Karthik Gopakumar, Vivekananda Samantaray, Mithun Kumar Prusty, Lopita Swain and Rajeev Ramanan
Chem. Commun., 2023,59, 13054-13057
DOI:
10.1039/D3CC04283A,
Communication
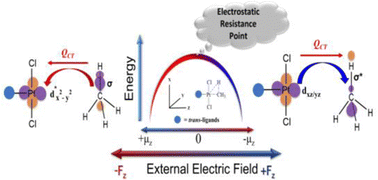
In a metal-catalyzed oxidative addition, an oriented external electric field (EEF) catalyzes the reaction along one direction and inhibits it when applied in the opposite direction. Beyond a threshold value, the inhibitory direction becomes catalyzing by swapping the metal-to-ligand charge transfer (MLCT) to ligand-to-metal charge-transfer (LMCT) or vice versa. The change in direction of the charge-transfer mechanism triggers the inversion of the dipole moment along the reaction axis, that results in the resurgence of catalysis. The charge-transfer mechanism in metal-catalyzed oxidative addition is tunable by EEF.