An organophosphate 3d–4f heterometallic polyoxoniobate nanowire†
Jin-Ai
Fan‡
a,
Hao
Yu‡
a,
Yu-Diao
Lin
ab,
Ming-Qiang
Qi
c,
Xiang-Jian
Kong
 c,
Cai
Sun
c,
Cai
Sun
 *a and
Shou-Tian
Zheng
*a and
Shou-Tian
Zheng
 *a
*a
aFujian Provincial Key Laboratory of Advanced Inorganic Oxygenated Materials, College of Chemistry, Fuzhou University, Fuzhou, Fujian 350108, China. E-mail: csun@fzu.edu.cn; stzheng@fzu.edu.cn
bFujian Provincial Key Laboratory of Coastal Basin Environment, Fujian Polytechnic Normal University, Fuqing, Fujian 350300, China
cState Key Laboratory of Physical Chemistry of Solid Surface, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005, China
First published on 5th June 2024
Abstract
Four different structural compositions of organophosphate, 3d transition metal, 4f lanthanide and polyoxoniobate (PONb) are unified in a system for the first time to form a new type of organophosphate 3d–4f heterometallic inorganic–organic hybrid PONb nanowire. Interesting magnetic anisotropy and slow magnetic relaxation are found in the PONb nanowire.
Polyoxometalates (POMs) typically refer to a class of cluster structures assembled from high-valent transition metals V, Mo, W, Nb, and Ta with oxygen atoms. By virtue of their controllable structures by introducing heterometallic ions, POMs have potential applications in optics, magnetism, catalysis, and functional materials and have attracted the attention of numerous researchers.1 The introduction of 3d transition metals and 4f lanthanides in POMs can form 3d transition metal-substituted polyoxometalates (3d POMs) and 4f lanthanide-substituted polyoxometalates (4f POMs), respectively.2,3 To date, extensive studies with rich applications has documented research on 3d POMs and 4f POMs.4 Furthermore, 3d–4f heterometallic POMs (3d–4f POMs) exhibit unique configurations, superior physicochemical properties, and intriguing synergistic interactions between transition metals and lanthanide metals, sparking researchers’ interest in exploring their potential applications in emerging technologies.5 However, the development of 3d–4f POMs lags significantly behind 3d POMs and 4f POMs.6
Until now, reports on 3d–4f heterometallic substituted POMs have focused on polyoxotungstates (POWs) and polyoxomolybdates (POMos)7 such as two giant 3d–4f POW clusters [LaNi12W35Sb3P3O139(OH)6]23− and [La10Ni48W140Sb16P12O568(OH)24(H2O)20]86− from Kong group8 two new classes of highly reduced mix-valence 3d–4f POMos [Mo64Ni8Ln6H26O200(H2O)30]8− from Cronin group.9 In contrast, the development of 3d–4f heterometallic substituted polyoxoniobate (PONb) remains a challenge. Firstly, the lack of soluble niobate oxoanion precursors, low activity and the narrow working pH region ranging 10–12 of niobate species are major limiting factors for the synthesis of PONbs.10 Secondly, alkaline working pH triggers the hydrolysis of lanthanides and transition metal ions, leading to the precipitation of oxide compounds. Thirdly, the different oxophilicity of 4f lanthanides and 3d transition metals inevitably lead to a competitive reaction, making it difficult to connect to PONb clusters simultaneously.11
Recently, we found that the hydrolysis of lanthanide ions in alkaline environments can be effectively restrained through the synergistic coordination of an organophosphate and a sodium carbonate buffer system and enabled the successful construction of a series of organophosphate–Ln–PONb composite clusters.12 Building upon the knowledge acquired in our previous studies, we are very interested in the challenge of synthesizing the PONb with 3d and 4f metal ions simultaneously and developing a research area that holds great potential for advancement.
Herein, four different structural compositions of organophosphate, 3d transition metal, 4f lanthanide and PONb are unified in a system for the first time, forming a new type of organophosphate 3d–4f heterometallic inorganic–organic hybrid PONb nanowire, denoted as: H33K18Na5{[Co(H2O)4]2[Dy4(CO3)4(EA)2]2[Nb32O92(H2O)4]2}·104H2O (1, EA = etidronic acid). The successful realization of 1 demonstrates the possibility of fusing four separate research areas of PONb chemistry, lanthanide chemistry, transition metal chemistry, and organophosphate chemistry. Such a multi-component system can lead to great compositional and topological diversity, showing a general strategy toward the construction of rich organophosphate–TM–Ln–PONb composite clusters.
PONb 1 was synthesized by reacting Lindqvist-type PONb precursor K7HNb6O19·13H2O with Dy(Ac)3·6H2O, cobalt acetylacetonate and EA in a Na2CO3/NaHCO3 buffer solution (pH 11.5) and 80 °C for 3 days (see the Experimental section in the ESI†). Powder X-ray diffraction (PXRD) patterns indicate that as-synthesized crystalline samples had a pure phase (Fig. S1†). The water content in the crystal was determined by thermogravimetric analysis (Fig. S2†).
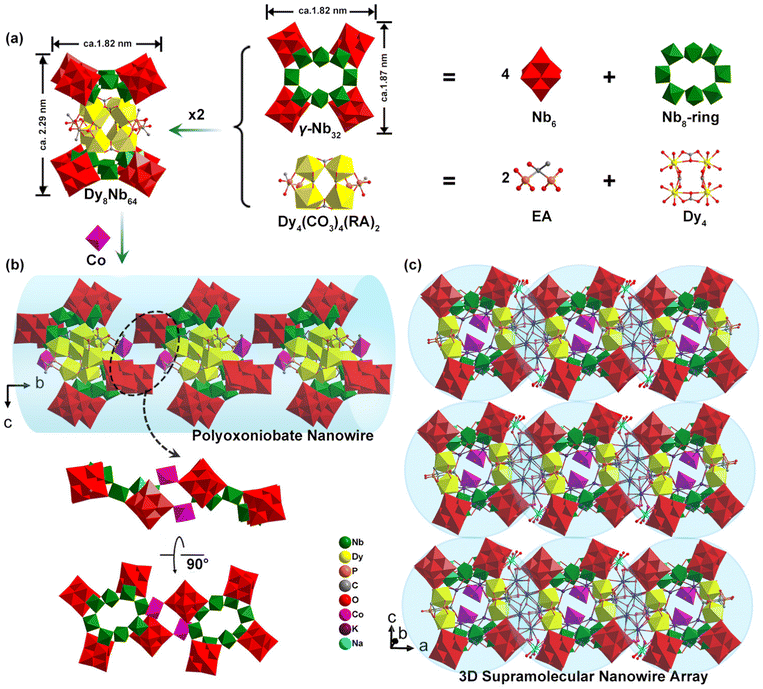
Single-crystal X-ray diffraction analyses revealed that 1 crystallizes in triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and its crystallographic data and structure refinement are summarized in Table S1.† As shown in Fig. 1a, the polyoxoanion {[Dy4(CO3)4(EA)2]2[Nb32O92(H2O)4]2} (Dy8Nb64) as a secondary building unit (SBU) is constructed by two {γ-Nb32O92(H2O)4} (γ-Nb32) fragments joined together with two mutually centrosymmetric [Dy4(CO3)4(EA)2]2 motifs and displays an interesting sandwich-like arrangement. γ-Nb32 is a classical four-membered cyclic PONb macrocyclic fragment, the structural details of three types of Nb32 fragments (α-, β-, γ-), which have been discussed in detail in our previous work,12 ultimately differ in size and symmetry due to the difference in the connection of the central Nb8 ring and the distribution position of the peripheral Nb6 cluster blocks (Fig. S5†).
and its crystallographic data and structure refinement are summarized in Table S1.† As shown in Fig. 1a, the polyoxoanion {[Dy4(CO3)4(EA)2]2[Nb32O92(H2O)4]2} (Dy8Nb64) as a secondary building unit (SBU) is constructed by two {γ-Nb32O92(H2O)4} (γ-Nb32) fragments joined together with two mutually centrosymmetric [Dy4(CO3)4(EA)2]2 motifs and displays an interesting sandwich-like arrangement. γ-Nb32 is a classical four-membered cyclic PONb macrocyclic fragment, the structural details of three types of Nb32 fragments (α-, β-, γ-), which have been discussed in detail in our previous work,12 ultimately differ in size and symmetry due to the difference in the connection of the central Nb8 ring and the distribution position of the peripheral Nb6 cluster blocks (Fig. S5†).
The [Dy4(CO3)4(EA)2] motif consists of four regular DyO8 bicapped trigonal prisms that are connected in a corner-sharing fashion, which are constructed with four oxygen atoms provided by two CO32−, two oxygen atoms provided by an EA ligand, with the remaining coordination site occupied by two terminal oxygen atoms stem from γ-Nb32. The four DyIII ions form a square plane with two pairs of CO32− ions arranged orthogonally above and below the square plane. The CO32− ions are encapsulated by Dy4 and connected to two DyIII ions through the two two-coordinated oxygen atoms and one three-coordinated oxygen atom. The Dy4 clusters are further linked by two EA ligands positioned at opposing edges of the square, where each EA ligand is connected to two DyIII ions through two phosphonic acid groups. Additionally, two [Dy4(CO3)4(EA)2] motifs are embedded in a sandwich formed by two opposing γ-Nb32 clusters. Each [Dy4(CO3)4(EA)2] motif is linked with NbO6 octahedra via eight Dy–O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Nb bonds [Dy–O bonds, 2.267(2)–2.498(3) Å; Nb
Nb bonds [Dy–O bonds, 2.267(2)–2.498(3) Å; Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, 1.715(3)–2.466(2) Å]; Fig. 1a, generating a huge inorganic–organic hybrid three-layered PONb SBU-like “hamburger” with the dimensions of ca. 2.29 × 1.87 × 1.82 nm3. The oxidation states of all Nb, Co and Dy atoms in 1 were confirmed as +5, +2 and +3 by Bond Valence Sum calculations (Tables S2–S4†).
O bonds, 1.715(3)–2.466(2) Å]; Fig. 1a, generating a huge inorganic–organic hybrid three-layered PONb SBU-like “hamburger” with the dimensions of ca. 2.29 × 1.87 × 1.82 nm3. The oxidation states of all Nb, Co and Dy atoms in 1 were confirmed as +5, +2 and +3 by Bond Valence Sum calculations (Tables S2–S4†).
Interestingly, each SBU is linked to adjacent one by two Co-centered distorted octahedra [CoO6] to give a 1D nanowire along the b direction, where each CoII linker is coordinated by four water ligands and two terminal Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O oxo atoms (Co–O bonds: 2.094(7)–2.289(8) Å) (Fig. 1b and S6–S8†). The two Nb
O oxo atoms (Co–O bonds: 2.094(7)–2.289(8) Å) (Fig. 1b and S6–S8†). The two Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O oxo atoms stem from Nb6 and Nb8-ring at the top and bottom of the adjacent SBUs, respectively. Finally, the 1D PONb nanowires further give rise to a 3D supramolecular nanowire array (Fig. 1c) through hydrogen bonds and electrostatic interactions between the hydrated sodium and potassium complex.
O oxo atoms stem from Nb6 and Nb8-ring at the top and bottom of the adjacent SBUs, respectively. Finally, the 1D PONb nanowires further give rise to a 3D supramolecular nanowire array (Fig. 1c) through hydrogen bonds and electrostatic interactions between the hydrated sodium and potassium complex.
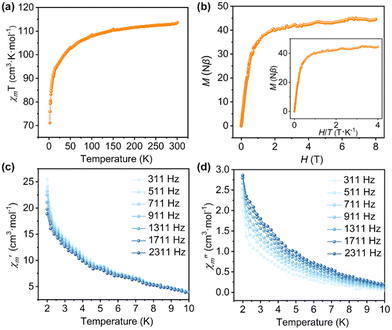
Motivated by the spin of CoII, high spin–orbit coupling of DyIII, and their possible magnetic interactions, we explored the magnetic properties of 1. Firstly, the direct current (DC) magnetic susceptibility (χm) of 1 was measured at an external magnetic field of 0.1 T with a temperature range of 2–300 K. The plots of χmT vs. T are shown in Fig. 2a, the observed χmT value at 300 K for 1 is 113.68 cm3 K mol−1, which is slightly lower than the theoretical value of 117.11 cm3 K mol−1 calculated for eight Dy3+ ions (14.17 cm3 K mol−1) and two Co2+ (1.875 cm3 K mol−1), probably resulting from weak antiferromagnetic interactions within the nanochain.14,15 As the temperature drops, the χmT value drops smoothly from 300 K to 100 K and declines rapidly to a minimum of 71.23 cm3 K mol−1 at 2 K. The result indicates the presence of magnetic anisotropy of the isolated CoII and DyIII ions or antiferromagnetic coupling interactions between adjacent DyIII ions.13 The field-dependent isothermal magnetization (M) of 1 was investigated at 2 K with the field strength (H) varying from 0 T to 8 T. Fig. 2b shows the rapid increase in M values at low fields, reaching 38.53Nβ at 1 T under 2 K, then followed by a slight rise to a maximum value of 44.57Nβ, which is less than the predicted saturation value of 86Nβ. This is most likely due to the large magnetic anisotropy or crystal-field effects at the DyIII ions, eliminating the 16-fold degeneracy of the 6H15/2 ground state.16
Furthermore, the combined effect of 3d–4f interactions can enhance the properties of a single molecule magnet.17 To study the dynamic properties of 1, alternating-current (AC) magnetic susceptibilities were investigated with frequencies ranging from 311 to 2311 Hz under an applied magnetic field of 3 Oe and zero direct-current fields. Notably, the observed out-of-phase (χ′′m) signals of 1 show frequency dependence with the varying frequency, and clearly indicate a slow magnetic relaxation below 10 K (Fig. 2c and d). The behavior of 1 aligns with the Debye model, characterized by one energy barrier and one-time constant based on the relationship: ln(χ′′m/χ′m) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ωτ0 + Ueff/T,18 which allows for a rough estimation of the energy barrier of the relaxation process (Table S5 and Fig. S9†). The smaller energy barrier of the slow magnetic relaxation may be caused by quantum tunneling.
ωτ0 + Ueff/T,18 which allows for a rough estimation of the energy barrier of the relaxation process (Table S5 and Fig. S9†). The smaller energy barrier of the slow magnetic relaxation may be caused by quantum tunneling.
Conclusions
In summary, we successfully obtained an organophosphate 3d–4f heterometallic inorganic–organic hybrid PONb nanowire, unifying organophosphate, 3d transition metal, 4f lanthanide and PONb in a system for the first time. This hybrid PONb not only exhibits significant magnetic anisotropy but also demonstrates a noticeable slow magnetic relaxation. This work opens avenues for further exploration in the realm of multicomponent functional PONb.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22371045, 22371046), Fujian Natural Science Youth Innovation Project (2021J05111, 2023J05211), Fujian Provincial Chemistry Discipline Alliance Foundation (50025401).Notes and references
- (a) B. Qin, H. Y. Chen, H. Liang, L. Fu, X. F. Liu, X. H. Qiu, S. Q. Liu, R. Song and Z. Y. Tang, J. Am. Chem. Soc., 2010, 132, 2886–2888 CrossRef CAS PubMed; (b) Y. D. Lin, R. Ge, C. B. Tian, C. Sun, Y. Q. Sun, Q. X. Zeng, X. X. Li and S. T. Zheng, Chem. Commun., 2021, 57, 8624 RSC; (c) N. Li, J. Liu, B. X. Dong and Y. Q. Lan, Angew. Chem., Int. Ed., 2020, 59, 20779–20793 CrossRef CAS PubMed; (d) X. M. Luo, N. F. Li, Z. B. Hu, J. P. Cao, C. H. Cui, Q. F. Lin and Y. Xu, Inorg. Chem., 2019, 58, 2463–2470 CrossRef CAS PubMed.
- (a) Z. W. Guo, L. H. Lin, J. P. Ye, Y. Chen, X. X. Li, S. Lin, J. D. Huang and S. T. Zheng, Angew. Chem., Int. Ed., 2023, 62, e202305260 CrossRef CAS PubMed; (b) S. S. Zhang, O. Oms;, L. Hao, R. J. Liu, M. Wang, Y. Q. Zhang, H. Y. He, A. Dolbecq, J. Marrot, B. Keita, L. J. Zhi, P. Mialane, B. Li and G. J. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 38486–38498 CrossRef CAS PubMed; (c) J. Zhen, W. Y. Wang, R. Wan, X. Y. Ma, Y. Y. Qiao, Y. F. Bai, P. T. Ma, J. Y. Niu and J. P. Wang, Inorg. Chem., 2022, 61, 16528–16532 CrossRef PubMed.
- S. M. Liu, Z. Zhang, X. H. Li, H. J. Jia and S. X. Liu, J. Alloys Compd., 2018, 761, 52–57 CrossRef CAS.
- (a) G. Y. Zhang, F. Wang, T. Tubul, M. Baranov, N. Leffler, A. Neyman, J. M. Poblet and I. A. Weinstock, Angew. Chem., Int. Ed., 2022, 61, e202213162 CrossRef CAS PubMed; (b) Z. K. Zhu, Y. Y. Lin, R. D. Lai, X. X. Li, Y. Q. Sun and S. T. Zheng, Chin. Chem. Lett., 2023, 34, 107773 CrossRef CAS; (c) D. D. Li, P. T. Ma, J. Y. Niu and J. P. Wang, Coord. Chem. Rev., 2019, 392, 49–80 CrossRef CAS; (d) C. Boskovic, Acc. Chem. Res., 2017, 50, 2205–2214 CrossRef CAS PubMed.
- (a) Y. N. Gu, Y. Chen, Y. L. Wu, S. T. Zheng and X. X. Li, Inorg. Chem., 2018, 57, 2472–2479 CrossRef CAS PubMed; (b) M. Ibrahim, V. Mereacre, N. Leblanc, W. Wernsdorfer, C. E. Anson and A. K. Powell, Angew. Chem., Int. Ed., 2015, 54, 15574–15578 CrossRef CAS PubMed; (c) Y. H. Chen, L. H. Sun, S. Z. Chang, L. J. Chen and J. W. Zhao, Inorg. Chem., 2018, 57, 15079–15092 CrossRef CAS PubMed.
- V. Das, R. Kaushik and F. Hussain, Coord. Chem. Rev., 2020, 143, 213271 CrossRef.
- (a) Y. N. Gu, H. Yu, L. D. Lin, Y. L. Wu, Z. Li, W. Y. Pan, J. He, L. Chen, Q. Li and X. X. Li, New J. Chem., 2019, 43, 3011–3016 RSC; (b) E. Tanuhadi, E. Al-Sayed, G. Novitchi, A. Roller, G. Giester and A. Rompel, Inorg. Chem., 2020, 59, 8461–8467 CrossRef CAS PubMed; (c) J. Cai, X. Y. Zheng, J. Xie, Z. H. Yan, X. J. Kong, Y. P. Ren, L. S. Long and L. S. Zheng, Inorg. Chem., 2017, 56, 8439–8445 CrossRef CAS PubMed.
- S. R. Li, H. Y. Wang, H. F. Su, H. J. Chen, M. H. Du, L. S. Long, X. J. Kong and L. S. Zheng, Small Methods, 2020, 2000777 Search PubMed.
- E. G. Ribó, L. B. Nicola, D. L. Long and L. Cronin, Angew. Chem., Int. Ed., 2022, 61, e202201672 CrossRef PubMed.
- Y. L. Wu, X. X. Li, Y. J. Qi, H. Yu, L. Jin and S. T. Zheng, Angew. Chem., Int. Ed., 2018, 57, 8572–8576 CrossRef CAS PubMed.
- J. C. Liu, Q. Han, L. J. Chen and J. W. Zhao, CrystEngComm, 2016, 18, 842–862 RSC.
- (a) H. Yu, Y. D. Lin, S. L. Huang, X. X. Li, C. Sun and S. T. Zheng, Angew. Chem., Int. Ed., 2023, e202302111 CAS; (b) P. Huang, C. Qin, Z. M. Su, Y. Xing, X. L. Wang, K. Z. Shao, Y. Q. Lan and E. B. Wang, J. Am. Chem. Soc., 2012, 134, 14004–14010 CrossRef CAS PubMed; (c) Z. K. Zhu, Y. Y. Lin, X. X. Li, D. Zhao and S. T. Zheng, Inorg. Chem. Front., 2021, 8, 1297–1302 RSC.
- L. Zhang, L. Zhao, P. Zhang, C. Wang, S. W. Yuan and J. K. Tang, Inorg. Chem., 2015, 54, 11535–11541 CrossRef CAS PubMed.
- L. F. Wang, J. Z. Qiu, S. G. Wu, Y. C. Chen, C. J. Li, Q. W. Li, J. L. Liu and M. L. Tong, Inorg. Chem., 2018, 57, 4070–4076 CrossRef CAS PubMed.
- X. N. Yao, J. Z. Du, Y. Q. Zhang, X. B. Leng, M. W. Yang, S. D. Jiang, Z. X. Wang, Z. W. Ouyang, L. Deng, B. W. Wang and S. Gao, J. Am. Chem. Soc., 2017, 139, 373–380 CrossRef CAS PubMed.
- J. F. Wu, L. Zhao, L. Zhang, X. L. Li, M. Guo, A. K. Powell and J. K. Tang, Angew. Chem., Int. Ed., 2016, 55, 15574–15578 CrossRef CAS PubMed.
- Y. Peng and A. K. Powell, Coord. Chem. Rev., 2021, 426, 213490 CrossRef CAS.
- J. Bartolome, G. Filoti, V. Kuncser, G. Schinteie, V. Mereacre, C. E. Anson, A. K. Powell, D. Prodius and C. Turta, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 014430 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2327331. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4nr01619j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |