Kaushik Chakrabarti, Anju Mishra, Dibyajyoti Panja, Bhaskar Paul and Sabuj Kundu
Green Chem., 2018,20, 3339-3345
DOI:
10.1039/C8GC00863A,
Paper
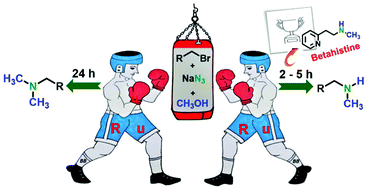
A Ru(II) complex mediated synthesis of various N,N-dimethyl and N-monomethyl amines from organic azides using methanol as a methylating agent is reported. This methodology was successfully applied for a one-pot reaction of bromide derivatives and sodium azide in methanol. Notably, by controlling the reaction time several N-monomethylated and N,N-dimethylated amines were synthesized selectively. The practical applicability of this tandem process was revealed by preparative scale reactions with different organic azides and synthesis of an anti-vertigo drug betahistine. Several kinetic experiments and DFT studies were carried out to understand the mechanism of this transformation.