Anionic vacancy engineering in the development of electrocatalysts for hydrogen evolution reaction: a review
Junheng
Tang
a,
Xiaobin
Liu
*ab,
Xinping
Wang
a,
Jingqi
Chi
a,
Zhenyu
Xiao
 *a,
Zexing
Wu
*a,
Zexing
Wu
 a and
Lei
Wang
a and
Lei
Wang
 *a
*a
aKey Laboratory of Eco-chemical Engineering, International Science and Technology Cooperation Base of Eco-chemical Engineering and Green Manufacturing, College of Marine Science and Biological Engineering, Qingdao University of Science and Technology, Qingdao 266042, PR China. E-mail: inorchemwl@qust.edu.cn; liuxb@qust.edu.cn; inorgxiaozhenyu@163.com
bCollege of Environment and Safety Engineering, Qingdao University of Science and Technology, Qingdao 266042, PR China
First published on 18th July 2024
Abstract
Water splitting, one of the emerging ways to produce hydrogen, has the advantages of being clean, simple and sustainable. The hydrogen evolution reaction (HER), as one of the half-reactions of water splitting, is considered to be the simplest electrochemical reaction as well as the basis for the study of more complex electrochemical reactions. Therefore, modification strategies for HER catalysts have also been widely investigated. Vacancy engineering, as one of the modification strategies for catalysts, has been widely used due to the advantages of its simple strategy and various modulation possibilities. This review aims to provide a comprehensive overview of recent advances in the field of anionic vacancies in HER catalysts. First, the characterization, introduction, and detection methods of vacancies are systematically described. Second, a list of common types of anionic vacancies available and their applications is given. An in-depth exploration of specific modification methods for anionic vacancies in HER catalysts follows. Finally, the prospects and challenges of anionic vacancies in the field of HER catalysts are discussed.
1. Introduction
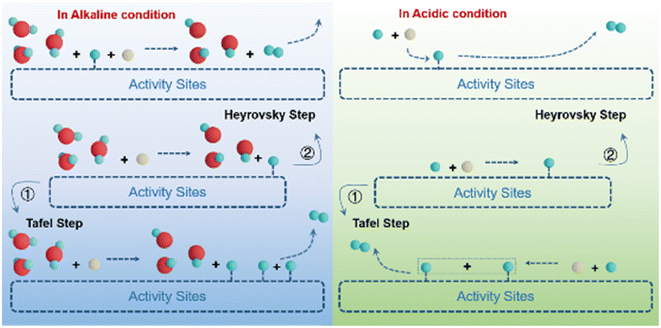
Hydrogen, as an alternative to traditional fossil energy sources, has the advantages of high energy density, clean and renewable products.1–4 Hydrogen production from water splitting using renewable energy sources is the most promising hydrogen production technology for sustainable development.5–7 As one of the half-reactions of water electrolysis, the hydrogen evolution reaction (HER) is a different reaction pathway in acidic and alkaline media (Fig. 1).8–11In acidic media, the HER follows the following steps:
| Volmer step: H3O+ + e− → Hads + H2O |
| Heyrovsky step: Hads + H3O+ + e− → H2 + H2O |
| Tafel step: Hads + Hads → H2 |
In alkaline media, the HER follows the following steps:
| Volmer step: H2O + e− → Hads + OH− |
| Heyrovsky step: Hads + H2O + e− → H2 + OH− |
| Tafel step: Hads + Hads → H2 |
The HER proceeds according to the Volmer–Heyrovsky/Volmer–Tafel procedure in both acidic and basic media.12 In large-scale industrial applications, the HER is usually carried out in alkaline media, as this provides maximum protection for the electrodes against corrosion.13–15 However, the overall reaction rate in alkaline media is several magnitudes slower than that of the HER in acidic media due to the absence of hydrogen ions.16,17 Even in acidic media, the HER activity of the catalysts is still unsatisfactory due to the catalyst morphology, its own electronic structure, and electrical conductivity.18,19 Therefore, both acidic and basic HER require the development of efficient electrocatalysts for the economic viability of hydrogen production from water electrolysis.20 In the past decades of development, Pt remains the most effective catalyst for the HER due to its position at the center of the catalyst volcano diagram.21,22 However, the expensive price, scarce reserves, and unfriendly alkaline HER properties of Pt have limited its development and application.23,24 Non-precious metals such as Fe, Co, Ni, and precious metals such as Ru and Rh have been studied more and more extensively as substitutes for Pt in recent years.8,25–29 However, the intrinsic activity of these catalysts still needs to be improved.
Strategies such as designing unique nanostructures, altering the electronic structure and bonding through heteroatom doping, designing unique heterojunctions for catalytic partitioning, and defect engineering can effectively improve the intrinsic activity of catalysts (Fig. 2).30–33 Nanostructure design, as one of the catalyst modification strategies, can optimize the HER activity of catalysts by exposing more active sites on the catalyst surface through unique morphology modulation.34–36 Asefa et al. synthesized cobalt-embedded nitrogen-rich carbon nanotubes (NRCNTs) from inexpensive starting materials (dicyandiamide and cobalt chloride) to achieve full pH electrocatalytic hydrogen precipitation.37 For example, Gao et al. prepared nanoflower catalysts with a Pt content of 15 wt% by pyrolysis of Hofmann-type metal–organic frameworks (MOFs), and the catalysts exhibited excellent HER performance.38 The experimental results indicate that the nanoflower-like multilevel structure has abundant edge-exposed bias alloy active sites, which are conducive to the promotion of basic HER catalytic activity. Heteroatom doping, as one of the effective modulation strategies, can effectively modulate the electronic structure while inevitably generating lattice distortions as well as defects.39,40 Han et al. explored the effect of doping on the adsorption behavior of intermediates by doping a series of 3d excess metals into three-dimensional Ni nanofilms.41 Doping with heteroatoms in carbon nanomaterials and MOFs can also serve to tailor their physicochemical properties.42–45 The combination of precious metals with suitable non-metallic substrates will also have an impact on enhancing the activity of the catalysts towards the HER.46 Thus, a heterogeneous structure can generate electron redistribution at the interface of the two phases and promote the synergistic effect between the two phases to achieve efficient HER.47 Sathe et al. prepared Ni/NiO@rGO nanostructures by chemical synthesis for overall water decomposition reaction with excellent electrocatalytic activity, in which the synergistic effect of Ni and NiO enhanced both the stability as well as the activity.48 Fan's group prepared a Mo8O26-NbNxOy heterostructure through hydrothermal synthesis and N-processing, achieving efficient hydrogen production from water electrolysis at full pH.49 Not only these modification strategies, but also some other electrocatalytic reactions can also affect the performance of HER catalysts. Sathe et al. sensitized silver nanoparticles-sensitized C60 for hydrazine oxidation and also explored its effect on the HER.50 Although these strategies are effective in modulating the intrinsic activity of catalysts, they still have some shortcomings. For example, the design of some nanostructures often leads to structural changes in the catalyst, affecting catalytic stability; too much heteroatom doping occasionally leads to a decrease in catalyst conductivity and mechanical strength; it takes too long to select the right two-phase substance to build the desired heterogeneous structure; and so on.51,52 Anionic vacancies, as a form of defect engineering, tend to introduce lattice mismatches in crystals, change the work function of the catalyst, and enhance electrical conductivity.52 Yan et al. accelerated the migration as well as the accumulation of actives by loading Pt species on MgO nanosheets with oxygen-rich vacancies, where the oxygen vacancies favored the generation of electron-rich Pt sites.53 In addition, Li's group explored the positive role of P vacancies in promoting the HER by studying the effect of P vacancies on the electronic structure of catalysts in MoP.54 Similar to O and P vacancies, anionic vacancies such as S, Se and N are also effective in increasing the intrinsic activity of catalysts.55–57 Nowadays, with the continuous development of characterization techniques, it is possible to discuss the application of anionic vacancies in HER in a more comprehensive way.
 | ||
| Fig. 2 Schematic of different modulation strategies for catalysts.58–63 Reproduced from ref. 58 with permission from Wiley, copyright 2022. Reproduced from ref. 59 with permission from Wiley, copyright 2022. Reproduced from ref. 60 with permission from Nature Publishing Group, copyright 2023. Reproduced from ref. 61 with permission from Wiley, copyright 2022. Reproduced from ref. 62 with permission from Nature Publishing Group, copyright 2022. Reproduced from ref. 63 with permission from Wiley, copyright 2023. | ||
In this review, the mode of introduction of anionic vacancies is explored and the means of characterization of anionic vacancies are enumerated. The synthesis methods, design principles, and performance tests of HER electrocatalysts with anionic vacancies are summarized, and the mechanism of the anionic vacancy-promoted HER is discussed in detail. Finally, this paper summarizes the problems and challenges in the application of anionic vacancies in HER catalysts, and at the same time looks forward to the future development of anionic vacancies in HER catalysts. This review provides new ideas for further studies of anionic vacancies in HER catalysts.
2. How anionic vacancies are introduced
Overall, the methods for introducing vacancies can be broadly categorized as follows: chemical or electrochemical reduction, plasma treatment, charge compensation, and a number of other methods with similar effects. We display in brief in Fig. 3 some of the methods for the introduction of anionic vacancies, including the construction of reducing environments, charge compensation, plasma treatment, and other unconventional methods.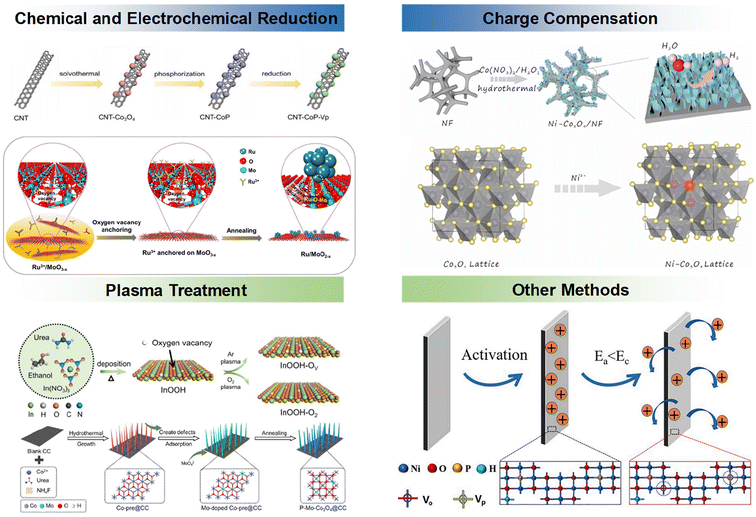 | ||
| Fig. 3 Summary of methods for introducing anionic vacancies using different strategies.64–68 Reproduced from ref. 64 with permission from Wiley, copyright 2022. Reproduced from ref. 65 with permission from Elsevier, copyright 2022. Reproduced from ref. 66 with permission from Elsevier, copyright 2022. Reproduced from ref. 67 with permission from Nature Publishing Group, copyright 2023. Reproduced from ref. 68 with permission from Wiley, copyright 2022. | ||
2.1. Chemical reduction and electrochemical reduction
Chemical reduction is a simple and feasible strategy for introducing anion vacancies into electrocatalysts via various reductants. Reductants are usually divided into three types: gas (such as H2, NH3, etc.), liquid (such as NaBH4, N2H4), and solid (Al, Zn). Bai et al. prepared CNT-CoP containing different P vacancy concentrations by immersion in NaBH4.64 Hou et al. used the reducibility of H2 to prepare oxygen vacancy-rich molybdenum trioxide nanosheets by a tube furnace annealing process (Ru/MoO2−x). Compared with traditional chemical reduction, electrochemical reduction is a milder way to generate anion vacancies in electrocatalysts, and the number of anion vacancies can be controlled by adjusting the current and time.65 For example, Liang et al. reconstructed the surface structure of In2O3 by linear voltammetry (LSV), and successfully synthesized In/In2O3−x containing a certain amount of oxygen vacancies.69 During the reduction process, the concentration of vacancies is often controlled by controlling the time of chemical reduction. Li et al. successfully prepared δ-MnO2 zinc ion battery cathode materials with different vacancy contents by controlling the reduction time of KBH4.702.2. Plasma treatment
The plasmon can interact with the surface atoms in the electrocatalyst structure, and the energy of the plasmon will be transferred from the energetic particles to the surface atoms, breaking the covalent bonds and causing the surface atoms to pop out to form vacancies, and commonly used plasmas include Ar plasma and the like. In addition to Ar plasma, plasma treatment of reactive gases such as H2 and NH3 can make surface atoms react with free radicals in the gas, effectively creating vacancies. Tang et al. successfully prepared heteroatom Mo-substituted and oxygen vacancy-enriched Co3O4 porous nanopin array catalysts using an Ar plasma treatment (P-assisted) strategy.68 Fang et al. used the same method to prepare InOOH nanosheets with different oxygen vacancy content by plasma treatment, which successfully improve the intrinsic activity of the catalyst.67 Sun et al. modulated the oxygen vacancy concentration of the samples by irradiating Bi2O3 under N2 plasma for different times.712.3. Charge compensation
When a metal ion replaces another metal ion in the lattice, it will occupy the position of the original ion in the lattice. After replacing the original ion, ion vacancies will be generated to maintain charge neutrality. This method is called charge compensation. Local lattice distortion can also induce the redistribution of electrons in the catalyst, thereby optimizing the electronic structure, thus improving the catalytic activity of catalyst. As described below, oxygen vacancies were successfully introduced into Ni-doped Co3O4 nanosheets by introducing Ni2+ into Co3O4, as reported by Zhong et al.722.4. Other methods
In addition to the abovementioned anion vacancy introduction methods, anion vacancies can also be produced in electrocatalysts by heat treatment and low-valent metal doping. For example, Hao et al. used P-NiO, which underwent an electrically derived reduction reaction at low applied voltages, resulting in the formation of P vacancies and O vacancies. Ramasubramaniam et al. successfully introduced Se vacancies in MoSe2 by doping with transition metal atoms (Fe, Mn and Ni).73 As the electron destabilization and hydrogen adsorption of Se atoms near the electron-rich transition metal dopant weakens the metal–Se bond, the formation of Se vacancies on the basal surface is thermodynamically more feasible.In summary, the formation mechanism of anionic vacancies is as follows, using CeO2 as an example to illustrate the formation mechanism of oxygen vacancies. For CeO2, each Ce provides 4 electrons to fill the p-orbitals of O. When a reducing atmosphere occurs, the oxygen atoms leave the lattice, i.e., oxygen vacancies. Meanwhile, when the lattice of CeO2 is doped with metal ions of other valence states, CeO2−x with non-stoichiometric ratio will be formed, resulting in the generation of oxygen vacancies.
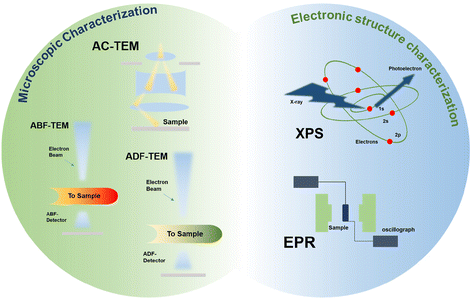
3. Methods for detecting anion vacancies
The detection methods for vacancies can be broadly classified into two categories, microscopic characterization methods and electronic structure characterization methods (Fig. 4).Microscopic characterization techniques have been considered to be the most direct way to observe vacancies. High-resolution spherical aberration-corrected transmission electron microscopy (AC-TEM) can provide structural information, on the atomic scale, of catalysts and is a powerful tool for observing anion vacancies. For example, Wu et al. detected the presence of C vacancies in the catalyst using the AC-STEM technique (Fig. 5a and b).74 Photon-sensitive annular bright-field scanning TEM (ABF-STEM) and atomic-resolution annular dark-field scanning TEM (ADF-STEM) can also be used to distinguish vacancies in electrocatalysts. For example, Lin et al. applied ABF-STEM to detect O vacancies in Li2MnO3 samples, and it can be clearly observed that the loss of O in the crystal leads to the existence and structural distortion of O vacancies in Li2MnO3 (Fig. 5c).75 Scanning tunneling microscopy (STEM) has been widely used to detect vacancies due to its high spatial resolution capability, which can visually present the atomic vacancies in electrocatalysts. Zhang et al. detected the absence of Ti atoms in Ti3C2Tx using STEM characterization (Fig. 5d).76 Although microscopic techniques can be used to visually distinguish vacancies, spectroscopic techniques are indispensable in analyzing the atomic structure, which provides deeper information on anionic vacancies. X-ray photoelectron spectroscopy (XPS) is a facile technique for examining the chemical state and elemental composition of the surface of materials. The work of Guo et al. as well as Long et al. used XPS tests to characterize the O 1s orbitals, confirming the presence of oxygen vacancies (Fig. 5e and f).77,78 If there are vacancies on the surface of the material, the chemical environment and electronic structure of each element may change, manifested as various differences in XPS spectra, such as new peaks, peak shifts, intensity enhancement and weakening, etc. In addition, spectroscopic techniques such as electron paramagnetic resonance (EPR) and electron spin resonance (ESR) are also helpful in analyzing the electronic environment around vacancies. For example, Mi et al. detected abundant Se vacancies in VSe2−x/C using the EPR technique. The peak at g = 2.003 indicates the existence of electrons in two states where the spin magnetic moment is in the same opposite direction to the magnetic field, which successfully demonstrates the successful introduction of vacancies (Fig. 5g).79
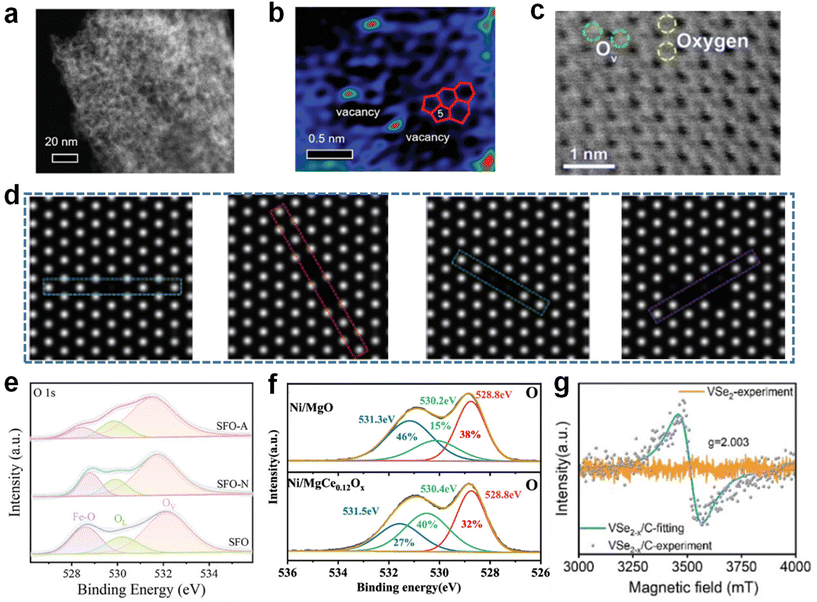 | ||
| Fig. 5 (a) The AC-TEM image and (b) filtered image after Fourier transformation of TC-900. Reproduced from ref. 74 with permission from Wiley, copyright 2024. (c) The ABF-STEM image of Li2MnO3. Reproduced from ref. 75 with permission from Elsevier, copyright 2023. (d) The STEM image of Ti2C2Tx. Reproduced from ref. 76 with permission from Springer, copyright 2024. (e) XPS spectra of O 1s in SFO-A, SFO-N and SFO. Reproduced from ref. 77 with permission from American Chemical Society, copyright 2022. (f) XPS spectra of O 1s in Ni/MgO and Ni/MgCe0.12Ox. Reproduced from ref. 78 with permission from Wiley, copyright 2024. (g) EPR spectra of VSe2−x/C. Reproduced from ref. 79 with permission from Wiley, copyright 2024. | ||
At the same time, Raman spectroscopy and X-ray absorption fine structure (XAFS) have been widely used in the detection of vacancies. Tong et al. synthesized Eu-doped CeO2, which promoted the formation of surface defects.80 Meanwhile, Raman spectroscopy was used to characterize the oxygen vacancies in the materials. The characteristic peaks around 600 cm−1 in the Raman spectrum are oxygen vacancies introduced due to the presence of Ce3+ as well as Eu in CeO2. Similarly, Ye et al. demonstrated the presence of oxygen vacancies in the material by detecting a characteristic peak located near 566 cm−1 in Raman spectra.81 Li et al. demonstrated an increase in electron density as well as a decrease in the number of coordination sites in Co3O4 samples after UV-induced treatments by means of the XAFS technique, demonstrating the formation of oxygen vacancies together with ESR.82
Structure–property correlation is an important point for catalysts.83–85 Metal oxygen and compounds of the same main group (M–O (S, Se, Te…)) have been shown in previous studies to have certain catalytic activity and modification capabilities due to their complex valence states, variable crystal structures, rich ligand relationships and large electronic tunability.86,87 However, their catalytic activity is still lacking. Therefore, vacancy engineering has achieved a wide range of applications in related catalysts. Common types of anionic vacancies are O vacancies, P vacancies, S vacancies, and Se vacancies.88,89
4. Overview of different types of anionic vacancies
4.1. O vacancy
Transition metal oxides have superior catalytic potential relative to semiconductor materials and have made great progress in HER catalysis in recent years. However, as HER catalysts, the activity of conventional transition metal oxides still cannot meet the demand.90 HER catalysts modulated by oxygen vacancies are starting to come into view.TiO2 catalysts with Pt nanoclusters loaded with oxygen vacancies (Pt/TiO2-Vo) were prepared by solvent-free microwave and low-temperature chemical plating by Ma et al. (Fig. 6a).91 The introduction of oxygen vacancies successfully enhanced the catalyst activity, and the HER performance was superior to that of conventional Pt/C (Fig. 6b). The presence of oxygen vacancies enabled Pt/TiO2−x to obtain an overpotential enhancement of 21 mV at a current density of 10 mA cm−2 (18 mV vs. 39 mV). Liu et al. also used this method of heteroatom doping to generate vacancies. La-RuO2 nanocrystals were successfully prepared by doping rare earth elements in noble metals by using a combination of heteroatom doping and oxygen vacancies, and the activity of RuO2 with reduced noble metal dosage was enhanced.92 Notably, lattice defects due to oxygen vacancy generation were also found at the RuO2 lattice edge (Fig. 6c). Typically, when ions with lower oxidation states are used to replace ions with higher oxidation states, oxygen vacancies and lattice distortions tend to be generated in the catalyst to maintain electrical neutrality, which is called charge compensation.93,94 In 2022, Shen et al. successfully prepared Ni-Co3O4 as an efficient HER electrocatalyst by doping Ni2+ into the Co3O4 lattice using a charge compensation method. When Ni2+ ions occupy the octahedral sites in Ni-Co3O4, oxygen vacancies and lattice distortion will be generated to maintain the electronic neutrality of the catalyst (Fig. 6d).95,96
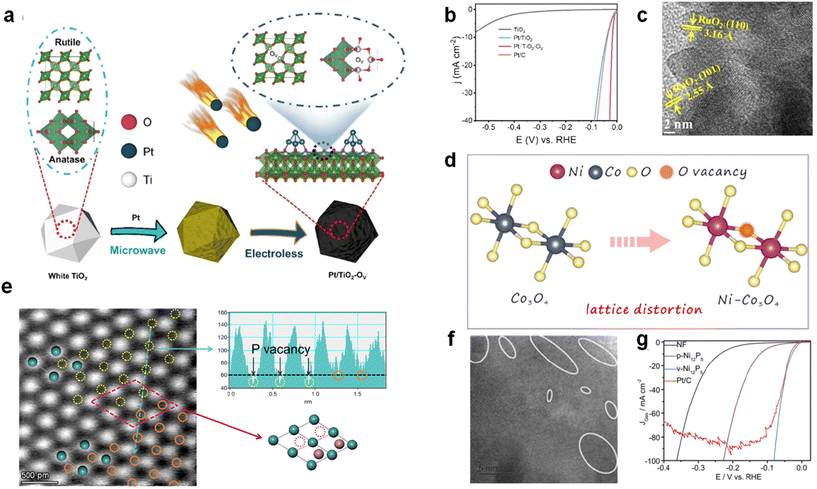 | ||
| Fig. 6 (a) Schematic diagram of Pt/TiO2-Vo synthesis. (b) LSV curve of Pt/TiO2-Vo. Reproduced from ref. 91 with permission from Wiley, copyright 2023. (c) SEM of La-RuO2. Reproduced from ref. 92 with permission from Elsevier, copyright 2022. (d) Schematic representation of the Co3O4 and Ni-Co3O4 lattice structures. Reproduced from ref. 95 with permission from Elsevier, copyright 2016. (e) HAADF-STM of MoP-Vp. Reproduced from ref. 98 with permission from Wiley, copyright 2024. (f) High-resolution TEM image; white circles mark the possible Pv areas. (g) LSV curves of different sample. Reproduced from ref. 99 with permission from Wiley, copyright 2020. | ||
4.2. P vacancy
P, as a representative element of main group V, has many similarities with the oxygen group elements in terms of electronic structure and other aspects. Therefore, the P vacancy is also one of the commonly used anionic vacancies.97A considerable number of modification strategies have been developed for the recently emerging HER catalyst MoP. Li's group prepared MoP enriched with phosphorus vacancies by H2 reduction in a negative pressure environment,98 and the presence of P vacancies in the catalyst was confirmed by HAADF-STM (where the yellow and orange regions represent the absence of P as well as the presence of P, respectively) (Fig. 6e). Qiao's group used annealing in commonly used NiP catalysts to generate P vacancies,99 which can be found in the HRTEM mapping in Fig. 6f, where the white regions indicate the presence of certain defects in the lattice. The enhancement of P vacancies for catalyst activity was also demonstrated in LSV tests (Fig. 6g).
4.3. S and Se vacancy
Defect engineering is often also applied to the modification of transition metal dichalcogenide (TMD) catalysts.100 For example, MoS2 catalysts with uniformly distributed monoatomic S vacancies were prepared by a simple chemical etching method, as reported by Kang et al.101 It was confirmed that single-atom S vacancies have superior HER properties than cluster-type S vacancies (Fig. 7a). Similarly, Xue et al. prepared SnS2 substrates by the chemical vapor deposition (CVD) technique and continued treatment with reducing gas at different temperatures to prepare SnS2 with different S vacancy concentrations.102 As can be seen in the HAADF-STEM image of Fig. 7b, the concentration of S vacancies increases in this way as the annealing temperature increases. The significant improvement in HER performance by vacancy concentration modulation is evident in the LSV curves of Fig. 7c. Liu et al. optimized molybdenum dichalcogenides with a hexagonal structure by defect engineering,62 and synthesized 2H-MoS2-VS bulk crystals with S vacancies using topochemical reactions as well as chemical etching that displayed excellent HER properties. DFT calculations of the system MoS2 were performed using a model containing 36 atoms by Liu et al., in which a vacuum plate of 15 Å was inserted in the z-axis direction for surface isolation, which was used to eliminate periodic interactions. From the differential charge densities in Fig. 7d, it can be seen that the presence of vacancies changes the charge state of the atoms surrounding the vacancies, which in turn has an effect on the HER performance.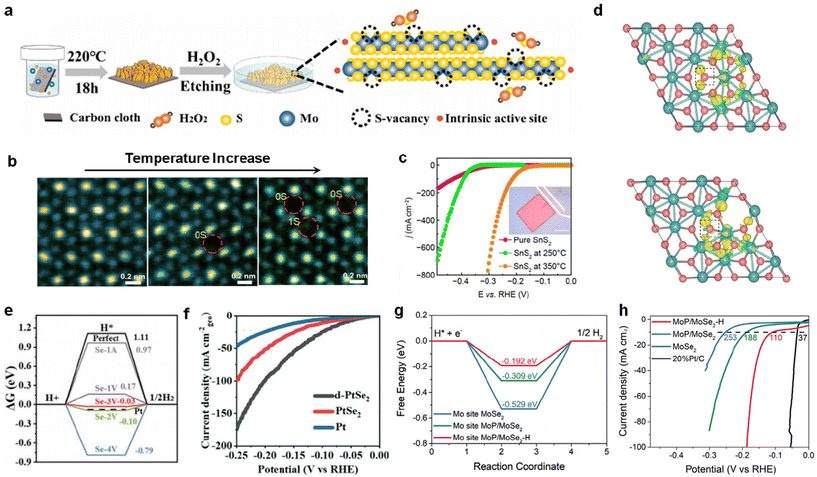 | ||
| Fig. 7 (a) Schematic of the etching project with the introduction of a single S-vacancy. Reproduced from ref. 101 with permission from American Chemical Society, copyright 2020. (b) Atomic resolution HAADF-STEM image of the catalyst with increasing temperature. Reproduced from ref. 102 with permission from Springer, copyright 2022. (c) LSV curves of different catalysts. (d) Calculated differential charge densities for different S sites. Reproduced from ref. 62 with permission from Nature Publishing Group, copyright 2022. (e) Free energy during HER of different Se vacancy configurations. (f) LSV curve of d-PtSe2, PtSe2 and Pt in 1 M KOH. Reproduced from ref. 104 with permission from Wiley, copyright 2022. (g) Free energy diagram for Mo sites of MoSe2, MoP/MoSe2, and MoP/MoSe2-H. (h) LSV curves of MoSe2, MoP/MoSe2, and MoP/MoSe2-H. Reproduced from ref. 63 with permission from Wiley, copyright 2023. | ||
Se is an element of the same main group as O and S, therefore Se vacancies are naturally similar to O and S vacancies.103 However, the application of Se vacancies alone in HER catalysts has rarely been reported. Gao et al. succeeded in trapping the local charge in favor of the HER by introducing Se vacancies in PtSe2.104 An 8 × 8 × 1 PtSe2 superunit under the gamma point was used for the calculations, which ensured sufficient spacing between the periodic vacancies and took into account the spin-polarization effect due to defects. The introduction of vacancies improved the adsorption behavior of H* on the active site (Fig. 7e), resulting in excellent HER kinetics for the catalyst (Fig. 7f). Se vacancies are often also reported to act synergistically with other modification strategies to promote the HER.105 Song et al. prepared MoP/MoSe2-H catalysts with a large number of active sites as well as excellent charge transfer capability by combining the strategies of defect engineering and heterogeneous structure design.63 The designed heterogeneous structure contributes to the apparent activity of the catalyst, while the binary vacancies of P and Se enhance the intrinsic activity of the catalyst. The introduction of double vacancies improved the intermediate adsorption energy of the surrounding Mo sites, thus increasing HER activity (Fig. 7g and h).
4.4. Unusual anionic vacancies
In addition to O, S, Se and P vacancies, a number of other anionic vacancies have been applied to HER catalyst modification.106 Te, as a congener element of O, can also alter the intrinsic activity of the catalyst by generating anionic vacancies.107 MoTe2 has excellent HER potential as a typical layered TMDs material (1.0 eV) with a small band gap.108 Based on this property, Liu et al. prepared single MoTe2 nanosheets as electrocatalysts using a semiconductor micro-nano process, and laser irradiation was employed to introduce Te vacancies within the 2H-MoTe2 2D plane (Fig. 8a).109 Firstly, theoretical calculations were carried out on the 2H-MoTe2, and it was found that after the introduction of Te vacancies, additional defect energy levels were created that reduce the band gap and improve the conductivity of the catalysts (Fig. 8b). Secondly, the introduction of Te vacancies led to an out-of-domain charge distribution of Mo atoms around the Te vacancies, which helped to promote the adsorption of intermediates during the HER process (Fig. 8c). On the experimental side, Mo's K-edge XAFS test showed a significant reduction in the negative displacement of the plots after the laser treatment. This indicates a reduced splitting of the molecular orbitals and represents a weaker coordination capacity, and this weakening of the coordination capacity of Mo may be due to the introduction of Te vacancies (Fig. 8d). From the LSV curves in Fig. 8e, it is found that the performance of the catalyst shows a significant improvement after the introduction of Te vacancies. After the introduction of the Te vacancy, the overpotential was raised from 0.41 V to 0.32 V at a current density of 10 mA cm−2, while the Tafel slope was reduced to 155 mV dec−1. DFT calculations were performed for the catalysts with respect to the adsorption capacity of H* intermediates. The Te and Mo sites in P-MoTe2 both have considerable ΔGH* when no vacancies were introduced, and this large ΔGH* is not favorable for the adsorption of hydrogen intermediates. After the introduction of the Te vacancy, the ΔGH* of the Mo site around the Te vacancy was reduced to −0.27 eV, which significantly optimized the adsorption behavior of the intermediate. The improvement of intrinsic activity of the catalyst is ascribed to Te vacancies, and this phenomenon is in line with other previous studies on the enhancement of catalyst activity by anionic vacancies (Fig. 8f).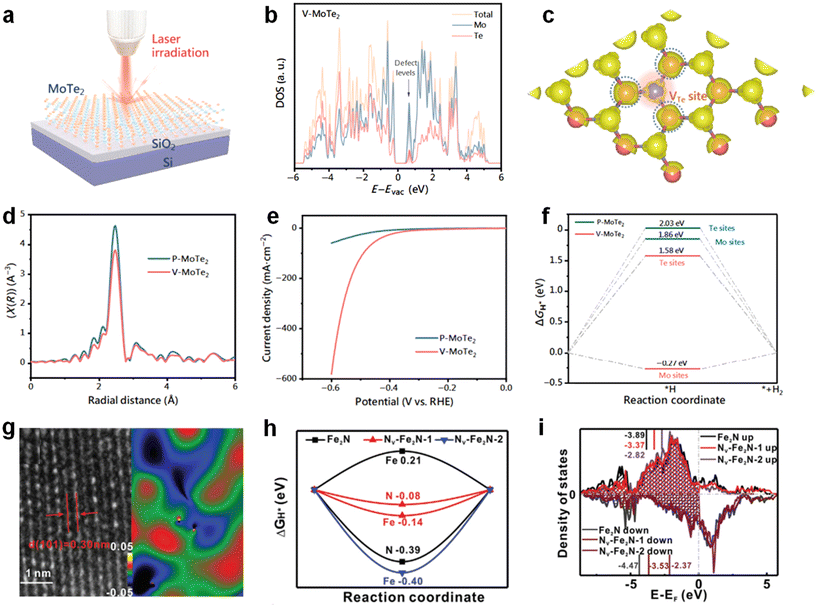 | ||
| Fig. 8 (a) Schematic of how to introduce empty space by laser method. (b) Calculated DOS for V-MoTe2. (c) Charge density distribution calculation of V-MoTe2. (d) The FT-EXAFS spectra of Mo K-edge. (e) LSV curves of different samples. (f) Free energy profiles for H adsorption on different sites. Reproduced from ref. 109 with permission from Springer, copyright 2021. (g) HRTEM image of Nv-Fe2N and a schematic of the corresponding lattice distortion. (h) H adsorption free energy on different samples. (i) The density of states (DOS) of different samples. Reproduced from ref. 111 with permission from Wiley, copyright 2020. | ||
Transition metal nitrides have a wide range of applications in electrocatalysis as well as in batteries due to their excellent electrical conductivity, excellent stability and other advantages.110 Zheng et al. employed a simple N vacancy-mediated orbital orientation strategy to rationally regulate the electronic coupling between Fe2N and adsorbed H to further optimize the HER performance.111 As can be seen in Fig. 8g, there is a lattice distortion in Nv-Fe2N due to hydrogen treatment, which is caused by the generation of N vacancies. As can be seen from the LSV curves, the HER activity of Nv-Fe2N is significantly better than that of the other catalysts, which proves the promotion of the HER by N vacancies. In order to explore how Nv affects the HER performance of the catalyst at a deeper level, DFT calculations were performed on the system. It can be seen that Nv-Fe2N-1 has a H closer to thermal neutrality relative to Nv-Fe2N-2, which suggests that Nv facilitates the optimization of the ΔGH* adsorption behavior of the catalyst (Fig. 8h). From the densities of states of different catalysts close to the Fermi energy level, it can be seen that there is an upward shift of the valence band center with the increase of Nv concentration, which suggests a decrease in the electron filling of the antibonding state, which leads to a change in the strength of the intermediate adsorption (Fig. 8i). The introduction of N vacancies makes the overpotential of Nv-Fe2N-350 at a current density of 10 mA cm−2 only 51 mV, which is much lower than that of Fe2N at 161 mV. This confirms the modulation of catalyst performance by the introduction of anionic vacancies.
The above results indicate that the introduction of anionic vacancies can effectively enhance the activity of the catalyst.
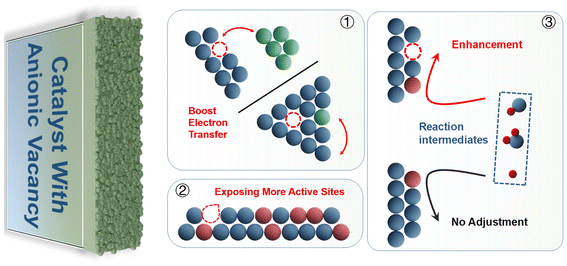
5. Influence of anion vacancies on catalyst
The mechanisms of anion vacancy modification in HER catalysts can be broadly categorized into three types: effects on the lattice structure, effects on the electronic structure, and effects on the adsorption capacity of the reaction intermediates (Fig. 9). These three regulatory mechanisms synergize and promote each other to enhance the intrinsic activity of the catalyst.5.1. Effect of vacancies on lattice structure
In order to explore in more depth the specific effect of anion vacancies on catalyst activity, we further discuss the specific changes that occur when oxygen vacancies are introduced inside the catalyst.The crystal lattice signifies the orderly arrangement of atoms within a crystal. Introducing anion vacancies often triggers alterations in this set structure. In a study conducted by Su's group, CoMoO4 nanosheets with varying oxygen vacancy concentrations were successfully fabricated. They achieved this by first securing the CoMoO4 nanosheets onto nickel foam via a hydrothermal method, and subsequently annealing these under a hydrogen–argon mixed atmosphere for different durations.98 As can be seen in Fig. 10a, a significant decrease in crystallinity occurs in CoMoO4 with the introduction of vacancies, which is responsible for the structural deformation due to oxygen vacancies. Our work introduced P vacancies in Fe-Ni2P and found that the introduction of P vacancies also changed the lattice structure.112 In the HRTEM of Fig. 10b, it can be seen that the lattice stripe spacing increases from 0.22 nm to 0.23 nm after the introduction of vacancies, which indicates that some degree of aggregation or stretching of atoms around the vacancy occurs. Liu's group prepared Co@CoO catalysts at different annealing temperatures,113 and the prepared catalysts had different vacancy concentrations (Fig. 10c). The shift of the A1g vibrational mode peak attributed to Co–O in Raman confirms the presence of altered Co–O bonding (Fig. 10d). XRD tests were carried out on samples with different vacancy concentrations, and it was found that the (200) crystallographic peaks of CoO in samples with the presence of O vacancies are all shifted in the direction of the lower binding energy relative to the pure CoO phase, and the amount of the shift is proportional to the concentration of the vacancies (Fig. 10e). This phenomenon tentatively confirms that the introduction of vacancies led to a lattice expansion of CoO. Not only that, but the samples were also subjected to XAS testing, and from the FT-EXAFS, it can be seen that the Co–Co peak length of Co@CoO-350 grows with respect to Co/CoO, suggesting that the presence of preferred vacancies leads to lattice expansion within CoO (Fig. 10f). This alteration of the lattice can often change the intrinsic activity of the catalyst, leading to richer active sites.114 The electrochemical double-layer capacitance (Cdl) of the catalyst and the turnover frequency (TOF) are often used to assess the number of active sites as well as the activity of the catalyst. In the work of our group, it can be seen that the presence of P vacancies brings greater Cdl and TOF to the catalyst, indicating that Fe-Ni2Pv has a richer active center (Fig. 10g and h). Tests on Faraday efficiency (FE) show that the average value of Faraday's efficiency is 97.8%.112 Li et al. activated inert CeO2 by using a rare earth metal and overmetal double doping strategy to induce lattice strain and oxygen vacancies, and also explored the interactions between compressive strain and oxygen vacancies in the material. This simultaneous presence of lattice strain and oxygen vacancies enhances the alkaline HER performance of the catalyst.115
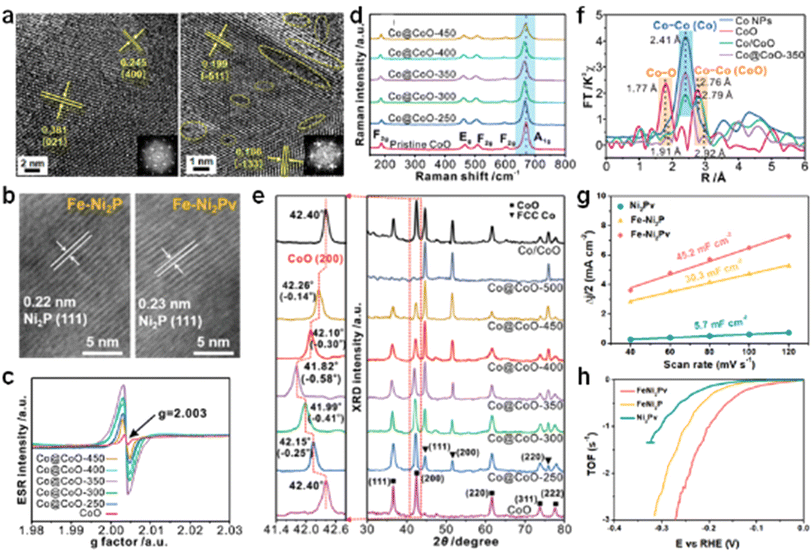 | ||
| Fig. 10 (a) TEM images of AP-CoMoO4 and 30-CoMoO4. Reproduced from ref. 98 with permission from Wiley, copyright 2024. (b) HRTEM images of Fe-Ni2P and Fe-Ni2Pv. Reproduced from ref. 112 with permission from Wiley, copyright 2024. (c) EPR spectra, (d) Raman spectra and (e) XRD spectra of Co@CoO-T. (f) FT-EXAFS of Co@CoO-T. Reproduced from ref. 113 with permission from Wiley, copyright 2021. (g) The capacitive current as a function of the scan rate for different samples. (h) The capacitive current as a function of the scan rate for different samples towards HER. Reproduced from ref. 112 with permission from Wiley, copyright 2024. | ||
At the same time, changes in the lattice structure tend to change the coordination of the catalyst, altering the spatial distribution of forces on the electron cloud orbitals of the atom at the center of the catalyst, and thus affecting the electron spin state. After the anionic vacancy changes the catalyst lattice structure, it will change the coordination mode of the central atom, thus optimizing the d-band center and further affecting the adsorption state of the intermediate. This may also be one of the ways in which anionic vacancy modulates the catalyst activity.116,117
5.2. Implications for electronic structure
Changes in the lattice structure due to the introduction of vacancies usually alter the coordination structure as well as parameters such as bond angles.118,119 Also, the change of these parameters induces a redistribution of the electron cloud and better electron transfer kinetics.120For example, Wang et al. prepared dual functional catalysts consisting of amorphous RuO2 and crystalline NiO for the HER as well as the urea oxidation reaction (UOR).121 The addition of amorphous RuO2 increased the oxygen vacancies in the catalyst (Fig. 11a). In order to further explore the electronic structure of the catalyst, the total density of states (TDOS) and projected density of states (PDOS) were conducted. It was found from Fig. 11b that after the introduction of amorphous RuO2, the catalyst had more electronic states near the Fermi level, indicating that the increase of oxygen vacancies improved the conductivity of the catalyst and accelerated the electron transfer. Meanwhile, an asymmetric spin-electronic behavior was observed in PDOS with or without the introduction of amorphous RuO2, and calculations showed that the amorphous RuO2 coupling increased the net spin by 0.03e−, which also increased the total magnetization strength (Fig. 11c). This suggests that the introduction of vacancies changes the polarization of the metal atoms and optimizes the electronic structure of the catalyst.
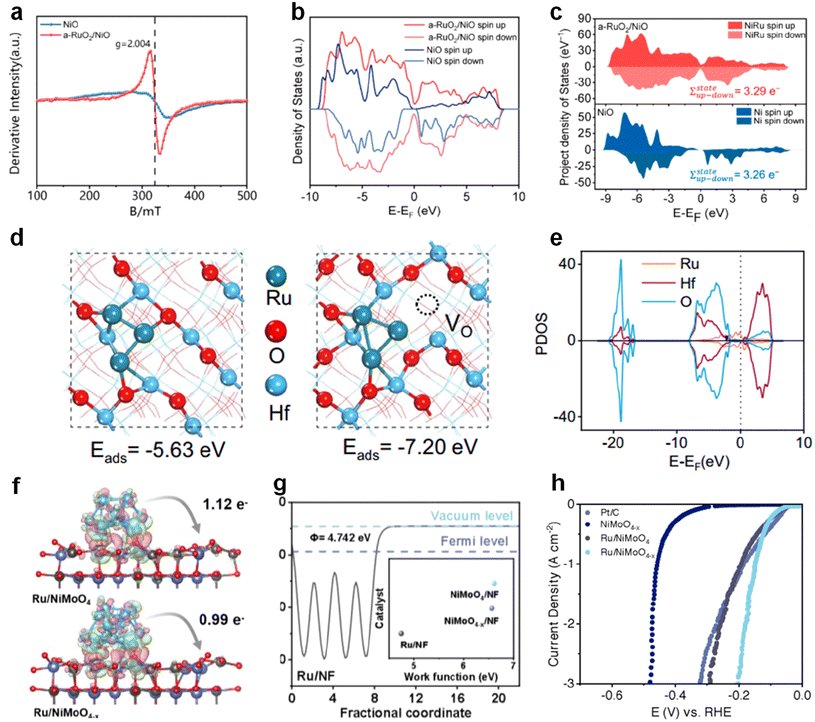 | ||
| Fig. 11 (a) The EPR spectra of RuO2/NiO and NiO. (b) TDOS and (c) PDOS of different samples. Reproduced from ref. 121 with permission from American Chemical Society, copyright 2024. (d) The most stable structure and adsorption energy of the Ru3 cluster adsorbed on a HfO2-OP and VO-HfO2-OP. (e) PDOS of VO-HfO2-OP. Reproduced from ref. 122 with permission from Nature Publishing Group, copyright 2022. (f) The differential charge density distributions between Ru and NiMoO4/NiMoO4−x. (g) Work function of different samples. (h) LSV curves. Reproduced from ref. 123 with permission from Wiley, copyright 2024. | ||
Anion vacancies are also extensively employed to modify the electronic structure of metal carrier-type catalysts. A notable example is the work by Qin and colleagues, where they developed an alkaline HER catalyst featuring ruthenium (Ru) nanoparticles loaded onto Vo-HfO2.122 In Fig. 11d, the degree of stabilization of Ru clusters was determined by calculating the adsorption energy of Ru on different samples, and the results showed that oxygen vacancies stabilized the Ru clusters in the catalysts. It is also demonstrated by PDOS that there is a strong overlap between different orbitals of different atoms, suggesting that vacancies enhance the interaction between Ru and the substrate to some extent (Fig. 11e). Wang's team loaded Ru onto vacancy-rich NiMoO4, achieving an efficient hydrogen spillover effect by optimizing the electronic structure of the two phases to enhance HER performance.123 From the differential charge densities, it is evident that the introduction of oxygen vacancies successfully reduces the electron transfer between the two phases, allowing more electrons to be retained on Ru and thereby facilitating the hydrogen transfer step (Fig. 11f). This transfer is attributed to the introduction of oxygen vacancies, which reduce the work function of NiMoO4 and enhance the driving force for electron transfer from NiMoO4 to the Ru phase (Fig. 11g). This vacancy-driven electron transfer kinetics successfully accelerated the reaction kinetics of the HER to obtain excellent hydrogen transport activity (Fig. 11h).
Similarly, Zhang et al. successfully synthesized Co9S8 nanosheets with abundant S vacancies to enhance the HER activity of the catalyst,124 revealing the formation of sulfur vacancies (S vacancies) in the HER and identifying the active center of catalyst activity. Sv-Co9S8 was synthesized by subjecting the inorganic–organic hybrid precursor, Co9S8-TETA, to an Ar plasma atmosphere. Following this Argon plasma treatment, the triethylenetetramine (TETA) within the precursor is entirely removed. During this process, some S atoms in Co9S8 can also be removed, inducing the formation of S vacancies (Fig. 12a), and the EPR results in the literature also demonstrate the existence of S vacancies (Fig. 12b). From the charge density difference analysis in Fig. 12c, it can be seen that around the Co3c site, the charge is shared by Co and S atoms, so the adsorption capacity of H on this site is weak. After the formation of the Co(OH)2/Sv-Co9S8 heterostructure mentioned above, the charges previously enriched on the S atom and the O atom in Co(OH)2 will be transferred to the Co3c site, enhancing the adsorption ability of the active H intermediate and promoting the occurrence of the HER. The specific HER performance is shown in Fig. 12d; it has the lowest overpotential at a current density of 100 mA cm−2. Tests of Faraday efficiency showed that the Faraday efficiency of the system was close to 100%, indicating that no electrons were supplied to other side reactions. Hu et al. successfully prepared P-doped NiS2 nanorods by combining two modulations of heteroatom doping and S vacancy engineering, achieving efficient water electrolysis.125 The preparation process is shown in Fig. 12e. The P doping in NiS2 triggers more S vacancies in the P-NiS2-500 compared with the pure NiS2 phase (Fig. 12f). The electronic structure of the catalyst was then analyzed by DFT calculations. DFT calculations were performed using a NiS2 model with 114 atoms extending along the (001) direction. From left to right, Fig. 12g shows the Bader charge schematic of NiS2, Vs-P-NiS2 and the charge density schematic of Vs-P-NiS2, respectively. In Vs-P-NiS2, the q-value of S atoms near the S vacancy and the connection of Ni to P atoms is reduced compared with pure NiS2. This suggests that the presence of S vacancies increases the number of electrons on the Ni atom, thereby enhancing Ni's ability to leave the electron domains and improving its frontal electrical conductivity. This indicates that optimizing the electronic structure of the catalyst through anionic vacancies may be one of the reasons for the improved intrinsic activity of the catalyst. The results also demonstrated that the H* adsorption behavior at the Ni site was significantly optimized (closer to 0) after the introduction of S vacancies. Although the HER activity at the S site was slightly reduced, the activity at the Ni site was notably enhanced.
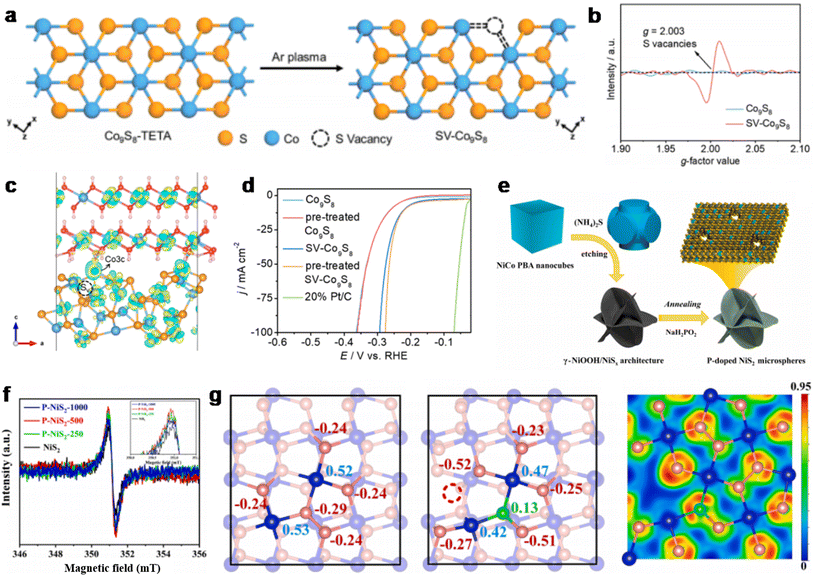 | ||
| Fig. 12 (a) Schematic diagram of the synthesis of SV-Co9S8. (b) The EPR spectra of SV-Co9S8. (c) Schematic representation of the differential charge density of SV-Co9S8. (d) LSV curves of different catalysts. Reproduced from ref. 124 with permission from American Chemical Society, copyright 2022. (e) Schematic diagram of the synthesis of Vs-P-NiS2. (f) The EPR spectra of Vs-P-NiS2. (g) The Bader charge numbers of atoms in pure NiS2, Vs-P-NiS2 and charge density distribution of Vs-P-NiS2. Reproduced from ref. 125 with permission from Elsevier, copyright 2021. | ||
The evidence presented underscores the potential of introducing anionic vacancies as a means to alter the electron distribution within catalysts and fine-tune their energy band structure. Such modifications have been demonstrated to enhance the HER performance. While these findings are promising, the method for optimizing this modulation for the purpose of maximizing HER activity warrants further in-depth investigation.
5.3. Effect of anions on the adsorption behavior of intermediates
Changes in the electronic structure around the active site often lead to changes in the reaction kinetics at the site.126,127 Moreover, the adsorption behavior of intermediates at the active sites during the HER process is a critical factor influencing electrocatalytic kinetics. Consequently, exploring the “anion vacancy–electronic structure–active site adsorption behavior” pathway has emerged as a prominent research direction in recent years. This increasing focus aims to provide deeper insights into the mechanism and enhance our ability to control the HER process effectively.128In the previously described P vacancy-containing Ni12-P5 (v-Ni12-P5) reported by Qiao et al.,99 the catalysts showed significant electron redistribution due to the introduction of P vacancies. Obvious regions of electron loss and electron accumulation can be seen from the electron distribution diagrams (Fig. 13a), with electron-rich areas marked in red and electron-deficient regions marked in blue. The crystal structure of Ni12P5 has been constructed based on the single cell of I4/m Ni12P5 in DFT calculations and its (420) crystallographic plane has been studied. The Ni 3d DOS of v-Ni12-P5 is slightly left-shifted with respect to p-Ni12-P5, which suggests that Ni in v-Ni12-P5 has a higher electron density, probably because the P vacancy attenuates the hybridization of the Ni 3d and P 2p orbitals (Fig. 13b). DFT calculations show that these changes in electronic structure alter the energy barrier of the HER path. The free energy change (ΔG) is a key factor in characterizing the HER activity of the catalyst. Since v-Ni12-P5 has a ΔG value closer to thermal neutrality, it is known that the P vacancy promotes the H* resolution step, which significantly enhances the intrinsic activity of the catalyst (Fig. 13c and d). The P vacancies enrich the electron density on Ni and P atoms and kinetically reduce the limiting potential of the HER. Sabatier's principle states that the interaction between the catalyst and the substrate must be appropriate; if the adsorption is too weak, the molecule cannot bind to the catalyst to react, and if the interaction is too strong, the reaction product will not be able to detach from the catalyst. For the HER, this principle can then be mapped to the adsorption state of the catalyst on intermediates. Yao et al. prepared MoOx/Ni3S2/NF catalysts using a one-step solvothermal method, confirming the presence of oxygen vacancies through EPR testing.129 From the differential charge densities, it can be seen that in the samples where oxygen vacancies are present, there is a stronger electron transfer between MoOx and Ni3S2, where the O site acquires more electrons, while causing the Mo site to assume an absence of electronic state (Fig. 13e). This change in electronic state is key to optimizing the adsorption behavior of H* on the catalyst. Subsequently, DFT calculations of the adsorption energy of H* were performed for different sites on each sample (Fig. 13f). It can be seen that the adsorption behavior of hydrogen intermediates at the Mo sites around the vacancies is greatly improved after the introduction of the vacancies. The results show that after modification, the Faraday efficiency is close to 100%.
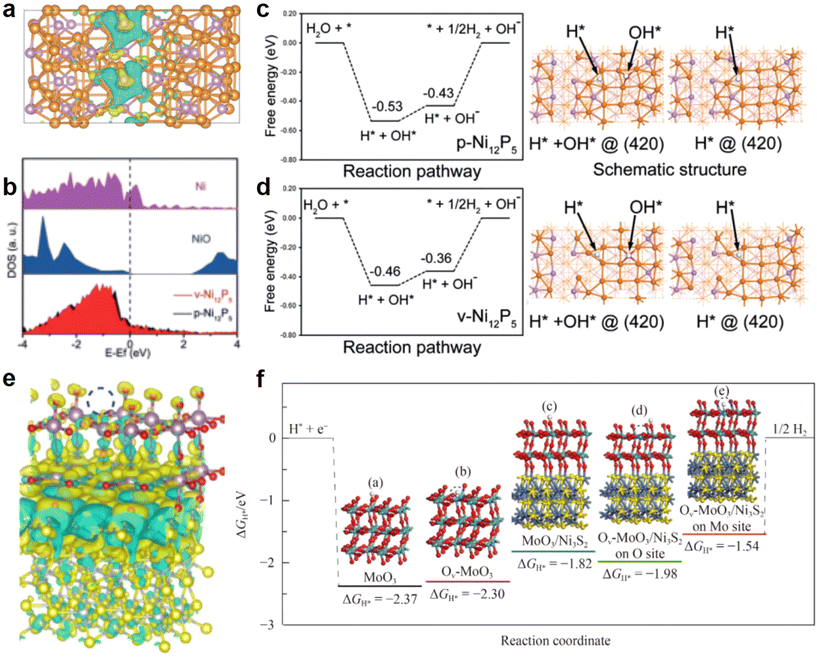 | ||
| Fig. 13 (a) Electron distribution; yellow area indicates electron accumulation while cyan means electron depletion. (b) DOS of Ni 3d. (c) DFT calculated of reaction pathways and the adsorption modeling of p-Ni12P5. (d) DFT calculated of reaction pathways and the adsorption modeling of v-Ni12P5. Reproduced from ref. 99 with permission from Wiley, copyright 2020. (e) Different charge density distribution of Ov-MoOx/Ni3S2/NF. (f) Free energy calculations for different H* adsorption states on different catalysts. Reproduced from ref. 129 with permission from Springer, copyright 2023. | ||
Based on the same idea, Zhu et al. synthesized 1T-PtSe2 ultrathin ribbons on gold foil by chemical vapor deposition.130 DFT calculations were performed at the base, edges of the structure, and the Se vacancies (Fig. 14a and b). The experimental results show that ΔGH* is relatively low at the edge positions as well as at the vacancies, which suggests that Se vacancies can be introduced at the basal plane to improve the catalyst activity (Fig. 14c). The situation related to P vacancies has also been explored in depth by Tao et al.131 Ni0.96Co0.04P electrocatalysts were prepared by introducing Co atoms for surface modulation of NiP and generating a large number of P vacancies with finely tuned Co content. The successful synthesis of two-phase heterostructures was first demonstrated by HRTEM (Fig. 14d). The catalyst was then characterized on an atomic scale, and the presence of P vacancies was successfully demonstrated by atomic intensity analysis of the red region in Fig. 14e and f. In electrochemical tests, it can be seen that superior electrochemical properties are obtained in samples where Co introduction leads to the creation of P vacancies (Fig. 14g). Continuing with the DFT calculations on the samples, the introduction of P vacancies optimizes the adsorption behavior of H* intermediates on the active site both for the Ni5P4 phase and the Ni2P phase, in keeping with the previous results (Fig. 14h and i).
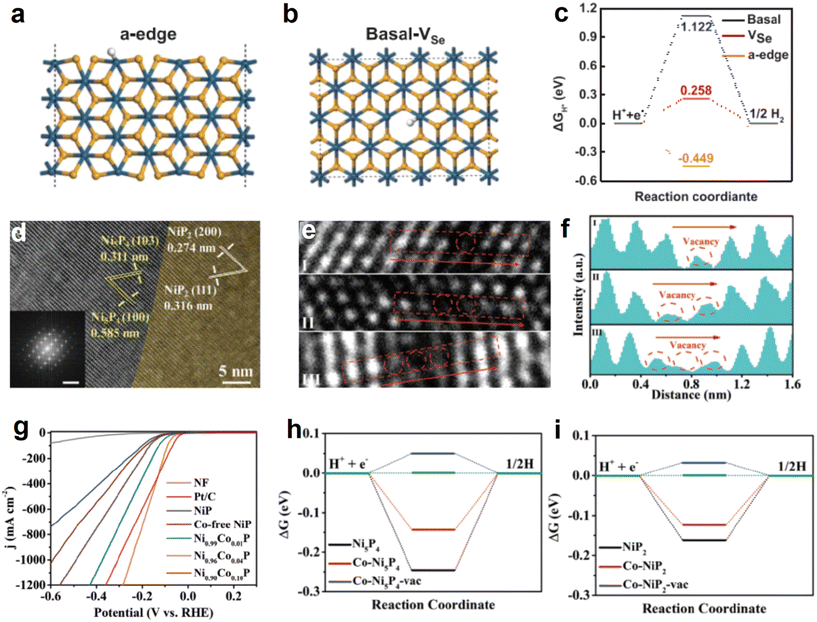 | ||
| Fig. 14 (a) Schematic representation of the adsorbed H structure at the edges in 1T-PtSe2. (b) Schematic representation of the Se vacancy adsorption H structure in 1T-PtSe2. (c) Hydrogen adsorption energy at different sites in theoretical calculations. Reproduced from ref. 130 with permission from Wiley, copyright 2023. (d) HRTEM images of Ni5P4 as well as NiP2, insets are FFT-transformed images. (e) Atomic-scale images of Ni0.96Co0.04P, where red circles are P vacancies. (f) Intensity profile recorded of corresponding areas in Fig. 13e. (g) LSV curves of different catalysts. Free energy diagram for (h) Ni5P4 and (i) Ni2P systems. Reproduced from ref. 131 with permission from Wiley, copyright 2023. | ||
Qin et al. explored the effect of oxygen vacancies on the adsorption behavior of HER intermediates by anchoring Ru clusters on trace P-doped TiO2 substrates.61 The Ru/P-TiO2 NPs catalysts were first prepared in two steps, hydrothermal and phosphorylation, in which P was introduced into the TiO2 lattice, replacing part of the Ti4+, and a large number of oxygen vacancies were generated during the P doping process. The lattice striations of both orthorhombic anatase TiO2 as well as rutile TiO2 crystalline phases can be seen in the HRTEM images of Fig. 15a and b, in which a series of blurring and missing lattice sites were found, proving the presence of oxygen vacancies in the lattice. Transformation of the HRTEM image in Fig. 15b yields Fig. 15c, which reveals the absence of obvious lattice stripes, which further confirms the presence of oxygen vacancies or even lattice defects. In order to corroborate how the introduction of P increases the lattice defects, the catalyst was subjected to EXAFS tests (Fig. 15d). The K-edge EXAFS of Ru in Ru/TiO2 can be seen to present two main peaks at 1.65 and 2.31 Å, which can correspond to the Ru–Ru coordination as well as the Ru–O–Ti coordination. It can be seen that the first peak of Ru/P-TiO2 is positively shifted, which is obtained as a result of the substitution of Ti4+ by P5+. The fitting of the EXAFS curve was continued to obtain explicit lattice parameters. After the introduction of O vacancies by P doping, the average coordination number of Ru decreases from 8.1 to 4.88, while the lengths of Ru–Ru and Ru–O bonds increase, which indicates the presence of vacancy defects within the lattice as well as the altered electron density. The above results confirm the effect of oxygen vacancies firstly on the overall electronic structure of the catalyst. In order to investigate the intrinsic effect of the altered electronic structure induced by the oxygen vacancies on the basic HER, DFT calculations were performed on the system. The plane-wave basis set with a kinetic energy cutoff of 400 eV, the energy convergence criterion of 10–5 eV, the force convergence criterion of 0.02 eV Å−1, and a (2 × 2 × 1) Monkhorst–Pack kpoint sampling were employed for structure relaxation. It can be seen that the adsorption energy for H2O is −0.59 eV for Ru/P-(R)TiO2 and −0.62 eV for Ru/P-(A)TiO2, whereas after the introduction of vacancies in the system for the calculations, the binding energy of the catalysts for H2O increases dramatically, to −0.87 eV (Fig. 15e). While it has been demonstrated in previous narratives that the presence of vacancies modifies the adsorption capacity of H*, the present work proves that the oxygen vacancies also have a significant positive effect on the water adsorption process. Subsequently, the system was tested for differential charge density, and it can be seen that after the introduction of the O vacancy, the electronic situation within the system changed, with more electrons enriched on Ru, allowing better dissociation of H2O at the Ru site (Fig. 15f). As illustrated in Fig. 15g, Ru/(R)-TiO2 exhibits a high energy barrier for water dissociation, which makes the transformation of adsorbed H2O into H* intermediates challenging. However, with the introduction of vacancies on (R)-TiO2, the energy required for H2O dissociation significantly decreases to 0.63 eV in the case of Ru/P(R)-TiO2-Vo. This observation underscores that electron-rich Ru clusters can execute the water dissociation step more efficiently due to the presence of oxygen vacancies. Moreover, Qin et al. have provided additional evidence emphasizing that anionic vacancies could potentially lower the reaction energy barrier associated with the Volmer step in the HER. Such a reduction in energy requirement would effectively accelerate the rate of the HER.
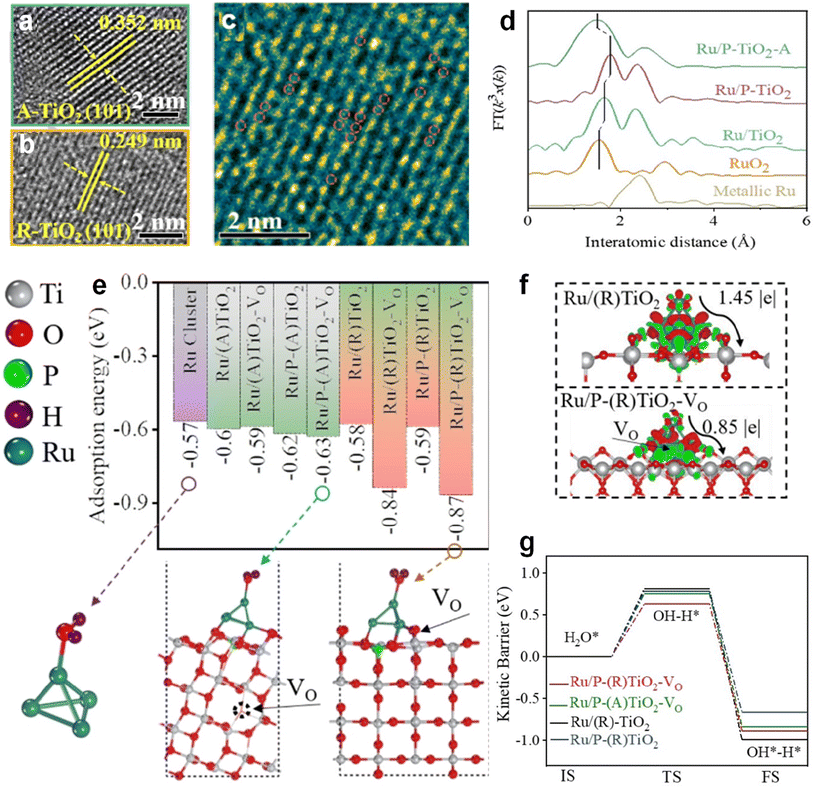 | ||
| Fig. 15 HRTEM image of Ru/P-TiO2, where (a) is A-TiO2 and (b) is R-TiO2. (c) Filtered image within area of (b). (d) Fourier transforms of Ru K-edge EXAFS spectra of Ru/TiO2, Ru/P-TiO2, Ru/P-TiO2-A in reference to commercially metallic Ru and RuO2. (e) Water adsorption energy on the active sites of different catalysts. (f) The differential charge density distributions between adsorbed Ru and R-TiO2, Ru and P-(R)TiO2-VO (down) with the isovalue of 0.008 e Å−3. Red represents negative charges and green represents positive charges. (g) Kinetic barrier of water dissociation on the active sites of different catalysts. IS, TS and FS represent initial, transition state and final state, respectively. Reproduced from ref. 61 with permission from Wiley, copyright 2022. | ||
Zhang et al. prepared S vacancy-rich Co3S4 PNSvac by plasma-induced exfoliation of layered Co3S4/TETA precursors (Fig. 16a).132 The existence of S vacancies was also demonstrated by EPR tests (Fig. 16b). DFT calculations were performed on the system to analyze the intrinsic reasons for the better HER activity of Co3S4 PNSvac (Fig. 16c). Firstly, regarding the H2O adsorption capacity of the system, it can be seen that Co3S4 PNSvac has a larger H2O adsorption energy (1.26 eV, absolute value) relative to Co3S4 NP (0.41 eV, absolute value) and Co3S4 NS (0.49 eV, absolute value), which is in agreement with the previous results that anionic vacancies are effective in accelerating the Volmer step of the initial HER process. Not only that, the calculation of the H2O dissociation reaction energy barriers reveals that Co3S4 PNSvac has the lowest energy barriers, and the combination of stronger H2O adsorption capacity and H2O dissociation capacity, which tells us that the introduction of S vacancies indeed accelerates the Volmer step in the HER (Fig. 16d). Mo-doped NiS/Ni3S2 polycrystalline heterostructures with sulfur-rich vacancies were prepared by a hydrothermal acid sulfide-assisted strategy by Yu et al.133 From the electrochemical tests, it can be seen that the S vacancies significantly enhanced the HER performance of the catalyst (Fig. 16e). The reason for the increase in catalyst activity was also explored by DFT calculations. Regardless of the presence of S vacancies in either the NiS phase or the Ni3S2 phase, there is a significant optimization of ΔGH* by S vacancies (closer to 0) relative to samples without vacancies (Fig. 16f). The S-3p energy band centers (εp) of the samples were subsequently calculated, which can be used as an indicator for evaluating the H* adsorption capacity. It can be seen that the presence of S vacancies optimizes the adsorption capacity of the catalyst for H* intermediates, which is in line with previous results.
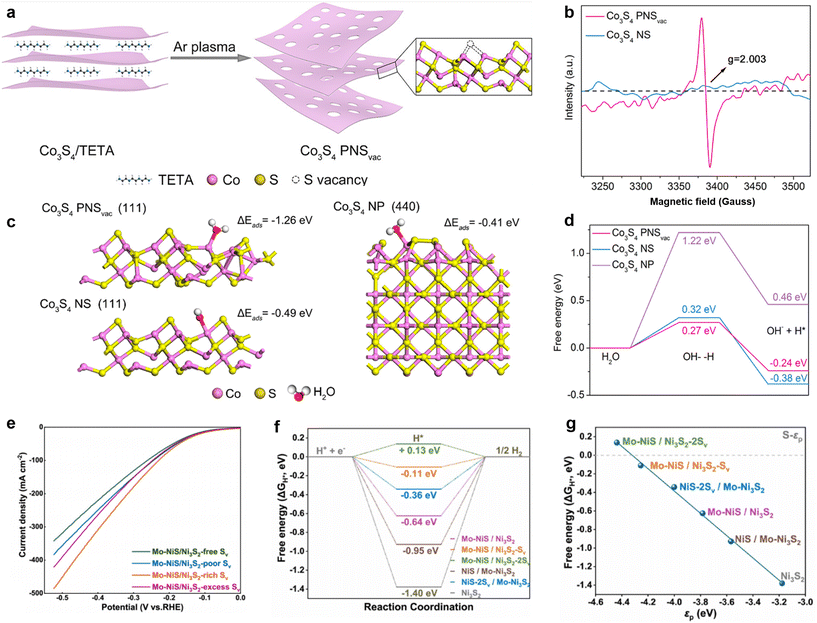 | ||
| Fig. 16 (a) Scheme for the preparation of Co3S4 PNSvac. (b) EPR spectra of the catalysts. The adsorption energies (ΔEads) (c) and the activation energy barriers (d) of H2O molecule on three models surfaces. Reproduced from ref. 132 with permission from American Chemical Society, copyright 2018. (e) LSV curves for different samples. (f) HER free-energy diagram calculated for different samples. (g) Dependence of free energies of hydrogen adsorption on the p-band centers (εp) of S-3p state. Reproduced from ref. 133 with permission from Elsevier, copyright 2023. | ||
All the above conclusions demonstrate that the introduction of anionic vacancies primarily causes a change in the electronic structure inside the catalyst, which tends to alter the original electron flow. Since the intrinsic adsorption capacity of the catalyst is often sub-optimal prior to the introduction of anionic vacancy modulation—resulting in either excessively strong or weak intermediate adsorption—the presence of anionic vacancies can help optimize this balance and enhance the overall catalytic performance.128,134 And most of the current HER catalysts applying anionic vacancy modification strategies follow roughly the same idea. The introduction of anionic vacancies initially induces a change in the lattice structure parameters and subsequently alters the electronic structure of the catalyst. This alteration continues to modify the adsorption energy of intermediates, thereby enhancing the intrinsic activity of the catalyst.
Not only that, anionic vacancy modification was used in combination with other strategies to enhance the intrinsic HER activity of the catalysts. Li et al. successfully synthesized a CoP@CoOOH with a core–shell heterogeneous structure and containing oxygen vacancies as an efficient catalyst for water electrolysis.135 Co(OH)F is grown directly on carbon paper (CP) by a simple hydrothermal method, and Co(OH)F/CP is phosphorylated in situ under an N2 atmosphere to form black CoP/CP. Furthermore, CoP/CP was oxidized in NaOH and H2O2 leading to an oxygen-rich CoOOH shell in situ formed on the CoP nucleus, resulting in a dark brown CoP@CoOOH/CP with abundant oxygen vacancies (Fig. 17a). Fig. 17b is a CoP@CoOOH SEM image, and it can be seen that CoP@CoOOH/CP exhibits a nanosheet-like structure of ripples and corrugations. The TEM image in Fig. 17c demonstrates the successful synthesis of the heterogeneous structure. In the XPS spectrum of the O 1s orbital in the CoP (Fig. 17d), the two peaks at 531.9 and 533.2 eV correspond to hydroxyl groups (Co-OH) and adsorption O, respectively. After H2O2 and NaOH treatment, two new peaks appeared in the XPS spectrum of the O 1s orbit in CoP@CoOOH/CP, located at 529.5 and 530.5 eV, respectively, which can be attributed to Co–O formation and surface vacancy. These results reflect the change of the electronic structure of the compound, which can effectively reduce the chemical interaction between the catalyst surface and the intermediate and enhance the electrocatalytic kinetics. As can be seen from the LSV curves in Fig. 17e and the Tafel slope in Fig. 17f, the most excellent HER activity was obtained for CoP@CoOOH/CP after dual modification with oxygen vacancies and heterostructure. The catalyst can effectively promote charge transfer and material transfer, optimize the binding energy of intermediates, and reduce the reaction activation energy to achieve the purpose of improving the activity of the catalyst, due to the synergistic effect of heterogeneous structures and oxygen vacancies. The Faraday efficiency of CoP@CoOOH/CP was proved to be close to 100% by practical measurements.
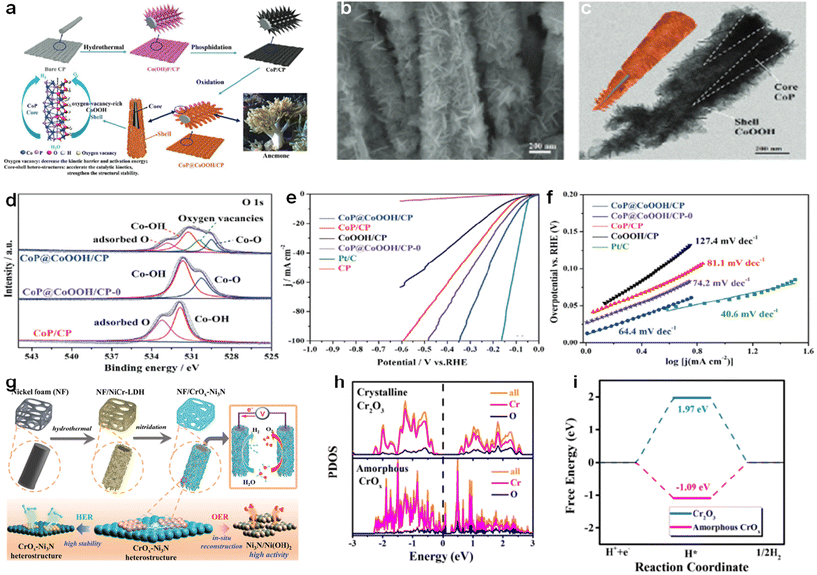 | ||
| Fig. 17 (a) Synthesis procedure and mechanism. Schematic illustration of the synthesis procedure of anemone-like CoP@CoOOH/CP heterostructures. (b) SEM images of CoP@CoOOH/CP. (c) Low-resolution HRTEM image of CoP@CoOOH/CP. (d) O 1s XPS spectra. (e) HER polarization curves of different catalysts in 1.0 M PBS at a scan rate of 5 mV s−1. (f) Corresponding Tafel plots of the different catalysts. Reproduced from ref. 135 with permission from Wiley, copyright 2022. (g) Schematic illustration of the preparation of the CrOx-Ni3N heterostructure nanosheets and its reaction mechanism for overall water splitting. (h) The calculated partial density of states. (i) H adsorption energies of crystalline Cr2O3 and amorphous CrOx. Reproduced from ref. 136 with permission from Wiley, copyright 2022. | ||
Similarly, Liu et al. synthesized an amorphous/crystalline CrOx-Ni3N heterostructure with oxygen vacancies as an efficient catalyst for water electrolysis.136 As shown in Fig. 17g, nano-Ni(OH)2 precursors are first generated on NF by a hydrothermal method, and nitridation is performed to convert the precursors into amorphous/crystalline CrOx-Ni3N heterostructure nanosheets. In DFT calculation, the effect of amorphous/crystalline CrOx-Ni3N heterostructure on the basic HER was studied. In the partial density of states (PDOS) calculated in Fig. 17h, the amorphous CrOx has a significantly enhanced carrier density at the Fermi level, which also shows that the conductivity of the amorphous CrOx has increased. As can be seen from Fig. 17i, the amorphous CrOx has a lower |ΔGH| value than the crystalline CrOx, indicating that the amorphous CrOx has better hydrogen adsorption and desorption ability. The electrochemical performance of the catalyst in 1.0 M KOH medium was also tested. The catalytic performance of CrOx-Ni3N requires only an overpotential of 53 mV at a current density of −10 mA cm−2, while Ni3N requires 86 mV and CrOx requires 220 mV. Moreover, the introduction of amorphous CrOx accelerates the reaction kinetics, and as the Cr content increases, CrOx-Ni3N nanosheets with different compositions exhibit a Tafel slope from 76.4 to 73.4 mV dec−1, which is much lower than the Tafel slope of Ni3N (95.0 mV dec−1).
In a nutshell, the strategic manipulation of anionic vacancies within the catalyst's electronic structure, compounded with the anchoring of metal heteroatoms, enables the design of an optimal heterostructure. This approach not only bolsters electron transport and material transfer rates but also significantly augments overall catalytic performance. Furthermore, employing the heterostructure technique within metal oxides presents another viable pathway to amplify the activity of the catalyst.
5.4. Effect of vacancy concentration on catalyst performance
When different concentrations of oxygen vacancies are introduced, there is bound to be some effect on the HER performance of the catalyst. In order to investigate the effect of vacancy concentration on catalyst activity, WOx catalysts containing different concentrations of oxygen vacancies were prepared by Yin et al.137 The relationship between catalyst activity and oxygen vacancy concentration is revealed by studying the difference in HER performance of these catalysts. Since O is more electronegative than elements such as C, N, P, S, and Se elements, it limits the metal electron transfer and reduces the reaction kinetics. After these catalysts were prepared, the concentration of oxygen vacancies was calculated by eqn (1), where the contents of W5+ and W4+ were obtained from the W content in the XPS results.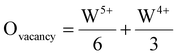 | (1) |
The correspondence between the ratios of different W ions and oxygen vacancies can be seen in Table 1. The correspondence of the HER performance relationship with the concentration of vacancies is listed in Fig. 18a. Experimentally, it was demonstrated that the vacancy concentration seems to be positively related to the HER performance. In 2022, Liao's group prepared a series of inert materials enriched with oxygen vacancies and further discussed the relationship between vacancy concentration and HER performance through experiments as well as theoretical calculations (Fig. 18b).71 EPR tests demonstrated the successful synthesis of samples with different oxygen vacancy concentrations (Fig. 18c). From the LSV curves in Fig. 18d, it can be seen that the HER performance instead decays when the vacancy concentration is at its maximum, suggesting that there is also an optimum value for the oxygen vacancy concentration. To illustrate why this happens, Liao et al. performed DFT calculations on the system. To better quantify the contribution of oxygen vacancy concentration to HER activity, changes in VO concentration measured in XPS, EPR, and M–S tests were combined with overpotential (Fig. 18e). It can be seen that the PI-30 sample has the lowest overpotential, which is consistent with previous LSV curve results. Supercells in the defect-free state or involving one/two oxygen vacancies were constructed to study the adsorption capacity, and a vacuum space of 15 Å was designed along the C direction of the supercell to avoid interactions. As shown in Fig. 18f, the models used in the DFT calculations are a single oxygen vacancy, two oxygen vacancies, and an oxygen site. The calculated density of states (DOS) showed that an increase in the concentration of oxygen vacancies led to a decrease in the band gap of the catalyst, which helped to promote electron transfer in the HER (Fig. 18g). Also, this change in electronic state leads to different intermediate adsorption capacities, with too strong intermediate adsorption being detrimental to hydrogen desorption, and too weak intermediate adsorption being detrimental to intermediate adsorption to the active site to participate in the reaction. In summary, although the introduction of vacancies can improve the HER performance of the catalysts to a certain extent, it is difficult to enhance the binding energy of hydrogen adsorption in a larger dimension just by increasing the content of oxygen vacancies.
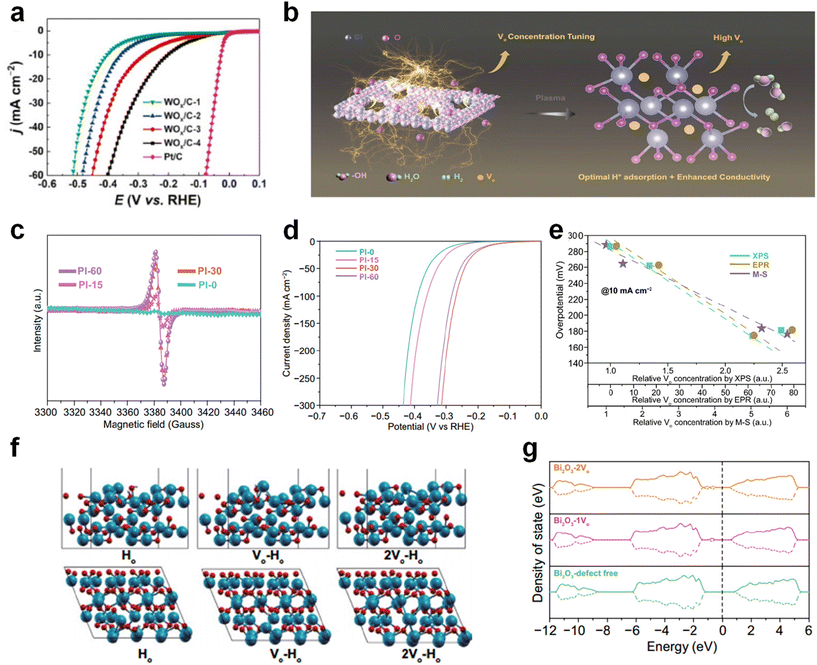 | ||
Fig. 18 (a) LSV cruse of WO3/C and Pt/C. Reproduced from ref. 137 with permission from Elsevier, copyright 2021. (b) EPR test and (c) LSV cruse of PI with different oxygen vacancy content. (d) The spin-polarized electronic density of states of Bi2O3 surfaces with different oxygen vacancies coverage. (e) The relationship of the overpotentials needed to reach j![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 mA cm−2 and the relative oxygen vacancy concentrations obtained from EPR, XPS and EPR measurements. (f) Hydrogen atom adsorbed Bi2O3 (010) surface models in defect-free state and with one or two oxygen vacancy incorporated. (g) The spin-polarized electronic density of states of Bi2O3 surfaces with different oxygen vacancies coverage. Reproduced from ref. 71 with permission from Springer, copyright 2022. 10 mA cm−2 and the relative oxygen vacancy concentrations obtained from EPR, XPS and EPR measurements. (f) Hydrogen atom adsorbed Bi2O3 (010) surface models in defect-free state and with one or two oxygen vacancy incorporated. (g) The spin-polarized electronic density of states of Bi2O3 surfaces with different oxygen vacancies coverage. Reproduced from ref. 71 with permission from Springer, copyright 2022. | ||
| W6+ (%) | W5+ (%) | W4+ (%) | Ovacancy (%) | |
|---|---|---|---|---|
| WOx/C-1 | 96.14 | 3.86 | — | 0.64 |
| WOx/C-2 | 91.32 | 8.68 | — | 1.45 |
| WOx/C-3 | 89.94 | 10.06 | — | 1.68 |
| WOx/C-4 | 70.99 | 6.86 | 22.15 | 8.53 |
5.5. Methods for stabilizing vacancies
Previous studies have shown that the introduction of oxygen vacancies leads to changes in the lattice structure.138–140 Since the HER is a reduction reaction, whether the oxygen vacancies change during the reaction in such a bad surrounding is still a question to be considered.141 Liu et al. modulated the electronic structure of the active site by anchoring coordination polyhedra to the catalyst surface, thereby stabilizing the oxygen vacancies and effectively inhibiting OH− corrosion of the catalyst.142Firstly, α-Co(OH)2 catalysts with a layered crystal structure and interlayer ions of NO3− were prepared by the hydrothermal method. From Fig. 19a, it can be seen that the performance of the catalyst in 1.0 M KOH showed a significant degradation after 300 rounds of CV testing, whereas there was no significant degradation of the performance after the addition of MoO42− to the medium. It was found by Raman test that the peaks belonging to Co(OH)2 disappear after CV cycles in KOH, which does not happen in MoO42− (Fig. 19b). Meanwhile, in the post-reaction SEM image, it can be seen that the nanosheet structure is damaged after CV testing in KOH, whereas the nanosheet structure is well maintained after the addition of MoO42− to the medium (Fig. 19c and d). The area ratios of the different peaks in the XPS of the O 1s orbitals before and after the reaction were analyzed to obtain information on the content of the remaining oxygen vacancies in the catalyst (Table 2). Maximum retention of oxygen vacancies was achieved with the addition of MoO42− to the medium, which demonstrates the stabilization of oxygen vacancies by MoO42−.
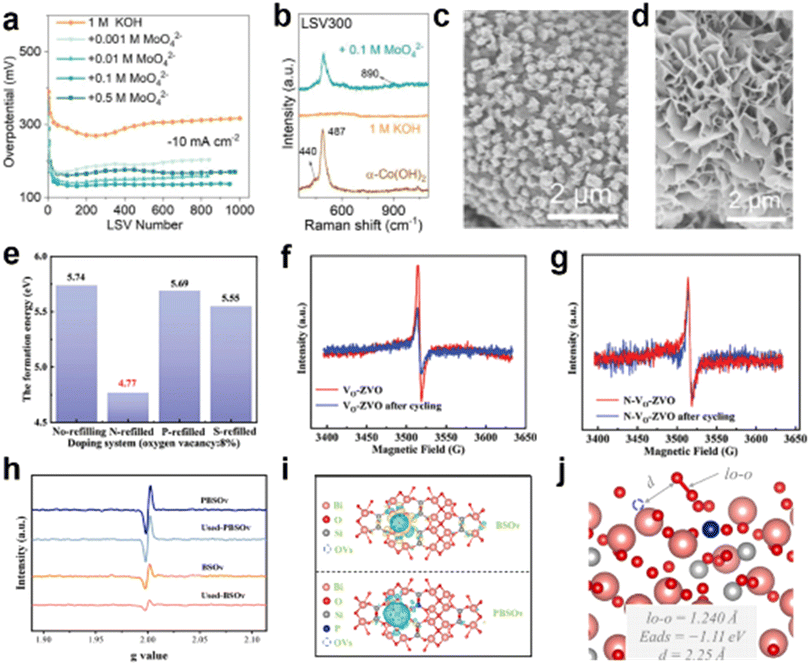 | ||
| Fig. 19 (a) Overpotential at 10 mA cm−2 after different levels of CV testing in different media. (b) Raman spectrum of α-Co(OH)2 after 300 cycles of CV. (c) and (d) SEM image after 300 cycles of CV test in KOH and MoO42−. Reproduced from ref. 142 with permission from Wiley, copyright 2022. (e) The formation energy of oxygen vacancy with doping of different atoms. (f) Change in EPR of Vo-ZVO and (g) N-Vo-ZVO after CV cycle. Reproduced from ref. 143 with permission from Wiley, copyright 2023. (h) Comparison of EPR before and after PBSOvvs. BSOv tests. (i) Charge difference distribution between Bi2SiO5 nanosheets and oxygen vacancy. (j) The activation of O2 over the surface of PBSOv. Reproduced from ref. 144 with permission from Elsevier, copyright 2022. | ||
| O 1s | Ov |
|---|---|
| Before HER | 23.7% |
| KOH | 1.9% |
| KOH + MoO42− | 17.3% |
Methods for protecting oxygen vacancies in other fields may shed light on how to stabilize oxygen vacancies in the HER. In the field of zinc ion batteries (ZIB), Zhao et al. prepared N-Vo-ZVO by filling N and other heteroatoms back into Zn3V2O7(OH)2 2H2O, obtaining more stable oxygen vacancies as well as better performance.143 Calculations show that the N-backfilled catalyst has the lowest energy of formation of oxygen vacancies, indicating that it is the most stable catalyst in the reaction (Fig. 19e). Also, the EPR test results demonstrated that the oxygen vacancy loss in the N-backfilled samples was almost negligible with increasing number of CV (Fig. 19f and g). Changing the local electronic structure is likewise one way to stabilize the oxygen vacancy. Sun et al. introduced PO4 doping in Bi2SiO5 with abundant oxygen vacancies, inducing an unbalanced local charge distribution.144 The prepared PBSOv showed much better oxygen vacancy stability relative to BSOv (Fig. 19h). Bi2SiO5 was modeled by using a periodic four-layer slab recurring in a 2 × 2 × 1 surface unit cell with optimized bulk parameters. The slab was blocked by a vacuum layer of 15 Å thickness. As shown in Fig. 19i, differential charge densities show an increase in the local charge density near the Ov and its surrounding Bi atoms after the introduction of the phosphate, which demonstrates that the introduction of the dopant exacerbates the uneven distribution of charge. From the values calculated by the model, it is known that the distance between adsorbed O2 molecules and oxygen vacancies in PBSOv increases, and O2 is more inclined to be adsorbed on the doped sites, thus enhancing the stability of Ov (Fig. 19j). While enhancing the HER catalyst activity through oxygen vacancies, stabilizing the oxygen vacancies using the above methods can help to enhance the HER stability as well as the catalytic activity of the catalyst.
6. Summary and prospects
In recent years, metal oxide materials, such as TMDs, TMSes, and TMPs have been more widely used in the field of water splitting due to their mature synthesis methods, unique electronic structure, large specific surface area, and flexible phase transitions. In the field of HER, in order to replace costly Pt-based catalysts that have scarce reserves, modification strategies for these kinds of catalysts have been developed, such as heteroatom doping, heterogeneous interface design, and vacancy engineering. Among them, vacancy engineering has been widely used due to its advantages such as easy design and obvious modulation effect on the electronic structure of catalysts, while vacancies are also introduced within the catalysts when constructing heterogeneous interfaces as well as doping heteroatoms, and thus the combined application of vacancy engineering with other modulation strategies has been widely investigated.In this review, we present a systematic summary of the application of anionic vacancies in HER catalysts. We first discuss methods for the introduction of anionic vacancies, in which a reducing environment as well as changes in lattice structure due to atomic substitutions are the basic principles for the generation of vacancies in catalysts. We list and compare the currently used characterization methods for vacancies, and the common ones are microscopic characterization and the two main categories of spectroscopic characterization for electronic structure analysis, such as AC-TEM, EPR, etc. After summarizing and generalizing several common anionic vacancies, we discuss how anionic vacancies can enhance the intrinsic activity of a catalyst. Overall, the introduction of vacancies into a catalyst often leads to changes in the lattice structure of the catalyst, such as lattice expansion and lattice mismatch, which to some extent will expose more active sites. In addition to the change in lattice structure, the electronic structure of the catalyst is also rearranged, in which the anionic vacancies tend to act as electron donors. The anionic vacancies can regulate the electron flow by adjusting the work function of the system. After causing the electronic rearrangement of different active sites on the catalyst, this leads to a change in the adsorption behavior of the intermediates, which is conducive to optimizing the adsorption of HER-active intermediates that are too strong or too weak, as well as changing the conductivity of the catalysts to a certain extent. These are all key factors in the ability of anionic vacancies to enhance the intrinsic activity of the catalyst.
The more common O, S, P, and Se vacancies are currently the ones that have been more extensively studied relative to the uncommon anionic vacancies such as N and Te. We show in Fig. 20 the development of anionic vacancies in recent years. Currently, the mainstream HER catalysts are still noble metal catalysts, such as Pt, Ru, etc. The design of non-precious metal catalysts with anionic vacancies, such as transition metal sulfur compounds, transition metal hydroxides and carbon-based catalysts, has become a hot research issue for the future. These emerging electrocatalysts will promote the development of anionic vacancies in the field of HER. As a result, the roles played by more and more anionic vacancies will be explored; therefore, it is of great importance to further investigate the linkage between different vacancy characteristics and catalytic activity for different anionic vacancies in the future. In the future, HER electrocatalysts with anionic vacancies could be precisely synthesized using advanced preparation methods. The activity and stability of the catalysts can be effectively enhanced by optimizing the number of anionic vacancies and controlling their position and distribution. Moreover, HER electrocatalysts with anionic vacancies often exhibit excellent performance not only in the HER but also in other electrocatalytic reactions. As research progresses, composite catalysts with multiple functions will be widely used to meet the needs of different electrocatalytic reactions. At the same time, vacancy engineering that has been developing for more than 50 years, such as combining anionic vacancies with emerging catalytic technologies, also needs to be explored, for example graphene-loaded quantum dot catalysts, and atomically dispersed catalysts with a theoretical 100 percent atom utilization, which have recently been explored to some extent in the HER field. How to introduce an anionic vacancy regulation mechanism in these new catalysts is also an important point for the future development of anionic vacancies. Not only that, more advanced in situ detection techniques can be used to trace the formation process of anionic vacancies in catalysts, in order to explain more rationally the specific reasons for the role played by anionic vacancies. Although anionic vacancies have great promise for application in HER catalysts, further studies are needed to fully understand the mechanism of their effect on catalytic performance and to further optimize the design of catalysts.
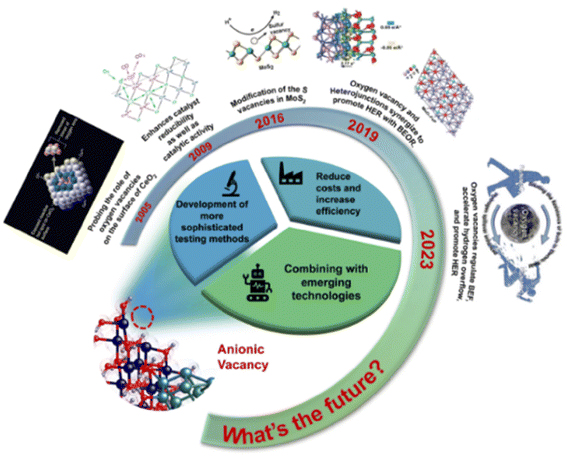 | ||
| Fig. 20 The evolution of anionic vacancies in HER catalysts and future perspectives.123,145–148 Reproduced from ref. 123 with permission from Wiley, copyright 2024. Reproduced from ref. 145 with permission from American Association for the Advancement of Science, copyright 2005. Reproduced from ref. 146 with permission from American Chemical Society, copyright 2009. Reproduced from ref. 147 with permission from American Chemical Society, copyright 2016. Reproduced from ref. 148 with permission from Wiley, copyright 2020. | ||
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Natural Science Foundation of China (21971132, 52072197 and 52372205), Postdoctoral Innovation Project of Shandong Province (202102039), Postdoctoral Applied Research Project of Qingdao, Youth Innovation and Technology Foundation of Shandong Higher Education Institutions, China (2019KJC004), Major Scientific and Technological Innovation Project (2019JZZY020405), Major Basic Research Program of Natural Science Foundation of Shandong Province under Grant (ZR2020ZD09), the 111 Project of China (D20017), Taishan Scholar Young Talent Program (tsqn201909114) and the Natural Science Foundation of Shandong Province, China (ZR2023MB142).References
- J. Guo, Y. Zhang, A. Zavabeti, K. Chen, Y. Guo, G. Hu, X. Fan and G. K. Li, Hydrogen production from the air, Nat. Commun., 2022, 13, 5046 CrossRef CAS.
- J. A. Turner, Sustainable Hydrogen Production, Science, 2004, 305, 972–974 CrossRef CAS PubMed.
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Nørskov and T. F. Jaramillo, Combining theory and experiment in electrocatalysis: Insights into materials design, Science, 2017, 355, eaad4998 CrossRef PubMed.
- Y. Wan, L. Zhou and R. Lv, Rational design of efficient electrocatalysts for hydrogen production by water electrolysis at high current density, Mater. Chem. Front., 2023, 7, 6035–6060 RSC.
- P. Da, Y. Zheng, Y. Hu, Z. Wu, H. Zhao, Y. Wei, L. Guo, J. Wang, Y. Wei, S. Xi, C.-H. Yan and P. Xi, Synthesis of Bandgap-tunable Transition Metal Sulfides through Gas-phase Cation Exchange-induced Topological Transformation, Angew. Chem., Int. Ed., 2023, 62, e202301802 CrossRef CAS PubMed.
- J. Wang, S. Xin, Y. Xiao, Z. Zhang, Z. Li, W. Zhang, C. Li, R. Bao, J. Peng, J. Yi and S. Chou, Manipulating the Water Dissociation Electrocatalytic Sites of Bimetallic Nickel-Based Alloys for Highly Efficient Alkaline Hydrogen Evolution, Angew. Chem., Int. Ed., 2022, 61, e202202518 CrossRef CAS.
- X. Zheng, X. Shi, H. Ning, R. Yang, B. Lu, Q. Luo, S. Mao, L. Xi and Y. Wang, Tailoring a local acid-like microenvironment for efficient neutral hydrogen evolution, Nat. Commun., 2023, 14, 4209 CrossRef CAS PubMed.
- X. Chen, X.-T. Wang, J.-B. Le, S.-M. Li, X. Wang, Y.-J. Zhang, P. Radjenovic, Y. Zhao, Y.-H. Wang, X.-M. Lin, J.-C. Dong and J.-F. Li, Revealing the role of interfacial water and key intermediates at ruthenium surfaces in the alkaline hydrogen evolution reaction, Nat. Commun., 2023, 14, 5289 CrossRef CAS PubMed.
- R.-Y. Shao, L.-W. Chen, Q.-Q. Yan, W.-J. Zeng, P. Yin and H.-W. Liang, Is Pt/C More Electrocatalytic than Ru/C for Hydrogen Evolution in Alkaline Electrolytes?, ACS Appl. Energy Mater., 2021, 4, 4284–4289 CrossRef CAS.
- H. Sun, Z. Yan, F. Liu, W. Xu, F. Cheng and J. Chen, Self-Supported Transition-Metal-Based Electrocatalysts for Hydrogen and Oxygen Evolution, Adv. Mater., 2020, 32, 1806326 CrossRef CAS.
- W. Zhan, X. Zhai, Y. Li, M. Wang, H. Wang, L. Wu, X. Tang, H. Zhang, B. Ye, K. Tang, G. Wang and M. Zhou, Regulating Local Atomic Environment around Vacancies for Efficient Hydrogen Evolution, ACS Nano, 2024, 18, 10312–10323 CrossRef CAS.
- J. N. Hansen, H. Prats, K. K. Toudahl, N. M. Secher, K. Chan, J. Kibsgaard and I. Chorkendorff, Is There Anything Better than Pt for HER?, ACS Energy Lett., 2021, 6, 1175–1180 CrossRef CAS.
- Y. Luo, Z. Zhang, F. Yang, J. Li, Z. Liu, W. Ren, S. Zhang and B. Liu, Stabilized hydroxide-mediated nickel-based electrocatalysts for high-current-density hydrogen evolution in alkaline media, Energy Environ. Sci., 2021, 14, 4610–4619 RSC.
- M. Carmo, D. L. Fritz, J. Mergel and D. Stolten, A comprehensive review on PEM water electrolysis, Int. J. Hydrogen Energy, 2013, 38, 4901–4934 CrossRef CAS.
- B. Zhang, J. Liu, J. Wang, Y. Ruan, X. Ji, K. Xu, C. Chen, H. Wan, L. Miao and J. Jiang, Interface engineering: The Ni(OH)2/MoS2 heterostructure for highly efficient alkaline hydrogen evolution, Nano Energy, 2017, 37, 74–80 CrossRef CAS.
- J. Wu, J. Fan, X. Zhao, Y. Wang, D. Wang, H. Liu, L. Gu, Q. Zhang, L. Zheng, D. J. Singh, X. Cui and W. Zheng, Atomically Dispersed MoOx on Rhodium Metallene Boosts Electrocatalyzed Alkaline Hydrogen Evolution, Angew. Chem., Int. Ed., 2022, 61, e202207512 CrossRef CAS.
- Y. Zheng, Y. Jiao, A. Vasileff and S.-Z. Qiao, The Hydrogen Evolution Reaction in Alkaline Solution: From Theory, Single Crystal Models, to Practical Electrocatalysts, Angew. Chem., Int. Ed., 2018, 57, 7568–7579 CrossRef CAS PubMed.
- Z. Wang, K. Chi, S. Yang, J. Xiao, F. Xiao, X. Zhao and S. Wang, Optimizing the Electronic Structure of Atomically Dispersed Ru Sites with CoP for Highly Efficient Hydrogen Evolution in both Alkaline and Acidic Media, Small, 2023, 19, 2301403 CrossRef CAS PubMed.
- K. Wu, K. Sun, S. Liu, W.-C. Cheong, Z. Chen, C. Zhang, Y. Pan, Y. Cheng, Z. Zhuang, X. Wei, Y. Wang, L. Zheng, Q. Zhang, D. Wang, Q. Peng, C. Chen and Y. Li, Atomically dispersed Ni–Ru–P interface sites for high-efficiency pH-universal electrocatalysis of hydrogen evolution, Nano Energy, 2021, 80, 105467 CrossRef CAS.
- H. Fei, R. Liu, Y. Zhang, H. Wang, M. Wang, S. Wang, M. Ni, Z. Wu and J. Wang, Extending MoS2-based materials into the catalysis of non-acidic hydrogen evolution: challenges, progress, and perspectives, Mater. Futures, 2023, 2, 022103 CrossRef CAS.
- K. L. Zhou, Z. Wang, C. B. Han, X. Ke, C. Wang, Y. Jin, Q. Zhang, J. Liu, H. Wang and H. Yan, Platinum single-atom catalyst coupled with transition metal/metal oxide heterostructure for accelerating alkaline hydrogen evolution reaction, Nat. Commun., 2021, 12, 3783 CrossRef CAS.
- Z. Shi, X. Zhang, X. Lin, G. Liu, C. Ling, S. Xi, B. Chen, Y. Ge, C. Tan, Z. Lai, Z. Huang, X. Ruan, L. Zhai, L. Li, Z. Li, X. Wang, G.-H. Nam, J. Liu, Q. He, Z. Guan, J. Wang, C.-S. Lee, A. R. J. Kucernak and H. Zhang, Phase-dependent growth of Pt on MoS2 for highly efficient H2 evolution, Nature, 2023, 621, 300–305 CrossRef CAS PubMed.
- X. Fan, C. Liu, B. Gao, H. Li, Y. Zhang, H. Zhang, Q. Gao, X. Cao and Y. Tang, Electronic Structure Engineering of Pt Species over Pt/WO3 toward Highly Efficient Electrocatalytic Hydrogen Evolution, Small, 2023, 19, 2301178 CrossRef CAS.
- X. Wei, S. Song, W. Cai, Y. Kang, Q. Fang, L. Ling, Y. Zhao, Z. Wu, X. Song, X. Xu, S. M. Osman, W. Song, T. Asahi, Y. Yamauchi and C. Zhu, Pt Nanoparticle–Mn Single-Atom Pairs for Enhanced Oxygen Reduction, ACS Nano, 2024, 18, 4308–4319 CrossRef CAS.
- H.-J. Liu, S. Zhang, Y.-M. Chai and B. Dong, Ligand Modulation of Active Sites to Promote Cobalt-Doped 1T-MoS2 Electrocatalytic Hydrogen Evolution in Alkaline Media, Angew. Chem., Int. Ed., 2023, 62, e202313845 CrossRef CAS.
- P. Ji, R. Yu, P. Wang, X. Pan, H. Jin, D. Zheng, D. Chen, J. Zhu, Z. Pu, J. Wu and S. Mu, Ultra-Fast and In-Depth Reconstruction of Transition Metal Fluorides in Electrocatalytic Hydrogen Evolution Processes, Adv. Sci., 2022, 9, 2103567 CrossRef CAS.
- H. Yang, P. Guo, R. Wang, Z. Chen, H. Xu, H. Pan, D. Sun, F. Fang and R. Wu, Sequential Phase Conversion-Induced Phosphides Heteronanorod Arrays for Superior Hydrogen Evolution Performance to Pt in Wide pH Media, Adv. Mater., 2022, 34, 2107548 CrossRef CAS.
- Z. Fan, F. Liao, Y. Ji, Y. Liu, H. Huang, D. Wang, K. Yin, H. Yang, M. Ma, W. Zhu, M. Wang, Z. Kang, Y. Li, M. Shao, Z. Hu and Q. Shao, Coupling of nanocrystal hexagonal array and two-dimensional metastable substrate boosts H2-production, Nat. Commun., 2022, 13, 5828 CrossRef CAS PubMed.
- M. S. A. Sher Shah, G. Y. Jang, K. Zhang and J. H. Park, Transition metal carbide-based nanostructures for electrochemical hydrogen and oxygen evolution reactions, EcoEnergy, 2023, 1, 344–374 CrossRef.
- H. Huang, H. Jung, C.-Y. Park, S. Kim, A. Lee, H. Jun, J. Choi, J. W. Han and J. Lee, Surface conversion derived core-shell nanostructures of Co particles@RuCo alloy for superior hydrogen evolution in alkali and seawater, Appl. Catal., B, 2022, 315, 121554 CrossRef CAS.
- T. U. Haq, M. Pasha, Y. Tong, S. A. Mansour and Y. Haik, Au nanocluster coupling with Gd-Co2B nanoflakes embedded in reduced TiO2 nanosheets: Seawater electrolysis at low cell voltage with high selectivity and corrosion resistance, Appl. Catal., B, 2022, 301, 120836 CrossRef CAS.
- X. H. Wang, Y. Ling, B. Wu, B. L. Li, X. L. Li, J. L. Lei, N. B. Li and H. Q. Luo, Doping modification, defects construction, and surface engineering: Design of cost-effective high-performance electrocatalysts and their application in alkaline seawater splitting, Nano Energy, 2021, 87, 106160 CrossRef CAS.
- F. Lyu, S. Zeng, Z. Jia, F.-X. Ma, L. Sun, L. Cheng, J. Pan, Y. Bao, Z. Mao, Y. Bu, Y. Y. Li and J. Lu, Two-dimensional mineral hydrogel-derived single atoms-anchored heterostructures for ultrastable hydrogen evolution, Nat. Commun., 2022, 13, 6249 CrossRef CAS PubMed.
- C. Wan, Z. Zhang, J. Dong, M. Xu, H. Pu, D. Baumann, Z. Lin, S. Wang, J. Huang, A. H. Shah, X. Pan, T. Hu, A. N. Alexandrova, Y. Huang and X. Duan, Amorphous nickel hydroxide shell tailors local chemical environment on platinum surface for alkaline hydrogen evolution reaction, Nat. Mater., 2023, 22, 1022–1029 CrossRef CAS.
- C. Li, Z. Wang, M. Liu, E. Wang, B. Wang, L. Xu, K. Jiang, S. Fan, Y. Sun, J. Li and K. Liu, Ultrafast self-heating synthesis of robust heterogeneous nanocarbides for high current density hydrogen evolution reaction, Nat. Commun., 2022, 13, 3338 Search PubMed.
- R. V. Digraskar, B. B. Mulik, P. S. Walke, A. V. Ghule and B. R. Sathe, Enhanced Hydrogen Evolution Reactions on Nanostructured Cu2ZnSnS4 (CZTS) Electrocatalyst, Appl. Surf. Sci., 2017, 412, 475–481 CrossRef CAS.
- X. Zou, X. Huang, A. Goswami, R. Silva, B. R. Sathe, E. Mikmeková and T. Asefa, Cobalt–Embedded Nitrogen–Rich Carbon Nanotubes Efficiently Catalyze Hydrogen Evolution Reaction at All pH Values, Angew. Chem., Int. Ed., 2014, 53, 4372–4376 CrossRef CAS PubMed.
- Y. Pan, J. Gao, E. Lv, T. Li, H. Xu, L. Sun, A. Nairan and Q. Zhang, Integration of Alloy Segregation and Surface Co-O Hybridization in Carbon-Encapsulated CoNiPt Alloy Catalyst for Superior Alkaline Hydrogen Evolution, Adv. Funct. Mater., 2023, 33, 2303833 CrossRef CAS.
- Y. Zhu, M. Klingenhof, C. Gao, T. Koketsu, G. Weiser, Y. Pi, S. Liu, L. Sui, J. Hou, J. Li, H. Jiang, L. Xu, W.-H. Huang, C.-W. Pao, M. Yang, Z. Hu, P. Strasser and J. Ma, Facilitating alkaline hydrogen evolution reaction on the hetero-interfaced Ru/RuO2 through Pt single atoms doping, Nat. Commun., 2024, 15, 1447 CrossRef CAS.
- Z. Wei, Y. Liu, J. Ding, Q. He, Q. Zhang and Y. Zhai, Promoting Electrocatalytic CO2 Reduction to CO via Sulfur-Doped Co-N–C Single-Atom Catalyst†, Chin. J. Chem., 2023, 41, 3553–3559 CrossRef CAS.
- J. Kim, H. Jung, S.-M. Jung, J. Hwang, D. Y. Kim, N. Lee, K.-S. Kim, H. Kwon, Y.-T. Kim, J. W. Han and J. K. Kim, Tailoring Binding Abilities by Incorporating Oxophilic Transition Metals on 3D Nanostructured Ni Arrays for Accelerated Alkaline Hydrogen Evolution Reaction, J. Am. Chem. Soc., 2021, 143, 1399–1408 CAS.
- B. R. Sathe, X. Zou and T. Asefa, Metal-free B-doped graphene with efficient electrocatalytic activity for hydrogen evolution reaction, Catal. Sci. Technol., 2014, 4, 2023–2030 RSC.
- R. V. Digraskar, V. S. Sapner, S. M. Mali, S. S. Narwade, A. V. Ghule and B. R. Sathe, CZTS Decorated on Graphene Oxide as an Efficient Electrocatalyst for High-Performance Hydrogen Evolution Reaction, ACS Omega, 2019, 4, 7650–7657 CrossRef CAS PubMed.
- V. S. Sapner, P. P. Chavan, A. V. Munde, U. S. Sayyad and B. R. Sathe, Heteroatom (N, O, and S)-Based Biomolecule-Functionalized Graphene Oxide: A Bifunctional Electrocatalyst for Enhancing Hydrazine Oxidation and Oxygen Reduction Reactions, Energy Fuels, 2021, 35, 6823–6834 CrossRef CAS.
- H. Gunaseelan, A. V. Munde, R. Patel and B. R. Sathe, Metal-organic framework derived carbon-based electrocatalysis for hydrogen evolution reactions: A review, Mater. Today Sustainability, 2023, 22, 100371 CrossRef.
- S. S. Narwade, S. M. Mali, V. S. Sapner and B. R. Sathe, Graphene Oxide Decorated with Rh Nanospheres for Electrocatalytic Water Splitting, ACS Appl. Nano Mater., 2020, 3, 12288–12296 CrossRef CAS.
- Z. Sun, L. Lin, M. Yuan, H. Yao, Y. Deng, B. Huang, H. Li, G. Sun and J. Zhu, Mott–Schottky heterostructure induce the interfacial electron redistribution of MoS2 for boosting pH-universal hydrogen evolution with Pt-like activity, Nano Energy, 2022, 101, 107563 CrossRef CAS.
- S. S. Narwade, S. M. Mali, R. V. Digraskar, V. S. Sapner and B. R. Sathe, Ni/NiO@rGO as an efficient bifunctional electrocatalyst for enhanced overall water splitting reactions, Int. J. Hydrogen Energy, 2019, 44, 27001–27009 CrossRef CAS.
- Y. Yang, L. Liu, S. Chen, W. Yan, H. Zhou, X.-M. Zhang and X. Fan, Tuning Binding Strength of Multiple Intermediates towards Efficient pH-universal Electrocatalytic Hydrogen Evolution by Mo8O26-NbNxOy Heterocatalysts, Angew. Chem., Int. Ed., 2023, 62, e202306896 CrossRef CAS.
- S. S. Narwade, B. B. Mulik, S. M. Mali and B. R. Sathe, Silver nanoparticles sensitized C60(Ag@C60) as efficient electrocatalysts for hydrazine oxidation: Implication for hydrogen generation reaction, Appl. Surf. Sci., 2017, 396, 939–944 CrossRef CAS.
- F.-Y. Chen, Z.-Y. Wu, Z. Adler and H. Wang, Stability challenges of electrocatalytic oxygen evolution reaction: From mechanistic understanding to reactor design, Joule, 2021, 5, 1704–1731 CrossRef CAS.
- Q. Gan, H. He, Y. Zhu, Z. Wang, N. Qin, S. Gu, Z. Li, W. Luo and Z. Lu, Defect-Assisted Selective Surface Phosphorus Doping to Enhance Rate Capability of Titanium Dioxide for Sodium Ion Batteries, ACS Nano, 2019, 13, 9247–9258 CrossRef CAS.
- H. Tan, B. Tang, Y. Lu, Q. Ji, L. Lv, H. Duan, N. Li, Y. Wang, S. Feng, Z. Li, C. Wang, F. Hu, Z. Sun and W. Yan, Engineering a local acid-like environment in alkaline medium for efficient hydrogen evolution reaction, Nat. Commun., 2022, 13, 2024 CrossRef CAS.
- H. Ma, W. Yan, Y. Yu, L. Deng, Z. Hong, L. Song and L. Li, Phosphorus vacancies improve the hydrogen evolution of MoP electrocatalysts, Nanoscale, 2023, 15, 1357–1364 RSC.
- A. T. Garcia-Esparza, S. Park, H. Abroshan, O. A. Paredes Mellone, J. Vinson, B. Abraham, T. R. Kim, D. Nordlund, A. Gallo, R. Alonso-Mori, X. Zheng and D. Sokaras, Local Structure of Sulfur Vacancies on the Basal Plane of Monolayer MoS2, ACS Nano, 2022, 16, 6725–6733 CrossRef CAS.
- T. D. Nguyen, J. Jiang, B. Song, M. D. Tran, W. Choi, J. H. Kim, Y.-M. Kim, D. L. Duong and Y. H. Lee, Gate-Tunable Magnetism via Resonant Se-Vacancy Levels in WSe2, Adv. Sci., 2021, 8, 2102911 CrossRef CAS.
- D. Zhang, H. Li, A. Riaz, A. Sharma, W. Liang, Y. Wang, H. Chen, K. Vora, D. Yan, Z. Su, A. Tricoli, C. Zhao, F. J. Beck, K. Reuter, K. Catchpole and S. Karuturi, Unconventional direct synthesis of Ni3N/Ni with N-vacancies for efficient and stable hydrogen evolution, Energy Environ. Sci., 2022, 15, 185–195 RSC.
- D. Chen, R. Lu, R. Yu, Y. Dai, H. Zhao, D. Wu, P. Wang, J. Zhu, Z. Pu, L. Chen, J. Yu and S. Mu, Work-function-induced Interfacial Built-in Electric Fields in Os-OsSe2 Heterostructures for Active Acidic and Alkaline Hydrogen Evolution, Angew. Chem., Int. Ed., 2022, 61, e202208642 CrossRef CAS.
- Y. Liu, Y. Chen, Y. Tian, T. Sakthivel, H. Liu, S. Guo, H. Zeng and Z. Dai, Synergizing Hydrogen Spillover and Deprotonation by the Internal Polarization Field in a MoS2/NiPS3 Vertical Heterostructure for Boosted Water Electrolysis, Adv. Mater., 2022, 34, 2203615 CrossRef CAS.
- R. Wu, J. Xu, C.-L. Zhao, X.-Z. Su, X.-L. Zhang, Y.-R. Zheng, F.-Y. Yang, X.-S. Zheng, J.-F. Zhu, J. Luo, W.-X. Li, M.-R. Gao and S.-H. Yu, Dopant triggered atomic configuration activates water splitting to hydrogen, Nat. Commun., 2023, 14, 2306 CrossRef CAS.
- S. Zhou, H. Jang, Q. Qin, L. Hou, M. G. Kim, S. Liu, X. Liu and J. Cho, Boosting Hydrogen Evolution Reaction by Phase Engineering and Phosphorus Doping on Ru/P-TiO2, Angew. Chem., Int. Ed., 2022, 61, e202212196 CrossRef CAS.
- X. Guo, E. Song, W. Zhao, S. Xu, W. Zhao, Y. Lei, Y. Fang, J. Liu and F. Huang, Charge self-regulation in 1T′′′-MoS2 structure with rich S vacancies for enhanced hydrogen evolution activity, Nat. Commun., 2022, 13, 5954 CrossRef.
- W. Yan, H. Ma, X. Zhao, Y. Zhang, P. Vishniakov, X. Wang, X. Zhong, Z. Hong, M. Y. Maximov, L. Song, S. Peng and L. Li, P and Se Binary Vacancies and Heterostructures Modulated MoP/MoSe2 Electrocatalysts for Improving Hydrogen Evolution and Coupling Electricity Generation, Small, 2023, 19, 2208270 CrossRef CAS.
- R. Sun, Y. Bai, Z. Bai, L. Peng, M. Luo, M. Qu, Y. Gao, Z. Wang, W. Sun and K. Sun, Phosphorus Vacancies as Effective Polysulfide Promoter for High-Energy-Density Lithium–Sulfur Batteries, Adv. Energy Mater., 2022, 12, 2102739 CrossRef CAS.
- C. Li, H. Jang, M. G. Kim, L. Hou, X. Liu and J. Cho, Ru-incorporated oxygen-vacancy-enriched MoO2 electrocatalysts for hydrogen evolution reaction, Appl. Catal., B, 2022, 307, 121204 CrossRef CAS.
- K. Zhao, W. Yang, L. Li, S. Wang, L. Wang, Z. Qi, Y. Yang, Z. Chen, J. Zhuang, J. Hao and W. Shi, Discharge Induced-Activation of Phosphorus-Doped Nickel Oxyhydroxide for Oxygen Evolution Reaction, Chem. Eng. J., 2022, 435, 135049 CrossRef CAS.
- F. Ye, S. Zhang, Q. Cheng, Y. Long, D. Liu, R. Paul, Y. Fang, Y. Su, L. Qu, L. Dai and C. Hu, The role of oxygen-vacancy in bifunctional indium oxyhydroxide catalysts for electrochemical coupling of biomass valorization with CO2 conversion, Nat. Commun., 2023, 14, 2040 CrossRef CAS PubMed.
- Y. Huang, M. Li, F. Pan, Z. Zhu, H. Sun, Y. Tang and G. Fu, Plasma–induced Mo–doped Co3O4 with enriched oxygen vacancies for electrocatalytic oxygen evolution in water splitting, Carbon Energy, 2022, 5, e279 CrossRef.
- Y. Liang, W. Zhou, Y. Shi, C. Liu and B. Zhang, Unveiling in situ evolved In/In2O3−x heterostructure as the active phase of In2O3 toward efficient electroreduction of CO2 to formate, Sci. Bull., 2020, 65, 1547–1554 CrossRef CAS.
- Y. Wang, Y. Zhang, G. Gao, Y. Fan, R. Wang, J. Feng, L. Yang, A. Meng, J. Zhao and Z. Li, Effectively Modulating Oxygen Vacancies in Flower-Like δ-MnO2 Nanostructures for Large Capacity and High-Rate Zinc-Ion Storage, Nano-Micro Lett., 2023, 15, 219 CrossRef CAS PubMed.
- Z. Wu, T. Liao, S. Wang, J. A. Mudiyanselage, A. S. Micallef, W. Li, A. P. O'Mullane, J. Yang, W. Luo, K. Ostrikov, Y. Gu and Z. Sun, Conversion of Catalytically Inert 2D Bismuth Oxide Nanosheets for Effective Electrochemical Hydrogen Evolution Reaction Catalysis via Oxygen Vacancy Concentration Modulation, Nano-Micro Lett., 2022, 14, 90 CrossRef CAS PubMed.
- W. Zhong, C. Yang, J. Wu, W. Xu, R. Zhao, H. Xiang, K. Shen, Q. Zhang and X. Li, Oxygen vacancies induced by charge compensation tailoring Ni-doped Co3O4 nanoflakes for efficient hydrogen evolution, Chem. Eng. J., 2022, 436, 134813 CrossRef CAS.
- A. Jain, M. B. Sadan and A. Ramasubramaniam, Promoting Active Sites for Hydrogen Evolution in MoSe2 via Transition-Metal Doping, J. Phys. Chem. C, 2020, 124, 12324–12336 CrossRef CAS.
- L. Yuan, Q. Zhang, Y. Pu, X. Qiu, C. Liu and H. Wu, Modulating Intrinsic Defect Structure of Fibrous Hard Carbon for Super-Fast and High-Areal Sodium Energy Storage, Adv. Energy Mater., 2024, 14, 2400125 CrossRef CAS.
- J.-E. Zhou, Y. Peng, X. Sang, C. Wu, Y. Liu, Z. Peng, H. Ou, Y. Wu, X. Lin and Y. Cai, A versatile strategy to activate self-sacrificial templated Li2MnO3 by defect engineering toward advanced lithium storage, J. Energy Chem., 2023, 85, 164–180 CrossRef CAS.
- T. Chu, L. Zhou, B. Zhang and F.-Z. Xuan, Accurate atomic scanning transmission electron microscopy analysis enabled by deep learning, Nano Res., 2024, 17, 2971–2980 CrossRef.
- X. Ding, Y. Yang, Z. Li, P. Huang, X. Liu, Y. Guo and Y. Wang, Engineering a Nickel–Oxygen Vacancy Interface for Enhanced Dry Reforming of Methane: A Promoted Effect of CeO2 Introduction into Ni/MgO, ACS Catal., 2023, 13, 15535–15545 CrossRef CAS.
- N. Wang, W.-H. Yang, R.-X. Wang, Z.-J. Li, X.-F. Xu, Y.-Z. Long and H.-D. Zhang, Oxygen Vacancy-Enhanced Centrosymmetric Breaking of SrFeO3−x for Piezoelectric-Catalyzed Synthesis of H2O2, Small, 2024, 20, 2307291 CrossRef CAS PubMed.
- D. Ma, Z. Zhao, Y. Wang, X. Yang, M. Yang, Y. Chen, J. Zhu, H. Mi and P. Zhang, Unlocking the Design Paradigm of In-Plane Heterojunction with Built-in Bifunctional Anion Vacancy for Unexpectedly Fast Sodium Storage, Adv. Mater., 2024, 36, 2310336 CrossRef CAS PubMed.
- Y. Huang, B. Long, M. Tang, Z. Rui, M.-S. Balogun, Y. Tong and H. Ji, Bifunctional catalytic material: An ultrastable and high-performance surface defect CeO2 nanosheets for formaldehyde thermal oxidation and photocatalytic oxidation, Appl. Catal., B, 2016, 181, 779–787 CrossRef CAS.
- J. Zhang, K. Wu, J. Xiong, Q. Ren, J. Zhong, H. Cai, H. Huang, P. Chen, J. Wu, L. Chen, M. Fu and D. Ye, Static and dynamic quantification tracking of asymmetric oxygen vacancies in copper-ceria catalysts with superior catalytic activity, Appl. Catal., B, 2022, 316, 121620 CrossRef CAS.
- R. Su, Y. Gao, L. Chen, Y. Chen, N. Li, W. Liu, B. Gao and Q. Li, Utilizing the oxygen-atom trapping effect of Co3O4 with oxygen vacancies to promote chlorite activation for water decontamination, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2319427121 CrossRef.
- Z. Wang, C. Wang, S. Mao, B. Lu, Y. Chen, X. Zhang, Z. Chen and Y. Wang, Decoupling the electronic and geometric effects of Pt catalysts in selective hydrogenation reaction, Nat. Commun., 2022, 13, 3561 CrossRef CAS PubMed.
- C. Liu, J. Xu, C. Jiang, H. Gao, X. Ren and L. Lu, The Microstructure–Activity Relationship of Metal–Organic Framework-Based Electrocatalysts for the Oxygen Evolution Reaction at the Single-Particle Level, ACS Mater. Lett., 2023, 5, 1902–1910 CrossRef CAS.
- H. Wang, X.-K. Gu, X. Zheng, H. Pan, J. Zhu, S. Chen, L. Cao, W.-X. Li and J. Lu, Disentangling the size-dependent geometric and electronic effects of palladium nanocatalysts beyond selectivity, Sci. Adv., 2019, 5, eaat6413 CrossRef.
- M. Chen, N. Kitiphatpiboon, C. Feng, A. Abudula, Y. Ma and G. Guan, Recent progress in transition-metal-oxide-based electrocatalysts for the oxygen evolution reaction in natural seawater splitting: A critical review, eScience, 2023, 3, 100111 CrossRef.
- H. Wang, T. Zhai, Y. Wu, T. Zhou, B. Zhou, C. Shang and Z. Guo, High-Valence Oxides for High Performance Oxygen Evolution Electrocatalysis, Adv. Sci., 2023, 10, 2301706 CrossRef CAS.
- Y. Gao, C. Liu, W. Zhou, S. Lu and B. Zhang, Anion Vacancy Engineering in Electrocatalytic Water Splitting, ChemNanoMat, 2021, 7, 102–109 CrossRef CAS.
- F. Gong, Y. Liu, Y. Zhao, W. Liu, G. Zeng, G. Wang, Y. Zhang, L. Gong and J. Liu, Universal Sub-Nanoreactor Strategy for Synthesis of Yolk-Shell MoS2 Supported Single Atom Electrocatalysts toward Robust Hydrogen Evolution Reaction, Angew. Chem., Int. Ed., 2023, 62, e202308091 CrossRef CAS PubMed.
- H. Sun, Z. Yan, C. Tian, C. Li, X. Feng, R. Huang, Y. Lan, J. Chen, C.-P. Li, Z. Zhang and M. Du, Bixbyite-type Ln2O3 as promoters of metallic Ni for alkaline electrocatalytic hydrogen evolution, Nat. Commun., 2022, 13, 3857 CrossRef CAS PubMed.
- Z. Wu, P. Yang, Q. Li, W. Xiao, Z. Li, G. Xu, F. Liu, B. Jia, T. Ma, S. Feng and L. Wang, Microwave Synthesis of Pt Clusters on Black TiO2 with Abundant Oxygen Vacancies for Efficient Acidic Electrocatalytic Hydrogen Evolution, Angew. Chem., Int. Ed., 2023, 62, e202300406 CrossRef CAS PubMed.
- Y. Wu, R. Yao, Q. Zhao, J. Li and G. Liu, La-RuO2 nanocrystals with efficient electrocatalytic activity for overall water splitting in acidic media: Synergistic effect of La doping and oxygen vacancy, Chem. Eng. J., 2022, 439, 135699 CrossRef CAS.
- T. Nakamura, H. Gao, K. Ohta, Y. Kimura, Y. Tamenori, K. Nitta, T. Ina, M. Oishi and K. Amezawa, Defect chemical studies on oxygen release from the Li-rich cathode material Li1.2Mn0.6Ni0.2O2−δ, J. Mater. Chem. A, 2019, 7, 5009–5019 RSC.
- K. Luo, M. R. Roberts, R. Hao, N. Guerrini, D. M. Pickup, Y.-S. Liu, K. Edström, J. Guo, A. V. Chadwick, L. C. Duda and P. G. Bruce, Charge-compensation in 3d-transition-metal-oxide intercalation cathodes through the generation of localized electron holes on oxygen, Nat. Chem., 2016, 8, 684–691 CrossRef CAS.
- Z. Ren, Z. Wu, W. Song, W. Xiao, Y. Guo, J. Ding, S. L. Suib and P.-X. Gao, Low temperature propane oxidation over Co3O4 based nano-array catalysts: Ni dopant effect, reaction mechanism and structural stability, Appl. Catal., B, 2016, 180, 150–160 CrossRef CAS.
- T. Jin, P.-F. Wang, Q.-C. Wang, K. Zhu, T. Deng, J. Zhang, W. Zhang, X.-Q. Yang, L. Jiao and C. Wang, Realizing Complete Solid-Solution Reaction in High Sodium Content P2-Type Cathode for High-Performance Sodium-Ion Batteries, Angew. Chem., Int. Ed., 2020, 59, 14511–14516 CrossRef CAS.
- K. Wang, S. He, B. Li, H. Du, T. Wang, Z. Du, L. Xie and W. Ai, Relaying alkaline hydrogen evolution over locally amorphous Ni/Co-based phosphides constructed by diffusion-limited phase-transition, Appl. Catal., B, 2023, 339, 123136 CrossRef CAS.
- H. Xiao, K. Chi, H. Yin, X. Zhou, P. Lei, P. Liu, J. Fang, X. Li, S. Yuan, Z. Zhang, Y. Su, J. Guo and L. Qian, Excess Activity Tuned by Distorted Tetrahedron in CoMoO4 for Oxygen Evolution, Energy Environ. Mater., 2024, 7, e12495 CrossRef CAS.
- J. Duan, S. Chen, C. A. Ortíz-Ledón, M. Jaroniec and S.-Z. Qiao, Phosphorus Vacancies that Boost Electrocatalytic Hydrogen Evolution by Two Orders of Magnitude, Angew. Chem., Int. Ed., 2020, 59, 8181–8186 CrossRef CAS.
- X. Wang, J. Wu, Y. Zhang, Y. Sun, K. Ma, Y. Xie, W. Zheng, Z. Tian, Z. Kang and Y. Zhang, Vacancy Defects in 2D Transition Metal Dichalcogenide Electrocatalysts: From Aggregated to Atomic Configuration, Adv. Mater., 2023, 35, 2206576 CrossRef CAS.
- X. Wang, Y. Zhang, H. Si, Q. Zhang, J. Wu, L. Gao, X. Wei, Y. Sun, Q. Liao, Z. Zhang, K. Ammarah, L. Gu, Z. Kang and Y. Zhang, Single-Atom Vacancy Defect to Trigger High-Efficiency Hydrogen Evolution of MoS2, J. Am. Chem. Soc., 2020, 142, 4298–4308 CrossRef CAS PubMed.
- G. Shao, H. Xiang, M. Huang, Y. Zong, J. Luo, Y. Feng, X.-X. Xue, J. Xu, S. Liu and Z. Zhou, S vacancies in 2D SnS2 accelerating hydrogen evolution reaction, Sci. China Mater., 2022, 65, 1833–1841 CrossRef CAS.
- I. S. Kwon, I. H. Kwak, G. M. Zewdie, S. J. Lee, J. Y. Kim, S. J. Yoo, J.-G. Kim, J. Park and H. S. Kang, MoSe2–VSe2–NbSe2 Ternary Alloy Nanosheets to Boost Electrocatalytic Hydrogen Evolution Reaction, Adv. Mater., 2022, 34, 2205524 CrossRef CAS.
- Y. Chang, P. Zhai, J. Hou, J. Zhao and J. Gao, Excellent HER and OER Catalyzing Performance of Se-Vacancies in Defects-Engineered PtSe2: From Simulation to Experiment, Adv. Energy Mater., 2022, 12, 2102359 CrossRef CAS.
- R. Zeng, C. Cheng, F. Xing, Y. Zou, K. Ding and C. Huang, Dual vacancies induced local polarization electric field for high-performance photocatalytic H2 production, Appl. Catal., B, 2022, 316, 121680 CrossRef CAS.
- M. Yang, J. Liu, H. Xu, Y. Pei, C. Jiang, D. He and X. Xiao, Intrinsic defects of nonprecious metal electrocatalysts for energy conversion: Synthesis, advanced characterization, and fundamentals, Chem. Phys. Mater., 2022, 1, 155–182 Search PubMed.
- Y. Zuo, N. Antonatos, L. Děkanovský, J. Luxa, J. D. Elliott, D. Gianolio, J. Šturala, F. Guzzetta, S. Mourdikoudis, J. Regner, R. Málek and Z. Sofer, Defect Engineering in Two-Dimensional Layered PdTe2 for Enhanced Hydrogen Evolution Reaction, ACS Catal., 2023, 13, 2601–2609 CrossRef CAS.
- B. Dai, Y. Su, Y. Guo, C. Wu and Y. Xie, Recent Strategies for the Synthesis of Phase-Pure Ultrathin 1T/1T′ Transition Metal Dichalcogenide Nanosheets, Chem. Rev., 2024, 124, 420–454 CrossRef CAS.
- H. Yang, Y. Zhao, Q. Wen, Y. Mi, Y. Liu, H. Li and T. Zhai, Single MoTe2 sheet electrocatalytic microdevice for in situ revealing the activated basal plane sites by vacancies engineering, Nano Res., 2021, 14, 4814–4821 CrossRef CAS.
- Q. Luo, C. Lu, L. Liu and M. Zhu, A review on the synthesis of transition metal nitride nanostructures and their energy related applications, Green Energy Environ., 2023, 8, 406–437 CrossRef CAS.
- Y. Wu, J. Cai, Y. Xie, S. Niu, Y. Zang, S. Wu, Y. Liu, Z. Lu, Y. Fang, Y. Guan, X. Zheng, J. Zhu, X. Liu, G. Wang and Y. Qian, Regulating the Interfacial Electronic Coupling of Fe2N via Orbital Steering for Hydrogen Evolution Catalysis, Adv. Mater., 2020, 32, 1904346 CrossRef CAS.
- X. Liu, Q. Yu, X. Qu, X. Wang, J. Chi and L. Wang, Manipulating Electron Redistribution in Ni2P for Enhanced Alkaline Seawater Electrolysis, Adv. Mater., 2024, 36, 2307395 CrossRef CAS.
- K. Qian, Y. Yan, S. Xi, T. Wei, Y. Dai, X. Yan, H. Kobayashi, S. Wang, W. Liu and R. Li, Elucidating the Strain–Vacancy–Activity Relationship on Structurally Deformed Co@CoO Nanosheets for Aqueous Phase Reforming of Formaldehyde, Small, 2021, 17, 2102970 CrossRef CAS.
- H. Pang, Z. Yu, X. Qin, B. Fan, R. Jiang, S. Li, Y. Hou, W. Tang, M. Wang and Z. Shi, Adjusting the valence band center of Co-Ni-bimetallic sulfides through lattice expansion and stacking faults triggered by strain engineering to boost oxygen evolution reaction, J. Colloid Interface Sci., 2023, 646, 503–516 CrossRef CAS.
- X. Liu, S. Wei, S. Cao, Y. Zhang, W. Xue, Y. Wang, G. Liu and J. Li, Lattice Strain with Stabilized Oxygen Vacancies Boosts Ceria for Robust Alkaline Hydrogen Evolution Outperforming Benchmark Pt, Adv. Mater., 2024, 2405970 CrossRef.
- Z. Du, Z. Meng, X. Gong, Z. Hao, X. Li, H. Sun, X. Hu, S. Yu and H. Tian, Rapid Surface Reconstruction of Pentlandite by High–Spin State Iron for Efficient Oxygen Evolution Reaction, Angew. Chem., Int. Ed., 2024, 63, e202317022 CrossRef CAS.
- Y. Ye, J. Xu, X. Li, Y. Jian, F. Xie, J. Chen, Y. Jin, X. Yu, M.-H. Lee, N. Wang, S. Sun and H. Meng, Orbital Occupancy Modulation to Optimize Intermediate Absorption for Efficient Electrocatalysts in Water Electrolysis and Zinc–Ethanol–Air Battery, Adv. Mater., 2024, 36, 2312618 CrossRef CAS.
- C. Jia, S. Li, Y. Zhao, R. K. Hocking, W. Ren, X. Chen, Z. Su, W. Yang, Y. Wang, S. Zheng, F. Pan and C. Zhao, Nitrogen Vacancy Induced Coordinative Reconstruction of Single-Atom Ni Catalyst for Efficient Electrochemical CO2 Reduction, Adv. Funct. Mater., 2021, 31, 2107072 CrossRef CAS.
- J. Wang, C. Yang, L. Mao, X. Cai, Z. Geng, H. Zhang, J. Zhang, X. Tan, J. Ye and T. Yu, Regulating the Metallic Cu–Ga Bond by S Vacancy for Improved Photocatalytic CO2 Reduction to C2H4, Adv. Funct. Mater., 2023, 33, 2213901 CrossRef CAS.
- S. Mondal, D. Bagchi, M. Riyaz, S. Sarkar, A. K. Singh, C. P. Vinod and S. C. Peter, In Situ Mechanistic Insights for the Oxygen Reduction Reaction in Chemically Modulated Ordered Intermetallic Catalyst Promoting Complete Electron Transfer, J. Am. Chem. Soc., 2022, 144, 11859–11869 CrossRef CAS PubMed.
- L. Li, X. Zhang, M. Humayun, X. Xu, Z. Shang, Z. Li, M. F. Yuen, C. Hong, Z. Chen, J. Zeng, M. Bououdina, K. Temst, X. Wang and C. Wang, Manipulation of Electron Spins with Oxygen Vacancy on Amorphous/Crystalline Composite-Type Catalyst, ACS Nano, 2024, 18, 1214–1225 CrossRef CAS PubMed.
- G. Li, H. Jang, S. Liu, Z. Li, M. G. Kim, Q. Qin, X. Liu and J. Cho, The synergistic effect of Hf-O-Ru bonds and oxygen vacancies in Ru/HfO2 for enhanced hydrogen evolution, Nat. Commun., 2022, 13, 1270 CrossRef CAS PubMed.
- X. Liu, X. Wang, K. Li, J. Tang, J. Zhu, J. Chi, J. Lai and L. Wang, Diluting the Resistance of Built-in Electric Fields in Oxygen Vacancy-enriched Ru/NiMoO4−x for Enhanced Hydrogen Spillover in Alkaline Seawater Splitting, Angew. Chem., Int. Ed., 2024, 63, e202316319 CrossRef CAS PubMed.
- F. Wu, R. Yang, S. Lu, W. Du, B. Zhang and Y. Shi, Unveiling Partial Transformation and Activity Origin of Sulfur Vacancies for Hydrogen Evolution, ACS Energy Lett., 2022, 7, 4198–4203 CrossRef CAS.
- S. Huang, Z. Jin, P. Ning, C. Gao, Y. Wu, X. Liu, P. Xin, Z. Chen, Y. Jiang, Z. Hu and Z. Chen, Synergistically modulating electronic structure of NiS2 hierarchical architectures by phosphorus doping and sulfur-vacancies defect engineering enables efficient electrocatalytic water splitting, Chem. Eng. J., 2021, 420, 127630 CrossRef CAS.
- Y. Cao, S. Zhang, B. Zhang, C. Han, Y. Zhang, X. Wang, S. Liu, H. Gong, X. Liu, S. Fang, F. Pan and J. Sun, Local Electric Field Promoted Kinetics and Interfacial Stability of a Phosphorus Anode with Ionic Covalent Organic Frameworks, Adv. Mater., 2023, 35, 2208514 CrossRef CAS.
- X. Wang, D. Du, Y. Yan, L. Ren, H. Xu, X. Wen, T. Zeng, G. Tian, S. Liu, F. Fan and C. Shu, Molecular cleavage strategy enabling optimized local electron structure of Co-based metal-organic framework to accelerate the kinetics of oxygen electrode reactions in lithium-oxygen battery, Energy Storage Mater., 2023, 63, 103033 CrossRef.
- Y. Zhao, H. Wang, J. Li, Y. Fang, Y. Kang, T. Zhao and C. Zhao, Regulating the Spin-State of Rare-Earth Ce Single Atom Catalyst for Boosted Oxygen Reduction in Neutral Medium, Adv. Funct. Mater., 2023, 33, 2305268 CrossRef CAS.
- Z. Yu, H. Yan, C. Wang, Z. Wang, H. Yao, R. Liu, C. Li and S. Ma, Oxygen-deficient MoOx/Ni3S2 heterostructure grown on nickel foam as efficient and durable self-supported electrocatalysts for hydrogen evolution reaction, Front. Chem. Sci. Eng., 2023, 17, 437–448 CrossRef CAS.
- Z. Li, M. Huang, J. Li and H. Zhu, Large-Scale, Controllable Synthesis of Ultrathin Platinum Diselenide Ribbons for Efficient Electrocatalytic Hydrogen Evolution, Adv. Funct. Mater., 2023, 33, 2300376 CrossRef CAS.
- X. Lv, S. Wan, T. Mou, X. Han, Y. Zhang, Z. Wang and X. Tao, Atomic-Level Surface Engineering of Nickel Phosphide Nanoarrays for Efficient Electrocatalytic Water Splitting at Large Current Density, Adv. Funct. Mater., 2023, 33, 2205161 CrossRef CAS.
- C. Zhang, Y. Shi, Y. Yu, Y. Du and B. Zhang, Engineering Sulfur Defects, Atomic Thickness, and Porous Structures into Cobalt Sulfide Nanosheets for Efficient Electrocatalytic Alkaline Hydrogen Evolution, ACS Catal., 2018, 8, 8077–8083 CrossRef CAS.
- K. Zhang, Y. Duan, N. Graham and W. Yu, Unveiling the synergy of polymorph heterointerface and sulfur vacancy in NiS/Ni3S2 electrocatalyst to promote alkaline hydrogen evolution reaction, Appl. Catal., B, 2023, 323, 122144 CrossRef CAS.
- J. Dai, Y. Zhu, Y. Chen, X. Wen, M. Long, X. Wu, Z. Hu, D. Guan, X. Wang, C. Zhou, Q. Lin, Y. Sun, S.-C. Weng, H. Wang, W. Zhou and Z. Shao, Hydrogen spillover in complex oxide multifunctional sites improves acidic hydrogen evolution electrocatalysis, Nat. Commun., 2022, 13, 1189 CrossRef CAS PubMed.
- B. Zhang, J. Shan, W. Wang, P. Tsiakaras and Y. Li, Oxygen Vacancy and Core–Shell Heterojunction Engineering of Anemone-Like CoP@CoOOH Bifunctional Electrocatalyst for Efficient Overall Water Splitting, Small, 2022, 18, 2106012 CrossRef CAS PubMed.
- M. Yang, M. Zhao, J. Yuan, J. Luo, J. Zhang, Z. Lu, D. Chen, X. Fu, L. Wang and C. Liu, Oxygen Vacancies and Interface Engineering on Amorphous/Crystalline CrOx-Ni3N Heterostructures toward High-Durability and Kinetically Accelerated Water Splitting, Small, 2022, 18, 2106554 CrossRef CAS.
- J. Lu, G. Qian, L. Luo, H. He and S. Yin, Contributions of oxygen vacancies to the hydrogen evolution catalytic activity of tungsten oxides, Int. J. Hydrogen Energy, 2021, 46, 676–682 CrossRef CAS.
- Y. Zhang, J. Lu, L. Zhang, T. Fu, J. Zhang, X. Zhu, X. Gao, D. He, Y. Luo, D. D. Dionysiou and W. Zhu, Investigation into the catalytic roles of oxygen vacancies during gaseous styrene degradation process via CeO2 catalysts with four different morphologies, Appl. Catal., B, 2022, 309, 121249 CrossRef CAS.
- J. Qu, W. Liu, R. Liu, J. He, D. Liu, Z. Feng, Z. Feng, R. Li and C. Li, Evolution of oxygen vacancies in cerium dioxide at atomic scale under CO2 reduction, Chem Catal., 2023, 3, 100759 CrossRef CAS.
- Z.-F. Huang, S. Xi, J. Song, S. Dou, X. Li, Y. Du, C. Diao, Z. J. Xu and X. Wang, Tuning of lattice oxygen reactivity and scaling relation to construct better oxygen evolution electrocatalyst, Nat. Commun., 2021, 12, 3992 CrossRef CAS.
- K. Wang, S. He, Y. Lin, X. Chen, W. Dai and X. Fu, Photo-enhanced thermal catalytic CO2 methanation activity and stability over oxygen-deficient Ru/TiO2 with exposed TiO2 {001} facets: Adjusting photogenerated electron behaviors by metal-support interactions, Chin. J. Catal., 2022, 43, 391–402 CrossRef CAS.
- L.-W. Jiang, Y. Huang, Y. Zou, C. Meng, Y. Xiao, H. Liu and J.-J. Wang, Boosting the Stability of Oxygen Vacancies in α-Co(OH)2 Nanosheets with Coordination Polyhedrons as Rivets for High-Performance Alkaline Hydrogen Evolution Electrocatalyst, Adv. Energy Mater., 2022, 12, 2202351 CrossRef CAS.
- M. Wang, G. Zhao, X. Bai, W. Yu, C. Zhao, Z. Gao, P. Lyu, Z. Chen and N. Zhang, Gradient Concentration Refilling of N Stabilizes Oxygen Vacancies for Enhanced Zn2+ Storage, Adv. Energy Mater., 2023, 13, 2301730 CrossRef CAS.
- H. Ma, X. Wang, T. Tan, X. Zhou, F. Dong and Y. Sun, Stabilize the oxygen vacancies in Bi2SiO5 for durable photocatalysis via altering local electronic structure with phosphate dopant, Appl. Catal., B, 2022, 319, 121911 CrossRef CAS.
- C. T. Campbell and C. H. F. Peden, Oxygen Vacancies and Catalysis on Ceria Surfaces, Science, 2005, 309, 713–714 CrossRef CAS PubMed.
- X. Liu, K. Zhou, L. Wang, B. Wang and Y. Li, Oxygen Vacancy Clusters Promoting Reducibility and Activity of Ceria Nanorods, J. Am. Chem. Soc., 2009, 131, 3140–3141 CrossRef CAS PubMed.
- G. Li, D. Zhang, Q. Qiao, Y. Yu, D. Peterson, A. Zafar, R. Kumar, S. Curtarolo, F. Hunte, S. Shannon, Y. Zhu, W. Yang and L. Cao, All The Catalytic Active Sites of MoS2 for Hydrogen Evolution, J. Am. Chem. Soc., 2016, 138, 16632–16638 CrossRef CAS PubMed.
- G. Yang, Y. Jiao, H. Yan, Y. Xie, A. Wu, X. Dong, D. Guo, C. Tian and H. Fu, Interfacial Engineering of MoO2-FeP Heterojunction for Highly Efficient Hydrogen Evolution Coupled with Biomass Electrooxidation, Adv. Mater., 2020, 32, 2000455 CrossRef CAS.
| This journal is © the Partner Organisations 2024 |