Regulation of band gap and localized surface plasmon resonance by loading Au nanorods on violet phosphene nanosheets for photodynamic/photothermal synergistic anti-infective therapy†
Qiudi
Shen
a,
Zhihao
Li
b,
Haoran
Bai
a,
Mengyue
Gu
c,
Jing
Kang
*a,
Ran
Jia
 d,
Jinying
Zhang
d,
Jinying
Zhang
 *c and
Alideertu
Dong
*c and
Alideertu
Dong
 *a
*a
aCollege of Chemistry and Chemical Engineering, Engineering Research Center of Dairy Quality and Safety Control Technology, Ministry of Education, Inner Mongolia University, 235 University West Street, Hohhot 010021, China. E-mail: dongali@imu.edu.cn
bDepartment of Chemistry, College of Sciences, Northeastern University, Shenyang 110819, China
cState Key Laboratory of Electrical Insulation and Power Equipment, Center of Nanomaterials for Renewable Energy (CNRE), School of Electrical Engineering, Xi’an Jiaotong University, Xi’an 710049, China
dInstitute of Theoretical Chemistry, College of Chemistry, Jilin University, 130023 Changchun, P. R. China
First published on 12th March 2024
Abstract
In the face of the serious threat to human health and the economic burden caused by bacterial antibiotic resistance, 2D phosphorus nanomaterials have been widely used as antibacterial agents. Violet phosphorus nanosheets (VPNSs) are an exciting bandgap-adjustable 2D nanomaterial due to their good physicochemical properties, yet the study of VPNS-based antibiotics is still in its infancy. Here, a composite of gold nanorods (AuNRs) loaded onto VPNS platforms (VPNS/AuNR) is constructed to maximize the potential of VPNSs for antimicrobial applications. The loading with AuNRs not only enhances the photothermal performance via a localized surface plasmon resonance (LSPR) effect, but also enhances the light absorption capacity due to the narrowing of the band gap of the VPNSs, thus increasing the ROS generation capacity. The results demonstrate that VPNS/AuNR exhibits outstanding antibacterial properties and good biocompatibility. Attractively, VPNS/AuNR is then extensively tested for treating skin wound infections, suggesting promising in vivo antibacterial and wound-healing features. Our findings may open a novel direction to develop a versatile VPNS-based treatment platform, which can significantly boost the progress of VPNS exploration.
Introduction
As one of the indispensable elements in living organisms, phosphorus has a significant impact on life.1–3 Phosphorus exists in all cells of the human body and participates in a lot of physiological chemical reactions.4–10 Recently, two-dimensional black phosphorus (BP) has been developed for biological applications due to its low toxicity and biodegradability. Upon light irradiation, reactive oxygen species (ROS) or heat can be generated for disease therapy and antibacterial activity.11–18 In the post-antibiotics era, bacterial resistance has seriously threatened human life and health,19–21 and there is an urgent need to explore new antimicrobial agents with multimodal antimicrobial mechanisms to combat drug-resistant bacteria. BP has shown excellent antibacterial ability via photodynamic therapy (PDT) and photothermal therapy (PTT) effects. However, BP is easily degraded in vivo, which leads to a decrease in the PDT performance of BP, as well as causing a local high concentration of phosphorus in vivo, further inducing biotoxicity in the body.22–25Recently, the newly synthesized violet phosphorus nanosheets (VPNSs) have attracted extensive attention across various fields, owing to their good stability, biocompatibility, tunable band gap, high charge carrier mobility, and unique in-plane anisotropy.26–35 In particular, VPNSs have demonstrated potential as a novel antibacterial agent to combat pathogenic bacteria through PDT without inducing bacterial resistance. Meanwhile, VPNSs show higher stability than BP due to their unique structure, which can avoid the issue of a local high concentration of phosphorus in vivo and the decrease in PDT performance caused by the degradation of BP in the body.26,36 VPNSs can produce ROS under irradiation conditions, generating them via a thickness-dependent adjustable band gap (1.7–2.3 eV).26,37,38 These light-produced ROS can disrupt the integrity of pathogens, leading to bacterial death.39–41 However, the rapid photo-induced recombination of photoexcited electron–hole pairs and the low absorption of light impose limitations on the generation of ROS, which is closely associated with antibacterial efficiency.42,43 To overcome these limitations, a strategy is proposed of loading the VPNSs with metal nanoparticles, which can inhibit the recombination of free charges by accelerating the separation and transport of photogenerated electron–hole pairs to promote increased ROS production.44–46 In particular, VPNSs have a high specific surface area and folded structure, which provide a favorable substrate for metal loading.
Nanomaterials have attracted much attention for their specific surface–interface interactions at the nanoscale, where one of the most fascinating properties of precious metals such as Au and Ag is their localized surface plasmon resonance (LSPR) effect, which is generated by conducting electrons in response to collective oscillations of incident light under resonant conditions.47–49 The combination of plasmonic precious metals and semiconductors provides a new opportunity to overcome low photocatalytic antibacterial activity, due to the fact that plasmonic precious metals can effectively improve the photocatalytic performance of semiconductors by enhancing the absorption in the visible and near-infrared regions and inhibiting the electron–hole recombination.50–53 Au nanorods (AuNRs) have been shown to enhance the direct electron transfer effect and thus the photosensitization process due to the strong local field around the tip of these types of anisotropic structures.54,55 In addition, the conduction electrons within AuNR nanostructures could induce LSPR, which facilitates the occurrence of nonradiative relaxation through electron–phonon and phonon–phonon coupling and thereby generates localized hyperthermia. The photothermal effects induced by AuNRs can be controlled by changing their size, shape, and degree of aggregation.56–58 PTT can damage the integrity of pathogenic bacteria under NIR irradiation;59,60 meanwhile, it is difficult for bacteria to develop resistance to PTT by blocking or reducing absorption, increasing metabolism, and drug excretion.61,62 Hence, AuNRs are a perfect candidate for photothermal therapeutics in biomedicine.
Due to the rapid photoinduced recombination of electron–hole pairs and the phenomenon of low light absorption in VPNSs, the antibacterial efficiency is limited. In this work, we aim to construct a synergistic antibacterial platform with enhanced photodynamic and photothermal antibacterial mechanisms by loading AuNRs with enhanced photocatalytic performance and excellent photothermal performance onto the VPNSs. The results show that the loading with AuNRs effectively improves the antimicrobial activity of VPNSs by generating more ROS and providing excellent photothermal properties. Fortunately, the antimicrobial nanocomposites could be synthesised in a facile, environmentally benign manner, and were successfully utilized for treating wound infections in vivo, thus offering potential applications in designing smart and multifunctional VP-based nanocomposites for biomedical research (Scheme 1).
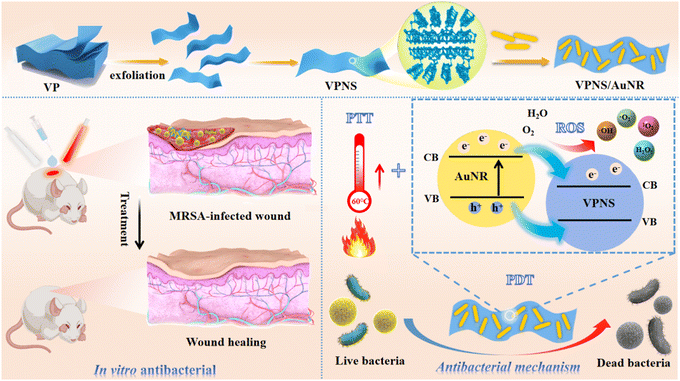 | ||
| Scheme 1 The preparation process of VPNS/AuNR and its synergistic antibacterial mechanism against bacteria via PDT and PTT for the treatment of wound infection. | ||
Results and discussion
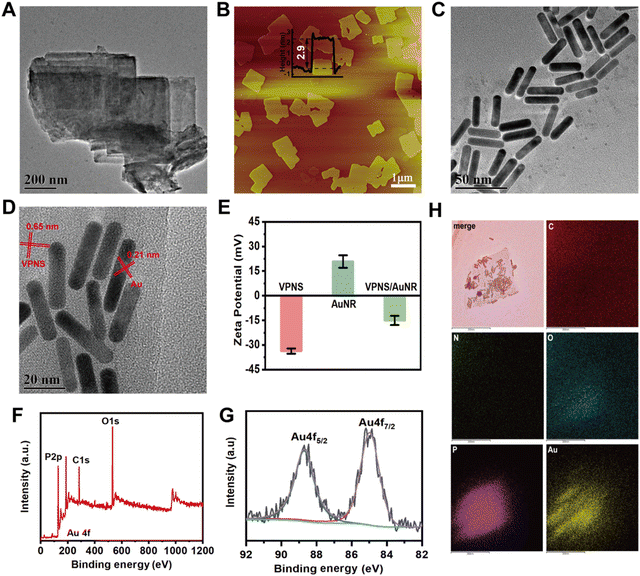
As illustrated in Fig. S1 (ESI†), scanning electron microscopy (SEM) shows that bulk violet phosphorus (VP) has a multilayer structure. The VPNSs were obtained via a solvent exfoliation method from bulk VP under mild conditions. Transmission electron microscopy (TEM) shows that VPNSs have a high specific surface area and their edges are irregular and rough after solution exfoliation (Fig. 1A). As shown by atomic force microscopy (AFM), the average thickness of the as-exfoliated VPNSs is around 2.9 nm (Fig. 1B). As shown in Fig. 1C and D, AuNRs were synthesized and characterized via TEM and ultraviolet-visible (UV-vis) absorption spectroscopy. The TEM image shows AuNRs with a size of about 30 nm × 8 nm and good monodispersity (Fig. 1C and Fig. S2, ESI†). The UV-vis spectrum reveals that the AuNRs have a characteristic absorption peak at 845 nm, which indicates their strong NIR absorption ability (Fig. S3, ESI†). VPNS/AuNR was obtained by successfully loading AuNRs on the VPNS surface through electrostatic interactions (Fig. S4, ESI†). The HRTEM image shows that the lattice spacings of the VPNSs and AuNRs are 0.65 nm and 0.21 nm, respectively (Fig. 1D).The VPNS/AuNR powder and its suspension in water are shown in Fig. S5 (ESI†). We have also compared the zeta potentials. VPNSs are negatively charged, while AuNRs are positive. The zeta potential of VPNSs changes significantly from −33.7 mV to −14.9 mV after the conjugation with the AuNRs (Fig. 1E). X-ray photoelectron spectroscopy (XPS) (Fig. 1F) clearly shows the Au signal detected from the VPNS/AuNR nanocomposite, indicating that AuNRs were successfully loaded onto the surface of the VPNSs. The XPS spectrum of Au 4f (Fig. 1G) shows doublets at 84.9 eV (4f7/2) and 88.7 eV (4f5/2), characteristic of metallic Au. TEM mapping analyses (Fig. 1H) reveal the existence and homogeneous distribution of phosphorus and gold elements across the whole of the samples, further indicating that the AuNRs were successfully loaded on the surface of the VPNSs.
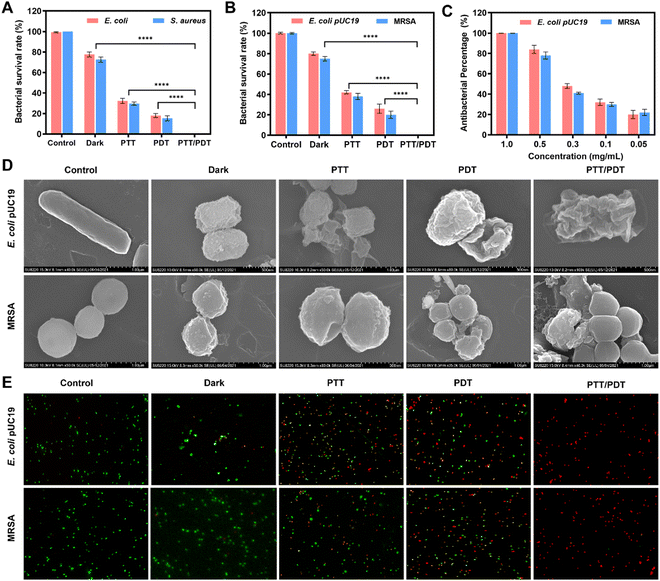
Based on VPNSs’ excellent photodynamic performance and AuNRs’ outstanding photothermal capability, we screened the functionalized VPNS/AuNR as a potential antibiotic by determining the bacterial survival rate using the colony-counting method. In particular, we chose 107 colony-forming units (CFU per mL) of Gram-negative Escherichia coli (E. coli) and Gram-positive Staphylococcus aureus (S. aureus), along with multidrug-resistant (MDR) counterparts (methicillin-resistant Staphylococcus aureus (MRSA) and Escherichia coli pUC19 (E. coli pUC19)) as model bacteria. To explore the synergistic antibacterial ability of VPNS/AuNR, VPNS/AuNR was applied with the following different treatment conditions: (1) control, (2) dark, (3) PTT (808 nm, 10 min), (4) PDT (LED, 60 min), and (5) PTT/PDT (808 nm, 10 min + LED, 60 min). As shown in Fig. 2A and B, after application of 1.0 mg mL−1 of VPNS/AuNR in the dark, the results indicated that VPNS/AuNR scarcely had a sterilizing impact on the bacterial survival ability of the model bacteria without any laser irradiation. The bacterial survival rates of the PTT group and PDT group were approximately 40% and 20%, respectively. Meanwhile, almost no bacteria survived in the PTT/PDT group, with the antibacterial efficiency of the VPNS/AuNR being 99.99% under the combined 808 nm laser and LED light irradiation, which indicated that the synergism of PTT and PDT could greatly improve the antibacterial efficiency. The corresponding agar plate digital photos further detected that no colonies were observed in the PTT/PDT group after the synergistic antibacterial action of VPNS/AuNR, while some bacteria still survived for the other groups (Fig. S6, ESI†). We also evaluated the effect of LED light exposure time on the antimicrobial performance of VPNS/AuNR. As shown in Fig. S7 (ESI†), the antibacterial efficiency gradually increased with the increase in light exposure time. When the LED light irradiation is applied for 60 min, an antibacterial efficiency of 99.0% can be achieved. As shown in Fig. 2C, dynamic testing also revealed that the antibacterial properties of VPNS/AuNR against E. coli pUC19 and MRSA were concentration-dependent.
We also observed the morphological changes and membrane integrity of E. coli pUC19 and MRSA treated with VPNS/AuNR under different conditions, using scanning electron microscopy (SEM). As shown in Fig. 2D, the bacteria in the control group showed smooth surfaces, intact cell membranes and no cytoplasmic leakage, indicating the bacteria survived well. Meanwhile, the bacterial membranes of other treated groups showed different degrees of wrinkling and damage, among which the PTT/PDT group, exposed to NIR laser and LED light irradiation at the same time, was the most damaged, indicating that the combination of PTT and PDT had the best effect on membrane destruction and could completely kill the bacteria. In addition, VPNS/AuNR-affected membranes were further observed using a live/dead BacLight Kit; dead bacteria were stained red with propidium iodide (PI) and live bacteria were stained green with SYTO 9. In Fig. 2E, strong green fluorescence dots correspond to controls with live bacteria, while increasingly intense red fluorescence dots were detected after NIR laser and LED light irradiation, revealing that bacteria had been killed. These results further confirm the high toxicity of VPNS/AuNR to pathogens under the synergistic mechanism of PDT and PTT, which is consistent with the plate counting method described above.
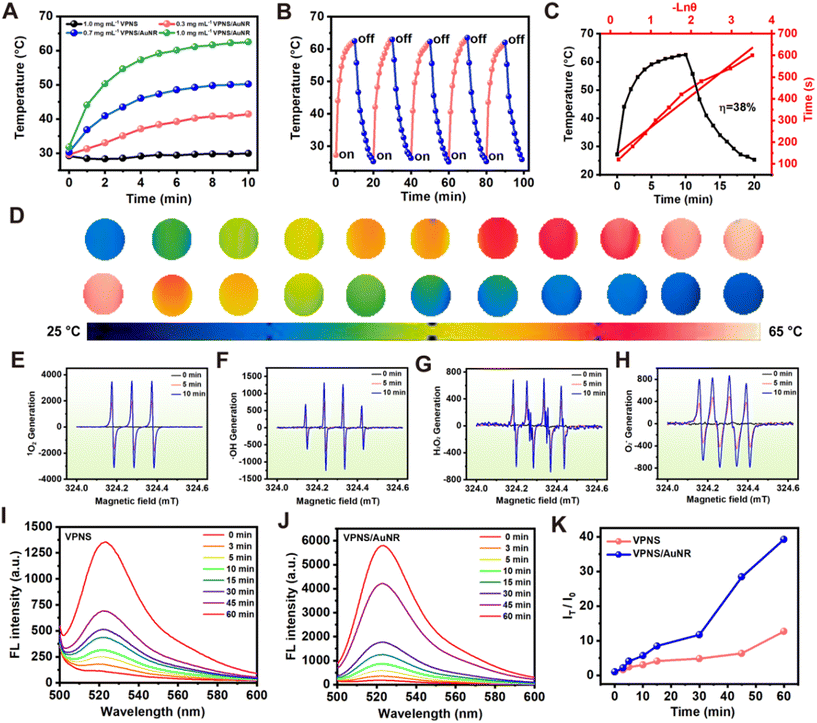
To assess the photothermal effect of VPNS/AuNR, we exposed aqueous dispersions of VPNS/AuNR at varying concentrations to an 808 nm laser at a power intensity of 1.0 W cm−2 for 10 min. Fig. 3A shows the concentration-dependent photothermal effect of the VPNS/AuNR suspensions. When VPNS/AuNR at a low concentration of 0.3 mg mL−1 is irradiated with the 808 nm laser for 10 min, the temperature of VPNS/AuNR rapidly increases to 40 °C. Subsequently, as the concentration rises to 1.0 mg mL−1, the temperature further escalates to 63 °C. The photothermal properties of the AuNRs are concentration-dependent. When the AuNRs at a low concentration of 0.3 nM are irradiated with the 808 laser for 10 min, the temperature of AuNR rapidly increases to about 60 °C, indicating that AuNRs have excellent photothermal properties (Fig. S8, ESI†). Meanwhile VPNSs have poor photothermal performance, only increasing slightly in temperature (∼30 °C) at the same concentration, which indicates that the loading with the gold nanorods greatly improves the photothermal performance. The photothermal stability of VPNS/AuNR was assessed as depicted in Fig. 3B. There was no obvious change in the photothermal effect of the VPNS/AuNR nanocomposite during five “on–off” heating cycles; the corresponding thermal imaging of a photothermal cycle is shown in Fig. 3D.
The photothermal conversion efficiency (PCE) of VPNS/AuNR is about 38% (Fig. 3C). Analysis of the time-dependent photothermal stability further showed that VPNS/AuNR could still reach about 60 °C even after 120 h storage, with no significant difference from the initial period (Fig. S9, ESI†). Electron spin resonance (ESR) spectroscopy was also employed to detect the potential generation of ROS by VPNS/AuNR under both dark and light-irradiation conditions. As illustrated in Fig. 3E–H, VPNSs exhibited the generation of four distinct ROS signals, namely those for singlet oxygen (1O2), hydroxyl radicals (˙OH), hydrogen peroxide (H2O2), and superoxide anion radicals (˙O2−), specifically under light exposure. Intriguingly, no ROS signals were detected in the absence of light irradiation. Furthermore, the intensity of these ROS signals demonstrated an increase with prolonged light exposure, suggesting a correlation between ROS production and aging time.
The photodynamic effects of VPNSs and VPNS/AuNR were evaluated with a typical ROS probe, 2,7-DCFH-DA (2′,7′-dichlorodihydrofluorescein diacetate), which can be converted into fluorescent 2-(3,6-diacetoxy-2,7-dichloro-9H-xanthen-9-yl)benzoic acid (DCF) in the presence of ROS. In the co-incubation of VPNS with DCFH-DA under LED irradiation, the oxidized DCF exhibited a strong fluorescence peak around 525 nm. Notably, the fluorescence intensity demonstrated a positive correlation with the extension of irradiation time (Fig. 3I), indicating the continuous accumulation of ROS with extended illumination. Importantly, it is noteworthy that the VPNS/AuNR group exhibited a stronger fluorescence intensity compared to the VPNS group under the same conditions (Fig. 3J). To quantify this difference, we calculated the fluorescence intensity ratio (IT/I0) between VPNS/AuNR and VPNS, revealing that the ability of VPNS/AuNR to generate ROS was four times that of VPNS alone (Fig. 3K). The results showed that VPNS/AuNR had a more potent photodynamic effect for the killing of bacteria.
To further identify the type of ROS, 9,10-anthracenediylbis(methylene)dimalonic acid (ABDA), dihydrorhodamine 123 (DHR 123), and hydroxyphenyl fluorescein (HPF) were utilized to detect 1O2, ˙O2−, and ˙OH, respectively. The absorbance of ABDA solutions remained constant after LED irradiation for 60 min (Fig. S10A, ESI†). In contrast, the absorption intensity of ABDA decreased in the presence of VPNS/AuNR, suggesting that VPNS/AuNR possibly produced 1O2 (Fig. S10B and C, ESI†). Meanwhile, the fluorescence intensities of DHR 123 and HPF were also measured. No significant fluorescence increase was observed in the DHR-123-only or HPF-only group (Fig. S11A and S12A, ESI†). However, the fluorescence intensity significantly increased within 60 min upon the addition of VPNS/AuNR under LED irradiation, reflecting the generation of ˙O2−, and ˙OH (Fig. S11B and S12B, ESI†).
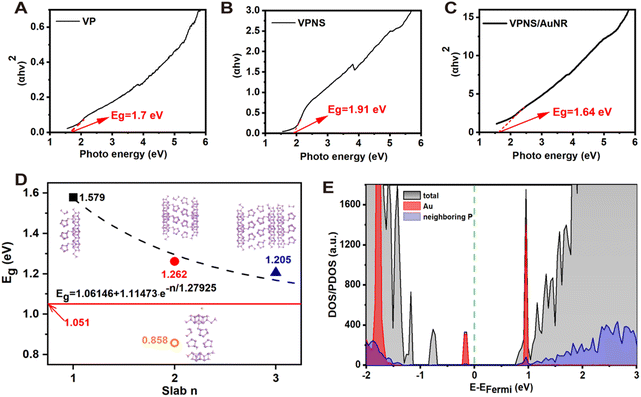
We sought to further explore the reasons why VPNS/AuNR generates more ROS than VPNSs. Firstly, UV-vis spectra of VP, VPNSs, and VPNS/AuNR were measured (Fig. S13, ESI†) and the Tauc curves were analyzed. As shown in Fig. 4A, the band gap of VP is 1.70 eV. With its decreased number of layers, the band gap of VPNSs increases to 1.91 eV (Fig. 4B). However, the band gap of the gold-loaded VPNS/AuNR system is significantly reduced to 1.64 eV, which is even lower than the VP band gap (Fig. 4C). We speculate that the decrease in the VPNS/AuNR band gap is the main reason for producing more ROS.
Furthermore, we proceed to validate the impact of loading with AuNR on the system's band gap via theoretical calculations. As exhibited in Fig. S14 (ESI†), the bulk violet phosphorus features stacked layered phosphorus atoms, where a single layer of violet phosphorus actually contains two sublayers. According to our calculations, the bond length between two sublayers in the bulk system is around 2.167 Å, where the interlayer distance is only 1.096 Å. The bond lengths between two sublayers in slab systems with 3, 2, and 1 layer(s) are 2.171 Å, 2.171 Å, and 2.175 Å, respectively. However, the interlayer distances in tri- and bi-layer systems increase significantly to 1.476 Å and 1.471 Å, respectively. Note that the interlayer distance is defined here as the height difference between the lowest and highest P atoms of two adjacent layers in their normal direction. In order to avoid a tedious simulation, a single Au atom is deposited onto the surface of the bilayer violet phosphorus system for a qualitative evaluation. As shown in Fig. 4D, the single Au atom is not embedded into the phosphorus layer. The corresponding adsorption energy is just −0.134 eV, in the range of typical physisorption. The distance between the Au atom and its nearest neighboring P atom is around 3.796 Å, which is obviously longer than the common Au–P bonds of around 2.4 Å.63
According to our calculations at the PBE level, the band gaps of the VP-slab models decrease exponentially to the one of the bulk VP-system (∼1.051 eV), as shown in Fig. 4D. Decoration with a single Au atom can significantly reduce the band gap of the slab-2 system to 0.858 eV, which is even lower than that of the bulk system. Although the PBE functional always underestimates the band gap of semiconductors, the results obtained at the same theoretical level can still be used for qualitative comparisons. This trend in the band gap variations is indeed consistent with the experimental observations. The rapid reduction of the band gap in the slab-2 system decorated with an Au atom just occurs due to the defect level. As shown in the DOS profiles (Fig. 4E), a new valence band maximum (VBM) is introduced by the Au atom, while the conduction band minimum (CBM) is still contributed by the P atoms far from the Au atom, which is in accordance with the experimental results.
Therefore, based on the density functional theory (DFT) calculations, it was found that the light absorption capacity of VPNS/AuNR was improved after electrostatic interaction with AuNRs. In summary, these theoretical results show that AuNRs were anchored on the surface of VPNSs through electrostatic interactions, which resulted in the emergence of a new intermediate state that appeared to change the band gap, resulting in improved light absorption of the VPNS/AuNR nanocomposite.
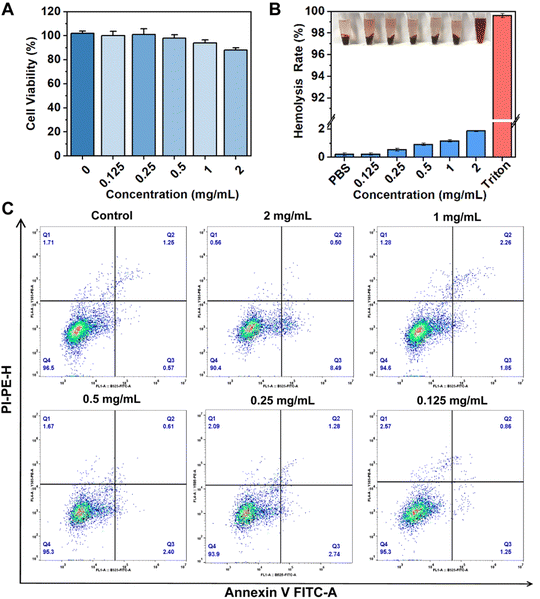
Based on the excellent antibacterial ability of VPNS/AuNR, it is crucial to evaluate the biocompatibility of VPNS/AuNR before its wider application in vivo. First, the cell viability of VPNS/AuNR was evaluated using the CCK-8 method (Fig. 5A). The cell viability of NIH 3T3 cells was above 80% at VPNS/AuNR concentrations ranging from 0.125 to 2.0 mg mL−1, showing that VPNS/AuNR showed low toxicity to NIH 3T3 cells and had good biocompatibility. The hemolysis test also serves as a crucial indicator in validating the applicability of VPNS/AuNR in vivo. We designated PBS and Triton X-100 as negative and positive controls, respectively. As shown in Fig. 5B, following the incubation of blood cells with VPNS/AuNR and subsequent centrifugation, the supernatant was clear and transparent, indicating that the integrity of red blood cells remained intact.
Notably, even at a concentration of 2.0 mg mL−1, the hemolysis rate of VPNS/AuNR was only 1.9%, which is far lower than the ASTM standard of the hemolysis rate of biological materials (<5%). Furthermore, the effect of VPNS on cell viability was assessed using flow cytometry analysis. In the flow cytometry plots, the lower left quadrant delineates the population of surviving cells, while the remaining quadrants indicate cells in early apoptosis (lower right quadrant) and late apoptosis (upper right quadrant) and necrotic cells (upper left quadrant), respectively. After the VPNS/AuNR was incubated with NIH 3T3 cells for 24 h, the annexin V-FITC/PI co-staining showed that the proportion of cells appearing in the lower left quadrant was above 90%, which showed no difference from the control group, indicating that VPNS/AuNR did not induce apoptosis and had good cell compatibility (Fig. 5C).
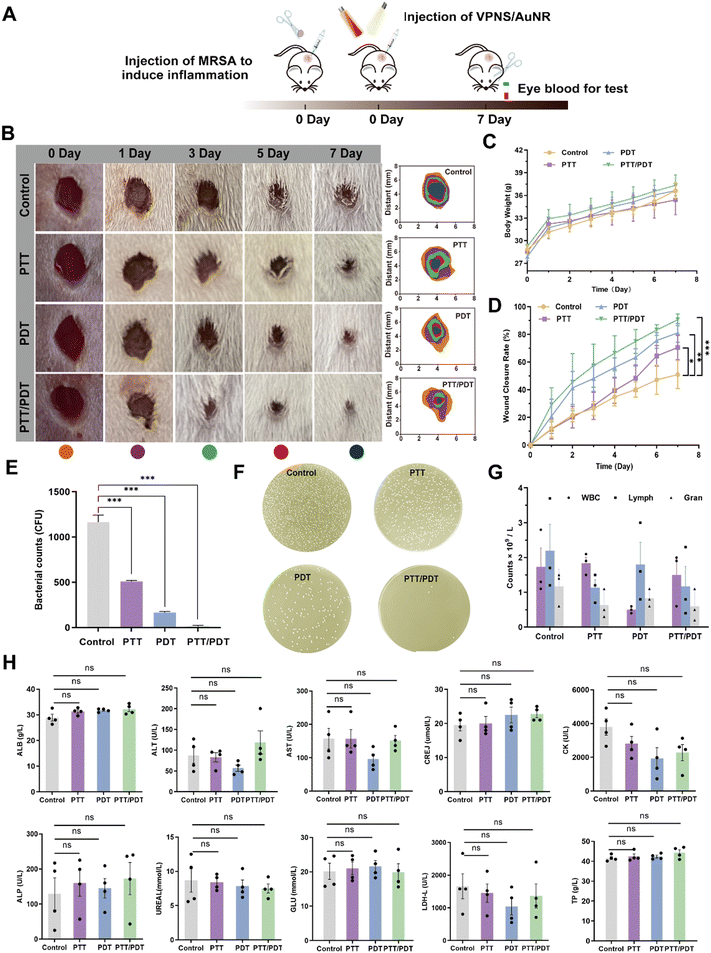
To demonstrate the potential application of VPNS/AuNR to combat bacterial infections in vivo, we first applied VPNS/AuNR in a mouse skin-infection model. A wound-infection model in mice was established using MRSA (107 CFU per mL), which was followed by different treatments with VPNS/AuNR (Fig. 6A). In order to determine the synergistic antibacterial effect of VPNS/AuNR, the mice were freely divided into the following four groups: (1) control (PBS), (2) PTT (808 nm, 10 min), (3) PDT (LED, 60 min) and (4) PTT/PDT (808 nm, 10 min + LED, 60 min), and the four groups of mice were treated for 7 days. The wound temperature of the mice increased to 47.5 °C after 10 min irradiation with an 808 nm laser, indicating that VPNS/AuNR has good photothermal effects in vivo (Fig. S15, ESI†). The body weight of the mice steadily increased in line with their growth pattern over 7 days (Fig. 6C). As shown in the digital pictures of wound recovery in Fig. 6B, the wound healing effect in the PTT, PDT and PTT/PDT groups irradiated with light was significantly faster than that in the control group. In particular, the PTT/PDT group treated with the combined irradiation of an 808 nm laser and LED light had the fastest healing rate of almost 85%, much higher than that of the control group with a healing rate of 40% (Fig. 6D).
The colony-counting method was also used to assess bacterial survival at the site of wound infection on day 7. In Fig. 6E and F, the lowest survival rate of bacteria in the PTT/PDT group indicated that VPNS/AuNR had the best antibacterial effect and healing ability under the combined 808 nm laser and LED light irradiation. In addition, there was no significant difference between the experimental group and the healthy group in the blood routine indices related to inflammation, including white blood cells (WBC), lymphocytes (Lymph), and neutrophils (Gran), indicating that VPNS/AuNR had anti-infection ability in vivo (Fig. 6G). The biosafety of VPNS/AuNR in vivo was also evaluated by detecting the biochemical indices of the mice (Fig. 6H), namely albumin (ALB), glutamic–pyruvic transaminase (ALT), glutamic oxaloacetic transaminase (AST), creatinine (GREJ), creatine kinase (CK), alkaline phosphatase (ALP), urea (UREAL), glucose (GLU), lactate dehydrogenase (LDH-L), and total protein (TP). The experimental results showed that the biochemical indices of the experimental group were no different from those of the control group, indicating that VPNS/AuNR had high biocompatibility and could be safely used in vivo.
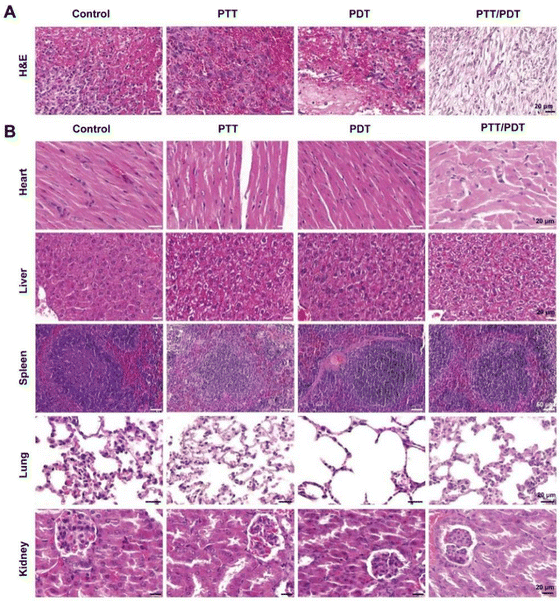
Hematoxylin–eosin (H&E) staining revealed wound healing in different treatment groups at the histological level. As shown in Fig. 7A, in the control group, there was no granulation tissue growth in the central area of the wound, obvious edema, bleeding, and cellulose exudation were observed, and neutrophils diffuse and moist within the wound indicate an inflammatory response. Wound tissue in the PDT and PTT groups showed mild congestion and less diffuse neutrophil infiltration compared to the control group. In the PTT/PDT group, the wound was filled with mature granulation tissue and the epidermis was completely regenerated, indicating the best wound healing. We also examined the major organs of the mice, namely the heart, liver, spleen, lungs, and kidneys, to further verify the biosafety of VPNS/AuNR in vivo using the H&E staining method. Fig. 7B shows that each organ has a clear outline, complete structure, orderly arrangement, and obvious marginal area, and no obvious lesions are observed, indicating that VPNS/AuNR has excellent biocompatibility and can be used safely in vivo.
Conclusions
In summary, we constructed a synergistic antibacterial nanosystem, VPNS/AuNR, for combined photothermal and photodynamic wound infection treatment in vivo by loading AuNRs with excellent photothermal properties onto VPNSs. Surprisingly, the loading with AuNRs not only enhances the photothermal performance but also narrows the band gap of the VPNSs, leading to increased generation of ROS, closely associated with antibacterial efficacy. The experimental findings demonstrate that VPNS/AuNR exhibits outstanding antibacterial properties and good biocompatibility, highlighting its potential for accelerating wound healing in vivo. This research provides a new strategy for constructing effective nanotherapeutic systems and is expected to provide new insights for more biological applications.Author contributions
Q. S., J. K., and A. D. designed the research plan. Q. S. and R. J performed the experiments and analyzed the data. Q. S., Z. L., H. B., and A. D. worked on the figures. Q. S. contributed to the writing of the manuscript. J. K. and A. D. refined the draft. M. G. and J. Z. provided technical support and conceptual advice. All authors discussed the results and implications and edited the manuscript at all stages.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (22062017), the Natural Science Foundation of Inner Mongolia Autonomous Region (2023QN02011), the Ordos City Program for Key Science and Technology (2022YY003), the Program of Higher-Level Talents of Inner Mongolia University (10000-22311201/035), and the Research Program of Science and Technology at Universities of Inner Mongolia Autonomous Region (NJZZ23091).References
- M. S. Calvo and C. J. Lamberg-Allardt, Adv. Nutr., 2015, 6, 860–862 CrossRef PubMed.
- C. Huesa, M. C. Yadav, M. A. Finnila, S. R. Goodyear, S. P. Robins, K. E. Tanner, R. M. Aspden, J. L. Millan and C. Farquharson, Bone, 2011, 48, 1066–1074 CrossRef CAS PubMed.
- S. Comber, M. Gardner, K. Georges, D. Blackwood and D. Gilmour, Environ. Technol., 2013, 34, 1349–1358 CrossRef CAS PubMed.
- C. M. Heyer, E. Weiss, S. Schmucker, M. Rodehutscord, L. E. Hoelzle, R. Mosenthin and V. Stefanski, Nutr. Res. Rev., 2015, 28, 67–82 CrossRef CAS PubMed.
- K. U. Sorensen, M. C. Kruger, J. Hansen-Moller and H. D. Poulsen, J. Anim. Sci., 2018, 96, 4693–4703 CrossRef PubMed.
- S. Akhtar Ali, A. Mathalikunnel, V. Bhardwaj, M. Braskett and P. Pitukcheewanont, Osteoporos Int., 2019, 30, 1887–1891 CrossRef CAS PubMed.
- K. Zhang, Y. Zhou, C. Xiao, W. Zhao, H. Wu, J. Tang, Z. Li, S. Yu, X. Li, L. Min, Z. Yu, G. Wang, L. Wang, K. Zhang, X. Yang, X. Zhu, C. Tu and X. Zhang, Sci. Adv., 2019, 5, eaax6946 CrossRef CAS PubMed.
- L. Cui, D. A. Houston, C. Farquharson and V. E. MacRae, Bone, 2016, 87, 147–158 CrossRef CAS PubMed.
- C. Liu, J. Shin, S. Son, Y. Choe, N. Farokhzad, Z. Tang, Y. Xiao, N. Kong, T. Xie, J. S. Kim and W. Tao, Chem. Soc. Rev., 2021, 50, 2260–2279 RSC.
- Y. Zhu, Z. J. Xie, J. F. Li, Y. Y. Liu, C. Z. Li, W. Y. Liang, W. C. Huang, J. L. Kang, F. L. Cheng, L. Kang, O. A. Al-Hartomy, A. Al-Ghamdi, S. Wageh, J. F. Xu, D. F. Li and H. Zhang, Coord. Chem. Rev., 2021, 446, 214110 CrossRef CAS.
- M. Qiu, W. X. Ren, T. Jeong, M. Won, G. Y. Park, D. K. Sang, L. P. Liu, H. Zhang and J. S. Kim, Chem. Soc. Rev., 2018, 47, 5588–5601 RSC.
- X. Zhang, J. Tang, C. Li, Y. Lu, L. Cheng and J. Liu, Bioact. Mater., 2021, 6, 472–489 CAS.
- W. Chen, J. Ouyang, H. Liu, M. Chen, K. Zeng, J. Sheng, Z. Liu, Y. Han, L. Wang, J. Li, L. Deng, Y. N. Liu and S. Guo, Adv. Mater., 2017, 29, 1603864 CrossRef PubMed.
- J. Huang, B. He, Z. Zhang, Y. Li, M. Kang, Y. Wang, K. Li, D. Wang and B. Z. Tang, Adv. Mater., 2020, 32, e2003382 CrossRef PubMed.
- J. Zeng, C. Gu, X. Geng, K. Lin, Y. Xie and X. Chen, Biomaterials, 2023, 297, 122122 CrossRef CAS PubMed.
- J. Hou, H. Wang, Z. Ge, T. Zuo, Q. Chen, X. Liu, S. Mou, C. Fan, Y. Xie and L. Wang, Nano Lett., 2020, 20, 1447–1454 CrossRef CAS PubMed.
- Q. Wu, G. Chen, K. K. Gong, J. Wang, X. X. Ge, X. Q. Liu, S. J. Guo and F. Wang, Matter, 2019, 1, 496–512 CrossRef.
- G. Qu, T. Xia, W. Zhou, X. Zhang, H. Zhang, L. Hu, J. Shi, X. F. Yu and G. Jiang, Chem. Rev., 2020, 120, 2288–2346 CrossRef CAS PubMed.
- W. P. J. Smith, B. R. Wucher, C. D. Nadell and K. R. Foster, Nat. Rev. Microbiol., 2023, 21, 519–534 CrossRef CAS PubMed.
- Antimicrobial Resistance Collaborators, Lancet, 2022, 399, 629–655 CrossRef PubMed.
- D. Şen Karaman, U. K. Ercan, E. Bakay, N. Topaloğlu and J. M. Rosenholm, Adv. Funct. Mater., 2020, 30, 1908783 CrossRef.
- T. Zhang, Y. Wan, H. Xie, Y. Mu, P. Du, D. Wang, X. Wu, H. Ji and L. Wan, J. Am. Chem. Soc., 2018, 140, 7561–7567 CrossRef CAS PubMed.
- M. Kim, H. G. Kim, S. Park, J. S. Kim, H. J. Choi, S. Im, H. Lee, T. Kim and Y. Yi, Angew. Chem., Int. Ed., 2019, 58, 3754–3758 CrossRef CAS PubMed.
- Y. Abate, D. Akinwande, S. Gamage, H. Wang, M. Snure, N. Poudel and S. B. Cronin, Adv. Mater., 2018, 30, 1704749 CrossRef PubMed.
- Y. Y. Li, P. C. Feng, C. Wang, W. J. Miao and H. Huang, Chem. Eng. J., 2020, 400, 125851 CrossRef CAS.
- L. Zhang, H. Huang, B. Zhang, M. Gu, D. Zhao, X. Zhao, L. Li, J. Zhou, K. Wu, Y. Cheng and J. Zhang, Angew. Chem., Int. Ed., 2020, 59, 1074–1080 CrossRef CAS PubMed.
- M. Qi, X. W. Zhao, X. Y. Zhao, H. Y. Zhang, Z. Li, X. Y. Zhang, R. Z. Fan, Q. S. Li, J. Y. Zhang and D. H. Xu, Chem. Eng. J., 2023, 475, 145884 CrossRef CAS.
- C. Zhao, X. Han, S. Wang, Z. Pan, X. Tang and Z. Jiang, Adv. Healthcare Mater., 2023, 12, e2201995 CrossRef PubMed.
- S. T. Wang, X. W. Zhao, Z. J. Liu, X. L. Yang, B. L. Pang, Y. T. Gao, R. W. Zhou, D. H. Xu, J. Y. Zhang, T. Q. Zhang and M. G. Kong, Chem. Eng. J., 2023, 452, 139481 CrossRef CAS.
- D. Zhao, L. Zhang, C. Fu, J. Zhang and C. Niu, Nano Res., 2019, 12, 1–17 CrossRef.
- L. H. Zhang, H. Y. Huang, Z. X. Lv, L. R. Li, M. Y. Gu, X. W. Zhao, B. Zhang, Y. H. Cheng and J. Y. Zhang, ACS Appl. Electron. Mater., 2021, 3, 1043–1049 CrossRef CAS.
- L. H. Zhang, X. B. Li, F. F. Yao, L. R. Li, H. Y. Huang, X. W. Zhao, S. H. Liu, Y. H. Cheng, H. Xu and J. Y. Zhang, Adv. Funct. Mater., 2022, 32, 2111057 CrossRef CAS.
- Y. Li, K. Gao, Y. Zhang, J. Jiao, L. Zhang and G. Xie, Nano Lett., 2023, 23, 6292–6300 CrossRef CAS PubMed.
- L. H. Jin, R. S. Guo, T. Han, R. B. Wang and Y. Zhang, Adv. Funct. Mater., 2023, 33, 2213583 CrossRef CAS.
- H. Y. Huang, L. H. Zhang, B. Xiao, Y. H. Cheng and J. Y. Zhang, Appl. Phys. Lett., 2019, 115, 163101 CrossRef.
- B. Zhang, L. H. Zhang, Z. Y. Wang, Y. F. Li, Y. H. Cheng, L. F. Ma and J. Y. Zhang, J. Mater. Chem. A, 2021, 9, 13855–13860 RSC.
- B. Zhang, Z. Y. Wang, H. Y. Huang, L. H. Zhang, M. Y. Gu, Y. H. Cheng, K. Wu, J. Zhou and J. Y. Zhang, J. Mater. Chem. A, 2020, 8, 8586–8592 RSC.
- A. G. Ricciardulli, Y. Wang, S. Yang and P. Samori, J. Am. Chem. Soc., 2022, 144, 3660–3666 CrossRef CAS PubMed.
- B. Li, Y. Luo, Y. F. Zheng, X. M. Liu, L. Tan and S. L. Wu, Prog. Mater. Sci., 2022, 130, 100976 CrossRef CAS.
- X. J. Hu, H. Zhang, Y. T. Wang, B. C. Shiu, J. H. Lin, S. J. Zhang, C. W. Lou and T. T. Li, Chem. Eng. J., 2022, 450, 138129 CrossRef CAS.
- F. Chen, Y. Luo, X. Liu, Y. Zheng, Y. Han, D. Yang and S. Wu, Adv. Healthcare Mater., 2022, 11, e2200360 CrossRef PubMed.
- X. Y. Kong, X. M. Liu, Y. F. Zheng, P. K. Chu, Y. Zhang and S. L. Wu, Mater. Sci. Eng., R, 2021, 145, 100610 CrossRef.
- B. Ran, L. Ran, Z. Wang, J. Liao, D. Li, K. Chen, W. Cai, J. Hou and X. Peng, Chem. Rev., 2023, 123, 12371–12430 CrossRef CAS PubMed.
- M. Liang, M. Zhang, S. Yu, Q. Wu, K. Ma, Y. Chen, X. Liu, C. Li and F. Wang, Small, 2020, 16, e1905938 CrossRef PubMed.
- D. Zhang, H. M. Liu, X. Shu, J. Feng, P. Yang, P. Dong, X. Xie and Q. Shi, J. Hazard. Mater., 2020, 393, 122317 CrossRef CAS PubMed.
- Q. Wu, M. Liang, S. Zhang, X. Liu and F. Wang, Nanoscale, 2018, 10, 10428–10435 RSC.
- J. Xu, Y. Zhang, T. T. Zhai, Z. Kuang, J. Li, Y. Wang, Z. Gao, Y. Y. Song and X. H. Xia, ACS Nano, 2018, 12, 6895–6903 CrossRef CAS PubMed.
- H. Tian, X. Liu, Z. Liang, P. Qiu, X. Qian, H. Cui and J. Tian, J. Colloid Interface Sci., 2019, 557, 700–708 CrossRef CAS PubMed.
- J. Pan, L. L. Zhang, S. T. Zhang, Z. Shi, X. Wang, S. Y. Song and H. J. Zhang, ACS Appl. Nano Mater., 2019, 2, 1516–1524 CrossRef CAS.
- L. Marín-Caba, G. Bodelón, Y. Negrín-Montecelo and M. A. Correa-Duarte, Adv. Funct. Mater., 2021, 31, 2105807 CrossRef.
- Z. Li, Q. Fu, J. Ye, X. Ge, J. Wang, J. Song and H. Yang, Angew. Chem., Int. Ed., 2020, 59, 22202–22209 CrossRef CAS PubMed.
- H. Huang, Q. L. Xiao, J. H. Wang, X. F. Yu, H. Y. Wang, H. Zhang and P. K. Chu, npj 2D Mater. Appl., 2017, 1, 20 CrossRef.
- I. Aksoy, H. Kucukkececi, F. Sevgi, O. Metin and I. Hatay Patir, ACS Appl. Mater. Interfaces, 2020, 12, 26822–26831 CrossRef CAS PubMed.
- A. Sousa-Castillo, M. Comesaña-Hermo, B. Rodríguez-González, M. Pérez-Lorenzo, Z. M. Wang, X. T. Kong, A. O. Govorov and M. A. Correa-Duarte, J. Phys. Chem. C, 2016, 120, 11690–11699 CrossRef CAS.
- J. Zheng, X. Cheng, H. Zhang, X. Bai, R. Ai, L. Shao and J. Wang, Chem. Rev., 2021, 121, 13342–13453 CrossRef CAS PubMed.
- Y. Liu, M. Yang, J. Zhang, X. Zhi, C. Li, C. Zhang, F. Pan, K. Wang, Y. Yang, J. Martinez de la Fuentea and D. Cui, ACS Nano, 2016, 10, 2375–2385 CrossRef CAS PubMed.
- H. Y. Zhang, Y. W. Liu, M. F. S. Shahidan, C. Kinnear, F. Maasoumi, J. Cadusch, E. M. Akinoglu, T. D. James, A. Widmer-Cooper, A. Roberts and P. Mulvaney, Adv. Funct. Mater., 2021, 31, 2006753 CrossRef CAS.
- P. Kesharwani, R. Ma, L. Sang, M. Fatima, A. Sheikh, M. A. S. Abourehab, N. Gupta, Z. S. Chen and Y. Zhou, Mol. Cancer, 2023, 22, 98 CrossRef CAS PubMed.
- Y. Q. Wang, Y. Y. Jin, W. Chen, J. J. Wang, H. Chen, L. Sun, X. Li, J. Ji, Q. Yu, L. Y. Shen and B. L. Wang, Chem. Eng. J., 2019, 358, 74–90 CrossRef CAS.
- J. Huo, Q. Jia, H. Huang, J. Zhang, P. Li, X. Dong and W. Huang, Chem. Soc. Rev., 2021, 50, 8762–8789 RSC.
- J. W. Xu, K. Yao and Z. K. Xu, Nanoscale, 2019, 11, 8680–8691 RSC.
- C. Xu and K. Pu, Chem. Soc. Rev., 2021, 50, 1111–1137 RSC.
- P. Schwerdtfeger, H. L. Hermann and H. Schmidbaur, Inorg. Chem., 2003, 42, 1334–1342 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb00105b |
| This journal is © The Royal Society of Chemistry 2024 |