Extractive denitrogenation of fuel oils using ionic liquids: a review
Rashid Abro
a,
Masroor Abroa,
Shurong Gao a,
Abdul Waheed Bhutto
b,
Zeenat M. Alic,
Asif Shahd,
Xiaochun Chen
*a and
Guangren Yu
*a
a,
Abdul Waheed Bhutto
b,
Zeenat M. Alic,
Asif Shahd,
Xiaochun Chen
*a and
Guangren Yu
*a
aBeijing Key Laboratory of Membrane Science & Technology, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: gryu@mail.buct.edu.cn; chenxc@mail.buct.edu.cn; Fax: +86-10-6443-4892; Tel: +86-10-6443-4892
bDepartment of Chemical Engineering, Dawood University of Engineering and Technology, Karachi, Pakistan
cDepartment of Chemical Engineering, Mehran University of Engineering and Technology, Jamshoro, Pakistan
dKey Laboratory of Materials Modification by Laser, Ion, and Electron Beams (Ministry of Education), School of Materials Science and Engineering, Dalian University of Technology, Liaoning, 116024, P. R. China
First published on 23rd September 2016
Abstract
Elimination of nitrogen (N) compounds contained in fuel oils is one of the essential processes for petroleum refinery due of their hindering consequences on the hydrodesulfurization (HDS) process. Traditional hydrodenitrogenation (HDN) techniques have some barriers to produce lower-N or N-free fuel oils, e.g., HDN is less effective to remove some cyclic N-compounds; HDN is expensive because of operating conditions such as high pressure and high temperature, and also requires the presence of an expensive catalyst and hydrogen. Application of ionic liquids (ILs) for the purpose of fuel oil extractive denitrogenation (EDN) has been an important part of research in recent years, and it has shown huge potential as an effective substitute or supplemental technique to HDN. In the present review, we studied research results of EDN using ILs and have discussed widely the diversified factors influencing denitrogenation. This review concludes that EDN employing ILs has a promising future owing to the ideal physical and chemical characteristics of ILs; though for such a new technology there are some challenges, for which a discussion is also given. This review contributes proposals for possible commercial application of ILs in EDN.
1. Introduction
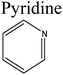
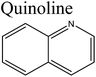
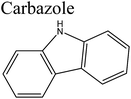
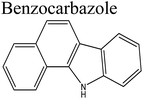
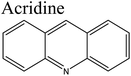
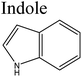
Removal of nitrogen (N) compounds in light petroleum fractions is very important due to their hindering influence on hydrodesulfurization (HDS) technology and the pollution resulting from NOX emissions in combustion processes.1–5 The N-species in fuel oils are either basic (where a lone pair of electrons of a ring-nitrogen atom is not in association with the aromatic system such as in pyridine, quinolone, acridine etc.) or non-basic (the aromatic rings and the electron lone pair of the nitrogen atom are part of the aromatic π electron system and where nitrogen atom is bound to hydrogen, such as in carbazole, pyrrole, indole etc.).6–8 Structures and properties of these N-compounds are given in Table 1. Legislation limiting N-content in fuel oils is strict in developed as well as in developing countries, e.g., in the case of diesel fuel, N-content has been limited from <70 ppm in 2003 to <1 ppm in 2010 in the USA.3 The traditional hydrodenitrogenation (HDN) technology broadly used in the petroleum refinery is not fully able to approach the continuously mounting needs of N-content in fuel oils because of its inability in eliminating some heterocyclic N-species due to steric factors affecting the catalyst performance. Furthermore, HDN requires relatively unfavorable operating conditions (e.g. noble catalyst, high pressure and high temperature) and so requires amendment or replacement by other denitrogenation methods.2,9,10In consideration of this, other methods such as extraction denitrogenation (EDN), oxidation denitrogenation (ODN), adsorptive denitrogenation (ADN) and biodenitrogenation (BDN), are currently under study.11–18 Among these, EDN is a desirable method because it only needs moderate operating conditions and maintains the structures of compounds in fuel oils. Previously, a few molecular solvents such as pyrimidinone, polyalkylene glycol, imidazolidinone, dimethyl sulfoxide and polyethylene glycol were analysed in EDN; however, the volatile behaviour of these solvents, combined with the small number of suitable extractants, prevented their utilization in industry owing the cost of loss of volatile solvent and difficulty in regeneration.
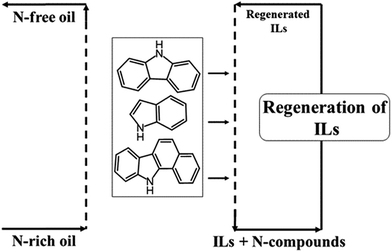
Ionic liquids (ILs) are also a candidate for EDN. ILs differ from traditional molecular compounds as they contain organic cations and organic/inorganic anions; as a result ILs have an insignificant vapour pressure. Moreover, most ILs have negligible or zero volatility (and thus ILs are considered as “green solvents”). In addition, ILs are highly stable both thermally as well as chemically, with a fabulous capability to dissolve and extract organic or inorganic compounds, they show a widespread liquidus range, are non-flammable and are easily recyclable. All these features favour ILs as solvents in EDN. Fig. 1 illustrates the flow diagram of general EDN using ILs, where extraction of N-compounds from the oil phase to the IL phase is conducted in the first stage and then either simple distillation is carried out for their removal from the IL phase (IL regeneration), taking advantage of the fact that ILs are non-volatile, or an alternative solvent is used for re-extraction employing adjustable solubility between the IL and the solvent.19–24
In recent years, EDN using ILs has been studied intensively. The research observations show good prospects for EDN using ILs as a substitute or supplemental method to HDN though some problems still occur. Several review articles have been published concerning denitrogenation of fuel oils,25–28 for example, Martinez-Palou et al.25 wrote a review article, concerning the applications of ILs for the removal of all types of pollutants including N-compounds from refinery feedstocks. Khan et al.26 summarized the already existing literature on the adsorptive removal of various hazardous compounds including N-compounds from fuel oils in his review paper using air by pristine or modified MOF materials. However, no review articles have focussed on EDN using ILs in detail. In this review paper, we have presented the research results of EDN using ILs and give wide-ranging discussions on a variety of factors for EDN, such as nature of ILs, cross solubility of ILs and oils, time, temperature, mass ratio of ILs and fuel oils, initial N-content, multiple extraction and regeneration. This review can be regarded as significant because of its possible contribution to not only experimental work on EDN using ILs but also on possible commercial application.
2. Extractive denitrogenation (EDN)
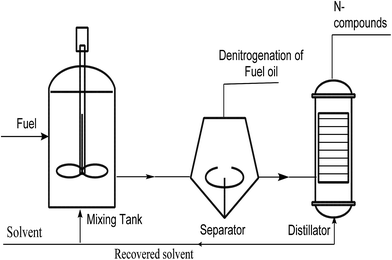
EDN is a simple and desirable technology, where N-compounds are separated from fuel oils without catalyst or hydrogen at mild temperature and pressure, as shown in Fig. 2. Additionally, EDN technology does not alter the structure of other compounds in fuel oils, and leads to the extraction of N-compounds which can be re-used. EDN is considered on the basis of different partitioning of N-compounds in both extractant as well as the oil phase. The proper selection of solvent for extraction is one of the major issues in EDN. As already discussed, there are certain issues when volatile solvents such as imidazolidinone, polyethylene glycol, pyrimidinone and dimethyl sulfoxide are used. The suitability of a solvent is determined on the basis of its efficient nature, reusability and recyclability, and due to these reasons researchers focus their aim on replacement of volatile organic compounds with green solvents. ILs as solvents exhibit a multiplicity of applications in purification/separation operations and a variety of ILs have been studied as solvents in EDN. Table 2 shows efficiencies of different ILs and other organic solvents for extractive denitrogenation.29–33| IL/organic solvent | Oil | T/°C | IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil oil |
N-Rem. (%) | Ref. |
|---|---|---|---|---|---|
| [Bmim]BF4 | Model diesel oil | RT | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60.0 | 30 |
| [Bmim]OcSO4 | Model diesel oil | RT | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
41.2 | 31 |
| [Emim]EtSO4 | Model diesel oil | RT | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
54.5 | 31 |
| [Bmim]Cl | Straight-run diesel feed (carbazole) | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
48.0 | 32 |
| [OPy]Cl | Straight-run diesel feed (carbazole) | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
58.0 | 32 |
| [Bmim]BF4 | Model oil (pyridine in n-C12) | RT | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
45 | 32 |
| [Emim]N(CN2) | Model oil (pyridine in n-hexane) | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
69.1 | 29 |
| [EtMe2S]N(CN2) | Model oil (pyridine in n-hexane) | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
59.8 | 29 |
| [S2]N(CN2) | Model oil (pyridine in n-hexane) | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
63.5 | 29 |
| [Bmim]N(CN2) | Model oil (pyridine in n-hexane) | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
72.7 | 29 |
| DMSO | Pyridine | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98.6 | 33 |
| Carbazole | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
79.9 | ||
| DMF | Pyridine | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96.4 | 33 |
| Carbazole | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
81.8 | ||
| Acetic acid | Pyridine | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
62.8 | 33 |
| Carbazole | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 |
3. Ionic liquids (ILs)
ILs, an emerging category of green solvents, are composed of organic/inorganic anions and organic cations. Being ionic and exhibiting non-volatility, ILs are dissimilar from organic molecular solvents. Furthermore, they are excellent solvents, interacting with organic as well as inorganic compounds, with a large liquidus range initiating from below ambient to well over 300 to 400 °C, as well as being highly chemically and thermally stable, and easily recycled. In historical context, the Friedel–Crafts acylation reaction was of great interest being central in the production of several medicines.34 Since its first report in 1887, this reaction was recognized as the method of choice to perform the alkylation of arenes and heteroarenes and form C–C and C-heteroatom bonds. Even though the Friedel–Crafts reaction is usually catalyzed by Lewis acids, it was also possible to accelerate the reaction by use of strong Brønsted acids (more environmentally benign choices involving alkylation by means of alcohols and styrenes have been implemented recently as an alternative to alkyl chlorides). The environmental aspects were however poor owing to the large amounts of VOCs generally utilized in these reactions. In this context, ILs soon started to be explored in this kind of reaction with interesting results. In 1914, Von Paul Walden synthesized ethylammonium nitrate ([EtNH3]NO3) as a molten salt35 while low melting salts with chloroaluminate ions for electroplating aluminum at decreased temperature were developed in 1951.36 In the 1970s and 80s, these ILs were investigated primarily for their role in electrochemical applications.37–41 Similarly, ILs with low melting point were suggested for use as solvents in organic synthesis. In the 1990s, it was increasingly appreciated that molten salts (mp < 100 °C) are unique media for chemical reactions, often being denoted by the term ‘room temperature ILs’ (RTILs). The properties of ILs according to a modern definition are summarized in Table 3.| Property | Description |
|---|---|
| Composition | Cation and/or anion quite large |
| Melting point | Preferably <100 °C |
| Liquidus range | Usually >200 °C |
| Thermal stability | Usually high |
| Chemical stability | Usually high |
| Viscosity | High, <100 cP workable in liquid–liquid extraction |
| Dielectric constant | Implied <30 |
| Polarity | High to moderate |
| Specific conductivity | Usually <10 mS cm−1 |
| Molar conductivity | <10 S cm2 mol−1 |
| Electrochemical window | >2 V |
| Solvent and/or catalyst | Excellent for many organic reactions |
| Vapor pressure | Often negligible |
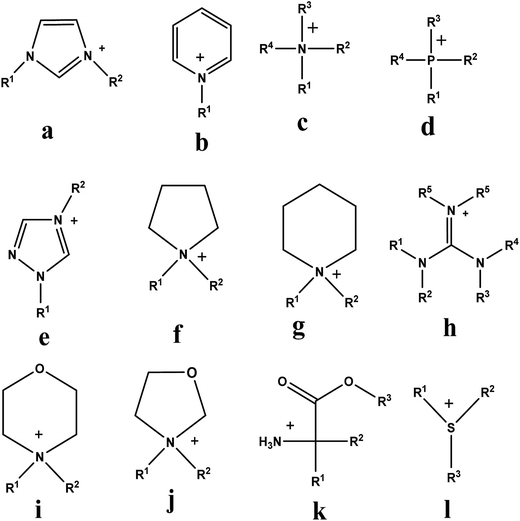
Fig. 3 shows common cation families of ILs while common anions include Cl/[AlCl3]−, N(CN2)−, Br−, Cl−, I−, NO3−, SO4−, CF3COO−, CF3SO2−, BF4−, PF6−, HSO4−, H2PO4− and CF3(SO2)2N−. In recent years, a number of publications have appeared which provide the results of EDN using ILs;29,30,30,32,42–51 ILs used in EDN are listed in Table 4.
| IL | Formula | Ref. |
|---|---|---|
| 1-Butyl-3-methylimidazolium dicyanamide | [Bmim]N(CN)2 | 29 |
| 1-Ethyl-3-methylimidazolium dicyanamide | [Emim]N(CN)2 | 29 |
| Ethylated tetrahydrothiophenium dicyanamide | [S2]N(CN)2 | 29 |
| Ethyldimethylsulfonium dicyanamide | [EtMe2S]N(CN)2 | 29 |
| 1-Butyl-3-methylimidazolium chloride | [Bmim]Cl | 32 |
| 1-Octylpyridinium chloride | [OcPy]Cl | 32 |
| 1-Butyl-3-methylimidazolium tetrafluoroborate | [Bmim]BF4 | 30 |
| 1-Butyl-3-methylimidazolium octyl sulfate | [Bmim]OcSO4 | 31 |
| 1-Ethyl-3-methylimidazolium ethyl sulfate | [Emim]EtSO4 | 31 |
| 1-Butyl-3-methylimidazolium chloride/ZnCl2 | [Bmim]Cl/ZnCl2 | 42 |
| 1-Butyl-3-methylimidazolium hydrogen sulfate | [Bmim]HSO4 | 42 |
| 1-Hexyl-3-methylimidazolium hydrogen sulfate | [Hmim]HSO4 | 42 |
| 1-Ethyl-3-methylimidazolium tetrafluoroborate | [Emim]BF4 | 30 |
| 1-Ethyl-3-methylimidazolium ethyl sulfate | [Emim]EtSO4 | 43 |
| 1-Pentyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide | [Pmim]Tf2N | 43 |
| 1-Hexyl-3,5-dimethylpyridinium bis(trifluoromethylsulfonyl)imide | [HmmPy]Tf2N | 43 |
| 1-Benzyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide | [Bzmim]Tf2N | 43 |
| 1-Heptyl-2,3-dimethylimidazolium bis(trifluoromethylsulfonyl)imide | [Hpmmim]Tf2N | 43 |
| 1-Decyl-2,3-dimethylimidazolium bis(trifluoromethylsulfonyl)imide | [Ocmmim]Tf2N | 43 |
| 3-Methyl-N-butylpyridinium dicyanamide | [3-mebupy]N(CN)2 | 44 |
| 4-Methyl-N-butylpyridinium dicyanamide | [4-mebupy]N(CN)2 | 44 |
| 4-Methyl-N-butylpyridinium trithiocyanate | [4-mebupy]C(CN)3 | 44 |
| 1-Ethyl-3-methylimidazolium ethyl sulfate–zinc chloride | [Emim]ZnCl2(EtSO4) | 45 |
| 1-Butyl-3-methylimidazoliumtrifluoromethanesulfonate | [Bmim]OTf | 46 |
| 1-Ethyl-3-methylimidazole acetate | [Emim]OAc | 47 |
| 1-Ethyl-3-methylimidazolium ethyl sulfate | [Emim]EtSO4 | 47 |
| 1-Ethyl-3-methylimidazolium methanesulfonate | [Emim]MeSO4 | 47 |
| 1-Butyl-3-methylimidazolium tricyanomethanide | [Bmim]TCM | 48 |
| 1-Butyl-1-methylmorpholinium tricyanomethanide | [Bmomr]TCM | 48 |
| 1-Butyl-1-methylpyridinium tricyanomethanide | [Bmpy]TCM | 48 |
| N-Butylmethylimidazolium dihydrogen phosphate | [Bmim]H2PO4 | 49 |
| 1-Methyl-3-(3-sulfopropyl)imidazolium dihydrogen phosphate | [Mimps]H2PO4 | 49 |
| 1-Ethyl-3-methylimidazolium ethyl sulfate | [Emim]EtSO4 | 50 |
| 1-Ethyl-3-methylpyridinium ethyl sulfate | [Empy]EtSO4 | 50 |
| 1-Ethyl-3,5-dimethyl-2-pentylpyridinium bis(trifluoromethylsulfonyl)imide | [1B3M5M2PPy]NTf2 | 51 |
| 1-Butyl-3,5-dimethyl-2-pentylpyridinium dicyanamide | [1B3M5M2PPy]N(CN)2 | 51 |
4. ILs in EDN: a short history
Eber et al. (2004), investigated for the first time the N-removal efficiency using ILs [Bmim]OcSO4 and [Emim]EtSO4 from fuel oils. The investigation was based on indole, piperidine and pyridine N-compounds.31 It was found that indole N-compounds were removed efficiently with high partition coefficient (KN) 340 mg(N) kg(IL)−1 per mg(N) kg(oil)−1. In the same year, Zhang et al. studied N-removal efficiency of fuel oils using BF4, PF6 and AlCl3-TMAC anion based ILs.30 Pyridine and 2-picoline were observed to be fully dissolvable with [Bmim]BF4, while the corresponding saturated compounds, piperidine and 2-methylpiperidine showed only a small degree of extraction. The AlCl3-TMAC IL was found to have outstanding capacities for extracting aromatics. Significant reduction in the absorption capacity was also found if a methyl group was present on the aromatic rings, which might be possible due to a steric effect. Similar results were obtained with chloride based-ILs i.e., [Bmim]Cl, 1-allyl-3-[Almim]Cl, [Bzmim]Cl and [Ocmim]Cl when investigated for EDN applications.32EDN efficiency of low viscous cyanamide-based ILs with imidazolium, thiophenium, tetrahedral trialkylsulfonium and pyridinium cations was also investigated by Asumana et al. and Hansmeier et al. Both results supported the fact that ILs show high capability to purify fuel oils from N-compounds. Moreover, carbazole was extracted with more efficiency in comparison to pyridine. This extraction capability of imidazolium and pyridinium ILs was better than for other cation based ILs.
Bronsted acid ILs have also been reported for EDN. Chen et al.42 and Wang et al.49 investigated the EDN efficiency of Bronsted acid ILs [Bmim]Cl/ZnCl2, [Bmim]Cl/2ZnCl2, [Bmim]HSO4, [Hmim]HSO4, [Bmim]H2PO4 and [Mimps]H2PO4 using pyridine, neutral carbazole and quinoline as representative N-compounds. It was determined that these ILs have high capability to remove the N-compounds from fuel oils. Typically, using [Bmim]Cl/ZnCl2 in extraction (temperature: 25 °C; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) IL
1 (w/w) IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil), 93.8% of carbazole and 98% pyridine removal was recorded after only a single stage extraction and the N-contents were become indiscernible after two-stage extraction, while ILs ([Bmim]H2PO4, and [Mimps]H2PO4) were also found to be efficient for quinolone extraction. Experimental results thus show the acidity of ILs also contributes to their EDN efficiency.42,49
oil), 93.8% of carbazole and 98% pyridine removal was recorded after only a single stage extraction and the N-contents were become indiscernible after two-stage extraction, while ILs ([Bmim]H2PO4, and [Mimps]H2PO4) were also found to be efficient for quinolone extraction. Experimental results thus show the acidity of ILs also contributes to their EDN efficiency.42,49
Vilas et al.51 measured the liquid–liquid equilibrium (LLE) data for ternary mixtures of heptane, nitrogen compound and IL at a temperature of 298.15 K and atmospheric pressure. The variation of selectivity (S) and solute distribution ratio (β) vs. aromatic compound content in the IL-rich phase is plotted in Fig. 4, which shows an increase in aromatic content leads to decreasing S and β values. Here, β is defined as the ratio of solute concentration in the extract phase to that of in the raffinate phase as given in eqn (1) and S is defined as the ratio of solute distribution coefficient to the distribution coefficient of hydrocarbon (HC) as given in eqn (2).
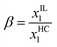 | (1) |
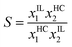 | (2) |
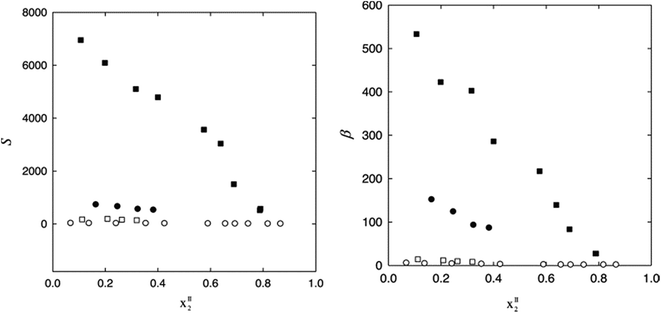 | ||
| Fig. 4 Selectivity, S, and solute distribution ratio, β, as a function of the aromatic compound mole fraction in the IL-rich phase, for the ternary systems: (○) {heptane (1) + thiophene (2) + [1B3M5M2PPy][NTf2] (3); (●) {heptane (1) + pyrrole (2) + [1B3M5M2PPy][NTf2] (3)}; (■) {heptane (1) + pyrrole (2) + [1B3M5M2PPy][N(CN)2] (3)} and (□) {heptane (1) + pyridine (2) + [1B3M5M2PPy][N(CN)2] (3)} at T = 298.15 K.51 | ||
Fig. 4 shows that the {heptane (1) + pyrrole (2) + [1B3M5M2PPy][N(CN)2] (3)} system achieved highest S and β values.51 The corresponding triangular diagram is shown in Fig. 5. As expected, heptane is less soluble than pyrrole in the studied ILs, which could be attributable to π–π interactions between pyrrole and the aromatic core of the IL cations.
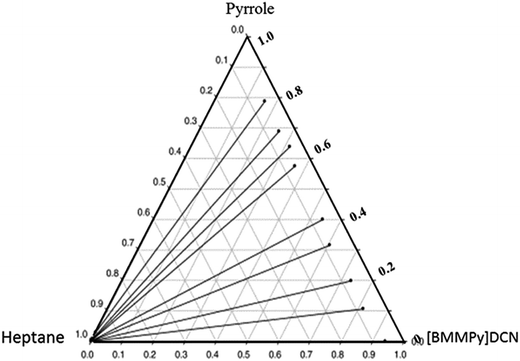 | ||
| Fig. 5 Experimental tie-lines for the LLE for the ternary mixture {heptane (1) + pyrrole (2) + [1B3M5M2PPy][DCN] (3)} at T = 298.15 K.51 | ||
According to the experimental studies by Domanska and Lukoshko, equilibrium compositions of the experimental tie-line ends in three ternary systems {IL (1) + pyridine (2) + heptane (3)} at T = 298.15 K and ambient pressure revealed almost the absence of IL in the raffinate (heptane) layer after LLE experiment which suggest that “entrainer lost” should not be expected.48 Absence of ILs in the raffinate phase is favourable for industrial applications because fewer steps, and ultimately less energy, is required to recover the solvent for recycling.52
The order of the extraction applicability given by Domańska and Lukoshko is: [Bmmor]TCM (S and β are in range 5.9–609.3 and 1.38–9.20, respectively) > [Bmpy]TCM (S and β are in range of 10.7–578.8 and 1.46–9.42, respectively) > [Bmim]TCM (S and β are in range of 30.3–540.3 and 2.47–11.30, respectively).48
In the non-random two-liquid (NRTL) model, the non-randomness of the mixture is measured by the non-randomness parameter (αij). The mixture is considered to be an ideal solution (i.e. completely random) when the value of αij is zero. LLE data for ternary systems of different ILs with different nitrogen compounds and model fuels were determined both experimentally and by the NRTL model by Domanska et al. with [Bmim]OTf and [Omim]NTf2 and tricyanomethanide-based ILs of [Bmim], [Bmmor] and [Bmpy] cations. It was observed that all these ILs have good capacity for denitrogenation. They enable full denitrogenation from fuel oils in three/four extraction steps with low amount of IL.
Ceron et al.53 synthesized 56 ILs in parallel under microwave irradiation and they were evaluated as extracting agents for N-containing compounds from a real fuel feed before being subjected to HDS to acquire zero-sulfur oil. Halogenated ILs are found as an excellent alternative since these ILs are relatively inexpensive, affirming a high selectivity for the extraction of N-containing compounds with positive regeneration and recycling ability.
5. Interaction between ILs and N-compounds
ILs are considered to have grids of both the anions and cations structured together by dispersive, coulombic forces and hydrogen bonds. The presence of these strong interactions between the ions causes moderately higher viscosity of ILs as compared to an organic solvent. Influence of solvent on chemical processes is understood in terms of its polarity. Though a sufficient literature on the experimental measurement of solvent polarity is available, still the nature of interactions is needed to be clarified. To the best of our knowledge, there are several studies investigating the denitrogenation efficiency of ILs, whereas literature relevant to interaction between ILs and N-compounds is limited.Chen et al.42 investigated EDN efficiency of acidic ILs for basic (i.e., pyridine) and non-basic (i.e., carbazole) N-compounds. An interesting result was found: whereas for the basic pyridine, EDN efficiency is 97%, for the non-basic carbazole, the efficiency is variable: 93.2, 90.1, 71.2 and 24.2% for [Bmim]Cl/ZnCl2, [Bmim]HSO4, [Bmim]Cl/2ZnCl2 and [Hmim]HSO4, respectively; such different extractive capabilities for the non-basic compound might be ascribed to their different anionic nature and length of alkyl side chain on the cation.42
Ramalingam et al.54 predicted the energetic and structural characteristics of the complexes between [EMIM]EtSO4 and various N-compounds by application of quantum chemical calculations. [EMIM]EtSO4 IL can be easily synthesized, recovered and regenerated under the prescribed conditions by dilution with water followed by distillation ([EMIM]EtSO4: molecular weight; 236 g, flash point; 162 °C, density; 1.24 g mL−1, refraction index; 1.481, melting point; <−30 °C, polar surface area; 8.81 Å). In that study an ab initio method was used for the determination of the interaction energies, structure, partial charges of atom in the molecules, highest occupied molecular orbital (HOMO) energy, lowest unoccupied molecular orbital (LUMO), chemical potential, chemical hardness and softness, electronegativity and electrophilicity index of Emim, EtSO4 and [Emim]EtSO4 interacting with pyrrole, quinoline, benzoquinoline, pyrrole, indole, indoline, carbazole and benzocarbazole. During the formation of interactive bonds between similar and dissimilar molecules their structure is of central importance. Charges in arrangement in the surrounding of molecules is found to be influential towards the absorption capacity. Both HOMO and LUMO energy play an important role in the interaction between low-reactivity N-molecules and [EMIM]EtSO4. For effective interaction between [EMIM]EtSO4 and N-molecules, orbital level interaction has been proved to be among the main parameters. Electronegativity, chemical hardness, chemical potential and electrophilicity index of all N-compounds also influence their behavior and interaction will depend on ease of polarization of both electron donors and acceptors. After the separation process, the easy recovery and regeneration of [Emim]EtSO4 under the supposed conditions has been indicated by the low chemical softness of individual molecules as compared to the complexes. Based on that, [Emim]EtSO4 IL is regarded as a potential solvent to separate all types of N-compounds contained in diesel oil under normal conditions. Xie et al.,55 in their study, focused upon the possible significance of hydrogen bond basicity of chloride-based ILs to achieve the separation of compounds with a hydrogen-donor group (i.e. neutral N-compounds) existing in hydrocarbon feeds with a high selectivity towards basic N-compounds and sulfur-containing compounds.
Hizaddin et al.52 performed chemical calculations using ab initio quantum methods to determine the optimized geometry of individual cations, anions, heterocyclic N-compounds, complexes of ILs, and complexes of heterocyclic N-compounds with eighteen ILs. The interaction between ILs and heterocyclic N-compounds due to CH–π and π–π bonding between the cations and N-heterocycles and hydrogen bonding between anion heteroatoms and heterocyclic N-compounds has been verified by their optimized structure with ILs. Calculation of partial charge transfer, global scalar properties, orbital energy values and interaction energy has been done for each individual species as well as in the complexes. According to the obtained results ILs are identified as being suitable for the denitrification of fuel oils by extraction. The obtained results strongly suggest all of the eighteen ILs mentioned in present work as potential solvents for denitrification of fuel oils due to favorable interactions as indicated by orbital energies, global scalar properties, interaction energy, partial charge transfer and sigma profiles. Although, some ILs undergo effective interaction with heterocyclic N-compounds on the basis of each parameter investigated, each parameter also serves to rank ILs on the basis of favorable interaction with N-compounds. Based on LUMO energy values, HOMO–LUMO gap, global softness and electrophilicity index, [Epy]EtSO4 is the most favorable IL for heterocyclic N-compound extraction. However, on the basis of interaction energy and partial charge transfer, [TMPYRA][Ac] is a more preferable IL. The σ-profile and σ-potential of each cation, anion and N-heterocycle confirm mutual interaction among the three species. Based on σ-profile and σ-potential, ILs with aromatic ring cations ([Emim], [Epy] and [TMPYRA]) have better capacity in donating hydrogen bonds as compared to saturated cations, thus, enabling better CH–π interaction with heterocyclic N-compounds.
6. Key factors for EDN in ILs
Overall literature have suggested that ILs are efficient for extracting N-compounds from fuel oils. However optimization of factors influencing EDN using ILs requires more research. These factors include the nature of the ILs, cross solubility, regeneration capability, time, temperature, multiple extraction, initial N-contents and oil/IL mass ratio. The following sub-divisions discuss EDN using ILs in view of these factors.6.1. Extractive equilibrium time (EET)
EET is an imperative factor of extraction because short extraction times favor greater yield of product or reduced size contact device in industry. As per observations from literature, the EET of EDN using ILs is related to IL viscosity and nature of anion–cation. Most high viscous ILs required >15 min to reach equilibrium. These include [Bmim]ZnCl2, [Bmim]2ZnCl2, [Bmim]HSO4, [Hmim]HSO4 (Fig. 6 and 7),42 whereas low viscous ILs such as [Bmim]N(CN)2, [EtMeS]N(CN)2, [S2]SCN and [Emim]N(CN)2 attain equilibrium in approximately 5–10 min (Fig. 8 and 9).29 A likely conclusive reason behind such differences could be that the low viscous ILs have better dispersion rate in fuel oils as compared to high viscous ILs, which causes rapid mass transfer of N-species to the IL phase from the fuel oil phase.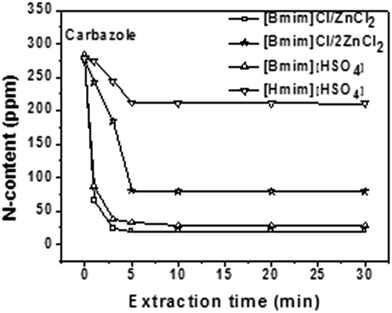 | ||
| Fig. 6 Time for the extractive removal of carbazole from fuel oils using high viscous ILs.42 | ||
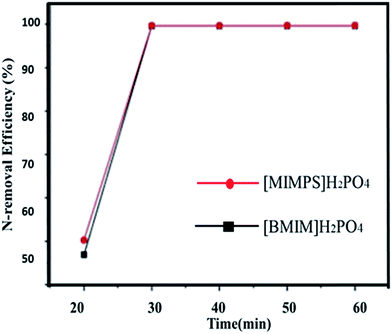 | ||
Fig. 7 Effect of time on N-removal efficiency (quinolone). Conditions: IL/oil = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, IL/H2O: 2 10, IL/H2O: 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, T = 25 °C, settlement time = 30 min.49 1, T = 25 °C, settlement time = 30 min.49 | ||
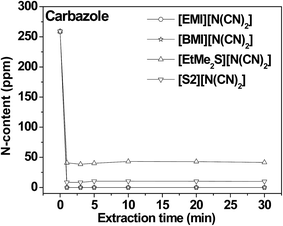 | ||
| Fig. 8 Time for the extractive removal of carbazole from fuel oils using low viscous ILs.29 | ||
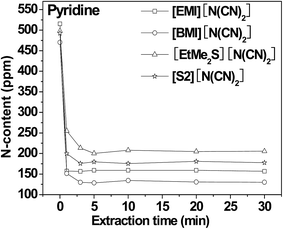 | ||
| Fig. 9 Time for the extractive removal of pyridine fuel oils using low viscous ILs.29 | ||
6.2. Nernst partition coefficient (KN)
KN is an important physiochemical equilibrium constant parameter, being the ratio of the concentrations of each compound within the two phases. Mathematically it can be calculated as:
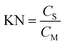 | (3) |
Dearden et al.56 examined the factors that can affect the measurement of partition coefficients. It was reported that the accuracy of partition coefficient determination can be affected by temperature, lack of mutual phase saturation, pH, buffer type and concentration, phase miscibility, solute concentration, solute solvent purity, solute stability, phase volume ratio, solute adsorption and failure to reach equilibrium conditions.
Overall it is found from the literature of EDN using ILs, that the N-Nernst partition coefficient is not too sensitive to the mass ratio of IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil in EDN under different conditions. This kind of inertness is helpful for industrial application because high N-removal can then be possible with smaller IL
oil in EDN under different conditions. This kind of inertness is helpful for industrial application because high N-removal can then be possible with smaller IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil ratio (i.e., economizes on ILs). Fig. 10 and 11 depict the N-Nernst partition coefficient of [Bmim]N(CN)229 and oil at different initial N-contents and temperatures, respectively.
oil ratio (i.e., economizes on ILs). Fig. 10 and 11 depict the N-Nernst partition coefficient of [Bmim]N(CN)229 and oil at different initial N-contents and temperatures, respectively.
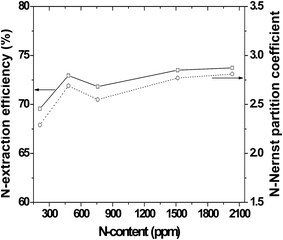 | ||
| Fig. 10 N-Extraction efficiency and KN-Nernst partition coefficient by [Bmim]N(CN)2 for pyridine-containing fuel oil at different initial N-contents.29 | ||
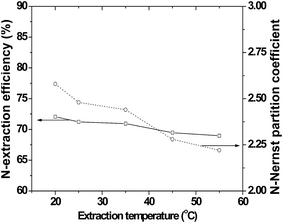 | ||
| Fig. 11 N-Extraction efficiency and KN-Nernst partition coefficient by [Bmim]N(CN)2 for pyridine-containing fuel oil at different extraction temperatures.29 | ||
6.3. IL/oil mass ratio
IL/oil mass ratio is another significant factor to select ILs for EDN. Since ILs are comparatively expensive as compared to other solvents, use of as small amounts as possible in fuel oil EDN should be aimed at. However, the literature reveals the increasing efficiency of EDN with increasing IL/oil mass ratio. Table 5 shows the effect on EDN with increasing IL/oil mass ratio. For example, [Bmim]N(CN2)2–oil mass ratios were analysed under mild conditions wherein descending N-extraction efficiency was found with the order of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 > 1
1 > 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 > 1
1 > 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 > 1
2 > 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 > 1
3 > 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 > 1
4 > 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 IL/oil mass ratios, while the N-partition coefficients were not shown to be highly sensitive to the IL/oil mass ratio. Similar results were observed when other ILs were used to predict the influence of IL/oil mass ratio.29 Notwithstanding increasing level of extraction efficiency by the enhancing IL/oil mass ratio, it was observed that the degree of increase is not at an identical rate for different ILs. This usually depends on the specific chemical nature of ILs in correspondence with their abilities of extraction.
5 IL/oil mass ratios, while the N-partition coefficients were not shown to be highly sensitive to the IL/oil mass ratio. Similar results were observed when other ILs were used to predict the influence of IL/oil mass ratio.29 Notwithstanding increasing level of extraction efficiency by the enhancing IL/oil mass ratio, it was observed that the degree of increase is not at an identical rate for different ILs. This usually depends on the specific chemical nature of ILs in correspondence with their abilities of extraction.
| IL | N-Compound | N-Extraction efficiency for IL–oil mass ratio | Conditions | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
||||
| a INC is initial N-content. | ||||||||
| [Bmim]Cl/ZnCl2 | Pyridine | 98 | 98 | 97 | 97 | 97 | INC: 483 ppm, fuel oil: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) IL 1 (w/w) IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil; t: 20 min, T: 298 K oil; t: 20 min, T: 298 K |
42 |
| [Bmim]Cl/ZnCl2 | Carbazole | 98 | 93 | 86 | 81 | 71 | INC: 279 ppm, fuel oil: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) IL 1 (w/w) IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil; t: 20 min, T: 298 K oil; t: 20 min, T: 298 K |
42 |
| [Bmim]N(CN)2 | Pyridine | 83 | 72.1 | 57 | 46 | 36 | INC: 483 ppm, fuel oil: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) IL 1 (w/w) IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil; t: 20 min, T: 298 K oil; t: 20 min, T: 298 K |
29 |
| [Bmim]N(CN)2 | Carbazole | n/a | n/a | 95 | 92 | 87 | INC: 258 ppm, fuel oil: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) IL 1 (w/w) IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil; t: 20 min, T: 298 K oil; t: 20 min, T: 298 K |
29 |
| [Pmim]Tf2N | Pyridine | n/a | 70 | 57 | n/a | ∼40 | Fuel oil: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) IL 1 (w/w) IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil; t: 20 min, T: 298 K oil; t: 20 min, T: 298 K |
43 |
| [HmmPy]Tf2N | Pyridine | n/a | 68 | 51 | n/a | 39 | Fuel oil: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) IL 1 (w/w) IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil; t: 20 min, T: 298 K oil; t: 20 min, T: 298 K |
43 |
In addition to the positive effect of enhancing IL/oil mass ratio, it has to be selected with care on the basis of a compromise between N-removal and fuel oil recapture. Furthermore the capacity to regenerate ILs must also be taken into consideration since it vastly contributes the total cost of process.
6.4. Initial N-content
For an ideal EDN using ILs, the actually existing N-content in the oil should have little or no influence on the efficiency of extraction. Asumana et al. studied the N-extraction efficiency of [Bmim]N(CN)2,29 for pyridine-containing fuel oil of initial N-content of 200, 500, 800, 1500 and 2000 ppm. The result is shown in Fig. 10 which clearly shows that the efficiency to remove nitrogen does alter with different N-contents in oil, but that the change is not significant; there is a variance of only 4.2% N-removal efficiency between 200 and 2000 ppm. This insensitivity of N-content to N-extraction is valuable to industrially process fuel oils containing a broad range of N-compounds.296.5. Temperature
Temperature is also a significant factor while selecting ILs for EDN. It is preferable to achieve maximum efficiency in any process at or near room temperature. The literature on EDN using ILs show that there is very small influence of temperature on N-extraction efficiency, which is a very positive attribute for the EDN process. The influence of temperature on EDN using ILs are displayed in Table 6, which shows that low viscous ILs, such as [Bmim]N(CN)2 and [Emim]N(CN)2,29 and Bronsted acidic ILs such as [Bmim]H2PO4 and [Mimps]H2PO4,49 show either a slight decline or increase for N-extraction efficiency and the corresponding S-partition coefficient, with increasing temperature.| IL | N-Compound | Time/min | N-Extraction efficiency for temperatures (%) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| 20 °C | 25 °C | 35 °C | 45 °C | 55 °C | ||||
| [Bmim]/Cl/ZnCl2 | Pyridine | 20 | 98 | 98 | 97.2 | 96 | 95.5 | 42 |
| Carbazole | 20 | 95 | 94 | 93.8 | 93.3 | 91 | 42 | |
| [Emim]N(CN)2 | Pyridine | 20 | 72 | 71.2 | 70.4 | 69.5 | 68.9 | 29 |
| [Bmim]H2PO4 | Quinoline | 20 | 99 | 99.6 | 99.6 | 99.7 | 98.3 | 49 |
| [Mimps]H2PO4 | Quinoline | 20 | 99 | 99.6 | 99.7 | 99.7 | 98.4 | 49 |
6.6. Multiple extractions
In single-step EDN, it is extremely challenging to diminish the N-species in fuel oils to the legislative requirement, a fact that has imposed more than one extraction step. This is because of N-compound partitioning between fuel oils and ILs which makes perfect removal of N-species in a single extraction impossible. [Emim]N(CN)2 reduced pyridine N-compounds in model diesel from ∼480 to ∼134 ppm (74% efficiency) in a single extraction at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) IL–oil, 25 °C for 20 min, and ∼15 ppm (about 97% efficiency) was achieved after completion of three extraction cycles. In the same mixture, [Bmim]Cl/ZnCl2 realized an almost 98% S-removal after two cycles as opposed to 87% in a single extraction. [Emim]EtSO4, [Bmim]N(CN)2 and [HmmPy]Tf2N removed pyridine completely from model fuel oils in three steps. All these researches show that a multiple-extractive process is an outstanding way to lessen N-content to near or below the desirable extent as indicated in Table 7.
1 (w/w) IL–oil, 25 °C for 20 min, and ∼15 ppm (about 97% efficiency) was achieved after completion of three extraction cycles. In the same mixture, [Bmim]Cl/ZnCl2 realized an almost 98% S-removal after two cycles as opposed to 87% in a single extraction. [Emim]EtSO4, [Bmim]N(CN)2 and [HmmPy]Tf2N removed pyridine completely from model fuel oils in three steps. All these researches show that a multiple-extractive process is an outstanding way to lessen N-content to near or below the desirable extent as indicated in Table 7.
| IL | Oil | N-Compound | Initial N-content | IL–oil mass ratio | Time/min | N-Extraction efficiency for multiple steps (%) | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||||
| [Bmim]N(CN)2 | Diesel | Pyridine | 479 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 | 74 | 42.1 | 96.8 | n/a | 29 |
| [Emim]EtSO4 | Gasoline | Pyridine | n/a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
20 | 27.5 | 49.1 | 70.5 | 70.5 | 29 |
| Diesel | Pyridine | n/a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
20 | 30.2 | 45.4 | 66.1 | 86.2 | ||
| [Pmim]Tf2N | Gasoline | Pyridine | n/a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
20 | 45.6 | 58.6 | 70.2 | 78.6 | 43 |
| Diesel | Pyridine | n/a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
20 | 35.1 | 49.6 | 76.9 | 87.8 | 43 | |
| [HmmPy]Tf2N | Gasoline | Pyridine | n/a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
20 | 39.2 | 45.8 | 65.7 | 75.7 | 43 |
| Diesel | Pyridine | n/a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
20 | 7.43 | 58.7 | 71.6 | 98.9 | 43 | |
| [BZmim]Tf2N | Gasoline | Pyridine | n/a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
20 | 36.2 | 54.7 | 68.4 | 70.2 | 43 |
| Diesel | Pyridine | n/a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
20 | 42.8 | 62.6 | 72.6 | 80.6 | 43 | |
| [HPmmim]Tf2N | Gasoline | Pyridine | n/a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
20 | 32.1 | 53.3 | 67.6 | 70.6 | 43 |
| Diesel | Pyridine | n/a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
20 | 41.1 | 65.5 | 78.7 | 81.0 | 43 | |
| [Dmmim]Tf2N | Gasoline | Pyridine | n/a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
20 | 32.5 | 54.3 | 67 | 77.1 | 43 |
| Diesel | Pyridine | n/a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
20 | 38.9 | 60.4 | 74.0 | 81.5 | 43 | |
| [Bmim]Cl/ZnCl2 | Pyridine | n/a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
30 | 99 | n/a | n/a | n/a | 42 | |
| Carbazole | 279 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
30 | 87.5 | n/a | n/a | n/a | 42 | ||
7. Regeneration and reuse of ILs
Regeneration of ILs after any process is a major feature for selectivity of ILs. However, while the cost of ILs is high, it can be recompensed by their recyclability and regeneration. There are various techniques to regenerate ILs, which depend on the nature of the ILs and N-compounds. Hydrophobic ILs are usually regenerated by distillation coupled by adsorption, and hydrophilic ILs are regenerated by dilution with water followed by distillation. In most situations, the actual structure of ILs is found to be unaffected after regeneration. After regeneration, ILs can be reused without a noticeable decrease in efficiency for model oils as presented in the literature. ILs such as [Bmim]N(CN)2, [Emim]N(CN)2, [Bmim]Cl/ZnCl2, [Mbpy]N(CN)2, [Bmim]H2PO4 and [Mimps]H2PO4 showed remarkable results after their regeneration. Furthermore it is also found in the literature that hydrophilic ILs such as [Bmim]H2PO4, [Mimps]H2PO4 and [Bmim]Cl/ZnCl2 can be more easily regenerated as compared to hydrophobic ILs.42,49 The influence of regeneration of low viscous ILs on EDN is shown in Fig. 12 and 13.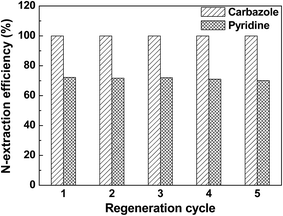 | ||
Fig. 12 N-Extraction efficiency vs. regeneration cycle of [Bmim][N(CN)2] for carbazole- and pyridine-containing fuel oils (temperature: 25 °C; mass ratio of IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil = 1 oil = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; extraction time: 20 min).29 1; extraction time: 20 min).29 | ||
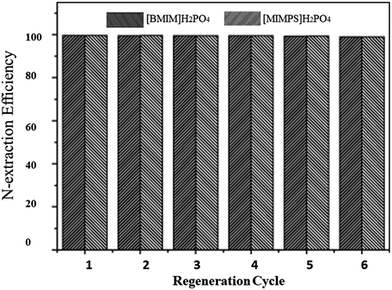 | ||
Fig. 13 N-Extraction efficiency vs. regeneration cycle of [Bmim]H2PO4 and [Mimps]H2PO4 for quinolone – containing fuel oils (temperature: 25 °C; mass ratio of IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil = 1 oil = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10; extraction time: 30 min).49 10; extraction time: 30 min).49 | ||
As shown in Fig. 12, [BMI]N(CN)2; after five cycles, 100% N-extraction efficiency for carbazole-containing fuel oil is maintained, while for pyridine, N-extraction efficiency is slightly reduced from 72 to 70%.29 Similar trends were observed for other studies of EDN using ILs. Therefore ILs have proven to be not only good extractants but also good candidates for industrial application.
8. Cross solubility
Due to two main reasons, the cross solubility of fuel oils and ILs is a considered as a vital feature in EDN using ILs. These reasons are (1) obvious dissolution of ILs in fuel oils causes loss of ILs and contamination of fuel oils, which may vary their composition and amount; (2) solubility of fuel oils in ILs results an increase in the cost of the process and loss of fuel oil. Various methods have been used to measure the cross solubility, such as vacuum drying, liquid chromatography and gravimetry. For application of ILs as solvents in fuel oil, zero or very minor cross solubility of fuel oils in ILs is required.32,57–65Many researchers have investigated ILs efficiency for extractions of impurities from fuel oils, but few had addressed this important issue that could circumvent the applicability of certain ILs. Overall, it is simply presumed that ILs do not dissolve into fuel oils due to their ionic and high polar nature. However, some researchers observed a degree of solubility of ILs with fuel oils, as shown in Table 8. Jiang et al. calculated model gasoline solubility in ILs, [Mmim]DMP 1.1%, [Emim]DEP 4.2% and [Bmim]DBP 20.6 wt%, at 298 K. Also, solubility values of gasoline, 2.06 wt% in [Emim]DMP, 5.81 in [Emim]DEP, 26.69 in [Beim]DBP, 3.53 in [Bmim]DMP and 10.2 in [Beim]DEP were obtained. While analyzing solubility of model oil in [Bmim]N(CN)2 and [Emim]N(CN)2, it was observed to be insignificantly soluble at a low content of toluene but it was found to raise by increasing the concentration of toluene in model oil (e.g., for [Bmim]N(CN)2, 6.7 wt% for 20% mass fraction of toluene; for [Emim]N(CN)2, 3.08 wt% for 15% mass fraction of toluene). Hansmeier et al. observed that the solubility of [Bmpy]BF4 and [Emim]EtSO4 in n-heptane was 0.3 and 0.9 mol%, respectively. The study of solubility of various ILs with different organic compounds has also been carried out. Most ILs have a degree of solubility in a few organic compounds such as acetone, ethanol, methanol and water, but much less so in hexane, toluene or heptane. The literature regarding cross solubility of liquid fuels and ILs suggests that cross solubility of ILs with fuel oils has to be given more attention in studies to optimize various future applications.
| IL | Fuel | Oil solubility in IL (wt% or ppm) | IL solubility in oil (wt%) | Ref. |
|---|---|---|---|---|
| [Mmp]DMP | n-Octane | 1.17 | 0.04 | 57 |
| [Mep]DEP | n-Octane | 5.83 | 0.14 | 57 |
| [Hmp]DMP | n-Octane | 0.46 | n/a | 57 |
| [Hmp]DMP | n-Octane | 4.56 | 0.09 | 57 |
| [Emim]DMP | Gasoline | 20.6 | n/a | 58 |
| [Emim]DEP | Gasoline | 5.81 | n/a | 58 |
| [Beim]DBP | Gasoline | 26.9 | n/a | 58 |
| [Mmim]DMP | Gasoline | 11.2 | n/a | 58 |
| [Emim]DEP | Gasoline | 42.5 | n/a | 58 |
| [Bmim]DBP | Gasoline | 20.6 | n/a | 58 |
| [Emim]DEP | Gasoline | 2.25 | n/a | 59 |
| [Bmim]DBP | Gasoline | 20.6 | n/a | 59 |
| [Mmim]DMP | Gasoline | 1.12 | n/a | 60 |
| [Emim]DEP | Diesel | 4.25 | n/a | 60 |
| [Bmim]DBP | Diesel | 20.6 | n/a | 60 |
| [Bmim]DMP | Diesel | 35.5 | n/a | 61 |
| [Bpy]BF4 | Model oil | 0.49 | n/a | 62 |
| [Opy]BF4 | Diesel | 1.97 | n/a | 62 |
| [Bmim]/Cl | Diesel | 25 ppm | n/a | 32 |
| [Almim]Cl | Diesel | 25 ppm | n/a | 32 |
| [Bzmim]Cl | Diesel | 35 ppm | n/a | 32 |
| [Ocmim]Cl | Diesel | 370 ppm | n/a | 32 |
| [OcPy]Cl | Diesel | 15 ppm | n/a | 32 |
| [Bmim]OCSO4 | n-Dodecane | 4.0 | n/a | 31 |
| 1-Octane | 22 | n/a | 31 | |
| [Emim]ETSO4 | n-Dodecane | 1 | n/a | 31 |
| 1-Octane | 2.8 | n/a | 31 | |
| [Bmim]N(CN)2 | Gasoline | 6.66 | n/a | 29 |
| Diesel | 1.97 | n/a | 29 | |
| [Emim]N(CN)2 | Gasoline | 5.64 | n/a | 29 |
| Diesel | 1.93 | n/a | 29 | |
| Et3NNHCl3·FeCl3 | Model oil | 0.27 | n/a | 63 |
| Real gasoline | 2.45 | n/a | ||
| [Bmpy]BF4 | n-Dodecane | 6.1 | n/a | 64 |
| [Hmpy]BF4 | n-Dodecane | 7.6 | n/a | 64 |
| [Ompy]BF4 | n-Dodecane | 9.5 | n/a | 64 |
| [Bmin]DCNM | n-Hexane | 4.19 | n/a | 65 |
9. Prospects and challenges
As the requirement for cleaner fuel oils rises, standards to lower N-contents in fuel oils will be implemented, and substitute technologies will be utilized due to limitations of the hydrotreating process to meet rising demands. Recently, ILs have proven and earned flourishing global interest as model solvents in different separation operations due to their ideal behaviour relative to traditional organic solvents. More experiments using ILs are expected to be reported as is evident from the massive number of publications.In contemporary studies, the N-extraction efficiency is not ideal for ILs. Generally 3–5 re-extraction steps with high oil–ILs mass ratios are required to lower the N-content to <10 ppm. Keeping in view the fact of ILs are more expensive than other organic solvents, a variety of factors need to be considered during their selection such as chemical/thermal stability, cross solubility with fuel oils, and ease of regeneration. Consequently further attention is required to be focused towards rationally designing task-specific ILs especially for producing environment friendly fuel oils.
Previous literature suggests some ILs are not truly green solvents66,67 and doubts in their sustainable development impede their applications as real green solvents. Wide-ranging research concerning the behavior of ILs is still required to be done. The significant features which should be considered to acquire an in-depth perception into environmental consequences of ILs are as given below. (1) A basic understanding into the mechanism for IL-induced toxicity at different levels of complexity as the underlying mechanisms of IL toxicity have rarely been studied. (2) Investigating the biodegradability of cationic and anionic compartments and toxicity of their degradation intermediates. This may provide useful information for designing safe and efficient ILs. (3) Developing the database of environmentally benign structure moieties of ILs on the basis of toxicological and biodegradation information, which would be practically useful as a reference for manufacturers and regulators to properly develop and regulate the use of ILs.
In the literature, mostly model oils are analysed with different ILs, which is compulsory for initial assessment. More real fuel oils are required to be studied because besides N-species studied in model fuel oils, there are various additional N-species that may impede the EDN process. This will be essential when shifting from laboratory scale work to pilot plant production.
10. Concluding remarks
In this review paper, published data of EDN of fuel oils using ILs are summarized. This includes the effect of time, temperature, cross solubility, IL regeneration, IL–oil mass ratio, multiple extraction steps and initial N-contents. It is concluded that: (1) cross solubility of ILs in fuel oils are negligible and that of fuel oils in ILs are affected by the nature of the oil; (2) regeneration of ILs with negligible activity loss can be achieved after a limited number of cycles. (3) Multiple extraction can be effective to result in complete N-removal; (4) initial N-content is found to have little effect; (5) EDN is essentially a temperature independent process, and equilibrium times are not an obstacle when low viscous ILs such as [Emim]N(CN)2 and [Bmim]N(CN)2 are used; (6) increased IL–oil mass ratio results a higher N-removal in one step, also variation in the partition coefficients (KN) does not occur. Optimization of ILs can be easily performed to lead to better performance of denitrogenation for real fuel oils in the future and is an effective technique to eliminate N-species. More studies such as expanding IL species, further studies with real fuel oils, and study of the denitrogenation mechanism are needed for promoting their ultimate commercialization.Acknowledgements
This work is financially supported by National Natural Science Foundation of China (21176021, 21276020).References
- L. C. Gutberlet and R. J. Bertolacini, Ind. Eng. Chem. Prod. Res. Dev., 1983, 22, 246–250 CrossRef CAS.
- H. Yang, J. W. Chen, Y. Briker, R. Szynkarczuk and Z. Ring, Catal. Today, 2005, 109, 16–23 CrossRef CAS.
- A. Jayaraman, F. H. Yang and R. T. Yang, Energy Fuels, 2006, 20, 909–914 CrossRef CAS.
- I. Ahmed and S. H. Jhung, J. Hazard. Mater., 2016, 301, 259–276 CrossRef CAS PubMed.
- M. A. G. Nunes, P. A. Mello, C. A. Bizzi, L. O. Diehl, E. M. Moreira, W. F. Souza, E. C. Gaudino, G. Cravotto and E. M. M. Flores, Fuel Process. Technol., 2014, 126, 521–527 CrossRef CAS.
- Y. Briker, Z. Ring, A. Iacchelli and N. McLean, Fuel, 2003, 82, 1621–1631 CrossRef CAS.
- I. Ahmed and S. H. Jhung, J. Hazard. Mater., 2016, 314, 318–325 CrossRef CAS PubMed.
- L. Alonso, A. Arce, M. Francisco and A. Soto, J. Chem. Eng. Data, 2010, 55, 3262–3267 CrossRef CAS.
- M. J. Girgis and B. C. Gates, Ind. Eng. Chem. Res., 1991, 30, 2021–2058 CrossRef CAS.
- J. R. Katzer and R. Sivasubramanian, Catal. Rev., 1979, 20, 155–208 CAS.
- H. F. Hizaddin, M. K. Hadj-Kali, A. Ramalingam and M. A. Hashim, J. Chem. Thermodyn., 2016, 95, 164–173 CrossRef CAS.
- J.-M. Kwon, J.-H. Moon, Y.-S. Bae, D.-G. Lee, H.-C. Sohn and C.-H. Lee, ChemSusChem, 2008, 1, 307–309 CrossRef CAS PubMed.
- A. Koriakin, K. M. Ponvel and C.-H. Lee, Chem. Eng. J., 2010, 162, 649–655 CrossRef CAS.
- S. Le Borgne and R. Quintero, Fuel Process. Technol., 2003, 81, 155–169 CrossRef CAS.
- I. Ahmed, N. A. Khan and S. H. Jhung, Inorg. Chem., 2013, 52, 14155–14161 CrossRef CAS PubMed.
- A. Akbari, M. Omidkhah and J. T. Darian, Chem. Ind. Chem. Eng. Q., 2014, 20, 315–323 CrossRef.
- M. Matsumoto, M. Mikami and K. Kondo, J. Jpn. Pet. Inst., 2006, 49, 256–261 CrossRef CAS.
- X. Feng, X. Ma, N. Li, C. Shang, X. Yang and X. D. Chen, RSC Adv., 2015, 5, 74684–74691 RSC.
- R. Abro, A. A. Abdeltawab, S. S. Al-Deyab, G. Yu, A. B. Qazi, S. Gao and X. Chen, RSC Adv., 2014, 4, 35302–35317 RSC.
- R. Abro, S. Gao, X. Chen, G. Yu, A. A. Abdeltawab and S. S. Al-Deyab, J. Braz. Chem. Soc., 2016, 27, 998–1006 Search PubMed.
- A. W. Bhutto, R. Abro, S. Gao, T. Abbas, X. Chen and G. Yu, J. Taiwan Inst. Chem. Eng., 2016, 62, 84–97 CrossRef CAS.
- A. W. Bhutto, K. Qureshi, R. Abro, K. Harijan, Z. Zhao, A. A. Bazmi, T. Abbas and G. Yu, RSC Adv., 2016, 6, 32140–32170 RSC.
- S. Gao, X. Chen, R. Abro, A. A. Abdeltawab, S. S. Al-Deyab and G. Yu, Ind. Eng. Chem. Res., 2015, 54, 9421–9430 CrossRef CAS.
- S. Gao, G. Yu, R. Abro, A. A. Abdeltawab, S. S. Al-Deyab and X. Chen, Fuel, 2016, 173, 164–171 CrossRef CAS.
- R. Martinez-Palou and R. Luque, Energy Environ. Sci., 2014, 7, 2414–2447 CAS.
- N. A. Khan, Z. Hasan and S. H. Jhung, J. Hazard. Mater., 2013, 244, 444–456 CrossRef PubMed.
- G. C. Laredo, P. M. Vega-Merino, F. Trejo-Zarraga and J. Castillo, Fuel Process. Technol., 2013, 106, 21–32 CrossRef CAS.
- A. G. Okunev, E. V. Parkhomchuk, A. I. Lysikov, P. D. Parunin, V. S. Semeykina and V. N. Parmon, Russ. Chem. Rev., 2015, 84, 981–999 CrossRef CAS.
- C. Asumana, G. Yu, Y. Guan, S. Yang, S. Zhou and X. Chen, Green Chem., 2011, 13, 3300–3305 RSC.
- S. G. Zhang, Q. L. Zhang and Z. C. Zhang, Ind. Eng. Chem. Res., 2004, 43, 614–622 CrossRef CAS.
- P. Wasserscheid and A. Jess, Green Chem., 2004, 6, 316–322 RSC.
- L.-L. Xie, A. Favre-Reguillon, S. Pellet-Rostaing, X.-X. Wang, X. Fu, J. Estager, M. Vrinat and M. Lemaire, Ind. Eng. Chem. Res., 2008, 47, 8801–8807 CrossRef CAS.
- M. C. Ali, Q. Yang, A. A. Fine, W. Jin, Z. Zhang, H. Xing and Q. Ren, Green Chem., 2016, 18, 157–164 RSC.
- Z. C. Liu, X. H. Meng, R. Zhang and C. M. Xu, Pet. Sci. Technol., 2009, 27, 226–237 CrossRef CAS.
- Y. Marcus, in Ionic Liquid Properties: From Molten Salts to RTILs, Springer International Publishing, Cham, 2016, pp. 123–220, DOI:10.1007/978-3-319-30313-0_6.
- F. H. Hurley and T. P. Wier, J. Electrochem. Soc., 1951, 98, 207–212 CrossRef CAS.
- S. E. Fry and N. J. Pienta, J. Am. Chem. Soc., 1985, 107, 6399–6400 CrossRef CAS.
- M. J. Earle and K. R. Seddon, Clean Solvents: Alternative Media for Chemical Reactions and Processing, ACS Symp. Ser., 2002, 819, 10–25 CrossRef CAS.
- A. Berthod, M. Ruiz-Angel and S. Carda-Broch, J. Chromatogr. A, 2008, 1184, 6–18 CrossRef CAS PubMed.
- M. Wlazlo, J. Gawkowska and U. Domanska, Ind. Eng. Chem. Res., 2016, 55, 5054–5062 CrossRef CAS.
- M. Wlazlo, M. Karpinska and U. Domanska, J. Chem. Thermodyn., 2016, 97, 260–267 CrossRef.
- X. Chen, S. Yuan, A. A. Abdeltawab, S. S. Al-Deyab, J. Zhang, L. Yu and G. Yu, Sep. Purif. Technol., 2014, 133, 187–193 CrossRef CAS.
- B. Gabric, A. Sander, M. C. Bubalo and D. Macut, Sci. World J., 2013 DOI:10.1155/2013/512953.
- A. R. Hansmeier, G. W. Meindersma and A. B. de Haan, Green Chem., 2011, 13, 1907–1913 RSC.
- E. S. Huh, A. Zazybin, J. Palgunadi, S. Ahn, J. Hong, H. S. Kim, M. Cheong and B. S. Ahn, Energy Fuels, 2009, 23, 3032–3038 CrossRef CAS.
- K. Kedra-Krolik, F. Mutelet, J.-C. Moise and J.-N. Jaubert, Energy Fuels, 2011, 25, 1559–1565 CrossRef CAS.
- R. Anantharaj and T. Banerjee, J. Chem. Eng. Data, 2013, 58, 829–837 CrossRef CAS.
- U. Domanska and E. V. Lukoshko, Fluid Phase Equilib., 2015, 395, 9–14 CrossRef CAS.
- H. Wang, C. Xie, S. Yu and F. Liu, Chem. Eng. J., 2014, 237, 286–290 CrossRef CAS.
- N. H. Chan, H. F. Hizaddin, R. Anantharaj and T. Banerjee, Chem. Prod. Process Model., 2015, 10, 161–171 CrossRef.
- M. Vilas, E. J. Gonzalez and E. Tojo, Fluid Phase Equilib., 2015, 396, 66–73 CrossRef CAS.
- H. F. Hizaddin, M. K. Hadj-Kali, A. Ramalingam and M. A. Hashim, Fluid Phase Equilib., 2015, 405, 55–67 CrossRef CAS.
- M. A. Ceron, D. J. Guzman-Lucero, J. F. Palomeque and R. Martinez-Palou, Comb. Chem. High Throughput Screening, 2012, 15, 427–432 CrossRef CAS PubMed.
- A. Ramalingam, H. F. Hizaddin, N. S. Jayakumar and M. A. B. Hashim, J. Innovative Res. Eng. Sci., 2013, 1, 3 Search PubMed.
- L.-L. Xie, X. Chen, X.-X. Wang, X.-Z. Fu, A. Favre-Reguillon, S. Pellet-Rostaing and M. Lemaire, Chin. J. Inorg. Chem., 2008, 24, 919–925 CAS.
- J. C. Dearden and G. M. Bresnen, Quant. Struct.–Act. Relat., 1988, 7, 133–144 CrossRef CAS.
- C. Liu, Q. He, Z. Zhang, Y. Su, R. Xu, B. Hu, C. Liu, Q. He, Z. Zhang and Y. Su, Chin. J. Chem., 2014, 32, 410–416 CrossRef CAS.
- X. Jiang, Y. Nie, C. Li and Z. Wang, Fuel, 2008, 87, 79–84 CrossRef CAS.
- A. Santanu Paria and P. K. Yuet, Ind. Eng. Chem. Res., 2007, 46, 108–113 CrossRef.
- Y. Nie, C. Li, A. Sun, H. Meng and Z. Wang, Energy Fuels, 2006, 20, 2083–2087 CrossRef CAS.
- N. Yi, C. Li, M. Hong and Z. Wang, Fuel Process. Technol., 2008, 89, 978–983 CrossRef.
- H. Gao, M. Luo, J. Xing, Y. Wu, Y. Li, W. Li, Q. Liu and H. Liu, Ind. Eng. Chem. Res., 2008, 47, 1323–1331 Search PubMed.
- F. Li, Y. Liu, Z. Sun, L. Chen, D. Zhao, R. Liu and C. Kou, Energy Fuels, 2010, 24, 4285–4289 CrossRef CAS.
- H. Gao, Y. Li, Y. Wu, M. Luo, Q. Li, J. Xing and H. Liu, Energy Fuels, 2009, 23, 2690–2694 CrossRef CAS.
- J. J. Ibrahim, S. Gao, A. A. Abdeltawab, S. S. Al-Deyab, L. Yu, G. Yu, X. Chen and X. Yong, Sep. Sci. Technol., 2015, 50, 1166–1174 CrossRef CAS.
- T. P. T. Pham, C. W. Cho and Y. S. Yun, Water Res., 2010, 44, 352–372 CrossRef CAS PubMed.
- Y. Zhao, J. Zhao, H. Ying, Q. Zhou, X. Zhang and S. Zhang, J. Hazard. Mater., 2014, 278, 320–329 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |