Makoto Shimizu, Takayoshi Morimoto, Yusuke Yanagi, Isao Mizota and Yusong Zhu
RSC Adv., 2020,10, 9955-9963
DOI:
10.1039/D0RA01152E,
Paper
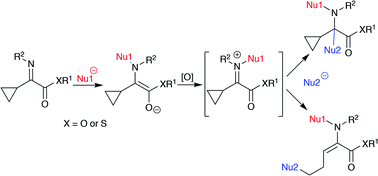
An umpolung N-alkylation reaction of α-cyclopropyl α-iminothioesters with diethylaluminum chloride or ethylmagnesium bromide affords the corresponding N-ethylated α-aminothioesters in good yields. Subsequent oxidation and reaction of the N-ethylated product with a thiolate or a chloride anion proceed effectively to give the ring-opened products in good yields. In contrast, relatively “hard” nucleophiles did not give the ring-opened products but gave the addition products to the iminium carbon.