Modulated assembly and structural diversity of heterometallic Sn–Ti oxo clusters from inorganic tin precursors†
Hui-Fang
Zhao
a,
Fang-Fang
Liu
a,
Qing-Rong
Ding
a,
Di
Wang
a,
Jian
Zhang
 a and
Lei
Zhang
a and
Lei
Zhang
 *ab
*ab
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, China
bInstitute of Modern Optics, College of Electronic Information and Optical Engineering, Nankai University, Tianjin 300350, China. E-mail: zhanglei3915@nankai.edu.cn
First published on 10th August 2024
Abstract
Through modulating the multidentate ligands, solvent environments, and inorganic tin precursors during the synthesis processes, we have successfully prepared a series of unprecedented heterometallic Sn–Ti oxo clusters with structural diversity and different physiochemical attributes. Initially, two Sn6Ti10 clusters were synthesized using trimethylolpropane as a structure-oriented ligand and SnCl4·5H2O as a tin source. Then, when a larger pentadentate ligand di(trimethylolpropane) was used instead of trimethylolpropane and aprotic acetonitrile solvent was introduced into the reaction system, four low-nuclearity Sn–Ti oxo clusters were discovered, including two Sn1Ti1, one Sn2Ti2 and one Sn2Ti6. Finally, two mixed-valence state clusters, SnII4SnIV2TiIV14 and SnII4SnIV4TiIV20, were obtained by transforming the tin precursor from SnCl4·5H2O to SnCl2·2H2O and adjusting the acetonitrile solution with trace acetic acid/formic acid. Sn8Ti20 is the highest-nuclearity heterometallic Sn–Ti oxo cluster to date. Moreover, comparative electrocatalytic CO2 reduction experiments were carried out, and it was concluded that the Sn8Ti20-decorated electrode showed the most satisfactory performance due to the influence of mixed-valence states of the Sn atoms and the charging effects provided by 20 Ti4+ ions. This study presents important guiding significance for the design, synthesis and application optimization of functional heterometallic nanoclusters.
Introduction
Studying the formation mechanisms, structures, and properties of clusters not only builds a bridge between atoms/molecules and condensed states of matter, but also helps us to conduct precise research on molecular materials.1 Therefore, in recent years, the chemistry of metal oxo clusters (MOCs) has developed rapidly and MOCs with accurate atomic structural information have been synthesized continuously.2–6 Doping heteroatoms with metal oxides is considered one of the most effective ways to improve the catalytic performance of materials.7,8 This might occur because the composite material produces synergistic effects to improve its catalytic performance.9,10 Similarly, heterometallic oxo clusters combine the characteristics of MOCs and the interaction between different kinds of metals in their composition, thus giving the materials unique physical and chemical properties.11–16 However, although both homometallic Ti- and Sn-oxo clusters have been extensively reported, the number of heterometallic Sn–Ti oxo clusters is relatively small at present. Extensive research in this field has been limited to the discovery of a few Sn–Ti oxo clusters with low nuclearity (≤11), such as {Sn1Ti1},17,18 {Sn1Ti2},19,20 {Sn3Ti1},21 {Sn4Ti1},22 {Sn2Ti6},23 {Sn4Ti6}24 and {Sn6Ti5}.25 Very recently, we prepared three dual-layered Sn–Ti oxo clusters, two {Sn2Ti12} and one {Sn6Ti14}, which were functionalized with 1,10-phenanthroline and showed interesting optical limiting effects.26 Therefore, it is necessary to develop high-nuclearity Sn–Ti oxo clusters to enrich their structures and expand applications.In the synthesis of tin oxo clusters, organotin compounds have been used as the main tin sources, while a few examples have been prepared using inorganic tin sources. There is a significant difference between organotin and inorganic tin precursors in the synthesis reactions.27–30 Moreover, unlike the single valence state of organic tin sources, inorganic tin precursors have two different valence states: +2 and +4.23,31,32 In addition, an important advantage of MOCs is that their modifiable surface organic ligands can regulate the structures of the obtained clusters and improve their properties.33–35 Compared with organotin precursors, inorganic tin precursors not only are more environmentally friendly, but also more easily break Sn–X (F, Cl, Br, I, etc.) bonds, which are more likely to be modified by functional organic ligands during the reaction process.30
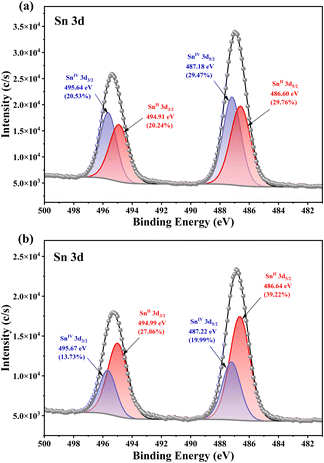
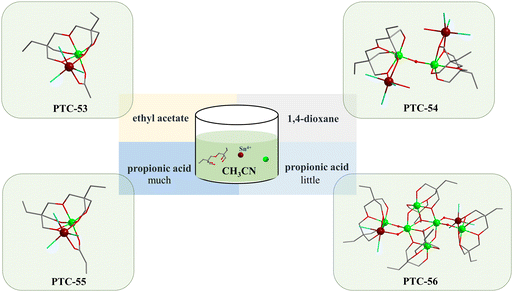
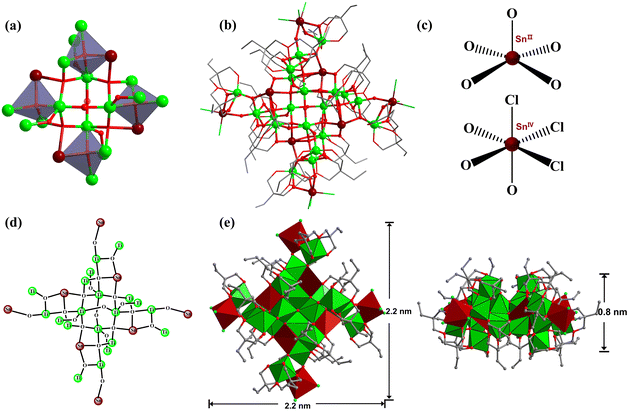
Usually, the multiple coordination modes of multidentate ligands can meet the spatial requirements and stabilize MOCs to the greatest extent.36–38 Herein, polyhydroxyalkanes with multiple mildly acidic hydroxyl groups were selected as the protecting ligands for the construction of heterometallic Sn–Ti oxo clusters, including trimethylolpropane (H3TPP) and di(trimethylolpropane) (H4DTPP). Through comprehensively adjusting solvents, tin precursors, temperature and other synthetic factors, the modulated assembly of a series of Sn–Ti oxo clusters was achieved (Table 1). As a result, seven intermediates were successfully identified, including two Sn6Ti10 clusters (TOC-51 and TOC-52), two Sn1Ti1 clusters (TOC-53 and TOC-54), one Sn2Ti2 cluster (TOC-55), one Sn2Ti6 cluster (TOC-56), and one Sn6Ti14 cluster (TOC-57), guiding to the targeted Sn8Ti20 cluster (TOC-58) eventually (Scheme 1). Among these, TOC-57 and TOC-58 show interesting mixed Sn2+/Sn4+ valences which were proved by bond-valence sum (BVS) calculations (Tables S5 and S6†), X-ray photoelectron spectroscopy (XPS) analysis (Fig. 1) and the charge balancing principle. The windmill-like structure of TOC-58 embodies a unique structural aesthetic. The core size of TOC-58 is ∼0.8 × 2.2 nm, and its nuclearity of 28 exceeds those of currently known heterometallic Sn–Ti oxo clusters (≤20). Electrocatalytic CO2 reduction (CO2RR) studies were conducted on three large clusters (TOC-51, TOC-57 and TOC-58), and the experimental results showed that the formate Faradaic efficiency (FEformate) of the electrode derived from TOC-58 with the largest {Sn8Ti20} core and mixed Sn2+/Sn4+ valences reached above 66% at −1.0 (vs. RHE).
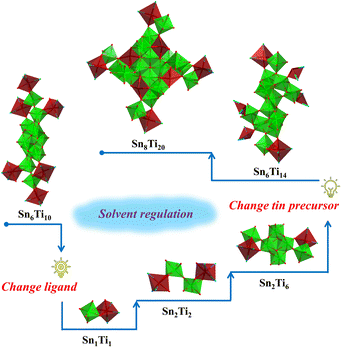 | ||
| Scheme 1 Illustration of the synthetic route from intermediates (Sn6Ti10, Sn1Ti1, Sn2Ti2, Sn2Ti6, and Sn6Ti14) to the largest Sn8Ti20. | ||
| Compound | Ligand | Sn source | Solvent | Formula | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbreviations: H3TPP = trimethylolpropane; H4DTPP = di(trimethylolpropane); HPr = propionic acid; HAc = acetic acid; 1,4-D = 1,4-dioxane. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TOC-51 | H3TPP | SnCl4·5H2O | 1,4-Dioxane | Ethyl acetate | [SnIV6TiIV10(μ4-O)2(μ3-O)2(μ2-O)4(μ2-OH)2(TPP)6(HTPP)4(Ac)2(H2O)2 Cl18]·2(1,4-D) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TOC-52 | H3TPP | Propionic acid | [SnIV6TiIV10(μ4-O)2(μ3-O)2(μ2-O)4(μ2-OH)2(TPP)6(HTPP)4(Pr)2(H2O)2 Cl18]·2(1,4-D) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TOC-53 | H4DTPP | Acetonitrile | Ethyl acetate | [SnIVTiIV (DTPP)(Ac)Cl3] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TOC-54 | H4DTPP | Propionic acid | [SnIVTiIV (DTPP)(Pr)Cl3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TOC-55 | H4DTPP | 1,4-Dioxane | [SnIV2TiIV2(μ2-O)(DTPP)2(H2O)2Cl6] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TOC-56 | H4DTPs | Propionic acid | H2[SnIV2TiIV6(μ2-O)6(DTPP)4Cl6] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TOC-57 | H4DTPP | SnCl2·2H2O | Acetic acid | [SnII4SnIV2TiIV14(μ4-O)2(μ3-O)4(μ2-O)8(DTPP)6(HDTPP)2Cl14] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TOC-58 | H4DTPP | Formic acid | [SnII4SnIV4TiIV20(μ4-O)8(μ3-O)4(μ2-O)10(DTPP)12Cl12] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Results and discussion
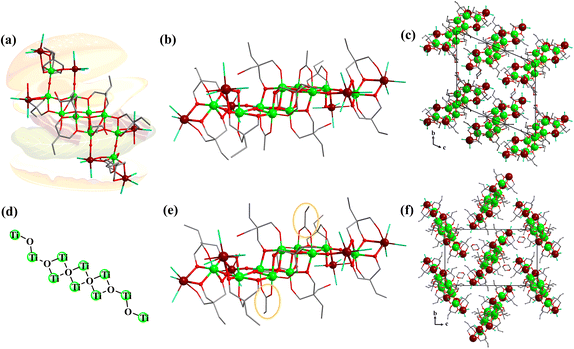
In the initial stage of this research, using SnCl4·5H2O, trimethylolpropane and Ti(OiPr)4 as raw materials, a colorless crystal of TOC-51 was synthesized by allowing it to stand at room temperature for 6 days after heating at 80 °C for 3 days in a mixture of 1,4-dioxane solvent and ethyl acetate. Ethyl acetate is easily hydrolyzed and will gradually produce acetic acid and ethanol during the reaction. As shown in Fig. 2a, single-crystal X-ray diffraction analysis indicates that trimethylolpropane collaborates with the auxiliary ligand HAc to form the hamburger-like structure of TOC-51. The protected inorganic Sn2Ti8 core is linked with two {Sn2Ti(TPP)(HTPP)Cl6} units by four μ2-O to form the whole TOC-51 cluster. The bread slices of the hamburger are {Sn2Ti(TPP)(HTPP)Cl6} moieties. The Sn2Ti8 core in the middle of the structure is composed of two μ4-O and two μ3-O connecting eight Ti atoms, while two Sn atoms are bonded to the Ti atoms by μ2-OH. In fact, there are two ways for O atoms in H3TPP to participate in the coordination. One is that two O atoms take part in the coordination and the other is that all three O atoms take part in the coordination. The Sn2Ti8 core is surrounded by six trimethylolpropanes, two acetic acids, and six Cl atoms. For the two Sn atoms in Sn2Ti8, there are two H2O molecules participating in their 6-coordination, respectively. Meanwhile, the acetic acid in TOC-51 can be replaced with propionic acid to form another new cluster TOC-52 (Fig. 2e). Although the two clusters are structurally identical except for modifying the same sites with different carboxylic acids, they are different in the packing modes (Fig. 2c and f). Both Sn atoms and Ti atoms exist with 6-coordination and +4 valence in the above two Sn6Ti10 clusters (TOC-51 and TOC-52).When only replacing the small-sized tridentate ligand H3TPP with the larger pentadentate ligand H4DTPP, there was no crystal growth in the original solvent environment. Through debugging the solvent, it was found that when aprotic solvent CH3CN was used, four new heterometallic Sn–Ti oxo clusters (TOC-53–TOC-56) were constructed with different auxiliary solvents (Fig. 3). TOC-53 was formed by the synergistic coordination of the organic ligand H4DTPP and the auxiliary ligand HAc from the decomposition of ethyl acetate solvent molecules. Just as the acetic acid in TOC-51 can be substituted by propionic acid to obtain TOC-52, the acetic acid in TOC-53 also can be replaced by propionic acid to give rise to TOC-54. The packing diagrams of TOC-53 and TOC-54 are also dissimilar (Fig. S1b and S2b†). TOC-55 can be seen as the connection of two TOC-51 clusters through a μ2-O between two Ti atoms, relying on H2O molecules to stabilize the 6-coordinate mode of Sn atoms. When we reduced the dosage of propionic acid to 50 μL while keeping the other conditions unchanged, TOC-56 was found. As shown in Fig. 3, TOC-56 can be regarded as the insertion of two DTPP ligands protecting four Ti atoms in the middle of the TOC-55 structure. In these four Sn–Ti heterometallic oxo clusters (TOC-53–TOC-56), Sn atoms and Ti atoms also exist in 6-coordinated and +4 valence states. Furthermore, energy dispersive spectroscopy (EDS) and plasma emission spectroscopy (ICP) analyses matched well with the obtained crystallographic results (Fig. S12–S15 and Table S7†).
Nevertheless, the cluster nuclearity was unable to further increase through continually adjusting the dosage of propionic acid or replacing propionic acid with other stronger/weaker acids. To break this deadlock, we considered introducing an inorganic tin raw material as SnCl2·2H2O with better reactivity. Fortunately, TOC-57 with a larger Sn6Ti14 core was obtained by changing the tin source from SnCl4·5H2O to SnCl2·2H2O and adjusting the acetonitrile solution with trace acetic acid (Fig. 4a). The cluster structure of TOC-57 can be seen to transform the four Ti atoms protected by DTPP ligands in the middle of TOC-56 into a Sn4Ti12 inorganic core encircled by DTPP ligands, to achieve a further increase in the number of cluster cores. Interestingly, BVS calculations (Table S5†) and XPS analysis (Fig. 1a) showed that the tin ions in TOC-57 were in mixed-valence states due to the partial oxidation of the initial Sn2+ ions during the synthesis process. The four Sn atoms in the Sn4Ti12 inorganic core display 3-coordination and +2 valence states. Therein, two Sn2+ ions are bridged with Ti atoms by two O atoms from two DTPP ligands and the other two Sn2+ ions are linked with Ti atoms by a μ2-O. The 12 Ti atoms in the inorganic core Sn4Ti12 are connected together by 4 μ2-O, 2 μ3-O, and 2 μ4-O. There are 6- and 7-coordination modes for Ti atoms, with two out of 14 Ti atoms being 7-coordinated.
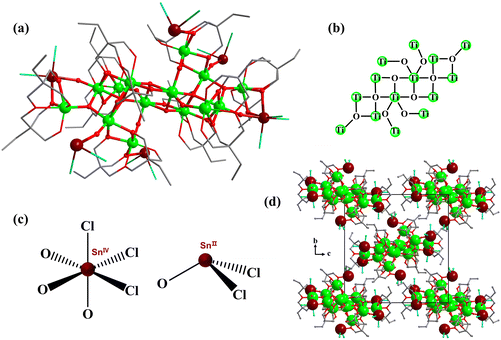
Based on the above synthesis results, we can clearly see that the assembly of heterometallic Sn–Ti oxo clusters was greatly affected by solvents and the pH value of the solution also played an important role in the assembly of the cluster core in this system. Accordingly, when we further replaced acetic acid with more acidic formic acid and the other conditions remained unchanged from those for TOC-57, a highly symmetric high-nuclearity Sn–Ti oxo cluster TOC-58 was synthesized (Fig. 5b). The cluster core of TOC-58 is Sn8Ti20, which represents the nuclearity record in the existing heterometallic Sn–Ti oxo clusters. The results of single-crystal X-ray diffraction show that it crystallizes in a cubic system with the space group I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3d. The atomic ratio of Sn to Ti was determined to be 8
3d. The atomic ratio of Sn to Ti was determined to be 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20.09 through ICP analysis (Table S7†), which is consistent with the structural analysis results. The cluster core size of TOC-58 is ∼2.2 × 0.8 nm (Fig. 5e). The crystal structure analysis reveals that TOC-58 has a central scale structure, and the whole cluster is like a windmill. At the top of the four blades of the windmill there are {SnTi(DTPP)Cl3} units, and the internal inorganic core is Sn4Ti16 (Fig. 5a). The Sn4Ti16 inorganic core of TOC-58 consists of four μ4-O bridged {SnTi3} tetranuclear parts and four Ti atoms are connected by four μ2-O. The two adjacent {SnTi3} tetranuclear moieties are connected by a μ4-O, and the linker between two opposite {SnTi3} tetranuclear parts is one μ2-O bridge. Moreover, each of the four Ti atoms forming the central scale has a μ2-O attached to a Ti atom. Four Ti atoms and four {SnTi3} tetranuclear moieties are distributed before and after in a regular pattern. The Sn4Ti16 inorganic core is surrounded by eight DTPP ligands. The tin atoms in TOC-58 are also in mixed-valence states as proved by BVS calculations (Table S6†) and XPS analysis (Fig. 1b). Different from the 3-coordination mode of Sn2+ ions in TOC-57 (Fig. 4c), the Sn2+ ions in TOC-58 adopt the 5-coordination mode (Fig. 5c). The 5-coordination tin atoms are connected to the 7-coordination Ti atoms by a μ4-O. In addition, the Ti4+ ions in TOC-58 are located in the {TiO7} or {TiO6} coordination geometry. Among the 20 Ti atoms, four are 7-coordinated which are distributed in four cubes, respectively. Because inorganic tin precursors are used, the Sn4+ ions in all clusters (TOC-51–TOC-58) are located in the {SnO3Cl3} coordination octahedra. By analysing the TG curve of TOC-58 (Fig. S19d†), it was observed that the skeleton of the compound remained relatively stable until ∼300 °C. Beyond this temperature, ligand detachment and structural decomposition occurred.
20.09 through ICP analysis (Table S7†), which is consistent with the structural analysis results. The cluster core size of TOC-58 is ∼2.2 × 0.8 nm (Fig. 5e). The crystal structure analysis reveals that TOC-58 has a central scale structure, and the whole cluster is like a windmill. At the top of the four blades of the windmill there are {SnTi(DTPP)Cl3} units, and the internal inorganic core is Sn4Ti16 (Fig. 5a). The Sn4Ti16 inorganic core of TOC-58 consists of four μ4-O bridged {SnTi3} tetranuclear parts and four Ti atoms are connected by four μ2-O. The two adjacent {SnTi3} tetranuclear moieties are connected by a μ4-O, and the linker between two opposite {SnTi3} tetranuclear parts is one μ2-O bridge. Moreover, each of the four Ti atoms forming the central scale has a μ2-O attached to a Ti atom. Four Ti atoms and four {SnTi3} tetranuclear moieties are distributed before and after in a regular pattern. The Sn4Ti16 inorganic core is surrounded by eight DTPP ligands. The tin atoms in TOC-58 are also in mixed-valence states as proved by BVS calculations (Table S6†) and XPS analysis (Fig. 1b). Different from the 3-coordination mode of Sn2+ ions in TOC-57 (Fig. 4c), the Sn2+ ions in TOC-58 adopt the 5-coordination mode (Fig. 5c). The 5-coordination tin atoms are connected to the 7-coordination Ti atoms by a μ4-O. In addition, the Ti4+ ions in TOC-58 are located in the {TiO7} or {TiO6} coordination geometry. Among the 20 Ti atoms, four are 7-coordinated which are distributed in four cubes, respectively. Because inorganic tin precursors are used, the Sn4+ ions in all clusters (TOC-51–TOC-58) are located in the {SnO3Cl3} coordination octahedra. By analysing the TG curve of TOC-58 (Fig. S19d†), it was observed that the skeleton of the compound remained relatively stable until ∼300 °C. Beyond this temperature, ligand detachment and structural decomposition occurred.
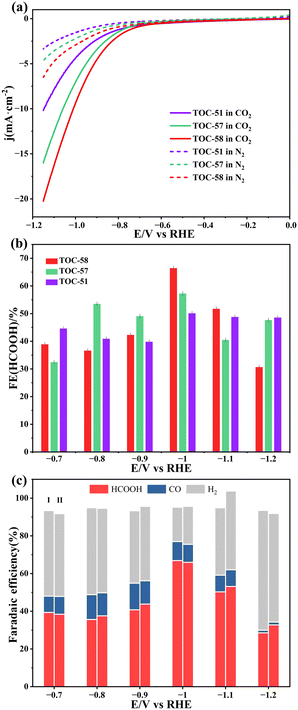
Sn containing materials are regarded as suitable CO2RR electrocatalysts to produce formic acid.39–46 Considering that TOC-51, TOC-57 and TOC-58 present potential active sites of Sn atoms (Fig. S7†), their applications in electrocatalysis were studied. First, linear sweep voltammetry (LSV) profiles of the three sample-modified working electrodes were measured in 0.5 M KHCO3 solution filled with N2 or CO2 (Fig. 6a). It was clear that the current densities of TOC-51-, TOC-57- and TOC-58-derived electrodes in CO2-saturated electrolyte were higher than those in N2-saturated electrolyte, revealing the capacity of the CO2RR. The TOC-58-modified electrode displayed the lowest initial potential, indicating the fastest electrocatalytic kinetics. The liquid product of the CO2RR was detected by nuclear magnetic resonance spectroscopy (Fig. S25†), and the analysis demonstrated that formate was the only liquid product. The FEformic was measured by ion chromatography (Fig. S26†), and the values for H2 (Fig. S27†) and CO (Fig. S28†) were measured by gas chromatography.
As shown in Fig. 6b, the FEformate of the TOC-51-modified electrode shows little difference under different voltages with the highest value being ∼51% at −1.0 V (vs. RHE). The electrode modified with TOC-57 exhibits the lowest FEformate at −0.7 (vs RHE) and it increases to around 57% at −1.0 V (vs. RHE). The highest FEformate of the electrode modified with TOC-58 exceeds 66% at −1.0 (vs. RHE). Although the number of Sn active sites in TOC-58 is less than that in TOC-57, it contains more Ti4+ to achieve better charging effects.24 Meanwhile, the mixed-valence states of Sn atoms show better catalytic activity than the single valence state.31 These comprehensive factors make TOC-58 exhibit the best catalytic effect. The stability of these cluster decorated electrodes was confirmed by the well-maintained mass spectrometry and XPS spectra after electrocatalysis (Fig. S31–S33†).
Conclusions
In conclusion, we used inorganic tin precursors and functional tridentate H3DPP and pentadentate H4DTPP ligands to direct the assembly of heterometallic Sn–Ti oxo clusters. A family of compounds with various nuclearities was successfully constructed by adjusting the solvents and changing the inorganic tin sources. Therein, Sn8Ti20 (TOC-58) presents the highest nuclearity and the largest size in the heterometallic Sn–Ti oxo cluster field. Moreover, mixed Sn2+/Sn4+ valent states have been achieved by the introduction of SnCl2·2H2O during synthesis. Both the mixed-valence states of Sn atoms and the charging effects of Ti4+ ions show influence on the electrocatalytic activity of these heterometallic Sn–Ti oxo clusters, with the TOC-58-decorated electrode exhibiting the highest FEformate (over 66%). This work not only enriches the structural diversity of Sn–Ti oxo clusters, but also provides an efficient strategy for improving their physiochemical performance.Data availability
The data supporting this article have been included as part of the ESI.†Crystallographic data for TOC-51 to TOC-58 have been deposited at the Cambridge Crystallographic Data Centre (CCDC) deposition numbers 2365528 to 2365535.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Natural Science Foundation of Fujian Province (2022J02013) and the National Natural Science Foundation of China (22271284).References
- L. Liu and A. Corma, Chem. Rev., 2018, 118, 4981–5079 CrossRef CAS PubMed.
- J. C. Liu, J. F. Wang, Q. Han, P. Shangguan, L. L. Liu, L. J. Chen, J. W. Zhao, C. Streb and Y. F. Song, Angew. Chem., Int. Ed., 2021, 60, 11153–11157 CrossRef CAS PubMed.
- N. I. Gumerova and A. Rompel, Chem. Soc. Rev., 2020, 49, 7568–7601 RSC.
- X. Y. Zheng, Y. H. Jiang, G. L. Zhuang, D. P. Liu, H. G. Liao, X. J. Kong, L. S. Long and L. S. Zheng, J. Am. Chem. Soc., 2017, 139, 18178–18181 CrossRef CAS PubMed.
- D. Wang, X. F. Yi and L. Zhang, Sci. China: Chem., 2021, 65, 114–119 CrossRef.
- P. Yang and U. Kortz, Acc. Chem. Res., 2018, 51, 1599–1608 CrossRef CAS PubMed.
- L. Yang, J. Lei, J. M. Fan, R. M. Yuan, M. S. Zheng, J. J. Chen and Q. F. Dong, Adv. Mater., 2021, 33, e2005019 CrossRef PubMed.
- X. Yang, D. Schipper, R. A. Jones, L. A. Lytwak, B. J. Holliday and S. Huang, J. Am. Chem. Soc., 2013, 135, 8468–8471 CrossRef CAS PubMed.
- G. Soldan, M. A. Aljuhani, M. S. Bootharaju, L. G. AbdulHalim, M. R. Parida, A. H. Emwas, O. F. Mohammed and O. M. Bakr, Angew. Chem., Int. Ed., 2016, 55, 5749–5753 CrossRef CAS PubMed.
- H. Zhang, M. Jin and Y. Xia, Chem. Soc. Rev., 2012, 41, 8035–8049 RSC.
- F. F. Liu, D. Wang, G. H. Chen, Y. Qiao, F. Luo, J. Zhang and L. Zhang, Sci. China: Chem., 2023, 66, 1731–1736 CrossRef CAS.
- J. X. Liu, X. B. Zhang, Y. L. Li, S. L. Huang and G. Y. Yang, Coord. Chem. Rev., 2020, 414, 213260 CrossRef CAS.
- Y. Liu, Y. Li, Y. Yang, J. Zhu and K. Wu, Sci. China: Chem., 2023, 66, 3628–3635 CrossRef CAS.
- B. L. Han, Z. Wang, R. K. Gupta, L. Feng, S. Wang, M. Kurmoo, Z. Y. Gao, S. Schein, C. H. Tung and D. Sun, ACS Nano, 2021, 15, 8733–8741 CrossRef CAS PubMed.
- Z. Wang, R. Senanayake, C. M. Aikens, W. M. Chen, C. H. Tung and D. Sun, Nanoscale, 2016, 8, 18905–18911 RSC.
- G. Zhang, M. Baranov, F. Wang, J. M. Poblet, S. Kozuch, N. Leffler, A. I. Shames, J. M. Clemente-Juan, A. Neyman and I. A. Weinstock, J. Am. Chem. Soc., 2021, 143, 20769–20778 CrossRef CAS PubMed.
- S. Mishra, E. Jeanneau, S. Mangematin, H. Chermette, M. Poor Kalhor, G. Bonnefont, G. Fantozzi, S. Le Floch, S. Pailhes and S. Daniele, Dalton Trans., 2015, 44, 6848–6862 RSC.
- S. Mishra, E. Jeanneau, L. Tian, I. Nuta, E. Blanquet, D. Singh, R. Ahuja, C. Marichy and S. Daniele, Cryst. Growth Des., 2021, 22, 54–59 CrossRef.
- M. Veith, S. Mathur, C. Mathur and V. Huch, Organometallics, 1998, 17, 1044–1051 CrossRef CAS.
- S. Mishra, E. Jeanneau, M. H. Berger, J. F. Hochepied and S. Daniele, Inorg. Chem., 2010, 49, 11184–11189 CrossRef CAS PubMed.
- T. J. Boyle, J. M. Segall, T. M. Alam, M. A. Rodriguez and J. M. Santana, J. Am. Chem. Soc., 2002, 124, 6904–6913 CrossRef CAS PubMed.
- Q. Zhang, H. D. Lai and Q. P. Lin, J. Solid State Chem., 2021, 297, 122056 CrossRef CAS.
- D. Wang, G. H. Chen, L. B. Yuan, C. C. Feng, J. Zhang and L. Zhang, Chem. – Eur. J., 2021, 27, 16117–16120 CrossRef CAS PubMed.
- D. Wang, Z. N. Chen, Q. R. Ding, C. C. Feng, S. T. Wang, W. Zhuang and L. Zhang, CCS Chem., 2020, 2, 2607–2616 Search PubMed.
- J. Wang, M. Luo and Q. P. Lin, J. Solid State Chem., 2024, 335, 124723 CrossRef CAS.
- H. F. Zhao, W. Z. Chen, S. T. Wang, S. Chen, J. Zhang and L. Zhang, J. Mater. Chem. C, 2024, 12, 4771–4778 RSC.
- M. Mehring, M. Schürmann, K. Jurkschat, H. Reuter and D. Dakternieks, Angew. Chem., Int. Ed., 1997, 109, 1150–1152 CrossRef.
- S. Saha, D. H. Park, D. C. Hutchison, M. R. Olsen, L. N. Zakharov, D. Marsh, S. Goberna-Ferron, R. T. Frederick, J. T. Diulus, N. Kenane, G. S. Herman, D. W. Johnson, D. A. Keszler and M. Nyman, Angew. Chem., Int. Ed., 2017, 56, 10140–10144 CrossRef CAS PubMed.
- X. J. Tian, Y. Z. Yu, Q. Lu and X. M. Zhang, Inorg. Chem., 2022, 61, 6037–6044 CrossRef CAS PubMed.
- B. Peters, N. Lichtenberger, E. Dornsiepen and S. Dehnen, Chem. Sci., 2020, 11, 16–26 RSC.
- D. Wang, G. H. Chen, S. T. Wang, J. Zhang and L. Zhang, Chem. Commun., 2022, 58, 4759–4762 RSC.
- K. Suzuki, T. Hanaya, R. Sato, T. Minato, K. Yamaguchi and N. Mizuno, Chem. Commun., 2016, 52, 10688–10691 RSC.
- S. Bhattacharya, U. Basu, M. Haouas, P. Su, M. F. Espenship, F. Wang, A. Sole-Daura, D. H. Taffa, M. Wark, J. M. Poblet, J. Laskin, E. Cadot and U. Kortz, Angew. Chem., Int. Ed., 2021, 60, 3632–3639 CrossRef CAS PubMed.
- C. Zhao, Y. Z. Han, S. Dai, X. Chen, J. Yan, W. Zhang, H. Su, S. Lin, Z. Tang, B. K. Teo and N. Zheng, Angew. Chem., Int. Ed., 2017, 56, 16252–16256 CrossRef CAS PubMed.
- W. Huang, W. Chen, Q. Bai, Z. Zhang, M. Feng and Z. Zheng, Angew. Chem., Int. Ed., 2022, 61, e202205385 CrossRef CAS PubMed.
- Q. R. Ding, Y. Yu, C. Cao, J. Zhang and L. Zhang, Chem. Sci., 2022, 13, 3395–3401 RSC.
- R. D. Lai, J. Zhang, X. X. Li, S. T. Zheng and G. Y. Yang, J. Am. Chem. Soc., 2022, 144, 19603–19610 CrossRef CAS PubMed.
- S. Bhattacharya, U. Basu, M. Haouas, P. Su, M. F. Espenship, F. Wang, A. Sole-Daura, D. H. Taffa, M. Wark, J. M. Poblet, J. Laskin, E. Cadot and U. Kortz, Angew. Chem., Int. Ed., 2021, 60, 3632–3639 CrossRef CAS PubMed.
- L. Fan, Z. Xia, M. Xu, Y. Lu and Z. Li, Adv. Funct. Mater., 2018, 28, 1706289 CrossRef.
- J. T. Feaster, C. Shi, E. R. Cave, T. Hatsukade, D. N. Abram, K. P. Kuhl, C. Hahn, J. K. Nørskov and T. F. Jaramillo, ACS Catal., 2017, 7, 4822–4827 CrossRef CAS.
- D. D. Zhu, J. L. Liu and S. Z. Qiao, Adv. Mater., 2016, 28, 3423–3452 CrossRef CAS PubMed.
- Y. Deng, J. Zhao, S. Wang, R. Chen, J. Ding, H. J. Tsai, W. J. Zeng, S. F. Hung, W. Xu, J. Wang, F. Jaouen, X. Li, Y. Huang and B. Liu, J. Am. Chem. Soc., 2023, 145, 7242–7251 CrossRef CAS PubMed.
- R. Samanta, M. Kempasiddaiah, R. K. Trivedi, B. Chakraborty and S. Barman, ACS Appl. Energy Mater., 2024, 7, 5359–5370 CrossRef CAS.
- B. Sun, Z. Li, D. Xiao, H. Liu, K. Song, Z. Wang, Y. Liu, Z. Zheng, P. Wang, Y. Dai, B. Huang, A. Thomas and H. Cheng, Angew. Chem., Int. Ed., 2024, 63, e202318874 CrossRef CAS PubMed.
- Z. Guo, Y. Yu, C. Li, E. Campos dos Santos, T. Wang, H. Li, J. Xu, C. Liu and H. Li, Angew. Chem., Int. Ed., 2024, 63, e202319913 CrossRef CAS PubMed.
- X. Mei, C. Liu, D. Zhang, J. Cao, R. Ge, J. Wang and W. Xu, Adv. Energy Mater., 2024, 14, 2303889 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2365528–2365535 (TOC-51–TOC-58). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4nr02644f |
| This journal is © The Royal Society of Chemistry 2024 |