, B, ESI†), a signature of strong electromagnetic coupling inside the confined plasmonic shell. The strong plasmonic coupling maximises heat generation (AuNCs’ responsiveness) under NIR illumination.Under 830 nm multiphoton excitation the particles generate bright, concentration- and power-dependent luminescence (cf., SectionS1 and Fig. S1C, ESI†), enabling intrinsic imaging29 without additional labels. Furthermore, AuNC concentration and 2PE power jointly determine heat generation and killing efficiency in 3D tumorspheres (SectionS2 and Fig. S2A, ESI†). The photothermal efficiency under pulsed 2PE (830 nm, 600 ns 2PE pulses) is a thoroughly discussed qualitatively in the ESI† (Section S2 and Fig. S2A, ESI†). When an aqueous bulk dispersion (50 μg Au mL−1) was irradiated with an 806 nm continuous-wave laser at 1 W cm−2, the temperature rose from 22 °C to 31 °C within six minutes and returned to baseline after the beam was blocked (Fig. 1D). Applying these data to eqn (1) yielded a photothermal conversion efficiency of 37%, comparable to the best values reported for plasmonically coupled Au assemblies and markedly higher than that of isolated 20 nm Au nanoparticles.74 The enhanced performance stems from collective plasmon damping and efficient non-radiative decay within the tightly packed Au monolayer.70
Beyond their synthetic simplicity, the capsules confer distinct biological advantages. Large (∼400 nm) AuNCs are rapidly internalised and progress more slowly through the endo-lysosomal pathway than smaller Au nanoparticles, which prolongs their intracellular residence time and extends the therapeutic window, an effect first demonstrated for polyelectrolyte capsules by Kastl et al.75 Our recent study using 3D melanoma models showed that the hollow cavity can encapsulate fluorescent payloads while the densely packed inner Au nano-islands provide bright two-photon luminescence, enabling simultaneous deep-tissue imaging and heat delivery.29 Moreover, the NCs efficiently target tumor biomarkers in vivo36 thus enhancing chemotherapeutic availability. Taken together, the structural integrity, broadband NIR absorption, bright multiphoton luminescence, high light-to-heat conversion efficiency, targetability, and biocompatibility, identify these ∼400 nm AuNCs as powerful theranostic agents for image-guided photothermal ablation of solid tumours.
Biological characterization of photoresponsive AuNCs in melanoma in vitro models
We next evaluated the photothermal performance of AuNCs in oncologically relevant B16-F10 melanoma spheroids.76 Tumor spheres were incubated with 0.1–80 μg mL−1 AuNCs and irradiated for 10 min with two-photon (2P) light at 830 nm at 3, 6, or 12 mW. Twenty-four hours later, live/dead staining and confocal imaging (SPL) revealed a clear, biparametric dependence. Viability decreased monotonically with both nanoparticle dose and laser power, failing to ∼48% at 80 μg mL−1 and 12 mW (Fig. 2A and B). Two-photon luminescence (2PL, cyan) acquired simultaneously provided an intrinsic, real-time map of AuNC distribution (Fig. 2B). Regions that emitted a lower 2PL signal (i.e., cyan luminescence recorded under identical imaging settings) contained fewer AuNCs and, consequently, experienced weaker inter-particle coupling and less photothermal damage. This result underscores that local particle density, rather than laser fluence alone, determines therapeutic outcome. Three photothermal regimes emerged: (i) high 2PL/high mortality (≥1 μg mL−1 AuNCs, 12 mW, 22 × 106 W cm−2), (ii) high 2PL/low mortality (10–80 μg mL−1, 6 mW; 11 × 106 W cm−2), and (iii) high 2PL/no mortality (10–80 μg mL−1, 3 mW; 5.5 × 106 W cm−2). Similar power-dependent 2PL amplification has been reported for thinner AuNC shells,29 confirming that greater particle loading and stronger excitation synergistically enhance local heating.To resolve how AuNC-mediated PTT (nPTT) ablates chemoresistant B16-F10 spheroids, we mapped cell viability as a function of nanoparticle dose, laser power and exposure time (Fig. S2A, ESI†). Controls in which either the laser (830 nm, 2PE) or the AuNCs were omitted compromised viability, confirming that all cytotoxicity stemmed from nPTT. Mortality scaled synergistically with both AuNC concentration and irradiation time, defining low (90–60% viability), moderate (60–30% viability) and high (<30% viability) nPTT windows. Unless specified otherwise, subsequent mechanistic experiments were performed under “low-nPTT” conditions, 5 μg mL−1 AuNCs, 600 ns pulses at 830 nm, and 3 mW (5.5 × 106 W cm−2), which inflict sub-lethal heat stress while preserving overall spheroid architecture. Live/dead imaging after high-nPTT confirmed extensive propidium iodide uptake and membrane poration, indicating that thermoablation proceeds via severe plasma membrane damage rather than apoptosis alone.
To benchmark our capsules, we synthesised surfactant-free gold nanostars (AuNSs, ∼110 nm, LSPR ≈ 830 nm), known to be an excellent photosensitive nanomaterial.77 At equivalent total Au, AuNCs induced higher apoptosis/necrosis than AuNSs (Fig. S2B, ESI†), despite the stronger far-field extinction of the stars. This superiority likely stems from the contiguous, hollow plasmonic layer in AuNCs, which concentrates thermal energy at the particle–cell interface and causes catastrophic membrane damage (Fig. S2C, ESI†). Collectively, these data position AuNCs as a robust 2PL-traceable platform for eradicating chemoresistant melanoma spheroids and for augmenting conventional chemotherapy.
Melanoma spheroids are notoriously chemoresistant because of a drug-efflux-active side-population.78–82 Consistently, paclitaxel (PTX) at up to 100 μM reduced spheroid viability by only 8% (Fig. S3, ESI†), whereas the same drug was cytotoxic to adherent B16-F10 cells, illustrating the protective effect of the 3D architecture.29 Importantly, combining low-dose PTX with sub-ablative nPTT (5 μg mL−1 AuNCs, 3 mW, 5.5 × 106 W cm−2) significantly potentiated cytotoxicity (Fig. S4, ESI†), mirroring the chemo-photothermal synergy described for other plasmonic constructs.83
Together, these data establish AuNCs as a potent, 2PL-traceable platform for eradicating chemoresistant melanoma spheroids and potentiating chemotherapy.
nPTT induces rapid oxidative stress and membrane destabilization in B16-F10 melanoma models
To evaluate the mechanisms of cell death triggered by nPTT, we monitored B16-F10 melanoma spheroids using live-cell and high-resolution imaging modalities, as outlined in Fig. 3A. Rhodamine B-loaded AuNCs were used to track nanoparticle distribution (λex = 543 nm). Coupled with RhB's minimal absorbance at 830 nm (ε < ε < 20 M−1 cm−1) and the viability observed in control spheroids (Fig. 3D and Fig. S6, ESI;† ref. 84), these findings exclude RhB-induced phototoxicity and demonstrate that cytotoxicity stems solely from the photothermal action of the Au shells.Upon treatment with 30 μg mL−1 AuNCs and 2PE irradiation (830 nm, 3 mW (5.5 × 106 W cm−2), 10 min), we observed marked cell death in 3D spheroids (Fig. 3B) and 2D cultures (Fig. 3C). In both models, nPTT induced phosphatidylserine (PS) externalization (green) and strong propidium iodide (PI) nuclear staining (red), indicative of apoptosis and necrosis, respectively. In parallel, cytoplasmic ROS (ROScyt) accumulation was detected shortly after nPTT in both 3D and 2D cultures (magenta, Fig. 3B, C and Fig. S5, ESI†). Control spheroids displayed negligible ROS levels and no PS signal (Fig. S5A, ESI†), while ROScyt increased sharply within 5 min post-nPTT (Fig. S5B, ESI†), preceding the onset of PS positivity and morphological swelling. Notably, once cells became apoptotic, ROScyt levels did not continue to rise, suggesting that oxidative stress may act as a trigger rather than a consequence of apoptosis. The high PS signal detected just 10 min after irradiation could also reflect increased plasma membrane permeability, rather than de novo apoptosis, as PS probes may more easily diffuse into the cytosol under membrane-disruptive conditions. This is consistent with literature describing pore formation and local membrane destabilization because of rapid, confined heating.85–87 Furthermore, given the intrinsic photocatalytic activity of gold nanostructures, the possibility of a combined photothermal and photodynamic effect cannot be excluded.51,88–92
To investigate subcellular effects in more detail, we used cryo-correlative epifluorescence (Cryo-epiFL) and soft X-ray tomography (cryoSXT). This is an imaging approach that allows analyzing the 3D cartography of a cell in its native state at organelle resolution level.71,72 The high electron density of AuNCs enables their use as contrast agents. With this approach, we assessed the cellular architecture under mild nPTT conditions (30 μg mL−1 AuNCs, 830 nm, 3 mW, 5.5 × 106 W cm−2), where gross viability remains largely intact (Fig. 3D). While untreated and laser-only cells showed normal ultrastructure, the nPTT group exhibited plasma membrane perforation, AuNC decomposition, and the loss of cytoplasmic and nuclear components. Red arrows denote electron-dense Au debris, while yellow arrows highlight membrane pores of approximately 1–1.5 μm (Fig. S6, ESI†). A close inspection of the virtual tomograms therefore points to early membrane rupture and in situ AuNC disintegration, phenomena that require transient local temperatures exceeding the 45–50 °C threshold for lipid bilayer melting and protein denaturation.49,93,94 Cryo-epiFL imaging of these samples confirmed massive PS exposure and the apparent absence of ROScyt, likely due to leakage of the ROS probe through permeabilized membranes.
Collectively, these observations support a model in which nPTT triggers inside-out hyperthermia, a highly localized heating mechanism that rapidly destabilizes the plasma membrane. This mechanism mirrors the membrane-rupturing activity observed in magnetic hyperthermia with iron oxide nanoparticles and may help circumvent chemoresistance by increasing drug access to the intracellular space.
Intratumoral AuNCs drive uniform cytotoxic hyperthermia and durable B16-F10 melanoma remission in vivo
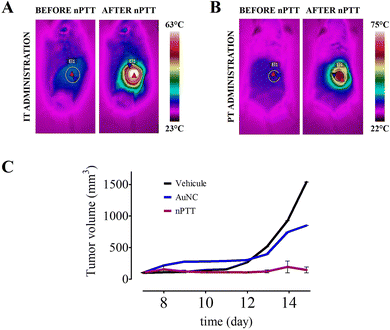
Having established the in vitro performance of the AuNCs, we next assessed their therapeutic capacity in vivo using B16-F10 melanoma xenografts in C57BL/6 mice.95 Tumours were treated once they reached ∼100 mm3 (12–14 days post-inoculation). AuNCs (1 mg; 28.5 mg mL−1) were injected either intratumourally (IT) one bolus into the tumour core, or peritumorally (PT), four deposits circumscribing the lesion. In both cases the AuNCs were allowed to diffuse for 24 h; shorter distribution times (1 h) produced heterogeneous heating and tumour relapse (data not shown). We treated each tumor with a single 4-min laser irradiation (806 nm laser, 1 W cm−2) (treatment procedure shown in Fig. S7, ESI†).Real-time thermography showed surface temperatures rising to ∼55 °C in AuNC-laden tumors, compared with sub-lethal <38 °C in laser-only controls (Fig. S8, ESI†). At the end of irradiation, the surface temperature of IT-treated lesions reached 64 °C, whereas PT-treated lesions peaked at 62 °C. In both cases the minimum temperature within the heated area was ≈55–56 °C, yielding a nearly identical ΔT of ≈26 °C (Fig. 4A and B; sequences in Fig. S9 and S10 (ESI†), quantitative summary in Table S1, ESI†). Because conductive losses typically reduce the IT temperature by 8–12 °C,96,97 the bulk of the tumor was held at 45–50 °C for at least the final minutes of irradiation, which is well within the cytotoxic hyperthermia window (≥43 °C maintained for ≥4 min).98,99
Despite similar surface profiles, therapeutic outcome diverged markedly. IT-nPTT eradicated every tumor, with volumes regressing to an imperceptible scar over the 21-day observation period (Fig. 4C and Table S1, ESI†). In contrast, PT-nPTT produced a striking superficial hot-spot but left a viable core that later resumed growth. The difference arises from nanoparticle distribution, rather than peak surface temperature. PT injection concentrates AuNCs near the skin, photon absorption occurs there, thus creating a steep gradient (hot rim → cool core) that spares deeper cells. Conversely, IT delivery disperses them throughout the parenchyma, ensuring the entire tumor volume absorbs sufficient heat, thus enabling uniform heat deposition for irreversible damage, and sparing the overlying skin from visible thermal injury.
This behavior agrees with other reports in which tightly clustered gold nanoparticles convert light to heat efficiently and that homogeneous intratumoral coverage is critical for cure. Examples include polyethyleneimine-induced AuNP clusters that eradicate CT26 tumours under 680 nm irradiation,100 Au nanorod/glutathione-Au-cluster hybrids that outperform single rods against HeLa cells,101 and 5-fluorouracil-templated Au clusters that enhance immunogenic hyperthermia in colorectal carcinomatosis.102 The densely packed inner shell of our AuNCs (Fig. 1A) provides an analogous architecture, underscoring their suitability for single-session photothermal eradication of melanoma.
No off-target burns, weight loss, or behavioural changes were observed, underscoring the safety of a single, image-guided AuNC injection followed by short NIR irradiation. Taken together, the data in Fig. 4 and the supporting thermographic analyses (Fig. S7 to S10 and Table S1, ESI†) show that an optimized IT protocol with AuNCs produces homogeneous cytotoxic hyperthermia (≈45–50 °C intratumorally) and achieves durable control of aggressive melanoma in a single session, setting the stage for the biodistribution and fragmentation-driven clearance analysis of AuNCs presented next.
Gold biodistribution after nPTT is dictated by the injection route
To elucidate the in vivo fate of the contrast agent and its relationship to therapeutic efficacy, we quantified elemental gold 24 h after treatment using ICP-MS (Fig. 5 and Fig. S11, ESI†). The technique vaporises and ionises the sample, then separates mono-charged ions by mass, allowing ppb-level metal quantification.103 In the IT group (Fig. 5A) only ≈4% of the injected dose was recoverable and >99% of that resided in the post-treatment scar. Although no charring was visible at the time of irradiation, a haemorrhagic scab formed within 24 h, evidence of sub-cutaneous necrosis caused by the transient 45–50 °C IT temperature rise. Liver, spleen and kidney together contained <0.05% of the dose, indicating minimal systemic dissemination.The pattern was reversed after PT administration (Fig. 5B). ≈65% of the dose was still detectable, but only 0.01% remained in the residual tumour; no scar formed, consistent with the superficial heating profile. Instead, gold accumulated in the spleen (48%) and liver (17%), classical sinks of the mononuclear phagocyte system. The large (∼400 nm) intact AuNCs injected around the lesion likely entered the leaky peri-tumoral vasculature and were rapidly cleared by circulating and resident macrophages, whereas those delivered intratumorally became trapped within the irregular melanoma vasculature104 or immobilised in the necrotic scar.
A trace kidney signal (0.02–0.07% in both groups) indicates that laser heating fragmented a small fraction of AuNCs into sub-6 nm debris capable of glomerular filtration. Such shock-wave-driven disassembly of plasmonic nanoparticles under nanosecond pulses has been reported previously41,105 and is corroborated here by cryo-SXT, which shows sub-10 nm shards dispersed in the cell cytoplasm (Fig. 3 and Fig. S11, ESI†).
Taken together, the data indicate that the injection route dictates AuNC retention versus systemic clearance. Although the PT route leaves ∼65% of the injected gold in the spleen and liver, prior studies show that comparable hepatic/splenic burdens of dextran-coated 20–30 nm AuNPs cause no histopathological changes or elevations in AST, ALT, creatinine or pro-inflammatory cytokines up to 4 weeks post-exposure.63 Likewise, transient IL-6 and TNF-α spikes reported for intravenously delivered gold colloids normalise within 96 h, suggesting a self-limited innate response. In our study, animals exhibited normal behaviour, weight gain and grooming throughout the 21-day follow-up, further indicating an absence of overt toxicity. Nevertheless, long-term (≥3-month) studies with comprehensive serum chemistry and organ histology are warranted to definitively rule out chronic effects. IT administration confines most gold, both intact AuNCs and photothermally generated fragments, to the treatment site, explaining the uniform deep heating and complete tumour ablation. PT delivery enables widespread escape, reducing IT gold concentration, limiting heating at depth, and ultimately permitting tumour regrowth. The small but consistent renal signal in both groups further suggests that photothermal fragmentation generates ultra-small gold species that can be renally excreted, consistent with the size-dependent urinary clearance threshold (<6 nm) reported by Choi et al.103 Thus, nanoparticle retention, rather than the total injected dose, emerges as the decisive factor for durable photothermal tumour control, while laser-induced fragmentation provides a plausible pathway for long-term elimination of residual gold from the body.
Conclusions
In this study, we demonstrated for the first time that the plasmonic photoresponsive properties of AuNCs can be exploited for two-photon luminescence-assisted nPTT. We validated AuNCs as an effective photothermal probe using paclitaxel-resistant B16-F10 melanoma spheroids, a three-dimensional model of chemoresistant melanoma. AuNCs alone were biocompatible with no measurable cytotoxicity in spheroids, consistent with prior reports on similar nanocapsules. Under two-photon excitation at 830 nm, AuNCs produced bright luminescence and efficient heat generation, with both signals increasing as AuNC concentration and laser power were increased (up to an optimal 80 μg mL−1 and 12 mW). This photothermal effect led to significant melanoma cell ablation in vitro. Notably, at equivalent gold doses, AuNCs outperformed gold nanostars, an established photothermal agent, in treating chemoresistant melanoma spheroids. Correlative epifluorescence imaging and cryo-soft X-ray microscopy confirmed that AuNCs disintegrated under these laser treatment conditions and induced nanoscale pores in cell membranes. We also observed elevated ROS during AuNC phototherapy, suggesting a combined photothermal and photodynamic mechanism of cancer cell killing. In vivo results further underscore the promise of this approach. In a murine subcutaneous melanoma model, AuNC-mediated PTT yielded complete tumor elimination with both intratumoral and peritumoral nanoparticle administration. However, the route of administration significantly influenced AuNC biodistribution. Intratumoral injection followed by PTT caused a localized necrotic tumor scab that effectively sequestered the bulk of the nanoparticles at the treatment site, limiting their spread. As a result, gold from intratumoral therapy remained largely at the tumor and was shed with the scab, with only minimal traces detected in the liver and spleen. By contrast, peritumoral injection (where no necrotic scab formed) still achieved tumor clearance but allowed more AuNCs to enter circulation, leading to appreciable gold accumulation in off-target organs such as the spleen and liver after treatment. In the intratumoral treatment scenario, detection of gold in the kidneys indicates that some heat-damaged AuNC fragments became small enough for renal clearance, a favorable outcome for nanoparticle elimination. Overall, our findings highlight AuNCs as a powerful nanoparticle-assisted PTT platform for chemotherapy-resistant melanoma. The ability of these nanocapsules to eradicate tumors under NIR excitation, while largely confining the particles to the tumor site (especially with intratumoral delivery), points to a potentially safer profile compared to many systemic nanotherapies. For clinical translation, the choice of delivery route will be important to balance maximal tumor destruction with minimal systemic exposure. This AuNC-based 2PL-guided phototherapy could be further enhanced by incorporating drug delivery (capitalizing on the AuNCs’ cargo-loading capability) or by combining with other treatments. Our study lays the groundwork for advanced two-photon guided chemo-PTT strategies and supports the continued development of AuNCs toward effective, targeted melanoma treatment.Author contributions
PZP, QS, and HDS performed the experiments. JJC assisted with the measurements at Synchrotron. MCIC, NF and DJ assisted with the in vivo experiments. PRG conceived the work, analysed the results, prepared the figures, and wrote the manuscript. All authors reviewed the manuscript.Conflicts of interest
The authors declare that they have no competing interests.Ethics approval and consent to participate
All the experiments were conducted in accordance with the European Union directives 63/2010UE and Spanish regulation RD 53/2013. The use of these animals was also approved by the Animal Ethics Committee of the Universidad Autónoma de Madrid (Madrid, Spain).Abbreviations
| AuNCs | Gold nanocapsules |
| CLSM | Confocal laser scanning microscopy |
| CW | Continuous wave |
| cryoSXT | Cryo-soft X-ray microscopy |
| GM | Growth medium |
| HEDFM | Hyperspectral-enhanced dark field microscopy |
| ICP-MS | Inductively coupled plasma atomic mass spectrometry |
| IT | Intratumorally |
| nPTT | Nanoparticle-assisted photothermal therapy |
| MPE | Multiphoton excitation |
| NIR | Near-infrared |
| 2PE | Two-photon excitation |
| 2PL | Two-photo luminescence |
| PI | Propidium iodide |
| PS | Phosphatidylserine |
| PTX | Paclitaxel |
| PT | Peritumoral |
| ROS | Reactive oxygen species |
| ROScyt | Cytoplasmatic reactive oxygen species |
| RT | Room temperature |
| SPL | Single photon luminescence |
Data availability
All data generated or analysed during this study are included in this published article (and its ESI†). The supporting information file contains additional information on the methodology and results.Acknowledgements
The study of the nanophotothermal therapy (nPTT) effects of gold nanocapsules (AuNCs) on melanoma cells using cryo soft X-ray tomography (CSXT) was conducted at the MISTRAL beamline of the ALBA Synchrotron (Project number: 2016091853). PRG acknowledges the Ministry of Science, Innovation and Universities (AEI-PID2022-140423NB-I00/AEI/10.13039/501100011033 and CNS2023 – 143700) and the AGAUR (2021 SGR 00175) for financial support. D. Jaque acknowledges the Ministry of Science, Innovation and Universities of Spain (PID2023-146775OB-I00). The authors thank Dr Vladimir Mulens Arias for fruitful discussions and Alba Riba for her contribution (Fig. S2A, ESI†).References
- F. Bray, M. Laversanne, H. Sung, J. Ferlay, R. L. Siegel, I. Soerjomataram and A. Jemal, Ca-Cancer J. Clin., 2024, 74, 229–263 CrossRef PubMed.
- M. Arnold, D. Singh, M. Laversanne, J. Vignat, S. Vaccarella, F. Meheus, A. E. Cust, E. de Vries, D. C. Whiteman and F. Bray, JAMA Dermatol., 2022, 158, 495–503 CrossRef PubMed.
- C. Karimkhani, A. C. Green, T. Nijsten, M. A. Weinstock, R. P. Dellavalle, M. Naghavi and C. Fitzmaurice, Br. J. Dermatol., 2017, 177, 134–140 CrossRef CAS PubMed.
- Y. Sun, Y. Shen, Q. Liu, H. Zhang, L. Jia, Y. Chai, H. Jiang, M. Wu and Y. Li, J. Am. Acad. Dermatol., 2025, 92, 100–107 CrossRef PubMed.
- Melanoma of the Skin – Cancer Stat Facts, https://seer.cancer.gov/statfacts/html/melan.html, (accessed May 13, 2025).
- J. Larkin, V. Chiarion-Sileni, R. Gonzalez, J.-J. Grob, P. Rutkowski, C. D. Lao, C. L. Cowey, D. Schadendorf, J. Wagstaff, R. Dummer, P. F. Ferrucci, M. Smylie, D. Hogg, A. Hill, I. Márquez-Rodas, J. Haanen, M. Guidoboni, M. Maio, P. Schöffski, M. S. Carlino, C. Lebbé, G. McArthur, P. A. Ascierto, G. A. Daniels, G. V. Long, L. Bastholt, J. I. Rizzo, A. Balogh, A. Moshyk, F. S. Hodi and J. D. Wolchok, N. Engl. J. Med., 2019, 381, 1535–1546 CrossRef CAS PubMed.
- H. A. Tawbi, D. Schadendorf, E. J. Lipson, P. A. Ascierto, L. Matamala, E. C. Gutiérrez, P. Rutkowski, H. J. Gogas, C. D. Lao, J. J. D. Menezes, S. Dalle, A. Arance, J.-J. Grob, S. Srivastava, M. Abaskharoun, M. Hamilton, S. Keidel, K. L. Simonsen, A. M. Sobiesk, B. Li, F. S. Hodi and G. V. Long, N. Engl. J. Med., 2022, 386, 24–34 CrossRef CAS PubMed.
- Center for Drug Evaluation and Research, U.S. Food and Drug Administration (FDA).
- G. V. Long, D. Stroyakovskiy, H. Gogas, E. Levchenko, F. de Braud, J. Larkin, C. Garbe, T. Jouary, A. Hauschild, J. J. Grob, V. Chiarion Sileni, C. Lebbe, M. Mandalà, M. Millward, A. Arance, I. Bondarenko, J. B. A. G. Haanen, J. Hansson, J. Utikal, V. Ferraresi, N. Kovalenko, P. Mohr, V. Probachai, D. Schadendorf, P. Nathan, C. Robert, A. Ribas, D. J. DeMarini, J. G. Irani, M. Casey, D. Ouellet, A.-M. Martin, N. Le, K. Patel and K. Flaherty, N. Engl. J. Med., 2014, 371, 1877–1888 CrossRef PubMed.
- M. A. Postow, R. Sidlow and M. D. Hellmann, N. Engl. J. Med., 2018, 378, 158–168 CrossRef CAS PubMed.
- R. D. Issels and M. H. Falk, Int. J. Hyperthermia, 2001, 17, 1–18 CrossRef.
- N. M. Bleehen, Br. J. Cancer, Suppl., 1982, 5, 96–100 CAS.
- C.-H. Hou, F.-L. Lin, S.-M. Hou and J.-F. Liu, Int. J. Mol. Sci., 2014, 15, 17380–17395 CrossRef PubMed.
- T. Mantso, S. Vasileiadis, I. Anestopoulos, G. P. Voulgaridou, E. Lampri, S. Botaitis, E. N. Kontomanolis, C. Simopoulos, G. Goussetis, R. Franco, K. Chlichlia, A. Pappa and M. I. Panayiotidis, Sci. Rep., 2018, 8, 10724 CrossRef CAS PubMed.
- J. P. Fruehauf and V. Trapp, Expert Rev. Anticancer Ther., 2008, 8, 1751–1757 CrossRef CAS PubMed.
- H. Zhao, H. Chen, Z. Guo, W. Zhang, H. Yu, Z. Zhuang, H. Zhong and Z. Liu, Chem. Eng. J., 2020, 394, 124314 CrossRef CAS.
- X. Duan, C. Chan and W. Lin, Angew. Chem., Int. Ed., 2019, 58, 670–680 CrossRef CAS PubMed.
- Q. Chen, Q. Hu, E. Dukhovlinova, G. Chen, S. Ahn, C. Wang, E. A. Ogunnaike, F. S. Ligler, G. Dotti and Z. Gu, Adv. Mater., 2019, 31, 1900192 CrossRef PubMed.
- D. Chang, M. Lim, J. A. C. M. Goos, R. Qiao, Y. Y. Ng, F. M. Mansfeld, M. Jackson, T. P. Davis and M. Kavallaris, Front. Pharmacol., 2018, 2(9), 831 CrossRef PubMed.
- J. Beik, Z. Abed, F. S. Ghoreishi, S. Hosseini-Nami, S. Mehrzadi, A. Shakeri-Zadeh and S. K. Kamrava, J. Controlled Release, 2016, 235, 205–221 CrossRef CAS PubMed.
- N. R. Datta, H. P. Kok, H. Crezee, U. S. Gaipl and S. Bodis, Front. Oncol., 2020, 12(10), 819 CrossRef PubMed.
- J. D. Rybka, Rep Pract Oncol Radiother., 2019, 24, 152–157 CrossRef PubMed.
- L. R. Hirsch, R. J. Stafford, J. A. Bankson, S. R. Sershen, B. Rivera, R. E. Price, J. D. Hazle, N. J. Halas and J. L. West, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 13549–13554 CrossRef CAS PubMed.
- J. Li, X. Zhen, Y. Lyu, Y. Jiang, J. Huang and K. Pu, ACS Nano, 2018, 12, 8520–8530 CrossRef CAS PubMed.
- Y. Li, W. Lu, Q. Huang, C. Li and W. Chen, Nanomedicine, 2010, 5, 1161–1171 CrossRef CAS PubMed.
- G. Dacarro, A. Taglietti and P. Pallavicini, Molecules, 2018, 23, 1414 CrossRef PubMed.
- P. Zhao, Y. Xu, W. Ji, S. Zhou, L. Li, L. Qiu, Z. Qian, X. Wang and H. Zhang, J. Nanobiotechnol., 2021, 19, 181 CrossRef CAS PubMed.
- Y. Zhang, X. Zhan, J. Xiong, S. Peng, W. Huang, R. Joshi, Y. Cai, Y. Liu, R. Li, K. Yuan, N. Zhou and W. Min, Sci. Rep., 2018, 8, 8720 CrossRef PubMed.
- P. Zamora-Perez, C. Xiao, M. Sanles-Sobrido, M. Rovira-Esteva, J. J. Conesa, V. Mulens-Arias, D. Jaque and P. Rivera-Gil, Acta Biomater., 2022, 142, 308–319 CrossRef CAS PubMed.
- R. Di Corato, D. Palumberi, R. Marotta, M. Scotto, S. Carregal-Romero, P. Rivera Gil, W. J. Parak and T. Pellegrino, Small, 2012, 8, 2731–2742 CrossRef CAS PubMed.
- P. Singh, P. Haloi, K. Singh, S. Roy, A. Sarkar, S. L. B. R. Choudhary, C. Mohite, S. Chawla, V. B. Konkimalla, P. Sanpui and A. Jaiswal, ACS Appl. Mater. Interfaces, 2023, 15, 39081–39098 CrossRef CAS PubMed.
- L. Li, C. Chen, H. Liu, C. Fu, L. Tan, S. Wang, S. Fu, X. Liu, X. Meng and H. Liu, Adv. Funct. Mater., 2016, 26, 4252–4261 CrossRef CAS.
- I. Grabowska-Jadach, D. Kalinowska, M. Drozd and M. Pietrzak, Biomed. Pharmacother., 2019, 111, 1147–1155 CrossRef CAS PubMed.
- W. Chen, M. Qin, X. Chen, Q. Wang, Z. Zhang and X. Sun, Theranostics, 2018, 8, 2229–2241 CrossRef CAS PubMed.
- A. S. Bear, L. C. Kennedy, J. K. Young, S. K. Perna, J. P. M. Almeida, A. Y. Lin, P. C. Eckels, R. A. Drezek and A. E. Foster, PLoS One, 2013, 8, e69073 CrossRef CAS PubMed.
- R. Xu, N. Martinez-Bosch, F. Rivera-Hueto, V. Mulens-Arias, F. Rubio-Moscardo, J. Javier Conesa, P. Navarro, R. Vicente and P. Rivera-Gil, J. Drug Targeting, 2025, 33, 143–155 CrossRef CAS PubMed.
- X. Huang and M. A. El-Sayed, J. Adv. Res., 2010, 1, 13–28 CrossRef.
- Y. Wu, M. R. K. Ali, K. Chen, N. Fang and M. A. El-Sayed, Nano Today, 2019, 24, 120–140 CrossRef CAS.
- L. A. Austin, M. A. Mackey, E. C. Dreaden and M. A. El-Sayed, Arch. Toxicol., 2014, 88, 1391–1417 CrossRef CAS PubMed.
- S. Ashraf, B. Pelaz, P. del Pino, M. Carril, A. Escudero, W. J. Parak, M. G. Soliman, Q. Zhang and C. Carrillo-Carrion, in Light-Responsive Nanostructured Systems for Applications in Nanomedicine, ed. S. Sortino, Springer International Publishing, Cham, 2016, pp. 169–202 Search PubMed.
- P. Rivera Gil, D. Hühn, L. L. del Mercato, D. Sasse and W. J. Parak, Pharmacol. Res., 2010, 62, 115–125 CrossRef CAS PubMed.
- S. Carregal-Romero, M. Ochs, P. Rivera-Gil, C. Ganas, A. M. Pavlov, G. B. Sukhorukov and W. J. Parak, J. Controlled Release, 2012, 159, 120–127 CrossRef CAS PubMed.
- P. K. Jain and M. A. El-Sayed, Chem. Phys. Lett., 2010, 487, 153–164 CrossRef CAS.
- P. K. Jain, X. Huang, I. H. El-Sayed and M. A. El-Sayed, Acc. Chem. Res., 2008, 41, 1578–1586 CrossRef CAS PubMed.
- S. Eustis and M. A. El-Sayed, Chem. Soc. Rev., 2006, 35, 209–217 RSC.
- P. Zamora-Perez, B. Pelaz, D. Tsoutsi, M. G. Soliman, W. J. Parak and P. Rivera-Gil, Nanoscale, 2021, 13, 13256–13272 RSC.
- P. Zamora-Perez, D. Tsoutsi, R. Xu and P. Rivera_Gil, Materials, 2018, 11, 243 CrossRef PubMed.
- E. Hemmer, A. Benayas, F. Légaré and F. Vetrone, Nanoscale Horiz., 2016, 1, 168–184 RSC.
- D. Jaque, L. M. Maestro, B. del Rosal, P. Haro-Gonzalez, A. Benayas, J. L. Plaza, E. M. Rodríguez and J. G. Solé, Nanoscale, 2014, 6, 9494–9530 RSC.
- Z. Bao, X. Liu, Y. Liu, H. Liu and K. Zhao, Asian J. Pharm. Sci., 2016, 11, 349–364 Search PubMed.
- V. Guerrero-Florez, S. C. Mendez-Sanchez, O. A. Patrón-Soberano, V. Rodríguez-González, D. Blach and F. Martínez, J. Mater. Chem. B, 2020, 8, 2862–2875 RSC.
- S. Bhana, G. Lin, L. Wang, H. Starring, S. R. Mishra, G. Liu and X. Huang, ACS Appl. Mater. Interfaces, 2015, 7, 11637–11647 CrossRef CAS PubMed.
- R. Vankayala, C.-C. Lin, P. Kalluru, C.-S. Chiang and K. C. Hwang, Biomaterials, 2014, 35, 5527–5538 CrossRef CAS PubMed.
- S. M. H. AL-Jawad, A. A. Taha, M. M. F. Al-Halbosiy and L. F. A. AL-Barram, Photodiagn. Photodyn. Ther., 2018, 21, 201–210 CrossRef CAS PubMed.
- H. S. Kim and D. Y. Lee, J. Pharm. Invest., 2017, 47, 19–26 CrossRef CAS.
- H. S. Kim and D. Y. Lee, Polymers, 2018, 10, 961 CrossRef PubMed.
- R. S. Riley and E. S. Day, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2017, 9(4) DOI:10.1002/wnan.1449.
- Q. Chen, L. Xu, C. Liang, C. Wang, R. Peng and Z. Liu, Nat. Commun., 2016, 7, 13193 CrossRef CAS PubMed.
- N. Zhang, J. Song, Y. Liu, M. Liu, L. Zhang, D. Sheng, L. Deng, H. Yi, M. Wu, Y. Zheng, Z. Wang and Z. Yang, J. Controlled Release, 2019, 306, 15–28 CrossRef CAS PubMed.
- P. Rivera Gil, C. Vazquez-Vazquez, V. Giannini, M. P. Callao, W. J. Parak, M. A. Correa-Duarte and R. A. Alvarez-Puebla, Angew. Chem., Int. Ed., 2013, 52, 13694–13698 CrossRef CAS PubMed.
- T. Zhou, R. Vicente and P. Rivera-Gil, ACS Omega, 2024, 9, 42183–42192 CrossRef CAS PubMed.
- C. Xiao, V. Izquierdo-Roca and P. Rivera-Gil, ACS Mater. Au, 2023, 3, 164–175 CrossRef CAS PubMed.
- A. Bailly, F. Correard, A. Popov, G. Tselikov, F. Chaspoul, R. Appay, A. Al-Kattan, A. Kabashim, D. Braguer and M. Esteve, Sci. Rep., 2019, 9, 12890 CrossRef PubMed.
- E. Alexander and K. W. Leong, Front. Nanotechnol., 2025, 7, 1512622 CrossRef.
- X.-D. Zhang, Z. Luo, J. Chen, S. Song, X. Yuan, X. Shen, H. Wang, Y. Sun, K. Gao, L. Zhang, S. Fan, D. T. Leong, M. Guo and J. Xie, Sci. Rep., 2015, 5, 8669 CrossRef CAS PubMed.
- M. Sanles-Sobrido, W. Exner, L. Rodríguez-Lorenzo, B. Rodríguez-González, M. A. Correa-Duarte, R. A. Álvarez-Puebla and L. M. Liz-Marzán, J. Am. Chem. Soc., 2009, 131, 2699–2705 CrossRef CAS PubMed.
- C. Xiao, V. Izquierdo-Roca and P. Rivera-Gil, ACS Mater. Au, 2023, 3, 164–175 CrossRef CAS PubMed.
- X. Wang, G. Li, Y. Ding and S. Sun, RSC Adv., 2014, 4, 30375–30383 RSC.
- D. N. Mastronarde and S. R. Held, J. Struct. Biol., 2017, 197, 102–113 CrossRef PubMed.
- W. W. Overwijk and N. P. Restifo, Curr. Protoc. Immunol., 2000, 39, 1–29 Search PubMed.
- R. Carzaniga, M.-C. Domart, L. M. Collinson and E. Duke, Protoplasma, 2014, 251, 449–458 CrossRef CAS PubMed.
- M. Chiappi, J. J. Conesa, E. Pereiro, C. O. S. Sorzano, M. J. Rodríguez, K. Henzler, G. Schneider, F. J. Chichón and J. L. Carrascosa, J. Nanobiotechnol., 2016, 14, 15 CrossRef PubMed.
- R. A. Odion, Y. Liu and T. Vo-Dinh, Cancers, 2022, 14, 5737 CrossRef CAS PubMed.
- J. Wang, Y. Zhang, N. Jin, C. Mao and M. Yang, ACS Appl. Mater. Interfaces, 2019, 11, 11136–11143 CrossRef CAS PubMed.
- L. Kastl, D. Sasse, V. Wulf, R. Hartmann, J. Mircheski, C. Ranke, S. Carregal-Romero, J. A. Martínez-López, R. Fernández-Chacón, W. J. Parak, H.-P. Elsasser and P. Rivera_Gil, ACS Nano, 2013, 7, 6605–6618 CrossRef CAS PubMed.
- H. Lu and M. H. Stenzel, Small, 2018, 14, 1702858 CrossRef PubMed.
- G. Chandrasekar, K. Mougin, H. Haidara, L. Vidal and E. Gnecco, Appl. Surf. Sci., 2011, 257, 4175–4179 CrossRef CAS.
- M. Zanoni, F. Piccinini, C. Arienti, A. Zamagni, S. Santi, R. Polico, A. Bevilacqua and A. Tesei, Sci. Rep., 2016, 6, 19103 CrossRef CAS PubMed.
- I. F. Hermans, T. W. Chong, M. J. Palmowski, A. L. Harris and V. Cerundolo, Cancer Res., 2003, 63, 8408–8413 CAS.
- Y. Kalechman, D. L. Longo, R. Catane, A. Shani, M. Albeck and B. Sredni, Int. J. Cancer, 2000, 86, 281–288 CrossRef CAS PubMed.
- A. S. Nunes, A. S. Barros, E. C. Costa, A. F. Moreira and I. J. Correia, Biotechnol. Bioeng., 2019, 116, 206–226 CrossRef CAS PubMed.
- S. M. Mousavi, M. Zarei, S. A. Hashemi, S. Ramakrishna, W.-H. Chiang, C. W. Lai and A. Gholami, Drug Metab. Rev., 2020, 52, 299–318 CrossRef CAS PubMed.
- M. Deinavizadeh, A. R. Kiasat, M. Shafiei, M. Sabaeian, R. Mirzajani, S. M. Zahraei, F. Khalili, M. Shao, A. Wu, P. Makvandi and N. Hooshmand, Sci. Rep., 2024, 14, 4373 CrossRef CAS PubMed.
- P. Zamora-Perez, C. Xiao, M. Sanles-Sobrido, M. Rovira-Esteva, J. J. Conesa, V. Mulens-Arias, D. Jaque and P. Rivera-Gil, Acta Biomater., 2022, 142, 308–319 CrossRef CAS PubMed.
- R. R. Letfullin, C. Joenathan, T. F. George and V. P. Zharov, Nanomedicine, 2006, 1, 473–480 CrossRef CAS PubMed.
- X. Wang, X. Lu, R. Zhu, K. Zhang, S. Li, Z. Chen and L. Li, Neurochem. Res., 2017, 42, 1130–1140 CrossRef CAS PubMed.
- D. K. Chatterjee, P. Diagaradjane and S. Krishnan, Ther. Delivery, 2011, 2, 1001–1014 CrossRef CAS PubMed.
- S. Hwang, J. Nam, S. Jung, J. Song, H. Doh and S. Kim, Nanomedicine, 2014, 9, 2003–2022 CrossRef CAS PubMed.
- R. S. Riley and E. S. Day, WIREs Nanomed. Nanobiotechnol., 2017, 9, e1449 CrossRef PubMed.
- L. Minai, D. Yeheskely-Hayon and D. Yelin, Sci. Rep., 2013, 3, 2146 CrossRef PubMed.
- A. Manke, L. Wang and Y. Rojanasakul, BioMed Res. Int., 2013, e942916 Search PubMed.
- L. Cui, W. Bu, J. Song, L. Feng, T. Xu, D. Liu, W. Ding, J. Wang, C. Li, B. Ma, Y. Luo, Z. Jiang, C. Wang, J. Chen, J. Hou, H. Yan, L. Yang and X. Jia, Arch. Pharm. Res., 2018, 41, 299–313 CrossRef CAS PubMed.
- H. D. A. Santos, E. C. Ximendes, M. del, C. Iglesias-de la Cruz, I. Chaves-Coira, B. del Rosal, C. Jacinto, L. Monge, I. Rubia-Rodríguez, D. Ortega, S. Mateos, J. GarcíaSolé, D. Jaque and N. Fernández, Adv. Funct. Mater., 2018, 28, 1803924 CrossRef.
- B. Sanz, M. P. Calatayud, T. E. Torres, M. L. Fanarraga, M. R. Ibarra and G. F. Goya, Biomaterials, 2017, 114, 62–70 CrossRef CAS PubMed.
- V. Mulens-Arias, A. Nicolás-Boluda, A. Gehanno, A. Balfourier, F. Carn and F. Gazeau, Nanoscale, 2019, 11, 3344–3359 RSC.
- D. Kim, J. Paik and H. Kim, Sci. Rep., 2023, 13, 12135 CrossRef PubMed.
- M. M. Rahman, I. Pop and M. Z. Saghir, Int. J. Heat Mass Transfer, 2019, 129, 198–211 CrossRef CAS.
- Z. A. Shahabad, C. B. Avci, F. Bani, A. Zarebkohan, M. Sadeghizadeh, R. Salehi, M. Ghafarkhani, R. Rahbarghazi, B. G. Bagca and N. P. Ozates, Sci. Rep., 2022, 12, 11774 CrossRef CAS PubMed.
- B. E. Kennedy, E. B. Noftall, C. Dean, A. Roth, K. N. Clark, D. Rowles, K. Singh, L. Pagliaro and C. A. Giacomantonio, Front. Immunol., 2025, 15, 1512543 CrossRef PubMed.
- S. Liu, J. Wang, Y. Song, S. He and H. Tan, Pharmaceutics, 2022, 14, 2451 CrossRef CAS PubMed.
- V. Mulens-Arias, A. Nicolás-Boluda, A. Pinto, A. Balfourier, F. Carn, A. K. A. Silva, M. Pocard and F. Gazeau, ACS Nano, 2021, 15, 3330–3348 CrossRef CAS PubMed.
- E. C. Mazarakioti, A. Zotos, A.-A. Thomatou, A. Kontogeorgos, A. Patakas and A. Ladavos, Foods, 2022, 11, 3705 CrossRef CAS PubMed.
- H. S. Choi, W. Liu, P. Misra, E. Tanaka, J. P. Zimmer, B. I. Ipe, M. G. Bawendi and J. V. Frangioni, Nat. Biotechnol., 2007, 25, 1165–1170 CrossRef CAS PubMed.
- W. Ngo, S. Ahmed, C. Blackadar, B. Bussin, Q. Ji, S. M. Mladjenovic, Z. Sepahi and W. C. W. Chan, Adv. Drug Delivery Rev., 2022, 185, 114238 CrossRef CAS PubMed.
- R. R. Letfullin, C. Joenathan, T. F. George and V. P. Zharov, Nanomedicine, 2006, 1, 473–480 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5tb00167f |
| ‡ Equally contributing authors. |
| This journal is © The Royal Society of Chemistry 2025 |