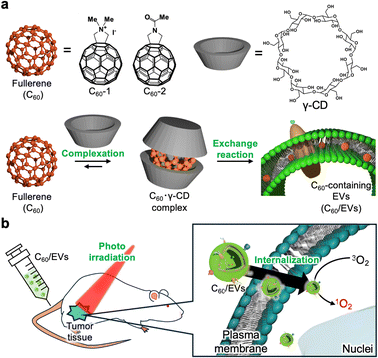
Extracellular vesicles containing fullerene derivatives prepared by an exchange reaction for photodynamic therapy†
Nanami
Kono‡
a,
Riku
Kawasaki‡
 *a,
Ayano
Oshige
a,
Kotaro
Nishimura
a,
Keita
Yamana
a,
Dilimulati
Yimiti
b,
Shigeru
Miyaki
b,
Nobuo
Adachi
b,
Naoki
Takabayashi
c,
Takeshi
Nagasaki
c and
Atsushi
Ikeda
*a,
Ayano
Oshige
a,
Kotaro
Nishimura
a,
Keita
Yamana
a,
Dilimulati
Yimiti
b,
Shigeru
Miyaki
b,
Nobuo
Adachi
b,
Naoki
Takabayashi
c,
Takeshi
Nagasaki
c and
Atsushi
Ikeda
 *a
*a
aProgram of Applied Chemistry, Graduate School of Advanced Science and Engineering, Hiroshima University, 1-4-1 Kagamiyama, Higashi Hiroshima, 739-8527, Japan. E-mail: riku0528@hiroshima-u.ac.jp; aikeda@hiroshima-u.ac.jp
bDepartment of Orthopaedic Surgery, Graduate School of Biomedical and Health Sciences, Hiroshima University, 1-2-3 Kasumi Minami-ku, Hiroshima, 734–8551, Japan
cDepartment of Applied Chemistry and Bioengineering, Graduate School of Osaka Metropolitan University, 3-3-138 Sugimoto, Sumiyoshi-ku, Osaka, 558-8585, Japan
First published on 31st August 2024
Abstract
Extracellular vesicles (EVs) have excellent biocompatibility and long retention times in the circulation and have consequently been expected to be useful as drug-delivery systems. However, their applications have been limited because of the inability to introduce hydrophobic compounds to EVs without the use of harmful organic solvents. Herein, we developed an organic-solvent-free drug-loading technique based on the host exchange reaction. We demonstrated that the exchange reaction enabled quantitative loading of EVs with highly concentrated (0.1 mM) hydrophobic fullerene derivatives. Fullerene derivative–loaded EVs (EVs/C60) could eliminate cancer cell lines more efficiently than fullerene derivative-loaded liposomes (Lip/C60). Moreover, the photodynamic activity of EVs/C60 was fivefold higher than that of the clinically available photosensitizer photofrin. EVs/C60 could efficiently suppress tumor growth in tumor-xenograft model mice.
Introduction
With low immunogenicity and targeting properties, extracellular vesicles (EVs)1,2 are expected to be used as bio-based drug-delivery systems. Moreover, EVs can provide internal spaces that can entrap hydrophilic and hydrophobic pharmaceuticals in the same manner as liposomes. EVs derived from plants are also emerging as drug-delivery platforms.3,4 Large masses of EVs can be derived from plants and could potentially solve some of the problems encountered in the formulation of EV-based pharmaceuticals in clinical applications. To expand their applicability as drug-delivery platforms, the development of drug-loading methods is indispensable. Although a large proportion of clinically available drugs are hydrophobic, the technology to encapsulate hydrophobic compounds for EVs remains challenging.4,5 Existing encapsulation methods for EVs include physical techniques such as sonication, electroporation, extrusion, and agitation; however, concerns remain that external stimuli can disrupt the structure of EVs.4 In loading hydrophobic compounds to EVs, these compounds have to be initially dissolved in organic solvents such as DMSO and ethanol that can mix with water arbitrarily and the resulting solution should be injected into the EV dispersion. However, the molecules that can dissolve in these organic solvents are limited and they can easily develop undesirable aggregates after injection into aqueous media. Moreover, organic solvents can induce the denature of membrane proteins in EVs, disorganize the membrane structure of EVs, and have harmful effects on biologics.6,7 Consequently, the development of organic-solvent free techniques to introduce hydrophobic compounds to EVs is required to expand their applicability as drug carriers.4Our group has established the loading methodology of hydrophobic compounds for liposomes based on host exchange, and the exchange reaction can efficiently encapsulate compounds such as fullerenes and porphyrins into lipid bilayersquantitatively at high concentration without using organic solvents.8–10 Moreover, the encapsulated molecules maintain their pharmacological activities, such as photo-reactivity, photodynamic activity, and anticancer effects.8 These advantages in the exchange reaction encouraged us to employ current methods to introduce fullerene, a hydrophobic compound, into EVs and to demonstrate the photodynamic activity of fullerene derivatives loaded into EVs for photo-triggered cancer treatment.
Photodynamic therapy (PDT)11,12 is a photochemistry-based cancer treatment that is minimally invasive because the light can be controlled with spatiotemporal high resolution. Cancer cell destruction in PDT is achieved by cytotoxic reactive oxygen species (ROS), including singlet oxygen (1O2), generated by photosensitizers13via laser irradiation. Since the lifetime of these ROS is short,14 the deliverability of photosensitizers to cancer cells is a key technology. Fullerenes,9,15 porphyrins,10,16 and phthalocyanines17 have been studied as photosensitizers for biologics because of their excellent conversion rate and absorbability in red light regions that can travel deeper from the tissue surface; however, their biomedical use is limited because of the hydrophobicity of the compounds, instability in aqueous media, and poor tumor-targeting properties. To address these issues, modification of hydrophilic moieties such as sulfonyl groups and ethylene glycol units to the compounds has been employed, although these conjugations can easily reduce the intended photodynamic activity. However, encapsulation of photosensitizers into drug-delivery systems is a powerful means of enhancing the water dispersibility and cancer-targeting properties of photosensitizers. Therefore, EVs are considered attractive platforms to establish photosensitizers with high anti-tumor efficacy.
Herein, we developed an organic solvent free drug-loading method for EVs via an exchange reaction and demonstrated the photodynamic activity of fullerene-loaded lemon-derived EVs for cancer treatment in vitro and in vivo (Fig. 1).
Results and discussion
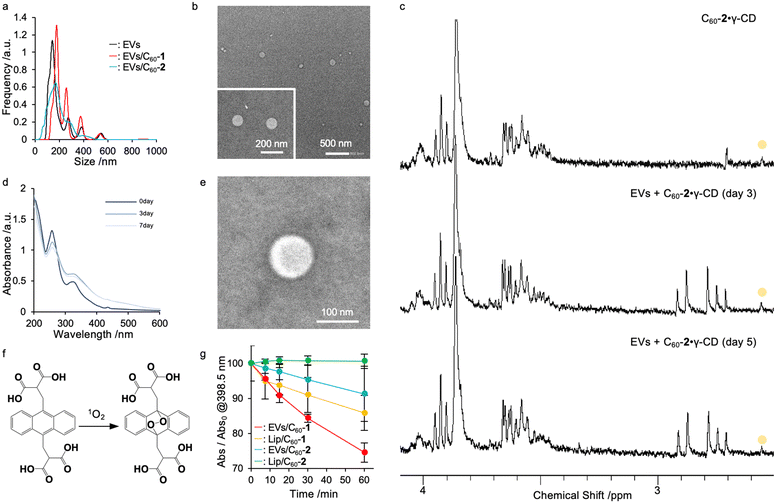
Preparation of EVs incorporating fullerenes via a host molecule exchange reaction
Plant-derived EVs are easily extractable and exhibit several benefits for human health for treating cancer and inflammation because they contain plant-derived biologically active molecules,3,4 such as polyphenols, flavonoids, and ceramides. Therefore, plant-derived EVs have been expected to be candidate delivery platforms for pharmaceuticals. Herein, we focused on lemon, which is one of the major fruits produced in Hiroshima, Japan. EVs were isolated by fractional centrifugation of lemon juice from squeezed lemons (purchased from a local supermarket) as reported by other groups.18 The size distribution of the EVs was confirmed by nano-tracking analysis (NTA), and the morphology of the EVs was observed by transmission electron microscopy (TEM). The NTA-derived average particle size was approximately 180 nm (Fig. 2a, black line) while spherical structures with a diameter of 150–200 nm were observed via transmission electron microscopy (TEM) (Fig. 2b). A total of 2.4 × 1012 particles were obtained from 100 g of lemons used for isolation of EVs and contained 0.5 mg mL−1 protein and 7.5 mg dL−1 lipid. Thus, the current method enabled the preparation of large amounts of EVs, and such scalability is appealing for formulation compared with other EV sources such as cultured cell lines. In this study, we employed liposomes as a control, which were prepared using 1,2-dimyristoyl-sn-glycero-3-phosphocholine using the freeze–thaw method. After size extrusion, the size distribution of liposomes was evaluated via dynamic light scattering (DLS), and their size was determined to be approximately 100 nm (polydispersity index, 0.09; Table 1).| [C60-X]a/mM | D hy /nm | Average diameterc/nm | ζ-potentiald/mV | |
|---|---|---|---|---|
| a Concentrations of introduced fullerene C60 derivatives were determined by absorbance. b D hy was determined for liposomes via DLS measurements performed at 25 °C. c Average diameter of EVs was calculated via NTA. d ζ-potential was measured using a capillary cell at 25 °C, pH 7.4. | ||||
| EVs | 0 | 180 | −14.0 ± 6 | |
| EVs/C60-1 | 0.1 | 140 | −18.7 ± 0.3 | |
| EVs/C60-2 | 0.1 | 170 | −25.4 ± 0.9 | |
| Lip/C60-1 | 0.1 | 190 ± 20 (0.24) | +1.46 ± 2 | |
| Lip/C60-2 | 0.1 | 460 ± 16 (0.41) | −5.97 ± 1 | |
Since a highly concentrated fullerene derivative solution is required to introduce fullerene derivatives into EVs, we initially prepared a fullerene–γ-cyclodextrin complex (C60·γ-CD) (C60-1, 2.7 mg; 3 μmol: C60-2, 2.4 mg; 3 μmol: γ-CD, 15.6 mg; 12 μmol) via high speed vibration milling (HSVM) as previously reported.8,9,19 The complexation of fullerene derivatives with γ-CD (C60-1·γ-CD and C60-2·γ-CD) was confirmed via1H-nuclear magnetic resonance (NMR) spectroscopy (Fig. 2c top panel and Fig. S1 top panel, ESI†). For C60-1, methyl groups in C60-1 were found at 2.2 ppm. Similarly, a representative peak at 2.1 ppm from the methyl group in C60-2 appeared after complexation. These results indicate that the fullerene derivatives were successfully dissolved in water. We evaluated the concentration of fullerene derivatives in each solution by measuring UV-Vis absorption spectra (Fig. 2d and Fig. S2, ESI†), and the concentrations of C60-1·γ-CD and C60-2·γ-CD were determined to be 0.7 and 0.61 mM, respectively (C60-1, ε322 = 4.31 × 104 cm−1 mol−1 dm3; C60-2; ε324 = 4.02 × 104 cm−1 mol−1 dm3). As previously reported,9,19 we obtained highly concentrated fullerene solutions for the exchange reaction.
We next conducted an exchange reaction with EVs using the complex of fullerene derivatives with γ-CD. The exchange reaction can proceed by mixing EVs and C60·γ-CD complex (C60, 0.20 mM; EVs, 0.25 mg mL−1) in aqueous media at 40 °C. The progress of the exchange reaction was traced by evaluating 1H-NMR and UV-vis spectra at each time point (0, 3, and 7 days). When the fullerenes were translocated from cyclodextrin complex to EVs, their diffusion got slower, resulting in the peaks from fullerenes in NMR get broadened and disappeared. The peaks assignable to C60-2·γ-CD at 2.05 ppm gradually broadened with time (Fig. 2c middle and bottom panel), as we previously reported in the case of the liposomal system,8,9,20 indicating the fullerenes were successfully loaded into EVs whose diffusion constant is larger than γ-CD complex. Moreover, the UV-vis absorption spectra of C60-2 broadened with time (Fig. 2d). Similar trends were found in the case of C60-1 (Fig. S1 and S2, ESI†). Here, ultracentrifugation for C60/EVs could remove all the γ-CD (Fig. S3, ESI†), indicating the fullerene derivatives were introduced to EVs via not γ-CD complex form but free form. In addition, we prepared fullerene derivatives encapsulated in liposomes (Lip/C60-1 and Lip/C60-2) by exchanging reactions as previously reported (C60, 0.20 mM; EVs, 0.25 mg mL−1; 80 °C). The exchange reaction was completed within 2 h (Fig. S4, ESI†). DLS measurements revealed that the hydrodynamic diameter (Dhy) increased from 100 to 190 nm. The time for exchanging reactions for EVs took much longer than that for the liposomal system. As we incubated the mixture of EVs and C60·γ-CD at 40 °C to avoid undesirable denaturing of proteins expressed in EVs, the thermal energy was insufficient to decompose the inclusion complex, which is the dominant driving force for the exchange reaction. NTA measurements revealed that the size distribution of EVs and the number of EVs did not significantly change (Fig. 2a red and blue line), and the spherical morphology of EVs was preserved even after the exchange reaction (Fig. 2e and Fig. S5, ESI†). In addition, there are not significant changes in protein concentration between EVs dispersion and resulting dispersion prepared by exchanging reaction. These results clearly indicate that the loading of C60 derivatives can be achieved without forming undesirable aggregates and precipitates under the current conditions. For these results, 8.0 × 105 molecules of fullerene derivatives were trapped in each EVs.
To demonstrate the photodynamic activity of EVs/C60 and Lip/C60, the 1O2-generation capacity was determined by the conversion of 9,10-anthracenediyl-bis(methylene)dimalonic acid (ABDA) to endoperoxide (Fig. 2f).21 When ABDA was consumed by oxidation by the generated 1O2, the absorbance from anthracene was quenched, as shown in Fig. S5 (ESI†). EVs/C60-1, EVs/C60-2, Lip/C60-1, and Lip/C60-2 (C60-1, C60-2; 20 μM) were co-incubated with ABDA (12.5 μM) and irradiated with light (>620 nm). At 60 min of photoirradiation, EVs/C60-1, EVs/C60-2, Lip/C60-1, and Lip/C60-2 consumed 28%, 8%, 12%, and 0% of ABDA via photoirradiation, respectively (Fig. 2g and Fig. S6, ESI†). EVs/C60-1 exhibited the highest photodynamic activity among these four systems. Moreover, none of systems exhibited precipitation, and the absorption from fullerene derivatives did not decrease during photoirradiation (Fig. S7, ESI†), indicating that these systems were stable with exposure to light. As more polarized fullerenes can be located on the surface where dissolved oxygen can address to achieve energy transfer, C60-1 systems could convert 3O2 into 1O2 more efficiently than C60-2 systems. In addition, EV systems showed higher activity than the liposomal system. Consequently, EVs systems are expected to be excellent delivery platforms for fullerene derivatives by manipulating the location in EVs components. To determine quantum yields (Φ) for these systems, we further examined ABDA bleaching test for water soluble photosensitizer, methylene blue (MB; Φ, 0.52;22 Fig. S8, ESI†). By comparing MB, the Φ of EVs/C60-1, EVs/C60-2, Lip/C60-1, and Lip/C60-1 were determined to be 0.043, 0.00029, 0.043, and 0.000024, respectively.
Therapeutic efficacy of PDT using EVs incorporating fullerenes in vitro
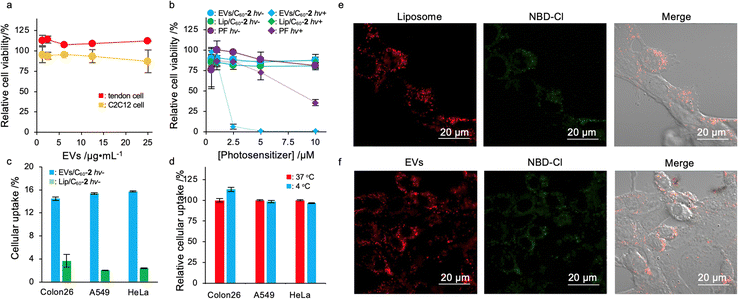
To address the suitability of EV-based delivery systems for fullerenes, we examined the cytotoxicity of EVs toward murine myoblast cells (C2C12) and murine tendon cells as healthy tissue-derived cell lines. After 24 h of incubation, EVs did not induce severe cytotoxicity toward either of these cell lines (Fig. 3a), indicating that current systems have low toxicity and high biocompatibility as drug carriers. We next investigated the photodynamic activity of EVs/C60 and Lip/C60 toward several cancer cells, including murine colon cancer (Colon26), human cervical cancer (HeLa), and human adenocarcinoma cells (A549). As a control for the experiment, we employed a clinically available photosensitizer based on a water dispersible porphyrin oligomer, PF. Although EVs/C60-1 and Lip/C60-1 showed higher photodynamic activity than EVs/C60-2 or Lip/C60-2, cytotoxicity was found even in dark conditions (Fig. S9, ESI†), which can induce side effects. The cytotoxicity may be caused by the cationic character of C60-1, which can induce damage to the anionic plasma membrane and mitochondrial membrane.23 However, neither the EVs/C60-2 nor Lip/C60-2 systems showed any apparent cytotoxicity in dark conditions (Fig. 3b and Fig. S10, ESI†). These results suggest that C60-2–loading systems are more appropriate as photosensitizers than C60-1–loading systems because of their sensitivity to light, which can lessen side effects. Although cytotoxicity under dark conditions decreased, Lip/C60-2 could not kill these three cancer cell lines even at the highest concentration under light irradiation. Conversely, photoirradiation of cancer cells treated with EVs/C60-2 enabled induction of cell death in the cell lines. Moreover, the photodynamic activity of EVs/C60-2 toward Colon26 and A549 higher photodynamic activity than that of the clinically available photosensitizer PF (Table 2). No significant differences in IC50 value were present for HeLa cells between PF and EVs/C60-2 treatments. These results clearly indicate that EV/C60-2 systems can be widely used as photosensitizers for cancer treatment.| EVs/C60-2/μM | Lip/C60-2/μM | PF/μM | |
|---|---|---|---|
| Colon26 | 1.7 | 10< | 8.0 |
| HeLa | 0.92 | 10< | 0.77 |
| A549 | 2.3 | 10< | 8.4 |
To address their excellent photodynamic activity in vitro, we verified the cellular uptake amount of cargo delivered by EVs using 4-chloro-7-nitro-2,1,3-benzoxadiazole (NBD) as a model fluorophore. NBD was also introduced to EVs via an exchange reaction as mentioned above.24 Briefly, NBD hydroxypropyl-β-cyclodextrin (HP-β-CDx) was prepared by HSVM (HP-β-CDx, 15.0 mg; 10 μmol: NBD, 1 mg; 5 μmol). The complex of NBD with HP-β-CDx was co-incubated with EVs (NBD, 0.20 mM; EVs, 0.25 mg mL−1) at 40 °C. As NBD is sensitive to the hydrophobicity of the microenvironment, the exchange process was confirmed by measuring the fluorescence spectra.25 After 7 days of incubation, the fluorescence from NBD increased (Fig. S11, ESI†), indicating that NBD molecules were translocated to a more hydrophobic environment, such as that on lipid membranes, from the cyclodextrin cavity. This clearly supports that the exchange reaction was successfully achieved with NBD.
To investigate the cellular uptake efficiencies of cargo molecules, EVs/NBD or Lip/NBD were exposed to Colon26, HeLa, and A549 cells. After 24 h of incubation, the cellular uptake amounts of NBD were quantified by measuring the fluorescence of NBD in the lysate of the treated cells. As shown in Fig. 3c, the EV system could deliver three- to fourfold more cargo molecules than liposomal systems to these three cancer cell lines. The excellent deliverability of EVs enabled the enhancement of photodynamic activity in vitro, and this feature is attractive for establishing delivery platforms for cancer therapy. We further addressed the cellular uptake mechanism of the EV system by maintaining the cells at 4 °C to inhibit energy-dependent cellular events, including endocytosis.26 Interestingly, our systems could deliver cargo molecules toward these three cell lines even under endocytosis inhibitory conditions at the same level as the cells actively uptake exogenous interests via endocytosis (Fig. 3d). This suggests that our system can be taken up in an endocytosis-independent manner, such as by membrane fusion and direct penetration.27 The features in the cellular uptake of EVs may affect the efficient accumulation of cargo molecules in cancer cells. We used a confocal laser scanning microscope to investigate the cellular uptake of the current system with a commercially available lipophilic dye, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), To visualize the distribution of liposomes and EVs, Colon26 cells were coincubated with DiI-stained NBD/Lip (DiI-NBD/Lip) or NBD/EVs (DiI-NBD/EVs) for 24 h. As shown in Fig. 3e, fluorescence signals from DiI and NBD were found within cells and highly overlapped, indicating that a large part of the delivered cargo molecules remained trapped in liposomes. For DiI-NBD/EVs, both fluorescence signals were also detected within cells as well as in the liposomal system (Fig. 3f); however, the overlap efficiencies between NBD and DiI were lower than those delivered by liposomes, suggesting that the EV system is more efficient than liposomal systems at releasing cargo molecules. This may imply differences in the integrated area of cargo molecules. For liposomes, almost all the hydrophobic compounds should be trapped by the liposomal membrane and the cargo will be highly stabilized, which can strongly bind cargo molecules within liposomal systems. Conversely, EVs not only provide a membrane but also a highly dense polysaccharide layer and membrane proteins for integration, which can easily release interests within cells by interacting with cytosolic biomolecules such as membrane structures and highly concentrated proteins. To address detailed subcellular distribution of cargo molecules delivered by EVs, lysosomes were stained by commercially available lysosome staining reagent, Lysotracker Red. As a result, delivered NBD-Cl were rarely overlapped with Lysotracker Red, indicating that cargo molecules are located in cytosol (Fig. S12, ESI†). The cytosolic delivery is advantageous in inducing oxidative stress to mitochondria and nuclei, which is considered as one of the dominant factors in killing cancer cells in PDT. These facts in deliverability of EVs should enhanced photodynamic activity of fullerene derivatives.
Therapeutic efficacy of PDT using EVs incorporating fullerenes in vivo
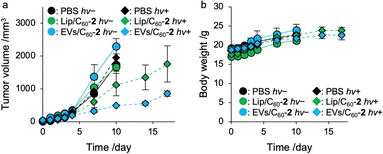
Finally, we demonstrated photodynamic activity of C60 encapsulated by EVs toward tumor xenograft mice. Tumor xenograft mice were established by transplantation of Colon26 cells (1.0 × 105 cells) to the right femur of Balb/c mice (male, 4-week-old, 18 g)28 and the mice were incubated for 7 days. When the tumor volume was grown up to approximately 20 mm3, PBS, Lip/C60-2, or EVs/C60-2 (C60, 0.20 mM; 100 μL) were administrated via tail vein. At 24 h-post injection, photoirradiation was carried out to the tumor tissue for 1 h (>600 nm, 15 mW) and tumor volume and body weight was measured at each time point (Fig. S13, ESI†). At 14 days post irradiation, photodynamic activity of EVs/C60-2 were 2.6-fold higher than that of Lip/C60-2 (Fig. 4a). During the period, no obvious body weight changes were found in all the group (Fig. 4b), indicating all systems can lessen side effects including photoinduced toxicity. These results clearly indicate that EVs systems are effective photosensitizer for PDT.Conclusion
We developed a hydrophobic compound–loading technique for EVs without using an organic solvent, which can potentially disrupt EV structures. We successfully introduced fullerenes to EVs quantitatively without precipitation by mixing EVs and C60·γ-CD complexes in aqueous media. 1H-NMR spectra showed that the fullerenes were inserted into the EVs by an exchange reaction as indicated by the disappearance of the C60/γ-CD specific peak. The prepared EVs/C60 showed an (1O2)-generating capacity and photodynamic activity comparable or superior with that of Lip/C60 prepared using a similar procedure. In addition, the photodynamic activity of EVs/C60-2 on cancer cells was observed to be superior to that of Lip/C60-2 and the commercially available photosensitizer PF. Overall, the exchange reaction is a powerful means to introduce hydrophobic compounds to EVs without using harmful organic solvents, and we expect our system to be a photosensitizer that can be applied to PDT.Author contributions
R. K., N. K., and A. O. mainly performed all the experiments. K. N., K. Y., and D. Y. supported the biological assays. S. M., N. A., A. I., and R. K. designed the systems. N. T. and T. N. conducted animal experiments. N. K., R. K., and A. I. mainly wrote this manuscript. All authors have conducted and approved the final manuscript.Data availability
No primary research results, software or code have been included and no new data were generated by this paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Japan Society for the Promotion of Science, KAKENHI (R. K., JP22K18196). Experiments using transmission electron microscopy were conducted at the Natural Science Center for Basic Research and Development (N-BARD). The author would like to thank Enago (https://www.enago.jp) for the English language review.Notes and references
- I. K. Herrmann, M. J. A. Wood and G. Fuhrman, Nat. Nanotechnol., 2021, 16, 748–759 CrossRef CAS PubMed.
- P. E. M. Castilla, L. Tong, C. Huang, A. M. Sofias, G. Pastorin, X. Chen, G. Storm, R. M. Schiffelers and J.-W. Wang, Adv. Drug Delivery Rev., 2021, 175, 113801 CrossRef PubMed.
- K.-J. Lo, M.-H. Wang, C.-T. Ho and M.-H. Pan, J. Agri. Food Chem., 2024, 72, 2853–2878 CrossRef CAS PubMed.
- M. Cao, N. Diao, X. Chen, Y. Xiao, C. Guo, D. Chen and X. Zhang, Mater. Horiz., 2023, 10, 3879–3894 RSC.
- R. Kar, R. Dhar, S. Mukherjee, S. Nag, S. Gorai, N. Mukerjee, D. Mukherjee, R. Vatsa, M. Chandrakanth Jadhav, A. Ghosh, A. Devi, A. Krishnan and N. D. Thorat, ACS Biomater. Sci. Eng., 2023, 9, 577–594 CrossRef CAS PubMed.
- M. Ruzycka-Ayoush, A. M. Nowicka, A. Kowalczyk, A. Gluchowska, A. Targonska, G. Mosieniak, K. Sobczak, M. Donten and I. P. Grudzinski, Eur. J. Pharm. Sci., 2023, 181, 106369 CrossRef CAS.
- S. Kartik Kumar and W. V. Sasidhar, Mol. Pharm., 2023, 20(8), 3829–3842 CrossRef.
- R. Kawasaki and A. Ikeda, ChemBioChem, 2023, 24, e202300455 CrossRef CAS.
- R. Shimada, S. Hino, K. Yamana, R. Kawasaki, T. Konishi and A. Ikeda, ACS Med. Chem. Lett., 2022, 13(4), 641–647 CrossRef CAS PubMed.
- K. Nishimura, K. Yamana, R. Kawasaki and A. Ikeda, Org. Biomol. Chem., 2023, 21, 4810–4816 RSC.
- J. Chen, T. Fan, Z. Xie, Q. Zeng, P. Xue, T. Zheng, Y. Chen, X. Luo and H. Zhang, Biomaterials, 2020, 237, 119827 CrossRef CAS.
- S. Li, F. Yang, Y. Wamng, T. Du and X. Hou, Chem. Eng. J., 2023, 451, 138621 CrossRef CAS.
- T. C. Pham, V.-N. Nguyen, Y. Choi, S. Lee and J. Yoon, Chem. Rev., 2021, 121(21), 13454–13619 CrossRef CAS PubMed.
- J. Zou, L. Li, J. Zhu, X. Li, Z. Yang, W. Huang and X. Chen, Adv. Mater., 2021, 33, e2103627 CrossRef PubMed.
- E. Öztürk Gündüz, M. E. Gedik, G. Günaydın and E. Okutan, ChemMedChem, 2022, 17, e202100693 CrossRef PubMed.
- A. Akbar, S. Khan, T. Chatterjee and M. Ghosh, J. Photochem. Photobiol., B, 2023, 248, 112796 CrossRef CAS PubMed.
- D. Li, S. Cai, P. Wang, H. Cheng, B. Cheng, Y. Zhang and G. Liu, Adv. Healthcare Mater., 2023, 12, 230063 Search PubMed.
- C. Lei, Y. Teng, L. He, M. Sayed, J. Mu, F. Xu, X. Zhang, A. Kumar, K. Sundaram, M. K. Sriwastva, L. Zhang, S. Y. Chen, W. Feng, S. Zhang, J. Yan, J. W. Park, M. K. Merchant, X. Zhang and H.-G. Zhang, iScience, 2021, 24, 102511 CrossRef CAS.
- S. Raimondo, O. Urzì, S. Meraviglia, M. D. Simone, A. M. Corsale, N. R. Ganji, A. P. Piccionello, G. Polito, E. L. Presti, F. Dieli, A. Conigliaro and R. Alessandro, J. Cell. Mol. Med., 2022, 9, 2105274 Search PubMed.
- A. Ikeda, T. Iizuka, N. Maekubo, R. Aono, J. Kikuchi, M. Akiyama, T. Konishi, T. Ogawa, N. Ishida-Kitagawa, H. Tanabe and K. Shiozaki, ACS Med. Chem. Lett., 2013, 4(8), 752–756 CrossRef CAS PubMed.
- T. Entradas, S. Waldron and M. Volk, J. Photochem. Photobiol., B, 2020, 204, 111787 CrossRef CAS PubMed.
- D. Gabrielli, E. Belisle, D. Severino, A. J. Kowaltowski and M. S. Baptista, Photochem. Photobiol., 2004, 79, 227–232 CrossRef CAS PubMed.
- T. Xia, M. Kovochich, M. Liong, J. I. Zink and A. E. Nel, ASC Nano, 2008, 2(1), 85–96 CrossRef CAS PubMed.
- R. Kawasaki, A. Oshige, K. Yamana, H. Hirano, K. Nishimura, Y. Miura, R. Yorioka, Y. Sanada, K. Bando, A. Tabata, K. Yasuhara, Y. Miyazaki, W. Shinoda, T. Nishimura, H. Azuma, T. Takata, Y. Sakurai, H. Tanaka, M. Suzuki, T. Nagasaki and A. Ikeda, Chem. – Eur. J., 2023, 29, e202302486 CrossRef CAS PubMed.
- Z. Ma, Y. Lin, H. Chen, L. Du and M. Li, RSC Adv., 2016, 6, 102773 RSC.
- J. J. Rennik, A. P. R. Johnston and R. G. Parton, Nat. Nanotechnol., 2021, 16, 266–276 CrossRef PubMed.
- N. Kastelowitz and H. Yin, ChemBioChem, 2014, 15, 923–928 CrossRef CAS PubMed.
- R. Kawasaki, R. Ohdake, K. Yamana, T. Eto, K. Sugikawa and A. Ikeda, J. Mater. Chem. B, 2021, 9, 6357–6363 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of the experimental procedure and analytical data. See DOI: https://doi.org/10.1039/d4tb00416g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |