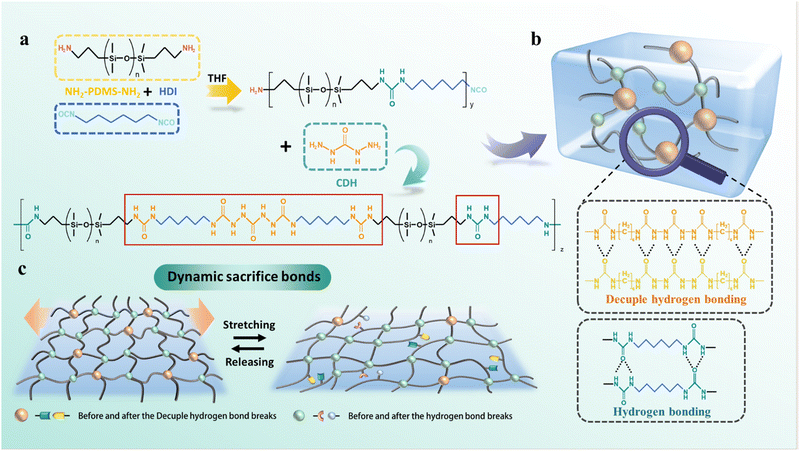
High-toughness, extensile and self-healing PDMS elastomers constructed by decuple hydrogen bonding†
Jing-Han
Gao
a,
Baoquan
Wan
a,
Ming-Sheng
Zheng
a,
Longbo
Luo
*c,
Hongkuan
Zhang
d,
Quan-Liang
Zhao
 *d,
George
Chen
e and
Jun-Wei
Zha
*d,
George
Chen
e and
Jun-Wei
Zha
 *ab
*ab
aBeijing Advanced Innovation Centre for Materials Genome Engineering, School of Chemistry and Biological Engineering, University of Science and Technology Beijing, Beijing, 100083, P. R. China. E-mail: zhajw@ustb.edu.cn
bShunde Graduate School of University of Science and Technology Beijing, Foshan, 528300, P. R. China
cState Key Laboratory of Polymer Material and Engineering, College of Polymer Science and Engineering, Sichuan University, Chengdu, 610065, P. R. China
dSchool of Mechanical and Materials Engineering, North China University of Technology, Beijing, 100041, China
eDepartment of Electronics and Computer Science, University of Southampton, Southampton SO17 1BJ, UK
First published on 19th December 2023
Abstract
Elastomers are widely used in traditional industries and new intelligent fields. However, they are inevitably damaged by electricity, heat, force, etc. during the working process. With the continuous improvement of reliability and environmental protection requirements in human production and living, it is vital to develop elastomer materials with good mechanical properties that are not easily damaged and can self-heal after being damaged. Nevertheless, there are often contradictions between mechanical properties and self-healing as well as toughness, strength, and ductility. Herein, a strong and dynamic decuple hydrogen bonding based on carbon hydrazide (CHZ) is reported, accompanied with soft polydimethylsiloxane (PDMS) chains to prepare self-healing (efficiency 98.7%), recyclable, and robust elastomers (CHZ–PDMS). The strategy of decuple hydrogen bonding will significantly impact the study of the mechanical properties of elastomers. High stretchability (1731%) and a high toughness of 23.31 MJ m−3 are achieved due to the phase-separated structure and energy dissipation. The recyclability of CHZ–PDMS further supports the concept of environmental protection. The application of CHZ–PDMS as a flexible strain sensor exhibited high sensitivity.
New conceptsHerein, we propose a strong and dynamic decuple hydrogen bonding based on carbon hydrazide (CHZ). The decuple hydrogen bonding was combined with soft polydimethylsiloxane (PDMS) chains to prepare a self-healing (efficiency 98.7%), recyclable, and robust elastomer (CHZ–PDMS). The strategy of decuple hydrogen bonding will significantly impact the study of the mechanical properties of elastomers. High stretchability (1731%) and a high toughness of 23.31 MJ m−3 are achieved due to the phase-separated structure and energy dissipation. The dynamic reversibility of hydrogen bonds endows the excellent self-healing and recyclable capability of CHZ–PDMS. The self-healing efficiency could reach almost 100%. The PDMS elastomers with decuple hydrogen bonds overcame various conflicts in self-healing elastomers and perfectly exhibited the combination of self-healing, good strength, high toughness, and outstanding stretchability. Furthermore, the application of CHZ–PDMS is exhibited as a flexible strain sensor with high sensitivity. |
1 Introduction
Elastomers have become an irreplaceable part of the industry due to their good elasticity, high toughness and strong plasticity, and include materials such as polydimethylsiloxane (PDMS),1,2 polyurethane (TPU),3–5 polyamide (TPA).6,7 In recent years, elastomers have also been widely used in electronic skins, flexible electronic devices, soft robots, etc. with the development of the intelligence field.8–15 However, the elastomers are easily damaged in the working process, resulting in degraded or even lost performance.16–18 Self-healing can prevent cracks of the material from expanding and repair the formed internal cracks.19,20 Moreover, it can extend the service life and effectively guarantee the security of materials. Intrinsic self-healing is achieved by designing the molecular structure of materials and introducing reversible chemical bonds or dynamic interactions into the molecular chain.21–23 Based on these advantages, self-healing has recently attracted extensive attention and has become one of the hot spots in the emerging intelligence field.24–26However, the continuous promotion of self-healing research also exposes a number of key problems. The mechanical property is an important criterion for evaluating materials.27 The contradiction between the self-healing ability and mechanical properties is one of the most noticeable problems among them. Strong covalent bonds lead to increased material strength but decreased healing efficiency, whereas weak bonds ensure effective healing ability but low strength.28 Researchers have tried to balance the self-healing ability and mechanical strength by combining strong covalent bonds with relatively weak and dynamic non-covalent bonds in polymers.29–31 Yu et al.32 combined covalent B–O bonds and non-covalent hydrogen bonds into PDMS. The mechanical property is regulated by adjusting the molar ratio of boroxine (BE) and 2-ureido-4[1H]-pyrimidinone (UPy). The outstanding tensile strength and self-healing ability arises from the excellent synergy of the reversible H-bonds and B–O bonds. In addition, the combination of high toughness and great strength in elastomers is also a long-standing topic. In the elastic networks, there is a trade-off between strength and toughness.33 Strong covalent crosslinking enhances the strength of the material but correspondingly reduces the toughness. The sacrificial bonds are introduced in the elastomer to achieve the effect of toughening, such as hydrogen bonds,34–37 metal coordination bonds,29,38–41 π–π stacking interactions.42–44 The preferential fracture of sacrificial bonds during stretching can dissipate the energy of stress. However, the sacrifice bond is usually weak, which is very detrimental to the strength of the elastomers. The degree of crosslinking also plays an important role in the ductility of the elastomers. Strong covalent crosslinking increases strength, while reducing the stretchability of the materials. The dynamic crosslinking can raise the elongation at the break of elastomers through the fracture and the recombination during the tensile process.
In order to regulate the advantages and disadvantages between the strength, toughness, and tensile properties of the elastomers, multiple dynamic strong hydrogen bonds have been applied, such as double,34,45 triple,46 quadruple,31,36,47 sextuple,48,49 and even octuple35 hydrogen bonding. The strength of multiple hydrogen bonds can enhance the tensile strength of materials and dissipate energy effectively. Meanwhile, the dynamic of hydrogen bonding also ensures the self-healing ability of materials. For instance, Dai et al.36 introduced quadruple hydrogen bonding (UPy) moieties into polymer networks to construct a self-healable electrification layer. The carbon nanotubes were further incorporated into a healable polymer to obtain a conductive nanocomposite that was applied in flexible and wearable electronic devices. Moreover, owing to the difference in the thermodynamic properties, combining soft and hard segments into one copolymer results in a phase-separated morphology.50 The physical cross-linking of multiple hydrogen bonds promotes the ordered packing and crystallization of hard segments to form the hard phase microdomains, which leads to the generation of microphase separation.51,52 The phase-separated structure acts as a reinforcing filler, significantly improving the mechanical strength and toughness of the polymers.40,53,54 Wang et al. reported a method for toughening thermoplastic elastomers by rationally tailoring hard–soft phase separation structures containing rigid and flexible supramolecular segments, demonstrating a record toughness (1.2 GJ m−3) and an ultrahigh true stress at break (2.3 GPa).50 Finally, PDMS is also commonly used in the manufacturing of sensors.55,56
In this work, we propose a more powerful strategy for the first time by building decuple hydrogen bonding. The decuple hydrogen bonds were introduced into the PDMS molecular chains and cooperated with the quadruple hydrogen bonds formed by the urea groups. This synergistic effect of strong and weak hydrogen bonds established a dynamic cross-linked network (CHZ–PDMS). After further characterization and calculation, it was confirmed that the multiple hydrogen bonds of the CHZ–PDMS acted as sacrificial bonds that dissipated energy, effectively leading to the improved toughness. Moreover, the strong decuple hydrogen bonding induced the generation of phase-separated structures, which enhanced the mechanical strength of the elastomers. The breaking-regeneration of the dynamic hydrogen bonds endowed the elastomers with exceptional ductility and self-healing ability. The elastomers with decuple hydrogen bonds overcame various conflicts in self-healing elastomers, and perfectly exhibited the combination of self-healing, good strength, high toughness, and outstanding stretchability. Finally, CHZ–PDMS also will be well applied to intelligent fields such as signal sensing.
2 Results and discussion
2.1 Structure design and characterization of CHZ–PDMS
The design and synthesis process of CHZ–PDMS elastomers are shown in Fig. 1a and Fig. S1 (ESI†). First, the functional group-terminated NH2-PDMS-NH2 was selected to react with hexamethylene diisocyanate (HDI) to prepare the PDMS prepolymer. Table S1 (ESI†) shows the dosage of NH2-PDMS-NH2, HDI, and CHZ, and the molecular weight (Mn) of PDMS-0, CHZ–PDMS-1, CHZ–PDMS-2 and CHZ–PDMS-3. The length of the chains was greatly extended, and the hydrogen bonds were formed because of the generation of urea groups. To increase the number and strength of hydrogen bonds, carbohydrazide with multiple hydrogen bond formation sites was introduced into the prepolymer. The carbohydrazide could react with –NCO to generate the decuple hydrogen bonding structure, as shown in Fig. 1b. The PDMS dynamic cross-linked network was composed of two parts, strong decuple hydrogen bonds and weaker quadruple hydrogen bonds (Fig. 1c). The content of the two parts could be regulated by changing the molar ratio of HDI to carbohydrazide to further adjust the mechanical properties. Moreover, the strong and weak dynamic cross-linking structure also endowed CHZ–PDMS with excellent self-healing properties. From the Fourier transform infrared (FTIR) spectroscopy shown in Fig. S2 (ESI†), it can be seen that there were no –NCO characteristic peaks at 2260–2280 cm−1 in the four spectral lines, indicating the complete reaction of HDI. Absorption bands at 3330 cm−1 and 1575 cm−1 are respectively –NH stretching vibration and bending vibration. The 1630 cm−1 absorption band was observed, belonging to the stretching vibration peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. Combined with 1H nuclear magnetic resonance (NMR) spectroscopy (Fig. S3, ESI†), it was demonstrated that PDMS-0 and CHZ–PDMS were successfully synthesized. The differential scanning calorimetry (DSC) curves of CHZ–PDMS are shown in Fig. S4 (ESI†). The Tg of the elastomer increased after the addition of carbohydrazide, which was attributed to the raised degree of cross-linking caused by the decuple hydrogen bonding. It can be seen from the thermogravimetric analysis (TGA) and derivative thermogravimetry (DTG) curves in Fig. S5 (ESI†) that the introduction of the decuple hydrogen bonding had almost no effect on the thermal stability of the elastomers. There was also no significant weight loss under 320 °C, indicating that the material maintained good thermal stability.
O. Combined with 1H nuclear magnetic resonance (NMR) spectroscopy (Fig. S3, ESI†), it was demonstrated that PDMS-0 and CHZ–PDMS were successfully synthesized. The differential scanning calorimetry (DSC) curves of CHZ–PDMS are shown in Fig. S4 (ESI†). The Tg of the elastomer increased after the addition of carbohydrazide, which was attributed to the raised degree of cross-linking caused by the decuple hydrogen bonding. It can be seen from the thermogravimetric analysis (TGA) and derivative thermogravimetry (DTG) curves in Fig. S5 (ESI†) that the introduction of the decuple hydrogen bonding had almost no effect on the thermal stability of the elastomers. There was also no significant weight loss under 320 °C, indicating that the material maintained good thermal stability.
2.2 Mechanical properties of the CHZ–PDMS elastomers
Mechanical properties tests were performed on the elastomers at a rate of 13 mm min−1. First, the stress–strain curves of different elastomers were obtained, as shown in Fig. 2a. It can be seen that the elastomer had excellent tensile strength and elongation at break (Fig. 2b). With the raised carbohydrazide content, the tensile strength of the elastomer increased gradually and the elongation at break reached the maximum value of 1731% at CHZ–PDMS-2, showing remarkable improvement compared to PDMS-0. In general, increasing the crosslinking degree by strong covalent bonds can significantly enhance the tensile strength of the material, but at the same time, the stretchability of the material will be sacrificed. Herein, CHZ–PDMS achieved a good combination of tensile strength and ductility by using the strong and dynamic decuple hydrogen bonds, and adjusting the ratio of strong and weak hydrogen bonds. The high strength of the decuple hydrogen bonds guaranteed the tensile strength, and the breaking-recombination of the dynamic hydrogen bonds during stretching improved the ductility.In addition, the Young's modulus and toughness of the four groups of samples were calculated and compared in Fig. 2c. The Young's modulus also increased with the raised decuple hydrogen bond content, and the toughness reached the maximum at CHZ–PDMS-2 accordingly. The rigidity of the materials had been improved because of the high strength of the decuple hydrogen bonding. The energy dissipation system was constructed through the sacrificial bond effect of combined strong and weak hydrogen bonds, so that the elastomer exhibited superior toughness, which could reach 23.31 MJ m−3. In order to explore and quantify the key role of decuple hydrogen bonds as a sacrificial bond in toughening elastomers, tensile cycling experiments were carried out with CHZ–PDMS-1 and CHZ–PDMS-2. Their dissipated energy and strain recovery were calculated and compared (Fig. S6, ESI†).30 With the added decuple hydrogen bond content, the energy dissipation, energy dissipation ratio showed a rising trend. Furthermore, the recovered strain ratio of both was above 75%, manifesting preeminent fatigue resistance. At small strains, the stress during stretching led to the fracture of part of the hydrogen bonds, dissipating some energy. Owing to the dynamics of the hydrogen bonds, the broken bonds can be recovered when the mechanical strain is removed, restoring the mechanical properties of the elastomers. More decuple hydrogen bonds led to the dissipation of more strain energy, proving the critical role of decuple hydrogen bonds on toughening. The CHZ–PDMS-2 with the best comprehensive mechanical properties was further explored. The stress–strain curves of CHZ–PDMS-2 at different stretching rates are shown in Fig. 2d. The tensile strength decreased and the elongation at break increased with the incremental tensile rate, which met the laws of mechanics. Fig. 2d shows the cyclic loading/unloading curves with 100%, 300% and 500% maximum tensile strains (Fig. 2e). The situation of their energy dissipation and recovered strain was also calculated and compared, as shown in Fig. S7 (ESI†). The energy dissipation and dissipation ratio increased with the added maximum tensile strains. It is attributed to there being more fractured hydrogen bonds caused by the increased strain, and therefore more energy to dissipate. Moreover, the CHZ–PDMS-2 with a relatively larger strain of 500% also showed a >80% recovered strain ratio, indicating excellent fatigue resistance characteristics. The CHZ–PDMS-2 elastomer was subjected to five consecutive 100% stretch cycle tests. It can be seen in Fig. 2f that the fifth cycle curve deviated greatly from the original curve because of the plastic deformation. The other group of samples rested for 5 min after each cycle to allow sufficient time for the broken hydrogen bonds to recombine. The cycle curve after resting was obviously recovered. It is further proved that the dynamic hydrogen bond acts as a sacrificial bond, and plays a key role in dissipating energy during the stretching process.
Surprisingly, this material with extraordinary toughness had a distinct advantage over other publications of silicone elastomer, as shown in Fig. 3a. In order to probe the intrinsic mechanism of the excellent mechanical properties, further characterizations were performed on the CHZ–PDMS elastomer. The temperature-dependent FTIR spectra are shown in Fig. 3b. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration peak of 1630 cm−1 experiences a red shift and the N–H bending vibration peak of 1575 cm−1 experiences a blue shift. These shifts are attributed to the generation of a large number of hydrogen bonds by C
O stretching vibration peak of 1630 cm−1 experiences a red shift and the N–H bending vibration peak of 1575 cm−1 experiences a blue shift. These shifts are attributed to the generation of a large number of hydrogen bonds by C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H in the system. The incremental temperature change led to the dissociation of hydrogen bonds, and the dissociation of hydrogen bonds caused a change of the C
O and N–H in the system. The incremental temperature change led to the dissociation of hydrogen bonds, and the dissociation of hydrogen bonds caused a change of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H groups from bonded to free. The C
O and N–H groups from bonded to free. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O absorption band under 25 °C and 100 °C are further deconvoluted into seven subpeaks (Fig. S8, ESI†). These subpeaks are very likely assigned to the free, disordered, and ordered H-bonds formed by C
O absorption band under 25 °C and 100 °C are further deconvoluted into seven subpeaks (Fig. S8, ESI†). These subpeaks are very likely assigned to the free, disordered, and ordered H-bonds formed by C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in different urea groups. The ratio of free H-bonds distinctly increased, while the ratio of ordered H-bonds did not decrease. This observation is attributed to the damage from the increasing temperature on the hydrogen bonds and on the ordered arrangement of the hydrogen bond. Moreover, as shown in Fig. 3c, the PDMS molecular chains were “locked” by combining to form strong decuple hydrogen bonds, storing a portion of the deformation (red area in Fig. 3c). With the small strain, the molecular chains extended towards the tensile direction and the weaker quadruple hydrogen bond broke first, dissipating a portion of energy. The fixed parts by the decuple hydrogen bond only undergo a slight deformation. With the increasing strain, the strong decuple hydrogen bonds were broken, and then the fixed molecular chains were “unlocked”. The elastomer released the stored deformation and strain energy, which gave rise to the further incremental ductility. When external forces were removed, they could recombine because of the dynamic performance of the hydrogen bonds. The performance of the materials was partially recovered, which is also the reason why the material has excellent fatigue resistance. However, the broken molecular chains were difficult to recover on account of the excessive external force. This may be due to the thermodynamic incompatibility between the soft segment of polydimethylsiloxane and the hard segment formed by the aggregation of multiple hydrogen bonds, which may form a phase-separated structure. Phase separation is an effective method to improve toughness and strength.52,57 The AFM images in Fig. 3d show that CHZ–PDMS-2 possesses a microphase separation structure resulting from the aggregation of the soft segments (PDMS, dark areas) and hard segments (decuple hydrogen bonding, bright areas). As shown in Fig. S9 (ESI†), the scanning electron microscope (SEM) image of the brittle fracture section of the elastomer showed that the fracture surface of PDMS-0 without carbohydrazide was smooth, while the fracture surface of CHZ–PDMS-2 was relatively rough. The contrast between them also confirms the phase separation structure of CHZ–PDMS. The X-ray diffraction (XRD) image of CHZ–PDMS-2 shows a strong central absorption peak and a broad central absorption peak. The diffraction peak at 2θ = 12° is attributed to the ordered structure of hard domains formed by the decuple hydrogen-bonded segments, and the diffraction peak at 2θ = 20° is due to the amorphous phase of polysiloxane (Fig. S10, ESI†). In addition, a distinct broad scattering peak was displayed in the 1D small-angle X-ray scattering (SAXS) spectrum of CHZ–PDMS-2, while the peak of PDMS-0 is not obvious. The 2D SAXS diffraction signal shows a uniform annular diffraction pattern. The diameter (d) of the separation domain was calculated to be about 7.85 nm (Fig. 3e), proving the appearance of the phase separation domain. To further exhibit the important role of the decuple hydrogen bonds, the tensile forces for the formation of quadruple bonds and decuple hydrogen bonded polymers were calculated by density functional theory (DFT) (the structure is shown in Fig. 3f).31 It can be observed that the tensile force of the decuple hydrogen bonding was much greater than that of the quadruple hydrogen bonding, which was consistent with the actual tests (Fig. 3g).
O in different urea groups. The ratio of free H-bonds distinctly increased, while the ratio of ordered H-bonds did not decrease. This observation is attributed to the damage from the increasing temperature on the hydrogen bonds and on the ordered arrangement of the hydrogen bond. Moreover, as shown in Fig. 3c, the PDMS molecular chains were “locked” by combining to form strong decuple hydrogen bonds, storing a portion of the deformation (red area in Fig. 3c). With the small strain, the molecular chains extended towards the tensile direction and the weaker quadruple hydrogen bond broke first, dissipating a portion of energy. The fixed parts by the decuple hydrogen bond only undergo a slight deformation. With the increasing strain, the strong decuple hydrogen bonds were broken, and then the fixed molecular chains were “unlocked”. The elastomer released the stored deformation and strain energy, which gave rise to the further incremental ductility. When external forces were removed, they could recombine because of the dynamic performance of the hydrogen bonds. The performance of the materials was partially recovered, which is also the reason why the material has excellent fatigue resistance. However, the broken molecular chains were difficult to recover on account of the excessive external force. This may be due to the thermodynamic incompatibility between the soft segment of polydimethylsiloxane and the hard segment formed by the aggregation of multiple hydrogen bonds, which may form a phase-separated structure. Phase separation is an effective method to improve toughness and strength.52,57 The AFM images in Fig. 3d show that CHZ–PDMS-2 possesses a microphase separation structure resulting from the aggregation of the soft segments (PDMS, dark areas) and hard segments (decuple hydrogen bonding, bright areas). As shown in Fig. S9 (ESI†), the scanning electron microscope (SEM) image of the brittle fracture section of the elastomer showed that the fracture surface of PDMS-0 without carbohydrazide was smooth, while the fracture surface of CHZ–PDMS-2 was relatively rough. The contrast between them also confirms the phase separation structure of CHZ–PDMS. The X-ray diffraction (XRD) image of CHZ–PDMS-2 shows a strong central absorption peak and a broad central absorption peak. The diffraction peak at 2θ = 12° is attributed to the ordered structure of hard domains formed by the decuple hydrogen-bonded segments, and the diffraction peak at 2θ = 20° is due to the amorphous phase of polysiloxane (Fig. S10, ESI†). In addition, a distinct broad scattering peak was displayed in the 1D small-angle X-ray scattering (SAXS) spectrum of CHZ–PDMS-2, while the peak of PDMS-0 is not obvious. The 2D SAXS diffraction signal shows a uniform annular diffraction pattern. The diameter (d) of the separation domain was calculated to be about 7.85 nm (Fig. 3e), proving the appearance of the phase separation domain. To further exhibit the important role of the decuple hydrogen bonds, the tensile forces for the formation of quadruple bonds and decuple hydrogen bonded polymers were calculated by density functional theory (DFT) (the structure is shown in Fig. 3f).31 It can be observed that the tensile force of the decuple hydrogen bonding was much greater than that of the quadruple hydrogen bonding, which was consistent with the actual tests (Fig. 3g).
 | ||
| Fig. 3 (a) Comparison of toughness with other publications on PDMS elastomers.38,58–62 (b) FTIR spectra at an incremental temperature from 25 °C to 145 °C of CHZ–PDMS-2. (c) Diagram of the tensile process of elastomers. (d) AFM phase images of PDMS-0 and CHZ–PDMS-2, showing the distinct micro-phase-separation. (e) The 1D SAXS spectra of PDMS-0 and CHZ–PDMS-2. The 2D SAXS spectrum of CHZ–PDMS-2. (f) DFT calculation of the molecular configuration of (i) quadruple hydrogen bonding and (ii) decuple hydrogen bonding. (g) DFT calculation results of decuple hydrogen bonds represented by the orange curve and quadruple hydrogen bonds represented by the blue curve. | ||
2.3 Self-healing ability and recyclability of CHZ–PDMS elastomers
Considering the dynamic properties of hydrogen bonds, we conducted detailed tests to study the self-healing ability of the CHZ–PDMS elastomers. The CHZ–PDMS-2 with the best comprehensive mechanical properties was selected for cutting, and the fracture surface was recontacted to heal to evaluate its healing efficiency (η). The healing efficiency (η) was calculated by toughness. The cracked samples were repaired at different temperatures for 12 h, and further subjected to tensile testing. The stress–strain curves were obtained, as shown in Fig. 4a. Fig. 4b shows the calculation and comparison of the repair efficiency at different temperatures. It is evident that the self-healing efficiency gradually increased with the increase of temperature. The self-healing efficiency was the highest at 70 °C, which could reach 91.4%. Thus, tensile tests were performed on CHZ–PDMS-2 with different healing times at 70 °C. As shown in Fig. 4d, the repair efficiency increased with the extended healing time. After healing for 24 h, the healing efficiency reached ≈100%, revealing excellent self-healing capability. There is no doubt that the rising repair temperature and time can promote the diffusion of polymer chains at the fracture interface and the activity of dynamic bonds, which is beneficial to self-healing. However, it is worth noting that the siloxane elastomer with such excellent mechanical properties can also maintain high healing efficiency. The images from before and after the elastomer repair step were thus acquired to clearly exhibit the self-healing performance of CHZ–PDMS-2. In Fig. 4e, the crack clearly healed after a period of repair, and the repaired sample did not break even after applying a certain external force. Under the optical microscope, the healing of the incision could be displayed more clearly. The crack gradually fades with the extension of healing time at 70 °C (Fig. 4f). For observing the healing inside the crack, the longitudinal section of the healed part of the material was photographed by SEM. As shown in Fig. S11 (ESI†), the internal cracks of the elastomer almost completely disappeared after healing. The excellent self-healing properties of CHZ–PDMS elastomers was attributed to the dynamic reversibility of the hydrogen bonds in the polymer network (Fig. 4g). It is well known that the self-healing of elastomers is based on the fluidity of the chains. The high degree of cross-linking of CHZ–PDMS restricts the motion of polymer chains, which results in the need for the temperature to improve the mobility of the chains, ensuring high self-healing efficiency. Polymer chains diffuse and rearrange across the fracture interface. Hydrogen bonds are then broken at a higher temperature and reorganize during chain diffusion. Dynamic hydrogen bonds then regenerated when the temperature was reduced, allowing the materials to be healed. The dynamic mechanical analysis (DMA) testing results in Fig. S12 (ESI†) show that the viscous flow temperature (Tf) is about 100 °C. Therefore, the self-healing temperature of 70 °C does not affect the normal use of the elastomer.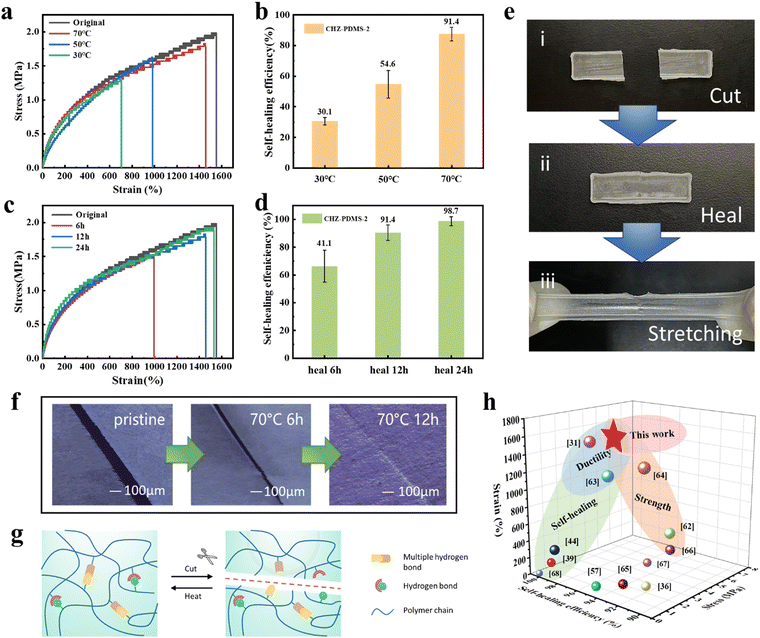 | ||
| Fig. 4 (a) The stress–strain curves of CHZ–PDMS-2 samples before and after self-healing at different temperatures for 12 h. (b) Self-healing efficiency of the fractured CHZ–PDMS healed at different healing temperatures for 12 h. (c) The stress–strain curves of CHZ–PDMS-2 samples before and after self-healing at 70 °C for different times. (d) Self-healing efficiency of the fractured CHZ–PDMS healed at for different times. (e) Photographs of the (i) fracture sample and (ii) healed sample of CHZ–PDMS-2. CHZ–PDMS-2 self-healed at 70 °C for 12 h. (f) Optical microscopy pictures of CHZ–PDMS-2 before and after 6 h and 12 h self-healing at 70 °C. (g) Schematic diagram of the self-healing mechanism. (h) The self-healing efficiency, tensile strength, and elongation at break of CHZ–PDMS compared with other reports.31,36,39,44,57,62–68 | ||
CHZ–PDMS-2 was taken to study the recyclability. The elastomer was soaked in tetrahydrofuran (THF) at room temperature. CHZ–PDMS-2 dissolved in THF solution, and was completely dissolved after 2 h (Fig. S13, ESI†). The dissolved solution was poured into the molds, and the CHZ–PDMS-2 elastomer was obtained again after the solvent was evaporated. The mechanical properties of the elastomers were tested after one recycle step and two recycle steps. As shown in Fig. S14 (ESI†), the stress–strain curves of the recycled elastomers were almost the same as the original curve, and even the sample recycled twice still exhibited similar tensile properties to the original. The FTIR spectra of the sample after two recycles is consistent with the original (Fig. S15, ESI†), and the storage modulus also seems to show no distinct change (Fig. S16, ESI†). This recycling was simple and environmentally friendly without heating or the addition of any other chemicals. CHZ–PDMS was cross-linked by hydrogen bonds. The reason why CHZ–PDMS-2 could be dissolved in THF is because the oxygen atoms of THF break the hydrogen bonds between the molecular chains, so that the molecular chains were dissociated and dissolved. This recycling process maintained the integrity of the main chains without dissociating the polymer into oligomers or monomers. Subsequently, CHZ–PDMS was combined with electronic components to fabricate wearable electronic systems with sensing, health monitoring or other functions. The electronic components could be separated from the material by soaking at room temperature for a period of time, realizing the recyclability and reuse of the electronic components (Fig. S17, ESI†). Thus, CHZ–PDMS balanced the relationship between the self-healing and mechanical properties, standing out among many self-healing materials. It has high self-healing efficiency, while combining with higher ductility and strength, as shown in Fig. 4h.
2.4 Application of CHZ–PDMS elastomers
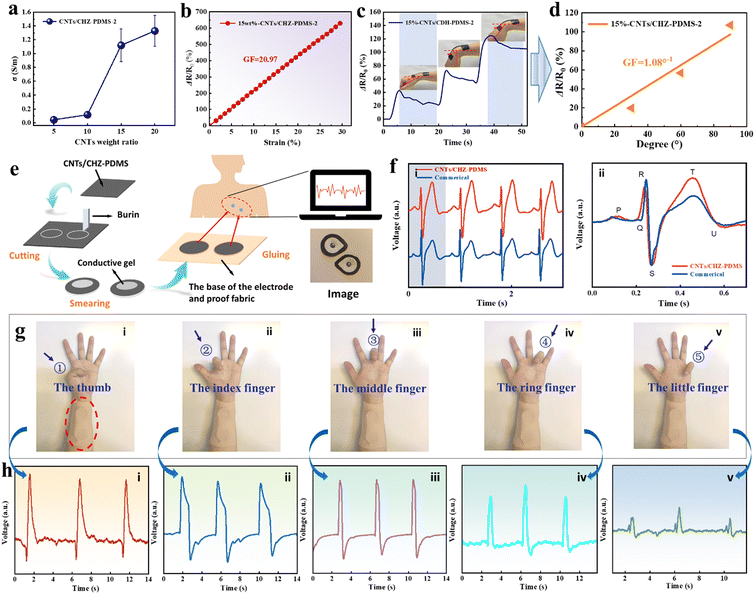
CHZ–PDMS with outstanding mechanical and self-healing properties had the potential to manufacture flexible sensors. Therefore, we doped CHZ–PDMS-2 with carboxylated carbon nanotubes (CNTs) to prepare conductive composites, which were further fabricated into simple flexible resistive sensors. CNTs/CHZ–PDMS-2 is shown in Fig. S18 (ESI†). The CNTs could improve the conductivity of CHZ–PDMS, thereby reducing the resistance. Moreover, the carboxyl group could form hydrogen bonds with the polymer, enhancing the binding force between CNTs and polymer matrix. The mechanical properties of the elastomers with different contents of CNTs (5 wt%, 10 wt%, 15 wt% and 20 wt%) were tested. It was found that the mechanical of the elastomers decreased after doping with CNTs (Fig. S19, ESI†), which was ascribed to the agglomeration of the CNTs. In addition, the conductivity increases dramatically when the doping amount increases from 10 wt% to 15 wt%, which may be related to the percolation threshold (Fig. 5a). When the content of doped filler closely approached the percolation threshold, the conductivity of the composite increased rapidly.69,70 Connecting with a power supply of 3 V, the minimum doping amount of CNTs/CHZ–PDMS-2 for lighting a commercial LED was 15 wt% (Fig. S20, ESI†). In addition, Fig. 5b shows that the relative resistance change ratio (ΔR/R0) increases linearly with the strain, and the sensitivity (Gauge factor, GF) could reach 20.97, which was very suitable as a strain sensor. According to Fig. 5a, a higher doping amount may lead to a lower sensitivity of the sensor when the doping amount exceeds the percolation threshold.71 Therefore, 15 wt%-CNTs/CHZ–PDMS-2 with 186% elongation at break and appropriate electrical conductivity was selected to be fabricated into strain sensors. The ability of the conductive elastomers to sense the movement of human joints was then tested by immobilizing the samples on volunteers' wrist (Fig. 5c). When the wrist joint was bent at different angles, the resistance of 15 wt%-CNTs/CHZ–PDMS changed with the bending action, accurately sensing the movement of different bending angles. It was shown that ΔR/R0 is in good linear relationship with the bending angle, and GF is about 1.08°−1 (Fig. 5d).In addition to strain sensor, CNTs/CHZ–PDMS could be applied to monitor human electrophysiology.72 Generally, traditional commercial Ag/AgCl gel electrodes are used in human electrocardiograms (ECG) and electromyograms (EMG). However, conductive gels dry out easily, resulting in decreased electrical conductivity and adhesion to the skin. It may also cause skin allergies with extended use. Therefore, CNTs/CHZ–PDMS-2 could be used as dry electrodes to test human ECG and EMG. The fabrication of the devices and the testing process are shown in Fig. 5e and Fig. S21 (ESI†), respectively. ECG and EMG signals were detected by a wireless module (BMD101, NeuroSky) through a medical adhesive tape (3M, 2733). For comparison, commercial Ag/AgCl gels and CNTs/CHZ–PDMS-2 were used as electrodes, respectively. As shown in Fig. 5f, typical ECG peaks (P, Q, R, S, T, and U) were observed in both curves, which indicated that there was no discernable distinction between them. In addition, the device was attached to the forearm of the volunteer to monitor EMG. Fig. 5g(i)–(v) shows the pictures of the five fingers bending, respectively, corresponding to the EMG signals in Fig. 5h(i)–(v). There is a strong peak when the fingers are bent and relaxed, which then returns to the flat state. Each finger movement showed an obviously different response signal. These applications were of great value in the field of medicine and kinematics.
Furthermore, the hydrophobicity of PDMS-0, CHZ–PDMS-2 and CNTs/CHZ–PDMS-2 were tested to explore the impact of decuple hydrogen bonding and CNTs. As shown in Fig. S22 (ESI†), the hydrophobic angle of PDMS-0 was 99.8°, reflecting good hydrophobicity. The hydrophobic angle of CHZ–PDMS-2 reached 104.8°, which was improved compared with the sample without decuple hydrogen bonding. The improvement was due to the formation of a stronger cross-linked network of PDMS, leading to the enhanced molecular cohesion. The movement of the flexible segment was hindered, which reduced the distance between the points of the molecular space network, resulting in less penetration of water molecules. Moreover, water, sweat, and other liquids are prone to produce a wetting effect of the flexible conductive materials. The hydrophobic angle of CNTs/CHZ–PDMS-2 was 96.6° (>90°) and still maintained hydrophobicity, protecting the weak electrical signals from interference in complex external environments. The sensitivity and accuracy of its response signals were well guaranteed. Finally, the performance of CNTs/CHZ–PDMS-2 was compared with other similar PDMS strain sensing materials (Table S2, ESI†). CNTs/CHZ–PDMS-2 with its expected conductivity, gauge factor, strain, self-healing ability and hydrophobicity exhibits excellent comprehensive capabilities, making it an ideal candidate material of sensors.
3 Conclusions
In summary, we described a strategy to construct the decuple hydrogen bonding via carbonyl hydrazine for the first time. The decuple hydrogen bonding was introduced into the PDMS chains, and combined with quadruple hydrogen bonding based on urea groups to establish a dynamic cross-linked network. The tensile strength and elongation at break of the CHZ–PDMS were significantly improved. The toughness could reach 23.31 MJ m−3, which is very prominent compared to that in the previous PDMS elastomer research. In addition, the PDMS elastomers had excellent self-healing properties based on the dynamic reversibility of the hydrogen bonds. The self-healing efficiency achieved almost 100%. The strong and dynamic decuple hydrogen bond overcomes the contradiction between the mechanical properties and self-healing. Furthermore, the recyclable performance of CHZ–PDMS could realize the recycling of electronic components, which is environmentally friendly and avoids waste. We doped CNTs into the CHZ–PDMS elastomer to prepare the CNTs/CHZ–PDMS conductive composites. The flexible strain sensor made by a simple compound with CNTs had high sensitivity and can be fitted on the wrist to sense the movement of human joints. The proposal of the decuple hydrogen bonding strategy is of great significance for the development of elastomers.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 52277022) and Scientific and Technological Innovation Foundation of Foshan (BK21BE006).Notes and references
- F. Madsen, L. Yu and A. Skov, ACS Macro Lett., 2016, 5(11), 1196–1200 CrossRef CAS PubMed.
- E. Ogliani, L. Yu, I. Javakhishvili and A. Skov, RSC Adv., 2018, 8(15), 8285–8291 RSC.
- Q. Qu, J. He, Y. Da, M. Zhu, Y. Liu, X. Li, X. Tian and H. Wang, Macromolecules, 2021, 17, 8243–8254 CrossRef.
- H. Li, J. T. Sun, C. Wang, S. Liu, D. Yuan, X. Zhou, J. Tan, L. Stubbs and C. He, ACS Sustainable Chem. Eng., 2017, 5, 7942–7949 CrossRef CAS.
- Z. Liu, L. Zhang, Q. Guan, Y. Guo, J. Lou, D. Lei, S. Wang, S. Chen, L. Sun, H. Xuan, E. M. Jeffries, C. He, F. L. Qing and Z. You, Adv. Funct. Mater., 2019, 29(28), 1901058 CrossRef.
- E. Linul, L. Marsavina and D. I. Stoia, Theor. Appl. Fract. Mech., 2020, 106, 102497 CrossRef CAS.
- W. Wang, X. Ma, D. Sun, X. Qi, J. Yang and Y. Wang, Composites, Part B, 2020, 128, 105671 CrossRef CAS.
- Z. Yang, H. Li, L. Zhang, X. Lai and X. Zeng, J. Colloid Interface Sci., 2020, 570, 1–10 CrossRef CAS PubMed.
- X. Liu, H. Sun, S. Liu, Y. Jiang, Z. Yin, B. Yu, N. Ning, M. Tian and L. Zhang, Composites, Part B, 2021, 223, 109103 CrossRef CAS.
- R. Kanno, S. Watanabe, K. Shimizu and J. Shintake, IEEE Robot. Autom. Lett., 2021, 4, 6274–6280 Search PubMed.
- Y. Zhao, L. J. Yin, S. L. Zhong, J. W. Zha and Z. M. Dang, IET Nanodielectrics, 2020, 3, 99–106 CrossRef.
- J. H. Zhang, Z. Li, J. Xu, J. Lin, K. Yan, W. Cheng, M. Xin, T. Zhu, J. Du, S. Chen, X. An, Z. Zhou, L. Cheng, S. Ying, J. Zhang, X. Gao, Q. Zhang, X. Jia, Y. Shi and L. Pan, Nat. Commun., 2022, 13, 1–10 Search PubMed.
- Y. Kim, H. Yuk, R. Zhao, S. A. Chester and X. Zhao, Nature, 2018, 558(7709), 274–279 CrossRef CAS.
- N. Ebrahimi, C. Bi, D. Cappelleri, G. Ciuti, A. Conn, D. Faivre, N. Habibi, A. Hošovský, V. Iacovacci, I. Khalil, V. Magdanz, S. Misra, C. Pawashe, R. Rashidifar, P. Soto-Rodriguez, Z. Fekete and A. Jafari, Adv. Funct. Mater., 2021, 31(11), 2005137 CrossRef CAS.
- S. Jeong, A. Daugaard and A. Skov, Electroactive Polymer Actuators and Devices (EAPAD) XXIII, SPIE, 2021, 11587, 155–161 Search PubMed.
- Y. Zhang, C. Ellingford, R. Zhang, J. Roscow, M. Hopkins, P. Keogh, T. McNally, C. Bowen and C. Wan, Adv. Funct. Mater., 2019, 29, 1808431 CrossRef.
- Y. Fu, F. Xu, D. Weng, X. Li, Y. Li and J. Sun, ACS Appl. Mater. Interfaces, 2019, 11, 37285–37294 CrossRef CAS.
- M. Bleszynski and M. Kumosa, Compos. Sci. Technol., 2018, 164, 74–81 CrossRef CAS.
- S. Wang and M. W. Urban, Nat. Rev. Mater., 2020, 5, 562–583 CrossRef CAS.
- Y. Yang, Z. M. Dang, Q. Li and J. He, Adv. Sci., 2020, 7, 2002131 CrossRef CAS PubMed.
- Z. Wang, X. Lu, S. Sun, C. Yu and H. Xia, J. Mater. Chem. B, 2019, 7, 4876–4926 RSC.
- W. Li, B. Dong, Z. Yang, J. Xu, Q. Chen, H. Li, F. Xing and Z. Jiang, Adv. Mater., 2018, 30, 1705679 CrossRef.
- X. Yang, X. Zhong, J. Zhang and J. Gu, J. Mater. Sci. Technol., 2021, 68, 209–215 CrossRef CAS.
- B. Wan, X. Yang, X. Dong, M. S. Zheng, Q. Zhao, H. Zhang, G. Chen and J. W. Zha, Adv. Mater., 2022, 2207451 Search PubMed.
- L. Zhang, J. Liang, C. Jiang, Z. Liu, L. Sun, S. Chen, H. Xuan, D. Lei, Q. Guan, X. Ye and Z. You, Nat. Sci. Rev., 2021, 8(5), nwaa154 CrossRef CAS.
- W. Sun, L. Zhang, S. Wang, J. Mao, J. Luo, Y. Chen and Y. Cheng, J. Mater. Chem. C, 2021, 9(27), 8579–8588 RSC.
- K. Ruan, Y. Guo and J. Gu, Macromolecules, 2021, 54, 4934–4944 CrossRef CAS.
- M. D. Hager, P. Greil, C. Leyens, S. van der Zwaag and U. S. Schubert, Adv. Mater., 2010, 22, 5424–5430 CrossRef CAS.
- J. C. Lai, X. Y. Jia, D. P. Wang, Y. B. Deng, P. Zheng, C. H. Li, J. L. Zuo and Z. Bao, Nat. Commun., 2019, 10, 1164 CrossRef PubMed.
- Q. Zhang, S. Niu, L. Wang, J. Lopez, S. Chen, Y. Cai, R. Du, Y. Liu, J. C. Lai, L. Liu and C. H. Li, Adv. Mater., 2018, 30, 1801435 CrossRef PubMed.
- H. Yu, Y. Feng, L. Gao, C. Chen, Z. Zhang and W. Feng, Macromolecules, 2020, 53, 7161–7170 CrossRef CAS.
- C. Creton, Macromolecules, 2017, 50, 8297–8316 CrossRef CAS.
- J. Kang, D. Son, G. J. N. Wang, Y. Liu, J. Lopez, Y. Kim, J. Y. Oh, T. Katsumata, J. Mun, Y. Lee and L. Jin, Adv. Mater., 2018, 30, 1706846 CrossRef PubMed.
- Y. Zhuo, Z. Xia, Y. Qi, T. Sumigawa, J. Wu, P. Šesták, Y. Lu, V. Håkonsen, T. Li, F. Wang and W. Chen, Adv. Mater., 2021, 33, 2008523 CrossRef CAS PubMed.
- X. Dai, L. B. Huang, Y. Du, J. Han, Q. Zheng, J. Kong and J. Hao, Adv. Funct. Mater., 2020, 30, 1910723 CrossRef CAS.
- J. H. Gao, B. Wan, M. S. Zheng and J. W. Zha, Polym. Chem., 2022, 13, 5412–5421 RSC.
- J. C. Lai, L. Li, D. P. Wang, M. H. Zhang, S. R. Mo, X. Wang, K. Y. Zeng, C. H. Li, Q. Jiang, X. Z. You and J. L. Zuo, Nat. Commun., 2018, 9, 1–9 CAS.
- H. Sun, X. Liu, S. Liu, B. Yu, N. Ning, M. Tian and L. Zhang, J. Mater. Chem. A, 2020, 8, 23330–23343 RSC.
- X. Wang, S. Zhan, Z. Lu, J. Li, X. Yang, Y. Qiao, Y. Men and J. Sun, Adv. Mater., 2020, 32, 2005759 CrossRef CAS.
- S. Burattini, H. M. Colquhoun, J. D. Fox, D. Friedmann, B. W. Greenland, P. J. Harris, W. Hayes, M. E. Mackay and S. J. Rowan, Chem. Commun., 2009, 6717–6719 RSC.
- L. Zhang, Z. Liu, X. Wu, Q. Guan, S. Chen, L. Sun, Y. Guo, S. Wang, J. Song, E. M. Jeffries, C. He, F. L. Qing, X. Bao and Z. You, Adv. Mater., 2019, 31(23), 1901402 CrossRef PubMed.
- J. F. Mei, X. Y. Jia, J. C. Lai, Y. Sun, C. H. Li, J. H. Wu, Y. Cao, X. Z. You and Z. Bao, Macromol. Rapid Commun., 2016, 37, 1667–1675 CrossRef CAS.
- J. Fan, J. Huang, M. Yan, Z. Gong, L. Cao and Y. Chen, J. Mater. Chem. A, 2020, 8, 16376–16384 RSC.
- H. P. Xiang, M. Z. Rong and M. Q. Zhang, Polymer, 2017, 108, 339–347 CrossRef CAS.
- A. Gooch, N. S. Murphy, N. H. Thomson and A. J. Wilson, Macromolecules, 2013, 46, 9634–9641 CrossRef CAS PubMed.
- P. Song and H. Wang, Adv. Mater., 2020, 32, 1901244 CrossRef CAS.
- J. Tellers, S. Canossa, R. Pinalli, M. Soliman, J. Vachon and E. Dalcanale, Macromolecules, 2018, 51, 7680–7691 CrossRef CAS.
- C. C. Cheng, I. H. Lin, Y. C. Yen, C. W. Chu, F. H. Ko, X. Wang and F. C. Chang, RSC Adv., 2012, 2, 9952–9957 RSC.
- T. Choi, J. Weksler, A. Padsalgikar and J. Runt, Polymer, 2009, 50, 2320–2327 CrossRef CAS.
- L. Wang, L. Guo, K. Zhang, Y. Xia, J. Hao and X. Wang, Angew. Chem., Int. Ed., 2023, e202301762 CAS.
- E. Delebecq, J. P. Pascault, B. Boutevin and F. Ganachaud, Chem. Rev., 2013, 113, 80–118 CrossRef CAS PubMed.
- Z. Li, Y. L. Zhu, W. Niu, X. Yang, Z. Jiang, Z. Y. Lu, X. Liu and J. Sun, Adv. Mater., 2021, 33, 2101498 CrossRef CAS.
- D. Wang, Z. Wang, S. Ren, J. Xu, C. Wang, P. Hu and J. Fu, Mater. Horiz., 2021, 8, 2238–2250 RSC.
- Z. Yang, H. Li, L. Zhang, X. Lai and X. Zeng, J. Colloid Interface Sci., 2020, 570, 1–10 CrossRef CAS PubMed.
- F. L. Gao, P. Min, X. Z. Gao, C. Li, T. Zhang, Z. Z. Yu and X. Li, J. Mater. Chem. A, 2022, 10(35), 18256–18266 RSC.
- D. Song, X. Li, X. P. Li, X. Jia, P. Min and Z. Z. Yu, J. Colloid Interface Sci., 2019, 555, 751–758 CrossRef CAS.
- C. Chen, W. B. Ying, J. Li, Z. Kong, F. Li, H. Hu, Y. Tian, D. H. Kim, R. Zhang and J. Zhu, Adv. Funct. Mater., 2022, 32, 2106341 CrossRef CAS.
- H. Chen, J. J. Koh, M. Liu, P. Li, X. Fan, S. Liu, J. C. C. Yeo, Y. Tan, B. C. K. Tee and C. He, ACS Appl. Mater. Interfaces, 2020, 12, 31975–31983 CrossRef CAS PubMed.
- C. H. Li, C. Wang, C. Keplinger, J. L. Zuo, L. Jin, Y. Sun, P. Zheng, Y. Cao, F. Lissel, C. Linder, X. Z. You and Z. Bao, Nat. Chem., 2016, 8, 618–624 CrossRef CAS PubMed.
- S. Liu, S. Chen, W. Shi, Z. Peng, K. Luo, S. Xing, J. Li and L. Liu, Adv. Funct. Mater., 2021, 31, 2102225 CrossRef CAS.
- Q. Chen, X. Zhao, B. Li, A. P. Sokolov, M. Tian, R. C. Advincula and P. F. Cao, Exceptionally recyclable, extremely tough, vitrimer-like polydimethylsiloxane elastomers via rational network design, Matter, 2023, 6, 3378–3393 CrossRef CAS.
- C. Lv, J. Wang, Z. Li, K. Zhao and J. Zheng, Composites, Part B, 2019, 177, 107270 CrossRef CAS.
- J. Zhao, R. Xu, G. Luo, J. Wu and H. Xia, J. Mater. Chem. B, 2016, 4, 982–989 RSC.
- Z. Feng, B. Yu, J. Hu, H. Zuo, J. Li, H. Sun, N. Ning, M. Tian and L. Zhang, Ind. Eng. Chem. Res., 2019, 58, 1212–1221 CrossRef CAS.
- H. Guo, Y. Han, W. Zhao, J. Yang and L. Zhang, Nat. Commun., 2020, 11, 1–9 CrossRef.
- S. Kirkpatrick, Rev. Mod. Phys., 1973, 45, 574–588 CrossRef.
- W. Sun, L. Zhang, S. Wang, J. Mao, J. Luo, Y. Chen and Y. Cheng, J. Mater. Chem. C, 2021, 9(27), 8579–8588 RSC.
- E. Ogliani, L. Yu, I. Javakhishvili and A. L. Skov, RSC Adv., 2018, 8(15), 8285–8291 RSC.
- R. Taherian, ECS J. Solid State Sci. Technol., 2014, 3, M26–M38 CrossRef CAS.
- H. Liu, Q. Li, S. Zhang, R. Yin, X. Liu, Y. He, K. Dai, C. Shan, J. Guo, C. Liu, C. Shen, X. Wang, N. Wang, Z. Wang, R. Wei and Z. Guo, J. Mater. Chem. C, 2018, 6, 12121–12141 RSC.
- L. Duan, M. Spoerk, T. Wieme, P. Cornillie, H. Xia, J. Zhang, L. Cardona and D. R. D'hooge, Compos. Sci. Technol., 2019, 171, 78–85 CrossRef CAS.
- Q. Zhao, Z. Wang, J. Chen, S. Liu, Y. Wang, M. Zhang, J. Di, G. He, L. Zhao, T. Su and J. Zhang, Nanoscale, 2021, 13, 10798–10806 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3mh01265d |
| This journal is © The Royal Society of Chemistry 2024 |