Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The structure and application portfolio of intricately architected silver cluster-assembled materials
Riki
Nakatani
 a,
Saikat
Das
a,
Saikat
Das
 *b and
Yuichi
Negishi
*b and
Yuichi
Negishi
 *ab
*ab
aDepartment of Applied Chemistry, Faculty of Science, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: negishi@rs.tus.ac.jp
bResearch Institute for Science & Technology, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: saikatdas@rs.tus.ac.jp
First published on 8th April 2024
Abstract
Silver Cluster-Assembled Materials (SCAMs) represent a new frontier of crystalline extended solids hallmarked by their customizable structures, commendable stabilities, and unique physical/chemical properties. Since their discovery in 2017, the diversity of organic linkers has endowed SCAMs with ingenious architectures and the application scenario has expanded beyond photoluminescence sensing to environmental sustainability and biomedical applications. It is critically important to chronicle these recent key advances and review the progress of SCAMs that can enable translating the material discoveries into real implementation. Herein, we provide a succinct overview of the trajectory of SCAM research, with crucial insights into atomic-level structural correlations with the phenomena at the nanoscale and discuss the gaps and opportunities that are still open in addition to charting a roadmap for future research directions.
1 Introduction
Nanoclusters are aggregates of metal atoms and their diameters are mostly 1–3 nm; they have been drawing attention as nanomaterials since the late 20th century because they demonstrate characteristics different from those of single metal atoms or bulk.1,2 Looking more closely at the structure of a nanocluster, the structure consists of a core composed of metal atoms and a shell formed of ligands around the core. The core can not only be composed of a single metal species, but also of multiple metal species, which can have a great impact on stability, optoelectronic properties, catalytic performance and so on.3,4 On the other hand, the shell has been of great importance because it not only contributes to the stabilization of the nanocluster structure, but also prevents aggregation between clusters and determines their properties and structures.5 However, nanoclusters have several shortcomings: they are notorious for their low yields, and the purity of the nanoclusters obtained is not high, making their purification and characterization considerably more difficult than for simple metal complexes.6 Therefore, the separation of clusters has also been the subject of substantial research in the field of nanomaterials.7 In addition to separation, there have been intensive studies on how to extend the use of clusters. Nanoclusters are largely used in a diverse array of applications, including photochemistry, electrochemistry, catalysis, sensing and others.8–11 Among them, silver has outstanding physical properties and is cheaper and more plentiful as a resource than gold, which is also a kind of precious metal.12 Numerous attempts have been made to synthesize silver nanoclusters with unique optical absorption properties.13–16 However, silver has a critical drawback of being easily oxidized by exposure to air and light that has greatly limited its range of applications compared to other precious metals.17In light to the foregoing, cutting-edge materials that can solve the most important issue of stability of silver clusters are silver cluster-assembled materials (SCAMs). SCAMs are crystalline extended structures connecting silver cluster nodes and organic linkers through coordination bonds. [(Ag12(StBu)8(CF3COO)4(bpy)4)]n (henceforth denoted as Ag12bpy) SCAM was prepared by Zang et al. upon substituting 4,4′-bipyridine (bpy) for coordinated acetonitrile (CH3CN) ligands of Ag12 nanoclusters.18 Ag12bpy showed stability far superior to that of discrete silver nanoclusters under many conditions, including prolonged exposure to air and light irradiation, and the luminescence quantum yield was dramatically improved. The organic linkers used for cross-linking not only stabilize the structure by linking clusters together, but also contribute to the SCAM properties.19 The structure of SCAMs can also be modified by changing the shape of the linker to form unique structures. Thereafter, many SCAMs utilizing bidentate, tridentate, and tetradentate linkers other than bpy have been reported.20–24 On the other hand, in terms of silver nanoclusters, which are the nodes of SCAMs, there have been reports of SCAMs constituted by nanoclusters of different numbers of silver atoms such as Ag9, Ag10, Ag27,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21,25,26etc., in addition to the more commonly known Ag12 cluster-based SCAMs. Hence, it can be said that SCAMs can not only resolve the stability problem of silver nanoclusters through metal–ligand coordination, but also display structural diversity. In addition to linking the silver cluster nodes via organic linkers, there have been instances of linking the silver cluster nodes without linker molecules to form one-dimensional (1D),27,28 two-dimensional (2D)28,29 and three-dimensional (3D) frameworks.28–31
21,25,26etc., in addition to the more commonly known Ag12 cluster-based SCAMs. Hence, it can be said that SCAMs can not only resolve the stability problem of silver nanoclusters through metal–ligand coordination, but also display structural diversity. In addition to linking the silver cluster nodes via organic linkers, there have been instances of linking the silver cluster nodes without linker molecules to form one-dimensional (1D),27,28 two-dimensional (2D)28,29 and three-dimensional (3D) frameworks.28–31
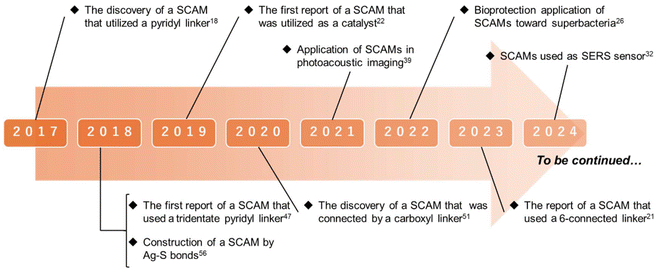
In this review, we briefly describe the crystalline structures, synthetic procedures, and applicability of SCAMs, which, as mentioned before, exhibit wide structural, morphological and functional diversity. Notably, most SCAMs yet reported have been constructed from pyridyl linkers; however, there are a few reports on structures built from carboxyl and thiourea linkers.20 From the viewpoint of synthetic procedures, it is difficult to predict the architectural complexity of the SCAM structures, as it may be possible to synthesize SCAMs by relatively facile routes such as the one-pot approach in which the organic linker is directly added to the solution of the metal precursor under stirring or the liquid–liquid diffusion method in which the reaction occurs at the interface between the inorganic and organic phases.26,32 In terms of application, SCAMs have shown use for a myriad of applications. The primary ones are the applications associated with luminescence. Intriguingly, many applications utilizing this property revealed that the emission peaks of SCAMs are different from those of linkers.21,33 In addition, there are also reports of SCAMs being used as sensors by harnessing the advantage of their structural stability as well as change in their luminescence behavior upon interaction of the analytes with the framework.21,34 Benefitting from the extended network structures that promote access to active sites and substrate diffusion into the active site as well as cooperative functions of silver clusters and organic linkers, SCAMs have been used for catalytic and bioprotection applications.19,22,26 The molecular-level designability, structural versatility and tunability of emission properties have enabled the emergence of SCAMs as optically active nanomaterials. Therefore, this article aims to provide a systematic overview of the unique structural and functional characteristics of SCAMs and their distinct features as an assembly relative to the building blocks from the perspective of systems chemistry and condensed matter chemistry, the correlation between structure and properties of these materials, and the attributes of individual structural components in the obtained networks and their interactions that add up to the sum total property of the entire framework i.e. how the extended frameworks in the condensed phase can not only inherit the properties from building blocks and local structures but also bring forth its uniqueness in achieving luminescence behavior and catalytic activity. Fig. 1 illustrates the milestones in SCAM research.
2 Structures of SCAMs
The structure of SCAMs is vast, and since the structures are dictated by multiple factors, including the structure of the linker, the number and position of sites that can form coordination bonds, the steric hindrance, and the number of silver atoms that make up the silver nanocluster, even a slight difference in the structure of the constituent units leads to a great diversity of SCAM structures. The structure of SCAMs can be deciphered by single-crystal X-ray diffraction (SC-XRD) analysis. In this section, we introduce the currently reported structures of SCAMs on the basis of the coordination number of the linker and the type of coordination bond.2.1 SCAMs formed by Ag–N bonds
There are many structures of SCAMs with Ag–N bonds. Here we introduce some of them.![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m. Thus, Ag12bpy has a 2D structure that extends into the ab plane (Fig. 5a). Besides, the layers of the SCAM are stacked in the ABAB fashion. The crystal structure of Ag12bpy after 10 hours of exposure to 420 nm light, after adsorption experiments of oxygen, nitrogen, and ethanol gases, and after one year was evaluated using powder X-ray diffraction (PXRD) with no detectable peak changes, implying that the structure is extremely stable.
2m. Thus, Ag12bpy has a 2D structure that extends into the ab plane (Fig. 5a). Besides, the layers of the SCAM are stacked in the ABAB fashion. The crystal structure of Ag12bpy after 10 hours of exposure to 420 nm light, after adsorption experiments of oxygen, nitrogen, and ethanol gases, and after one year was evaluated using powder X-ray diffraction (PXRD) with no detectable peak changes, implying that the structure is extremely stable.
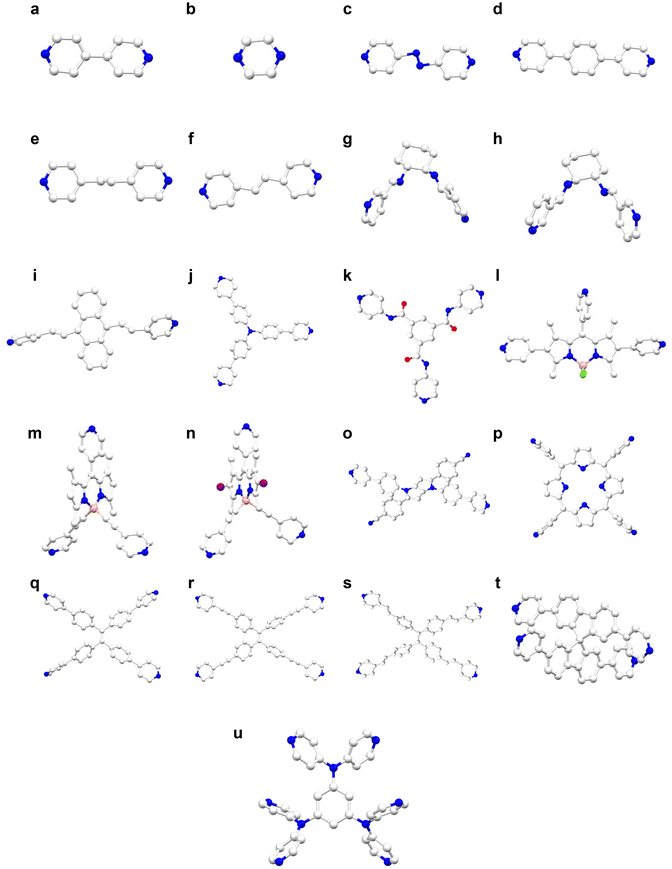 | ||
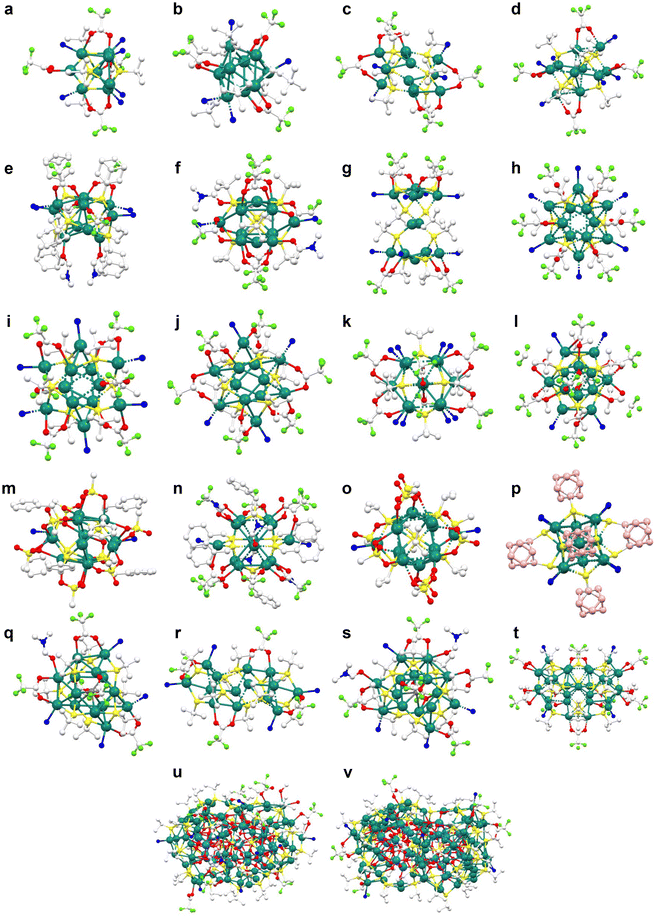
| Fig. 3 (a)–(u) Structural representation of pyridyl linkers used in the construction of SCAMs (white: C, blue: N, red: O, pink: B, light green: F, and purple: I). | ||
Zang et al. reported four new SCAMs, SCAM-1,2,3,4 (SCAM-1 = {[Ag14(C2B10H10S2)6(Pyrazine)6.5(DMAc)(CH3CN)0.5]·2DMAc}n, SCAM-2 = {[Ag14(C2B10H10S2)6(dpd)2(CH3CN)4]·DMAc}n, SCAM-3 = {[Ag14(C2B10H10S2)6(bpy)4]}n, and SCAM-4 = {[Ag14(C2B10H10S2)6 (dpbz)4]}n), in 2018.35 In these syntheses, [Ag2(C2B10H10S2) (CH3CN)2]n was used as a precursor. Focusing on the structure of each SCAM, each reported SCAM node is composed of Ag14(C2B10H10S2)6 that consists of six protecting ligands located on the vertex of the octahedron. In these SCAMs, there are eight possible sites for linker coordination, which correspond to the apex of the cube (Fig. 2p). SCAM-1 comprises six and a half pyrazine (Fig. 3b), one DMAc, and half a CH3CN molecules. However, due to the small size of the pyrazine molecules, they are sterically hindered and cannot form a higher dimensional structure, so only the diagonal pyrazines are coordinated to form a 1D structure that extends along the c-axis. In SCAM-2, four dipyridin-4-yl-diazene (Fig. 3c) and four CH3CN are coordination bonded. Unlike the pyrazine used in SCAM-1, its large molecular size makes it suitable for cross-linking, and all four dipyridin-4-yl-diazene cross-link clusters together. As a result, SCAM-2 forms a 2D structure that extends over the bc plane. Regarding SCAM-3, some of the coordination sites are not solvent molecules as in SCAM-1 and 2, but all eight are composed of bpy (Fig. 3a), which is used for cross-linking. As a result, the secondary building unit (SBU) formed an octahedral structure, while SCAM-3 formed a structure extending to 3D space. SCAM-4 consists of a 1,4-bis(4-pyridyl) benzene linker (Fig. 3d), which has a longer molecular length than that of SCAM-3. Like SCAM-3, the SCAM-4 structure adopts a 3D structure with eight linkers attached to the node. This structure showed high stability owing to interpenetration, which was confirmed to be maintained even after nitrogen adsorption experiments. The structure was also highly stable at elevated temperatures up to ca. 220 °C, as corroborated by variable-temperature PXRD. In terms of crystal systems and space groups, SCAM-1 was trigonal and P32, SCAM-2 was monoclinic and C2/m, SCAM-3 was tetragonal and P4/mnc, and SCAM-4 was tetragonal and P41/a, respectively.
In the same year, Zang et al. reported that Ag12bpy was converted to [(Ag12(StBu)6(CF3COO)6(bpy)3)]n (Ag12bpy-2) by adding Ag12bpy to a mixture of DMAc and toluene, and conversely, Ag12bpy-2 to a mixture of ethanol and CH3CN.36 First, a comparison in the core structure of the clusters showed that the Ag12 core in Ag12bpy formed D2d symmetry (Fig. 2g), while the Ag12 core in Ag12bpy-2 underwent a symmetry change to C3v (Fig. 2h). This change in the core structure was found to have caused a variety of structural changes, including dipole moments, the number of bonds between Ag atoms, and the strength of argentophilic interactions. It was also confirmed that the structure of SCAM changes depending on the type of solvent used in the synthesis. These changes were confirmed by SC-XRD, which showed that the crystal system was tetragonal in Ag12bpy but changed to trigonal in Ag12bpy-2, and that the space group changed from I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m in Ag12bpy to P
2m in Ag12bpy to P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) in Ag12bpy-2.
in Ag12bpy-2.
A novel 1D SCAM [Ag10(StBu)6(CF3CF2COO)4(CH3CN)2(bpy)2]n (denoted as Ag10bpy-CH3CN) (Fig. 2c) was discovered by Zang et al.34 This SCAM showcased an unique feature where the crystal structure changes to [Ag10(StBu)6(CF3CF2COO)4(bpy)2]n (Ag10bpy) after removal of the coordinating CH3CN molecules, and like Ag12bpy described above, reversible transformation between Ag10bpy and Ag10bpy-CH3CN. Both cluster nodes had Ag10S6 as the core and four CF3CF2COO− anions were used as anionic ligands, bridged by two bpy linkers (four bpy in total). The crystal system and space group of the SCAM were identified as triclinic and P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , respectively.
, respectively.
In 2019, Zang et al. reported [Ag12(StBu)6(CF3COO)6(bpa)3(DMAc)12]n (Ag12bpa) and [Ag12(StBu)6(CF3COO)6(bpe)3(DMAc)14.7]n (Ag12bpe) with Ag12 cluster nodes bridged by 1,2-bis(4-pyridyl) ethane (bpa, Fig. 3e) and 1,2-bis(4-pyridyl) ethylene (bpe, Fig. 3f).23 The nodes of these two SCAMs (Fig. 2h) have structural similarity with that of Ag12bpy-2, and both the crystal system and space groups were trigonal and P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) . Immersion of both of them in CH3CN entailed a phenomenal change in both: the structure of 2D Ag12bpa was transformed into a 1D structure, Ag10bpa (Fig. 2c). This change made the crystal system triclinic and the space group P
. Immersion of both of them in CH3CN entailed a phenomenal change in both: the structure of 2D Ag12bpa was transformed into a 1D structure, Ag10bpa (Fig. 2c). This change made the crystal system triclinic and the space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . On the other hand, Ag12bpe was converted to Ag12bpe-1b, accompanied by minor distortion of the Ag12S6 node caused by the contact of Ag12bpe with CH3CN and changes in stacking patterns (Fig. 2i). The crystal system and space group of Ag12bpe-1b also changed to monoclinic and P21/c, respectively. In addition, Ag12bpe-1b could be irreversibly converted to Ag10bpa by immersion in a CH3CN solution of bpa. This study demonstrated that the structural conversion of SCAM depends on the flexibility of the linker used.
. On the other hand, Ag12bpe was converted to Ag12bpe-1b, accompanied by minor distortion of the Ag12S6 node caused by the contact of Ag12bpe with CH3CN and changes in stacking patterns (Fig. 2i). The crystal system and space group of Ag12bpe-1b also changed to monoclinic and P21/c, respectively. In addition, Ag12bpe-1b could be irreversibly converted to Ag10bpa by immersion in a CH3CN solution of bpa. This study demonstrated that the structural conversion of SCAM depends on the flexibility of the linker used.
In the same year, Bakr and co-workers reported [Ag15Cl(StBu)8(CF3COO)5.67(NO3)0.33(bpy)2(DMF)2]·4.3-(DMF)·H2O (hereinafter 1-D NCF) and [Ag14Cl(StBu)8(CF3COO)5(bpy)2(DMF)]·2(DMF) (hereinafter 2-D NCF), which contain Cl anions in the cluster.37 The interesting aspect of this report was that the synthesis can be accomplished by adding bpy to the synthesis scheme of Ag16 nanoclusters using Cl anions as the template. 1-D NCF's Ag15 core structure was Ag15S8Cl, and the structure was asymmetric because one Ag atom is absent from the Ag16 nanocluster. Consequently, the number of Ag atoms bridged by six S atoms is four and the number of Ag atoms bridged by two S atoms is three. Among the six CF3COO− ions present as protective ligands around the core, four CF3COO− ions located equatorially form bonds to the two Ag atoms. The remaining two CF3COO− ions are located in the axial positions, one bridging to three Ag atoms and one bridging to two Ag atoms. Of the CF3COO− ions in the equatorial position, there is one with an occupancy of 0.67, and the remaining 0.33 is occupied by a NO3 anion ligand believed to be appearing from the remaining silver nitrate in AgStBu. Out of 4 bpy linkers linking one Ag cluster with three adjacent Ag clusters, 2 bpy linkers link another two Ag clusters in one dimension and the remaining two bpy ligands form bridged structures with the third cluster (Fig. 2s). The crystal system of 1-D NCF was triclinic, and its space group was P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . Next, looking at the structure of 2-D NCF, the node Ag14 nanocluster has a structure such that two Ag atoms are missing from the Ag16 nanocluster, and the core was Ag14S8Cl. 4 S atoms form coordination bonds with 4 different Ag atoms, and the remaining S atoms form coordination bonds with three Ag atoms. Out of the five CF3COO− ligands protecting the Ag14 core, all of the five CF3COO− ions are coordinated to two Ag atoms, and only the CF3COO− ligand in the axial position showed different types of coordination modes. This is also like 1-D NCF, the Ag14 nanoclusters in 2-D NCF are bridged to three different nanoclusters by four bpy linkers (Fig. 2q). The crystal system and space group of this crystal structure were determined as triclinic and P
. Next, looking at the structure of 2-D NCF, the node Ag14 nanocluster has a structure such that two Ag atoms are missing from the Ag16 nanocluster, and the core was Ag14S8Cl. 4 S atoms form coordination bonds with 4 different Ag atoms, and the remaining S atoms form coordination bonds with three Ag atoms. Out of the five CF3COO− ligands protecting the Ag14 core, all of the five CF3COO− ions are coordinated to two Ag atoms, and only the CF3COO− ligand in the axial position showed different types of coordination modes. This is also like 1-D NCF, the Ag14 nanoclusters in 2-D NCF are bridged to three different nanoclusters by four bpy linkers (Fig. 2q). The crystal system and space group of this crystal structure were determined as triclinic and P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , respectively. Thermogravimetric analysis (TGA) studies revealed that the thermal stability of the SCAMs increased with the complexity of the structures.
, respectively. Thermogravimetric analysis (TGA) studies revealed that the thermal stability of the SCAMs increased with the complexity of the structures.
A study by Zang and colleagues presented the postsynthetic modification strategy of the parent SCAM utilizing the functional group of the linker.38 First, an amine-substituted linker, 1,4-bis(pyrid-4-yl) benzenamine (NH2-bpz), was used to prepare a SCAM named NH2-Ag12bpz. The amine group was then covalently bonded to methacrylic anhydride (MA) and polymerized by a photoinduced free radical reaction using butyl methacrylate (BMA) or triethylene glycol dimethacrylate (TEGDMA) as the acrylate monomer. In the SCAM membranes developed using varying polymerization ratios of BMA and TEGDMA, BMA enhanced the mechanical strength of the membranes while TEGDMA imparted the membranes with flexibility. The resulting structure with both coordination and covalent bonds is rewarding for higher structural complexity as well as synergistic effects.
In 2021, Mandal et al. reported a newly synthesized SCAM, [Ag14(StBu)10(CF3COO)4(4,4′-azopyridine)2] (Ag14CAM), with the Ag14S10 nanocluster core having a distorted polyhedral shape composed of eight square and eight triangular faces, and surrounded by ten StBu and four CF3COO− ligands (Fig. 2r).39 Ag14CAM adopted a 2D structure and had an ABC stacking pattern in the ac plane. Another intriguing feature of this study is that ethanol and acetonitrile were used as solvents in the synthesis of Ag14CAM, but crystals with different shapes were obtained even when ethanol and chloroform were used. However, the latter SCAM was found to have the same crystalline structure as the former (crystal system: monoclinic, space group: C2/c), as evaluated by SC-XRD and PXRD analyses.
In the same year, Dong, Li and co-workers succeeded in synthesizing a novel SCAM by bringing further innovations to the linker.40 Cucurbit[6]urils-N,N‘-hexamethylene-bis(pyrazinylhexafluorophosphate) (BPHF@CB[6]), which was used as the linker of the reported SCAM, is a complex composed of CB and BPHF through host–guest interactions. The incorporation of CB into linear BPHF is known to exhibit unprecedented structural and chemical properties. The use of BPHF@CB[6] as a bidentate linker has led to the formation of three new SCAMs: [Ag12(SC7H7)6(CH3SO3)8(BPHF@CB[6])]n, [Ag12(SC7H7)6(CF3COO)8(DMF)3(CH3OH)(BPHF@CB[6])]n, and [Ag12(iPrS)6(CH3SO3)8(BPHF@CB[6])]n, hereinafter denoted as SCM 1, SCM 2, and SCM 3, respectively. The silver cluster core of SCM 1 is protected by a CH3SO3 anion ligand and SC7H7, and BPHF@CB[6] formed a 1D wave-like structure by forming Ag–N coordination bonds at two of the six Ag atoms in the asymmetric position of the intermediate layer (Fig. 2m). The crystal system of SCM 1 was ascertained as monoclinic, and the space group as P2/c. The CF3COO− ligands protecting the core of SCM 2 have two kinds of protecting patterns: two CF3COO− protect one Ag atom and six of them protect two Ag atoms. BPHF@CB[6] generated a linear 1D SCM 2 by forming two Ag–N coordination bonds with respect to the symmetric position of the intermediate layer in the cluster node (Fig. 2n). The crystal system of SCM 2 was characterized as monoclinic, as in SCM 1, but the space group was P21/n. Similar to SCM 1 and 2, SCM 3 also featured a 1D structure (Fig. 5b), with a BPHF@CB[6] linker bridging the clusters, each of the six iPrS− ligands protecting four Ag atoms and eight CH3SO3− ligands protecting the cluster node (Fig. 2o). Unlike SCM 1 and SCM 2, the crystal system of SCM 3 was identified as triclinic and the space group was P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) .
.
A work introducing a new 1D SCAM [Ag11(AdmS)6(CF3COO)4(apy)2(DMF)2][NO3] (Ag11CAM, AdmSH = adamantanethiol, apy = 4,4′-azopyridine) using the Ag11 cluster node was reported by Mandal et al.41 The cluster core was composed of Ag11S6, surrounded by six AdmS− and four CF3COO− ligands. This is the first reported SCAM in which AdmS was utilized as a protective ligand. The clusters were bridged by two apy linkers, and there were four Ag–N coordination bonds per cluster (Fig. 2e). The Ag11CAM stacked in the ABA pattern. NO3− ions were found to be accommodated as counter anions in the lattice spacing.
Another paper by Mandal et al. reported 1D [Ag2(PhPO3H)2(apy)2] (apy = 4,4′-azopyridine) (hereinafter referred to as Ag2apy), which did not use thiols as stabilizing ligands.42 The two PhPO3H− ligands form coordination bonds opposite to each other with both Ag atoms constituting the cluster nodes. The two apy linkers connecting the clusters are aligned parallel to each other at an average distance of 3.74 Å. The crystal system of the Ag2apy SCAM was triclinic and the space group was P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) .
.
In 2021, Teo, Zheng and colleagues used a chiral Ag14 nanocluster as the node and two chiral bidentate linkers, (1R,2R,N1E,N2E)-N1,N2-bis(pyridine-3-ylmethylene)-cyclohexane-1,2-diamine (hereinafter LR) (Fig. 3g) and (1S,2S, N1E,N2E)-N1,N2-bis(pyridine-3-ylmethylene)-cyclohexane-1,2-diamine (hereafter LS) (Fig. 3h) to construct [Ag14(SPh(CF3)2)12(PPh3)4(LR)2]n (denoted as Ag14-LR) and [Ag14(SPh(CF3)2)12(PPh3)4(LS)2]n (denoted as Ag14-LS) enantiomeric SCAMs (Fig. 4a and b).43 Ag14-LR/LS adopted a 3D structure constructed by the replacement of four DMF molecules (originally coordinated to the nanoclusters) by linkers. Both crystal systems were hexagonal, and the space groups were P6122 for Ag14-LR and P6522 for Ag14-LS. The thermal durability of both SCAMs up to ca. 150 °C was validated by PXRD studies. It was also confirmed that the Ag14 nanoclusters building Ag14-LR/LS were homochiral. In other words, the racemic Ag14 clusters were transformed into homochiral building units that led to the SCAMs. Moving further, the racemic form of the linker LR/LS (LRS) was used. The SCAM crystal structure obtained by using this racemic form contained R-Ag14 and S-Ag14 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Unlike Ag14-LR/LS, the crystal system of Ag14-LRS was orthorhombic and the space group was Pnnn.
1 ratio. Unlike Ag14-LR/LS, the crystal system of Ag14-LRS was orthorhombic and the space group was Pnnn.
In 2022, Sun and co-workers presented {[(PW9O34)2@Ag72S(iPrS)41(CF3COO)8(bipy)5.5(CH3OH)(H2O)]·3CF3COO}n (hereinafter referred to as SD/Ag72a) (bipy: 4,4′-bipyridine) and {[(PW9O34)2@Ag72S(iPrS)42(CF3COO)7(pi-bipy)4.5(CH3OH)]·3CF3COO}n (hereinafter referred to as SD/Ag72c) (pi-bipy: 1,4-bis(4-pyridinylmethyl)piperazine) obtained from the bipy/pi-bipy linker and [PW9O34@Ag51] clusters.44 SD/Ag72a exhibited a 2D structure, while SD/Ag72c showed a 3D structure. Their respective space groups are P21/n and P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , respectively. Each node of SD/Ag72a forms a bond with the other four nodes via a bipy linker, forming a network structure with a total of 11 bipy linkers (Fig. 2u). On the other hand, each node of SD/Ag72c forms a structure via a pi-bipy linker with six different nodes, with four clusters bridged by one pi-bipy, one cluster bridged by two pi-bipy, and one cluster bridged by three pi-bipy linkers (Fig. 2v).
, respectively. Each node of SD/Ag72a forms a bond with the other four nodes via a bipy linker, forming a network structure with a total of 11 bipy linkers (Fig. 2u). On the other hand, each node of SD/Ag72c forms a structure via a pi-bipy linker with six different nodes, with four clusters bridged by one pi-bipy, one cluster bridged by two pi-bipy, and one cluster bridged by three pi-bipy linkers (Fig. 2v).
In 2023, Das, Negishi and colleagues reported the synthesis of a 2D SCAM, [Ag12(StBu)6(CF3COO)6(bpva)3]n (bpva = 9,10-bis((2-pyridin-4-yl)vinyl)anthracene) (Fig. 3i) (hereinafter Ag12bpva), and an 1D SCAM, {[Ag 12(StBu)6(CF3COO)6(bpb)(DMAc)2(H2O)2](DMAc)2}n (bpb = 1,4-bis(4-pyridyl)benzene) (Fig. 3d) (hereinafter Ag12bpb).21 Each node is an Ag12 cluster and the core is Ag12S6, with S bridged to four different Ag atoms. The Ag12 nanoclusters in Ag12bpva form a three-layered Ag3S3–Ag6–Ag3S3 core, with the six Ag atoms in the middle layer acting as a six-linked node (Fig. 2h). The crystal system of this SCAM was monoclinic, and the space group was P21/n. On the other hand, the core of Ag12bpb has a three-layer structure of Ag5S2–Ag2S2–Ag5S2 and is protected by two DMAc molecules and two water molecules, in addition to six CF3COO− ligands (Fig. 2f). The crystal system and space group of Ag12bpb were the same as Ag12bpva, monoclinic and P21/n. Both SCAMs were immersed in a variety of organic solvents followed by PXRD analyses, which corroborated the preservation of the crystalline structures after solvent treatment.
In the same year, Zang et al. reported a chiral SCAM, {[(Ag12(iSPr)6(D/L-CSA)6(An2Py)3)]·(H2O)2}n (CSA = camphor sulfonic acid) (hereinafter D-Ag12An2Py, L-Ag12An2Py),45 by utilizing the bpva linker used by Das and Negishi et al. The node is composed of Ag12 nanoclusters, which form six coordination bonds and a net structure with 4 different nodes along the 2D space (Fig. 4c and d). The 2D SCAMs displayed the AB stacking pattern. The space group of the SCAMs was P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and the topology was characterized as sql.
and the topology was characterized as sql.
A paper from Zang et al. reported the synthesis of [(CO3)@Ag20(tBuS)10(R/S-THF-COO)8bpy2] (hereinafter R/S-Ag20bpy).46 The node of this SCAM is composed of Ag20 nanoclusters, with CO3 derived from atmospheric CO2 incorporated into the structure as an anion template. The cluster core is protected by ten tBuS− ligands, each forming a bond with four different Ag atoms. The core is also protected by eight R/S-THF-COO− ligands. Each node forms four coordination bonds (Fig. 4e and f). The authors also reported that a similar structure could be obtained by using bpy-NH2 instead of bpy.
Thus, although only a short time has passed since the discovery of SCAMs, a variety of SCAMs with different structures have been reported. SCAMs using bidentate linkers revealed new structures upon changing the structure of the linker, structural transformation accomplished under specific conditions, and the construction of hybrid structures using SCAMs.
In 2018, Wang, Zang and colleagues discovered [Ag12(StBu)6(CF3COO)6(TPPA)6-(DMAc)x]n (denoted as Ag12TPPA·AA), a 2D SCAM, by using the tridentate linker tris(4-pyridylphenyl)amine (TPPA) (Fig. 2h and 3j).47 This 2D SCAM is stacked in the AA fashion. However, there was a change in the PXRD peaks observed between the crystals in the mother solvent and after drying in air for some time. Intriguingly, this change was believed to be caused by the displacement of SBUs and the collapse of the AA stacking mode due to the desorption of DMAc from the structure upon drying in air. This was confirmed by SC-XRD measurements on the crystals after drying, which revealed that structural shrinkage occurred, resulting in a change in the AB stacking mode. More interestingly, after 6 hours of growth in the other solvent, this structure changed to an ABC stacking structure. Structural analysis of these crystals revealed that changes in the stacking mode caused changes in the crystal system and space group: the crystal system of Ag12TPPA·AA was trigonal and the space group was P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , for Ag12TPPA·AB they were hexagonal and P63/mmc, and for Ag12TPPA·ABC they were trigonal and R
, for Ag12TPPA·AB they were hexagonal and P63/mmc, and for Ag12TPPA·ABC they were trigonal and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. The distance between the layers became gradually shorter with each change to the AA, AB, or ABC stacking mode.
m. The distance between the layers became gradually shorter with each change to the AA, AB, or ABC stacking mode.
In 2023, Das and Negishi et al. reported [Ag12(StBu)6(CF3COO)6(TPBTC)6]n (TUS 3) using the tridentate ligand, benzene-1,3,5-tricarboxylic acid tris-pyridin-4-ylamde (TPBTC) (Fig. 3k).19 TUS-3 has linkers that form three coordination bonds, while the Ag12 nanoclusters form six bonds (Fig. 2h). The kgd topology was reported for the first time in SCAMs. SC-XRD analysis illustrated that the crystal system of TUS 3 was trigonal, and the space group was R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) . Uniaxial open channels arising from stacked 2D layers enabled an impressive Brunauer–Emmett–Teller (BET) surface area of 394 m2 g−1. PXRD analysis showed that the structure of TUS 3 did not change when TUS 3 was exposed to dichloromethane, ethanol, 0.1 M NaOH, and 0.1 M HCl, suggestive of its structural stability.
. Uniaxial open channels arising from stacked 2D layers enabled an impressive Brunauer–Emmett–Teller (BET) surface area of 394 m2 g−1. PXRD analysis showed that the structure of TUS 3 did not change when TUS 3 was exposed to dichloromethane, ethanol, 0.1 M NaOH, and 0.1 M HCl, suggestive of its structural stability.
In the same year, Wang, Zhang and colleagues reported [Ag12(StBu)6(CF3COO)6(BDP-Py)2]n, [Ag12(SiPr)6(CF3COO)6(BDP-H)2]n, and [Ag12(SiPr)6(CF3COO)6(BDP-I)2]n (hereinafter Ag12-BDP-Py, Ag12-BDP-H, and Ag12BDP-I).48 The cluster node in each SCAM is composed of 12 Ag atoms, and each node forms coordination bonds with six different linkers. Each Ag12 nanocluster is stabilized by six StBu− or SiPr− and six CF3COO− ligands. The net structures of Ag12-BDP-Py (Fig. 3l), Ag12BDP-H (Fig. 3m) and Ag12BDP-I (Fig. 3n) are all forming 2D structures and all of their space groups are P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . From the viewpoint of topologies, that of Ag12-BDP-H and Ag12BDP-I was kgd and the stacking mode was AB stacking. Besides, the thermal stability of Ag12-BDP-H and Ag12BDP-I until 163 °C and 175 °C were assessed by TGA measurements.
. From the viewpoint of topologies, that of Ag12-BDP-H and Ag12BDP-I was kgd and the stacking mode was AB stacking. Besides, the thermal stability of Ag12-BDP-H and Ag12BDP-I until 163 °C and 175 °C were assessed by TGA measurements.
Thus, there are considerably fewer reported cases of SCAMs using a tridentate linker compared to the bidentate linker and the tetradentate linker. Therefore, SCAMs using tridentate linkers are novel and have great potential to become a hotspot of SCAMs in the future.
In 2019, Wang, Zang and colleagues successfully synthesized [Ag12(StBu)6(CF3COO)3(TPyP)]n (Ag12TPyP, TPyP = 5,10,15,20-tetra(4-pyridyl)porphyrin, Fig. 3p).22 The Ag12S6 cluster core of Ag12TPyP showed a structure forming only four coordination bonds (Fig. 2j), unlike the usual core forming six coordination bonds. Ag12TPyP formed a 2D structure by coordination bonding of the linker to four different cluster nodes and showed an AB stacking pattern. The crystal system of Ag12TPyP was triclinic and the space group was P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The crystal structure of Ag12TPyP after immersion in many organic solvents for 36 hours or left in the air for 3 months did not collapse, indicative of the high stability of the structure.
. The crystal structure of Ag12TPyP after immersion in many organic solvents for 36 hours or left in the air for 3 months did not collapse, indicative of the high stability of the structure.
In the same year, the synthesis of {[Ag12(StBu)6(CF3CO2)6(tppe)1.5](DMAc)39}n (tppe = 1,1,2,2-tetrakis(4-(pyridine-4-yl)phenyl)ethane, Fig. 3q) (hereinafter named Ag12tppe ⊃ DMAc) was reported by Li, Zang and colleagues.24 This structure consists of a node with an Ag12S6 core and a tetradentate linker TPPE, but what is remarkable is the large pore size (32 Å). 1H NMR spectra obtained after the incorporation of D-menthol, L-menthol, 4,4-dimethoxybenzil, and 3,6-di(2-thienyl)diketopyrrolopyrrole as guest molecules showed that the guest molecules were encapsulated in the SCAM structure; however, PXRD observations indicated no structural change with the introduction of any of the guest molecules. The structural analysis by SC-XRD showed that the crystal system of Ag12tppe ⊃ DMAc was cubic and the space group was Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m.
m.
In the same year, Zang, Tang and colleagues reported the successful synthesis of an innovative SCAM. The SCAM stands out owing to the existence of two kinds of Ag nanocluster nodes.50 The chemical formula of this SCAM is {[Ag12(StBu)6(CF3CO2)6]0.5[Ag8(StBu)4(CF3CO2)4](tppe)2(DMAc)10}n (hereinafter Ag12Ag8tppe). The number of DMAc molecules in the SCAM structure was determined by TGA, elemental analysis, and 1H NMR spectroscopy. Ag8 nanocluster nodes are forming six coordination bonds (Fig. 2a). On the other hand, the Ag12 cluster is a three-layered node of Ag3S3–Ag6–Ag3S3, with six StBu− and six CF3COO− as ligands, forming four coordination bonds with tppe, as evidenced by structural analysis (Fig. 2l). The tppe bridging clusters formed a 3D structure by coordinating with three Ag8 clusters and one Ag12 cluster per linker molecule; the type of geometric structure of Ag12Ag8tppe was {42·84}{43·62·8}4{49·66}2. Different from Ag12tppe, Ag12Ag8tppe displayed the crystal system and space group as triclinic and P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , respectively.
, respectively.
In 2020, Cui, Sun and co-workers successfully synthesized {[Ag27S2(tBuS)14(CF3COO)8(TPyP-H2)](CF3COO)}n (Ag27-MOF) using a TPyP linker,25 which Zang et al. used to synthesize another SCAM.22 This SCAM has an Ag27 cluster core protected by 14 StBu− and 8 CF3COO− ligands (Fig. 2t). To reduce the steric hindrance of TPyP, only four of the 27 silver atoms form coordination bonds with the linker, and the structure is expanded along 2D space. PXRD measurements were also performed to determine whether the crystal structure is affected by aging (4 months) and solvent immersion, and it was confirmed that none of the peaks changed. Structural analysis showed that the crystal system of the Ag27-MOF was orthorhombic, and the space group was Cmcm.
In 2022, Wang, Zang and colleagues reported [Ag9(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)6(CF3COO)3(AgTPyP)]n, (hereinafter Ag9-AgTPyP).26 This structure was found to be 2D composed of Ag9 clusters (Fig. 2b) as a node forming four coordination bonds and having an AB stacking structure (Fig. 5c). Structural analysis revealed that this node has six tBuC
C)6(CF3COO)3(AgTPyP)]n, (hereinafter Ag9-AgTPyP).26 This structure was found to be 2D composed of Ag9 clusters (Fig. 2b) as a node forming four coordination bonds and having an AB stacking structure (Fig. 5c). Structural analysis revealed that this node has six tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C ligands in two types of bonding modes, σ-type and π-type, and three CF3COO− ligands are coordinated to two Ag atoms. On the other hand, focusing on the tetradentate linker TPyP, a very interesting structural change occurred. The Ag atom forms a complex with TPyP at the synthesis stage. The crystal system of this SCAM was monoclinic, and the space group was C2/c. PXRD measurements of Ag9-AgTPyP after immersion in water for 24 hours and in air for 5 months showed no detectable change in its peaks.
C ligands in two types of bonding modes, σ-type and π-type, and three CF3COO− ligands are coordinated to two Ag atoms. On the other hand, focusing on the tetradentate linker TPyP, a very interesting structural change occurred. The Ag atom forms a complex with TPyP at the synthesis stage. The crystal system of this SCAM was monoclinic, and the space group was C2/c. PXRD measurements of Ag9-AgTPyP after immersion in water for 24 hours and in air for 5 months showed no detectable change in its peaks.
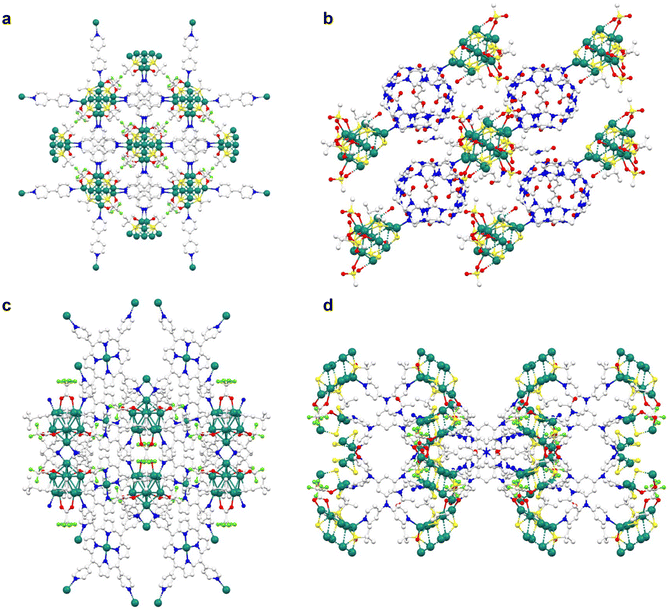 | ||
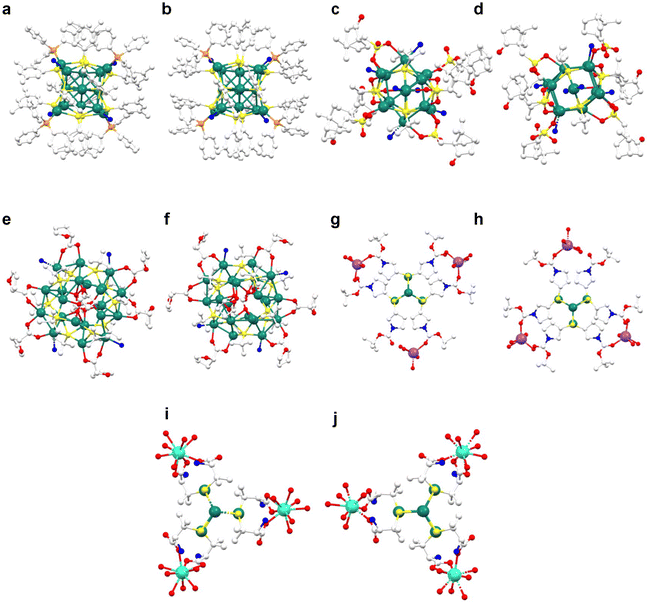
| Fig. 5 Extended structure of SCAMs; (a) Ag12bpy (2D), (b) SCM 3 (1D), (c) Ag9-AgTPyP (2D), and (d) Ag10hpbt (3D) (white: C, blue: N, red: O, light green: F, green: Ag, and yellow: S). | ||
In 2023, Das and Negishi et al. used the linkers 1,1,2,2-tetrakis(4-(pyridin-4-ylethynyl)phenyl)ethane (TPEPE, Fig. 3r) and 1,1,2,2-tetrakis(4-((E)-2-(pyridin-4-yl)vinyl)phenyl)ethane (TPVPE, Fig. 3s) to prepare [Ag12(StBu)6(CF3COO)6(TPEPE)6]n (TUS 1) and [Ag12(StBu)6(CF3COO)6(TPVPE)6]n (TUS 2).33 Both SCAMs revealed 3D structures, and both form Ag12 nodes of the same shape, allowing for the formation of six coordination bonds with the linkers (Fig. 2h). SCXRD structural analysis showed that the crystal system of both TUS 1 and TUS 2 was trigonal and the space group was R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c. To confirm the structural stability against solvents, TUS 1 and TUS 2 were placed in toluene, dioxane, chloroform, acetone, and water followed by PXRD studies which displayed no obvious change in the peaks.
c. To confirm the structural stability against solvents, TUS 1 and TUS 2 were placed in toluene, dioxane, chloroform, acetone, and water followed by PXRD studies which displayed no obvious change in the peaks.
In 2024, the same research group presented the synthesis of 3D [Ag12(StBu)6(CF3COO)6(TPSBF)6]n (designated as TUS 4) (TPSBF = 2,2′,7,7′-tetra(pyridine-4-yl)-9,9′-spirobi(fluorene)) (Fig. 3t).32 The Ag12 cluster core has a three-layer structure of Ag3S3–Ag6–Ag3S3, with the six Ag atoms in the middle layer, cubic and the space group was Pn![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n. The SCAM structure was found to collapse after grinding. This was determined by changes in PXRD before and after grinding. Solid-state UV-vis absorption spectra, solid-state emission spectra and Raman spectra of the ground SCAM sample were comparable to the respective spectra of the linker. In addition, it was necessary to add NaBH4 to TUS-4 in order to use it for surface-enhanced Raman scattering (SERS) application, but the PXRD peaks did not change significantly before and after the addition of NaBH4, indicating that the crystal structure was not changed by the addition of the NaBH4 reducing agent.
n. The SCAM structure was found to collapse after grinding. This was determined by changes in PXRD before and after grinding. Solid-state UV-vis absorption spectra, solid-state emission spectra and Raman spectra of the ground SCAM sample were comparable to the respective spectra of the linker. In addition, it was necessary to add NaBH4 to TUS-4 in order to use it for surface-enhanced Raman scattering (SERS) application, but the PXRD peaks did not change significantly before and after the addition of NaBH4, indicating that the crystal structure was not changed by the addition of the NaBH4 reducing agent.
As described above, it was confirmed that SCAMs using tetradentate linkers are no less than SCAMs using bidentate linkers. The structure of SCAMs with tetradentate linkers can be 2D or 3D, making them more complex than with bidentate linkers.
Currently, only bidentate, tridentate, tetradentate, and hexadentate linkers have been reported in SCAMs, and there is only one reported case of a SCAM constructed from a hexadentate linker. Therefore, the SCAM formed by using a hexadentate linker features high novelty. In addition, from the viewpoint of linker symmetry, there is a good possibility that SCAMs can be synthesized using linkers with even higher denticities.
2.2 SCAMs formed by Ag–O bonds
The number of reported examples of SCAMs with Ag–O bonds is small compared to SCAMs constructed with Ag–N bonds. Despite that some SCAMs synthesized by using pyridyl linkers show good stability, a lack of generalizability exists.20 On this account, using carboxyl groups can augment the stability of the SCAMs than when using a pyridyl linker.20 Here we describe the reported SCAM structures constructed from Ag–O bonds.In 2021, Morsali et al. reported the successful synthesis of {Ag14[O2C-(CF2)4CO2]4(SPri)6(DMF)6}n (SSc-2).52 This structure was found to consist of Ag14 cluster nodes bridged via octafluoroadipic acid linkers. SC-XRD analysis revealed that the cluster core was composed of 14 Ag atoms and six S atoms of the SPri− ligands. The SCAM adopted the orthorhombic crystal system and the space group Pccn.
In another study, the same research group reported the synthesis of SCAM with the formula [Ag14(SPri)6(C9F14O4)4(DMF)8]n (SPc).53 Like SSc-2, its core is composed of 14 Ag atoms and six S atoms of the SPri− ligands, which are protected by eight DMF molecules. The 2D SCAM sheets extend in the ac-plane, with no interpenetration between the sheets and the distance between the 2D sheets as 1.02 nm. The crystal system of SPc was triclinic, and the space group was P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) .
.
In 2023, [Ag12(SiPr)6(D/L-CSA)6(MeOH)4]n was reported by Duan, Zang and colleagues.45 This SCAM structure is spread over one dimension. Each SiPr− ligand is bonded to four different Ag atoms, and each CSA− forms bonds with two or three silver atoms. Structural analysis revealed that each cluster node is bridged by two CSA− and the number of CSA− that connected with each node is four.
As shown above, the number of SCAMs using bidentate carboxyl linkers is far fewer than those using pyridine linkers. Besides, the reports are relatively recent. Therefore, SCAMs synthesized by using bidentate carboxyl linkers are of great novelty, and further development is expected in the future.
In the same year, Wang, Zang and co-workers reported the synthesis of [(HPO4)4(H2PO4)2@Ag54(iPrS)32(CF3COO)6(CH3CN)2(H2TCPP)(TCPP)]n, (hereinafter ZZU-601; H2TCPP = tetrakis(4-carboxyphenyl)porphyrin) and {[(HPO4)2@Ag18(iPrS)8(H2PO4)4(H2ZnTCPP(C2H5OH))]·2CH3CN}n, (hereinafter ZZU-602; ZnTCPP = 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrinato-Zn(II)).55 ZZU-601 had four [HPO4]2− ions and two [H2PO4]− ions acting as anion templates for 36 silver atoms, and the Ag54 cluster node was protected by 32 iPrS−. Although there was a total of eight TCPP linkers bridging the Ag54 nanoclusters, the coordination of four TCPP linkers in two planes resulted in a 2D planar extension, finally forming a 3D structure for ZZU-601 as a whole. On the other hand, in ZZU-602, two [HPO4]2− acted as anion templates and the core was protected by 10 iPrS− ions and four [H2PO4]− ions. Overall, this SCAM structure exhibited 2D extension after being connected by ZnTCPP linkers. The crystal system of both ZZU-601 and ZZU-602 was triclinic, and the space group was P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) .
.
Thus, there are only a few reported examples of the synthesis of SCAMs utilizing carboxylic acids as tetradentate linkers in the last one or two years. Therefore, it can be said that SCAMs using tetradentate linkers that make Ag–O bonds have a short history and are still a developing field.
2.3 SCAMs formed by Ag–S bonds
Attempts have been made to synthesize SCAMs by using S as an electron-donating group like N, but the HSAB rule theoretically makes it impossible to synthesize SCAMs with Ag–S bonds due to the problem that precipitation occurs too rapidly.20However, in 2018, Duan et al. created a stir against this status quo. They were the first to report an Ag–S-bonded SCAM by using the tridentate thiourea linker 2,2′,2′′-((((1,3,5-triazine-2,4,6-triyl)tris(azanedidiyl))tris(benzene-4,1-diyl))tris(ethan-1-yl-1-ylidene))tris(hydrazinecarbothioamide) to form TNS-Ag8 SCAM and 2,2′,2′′-((((1,3,5-triazine-2,4,6-triyl)tris(oxy))tris(benzene-4,1-diyl))tris(methanylidene))tris(hydrazine carbothioamide) to form the TOS-Ag4 SCAM.56 The Ag atoms in TNS-Ag8 are bonded to three different S atoms and are located at the center of a plane consisting of three S atoms. The clusters are also bridged by forming Ag–S bonds, but the S atoms are bridged to two Ag atoms. The core of the TNS-Ag8 cluster is Ag8S12, which means that 12 ligands are coordinated to the eight Ag atoms. Two linkers are used to connect three different clusters. In other words, the clusters are nodes that form coordination bonds with six pairs of linkers. This SCAM has a 3D structure, and the clusters can be regarded as a six-linked node. Besides, there are two types of pores due to this particular structure. Structural analysis revealed that the crystal system of TNS-Ag8 is cubic, and the space group is Pa![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) . On the other hand, in TOS-Ag4, tetrahedral clusters form coordination bonds as 3-linked nodes, forming a doubly interpenetrated 3D structure. The core of this cluster is Ag4S6, and the Ag atoms form coordination bonds with the six S atoms. Each S atom is located on an edge of the tetrahedron and is bridged to two Ag atoms. The number of linkers bridging the clusters is six per cluster. This means that two linkers form a structure bridging three clusters, which is also the same as in TNS-Ag8. The resulting SCAM structure is 3D, and the silver nanoclusters can be said to behave as a three-linked node. As a result, TNS-Ag4 also has two types of pores. Structural analysis showed that the crystal system of TOS-Ag4 is cubic, as in TNS-Ag8, and the space group is P213.
. On the other hand, in TOS-Ag4, tetrahedral clusters form coordination bonds as 3-linked nodes, forming a doubly interpenetrated 3D structure. The core of this cluster is Ag4S6, and the Ag atoms form coordination bonds with the six S atoms. Each S atom is located on an edge of the tetrahedron and is bridged to two Ag atoms. The number of linkers bridging the clusters is six per cluster. This means that two linkers form a structure bridging three clusters, which is also the same as in TNS-Ag8. The resulting SCAM structure is 3D, and the silver nanoclusters can be said to behave as a three-linked node. As a result, TNS-Ag4 also has two types of pores. Structural analysis showed that the crystal system of TOS-Ag4 is cubic, as in TNS-Ag8, and the space group is P213.
In 2022, Si, Zang and co-workers succeeded in synthesizing a SCAM with Ag–S bonds by developing a new approach. The reported SCAM has the structure [NaAg5(R/S-L)6(H2O)2(CH3OH)]Na[Ag5(R/S-L)6] (R-Ag5-1, S-Ag5-1) (R-L,S-L = R/S-tert-butyl-3-mercaptopyrrolidine-1-carboxylate), which is a 2D structure of Ag5 nanoclusters cross-linked by forming coordination bonds with Na ions (Fig. 4g and h).57 The 2D structures were stacked in the AA fashion to form channels along the c-axis. Looking at the nanoclusters composing the node, its core structure was Ag5S6, with a triangular prism formed by six S atoms. Each S atom formed a coordination bond with two different Ag atoms, and the five Ag atoms were located at the center of the top and bottom faces or at the center of the sides of the triangular prism composed of six S atoms, thus forming a twisted triangular biconical structure. On the other hand, the Na ions forming the bridging structure formed coordination bonds with the two carboxyl groups of the ligands of the two Ag5 nanoclusters, two molecules of water, and one molecule of methanol, for a total of five molecules. There are six ligands with carboxyl groups around the cluster that can coordinate with sodium, and three of them form coordination bonds with sodium: two from the ligands on the same base of the triangular prism and one from the ligand on the other base. The remaining three carboxyl groups that are not used for coordination bonds with sodium may form hydrogen bonds with water molecules. Interestingly, in addition to the Ag5 nanoclusters that make up a SCAM, there are also Ag5 nanoclusters in the channels, which are stabilized by forming hydrogen bonds with the backbone of the structure. The crystal system of both was trigonal and the space group was P3. The PXRD peaks of this SCAM showed that the structure changed to Na[Ag5(R/S-L)6] by desorption of the water molecules and methanol molecules that form the backbone upon drying.
In 2023, taking a similar approach to Zang et al., Cao, Zheng and colleagues synthesized a new SCAM with an Ag–S bond. They used lanthanides, instead of sodium, to obtain {[LnAg5(L-HL)6]·(ClO4)2·xH2O}n (L-/D-HL = L-/D-penicillamine) (L-LnAg5-1: Ln = Gd, x = 15, L-LnAg5-2: Ln = Eu, x = 15, L-LnAg5-3: Ln = Tb, x = 11) and {[LnAg5(D-HL)6]·(ClO4)2·yH2O}n (D-LnAg5-1: Ln = Gd, y = 12, D-LnAg5-2: Ln = Eu, y = 24, D-LnAg5-3: Ln = Tb, y = 12) (Fig. 4i and j).58 The SCAMs have a chiral Ag5 cluster with C3 symmetry bridged by three different lanthanides as inorganic ligands. The core structure of the cluster is Ag5S6, in which the five Ag atoms are arranged to form a shape like two triangular pyramids combined, and the six linkers with S bind two different Ag atoms. The Gd in L-LnAg5-1 is coordinated by nine O atoms, which are derived from the carboxyl groups possessed by the six L-HL− ligands. Next, L-LnAg5-1 was immersed in a wide variety of organic solvents, and PXRD showed that no structural changes occurred. The crystal system and space group of all L-LnAg5-1, L-LnAg5-2, L-LnAg5-3, D-LnAg5-1, D-LnAg5-2, and D-LnAg5-3 are cubic and P213.
As described above, SCAMs with Ag–S bonds can be divided into two main types: one is bridged by thiourea linkers, which was the first SCAM to be bridged by Ag–S bonds. The other is a thiol-protected ligand that acts as an inorganic linker by using a different metal to expand the structure of the ligand. Both SCAMs have very interesting structures, and the latter is expected to be diverse depending on the type of thiol ligand used and the metal species used to act as the inorganic linker.
2.4 SCAMs formed by special types of coordination bonds
The SCAMs shown so far were discussed separately in terms of the type of bonding. Here we discuss SCAMs that have additional intricacy by combining two different types of bonding.In 2019, Liu, Chen and colleagues reported [Ag7(tza)3(Htza)2(H2tza)(H2O)] (hereafter Ag7tza(H2O)), a new SCAM with unprecedented complexity by using 1H-tetrazole-5-acetic acid (H2tza).59 Silver atoms belonging to the Ag7 cluster, which corresponds to the node of this SCAM, have three different states: the first is silver atoms coordinating with N atoms having four different linkers. There are two atoms of this type. The second is a silver atom coordinating with the N atoms of three different linkers and there are two silver atoms that have this arrangement; the third is a silver atom in coordination bonds with both N and O atoms and the remaining three are silver atoms that fall into this category. This silver atom forms coordination bonds with three N atoms and one O atom, and the silver atom is located in the distorted triangular pyramid. In contrast, the next step is to look at the bonding mode of H2tza. It was found that the coordination structure of the linker, H2tza, also has three different states. There are three types of coordination structures: a structure in which four N atoms in the tetrazole ring bond to four different silver atoms, a structure in which three out of four N atoms of the tetrazole ring bond to three different silver atoms, and a structure that connected with five different Ag atoms bonding with a carboxyl group in addition to the tetrazole ring. The coordination structures of the silver atoms and linkers in these various states create a unique 3D structure. This crystal system was triclinic, and the space group was P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) .
.
3 Applications of SCAMs
3.1 In sensing
In 2017, Zang and co-workers reported a Ag12bpy SCAM with unique turn-off fluorescence switching in response to oxygen and turn-on vapochromic switching in response to volatile organic compounds.18 On the basis of the Stern–Volmer plot, the SCAM exhibited a limit of detection (LOD) of 32 Pa for oxygen at 1% quenching. Moreover, Ag12bpy treated with ethanol vapor showed a significant increase in photoluminescence intensity by 26 times compared to air (off state). In 2018, the same research group presented Ag10bpy as a solvatochromic sensor for the detection of chloromethanes.60 Each chloromethane showed a different fluorescence response, thereby enabling the SCAM to distinguish the chemicals. This turn-on luminescence switching was caused by the gate-opening effect. Hence, Ag10bpy with solvents manifested different chromic variations within 83–293 K. In 2019, they reported Ag18bpy-NH2 which showed different photoluminescence behavior after grinding, and the blue emission of the pristine sample was recovered again after fuming with EtOH and acetone.61 Pristine Ag18bpy-NH2 demonstrated an emission spectrum centered at 458 nm, and the emission peak was shifted to 491 nm post-grinding. This phenomenon was assumed to stem from inter-chain bpy-NH2 interaction. As substantiated by PXRD analysis, the crystallinity of the SCAM could be recovered again after treatment with EtOH/acetone. In another study, reversibly photoswitchable Ag10bpy was utilized for the sensing of acetonitrile (CH3CN).34 Ag10bpy soaked with CH3CN showed green emission; however, the intensity of the emission declined gradually up to quenching after removing the CH3CN which could be recovered instantaneously upon treating the sample with CH3CN. The cycling tests for its switchable turn-on/off luminescence divulged that the SCAM could undergo reversible structural change for at least 20 cycles. In 2020, the Ag12bpy-NH2 SCAM was adopted for oxygen sensing.62 Owing to the dual-emitting (fluorescence and phosphorescence) behavior of Ag12bpy-NH2, the ratio of fluorescence to phosphorescence (that depends on oxygen concentrations) was checked at different oxygen levels. As a result, a detection limit of 11.4 mPa was attained (this LOD was only 3.56 × 10−6% of Ag12bpy). In 2022, Pradeep et al. utilized the AgNC-MOF for the fluorescence sensing of nitroaromatics.63 The AgNC-MOF was soaked in some solvents, and the photoluminescence results showed that the intensity dramatically decreased when it was exposed to 2,4-dinitrotoluene and 2-nitrotoluene. Furthermore, after it was placed in an oven for 20 min at 45 °C, its emission was recovered. In 2023, Das, Negishi and colleagues introduced two SCAMs designated as TUS 1 and TUS 2 for the detection of Fe3+ in water.33 The LOD of Fe3+ was evaluated to be 0.05 nM L−1 for TUS 1 and 0.86 nM L−1 for TUS 2. These SCAMs were used to identify other metal ions as well, albeit finally futile. Accordingly, these SCAMs revealed superb selectivity towards Fe3+. In 2024, the same laboratory reported TUS 4 for detecting Hg2+.32 This detection of Hg2+ ions was verified by SERS, and TUS-4 showed the LOD of 0.07 pg mL−1. The Raman spectral intensity with different ions was also inspected using the same protocol, and the results suggested that only after adding Hg2+, the intensity was changed.3.2 In catalysis
During World War I, mustard gas was used as a life-threatening warfare agent by the German military and brought over 4000 military deaths; hence, detoxification of this toxic gas is important for our health.64 In 2019, Zang and colleagues reported the Ag12TPyP SCAM as a photocatalyst for detoxifying 2-chloroethyl ethyl sulfide (CEES).22 Under LED light irradiation and an O2 atmosphere (t1/2 = 1.5 min), this SCAM showed exemplary performance in converting 98% of CEES (1% loading) to CEESO within only 4 min. Besides, the catalytic activities of the Ag12 nanocluster, Ag12bpy-2, TPyP and Ag-TPyP were also assessed for comparative analysis. As a result, Ag12TPyP capably showed superlative performance and the conversion rate reached 100% at only 5 min, which revealed that the interaction between the Ag12 cluster and TPyP molecules is crucial to 1O2 generation.As a greenhouse gas, CO2 has a pronounced impact on global warming; so many research efforts have been devoted to using CO2 as the starting material for organic reactions.65 Sun et al. adopted the Ag27-MOF as a catalyst for CO2-cycloaddition organic reactions.25 The exposed silver nodes promoted the Ag⋯π interactions which in turn helped in activation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. The result of CO2-cycloaddition of the benzylprop-2-ynylamine reaction showed that the Ag27-MOF indicated higher activities than other Ag compounds and precursors. Additionally, the Ag27-MOF could be reused as a catalyst at least four times with the yield maintained over 90%. Various propargylamine (PA) substrates were used as the starting materials of the reaction and the following correlations were found: terminal PA substrates that have N-alkyl groups could get products in high yields, internal PA substrates also reacted and the products were the Z isomers.
C bonds. The result of CO2-cycloaddition of the benzylprop-2-ynylamine reaction showed that the Ag27-MOF indicated higher activities than other Ag compounds and precursors. Additionally, the Ag27-MOF could be reused as a catalyst at least four times with the yield maintained over 90%. Various propargylamine (PA) substrates were used as the starting materials of the reaction and the following correlations were found: terminal PA substrates that have N-alkyl groups could get products in high yields, internal PA substrates also reacted and the products were the Z isomers.
A recent paper from Das, Negishi and co-workers reported the TUS 3 SCAM as a catalyst for reduction of Fe(CN)63− utilizing NaBH4 in an aqueous medium.19 Deriving from the structural order of the framework, aggregation problems usually occurring with silver nanoparticles as catalysts could be avoided and the stability enhanced. The reaction was monitored by UV-Vis absorption spectroscopy which was able to record the diminution of the Fe(CN)63− peak. When the concentration of this SCAM was increased from 0.1 to 1 nM, the reaction rate was obviously increased. Furthermore, the recyclability of the catalyst was validated by Fourier-transform infrared spectroscopy and powder X-ray diffraction which confirmed that TUS 3 could be used at least 4 times.
3.3 In biomedicine
Bacterial resistance ensuing from the misuse and overuse of antibiotics has put public health in serious peril and accounted for 1.27 million deaths globally in 2019.66,67 A fascinating work from Zang and co-workers reported the synthesis of Ag9-AgTPyP SCAM comprised of silver porphyrin units that harvest visible light for electron transfer to the Ag9 clusters so as to allow O2 activation to generate reactive oxygen species (ROS).26 A surge in ROS concentrations could result in oxidative stress damage to lipids, proteins, nucleic acids and other cellular components, leading to bacterial cell death. Consequently, Ag9-AgTPyP demonstrated notable antibacterial efficacy with 99.99999% against S. aureus after 90 min and 99.999% against E. coli after 120 min of visible light exposure. Taking a step further, this paper considered the competency of the SCAM film in personal protective equipment that also exhibited prominent antibacterial activity against multidrug resistant superbugs.Integrating the high sensitivity and contrast of optical imaging with the high penetration depth and spatial resolution of ultrasound imaging,68,69 photoacoustic imaging is a non-invasive diagnostic imaging technique which produces ultrasonic waves upon irradiation of the tissue by pulsed laser irradiation and reconstructs the absorption distribution of the tissue.68,70 The applicability of a Ag14 cluster based SCAM in photoacoustic imaging was studied by Mandal and colleagues.39 Strategically, a continuous-wave laser beam was utilized together with a pulsed laser beam to irradiate the sample in order to elevate the temperature of the sample selectively in a predetermined region of interest,71 which thereby led to photoacoustic signal amplification over 85%.39 A transparent glass tube loaded with the SCAM dissolved in ethanol/acetonitrile was inserted through a chicken breast tissue at a depth of ca. 2 mm to visualize the blood-vessel mimicking ability of the SCAM inside the tissue.39
The detection of nucleic acids is critical to ensuring point-of-care diagnostics and in genomics research. The majority of the detection approaches are label-based that furnish very good sensitivity. On the other hand, the label-free detection approaches have made the analytical method easier by excluding labeling steps, thereby showing superiority in speed, convenience and cost-efficiency.72 Negishi and co-workers achieved label-free detection of HIV-1 DNA by allowing a single-stranded DNA (ssDNA) probe to hybridize to target DNA sequences and two novel SCAMs in the role of a quencher to reduce the large background signal of ssDNA probes with SYBR Green I.73
4 Future vision
In the final section, we outline some potential research directions that can enable the future progress of this research field:4.1 Understanding SCAM crystallization at the molecular level
Even though several SCAMs have been studied by many researchers, the crystallization mechanism underpinning SCAM synthesis still remains abstruse owing to the inadequacy of systematic assessment of the SCAM crystal nucleation and growth process. To overcome this lacuna, we can track the crystallization pathway by in situ/operando characterization approaches such as in situ XRD, in situ NMR spectroscopy, in situ transmission electron microscopy (TEM), in situ time-resolved high-resolution mass spectrometry, etc. The newly emerged CLASSIC NMR (Combined Liquid- And Solid-State In situ Crystallization NMR) technique enables one to get information on the evolution of solid and liquid phases concomitantly as a function of time throughout crystallization.74,75 Furthermore, the lack of comprehension and control of SCAM crystallization poses a challenge to growing single crystals of adequate size to get good diffraction data by SCXRD. The advent of the cryo-electron microscopy-based technique viz. microcrystal electron diffraction (MicroED) enabled fetching of the structural information even if the crystal sizes are one-billionth the size required for X-ray diffraction.764.2 Photoluminescence of SCAMs
From a knowledge of the average lifetime and quantum yield of SCAMs determined using time-correlated single-photon counting (TCSPC), it is possible to categorize their luminescence as either phosphorescence or fluorescence. If the luminescence behavior of the SCAMs is characterized as phosphorescence, this is associated with a spin multiplicity of 3 which can be utilized for singlet oxygen generation and photon upconversion. The flexibility of the organic linkers plays a major role in internal conversion (IC) which affects the nonradiative rate constant, which in turn largely dictates if a SCAM can exhibit photoluminescence or not.4.3 Introduction of silver clusters into covalent organic frameworks (COFs) to develop silver cluster–covalent organic frameworks (SCCOFs)
The coordination bond does not impart the SCAMs with adequate durability to withstand harsh operating conditions. To bring the open metal sites of SCAMs performing as absorption or active sites and the robustness of covalent bonds together under one roof, it might be rewarding to integrate silver clusters into COFs to construct SCCOFs. Given that the conventional synthesis of COFs relies on the solvothermal method which involves a high temperature (85–250 °C) while SCAMs are commonly prepared by slow evaporation of the reaction mixture at room temperature or low temperature (5–10 °C), a one-pot synthesis route might not be desirable for the preparation of SCCOFs owing to the thermal stability and solubility issues of silver precursors. As another option, post-synthetic metalation on a pre-synthesized COF network comprising linkers and bonds that contain electronegative elements (N, S, O) serving as metal binding sites confers good potential to obtain SCCOFs. More particularly, pre-synthesized COFs can be added to a solution of silver precursors, which can be reduced to produce silver nanoclusters within the COF network.4.4 Multicomponent SCAMs
Borrowing ideas from biological systems like nucleic acids, lipids, proteins, etc. which are made of multiple building blocks, multicomponent SCAMs i.e. SCAMs composed of other metal clusters in addition to silver clusters or multiple kinds of linkers can bring in heterogeneity (complexity) without compromising structural order, thereby giving rise to unprecedented functional properties beyond the purview of regular SCAMs. Multicomponent SCAMs show great potential for photoluminescence sensing and cooperative catalysis owing to the synergistic influence of multiple components. However, the major challenge lies in suppressing the competing phases that can lead to other thermodynamically favorable single-component SCAMs as the main product rather than the desired multicomponent SCAMs. This can be accomplished by choosing suitable building units and effectively controlling the reaction conditions.4.5 Cutting-edge applications
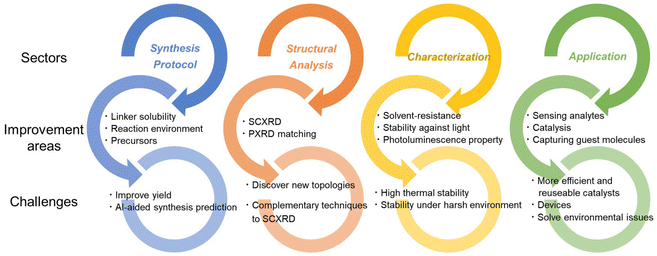
The rich plasmonic properties of silver clusters constituting SCAMs can be considered as a viable option for electronic communication applications.77 Often, the uniformity of the surface or the distribution of the nanoparticles influences the material's performance. SCAMs could be crucial to preparing highly uniform materials with tunable properties. Regarding catalysis, more efficient and reusable SCAM-based catalysts are highly sought after. Optoelectronic device fabrication and integration techniques using SCAMs are imperative to practical applications.The challenges and areas for improvement are briefly summarized in Fig. 6.
5. Final thoughts
Ever since their inception, SCAMs have been rapidly gaining momentum mainly driven by the flexible coordination nodes along with varying geometry and functionality of organic linker species to control framework connectivity. Hitherto, around 60 SCAMs have been reported and a significant portion of them display intense luminescence characteristics from electronic excited states and distinct luminescent responses to different analytes useful for selective sensing. SCAMs still have immense potential to accomplish exceptional optical properties when integrated into devices for leading-edge display and lighting technology, and intelligent switching and sensor platforms. Besides, chiral SCAMs are poised for significant impact in chiroptical detection systems.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge funding from the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant no. 20H02698, 20H02552), the Scientific Research on Innovative Areas “Aquatic Functional Materials” (grant no. 22H04562), the Yazaki Memorial Foundation for Science and Technology, and the Ogasawara Foundation for the Promotion of Science and Engineering.References
- J. D. Aiken and R. G. Finke, J. Mol. Catal. A: Chem., 1999, 145, 1–44 CrossRef CAS.
- Y. Li, M. Zhou, Y. Song, T. Higaki, H. Wang and R. Jin, Nature, 2021, 594, 380–384 CrossRef CAS PubMed.
- H. Qian, D.-e. Jiang, G. Li, C. Gayathri, A. Das, R. R. Gil and R. Jin, J. Am. Chem. Soc., 2012, 134, 16159–16162 CrossRef CAS PubMed.
- H. Zhang, T. Watanabe, M. Okumura, M. Haruta and N. Toshima, Nat. Mater., 2012, 11, 49–52 CrossRef PubMed.
- Z. Lei, X.-K. Wan, S.-F. Yuan, Z.-J. Guan and Q.-M. Wang, Acc. Chem. Res., 2018, 51, 2465–2474 CrossRef CAS PubMed.
- A. W. Cook and T. W. Hayton, Acc. Chem. Res., 2018, 51, 2456–2464 CrossRef CAS PubMed.
- D. Li, B. Kumari, X. Zhang, C. Wang, X. Mei and V. M. Rotello, Adv. Colloid Interface Sci., 2020, 276, 102090 CrossRef CAS PubMed.
- Y. Akinaga, T. Kawawaki, H. Kameko, Y. Yamazaki, K. Yamazaki, Y. Nakayasu, K. Kato, Y. Tanaka, A. T. Hanindriyo, M. Takagi, T. Shimazaki, M. Tachikawa, A. Yamakata and Y. Negishi, Adv. Funct. Mater., 2023, 33, 2303321 CrossRef CAS.
- T. Kawawaki, Y. Mitomi, N. Nishi, R. Kurosaki, K. Oiwa, T. Tanaka, H. Hirase, S. Miyajima, Y. Niihori, D. J. Osborn, T. Koitaya, G. F. Metha, T. Yokoyama, K. Iida and Y. Negishi, Nanoscale, 2023, 15, 7272–7279 RSC.
- T. N. Gieshoff, U. Chakraborty, M. Villa and A. J. von Wangelin, Angew. Chem., Int. Ed., 2017, 56, 3585–3589 CrossRef CAS PubMed.
- J. N. Anker, W. P. Hall, O. Lyandres, N. C. Shah, J. Zhao and R. P. Van Duyne, Nat. Mater., 2008, 7, 442–453 CrossRef CAS PubMed.
- A. Desireddy, B. E. Conn, J. Guo, B. Yoon, R. N. Barnett, B. M. Monahan, K. Kirschbaum, W. P. Griffith, R. L. Whetten, U. Landman and T. P. Bigioni, Nature, 2013, 501, 399–402 CrossRef CAS PubMed.
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346–10413 CrossRef CAS PubMed.
- W. Du, S. Jin, L. Xiong, M. Chen, J. Zhang, X. Zou, Y. Pei, S. Wang and M. Zhu, J. Am. Chem. Soc., 2017, 139, 1618–1624 CrossRef CAS PubMed.
- A. Ma, W. Du, J. Wang, K. Jiang, C. Zhang, W. Sheng, H. Zheng, R. Jin and S. Wang, J. Phys. Chem. Lett., 2023, 14, 5095–5101 CrossRef CAS PubMed.
- X. Wang, B. Yin, L. Jiang, C. Yang, Y. Liu, G. Zou, S. Chen and M. Zhu, Science, 2023, 381, 784–790 CrossRef CAS PubMed.
- S. Biswas, S. Das and Y. Negishi, Coord. Chem. Rev., 2023, 492, 215255 CrossRef CAS.
- R.-W. Huang, Y.-S. Wei, X.-Y. Dong, X.-H. Wu, C.-X. Du, S.-Q. Zang and T. C. W. Mak, Nat. Chem., 2017, 9, 689–697 CrossRef CAS PubMed.
- R. Nakatani, S. Biswas, T. Irie, J. Sakai, D. Hirayama, T. Kawawaki, Y. Niihori, S. Das and Y. Negishi, Nanoscale, 2023, 15, 16299–16306 RSC.
- Z.-Y. Wang and S.-Q. Zang, in Atomically Precise Nanochemistry, 2023, pp. 479–501, DOI:10.1002/9781119788676.ch16.
- T. Sekine, J. Sakai, Y. Horita, H. Mabuchi, T. Irie, S. Hossain, T. Kawawaki, S. Das, S. Takahashi, S. Das and Y. Negishi, Chem. – Eur. J., 2023, 29, e202300706 CrossRef CAS PubMed.
- M. Cao, R. Pang, Q.-Y. Wang, Z. Han, Z.-Y. Wang, X.-Y. Dong, S.-F. Li, S.-Q. Zang and T. C. W. Mak, J. Am. Chem. Soc., 2019, 141, 14505–14509 CrossRef CAS PubMed.
- J.-Y. Wang, R.-W. Huang, Z. Wei, X.-J. Xi, X.-Y. Dong and S.-Q. Zang, Chem. – Eur. J., 2019, 25, 3376–3381 CrossRef CAS PubMed.
- X.-H. Wu, Z. Wei, B.-J. Yan, R.-W. Huang, Y.-Y. Liu, K. Li, S.-Q. Zang and T. C. W. Mak, CCS Chem., 2019, 1, 553–560 CrossRef CAS.
- M. Zhao, S. Huang, Q. Fu, W. Li, R. Guo, Q. Yao, F. Wang, P. Cui, C.-H. Tung and D. Sun, Angew. Chem., Int. Ed., 2020, 59, 20031–20036 CrossRef CAS PubMed.
- M. Cao, S. Wang, J.-H. Hu, B.-H. Lu, Q.-Y. Wang and S.-Q. Zang, Adv. Sci., 2022, 9, 2103721 CrossRef CAS PubMed.
- K. Zhou, C. Qin, X.-L. Wang, K.-Z. Shao, L.-K. Yan and Z.-M. Su, CrystEngComm, 2014, 16, 7860–7864 RSC.
- X. Wei, X. Kang, Z. Zuo, F. Song, S. Wang and M. Zhu, Natl. Sci. Rev., 2021, 8, nwaa077 CrossRef CAS PubMed.
- Z. Wang, X.-Y. Li, L.-W. Liu, S.-Q. Yu, Z.-Y. Feng, C.-H. Tung and D. Sun, Chem. – Eur. J., 2016, 22, 6830–6836 CrossRef CAS PubMed.
- Z.-Y. Chen, D. Y. S. Tam, L. L.-M. Zhang and T. C. W. Mak, Chem. – Asian J., 2017, 12, 2763–2769 CrossRef CAS PubMed.
- S. Chen, W. Du, C. Qin, D. Liu, L. Tang, Y. Liu, S. Wang and M. Zhu, Angew. Chem., Int. Ed., 2020, 59, 7542–7547 CrossRef CAS PubMed.
- R. Nakatani, S. Biswas, T. Irie, Y. Niihori, S. Das and Y. Negishi, ACS Mater. Lett., 2024, 6, 438–445 CrossRef CAS.
- J. Sakai, S. Biswas, T. Irie, H. Mabuchi, T. Sekine, Y. Niihori, S. Das and Y. Negishi, Nanoscale, 2023, 15, 12227–12234 RSC.
- Y.-H. Li, R.-W. Huang, P. Luo, M. Cao, H. Xu, S.-Q. Zang and T. C. W. Mak, Sci. China: Chem., 2019, 62, 331–335 CrossRef CAS.
- Z.-Y. Wang, M.-Q. Wang, Y.-L. Li, P. Luo, T.-T. Jia, R.-W. Huang, S.-Q. Zang and T. C. W. Mak, J. Am. Chem. Soc., 2018, 140, 1069–1076 CrossRef CAS PubMed.
- R.-W. Huang, X.-Y. Dong, B.-J. Yan, X.-S. Du, D.-H. Wei, S.-Q. Zang and T. C. W. Mak, Angew. Chem., Int. Ed., 2018, 57, 8560–8566 CrossRef CAS PubMed.
- M. J. Alhilaly, R.-W. Huang, R. Naphade, B. Alamer, M. N. Hedhili, A.-H. Emwas, P. Maity, J. Yin, A. Shkurenko, O. F. Mohammed, M. Eddaoudi and O. M. Bakr, J. Am. Chem. Soc., 2019, 141, 9585–9592 CrossRef CAS PubMed.
- Y.-M. Wang, J.-W. Zhang, Q.-Y. Wang, H.-Y. Li, X.-Y. Dong, S. Wang and S.-Q. Zang, Chem. Commun., 2019, 55, 14677–14680 RSC.
- A. K. Das, S. Biswas, A. Thomas, S. Paul, A. S. Nair, B. Pathak, M. S. Singh and S. Mandal, Mater. Chem. Front., 2021, 5, 8380–8386 RSC.
- N.-N. Li, M. Yang, X.-J. Xu, X.-Y. Dong, S. Li and S.-Q. Zang, Dalton Trans., 2021, 50, 15267–15273 RSC.
- A. K. Das, S. Biswas, S. S. Manna, B. Pathak and S. Mandal, Inorg. Chem., 2021, 60, 18234–18241 CrossRef CAS PubMed.
- S. Biswas, A. K. Das, A. Nath, S. Paul, M. S. Singh and S. Mandal, Nanoscale, 2021, 13, 17325–17330 RSC.
- G. Deng, B. K. Teo and N. Zheng, J. Am. Chem. Soc., 2021, 143, 10214–10220 CrossRef CAS PubMed.
- Z. Wang, Y.-J. Zhu, Y.-Z. Li, G.-L. Zhuang, K.-P. Song, Z.-Y. Gao, J.-M. Dou, M. Kurmoo, C.-H. Tung and D. Sun, Nat. Commun., 2022, 13, 1802 CrossRef CAS PubMed.
- J.-Y. Wang, Y. Si, X.-M. Luo, Z.-Y. Wang, X.-Y. Dong, P. Luo, C. Zhang, C. Duan and S.-Q. Zang, Adv. Sci., 2023, 10, 2207660 CrossRef CAS PubMed.
- J.-Y. Wang, J.-W. Yuan, X.-M. Liu, Y.-J. Liu, F. Bai, X.-Y. Dong and S.-Q. Zang, Aggregate, 2024, e508 CrossRef.
- X.-S. Du, B.-J. Yan, J.-Y. Wang, X.-J. Xi, Z.-Y. Wang and S.-Q. Zang, Chem. Commun., 2018, 54, 5361–5364 RSC.
- M. Zhang, M. Cao, Z.-B. Sun, Z. Han, Z.-Y. Wang, Q.-Y. Wang and S.-Q. Zang, Chem. Mater., 2023, 35, 10238–10246 CrossRef CAS.
- Z. Wei, X.-H. Wu, P. Luo, J.-Y. Wang, K. Li and S.-Q. Zang, Chem. – Eur. J., 2019, 25, 2750–2756 CrossRef CAS PubMed.
- X.-H. Wu, P. Luo, Z. Wei, Y.-Y. Li, R.-W. Huang, X.-Y. Dong, K. Li, S.-Q. Zang and B. Z. Tang, Adv. Sci., 2019, 6, 1801304 CrossRef PubMed.
- J.-Y. Wang, W.-H. Li, Z. Wei, C. Zhang, Y.-H. Li, X.-Y. Dong, G. Xu and S.-Q. Zang, Chem. Commun., 2020, 56, 2091–2094 RSC.
- Q.-S. Wu, F. Bigdeli, F. Rouhani, X.-M. Gao, H. Kaviani, H.-J. Li, W. Wang, K.-G. Liu, M.-L. Hu, X.-Q. Cai and A. Morsali, Inorg. Chem., 2021, 60, 1523–1532 CrossRef CAS PubMed.
- J. Zhuge, F. Rouhani, F. Bigdeli, X.-M. Gao, H. Kaviani, H.-J. Li, W. Wang, M.-L. Hu, K.-G. Liu and A. Morsali, Dalton Trans., 2021, 50, 2606–2615 RSC.
- C.-H. Gong, X.-Z. Hu, Z. Han, X.-F. Liu, M.-Z. Yang and S.-Q. Zang, Chem. Commun., 2022, 58, 1788–1791 RSC.
- C.-H. Gong, Z.-B. Sun, M. Cao, X.-M. Luo, J. Wu, Q.-Y. Wang, S.-Q. Zang and T. C. W. Mak, Chem. Commun., 2022, 58, 9806–9809 RSC.
- Z. Chang, X. Jing, C. He, X. Liu and C. Duan, ACS Catal., 2018, 8, 1384–1391 CrossRef CAS.
- Q.-X. Zang, Z.-Y. Wang, Y. Li, X.-M. Luo, H.-Y. Li, Y.-N. Si and S.-Q. Zang, Chem. Commun., 2022, 58, 11985–11988 RSC.
- S.-R. He, H. Xu, C. Chen, X.-T. Wang, T.-Q. Lu, L. Cao, J. Zheng and X.-Y. Zheng, Nanoscale, 2023, 15, 15730–15738 RSC.
- X. Ma, C. Cai, W. Sun, W. Song, Y. Ma, X. Liu, G. Xie, S. Chen and S. Gao, ACS Appl. Mater. Interfaces, 2019, 11, 9233–9238 CrossRef CAS PubMed.
- X.-Y. Dong, H.-L. Huang, J.-Y. Wang, H.-Y. Li and S.-Q. Zang, Chem. Mater., 2018, 30, 2160–2167 CrossRef CAS.
- X.-H. Ma, J.-Y. Wang, J.-J. Guo, Z.-Y. Wang and S.-Q. Zang, Chin. J. Chem., 2019, 37, 1120–1124 CrossRef CAS.
- X.-Y. Dong, Y. Si, J.-S. Yang, C. Zhang, Z. Han, P. Luo, Z.-Y. Wang, S.-Q. Zang and T. C. W. Mak, Nat. Commun., 2020, 11, 3678 CrossRef CAS PubMed.
- W. A. Dar, A. Jana, K. S. Sugi, G. Paramasivam, M. Bodiuzzaman, E. Khatun, A. Som, A. Mahendranath, A. Chakraborty and T. Pradeep, Chem. Mater., 2022, 34, 4703–4711 CrossRef CAS.
- K. Ghabili, P. S. Agutter, M. Ghanei, K. Ansarin and M. M. Shoja, J. Appl. Toxicol., 2010, 30, 627–643 CrossRef CAS PubMed.
- J. Kim, S.-N. Kim, H.-G. Jang, G. Seo and W.-S. Ahn, Appl. Catal., A, 2013, 453, 175–180 CrossRef CAS.
- https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance#:~:text=It%20is%20estimated%20that%20bacterial,development%20of%20drug-resistant% .
- C. J. L. Murray, K. S. Ikuta, F. Sharara, L. Swetschinski, G. R. Aguilar, A. Gray, C. Han, C. Bisignano, P. Rao, E. Wool, S. C. Johnson, A. J. Browne, M. G. Chipeta, F. Fell, S. Hackett, G. Haines-Woodhouse, B. H. K. Hamadani, E. A. P. Kumaran, B. McManigal, S. Achalapong, R. Agarwal, S. Akech, S. Albertson, J. Amuasi, J. Andrews, A. Aravkin, E. Ashley, F.-X. Babin, F. Bailey, S. Baker, B. Basnyat, A. Bekker, R. Bender, J. A. Berkley, A. Bethou, J. Bielicki, S. Boonkasidecha, J. Bukosia, C. Carvalheiro, C. Castañeda-Orjuela, V. Chansamouth, S. Chaurasia, S. Chiurchiù, F. Chowdhury, R. C. Donatien, A. J. Cook, B. Cooper, T. R. Cressey, E. Criollo-Mora, M. Cunningham, S. Darboe, N. P. J. Day, M. D. Luca, K. Dokova, A. Dramowski, S. J. Dunachie, T. D. Bich, T. Eckmanns, D. Eibach, A. Emami, N. Feasey, N. Fisher-Pearson, K. Forrest, C. Garcia, D. Garrett, P. Gastmeier, A. Z. Giref, R. C. Greer, V. Gupta, S. Haller, A. Haselbeck, S. I. Hay, M. Holm, S. Hopkins, Y. Hsia, K. C. Iregbu, J. Jacobs, D. Jarovsky, F. Javanmardi, A. W. J. Jenney, M. Khorana, S. Khusuwan, N. Kissoon, E. Kobeissi, T. Kostyanev, F. Krapp, R. Krumkamp, A. Kumar, H. H. Kyu, C. Lim, K. Lim, D. Limmathurotsakul, M. J. Loftus, M. Lunn, J. Ma, A. Manoharan, F. Marks, J. May, M. Mayxay, N. Mturi, T. Munera-Huertas, P. Musicha, L. A. Musila, M. M. Mussi-Pinhata, R. N. Naidu, T. Nakamura, R. Nanavati, S. Nangia, P. Newton, C. Ngoun, A. Novotney, D. Nwakanma, C. W. Obiero, T. J. Ochoa, A. Olivas-Martinez, P. Olliaro, E. Ooko, E. Ortiz-Brizuela, P. Ounchanum, G. D. Pak, J. L. Paredes, A. Y. Peleg, C. Perrone, T. Phe, K. Phommasone, N. Plakkal, A. Ponce-de-Leon, M. Raad, T. Ramdin, S. Rattanavong, A. Riddell, T. Roberts, J. V. Robotham, A. Roca, V. D. Rosenthal, K. E. Rudd, N. Russell, H. S. Sader, W. Saengchan, J. Schnall, J. A. G. Scott, S. Seekaew, M. Sharland, M. Shivamallappa, J. Sifuentes-Osornio, A. J. Simpson, N. Steenkeste, A. J. Stewardson, T. Stoeva, N. Tasak, A. Thaiprakong, G. Thwaites, C. Tigoi, C. Turner, P. Turner, H. R. van Doorn, S. Velaphi, A. Vongpradith, M. Vongsouvath, H. Vu, T. Walsh, J. L. Walson, S. Waner, T. Wangrangsimakul, P. Wannapinij, T. Wozniak, T. E. M. W. Young-Sharma, K. C. Yu, P. Zheng, B. Sartorius, A. D. Lopez, A. Stergachis, C. Moore, C. Dolecek and M. Naghavi, Lancet, 2022, 399, 629–655 CrossRef CAS PubMed.
- P. Beard, Interface Focus, 2011, 1, 602–631 CrossRef PubMed.
- Q. Fan, K. Cheng, Z. Yang, R. Zhang, M. Yang, X. Hu, X. Ma, L. Bu, X. Lu, X. Xiong, W. Huang, H. Zhao and Z. Cheng, Adv. Mater., 2015, 27, 843–847 CrossRef CAS PubMed.
- S. Xu, X. Shi, C. Chu and G. Liu, in Methods Enzymol, ed. J. Chan, Academic Press, 2021, vol. 657, pp. 145–156 Search PubMed.
- A. Thomas, S. Paul, J. Mitra and M. S. Singh, Sensors, 2021, 21, 1190 CrossRef CAS PubMed.
- A. Star, E. Tu, J. Niemann, J.-C. P. Gabriel, C. S. Joiner and C. Valcke, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 921–926 CrossRef CAS PubMed.
- S. Das, T. Sekine, H. Mabuchi, S. Hossain, S. Das, S. Aoki, S. Takahashi and Y. Negishi, Chem. Commun., 2023, 59, 4000–4003 RSC.
- C. E. Hughes, P. A. Williams and K. D. M. Harris, Angew. Chem., Int. Ed., 2014, 53, 8939–8943 CrossRef CAS PubMed.
- K. D. M. Harris, C. E. Hughes, P. A. Williams and G. R. Edwards-Gau, Acta Crystallogr., Sect. C: Struct. Chem., 2017, 73, 137–148 CrossRef CAS PubMed.
- C. G. Jones, M. W. Martynowycz, J. Hattne, T. J. Fulton, B. M. Stoltz, J. A. Rodriguez, H. M. Nelson and T. Gonen, ACS Cent. Sci., 2018, 4, 1587–1592 CrossRef CAS PubMed.
- A. S. Baburin, A. M. Merzlikin, A. V. Baryshev, I. A. Ryzhikov, Y. V. Panfilov and I. A. Rodionov, Opt. Mater. Express, 2019, 9, 611–642 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |