Atomically precise nanocluster-catalyzed coupling reactions
Jinhui
Hu
,
Yi-Ming
Li
,
Bei
Zhang
,
Xi
Kang
 * and
Manzhou
Zhu
* and
Manzhou
Zhu
 *
*
Department of Chemistry and Centre for Atomic Engineering of Advanced Materials, Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials, Anhui University, Hefei, Anhui 230601, China. E-mail: zmz@ahu.edu.cn
First published on 1st July 2024
Abstract
The application of atomically precise nanocluster-based catalysts in organic synthesis has been practiced for decades. Such nanoclusters have been used as ideal catalysts as their structures are designable at the atomic level, their confined structures are conducive to mechanistic study, and most of them can be recycled during the synthesis process. The catalysis of coupling reactions using nanoclusters is an advanced methodology to build complicated carbohydrate scaffolds in one step. The history of atomically precise nanocluster-catalyzed coupling reactions is short; however, the past decade has witnessed the prosperity of this field-atomically precise nanocluster-catalyzed carbon–carbon coupling reactions including many named reactions, carbon–heteroatom coupling reactions, and multi-component coupling reactions have been reported and extensively applied in medicinal chemistry and materials science. The components and geometries of metal nanoclusters, such as ligands, motifs, metal cores, and supports, affect their catalytic abilities synergistically. This review summarizes carbon–carbon and carbon–heteroatom coupling reactions over atomically precise nanoclusters and highlights the correlations between nanoclusters’ catalytic properties and their specific components. Guidance for choosing suitable nanoclusters for specific coupling reactions and possible research directions in this field have been proposed. We hope that this review will provide researchers attempting to study the coupling reactions catalyzed by metal nanoclusters with a comprehensively catalytic toolbox and insightful research fundamentals, so as to provide tailor-made approaches to achieve more efficient cluster-based catalysts towards coupling reactions.
1. Introduction
Coupling reactions, in general, connect molecules by constructing carbon–carbon bonds or carbon–heteroatom bonds and are usually catalyzed by metals (metal salts,1 metal complexes,2 metal nanoparticles,3 and metal nanoclusters4). This type of chemistry might be one of the most significant organic reactions since complicated carbohydrate scaffolds can be built in one step. Many coupling reactions have been named after scientists to recognize their contributions to the development of organic chemistry. The Nobel Prize was awarded to chemists who discovered and developed coupling reactions.5 Coupling reactions continue to draw the attention of researchers, motivated by the discovery of better catalysis systems, accurate step-by-step mechanistic study, and the need for waste-reducing and energy-saving methodologies, where atomically precise nanoclusters are prominent because (i) they can be designed and engineered at the atomic level for catalytic performance screening; (ii) the well-defined structure determined by X-ray single-crystal diffraction can be used to unveil the catalytic mechanism both theoretically and experimentally; (iii) nanoclusters, in particular supported nanoclusters, can be recycled easily during catalytic reactions to build a greener catalytic system.6Nanoclusters are optimal catalysts for various homogenous and heterogenous catalytic reactions with their distinct morphology.6,7 Cross-coupling reactions over supported gold nanoclusters have been reviewed.8,9 However, many new examples have been reported recently. Thus, it is our intention to highlight the most relevant and recent advances by selecting studies that best represent vital concepts, so as to show the nanocluster family's catalytic potential for organic chemistry. For each report, the assessment is focused on reaction conditions, overall yields, and the relationship between nanoclusters’ structures and their catalytic mechanisms. Examples of nanoclusters with specific metal numbers but no exact chemical formulas are also included in this review to provide a straightforward understanding of the nanocluster catalysis system. Some well-reviewed examples of coupling reactions over nanoclusters are also included but are summarized from different angles and perspectives for the rationalization and completion of the review.
Carbon–carbon and carbon–heteroatom coupling reactions catalyzed by atomically precise nanoclusters are thoroughly reviewed in this article. Most of the examples in each section are listed chronologically to see the development of each system. The catalytic properties of each atomically precise nanocluster are evaluated, and each mechanism is discussed. The application in coupling reactions catalyzed by metal nanoclusters provides opportunities for investigating the correlation between confined structures and physicochemical properties at the atomic level. In this context, this article is intended to guide you in choosing suitable nanoclusters for coupling reactions regarding the metal core, the protecting ligand, and the immobilization support and predict proper catalytic abilities for newly synthesized nanoclusters (Scheme 1).
2. Factors altering nanoclusters’ catalytic properties
The ligand effect, heteroatom doping effect, nanocluster-support synergism, and defective effect have been exploited to dictate the catalytic properties of atomically precise nanoclusters. For instance, homologous gold nanoclusters and gold-base bimetallic nanoclusters behave differently in catalytic performance due to the synergetic effects between gold and foreign atoms.10,11 Gold-base bimetallic nanoclusters with different foreign atoms show diverse reaction performances.12 Herein, we list the factors that can alter nanoclusters’ catalytic properties and introduce each of them briefly (Scheme 2).2.1 Protecting ligand effect
Protecting ligands as the outmost part of morphology is an essential part of atomically precise nanoclusters to prevent the aggregation of nanoclusters and protect metal motifs and kernels. Commonly used ligands are thiolates, alkynyls, phosphines, and their combinations. Inorganic ligands have also been reported.13 All these ligands coordinate with nanoclusters by only one or two atoms (S, P, or C) with mono- or multi-chelation modes. Different protecting ligands could largely influence nanoclusters’ shape and size, as well as their related properties.14 Engineering nanoclusters by changing their ligands could alter their electronic properties and further influence their catalytic properties.15 Nanoclusters with different capping ligands could have different total conversion rates, product selectivity, and even reaction directions. There are countless methods of welding capping ligands on nanoclusters, such as simple polydispersity16 and ligand exchange,17 indicating that changing the protecting ligands is one of the easiest ways to manipulate nanoclusters’ catalytic properties.2.2 Doping effect
Alloy is a well-known concept in modern industry to enhance metals’ properties by adding other metal or non-metal materials. The hybrid metal usually represents better oxygen, water, and salt resistance and higher mechanical strength. The same strategy can be extended in nanoscience with various remarkable applications.18,19 This versatile alloying strategy can drastically increase the nanocluster family's diversity. Sequentially, atomically precise alloy nanocluster-related physicochemical properties can be evaluated at the atomic level owing to their atomically confined structures. Advanced techniques allow scientists to change nanoclusters’ metal atoms one by one. Therefore, some series of nanoclusters can be listed as follows: (1) doping the same parent metal with one hetero-metal atom, for instance, X1Au24, where X is a foreign metal atom that can be Hg,20 Pd,21 Pt,22 Cd,23etc., based on synthesis procedures and Au is the parent metal atom and (2) doping different numbers of the same foreign metal atom in parent nanoclusters such as Au25−nAgn where Au25 is the parent metal kernel, while different numbers of Ag can be doped in it.24,25 The synergistic effect of hetero atoms is the main reason why their properties changed. Thus, doping hetero-metal atoms to corresponding homologous nanoclusters can improve the physicochemical performance of nanoclusters. Alloying different hetero-metal atoms to the same parent nanocluster could result in different catalytic properties. To date, metals such as Au, Ag, Pt, Pd, Cu, and Cd can be doped into parent homologous nanoclusters to generate bi-, tri-, and multi-metallic alloy nanoclusters.26 The catalytic properties of the alloy nanoclusters can be assessed and predicted according to the metal components before applying them to reactions for saving time and cost.2.3 Support effect
One of the major limitations of atomically precise nanoclusters is their unstable nature. Even stable nanoclusters can be aggregated, decomposed, or deactivated when they are applied to chemical reaction conditions such as higher or lower PH, extremely high reaction temperature, and long-time exposure to solvent or optical radiation. Immobilizing nanoclusters in support is the most frequently used approach to solve this issue. Common supports are metal oxides such as Ce2O, Ti2O, and MgO, porous materials such as activated carbons and carbon nanotubes, and high polymer materials such as poly(N-vinylpyrrolidone). Mounting nanoclusters on supports turns the material into a heterogenous catalyst, which prevents the decomposition of nanoclusters during chemical reactions and keeps them intact during recyclization. Moreover, the porous structure of the support can largely enhance the catalytic properties of nanoclusters by dramatically increasing the contact area between the catalyst and reactant molecules. Some catalytic reactions cannot push forward without the assistance of supports. Moreover, the synergistic effect between nanoclusters and supports can regulate the target reaction's conversion rate and selectivity.27–29 This approach makes nanoclusters better catalysts with higher efficiency, lower cost, and higher stability.2.4 Defective effects
Defect is a solid material terminology. It means interruption in the homogenous arrangement of solid building blocks caused by vacancies or other components. The phenomenon results in property changes including electrical and magnetic, which can be utilized to improve their performance. The application of defects is smoothly extended from the macroscopic to the microscopic level, where defect engineering is considered a splendid method to direct electrical, optical, magnetic, and catalytic regulation in nanotechnologies.30 As for nanoclusters, the defect is still a coinage and it usually represents the surface vacancy of nanocluster metal motifs and kernels leading to changes in nanoclusters’ active sites.31–33 Catalytic reactions occur at the interface between nanoclusters’ metal active sites and reactive species. Hence, defects can directly influence nanoclusters’ catalytic properties by altering the interfacial interaction such as surface adsorption and desorption.34 The imperfection at the atomic level offers new insights into defect chemistry and nanocluster design.Other characteristics of nanoclusters can also change their catalytic properties, for instance, nanoclusters with less than 10 metal atoms perform differently in chemical reactions from nanoclusters with more than 10 metal atoms due to their coreless nature.35 Nanoclusters catalyze chemical reactions as a whole with their specific morphology including capping ligands, motifs, kernels, and even support. The above-mentioned factors influence nanoclusters’ physicochemical properties collectively. The design and analysis of atomically precise nanoclusters for catalytic coupling reactions require scientists to oversee the catalytic patterns coming from each factor. Their collaborations finally give rise to individual nanocluster's specific catalytic properties and their performance is discussed in section 3 case by case.
3. Catalytic performance
3.1 Carbon–carbon coupling
Carbon–carbon bond formation via coupling reactions is the most versatile and extensively used method to construct carbon scaffolds of organic molecules in organic synthesis. With the rapid development of scientific research, carbon–carbon coupling reactions including Csp3–Csp3, Csp3–Csp2, Csp3–Csp, Csp2–Csp2, and Csp–Csp bond formation catalyzed by noble metals, inexpensive metals and even without metal have been documented.36 To extend the application of the newly discovered nanomaterial, atomically precise nanoclusters were utilized in carbon–carbon coupling systems to unveil their catalytic abilities.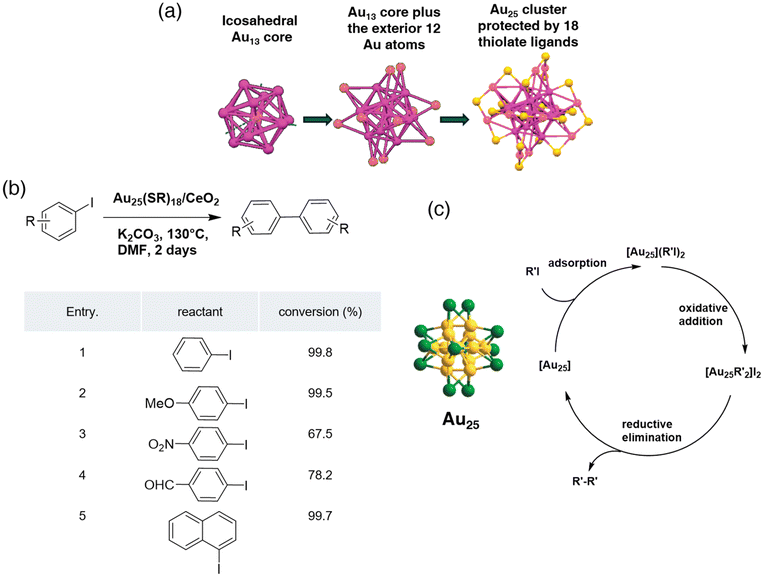 | ||
| Fig. 1 (a) Crystal structure of an Au25(SR)18 cluster (R is the phenylethyl group); (for clarity, only S was shown for ligands; magenta: Au; yellow: S). Reprinted with permission from ref. 38. Copyright 2008 American Chemical Society. (b) Homocoupling of aryl iodides using the Au25(SR)18/CeO2 catalyst. (c) Proposed mechanism for the Ullmann-type homocoupling reaction of iodobenzene catalyzed by Au25(SR)18 nanoclusters (green: surface Au atoms; yellow: core Au atoms; the thiol ligands are omitted for clarity). Reprinted with permission from ref. 41. Copyright 2012, the Royal Society of Chemistry. | ||
Later the same group investigated the effects of ligand engineering regarding Au25 nanoclusters’ catalytic performance.11 The model reaction they used was the Ullmann hetero coupling reaction between 4-methyl-iodobenzene and 4-nitro-iodobenzene. Au25 nanoclusters with different thiolate ligands were tested under optimized conditions, and the total conversion rate and selectivity towards heterocoupling products were determined (Fig. 2b). According to the results, Au25 protected by aromatic ligands (Fig. 2a) gave the best conversion rate and selectivity, while nanoclusters protected by carbon-chain ligands tended to generate homocoupling products. DFT calculations were used to understand the ligand effects of this system. Jin believed one thiolate ligand was lost, and two gold atoms were exposed to the starting materials during the experiment. Hence, model systems of the iodobenzene with the exposed gold atom of Au25(SCH3)2(SH)15 and Au25(SNap)2(SH)15 (SNap = 1-naphthalenethiolate) were calculated to reduce the computational costs. Aromatic thiolate ligands decreased the activation energy of the C–I bond formation from −4.3 to −7.2 kcal mol−1 compared to aliphatic ligands, making the Ullmann heterocoupling reaction favorable, and meeting the experimental results.
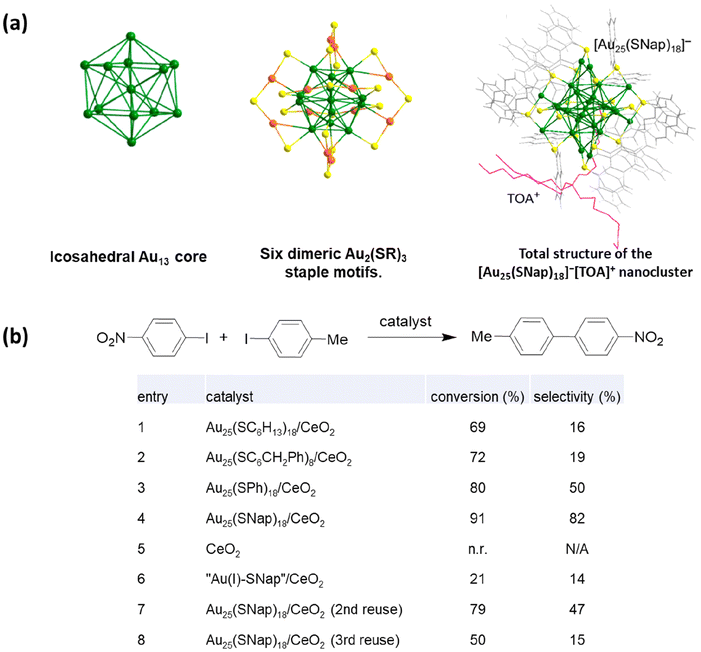 | ||
| Fig. 2 (a) Structure of the [Au25(SNap)18]−[TOA]+ nanocluster. Au25S18 is shown in ball-and-stick mode, thiolate ligands, and TOA+ in wire-and-stick mode. Gold: green, or orange; sulfur: yellow; carbon: gray; hydrogen: white; TOA+: magenta. (b) Catalytic performance of Au25(SR)18/CeO2 catalysts for Ullmann heterocoupling between 4-methyl-iodobenzene and 4-nitro-iodobenzene. Reprinted with permission from ref. 15. Copyright 2016 American Chemical Society. | ||
The doping effect of the AuxM(25−x) alloy nanoclusters in the Ullmann coupling reactions has also been explored.44 Li et al. synthesized a group of TiO2-supported gold-based bimetallic nanoclusters,44,45 and matrix-assisted laser desorption/ionization-mass spectrum (MALDI-MS) was used to determine their precise formula. Among them, AgxAu(25−x) nanoclusters (x = 0–5) and CuxAu(25−x) nanoclusters (x = 0–6) were mixtures, while the PtxAu(25−x) nanocluster had only one component: Pt1Au24. They were treated with p-iodoanisole and phenylacetylene in an N2 atmosphere at 160 °C (Fig. 3a). The Ullmann homocoupling reaction and Sonogashira cross-coupling occurred simultaneously and with different doping hetero atoms, the total conversion rate and selectivity varied. Regarding the Ullmann coupling reaction, none of these bimetallic nanoclusters gave good results except CuxAu(25−x) nanoclusters. It is probably because copper salts and complexes, by themselves, are excellent catalysts for the Ullmann coupling reactions.46 The PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–H/Ph–I pairs are adsorbed on the Au3 open facet, which leads to the Ullmann homo-coupling reactions (Fig. 3b). The details of the structures of these nanoclusters and the mechanisms of the coupling reactions will be discussed in the Sonogashira coupling reaction section. The results of this study confirmed that doping foreign metal atoms into nanoclusters led to changes in their catalytic performance.
C–H/Ph–I pairs are adsorbed on the Au3 open facet, which leads to the Ullmann homo-coupling reactions (Fig. 3b). The details of the structures of these nanoclusters and the mechanisms of the coupling reactions will be discussed in the Sonogashira coupling reaction section. The results of this study confirmed that doping foreign metal atoms into nanoclusters led to changes in their catalytic performance.
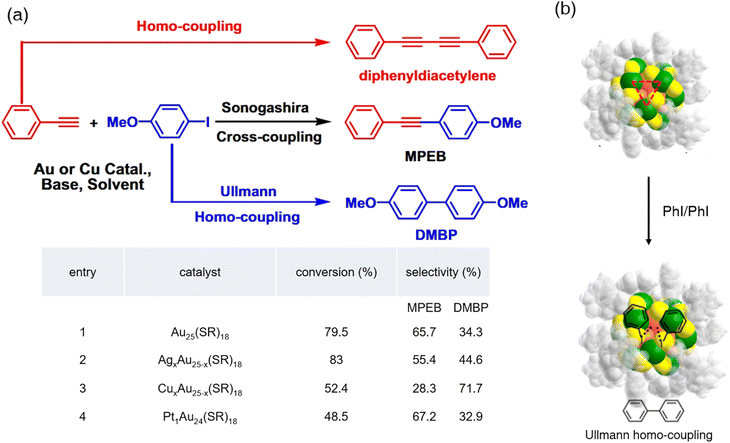 | ||
| Fig. 3 (a) Catalytic performances of TiO2-supported MxAu25−x(SR)18 nanocluster catalysts for the carbon–carbon coupling reaction between p-iodoanisole and phenylacetylene. (b) Proposed mechanism for the MxAu25−x(SR)18-catalyzed-Ullmann coupling reaction. Reprinted with permission from ref. 44. Copyright 2016 Chinese Materials Research Society. Published by Elsevier B.V. | ||
Ehara, Sakurai, and colleagues reported the first Ullmann coupling reactions of chloroarenes under ambient conditions in 2012.47 A series of bimetallic nanoclusters AuxPd(20−x) supported by poly(N-vinylpyrrolidone) (PVP) were synthesized and tested for Ullmann coupling of 4-chlorotoluenen. Au16Pd4, Au10Pd10, and Au4Pd16 showed catalytic abilities under base conditions. With 300 mol% KOH, the Au10Pd10 nanocluster gave the best result: yielding 98% at room temperature in 24 h. The scope of this methodology had been expanded with substituted chloroarenes. Most cases gave excellent yields under optimized conditions within 6–24 h. To further investigate the low-energy cost of this catalyst, computational studies were conducted. The oxidative addition step of the reaction pathway was calculated with model systems of Au20-, Au16Pd4-, and Au10Pd10. The energy diagram showed that the alloy nanocluster stabilized the intermediate and dramatically reduced the activation energy, while homologous Au20 nanoclusters showed no catalytic ability of this Ullmann coupling reaction at all. It is also worth noting that N,N-dimethylformamide (DMF) played an essential role in this experiment as both a co-solvent and a reductant.
Another smaller gold nanocluster-catalyzed Ullmann coupling reaction has been reported recently.48 Mandal's group has synthesized and elucidated the structure of a smaller Au nanocluster, Au11(PPh3)7I3, in detail (Fig. 4a). Since Au11 has a symmetrical core like the Au13 kernel in Au25 nanoclusters,38 the Ullmann C–C coupling reactions were used to test the catalytic ability of the chemical (Fig. 4b). The nanoclusters were supported by CeO2 and underwent both homocoupling and heterocoupling reactions. The homocoupling reactions followed the same pattern as the Au25 nanoclusters’ catalytic ability with a better total conversion rate. The heterocoupling reactions, however, produced a trace amount of hetero-coupled product. These results were consistent with the ligand effect study conducted by Jin,15 which confirmed that the protecting ligand of nanoclusters plays an important role in coupling reaction selectivity. DFT calculations have been managed to discover the mechanism of coupling reactions. A simulation of the reaction pathway of C–I bond dissociation was conducted showing that PhI intended to bind the atop site of the optimized Au11 hexagonal close-packed (hcp) structure.49 Unlike Au25 nanoclusters’ catalytic performance, the results indicated that the mechanism of this reaction follows the single-atom catalytic behavior, where intermediate I–Au11–Ph is generated. The Au11 nanoclusters cannot bind two aryl compounds simultaneously, which explains why the heterocoupling product was not observed as the key intermediate of the heterocoupling reaction cannot be formed (Fig. 4c).
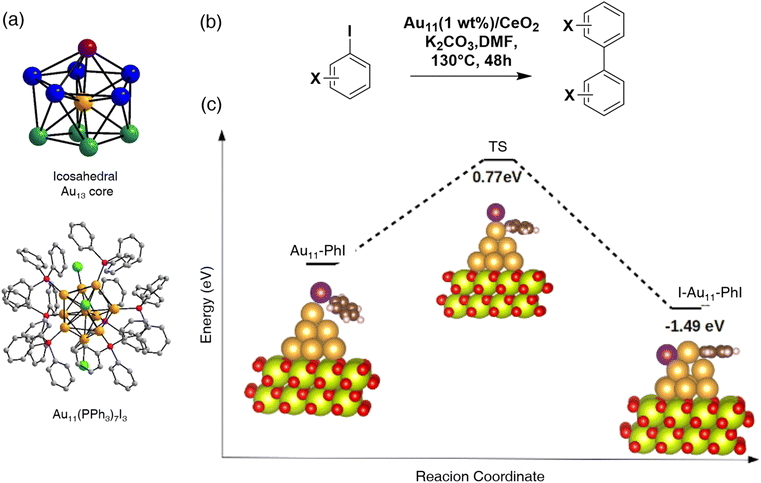 | ||
| Fig. 4 (a) Total crystal structure of the Au11(PPh3)7I3 nanocluster (color legends: Au, yellow, purple, blue, bluish-green; P, magenta; I, green; C, gray; H atoms are missing for clarity). (b) Ullmann C–C coupling reaction over Au11. (c) Proposed reaction pathway of C–I bond dissociation using the Au11@CeO2 catalyst. Reprinted with permission from ref. 48. Copyright 2020 American Chemical Society. | ||
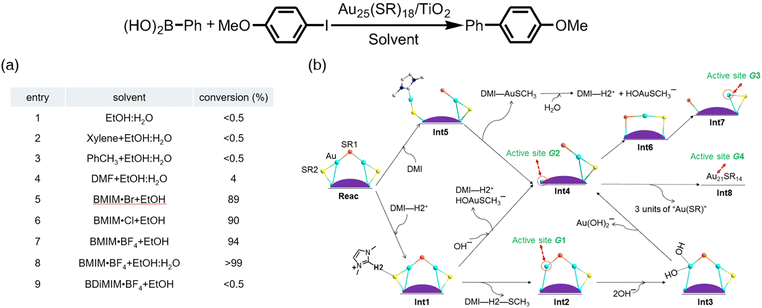 | ||
| Fig. 5 (a) Catalytic performance of thiolate-capped Au25 clusters supported on titania as catalysts for the Suzuki cross-coupling reaction of iodoanisole and phenylboronic acid. (b) Proposed reaction mechanism for the formation of catalytically active sites on the Au25 cluster promoted by imidazolium-based ionic liquids. Reprinted with permission from ref. 55. Copyright 2016 Elsevier Inc. | ||
| Reaction type | Catalyst | Additives | Conditions | Yield (%) | Ref. |
|---|---|---|---|---|---|
| Ullmann C–C coupling | Au25(PET)18/CeO2 | K2CO3 | 130 °C, 2 days | Up to 99.8 | 38 |
| CuxAu(25−x)/TiO2 | K2CO3 | 160 °C, 40 h | 71.7 | 44 | |
| Pd10Au10/PVP | K2CO3 | 40 °C, 24 h | Up to 98 | 47 | |
| Au11(PPh3)7I3 | K2CO3 | 130 °C, 2 days | >99 | 48 | |
| Suzuki–Miyaura coupling | Au25(PET)18/TiO2 | K2CO3, ionic liquid, H2O | 90 °C, 18 h | >99 | 55 |
| Pd3Cl(PPh2)2(PPh3)3 | K2CO3, H2O | r.t., less than 1 h | Up to 99 | 57 | |
| Heck reaction | PdCo/MgAl-LDH | K2CO3, H2O | 160 °C, 40 h | Up to 95 | 73 |
| Pd3(PPh3)3(PPh2)2/MSNs | NEt3 | 80 °C, 12 h | Up to 95 | 74 | |
| Sonogashira reaction | Au25(PET)18/CeO2 | K2CO3 | 160 °C, 40 h | Up to 98 | 27 |
| Pt1Au24/TiO2 | K2CO3 | 160 °C, 40 h | 67.2 | 44 | |
| Au13Cu2(PPh3)6(SC2H4Ph)6 | K3PO4 | N2, r.t., 8 h | 93 | 90 | |
| Cu28-PPh2Py | NaOMe | Blue LEDs, 30 °C, 24 h | Up to 82 | 33 | |
| Oxidative coupling | Au1Cu24/AC | Cs2CO3 | 30 °C, 10 h | Up to 99.7 | 103 |
| Cu1Au11(PPh3)7Cl2 | K2CO3 | 120 °C, 10 h | Up to 100 | 107 | |
| C–N coupling | Cu6(GS)2 | KOH | 120 °C, 7–15 h | Up to 96 | 123 |
| Cu61(StBu)26S6Cl6H14 | K3PO4 | N2, Blue LEDs, 34 °C, 24 h | Up to 85 | 126 | |
| C–O coupling | Au4Cu5(Dppm)2(C6H11S)6/AC | Cs2CO3 | 80 °C, 14 h | Up to 99 | 131 |
| A3 coupling | Au13{Sb(p-tolyl)3}8Cl4 | Piperidine | 50 °C, 12h | 92 | 143 |
| Au25(PET)18 | — | Ar, 60 °C, 24 h | Up to 99 | 144 | |
Au25(PPh3)10(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)5Cl2/TiO2 CPh)5Cl2/TiO2 |
Piperidine | N2, 100 °C, 18 h | 94 | 145 | |
| Au38(SC2H4Ph)24 | Piperidine | N2, 80 °C, 5 h | Up to 100 | 146 | |
| [Au13Cd2(PPh3)6(SC2H4Ph)6(NO3)2]2Cd(NO3)4 | — | r.t. 10 h | Up to 95 | 151 | |
| (Au1Ag16Cu12(SSR)12(PPh3)4) | — | 130 °C, 3 min | 99 | 154 | |
| AHA coupling | Cu6(SC7H4NO)6 | Cs2CO3 | 50 °C, 24 h | 99.6 | 166 |
Since the S–M coupling reaction is originally catalyzed by the Pd(0) complex, the Pd nanocluster is a rational catalyst candidate. Corma and colleagues found that regardless of the Pd source (salt, complex, or nanoparticles), the generation of small nanoclusters, particularly three or four Pd atom nanoclusters, is always a prerequisite for the C–C bond formation of halogenated benzenes (Suzuki, Sonogashira, Heck, and so on).56 Zhu et al. reported a S–M coupling reaction catalyzed by Pd3 nanoclusters in 2017.57 The trinuclear Pd nanocluster with a BF4-counteranion was identified decades ago but the crystal structure has not been reported yet.58 They composed a novel synthesis method to obtain [Pd3Cl(PPh2)2(PPh3)3]+[SbF6]−, and X-ray crystallography (Fig. 6b) was utilized to determine its absolute formula. The S–M cross-coupling reactions between various aryl bromides and aromatic boronic acid were conducted to test the catalytic ability of the Pd3Cl nanocluster. Unlike normal S–M reactions, this experiment required no extra heat, making it even more competitive. The scope of this strategy turned out to be surprisingly wide as substrates with either EWG or EDG gave results in high yields within one hour (Fig. 6a). To deeply understand the mechanism of the Pd3Cl-catalyzed coupling reaction, electrospray ionization-mass spectroscopy (ESI-MS) was performed. By monitoring the reaction process, intermediates have been captured and an intriguing Pd catalysis mechanism has been discovered (Fig. 6c). Instead of taking the oxidative addition step,59 the Pd3Cl nanocluster broke the C–B bond on phenylboronic acid and generated the intermediate Pd3Ar. Aryl bromide attacked the Pd3Ar intermediate to form the final diaryl product along with Pd3Br, which can be treated similar to Pd3Cl to participate in another catalytic cycle.
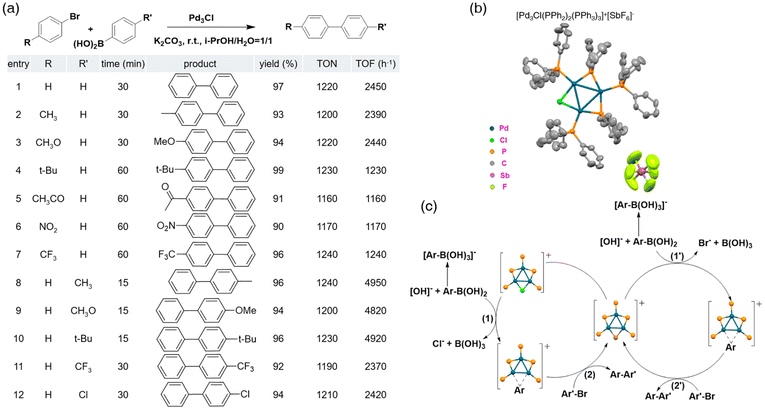 | ||
| Fig. 6 (a) Catalytic activity of [Pd3Cl(PPh2)2(PPh3)3]+ in a Suzuki coupling reaction. (b) Crystal structure of the Pd3Cl catalyst [Pd3Cl(PPh2)2(PPh3)3]+[SbF6]−. (c) Mechanism of the Suzuki coupling reaction catalyzed by the Pd3Cl cluster [Pd3Cl(PPh2)2(PPh3)3]+[SbF6]−. Reprinted with permission from ref. 57. Copyright 2017 American Chemical Society. | ||
The gas-phase nanocluster fabrication technique combined with size-selected soft-landing methods can produce single-sized nanocluster catalysts.60 Nakajima et al. applied the combined methodology to generate and separate Pd nanoclusters using a size-selection chamber and a quadrupole ion deflector. Unprotected Pdn nanoclusters from Pd1 to Pd55 have been fabricated, sifted, and soft-landed on strontium titanate (STO). These STO-supported Pd nanoclusters were added into an S–M coupling reaction system size by size to investigate the size effect61 on Pd nanoclusters’ catalytic ability. A Pd13 nanocluster represented an outstanding catalytic ability toward cross-coupling products while other sizes of Pd nanoclusters either gave lower yields or generated hydrolysis products. From the study, they have approved that the partially positive-charged Pd nanoclusters were the key factors of the S–M cross-coupling reaction and the catalytic activity increased as the size of the Pd nanoclusters increased. After comparing the catalytic ability of the Pd13 nanocluster with the MxPd(13−x) nanoclusters (M = Cu or W), the relationship between the size-specific activity for n = 13 and the electronic structure is still a myth. Therefore, the authors shed light on the specific geometry of Pd13 nanoclusters and noticed that most of the supported Pd13 nanoclusters consisted of a hemispherical oblate structure.62 The geometry of the Pd13 nanocluster supported on STO, however, was observed as a spherical structure. Hence, Nakajima concluded that the excellent performance of the Pd13 nanocluster was due to its site-specific distribution combined with the unique geometric structure.
Some other reports of S–M coupling reactions should be considered to see the big picture of nanocluster-catalyzed C–C coupling reactions between aryl halides and organoboron compounds. In the last decade, composite materials such as Pd/Ni bimetallic nanoclusters,63 PdO nanoclusters supported by WO3 nanosheet,64 α-Fe2O3 nanoclusters supported by graphene oxide,65 and copper-based mixed nanoclusters53,66 showed catalytic abilities for various S–M C–C coupling systems. Recently, a novel S–M coupling polymerization reaction to synthesize aromatic polyketones using Pd nanoclusters has been reported.67 Even though none of them have a precise crystal structure or an accurate chemical formula, they extended the use of nanoclusters in catalytic C–C coupling reactions.
Corma's theory was introduced previously,56 and they believed that this was also applied to the Heck reaction system. The Heck reactions between vinyl bromide and butyl acrylate with varieties of Pd catalysts were performed to prove their hypothesis. The reaction condition was heating in N-methylpyrrolidone (NMP) with an inorganic base, which was a common condition for the Heck reaction.72 Since the premade Pd NP (NP: nanoparticle) required a similar induction period with the Pd salts, they realized that the Pd NPs were not the active species for the Heck reaction. Water was a key ingredient in this recipe since it promoted the formation of Pd nanoclusters. ESI-MS and MALDI-TOF analyses were conducted, and it was found that the reaction started only when Pd nanoclusters with a mass below 500 Da were generated.Although they didn’t identify the exact catalyst for the reaction, they have proved that the active species in the Heck reaction were small Pd nanoclusters. Later, Zhang et al. reported the Heck reactions of iodobenzene and styrene catalyzed by various PdCo alloy nanoclusters in 2019.73 By immobilizing PdCor-PVP nanoclusters on layered double hydroxide (LDH), a group of PdCo alloy nanocluster composites, x-PdCor/MgAl-LDH, were synthesized (x: Pd loading; r: Co/Pd molar ratio). Among these various sizes of bimetallic nanoclusters, 0.81-PdCo0.10/MgAl-LDH (2.6 ± 0.6 nm), 0.86-PdCo0.28/MgAl-LDH (2.3 ± 0.7 nm), and 0.79-PdCo0.54/MgAl-LDH (3.2 ± 0.9 nm) showed significant catalytic activity for the Heck reaction compared to regular catalysts. According to their results, the smallest competitor 0.86-PdCo0.28 possessed the highest catalytic ability due to the maximum electron density of the Pd0 center and the strongest synergistic effect between the nanoclusters and the supports. Furthermore, the material exhibited great substrate tolerance and recyclability, yet the precise chemical formula has not been identified.
Recently, Gao et al. have reported that the mesoporous silica nanoparticle (MSN)-supported [Pd3(PPh3)3(PPh2)2]Cl (Pd3Cl/MSN) catalyzed the Heck reaction between iodobenzene and styrene.74 They have followed Zhu's synthesis method57 mentioned in the S–M coupling reaction section. Pd3Cl was mounted on MSNs by physically mixing and annealing at 100 °C in a vacuum oven before further treatment. Transmission electron microscopy (TEM) and energy-dispersive X-ray spectroscopy (EDX) were performed to confirm the homogenous dispersion of Pd3Cl on MSNs. During the experiment, the supported nanoclusters showed comparable catalytic activity since they gave the same results as PdCl2 and their performances were better than the commonly used heterogeneous catalyst Pd/C. It was still intact after three cycles of catalysis. Different substituted iodobenzenes and non-aromatic olefins were used to expand the scope of this protocol. Of note was that only the iodized benzenes gave excellent results, while brominated and chlorinated benzenes were not active in this system because they are not good leaving group in the Heck reaction.
The theoretical simulation was performed long before experimental research to pave the road for this catalytic system.82,83 A Au nanorod,84 as one of the Au nanoparticles with well-defined surfaces and morphologies, was selected to mimic the performance of Au nanoclusters in catalytic reactions.85 Based on Li's group's DFT studies, plausible cross-coupling pathways on the Au(100) and Au(111) facets were proposed.86 It was not until 2013 that the first example of the atomically precise nanocluster-catalyzed Sonogashira cross-coupling reaction was published, which revealed the magic power of thiolate-protected Au25(SR)18.27 Jin's group demonstrated that Au25(SR)18 nanoclusters are the “jack of all trades” since they can be used to catalyzed majorities of C–C coupling reactions.9 The protecting thiolate-ligand mediated in the Sonogashira cross-coupling reaction system was PET and the immobilizer was still an oxide (Ce2O, Ti2O, MgO, or SiO2). The optimized reaction condition was harsher than other coupling reactions (40 hours of reaction time at 160 °C under basic conditions). First, the support oxides were screened adequately and Au25/CeO2 gave the best result (Fig. 7a). The recyclability of the composites was examined, and the catalytic performance decreased from 88.1% to 64.5% after 5 cycles, showing that the Au25 nanoclusters cannot endure the harsh reaction conditions and gradually aggregated to larger particles.
 | ||
| Fig. 7 (a) Catalytic performance of Au25(SR)18 supported on various oxides as catalysts for the Sonogashira cross-coupling reaction of p-iodoanisole and phenylacetylene. (b) Proposed mechanism for the Sonogashira-type cross-coupling reaction catalyzed by [Au25] nanoclusters. For simplicity, [Au25] represents Au25(SCH2CH2Ph)18. Reprinted with permission from ref. 27. Copyright 2013 Elsevier Inc. | ||
As mentioned in the Ullmann coupling section, the Au25PET18 nanocluster consists of an Au13 kernel surrounded by six “-SR-Au-SR-Au-SR-” staple-like motifs and two facets where three external gold atoms are exposed to the ambience for reactant access. DFT calculations recognized the irreplaceable role of the Au387 open facet. Aryl iodide (PhI) and phenylacetylene (PA) prefer to adsorb on Au3 with a total adsorption energy of −0.90 eV. The ideal situation designed by the simulation was: on the Au3 open facet, (1) PhI adsorbs on one gold atom; (2) PA adsorbs on the adjacent gold atom; and (3) both the triple bond of PA and the halogen group of PhI point toward the third gold atom. The mechanism of this recipe was proposed accordingly (Fig. 7b). After the adsorption, the Ph–I bond was activated to Ph–Au–I, and the C–H bond of PA was activated to Au–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh. Then, reductive elimination happened to couple –Ph and –C
CPh. Then, reductive elimination happened to couple –Ph and –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh to form the final product.
CPh to form the final product.
The hetero metal atom doping effect of the Au25 nanocluster in terms of their catalytic abilities in the Sonogashira cross-coupling reaction has been evaluated by Li's group.44 Silver-, platinum-, and copper-doped Au25 nanoclusters were synthesized and mounted on TiO2. The observation suggested that Cu and Ag atoms preferred replacing gold atoms at the Au13 core, while Pt can only stay at the center of the cluster. These materials were treated with p-iodoanisole, phenylacetylene, and K2CO3, and stirred under N2 at 160 °C for 40 h. The Ullmann homocoupling product was the major byproduct in this system. Their catalytic abilities were sorted regarding the generation of cross-coupling products: AgxAu25−x(SR)18 ≈ Au25(SR)18 > Pt1Au24(SR)18 > CuxAu25−x(SR)18. Even though the doped Pt was located in the very center of the whole structure, it decreased the catalytic ability of Au25. Copper, as a good catalyst for the Ullmann homocoupling, directed the reaction the other way. In conclusion, foreign dopants turned out to drastically influence the catalytic ability of the Au25 nanoclusters, which was consistent with previous research studies.88,89
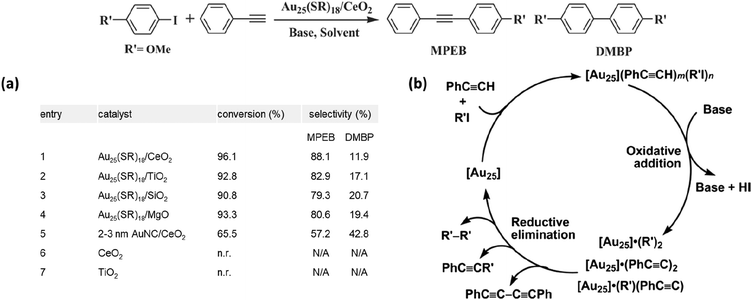
Wu et al. have recently reported another atomically precise Au-based bimetallic nanocluster-catalyzed Sonogashira reaction.90 They introduced a novel approach to prepare a copper-doped Au13 nanocluster through rapid synthesis. A PPh3-Cu complex, (Cu(PPh3)2NO3), was applied to decrease the reaction time, so that the decomposition of the Au13 intermediate can be neglected. The ESI-MS spectrum and single-crystal X-ray crystallography (SCXC) confirmed the composition to be [Au13Cu2(PPh3)6(SC2H4Ph)6]+[NO3]−, providing the atomically precise structure of Au13Cu2 (Fig. 8b). Unlike copper-doped Au25 nanoclusters, the copper atoms in Au13Cu2 did not touch the Au13 icosahedral framework but capped two triangular Au3 surfaces. They also noticed that the bimetallic nanocluster is much more stable than Au13, which led to better catalytic performance under harsh reaction conditions. In the Sonogashira reaction between p-bromoacetophenone and phenylacetylene, various catalysts were added respectively to highlight the catalytic ability of Au13Cu2 nanoclusters. Interestingly, the Sonogashira reaction catalyzed by Au13Cu2 seemed no longer sensitive to air even with a super low catalyst loading (Fig. 8a), showing that the bimetallic nanocluster catalyst was much more active and selective in the Sonogashira coupling reaction. To emphasize the excellent catalytic ability of the nanocluster, various substituted alkynes and aryl halides were tested and compared with regular Sonogashira catalysts. The new catalyst gave a decent yield in almost all cases even when regular catalysts showed bad results. A mechanistic study was proceeded by DFT calculations, and four potential active sites were identified. The energy barrier for the oxidative addition of aryl bromide on these potential active sites was 22.8, 35.0, 34.5, and 25.2 kcal mol−1 respectively. Moreover, the distance between Au1 and Cu4 is the closest compared to other gold atoms, which might accelerate the transmetalation step. A hypothesized mechanism was depicted considering the above-mentioned calculations (Fig. 8c). The synergetic effect of Au1 and Cu4 played an important role in catalyzing the cross-coupling reaction. Worthy to note is that the gram-scale synthesis of internal alkynes was successfully performed with a great yield and Au13Cu2 was still intact afterward.
 | ||
| Fig. 8 (a) Catalytic performance of Au13Cu2 and Pd(PPh3)2Cl2 + CuI in the Sonogashira reaction. (b) X-ray crystal structure of Au13Cu2 (purple: Au; blue: Cu; yellow: S; red: P). All H and C atoms are omitted for clarity. (c) Proposed mechanism of Sonogashira reaction catalyzed by Au13Cu2. Reprinted with permission from ref. 90. Copyright 2021 Elsevier Inc. | ||
Scientists recognized the significance of defects in nanotechnology91,92 and shed light on the surface-defect chemistry in nanoclusters with atomic precision. Very few studies of vacancy defects in well-defined nanoclusters were reported due to the challenges in synthesizing, isolating, and characterizing such defective metal nanoclusters.32 Zhu's group has successfully synthesized the defective atomically precise copper nanoclusters characterized as [Cu28(SC6H11)18(PPh2Py)3H8]2+ (denoted as Cu28-PPh2Py).33 Non-defective nanoclusters [Cu29(SAdm)15Cl3(P(Ph-Cl)3)4H10](PF6) and [Cu29(SC6H11)18(P(Ph-pMe)3)4H10](BF4) were synthesized subsequently to examine the surface-defect effect. The research confirmed that the surface defects change the physicochemical properties of the nanoclusters. However, nanoclusters with defectives have not been investigated as model catalysts. A light-induced Sonogashira cross-coupling reaction catalyzed by an atomically precise Copper nanocluster was reported this year by Bakr and Rueping's group.93 Another instance of a defective atomically precise copper nanocluster was synthesized and identified as [Cu28H10(C7H7S)18(TPP)3] (TPP: triphenylphosphine; C7H7S: o-thiocresol) (denoted as Cu28TPP). The overall structure of Cu28TTP is a tetrahedral missing in one of the vertices due to the defective copper atom and ligands (Fig. 9b). DFT calculations indicated that the surface vacancy in Cu28TTP can act as a catalytic active site and the Sonogashira cross-coupling reaction proceeded to confirm the simulation. Various catalysts were screened to prominent the catalytic ability of Cu28TPP in the aryl iodide and phenylacetylene. Cu28 gave the best yield and inhibited the formation of the byproduct. The scope and limit of the new methodology were investigated with substituted phenyl iodide and bromide (Fig. 9a). The substrate tolerance of this catalytic system was super broad with both EWG and EDG on aromatic rings. A mechanistic study was conducted and the light induction in the strategy indicated the involvement of the Cu-photoredox chemistry.94 Unlike the regular Sonogashira organometallic catalytic cycle, this reaction started with the single-electron-transfer (SET) step (Fig. 9c). Cu28TTP was excited by the blue light and an electron was then transferred to aryl iodide generating a phenyl radical. The triple bond of terminal alkynes could be activated by Cu28TTP via a Cu nanocluster-π-alkynyl complex,91 which was identified by the Zhu and Sheng's group.95 The phenyl radical attacked the activated carbon triple bond to form the vinylic radical intermediate following another SET process to bring Cu28TTP to the ground state. The final product was then generated under basic conditions. The above-mentioned radicals were captured and identified by GC-MS to confirm the radical mechanism.
 | ||
| Fig. 9 (a) Substrate scope of Cu28-catalyzed Sonogashira reactions. (b) Structure of Cu28. All carbon atoms of the surface ligands are omitted for clarity. (c) Proposed reaction mechanism of Cu28-catalyzed Sonogashira reactions. Reprinted with permission from ref. 93. Copyright 2023 Wiley-VCH GmbH. | ||
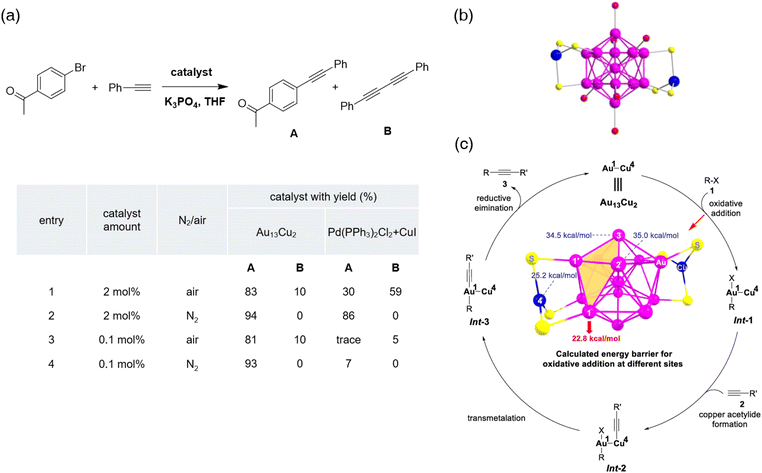
Therefore, copper nanoclusters with precision numbers are the most rational candidates for oxidative C(sp)–C(sp) coupling reactions. Li et al. approved the hypothesis by preparing a new tetranuclear Cu nanocluster protected by bidentate alkyne ligands.101 The synthesis route and characterization were provided in detail and the Cu nanocluster was identified as Cu4(PPh3)4(bis(prop-2-ynyloxy) biphenyl)2. According to the X-ray diffraction (XRD) analysis, the crystal consists of a new alkynyl-Cu motif and a butterfly-like framework (Fig. 10a). Common oxides were used to immobilize the copper nanoclusters before the composites were examined for the oxidative C(sp)–C(sp) coupling reaction. Cu4 supported by basic oxide NiO showed the best catalytic ability and recyclability. It is worth noting that if the Cu4 nanocluster were annealed at 300 °C for 2 h in air, the Cu(I) clusters would convert into the Cu(II) species ending up with zero catalytic ability toward the coupling reaction. Subsequently, several aliphatic alkynes and aromatic alkynes were applied to test this study's scope (Fig. 10b). The electron effect was observed during the experiment where EWG induced the coupling, while EDG decreased the conversion rate.
 | ||
| Fig. 10 (a) Structure of Cu4 and synthetic protocol of Cu4(PPh3)4L2 clusters from the CuI2L and triphenylphosphine ligands in the presence of excess copper powder. Color code: Cu atoms, turquoise; P atoms, pink; O atoms, yellow; C atoms, grey. All the H atoms are omitted for clarity. (b) Substrate scope of homocoupling of terminal alkynes over Cu4/NiO catalysts. Reprinted with permission from ref. 101. Copyright 2020 Wiley-VCH GmbH. (c) Structure of the optimized Cu6(GS)2 cluster with selected bond lengths (Å). (d) Substrate scopes for C(sp2)–N(sp3) bond formation reactions catalyzed by Cu6 nanoclusters. Reprinted with permission from ref. 123. Copyright 2022 American Chemical Society. | ||
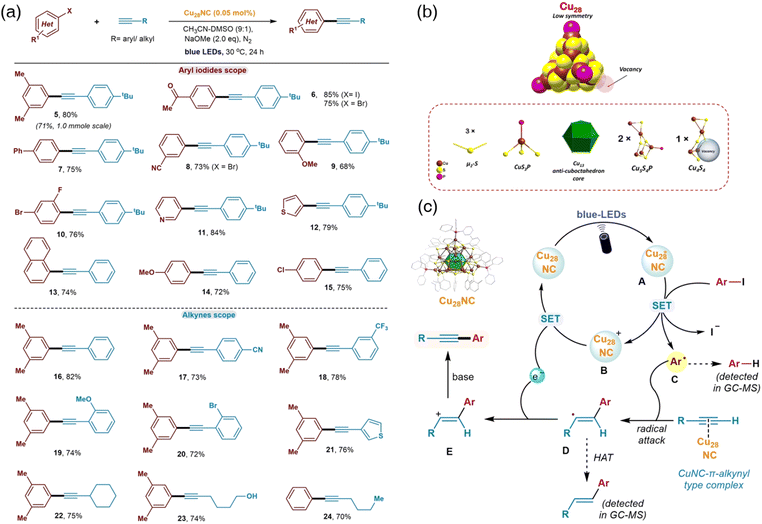
Very recently, Zhu and Sheng's group prepared copper-based bimetallic nanoclusters extending the application of atomically precise nanoclusters in making symmetric and asymmetric 1,3-diynes102,103 The synthetic route of AuxCu25−x nanoclusters with 12 (p-FPh)3P protecting ligands was reported by Zhu's group.104 One of the objectives of this study was to discover the influence on catalytic performance by the number of ligands on the surface of nanoclusters.105 Thus, Au1Cu24H22((p-FPh)3P)12 was synthesized, supported by activated charcoal (AC), and heated at different temperatures to make a group of catalysts Au1Cu24/AC-X (X is the heated temperature = 0, 200, 300, 500, and 800) (Fig. 11b). Based on XRD and thermogravimetric analysis (TGA), Au1Cu24 shed about two ligands at 200 °C, while all of the ligands collapsed after 350 °C. It turned out that the partially exposed nanoclusters, Au1Cu24/AC-200, showed the best catalytic efficiency of the homocoupling of phenylacetylene. Cu25 and Au25 nanoclusters were prepared accordingly to highlight the synergetic effect of copper and gold in this system. Compared to single-metal nanoclusters, the alloy nanoclusters represented the best catalytic ability and recyclability. Subsequently, various terminal alkynes were treated with optimized reaction conditions to test the adaptive capacity of this strategy (Fig. 11a, 1–6). The oxidative C(sp)–C(sp) cross-coupling reactions were applied to the experiment by overdosing one of the terminal alkynes. The data indicated that Au1Cu24/AC-200 gave decent yields of cross-coupling products while tolerating halogen groups (Fig. 11a, 7–16). The mechanism of this experiment was referred to previously reported mechanisms.106 It demonstrated that the unprotected part of the nanocluster core was the active site and oxygen was an indispensable component of this reaction (Fig. 11c).
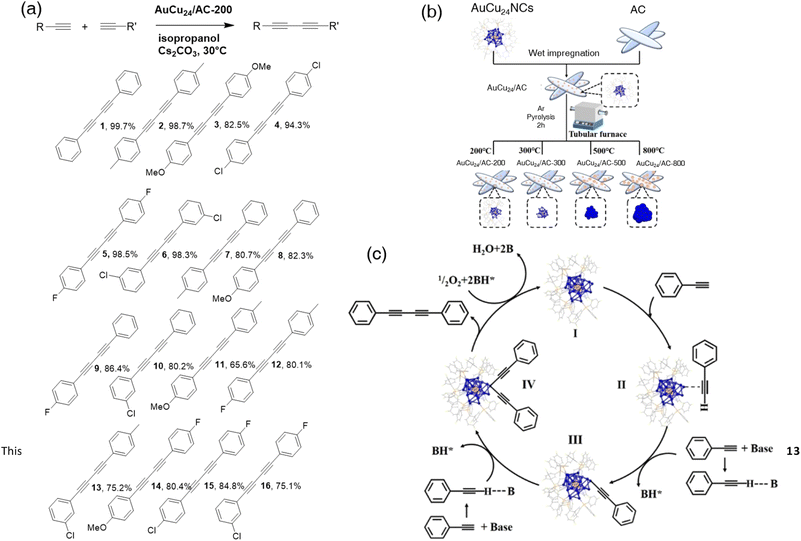 | ||
| Fig. 11 (a) Substrate scope of homocoupling and heterocoupling of terminal alkynes catalyzed by Au1Cu24/AC-200. (b) Schematic diagram of AuCu24/AC-200 and comparison of catalyst synthesis. (c) Proposed mechanism of the Au1Cu24/AC-200-catalyzed phenylacetylene oxidative homocoupling reaction. Reprinted with permission from ref. 103. Copyright 2023 Wiley-VCH GmbH. | ||
Copper-based nanoclusters showed their privilege in catalyzing the oxidative C(sp)–C(sp) coupling reaction. Furthermore, Mandal et al. have proved that a single copper dopant in nanoclusters could also do the trick by presenting another example of the foreign atom doping effect of atomically precise phosphine-protected Au nanoclusters.107 Phosphine-protected Au nanoclusters (AuPR3) are one of the earliest studied nanoclusters108,109 due to their straightforward Au–P coordination pattern. The applications of AuPR3,110,111 however, are limited because the weak Au–P bond lacks stability.112 Doping hetero metal atoms improves the stability and physiochemical properties of Au nanoclusters, which is well demonstrated previously.113 Hence, they managed to design and synthesize a novel single copper-doped bimetallic AuPR3 nanocluster (Cu1Au11(PPh3)7Cl2). The synthesis strategy of the nanoclusters was developed by Zhu.114 After characterization, the crystal structure of Cu1Au11(PPh3)7Cl2 was depicted as an undeca-gold Au11 core aside by a copper atom (Fig. 12a). Originally, the Sonogashira cross-coupling reaction of 4-methyl iodobenzene and phenylacetylene was used to test the catalytic properties of the newly generated nanoclusters supported by ceria. The results turned out to be a competition between cross-coupling products and homo-coupling of phenylacetylene (PA). For most of the cases, the homo-coupled product diphenylbuta-1,3-diyne was the predominant product, especially when phenylacetylene was substituted by EWG. Control experiments were conducted by synthesizing the parent nanocluster Au11(PPh3)7Cl3 and other related nanoclusters, and none of them showed good catalytic ability toward oxidative C(sp)–C(sp) coupling. Of note, the parent nanocluster Au11(PPh3)7Cl3 had no catalytic activity toward either homo-coupled products or hetero-coupled products whatsoever. DFT calculations were performed, and phenylacetylene adsorption on the nanocluster occurred at the unsaturated Cu atom. This explained why the parent Au11 nanocluster had no catalytic ability since all metal sites were ligated. The computational study also indicated a strong PA–PA interaction, which suggested that the homo-coupled product was the major product (Fig. 12b). This study once again proved that a tiny change in the nanocluster's metal core would lead to a drastic change in catalytic performance.
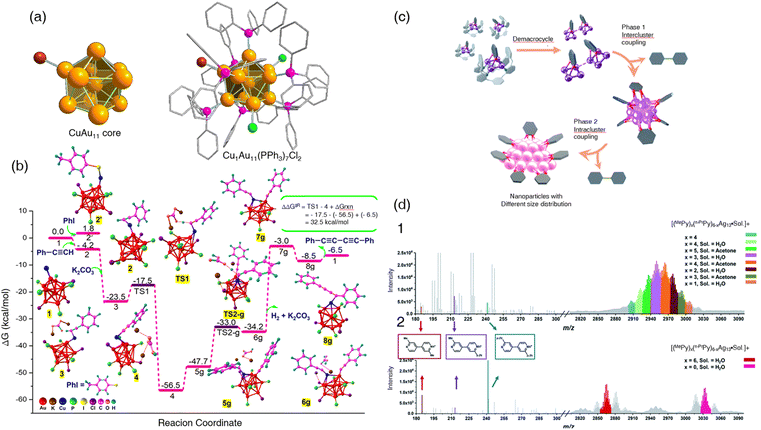 | ||
| Fig. 12 (a) Complete structure of Cu1Au11(PPh3)7Cl2 with halides and triphenyl phosphines attached to 10 outer gold atoms. Color code: Cu atoms, red; P atoms, pink; Au atoms, yellow. All hydrogen atoms are omitted from the phenyl rings for clarity. (b) Reaction free energy profile diagram for the Glaser coupling reaction. ΔΔG‡R and ΔGrxn denote the total reaction free energy barrier and total reaction free energy of the catalytic cycle, respectively. The methyl groups in ligands are omitted for the convenience of visualization. Here, g defines the Glaser coupling. Reprinted with permission from ref. 107. Copyright 2023 American Chemical Society. (c) Structural evolution of organosilver species from organic diide–Ag4 clusters through nanoclusters to nanoparticles along with the occurrence of coupling reactions at different stages. (d) ESI mass spectra of the acidifying solution samples containing equivalent (1) MePyAg5 and n-PrPyAg5 in acetone, and (2) MePyAg13 and n-PrPyAg13 in acetone. Reprinted with permission from ref. 115. Copyright 2023 Royal Society of Chemistry. | ||
To the best of our knowledge, oxidative coupling reactions other than the oxidative C(sp)–C(sp) coupling are hardly reported over atomically precise nanocluster systems. Zhao et al. reported an intriguing methodology for making Csp2–Csp2 oxidative coupling products while synthesizing atomically precise nanocluster systems.115 Instead of using atomically precise nanoclusters as catalysts, the oxidative coupling reaction occurred during the generation of nanoclusters. First, substituted pyridyl dicarbanion-bonded Ag4 clusters were synthesized from simple propargylamine and ketones with a special macrocycle ligand, octamethylazacalix[8]pyridine (Py[8]).116 By adding different ketones (acetone, 2-pentanone, and acetophenone respectively), three atomically precise Ag4 nanoclusters were synthesized: [Ag5(C6NH5)(Py[8])](CF3SO3)3 (denoted as MePyAg5), [Ag5(C8NH9)(Py[8])](CF3SO3)3 (denoted as n-PrPyAg5), and [Ag5(C11NH7)(Py[8])](CF3SO3)3 (denoted as PhPyAg5). Three corresponding Ag13 nanoclusters were produced using the Ag4 nanoclusters and denoted as MePyAg13, n-PrPyAg13, and PhPyAg13 respectively. According to the in situ ESI-MS monitoring, the cross-coupling product 2-methyl-2′-propyl-4,4′-bipyridine and the homo-coupling products 2,2′-dimethyl-4,4′-bipyridine and 2,2′dipropyl-4,4′-bipyridine were observed (Fig. 12d). The oxidative coupling of organic species during the in situ generation of metal subnano- or nano-clusters was proposed before, with uncertain mechanisms and unclear intermediates.117,118 In this experiment, the removal of the macrocycle Py[8] ligands by protonation led to Ag4 nanocluster combination. The Ag4 combination underwent a two-electron oxidative coupling to release the Csp2–Csp2 oxidative coupling products while generating low-valence silver atoms. It is worth noting that Ag13 can further aggregate to larger nanoparticles while generating homo-coupling bipyridine products via intra-cluster oxidative coupling (Fig. 12c). It is not a traditional atomically precise nanocluster-catalyzed oxidative coupling reaction, but this study has raised a new idea of generating complex chemicals during the synthesis of nanoclusters.
3.2 Carbon–heteroatom coupling
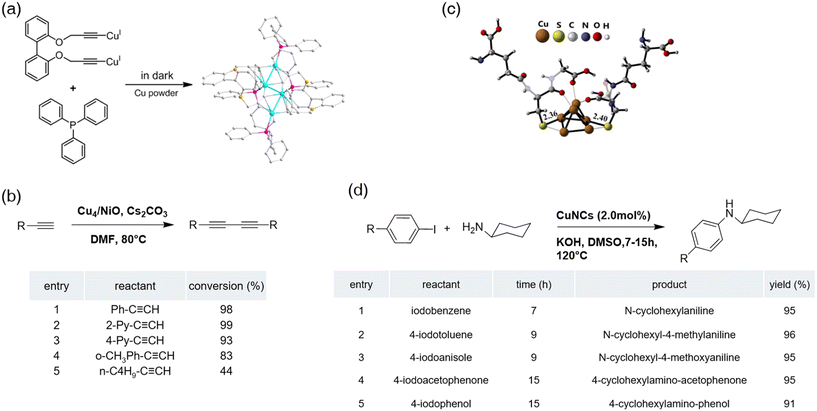
Compounds containing carbon–heteroatom bonds have extensive applications in agriculture, pharmaceuticals, manufacturing, and material sciences.119 The construction of carbon–heteroatom bonds has been an essential research field in chemistry, and transition metal-catalyzed cross-coupling reactions are the most common methods developed for decades.120 However, carbon–heteroatom cross-coupling catalyzed by nanoclusters is uncommon probably because the total development time of nanocluster catalysis is relatively short, let alone this type of reaction usually requires harsher reaction conditions leading to the degradation of nanoclusters.Before applying atomically precise nanoclusters, metal nanoclusters with uncertain structures have been proven to be active catalysts for carbon–heteroatom cross-coupling reactions. Even though the unclear formula of the nanoclusters restricts the innovation of these novel strategies, they draw scientists’ attention toward the application of nanoclusters in carbon–heteroatom cross-coupling systems. Shimizu et al. noticed that supported Pt nanoclusters showed excellent catalytic properties toward selective cross-coupling of amines compared to the regular strategy.28 They prepared a series of oxide-supported nanoclusters Mx/Oxides-D, where x is the content of (weight%) metal (M) and D is the particle size (nm), and treated them with morpholine and benzylamine.121 The results indicated that Pd nanoclusters with an average particle size of 1.8 nm supported by Al2O3 selectively produce cross-coupling products in a decent yield. Pd nanoclusters with larger average particle sizes would also catalyze this reaction with bad selectivity, while other metals such as Au, Ag, Pt, Rh, and Ru seldom catalyzed this reaction. The scope of the first example of reusable Pd nanocluster catalysts for cross-coupling of amines was expanded. Later, the same group found that alumina-supported Pt nanoclusters with an average particle size of 0.8 nm (Pt/Al2O3-0.8) were also effective catalysts for amine cross-coupling in the system of aniline and amine.28
Corma and Leyva-Pérez et al. reported that copper nanoclusters with Cu atoms less than seven have a tremendous ability to activate and catalyze C–N, C–O, C–S, and C–P bond formation.122 They noticed that the cross-coupling reaction between iodobenzene and amide did not occur if the solvent was changed from amide (in this case, NMP) to toluene or dioxane when the copper salt was used as the catalyst. Subsequent experiments confirmed the formation of Cu nanoclusters in an amide solvent and the authors proposed that the amide acted as a reducing agent for the Cu nanoclusters. They first proved that Cu nanoparticles were not the active species. Cu nanoclusters with an average size of 5 atoms were proved to be effective catalysts for carbon–heteroatom coupling reactions. This experiment proves the catalytic potential of nanoclusters, in particular Cu nanoclusters, in carbon–heteroatom coupling systems.
Copper nanoclusters are reported to have large absorption cross sections under visible light and possess long exited-state lifetimes.124,125 Rueping's group realized that these properties make Cu nanoclusters as potential modular catalysts for cross-coupling reactions induced by visible light. Their instincts were proved to be right by the research on the Sonogashira cross-coupling reaction catalyzed by atomically precise copper nanocluster mentioned previously.93 The same group also reported another example of using an atomically precise copper nanocluster to activate aryl chlorides to enable Ullmann C–N cross-coupling reactions.126 [Cu61(StBu)26S6Cl6H14]+ (denoted as Cu61, –StBu: tert-butyl thiolate) was synthesized127 and characterized (Fig. 13b). The nanocluster consists of a triangular gyrobicupola Cu19 core and a novel “18-crown-6” metal sulfide-like belt as the shell. The model reaction was designed as follows: amination of carbazole with p-bromobenzonitrile in acetonitrile protected by nitrogen gas at ambient temperature under basic conditions. After optimization, their best yield was 88%, which was much better than the copper salt-catalyzed reactions. The authors spent much time examining the scope of this novel protocol, demonstrating that the Cu61 nanocluster-catalyzed system showed great functional group tolerance (Fig. 13a). Of note, this methodology can be applied to aryl chlorides, which are much harder to activate than aryl bromides. A possible mechanism was proposed based on literature reports of copper-related photoredox chemistry94 and their confirmatory experiments (Fig. 13c). The amine adsorption on copper nanoclusters was the first step followed by blue-light irradiation to produce the photoexcited state. A SET process happened to donate an electron to aryl halide, generating an aryl radical species and bringing copper nanoclusters to the ground state spontaneously. The desired C–N coupled product was synthesized by radical–radical bounding, bringing the copper nanocluster to its original form. Copper nanoclusters exhibit a unique role in photocatalysts for coupling reactions. Thus, there is no doubt that more examples of copper nanocluster-related photocatalyst coupling reactions spring out sooner than later.
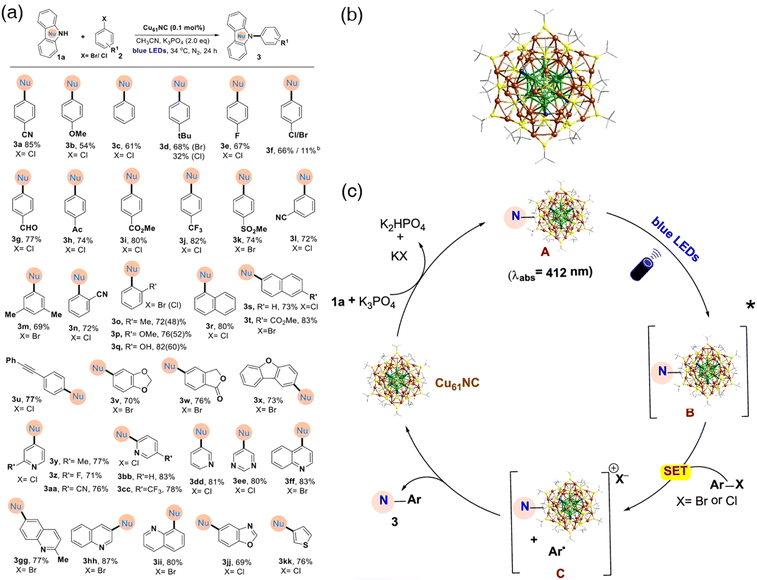 | ||
| Fig. 13 (a) Substrate scope of aryl halides catalyzed by Cu61. (b) Well-defined crystal structure of the Cu61 nanocluster. Color legend: green and brown, copper atoms of the core and the shell, respectively; yellow, sulfur; dark blue, chloride; and gray, carbon (c) proposed reaction mechanism of the C–N cross-coupling catalyzed by Cu61. Reprinted with permission from ref. 126. Copyright 2022 American Chemical Society. | ||
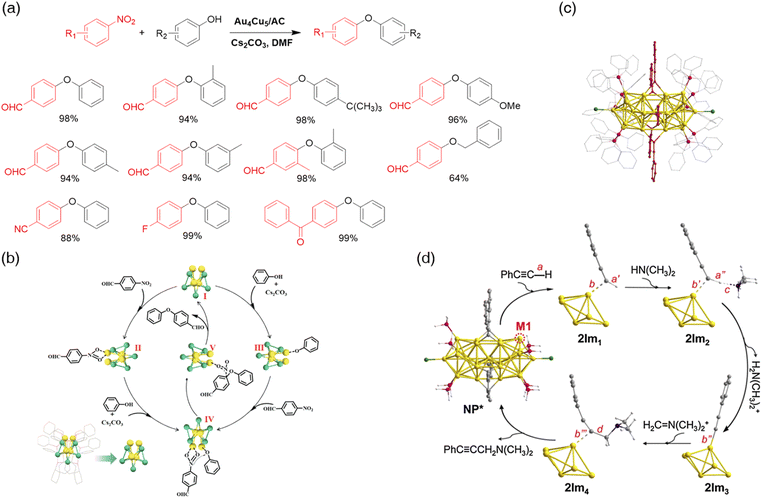 | ||
| Fig. 14 (a) Scope of the Au4Cu5/AC-catalyzed Ullmann C–O coupling. (b) Mechanism of the Ullmann C–O coupling reaction over the Au4Cu5 nanocluster. Reprinted with permission from ref. 131. Copyright 2023 Tsinghua University Press. (c) Structure of Au25(PPh3)10(PA)5X2. Color codes: Au, yellow; P, pink; C, gray; X (Cl/Br), green; phenylacetylide, red. (d) Mechanism of the catalyzed A3-coupling reaction over the Au25(PH3)10(PA)5Cl2 cluster. Color code: Au, yellow; P, purple; X, green; N, blue; C, gray; H, white. Only partial gold atoms are shown in 2Im1, 2Im2, 2Im3, and 2Im4, and other atoms are omitted for clarity. Reprinted with permission from ref. 145. Copyright 2021 Elsevier Inc. | ||
3.3 Multicomponent coupling reactions
The construction of complex molecules from simple and readily available substrates is a major challenge in organic chemistry.134 Multicomponent coupling reactions are effective methodologies for generating complex chemical frameworks from simple substrates in a single step. They provide a chance for the formation of multiple bonds simultaneously. Atomically precise nanoclusters seem to be capable of catalyzing this type of coupling reaction effectively owing to their abundant active sites for multiple bond formation. Moreover, the synergistic effect between hetero-metal atoms opens access to the carbon–heteroatom linkage in multicomponent coupling reaction systems.To our knowledge, almost all reported reactions of atomically precise nanoclusters related to A3 coupling are catalyzed by gold-based nanoclusters.143 The Au25 nanoclusters’ high status in nanocluster catalysis is still invincible. Two research groups reported Au25 nanocluster-catalyzed A3 coupling in 2016 independently.144,145 The protecting ligand used by Obora's group was phenylethanethiol ([Au25(PET)18]), while Li et al. mediated phosphine and phenylacetylide as the ligands (Au25(PPh3)10(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)5X2 (X = Cl/Br)) (Fig. 14c). Unlike Obora's strategy, Li immobilized Au25 nanoclusters on TiO2 but both plans have decent functional group tolerance. It is worth noting that even though both reactions required an external heating source, Li's protocol used water as the solvent, which has zero hazardous concerns compared to toluene. Additionally, Obora noticed that the initial substrate ratio played an important role in the coupling reaction. The access of alkyne increased the yield, while the yield decreased if too much amine was added. Oxygen is an essential additive in Au25(PET)18 systems, while Au25(PPh3)10(C
CPh)5X2 (X = Cl/Br)) (Fig. 14c). Unlike Obora's strategy, Li immobilized Au25 nanoclusters on TiO2 but both plans have decent functional group tolerance. It is worth noting that even though both reactions required an external heating source, Li's protocol used water as the solvent, which has zero hazardous concerns compared to toluene. Additionally, Obora noticed that the initial substrate ratio played an important role in the coupling reaction. The access of alkyne increased the yield, while the yield decreased if too much amine was added. Oxygen is an essential additive in Au25(PET)18 systems, while Au25(PPh3)10(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)5X2 required an aerophobia environment. Li's group shed light on the mechanism of the A3 coupling over gold nanoclusters by DFT calculations (Fig. 14d). They believed that one of the phosphine ligands was removed exposing the metal site for the catalysis. Terminal alkyne was activated by the open metal site making it easier to be deprotonated by a base. The iminium ion adsorbed on the open site was then attacked by –C
CPh)5X2 required an aerophobia environment. Li's group shed light on the mechanism of the A3 coupling over gold nanoclusters by DFT calculations (Fig. 14d). They believed that one of the phosphine ligands was removed exposing the metal site for the catalysis. Terminal alkyne was activated by the open metal site making it easier to be deprotonated by a base. The iminium ion adsorbed on the open site was then attacked by –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh to form the final product.
CPh to form the final product.
Another example of A3 coupling over pure gold nanoclusters was reported in the same year by Jin et al.146 A bigger thermally stable gold nanocluster Au38(SC2H4Ph)24 was synthesized147 and characterized. According to the X-ray crystallography, the nanocluster was composed of a bi-icosahedral Au23 core, six dimeric Au2(SR)3, and three monomeric Au(SR)2 surface motifs. The authors realized that the catalytic performance would decrease drastically if the protecting thiolate ligands were removed by thermal pretreatment.148,149 Moreover, the Au(I)-S-C2H4Ph complex was synthesized to apply to the same reaction while giving a significantly decreased conversion rate. These two comparison experiments demonstrated that the catalytic property of the Au38 nanoclusters was coming from the entire structure since both the synergistic effect of the thiolate-protected gold surface and the electron-rich Au23 kernel played important roles in their catalytic performance. Of note, this strategy can proceed without any solvent, making it an environmentally friendly method. Later, Leong and Li investigated a smaller-size Au nanocluster in A3 coupling and once again underlined the ligand effects on the nature of nanoclusters’ catalytic ability and efficiency.143 They have synthesized an Au13 nanocluster ([Au13{Sb(p-tolyl)3}8Cl4][Cl]) stabilized by stibine, which is not a common ligand for Au nanoclusters.150 The 13 gold atoms formed an icosahedral model with 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 disorder surrounded by eight SbPh3 and four chlorides. According to their comparative study, the Au13 nanoclusters have much better catalytic ability than that of previously mentioned Au25
10 disorder surrounded by eight SbPh3 and four chlorides. According to their comparative study, the Au13 nanoclusters have much better catalytic ability than that of previously mentioned Au25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 144,145 and Au38
144,145 and Au38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 146 nanoclusters protected by phosphines or thiolates. Au13 nanoclusters managed to catalyze the A3 coupling reaction of benzaldehyde, piperidine, and phenylacetylene with (1) low catalyst loadings; (2) short reaction periods; (3) solvent-free nature; (4) protection-gas-free nature; (5) low reaction temperatures; and (6) high conversion rates. However, the authors did not test the scope and limitations of this protocol leaving blank space for future discovery. We stay tuned for the additional application of this highly effective strategy.
146 nanoclusters protected by phosphines or thiolates. Au13 nanoclusters managed to catalyze the A3 coupling reaction of benzaldehyde, piperidine, and phenylacetylene with (1) low catalyst loadings; (2) short reaction periods; (3) solvent-free nature; (4) protection-gas-free nature; (5) low reaction temperatures; and (6) high conversion rates. However, the authors did not test the scope and limitations of this protocol leaving blank space for future discovery. We stay tuned for the additional application of this highly effective strategy.
The first atomically precise bimetallic nanocluster-catalyzed A3 coupling was discovered by Wu et al.151 They prepared [Au13Cd2(PPh3)6(SC2H4Ph)6(NO3)2]2Cd(NO3)4 (Au26Cd5) by peeling and doping the well-studied Au25(SCH2CH2Ph)18 nanocluster. Au25(SCH2CH2Ph)18 can be peeled to Au13(PPh3)8(SCH2CH2Ph)32+via ligand exchange of PPh3.152 Two Au13Cd2 units were formed and linked by Cd(NO3)4 when a cadmium salt gets involved. Even though Au25(SCH2CH2Ph)18 has no catalytic ability toward A3 coupling, Au26Cd5 showed exclusive catalytic performance and functional group tolerance. This methodology represented the importance of tuning the nanoclusters’ compositions and structures in improving their catalytic performance. Li et al. synthesized a coreless Au2Ag2(C10H6NO)4(Ph3P)2 bimetallic nanocluster with a well-elucidated crystal structure.153 The gold and silver atoms are located in a plane to form a rhombus. The two phosphine and two benzonitrile ligands coordinate with the silver atom through σ-bonds and π-bonds respectively, while the gold atom only coordinates with benzonitrile ligands via σ-bonds (Fig. 15a). Interestingly, the homologous Au4(C10H6NO)4(PPh3)2 and Ag4(C10H6NO)4(PPh3)2 clusters cannot be prepared via a similar synthetic route. Hence, the triple bond of benzonitrile binding to Au and Ag through σ- and π-bonds respectively, which is considered a novel alkyne-metal coordination mode, is the key to forming the alloy nanocluster. The nanocluster was then immobilized on TiO2 to catalyze a three-component coupling reaction of benzaldehyde, piperidine, and phenylacetylene. The result indicated that the Au2Ag2/TiO2 did a better job than phosphine-protected Au25 and Au13 nanoclusters owing to the full exposure of metal active sites.
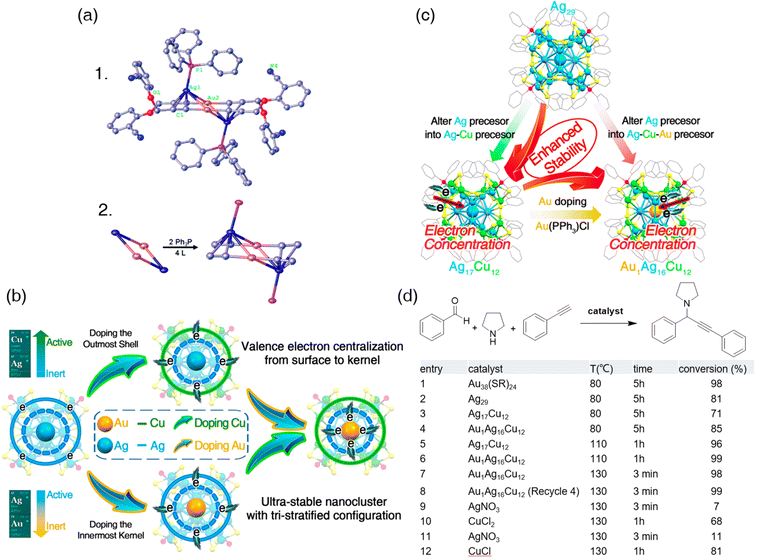 | ||
| Fig. 15 (a) 1. Full structure of Au2Ag2(L)4(Ph3P)2 with 30% probably ellipsoids. 2. Core structure and coordination model in Au2Ag2 clusters. Color code: Au, gold; Ag, dark blue; P, pink; O, red; N, blue; C, grey. H atoms are omitted for clarity. Reprinted with permission from ref. 151. Copyright 2017 Royal Society of Chemistry. (b) Illustration of the composition control (innermost kernel, icosahedral edge, and outmost shell) of core–shell nanoclusters to obtain multi-stratified alloy nanoclusters that display enhanced thermal stability, owing to the concentration of free valence electrons to the interior of nanoclusters. (c) Syntheses and crystal structures of monometallic Ag29, bimetallic Ag17Cu12, and trimetallic Au1Ag16Cu12 nanoclusters. (d) Multicomponent A3 coupling reaction of benzaldehyde, pyrrolidine, and phenylacetylene catalyzed by different nanoclusters. Reprinted with permission from ref. 154. Copyright 2019 American Chemical Society. | ||
The only reaction of A3 coupling over atomically precise nanoclusters that is not catalyzed by a Ag-based nanocluster was reported by Zhu and Kang's groups in 2019.154 The authors’ indication of designing the specific trimetallic nanocluster (Au1Ag16Cu12(SSR)12(PPh3)4) was to investigate whether rearrangements of free valence electrons could stabilize nanoclusters. Substituting the parent metal with hetero-metal atoms would change the electron distribution probably according to their electronegativity. In other words, doping higher electronegativity metal atoms into the kernel and exchanging the shell with lower electronegativity metal atoms could endow the core–shell nanocluster with a higher partial positive charge (δ+) on the surface and partial negative charge (δ−) on the kernel (i.e., metallic state M0). The highly positive-charged surface would make the nanocluster hard to be further oxidized, leading to better thermostability.154 The Ag nanoclusters are considered unstable, and doping inert metals into the kernel of Ag nanoclusters could secure thermal stability due to the electron distribution rearrangement.155,156 Therefore, the design thinking of the groups was manipulating nanoclusters’ stability through valence electron centralization by putting copper (electronegativity is 1.9) into the outmost shell of the core–shell Ag nanocluster (Ag's electronegativity is 1.93) and doping Ag (electronegativity is 2.54) in the innermost core of the Ag nanocluster (Fig. 15b). The bimetallic Ag17Cu12 and trimetallic Au1Ag16Cu12 nanoclusters were synthesized and determined respectively (Fig. 15c). Thermal stability tests were performed sequentially, showing that free valence electron centralization is a promising means to stabilize nanoclusters. The homologous Ag29 nanocluster lost its original form when heated at 130 °C under vacuum, while Ag17Cu12 remained intact at 150 °C under vacuum. Surprisingly, Au1Ag16Cu12 retained their original formation even at 175 °C under air. Hence, Au1Ag16Cu12 can be applied to catalytic reactions with high temperature (175 °C) without protection gas. Thus, a high-temperature three-component coupling reaction of benzaldehyde, pyrrolidine, and phenylacetylene was conducted to demonstrate the extraordinary catalytic nature of Au1Ag16Cu12 (Fig. 15d). The A3 coupling reaction catalyzed by Au1Ag16Cu12 can proceed at 130 °C, giving a 99% conversion rate within three minutes. Moreover, the catalytic ability of Au1Ag16Cu12 remained the same even after five cycles of high-temperature treatment. The intriguing methodology of thermally stabilizing nanoclusters and the stunning catalytic performance of the stabilized nanoclusters provide new insights into the composition-property collaborations that will keep catching scientists’ eyes in materials science.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 133 were synthesized, characterized (Fig. 16a) and supported by AC respectively. Three nanoclusters represented the intrinsic catalytic ability over AHA coupling of phenylacetylene, piperidine, and dichloromethane with a large TON at 50 °C (313.2, 345.8, and 353.5 respectively), while previously reported catalysts gave a super low TON (Fig. 16b). Cu6/AC was selected as the model catalyst for scope and limitation examination. It turned out that this composite bested almost all non-noble metal catalysts mentioned before in the total conversion rate, functional group tolerance, and TON. During the mechanistic study, GC MS detected methyleneammonium, which was probably generated by the amine and dichloromethane under basic conditions.169 Hence, the mechanism they proposed accordingly was depicted (Fig. 16c). The copper nanocluster activated the terminal alkyne, while methyleneammonium formed independently. The methyleneammonium was then adsorbed by the nanocluster, providing the site for interaction between methyleneammonium and activated alkyne. The final product was released after reductive elimination.
133 were synthesized, characterized (Fig. 16a) and supported by AC respectively. Three nanoclusters represented the intrinsic catalytic ability over AHA coupling of phenylacetylene, piperidine, and dichloromethane with a large TON at 50 °C (313.2, 345.8, and 353.5 respectively), while previously reported catalysts gave a super low TON (Fig. 16b). Cu6/AC was selected as the model catalyst for scope and limitation examination. It turned out that this composite bested almost all non-noble metal catalysts mentioned before in the total conversion rate, functional group tolerance, and TON. During the mechanistic study, GC MS detected methyleneammonium, which was probably generated by the amine and dichloromethane under basic conditions.169 Hence, the mechanism they proposed accordingly was depicted (Fig. 16c). The copper nanocluster activated the terminal alkyne, while methyleneammonium formed independently. The methyleneammonium was then adsorbed by the nanocluster, providing the site for interaction between methyleneammonium and activated alkyne. The final product was released after reductive elimination.
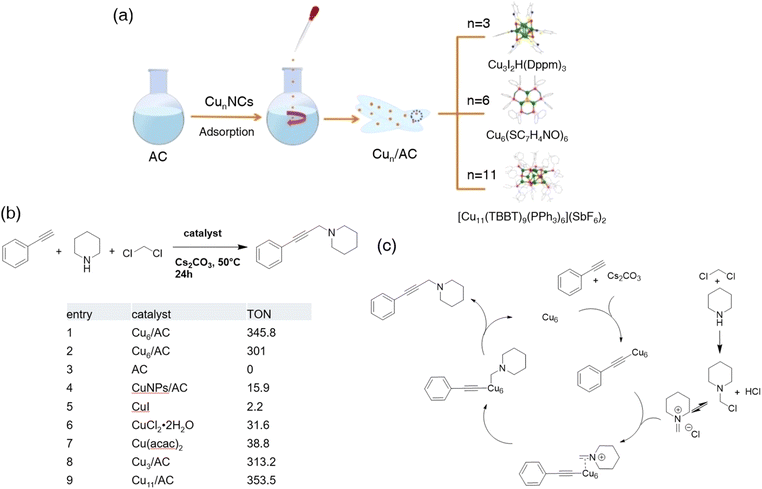 | ||
| Fig. 16 (a) Schematic illustration of the formation of Cun (n = 3, 6, and 11)/AC. (b) TON value of different catalysts for the AHA coupling reaction. Reaction conditions: 6.6 × 104 mmol Cu catalyst or 3.8 × 103 mmol copper metal species, phenylacetylene (0.25 mmol), Cs2CO3 (0.25 mmol), piperidine (0.56 mmol), dichloromethane (1.0 mL), 50 °C, reacting for 24 h under argon. (c) A plausible mechanism for the synthesis of propargylamines via the coupling of phenylacetylene, piperidine, and dichloromethane using Cu6/AC. Reprinted with permission from ref. 166. Copyright 2022 Wiley-VCH GmbH. | ||
4. Conclusion and perspectives
Atomically precise nanoclusters have demonstrated their essential position in nanomaterials by representing tremendous applications in thermal-, electrical-, and photo-catalysis reactions.6 These catalytic reactions over nanoclusters cover most of the research and application fields of advanced materials, which means atomically precise nanoclusters are all-rounders of academia, industry, agriculture, and pharmacy. Particularly, in the past two decades, the development of confined nanocluster catalysts in coupling reactions has delivered effective, low-cost, and environmentally friendly methodologies for synthesizing complex chemical species from simple molecules. To date, coupling reactions connecting Csp2–Csp2, Csp–Csp2, Csp–Csp, C–N, C–O, and C–N–C bonds can be mediated by atomically precise nanoclusters (Table 1). Au nanoclusters and Au-based alloy nanoclusters are the most prominent nanocluster catalysts as generalists of almost all coupling reactions mentioned in this review. The higher catalytic activity comes from the distinct electronic properties of Au8,154 and the superior synergism related to hetero-metal atoms, protecting ligands and supports. The Au–S metallic bond stabilizes the nanoclusters while leaving more room for catalytic active sites. This makes thiolate-protected Au nanoclusters more useful in coupling reactions. The doping effect of hetero-metal atoms makes Au-based alloy nanoclusters suitable for multiple coupling bond formation systems. Au–Cu alloy nanoclusters seem to be rising stars in this field because doping copper in Au nanoclusters decreases the total cost while showing even better catalytic abilities. Of note, CeO2 and TiO are probably the best assistants for Au nanoclusters under catalytic reaction conditions.170,171 Pd nanoclusters, in particular small Pd nanoclusters (metal number less than 10), have their unique position in nanocluster-based catalysts since they are still the only active nanoclusters for the Heck reaction, let alone their distinguish catalytic properties in other C–C and C–N connection systems. The development of nanocluster-catalyzed coupling reactions follow the same path as regular coupling reactions36 where the advanced catalysts are transforming from noble metal nanoclusters to non-noble metal nanoclusters. Copper nanoclusters as the most researched non-noble metal nanoclusters demonstrated extraordinary catalytic abilities in coupling reactions. As the price of precious metals continues to rise, copper nanoclusters are bound to become the most popular nanoclusters in the future.Based on different types of coupling reactions, capping ligands, motifs, kernels, and support influence nanoclusters’ catalytic properties collectively. In general, aromatic protecting ligands tend to give better results in coupling systems compared to aliphatic ligands including thiolate-, phosphine-, and alkyne-aromatic ligands. The synergistic effect between noble metal nanoclusters (Au, Pd, Pt, etc.) and the metal oxide support is obvious, while Cu nanoclusters are usually mounted on porous carbon materials. Nanoclusters with core–shell structures are more stable than coreless nanoclusters, while coreless nanoclusters provide more metal active sites. The hetero metal synergetic effects are an ice breaker in carbon-heteroatom coupling systems. It indicates that nanocluster-catalyzed carbon–heteroatom cross-coupling reactions are size- and support-specific, which emphasizes that the designing of the nanoclusters is the key to altering nanoclusters’ catalytic properties. The atomic-defined nature of atomically precise nanoclusters provides opportunities for trimming and fine-tuning their structures, so that their catalytic properties can be evaluated atom-by-atom and ligand-by-ligand.
Herein, several perspectives regarding atomically precise nanoclusters over coupling reactions are discussed below.
(i) Recommendations for synthesizing atomically precise nanoclusters toward target coupling reactions (and vice versa). Au25 and its alloy nanoclusters are the best shots for the Ullmann C–C coupling reactions. If the designed products are symmetric biaryls (homocoupling products), CuxAu(25−x) bimetallic nanoclusters or Au11 and other smaller Au nanoclusters172 that behave like single atoms should be considered for the systems. Ce2O should be considered as the immobilizer if a heterogenous catalyst is needed. As for heterocoupling reactions, PtxAu(25−x) nanoclusters protected by aromatic ligands are the top-one candidates. As for S–M coupling reaction systems, Pd nanoclusters are more effective than other metal nanoclusters. Pd alloy nanoclusters and small Pd nanoclusters should be better candidates for S–M coupling reactions. The Heck reaction is one of the most important paths for coupling sp2 carbon with hydrocarbons in one step. Hence, the development of atomically precise nanocluster-catalyzed Heck reaction systems calls for brainstorming despite insufficient examples. From my perspective, future exploration could still be focused on small Pdn nanoclusters (n = 2–10) and Pd-based alloy nanoclusters such as Pd–Cu nanoclusters. The development of the Sonogashira cross-coupling reaction catalyzed by atomically precise nanoclusters follows the same path as the traditional Sonogashira reaction by moving from expensive noble metal nanoclusters to cheap copper nanoclusters. For future reference, atomically precise copper nanoclusters are still the seeded players in the Sonogashira cross-coupling reaction system. The surface defective effect is calling for a deeper investigation to unveil the relationship between defects and catalytic ability. Copper nanoclusters with precision numbers are still the most rational candidates for oxidative C(sp)–C(sp) coupling reactions. It is still worth optimizing reaction conditions to reduce the reaction temperature and time.
The total development time of C-heteroatom coupling is relatively short. Hence, the time for summarization is not yet ripe. However, based on the limited examples, the atomically precise nanoclusters showed overwhelming catalytic abilities for carbon–heteroatom coupling. Future exploration could focus on coreless alloy nanoclusters since they are much easier to prepare than single-atom catalysts and core–shell nanoclusters. Corma, Leyva-Pérez, et al. have proved the catalytic ability of small Cu nanoclusters in carbon–heteroatom bond formation.122 Research related to C-heteroatom bond connection should pay more attention to small Cu and small Cu-based alloy nanoclusters. A3 coupling systems seem to need higher reaction temperatures for the best yield. Thus, thermally stable nanoclusters are the optimal candidates for multicomponent coupling reactions.
In general, even though the majority of reports use bigger nanoclusters with core–shell structures in catalytic systems, we believe that small atomically precise nanoclusters with metal numbers less than 10 will demonstrate a paramount role in catalyzed-coupling reactions in the future because (1) small nanocluster synthesis procedures are much easier than those of bigger nanoclusters; (2) full access to metal-core provides more active sites; (3) the synergistic effect of adjacent metal atoms cannot be interrupted by ligands; (4) calculations and simulations involving fewer atoms are much easier to be conducted for better mechanistic understanding.
(ii) There are still lots of coupling reactions that have not been explored over atomically precise nanoclusters such as Csp3–Csp3, Csp3–Csp2, and Csp3–Csp. The activation of Csp and Csp2 can be achieved by nanoclusters, while the example of Csp3 activation is less common over atomically precise nanoclusters. The sp3 C–H activation is generally accepted to be much more challenging owing to the low reactivity (high bond energy).173 Modern strategies of sp3 C–H functionalization usually rely on SET induced by light-activated catalysts and photoredox catalysis,174–176 which remind us of the optical properties124–126 of copper nanoclusters. Therefore, copper nanoclusters with large absorption cross-sections under visible light and longer excited-state lifetimes should be examined for sp3 C–H functionalization systems. Even though only C–N and C–O coupling reactions catalyzed by atomically precise nanoclusters have been discovered, other carbon-heteroatom bond formations such as C–S, C–P, and C–Si should be taken into consideration according to Corma's findings118 to test the catalytic ability of copper coreless alloy nanoclusters.
Catalytic asymmetric coupling reactions, especially the sterically specific Suzuki–Miyaura coupling are one the most useful methods to synthesize chiral biaryls. The most common ligands for asymmetric catalyst metal complexes are optical phosphine ligands,177 which can be utilized as nanoclusters’ capping ligands as well. Atomically precise nanoclusters possess distinct optical properties,178,179 while their optical properties can be altered by engineering protecting ligands.180 Therefore, the development of catalytic asymmetric coupling reactions over atomically precise nanoclusters with optical ligands should be taken into account.
(iii) Keeping nanoclusters stable and active during catalytic reactions are one of the major challenges in practical applications of atomically precise nanoclusters. Zhu and Kang's strategy of generating trimetallic nanoclusters is a prominent protocol to stabilize nanoclusters by rearrangement of free valence electrons.154 The core–shell nanocluster has a higher partial positive charge (δ+) on the surface and partial negative charge (δ−) on the kernel. This valence electron centralization pattern should be promoted for the future design of thermostable nanoclusters by doping higher electronegativity metal atoms into the innermost kernel and exchanging the outmost metal shell with lower electronegativity metal atoms.
Another strategy of stabilizing nanoclusters is to immobilize nanoclusters on supports such as metal oxides and porous carbon materials. The synergism effect and host–guest interaction enhance nanoclusters’ catalytic abilities while keeping the nanoclusters intact under various harsh reaction conditions. Modern technology of reticular chemistry offers novel and superior support materials for nanoclusters such as metal–organic frameworks (MOFs), covalent organic frameworks (COFs), and hydrogen-bonded organic frameworks (HOFs). Compared to regular supports, the reticular materials have accurate structures and specific porous sizes that can be designed and modified to cooperate with nanoclusters for optimal catalytic properties. Compositing nanoclusters with MOFs181 and COFs182 can largely enhance their photostability and photocatalytic performance. Even though there are very few publications on nanocluster/reticular materials in practical applications, we believe that reticular chemistry has great potential in collaboration with atomically precise nanoclusters to rationally design effective catalysts for coupling reactions.
In conclusion, atomically precise nanoclusters hold unlimited potential in catalyzing reactions and understanding reaction processes mechanistically. By designing specific protecting ligands, metal kernels, and support, an effective nanocluster catalyst could be generated for target coupling reactions. There are still some obstacles to apply nanocluster catalysts in complicated reaction conditions, including the instability of nanoclusters and the uncertain synergetic effect between components. With the development of atomically precise nanocluster chemistry and their related nanocomposites, the rational designing of nanoclusters for specific reaction systems could be more regulated and straightforward.
Data availability
This is a Review article, and no data supporting this review have been included. Thus, the section of “Data availability” is not applicable for this work.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support of the NSFC (22371003 and 22101001), the Ministry of Education, the University Synergy Innovation Program of Anhui Province (GXXT-2020-053), the Scientific Research Program of Universities in Anhui Province (2022AH030009), and the Research Program of Wuhu Hangfeng Techo Ltd (2024340104001629).References
- K. V. Arundhathi, P. Vaishnavi, T. Aneeja and G. Anilkumar, Copper-catalyzed Sonogashira reactions: advances and perspectives since 2014, RSC Adv., 2023, 13, 4823–4834 RSC.
- R. Malav and S. Ray, Carbon-carbon cross coupling reactions assisted by Schiff base complexes of Palladium, cobalt and copper: A brief overview, Inorg. Chim. Acta, 2023, 551, 121478 CrossRef CAS.
- M. Ashraf, M. S. Ahmad, Y. Inomata, N. Ullah, M. N. Tahir and T. Kida, Transition metal nanoparticles as nanocatalysts for Suzuki, Heck and Sonogashira cross-coupling reactions, Coord. Chem. Rev., 2023, 476, 214928 CrossRef CAS.
- Y. Du, H. Sheng, D. Astruc and M. Zhu, Atomically Precise Noble Metal Nanoclusters as Efficient Catalysts: A Bridge between Structure and Properties, Chem. Rev., 2020, 120, 526–622 CrossRef CAS PubMed.
- C. C. C. J. Seechurn, M. O. Kitching, T. J. Colacot and V. Sniechus, Palladium–Catalyzed Cross–Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize, Angew. Chem., Int. Ed., 2012, 51, 5062–5085 CrossRef PubMed.
- Y. Li, G. J. Stec, A. E. Thorarinsdottir, R. D. McGillicuddy, S.-L. Zheng and J. A. Mason, The role of metal accessibility on carbon dioxide electroreduction in atomically precise nanoclusters, Chem. Sci., 2023, 14, 12283–12291 RSC.
- E. C. Tyo and S. Vajda, Catalysis by Clusters with Precise Numbers of Atoms, Nat. Nanotechnol., 2015, 10, 577–588 CrossRef CAS PubMed.
- Q. Shi, Z. Qin, H. Xu and G. Li, Heterogeneous Cross-Coupling over Gold Nanoclusters, Nanomaterials, 2019, 9, 838 CrossRef PubMed.
- J. Zhao and R. Jin, Heterogeneous Catalysis by Gold and Gold-Based Bimetal Nanoclusters, Nano Today, 2018, 18, 86–102 CrossRef CAS.
- H. Li, K. Shin and G. Henkelman, Effects of ensembles, ligand, and strain on adsorbate binding to alloy surfaces, J. Chem. Phys., 2018, 149, 174705 CrossRef PubMed.
- C. L. Bracey, P. R. Ellis and G. J. Hutchings, Application of copper–gold alloys in catalysis: current status and future perspectives, Chem. Soc. Rev., 2009, 38, 2231 RSC.
- K. Kaizuka, H. Miyamura and S. Kobayashi, Remarkable Effect of Bimetallic Nanocluster Catalysts for Aerobic Oxidation of Alcohols: Combining Metals Changes the Activities and the Reaction Pathways to Aldehydes/Carboxylic Acids or Esters, J. Am. Chem. Soc., 2010, 132, 15096–15098 CrossRef CAS PubMed.
- S.-S. Zhang, F. Alkan, H.-F. Su, C. M. Aikens, C.-H. Yung and S. Sun, [Ag48(C≡C, tBu)20(CrO4)7]: An Atomically Precise Silver Nanocluster Co-protected by Inorganic and Organic Ligands, J. Am. Chem. Soc., 2019, 141, 4460–4467 CrossRef CAS PubMed.
- P. Liu, R. Qin, G. Fu and N. Zheng, Surface Coordination Chemistry of Metal Nanomaterials, J. Am. Chem. Soc., 2017, 139, 2122–2131 CrossRef CAS PubMed.
- G. Li, H. Abroshan, C. Liu, S. Zhuo, Z. Li, Y. Xie, H. Kim, N. Ros and R. Jin, Tailoring the Electronic and Catalytic Properties of Au25 Nanoclusters via Ligand Engineering, ACS Nano, 2016, 10, 7998–8005 CrossRef CAS PubMed.
- Y. Wang, Y. Zheng, C. Z. Huang and Y. Xia, Synthesis of Ag Nanocubes 18–32 nm in Edge Length: The Effects of Polyol on Reduction Kinetics, Size Control, and Reproducibility, J. Am. Chem. Soc., 2013, 135, 1941–1951 CrossRef CAS PubMed.
- X. Kang and M. Zhu, Transformation of Atomically Precise Nanoclusters by Ligand-Exchange, Chem. Mater., 2019, 31, 9939–9969 CrossRef CAS.
- C.-Z. Ning, L. Dou and P. Yang, Bandgap engineering in semiconductor alloy nanomaterials with widely tunable compositions, Nat. Rev. Mater., 2017, 2, 17070 CrossRef CAS.
- M. Luo and S. Guo, Strain-controlled electrocatalysis on multimetallic nanomaterials, Nat. Rev. Mater., 2017, 2, 17059 CrossRef CAS.
- L. Liao, S. Zhou, Y. Dai, L. Liu, C. Yao, C. Fu, J. Yang and Z. Wu, Mono-Mercury Doping of Au25 and the HOMO/LUMO Energies Evaluation Employing Differential Pulse Voltammetry, J. Am. Chem. Soc., 2015, 137, 9511–9514 CrossRef CAS PubMed.
- S. Tian, L. Liao, J. Yuan, C. Yao, J. Chen, J. Yang and Z. Wu, Structures and magnetism of mono-palladium and mono-platinum doped Au25(PET)18 nanoclusters, Chem. Commun., 2016, 52, 9873–9876 RSC.
- M. Suyama, S. Takano, T. Nakamura and T. Tsukuda, Stoichiometric Formation of Open-Shell [PtAu24 (SC2H4Ph)18]− via Spontaneous Electron Proportionation between [PtAu24(SC2H4Ph)18]2− and [PtAu24(SC2H4Ph)18]0, J. Am. Chem. Soc., 2019, 141, 14048–14051 CrossRef CAS PubMed.
- W. Fei, S. Antonello, T. Dainese, A. Dolmella, M. Lahtinen, K. Rissanen, A. Venzo and F. Maran, Metal Doping of Au25(SR)18 Clusters: Insights and Hindsights, J. Am. Chem. Soc., 2019, 141, 16033–16045 CrossRef CAS PubMed.
- Z. Wu, Anti–Galvanic Reduction of Thiolate–Protected Gold and Silver Nanoparticles, Angew. Chem., Int. Ed., 2012, 51, 2934–2938 CrossRef CAS PubMed.
- R. Jin, S. Zhao, C. Liu, M. Zhou, G. Panapitiya, Y. Xing, N. L. Rosi, J. P. Lewis and R. Jin, Controlling Ag-doping in [AgxAu25−x(SC6H11)18]− nanoclusters: cryogenic optical, electronic and electrocatalytic properties, Nanoscale, 2017, 9, 19183–19190 RSC.
- X. Kang, Y. Li, M. Zhu and R. Jin, Atomically Precise Alloy Nanoclusters: Syntheses, Structures, and Properties, Chem. Soc. Rev., 2020, 49, 6443–6514 RSC.
- G. Li, D. Jiang, C. Liu, C. Yu and R. Jin, Oxide-Supported Atomically Precise Gold Nanocluster for Catalyzing Sonogashira Cross-Coupling, J. Catal., 2013, 306, 177–183 CrossRef CAS.
- K. Shimizu, K. Ohshima, Y. Tai, M. Tamura and A. Stasuma, Size- and support-dependent selective amine cross-coupling with platinum nanocluster catalysts, Catal. Sci. Technol., 2012, 2, 730–738 RSC.
- M. Taleblou, M. F. Camellone, S. Fabris and S. Piccinin, CO Oxidation over Platinum Nanoclusters: Unraveling the Role of the Cluster Size and the Supporting Surface, J. Phys. Chem. C, 2023, 127, 21132–21149 CrossRef CAS.
- X. Wang, Y. Zhang, H. Si, Q. Zhang, J. Wu, L. Gao, X. Wei, Y. Sun, Q. Liao, Z. Zhang, K. Ammarah, L. Gu, Z. Kang and Y. Zhang, Single-Atom Vacancy Defect to Trigger High-Efficiency Hydrogen Evolution of MoS2, J. Am. Chem. Soc., 2020, 142, 4298–4308 CrossRef CAS PubMed.
- X. Wei, H. Shen, C. Xu, H. Li, S. Jin, X. Kang and M. Zhu, Ag48 and Ag50 Nanoclusters: Toward Active-Site Tailoring of Nanocluster Surface Structures, Inorg. Chem., 2021, 60, 5931–5936 CrossRef CAS PubMed.
- C. Dong, R.-W. Huang, C. Chen, J. Chen, S. Nematulloev, X. Guo, A. Ghosh, B. Alamer, M. N. Hedhili, T. T. Isimjan, Y. Han, O. F. Mohammed and O. M. Bakr, [Cu36H10(PET)24(PPh3)6Cl2] Reveals Surface Vacancy Defects in Ligand-Stabilized Metal Nanoclusters, J. Am. Chem. Soc., 2021, 143, 11026–11035 CrossRef CAS PubMed.
- Y. Bao, X. Wu, B. Yin, X. Kang, Z. Lin, H. Deng, H. Yu, S. Jin, S. Chen and M. Zhu, Structured Copper-hydride Nanoclusters Provide Insight into the Surface-Vacancy-Defect to Non-defect Structural Evolution, Chem. Sci., 2022, 13, 14357–14365 RSC.
- C. Xie, D. Yan, H. Li, S. Du, W. Chen, Y. Wang, Y. Zou, R. Chen and S. Wang, Defect Chemistry in Heterogeneous Catalysis: Recognition, Understanding, and Utilization, ACS Catal., 2020, 10, 11082–11098 CrossRef CAS.
- Y. Hua, J. Huang, Z. Shao, X. Luo, Z. Wang, J. Liu, X. Zhao, X. Chen and S. Zang, Composition–Dependent Enzyme Mimicking Activity and Radiosensitizing Effect of Bimetallic Clusters to Modulate Tumor Hypoxia for Enhanced Cancer Therapy, Adv. Mater., 2022, 34, 2203734 CrossRef CAS PubMed.
- J. J. Mousseau and A. B. Charette, Direct Functionalization Processes: A Journey from Palladium to Copper to Iron to Nickel to Metal-Free Coupling Reactions, Acc. Chem. Res., 2013, 46, 412–424 CrossRef CAS PubMed.
- M. Zhu, E. Lanni, N. Garg, M. E. Bier and R. Jin, Kinetically Controlled, High-Yield Synthesis of Au25 Clusters, J. Am. Chem. Soc., 2008, 130, 1138–1139 CrossRef CAS PubMed.
- M. Zhu, C. M. Aikens, F. J. Hollander, G. C. Schatz and R. Jin, Correlating the Crystal Structure of A Thiol-Protected Au25 Cluster and Optical Properties, J. Am. Chem. Soc., 2008, 130, 5883–5885 CrossRef CAS PubMed.
- M. Zhu, W. T. Eckenhoff, T. Pintauer and R. Jin, Conversion of Anionic [Au25(SCH2CH2Ph)18]− Cluster to Charge Neutral Cluster via Air Oxidation, J. Phys. Chem. C, 2008, 112, 14221–14224 CrossRef CAS.
- M. Zhu, C. M. Aikens, M. P. Hendrich, R. Gupta, H. Qian, G. C. Schatz and R. Jin, Reversible Switching of Magnetism in Thiolate-Protected Au 25 Superatoms, J. Am. Chem. Soc., 2009, 131, 2490–2492 CrossRef CAS PubMed.
- G. Li, C. Liu, Y. Lei and R. Jin, Au25 Nanocluster-Catalyzed Ullmann-Type Homocoupling Reaction of Aryl Iodides, Chem. Commun., 2012, 48, 12005 RSC.
- P. S. D. Robinson, G. N. Khairallah, G. da Silva, H. Lioe and R. A. J. O'Hair, Gold–Mediated C-I Bond Activation of Iodobenzene, Angew. Chem., Int. Ed., 2012, 51, 3812–3817 CrossRef CAS PubMed.
- H. Chen, C. Liu, M. Wang, C. Zhang, G. Li and F. Wang, Thermally robust silica-enclosed Au 25 nanocluster and its catalysis, Chin. J. Catal., 2016, 37, 1787–1793 CrossRef CAS.
- Z. Li, X. Yang, C. Liu, J. Wang and G. Li, Effects of doping in 25-atom bimetallic nanocluster catalysts for carbon-carbon coupling reaction of iodoanisole and phenylacetylene, Prog. Nat. Sci.: Mater. Int., 2016, 26, 477–482 CrossRef CAS.
- E. Gottlieb, H. Qian and R. Jin, Atomic–Level Alloying and De–alloying in Doped Gold Nanoparticles, Chem. – Eur. J., 2013, 19, 4238–4243 CrossRef CAS PubMed.
- F. Monnier and M. Taillefer, Catalytic C-C, C-N, and C-O Ullmann–Type Coupling Reactions: Copper Makes a Difference, Angew. Chem., Int. Ed., 2008, 47, 3096–3099 CrossRef CAS PubMed.
- R. N. Dhital, C. Kamonsatikul, E. Somsook, K. Bobuatong, M. Ehara, S. Karanjit and H. Sakurai, Low-Temperature Carbon–Chlorine Bond Activation by Bimetallic Gold/Palladium Alloy Nanoclusters: An Application to Ullmann Coupling, J. Am. Chem. Soc., 2012, 134, 20250–20253 CrossRef CAS PubMed.
- A. K. Das, S. Mukherjee, S. R. Sreehari, A. S. Nair, S. Bhandary, D. Chopra, D. Sanyal, B. Pathak and S. Mandal, Defects Engineering on Ceria and C-C Coupling Reactions Using [Au11(PPh3)7I3] Nanocluster: A Combined Experimental and Theoretical Study, ACS Nano, 2020, 14, 16681–16688 CrossRef CAS PubMed.
- C. Zhang, A. Michaelides, D. A. King and S. J. Jenkins, Positive Charge States and Possible Polymorphism of Gold Nanoclusters on Reduced Ceria, J. Am. Chem. Soc., 2010, 132, 2175–2182 CrossRef CAS PubMed.
- M. Norio and S. Akira, Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds, Chem. Rev., 1995, 95, 2457–2483 CrossRef.
- S. Saito, S. Oh-tani and N. Miyaura, Synthesis of Biaryls via a Nickel(0)-Catalyzed Cross-Coupling Reaction of Chloroarenes with Arylboronic Acids, J. Org. Chem., 1997, 62, 8024–8030 CrossRef CAS PubMed.
- T. Hatakeyama, T. Hashimoto, K. K. A. D. S. Kathriarachchi, T. Zenmyo, H. Seike and M. Nakamura, Iron–Catalyzed Alkyl–Alkyl Suzuki–Miyaura Coupling, Angew. Chem., Int. Ed., 2012, 51, 8834–8837 CrossRef CAS PubMed.
- M. B. Thathagar, J. Beckers and G. Rothenberg, Copper-Catalyzed Suzuki Cross-Coupling Using Mixed Nanocluster Catalysts, J. Am. Chem. Soc., 2002, 124, 11858–11859 CrossRef CAS PubMed.
- F.-S. Han, Transition-metal-catalyzed Suzuki–Miyaura cross-coupling reactions: a remarkable advance from palladium to nickel catalysts, Chem. Soc. Rev., 2013, 42, 5270 RSC.
- H. Abroshan, G. Li, J. Lin, H. Kim and R. Jin, Molecular mechanism for the activation of Au25(SCH2CH2Ph)18 nanoclusters by imidazolium-based ionic liquids for catalysis, J. Catal., 2016, 337, 72–79 CrossRef CAS.
- A. Leyva-Pérez, J. Oliver-Meseguer, P. Rubio-Marqués and A. Corma, Water-Stabilized Three- and Four-Atom Palladium Clusters as Highly Active Catalytic Species in Ligand-Free C-C Cross-Coupling Reactions, Angew. Chem., Int. Ed., 2013, 52, 11554–11559 CrossRef PubMed.
- F. Fu, J. Xiang, H. Cheng, L. Cheng, H. Chong, S. Wang, P. Li, S. Wei, M. Zhu and Y. Li, A Robust and Efficient Pd3 Cluster Catalyst for the Suzuki Reaction and Its Odd Mechanism, ACS Catal., 2017, 7, 1860–1867 CrossRef CAS.
- K. R. Dixon and A. D. Rattray, Trinuclear palladium clusters: synthesis and phosphorus-31 nuclear magnetic resonance spectra of [Pd3Cl(PPh2)2(PPh3)3][BF4] and related complexes, Inorg. Chem., 1978, 17, 1099–1103 CrossRef CAS.
- Modern Arene Chemistry, ed. D. Astruc, Wiley, 1st edn, 2002 Search PubMed.
- R. E. Palmer, S. Pratontep and H.-G. Boyen, Nanostructured surfaces from size-selected clusters, Nat. Mater., 2003, 2, 443–448 CrossRef CAS PubMed.
- H. Tsunoyama, Y. Yamano, C. Zhang, M. Komori, T. Eguchi and A. Nakajuma, Size-Effect on Electrochemical Hydrogen Evolution Reaction by Single-Size Platinum Nanocluster Catalysts Immobilized on Strontium Titanate, Top. Catal., 2018, 61, 126–135 CrossRef CAS.
- M. Moseler, M. Walter, B. Yoon, U. Landman, V. Habibpour, C. Harding, S. Kunz and U. Heiz, Oxidation State and Symmetry of Magnesia-Supported Pd13Ox Nanocatalysts Influence Activation Barriers of CO Oxidation, J. Am. Chem. Soc., 2012, 134, 7690–7699 CrossRef CAS PubMed.
- K. Seth, P. Purohit and A. Chakraborti, Cooperative Catalysis by Palladium-Nickel Binary Nanocluster for Suzuki-Miyaura Reaction of Ortho-Heterocycle-Tethered Sterically Hindered Aryl Bromides, Org. Lett., 2014, 16, 2334–2337 CrossRef CAS PubMed.
- D. Yim, F. Raza, J. Park, J. Lee, H. Kim, J. Yang, I. Hwang and J. Kim, Ultrathin WO3 Nanosheets Converted from Metallic WS2 Sheets by Spontaneous Formation and Deposition of PdO Nanoclusters for Visible Light-Driven C-C Coupling Reactions, ACS Appl. Mater. Interfaces, 2019, 11, 36960–36969 CrossRef CAS PubMed.
- C. Wang, L. Salmon, R. Ciganda, L. Yate, S. Moya, J. Ruiz and D. Astruc, An efficient parts-per-million α-Fe2O3 nanocluster/graphene oxide catalyst for Suzuki-Miyaura coupling reactions and 4-nitrophenol reduction in aqueous solution, Chem. Commun., 2017, 53, 644–646 RSC.
- M. B. Thathagar, J. Beckers and G. Rothenberg, Combinatorial Design of Copper-Based Mixed Nanoclusters: New Catalysts for Suzuki Cross-Coupling, Adv. Synth. Catal., 2003, 345, 979–985 CrossRef CAS.
- K. Maeyama, T. Tsukamoto, M. Suzuki, S. Higashibayashi and H. Sakurai, Synthesis of Aromatic Polyketones Bearing 1,1′-Binaphthyl-2,2′-dioxy Units through Suzuki-Miyaura Coupling Polymerization, Chem. Lett., 2011, 40, 1445–1446 CrossRef CAS.
- N. T. S. Phan, M. Van Der Sluys and C. W. Jones, On the Nature of the Active Species in Palladium Catalyzed Mizoroki–Heck and Suzuki–Miyaura Couplings–Homogeneous or Heterogeneous Catalysis, A Critical Review, Adv. Synth. Catal., 2006, 348, 609–679 CrossRef CAS.
- M. T. Reetz and J. G. De Vries, Ligand-free Heck reactions using low Pd-loading, Chem. Commun., 2004, 1559 RSC.
- X. Cui, J. Li, Z.-P. Zhang, Y. Fu, L. Liu and Q.-X. Guo, Pd(quinoline-8-carboxylate)2 as a Low-Priced, Phosphine-Free Catalyst for Heck and Suzuki Reactions, J. Org. Chem., 2007, 72, 9342–9345 CrossRef CAS PubMed.
- J. P. Stambuli, S. R. Stauffer, K. H. Shaughnessy and J. F. Hartwig, Screening of Homogeneous Catalysts by Fluorescence Resonance Energy Transfer. Identification of Catalysts for Room-Temperature Heck Reactions, J. Am. Chem. Soc., 2001, 123, 2677–2678 CrossRef CAS PubMed.
- D. A. Alonso and C. Nájera, Oxime-derived palladacycles as source of palladium nanoparticles, Chem. Soc. Rev., 2010, 39, 2891 RSC.
- J. Li, Y. Song, Y. Wang and H. Zhang, Enhanced Heck reaction on flower-like Co(Mg or Ni)Al layered double hydroxide supported ultrafine PdCo alloy nanocluster catalysts: the promotional effect of Co, Dalton Trans., 2019, 48, 17741–17751 RSC.
- T. Gao, Y. Zhao, Y. Zhou and Z. Kang, Mesoporous Silica Nanospheres Supported Atomically Precise Palladium Nanocluster: Highly Efficient and Recyclable Catalysts in the Reduction of 4-Nitrophenol and Heck Reactions, Appl. Organomet. Chem., 2022, 36, e6541 CrossRef CAS.
- H. Plenio, Catalysts for the Sonogashira Coupling—The Crownless Again Shall Be King, Angew. Chem., Int. Ed., 2008, 47, 6954–6956 CrossRef CAS PubMed.
- R. Chinchilla and C. Nájera, The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry, Chem. Rev., 2007, 107, 874–922 CrossRef CAS PubMed.
- P. Lei, Y. Wang, C. Zhang, Y. Hu, J. Feng, Z. Ma, X. Liu, R. Szostak and M. Szostak, Sonogashira Cross-Coupling of Aryl Ammonium Salts by Selective C–N Activation Catalyzed by Air- and Moisture-Stable, Highly Active [Pd(NHC)(3-CF3-An)Cl2] (An = Aniline) Precatalysts, Org. Lett., 2022, 24, 6310–6315 CrossRef CAS PubMed.
- A. M. Thomas, A. Sujatha and G. Anilkumar, Recent advances and perspectives in copper-catalyzed Sonogashira coupling reactions, RSC Adv., 2014, 4, 21688–21698 RSC.
- X.-Y. Dong, Y.-F. Zhang, C.-L. Ma, Q.-S. Gu, F.-L. Wang, Z.-L. Li, S.-P. Jiang and X.-Y. Liu, A general asymmetric copper-catalysed Sonogashira C(sp3)–C(sp) coupling, Nat. Chem., 2019, 11, 1158–1166 CrossRef CAS PubMed.
- X. Mo, B. Chen and G. Zhang, Copper–Catalyzed Enantioselective Sonogashira Type Coupling of Alkynes with α–Bromoamides, Angew. Chem., Int. Ed., 2020, 59, 13998–14002 CrossRef CAS PubMed.
- M. B. Thathagar, J. Beckers and G. Rothenberg, Palladium-Free and Ligand-Free Sonogashira Cross-Coupling, Green Chem., 2004, 6, 215 RSC.
- C. González-Arellano, A. Abad, A. Corma, H. Garcia, M. Iglesias and F. Sanchez, Catalysis by Gold(I) and Gold(III): A Parallelism between Homo– and Heterogeneous Catalysts for Copper–Free Sonogashira Cross–Coupling Reactions, Angew. Chem., Int. Ed., 2007, 46, 1536–1538 CrossRef PubMed.
- V. Kanuru, G. Kyriakou, S. Beaumont, A. Pafageorgiou, D. Watson and R. Lambert, Sonogashira Coupling on an Extended Gold Surface in Vacuo: Reaction of Phenylacetylene with Iodobenzene on Au(111), J. Am. Chem. Soc., 2010, 132, 8081–8086 CrossRef CAS PubMed.
- X. Bai, Y. Gao, H. Liu and L. Sheng, Synthesis of Amphiphilic Ionic Liquids Terminated Gold Nanorods and Their Superior Catalytic Activity for the Reduction of Nitro Compounds, J. Phys. Chem. C, 2009, 113, 17730–17736 CrossRef CAS.
- C. J. Murphy, A. M. Gole, S. E. Hunyadi, J. W. Stone, P. N. Sisco, A. Alkilany, B. E. Kinard and P. Hankins, Chemical sensing and imaging with metallic nanorods, Chem. Commun., 2008, 5, 544–557 RSC.
- J. Lin, H. Abroshan, C. Liu, M. Zhu, G. Li and M. Haruta, Sonogashira cross-coupling on the Au(111) and Au(100) facets of gold nanorod catalysts: Experimental and computational investigation, J. Catal., 2015, 330, 354–361 CrossRef CAS.
- C. Liu, Y. Tan, S. Lin, H. Li, X. Wu, L. Li, Y. Pei and X. C. Zeng, CO Self-Promoting Oxidation on Nanosized Gold Clusters: Triangular Au3 Active Site and CO Induced O–O Scission, J. Am. Chem. Soc., 2013, 135, 2583–2595 CrossRef CAS PubMed.
- S. Xie, H. Tsunoyama, W. Kurashige, Y. Hegishi and T. Tsukuda, Enhancement in Aerobic Alcohol Oxidation Catalysis of Au25 Clusters by Single Pd Atom Doping, ACS Catal., 2012, 2, 1519–1523 CrossRef CAS.
- H. Qian, D. Jiang, G. Li, C. Gayathri, A. Das, R. R. Gil and R. Jin, Monoplatinum Doping of Gold Nanoclusters and Catalytic Application, J. Am. Chem. Soc., 2012, 134, 16159–16162 CrossRef CAS PubMed.
- Y. Yang, C. Chen, G. Xu, J. Yuan, S. Ye, L. Chen, Q. Lv, G. Luo, J. Yang, M. Li and Z. Wu, An efficient nanocluster catalyst for Sonogashira reaction, J. Catal., 2021, 401, 206–213 CrossRef CAS.
- Y. Jia, K. Jiang, H. Wang and X. Yao, The Role of Defect Sites in Nanomaterials for Electrocatalytic Energy Conversion, Chem, 2019, 5, 1371–1397 CAS.
- R. Schmitt, A. Nenning, O. Kraynis, R. Korobko, A. I. Frenkel, I. Lubomirsky, S. M. Haile and J. L. M. Rupp, A review of defect structure and chemistry in ceria and its solid solutions, Chem. Soc. Rev., 2020, 49, 554–592 RSC.
- S. Nematulloev, A. Sagadevan, B. Alamer, A. Shkurenko, R. Huang, J. Yin, C. Dong, P. Yuan, K. Yorov, A. Karluk, W. Mir, B. Hasanov, M. Hedhili, N. Halappa, M. Eddaoudi, O. Mohammed, M. Rueping and O. Bakr, Atomically Precise Defective Copper Nanocluster Catalysts for Highly Selective C-C Cross-Coupling Reactions, Angew. Chem., Int. Ed., 2023, 62, e202303572 CrossRef CAS PubMed.
- A. Hossain, A. Bhattacharyya and O. Reiser, Copper's rapid ascent in visible-light photoredox catalysis, Science, 2019, 364, eaav9713 CrossRef PubMed.
- Y. Fang, K. Bao, P. Zhang, H. Sheng, Y. Yun, S.-X. Hu, D. Astruc and M. Zhu, Insight into the Mechanism of the CuAAC Reaction by Capturing the Crucial Au4Cu4−π-Alkyne Intermediate, J. Am. Chem. Soc., 2021, 143, 1768–1772 CrossRef CAS PubMed.
- Z. Huang, S. Tang and A. Lei, Oxidative Cross-Coupling: An Alternative Way for C–C Bond Formations, Sci. Bull., 2015, 60, 1391–1394 CrossRef CAS.
- Y. Liao, F. Liu and Z. Shi, Recent progress in the oxidative coupling of unactivated Csp3−H bonds with other C–H bonds, Chem. Commun., 2021, 57, 13288–13296 RSC.
- C. Glaser, Beiträge zur Kenntniss des Acetenylbenzols, Ber. Dtsch. Chem. Ges., 1869, 2, 422–424 CrossRef.
- K. Yin, C. Li, J. Li and X. Jia, CuCl-catalyzed green oxidative alkyne homocoupling without palladium, ligands and bases, Green Chem., 2011, 13, 591 RSC.
- X. Jia, K. Yin, C. Li, J. Li and H. Bian, Copper-catalyzed oxidative alkyne homocoupling without palladium, ligands and bases, Green Chem., 2011, 13, 2175 RSC.
- C. Yin, S. Liu, Z. Qin, Y. Zhang, G. Li and Z. Zhao, Butterfly-Like Tetranuclear Copper(I) Clusters for Efficient Alkyne Homocoupling Reactions, Eur. J. Inorg. Chem., 2021, 2021, 392–397 CrossRef CAS.
- X. Ma, N. V. Tzouras, M. Peng, K. Van Hecke and S. P. Nolan, Azolium Aurates as Pre-Catalysts for the Oxidative Coupling of Terminal Alkynes under Mild Conditions, J. Org. Chem., 2022, 87, 4883–4893 CrossRef CAS PubMed.
- N. Sun, Y. Yun, K. Li, X. Ni, Y. Qin, X. Yang, H. Sheng and M. Zhu, Highly Efficient Conversion of Homocoupling and Heterocoupling of Terminal Alkynes Catalyzed by AuCu24 /AC–200, Eur. J. Inorg. Chem., 2023, 26, e202300161 CrossRef CAS.
- A. Chen, X. Kang, S. Jin, W. Su, S. Wang and M. Zhu, Gram-Scale Preparation of Stable Hydride M@Cu24 (M = Au/Cu) Nanoclusters, J. Phys. Chem. Lett., 2019, 10, 6124–6128 CrossRef CAS PubMed.
- S. Pollitt, V. Truttmann, T. Haunold, C. Garcia, W. Olszewski, J. Llorca, N. Barrabes and G. Rupprechter, The Dynamic Structure of Au38SR24 Nanoclusters Supported on CeO2 upon Pretreatment and CO Oxidation, ACS Catal., 2020, 10, 6144–6148 CrossRef CAS PubMed.
- R. Chutia and B. Chetia, Ligand and additive free aerobic synthesis of diynes using Pd–CuFe2O4 magnetic nanoparticles as an efficient reusable catalyst, New J. Chem., 2020, 44, 18199–18207 RSC.
- S. Mukherjee, A. Das, A. Sheriff, K. Sunny, A. Nair, S. Bhandary, R. Bhowal, D. Chopra, B. Pathak, S. Yamazoe and S. Mandal, Single Cu Atom Doping on Au11 Nanocluster: Its Implication toward Selectivity in C-C Coupling Reaction, Chem. Mater., 2023, 35, 1659–1666 CrossRef CAS.
- L. Naldini, F. Cariati, G. Simonetta and L. Malatesta, Gold–tertiary phosphine derivatives with intermetallic bonds, Chem. Commun., 1966, 647–648 RSC.
- M. McPartlin, R. Mason and L. Malatesta, Novel cluster complexes of gold(0)–gold(I), J. Chem. Soc. D, 1969, 334–334 RSC.
- L. Wang, J. Peng, Z. Tang, X. Kang, M. Fu and S. Chen, Styrene oxidation catalyzed by Au11(PPh3)7Cl3 and [Au11(PPh3)8Cl2]Cl nanoclusters: Impacts of capping ligands, particle size and charge state, Appl. Catal., A, 2018, 557, 1–6 CrossRef CAS.
- Z. Qin, D. Zhao, L. Zhao, Q. Xiao, T. Wu, J. Zhang, C. Wan and G. Li, Tailoring the Stability, Photocatalysis and Photoluminescence Properties of Au11 Nanoclusters via Doping Engineering, Nanoscale Adv., 2019, 1, 2529–2536 RSC.
- L. C. McKenzie, T. O. Zaikova and J. E. Hutchison, Structurally Similar Triphenylphosphine-Stabilized Undecagolds, Au11(PPh3)7Cl3 and [Au11(PPh3)8Cl2]Cl, Exhibit Distinct Ligand Exchange Pathways with Glutathione, J. Am. Chem. Soc., 2014, 136, 13426–13435 CrossRef CAS PubMed.
- S. L. Christensen, M. A. MacDonald, A. Chatt, P. Zhang, H. Qian and R. Jin, Dopant Location, Local Structure, and Electronic Properties of Au24Pt(SR)18 Nanoclusters, J. Phys. Chem. C, 2012, 116, 26932–26937 CrossRef CAS.
- X. Kang, J. Xiang, Y. Lv, W. Du, H. Yu, S. Wang and M. Zhu, Synthesis and Structure of Self-Assembled Pd2Au23(PPh3)10Br7 Nanocluster: Exploiting Factors That Promote Assembly of Icosahedral Nano-Building-Blocks, Chem. Mater., 2017, 29, 6856–6862 CrossRef CAS.
- C. Li, S. Zhang, J. Tang, R. Jian, Y. Xia and L. Zhao, Pyridine dicarbanion-bonded Ag13 organometallic nanoclusters: synthesis and on-surface oxidative coupling reaction, Chem. Sci., 2022, 13, 8095–8103 RSC.
- E.-X. Zhang, D.-X. Wang, Q.-Y. Zheng and M.-X. Wang, Synthesis of Large Macrocyclic Azacalix[n]pyridines (n = 6–9) and Their Complexation with Fullerenes C60 and C70, Org. Lett., 2008, 10, 2565–2568 CrossRef CAS PubMed.
- M. S. Szulmanowicz, A. Gniewek, W. Gil and A. M. Trzeciak, Palladium(II) Complexes with Small N–Heterocyclic Carbene Ligands as Highly Active Catalysts for the Suzuki–Miyaura Cross–Coupling Reaction, ChemCatChem, 2013, 5, 1152–1160 CrossRef CAS.
- D. B. Eremin and V. P. Ananikov, Understanding active species in catalytic transformations: From molecular catalysis to nanoparticles, leaching, “Cocktails” of catalysts and dynamic systems, Coord. Chem. Rev., 2017, 346, 2–19 CrossRef CAS.
- Q. Zeng, L. Zhang and Y. Zhou, Advances in Selective Carbon–Heteroatom Coupling Reactions, Chem. Rec., 2018, 18, 1278–1291 CrossRef CAS PubMed.
- N. M. Camasso and M. S. Sanford, Design, synthesis, and carbon-heteroatom coupling reactions of organometallic nickel(IV) complexes, Science, 2015, 347, 1218–1220 CrossRef CAS PubMed.
- K. Shimizu, K. Shimura, K. Ohshima, M. Tamura and A. Stasuma, Selective cross-coupling of amines by alumina-supported palladium nanocluster catalysts, Green Chem., 2011, 13, 3096–3100 RSC.
- J. Oliver-Messeguer, L. Liu, S. García-García, C. Canos-Gimenez, I. Dominguez, R. Gavara, A. Domernech-Carbo, P. Concepsion, A. Leyva-Pérez and A. Corma, Stabilized Naked Sub-Nanometric Cu Clusters within a Polymeric Film Catalyze C–N, C–C, C–O, C–S, and C–P Bond-Forming Reactions, J. Am. Chem. Soc., 2015, 137, 3894–3900 CrossRef PubMed.
- B. Mondal, K. Basu, R. Jana, P. Mondal, B. Hansda, A. Datta and A. Banerjee, Copper Nanoclusters for Catalytic Carbon-Carbon and Carbon-Nitrogen Bond Formations, ACS Appl. Nano Mater., 2022, 5, 7932–7943 CrossRef CAS.
- R.-W. Huang, J. Yin, C. Dong, A. Ghosh, M. J. Alhilaly, X. Dong, M. N. Hedhili, E. Abou-Hamad, B. Alamer, S. Nematulloev, Y. Han, O. F. Mohammed and O. M. Bakr, [Cu81(PhS)46(t BuNH2)10H32]3+ Reveals the Coexistence of Large Planar Cores and Hemispherical Shells in High-Nuclearity Copper Nanoclusters, J. Am. Chem. Soc., 2020, 142, 8696–8705 CrossRef PubMed.
- Y. Song, Y. Li, M. Zhou, X. Liu, H. Li, H. Wang, Y. Shen, M. Zhu and R. Lin, Ultrabright Au@Cu14 nanoclusters: 71.3% phosphorescence quantum yield in non-degassed solution at room temperature, Sci. Adv., 2021, 7, eabd2091 CrossRef CAS PubMed.
- A. Sagadevan, A. Ghosh, P. Maity, O. F. Mohammed, O. M. Bakr and M. Rueping, Visible-Light Copper Nanocluster Catalysis for the C–N Coupling of Aryl Chlorides at Room Temperature, J. Am. Chem. Soc., 2022, 144, 12052–12061 CrossRef CAS PubMed.
- A. Ghosh, R.-W. Huang, B. Alamer, E. Abou-Hamad, M. N. Hedhili, O. F. Mohammed and O. M. Bakr, [Cu61(StBu)26S6Cl6H14]+ : A Core–Shell Superatom Nanocluster with a Quasi- J36Cu19 Core and an “18-Crown-6” Metal-Sulfide-like Stabilizing Belt, ACS Mater. Lett., 2019, 1, 297–302 CrossRef CAS.
- L. Sun, K. Shen, H. Sheng, Y. Yun, Y. Song, D. Pan, Y. Du, H. Yu, M. Chen and M. Zhu, Au-Ag Synergistic Effect in CF3-Ketone Alkynylation Catalyzed by Precise Nanoclusters, J. Catal., 2019, 378, 220–225 CrossRef CAS.
- M. Zhou, S. Jin, X. Wei, Q. Yuan, S. Wang, Y. Du and M. Zhu, Reversible Cu–S Motif Transformation and Au4 Distortion via Thiol Ligand Exchange Engineering, J. Phys. Chem. C, 2020, 124, 7531–7538 CrossRef CAS.
- W. Yu, D. Hu, L. Xiong, Y. Li, X. Kang, S. Chen, S. Wang, Y. Pei and M. Zhu, Isomer Structural Transformation in Au–Cu Alloy Nanoclusters: Water Ripple–Like Transfer of Thiol Ligands, Part. Part. Syst. Charact., 2019, 36, 1800494 CrossRef.
- Y. Yun, L. Li, M. Zhou, M. Li, N. Sun, H. Li, S. Jin, C. Zuo, S. Sheng and M. Zhu, Atomically Precise Coreless AuCu Bimetallic Nanoclusters for Ullmann C-O Coupling, Nano Res., 2023, 16, 10756–10762 CrossRef CAS.
- L. Liu, Y. Song, H. Chong, S. Yang, J. Xiang, S. Jin, X. Kang, J. Zhang, H. Yu and M. Zhu, Size-Confined Growth of Atom-Precise Nanoclusters in Metal-Organic Frameworks and Their Catalytic Applications, Nanoscale, 2016, 8, 1407–1412 RSC.
- H. Li, H. Zhai, C. Zhou, Y. Song, F. Ke, W. W. Xu and M. Zhu, Atomically Precise Copper Cluster with Intensely Near-Infrared Luminescence and Its Mechanism, J. Phys. Chem. Lett., 2020, 11, 4891–4896 CrossRef CAS PubMed.
- C. De Graaff, E. Ruijter and R. V. A. Orru, Recent developments in asymmetric multicomponent reactions, Chem. Soc. Rev., 2012, 41, 3969 RSC.
- C. Wei and C.-J. Li, Enantioselective Direct-Addition of Terminal Alkynes to Imines Catalyzed by Copper(I)pybox Complex in Water and in Toluene, J. Am. Chem. Soc., 2002, 124, 5638–5639 CrossRef CAS PubMed.
- V. A. Peshkov, O. P. Pereshivko and E. V. Van Der Eycken, A walk around the A3-coupling, Chem. Soc. Rev., 2012, 41, 3790 RSC.
- C.-J. Li and C. Wei, Highly efficient Grignard-type imine additions via C–H activation in water and under solvent-free conditions, Chem. Commun., 2002, 3, 268–269 RSC.
- T. Zeng, W.-W. Chen, C. M. Cirtiu, A. Moores, G. Song and C.-J. Li, Fe3O4 nanoparticles: a robust and magnetically recoverable catalyst for three-component coupling of aldehyde, alkyne and amine, Green Chem., 2010, 12, 570 RSC.
- K. Namitharan and K. Pitchumani, Nickel–Catalyzed Solvent–Free Three–Component Coupling of Aldehyde, Alkyne and Amine, Eur. J. Org. Chem., 2010, 411–415 CrossRef CAS.
- C. Wei, Z. Li and C.-J. Li, The First Silver-Catalyzed Three-Component Coupling of Aldehyde, Alkyne, and Amine, Org. Lett., 2003, 5, 4473–4475 CrossRef CAS PubMed.
- C. Wei and C.-J. Li, A Highly Efficient Three-Component Coupling of Aldehyde, Alkyne, and Amines via C−H Activation Catalyzed by Gold in Water, J. Am. Chem. Soc., 2003, 125, 9584–9585 CrossRef CAS PubMed.
- M.-B. Li, S.-K. Tian and Z. Wu, Catalyzed formation of α,β-unsaturated ketones or aldehydes from propargylic acetates by a recoverable and recyclable nanocluster catalyst, Nanoscale, 2014, 6, 5714 RSC.
- Y.-Z. Li and W. K. Leong, A Comparative Study on Atomically Precise Au Nanoclusters as Catalysts for the Aldehyde-Alkyne-Amine (A3) Coupling Reaction: Ligand Effects on the Nature of the Catalysis and Efficiency, RSC Adv., 2019, 9, 5475–5479 RSC.
- Y. Adachi, H. Kawasaki, T. Nagata and Y. Obora, Thiolate-protected Gold Nanoclusters Au25(phenylethanethiol)18: An Efficient Catalyst for the Synthesis of Propargylamines from Aldehydes, Amines, and Alkynes, Chem. Lett., 2016, 45, 1457–1459 CrossRef CAS.
- Y. Chen, C. Liu, H. Abroshan, Z. Li, J. Wang, G. Li and M. Haruta, Phosphine/Phenylacetylide-Ligated Au Clusters for Multicomponent Coupling Reactions, J. Catal., 2016, 340, 287–294 CrossRef CAS.
- Q. Li, A. Das, S. Wang, Y. Chen and R. Jin, Highly Efficient Three-Component Coupling Reaction Catalysed by Atomically Precise Ligand-Protected Au38(SC2H4Ph)24 Nanoclusters, Chem. Commun., 2016, 52, 14298–14301 RSC.
- H. Qian, W. T. Eckenhoff, Y. Zhu, T. Pintauer and R. Jin, Total Structure Determination of Thiolate-Protected Au38 Nanoparticles, J. Am. Chem. Soc., 2010, 132, 8280–8281 CrossRef CAS PubMed.
- X. Nie, C. Zeng, X. Ma, H. Qian, Q. Ge, H. Xe and R. Jin, CeO2-Supported Au38SR24 Nanocluster Catalysts for CO Oxidation: A Comparison of Ligand-on and -off Catalysts, Nanoscale, 2013, 5, 5912–5918 RSC.
- X. Wan, W. W. Xu, S. Yuan, Y. Gao, X. Zeng and Q. A. Wang, A Near–Infrared–Emissive Alkynyl–Protected Au24 Nanocluster, Angew. Chem., 2015, 127, 9819–9822 CrossRef.
- Y.-Z. Li, R. Ganguly, K. Y. Hong, Y. Li, M. E. Tessensohn, R. Webster and W. K. Leong, Stibine-protected Au13 nanoclusters: syntheses, properties and facile conversion to GSH-protected Au25 nanocluster, Chem. Sci., 2018, 9, 8723–8730 RSC.
- M. Li, S. Tian and Z. Wu, Improving the Catalytic Activity of Au25 Nanocluster by Peeling and Doping, Chin. J. Chem., 2017, 35, 567–571 CrossRef CAS.
- M.-B. Li, S.-K. Tian, Z. Wu and R. Jin, Peeling the Core–Shell Au25 Nanocluster by Reverse Ligand-Exchange, Chem. Mater., 2016, 28, 1022–1025 CrossRef CAS.
- Q. Shi, Z. Qin, G. Ping, S. Liu, H. Xu and G. Li, Alkynyl- and Phosphine-Ligated Quaternary Au2Ag2Clusters Featuring an Alkynyl-AuAg Motif for Multicomponent Coupling, RSC Adv., 2020, 10, 21650–21655 RSC.
- X. Kang, H. Abroshan, S. Wang and M. Zhu, Free Valence Electron Centralization Strategy for Preparing Ultrastable Nanoclusters and Their Catalytic Application, Inorg. Chem., 2019, 58, 11000–11009 CrossRef CAS PubMed.
- H. Yang, Y. Wang, H. Huang, L. Gell, L. Lehtovaara, S. Molola, H. Hakkinen and N. Zheng, All-thiol-stabilized Ag44 and Au12Ag32 nanoparticles with single-crystal structures, Nat. Commun., 2013, 4, 2422 CrossRef PubMed.
- G. Soldan, M. A. Aljuhani, M. S. Bootharaju, L. G. Abdulhalim, M. R. Parida, A. Emwas, O. F. Mohammed and O. M. Bakr, Gold Doping of Silver Nanoclusters: A 26−Fold Enhancement in the Luminescence Quantum Yield, Angew. Chem., Int. Ed., 2016, 55, 5749–5753 CrossRef CAS PubMed.
- D. Yu and Y. Zhang, Copper–Catalyzed Three–Component Coupling of Terminal Alkyne, Dihalomethane and Amine to Propargylic Amines, Adv. Synth. Catal., 2011, 353, 163–169 CrossRef CAS.
- S. Zeng, S. Xu, Y. Wang, M. Yu, L. Zhu and X. Yao, Copper Nanoparticles Catalyzed Three-Component Coupling of Alkyne, Dihalomethane and Amine for the Synthesis of Propargylic Amine, Chin. J. Org. Chem., 2015, 35, 827 CrossRef CAS.
- M. Rahman, A. Kr. Bagdi, A. Majee and A. Hajra, Nano indium oxide catalyzed efficient synthesis of propargylamines via C–H and C–Cl bond activations, Tetrahedron Lett., 2011, 52, 4437–4439 CrossRef CAS.
- J. Gao, Q.-W. Song, L.-N. He, Z.-Z. Yang and X.-Y. Dou, Efficient iron(III)-catalyzed three-component coupling reaction of alkynes, CH2Cl2 and amines to propargylamines, Chem. Commun., 2012, 48, 2024 RSC.
- Y. Tang, T. Xiao and L. Zhou, Cobalt-catalyzed alkyne–dihalomethane–amine coupling: an efficient route for propargylamines, Tetrahedron Lett., 2012, 53, 6199–6201 CrossRef CAS.
- X. Chen, T. Chen, Y. Zhou, C.-T. Au, L.-B. Han and S.-F. Yin, Efficient synthesis of propargylamines from terminal alkynes, dichloromethane and tertiary amines over silver catalysts, Org. Biomol. Chem., 2014, 12, 247–250 RSC.
- D. Aguilar, M. Contel and E. P. Urriolabeitia, Mechanistic Insights into the One–Pot Synthesis of Propargylamines from Terminal Alkynes and Amines in Chlorinated Solvents Catalyzed by Gold Compounds and Nanoparticles, Chem. – Eur. J., 2010, 16, 9287–9296 CrossRef CAS PubMed.
- A. Berrichi, R. Bachir, M. Benabdallah and N. Choukchou-Braham, Supported nano gold catalyzed three-component coupling reactions of amines, dichloromethane and terminal alkynes (AHA), Tetrahedron Lett., 2015, 56, 1302–1306 CrossRef CAS.
- V. S. Rawat, T. Bathini, S. Govardan and B. Sreedhar, Catalyst-free activation of methylene chloride and alkynes by amines in a three-component coupling reaction to synthesize propargylamines, Org. Biomol. Chem., 2014, 12, 6725 RSC.
- J. Yuan, Y. Yun, Z. Tao, Y. Zhu, L. Li, H. Sheng and M. Zhu, Atomically Precise Cun(n=3, 6 and 11) Nanocatalysts for Alkyne-Haloalkane-Amine (AHA) Coupling Reaction, Eur. J. Inorg. Chem., 2022, 2022, e202200159 CrossRef CAS.
- M. Amini, M. Nikkhoo, S. B. Tekantappeh, S. M. F. Farnis, G. Mahmoudi and O. Buyukgungor, Synthesis, Characterization and Catalytic Properties of a Copper Complex Containing Decavanadate Nanocluster, Na2[Cu(H2O)6]2{V10O28}4H2O, Inorg. Chem. Commun., 2017, 77, 72–76 CrossRef CAS.
- X. Gao, S. He, C. Zhang, C. Du, X. Chen, W. Xing, S. Chen, A. Clayborne and W. Chen, Single Crystal Sub–Nanometer Sized Cu6(SR)6 Clusters: Structure, Photophysical Properties, and Electrochemical Sensing, Adv. Sci., 2016, 3, 1600126 CrossRef PubMed.
- G. O. Nevstad, J. Songstad, B. Rodriguez, L. Morch and T. Norin, Solvent Properties of Dichloromethane. II. The Reactivity of Dichloromethane Toward Amines, Acta Chem. Scand., Ser. B, 1984, 38, 469–477 CrossRef.
- Z. Li, W. Li, H. Abroshan, Q. Ge, G. Li and R. Jin, Dual Effects of Water Vapor on Ceria-Supported Gold Clusters, Nanoscale, 2018, 10, 6558–6565 RSC.
- S. Guo, S. Zhang, Q. Fang, H. Abrosha, H. J. Kim, M. Haruta and G. Li, Gold–Palladium Nanoalloys Supported by Graphene Oxide and Lamellar TiO2 for Direct Synthesis of Hydrogen Peroxide, ACS Appl. Mater. Interfaces, 2018, 10, 40599–40607 CrossRef CAS PubMed.
- A. Nijamudheen and A. Datta, Mechanism for C–I Bond Dissociation in Iodoethane, Iodobenzene, and Iodoethene for the C–C Cross Coupling Reactions over Gold Clusters, J. Phys. Chem. C, 2013, 117, 21433–21440 CrossRef CAS.
- Z. Zuo, D. T. Ahneman, L. Chu, J. A. Terrett, A. G. Doyle and D. W. C. Macmillan, Merging photoredox with nickel catalysis: Coupling of α-carboxyl sp3-carbons with aryl halides, Science, 2014, 345, 437–440 CrossRef CAS PubMed.
- M. T. Pirnot, D. A. Rankic, D. B. C. Martin and D. W. C. Macmillan, Photoredox Activation for the Direct -Arylation of Ketones and Aldehydes, Science, 2013, 339, 1593–1596 CrossRef CAS PubMed.
- X. Zhang and D. W. C. MacMillan, Direct Aldehyde C–H Arylation and Alkylation via the Combination of Nickel, Hydrogen Atom Transfer, and Photoredox Catalysis, J. Am. Chem. Soc., 2017, 139, 11353–11356 CrossRef CAS PubMed.
- E. Mao and D. W. C. MacMillan, Late-Stage C(sp3)–H Methylation of Drug Molecules, J. Am. Chem. Soc., 2023, 145, 2787–2793 CrossRef CAS PubMed.
- K. Kapłon, S. Frynas, B. Mirosław, J. Lipkowski and O. M. Demchuk, An Efficient Asymmetric Cross-Coupling Reaction in Aqueous Media Mediated by Chiral Chelating Mono Phosphane Atropisomeric Biaryl Ligand, Catalysts, 2023, 13, 353 CrossRef.
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities, Chem. Rev., 2016, 116, 10346–10413 CrossRef CAS PubMed.
- I. Chakraborty and T. Pradeep, Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles, Chem. Rev., 2017, 117, 8208–8271 CrossRef CAS PubMed.
- Y. Zeng, S. Havenridge, M. Gharib, A. Baksi, K. L. D. M. Weerawardene, A. R. Ziefub, C. Strelow, C. Rehbock, A. Mews, S. Barcikowski, M. M. Kappes, W. J. Parak, C. M. Aikens and I. Chakraborty, Impact of Ligands on Structural and Optical Properties of Ag 29 Nanoclusters, J. Am. Chem. Soc., 2021, 143, 9405–9414 CrossRef CAS PubMed.
- Y.-M. Li, J. Hu and M. Zhu, Confining atomically precise nanoclusters in metal–organic frameworks for advanced catalysis, Coord. Chem. Rev., 2023, 495, 215364 CrossRef CAS.
- Y. Deng, Z. Zhang, P. Du, X. Ning, Y. Wang, D. Zhang, J. Liu, S. Zhang and X. Lu, Embedding Ultrasmall Au Clusters into the Pores of a Covalent Organic Framework for Enhanced Photostability and Photocatalytic Performance, Angew. Chem., Int. Ed., 2020, 59, 6082–6089 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2024 |