Recent developments and future perspectives of microfluidics and smart technologies in wearable devices
Sasikala
Apoorva
a,
Nam-Trung
Nguyen
 b and
Kamalalayam Rajan
Sreejith
*b
b and
Kamalalayam Rajan
Sreejith
*b
aUKF Centre for Advanced Research and Skill Development(UCARS), UKF College of Engineering and Technology, Kollam, Kerala, India, 691 302
bQueensland Micro and Nanotechnology Centre, Griffith University, 170 Kessels Road, Nathan, 4111, Queensland, Australia. E-mail: s.kamalalayamrajan@griffith.edu.au
First published on 28th February 2024
Abstract
Wearable devices are gaining popularity in the fields of health monitoring, diagnosis, and drug delivery. Recent advances in wearable technology have enabled real-time analysis of biofluids such as sweat, interstitial fluid, tears, saliva, wound fluid, and urine. The integration of microfluidics and emerging smart technologies, such as artificial intelligence (AI), machine learning (ML), and Internet of Things (IoT), into wearable devices offers great potential for accurate and non-invasive monitoring and diagnosis. This paper provides an overview of current trends and developments in microfluidics and smart technologies in wearable devices for analyzing body fluids. The paper discusses common microfluidic technologies in wearable devices and the challenges associated with analyzing each type of biofluid. The paper emphasizes the importance of combining smart technologies with microfluidics in wearable devices, and how they can aid diagnosis and therapy. Finally, the paper covers recent applications, trends, and future developments in the context of intelligent microfluidic wearable devices.
1. Introduction
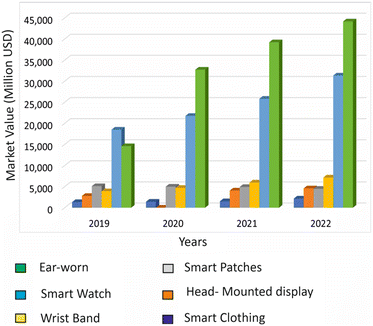
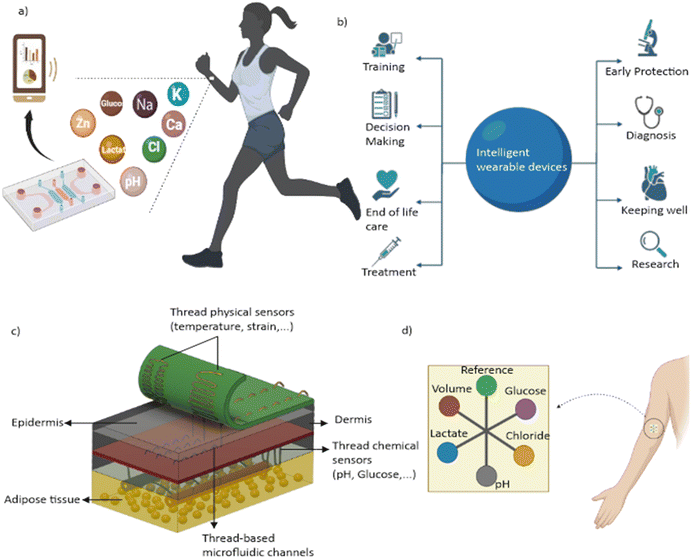
Wearable devices have transformed healthcare and personal wellness by providing affordable and convenient options for continuous patient monitoring.1 In contrast to traditional clinical diagnostics, wearable devices can provide real-time data on an individual's health using non-invasive or minimally invasive methods, making them an attractive option for healthcare monitoring.2 For instance, wearable devices such as Fitbit,3 Apple Watch,4 and Garmin5 are commonly used for tracking fitness metrics, while other devices such as continuous glucose monitors6 and ECG monitors7 are used for medical purposes such as monitoring blood sugar levels and heart activity. Although these devices initially struggled to gain acceptance, the COVID-19 pandemic has accelerated their adoption in healthcare.8 According to the forecast of Gartner Inc., the global end-user expenditure on wearable devices was forecasted to reach $93.8 billion in 2022, with most customers relying on smartwatches for fitness monitoring and smart patches for health monitoring (Fig. 1).9To address the need for more specific data collection from wearable devices, microfluidics has been incorporated into wearable sensors, allowing medical experts to continuously acquire specific high-quality data from patients.10,11 By combining microfluidic wearable sensors with other wearable technologies, such as gyroscopes, accelerometers, and temperature sensors, real-time bodily fluids can be analysed with continuous tracking.12 These integrated devices offer high throughput, high sensitivity, and low power consumption.13 Wearable sensors that attach to the skin surface can measure temperature, heart rate, blood sugar, and other vitals more accurately than other wearables, making them an attractive option for monitoring health.14 Microfluidics can also be used for on-site therapy and precise delivery of drugs or pharmaceuticals.15,16
Incorporating smart intelligent technologies such as artificial intelligence (AI), machine learning (ML), and Internet of Things (IoT) into wearable devices has several technical advantages. AI algorithms can analyse vast amounts of data collected by wearable sensors, allowing for a more accurate and detailed assessment of an individual's health status.17 This analysis takes into account not only an individual's vital signs, but also other relevant factors such as age, sex, and geographic region. By determining the normal range of vital signs for specific populations, healthcare professionals can provide more personalized recommendations for treatment and monitoring.18 This personalized approach leads to the development of individualized health profiles that provide better accuracy in diagnosing and treating diseases.19 The integration of AI with Internet of Things (IoT) platforms further enhances the capabilities of wearable devices, allowing for real-time monitoring and communication with healthcare professionals.20,21 The incorporation of these intelligent technologies in wearable devices can ultimately lead to the development of a more personalized approach to healthcare, with the ability to predict and prevent disease, improving overall wellness.17
These exciting developments make clear that the future of healthcare is strongly linked to the integration of smart technologies and microfluidics in wearable devices. The potential benefits of such integration are numerous.22 However, there are challenges to be addressed, including the development of algorithms that can accurately interpret and make sense of the vast amount of data generated by microfluidic wearable sensors, and ensuring that the technology is accessible and affordable to all patients.23
To fully realize the potential of smart microfluidic wearable devices in healthcare, it is crucial to continue investing in research and development, as well as promoting collaboration between experts in the fields of AI, microfluidics, and healthcare. Considering this objective, the present review focuses on non-invasive and minimally invasive microfluidic wearable sensors, for analysis, diagnosis and monitoring. The novelty of this review is that it emphasises the combination of emerging smart technologies and microfluidics, and how it can aid diagnosis and therapy of wearable devices. Furthermore, recent applications, recent trends, and future developments in smart microfluidic wearable devices are also provided.
2. Microfluidics in wearable devices
Microfluidics is a rapidly growing research field that focuses on the manipulation of fluids on the microscale, with typical dimensions of microchannels less than 1 mm.11,15,24 This field has been the subject of numerous theoretical studies aiming at more efficient processes and devices for applications in chemistry, biology, and medicine.25,26 Microfluidic technologies are well-suited for a variety of applications due to their advantages of low volumes, high sensitivity, rapid processing, high spatial resolution, and high integration with sensing components.15,27,28 The ease and low cost of fabrication, prototyping, and implementation have also played an important role in the success of microfluidic technology.In the field of wearable devices, microfluidics has seen significant growth, particularly for healthcare applications. By controlling and manipulating small amounts of bodily fluids on the microscale, microfluidics enables more accurate and precise analysis of these fluids, essential for continuously monitoring a patient's health.29 Small changes in bodily fluids can provide critical information about a patient's health status, making microfluidics a valuable tool in healthcare monitoring.30 As mentioned earlier, microfluidics can also be used for the precise delivery of drugs or other therapeutic agents, enhancing the effectiveness of treatment.
The miniaturization of microfluidic components makes integrating this technology into wearable devices possible, allowing for portability and ease of use.31 Microfluidic technology is offering a promising solution for non-invasive and real-time monitoring.13 Wearable devices that incorporate microfluidic channels can provide valuable information about a wearer's health status, including electrolyte levels and biomarkers, which enables the diagnosis and management of a range of health conditions.32
The potential applications of microfluidic wearable devices are not limited to healthcare alone. This technology has the potential to be utilized in environmental monitoring, food safety, and sports performance.33,34 For instance, microfluidic wearable devices can be used to monitor environmental pollutants or detect contaminants in food products. In sports, these devices can be used to monitor the electrolyte levels of athletes during training and competition.35
The proper functioning of a microfluidic device depends on careful consideration of each step of the development process, from design to fabrication, to analysis and to signal processing.36 The fabrication of a microfluidic device involves designing and manufacturing microchannels, chambers, and valves using materials such as glass, silicon, or polymers.25,37,38 Appropriate sample collection and storage methods are crucial to ensure accurate and reliable results. Various techniques can be employed depending on the type of sample being analysed. Sample analysis is the core component of a microfluidic device and involves a range of methods such as optical, electrochemical, and biological assays, to detect and quantify analytes such as biomarkers.39 Within microfluidic wearable devices, signal transduction and amplification processes play a crucial role in transforming subtle signals from samples into easily interpretable data. Concurrently, mechanical sensing mechanisms ensure the precise handling and delivery of these minuscule samples, significantly enhancing the accuracy of measurements and analyses. Finally, the device must be powered, which can be achieved through various means, such as batteries or external power sources.40,41
Polydimethylsiloxane (PDMS),42 paper microfluidics,25 and patches with microneedles43 are microfluidic technologies that have unique features and advantages for various applications. The following sections provide a detailed discussion on the applications of the aforementioned technologies and their corresponding features.
2.1. PDMS-based microfluidics
Microfluidic devices for manipulating small volumes of fluids are commonly made from polymers due to their biocompatibility, flexibility, and cost-effectiveness.44 Techniques for manufacturing polymer-based microfluidic devices include soft lithography,45 hot embossing,46 and injection moulding,47 with soft lithography being the most widely used.48 Polydimethylsiloxane (PDMS) is the most commonly used polymer for fabricating microfluidic devices.49 Other than PDMS, other polymers are polystyrene (PS),50 polyether ether ketone (PEEK),51 polyethylene terephthalate (PET),52 polyvinyl chloride (PVC),53 polymethylmethacrylate (PMMA),54 cyclic olefin copolymer (COC),55 polycarbonate (PC),56 and polyetherimide (PEI)57 that are used for making microfluidic devices.58PDMS belongs to the siloxane family and is a type of mineral organic polymer. PDMS is highly suitable for lab-on-a-chip (LOC) applications,59 and its unique properties make it a primary choice for researchers and developers working on microfluidic devices and wearable sensors. PDMS has a low Young's modulus, allowing it to easily conform to and wrap around curved surfaces.60 This property also makes it a popular choice for providing conformal contact with different surfaces. Its elasticity and flexibility are highly desirable properties of wearable devices.61 PDMS is relatively low-cost compared to other materials used for microfluidics. The transparency of PDMS allows for easy visualization of the fluid content and all other components.62 These features make PDMS ideal for serving as a foundational material for wearable electronic devices like fitness trackers. Researchers commonly use two types of PDMS, PDMS RTV-615 and PDMS Sylgard 184, for microfluidic applications.63 However, the exact composition of these two PDMS types is not disclosed by the vendors.
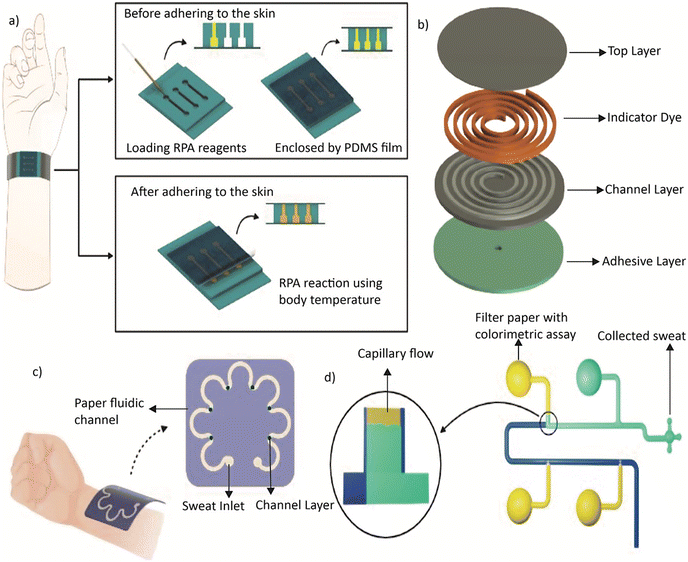
Researchers have developed innovative ways to utilize PDMS in wearable microdevices. For example, Trinh et al. developed a wearable microdevice that uses flexible and soft-contact PDMS for the amplification of nucleic acids through recombinase polymerase amplification (RPA).38 The PDMS was mixed with different ratios of the pre-polymer and a curing agent without altering the fundamental properties of PDMS. The team demonstrated that the basic characteristics of PDMS remained consistent despite the change in the prepolymer and curing agent ratio. The replica moulding technique was used to build the wearable PDMS microdevice with microchambers for RPA reagents. It required soft lithography to create a mould on a PET film, pouring and curing pre-polymer PDMS, and then adhering it to a thin PDMS film. For RPA tests, the flexible device adhered to human skin. The wearable RPA microdevice contained procedures for loading RPA reagents and carrying out reactions both before and after it was attached to a person's skin. To prevent sample loss during movement, a thin PDMS sheet was placed over the microdevice chamber during reactions. The microdevice was taken out after the reaction to be used for RPA result analysis. Fig. 2a shows the typical operation of the wearable PDMS device.
On the other hand, Heo et al. developed a collagen–PDMS composite material that maintains the soft elastomer properties needed for skin-interfaced microfluidics while reducing water evaporation.64 The skin-interfaced collagen–PDMS microfluidic device enhances sweat retention by 45% over a period of 9 hours compared to pure PDMS. Fig. 2b shows an exploded view of the device with layers including a cover layer, an indicator dye, a channel layer and adhesive.
While PDMS is widely used in the fabrication of microfluidic devices, the material has limitations. For instance, PDMS may age over time, limiting the intended performance. Additionally, PDMS is not highly compatible with many organic solvents, absorbing hydrophobic molecules and water vapour, which can be inadvertently emitted during experiments.65 One major drawback of PDMS microfluidic devices is the inability to integrate electrodes within the device.66 However, this can be overcome by placing electrodes on a glass cover slide instead of directly on the chip.
2.2. Paper-based microfluidics
Existing diagnostic technologies are often expensive and inaccessible. This bottleneck can be addressed by paper-based microfluidic devices.67 As an inexpensive and lightweight substrate, paper is an ideal option for microfluidic devices that are portable for immediate use.68 These devices are made by patterning paper with hydrophobic structures to define hydrophilic channels that transport fluid through capillary action.69 While most devices use colorimetric assays,70 electrochemical,71 chemiluminescence,72 and electrochemiluminescence71 methods can also be employed in paper-based devices. Techniques such as wax printing,73 inkjet printing,74 photolithography,75 flexographic printing,76 plasma treatment,77 laser treatment,78 wet etching,79 and screen printing80 have been utilised for fabricating paper-based microfluidic devices. Among these, wax printing is the simplest method.Mogera et al. introduced a wearable microfluidic system with plasmonic sensors on paper for continuous monitoring of sweat loss, sweat rate, and its constituent metabolites.81 This soft, flexible, and stretchable system covers the skin without causing any physical or chemical irritations. Fig. 2c shows the conceptual diagram of the device. Similarly, Abbasiasl et al. presented an easy-to-fabricate paper-integrated microfluidic device for sequential analysis of sweat that eliminates the need for air exits in each reservoir, thereby reducing the negative effects of sweat evaporation.82Fig. 2d illustrates the top and bottom views of this device. By directing liquid sequentially into the chambers using the high capillary force of filter paper, the device enables further chemical analysis. Researchers employed colorimetric assays to demonstrate the device's performance in chrono-analysis of glucose standard solutions and pH of sweat during exercise. The findings indicate the potential of this approach to sequentially analyse the concentration of biomarkers over a specific period.
Paper-based microfluidic devices have several advantages over traditional substrates such as glass and PDMS, including low cost, disposability, and portability.83 However, liquid transport with patterned channels can be challenging on paper compared to other substrates due to the fibrous and porous nature of paper.84 Despite this issue, the affordability and simplicity of paper-based microfluidic devices make them an attractive option for developing wearable devices and other portable diagnostic tools.
2.3. Microneedle-based transdermal microfluidics
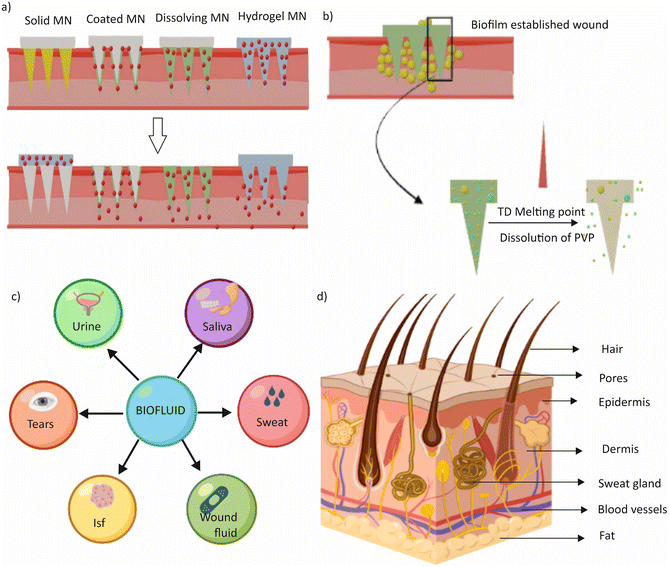
Microneedle-based transdermal microfluidics is a revolutionary technology that combines microneedles and microfluidics to deliver drugs and therapeutics transdermally.85 Microneedles, typically smaller than a millimetre, create tiny channels in the skin that allow for transdermal drug delivery, while microfluidics involves microchannels embedded in a patch to transport and deliver chemicals to the microneedles.86 The integration of microneedles with microfluidics offers several potential advantages over traditional drug delivery methods, such as increased efficiency, reduced side effects, and improved patient compliance.87There are four types of microneedles: solid, coated, polymeric (dissolvable), and hollow microneedles. Fig. 3a shows the schematic diagram of the different types of microneedles.88 Solid microneedles puncture the skin to create channels for drug delivery and cause less pain than traditional needles.89 Microneedles can be made from various materials such as silicon, metals, polymers, or ceramics. Following the insertion, a drug formulation can be applied to the skin using a patch or a topical cream. Hollow microneedles are used for drug infusion into the skin and can be present in a microfluidic device either singularly or in an array.90 Coated microneedles are covered with a water-soluble formulation of the drug before administration, which subsequently dissolves into the skin after puncturing.91 Dissolvable microneedles are made from polymers that dissolve completely after insertion, releasing the therapeutic agent.92 They are suitable for delivering thermo-sensitive drugs such as proteins and antigens and can be fabricated using micromoulds or in situ polymerization of liquid monomers.
Kang et al. reported wearable devices utilizing microneedle-based transdermal microfluidics for drug delivery and analyte collection.93 Hyaluronic acid (HA) is a particularly advantageous material due to its human autologous source, biocompatibility, strong water absorption, and viscoelasticity.94 The devices were employed for wound healing, targeted therapy, extraction of interstitial skin fluid (ISF), and drug preservation. Luzuriaga et al. presented biodegradable 3D printed polymer microneedles for transdermal drug delivery using polylactic acid, an FDA-approved biodegradable material.95 The needles have a tip size as small as 1 μm due to a post-fabrication chemical etching protocol that improved the feature size of the printed parts. Near-infrared (NIR) light-responsive microneedle patches have also been introduced for the on-demand release of antimicrobial peptides for the treatment of wound biofilms.96 The patch contains dissolvable poly(vinylpyrrolidone) (PVP) microneedles loaded with IR780 iodide as a photothermal conversion agent and molecularly engineered peptide W379 as an antimicrobial agent. Upon exposure to NIR light, IR780 converts light to heat, causing the phase change material 1-tetradecane (TD) to melt, releasing the loaded W379 peptide from the microneedles into the surrounding regions. The NIR light-responsive microneedle patches can program the release of antimicrobial peptides and show high antibacterial efficacy in vitro compared with traditional microneedle patches. Fig. 3b provides a schematic illustration of the above-mentioned microneedle patches.
While microneedles have numerous potential applications,97,98 only a few products have been so far commercialized. Safety and efficacy considerations are crucial when developing microneedles for delivering small and large molecules. Metallic microneedles may leave metal residues under the skin, leading to various side effects such as irritation, swelling, or discolouration. When microneedles are used repeatedly on the same location of the skin or on areas of the skin with different levels of thickness, the effectiveness of delivering substances through these microneedles can be influenced. This could lead to problems in how well the substances are absorbed by the body and potentially result in various complications or negative outcomes.86,89
3. Biofluids
Biofluids are fluids that can be excreted, secreted, or obtained through needle aspiration or as a result of pathological processes in the human body.99 These fluids contain biochemical components or biomarkers that directly relate to human health conditions.100 For effective target analysis, it is essential to collect and process biofluids that are typically secreted by the human body. Sweat, saliva, urine, tears, interstitial fluids of the skin, and wound fluids are examples of biofluids commonly analysed by microfluidics-based wearable devices.32,101Fig. 3c provides a schematic overview of various biofluids.102 The collection and analysis of biofluid samples such as blood and urine can be time-consuming and troublesome, when done in a laboratory. However, microfluidics-based wearable devices can help overcome these challenges by ensuring that biofluid collection and analysis are non-invasive, reliable, and accurate.103 Microfluidic technologies are capable of collecting biofluids in small amounts, utilizing their advantages on the microscale.11 This makes microfluidics-based wearable devices highly relevant in the current era.The following sections provide a discussion of the fundamental composition and physiological and pathological properties of biofluids, as well as the analytes present in them. Additionally, the latest microfluidics-based wearable technologies in this field and the potential for integrating artificial intelligence are addressed. We also discuss the challenges and prospects associated with each biofluid. For ease of further reading, Table 1 shows the typical concentration of analytes in each biofluid along with the associated health hazards. Table 2 shows various existing sensing methods used in wearable devices for different biofluids associated with corresponding limitations.
| Analytes | Concentration | Potential health hazards | Ref. | |
|---|---|---|---|---|
| Glucose | Sweat | 0.06 to 0.2 mM | Diabetes | 126, 144, 179, 200, 232 |
| Saliva | 0.5 to 1.00 mg/100 ml | |||
| ISF | 60 to 90 mg dL−1 | |||
| Urine | 25 mg dL−1 | |||
| Tears | 0.032 mmol L−1 | |||
| Sodium | Sweat | 70 mmol L−1 | Cystic fibrosis dehydration | 118, 119, 188, 197, 233 |
| Saliva | 8.7 to 24 mEq L−1 | |||
| ISF | −135–145 mmol L−1 | |||
| Urine | 20 mEq L−1 | |||
| Tears | 120 to 170 mM | |||
| Potassium | Sweat | 10 to 50 mmol L−1 | Muscle cramps | 115, 186, 196, 234 |
| Saliva | 13 to 16 mEq L−1 | |||
| ISF | 3.97 mM | |||
| Urine | 20 mEq L−1 | |||
| Tears | 20 mEq L−1 | |||
| Lactate | Sweat | 2 to 20 mmol L−1 | Anaerobic metabolism | 125, 149, 194, 301, 234 |
| Saliva | 0.1 to 2.5 mM | |||
| ISF | 1 to 2 mM | |||
| Urine | 4.5 to 19.8 mg dL−1 | |||
| Tears | 1 to 5 mM | |||
| Chloride | Sweat | 30–59 mmol L−1 | Cystic fibrosis dehydration | 117, 120, 189, 223 |
| Saliva | 5 to 40 mmol L−1 | |||
| ISF | 100–110 mmol L−1 | |||
| Urine | 110 to 250 mEq | |||
| Tears | IIO and 135 mEq L−1 | |||
| Uric acid | Sweat | 24.5 mmol L−1 | Renal dysfunction | 80, 131, 161 |
| Saliva | 199 ± 27 μmol L−1 | |||
| ISF | 0.1 to 0.3 mg dL−1 | |||
| Urine | 250 to 750 mg/24 hours | |||
| Tears | 25–150 μM | |||
| pH | Sweat | 6.3 | Skin disease | 134, 168, 191 |
| Saliva | 6.6 to 7.1 | |||
| ISF | 7.35 to 7.45 | |||
| Urine | 4.5 to 8 | |||
| Tears | 6.5 to 7.6 | |||
| Sensing method | Biofluid | Analyte | Limitations | Ref. |
|---|---|---|---|---|
| Amperometry sensors | Blood, interstitial fluid, saliva | Glucose, lactate | • Sensitive to interfering substances present in the biofluids | 126, 154, 180, 193 |
| • Accuracy may decrease over time due to electrode fouling | ||||
| Potentiometric sensors | Blood, sweat, saliva | pH, ions | • Limited dynamic range | 126, 187, 189, 191 |
| • May exhibit slower response times compared to other sensor types | ||||
| • pH variations in the biofluid may impact sensor performance | ||||
| Voltammetric sensors | Blood, interstitial fluid | Uric acid, vitamin C | • Need antifouling strategies | 192, 193 |
| • Require sophisticated algorithms or analysis techniques for accurate information extraction | ||||
| Colorimetric sensors | Blood, urine, sweat | pH, glucose, specific ions | • Limited quantitative analysis | 120, 127, 128, 147, 168 |
| • Colour changes can be influenced by external factors | ||||
| Fluorescence sensors | Blood, saliva, urine, interstitial fluid | Proteins, ions, pH | • Sensitive to environmental conditions | 235, 246, 305 |
| • Simultaneous detection of multiple analytes is challenging due to spectral overlap |
3.1. Sweat
Sweat is a hypotonic fluid produced by sweat glands as a part of bodily thermoregulation.104,105 An ordinary human adult can generate 500 to 700 ml of sweat per day.106 Compared to other bodily secretions, sweat can be collected non-invasively and contains a rich composition of biomarkers such as water, electrolytes (e.g., sodium, potassium, and chloride), metabolites (e.g., glucose and lactate), trace elements, lactic and uric acid, and drugs.107–109 The abundance of eccrine glands throughout the body makes sweat collection relatively straightforward. Its non-invasive nature and ready availability make sweat a popular easy-to-access sample.108Sweat contains a wealth of information that can be used for diagnosing various ailments and diseases. For instance, the correlation between glucose levels in blood and sweat enabled continuous monitoring of diabetes, while the amount of lactate in perspiration can provide insight into the severity of ischemia associated with certain diseases.110 Sodium and calcium levels in sweat can be used to identify and diagnose cystic fibrosis in newborns and other related conditions.111 Furthermore, skin temperature and sweat analysis can provide valuable information on the occurrence and progression of various skin diseases and conditions.109,112
Sweat is primarily collected from the eccrine glands; each sweat gland is connected to vascularized tubes that stretch from the dermis to the skin surface. Sweat is expelled onto the skin surface with the assistance of myoepithelial cells.106,113 There are two primary methods for collecting sweat: the passive approach and the active approach. The passive approach involves physical exercise or thermal stimuli, such as running, cycling, sauna, or skipping, while the active sweat collection method is commonly done through iontophoresis.114,115 Iontophoresis involves applying a voltage between two electrodes to stimulate a local area of the skin.109 However, this technique can be uncomfortable and is prone to electrode corrosion.
Compared to other biofluids, sweat has a lower concentration of biomarkers, which makes quantitative analysis challenging. Conventional sweat analysis devices use absorbent pads or fabrics, which require additional design for processing.1 These methods are also prone to low secretion rates and evaporation challenges. Microfluidics has emerged as a promising method for preparing and analysing sweat, as microfluidic devices require only a small volume of sweat and can be designed to be portable, low-cost, reusable, and disposable.108 Microfluidic components in wearable devices allow for transporting sweat in time after its detection, preventing contamination of sweat for subsequent analysis. Microchannels in the devices can drive and collect sweat in a well-designed manner, improving efficiency.11
Cystic fibrosis is associated with high levels of chloride ions in sweat, making them a crucial indicator of the disease. A potentiometric chloride ion biosensor was developed for the diagnosis and management of cystic fibrosis as explained by Grasta et al.119 The sensor uses ion-sensitive field-effect transistors to detect the presence of chloride ions in sweat. FEM-based modelling, which includes both semiconductors and electrochemistry, is used to develop the sensor. However, these biosensors can be expensive and require calibration, limiting their widespread adoption in clinical settings.
Similarly, Biswas et al. reported a novel transdermal patch for cystic fibrosis diagnosis.120 The patch, which looks like a conventional sticker, absorbs sweat through tiny canals. Pilocarpine, a drug that stimulates sweat production, is applied to the skin using a mild electric current. The resulting colour change indicates the presence of cystic fibrosis. While this approach is simple and non-invasive, the colorimetric readout can be difficult to interpret in low-light settings.
To address some of these challenges, a soft, epidermal microfluidic device (“sweat sticker”) has been designed for the simple and rapid collection and analysis of sweat.121 Intimate, conformal coupling with the skin supports nearly perfect efficiency in sweat collection without leakage. Real-time image analysis of chloride reagents allows for quantitative assessment of chloride concentrations using a smartphone camera, without requiring extraction of sweat or external analysis. However, the wearable microfluidic technologies and smartphone-based analytics reported here are still in the experimental stage and may require further refinement before they can be widely adopted in clinical settings.
Potassium (K+) is an important electrolyte present in sweat, along with sodium and chloride. Unlike sodium and chloride, K+ is independent of the sweat generation rate and has a strong correlation with blood concentration.116 Measuring K+ levels in sweat can be used as an indicator of the hydration status, as well as to monitor conditions such as renal disease and dehydration.122 The normal range of K+ in sweat is between 10 and 50 mmol L−1, but may vary depending on the individual.123
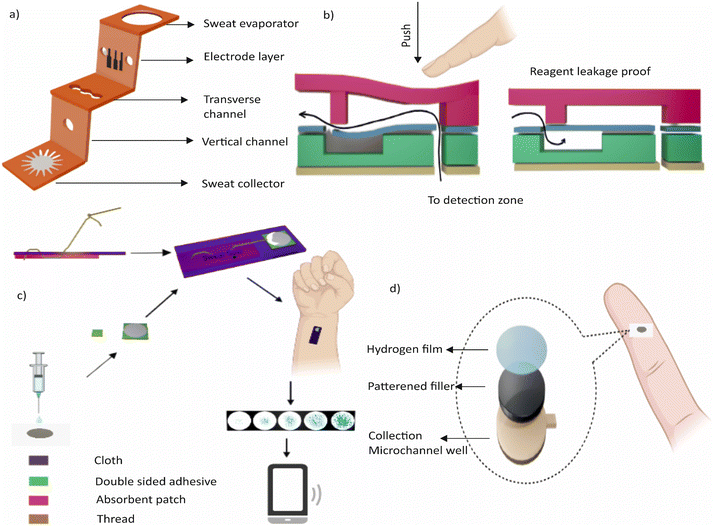
Recent advancements in wearable technology have led to the development of devices that can measure K+ levels in sweat in real-time. One of such devices is the SwEatch platform, which uses ion-sensitive electrodes (ISEs) fabricated and containing either poly(3,4-ethylenedioxythiophene) (PEDOT) or poly(3-octylthiophene-2,5-diyl) (POT) as a conductive polymer transducing component. The microfluidic unit of the device draws sweat from the skin through a passive capillary reaction and brings it into contact with the two electrodes. This technology is mainly focused on sport application and can provide immediate feedback on hydration levels of athletes. Liang et al. demonstrated an integrated three-dimensional paper-based microfluidic electrochemical device (3D-PMED) for real-time monitoring of sweat potassium.124Fig. 4a shows the schematic illustration of the device with channels, an electrode layer, and a sweat evaporator. The device includes a screen-printed potassium ion-selective sensor on a PET substrate and a paper-based microfluidic pad for sweat collection and detection. The 3D-PMED technology offers a detection range of 1–32 mM and has the potential to address the limitations of existing sweat monitoring methods.
While K+ as an indicator of the hydration status is a promising marker, there are limitations to this approach. For example, factors affecting the level of K+ in sweat are exercise, medication, and diet. Additionally, the accuracy of K+ measurement can be affected by factors such as skin temperature and sweat generation rate. To address these limitations, future research could explore the use of multi-sensor systems that integrate K+ measurement with other parameters such as skin temperature and sweat rate. Such systems could provide a more comprehensive assessment of an individual's hydration status and help to improve the accuracy of K+ measurement in sweat.
Lactate. Lactate is a hydrophilic metabolite and analyte that is present in sweat. During exercise and muscle activities, glycogen breakdown locally produces lactate in sweat. There is also a correlation with blood lactate levels. Typically, the lactate concentration in sweat ranges from 2 to 20 mmol L−1. While lactate is primarily used as a potential biomarker for exercise analysis in most wearable devices, lactate sensors are typically integrated with other sensors to provide multiplex and comprehensive health monitoring.
One example is the epidermal patch developed by Sempionatto et al., which allows for the simultaneous monitoring of haemodynamic and metabolic biomarkers.125 This non-invasive device utilises ultrasonic transducers and electrochemical sensors to monitor blood pressure and heart rate using multiple biomarkers, including lactate. Another example is a continuous sweat monitoring system that is integrated with wireless electronics in the form of wearable glasses.126 This real-time monitoring system has a chemical sensing platform capable of sensing electrolytes and metabolites in sweat such as lactate and potassium ions. The system includes an amperometry lactate biosensor and a potentiometric ion-selective electrode on the two nose bridge pads of the spectacles, with PET stickers used on the glass nose pads to monitor sweat metabolites and electrolytes. The entire setup is integrated into the arms of the glass for wireless electronic communication.
Glucose. Glucose is another crucial substance that is commonly evaluated in sweat. Though it is typically found in blood, glucose can also be detected in sweat, albeit at a much lower concentration. The normal range of glucose in sweat is between 0.06 and 0.2 mM, compared to 3.3 to 17.3 mM in blood. Factors such as diet, exercise, and stress levels can affect the concentration of glucose in sweat. The correlation between glucose in sweat and glucose in the blood is a subject of ongoing research. However, studies have shown that there is a strong correlation between the two in both type 1 and 2 diabetes cases, even during exercise.
The measurement of glucose levels in sweat has potential applications in monitoring diabetic patients and athletes during exercise. There is increasing interest in epidermal wearable devices for tracking sweat glucose. These devices offer a non-invasive and continuous way of monitoring glucose levels, which could be more convenient for patients compared to traditional blood glucose monitoring systems. Examples of sweat-based glucose monitoring devices include wristwatches, wearable patches, optical instruments, and stretchable tattoos. One of such devices is a microfluidics-based wearable colorimetric sensor designed to detect glucose in sweat demonstrated by Xiao et al.127Fig. 4b shows the schematic of the device. The sensor consists of five microfluidic channels connected to detection microchambers with a check valve in each channel to prevent the backflow of chemical reagents. The microchambers contain pre-embedded glucose oxidase (GOD)–peroxidase–o-dianisidine reagents that sense glucose in sweat. The sensor shows a more sensitive response to glucose than conventional GOD–peroxidase–KI systems and can perform five parallel detections simultaneously. The sensor has a linear range for sweat glucose of 0.1–0.5 mM with a limit of detection of 0.03 mM.
Another wearable device developed for non-invasive, quantitative, and in situ monitoring of human sweat glucose is the microfluidic thread/paper-based analytical device (μTPAD) reported by Xiao et al.128 (Fig. 4c). The device contains a cotton thread and functionalized filter paper that achieves high-performance colorimetric sensing of glucose. The μTPAD is integrated with an arm guard to sensitively detect glucose in human sweat, making it a low-cost and easy-to-use wearable device for human sweat analysis. In addition, a soft and flexible wearable sweat epidermal microfluidic device capable of simultaneously stimulating, collecting, and electrochemically analysing sweat was demonstrated by Bolat et al.129 The device integrates an iontophoretic pilocarpine delivery system around the inlet channels of the epidermal polydimethylsiloxane (PDMS) microfluidic device for sweat collection and analysis. The device eliminates the need for intense physical exercise as the freshly generated sweat is naturally pumped into the fluidic inlet. The on-body performance and layout of the device were optimized. The device was evaluated for detecting sweat glucose in several volunteers. Furthermore, the microfluidic monitoring device was integrated with a real-time wireless data transmission system using a flexible printed circuit board conformal with the body surface.
Uric acid. Uric acid is a waste product typically excreted in urine, but it can also be found in sweat at a measurable level.130 Monitoring the levels of uric acid in sweat provides valuable insights into an individual's health status, such as dehydration, increased physical activity, or hyperuricemia.131 However, the correlation between the levels of uric acid in sweat and blood is not always direct, and the concentration of uric acid can vary depending on the individual even without hyperuricemia.130,132 Despite these challenges, recent advances in wearable technology have led to the development of microfluidic-based electrochemical and plasmonic sensors for accurate and sensitive detection of uric acid in sweat.
Mogera et al. introduced a wearable plasmonic paper-based microfluidic system for continuous and simultaneous quantitative analysis of sweat loss, sweat rate, and metabolites in sweat.81 Plasmonic sensors based on label-free surface-enhanced Raman spectroscopy (SERS) provide chemical “fingerprint” information for analyte identification, enabling sensitive detection and quantification of uric acid in sweat at physiological and pathological concentrations. Xu et al. proposed a wearable microfluidics-based electrochemical sensor incorporating a conducting polymer PEDOT:PSS hydrogel for the accurate and sensitive detection of uric acid in sweat.133 The prepared flexible sensor shows an ultrahigh sensitivity and a low limit of detection and is capable of detecting uric acid levels in real human sweat samples. These microfluidic devices have promising applications in the construction of high-performance wearable sensors for monitoring biomarkers, metabolites, and nutrients. However, further research is required to fully understand the correlation between the levels of uric acid in sweat and blood.
Sweat pH. Sweat pH is one of the key parameters to monitor an individual's health. The pH of human sweat ranges typically between 4.5 and 7.0. The average pH of sweat for a healthy human is 6.3. The pH of sweat is influenced by various factors such as electrolyte concentration and bacterial activity. An imbalance in pH levels can lead to skin disorders and medical conditions. To address this, the National University of Singapore has developed a pH sweat sensor that offers a flexible and highly responsive method of detecting and analysing sweat pH-related issues.134 The sensor is made of polyaniline polymer, a cost-effective, durable, and flexible material that can change colour based on sweat pH. The sensor can be integrated with smartwatches or pulse oximeters to offer continuous monitoring of sweat pH.
A wearable wristband has been developed for collecting sweat from the skin and has a colour-based pH area for easy readout.135 Real-time data can be transmitted to smart applications through a Bluetooth interface. Additionally, a wearable patch has been developed for continuous sweat monitoring at rest, which has hydrophilic fillers for rapid sweat uptake into the sensing channel and is integrated with an electrochemical sensor for pH, Cl−, and levodopa monitoring.136Fig. 4d shows this wearable patch.
In addition to pH, sweat contains a wide range of analytes such as lipophilic molecules, steroid hormones, and legal and illicit drugs like heroin, morphine, and methadone.101 Sweat analysis has the potential to offer valuable insights into an individual's physical condition and health. Over 800 unique proteins and more than 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 endogenous peptides have been discovered in sweat,137 offering a promising avenue for further research.
000 endogenous peptides have been discovered in sweat,137 offering a promising avenue for further research.
One major challenge is the variation of sweat composition, which can be influenced by factors such as temperature, exercise, and the physiological status. This variation affects the accuracy and reliability of the analysis, especially in cases where sweat is exposed and contaminated. In addition, individual differences and variations in physiological and pathological states, such as gender, diet, and genetics, can further complicate the analysis and interpretation of results.
Another challenge is the limited applications of sweat analysis using microfluidic wearable devices, which are currently restricted to monitoring cystic fibrosis, diabetes, and performance of athletes during exercise. Despite providing valuable information about human physiology through biomarkers, the potential applications of sweat analysis remain restricted. Therefore, further research and development are needed to explore and expand the potential of these devices.
Overall, the challenges associated with sweat analysis using wearable microfluidic devices highlight the need for continued innovation and improvement in this field. Addressing these challenges will be crucial for reaping the full potential of sweat analysis as a non-invasive and continuous monitoring tool for physiological and pathological conditions.
3.2. Saliva
Saliva is a readily accessible biofluid produced by three major and numerous minor salivary glands, containing biomarkers similar to sweat.138 The minor glands produce 10% of total saliva, which contains more blood components, while the major glands, including the parotid, submandibular, and sublingual glands, produce 90% of total saliva. The individual production of saliva is 20%, 65%, and 7–8% for the respective glands.139 Saliva production varies depending on factors such as time of day, age, gender, taste, and smell stimulus. There are two types of saliva, stimulated and unstimulated. The production rate of saliva is controlled by the sympathetic and parasympathetic nervous systems. The saliva's composition includes hormones, proteins, electrolytes, mucus, enzymes, glycoproteins, inorganic and organic compounds, and antibacterial compounds, with the concentration of these components varying between stimulated and unstimulated saliva.140Saliva plays a crucial role in the human body by aiding in digestion, lubricating the mouth, protecting teeth from decay, and preventing infections.141 Analysing saliva can provide valuable information about a person's health status, including the diagnosis and monitoring of various diseases such as diabetes, autoimmune diseases, infections, and cancer. It can also be used to monitor drug levels and assess the effects of medication.142 Salivary analysis is a non-invasive, easy-to-collect, and cost-effective method compared to other biofluids such as blood or urine. Due to its potential in early disease detection and personalized medicine, the popularity of salivary analysis has increased in recent years.101
The concentration of the biomarkers in saliva may vary depending on the individual's physiological and pathological state, which makes it necessary to establish a correlation between salivary biomarkers and data of the human body.143 The analysis of saliva biomarkers can provide useful information on various physiological and pathological conditions, such as inflammation, hormone levels, oral health, and stress levels. Saliva has also been used in sports medicine to monitor athletes' hydration and electrolyte balance.144
Several studies have explored the development of wearable salivary biosensors for glucose monitoring. de Castro et al. introduced a paper microfluidic device for detecting salivary glucose and nitrate, Fig. 5a.146 This device consists of two interconnected detection zones through a microfluidic channel and has been integrated into a silicon mouthguard using a 3D-printed holder. However, the device has limitations due to its colorimetric evaluation.147
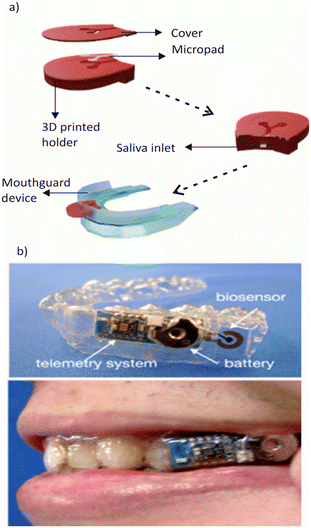 | ||
| Fig. 5 a) Conceptual diagram of a 3D-printed paper microfluidic device for detecting salivary glucose and nitrate. b) Schematic representation of a salivary biosensor that incorporates Pt and Ag/AgCl electrodes on a mouthguard support with an enzyme membrane. Reprinted with permission from ref. 143. Copyright 2020, American Chemical Society. | ||
Arakawa et al. developed a salivary biosensor that incorporates Pt and Ag/AgCl electrodes on a mouthguard support with an enzyme membrane, Fig. 5b.148 The electrodes are formed on the polyethylene terephthalate glycol (PETG) surface of the mouthguard, and the Pt working electrode is coated with a glucose oxidase (GOD) membrane. Although the biosensor seamlessly integrates with a glucose sensor and a wireless measurement system, the potential disadvantage of this technology is the form factor of the mouthguard, which may not be a convenient or comfortable option for some users.
García-Carmona et al. developed a portable saliva-based sensor for continuous monitoring of glucose levels in infants.149 The sensor uses a nontoxic polymeric nipple for saliva collection, making it more practical for infants compared to invasive methods or wearable devices. The glucose-oxidase enzyme is immobilized on the electrode using chitosan, and the resulting oxidation of glucose creates detectable changes in current that are read by a Prussian blue electrode transducer. Although the functionality of the sensor was tested in type I diabetic patients and showed comparable results to blood tests, the potential disadvantage of the device is that its response may be affected by changes in temperature and humidity, requiring frequent recalibration to maintain its accuracy over time.
Saliva is a promising biofluid for detecting lactate, a compound that indicates various medical conditions and physical performance.150 The concentration of lactate in the saliva is correlated with that in the blood, making it a non-invasive and convenient alternative to blood sampling. The normal range of salivary lactate concentration is 0.1 to 2.5 mM, with higher levels indicating metabolic abnormalities or exercise-induced stress.151
One potential application of salivary lactate measurement is diabetes management, as lactate and glucose have a close metabolic relationship.152 Monitoring salivary lactate levels provides insights into glucose metabolism and helps optimize treatment strategies. Additionally, athletes can benefit from salivary lactate measurement as it can help to determine lactate threshold and adjust their training intensity accordingly.142,153
Several biosensors have been developed for salivary lactate measurement, including wearable devices that offer real-time monitoring. Kim et al. developed a wearable device that uses lactate oxidase immobilized on a polymeric film for amperometry measurement.154 However, the field of microfluidics-based sensors for salivary lactate is still underdeveloped. Existing wearable technologies for saliva monitoring can be expensive, complex, and not well-suited for practical use.
Saliva analysis is a promising avenue for detecting various hormones in the body. Cortisol, the primary hormone detected in saliva, has a strong correlation with its concentration in the blood and is commonly studied for stress and anxiety monitoring.155 Salivary testosterone levels can also be used to study behaviour and sports endocrinology in both males and females, while salivary progesterone levels can be used to monitor menstrual cycles and pregnancy in females. In addition, salivary dihydroxyphenyl glycol and melatonin have also been studied for their potential uses in detecting catecholamine levels and pineal physiology in newborns, respectively.156–159
Despite the potential benefits of salivary hormone analysis, there is a lack of wearable devices that can detect these hormones accurately and conveniently in real time. Current devices with microfluidic technology are not flexible or wearable on the human body, and many require additional components for hormone analysis, making them more expensive and inconvenient for users. For instance, while a label-free paper-based electrical biosensor chip has been developed to quantify salivary cortisol at the point-of-care level, it still requires a lab-built, miniaturized PCB for electrical connection and wireless data transmission.160
To fully harness the potential of salivary hormone analysis, much research is needed to develop microfluidic wearable devices that can accurately and conveniently detect salivary hormones in real time without additional components. Such devices would enable continuous monitoring of hormone levels in a non-invasive manner, providing valuable insights into the physiological state of the body. Moreover, they would be particularly useful in fields such as sports endocrinology, where monitoring hormone levels can provide valuable information for optimizing training regimens and performance.
Uric acid is an abundant analyte in saliva, offering a non-invasive means of detection. Saliva testing is a useful technique for monitoring hyperuricemia, hypertension, metabolic syndrome, and cardiovascular risks, as there exists a linear relationship between blood and salivary uric acid.161 A healthy individual has a concentration of 199 ± 27 μmol L−1 uric acid in saliva. Kim et al. developed an instrumented mouthguard capable of non-invasively monitoring salivary uric acid levels using an enzyme-modified screen-printed electrode system integrated onto a mouthguard platform along with anatomically-miniaturized instrumentation electronics featuring a potentiometer, a microcontroller, and a Bluetooth Low Energy (BLE) transceiver.162 However, the device is not wearable because of the requirement of wearing the bulky mouthguard, which may be inconvenient and uncomfortable for some. The absence of microfluidic wearable devices in this field is a significant limitation toward continuous real-time monitoring of analytes such as uric acid.
Saliva contains immunoglobulin A (IgA) and immunoglobulin G (IgG), which are important in fighting pathogens such as viruses, fungi, bacteria, allergic components and parasite agents.163 The measurement of these two antibodies is crucial in assessing mucosal humoral immunity and various intestinal issues related to worms.164 IgA is actively transported into saliva, while IgG enters through passive leakage. Mannoor et al. developed an early wearable platform for detecting bacteria using saliva as a sample in 2012.165 The team used water-soluble silk with printed graphene to transfer it onto bovine tooth enamel, enabling the detection of bacteria even at a single-cell level through self-assembly of antimicrobial peptides on the graphene surface. This resonant-circuit-based device functions without onboard power and could be monitored wirelessly. The team tested the device on H. pylori, a bacterium that leads to duodenal ulcers, and demonstrated the detection of a low number of cells in a 1 μL sample, indicating its sensitivity and potential for remote monitoring of pathogenic bacteria. However, the semi-selectivity of the device might limit its application. Furthermore, since the structure and properties of bovine tooth enamel differ from those of human tooth enamel, it might not be ideal for human use as a substrate. In addition, saliva can also be used for detecting human immunodeficiency virus (HIV), hepatitis C virus, and SARS-CoV-2 (COVID-19) virus.166
The normal pH level of human saliva is 6.6 to 7.1. Real-time monitoring of pH in saliva would be beneficial to understand the health condition of the oral cavity and digestive system.167 Matzeu et al. developed an edible colorimetric pH sensor.168 The sensor shows different colours when exposed to saliva with various pH levels, allowing for easy observation of salivary pH levels with the naked eye. The controlled concentration of the pH indicator ensures biosafety and controlled cost. However, the disadvantage of this sensor is its inability to provide continuous and real-time monitoring of the pH changes in saliva, which is crucial for some applications. A wearable device for wireless real-time monitoring of salivary pH in patients has been developed by Mondal et al.,169 which includes a miniaturized battery-less passive transponder. The transponder consists of an RF front end with digital modulation circuitry and sensing electrodes for electrochemical detection and is capable of detecting changes in pH from 4 to 9. The digital circuitry converts sensor data into a bit sequence and provides the digital sensing data over the reflected backscattered signal. Although the sensor demonstrated a sensitivity of 49.5 mV pH−1, a potential limitation is the narrow pH range. Nevertheless, this technology has the potential for monitoring pH in soil, food, chemicals, and other areas beyond healthcare.
Saliva is a complex fluid that contains impurities, such as charged ions, enzymes, and microorganisms, which can interfere with or damage oral wearable sensors.155,156,167 Despite efforts to improve their reliability, current oral wearable sensors still struggle to meet user demands in this regard.113 The oral cavity is also home to numerous physiological activities that affect the accuracy of the sensor. Although patch or sticker-like oral cavity devices can help mitigate these issues, their removal and washability for hygiene purposes must also be considered. Nanotechnology and materials science may be leveraged to achieve biocompatibility and avoid toxicity.170
Despite the above challenges, wearable devices for analysing saliva are expected to advance to detect viruses like HIV and SARS-CoV-2. Wearable sensors are predicted to play a critical role in disease diagnosis and prevention by integrating with microfluidics and AI, ultimately leading to the diagnosis of future pandemics.
3.3. Interstitial fluid (ISF)
Interstitial fluid (ISF) is an increasingly popular biofluid for wearable medical devices due to its accessibility and similarity to blood composition.171,172 ISF is a colourless fluid found in the spaces between cells, and it can be sampled non-invasively through techniques such as microneedles. ISF is formed by the exchange of fluids and solutes between capillaries and cells through various types of diffusion.173 The accessibility of ISF makes it ideal for monitoring metabolic disorders, therapy assessments, and organ failure, among other medical conditions.101ISF analysis has been challenging in the past due to the difficulty in collecting and analysing samples. ISF is formed from capillaries, its composition is very similar to that of blood, with the highest correlation among all the biofluids in terms of concentration of analytes.174 ISF is found in many parts of the body, including the epidermis, around the salivary and sweat glands, and in other tissues. As a result, ISF is accessible through minimally invasive techniques such as microneedles.175 Recent advances in microfluidic technology have made it possible to develop wearable devices that can monitor ISF in real-time. Microneedle patches emerged as a useful analytical tool to address this information gap. These devices measure glucose levels, electrolytes, and other important biomarkers, providing continuous and non-invasive health monitoring.176 This breakthrough has the potential to transform medical technology, allowing healthcare providers to obtain valuable information about a patient's health status without invasive procedures. The following section presents details about each analyte, including their concentration in blood and associated disorders, along with the respective wearable devices.
Traditionally, measuring blood glucose levels requires a needle inserted into a vein or a finger prick, which can be uncomfortable and painful. Wearable devices that use ISF for continuous glucose monitoring are more practical and less intrusive for diabetic individuals who require frequent glucose monitoring. Takeuchi et al. developed a microfluidic chip with porous microneedles (MNs) to collect ISF.176Fig. 6a shows a conceptual illustration of the microfluidic chip with porous microneedles. The microfluidic chip with an interface for the MN array enabled liquid flow through the entire microfluidic structure. The team also designed a Na+ sensor and correction model to eliminate the effect of individual differences that cause fluctuations in the amount of ISF extracted. Additionally, they designed an electrochemical sensor with a 3D nanostructured working electrode surface to enable precise in situ glucose measurement.
A skin-worn, disposable, wireless electrochemical biosensor for extended non-invasive monitoring of glucose in interstitial fluid (ISF) has been developed, integrating a screen-printed iontophoretic electrode system for ISF extraction by reverse iontophoresis (RI), a printed three-electrode amperometry glucose biosensor, and an electronic interface for control and wireless communication.180 However, it is important to note that prolonged wear of these devices may cause skin irritation or allergic reactions, and frequent replacement of disposable components may increase the overall cost of long-term operation.
Currently, several options for continuous glucose monitoring using ISF are available in the market, including Freestyle Libre by Abbott,181 Dexcom G6 by Dexcom,182 Eversense by Senseonics,183 and Guardian Connect by Medtronic.184 These diagnostic systems use a small needle-like sensor and transmitter to measure glucose levels in the subcutaneous interstitial space. Some of these systems, such as MiniMed185 and Paradigm Revel by Medtronic and t:slim X2 by Tandem, are integrated with insulin pumps that can adjust insulin doses automatically based on glucose levels.
Potassium is one of the electrolytes found in ISF along with sodium, chloride and bicarbonates. As mentioned in sweat and saliva, the concentration of electrolytes such as potassium is important for the well-being of the human body.186 Potassium was found to be 4.37 mM in plasma and 3.97 mM in ISF. A new analytical all-solid-state platform for intradermal potentiometric detection of potassium in interstitial fluid is presented by Parrilla et al.187 This epidermal patch showed good analytical performance and the cell also demonstrated fast response time, selectivity, and reproducibility, making it appropriate for potassium analysis in ISF at both clinical and harmful levels. Miller et al. developed a microfluidic-microneedle platform that combines a hollow microneedle with a microfluidic chip to analyse the potassium content in interstitial fluid.188 To extract fluid through a channel, this system additionally contains a solid-state ion-selective electrode downstream.
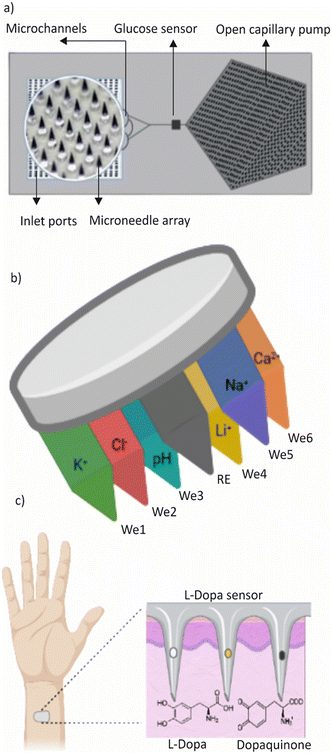
Electrolytes such as sodium, chloride, and calcium are essential for maintaining human health, along with potassium. Therefore, it is imperative to monitor their levels throughout the body. Microneedle sensors present a promising avenue for decentralized clinical analyses, allowing for real-time, on-body monitoring of multiple ions simultaneously.
To this end, Molinero-Fernández et al. demonstrated the potential of membrane-based microneedles for achieving transdermal multiplexed tracing of pH, Na+, K+, Ca2+, Li+, and Cl−.189Fig. 6b shows a graphical representation of the device. The device features an array of seven solid microneedles, externally modified to provide six indicator electrodes – each selective for a different ion – and a common reference electrode, all integrated into a wearable patch that can be read in potentiometric mode. The accuracy is assessed by benchmarking with gold standard techniques used to characterize collected dermal fluid, blood, and serum. The ability to detect multiple ions simultaneously is relevant for a more comprehensive and reliable assessment of the clinical status of a subject concerning electrolyte disorders and other related conditions.
Another system is a minimally invasive micro-needle-based potentiometric sensing system for continuous monitoring of Na+ and K+ levels in the interstitial fluid (ISF) of the skin.189 Li et al. designed this system with a miniaturized stainless steel hollow microneedle to prevent delamination and a set of microneedle electrodes for multiple monitoring. However, the accuracy of the measurements is affected by factors such as tissue heterogeneity, depth of insertion, and calibration difficulties. Thus, it is crucial to conduct a thorough evaluation and verification of the accuracy and dependability of these systems before employing them for clinical purposes.
Maintaining the pH level of interstitial fluid (ISF) is crucial for the proper functioning of cells.190 The pH levels in the blood and ISF are closely related to each other, and any changes in the blood pH level reflect in the ISF pH level. Normal ISF pH ranges between 7.35 and 7.45 levels.191 However, if the pH level becomes too acidic or alkaline, it can negatively affect cell functions such as cell division and protein synthesis. Therefore, it is important to monitor and maintain the pH level of ISF. García-Guzmán et al. developed microneedle (MN) potentiometric sensors for pH transdermal measurements.191 The initial assessment of the MN sensors demonstrated good analytical performance with a response range of 8.5 to 5.0 and a fast response time in both buffer media and artificial interstitial fluid (ISF). The MN sensors were also evaluated for their ability to resist skin insertions in ex vivo setups using animal skins. Researchers are currently in the early stages of studying ISF pH and there are only a few wearable technologies available that can monitor pH levels in vivo.
ISF-based monitoring of therapeutic and illicit drugs is an area of growing interest. Research has demonstrated a strong correlation between blood and concentrations of small drug molecules in ISF, making ISF a potential alternative for therapeutic drug monitoring.101 In a recent research study, Mishra et al. introduced a wearable microneedle sensor array capable of continuous electrochemical detection of opioid and organophosphate nerve agents on a single patch platform. The sensor array utilizes unmodified and organophosphorus hydrolase (OPH), enzyme-modified carbon paste (CP), and microneedle electrodes for square wave voltammetric (SWV) detection of fentanyl and nerve agent targets, respectively.192 The management of Parkinson's disease can be challenging, as the effectiveness of the most commonly used medication, levodopa, is currently assessed based on the patient's self-report of symptoms. Goud et al. developed a new microneedle sensing platform for continuous and minimally invasive monitoring of levodopa.193Fig. 6c shows the schematic illustration of the levodopa sensing device. The platform uses multiple microneedles on a single sensor array patch to simultaneously and independently detect levodopa through enzymatic-amperometry and nonenzymatic voltammetric methods. This technology has the potential to improve the monitoring and detection of drugs of abuse, as well as the exposure to chemical weapons in military and civilian settings. However, current studies in this area are limited.
To overcome these challenges, researchers are exploring the use of biodegradable and non-toxic materials in designing micro-needles for ISF extraction. They are also seeking to improve the durability and reusability of the devices to minimize frequent skin penetration. Incorporating multiplexed sensors into the device to measure multiple analytes, including glucose, hormones, and electrolytes, is another solution to broaden the scope of monitoring and provide more comprehensive data. Microfluidic technology combined with ISF detection can address most of these challenges and lead to significant progress in this field.
3.4. Tears
Tears are a clear, watery fluid that is secreted by the lacrimal glands, conjunctival goblet cells, or para lacrimal glands.195 Tears serve the purpose of lubricating and sterilizing the eyes and contain various components such as lysozyme, immunoglobulin, sugar, and inorganic salts, including numerous salts, proteins, enzymes, and lipids.196 Tears play an essential role in maintaining an individual's health, and a relationship exists between the concentration of glucose, sodium, potassium, and other analytes in blood and tears.197 Changes in the chemical composition of tears can predict or diagnose various health conditions or diseases, making it a valuable diagnostic tool.198 A significant breakthrough in tear analysis is its role in detecting breast cancer.199 Furthermore, changes in tear composition can predict diseases, such as diabetes and ocular diseases. Tear glucose concentration can also be used in the adjuvant treatment of diabetes.200Since tears are similar to sweat, they can be used for therapeutic drug monitoring. Drugs such as acetaminophen, lithium, anticonvulsants, methotrexate, minocycline, etc. can be analysed using tears.201 In addition, tears have unique merits for diagnostic purposes. Tears can be collected in a non-invasive manner, which is especially important for patients, who may be uncomfortable with drawing blood or other invasive procedures.197 Tear collection can also be performed quickly and easily, making it a practical and convenient method for screening and monitoring diseases. Moreover, tears for diagnosis are an emerging research field, and researchers are exploring tear biomarkers for a variety of applications, including the diagnosis and monitoring of cancer, infectious diseases, and neurodegenerative disorders. The following section discusses in detail each analyte in tears, including the corresponding concentration in blood and associated disorders, along with the respective wearable devices and challenges.
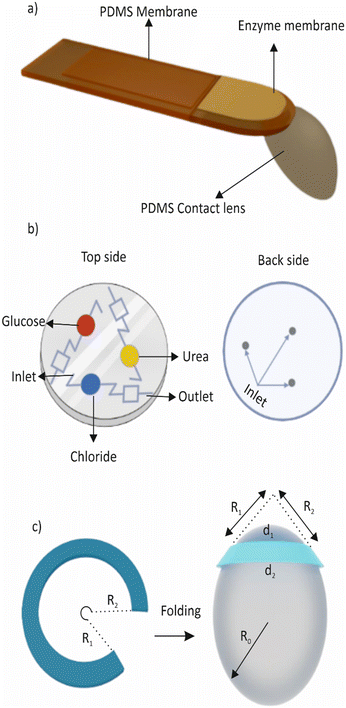
Other than glucose, monitoring the intraocular pressure (IOP) is crucial for maintaining good eye health as it aids in the detection and management of various eye disorders such as glaucoma,206 ocular hypertension, and other related conditions. IOP is the pressure inside the eye that is influenced by the equilibrium between the production and drainage of fluid in the eye.207 Elevated IOP can result in damage to the optic nerve, causing vision loss or blindness. The conventional method of measuring IOP involves using tonometry to measure the pressure on the eye's surface.208 However, this method has some limitations, such as being uncomfortable for patients and being influenced by the corneal thickness and other eye conditions. Tear-analysing biosensors, on the other hand, are a promising technology that offers a non-invasive and potentially more precise method of IOP measurement.209 These biosensors detect changes in specific proteins and biomarkers found in tears that are correlated with changes in IOP.210 As a result, tear-analysed biosensors have the potential to provide an accurate and convenient way of monitoring IOP, particularly for individuals with glaucoma or ocular hypertension who need regular IOP measurements.211 Yang et al. developed a microfluidic contact lens with a notched-ring structure that monitors intraocular pressure continuously. The lens employs a folding technique that enables the transformation of a planar microchannel from 2D to 3D.212Fig. 7c provides a conceptual illustration of the device. To ensure high sensitivity, the folding method is combined with an ultra-sensitive serpentine microchannel in a notched-ring configuration. An et al. reported a microfluidic contact lens sensor, which has the potential to continuously monitor intraocular pressure without the need for a power source or invasive procedures.213 The sensor consists of a micropatterned soft-elastomer sensing layer and a hard plastic reference layer. The device includes an annular sensing chamber filled with dyed liquid and a sensing microchannel that serves as the IOP transducer. When a pressure is applied, the deformation of the sensing layer causes a change in the volume of the sensing chamber, resulting in a displacement of the dyed liquid's interface in the sensing channel. This displacement can be optically observed using a smartphone camera, providing a non-invasive means for monitoring IOP.
A common problem for contact lens wearers is contact lens-induced dry eye (CLIDE), which occurs due to reduced tear volume, tear film instability, and increased tear osmolarity leading to inflammation and discomfort. Zhu et al. addressed this issue by developing an eye-blink mimicking system using a microfluidic hydrogel with integrated microchannels.214 Their in vitro study demonstrated that the system could enhance tear transport from the pre-lens tear film to the post-lens tear film by simulating the motion of an artificial eyelid in a pressure range similar to that of human eyelid pressure. The microchannels were made of poly(2-hydroxyethyl methacrylate) (poly(HEMA)). This study provides a proof of concept for the potential of the system to alleviate CLIDE.
Tear devices demonstrated utility beyond monitoring ocular health by enabling drug detection and delivery. The presence of various biomarkers and small molecules in tears makes them a suitable medium for detecting drugs in the body. Additionally, tears have been explored as a non-invasive means for drug delivery, which offers the advantages of bypassing the gastrointestinal and hepatic first-pass metabolism and yielding a more rapid onset of action. In a study by Sempionatto et al., a wearable platform for tear bioelectronics was developed.215 The platform integrates a microfluidic electrochemical detector into the nose-bridge pad of eyeglasses to monitor key tear biomarkers non-invasively. The biosensing fluidic system uses alcohol-oxidase (AOx) to collect and measure alcohol in real time from stimulated tears, making it the first wearable platform for tear alcohol monitoring. The platform is placed outside the eye region, addressing the limitations of sensor systems that require direct contact with the eye, such as contact lens. The wireless electronic circuitry is integrated into the eyeglasses frame, making it fully portable, convenient, and fashionable to use.
3.5. Urine
Urine is a complex and dynamic biofluid that contains a variety of chemical and biological analytes, including glucose, electrolytes, and hormones. The information provided by these analytes can be invaluable for monitoring an individual's health and detecting the presence of diseases or imbalances in the body.217 In addition, when a drug is consumed, it is metabolized by the body and excreted through urine. Therefore, urine can be analysed to identify the presence of drugs or their metabolites.201 Due to its non-invasive and painless collection process, as well as its abundance, urine is an attractive biofluid for wearable devices.Urinalysis is a technique that has been used for decades to analyse urine, and it involves examining the physical, chemical, and microscopic properties of the sample. Traditional methods of urinalysis include a urine culture, microscopy, and dipstick tests, which can provide information on the presence of bacteria, cells, proteins, and other substances.218 However, these methods have their limitations, including the need for subjective interpretation and the potential for inaccuracies in test results.
Emerging technologies such as biosensing and microfluidics have the potential to overcome these limitations and improve the accuracy and precision of urinalysis for wearable devices.217 Biosensing technologies involve biological receptors such as enzymes or antibodies to detect specific analytes in urine.219 Microfluidic technologies, on the other hand, involve the manipulation of small volumes of urine through microchannels and microreactors to perform analyses. A well-known application of microfluidics in urine is the use of paper-based devices for home pregnancy tests. These tests detect the concentration of the human chorionic gonadotropin (hCG) hormone in urine, which is a reliable indicator of pregnancy.220 Overall, the use of urine as a biofluid in wearable devices has significant potential for improving health outcomes by enabling real-time monitoring and early detection of diseases. While there are limitations to current urinalysis techniques, ongoing advancements in biosensing, microfluidics, and alternative specimen collection methods are paving the way for improved analysis of this valuable biofluid.
Sodium and potassium are two electrolytes that are commonly measured in urine. The balance of sodium and potassium in the body is important for the regulation of blood pressure and fluid balance.223 Typically, the kidneys regulate the amount of sodium and potassium excreted in urine to maintain a healthy balance in the body. However, certain medical conditions or medications can cause imbalances in sodium and potassium levels in urine, which can be determined through urine analysis. Urine also contains various hormones that can provide valuable information about a person's health.224 As mentioned earlier, human chorionic gonadotropin (hCG) is a hormone produced during pregnancy,220 and its presence in urine can confirm pregnancy. Similarly, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) are hormones that are involved in reproductive health, and their levels in urine can be used to monitor fertility.225,226 Cortisol, which is a stress hormone, and aldosterone, which regulates salt and water balance in the body, can also be detected in urine.227,228
Despite the presence of valuable analytes in urine, there are few wearable devices capable of detecting them. While portable urine analysis devices do exist,229–233 the number of continuous monitoring devices utilizing microfluidic technology is limited. Li et al. developed a flexible electrode array that can detect various biomarkers such as potassium ions, sodium ions, hydrogen peroxide, uric acid, and glucose, which are indicative of certain conditions, in urine samples.234 The array, which is about the size of a U.S. quarter, was connected to a circuit board with a Bluetooth module and a lithium-ion battery power source. When exposed to urine samples from volunteers, the device performed comparably to a commercial urine test system. The team then incorporated the array into a diaper and found that it could detect the biomarkers in the presence of urine. However, it should be noted that in a real-time setting, where dry diapers gradually become saturated with urine, the electrode array would require multiple measurements to obtain stable readings. Couto et al. introduced a microfluidic paper-based device that enables the screening and analysis of multiple biomarkers from urine samples on diapers.37 The device allows for testing five different biomarkers with the same sample by distributing the urine sample into multiple spatially segregated regions through capillary action, without the need for external pumps. The device includes a “self-locking” mechanism that closes the sample inlet in approximately four minutes to prevent contamination and continuous entrance of fluids. Moreover, the device provides comfort by maintaining a total thickness of 5.3 mm.
Another device was reported by Cho et al. A microfluidic paper analytical device (μPAD) was created to detect urinary tract infections (UTIs) caused by E. coli and sexually transmitted diseases (STDs) caused by Neisseria gonorrhoeae.235 The device consisted of paper microfluidic channels, with anti-E. coli or anti-N. gonorrhoeae antibodies conjugated to submicron particles, preloaded and dried in the centre of each channel. Undiluted human urine samples spiked with E. coli or N. gonorrhoeae were mixed with 1% Tween 80 for 5 minutes before being introduced into the μPAD, which then flowed through the channel via capillary force. The μPAD successfully filtered out urobilin, which is responsible for the yellow colour and green fluorescence of urine, reducing false-positive signals. Antibody-conjugated particles were then detected by angle-specific Mie scattering, which was quantified using a smartphone camera as a detector. The entire μPAD assay took less than 30 seconds to complete. However, the performance in detecting two specific pathogens in urine samples and the effectiveness for detecting other biomarkers or conditions may vary.
Moreover, urine is not easily accessible for continuous monitoring using wearable devices, as traditional methods for point-of-care urinalysis such as dipstick tests and microscopy are often inaccurate and time-consuming. Developing more affordable and accessible diagnostic solutions, such as combining electrochemical biosensors with microfluidics, could greatly improve access to urinalysis and enable earlier detection and diagnosis of diseases. Microfluidic biosensors could detect even low levels of biomarkers in urine and can lead to earlier cancer diagnosis and improved monitoring of chronic diseases, giving an increasing understanding of the pathophysiology of various diseases. However, the success of these devices requires further research and validation of reference concentrations of biomarkers in urine.
3.6. Wound fluid
Wound fluid is a biofluid that is produced by the body in response to an injury. Wound fluid plays an important role in the wound-healing process. It is composed of a complex mixture of water, electrolytes, proteins, and cellular components. Wound fluid can be analysed to gain insight into the wound healing process and to monitor the effectiveness of treatments.237 It helps to remove debris, bacteria, and other foreign particles from the wound site, and it provides a moist environment that is necessary for tissue repair and regeneration. Additionally, wound fluid contains growth factors and cytokines.238 The presence of certain cytokines in wound fluid can indicate the presence of inflammation or infection and can provide information about the repair and regeneration of the tissues. Analysing the composition of wound fluid will help to choose the suitable dressing technique, the need for antibiotics, etc. The exact composition of wound fluid can vary depending on the type and stage of the wound.239Conventional wound dressing involves several steps, beginning with the use of a saline solution or wound cleanser to remove debris and bacteria from the wound.240 Next, dead tissue may be removed using surgical debridement or debriding agents. Based on the wound type and stage, an appropriate dressing is selected and applied to the wound to promote healing and protect it from further injury. This dressing is typically changed every few days or as directed by a healthcare provider. To supplement the dressing, additional treatments such as antibiotics or growth factors may be used, and the dressing is secured with bandages or tape to maintain a sterile environment. While conventional wound dressing is still commonly used, newer technologies such as smart bandages and microfluidics-based devices offer additional benefits for wound healing. Microfluidics-based devices for wound healing have advantages over traditional wound healing methods and dressing techniques.241 These devices can deliver drugs in a targeted and controlled manner, monitor wound parameters in real time, can be customized to individual patient needs, reduce the risk of infection, and promote faster healing.
The following section provides insights into various analytes found in wound fluid, as well as their relevance in smart wearable devices and bandages for wound healing. The section also addresses some of the challenges associated with these devices.
The wound dressing also includes a microcontroller that processes the data obtained from the sensors and programs the drug release protocol for personalized treatment. This technology has the potential to revolutionize wound care by allowing for timely and individualized treatment to promote faster healing and improved outcomes. P. Rajput et al. developed a prototype of a smart bandage and tested it for its effectiveness in accelerating wound healing.245 It is capable of monitoring the wound status using pH and moisture sensors. The smart bandage consists of various components such as microchannels, electroosmotic mixers, drug reservoirs that release medication in response to heat stimuli, a DC motor mechanism, a porous material, and small sinks to collect used drugs. A recent study involved the development of a smart wound dressing using a hydrogel that can detect bacterial infections by monitoring pH changes.246 This is done through a fluorescence resonance energy transfer (FRET) transition between two dyes, Cyanine3 (Cy3) and Cyanine5 (Cy5), in the presence of bacteria. Additionally, the smart dressing is capable of treating bacterial infections through the release of antibiotics triggered by near-infrared (NIR) light. However, pH may not always be indicative of infection. Other factors, such as inflammation or tissue damage, can also affect wound pH levels, leading to false positives and unnecessary treatments.
The delivery of oxygen to a wound can accelerate and improve wound healing. Oxygen is required for the production of reactive oxygen species (ROS), which are involved in clearing debris and fighting off infections during the inflammatory phase of wound healing. Additionally, oxygen is necessary for angiogenesis and collagen synthesis, which are essential steps in the formation of new blood vessels and tissues. Insufficient oxygen supply can lead to delayed or impaired wound healing, causing chronic wounds or non-healing ulcers. By providing a sufficient supply of oxygen to the wound site, an oxygen delivery device can enhance cellular processes, resulting in faster wound closure and reduced complications. Oxygen delivery devices may be especially beneficial for patients with underlying health conditions that impair their ability to transport oxygen to the wound site.
Ochoa et al. developed a low-cost alternative for continuous delivery and sensing of oxygen. The platform was made of an inexpensive, biocompatible, and flexible paper-based substrate that generates and measures oxygen in a wound region. The system takes advantage of recent developments in flexible microsystems and inkjet printing technology. The platform was able to increase oxygen concentration in a gel substrate by 13% (5 ppm) in 1 hour and was capable of sensing oxygen in a range of 5–26 ppm. On the other hand, Lo et al. developed a new type of wound dressing that uses microfluidic diffusion to deliver oxygen.247 This bandage not only controls the amount of oxygen delivered but also creates a seal that conforms to the wound. When 100% oxygen is delivered, it enters the wound tissues and increases the oxygen levels at the site, thereby promoting healing.
Microfluidic platforms are utilized for delivering therapeutic agents such as drugs and growth factors directly to the wound site. As mentioned earlier, these platforms also help in studying cellular responses to mechanical and chemical stimuli under controlled conditions. The application of microfluidics in wound healing research has resulted in the creation of innovative techniques to facilitate wound closure and tissue regeneration.248 This includes the targeted delivery of oxygen and nutrients to the wound area and the generation of gradients of signalling molecules to direct cell migration and differentiation.248–251 Huang et al. developed a new method for creating fibres using microfluidic spinning technology that can carry two different types of cargo and release them when needed.252Fig. 8a provides an illustration of microfluidic spinning and collection of dual-cargo-loaded microfibers. This is achieved by combining biomaterials with hydrophobic and hydrophilic properties to form a bead-on-string microfibre structure. The team reported successful loading of bovine serum albumin (BSA) in the sodium alginate phase and ibuprofen in the polylactic acid (PLA) phase. The resulting fibres are biocompatible and can stop bleeding in live animals. When woven into a skin scaffold and loaded with antibacterial and anti-inflammatory agents, the fibres have the potential to promote faster wound healing. Fig. 8b shows a schematic representation of the oxygen sensing and delivery patch for foot ulcers along with a cross-sectional area of the wound.253 While microfluidic devices and smart bandages offer several advantages in wound healing, they may still require the supervision of a medical expert.
4. Smart wearable microfluidic devices
Following the introduction of digital hearing aids in the 1980s, wearables have undergone significant transformations, evolving in form and functionality.254 One area that has seen substantial growth is the integration of microfluidics into healthcare wearables. However, the development of wearable microfluidic devices for commercialization faces challenges related to miniaturization, integration, and intelligence. Recent advancements in microfluidics have explored the fusion of artificial intelligence (AI) technologies, leading to the emergence of intelligent microfluidic devices.255Fig. 9a illustrates examples of typical advanced wearable devices.102The healthcare industry has been benefitting from the rapid growth of cutting-edge technologies such as the Internet of Things (IoT),256 artificial intelligence (AI),257 and the Internet of Medical Things (IoMT).258 These technologies, once confined to the realm of science fiction, have become a tangible reality, affecting various aspects of our lives. Their integration has expanded the capabilities and functionalities of wearable devices. AI and IoT offer robust high-dimensional data processing capabilities, particularly in disease diagnostics and fatigue monitoring, paving the way for personalized medicine and improved patient outcomes. AI enables real-time analysis and decision-making, while IoT and IoMT facilitate seamless data exchange and remote monitoring. The integration of IoT into microfluidic devices has gained momentum, driven by the rapid growth of automation and wireless networks. This advancement enables the creation of remotely controlled and monitored microfluidic devices, facilitating on-site analysis and monitoring.259,260
The increasing dependence on AI in the healthcare sector is evident in the projected growth of global artificial intelligence in the healthcare market, which is expected to reach USD 31.3 billion by 2025, with a remarkable CAGR of 41.5% (according to Grand View Research, Inc.).261 This substantial growth reflects the expanding scope and potential of AI in improving healthcare outcomes and transforming the industry.
The following sections explore the recent developments in the integration of AI, IoT, IoMT and IoP in microfluidic wearable devices for on-site performance, address the challenges and limitations faced, and discuss potential future directions. By examining the convergence of these technologies, we can gain valuable insights into their impact on the development of intelligent, connected, and user-centric microfluidic-based wearable devices. For ease of reference, advantages of the intelligent wearable devices are illustrated in Fig. 9b.102
4.1. Artificial intelligence and microfluidic wearable devices
Artificial intelligence (AI) is the simulation of human intelligence processes by computer systems. These processes include learning (the acquisition of information and rules for using the information), reasoning (using the rules to reach approximate or definite conclusions), and self-correction.262 Artificial intelligence (AI) techniques, including machine learning and deep learning algorithms, have gained significant attention in recent years due to their ability to analyse complex data, recognize patterns, and make real-time decisions. In the context of microfluidics-based wearable devices, AI empowers these devices with intelligent data processing, predictive modelling, and adaptive control capabilities. By harnessing AI algorithms, these devices can extract valuable insights from the vast amount of data generated by microfluidic systems, leading to improved diagnostics, personalized treatment strategies, and real-time monitoring of health conditions.255,263AI in wearable microfluidic devices offers valuable applications in data analysis and decision-making. AI algorithms excel at extracting meaningful patterns and interpreting complex data, enabling precise diagnostics, continuous monitoring, and personalized treatment recommendations.264 Furthermore, AI's predictive analytics capabilities enable early disease detection, prognosis assessment, and preventive interventions based on historical data. Intelligent control and automation represent another area where AI enhances wearable devices, driving significant advancements in healthcare.265
In microfluidic wearable devices, conventional control systems typically operate using fixed algorithms and predetermined rules.266 These systems adhere to predefined instructions for tasks like drug release or parameter monitoring. While functional, they exhibit a lack of adaptability to dynamically changing conditions. In contrast, AI-based control systems introduce a transformative approach to microfluidic wearables. Rather than relying on static rules, these systems depend on machine learning algorithms to analyze real-time data.267 This empowers them to make decisions based on the current context, learning and adapting over time.268
AI-based control systems offer considerable advantages in the design of drug delivery devices, especially in the context of diabetes management. In comparison to conventional control systems that administer insulin at predetermined intervals, regardless of the wearer's immediate blood glucose levels, AI-based systems can dynamically adjust the insulin dosage based on real-time glucose monitoring.269 However, AI-based systems present a more sophisticated and responsive approach.270 These AI-driven systems can continuously monitor glucose levels, assimilate patterns over time, and dynamically adjust insulin dosages accordingly. This dynamic response ensures more effective blood sugar control, significantly reducing the risks associated with hypoglycemia or hyperglycemia. The benefits of AI-based control extend beyond adaptability, demonstrating notable strengths in personalization. These systems excel at tailoring responses based on individual user data, providing a level of customization that is challenging for conventional systems to achieve.
Moreover, the significance of AI-based control systems extends to critical tasks such as fluid mixing within microfluidic devices, where precise combinations of fluids are paramount.265 Traditional systems may encounter challenges in adapting to variations in fluid properties, concentrations, or environmental factors. Conversely, AI-driven microfluidic systems excel at optimizing mixing ratios, ensuring the achievement of accurate and consistent results. Similarly, in the context of flow control, AI-based systems demonstrate a heightened level of sophistication and responsiveness.271,272 These systems can dynamically regulate flow rates based on real-time data, adapting seamlessly to changes in fluid viscosity or external environmental conditions.273 This heightened adaptability contributes to the overall efficiency and performance of microfluidic wearables, offering a level of precision that is particularly crucial in processes such as drug delivery or diagnostic procedures.270 The dynamic adjustment of flow rates ensures that the microfluidic processes remain finely tuned, providing a more accurate and personalized experience within the wearable device.274
AI significantly contributes to personalized medicine as well by analysing patient-specific data, including genetic information, biomarker profiles, and physiological parameters. AI algorithms can develop tailored treatment plans.275 These plans consider individual characteristics, allowing for personalized therapies, precise dosages, and targeted interventions, ultimately leading to improved healthcare outcomes. Furthermore, they have the potential to revolutionize disease monitoring and management. Through continuous monitoring and real-time feedback, these devices detect changes in biomarkers, track disease progression, and trigger timely interventions. This proactive approach to healthcare management reduces the risk of complications and empowers both patients and healthcare providers with actionable insights. Moreover, AI facilitates the fusion and integration of data within wearable devices.265 These devices seamlessly integrate data from various sources, such as physiological measurements, genetic data, and environmental factors. By combining these diverse data streams, AI algorithms uncover hidden relationships and provide a comprehensive understanding of an individual's health. This comprehensive understanding enables healthcare professionals to make more informed decisions.
The integration of artificial intelligence (AI) with point-of-care microfluidic devices has indeed seen notable progress.270 However, the extension of this synergy to microfluidic wearable devices within the same domain faces certain limitations. Several factors contribute to the current absence of widespread AI-driven wearable microfluidic devices. The challenges lie in the complexity of merging AI algorithms with the miniaturized and portable nature of wearable microfluidic devices. While microfluidic devices at point-of-care settings benefit from integrated AI for improved diagnostics or treatment optimization, scaling down these capabilities for wearables introduces additional technical hurdles. In Table 3, various examples of microfluidic devices associated with AI-based control systems are likely presented. These could encompass devices designed for tasks such as flow control, mixing and particle manipulation. These devices leverage AI algorithms to process the data obtained from microfluidic components, allowing for more sophisticated and adaptive functionality.23
| Device | Biofluid | Technique used | Advantages | Ref. |
|---|---|---|---|---|
| μTPAD and 3D μPAD for glucose analysis | Artificial urine | Artificial neural network (ANN trained on CMYK colour data) | 1. High accuracy in classifying samples (94.4% for μTPAD, 91.2% for μPAD) | 265 |
| Rare hereditary hemolytic anemia (RHHA) study using microfluidics | Blood | Deep learning (DL) | 1. Enables user-independent, automatic analysis with image analysis modules | 284 |
| 2. Supports continuous monitoring using low-cost time-lapse microscopy (TLM) equipment | ||||
| Microfluidic chip for T-cell and B-cell isolation and detection | Blood | Support Vector Machine (SVM) with Histogram of Oriented Gradients (HOG) | 1. Efficient separation of T-cells and B-cells from sub-microliter blood samples | 267 |
| 2. Utilizes SVM with HOG and colour distribution features for fast and robust cell detection | ||||
| 3. Achieves high accuracy (94% for detection, 96% with cross-validation) in distinguishing T-cells and B-cells | ||||
| Enhanced immunoassay system | Serum | Convolutional neural network (CNN) | 1. Simultaneous detection of six cytokines with high sensitivity | 287 |
| 2. Short processing time | ||||
| 3. Rapid and accurate machine-learning-based image processing using CNN | ||||
| Soft microfluidic patch | Sweat | Guided image capture and automated analysis | 1. Real-time monitoring of sweat profiles | 273 |
| 2. Personalized fluid intake recommendations | ||||
| 3. Usable in a broad range of fitness levels and conditions |
4.2. Machine learning and deep learning in microfluidics
Machine Learning (ML) is an integral part of artificial intelligence and serves as its core. ML algorithms construct models using sample data, known as training data, enabling them to make predictions or decisions without explicit programming.276 The fundamental elements of ML are data, algorithms (models), and computational power. ML particularly excels in tasks involving high-dimensional data, such as classification, regression, and clustering.277 ML proves to be highly useful, as it learns from past computations and extracts patterns from extensive databases, resulting in reliable and consistent decision-making. The field of machine learning has seen remarkable progress in recent years, particularly in the context of wearable devices that collect high-dimensional data on users' health.278 Machine learning algorithms are capable of processing these data to extract insights and make predictions about an individual's health.Machine learning (ML) encompasses two primary approaches: supervised learning and unsupervised learning. Supervised learning involves training ML algorithms using labelled data, where each data point is accompanied by its corresponding target or outcome.279 The algorithm learns to map input variables to the desired output based on labelled training data. With this knowledge, ML can then make predictions or decisions for new, unseen data. Supervised learning is highly useful for tasks like classification and regression, where the goal is to predict a specific outcome. The advantage of supervised learning is that it can provide accurate predictions and make well-informed decisions based on labelled training data. On the other hand, unsupervised learning deals with unlabelled data, meaning that the input data does not come with predefined outcomes. Instead, the algorithm aims to find patterns, structures, or relationships within the data on its own. It does so by grouping similar data points or identifying underlying patterns that may exist. Unsupervised learning is often employed in tasks such as clustering or anomaly detection. One of the key advantages of unsupervised learning is its ability to discover hidden insights and uncover previously unknown patterns within data, providing valuable insights for further analysis.280–282
Both supervised and unsupervised learning methods have their respective advantages and use cases in machine learning.282 Supervised learning allows for accurate predictions and decision-making based on labelled data, which is particularly valuable in scenarios with known desired outcomes. Unsupervised learning, on the other hand, can uncover hidden patterns and relationships in unlabelled data, providing valuable insights and opportunities for further exploration.
Deep learning is a subfield of artificial intelligence (AI) that focuses on training artificial neural networks to learn and make predictions or decisions without explicit programming.283 The process involves training deep neural networks with multiple layers to extract and understand complex patterns and representations from large datasets. In the context of microfluidic wearable devices, deep learning can be utilized for various tasks such as data analysis, decision-making, and control. The training process involves feeding the neural network with labelled or unlabelled data, depending on whether it's supervised or unsupervised learning.284
Deep neural networks find significant applications in medical diagnostics, where the pivotal role of supervised learning becomes evident.285 In the context of wearable devices, supervised learning is harnessed to train deep learning models using labelled datasets. These datasets comprise input samples such as physiological measurements and environmental factors, paired with corresponding output labels indicating specific health conditions or disease states. Through this process, the deep learning model learns to associate input data with their respective labels, enabling accurate predictions or classifications on new, unseen data.
A critical element in supervised deep learning is the definition and utilization of a loss function.286 This function serves as a metric for the disparity between the model's predictions and the true labels in the dataset.286 The minimization of this loss involves backpropagation, a technique where the computed loss is systematically propagated backwards through the neural network, influencing the model's parameters.287 This iterative refinement allows the model to learn and enhance its performance over time. The ultimate objective is to update the model's parameters iteratively, improving its ability to make accurate predictions on unseen data.276
Microfluidic platforms, when coupled with deep learning algorithms, provide a versatile framework for simulating physiological conditions and studying various disorders. The application of deep learning in microfluidics allows for efficient data analysis and pattern recognition in dynamic biological systems. Comparative analyses of various deep learning architectures, including baseline convolutional neural networks (CNNs), SimpleNet, and CapsNet, reveal insights into their strengths and weaknesses.288 Recurrent neural networks (RNNs), known for their sequential data processing capabilities, find application in microchip design for diagnostics.289 Utilizing RNNs, particularly variants like long short-term memory (LSTM), allows for real-time processing of time-series data, showcasing the flexibility of deep learning in handling dynamic information.
On the other hand, unsupervised learning involves training the deep learning model using unlabelled data. Without explicit labels, the model focuses on discovering hidden patterns or structures within the data. Unsupervised learning is useful for tasks such as clustering, anomaly detection, and dimensionality reduction. In microfluidics, unsupervised learning can help to identify distinct groups or clusters of samples based on their characteristics or detect unusual patterns that may indicate abnormalities or outliers in the data.290,291
Both supervised and unsupervised learning methods have their advantages in wearable microfluidic devices. Supervised learning allows for targeted predictions or classifications based on known labels, which can be beneficial for specific diagnostic or monitoring applications. Unsupervised learning, on the other hand, provides a data-driven approach to uncovering patterns and relationships within complex microfluidic datasets, enabling researchers to gain insights and discover novel information without prior knowledge or assumptions.
Baker et al. present an updated wearable microfluidic sweat testing system for recreational athletes that includes a microfluidic patch and a smartphone app that can analyse sweat in real time using digital image processing algorithms (ML).292 The microfluidic patch can accommodate a broad range of sweating rates, and the smartphone app can analyse the sweat under different lighting conditions and patch orientations. In another study, Ning et al. reported a rapid segmentation and sensitive analysis of CRP with a paper-based microfluidic device using machine learning.293 The study involves the fabrication of multi-layer μPADs using the imprinting method for colorimetric detection of C-reactive protein (CRP). The detection-related performance of the μPADs is enhanced by utilizing a machine learning algorithm, specifically, the You Only Look Once (YOLO) model,294 which is capable of identifying the reaction areas in the μPADs under different lighting conditions and shooting angles of scenes. The YOLO model was trained in the study and was able to quickly and accurately identify all reaction areas without error. The reaction areas were then characterized by classification algorithms to determine the risk level of CRP concentration. A microfluidic ultrafine particle dosimeter using an electrical detection method with a machine-learning-aided algorithm for real-time monitoring of particle density and size distribution was also reported. Mostafalu et al. developed thread-based microfluidic networks that establish close connections with biological tissues in a three-dimensional manner, Fig. 9c.295 The research also involves the creation of a range of physical and chemical sensors, which are seamlessly integrated into these microfluidic networks. These sensors are fabricated using conductive threads infused with nanomaterials and are interconnected with electronic circuitry through flexible thread-based interconnects. This setup allows for the measurement and monitoring of physiochemical tissue properties, facilitating direct integration with tissues and enabling the implementation of a thread-based diagnostic device (TDD) platform.295 To showcase the capabilities of our integrated sensor suite, we conducted experiments using TDD platforms to measure parameters such as strain, gastric pH, and subcutaneous pH both in vitro and in vivo.
While the field of wearable devices incorporating microfluidics and artificial intelligence holds immense promise, the limited prevalence of such devices prompts a closer examination of the challenges impeding their widespread adoption. Within the complex landscape of AI and data science, a series of nuanced challenges must be navigated before these innovative devices can proliferate.270 One pivotal challenge lies in ensuring the generalizability of the AI models across diverse user populations. The ability of these models to adapt and perform effectively across various demographic and physiological characteristics is essential for their practical application.283 Achieving this requires a thorough understanding of the complexities associated with different user contexts and the development of robust algorithms that can accommodate these variations.
Model complexity considerations represent another significant hurdle. Striking the right balance in model complexity is crucial to prevent issues such as overfitting or underfitting.263 The intricacies of microfluidic data and the diverse nature of physiological measurements necessitate AI models that are sophisticated enough to capture relevant patterns yet not overly complex to hinder practical deployment. Ethical considerations add an additional layer of complexity.296,297 The deployment of AI in wearable devices raises concerns related to data privacy, bias detection, and transparency in decision-making processes. Safeguarding user data, ensuring fairness in algorithmic outcomes, and maintaining transparency are paramount for responsible AI deployment in healthcare.
4.3. Internet of Things and wearable microfluidic devices
The Internet of Things (IoT) is a network of physical devices, objects, and appliances equipped with sensors, software, and connectivity.298 The IoT enables them to collect and exchange data over the internet, creating a seamless connection between the physical and digital worlds. In IoT, devices use sensors to gather data about their surroundings or interactions. These data include temperature, motion, and biometrics.299 The devices process the data using internal processors, often in real time. IoT devices connect to the internet, allowing them to transmit data to cloud platforms or other devices. Communication occurs through protocols such as Wi-Fi, Bluetooth, or specialized IoT protocols. Advanced analytics and algorithms analyse data, generating valuable insights, detecting patterns, and triggering automated actions. Security is vital in IoT to protect data integrity and privacy.300 Encryption, authentication, and access control mechanisms ensure that data are secured. The IoT has diverse applications, including smart homes, industrial automation, agriculture, transportation systems, and healthcare monitoring.The integration of IoT technology with microfluidic wearable devices is transforming the healthcare industry.301 This combination of microfluidics and IoT connectivity brings new possibilities for diagnostics, monitoring, and personalized medicine. By connecting to the internet, microfluidic wearables can transmit real-time data, enabling remote monitoring of a patient's health.11 Equipped with sensors, these wearables capture vital health parameters like biomarkers or fluid flow rates. The data are wirelessly sent to healthcare providers or cloud-based platforms for in-depth analysis using advanced analytics.
Real-time monitoring becomes a reality as healthcare professionals receive continuous updates on patients' conditions. Immediate alerts can be triggered for critical events or abnormal readings, facilitating timely interventions that can potentially save lives.302 Remote monitoring is particularly beneficial for individuals with chronic conditions or those in remote areas.
The integration of IoT also brings data-driven insights and personalized healthcare. Advanced analytics algorithms process the collected data, identifying trends, patterns, and potential health issues.303 This information empowers healthcare providers to make accurate diagnoses, create personalized treatment plans, and deliver proactive care. Patients can actively engage in their healthcare by accessing health data through mobile applications, setting goals, and receiving tailored recommendations.304
Additionally, the integration of microfluidic wearables with IoT offers scalability and interoperability.305 These devices can be easily deployed across various healthcare settings, seamlessly integrating with existing systems, electronic health records, and other medical devices. The smooth exchange of data ensures a comprehensive understanding of a patient's health history and enables collaboration among healthcare professionals.306
Despite the numerous advantages and benefits of integrating IoT with microfluidic wearable devices, the availability of IoT-based microfluidic wearables is currently limited. However, Ardalan et al. have made significant progress in this area. The team developed a cellulose-based wearable patch, combined with a smartphone-based fluorescence imaging module and a custom smartphone app. This system allows for non-invasive and in situ multi-sensing of sweat biomarkers such as glucose, lactate, pH, chloride, and volume.307Fig. 9d shows the schematic illustration of the device. Their smart wearable sweat patch (SWSP) sensor consists of highly fluorescent sensing probes embedded in paper substrates, along with microfluidic channels made of cotton threads. These channels effectively collect sweat from the skin surface and transport it to the paper-based sensing probes. To capture digital images of the sensors, an imaging module was fabricated using a 3D printer and equipped with UV-LED lamps and an optical filter. These images are captured via a smartphone, which also features a specially designed app with a detection algorithm to quantify the concentration of the biomarkers. Furthermore, the researchers proposed an IoT-based model for their SWSP sensor, envisioning its potential applications in the future. Field studies involving human subjects were conducted to assess the feasibility of the developed SWSP sensor for analysing sweat biomarkers.
In addition to wearability, integrated Internet of Things (IoT) will enhance the functionality of microfluidic devices. For instance, Yuan et al. discussed the design, fabrication, and implementation of an IoT-based electrochemical microfluidic system, utilizing 3D printing technology, for detecting free calcium concentration.308 This system is capable of accurately measuring free calcium solutions within the desired concentration range of 0 to 40 μM and transmitting signals to the cloud for data sharing. Consequently, this system offers a precise and real-time monitoring solution for various biomedical samples.
4.4. Other smart technologies
Alongside artificial intelligence and the Internet of Things, there are other technologies with the potential to revolutionize healthcare when integrated with microfluidic wearable devices. Two notable examples include the Internet of Medical Things (IoMT) and the Internet of People (IoP). These technologies hold immense capabilities for transforming healthcare by connecting medical devices and individuals, respectively.The Internet of Medical Things (IoMT) facilitates the connection and communication between medical devices, sensors, and systems through the internet.258 When combined with microfluidic wearable devices, the IoMT enables seamless real-time monitoring and data collection. This integration allows healthcare professionals to continuously track vital signs, analyse biofluids, and monitor various health parameters remotely. By harnessing the power of IoMT, medical practitioners gain access to accurate and up-to-date information, leading to enhanced diagnostics, personalized treatments, and proactive preventive care.309 On the other hand, the Internet of People (IoP) focuses on interconnectivity between individuals and their surrounding devices. When merged with microfluidic wearable devices, the IoP empowers individuals to actively participate in managing their health.310 Through wearable devices integrated with IoP, users can effortlessly track their health metrics, receive personalized health recommendations, and even share their data with healthcare providers.311 This collaborative approach promotes proactive healthcare management, early detection of potential health issues, and seamless communication between patients and medical professionals.
4.5. Challenges and outlook
The integration of microfluidic technology with advanced technologies such as AI, IoT, IoMT, and IoP in wearable devices is an emerging and complex field that faces several challenges.312 As a result, the number of commercially available microfluidic wearable devices integrated with these technologies is currently limited. One key challenge is the complexity of integration. Microfluidic devices are intricate systems that require precise fabrication and assembly of fluidic channels, sensors, and actuators. Incorporating AI, IoT, IoMT, and IoP into these devices adds a layer of complexity in terms of hardware, software, and connectivity requirements.11 Developing reliable and robust integration methods necessitates extensive research and development efforts.Moreover, there are significant technological hurdles to overcome. One significant challenge revolves around power consumption, as these devices must operate efficiently within limited power constraints to ensure prolonged usability without frequent recharging. Achieving a balance between computational power and energy efficiency is imperative for the success of such wearable devices. Another crucial aspect is the miniaturization and seamless integration of AI components into the microfluidic platform. Given the restricted physical space available, designing and incorporating compact AI hardware without compromising overall device performance is a non-trivial task.287 The quality and quantity of data present additional hurdles. Gathering representative data from microfluidic experiments, while maintaining accuracy and relevance, is vital for training robust AI models. Optimization of algorithms specific to microfluidic data characteristics is also essential for effective model performance. Interdisciplinary collaboration is fundamental, as the development of AI-integrated microfluidic wearable devices requires expertise in microfluidics, AI, materials science, and other fields.264 Bridging the interdisciplinary gap is crucial for a comprehensive understanding and successful device development.
Security and privacy concerns must not be overlooked. The integration of various technologies, including ML, IoT, and AI, into microfluidic devices introduces a spectrum of ethical considerations and social security challenges. These multifaceted issues require careful examination to ensure responsible and equitable deployment.296 From an ethical standpoint, obtaining informed consent for data collection and addressing concerns related to data ownership and control are paramount.313 Transparency in ML algorithms, regardless of whether they involve AI, is crucial to build trust and mitigate biases that may affect medical diagnostics. Privacy standards must be rigorously upheld to protect sensitive health information collected by IoT-enabled microfluidic devices. Security measures become even more critical when considering the interconnected nature of IoT. Safeguarding against cyber threats and unauthorized access to data and devices is essential to maintain the integrity and confidentiality of information.314 Ensuring fairness in ML algorithms and preventing discrimination are an ethical imperative, especially when these technologies are applied in healthcare settings.
Additionally, regulatory and standard considerations play a vital role in the development and commercialization of medical devices, including microfluidic wearables. Adhering to regulatory guidelines and obtaining necessary certifications can be a time-consuming and resource-intensive process, further delaying the introduction of integrated microfluidic wearable devices to the market. Moreover, future research should focus on investigating potential biases that may arise from measurement sources, particularly considering the variability in fluid compositions among diverse user populations. Understanding how different demographic and physiological factors can introduce bias in microfluidic measurements is crucial for developing robust and unbiased AI algorithms. This exploration is essential not only for enhancing the accuracy and reliability of AI-integrated microfluidic devices but also for ensuring equitable and unbiased healthcare solutions across various user demographics.
Despite these challenges, ongoing research and technological advancements continue to pave the way for the future development and commercialization of microfluidic wearable devices integrated with AI, IoT, IoMT, and IoP. As the field progresses, we can anticipate increased innovation, improved integration methods, and a broader range of microfluidic wearable devices that leverage these advanced technologies to enhance healthcare outcomes.
5. Conclusion
Microfluidics has emerged as a truly exciting and promising technology that holds great potential in the field of healthcare. Its unique ability to handle small sample volumes, perform rapid reactions, and achieve high sensitivity and throughput data has opened up a world of possibilities across diverse fields.11The integration of microfluidics with advanced technologies like microelectronics, biosensors, soft materials, and artificial intelligence (AI) has led to the development of wearable microfluidics, a groundbreaking innovation with immense promise in the realm of medical diagnosis and personalized healthcare.254 By combining the power of microfluidics with AI-driven data analytics and machine learning (ML), we can now envision wearable devices that provide real-time diagnostic information to patients. This remarkable combination allows for high-throughput analysis of small sample volumes and advanced data processing, unlocking potential breakthroughs in cell sorting, biomolecular analysis, and many more.
Looking towards the future, the potential of microfluidics lies in the region of intelligent systems. As we venture into an interconnected era where energy, electronics, communication, computers, and sensors converge, intelligent microfluidic systems will serve as powerful platforms for biomedical analysis. Moreover, the integration of microfluidics with other cutting-edge technologies such as the Internet of Things (IoT), Internet of Medical Things (IoMT), and Internet of People (IoP) amplifies the transformative capabilities of these wearable devices.
While the integration of these advanced technologies with wearable microfluidic devices brings tremendous promise, it is crucial to acknowledge the challenges that lie ahead. Technical complexities, standardization efforts, regulatory considerations, and user acceptance all demand further attention and refinement to ensure successful implementation.
Looking ahead, we anticipate that intelligent microfluidics will play an increasingly crucial role in both research and industry. These integrated wearable devices offer the potential to revolutionize healthcare and personalized medicine, empowering individuals with accurate biofluid analysis, remote monitoring capabilities, and personalized treatments. As research progresses and we continue to push the boundaries, we can expect the development of even more sophisticated and interconnected wearable microfluidic devices. Ultimately, this progress will pave the way for improved healthcare outcomes, preventive care, and a better quality of life for individuals around the globe.
In summary, the integration of microfluidics with wearable technology and intelligence technologies such as AI, IoT, IoMT, and IoP represents an exciting frontier in healthcare and personalized medicine. By combining the elegance of microfluidics with these advanced technologies, we embark on a journey of innovation and transformation. The possibilities are vast, and as we advance, we move closer to a future where microfluidic wearable devices bring about tangible improvements in healthcare, revolutionizing the way we diagnose, monitor, and treat medical conditions.
Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
Kamalalayam Rajan Sreejith acknowledges the financial support provided by Griffith University through the Griffith University Postdoctoral Fellowship. Nam-Trung Nguyen acknowledges funding support from the Australian Research Council through the Australian Laureate Fellowship FL230100023.References
- J. Heikenfeld, A. Jajack, J. Rogers, P. Gutruf, L. Tian, T. Pan, R. Li, M. Khine, J. Kim, J. Wang and J. Kim, Lab Chip, 2018, 18, 217–248 RSC.
- G. Aroganam, N. Manivannan and D. Harrison, Sensors, 2019, 19, 1983 CrossRef PubMed.
- Fitbit Official Site for Activity Trackers and More, https://www.fitbit.com/global/in/home, (accessed 18 April 2023).
- Monitor your heart rate with Apple Watch, https://support.apple.com/en-in/HT204666, (accessed 18 April 2023).
- G. L. or its subsidiaries, Fitness Tracker|Wearables, https://www.garmin.co.in/products/wearables/, (accessed 18 April 2023).
- B. J. van Enter and E. von Hauff, Chem. Commun., 2018, 54, 5032–5045 RSC.
- P. Kamga, R. Mostafa and S. Zafar, Curr. Emerg. Hosp. Med. Rep., 2022, 10, 67–72 CrossRef PubMed.
- A. Channa, N. Popescu, J. Skibinska and R. Burget, Sensors, 2021, 21, 5787 CrossRef CAS PubMed.
- Gartner Forecasts Global Spending on Wearable Devices to Total $81.5 Billion in 2021, https://www.gartner.com/en/newsroom/press-releases/2021-01-11-gartner-forecasts-global-spending-on-wearable-devices-to-total-81-5-billion-in-2021, (accessed 2 April 2023).
- C. Wu, P. Jiang, W. Li, H. Guo, J. Wang, J. Chen, M. R. Prausnitz and Z. L. Wang, Adv. Funct. Mater., 2020, 30, 1907378 CrossRef CAS.
- J. R. Mejía-Salazar, K. Rodrigues Cruz, E. M. Materón Vásques and O. Novais de Oliveira Jr, Sensors, 2020, 20, 1951 CrossRef PubMed.
- Y. Xing, L. Zhao, Z. Cheng, C. Lv, F. Yu and F. Yu, ACS Appl. Bio Mater., 2021, 4, 2160–2191 CrossRef CAS PubMed.
- F. Gao, C. Liu, L. Zhang, T. Liu, Z. Wang, Z. Song, H. Cai, Z. Fang, J. Chen, J. Wang, M. Han, J. Wang, K. Lin, R. Wang, M. Li, Q. Mei, X. Ma, S. Liang, G. Gou and N. Xue, Microsyst. Nanoeng., 2023, 9, 1 CrossRef PubMed.
- J. Choi, A. J. Bandodkar, J. T. Reeder, T. R. Ray, A. Turnquist, S. B. Kim, N. Nyberg, A. Hourlier-Fargette, J. B. Model, A. J. Aranyosi, S. Xu, R. Ghaffari and J. A. Rogers, ACS Sens., 2019, 4, 379–388 CrossRef CAS PubMed.
- S. Damiati, U. Kompella, S. Damiati and R. Kodzius, Genes, 2018, 9, 103 CrossRef PubMed.
- T. Sun, J. Hui, L. Zhou, B. Lin, H. Sun, Y. Bai, J. Zhao and H. Mao, Sens. Actuators, B, 2022, 368, 132184 CrossRef CAS.
- J.-D. Huang, J. Wang, E. Ramsey, G. Leavey, T. J. A. Chico and J. Condell, Sensors, 2022, 22, 8002 CrossRef PubMed.
- B. R. Schatz, NPJ Digit. Med., 2018, 1, 20174 CrossRef PubMed.
- C. Y. Jin, IOP Conf. Ser.: Mater. Sci. Eng., 2019, 688, 044072 CrossRef.
- S. A. Amyx+McKinsey, Wearing Your Intelligence, https://www.wired.com/insights/2014/12/wearing-your-intelligence/, (accessed 18 April 2023).
- F. John Dian, R. Vahidnia and A. Rahmati, IEEE Access, 2020, 8, 69200–69211 Search PubMed.
- S. Radhakrishnan, M. Mathew and C. S. Rout, Mater. Adv., 2022, 3, 1874–1904 RSC.
- H.-F. Tsai, S. Podder and P.-Y. Chen, Micromachines, 2023, 14, 826 CrossRef PubMed.
- S. Shrivastava, T. Q. Trung and N.-E. Lee, Chem. Soc. Rev., 2020, 49, 1812–1866 RSC.
- Anushka, A. Bandopadhyay and P. K. Das, Eur. Phys. J.: Spec. Top., 2023, 232(6), 781–815 CAS.
- Y. Yang, E. Noviana, M. P. Nguyen, B. J. Geiss, D. S. Dandy and C. S. Henry, Anal. Chem., 2017, 89, 71–91 CrossRef CAS PubMed.
- X. Zhang, L. Li and C. Luo, Lab Chip, 2016, 16, 1757–1776 RSC.
- H. H. Caicedo and S. T. Brady, Trends Biotechnol., 2016, 34, 1–3 CrossRef CAS PubMed.
- W. Yang, W. Han, H. Gao, L. Zhang, S. Wang, L. Xing, Y. Zhang and X. Xue, Nanoscale, 2018, 10, 2099–2107 RSC.
- S. R. Corrie, J. W. Coffey, J. Islam, K. A. Markey and M. A. F. Kendall, Analyst, 2015, 140, 4350–4364 RSC.
- S. A. N. Gowers, V. F. Curto, C. A. Seneci, C. Wang, S. Anastasova, P. Vadgama, G.-Z. Yang and M. G. Boutelle, Anal. Chem., 2015, 87, 7763–7770 CrossRef CAS PubMed.
- M. Chung, G. Fortunato and N. Radacsi, J. R. Soc., Interface, 2019, 16, 20190217 CrossRef CAS PubMed.
- J. C. Jokerst, J. M. Emory and C. S. Henry, Analyst, 2012, 137, 24–34 RSC.
- S. He, N. Joseph, S. Feng, M. Jellicoe and C. L. Raston, Food Funct., 2020, 11, 5726–5737 RSC.
- H. Tabasum, N. Gill, R. Mishra and S. Lone, RSC Adv., 2022, 12, 8691–8707 RSC.
- S. Scott and Z. Ali, Micromachines, 2021, 12, 319 CrossRef PubMed.
- A. Couto and T. Dong, in 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), IEEE, Seogwipo, 2017, pp. 181–184 Search PubMed.
- K. T. L. Trinh and N. Y. Lee, Biosensors, 2022, 12, 72 CrossRef CAS PubMed.
- M. Sonker, V. Sahore and A. T. Woolley, Anal. Chim. Acta, 2017, 986, 1–11 CrossRef CAS PubMed.
- G. Rong, Y. Zheng and M. Sawan, Sensors, 2021, 21, 3806 CrossRef PubMed.
- M. Gao, P. Wang, L. Jiang, B. Wang, Y. Yao, S. Liu, D. Chu, W. Cheng and Y. Lu, Energy Environ. Sci., 2021, 14, 2114–2157 RSC.
- A. Shakeri, S. Khan and T. F. Didar, Lab Chip, 2021, 21, 3053–3075 RSC.
- K. Ganeson, A. H. Alias, V. Murugaiyah, A.-A. A. Amirul, S. Ramakrishna and S. Vigneswari, Pharmaceutics, 2023, 15, 744 CrossRef CAS PubMed.
- I. Miranda, A. Souza, P. Sousa, J. Ribeiro, E. M. S. Castanheira, R. Lima and G. Minas, JFB, 2021, 13, 2 CrossRef PubMed.
- J. C. Love, J. R. Anderson and G. M. Whitesides, MRS Bull., 2001, 26, 523–528 CrossRef.
- V. N. Goral, Y.-C. Hsieh, O. N. Petzold, R. A. Faris and P. K. Yuen, J. Micromech. Microeng., 2011, 21, 017002 CrossRef.
- U. N. Lee, X. Su, D. J. Guckenberger, A. M. Dostie, T. Zhang, E. Berthier and A. B. Theberge, Lab Chip, 2018, 18, 496–504 RSC.
- R. S. Kane, A. D. Stroock, N. Li Jeon, D. E. Ingber and G. M. Whitesides, in Optical Biosensors, Elsevier, 2002, pp. 571–595 Search PubMed.
- K. Raj M and S. Chakraborty, J. Appl. Polym. Sci., 2020, 137, 48958 CrossRef CAS.
- A. M. Pentecost and R. S. Martin, Anal. Methods, 2015, 7, 2968–2976 RSC.
- T. Hamada, S. Hasegawa, H. Fukasawa, S. Sawada, H. Koshikawa, A. Miyashita and Y. Maekawa, J. Mater. Chem. A, 2015, 3, 20983–20991 RSC.
- L. Yang, Z. Zhang and X. Wang, Micromachines, 2022, 13, 552 CrossRef PubMed.
- D. Voicu, G. Lestari, Y. Wang, M. DeBono, M. Seo, S. Cho and E. Kumacheva, RSC Adv., 2017, 7, 2884–2889 RSC.
- W. Zhang, S. Lin, C. Wang, J. Hu, C. Li, Z. Zhuang, Y. Zhou, R. A. Mathies and C. J. Yang, Lab Chip, 2009, 9, 3088 RSC.
- N. Keller, T. M. Nargang, M. Runck, F. Kotz, A. Striegel, K. Sachsenheimer, D. Klemm, K. Länge, M. Worgull, C. Richter, D. Helmer and B. E. Rapp, Lab Chip, 2016, 16, 1561–1564 RSC.
- D. Ogończyk, J. Węgrzyn, P. Jankowski, B. Dąbrowski and P. Garstecki, Lab Chip, 2010, 10, 1324 RSC.
- Polyetherimide (PEI) Polymer, https://omnexus.specialchem.com/selection-guide/polyetherimide-pei-high-heat-plastic, (accessed 19 April 2023).
- L. A. Damiati, M. El-Yaagoubi, S. A. Damiati, R. Kodzius, F. Sefat and S. Damiati, Polymer, 2022, 14, 5132 CAS.
- M. Figurova, D. Pudis, P. Gaso and I. Cimrak, in 2016 ELEKTRO, IEEE, Strbske Pleso, High Tatras, Slovakia, 2016, pp. 608–611 Search PubMed.
- G. Comina, A. Suska and D. Filippini, Lab Chip, 2014, 14, 424–430 RSC.
- I. Hoek, F. Tho and W. M. Arnold, Lab Chip, 2010, 10, 2283 RSC.
- Y. H. Ko, S. H. Lee, J. W. Leem and J. S. Yu, RSC Adv., 2014, 4, 10216 RSC.
- M. P. Wolf, G. B. Salieb-Beugelaar and P. Hunziker, Prog. Polym. Sci., 2018, 83, 97–134 CrossRef CAS.
- B. Heo, M. Fiola, J. H. Yang and A. Koh, Colloid Interface Sci. Commun., 2020, 38, 100301 CrossRef CAS.
- M. W. Toepke and D. J. Beebe, Lab Chip, 2006, 6, 1484 RSC.
- E. Team, Elveflow.
- A. K. Yetisen, M. S. Akram and C. R. Lowe, Lab Chip, 2013, 13, 2210 RSC.
- Z. Ye, Y. Yuan, S. Zhan, W. Liu, L. Fang and T. Li, Analyst, 2023, 148, 1175–1188 RSC.
- Z. Yao, P. Coatsworth, X. Shi, J. Zhi, L. Hu, R. Yan, F. Güder and H.-D. Yu, Sens. Diagn., 2022, 1, 312–342 RSC.
- S. G. Yedire, H. Khan, T. AbdelFatah, R. Siavash Moakhar and S. Mahshid, Sens. Diagn., 2023, 2, 763–780 RSC.
- W. Dungchai, O. Chailapakul and C. S. Henry, Anal. Chem., 2009, 81, 5821–5826 CrossRef CAS PubMed.
- B. Hu, J. Li, L. Mou, Y. Liu, J. Deng, W. Qian, J. Sun, R. Cha and X. Jiang, Lab Chip, 2017, 17, 2225–2234 RSC.
- E. Carrilho, A. W. Martinez and G. M. Whitesides, Anal. Chem., 2009, 81, 7091–7095 CrossRef CAS PubMed.
- H. Shibata, Y. Hiruta and D. Citterio, Analyst, 2019, 144, 1178–1186 RSC.
- L. Yu and Z. Z. Shi, Lab Chip, 2015, 15, 1642–1645 RSC.
- J. Olkkonen, K. Lehtinen and T. Erho, Anal. Chem., 2010, 82, 10246–10250 CrossRef CAS PubMed.
- X. Li, J. Tian, T. Nguyen and W. Shen, Anal. Chem., 2008, 80, 9131–9134 CrossRef CAS PubMed.
- G. Chitnis, Z. Ding, C.-L. Chang, C. A. Savran and B. Ziaie, Lab Chip, 2011, 11, 1161 RSC.
- L. Zhang, W. Wang, X.-J. Ju, R. Xie, Z. Liu and L.-Y. Chu, RSC Adv., 2015, 5, 5638–5646 RSC.
- Y. Sameenoi, P. N. Nongkai, S. Nouanthavong, C. S. Henry and D. Nacapricha, Analyst, 2014, 139, 6580–6588 RSC.
- U. Mogera, H. Guo, M. Namkoong, M. S. Rahman, T. Nguyen and L. Tian, Sci. Adv., 2022, 8, eabn1736 CrossRef CAS PubMed.
- T. Abbasiasl, F. Mirlou, E. Istif, H. Ceylan Koydemir and L. Beker, Sens. Diagn., 2022, 1, 775–786 RSC.
- S. Nishat, A. T. Jafry, A. W. Martinez and F. R. Awan, Sens. Actuators, B, 2021, 336, 129681 CrossRef CAS.
- E. Noviana, T. Ozer, C. S. Carrell, J. S. Link, C. McMahon, I. Jang and C. S. Henry, Chem. Rev., 2021, 121, 11835–11885 CrossRef CAS PubMed.
- S. Mdanda, P. Ubanako, P. P. D. Kondiah, P. Kumar and Y. E. Choonara, Polymer, 2021, 13, 2405 CAS.
- K. Ita, Pharmaceutics, 2015, 7, 90–105 CrossRef CAS PubMed.
- R. Maia, V. Carvalho, R. Lima, G. Minas and R. O. Rodrigues, Pharmaceutics, 2023, 15, 792 CrossRef CAS PubMed.
- J. H. Jung and S. G. Jin, J. Pharm. Invest., 2021, 51, 503–517 CrossRef PubMed.
- T. Waghule, G. Singhvi, S. K. Dubey, M. M. Pandey, G. Gupta, M. Singh and K. Dua, Biomed. Pharmacother., 2019, 109, 1249–1258 CrossRef CAS PubMed.
- P. M. Wang, M. Cornwell, J. Hill and M. R. Prausnitz, J. Invest. Dermatol., 2006, 126, 1080–1087 CrossRef CAS PubMed.
- H.-R. Jeong, H. Jun, H.-R. Cha, J. Lee and J.-H. Park, Micromachines, 2020, 11, 710 CrossRef PubMed.
- Y. Ye, J. Yu, D. Wen, A. R. Kahkoska and Z. Gu, Adv. Drug Delivery Rev., 2018, 127, 106–118 CrossRef CAS PubMed.
- H. Kang, Z. Zuo, R. Lin, M. Yao, Y. Han and J. Han, Drug Delivery, 2022, 29, 3087–3110 CrossRef CAS PubMed.
- A. Sionkowska, M. Gadomska, K. Musiał and J. Piątek, Molecules, 2020, 25, 4035 CrossRef CAS PubMed.
- M. A. Luzuriaga, D. R. Berry, J. C. Reagan, R. A. Smaldone and J. J. Gassensmith, Lab Chip, 2018, 18, 1223–1230 RSC.
- Y. Su, S. M. Andrabi, S. M. S. Shahriar, S. L. Wong, G. Wang and J. Xie, J. Controlled Release, 2023, 356, 131–141 CrossRef CAS PubMed.
- A. H. Sabri, Y. Kim, M. Marlow, D. J. Scurr, J. Segal, A. K. Banga, L. Kagan and J. B. Lee, Adv. Drug Delivery Rev., 2020, 153, 195–215 CrossRef CAS PubMed.
- Z. Zhao, Y. Chen and Y. Shi, RSC Adv., 2020, 10, 14040–14049 RSC.
- J. Zhao, H. Guo, J. Li, A. J. Bandodkar and J. A. Rogers, Trends Chem., 2019, 1, 559–571 CrossRef CAS.
- N. Kashaninejad and N.-T. Nguyen, Lab Chip, 2023, 23, 913–937 RSC.
- J. Heikenfeld, A. Jajack, B. Feldman, S. W. Granger, S. Gaitonde, G. Begtrup and B. A. Katchman, Nat. Biotechnol., 2019, 37, 407–419 CrossRef CAS PubMed.
- Scientific Image and Illustration Software|BioRender, https://www.biorender.com/, (accessed 25 July 2023).
- S. Solanki, C. M. Pandey, R. K. Gupta and B. D. Malhotra, Biotechnol. J., 2020, 15, 1900279 CrossRef CAS PubMed.
- L. B. Baker, Temperature, 2019, 6, 211–259 CrossRef PubMed.
- Y. Liu, T. Liu and D. Jiang, Curr. Res. Biotechnol., 2023, 6, 100143 CrossRef CAS.
- D. A. Kidwell, J. C. Holland and S. Athanaselis, J. Chromatogr. B: Biomed. Sci. Appl., 1998, 713, 111–135 CrossRef CAS PubMed.
- K. Van Hoovels, X. Xuan, M. Cuartero, M. Gijssel, M. Swarén and G. A. Crespo, ACS Sens., 2021, 6, 3496–3508 CrossRef CAS PubMed.
- Z. Sonner, E. Wilder, J. Heikenfeld, G. Kasting, F. Beyette, D. Swaile, F. Sherman, J. Joyce, J. Hagen, N. Kelley-Loughnane and R. Naik, Biomicrofluidics, 2015, 9, 031301 CrossRef CAS PubMed.
- H. Tabasum, N. Gill, R. Mishra and S. Lone, RSC Adv., 2022, 12, 8691–8707 RSC.
- T.-T. Luo, Z.-H. Sun, C.-X. Li, J.-L. Feng, Z.-X. Xiao and W.-D. Li, J. Physiol. Sci., 2021, 71, 26 CrossRef CAS PubMed.
- B. Schazmann, D. Morris, C. Slater, S. Beirne, C. Fay, R. Reuveny, N. Moyna and D. Diamond, Anal. Methods, 2010, 2, 342 RSC.
- H. Yoon, X. Xuan, S. Jeong and J. Y. Park, Biosens. Bioelectron., 2018, 117, 267–275 CrossRef CAS PubMed.
- H. Y. Nyein, W. Gao, Z. Shahpar, S. Emaminejad, S. Challa, K. Chen, H. M. Fahad, L. C. Tai, H. Ota, R. W. Davis and A. Javey, ACS Nano, 2016, 10(7), 7216–7224 CrossRef CAS PubMed.
- Y. Zhang, H. Guo, S. B. Kim, Y. Wu, D. Ostojich, S. H. Park, X. Wang, Z. Weng, R. Li, A. J. Bandodkar, Y. Sekine, J. Choi, S. Xu, S. Quaggin, R. Ghaffari and J. A. Rogers, Lab Chip, 2019, 19, 1545–1555 RSC.
- L. B. Baker, M. S. Seib, K. A. Barnes, S. D. Brown, M. A. King, P. J. D. De Chavez, S. Qu, J. Archer, A. S. Wolfe, J. R. Stofan, J. M. Carter, D. E. Wright, J. Wallace, D. S. Yang, S. Liu, J. Anderson, T. Fort, W. Li, J. A. Wright, S. P. Lee, J. B. Model, J. A. Rogers, A. J. Aranyosi and R. Ghaffari, Adv. Mater. Technol., 2022, 7, 2200249 CrossRef.
- P. Pirovano, M. Dorrian, A. Shinde, A. Donohoe, A. J. Brady, N. M. Moyna, G. Wallace, D. Diamond and M. McCaul, Talanta, 2020, 219, 121145 CrossRef CAS PubMed.
- H. Veeze, Neth. J. Med., 1995, 46, 271–274 CrossRef CAS PubMed.
- A. Mishra, R. Greaves, K. Smith, J. B. Carlin, A. Wootton, R. Stirling and J. Massie, J. Pediatr., 2008, 153, 758–763.e1 CrossRef PubMed.
- A. la Grasta, M. De Carlo, A. Di Nisio, F. Dell'Olio and V. M. N. Passaro, Sensors, 2023, 23, 2491 CrossRef CAS PubMed.
- R. Biswas, JBMOA, 2022, 10, 66–67 CrossRef.
- T. R. Ray, M. Ivanovic, P. M. Curtis, D. Franklin, K. Guventurk, W. J. Jeang, J. Chafetz, H. Gaertner, G. Young, S. Rebollo, J. B. Model, S. P. Lee, J. Ciraldo, J. T. Reeder, A. Hourlier-Fargette, A. J. Bandodkar, J. Choi, A. J. Aranyosi, R. Ghaffari, S. A. McColley, S. Haymond and J. A. Rogers, Sci. Transl. Med., 2021, 13, eabd8109 CrossRef CAS PubMed.
- L. B. Baker, Sports Med., 2017, 47, 111–128 CrossRef PubMed.
- Office of Dietary Supplements - Potassium, https://ods.od.nih.gov/factsheets/Potassium-HealthProfessional/, (accessed 24 April 2023).
- B. Liang, Q. Cao, X. Mao, W. Pan, T. Tu, L. Fang and X. Ye, IEEE Sens. J., 2021, 21, 9642–9648 CAS.
- J. R. Sempionatto, M. Lin, L. Yin, E. De la Paz, K. Pei, T. Sonsa-ard, A. N. de Loyola Silva, A. A. Khorshed, F. Zhang, N. Tostado, S. Xu and J. Wang, Nat. Biomed. Eng., 2021, 5, 737–748 CrossRef CAS PubMed.
- J. R. Sempionatto, T. Nakagawa, A. Pavinatto, S. T. Mensah, S. Imani, P. Mercier and J. Wang, Lab Chip, 2017, 17, 1834–1842 RSC.
- J. Xiao, Y. Liu, L. Su, D. Zhao, L. Zhao and X. Zhang, Anal. Chem., 2019, 91, 14803–14807 CrossRef CAS PubMed.
- G. Xiao, J. He, X. Chen, Y. Qiao, F. Wang, Q. Xia, L. Yu and Z. Lu, Cellulose, 2019, 26, 4553–4562 CrossRef CAS.
- G. Bolat, E. De La Paz, N. F. Azeredo, M. Kartolo, J. Kim, A. N. De Loyola, E. Silva, R. Rueda, C. Brown, L. Angnes, J. Wang and J. R. Sempionatto, Anal. Bioanal. Chem., 2022, 414, 5411–5421 CrossRef CAS PubMed.
- Y. Y. Al-Tamer, E. A. Hadi and I. E. I. Al-Badrani, Urol. Res., 1997, 25, 337–340 CrossRef CAS PubMed.
- X. Wang, S. Chen, X. Tang, D. Lin and P. Qiu, RSC Adv., 2019, 9, 36578–36585 RSC.
- D. I. Jalal, Curr. Med. Res. Opin., 2016, 32, 1863–1869 CrossRef CAS PubMed.
- Z. Xu, J. Song, B. Liu, S. Lv, F. Gao, X. Luo and P. Wang, Sens. Actuators, B, 2021, 348, 130674 CrossRef CAS.
- A. N. Balaji, C. Yuan, B. Wang, L.-S. Peh and H. Shao, in Proceedings of the 17th Annual International Conference on Mobile Systems, Applications, and Services, ACM, Seoul Republic of Korea, 2019, pp. 262–274 Search PubMed.
- P. Escobedo, C. E. Ramos-Lorente, A. Martínez-Olmos, M. A. Carvajal, M. Ortega-Muñoz, I. de Orbe-Payá, F. Hernández-Mateo, F. Santoyo-González, L. F. Capitán-Vallvey, A. J. Palma and M. M. Erenas, Sens. Actuators, B, 2021, 327, 128948 CrossRef CAS.
- H. Y. Y. Nyein, M. Bariya, B. Tran, C. H. Ahn, B. J. Brown, W. Ji, N. Davis and A. Javey, Nat. Commun., 2021, 12, 1823 CrossRef CAS PubMed.
- Y. Yu, I. Prassas, C. M. J. Muytjens and E. P. Diamandis, J. Proteomics, 2017, 155, 40–48 CrossRef CAS PubMed.
- K. Ngamchuea, K. Chaisiwamongkhol, C. Batchelor-McAuley and R. G. Compton, Analyst, 2018, 143, 81–99 RSC.
- E. Papacosta and G. P. Nassis, J. Sci. Med. Sport, 2011, 14, 424–434 CrossRef PubMed.
- G. Iorgulescu, J. Med. Life, 2009, 2, 303–307 Search PubMed.
- M. Dodds, S. Roland, M. Edgar and M. Thornhill, BDJ Team, 2015, 2, 15123 CrossRef.
- R. S. P. Malon, S. Sadir, M. Balakrishnan and E. P. Córcoles, Biomed Res. Int., 2014, 2014, 1–20 CrossRef PubMed.
- O. R. Barley, D. W. Chapman and C. R. Abbiss, J. Int. Soc. Sports Nutr., 2020, 17, 52 CrossRef PubMed.
- M. Villiger, R. Stoop, T. Vetsch, E. Hohenauer, M. Pini, P. Clarys, F. Pereira and R. Clijsen, Eur. J. Clin. Nutr., 2018, 72, 69–76 CrossRef CAS PubMed.
- G. Selvolini, A.-M. Drăgan, G. Melinte, C. Cristea and G. Marrazza, in Sensors and Microsystems, ed. G. Di Francia and C. Di Natale, Springer International Publishing, Cham, 2023, vol. 918, pp. 3–7 Search PubMed.
- L. F. de Castro, S. V. de Freitas, L. C. Duarte, J. A. C. de Souza, T. R. L. C. Paixão and W. K. T. Coltro, Anal. Bioanal. Chem., 2019, 411, 4919–4928 CrossRef CAS PubMed.
- M. J. Kangas, R. M. Burks, J. Atwater, R. M. Lukowicz, P. Williams and A. E. Holmes, Crit. Rev. Anal. Chem., 2017, 47, 138–153 CrossRef CAS PubMed.
- T. Arakawa, K. Tomoto, H. Nitta, K. Toma, S. Takeuchi, T. Sekita, S. Minakuchi and K. Mitsubayashi, Anal. Chem., 2020, 92, 12201–12207 CrossRef CAS PubMed.
- L. García-Carmona, A. Martín, J. R. Sempionatto, J. R. Moreto, M. C. González, J. Wang and A. Escarpa, Anal. Chem., 2019, 91, 13883–13891 CrossRef PubMed.
- J. Ballesta Claver, M. C. Valencia Mirón and L. F. Capitán-Vallvey, Analyst, 2009, 134, 1423 RSC.
- K. Suneetha and T. Rambabu, Indian J. Endocrinol. Metab., 2012, 16, 665 CrossRef CAS PubMed.
- P. Swetha, U. Balijapalli and S.-P. Feng, Electrochem. Commun., 2022, 140, 107314 CrossRef CAS.
- A.-M. Spehar-Délèze, S. Anastasova and P. Vadgama, Chemosensors, 2021, 9, 195 CrossRef.
- J. Kim, G. Valdés-Ramírez, A. J. Bandodkar, W. Jia, A. G. Martinez, J. Ramírez, P. Mercier and J. Wang, Analyst, 2014, 139, 1632–1636 RSC.
- Q. U. Khan, Cureus, 2020, 12(11), e11548 Search PubMed.
- L. F. Hofman, J. Nutr., 2001, 131, 1621S–1625S CrossRef CAS PubMed.
- M. A. Laine and A. O. Ojanotko, J. Steroid Biochem. Mol. Biol., 1999, 70, 109–113 CrossRef CAS PubMed.
- A. Diago-Galmés, C. Guillamón-Escudero, J. M. Tenías-Burillo, J. M. Soriano and J. Fernández-Garrido, Biology, 2021, 10, 93 CrossRef PubMed.
- B. Keevil, P. MacDonald, W. Macdowall, D. Lee, F. Wu and The NATSAL Team, Ann. Clin. Biochem., 2014, 51, 368–378 CrossRef CAS PubMed.
- M. S. Khan, S. K. Misra, Z. Wang, E. Daza, A. S. Schwartz-Duval, J. M. Kus, D. Pan and D. Pan, Anal. Chem., 2017, 89, 2107–2115 CrossRef CAS PubMed.
- R. D. Goll and B. K. Mookerjee, J. Dial., 1978, 2, 399–414 CrossRef CAS PubMed.
- J. Kim, S. Imani, W. R. de Araujo, J. Warchall, G. Valdés-Ramírez, T. R. L. C. Paixão, P. P. Mercier and J. Wang, Biosens. Bioelectron., 2015, 74, 1061–1068 CrossRef CAS PubMed.
- P. Brandtzaeg, J. Oral Microbiol., 2013, 5, 20401 CrossRef CAS PubMed.
- M. reviewed by D. C. L. D Pharm, Immunoglobulin Types and Their Functions, https://ameripharmaspecialty.com/immunoglobulins-ig/, (accessed 26 April 2023).
- M. S. Mannoor, H. Tao, J. D. Clayton, A. Sengupta, D. L. Kaplan, R. R. Naik, N. Verma, F. G. Omenetto and M. C. McAlpine, Nat. Commun., 2012, 3, 763 CrossRef PubMed.
- J. Ceron, E. Lamy, S. Martinez-Subiela, P. Lopez-Jornet, F. Capela-Silva, P. Eckersall and A. Tvarijonaviciute, JCM, 2020, 9, 1491 CrossRef CAS PubMed.
- S. Baliga, S. Muglikar and R. Kale, J. Indian Soc. Periodontol, 2013, 17, 461 CrossRef PubMed.
- G. Matzeu, G. R. S. Naveh, S. Agarwal, J. A. Roshko, N. A. Ostrovsky-Snider, B. S. Napier and F. G. Omenetto, Adv. Sci., 2021, 8, 2003416 CrossRef CAS PubMed.
- S. Mondal, S. Karuppuswami, R. Steinhorst and P. Chahal, in 2019 IEEE 69th Electronic Components and Technology Conference (ECTC), IEEE, Las Vegas, NV, USA, 2019, pp. 1240–1245 Search PubMed.
- M. Adabi, M. Naghibzadeh, M. Adabi, M. A. Zarrinfard, S. S. Esnaashari, A. M. Seifalian, R. Faridi-Majidi, H. Tanimowo Aiyelabegan and H. Ghanbari, Artif. Cells, Nanomed., Biotechnol., 2017, 45, 833–842 CrossRef CAS PubMed.
- J. Kim, A. S. Campbell, B. E.-F. De Ávila and J. Wang, Nat. Biotechnol., 2019, 37, 389–406 CrossRef CAS PubMed.
- A. J. Bandodkar, W. Jia, C. Yardımcı, X. Wang, J. Ramirez and J. Wang, Anal. Chem., 2015, 87, 394–398 CrossRef CAS PubMed.
- H. C. Ates, P. Q. Nguyen, L. Gonzalez-Macia, E. Morales-Narváez, F. Güder, J. J. Collins and C. Dincer, Nat. Rev. Mater., 2022, 7, 887–907 CrossRef PubMed.
- P. J. Stout, N. Peled, B. J. Erickson, M. E. Hilgers, J. R. Racchini and T. B. Hoegh, Diabetes Technol. Ther., 2001, 3, 81–90 CrossRef CAS PubMed.
- N. Kashaninejad, A. Munaz, H. Moghadas, S. Yadav, M. Umer and N.-T. Nguyen, Chemosensors, 2021, 9, 83 CrossRef CAS.
- K. Takeuchi, N. Takama, B. Kim, K. Sharma, O. Paul and P. Ruther, Biomed. Microdevices, 2019, 21, 28 CrossRef PubMed.
- J. Kim, J. R. Sempionatto, S. Imani, M. C. Hartel, A. Barfidokht, G. Tang, A. S. Campbell, P. P. Mercier and J. Wang, Adv. Sci., 2018, 5, 1800880 CrossRef PubMed.
- T. Siegmund, L. Heinemann, R. Kolassa and A. Thomas, J. Diabetes Sci. Technol., 2017, 11, 766–772 CrossRef PubMed.
- A. Facchinetti, G. Sparacino and C. Cobelli, J. Diabetes Sci. Technol., 2007, 1, 617–623 CrossRef PubMed.
- Z. Pu, X. Zhang, H. Yu, J. Tu, H. Chen, Y. Liu, X. Su, R. Wang, L. Zhang and D. Li, Sci. Adv., 2021, 7, eabd0199 CrossRef CAS PubMed.
- Join My Freestyle - FreeStyle Libre|Abbott, https://www.freestyle.abbott/in-en/myfreestyle.html, (accessed 8 April 2023).
- Dexcom G6 CGM System|No Fingersticks, No Scanning|Dexcom, https://www.dexcom.com/en-us/g6-cgm-system, (accessed 8 April 2023).
- Introducing the Eversense® E3 CGM System|Ascensia Diabetes Care, https://www.ascensiadiabetes.com/eversense/, (accessed 8 April 2023).
- Guardian Connect Continuous Glucose Monitoring|Medtronic, https://www.medtronicdiabetes.com/products/guardian-connect-continuous-glucose-monitoring-system, (accessed 8 April 2023).
- MiniMed Revel Insulin Pump|Medtronic Diabetes, https://www.medtronicdiabetes.com/customer-support/minimed-revel-insulin-pump, (accessed 8 April 2023).
- T. Hutter, T. S. Collings, G. Kostova and F. E. Karet Frankl, Sens. Diagn., 2022, 1, 614–626 RSC.
- M. Parrilla, M. Cuartero, S. Padrell Sánchez, M. Rajabi, N. Roxhed, F. Niklaus and G. A. Crespo, Anal. Chem., 2019, 91, 1578–1586 CrossRef CAS PubMed.
- P. R. Miller, X. Xiao, I. Brener, D. B. Burckel, R. Narayan and R. Polsky, Adv. Healthcare Mater., 2014, 3, 876–881 CrossRef CAS PubMed.
- Á. Molinero-Fernández, A. Casanova, Q. Wang, M. Cuartero and G. A. Crespo, ACS Sens., 2023, 8, 158–166 CrossRef PubMed.
- J. J. García-Guzmán, C. Pérez-Ràfols, M. Cuartero and G. A. Crespo, ACS Sens., 2021, 6, 1129–1137 CrossRef PubMed.
- J. J. García-Guzmán, C. Pérez-Ràfols, M. Cuartero and G. A. Crespo, ACS Sens., 2021, 6, 1129–1137 CrossRef PubMed.
- R. K. Mishra, K. Y. Goud, Z. Li, C. Moonla, M. A. Mohamed, F. Tehrani, H. Teymourian and J. Wang, J. Am. Chem. Soc., 2020, 142, 5991–5995 CrossRef CAS PubMed.
- K. Y. Goud, C. Moonla, R. K. Mishra, C. Yu, R. Narayan, I. Litvan and J. Wang, ACS Sens., 2019, 4, 2196–2204 CrossRef CAS PubMed.
- C. Cobelli, M. Schiavon, C. Dalla Man, A. Basu and R. Basu, Diabetes Technol. Ther., 2016, 18, 505–511 CrossRef CAS PubMed.
- D. Ami, A. Duse, P. Mereghetti, F. Cozza, F. Ambrosio, E. Ponzini, R. Grandori, C. Lunetta, S. Tavazzi, F. Pezzoli and A. Natalello, Anal. Chem., 2021, 93, 16995–17002 CrossRef CAS PubMed.
- Y. Shi, N. Jiang, P. Bikkannavar, M. F. Cordeiro and A. K. Yetisen, Analyst, 2021, 146, 6416–6444 RSC.
- A. K. Yetisen, N. Jiang, A. Tamayol, G. U. Ruiz-Esparza, Y. S. Zhang, S. Medina-Pando, A. Gupta, J. S. Wolffsohn, H. Butt, A. Khademhosseini and S.-H. Yun, Lab Chip, 2017, 17, 1137–1148 RSC.
- K. Yamada, S. Takaki, N. Komuro, K. Suzuki and D. Citterio, Analyst, 2014, 139, 1637 RSC.
- A. Daily, P. Ravishankar, S. Harms and V. S. Klimberg, PLoS One, 2022, 17, e0267676 CrossRef CAS PubMed.
- M. Elsherif, M. U. Hassan, A. K. Yetisen and H. Butt, ACS Nano, 2018, 12, 5452–5462 CrossRef CAS PubMed.
- H. Teymourian, M. Parrilla, J. R. Sempionatto, N. F. Montiel, A. Barfidokht, R. Van Echelpoel, K. De Wael and J. Wang, ACS Sens., 2020, 5, 2679–2700 CrossRef CAS PubMed.
- M. Aihara, N. Kubota, T. Minami, R. Shirakawa, Y. Sakurai, T. Hayashi, M. Iwamoto, I. Takamoto, T. Kubota, R. Suzuki, S. Usami, H. Jinnouchi, M. Aihara, T. Yamauchi, T. Sakata and T. Kadowaki, J. Diabetes Invest., 2021, 12, 266–276 CrossRef CAS PubMed.
- H. Kudo, T. Sawada, E. Kazawa, H. Yoshida, Y. Iwasaki and K. Mitsubayashi, Biosens. Bioelectron., 2006, 22, 558–562 CrossRef CAS PubMed.
- M. Chu, T. Shirai, D. Takahashi, T. Arakawa, H. Kudo, K. Sano, S. Sawada, K. Yano, Y. Iwasaki, K. Akiyoshi, M. Mochizuki and K. Mitsubayashi, Biomed. Microdevices, 2011, 13, 603–611 CrossRef CAS PubMed.
- X. Yang, H. Yao, G. Zhao, G. A. Ameer, W. Sun, J. Yang and S. Mi, J. Mater. Sci., 2020, 55, 9551–9561 CrossRef CAS.
- P. T. Khaw, BMJ, 2004, 328, 97–99 CrossRef CAS PubMed.
- S. Agaoglu, P. Diep, M. Martini, S. Kt, M. Baday and I. E. Araci, Lab Chip, 2018, 18, 3471–3483 RSC.
- J. Bader, M. Zeppieri and S. J. Havens, in StatPearls [Internet], StatPearls Publishing, 2022 Search PubMed.
- A. E. Kownacka, D. Vegelyte, M. Joosse, N. Anton, B. J. Toebes, J. Lauko, I. Buzzacchera, K. Lipinska, D. A. Wilson, N. Geelhoed-Duijvestijn and C. J. Wilson, Biomacromolecules, 2018, 19, 4504–4511 CrossRef CAS PubMed.
- S. R. Corrie, J. W. Coffey, J. Islam, K. A. Markey and M. A. F. Kendall, Analyst, 2015, 140, 4350–4364 RSC.
- M. H. M. Kouhani, J. Wu, A. Tavakoli, A. J. Weber and W. Li, Lab Chip, 2020, 20, 332–342 RSC.
- W. Yang, X. Zhang, Y. Wang, Q. Fan, S. Zhang, Y. Chen, X. Shen, M. Xie and X. Duan, Appl. Phys. Lett., 2021, 119, 193701 CrossRef CAS.
- H. An, L. Chen, X. Liu, B. Zhao, H. Zhang and Z. Wu, Sens. Actuators, A, 2019, 295, 177–187 CrossRef CAS.
- Y. Zhu, R. Nasiri, E. Davoodi, S. Zhang, S. Saha, M. Linn, L. Jiang, R. Haghniaz, M. C. Hartel, V. Jucaud, M. R. Dokmeci, A. Herland, E. Toyserkani and A. Khademhosseini, Small, 2023, 19, 2207017 CrossRef CAS PubMed.
- J. R. Sempionatto, L. C. Brazaca, L. García-Carmona, G. Bolat, A. S. Campbell, A. Martin, G. Tang, R. Shah, R. K. Mishra, J. Kim, V. Zucolotto, A. Escarpa and J. Wang, Biosens. Bioelectron., 2019, 137, 161–170 CrossRef CAS PubMed.
- J. Xu, X. Tao, X. Liu and L. Yang, Anal. Chem., 2022, 94, 8659–8667 CrossRef CAS PubMed.
- C.-C. Lin, C.-C. Tseng, T.-K. Chuang, D.-S. Lee and G.-B. Lee, Analyst, 2011, 136, 2669 RSC.
- Z. Zhang, J. Liu, Y. Cheng, J. Chen, H. Zhao and X. Ren, Front. Anal. Sci., 2022, 1, 812301 CrossRef.
- D. Martens, P. Ramirez-Priego, M. S. Murib, A. A. Elamin, A. B. Gonzalez-Guerrero, M. Stehr, F. Jonas, B. Anton, N. Hlawatsch, P. Soetaert, R. Vos, A. Stassen, S. Severi, W. Van Roy, R. Bockstaele, H. Becker, M. Singh, L. M. Lechuga and P. Bienstman, Anal. Methods, 2018, 10, 3066–3073 RSC.
- C. Gnoth and S. Johnson, Geburtshilfe Frauenheilkd., 2014, 74, 661–669 CrossRef CAS PubMed.
- N. K. Griffin, M. A. Smith, P. A. Jenkins, G. Werther and J. D. Baum, Arch. Dis. Child., 1979, 54, 371–374 CrossRef CAS PubMed.
- H. Blotner, JAMA, 1946, 131, 1109 CrossRef CAS PubMed.
- K. Phoonsawat, T. Ozer, W. Dungchai and C. S. Henry, Analyst, 2022, 147, 4517–4524 RSC.
- Y. Zhou and Z. Cai, Rapid Commun. Mass Spectrom., 2020, 34(S1) DOI:10.1002/rcm.8583.
- R. A. Leiva, T. P. Bouchard, S. H. Abdullah and R. Ecochard, Front. Public Health, 2017, 5, 320 CrossRef PubMed.
- A. Z. Steiner, D. L. Long, A. H. Herring, J. S. Kesner, J. W. Meadows and D. D. Baird, Reprod. Sci., 2013, 20, 549–556 CrossRef CAS PubMed.
- A. Plenis, L. Konieczna, I. Olędzka, P. Kowalski and T. Bączek, Mol. BioSyst., 2011, 7, 1487 RSC.
- O. Olivieri, D. Cecconi, A. Castagna, L. Chiecchi, P. Guarini, M. Gunasekaran, F. Morandini, P. Brazzarola, L. Zolla, A. D'Alessandro, F. Veglio, P. Mulatero and F. Pizzolo, Mol. BioSyst., 2014, 10, 1281 RSC.
- J. Ye, N. Li, Y. Lu, J. Cheng and Y. Xu, Anal. Methods, 2017, 9, 2464–2471 RSC.
- T. Liabsuetrakul, S. Srisook, K. Jandee and R. Mori, Hypertension Research in Pregnancy, 2021, 9, 75–81 CrossRef.
- M. Ra, M. S. Muhammad, C. Lim, S. Han, C. Jung and W.-Y. Kim, IEEE J. Transl. Eng. Health Med., 2018, 6, 1–11 Search PubMed.
- I. P. Korneeva, K. A. Kramar, T. M. Magrupov and E. A. Semenova, in 2021 International Conference on Information Science and Communications Technologies (ICISCT), IEEE, Tashkent, Uzbekistan, 2021, pp. 1–4 Search PubMed.
- G. Liu, N. Hu, Z. Ma and R. Li, J. Instrum., 2018, 13, T07002 CrossRef.
- X. Li, C. Zhan, Q. Huang, M. He, C. Yang, C. Yang, X. Huang, M. Chen, X. Xie and H.-J. Chen, ACS Appl. Nano Mater., 2022, 5, 4767–4778 CrossRef CAS.
- S. Cho, T. S. Park, T. G. Nahapetian and J.-Y. Yoon, Biosens. Bioelectron., 2015, 74, 601–611 CrossRef CAS PubMed.
- H. Li, S. Gu, Q. Zhang, E. Song, T. Kuang, F. Chen, X. Yu and L. Chang, Nanoscale, 2021, 13, 3436–3453 RSC.
- F. S. H. Krismastuti, W. L. A. Brooks, M. J. Sweetman, B. S. Sumerlin and N. H. Voelcker, J. Mater. Chem. B, 2014, 2, 3972–3983 RSC.
- S. Barrientos, H. Brem, O. Stojadinovic and M. Tomic-Canic, Wound Repair Regen, 2014, 22, 569–578 CrossRef PubMed.
- E. Rezvani Ghomi, S. Khalili, S. Nouri Khorasani, R. Esmaeely Neisiany and S. Ramakrishna, J. Appl. Polym. Sci., 2019, 136, 47738 CrossRef.
- S. Dhivya, V. V. Padma and E. Santhini, BioMed, 2015, 5, 22 CrossRef PubMed.
- G. Shabestani Monfared, P. Ertl and M. Rothbauer, Pharmaceutics, 2021, 13, 793 CrossRef PubMed.
- Y.-C. Chuang, V. Tyagi, R.-T. Liu, M. B. Chancellor and P. Tyagi, Urol. Sci., 2010, 21, 132–136 CrossRef CAS.
- R.-Y. Xu, H.-W. Liu, J.-L. Liu and J.-H. Dong, BMC Urol., 2014, 14, 45 CrossRef PubMed.
- P. Mostafalu, A. Tamayol, R. Rahimi, M. Ochoa, A. Khalilpour, G. Kiaee, I. K. Yazdi, S. Bagherifard, M. R. Dokmeci, B. Ziaie, S. R. Sonkusale and A. Khademhosseini, Small, 2018, 14, 1703509 CrossRef PubMed.
- P. Rajput, et al. , TURCOMAT, 2021, 12(5), 1650–1662 CrossRef.
- B. Qiao, Q. Pang, P. Yuan, Y. Luo and L. Ma, Biomater. Sci., 2020, 8, 1649–1657 RSC.
- J. F. Lo, M. Brennan, Z. Merchant, L. Chen, S. Guo, D. T. Eddington and L. A. DiPietro, Wound Repair Regen, 2013, 21, 226–234 CrossRef PubMed.
- S. Shaner, A. Savelyeva, A. Kvartuh, N. Jedrusik, L. Matter, J. Leal and M. Asplund, Lab Chip, 2023, 23, 1531–1546 RSC.
- Microfluidic spinning-induced heterotypic bead-on-string fibers for dual-cargo release and wound healing - Journal of Materials Chemistry B (RSC Publishing), https://rsc.66557.net/en/content/articlelanding/2021/tb/d0tb02305a, (accessed 13 April 2023).
- W. Zhao, Y. Zhang, L. Liu, Y. Gao, W. Sun, Y. Sun and Q. Ma, J. Mater. Chem. B, 2022, 10, 8357–8374 RSC.
- F. Hu, Q. Gao, J. Liu, W. Chen, C. Zheng, Q. Bai, N. Sun, W. Zhang, Y. Zhang and T. Lu, J. Mater. Chem. B, 2023, 11, 2830–2851 RSC.
- Q. Huang, F. He, J. Yu, J. Zhang, X. Du, Q. Li, G. Wang, Z. Yu and S. Chen, J. Mater. Chem. B, 2021, 9, 2727–2735 RSC.
- M. Ochoa, R. Rahimi, J. Zhou, H. Jiang, C. K. Yoon, D. Maddipatla, B. B. Narakathu, V. Jain, M. M. Oscai, T. J. Morken, R. H. Oliveira, G. L. Campana, O. W. Cummings, M. A. Zieger, R. Sood, M. Z. Atashbar and B. Ziaie, Microsyst. Nanoeng., 2020, 6, 46 CrossRef CAS PubMed.
- H. Levitt, Trends in Amplification, 2007, 11, 7–24 CrossRef PubMed.
- L. Liu, M. Bi, Y. Wang, J. Liu, X. Jiang, Z. Xu and X. Zhang, Nanoscale, 2021, 13, 19352–19366 RSC.
- Wearable Devices and the Internet of Things|Mouser, https://au.mouser.com/applications/article-iot-wearable-devices/, (accessed 8 July 2023).
- Age of AI: Everything you need to know about artificial intelligence|TechCrunch, https://techcrunch.com/2023/07/07/age-of-ai-everything-you-need-to-know-about-artificial-intelligence/, (accessed 8 July 2023).
- J. Srivastava, S. Routray, S. Ahmad and M. M. Waris, Comput. Intell. Neurosci., 2022, 2022, 1–17 Search PubMed.
- Wearables and AI will be The Game Changer in Healthcare - Digital Salutem, https://digitalsalutem.com/wearables-and-ai-in-healthcare/, (accessed 8 July 2023).
- S. Cheng, Z. Gu, L. Zhou, M. Hao, H. An, K. Song, X. Wu, K. Zhang, Z. Zhao, Y. Dong and Y. Wen, Front. Bioeng. Biotechnol., 2021, 9, 765987 CrossRef PubMed.
- Artificial Intelligence Market Size, Share, Growth Report 2030, https://www.grandviewresearch.com/industry-analysis/artificial-intelligence-ai-market, (accessed 8 July 2023).
- What is Artificial Intelligence (AI)?, https://www.ibm.com/topics/artificial-intelligence, (accessed 8 July 2023).
- H. Liu, L. Nan, F. Chen, Y. Zhao and Y. Zhao, Lab Chip, 2023, 23, 2497–2513 RSC.
- N. H. Bhuiyan, J. H. Hong, M. J. Uddin and J. S. Shim, Anal. Chem., 2022, 94, 3872–3880 CrossRef CAS PubMed.
- X. Ma, G. Guo, X. Wu, Q. Wu, F. Liu, H. Zhang, N. Shi and Y. Guan, Micromachines, 2023, 14, 972 CrossRef PubMed.
- W. Lee, A. Gonzalez, P. Arguelles, R. Guevara, M. J. Gonzalez-Guerrero and F. A. Gomez, Electrophoresis, 2018, 39(12), 1443–1451 CrossRef CAS PubMed.
- S. Shajari, K. Kuruvinashetti, A. Komeili and U. Sundararaj, Sensors, 2023, 23, 9498 CrossRef CAS PubMed.
- B. Turan, T. Masuda, W. Lei, A. M. Noor, K. Horio, T. I. Saito, Y. Miyata and F. Arai, ROBOMECH J., 2018, 5, 27 CrossRef.
- K. P. Seng, L.-M. Ang, E. Peter and A. Mmonyi, Electronics, 2023, 12, 1509 CrossRef.
- D. Nahavandi, R. Alizadehsani, A. Khosravi and U. R. Acharya, Comput. Methods Programs Biomed., 2022, 213, 106541 CrossRef PubMed.
- T. Abe, S. Oh-Hara and Y. Ukita, Biomicrofluidics, 2021, 15, 034101 CrossRef PubMed.
- M. Shayan, S. Bhattacharjee, Y.-A. Song, K. Chakrabarty and R. Karri, IEEE Trans. Very Large Scale Integr. (VLSI) Syst., 2019, 27, 2755–2766 Search PubMed.
- T. Abe, S. Oh-hara and Y. Ukita, Biomicrofluidics, 2022, 16, 024106 CrossRef CAS PubMed.
- L. B. Baker, M. S. Seib, K. A. Barnes, S. D. Brown, M. A. King, P. J. D. De Chavez, S. Qu, J. Archer, A. S. Wolfe, J. R. Stofan, J. M. Carter, D. E. Wright, J. Wallace, D. S. Yang, S. Liu, J. Anderson, T. Fort, W. Li, J. A. Wright, S. P. Lee, J. B. Model, J. A. Rogers, A. J. Aranyosi and R. Ghaffari, Adv. Mater. Technol., 2022, 7, 2200249 CrossRef.
- K. B. Johnson, W. Wei, D. Weeraratne, M. E. Frisse, K. Misulis, K. Rhee, J. Zhao and J. L. Snowdon, Clin. Transl. Sci., 2021, 14, 86–93 CrossRef PubMed.
- A. Lashkaripour, C. Rodriguez, N. Mehdipour, R. Mardian, D. McIntyre, L. Ortiz, J. Campbell and D. Densmore, Nat. Commun., 2021, 12, 25 CrossRef CAS PubMed.
- E. A. Galan, H. Zhao, X. Wang, Q. Dai, W. T. S. Huck and S. Ma, Matter, 2020, 3, 1893–1922 CrossRef.
- M. S. Mahdavinejad, M. Rezvan, M. Barekatain, P. Adibi, P. Barnaghi and A. P. Sheth, Digit Commun. Netw., 2018, 4, 161–175 CrossRef.
- Supervised vs. Unsupervised Learning: What's the Difference?|IBM, https://www.ibm.com/cloud/blog/supervised-vs-unsupervised-learning, (accessed 8 July 2023).
- What is Supervised Learning?|IBM, https://www.ibm.com/topics/supervised-learning, (accessed 8 July 2023).
- Supervised and Unsupervised Learning in (Machine Learning), https://www.simplilearn.com/tutorials/machine-learning-tutorial/supervised-and-unsupervised-learning, (accessed 8 July 2023).
- Supervised vs. Unsupervised Learning [Differences & Examples], https://www.v7labs.com/blog/supervised-vs-unsupervised-learning, (accessed 8 July 2023).
- Z. Ao, H. Cai, Z. Wu, L. Hu, A. Nunez, Z. Zhou, H. Liu, M. Bondesson, X. Lu, X. Lu, M. Dao and F. Guo, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2214569119 CrossRef CAS PubMed.
- S. Han, T. Kim, D. Kim, Y.-L. Park and S. Jo, IEEE Robot. Autom. Lett., 2018, 3, 873–880 Search PubMed.
- V. Rizzuto, A. Mencattini, B. Álvarez-González, D. Di Giuseppe, E. Martinelli, D. Beneitez-Pastor, M. D. M. Mañú-Pereira, M. J. Lopez-Martinez and J. Samitier, Sci. Rep., 2021, 11, 13553 CrossRef CAS PubMed.
- L. Alzubaidi, J. Zhang, A. J. Humaidi, A. Al-Dujaili, Y. Duan, O. Al-Shamma, J. Santamaría, M. A. Fadhel, M. Al-Amidie and L. Farhan, J. Big Data, 2021, 8, 53 CrossRef PubMed.
- D. McIntyre, A. Lashkaripour, P. Fordyce and D. Densmore, Lab Chip, 2022, 22, 2925–2937 RSC.
- Y. Song, J. Zhao, T. Cai, A. Stephens, S.-H. Su, E. Sandford, C. Flora, B. H. Singer, M. Ghosh, S. W. Choi, M. Tewari and K. Kurabayashi, Biosens. Bioelectron., 2021, 180, 113088 CrossRef CAS PubMed.
- C. Honrado, J. S. McGrath, R. Reale, P. Bisegna, N. S. Swami and F. Caselli, Anal. Bioanal. Chem., 2020, 412, 3835–3845 CrossRef CAS PubMed.
- S. Hussein, P. Kandel, C. W. Bolan, M. B. Wallace and U. Bagci, IEEE Trans. Med. Imaging, 2019, 38, 1777–1787 Search PubMed.
- M. Alloghani, D. Al-Jumeily, J. Mustafina, A. Hussain and A. J. Aljaaf, in Supervised and Unsupervised Learning for Data Science, ed. M. W. Berry, A. Mohamed and B. W. Yap, Springer International Publishing, Cham, 2020, pp. 3–21 Search PubMed.
- L. B. Baker, M. S. Seib, K. A. Barnes, S. D. Brown, M. A. King, P. J. D. De Chavez, S. Qu, J. Archer, A. S. Wolfe, J. R. Stofan, J. M. Carter, D. E. Wright, J. Wallace, D. S. Yang, S. Liu, J. Anderson, T. Fort, W. Li, J. A. Wright, S. P. Lee, J. B. Model, J. A. Rogers, A. J. Aranyosi and R. Ghaffari, Adv. Mater. Technol., 2022, 7, 2200249 CrossRef.
- Q. Ning, W. Zheng, H. Xu, A. Zhu, T. Li, Y. Cheng, S. Feng, L. Wang, D. Cui and K. Wang, Anal. Bioanal. Chem., 2022, 414, 3959–3970 CrossRef CAS PubMed.
- J. Redmon, S. Divvala, R. Girshick and A. Farhadi, You Only Look Once: Unified, Real-Time Object Detection, 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 2016, pp. 779–788, DOI:10.1109/CVPR.2016.91.
- P. Mostafalu, M. Akbari, K. A. Alberti, Q. Xu, A. Khademhosseini and S. R. Sonkusale, Microsyst. Nanoeng., 2016, 2, 16039 CrossRef PubMed.
- A. Sui, W. Sui, S. Liu and R. Rhodes, Digit. Health, 2023, 9, 205520762311537 CrossRef PubMed.
- Y. Li, P. Ni and V. Chang, Ethical problems of smart wearable devices, COMPLEXIS 2019 - Proceedings of the 4th International Conference on Complexity, Future Information Systems and Risk, SciTePress, 2019, pp. 121–129, DOI:10.5220/0007722000520058.
- S. Kumar, P. Tiwari and M. Zymbler, J. Big Data, 2019, 6, 111 CrossRef.
- S. Nižetić, P. Šolić, D. López-de-Ipiña González-de-Artaza and L. Patrono, J. Cleaner Prod., 2020, 274, 122877 CrossRef PubMed.
- C. Perera, R. Ranjan, L. Wang, S. U. Khan and A. Y. Zomaya, Big Data Privacy in the Internet of Things Era, in IT Professional, 2015, vol. 17(3), pp. 32–39, DOI:10.1109/MITP.2015.34.
- A. A. Dos-Reis-Delgado, A. Carmona-Dominguez, G. Sosa-Avalos, I. H. Jimenez-Saaib, K. E. Villegas-Cantu, R. C. Gallo-Villanueva and V. H. Perez-Gonzalez, Electrophoresis, 2023, 44, 268–297 CrossRef CAS PubMed.
- A. Haleem, M. Javaid, R. P. Singh and R. Suman, Sens. Int., 2021, 2, 100117 CrossRef PubMed.
- N. Al Bassam, S. A. Hussain, A. Al Qaraghuli, J. Khan, E. P. Sumesh and V. Lavanya, Inf. Med. Unlocked, 2021, 24, 100588 CrossRef PubMed.
- T. Poongodi, R. Krishnamurthi, R. Indrakumari, P. Suresh and B. Balusamy, in A Handbook of Internet of Things in Biomedical and Cyber Physical System, ed. V. E. Balas, V. K. Solanki, R. Kumar and Md. A. R. Ahad, Springer International Publishing, Cham, 2020, vol. 165, pp. 245–273 Search PubMed.
- M. Ibrahim, M. Gorlatova and K. Chakrabarty, in 2019 IEEE/ACM International Conference on Computer-Aided Design (ICCAD), IEEE, Westminster, CO, USA, 2019, pp. 1–8 Search PubMed.
- S. M. A. Iqbal, I. Mahgoub, E. Du, M. A. Leavitt and W. Asghar, npj Flex. Electron, 2021, 5, 9 CrossRef.
- S. Ardalan, M. Hosseinifard, M. Vosough and H. Golmohammadi, Biosens. Bioelectron., 2020, 168, 112450 CrossRef CAS PubMed.
- Y. Yuan, S. Feng, M. Alahi, A. Nag, N. Afsarimanesh, H. Zhang and S. He, Appl. Sci., 2018, 8, 1357 CrossRef.
- R. Dwivedi, D. Mehrotra and S. Chandra, J. Oral Biol. Craniofac. Res., 2022, 12, 302–318 CrossRef PubMed.
- M. Conti and A. Passarella, Comput. Commun., 2018, 131, 51–65 CrossRef.
- M. Conti, A. Passarella and S. K. Das, Pervasive Mob. Comput., 2017, 41, 1–27 CrossRef.
- F. Liu, J.-L. Han, J. Qi, Y. Zhang, J.-L. Yu, W.-P. Li, D. Lin, L.-X. Chen and B.-W. Li, Chin. J. Anal. Chem., 2021, 49, 159–171 CAS.
- B. Murdoch, BMC Med. Ethics, 2021, 22, 122 CrossRef PubMed.
- P. Esmaeilzadeh, BMC Med. Inform. Decis. Mak., 2020, 20, 170 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2024 |