Open Access Article
Open Access ArticleUnraveling non-radiative decay channels of exciplexes to construct efficient red emitters for organic light-emitting diodes†
Heng-Yuan
Zhang
a,
Ming
Zhang
a,
Hao
Zhuo
a,
Hao-Yu
Yang
a,
Bo
Han
 *b,
Yong-Hao
Zheng
*b,
Yong-Hao
Zheng
 a,
Hui
Wang
c,
Hui
Lin
a,
Hui
Wang
c,
Hui
Lin
 a,
Si-Lu
Tao
a,
Si-Lu
Tao
 a,
Cai-Jun
Zheng
a,
Cai-Jun
Zheng
 *a and
Xiao-Hong
Zhang
*a and
Xiao-Hong
Zhang
 *c
*c
aSchool of Optoelectronic Science and Engineering, University of Electronic Science and Technology of China, Chengdu 611731, P. R. China. E-mail: zhengcaijun@uestc.edu.cn
bChengdu University of Traditional Chinese Medicine, State Key Laboratory Southwestern Chinese Medicine Resources, Chengdu 611137, P. R. China. E-mail: hanbo@cdutcm.edu.cn
cInstitute of Functional Nano & Soft Materials (FUNSOM), Soochow University, Suzhou 215123, P. R. China. E-mail: xiaohong_zhang@suda.edu.cn
First published on 2nd August 2024
Abstract
Exciplex emitters naturally have thermally activated delayed fluorescence characteristics due to their spatially separated molecular orbitals. However, the intermolecular charge transfer potentially induces diverse non-radiative decay channels, severely hindering the construction of efficient red exciplexes. Thus, a thorough comprehension of this energy loss is of paramount importance. Herein, different factors, including molecular rigidity, donor–acceptor interactions and donor–donor/acceptor–acceptor interactions, that impact the non-radiative decay were systematically investigated using contrasting exciplex emitters. The exciplex with rigid components and intermolecular hydrogen bonds showed a photoluminescence quantum yield of 84.1% and a singlet non-radiative decay rate of 1.98 × 106 s−1 at an optimized mixing ratio, respectively, achieving a 3.3-fold increase and a 70% decrease compared to the comparison group. In the electroluminescent device, a maximum external quantum efficiency of 23.8% was achieved with an emission peak of 608 nm, which represents the state-of-the-art organic light-emitting diodes using exciplex emitters. Accordingly, a new strategy is finally proposed, exploiting system rigidification to construct efficient red exciplex emitters that suppress non-radiative decay.
Introduction
Excited complexes, known as exciplexes, have been considered detrimental to the efficiency of organic light-emitting diodes (OLEDs) since Karasz et al. first observed the unexpected and extra red-shifted emission in 1994.1 It was not until 2012 when Adachi and coworkers2 reported the thermally activated delayed fluorescence (TADF) characteristics of some exciplexes that researchers began to probe their applications in OLEDs.3–9 Formed by mixing electron donors and electron acceptors (D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A) to generate intermolecular charge-transfer (CT) states, the exciplexes possess small singlet–triplet energy gaps (ΔESTs) because of the spatially separated highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), which promotes the reverse intersystem crossing (RISC), converting dark triplet excitons to singlet excitons. Benefiting from the theoretical 100% exciton utilization, TADF exciplex emitters are promising to achieve efficient electroluminescence (EL).
A) to generate intermolecular charge-transfer (CT) states, the exciplexes possess small singlet–triplet energy gaps (ΔESTs) because of the spatially separated highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), which promotes the reverse intersystem crossing (RISC), converting dark triplet excitons to singlet excitons. Benefiting from the theoretical 100% exciton utilization, TADF exciplex emitters are promising to achieve efficient electroluminescence (EL).
Over the past decade, with considerable efforts in molecular design and combination optimization of D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A pairs, blue and green OLEDs that utilize exciplex emission have realized remarkable maximum external quantum efficiencies (EQEmaxs) of 20.43%10 and 26.4%,11 respectively. However, the efficiency of red exciplex emitters is far from satisfactory. The highest EL efficiency to date was contributed by Cheng et al., realizing an EQEmax of 14.6% with an emission peak at 592 nm.12 When the emission peak is limited to wavelengths redder than 600 nm, the highest EQEmax (8.68%) was achieved by using a manganese-based donor and a beryllium-based acceptor.13 Besides, researchers combined stronger donors and acceptors for deep-red and near-infrared emission, but the EQEmaxs were less than 5%.14–16 As summarized above, a clear trend can be observed, that is, a sharp decrease in efficiency with the redshift of emission, which can be mainly ascribed to the exponentially increasing non-radiative energy loss as the energy gap gets smaller.17 Therefore, the inhibition of such energy loss is imperative in realizing efficient red exciplexes, as evidenced by the extensive research on single-molecule red TADF emitters.18–20 Theoretically, the emission process of an exciplex can be described as:5
A pairs, blue and green OLEDs that utilize exciplex emission have realized remarkable maximum external quantum efficiencies (EQEmaxs) of 20.43%10 and 26.4%,11 respectively. However, the efficiency of red exciplex emitters is far from satisfactory. The highest EL efficiency to date was contributed by Cheng et al., realizing an EQEmax of 14.6% with an emission peak at 592 nm.12 When the emission peak is limited to wavelengths redder than 600 nm, the highest EQEmax (8.68%) was achieved by using a manganese-based donor and a beryllium-based acceptor.13 Besides, researchers combined stronger donors and acceptors for deep-red and near-infrared emission, but the EQEmaxs were less than 5%.14–16 As summarized above, a clear trend can be observed, that is, a sharp decrease in efficiency with the redshift of emission, which can be mainly ascribed to the exponentially increasing non-radiative energy loss as the energy gap gets smaller.17 Therefore, the inhibition of such energy loss is imperative in realizing efficient red exciplexes, as evidenced by the extensive research on single-molecule red TADF emitters.18–20 Theoretically, the emission process of an exciplex can be described as:5
| D + A + hν → A* + D or A + D* → (Aδ−Dδ+)* → hνexciplex + A + D | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
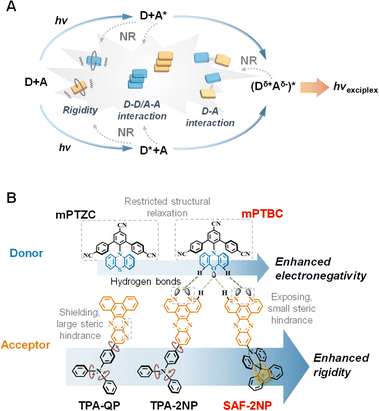
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A-type exciplex emitters potentially possess more non-radiative decay channels (Fig. 1A) compared with the single-molecule TADF emitters. And it is of vital significance to unravel specific paths and propose effective strategies to construct efficient red exciplex emitters.
A-type exciplex emitters potentially possess more non-radiative decay channels (Fig. 1A) compared with the single-molecule TADF emitters. And it is of vital significance to unravel specific paths and propose effective strategies to construct efficient red exciplex emitters.
In this work, we systematically investigate non-radiative decay channels of red exciplex emitters using contrasting D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A pairs and propose an effective strategy accordingly. As shown in Fig. 1B, two donors and three acceptors were developed using phenoxazine/phenothiazine (PXZ/PTZ) and N-heterocycles to achieve red emission, and all six exciplexes show emission peaks around 600 nm. The three acceptors are distinguished by their attachments that the spiro-locked SAF-2NP is much more rigid than triphenylamine (TPA)-based TPA-2NP and TPA-QP. Additionally, the regulation of intermolecular interactions between D and A molecules was achieved by strategic molecular design. Intermolecular hydrogen bonds (HBs) between PXZ and 2NP were found in mPTBC
A pairs and propose an effective strategy accordingly. As shown in Fig. 1B, two donors and three acceptors were developed using phenoxazine/phenothiazine (PXZ/PTZ) and N-heterocycles to achieve red emission, and all six exciplexes show emission peaks around 600 nm. The three acceptors are distinguished by their attachments that the spiro-locked SAF-2NP is much more rigid than triphenylamine (TPA)-based TPA-2NP and TPA-QP. Additionally, the regulation of intermolecular interactions between D and A molecules was achieved by strategic molecular design. Intermolecular hydrogen bonds (HBs) between PXZ and 2NP were found in mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP and mPTBC
SAF-2NP and mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP because of the exposed electronegative sites, while mPTBC
TPA-2NP because of the exposed electronegative sites, while mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-QP, mPTZC
TPA-QP, mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP, mPTZC
SAF-2NP, mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP and mPTZC
TPA-2NP and mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-QP lack the corresponding interactions. It was found that both suppressing the structural relaxation of exciplex components and strengthening the D–A interactions are beneficial for restricting non-radiative decay. Owing to the synergy of stable structures and intermolecular HBs, mPTBC
TPA-QP lack the corresponding interactions. It was found that both suppressing the structural relaxation of exciplex components and strengthening the D–A interactions are beneficial for restricting non-radiative decay. Owing to the synergy of stable structures and intermolecular HBs, mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP showed the lowest knr (singlet non-radiative decay rate, 1.98 × 106 s−1) and the largest photoluminescence quantum yield (PLQY, 84.1%) among the six exciplexes. Furthermore, the D–D/A–A interactions were studied. Suffering from exciton quenching caused by π–π stacking of SAF-2NP, the knr of mPTBC
SAF-2NP showed the lowest knr (singlet non-radiative decay rate, 1.98 × 106 s−1) and the largest photoluminescence quantum yield (PLQY, 84.1%) among the six exciplexes. Furthermore, the D–D/A–A interactions were studied. Suffering from exciton quenching caused by π–π stacking of SAF-2NP, the knr of mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP gradually increased as the acceptor concentration increased. The concentration quenching was much more severe in TPA-containing exciplex emitters. Moderate efficiency declines of mPTBC
SAF-2NP gradually increased as the acceptor concentration increased. The concentration quenching was much more severe in TPA-containing exciplex emitters. Moderate efficiency declines of mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP-based devices were observed where EQEmaxs maintain above 20% with the D
SAF-2NP-based devices were observed where EQEmaxs maintain above 20% with the D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratio from 9
A ratio from 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 6
1 to 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. At the optimized ratio, an outstanding EQEmax of 23.8% with an emission peak at 608 nm was obtained, which not only surpasses all previously reported red exciplex OLEDs but also ranks among state-of-the-art exciplex-emissive devices. Based on the above results, we elucidate that the molecular rigidity, D–D/A–A interactions and D–A interactions are three factors that impact the non-radiative decay. Accordingly, we propose a new strategy leveraging a rigid system featuring stiff structures, intermolecular HBs and optimized D
4. At the optimized ratio, an outstanding EQEmax of 23.8% with an emission peak at 608 nm was obtained, which not only surpasses all previously reported red exciplex OLEDs but also ranks among state-of-the-art exciplex-emissive devices. Based on the above results, we elucidate that the molecular rigidity, D–D/A–A interactions and D–A interactions are three factors that impact the non-radiative decay. Accordingly, we propose a new strategy leveraging a rigid system featuring stiff structures, intermolecular HBs and optimized D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratios to construct red exciplex emitters that effectively suppress the non-radiative decay. The present work provides perspectives to understand the non-radiative decay paths and is helpful in elucidating the emission mechanisms of exciplex emitters.
A ratios to construct red exciplex emitters that effectively suppress the non-radiative decay. The present work provides perspectives to understand the non-radiative decay paths and is helpful in elucidating the emission mechanisms of exciplex emitters.
Results and discussion
Exciplex construction and molecular characterization
The donor–acceptor structure was applied to construct exciplex constituents, which induces TADF characteristics and thus improves exciton utilization of exciplex systems. mPTBC was constructed by incorporating PXZ onto a sterically crowded group. The V-shaped tri-benzonitrile, on the one hand, obstructs the rotation of PXZ and minimizes the structure deformation, and on the other hand prevents planar PXZ from π–π stacking. The acceptor SAF-2NP was a bulky and planar N-heterocycle modified with an sp3 hybridized carbon mediated orthogonal spiro-acridine unit, which locks the rotational peripheries. To regulate the constituent rigidity and D–A intermolecular interactions, as for the donor, PTZ is substituted for PXZ to produce mPTZC, resulting in a decrease in electronegativity22 and a moderate alteration in the HOMO energy level. This greatly lowers the possibility of HB formation since weak HBs are dominated by electrostatic interactions.23 As for the acceptors, by replacing SAF with the TPA unit, the structural stability is greatly impaired because of the rotatable attachments and reduced steric hindrance between TPA and 2NP/QP.24 In addition, the position of nitrogen atoms of QP is elaborately modified from exposing to shielding sites of the molecule (Fig. 1B), generating large steric hindrance for HB formation compared with 2NP.2′-(10H-Phenoxazin-10-yl)-[1,1′:3′,1′′-terphenyl]-4,4′′,5′-tricarbonitrile (mPTBC),25 10-(dipyrido[3,2-a:2′,3′-c]phenazin-11-yl)-10H-spiro[acridine-9,9′-fluorene] (SAF-2NP),26 4-(dipyrido[3,2-a:2′,3′-c]phenazin-11-yl)-N,N-diphenylaniline (TPA-2NP)27 and 4-(dibenzo[f,h]pyrido[2,3-b]quinoxalin-12-yl)-N,N-diphenylaniline (TPA-QP)28 were synthesized according to previous reports. 2′-(10H-Phenoxazin-10-yl)-[1,1′:3′,1′′-terphenyl]-4,4′′,5′-tricarbonitrile (mPTZC) was readily synthesized using the same procedure as mPTBC, including the Suzuki coupling, diazotization reaction and C–N cross-coupling of tri-benzonitrile and PTZ. Column chromatography, recrystallization and sublimation were used to obtain appreciable purity. Its chemical structure was fully characterized through 1H and 13C nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry. The basic physical properties of donors and acceptors were characterized first (summarized in Table S1†). Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC; Fig. S1†) tests were conducted to evaluate the thermal stability of mPTZC. The decomposition temperature (corresponding to 5% weight loss) of mPTZC was calculated to be 381 °C, and no glass transition was observed. As shown in Fig. S2,†mPTZC, TPA-2NP and TPA-QP show clear CT absorption and emission peaks around 430 nm and 540 nm, respectively. According to the fluorescence and phosphorescence spectra, TPA-2NP and TPA-QP possess relatively large ΔESTs due to the large conjugation, similar to other TPA-based TADF emitters.19,29,30 In contrast, twisted structures of mPTBC, mPTZC and SAF-2NP induce tiny ΔESTs, which may further affect the fluorescence lifetime and RISC rates of the corresponding exciplexes. Estimated according to the reduction and oxidation curves (Fig. S3†) obtained by the cyclic voltammetry method, SAF-2NP, TPA-2NP and TPA-QP have similar LUMO energy levels of −3.32 eV, −3.27 eV and −3.31 eV, respectively. mPTBC and mPTZC show similar HOMO energy levels, making the corresponding exciplex emitters comparable. The fluorescence spectra of the six exciplex emitters and their electron-donating and electron-accepting components are displayed in Fig. S4 and S5.† Emission peaks at around 600 nm were observed for all six exciplexes, exhibiting clear red shifts compared with those of their constituents, confirming the exciplex formation.31 The emission energy is in accordance with the differences between HOMO energy levels of donors and LUMO energy levels of acceptors (listed in Table S1†).
Molecular rigidity and intermolecular interactions
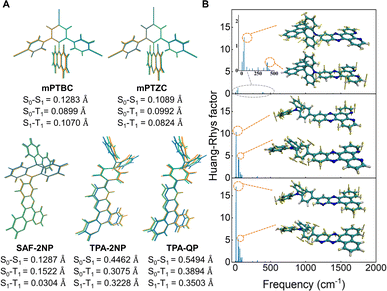
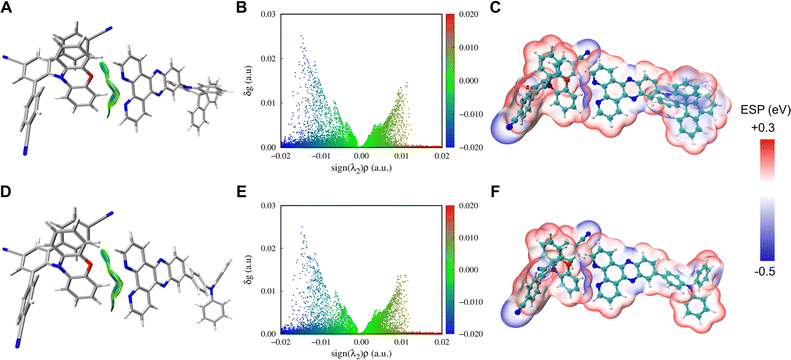
In order to theoretically distinguish the rigidity of these compounds, density functional theory (DFT) and time dependent DFT (TD-DFT) were conducted using Gaussian.32 As shown in Fig. 2A, optimized geometries of the ground state (S0), the lowest singlet excited state (S1) and the lowest triplet state (T1) as well as the corresponding root-mean-square displacements (RMSDs) of all five components were calculated using VMD33 in the first place. There is little geometry change between the ground state and excited states for mPTBC and mPTZC, indicating that the V-shaped tri-benzonitrile effectively stabilizes the molecules. As for the acceptors, RMSD show a prominently decreasing trend from TPA-QP to SAF-2NP, indicating suppressed structural relaxation. These larger values of TPA-modified acceptors mainly arise from the dihedral angle change between TPA and 2NP/QP as well as the rotation of peripheral phenyl. To further study the structural deformation, Huang–Rhys factors at different normal mode frequencies of the three acceptors were calculated according to the reorganization energy using the DUSHIN program,34 and the results are demonstrated in Fig. 2B and S6.† The Huang–Rhys factor of SAF-2NP is significantly smaller than those of TPA-2NP and TPA-QP. As shown by the inset in Fig. 2B, the most and second most representative normal modes of SAF-2NP correspond to wagging vibrations of the acridine unit (38 cm−1) and C–H as well as C–C twisting vibrations of the 2NP plane (396 cm−1). However TPA-2NP and TPA-QP possess similar twisting vibration modes resulting in rotations between the peripheral phenyl and the acceptor plane (12 cm−1) as well as rotations between the acceptor plane and adjacent phenyl (51 cm−1). Besides, the primary vibrations that contribute to the reorganization energy of SAF-2NP and TPA-derivatives, respectively, belong to the high-frequency region which corresponds to the bond length change and the low-frequency region that relates to the dihedral angle change (Fig. S6†).24 The relatively smaller reorganization energy of SAF-2NP is derived from the effective suppression of low-frequency vibrations. The above data well support our molecular design that SAF-2NP is rigidified by restricting D–A rotations using acridine with large steric hindrance and locking the flexible benzene with fluorene. Therefore, it is rational to explore the influence of structural stiffness on the non-radiative decay of exciplexes using SAF-2NP, TPA-2NP and TPA-QP as acceptors.Moreover, the D–A intermolecular interactions were investigated experimentally and theoretically. Based on our molecular design concept, mPTZC and TPA-QP cannot form the desired intermolecular HBs in exciplex systems. The assumption was initially verified using fitted atomic charges of the five molecules. As shown in Fig. S7,† although the maximum hydrogen charges of mPTBC and mPTZC are similar, respectively, located near oxygen and sulfur, the oxygen charge is two times more negative than the sulfur charge, providing feasibility for stronger interactions. For SAF-2NP and TPA-2NP, nitrogen atoms in phenanthroline have the most negative charges, and the exposed sites benefit the formation of intermolecular HBs between PXZ and 2NP in mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP and mPTBC
SAF-2NP and mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP. In contrast, the electronegative nitrogen atoms of TPA-QP are shielded by TPA and phenanthrene groups, generating large steric hindrance. The restrained electrostatic potential (RESP) was also used (Fig. S8†), which showed good consistency with the MK method. Fourier Transform Infrared (FT-IR) spectra were recorded to make experimental validation (Fig. S9†). New absorption peaks at 2967 and 2962 cm−1 are, respectively, observed for mPTBC
TPA-2NP. In contrast, the electronegative nitrogen atoms of TPA-QP are shielded by TPA and phenanthrene groups, generating large steric hindrance. The restrained electrostatic potential (RESP) was also used (Fig. S8†), which showed good consistency with the MK method. Fourier Transform Infrared (FT-IR) spectra were recorded to make experimental validation (Fig. S9†). New absorption peaks at 2967 and 2962 cm−1 are, respectively, observed for mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP and mPTBC
SAF-2NP and mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP, which are shifted from the C–H stretching modes (around 3059 cm−1), demonstrating the existence of intermolecular HBs.35,36 Meanwhile, the spectra of the other four blends are the superposition of the components. To better illustrate the intermolecular interactions of mPTBC
TPA-2NP, which are shifted from the C–H stretching modes (around 3059 cm−1), demonstrating the existence of intermolecular HBs.35,36 Meanwhile, the spectra of the other four blends are the superposition of the components. To better illustrate the intermolecular interactions of mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP and mPTBC
SAF-2NP and mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP, the independent gradient model based on Hirshfeld partition (IGMH)37 analysis was carried out using Multiwfn38 based on the optimized geometries. As shown in Fig. 3A, B, D and E, the blue region in the isosurface and scatter plots denotes attractive interaction and the green region represents dispersion-dominant interaction. Prominent C–H⋯N intermolecular HBs are observed with the participation of the active hydrogen next to oxygen and two strong-electronegative nitrogen atoms (Fig. S10†). The central area of the C–H⋯O isosurface appears light blue, indicating that the interactions are weaker than C–H⋯N, but it is also helpful to stabilize the exciplex system. The interaction energy was, respectively, calculated to be −10.09 and −10.19 kcal mol−1 for mPTBC
TPA-2NP, the independent gradient model based on Hirshfeld partition (IGMH)37 analysis was carried out using Multiwfn38 based on the optimized geometries. As shown in Fig. 3A, B, D and E, the blue region in the isosurface and scatter plots denotes attractive interaction and the green region represents dispersion-dominant interaction. Prominent C–H⋯N intermolecular HBs are observed with the participation of the active hydrogen next to oxygen and two strong-electronegative nitrogen atoms (Fig. S10†). The central area of the C–H⋯O isosurface appears light blue, indicating that the interactions are weaker than C–H⋯N, but it is also helpful to stabilize the exciplex system. The interaction energy was, respectively, calculated to be −10.09 and −10.19 kcal mol−1 for mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP and mPTBC
SAF-2NP and mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP considering the basis set superposition error (BBSE), which is in a range of weak intermolecular HBs.23 The interaction region in the electrostatic potential (ESP) map39 (Fig. 3C and F) exhibits red-blue crossings, in line with the previous report that weak HBs are dominated by electrostatic interaction. Intermolecular HBs may benefit the interactions between D and A molecules, facilitating the exciplex formation. Moreover, the exciplex systems can be stabilized, weakening the non-radiative decay.
TPA-2NP considering the basis set superposition error (BBSE), which is in a range of weak intermolecular HBs.23 The interaction region in the electrostatic potential (ESP) map39 (Fig. 3C and F) exhibits red-blue crossings, in line with the previous report that weak HBs are dominated by electrostatic interaction. Intermolecular HBs may benefit the interactions between D and A molecules, facilitating the exciplex formation. Moreover, the exciplex systems can be stabilized, weakening the non-radiative decay.
Based on the above analysis, molecular rigidity and intermolecular interactions are modified by elaborate molecular design. And the pairwise combination of D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A can be fitted into the following four scenarios. mPTBC
A can be fitted into the following four scenarios. mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-QP, mPTZC
TPA-QP, mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP and mPTZC
TPA-2NP and mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-QP contain relatively flexible TPA segments. Besides, the weak electronegativity of sulfur in mPTZC and shielding nitrogen in TPA-QP impede the formation of desired HBs. Therefore, these three exciplexes stand for circumstances that lack both constituent rigidity and effective intermolecular HBs. Likewise, mPTZC
TPA-QP contain relatively flexible TPA segments. Besides, the weak electronegativity of sulfur in mPTZC and shielding nitrogen in TPA-QP impede the formation of desired HBs. Therefore, these three exciplexes stand for circumstances that lack both constituent rigidity and effective intermolecular HBs. Likewise, mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP is expected to have no intermolecular HBs between PTZ and 2NP, while good rigidity is anticipated. mPTBC
SAF-2NP is expected to have no intermolecular HBs between PTZ and 2NP, while good rigidity is anticipated. mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP possesses favorable HBs but serious structural relaxation. Impressively, both were achieved for the proof-of-concept mPTBC
TPA-2NP possesses favorable HBs but serious structural relaxation. Impressively, both were achieved for the proof-of-concept mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP, which is expected to effectively inhibit the non-radiative decay and achieve efficient red emission.
SAF-2NP, which is expected to effectively inhibit the non-radiative decay and achieve efficient red emission.
Photophysical properties
The transient photophysical properties of the six exciplexes were then characterized to study the exciton dynamics. Given the twisted structure of donors and planar geometry of acceptors, the D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A mixing ratio for all six exciplexes was set to be 9
A mixing ratio for all six exciplexes was set to be 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
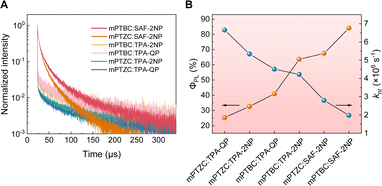
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the first place to avoid concentration quenching. As shown in Fig. 4A and S9† and Table 1, the nanosecond-scale prompt decay lifetime (τp) and microsecond-scale delayed fluorescence lifetime (τd) were obtained for all six exciplexes, validating their TADF characteristics. Note that the multiple RISC channels in all six exciplex systems lead to complexity of the emission processes.40 Herein, we focus on the overall decay characteristics rather than the classification of specific emitting species. TPA-containing exciplexes have larger τds, which can be ascribed to their larger ΔESTs (Fig. S12†). And the longer τds of TPA-2NP and TPA-QP compared with that of SAF-2NP (Fig. S13†) are also responsible for the longer τds of TPA-containing exciplexes since this leads to longer triplet lifetimes in the exciplex systems. The significantly smaller τd of SAF-2NP compared with those of TPA-based acceptors contributes to shorter τds of mPTBC
1 in the first place to avoid concentration quenching. As shown in Fig. 4A and S9† and Table 1, the nanosecond-scale prompt decay lifetime (τp) and microsecond-scale delayed fluorescence lifetime (τd) were obtained for all six exciplexes, validating their TADF characteristics. Note that the multiple RISC channels in all six exciplex systems lead to complexity of the emission processes.40 Herein, we focus on the overall decay characteristics rather than the classification of specific emitting species. TPA-containing exciplexes have larger τds, which can be ascribed to their larger ΔESTs (Fig. S12†). And the longer τds of TPA-2NP and TPA-QP compared with that of SAF-2NP (Fig. S13†) are also responsible for the longer τds of TPA-containing exciplexes since this leads to longer triplet lifetimes in the exciplex systems. The significantly smaller τd of SAF-2NP compared with those of TPA-based acceptors contributes to shorter τds of mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP (22.3 μs) and mPTZC
SAF-2NP (22.3 μs) and mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP (17.5 μs). An increasing trend of ΦPL and a decreasing trend of knr were observed from mPTZC
SAF-2NP (17.5 μs). An increasing trend of ΦPL and a decreasing trend of knr were observed from mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-QP to mPTBC
TPA-QP to mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP, as shown in Fig. 4B. mPTBC
SAF-2NP, as shown in Fig. 4B. mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-QP, mPTZC
TPA-QP, mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP and mPTZC
TPA-2NP and mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-QP have large knrs, and non-radiative decay is gradually reduced after introducing HBs (mPTBC
TPA-QP have large knrs, and non-radiative decay is gradually reduced after introducing HBs (mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP) and the stiff acceptor (mPTZC
TPA-2NP) and the stiff acceptor (mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP). mPTBC
SAF-2NP). mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP possesses the smallest knr. Specifically, in terms of structure rigidification, knrs are, respectively, decreased by 57.9% and 47.6% from mPTZC
SAF-2NP possesses the smallest knr. Specifically, in terms of structure rigidification, knrs are, respectively, decreased by 57.9% and 47.6% from mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-QP (6.65 × 106 s−1) and mPTZC
TPA-QP (6.65 × 106 s−1) and mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP (5.34 × 106 s−1) to mPTZC
TPA-2NP (5.34 × 106 s−1) to mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP (2.80 × 106 s−1). Similarly, a drop rate of 53.1% is obtained from mPTBC
SAF-2NP (2.80 × 106 s−1). Similarly, a drop rate of 53.1% is obtained from mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP to mPTBC
TPA-2NP to mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP. These improvements can be attributed to the bulky acridine and periphery-locking fluorene, which effectively curbs the structural deformation. TPA-QP-based exciplexes have larger knrs than those containing TPA-2NP, which can be ascribed to more severe structural relaxation of TPA-QP (Fig. 2). Comparatively, the introduction of HBs has less effect on knr. It is, respectively, decreased by 29.3% and 6.4% from mPTZC
SAF-2NP. These improvements can be attributed to the bulky acridine and periphery-locking fluorene, which effectively curbs the structural deformation. TPA-QP-based exciplexes have larger knrs than those containing TPA-2NP, which can be ascribed to more severe structural relaxation of TPA-QP (Fig. 2). Comparatively, the introduction of HBs has less effect on knr. It is, respectively, decreased by 29.3% and 6.4% from mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP (2.80 × 106 s−1) to mPTBC
SAF-2NP (2.80 × 106 s−1) to mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP (1.98 × 106 s−1) and from mPTBC
SAF-2NP (1.98 × 106 s−1) and from mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-QP (4.51 × 106 s−1) to mPTBC
TPA-QP (4.51 × 106 s−1) to mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP (4.22 × 106 s−1). This experimentally validates our assumption that intermolecular HBs can rigidify the exciplex systems and weaken non-radiative decay. The enhancement of kf of mPTBC
TPA-2NP (4.22 × 106 s−1). This experimentally validates our assumption that intermolecular HBs can rigidify the exciplex systems and weaken non-radiative decay. The enhancement of kf of mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP compared with that of mPTZC
TPA-2NP compared with that of mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP may originate from the large conjugation of TPA-2NP. Remarkably, mPTBC
SAF-2NP may originate from the large conjugation of TPA-2NP. Remarkably, mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP has the largest ΦPL of 84.1% and the smallest knr, showing a 3.3-fold increase and a 70% decrease compared with those of mPTZC
SAF-2NP has the largest ΦPL of 84.1% and the smallest knr, showing a 3.3-fold increase and a 70% decrease compared with those of mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-QP. According to these results, improving the component stiffness has a dramatic impact on the non-radiative decay of the exciplex systems, while intermolecular HBs have relatively moderate influence and barely work for systems that lack sufficient structural stability. The impressive performance of mPTBC
TPA-QP. According to these results, improving the component stiffness has a dramatic impact on the non-radiative decay of the exciplex systems, while intermolecular HBs have relatively moderate influence and barely work for systems that lack sufficient structural stability. The impressive performance of mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP highlights the significance of the synergy of rigid structures and strong intermolecular interactions that effectively suppress the non-radiative transition.
SAF-2NP highlights the significance of the synergy of rigid structures and strong intermolecular interactions that effectively suppress the non-radiative transition.
| Emitter | λ PL , [nm] | Φ PL , [%] | τ p [ns] | τ d [μs] | k f [106 s−1] | k ISC [107 s−1] | k RISC [105 s−1] | k nr [106 s−1] |
|---|---|---|---|---|---|---|---|---|
a Emission peaks of fluorescence spectra.
b Measured with exciplex solid films (D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A = 9 A = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
c Measured in a nitrogen atmosphere.
d Intersystem crossing rate constant.
e RISC rate constant.
f Singlet non-radiative decay rate constant. 1).
c Measured in a nitrogen atmosphere.
d Intersystem crossing rate constant.
e RISC rate constant.
f Singlet non-radiative decay rate constant.
|
||||||||
mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP SAF-2NP |
610 | 84.1 | 20.7 | 22.3 | 10.50 | 3.57 | 1.74 | 1.98 |
mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP SAF-2NP |
608 | 67.5 | 23.2 | 17.5 | 5.82 | 3.43 | 2.86 | 2.80 |
mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP TPA-2NP |
596 | 63.6 | 18.7 | 65.2 | 7.04 | 4.18 | 0.71 | 4.22 |
mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-QP TPA-QP |
610 | 40.9 | 21.3 | 59.6 | 3.12 | 3.92 | 1.03 | 4.51 |
mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP TPA-2NP |
600 | 32.6 | 15.5 | 77.7 | 2.58 | 5.65 | 1.05 | 5.34 |
mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-QP TPA-QP |
608 | 25.3 | 13.9 | 62.6 | 2.25 | 6.29 | 1.29 | 6.65 |
According to the above studies, the molecular rigidity of red exciplex emitters is the basis for suppressing non-radiative decay. Planar structures are therefore hard to avoid and the consequent concentration quenching would be no longer negligible. Steady-state PL spectra, ΦPL and transient PL curves of mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP with D
SAF-2NP with D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratios from 9
A ratios from 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 6
1 to 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 were measured. Both τp and τd remain nearly identical (Fig. S14 and Table S2†) with increasing concentration of SAF-2NP. However ΦPL shows a decreasing trend, making knr gradually increase from 1.98 × 106 s−1 to 2.72 × 106 s−1. The photophysical properties of other five exciplexes with different D
4 were measured. Both τp and τd remain nearly identical (Fig. S14 and Table S2†) with increasing concentration of SAF-2NP. However ΦPL shows a decreasing trend, making knr gradually increase from 1.98 × 106 s−1 to 2.72 × 106 s−1. The photophysical properties of other five exciplexes with different D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratios were also studied (Fig. S15 and Table S3†). The knrs of TPA-containing exciplexes are much more sensitive to the concentration, demonstrating 1.2–1.7-fold increases from D
A ratios were also studied (Fig. S15 and Table S3†). The knrs of TPA-containing exciplexes are much more sensitive to the concentration, demonstrating 1.2–1.7-fold increases from D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A = 9
A = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 8
1 to 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. However the concentration of SAF-2NP has smaller alterations.
2. However the concentration of SAF-2NP has smaller alterations.
To understand these results, the crystal structures and corresponding packing modes of mPTBC (CCDC 2343106), SAF-2NP (CCDC 2343111) and TPA-QP (CCDC 2370557) are demonstrated. As shown in Fig. S16,† for mPTBC, the stacking of PXZ is effectively avoided by the two carbonitrile arms. No π–π interactions but only C–H⋯N and C–H⋯π interactions were observed. For the acceptors (Fig. S17†), SAF-2NP has head-to-tail stacking with larger intermolecular distances of ∼3.33 and ∼3.45 Å, while TPA-QP shows head-to-head off-center parallel stacking with intermolecular distances of ∼3.27 Å. As the acceptor concentration increases, more serious π–π stacking and thus more severe energy loss can be anticipated. Therefore, all six exciplexes showed greater knr when the acceptor concentration increases. In particular, SAF-2NP-based exciplex emitters are less sensitive owing to the nearly perpendicular structure of SAF.
OLED devices
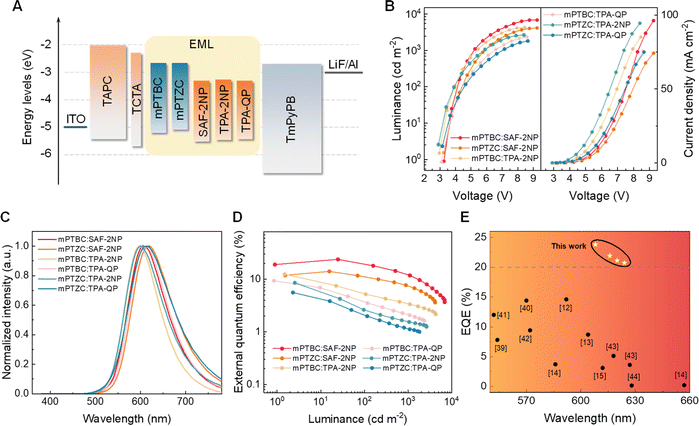
Considering the dramatic contrast in the PL properties of the six exciplexes, their EL properties were then investigated. As shown in Fig. 5A, a simple multi-layer device structure of ITO (110 nm)/TPAC (35 nm)/TCTA (10 nm)/emitting layer (EML, 20 nm)/TmPyPB (65 nm)/LiF (1 nm)/Al (100 nm) was used, where 4,4′-(cyclohexane-1,1-diyl)bis(N,N-di-p-tolylaniline) (TAPC), tris(4-(9H-carbazol-9-yl)phenyl)amine (TCTA) and 3,3′-(5′-(3-(pyridin-3-yl)phenyl)-[1,1′:3′,1′′-terphenyl]-3,3′′-diyl)dipyridine (TmPyPB) were hole transporting, electron blocking and electron transporting layers, respectively (for chemical structures, see Fig. S18†). Indium tin oxide (ITO) and Al, respectively, served as the anode and cathode. LiF improved electron injection. Devices A–F represent the EML using mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP, mPTZC
SAF-2NP, mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP, mPTBC
SAF-2NP, mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP, mPTBC
TPA-2NP, mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-QP, mPTZC
TPA-QP, mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP and mPTZC
TPA-2NP and mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-QP, respectively. As mentioned above, the D
TPA-QP, respectively. As mentioned above, the D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A weight ratio was set to be 9
A weight ratio was set to be 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the first place to suppress the π–π stacking of the acceptors. mPTBC
1 in the first place to suppress the π–π stacking of the acceptors. mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP-based OLEDs demonstrate the highest maximum luminance (Fig. 5B), which can be ascribed to their superior structural stability. The higher electron transport ability of TPA-2NP endows TPA-2NP-based devices with higher current density than those based on TPA-QP (Fig. S19†). According to the EL spectra in Fig. 5C, all six exciplex-based OLEDs exhibited red emission that peaked around 600 nm, similar to their PL spectra. Due to the serious non-radiative decay mainly induced by the flexible TPA group, devices D, E and F showed poor EQEmaxs of 9.34%, 8.55% and 5.58%, respectively. Excitingly, devices B and C demonstrated obvious performance enhancement and achieved a maximum CE/PE/EQE of 24.3 cd A−1/25.8 lm W−1/12.5% and 19.6 cd A−1/16.4 lm W−1/14.1%, respectively (Fig. 5D and Table 2). Consistent with what have been concluded from the PL properties, it is further verified that structural robustness is the priority in constructing high-performance red exciplex emitters, which is demonstrated by more significant improvements after rigidifying the molecules (from mPTZC
SAF-2NP-based OLEDs demonstrate the highest maximum luminance (Fig. 5B), which can be ascribed to their superior structural stability. The higher electron transport ability of TPA-2NP endows TPA-2NP-based devices with higher current density than those based on TPA-QP (Fig. S19†). According to the EL spectra in Fig. 5C, all six exciplex-based OLEDs exhibited red emission that peaked around 600 nm, similar to their PL spectra. Due to the serious non-radiative decay mainly induced by the flexible TPA group, devices D, E and F showed poor EQEmaxs of 9.34%, 8.55% and 5.58%, respectively. Excitingly, devices B and C demonstrated obvious performance enhancement and achieved a maximum CE/PE/EQE of 24.3 cd A−1/25.8 lm W−1/12.5% and 19.6 cd A−1/16.4 lm W−1/14.1%, respectively (Fig. 5D and Table 2). Consistent with what have been concluded from the PL properties, it is further verified that structural robustness is the priority in constructing high-performance red exciplex emitters, which is demonstrated by more significant improvements after rigidifying the molecules (from mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP to mPTZC
TPA-2NP to mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP), as compared with introducing HBs (from mPTBC
SAF-2NP), as compared with introducing HBs (from mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-QP to mPTBC
TPA-QP to mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP). The proof-of-concept mPTBC
TPA-2NP). The proof-of-concept mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP achieved exceptionally maximum CE, PE and EQE of 41.5 cd A−1, 34.7 lm W−1 and 23.8%, respectively, showing a 4.2-fold improvement in EQEmax compared with that of mPTZC
SAF-2NP achieved exceptionally maximum CE, PE and EQE of 41.5 cd A−1, 34.7 lm W−1 and 23.8%, respectively, showing a 4.2-fold improvement in EQEmax compared with that of mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-QP. These results not only take a huge lead to previously reported red OLEDs based on exciplex emitters (Fig. 5E)41–46 but also ranks among the state-of-the-art exciplex-emissive OLEDs (Fig. S20, Table S6†). Furthermore, OLEDs with different D
TPA-QP. These results not only take a huge lead to previously reported red OLEDs based on exciplex emitters (Fig. 5E)41–46 but also ranks among the state-of-the-art exciplex-emissive OLEDs (Fig. S20, Table S6†). Furthermore, OLEDs with different D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A mixing ratios were then fabricated to study the effect of D–D/A–A interactions on device performances. For mPTBC
A mixing ratios were then fabricated to study the effect of D–D/A–A interactions on device performances. For mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP, devices A1–A4 were fabricated with D
SAF-2NP, devices A1–A4 were fabricated with D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratios of 9
A ratios of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8
1, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 7
2, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 6
3 and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, respectively. The spectral redshifts are due to the synergistic effect of increased π–π interactions of SAF-2NP and the self-polarization-induced solid-state solvation effect caused by larger dipole moments of SAF-2NP (1.80 debye) than those of mPTBC (0.055 debye).29 The EQEmaxs gradually decreased with the increase in the acceptor concentration (Fig. S21,†Table 2), showing a similar trend to that observed in photophysical studies, which should be ascribed to exciton quenching due to the π–π interactions of SAF-2NP. Despite this, EQEmaxs of the four devices remain above 20%, with emission peaks ranging from 608 to 624 nm, demonstrating superiority to those concentration-sensitive single-component TADF red emitters.29,30,47–49 The device performances based on other five emitters with different D
4, respectively. The spectral redshifts are due to the synergistic effect of increased π–π interactions of SAF-2NP and the self-polarization-induced solid-state solvation effect caused by larger dipole moments of SAF-2NP (1.80 debye) than those of mPTBC (0.055 debye).29 The EQEmaxs gradually decreased with the increase in the acceptor concentration (Fig. S21,†Table 2), showing a similar trend to that observed in photophysical studies, which should be ascribed to exciton quenching due to the π–π interactions of SAF-2NP. Despite this, EQEmaxs of the four devices remain above 20%, with emission peaks ranging from 608 to 624 nm, demonstrating superiority to those concentration-sensitive single-component TADF red emitters.29,30,47–49 The device performances based on other five emitters with different D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A mixing ratios are shown in Fig. S22 and S23 and Table S5.† Similarly, mPTZC
A mixing ratios are shown in Fig. S22 and S23 and Table S5.† Similarly, mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP also shows moderate concentration quenching with EQEmaxs decreasing from 14.1% to 11.9% as the acceptor concentration increases to 20%. Conversely, TPA-containing exciplex emitters demonstrate significant EQEmax decline with drop rates ranging from 45.7% to 56.7%, indicating that TPA-based acceptors are much more sensitive to concentration and are prone to π–π stacking, which is consistent with their photophysical properties. Based on the above studies, the outstanding performance of device A1 is the result of effectively suppressing the non-radiative decay, including (1) applying stiff structures of the spiro-locked acceptor and the sterically restricted donor that minimize structural relaxation, (2) introducing intermolecular HBs that optimize the D–A interactions and (3) fine-tuned D
SAF-2NP also shows moderate concentration quenching with EQEmaxs decreasing from 14.1% to 11.9% as the acceptor concentration increases to 20%. Conversely, TPA-containing exciplex emitters demonstrate significant EQEmax decline with drop rates ranging from 45.7% to 56.7%, indicating that TPA-based acceptors are much more sensitive to concentration and are prone to π–π stacking, which is consistent with their photophysical properties. Based on the above studies, the outstanding performance of device A1 is the result of effectively suppressing the non-radiative decay, including (1) applying stiff structures of the spiro-locked acceptor and the sterically restricted donor that minimize structural relaxation, (2) introducing intermolecular HBs that optimize the D–A interactions and (3) fine-tuned D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A weight ratios which constrain concentration quenching. Apart from the higher EQEmaxs, it is noteworthy that devices A1 and B had small efficiency roll-offs of 18.9%/39.1%/49.2% and 21.3%/38.3%/47.5%, respectively, at 100/500/1000 cd m−2. In sharp contrast, EQEs of devices C–F decreased much faster that drop rates of EQE100 vary from 57.6% to 69.6%, which can be ascribed to the triplet–triplet annihilation and triplet–polaron annihilation induced by the longer τd of TPA-2NP- and TPA-QP-based exciplexes.
A weight ratios which constrain concentration quenching. Apart from the higher EQEmaxs, it is noteworthy that devices A1 and B had small efficiency roll-offs of 18.9%/39.1%/49.2% and 21.3%/38.3%/47.5%, respectively, at 100/500/1000 cd m−2. In sharp contrast, EQEs of devices C–F decreased much faster that drop rates of EQE100 vary from 57.6% to 69.6%, which can be ascribed to the triplet–triplet annihilation and triplet–polaron annihilation induced by the longer τd of TPA-2NP- and TPA-QP-based exciplexes.
| Device | Emitter | Weight ratio | V on [V] | λ EL [nm] | Maximum CE/PE/EQE [cd A−1/lm W−1/%] | EQE100/EQE500/EQE1000c [%] | CIEb [x, y] |
|---|---|---|---|---|---|---|---|
| a Driving voltage at 1 cd m−2. b At 500 cd m−2. c EQE at 100, 500 and 1000 cd m−2. | |||||||
| A1 |
mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP SAF-2NP |
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3.3 | 608 | 41.5/34.7/23.8 | 19.3/14.5/12.1 | (0.58, 0.42) |
| A2 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
3.1 | 616 | 31.2/27.2/21.9 | 17.6/13.4/11.2 | (0.61, 0.39) | |
| A3 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
3.0 | 620 | 26.8/24.0/21.1 | 17.1/13.3/11.3 | (0.62, 0.38) | |
| A4 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
3.0 | 624 | 24.3/21.8/20.7 | 17.3/13.4/11.3 | (0.63, 0.37) | |
| B |
mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP SAF-2NP |
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3.2 | 620 | 19.6/16.4/14.1 | 11.1/8.7/7.4 | (0.60, 0.40) |
| C |
mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP TPA-2NP |
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.9 | 596 | 24.3/25.8/12.5 | 5.3/3.9/3.4 | (0.56, 0.43) |
| D |
mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-QP TPA-QP |
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3.1 | 612 | 13.3/13.5/9.34 | 3.7/2.5/2.1 | (0.58, 0.41) |
| E |
mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP TPA-2NP |
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.8 | 600 | 14.5/15.7/8.55 | 2.6/1.8/1.6 | (0.56, 0.44) |
| F |
mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-QP TPA-QP |
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3.1 | 612 | 7.51/7.48/5.58 | 2.1/1.3/1.1 | (0.56, 0.43) |
To demonstrate the positive effect of TADF-characterized components on the exciton utilization, 11-([1,1′:3′,1′′-terphenyl]-5′-yl)dipyrido[3,2-a:2′,3′-c]phenazine (TPh-2NP) was designed and synthesized for comparison purposes (Fig. S24†) by replacing TPA with terphenyl. Only a nanosecond-scale lifetime was observed, suggesting its locally excited characteristics. Demonstrating a similar rigidity to that of TPA-2NP (Fig. S24a;† the relatively small RMSDS1–T1 may be due to both S1 and T1 being locally excited states), the EQEmax of the mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPh-2NP-based device is 5.2%, less than half of that of mPTBC
TPh-2NP-based device is 5.2%, less than half of that of mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP (Fig. S25†), indicating that the introduction of TADF components greatly improves the exciton utilization.50 Compared with blue and green emissions, such a positive effect may be amplified in the long-wavelength region where the non-radiative decay becomes overwhelming.8,16
TPA-2NP (Fig. S25†), indicating that the introduction of TADF components greatly improves the exciton utilization.50 Compared with blue and green emissions, such a positive effect may be amplified in the long-wavelength region where the non-radiative decay becomes overwhelming.8,16
Finally, the operational stability of the six exciplex emitters was studied using a device structure of ITO/NPB (40 nm)/Tris-PCz (10 nm)/EML (20 nm)/SF3-TRZ(10 nm)/SF3-TRZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Liq (7
Liq (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 50 nm)/Liq (2 nm)/Al (100 nm). The EML is consistent with those of devices A1 and B–F. As shown in Fig. S26,† LT50 (time corresponding to 50% of the initial luminance) values at 500 cd m−2 of mPTBC
3, 50 nm)/Liq (2 nm)/Al (100 nm). The EML is consistent with those of devices A1 and B–F. As shown in Fig. S26,† LT50 (time corresponding to 50% of the initial luminance) values at 500 cd m−2 of mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP, mPTZC
SAF-2NP, mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP, mPTBC
SAF-2NP, mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP, mPTBC
TPA-2NP, mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-QP, mPTZC
TPA-QP, mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-2NP and mPTZC
TPA-2NP and mPTZC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPA-QP are 349, 213, 186, 157, 153 and 92 hours, following the same trend as their EQEmaxs in Table 2. The longer lifetimes of SAF-2NP-based exciplex emitters may originate from their shorter exciton lifetimes51 as mentioned in the photophysical study.
TPA-QP are 349, 213, 186, 157, 153 and 92 hours, following the same trend as their EQEmaxs in Table 2. The longer lifetimes of SAF-2NP-based exciplex emitters may originate from their shorter exciton lifetimes51 as mentioned in the photophysical study.
Conclusions
In conclusion, we have elucidated that the rigidity of molecular structures, the interactions between D and A molecules as well as D–D/A–A interactions are influential in the non-radiative decay of exciplex emitters. By constructing a rigid system featuring stiff structures that inhibit structural relaxation, HBs which optimize intermolecular interactions and a fine-tuned D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A mixing ratio, the proof-of-concept exciplex emitter mPTBC
A mixing ratio, the proof-of-concept exciplex emitter mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP showed a 3.3-fold higher ΦPL (84.1%) and a 70% lower knr (1.98 × 106 s−1) than the comparison group. mPTBC
SAF-2NP showed a 3.3-fold higher ΦPL (84.1%) and a 70% lower knr (1.98 × 106 s−1) than the comparison group. mPTBC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SAF-2NP also achieved outstanding EL performance, showing a superior EQEmax of 23.8% with an emission peak of 608 nm, which represents a significant advancement over previously reported red OLEDs based on exciplex emitters. The present work provides a comprehensive understanding of non-radiative decay paths of exciplex emitters and can be a guide for developing efficient multicomponent emitting systems.
SAF-2NP also achieved outstanding EL performance, showing a superior EQEmax of 23.8% with an emission peak of 608 nm, which represents a significant advancement over previously reported red OLEDs based on exciplex emitters. The present work provides a comprehensive understanding of non-radiative decay paths of exciplex emitters and can be a guide for developing efficient multicomponent emitting systems.
Data availability
All experimental and calculational data are available from the corresponding author upon reasonable request.Author contributions
H.-Y. Z. led the investigation, conducted formal analysis and wrote the original draft. M. Z. and H. Z. helped with the data curation and validation. H.-Y. Y. helped with the investigation (synthesis). B. H. contributed to formal analysis and funding acquisition. Y.-H. Z. contributed to formal analysis and methodology. H. W. helped with the methodology. S.-L. T. and H. L. helped review and edit the manuscript. C.-J. Z. conceptualized, supervised and funded the project. X.-H. Z. helped review the manuscript and provided funding.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 62222503, 52073040 and 52130304), the Sichuan Science and Technology Program (Grant No. 2024NSFSC0012 and 2023NSFSC1973), the Collaborative Innovation Center of Suzhou Nano Science & Technology, and the Open Research Fund of Chengdu University of Traditional Chinese Medicine State Key Laboratory Southwestern Chinese Medicine Resources (SKLTCM202303).Notes and references
- B. Hu, Z. Yang and F. E. Karasz, J. Appl. Phys., 1994, 76, 2419–2422 CrossRef CAS.
- K. Goushi, K. Yoshida, K. Sato and C. Adachi, Nat. Photonics, 2012, 6, 253–258 CrossRef CAS.
- M. Zhang, C.-J. Zheng, H. Lin and S.-L. Tao, Mater. Horiz., 2021, 8, 401–425 RSC.
- M. Sarma, L.-M. Chen, Y.-S. Chen and K.-T. Wong, Mater. Sci. Eng., R, 2022, 150, 100689 CrossRef.
- X.-K. Liu, Z. Chen, C.-J. Zheng, C.-L. Liu, C.-S. Lee, F. Li, X.-M. Ou and X.-H. Zhang, Adv. Mater., 2015, 27, 2378–2383 CrossRef CAS PubMed.
- M. Wang, Y. Huang, K. Lin, T. Yeh, J. Duan, T. Ko, S. Liu, K. Wong and B. Hu, Adv. Mater., 2019, 31, 1904114 CrossRef CAS PubMed.
- T.-C. Lin, M. Sarma, Y.-T. Chen, S.-H. Liu, K.-T. Lin, P.-Y. Chiang, W.-T. Chuang, Y.-C. Liu, H.-F. Hsu, W.-Y. Hung, W.-C. Tang, K.-T. Wong and P.-T. Chou, Nat. Commun., 2018, 9, 3111 CrossRef PubMed.
- M. Zhang, W. Liu, C. Zheng, K. Wang, Y. Shi, X. Li, H. Lin, S. Tao and X. Zhang, Adv. Sci., 2019, 6, 1801938 CrossRef PubMed.
- J. Zhao, C. Zheng, Y. Zhou, C. Li, J. Ye, X. Du, W. Li, Z. He, M. Zhang, H. Lin, S. Tao and X. Zhang, Mater. Horiz., 2019, 6, 1425–1432 RSC.
- Z. Zhang, D. Dou, R. Xia, P. Wu, E. Spuling, K. Wang, J. Cao, B. Wei, X. Li, J. Zhang, S. Bräse and Z. Wang, Sci. Adv., 2023, 9, eadf4060 CrossRef PubMed.
- N. R. Al Amin, K. K. Kesavan, S. Biring, C.-C. Lee, T.-H. Yeh, T.-Y. Ko, S.-W. Liu and K.-T. Wong, ACS Appl. Electron. Mater., 2020, 2, 1011–1019 CrossRef CAS.
- T.-L. Wu, S.-Y. Liao, P.-Y. Huang, Z.-S. Hong, M.-P. Huang, C.-C. Lin, M.-J. Cheng and C.-H. Cheng, ACS Appl. Mater. Interfaces, 2019, 11, 19294–19300 CrossRef CAS PubMed.
- Y. Qin, P. Tao, L. Gao, P. She, S. Liu, X. Li, F. Li, H. Wang, Q. Zhao, Y. Miao and W. Huang, Adv. Opt. Mater., 2019, 7, 1801160 CrossRef.
- W.-Y. Hung, G.-C. Fang, S.-W. Lin, S.-H. Cheng, K.-T. Wong, T.-Y. Kuo and P.-T. Chou, Sci. Rep., 2014, 4, 5161 CrossRef CAS PubMed.
- M. Zhang, C.-J. Zheng, H.-Y. Zhang, H.-Y. Yang, K. Wang, Y.-Z. Shi, H. Lin, S.-L. Tao and X.-H. Zhang, J. Mater. Chem. C, 2022, 10, 15593–15600 RSC.
- Y. Hu, Y. Yu, Y. Yuan, Z. Jiang and L. Liao, Adv. Opt. Mater., 2020, 8, 1901917 CrossRef CAS.
- R. Englman and J. Jortner, Mol. Phys., 1970, 18, 145–164 CrossRef CAS.
- J. H. Kim, J. H. Yun and J. Y. Lee, Adv. Opt. Mater., 2018, 6, 1800255 CrossRef.
- Q. Zhang, H. Kuwabara, W. J. Potscavage, S. Huang, Y. Hatae, T. Shibata and C. Adachi, J. Am. Chem. Soc., 2014, 136, 18070–18081 CrossRef CAS PubMed.
- H.-Y. Yang, H. Zhang, M. Zhang, X. Fan, H. Lin, S.-L. Tao, C.-J. Zheng and X.-H. Zhang, Chem. Eng. J., 2022, 448, 137717 CrossRef CAS.
- C.-Y. Lin, C.-H. Hsu, C.-M. Hung, C.-C. Wu, Y.-H. Liu, E. H.-C. Shi, T.-H. Lin, Y.-C. Hu, W.-Y. Hung, K.-T. Wong and P.-T. Chou, Nat. Chem., 2024, 16, 98–106 CrossRef CAS PubMed.
- A. Chand, D. K. Sahoo, A. Rana, S. Jena and H. S. Biswal, Acc. Chem. Res., 2020, 53, 1580–1592 CrossRef CAS PubMed.
- S. Emamian, T. Lu, H. Kruse and H. Emamian, J. Comput. Chem., 2019, 40, 2868–2881 CrossRef CAS PubMed.
- R. Lin, J. Liu, W. Xu, Z. Liu, X. He, C. Zheng, M. Kang, X. Li, Z. Zhang, H.-T. Feng, J. W. Y. Lam, D. Wang, M. Chen and B. Z. Tang, Adv. Mater., 2023, 35, 2303212 CrossRef CAS PubMed.
- D.-Q. Wang, M. Zhang, K. Wang, C.-J. Zheng, Y.-Z. Shi, J.-X. Chen, H. Lin, S.-L. Tao and X.-H. Zhang, Dyes Pigm., 2017, 143, 62–70 CrossRef CAS.
- H.-Y. Zhang, H.-Y. Yang, M. Zhang, H. Lin, S.-L. Tao, C.-J. Zheng and X.-H. Zhang, Mater. Horiz., 2022, 9, 2425–2432 RSC.
- C. B. Larsen, H. van der Salm, C. A. Clark, A. B. S. Elliott, M. G. Fraser, R. Horvath, N. T. Lucas, X.-Z. Sun, M. W. George and K. C. Gordon, Inorg. Chem., 2014, 53, 1339–1354 CrossRef CAS PubMed.
- C. Si, A. K. Gupta, B. Basumatary, A. P. McKay, D. B. Cordes, A. M. Z. Slawin, I. D. W. Samuel and E. Zysman-Colman, Adv. Funct. Mater., 2024, 34, 2315935 CrossRef CAS.
- J. Xue, Q. Liang, R. Wang, J. Hou, W. Li, Q. Peng, Z. Shuai and J. Qiao, Adv. Mater., 2019, 31, 1808242 CrossRef PubMed.
- C. Li, R. Duan, B. Liang, G. Han, S. Wang, K. Ye, Y. Liu, Y. Yi and Y. Wang, Angew. Chem., Int. Ed., 2017, 56, 11525–11529 CrossRef CAS PubMed.
- M. Cocchi, D. Virgili, G. Giro, V. Fattori, P. Di Marco, J. Kalinowski and Y. Shirota, Appl. Phys. Lett., 2002, 80, 2401–2403 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16 Rev. A.03., Wallingford, CT, 2016 Search PubMed.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33–38 CrossRef CAS PubMed.
- J. R. Reimers, J. Chem. Phys., 2001, 115, 9103–9109 CrossRef CAS.
- M. Zhang, C. Zheng, K. Wang, Y. Shi, D. Wang, X. Li, H. Lin, S. Tao and X. Zhang, Adv. Funct. Mater., 2021, 31, 2010100 CrossRef CAS.
- X. Du, X. Lu, J. Zhao, Y. Zhang, X. Li, H. Lin, C. Zheng and S. Tao, Adv. Funct. Mater., 2019, 29, 1902078 CrossRef.
- T. Lu and Q. Chen, J. Comput. Chem., 2022, 43, 539–555 CrossRef CAS PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- J. Zhang and T. Lu, Phys. Chem. Chem. Phys., 2021, 23, 20323–20328 RSC.
- V. Jankus, P. Data, D. Graves, C. McGuinness, J. Santos, M. R. Bryce, F. B. Dias and A. P. Monkman, Adv. Funct. Mater., 2014, 24, 6178–6186 CrossRef CAS.
- W.-Y. Hung, G.-C. Fang, Y.-C. Chang, T.-Y. Kuo, P.-T. Chou, S.-W. Lin and K.-T. Wong, ACS Appl. Mater. Interfaces, 2013, 5, 6826–6831 CrossRef CAS PubMed.
- B. Zhao, T. Zhang, B. Chu, W. Li, Z. Su, Y. Luo, R. Li, X. Yan, F. Jin, Y. Gao and H. Wu, Org. Electron., 2015, 17, 15–21 CrossRef CAS.
- D. Chen, G. Xie, X. Cai, M. Liu, Y. Cao and S. Su, Adv. Mater., 2016, 28, 239–244 CrossRef CAS PubMed.
- G. Grybauskaite-Kaminskiene, K. Ivaniuk, G. Bagdziunas, P. Turyk, P. Stakhira, G. Baryshnikov, D. Volyniuk, V. Cherpak, B. Minaev, Z. Hotra, H. Ågren and J. V. Grazulevicius, J. Mater. Chem. C, 2018, 6, 1543–1550 RSC.
- G. Liu, T. Huang, H. Wang, C. Hsu, P. Chou, W. Hung and K. Wong, Chem.–Eur. J., 2023, 29, e202203660 CrossRef CAS PubMed.
- M. Chapran, P. Pander, M. Vasylieva, G. Wiosna-Salyga, J. Ulanski, F. B. Dias and P. Data, ACS Appl. Mater. Interfaces, 2019, 11, 13460–13471 CrossRef CAS PubMed.
- Y. Zhang, Q. Ran, Q. Wang, Y. Liu, C. Hänisch, S. Reineke, J. Fan and L. Liao, Adv. Mater., 2019, 31, 1902368 CrossRef CAS PubMed.
- Z. Cai, X. Wu, H. Liu, J. Guo, D. Yang, D. Ma, Z. Zhao and B. Z. Tang, Angew. Chem., Int. Ed., 2021, 60, 23635–23640 CrossRef CAS PubMed.
- H. Wang, K. Wang, J. Chen, X. Zhang, L. Zhou, X. Fan, Y. Cheng, X. Hao, J. Yu and X. Zhang, Adv. Funct. Mater., 2023, 33, 2304398 CrossRef CAS.
- W. Liu, J.-X. Chen, C.-J. Zheng, K. Wang, D.-Y. Chen, F. Li, Y.-P. Dong, C.-S. Lee, X.-M. Ou and X.-H. Zhang, Adv. Funct. Mater., 2016, 26, 2002–2008 CrossRef CAS.
- L.-S. Cui, A. J. Gillett, S.-F. Zhang, H. Ye, Y. Liu, X.-K. Chen, Z.-S. Lin, E. W. Evans, W. K. Myers, T. K. Ronson, H. Nakanotani, S. Reineke, J.-L. Bredas, C. Adachi and R. H. Friend, Nat. Photonics, 2020, 14, 636–642 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC mPTBC: 2343106; SAF-2NP: 2343111; TPA-QP: 2370557. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc03667k |
| This journal is © The Royal Society of Chemistry 2024 |