Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stereogenic-at-iron mesoionic carbene complex for enantioselective C–H amidation†
Nemrud
Demirel
,
Mahiob
Dawor
,
Greta
Nadler
,
Sergei I.
Ivlev
 and
Eric
Meggers
and
Eric
Meggers
 *
*
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Strasse 4, 35043 Marburg, Germany. E-mail: meggers@chemie.uni-marburg.de
First published on 10th September 2024
Abstract
Electronically tuned C2-symmetric stereogenic-at-iron complexes, featuring strongly σ-donating 1,2,3-triazolin-5-ylidene mesoionic carbene (MIC) ligands, exhibit enhanced catalytic efficiency compared to conventional imidazol-2-ylidene analogs, as demonstrated in nitrene-mediated ring-closing C(sp3)–H amidation reactions. Furthermore, a chiral pinene-derived pyridyl triazole ligand enables a highly diastereoselective synthesis of a non-racemic chiral iron catalyst, thereby controlling the absolute configuration at the metal center, as confirmed by NMR and X-ray crystallography. This pinene-modified stereogenic-at-iron MIC complex demonstrates high catalytic activity and a respectable asymmetric induction in the ring-closing C(sp3)–H amination of N-benzoyloxyurea, yielding 2-imidazolidinones with enantiomeric ratios of up to 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. These findings reflect the profound potential of this new class of mesoionic carbene iron complexes in further understanding and tuning the reactivity of iron-based catalysts.
8. These findings reflect the profound potential of this new class of mesoionic carbene iron complexes in further understanding and tuning the reactivity of iron-based catalysts.
Introduction
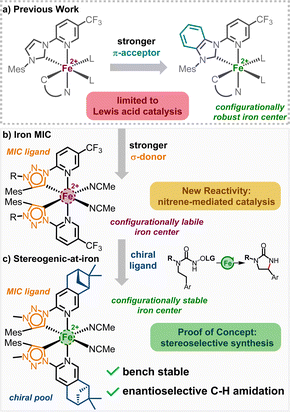
Iron-catalyzed reactions have recently gained significant attention in both academia and industry, driven by the increasing demand for nontoxic, sustainable, and cost-effective alternatives to commonly used precious metals.1–3 Given iron's versatility and the capabilities of its complexes in redox and radical chemistry, coupled with its high abundance in the Earth's crust, low toxicity, and its natural role as a catalytic metal, it is no surprise that iron emerges as a particularly promising candidate to fulfill these criteria. For example, the field of asymmetric iron catalysis has seen notable progress over the last two decades, resulting in a diverse array of chiral iron catalysts.4 However, for iron to rival its precious metal counterparts, numerous challenges persist, including improving catalytic performance, discovering new and unique catalytic processes, identifying new chiral iron catalyst scaffolds, and establishing economic syntheses for these chiral iron catalysts.With respect to the development of novel iron catalyst scaffolds, our group recently reported the first examples of chiral iron catalysts in which the overall chirality is exclusively due to a stereogenic iron center (Fig. 1a).5 This is a noteworthy accomplishment considering the general configurational lability of 3d-metals compared to its 4d- and 5d-congeners. In this chiral-at-iron catalyst scaffold, iron is coordinated by two chelating N-(2-pyridyl)-substituted N-heterocyclic carbenes (PyNHC) in a C2-symmetrical manner, resulting in a helical topology with an iron center possessing stereogenicity in either the Λ (left-handed helicity) or Δ (right-handed helicity) configuration.6–8
The coordination sphere is completed by two acetonitrile molecules, providing an overall octahedral geometry. In this arrangement, the PyNHC ligands are designed to be configurationally stable to preserve the stereochemical information, while the acetonitrile ligands are intended to be labile to facilitate catalysis. Configurational robustness was further improved by replacing the imidazol-2-ylidene carbene moieties with slightly more π-accepting benzimidazol-2-ylidenes.5c The obtained benzimidazol-2-ylidene chiral-at-iron complex was demonstrated to be an excellent catalyst for an asymmetric hetero-Diels–Alder reaction under open flask conditions. However, using this class of chiral-at-iron catalysts, thus far we only accomplished chiral Lewis acid catalysis.
To alter the catalytic activity, we envisioned to replace the normal NHC (nNHC) of our bidentate PyNHC ligands with a stronger σ-donating abnormal NHC (aNHC). For that reason, 1,2,3-triazolin-5-ylidene was chosen to substitute the imidazoline-2-ylidene moiety, as the triazole scaffold can be easily synthesized and modified (Fig. 1b).9 1,2,3-Triazolin-5-ylidenes belong to a subclass of aNHCs, which are known as mesoionic carbenes (MICs), due to the zwitterionic character of all its sensible mesomeric structures. Since the first report by Albrecht et al. in 2008,10 the triazole based aNHCs have been widely accepted as an addition to the toolbox of organometallic chemistry.9,11 Recently, several iron triazolin-5-ylidene complexes emerged in the literature, serving as catalyst for the intramolecular C–H amination of organic azides,12 or as photosensitizer with long excited-state lifetime.13 The simplicity of the triazole core synthesis via click chemistry has even been acknowledged in 2022 with a Nobel Prize in chemistry.
Herein, we demonstrate the influence of the strong σ-donating properties of the MIC ligands on the reactivity of the corresponding iron complexes in nitrene transfer catalysis and the modulation in the configurational stability of the corresponding iron complexes. While replacing the imidazol-2-ylidene carbene moieties with 1,2,3-triazolin-5-ylidenes significantly enhances the reactivity of the iron complexes towards a nitrene mediated intramolecular C(sp3)–H amidation reaction, the configurational stability is severely impacted. However, a combination of the electron rich 1,2,3-triazolin-5-ylidene MICs with a chiral pinene-modified pyridyl ligand gives rise to a new class of stereogenic-at-iron catalysts (Fig. 1c), which assemble from the ligands in a highly diastereoselective fashion. This new non-racemic stereogenic-at-iron catalyst catalyzes an asymmetric nitrene-mediated intramolecular C(sp3)–H amidation to afford chiral 2-imidazolidinones with high yield and satisfactory enantioselectivity.
Results and discussion
Design and synthesis of racemic FeMIC complexes
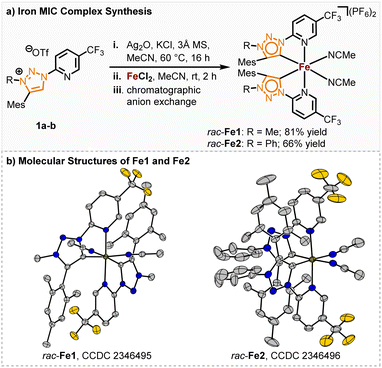
We started our investigation with the pyridyl triazole ligands 1a, b (Scheme 1). Among others, the research groups of Sarkar, Košmrlj and Albrecht developed multiple synthetic pathways to pyridyl triazoles,14 assessing their electronic properties15 and implementing them as ligands.16 MICs generally exhibit stronger σ-donating properties than traditional nNHCs, leading to a more electron-rich iron center and thereby potentially unlocking novel reactivities. Possessing nearly identical ligand geometries to their nNHC counterparts, altered catalytic properties of the corresponding iron complexes should therefore be traced back to the altered electronic properties of the ligands.Previously, we accomplished the synthesis of chiral-at-iron complexes bearing achiral nNHC ligands which could be synthesized through electrochemical or standard base induced methods.5 While the synthesis was simplified and even the configurational stability significantly enhanced through a fine-tuning of the electronic properties of the 2-pyridyl-NHC ligand, the catalytic reactivity of these complexes towards non-Lewis acid mediated reactions is thus far limited. In this work we investigated the influence of stronger σ-donating, but also weaker π-accepting MIC ligands on the catalytic performance of the corresponding chiral-at-iron complexes. The synthesis of the ligands 1a, b was performed following a slightly modified literature procedure of Košmrlj and co-workers.14 Both methylated and arylated triazoline ligands were synthesized for comparison (see ESI† for details of the ligand synthesis). The synthesis of the racemic iron(II) complexes was achieved via a silver carbene route and subsequent in situ complex formation after addition of FeCl2. After chromatographic anion exchange, the complexes rac-Fe1 and rac-Fe2 were obtained in 81% and 66% yields, respectively (Scheme 1a). Single crystal X-ray diffraction confirmed the C2-symmetry of these iron(II) complexes (Scheme 1b). Both racemic complexes exhibit robustness towards air and water and could be stored under ambient temperature over months and at 4 °C for over 2 years without any sign of decomposition.
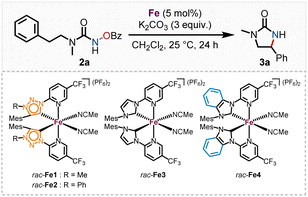
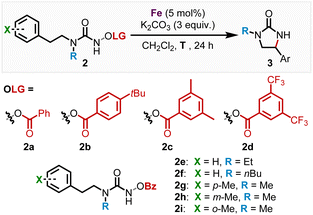
We subsequently discovered that the more electron rich iron(II) complexes rac-Fe1, 2 displayed significant catalytic activity in the nitrene-mediated ring-closing C(sp3)–H-amidation reaction of N-benzoyloxyurea 2 to 2-imidazolidinone 3 (Table 1).17 For example, at room temperature after 24 h, rac-Fe1 and rac-Fe2 at catalyst loadings of 5 mol% provided conversions of over 80% with product NMR yields of 40% and 55%, respectively (entries 1 and 2), while for the previous nNHC congeners rac-Fe3 and rac-Fe4 the conversions under identical reaction conditions were just 44% and 12% with NMR yields of 18% and 9%, respectively (entries 3 and 4).
| # | Catalyst | Conversionb (%) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: rac-Fe1–4 (5 mol%), urea 2 (0.05 mmol) and K2CO3 (0.15 mmol) were dissolved in distilled CH2Cl2 (1.00 mL) and stirred under indicated conditions for 24 h. b Yields and conversion were determined via1H NMR analysis with 1,1,2,2-tetrachloroethane as standard. | |||
| 1 | rac-Fe1 | 85 | 40 |
| 2 | rac-Fe2 | 86 | 55 |
| 3 | rac-Fe3 | 44 | 18 |
| 4 | rac-Fe4 | 12 | 9 |
Apparently, the more electron-rich MIC ligands are beneficial for such nitrene-mediated C–H amidation, presumably by increasing the rate of formation of the iron–nitrene intermediate. This aligns with findings of Maldivi and Latour, who asserted that nitrene formation is favored by electron-rich catalysts, while nitrene transfer requires a more electrophilic species.18 Investigation of the MIC ligands 1a, bvia Ganter's 77Se NMR method19 confirmed the expected decrease in π-accepting properties for the stronger σ-donating MIC ligands. The increase in σ-donation is further supported by the Szostak parameter20 (see ESI† for details).
Non-racemic iron MIC complexes
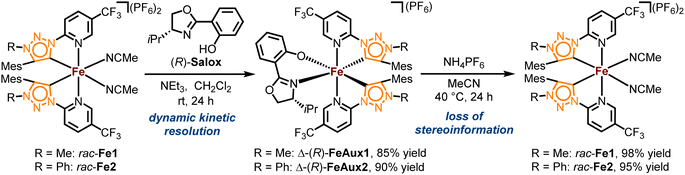
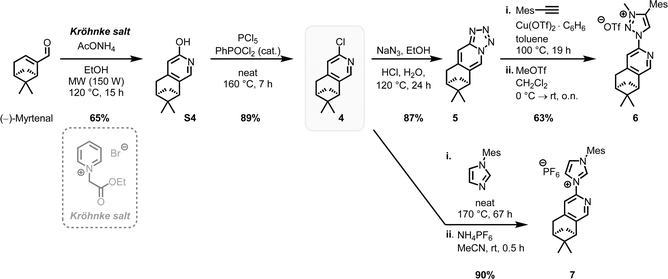
The complexes rac-Fe1, 2 contain only achiral ligands but are chiral due to the stereogenic iron center. With the aim to next investigate the enantioselective version of the nitrene-mediated ring-closing C(sp3)–H amidation, we were seeking to obtain non-racemic iron(II) triazolin-5-ylidene complexes. Unfortunately, a chiral resolution of rac-Fe1, 2 using our previously established auxiliary-mediated route5 failed due to an increased configurational lability of these iron MIC complexes compared to the related iron nNHC congeners. Accordingly, using the chiral auxiliary ligand (R)-Salox, Δ-(R)-FeAux1 and Δ-(R)-FeAux2 were obtained with yields of 85% and 90% respectively (Scheme 2), suggesting a dynamic kinetic resolution and hinting at a limited configurational stability of the iron center. It was therefore not surprising that after cleavage of the chiral auxiliary, the iron complexes were obtained as racemic mixtures. Also, exchanging the N-5-methyl with a phenyl, which is known to result in a more stable carbene,21 had no influence on the configurational stability of the corresponding iron complex. This configurational lability of the MIC complexes can be attributed to the imbalance in the electronic properties of the weaker π-accepting and stronger σ-donating MIC ligands. This is consistent with our previous finding, in which a slight decrease in σ-donating combined with an increase in π-accepting properties afforded a configurational more robust chiral-at-iron complex.5c Therefore, we next pursued a strategy employing chiral ligands to control the iron-centered configuration.22We designed the chiral bidentate MIC ligand 6 as illustrated in Scheme 3 which is based on pinene-modified chiral pyridine ligands previously developed by von Zelewsky.23 Considering the inter-ligand stacking between mesityl groups and coordinated pyridines seen in Fe1, 2 and similar complexes involving mesityl groups coordinating pyridines, we predicted that this pinene MIC ligand 6 would exhibit a strong preference for one diastereomer, consequently resulting in a specific metal-centered configuration (see ESI† for more details). For comparison, we also synthesized the corresponding pinene nNHC ligand 7. Both ligands were synthesized from the literature known chloro pyridyl precursor 4 which can be obtained in 2-steps from (−)-myrtenal, a readily available and cheap reagent from the “chiral pool”.23,24 We significantly optimized the reaction conditions for the Kröhnke annulation of (−)-myrtenal with the Kröhnke salt using a microwave-assisted setup. This enhancement increased the yield for S4 from 27%24b to 65% yield while also reducing the reaction time from 7 d to 15 h. The subsequent deoxochlorination with PCl5 and catalytic PhPOCl2 to obtain the chloro pyridyl 4 not only significantly improved the yield from 73%24a to 89% by utilizing the method from Zhu et al.,25 but was also now reliably reproducible.
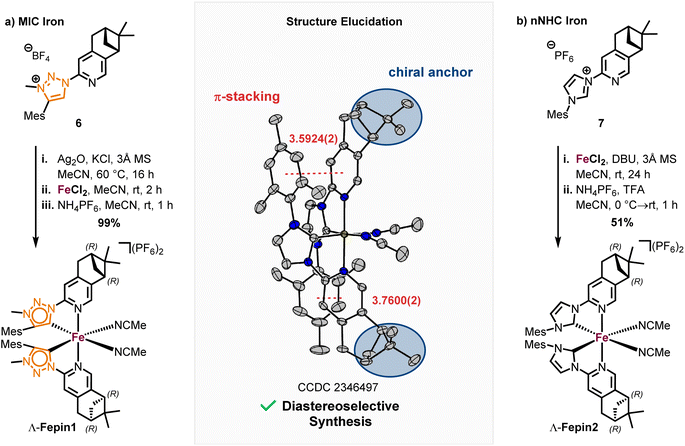
With the precursor 4 in hand, the MIC ligand 6 was synthesized via a tetrazole formation followed by a CuAAC and subsequent methylation to yield the desired ligand in 55% yield over 3 steps. Following a procedure form Chattopadhyay and co-workers,26 tetrazole 5 was formed by an SNAr with NaN3 under acidic conditions in 87% yield. The CuAAc was performed in toluene, a solvent with low dielectric constant, and high temperatures of 100 °C to shift the equilibrium of the tetrazole 5 to the open azide form, which then can take part in the CuAAc with mesitylene alkyne.14b As the optimal copper catalyst, Cu(OTf)2·C6H6 was chosen, as CuBr(PPh3)3 showed lower reactivity and higher rate of the undesired Glaser coupling product. In the final step, the triazole was methylated using MeOTf to obtain the desired triazolium ligand 6 with 63% yield over two steps. The corresponding nNHC ligand 7 was synthesized from the precursor 4via our previously established SNAr method5 with mesityl imidazole under neat conditions with an excellent yield of 90%. The chiral pinene-based MIC ligand 6 was converted to the complex Λ-Fepin1 following the silver carbene route in analogy to the racemic synthesis of rac-Fe1, 2 (Scheme 4). It turned out to be beneficial starting from the ligand 6 as its BF4 salt, as this ensured a clean conversion to the desired complex and easier purification on the silica column. The counterion of the complex was afterwards readily exchanged with PF6 by just stirring the complex with an excess of NH4PF6 in MeCN. With this, the chiral MIC iron complex Λ-Fepin1 was obtained in quantitative yield (Scheme 4a). 1H and 13C NMR spectroscopy are consistent with the formation of a single stereoisomer. Furthermore, CD spectroscopy revealed the optical activity of this complex. The corresponding nNHC complex Λ-Fepin2 was synthesized utilizing our previously developed organic base mediated method (Scheme 4b).5c However, due to the less acidic character of the NHC, a stronger base had to be used, in this case DBU.
Since we observed DBU coordinating to the newly formed complex, TFA was added afterwards under presence of additional NH4PF6 to ensure a complete dissociation of DBU and exchange with acetonitrile. After recrystallization from i-PrOH the complex was obtained in a yield of 51%. Also here, NMR spectroscopy revealed the formation of a single diastereomer. A structural confirmation via single crystal XRD showed, that only one enantiomer and diastereomer of the complex is present, due to the chiral anchor favoring the inter-ligand π-stacking from one site which leads to the formation of the Λ-configuration at the iron center (Scheme 4, see also ESI† for more details). With this, an enantiomerically and diastereomerically pure complex was formed without any chiral resolution necessary. For comparison, for previously reported rhodium based chiral-at-metal complexes bearing chiral pinene-based ligands, always both Λ- and Δ-diastereomers were obtained.27 Furthermore, the complexes showed no sign of configurational lability after 1 week in acetonitrile under air. However, in the non-coordinating solvent dichloromethane and in the absence of any potential exogeneous ligand, slow decomposition was observed, which we attribute to a dissociation of at least one MeCN ligand to provide a coordinatively unsaturated, less stable iron complex.28 The steric maps of the iron MIC/NHC complexes bearing achiral or chiral ligands, as well the formation of both possible configurational isomers of the chiral iron complexes are further discussed in the ESI.†
A highly diastereoselective formation of octahedral iron complexes from bidentate ligands is rare. For example, Scott reported the diastereoselective formation of fac-tris(iminopyridine) complexes,29 while Yamamoto used a binaphthyl-modified 2,9-phenanthroline for diastereoselective iron-coordination.30
Enantioselective catalysis
With the non-racemic stereogenic-at-iron complexes Λ-Fepin1, 2 in hand, we next investigated the asymmetric version of the nitrene-mediated ring-closing C(sp3)–H amidation to 2-imidazolidinones (Table 2). First, Λ-Fepin1 and Λ-Fepin2 were tested with standard substrate 2a showing a conversion of 97% and 75% NMR yield with an enantiomeric ratio of 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 for the MIC complex Λ-Fepin1 (entry 1), while for the nNHC complex Λ-Fepin2 a conversion of only 61% and NMR yield of just 36% was obtained (entry 2). As expected, the more electron-rich MIC complex displayed superior catalytic activity compared to the nNHC complex and provided an encouraging enantioselectivity. Furthermore, the higher catalytic activity of the MIC complex Λ-Fepin1 (Table 2, entry 1) compared to the MIC complex rac-Fe1 (Table 1, entry 1) can be rationalized with a higher electron density at the iron in Λ-Fepin1 due to a more electron rich pyridyl ligand (CF3 replaced with pinene moiety). Next, in order to optimize this conversion, the role of the benzoate leaving group was investigated (entries 3–6). As a result, the more electron rich and bulky leaving groups 4-tbu-Bz 2b and 3,5-Me2-Bz 2c showed both a diminished conversion of 42% and 48%, respectively, with an er of 82
18 for the MIC complex Λ-Fepin1 (entry 1), while for the nNHC complex Λ-Fepin2 a conversion of only 61% and NMR yield of just 36% was obtained (entry 2). As expected, the more electron-rich MIC complex displayed superior catalytic activity compared to the nNHC complex and provided an encouraging enantioselectivity. Furthermore, the higher catalytic activity of the MIC complex Λ-Fepin1 (Table 2, entry 1) compared to the MIC complex rac-Fe1 (Table 1, entry 1) can be rationalized with a higher electron density at the iron in Λ-Fepin1 due to a more electron rich pyridyl ligand (CF3 replaced with pinene moiety). Next, in order to optimize this conversion, the role of the benzoate leaving group was investigated (entries 3–6). As a result, the more electron rich and bulky leaving groups 4-tbu-Bz 2b and 3,5-Me2-Bz 2c showed both a diminished conversion of 42% and 48%, respectively, with an er of 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 for both leaving groups (entries 3 and 4). The electron withdrawing CF3-group 3,5-(CF3)2-Bz 2d showed almost full conversion of 97% with an NMR yield of 54% but a reduced er of 80
18 for both leaving groups (entries 3 and 4). The electron withdrawing CF3-group 3,5-(CF3)2-Bz 2d showed almost full conversion of 97% with an NMR yield of 54% but a reduced er of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 for Λ-Fepin1 (entry 5). Also, Λ-Fepin2 was tested with 3,5-(CF3)2-Bz 2d as the leaving group, revealing an increased conversion of 87% compared to the Bz leaving group. The NMR yield remained low with 41% but displaying an enantioselectivity of 83
20 for Λ-Fepin1 (entry 5). Also, Λ-Fepin2 was tested with 3,5-(CF3)2-Bz 2d as the leaving group, revealing an increased conversion of 87% compared to the Bz leaving group. The NMR yield remained low with 41% but displaying an enantioselectivity of 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 er comparable with Λ-Fepin1 (entry 6). The leaving group screen revealed, that altering the electronic properties of the leaving group only influenced the conversion and yield but had no effect on the enantioselectivity of the C–H amidation product, suggesting that the leaving group does not remain coordinated to the iron center after N–O bond cleavage. This is in contrast to previous work from our group, where utilizing an Fe–N4 catalyst showed that coordination of the carboxylate leaving group to the iron center greatly influences the enantioselectivity in the ring closing C–H amidation.17c Next, the influence of increased sterics on the methylated nitrogen was investigated by replacing the methyl moiety with either an ethyl (2e, entry 7) or n-butyl (2f, entry 8). Both reactions provided almost full conversion (97% for 2e and 95% 2f) with 75% and 67% NMR yield and 78
17 er comparable with Λ-Fepin1 (entry 6). The leaving group screen revealed, that altering the electronic properties of the leaving group only influenced the conversion and yield but had no effect on the enantioselectivity of the C–H amidation product, suggesting that the leaving group does not remain coordinated to the iron center after N–O bond cleavage. This is in contrast to previous work from our group, where utilizing an Fe–N4 catalyst showed that coordination of the carboxylate leaving group to the iron center greatly influences the enantioselectivity in the ring closing C–H amidation.17c Next, the influence of increased sterics on the methylated nitrogen was investigated by replacing the methyl moiety with either an ethyl (2e, entry 7) or n-butyl (2f, entry 8). Both reactions provided almost full conversion (97% for 2e and 95% 2f) with 75% and 67% NMR yield and 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 and 76
22 and 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 er, respectively. This revealed that an increase in steric bulk on the nitrogen leads diminished enantioselectivity, while reactivity remained almost unchanged. To our surprise, changing the counterion of the complex from PF6 to BF4 resulted in diminished product formation of 26% with almost full conversion, thus demonstrating a counterion effect for this ring-closing C–H amidation (entry 9). Lastly, we tested the influence of temperature on the reaction (entries 10 and 11). At 4 °C, full conversion was achieved with an increased NMR yield of 90% and slightly higher er of 84
24 er, respectively. This revealed that an increase in steric bulk on the nitrogen leads diminished enantioselectivity, while reactivity remained almost unchanged. To our surprise, changing the counterion of the complex from PF6 to BF4 resulted in diminished product formation of 26% with almost full conversion, thus demonstrating a counterion effect for this ring-closing C–H amidation (entry 9). Lastly, we tested the influence of temperature on the reaction (entries 10 and 11). At 4 °C, full conversion was achieved with an increased NMR yield of 90% and slightly higher er of 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 (entry 10). At −10 °C, full conversion was still maintained with a similar NMR yield of 89% and an er of 86
16 (entry 10). At −10 °C, full conversion was still maintained with a similar NMR yield of 89% and an er of 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 (entry 11). By lowering the temperature, we could observe an increased stability of the catalyst in solution, marked by a colored solution, enabling an efficient participation of the iron catalyst in the reaction pathway, which leads to an increased yield. As expected, even the enantiomeric ratio could be slightly increased from 82
14 (entry 11). By lowering the temperature, we could observe an increased stability of the catalyst in solution, marked by a colored solution, enabling an efficient participation of the iron catalyst in the reaction pathway, which leads to an increased yield. As expected, even the enantiomeric ratio could be slightly increased from 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 at 25 °C to 86
18 at 25 °C to 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 at −10 °C. Further decreasing the temperature was not feasible, as the reaction rate strongly decreased. In entries 12–14 steric effects were investigated by substituting a hydrogen in para-, meta-, or ortho-position of the phenyl ring with a methyl moiety, leading to er values of 86
14 at −10 °C. Further decreasing the temperature was not feasible, as the reaction rate strongly decreased. In entries 12–14 steric effects were investigated by substituting a hydrogen in para-, meta-, or ortho-position of the phenyl ring with a methyl moiety, leading to er values of 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 (2g), 84
14 (2g), 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 (2h) and 91
16 (2h) and 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (2i), respectively, with yields ranging from 80–90% at 4 °C. To our surprise, a steric increase in the ortho-position positively effected the enantiomeric ratio in contrary to our previous published results.17c The er could even be further increased to 92
9 (2i), respectively, with yields ranging from 80–90% at 4 °C. To our surprise, a steric increase in the ortho-position positively effected the enantiomeric ratio in contrary to our previous published results.17c The er could even be further increased to 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 at −10 °C (entry 15).
8 at −10 °C (entry 15).
| # | Catalyst | T (°C) | Conversionb (%) | Substr. | Yieldb (%) | erc |
|---|---|---|---|---|---|---|
| a Reaction conditions: Λ-Fepin1, 2 (5 mol%), urea 2a–i (0.05 mmol) and K2CO3 (0.15 mmol) were dissolved in distilled CH2Cl2 (1.00 mL) and stirred under indicated conditions for 24 h. b Yields and conversion were determined via1H NMR analysis with 1,1,2,2-tetrachloroethane as standard. c er were determined by HPLC analysis on a chiral stationary phase; nd = not determined. | ||||||
| 1 | Λ-Fepin1 | 25 | 97 | 2a | 75 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
| 2 | Λ-Fepin2 | 25 | 61 | 2a | 36 | nd |
| 3 | Λ-Fepin1 | 25 | 42 | 2b | nd | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
| 4 | Λ-Fepin1 | 25 | 48 | 2c | nd | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
| 5 | Λ-Fepin1 | 25 | 97 | 2d | 54 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 6 | Λ-Fepin2 | 25 | 87 | 2d | 41 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
| 7 | Λ-Fepin1 | 25 | 97 | 2e | 75 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
| 8 | Λ-Fepin1 | 25 | 95 | 2f | 67 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
| 9 | Λ-Fepin1BF4 | 25 | 97 | 2a | 26 | nd |
| 10 | Λ-Fepin1 | 4 | 100 | 2a | 90 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
| 11 | Λ-Fepin1 | −10 | 100 | 2a | 89 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 12 | Λ-Fepin1 | 4 | 100 | 2g | 90 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 13 | Λ-Fepin1 | 4 | 100 | 2h | 80 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
| 14 | Λ-Fepin1 | 4 | 100 | 2i | 80 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| 15 | Λ-Fepin1 | −10 | 100 | 2i | 81 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
Conclusions
In conclusion, this work investigated the influence of strongly σ-donating 1,2,3-triazolin-5-ylidene mesoionic carbene ligands (MICs) on the configurational stability and reactivity of stereogenic-at-iron complexes. C2-symmetric iron complexes with two coordinated pyridyl triazolin-5-ylidene ligands display an increased catalytic activity compared to their imidazol-2-ylidene congeners regarding a nitrene-mediated ring-closing C(sp3)–H amidation. However, the MIC ligands render the stereogenic iron center configurationally labile. To nevertheless achieve the generation of non-racemic complexes, the pyridyl moiety was functionalized with a chiral pinene moiety which resulted in the formation of a single diastereomer in the course of ligand coordination. We subsequently demonstrated that a non-racemic pinene-modified iron MIC complex can catalyze the ring-closing C–H amidation of N-benzoyloxyurea to 2-imidazolidinones with an enantiomeric ratio of up to 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. Future work will investigate other applications for this new class of chiral iron MIC catalysts.
8. Future work will investigate other applications for this new class of chiral iron MIC catalysts.
Data availability
The ESI† contains detailed synthetic procedures and complete characterization data for all new compounds, CD spectra of all non-racemic iron complexes, as well UV/vis absorbance spectra of the here discussed iron complexes. Investigation of the electronic properties of the MIC ligands and detailed steric maps of the iron complexes are further discussed in the ESI.†Author contributions
N. D. and E. M. conceived and designed the project. N. D. performed the majority of the synthetic experiments and analyzed the data. M. D. and G. N. contributed with synthetic experiments. S. I. I. performed the X-ray crystallography. N. D. and E. M. wrote the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 Research and Innovation Programme (grant agreement no. 883212).Notes and references
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- For recent special issues on the catalysis with earth-abundant metals, see: (a) P. Chirik and R. Morris, Acc. Chem. Res., 2015, 48, 2495 CrossRef CAS PubMed; (b) M. Beller, Chem. Rev., 2019, 119, 2089 CrossRef CAS PubMed.
- For selected reviews on iron catalysis, see: (a) C. Bolm, J. Legros, J. Le Paih and L. Zani, Chem. Rev., 2004, 104, 6217–6254 CrossRef CAS PubMed; (b) S. Enthaler, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2008, 47, 3317–3321 CrossRef CAS PubMed; (c) I. Bauer and H.-J. Knölker, Chem. Rev., 2015, 115, 3170–3387 CrossRef CAS PubMed; (d) A. Fürstner, ACS Cent. Sci., 2016, 2, 778–789 CrossRef PubMed; (e) R. Shang, L. Ilies and E. Nakamura, Chem. Rev., 2017, 117, 9086–9139 CrossRef CAS PubMed; (f) A. Piontek, E. Bisz and M. Szostak, Angew. Chem., Int. Ed., 2018, 57, 11116–11128 CrossRef CAS PubMed; (g) P. DaBell and S. P. Thomas, Synthesis, 2020, 52, 949–963 CrossRef CAS; (h) Y. Liu, T. You, H.-X. Wang, Z. Tang, C.-Y. Zhou and C.-M. Che, Chem. Soc. Rev., 2020, 49, 5310–5358 RSC; (i) G. M. Fusi, S. Gazzola and U. Piarulli, Adv. Synth. Catal., 2022, 364, 696–714 CrossRef CAS.
- For selected reviews on asymmetric iron catalysis, see: (a) K. Gopalaiah, Chem. Rev., 2013, 113, 3248–3296 CrossRef CAS PubMed; (b) T. Ollevier and H. Keipour, Enantioselective Iron Catalysts, in Iron Catalysis II, ed. E. Bauer, Springer International Publishing, Cham, 2015, pp. 259–309 Search PubMed; (c) H. Pellissier, Coord. Chem. Rev., 2019, 386, 1–31 CrossRef CAS; (d) A. Casnati, M. Lanzi and G. Cera, Molecules, 2020, 25, 3889 CrossRef CAS PubMed.
- For our recent work focusing on chiral-at-iron complexes, see: (a) Y. Hong, L. Jarrige, K. Harms and E. Meggers, J. Am. Chem. Soc., 2019, 141, 4569–4572 CrossRef CAS PubMed; (b) Y. Hong, T. Cui, S. Ivlev, X. Xie and E. Meggers, Chem.–Eur. J., 2021, 27, 8557–8563 CrossRef CAS PubMed; (c) N. Demirel, J. Haber, S. I. Ivlev and E. Meggers, Organometallics, 2022, 41, 3852–3860 CrossRef CAS PubMed.
- L. Zhang and E. Meggers, Chem.–Asian J., 2017, 12, 2335–2342 CrossRef CAS PubMed.
- L. Zhang and E. Meggers, Acc. Chem. Res., 2017, 50, 320–330 CrossRef CAS PubMed.
- P. S. Steinlandt, L. Zhang and E. Meggers, Chem. Rev., 2023, 123, 4764–4794 CrossRef CAS PubMed.
- For a comprehensive review on MICs and other abnormal NHCs, see: A. Vivancos, C. Segarra and M. Albrecht, Chem. Rev., 2018, 118, 9493–9586 CrossRef CAS PubMed.
- P. Mathew, A. Neels and M. Albrecht, J. Am. Chem. Soc., 2008, 130, 13534–13535 CrossRef CAS PubMed.
- R. Maity and B. Sarkar, JACS Au, 2022, 2, 22–57 CrossRef CAS PubMed.
- (a) W. Stroek, M. Keilwerth, D. M. Pividori, K. Meyer and M. Albrecht, J. Am. Chem. Soc., 2021, 143, 20157–20165 CrossRef CAS PubMed, For other iron scaffolds facilitating an intramolecular C–H amination utilizing organic azides, see: (b) E. T. Hennessy and T. A. Betley, Science, 2013, 340, 591–595 CrossRef CAS PubMed; (c) N. C. Thacker, Z. Lin, T. Zhang, J. C. Gilhula, C. W. Abney and W. Lin, J. Am. Chem. Soc., 2016, 138, 3501–3509 CrossRef CAS PubMed; (d) B. Bagh, D. L. J. Broere, V. Sinha, P. F. Kuijpers, N. P. van Leest, B. de Bruin, S. Demeshko, M. A. Siegler and J. I. van der Vlugt, J. Am. Chem. Soc., 2017, 139, 5117–5124 CrossRef CAS PubMed; (e) W. Stroek and M. Albrecht, Chem. Sci., 2023, 14, 2849–2859 RSC; (f) W. Stroek, L. Hoareau and M. Albrecht, Catal. Sci. Technol., 2023, 13, 958–962 RSC.
- P. Chábera, Y. Liu, O. Prakash, E. Thyrhaug, A. E. Nahhas, A. Honarfar, S. Essén, L. A. Fredin, T. C. B. Harlang, K. S. Kjær, K. Handrup, F. Ericson, H. Tatsuno, K. Morgan, J. Schnadt, L. Häggström, T. Ericsson, A. Sobkowiak, S. Lidin, P. Huang, S. Styring, J. Uhlig, J. Bendix, R. Lomoth, V. Sundström, P. Persson and K. A. Wärnmark, Nature, 2017, 543, 695–699 CrossRef PubMed.
- (a) A. Bolje and J. Košmrlj, Org. Lett., 2013, 15, 5084–5087 CrossRef CAS PubMed; (b) A. Bolje, D. Urankar and J. Košmrlj, Eur. J. Org Chem., 2014, 2014, 8167–8181 CrossRef CAS; (c) M. Virant and J. Košmrlj, J. Org. Chem., 2019, 84, 14030–14044 CrossRef CAS PubMed.
- (a) J. Beerhues, H. Aberhan, T.-N. Streit and B. Sarkar, Organometallics, 2020, 39, 4557–4564 CrossRef CAS; (b) J. Beerhues, M. Neubrand, S. Sobottka, N. I. Neuman, H. Aberhan, S. Chandra and B. Sarkar, Chem.–Eur. J., 2021, 27, 6557–6568 CrossRef CAS PubMed; (c) R. Maity and B. Sarkar, JACS Au, 2022, 2, 22–57 CrossRef CAS PubMed; (d) T. Bens, J. A. Kübler, R. R. M. Walter, J. Beerhues, O. S. Wenger and B. Sarkar, ACS Org. Inorg. Au, 2023, 3, 184–198 CrossRef CAS PubMed; (e) T. Bens and B. Sarkar, Inorganics, 2024, 12, 46–63 CrossRef CAS.
- (a) S. Hohloch, L. Suntrup and B. Sarkar, Organometallics, 2013, 32, 7376–7385 CrossRef CAS; (b) V. Leigh, W. Ghattas, R. Lalrempuia, H. Mueller-Bunz, M. T. Pryce and M. Albrecht, Inorg. Chem., 2013, 52, 5395–5402 CrossRef CAS PubMed; (c) A. Petronilho, A. Llobet and M. Albrecht, Inorg. Chem., 2014, 53, 12896–12901 CrossRef CAS PubMed; (d) A. Petrolnilho, J. A. Woods, H. Mueller-Bunz, S. Bernhard and M. Albrecht, Chem.–Eur. J., 2014, 20, 15775–15784 CrossRef PubMed; (e) A. Bolje, S. Hohloch, M. van der Meer, J. Košmrlj and B. Sarkar, Chem.–Eur. J., 2015, 21, 6756–6764 CrossRef CAS PubMed; (f) Y. Wei, S.-X. Liu, H. Mueller-Bunz and M. Albrecht, ACS Catal., 2016, 6, 8192–8200 CrossRef CAS; (g) C. Johnson and M. Albrecht, Organometallics, 2017, 36, 2902–2913 CrossRef CAS.
- (a) Z. Zhou, Y. Tan, T. Yamahira, S. Ivlev, X. Xie, R. Riedel, M. Hemming, M. Kimura and E. Meggers, Chem, 2020, 6, 2024–2034 CrossRef CAS; (b) L. Jarrige, Z. Zhou, M. Hemming and E. Meggers, Angew. Chem., Int. Ed., 2021, 60, 6314–6319 CrossRef CAS PubMed; (c) T. Cui, C.-X. Ye, J. Thelemann, D. Jenisch and E. Meggers, Chin. J. Chem., 2023, 41, 2065–2070 CrossRef CAS.
- (a) G. Coin and J.-M. Latour, J. Inorg. Biochem., 2021, 225, 111613 CrossRef CAS PubMed; (b) J. Donat, P. Dubourdeaux, M. Clémancey, J. Rendon, C. Gervasoni, M. Barbier, J. Barilone, J. Pécaut, S. Gambarelli, P. Maldivi and J.-M. Latour, Chem.–Eur. J., 2022, 28, e202201875 CrossRef CAS PubMed.
- (a) A. Liske, K. Verlinden, H. Buhl, K. Schaper and C. Ganter, Organometallics, 2013, 32, 5269–5272 CrossRef CAS; (b) K. Verlinden, H. Buhl, W. Frank and C. Ganter, Eur. J. Inorg. Chem., 2015, 2015, 2416–2425 CrossRef CAS; (c) S. V. C. Vummaleti, D. J. Nelson, A. Poater, A. Gomez-Suarez, D. B. Cordes, A. M. Z. Slawin, S. P. Nolan and L. Cavallo, Chem. Sci., 2015, 6, 1895–1904 RSC.
- G. Meng, L. Kakalis, S. P. Nolan and M. Szostak, Tetrahedron Lett., 2019, 60, 378–381 CrossRef CAS.
- (a) G. Guisado-Barrios, J. Bouffard, B. Donnadieu and G. Bertrand, Angew. Chem., Int. Ed., 2010, 49, 4759–4762 CrossRef CAS PubMed; (b) J. Bouffard, B. K. Keitz, R. Tonner, G. Guisado-Barrios, G. Frenking, R. H. Grubbs and G. Bertrand, Organometallics, 2011, 30, 2617–2627 CrossRef CAS PubMed.
- (a) H. Brunner, Angew. Chem., Int. Ed., 1999, 38, 1194–1208 CrossRef CAS; (b) U. Knof and A. von Zelewsky, Angew. Chem., Int. Ed., 1999, 38, 302–322 CrossRef; (c) P. D. Knight and P. Scott, Coord. Chem. Rev., 2003, 242, 125–143 CrossRef CAS; (d) E. Meggers, Eur. J. Inorg. Chem., 2011, 2011, 2911–2926 CrossRef CAS.
- (a) P. Hayoz and A. von Zelewsky, Tetrahedron Lett., 1992, 33, 5165–5168 CrossRef CAS; (b) P. Hayoz, A. von Zelewsky and H. Stoeckli-Evans, J. Am. Chem. Soc., 1993, 115, 5111–5114 CrossRef CAS.
- (a) A. V. Malkov, I. R. Baxendale, M. Bella, V. Langer, J. Fawcett, D. R. Russell, D. J. Mansfield, M. Valko and P. Kočovský, Organometallics, 2001, 20, 673–690 CrossRef CAS; (b) X. Sala, A. M. Rodríguez, M. Rodríguez, I. Romero, T. Parella, A. von Zelewsky, A. Llobet and J. Benet-Buchholz, J. Org. Chem., 2006, 71, 9283–9290 CrossRef CAS PubMed.
- J. Wu, J. Zhou, Y. Shi and J. Zhu, Synth. Commun., 2016, 46, 1619–1624 CrossRef CAS.
- S. Das, A. W. Ehlers, S. Patra, B. de Bruin and B. Chattopadhyay, J. Am. Chem. Soc., 2023, 145, 14599–14607 CrossRef CAS PubMed.
- (a) Y. Zheng, K. Harms, L. Zhang and E. Meggers, Chem.–Eur. J., 2016, 22, 11977–11981 CrossRef CAS PubMed; (b) J. Gong, Q. Wan and Q. Kang, Adv. Synth. Catal., 2018, 360, 4031–4036 CrossRef CAS.
- See the ESI† for experiments regarding stability and activity of the Fepin complexes.
- S. E. Howson, L. E. N. Allan, N. P. Chmel, G. J. Clarkson, R. van Gorkum and P. Scott, Chem. Commun., 2009, 1727–1729 RSC.
- Y. Nishikawa and H. Yamamoto, J. Am. Chem. Soc., 2011, 133, 8432–8435 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2346495–2346497. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc03504f |
| This journal is © The Royal Society of Chemistry 2024 |