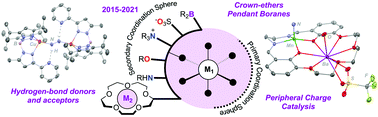
This tutorial review showcases recent (2015–2021) work describing ligand construction as it relates to the design of secondary coordination spheres (SCSs). Metalloenzymes, for example, utilize SCSs to stabilize reactive substrates, shuttle small molecules, and alter redox properties, promoting functional activity. In the realm of biomimetic chemistry, specific incorporation of SCS residues (e.g., Brønsted or Lewis acid/bases, crown ethers, redox groups etc.) has been shown to be equally critical to function. This contribution illustrates how fundamental advances in organic and inorganic chemistry have been used for the construction of such SCSs. These imaginative contributions have driven exciting findings in many transformations relevant to clean fuel generation, including small molecule (e.g., H+, N2, CO2, NOx, O2) reduction. In most cases, these reactions occur cooperatively, where both metal and ligand are requisite for substrate activation.