Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
π-extended pyrenes: from an antiaromatic buckybowl to doubly curved nanocarbons with gulf architectures†
Binbin
Liu‡
a,
Zhengxiong
Jin‡
a,
Xinyue
Liu
a,
Lanfei
Sun
c,
Cao
Yang
*b and
Lei
Zhang
 *a
*a
aBeijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: zhl@mail.buct.edu.cn
bSchool of Materials Science and Engineering, The Key Laboratory of Material Processing and Mold of Ministry of Education, Henan Key Laboratory of Advanced Nylon Materials and Application, Zhengzhou University, Zhengzhou 450001, P. R. China. E-mail: yc321@zzu.edu.cn
cShandong North Modern Chemistry Industry Co., Ltd, Jinan 252300, P. R. China
First published on 16th September 2024
Abstract
The synthesis of π-extended pyrenes keeps attracting considerable attention. In particular, frameworks containing nonbenzenoid rings might display intriguing properties. Here, we report a practical synthetic pathway to access a new buckybowl (1), which is composed of four five-membered rings externally fused to a pyrene core. The buckybowl 1 exhibits antiaromaticity involving 22 π-electrons, a rapid bowl-to-bowl interconversion, and a small band gap. Furthermore, this buckybowl could be subjected to Scholl cyclodehydrogenation to prepare the doubly curved nanocarbons (2rac and 2meso), which exist as two diastereomers, as demonstrated by X-ray crystal structure determination. Variable temperature 1H NMR measurements reveal that 2meso can isomerize into 2rac under thermal conditions, with an activation free energy of 27.1 kcal mol−1. Both the enantiomers of 2rac can be separated by chiral HPLC and their chiroptical properties are thoroughly examined. In addition, the nanocarbon 2meso with two gulf architectures facilitates host–guest chemistry with a variety of guests, including PDI, TDI, C60 and C70.
Introduction
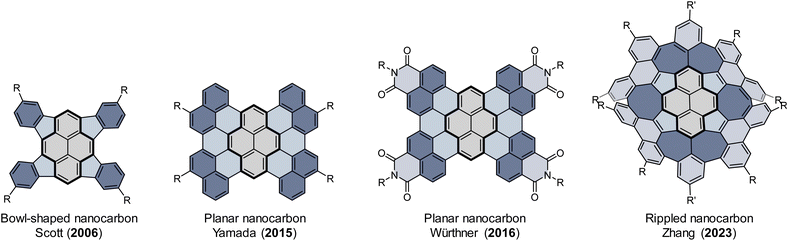
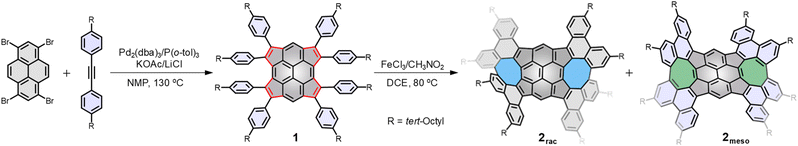
Incorporating nonbenzenoid rings into sp2 hexagonal nanographenes has emerged as a promising strategy to construct curved polycyclic aromatic hydrocarbons (PAHs).1–5 For example, the introduction of five-membered rings (pentagons) into a hexagonal network typically causes positive Gaussian curvature, leading to bowl-shaped PAHs (buckybowls or π-bowls),6–12 while seven- or eight-membered rings induce negative Gaussian curvature, leading to saddle-shaped PAHs.13–18 Recently, significant attention has focused on curved PAHs comprising a combination of different nonbenzenoid rings, which display both positive and negative curvatures.19–23 These nanostructures are attractive components of optoelectronic devices due not only to their unique electronic and photophysical properties, which result from the internal charge transfer and aromaticity between the differently sized rings,24–27 but also to their properties of molecular recognition towards various guests to assemble into highly ordered structures in the solid state.28–30 Additionally, the co-existence of both curved segments twists the molecular skeleton and induces chirality-an essential feature for some advancement in technological applications.31As the prototypical PAH, pyrene is among the most popular building blocks for complex nanocarbons.32 Increasing the size of pyrene by ring fusion or lateral extension of its π-system might result in novel electronic, photophysical, and supramolecular properties (Fig. 1).33–36 For example, Würthner reported the synthesis of a planar C64 nanographene, which is composed of four naphthalimide moieties fused to a pyrene core.37 This nanographene was shown to exhibit unique self-assembly with a variety of PAH guests as a result of the large π-surface and its bulky imide substituents.38,39 Although a growing number of π-extended pyrenes with fusion of benzenoid rings have been well-characterized,40–46 the π-extended pyrenes with fusion of nonbenzenoid rings are underexplored, presumably due to the σ-strain imposed on the pyrene skeleton from the externally fused nonbenzenoid rings.47–49 Very recently, we reported the synthesis of a rippled C84 molecular carbon, which contains ten nonbenzenoid rings that are contiguously fused to a pyrene core.50 These fused nonbenzenoid rings impart advantageous effects, such as high solubility, configurational stability, a narrow band gap, unique aromaticity, and ambipolar transport properties to the system. Herein we report the synthesis of a new buckybowl (1) and two doubly curved nanocarbons (2rac and 2meso) by the fusion of nonbenzenoid rings onto the pyrene core (Scheme 1). A palladium-catalyzed cyclopentannulation of 1,3,6,8-tetrabromopyrene with diphenylacetylenes afforded tetracyclopenta[cd,fg,jk,mn]pyrene (TPP), a bowl-shaped π system that was anticipated with potentially interesting antiaromatic properties.47,48 We choose the diphenylacetylenes as cyclopentannulation components because the resulting pendant aryl groups at the periphery of TPP not only protect the strained double bonds from unwanted reactions but are also enabled by the application of the Scholl reaction to give the desired nanocarbons, which are structurally fascinating, but their promise for structure dynamics investigation and binding affinity towards a series of guests are perhaps of greater interest.
Results and discussion
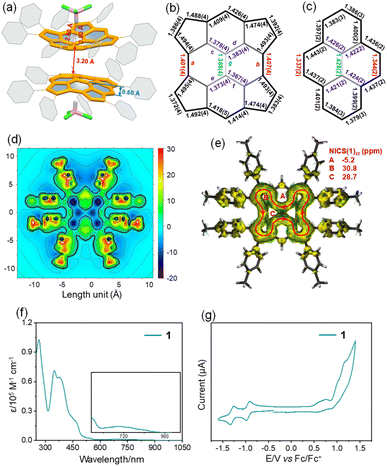
Scheme 1 shows the synthesis of 1, 2rac, and 2meso. The first key step was the generation of tetracyclopenta[cd,fg,jk,mn]pyrene 1, which was achieved by cyclopentannulation of 1,3,6,8-tetrabromopyrene with diphenylacetylenes by using Pd2(dba)3 as the catalyst with KOAc as the base and LiCl as the additive. This reaction process likely involves (1) reduction of the palladium(II) salt to Pd(0), (2) oxidative addition of the aryl bromide to Pd(0), (3) arylpalladation of the alkyne to produce an open vinylic palladium intermediate, which rapidly undergoes concerted metalation deprotonation to generate the desired cyclopentadienide derivative, and (4) reductive elimination of Pd(0) for catalytic recycling (Fig. S1†). This intermediate 1 bearing sterically demanding phenyl group, allows the final step to be carried out, oxidative C–C bond formation using FeCl3 at 80 °C to generate two quasi-[8]circulene moieties. As expected by looking at the structure of the double quasi-[8]circulenes,30 two possible diastereomers, consisting of chiral (P,P and M,M configurations) and meso (P,M configuration) forms,51 are synthetically feasible. Fortunately, we were able to isolate and identify them. The minor and major products were identified as the P,P/M,M (2rac) and M,P (2meso) configurations, respectively, which are unambiguously verified by single-crystal X-ray analysis.Single crystals of 1 suitable for X-ray diffraction analysis were obtained by slow diffusion of methanol into chloroform solution. Compound 1 crystallizes with chloroform molecules and displays bowl-shaped conformation (Fig. 2a). The bowl depth is measured to be 0.60 Å based on the original 2,7-positions of the pyrene, which is shallower than that of tetraindenopyrene36 (0.69 Å) calculated at the B3LYP/6-311G(d,p) level of theory. The presence of chloroform molecules prevents concave–convex stacking of 1 in the crystal. Instead, the molecules adopt a concave–concave dimeric stacking model, which is based on multiple C–H⋯π (2.80 Å) and C⋯C (3.20 Å) contacts. The C–C double bonds in the five-membered rings are nearly homogeneous ranging from 1.372(4) to 1.392(4) Å, larger than that of the typical olefins (1.350 Å), and the two C–C bonds linking each of these double bonds are much longer (1.474(4)–1.495(4) Å), indicating that the C–C double bonds have a localized olefinic character and may participate only in perimeter delocalization (Fig. 2b). The bonds a and b in 1 (1.401(4) and 1.407(4) Å) are much longer than those bonds in the pyrene substructure52 (1.334(2) and 1.337(2) Å), while the bonds c, d, e, f, and g are observed to be considerably shorter in 1 (1.367(4)–1.388(4) Å) relative to the pyrene substructure (1.421(2)–1.428(2) Å) (Fig. 2c).
2D isochemical shielding surface (ICSS) (Fig. 2d) shows a strongly deshielded chemical environment in the inner planes of four five-membered rings and two vertical six-membered rings in 1, which are also quantified by the nucleus-independent chemical shift (NICS), suggesting the strong antiaromaticity of these rings. In line with this, the current induced density (ACID) plot (Fig. 2e) reveals a paratropic current on the periphery of 1, which involves 22 π-electrons and disobeys any of the known aromaticity rules.53 However, taking Clar's model into consideration, this could be explained by the fact that there are ten equal resonance structures of 1, and each contains two Clar's sextets with a local conjugated circuit involving 16 π-electrons that agrees with Hückel's rule predicting its antiaromaticity (Fig. S2 and S3†).50 Since they are degenerate, the resulting conjugation is their interferences and combinations. These results are also validated with the UV-vis spectrum of 1, which shows much stronger absorption in the high energy spectral region (300–400 nm), but with a broad, low energy absorbance tail that extends into 960 nm (Fig. 2f), in agreement with its antiaromatic character.54 Time-dependent density functional theory (TD-DFT) calculations at the B3LYP/6-31G(d,p) level of theory predict that the low-energy absorbance tail corresponds to the symmetry forbidden of the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO) transition (λcalc = 960 nm, f = 0.0001) (Fig. S4 and Table S1†). Similar to cyclopenta-fused PAHs,551 shows almost no emission in solution and in the solid state, likely due to the presence of the cyclopenta-fused rings in 1, which might increase the rate of intersystem crossing.56 In addition, 1 in dichloromethane exhibits two reversible reduction and multiple irreversible oxidation waves (Fig. 2g). The presence of two reversible reduction waves at low potentials (Ered1/2 = −0.89 V vs. Fc/Fc+) is attributed to its high electron-accepting capacity to form cyclopentadienyl-like anions by accepting electrons, affording 4n + 2 aromatic systems.57,58
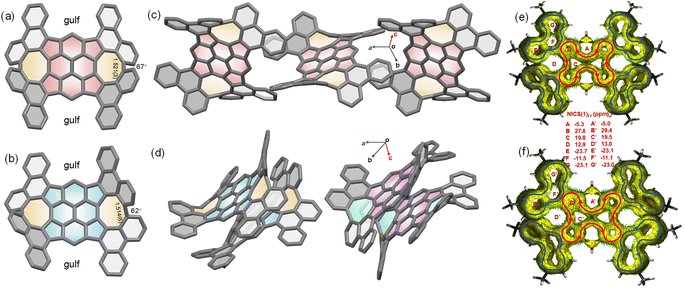
The crystals suitable for X-ray diffraction analysis were obtained by diffusion of methanol into chlorobenzene (2meso) and toluene (2rac). Both 2rac and 2meso display a doubly curved conformation, while much of the curvature is created by the disposition of the four phenanthrene subunits (Fig. 3a and b). In both cases, two phenanthrene subunits are directed to one side of the TPP core and the other two are directed to the other side, giving rise to the concave surfaces. The four phenanthrene subunits appear to be locked into a fixed orientation because of the steric interactions between the hydrogens of quasi-[8]circulenes. However, the central TPP cores in 2rac and 2meso are not bowl-shaped, as predicted by gas-phase density functional theory (DFT) calculations, but rather have a nearly planar conformation (Fig. S5†). This, however, is understandable in the TPP core because the two conformations interconvert through a vanishingly small energy barrier (Tables S2 and S3†). Thus, the preference in the crystal for the planar conformation is a result of packing forces. It is observed that the average bond lengths in TPP cores of 1, 2rac, and 2meso are essentially identical. The torsion angles of quasi-[8]circulenes in 2rac and 2meso are 62° and 67°, respectively, which could account for the elongated bond lengths in quasi-[8]circulenes, leading to the weak conjugation between the neighbouring phenanthrene subunits. In the crystal of 2meso, the presence of gulf renders an orthogonal arrangement of the neighbouring 2meso, which, in turn, leads to a similar, chainlike fashion (Fig. 3c). These 2meso chains pack loosely in the solid state and no any π–π interactions are observed. For 2rac, the crystal is a racemate composed of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of the enantiomers. The two neighbouring molecules adopt a homochiral dimer motif through C⋯C contacts. Each homochiral dimer engages in C–H⋯π interactions with the neighbouring enantiomeric dimers (Fig. 3d).
1 mixture of the enantiomers. The two neighbouring molecules adopt a homochiral dimer motif through C⋯C contacts. Each homochiral dimer engages in C–H⋯π interactions with the neighbouring enantiomeric dimers (Fig. 3d).
The calculated NICS values together with ACID plots for 2rac and 2meso show aromaticity in four phenanthrene subunits and antiaromaticity in the TPP core (Fig. 3e and f). Thus, five local ring currents are observed in 2rac and 2meso, which include four diatropic currents exclusively in the four phenanthrene subunits and one paratropic current in the TPP core, consistent with the weak interaction between phenanthrene subunits. According to the NICS values, the degree of aromaticity is nearly identical in 2rac and 2meso.
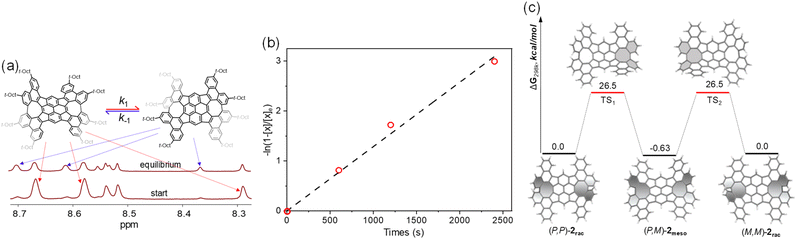
Furthermore, a control experiment was performed to evaluate the interconversion between 2rac and 2meso. Solution of pure 2meso was prepared in 1,1,2,2-tetrachloroethane-d2 in a sealed NMR tube and heated at 80 °C, and the signals assigned to the isomer 2rac began to emerge with time (Fig. 4a and S6†). After one hour, a solution containing each pure isomer comes to a final 2meso/2rac equilibrium state, the ratio of which is determined to be 1/0.7. Accordingly, the free energy ΔG‡ for the isomerization is determined to be 1.05 kJ mol−1, indicating that 2rac is less stable. The forward (k1) and reverse (k−1) rate constants can be estimated by using the equation −ln(1 – [x]/[x]e) = (k1 + k−1)t, here, [x] is the concentration of 2meso that has been depleted at a certain time, and [x]e is defined as [x] at the equilibrium state (Fig. 4b). Plotting −ln(1 – [x]/[x]e) versus time gives the equilibrium constant k1/k−1 of 1.4, and the rate constants k1 and k−1 at 80 °C of 5.3 × 10−4 s−1 and 7.6 × 10−4 s−1, respectively. Fitting the data by using the Eyring equation gives the activation free energy ΔG‡ of 26.1 kcal mol−1 for 2meso to 2rac isomerization, which is in agreement with the calculated ΔG‡ for isomerization (27.1 kcal mol−1). The DFT calculations also confirm that the racemization process between (P,P)-2rac and (M,M)-2rac proceeds via the intermediate (P,M)-2meso, with the racemization barrier of 26.5 kcal mol−1 (Fig. 4c and Table S4†). As expected, the racemic 2rac was readily resolved into two enantiomers by chiral high-performance liquid chromatography (HPLC) at room temperature (Fig. S7†).
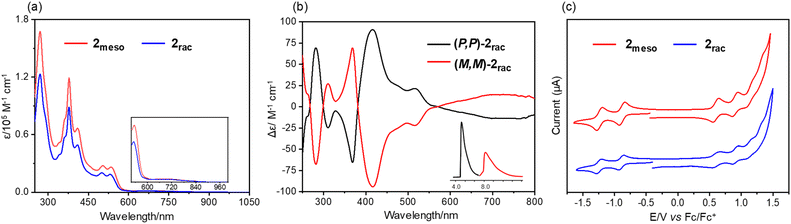
The UV-vis absorption spectrum of 2meso in chloroform features four characteristic absorption peaks at 378 nm, 410 nm, 503 nm, and 535 nm (Fig. 5a). The spectrum of 2rac was slightly blue-shifted but comparable to 2meso, with absorption peaks at 377 nm, 408 nm, 500 nm, and 530 nm. In addition, both compounds display a very low-intensity absorption tail extending to 960 nm. For 2rac and 2meso, TD-DFT calculations suggest that the low-energy absorbance tails originate from the symmetry forbidden HOMO to LUMO transition (Tables S5 and S6†). The shorter wavelength bands for 2rac and 2meso are also in accordance with the simulated spectra from the TD-DFT calculations. Similar to 1, 2rac and 2meso show almost no emission in solution and in the solid state. Furthermore, the chiroptical properties of the enantiomers of 2rac were investigated by circular dichroism (CD) measurement (Fig. 5b). The absolute configurations of the enantiomers were assigned using CD spectroscopy assisted by TD-DFT calculations (Fig. S8†). Multiple CD bands are present across the whole spectral range with |Δε| ∼ 10–85 M−1 cm−1. The Cotton effects correspond to the lowest energy absorption band with moderately strong chirooptical properties, as determined by the absorption anisotropy factor, |g| = |Δε|/ε = 0.012 at λ = 725 nm. Analysis of the electrochemical behaviours of 2rac and 2meso reveals two reversible reduction peaks, two reversible oxidation peaks, and two pseudoreversible oxidation peaks (Fig. 5c). The first oxidation and reduction potentials of 2rac and 2meso are almost identical, occurring at 0.50/−0.83 V and 0.53/−0.81 V (vs. Fc/Fc+), respectively, which are comparable to those of compound 1 (0.47/−0.89 V). The DFT-calculated energy levels are consistent with the trend of experimental values (Fig. S9†). Accordingly, the electrochemical HOMO–LUMO energy gaps are calculated to be 1.36 eV for 1, 1.34 eV for 2meso, and 1.33 eV for 2rac.
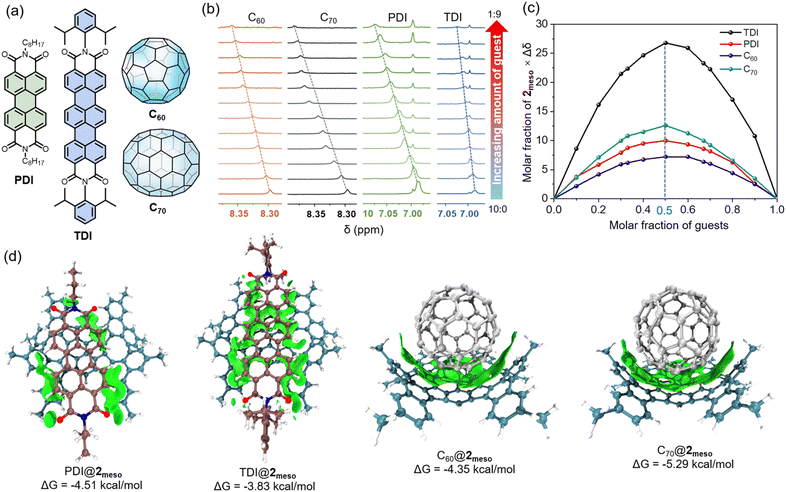
The unique gulf architecture and electron-rich concave surface of 2meso indicate that this nanocarbon might be able to host a range of electron-poor guests. The planar N,N′-dioctyl-3,4,9,10-perylenedicarboximide (PDI) was selected as a representative guest (Fig. 6a). The 1H NMR titration revealed that both aromatic protons on 2meso and PDI gradually changed upon adding PDI, indicating the presence of intermolecular interactions between 2meso and PDI (Fig. 6b and S10†). The Job plot based on the titration indicates the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex formation with the binding constant (Ka) of 2019 M−1 (Fig. 6c, S11 and S12†). In addition, a 2D 1H–1H NOESY spectrum confirmed the through-space correlations between four aromatic protons of PDI and the protons of phenanthrene subunits of 2meso (Fig. S13†). Furthermore, the other three guests, N,N′-di(2,6-diisopropylphenyl)-3,4,11,12 terrylenedicarboximide (TDI), C60, and C70 were selected to explore the binding ability of 2meso towards guests with different shapes and electronic natures. The titration data versus2meso for guests were fitted successfully to a 1
1 complex formation with the binding constant (Ka) of 2019 M−1 (Fig. 6c, S11 and S12†). In addition, a 2D 1H–1H NOESY spectrum confirmed the through-space correlations between four aromatic protons of PDI and the protons of phenanthrene subunits of 2meso (Fig. S13†). Furthermore, the other three guests, N,N′-di(2,6-diisopropylphenyl)-3,4,11,12 terrylenedicarboximide (TDI), C60, and C70 were selected to explore the binding ability of 2meso towards guests with different shapes and electronic natures. The titration data versus2meso for guests were fitted successfully to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding mode (Fig. 6b and c), yielding binding constants of 643 M−1 for TDI (Fig. S14–S16†), 1546 M−1 for C60 (Fig. S17–S19†), and 7361 M−1 for C70 (Fig. S20–S22†). Accordingly, the Gibbs free energy (ΔG) is calculated to be −4.51 kcal mol−1 for PDI@2meso, −3.83 kcal mol−1 for TDI@2meso, −4.35 kcal mol−1 for C60@2meso, and −5.29 kcal mol−1 for C70@2meso, respectively.
1 binding mode (Fig. 6b and c), yielding binding constants of 643 M−1 for TDI (Fig. S14–S16†), 1546 M−1 for C60 (Fig. S17–S19†), and 7361 M−1 for C70 (Fig. S20–S22†). Accordingly, the Gibbs free energy (ΔG) is calculated to be −4.51 kcal mol−1 for PDI@2meso, −3.83 kcal mol−1 for TDI@2meso, −4.35 kcal mol−1 for C60@2meso, and −5.29 kcal mol−1 for C70@2meso, respectively.
While X-ray crystal structures of the host–guest complexes could not be obtained, the relative stability of the conformers of the complexes was assessed by DFT calculations at the B3LYP/6-31G(d,p) level of theory (Fig. 6d). For PDI@2meso, the complex is formed by non-covalent interactions between the perylene core and the concave surface of 2meso, whereas in TDI@2meso, the terrylene core interacts with the concave surface of 2meso and the concave surface of the TPP core. DFT calculations also reveal short intermolecular contacts arising from C–H⋯O interactions between the C–Hs to the alkyl chains and carbonyl groups on adjacent PDI and TDI. The octyl and 2,6-diisopropylphenyl substituents at the imide positions occupy the gulf positions to alleviate the unfavourable electrostatic interactions found in neighbouring molecules. For C60@2meso and C70@2meso, Both C60 and C70 interact with the concave surface of 2meso and the concave surface of the TPP core. In C70@2meso, 2meso exhibits a preferential spatial alignment, which offers a perfect complementary shape and curvature match for the elongated side of C70. The distances between the centroids of the TPP core and C60 and C70 are 6.60 and 6.70 Å, respectively.
Conclusions
In conclusion, we have reported the synthesis of a new antiaromatic buckybowl via a facile palladium-catalyzed cyclopentannulation. This buckybowl can be further transformed to doubly curved nanocarbons by utilizing a Scholl cyclodehydrogenation. The two quasi-[8]circulene moieties in the newly synthesized nanocarbons lead to two diastereomers (2rac and 2meso), consisting of chiral and meso forms, respectively. DFT calculations and experimental studies reveal 2meso as the more stable isomer that can isomerize into 2rac at elevated temperature. The enantiomers of 2rac were separated by HPLC and the chiroptical properties were investigated with CD measurement. The nanocarbons exhibit unique doubly curved conformations, which possess intriguing host–guest properties, making them attractive for both supramolecular chemistry and more complex materials. These results indicate that incorporating nonbenzenoid rings into sp2 carbon nanostructures is a viable strategy to not only fundamentally influence both optoelectronic and supramolecular properties but also offer extensive structure elaboration.Data availability
Detailed synthesis and characterization of the related compounds, crystal data for 1, 2meso, and 2rac (Table S7† and Fig. S23–S25†), 1H NMR, 13C NMR, high-resolution mass spectra (HRMS) of the related compounds (Fig. S26–S35†), CV and UV measurements, NMR titration, and theoretical calculation details are available in the ESI.†Author contributions
L. Z and C. Y received and designed the project. B. L and Z. J synthesized and characterized the compounds. X. L and L. S performed DFT calculations. All authors discussed the results and contributed to the manuscript preparation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2022YFC2106100) and the National Natural Science Foundation of China (NSFC) (22175013).Notes and references
- M. Rickhaus, M. Mayor and M. Juríček, Chem. Soc. Rev., 2017, 46, 1643 RSC.
- S. H. Pun and Q. Miao, Acc. Chem. Res., 2018, 51, 1630 CrossRef PubMed.
- C. Chaolumen, I. A. Stepek, K. E. Yamada, H. Ito and K. Itami, Angew. Chem., Int. Ed., 2021, 60, 23508 Search PubMed.
- M.-W. Wang, W. Fan, X. Li, Y. Liu, Z. Li, W. Jiang, J. Wu and Z. Wang, ACS Nano, 2023, 17, 20734 CrossRef PubMed.
- Y. Zhang, S. H. Pun and Q. Miao, Chem. Rev., 2022, 122, 14554 CrossRef PubMed.
- Y. Wu and J. S. Siegel, Chem. Rev., 2006, 106, 4843 CrossRef PubMed.
- Y. Wu and J. S. Siegel, Top. Curr. Chem., 2014, 349, 63 CrossRef CAS PubMed.
- M. Saito, H. Shinokubo and H. Sakurai, Mater. Chem. Front., 2018, 2, 635 RSC.
- E. Nestoros and M. C. Stuparu, Chem. Commun., 2018, 54, 6503 RSC.
- G. Gao, M. Chen, J. Roberts, M. Feng, C. Xiao, G. Zhang, S. Parkin, C. Risko and L. Zhang, J. Am. Chem. Soc., 2020, 142, 2460 CrossRef CAS PubMed.
- M. Chen, Y. Duan, X. Liu, Q. Zhan, H. Hayashi, K. Matsuo, H. Yamada, G. Gao, Y. Zheng and L. Zhang, CCS Chem., 2024, 6, 353 CrossRef.
- X. Li, X. Li, F. Kang and M. Inagaki, Small, 2016, 12, 3206 CrossRef PubMed.
- G. González Miera, S. Matsubara, H. Kono, K. Murakami and K. Itami, Chem. Sci., 2022, 13, 1848 RSC.
- S. Matsubara, Y. Koga, Y. Segawa, K. Murakami and K. Itami, Nat. Catal., 2020, 3, 710 CrossRef.
- I. R. Márquez, S. Castro-Fernández, A. Millán and A. G. Campaña, Chem. Commun., 2018, 54, 6705 RSC.
- J. Wang, F. G. Gámez, J. Marín-Beloqui, A. Diaz-Andres, X. Miao, D. Casanova, J. Casado and J. Liu, Angew. Chem., Int. Ed., 2023, 62, e202217124 CrossRef PubMed.
- K. Y. Cheung, X. Xu and Q. Miao, J. Am. Chem. Soc., 2015, 137, 3910 CrossRef PubMed.
- C. M. Cruz, I. R. Márquez, S. Castro-Fernández, J. M. Cuerva, E. Maçôas and A. G. A Campaña, Angew. Chem., Int. Ed., 2019, 58, 8068 CrossRef PubMed.
- K. Kawasumi, Q. Zhang, Y. Segawa, L. T. Scott and K. Itami, Nat. Chem., 2013, 5, 739 CrossRef.
- T. Kirschbaum, F. Rominger and M. A. Mastalerz, Angew. Chem., Int. Ed., 2020, 59, 270 CrossRef.
- H. Luo and J. Liu, Angew. Chem., Int. Ed., 2023, 62, e202302761 CrossRef PubMed.
- M. Krzeszewski, Ł. Dobrzycki, A. L. Sobolewski, M. K. Cyrański and D. T. Gryko, Angew. Chem., Int. Ed., 2021, 60, 14998 CrossRef CAS.
- J. M. Fernández-García, P. J. Evans, S. M. Rivero, I. Fernández, D. García-Fresnadillo, J. Perles, J. Casado and N. J. Martín, Am. Chem. Soc., 2018, 140, 17188 CrossRef PubMed.
- E. C. Rascon, A. Riss, A. Matěj, A. Wiengarten, P. Mutombo, D. Soler, P. Jelinek and W. Auwärter, J. Am. Chem. Soc., 2023, 145, 967 CrossRef CAS PubMed.
- B. Mallada, B. de la Torre, J. I. Mendieta-Moreno, D. Nachtigallová, A. Matěj, M. Matoušek, P. Mutombo, J. Brabec, L. Veis, T. Cadart, M. Kotora and P. Jelínek, J. Am. Chem. Soc., 2021, 143, 14694 CrossRef CAS.
- J. Liu, S. Mishra, C. A. Pignedoli, D. Passerone, J. Urgel, A. Fabrizio, T. G. Lohr, J. Ma, H. Komber, M. Baumgarten, C. Corminboeuf, R. Berger, P. Ruffieux, K. Müllen, R. Fasel and X. Feng, J. Am. Chem. Soc., 2019, 141, 12011 CrossRef CAS PubMed.
- Y. Fei, Y. Fu, X. Bai, L. Du, Z. Li, H. Komber, K.-H. Low, S. Zhou, D. L. Phillips, X. Feng and J. Liu, J. Am. Chem. Soc., 2021, 143, 2353 CrossRef CAS.
- J. Liu, J. Hong, Z. Liao, J. Tan, H. Liu, E. Dmitrieva, L. Zhou, J. Ren, X.-Y. Cao, A. A. Popov, Y. Zou, A. Narita and Y. Hu, Angew. Chem., Int. Ed., 2024, 63, e202400172 CrossRef CAS.
- S. Zank, J. M. Fernández-García, A. J. Stasyuk, A. A. Voityuk, M. Krug, M. Solà, D. M. Guldi and N. Martín, Angew. Chem., Int. Ed., 2022, 61, e202112834 CrossRef CAS.
- Y. Duan, M. Chen, H. Hayashi, H. Yamada, X. Liu and L. Zhang, Chem. Sci., 2023, 14, 10420 RSC.
- J. R. Brandt, F. Salerno and M. J. Fuchter, Nat. Rev. Chem, 2017, 1, 0045 CrossRef.
- T. M. Figueira-Duarte and K. Müllen, Chem. Rev., 2011, 111, 7260 CrossRef.
- W. Yang, J. H. S. K. Monteiro, A. de Bettencourt-Dias, V. J. Catalano and W. A. Chalifoux, Angew. Chem., Int. Ed., 2016, 55, 10427 CrossRef PubMed.
- H. A. Wegner, H. Reisch, K. Rauch, A. Demeter, K. A. Zachariasse, A. de Meijere and L. T. Scott, J. Org. Chem., 2006, 71, 9080 CrossRef PubMed.
- A. Matsumoto, M. Suzuki, D. Kuzuhara, H. Hayashi, N. Aratani and H. Yamada, Angew. Chem., Int. Ed., 2015, 54, 8175 CrossRef PubMed.
- S. M. Elbert, A. Haidisch, T. Kirschbaum, F. Rominger, U. Zschieschang, H. Klauk and M. Mastalerz, Chem.–Eur. J., 2020, 26, 10585 CrossRef.
- S. Seifert, K. Shoyama, D. Schmidt and F. Würthner, Angew. Chem., Int. Ed., 2016, 55, 6390 CrossRef.
- M. A. Niyas, K. Shoyama and F. Würthner, Angew. Chem., Int. Ed., 2023, 62, e2023020 CrossRef PubMed.
- M. Mahl, M. A. Niyas, K. Shoyama and F. Würthner, Nat. Chem., 2022, 14, 457 CrossRef.
- Y. Li, Z. Jia, S. Xiao, H. Liu and Y. Li, Nat. Commun., 2016, 7, 11637 CrossRef.
- Y. Gu, R. Muñoz-Mármol, S. Wu, Y. Han, Y. Ni, M. A. Díaz-García, J. Casado and J. Wu, Angew. Chem., Int. Ed., 2020, 59, 8113 CrossRef PubMed.
- K. Ozaki, K. Kawasumi, M. Shibata, H. Ito and K. Itami, Nat. Commun., 2015, 6, 6251 CrossRef PubMed.
- R. K. Dubey, M. Melle-Franco and A. Mateo-Alonso, J. Am. Chem. Soc., 2021, 143, 6593 CrossRef CAS PubMed.
- F. Hernández-Culebras, M. Melle-Franco and A. Mateo-Alonso, Angew. Chem., Int. Ed., 2022, 61, e202205018 CrossRef.
- Y. Min, C. Dou, D. Liu, H. Dong and J. Liu, J. Am. Chem. Soc., 2019, 141, 17015 CrossRef.
- Y. Hu, G. M. Paternò, X.-Y. Wang, X.-C. Wang, M. Guizzardi, Q. Chen, D. Schollmeyer, X.-Y. Cao, G. Cerullo, F. Scotognella, K. Müllen and A. Narita, J. Am. Chem. Soc., 2019, 141, 12797 CrossRef.
- R. W. A. Havenith, H. Jiao, L. W. Jenneskens, J. H. van Lenthe, M. Sarobe, P. v. R. Schleyer, M. Kataoka, A. Necula and L. T. Scott, J. Am. Chem. Soc., 2002, 124, 2363 CrossRef PubMed.
- R. W. A. Havenith, J. H. van Lenthe, F. Dijkstra and L. W. Jenneskens, J. Phys. Chem. A, 2001, 105, 3838 CrossRef.
- J. Ma, Y. Fu, J. Liu and X. Feng, Beilstein J. Org. Chem., 2020, 16, 791 CrossRef CAS PubMed.
- B. Liu, M. Chen, X. Liu, R. Fu, Y. Zhao, Y. Duan and L. Zhang, J. Am. Chem. Soc., 2023, 145, 28137 CrossRef CAS.
- J. Luo, X. Xu, R. Mao and Q. Miao, J. Am. Chem. Soc., 2012, 134, 13796 CrossRef CAS PubMed.
- C. S. Frampton, K. S. Knight, N. Shankland and K. Shankland, J. Mol. Struct., 2000, 520, 29 CrossRef CAS.
- M. Solà, Nat. Chem., 2022, 14, 585 CrossRef PubMed.
- J.-Y. Shin, K. S. Kim, M.-C. Yoon, J. M. Lim, Z. S. Yoon, A. Osuka and D. Kim, Chem. Soc. Rev., 2010, 39, 2751 RSC.
- H. Dang, M. Levitus and M. A. Garcia-Garibay, J. Am. Chem. Soc., 2002, 124, 136 CrossRef CAS PubMed.
- B. F. Plummer, M. J. Hopkinson and J. H. Zoeller, J. Am. Chem. Soc., 1979, 101, 6779 CrossRef CAS.
- H. Dang and M. A. Garcia-Garibay, J. Am. Chem. Soc., 2001, 123, 355 CrossRef CAS PubMed.
- J. D. Wood, J. L. Jellison, A. D. Finke, L. Wang and K. N. Plunkett, J. Am. Chem. Soc., 2012, 134, 15783 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2353253 (for 1), 2353606 (for 2meso), and 2353623 (for 2rac). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc03460k |
| ‡ B. Liu and Z. Jin contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |