A guanidiniocarbonyl-pyrrole functionalized cucurbit[7]uril derivative as a cytomembrane disruptor for synergistic antibacterial therapy†
Ruixue
Han‡
a,
Kehan
Du‡
b,
Shengke
Li
 c,
Minzan
Zuo
c,
Minzan
Zuo
 a,
Ponmani
Jeyakkumar
a,
Hao
Jiang
a,
Ponmani
Jeyakkumar
a,
Hao
Jiang
 *b,
Leyong
Wang
*b,
Leyong
Wang
 d and
Xiao-Yu
Hu
d and
Xiao-Yu
Hu
 *ae
*ae
aCollege of Materials Science and Technology, Nanjing University of Aeronautics and Astronautics, Nanjing 211106, China. E-mail: huxy@nuaa.edu.cn
bHubei Engineering Research Center for Biomaterials and Medical Protective Materials, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Wuhan 430074, China. E-mail: hustjh@hust.edu.cn
cMacao Centre for Research and Development in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macau 999078, China
dSchool of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, China
eCollege of Chemistry and Chemical Engineering, Jiangxi Normal University, Nanchang 330022, China
First published on 15th October 2024
Abstract
The antibiotic resistance of bacterial membranes poses a significant threat to global public health, highlighting the urgent need for novel therapeutic agents and strategies to combat bacterial membranes. In response, we have developed a novel macrocyclic host molecule (GCPCB) based on guanidiniocarbonyl-pyrrole (GCP) functionalized cucurbit[7]uril with an aggregation-induced luminescence effect. GCPCB exhibits high antimicrobial potency against bacterial membranes, particularly demonstrating strong antibacterial activity against Gram-positive strains of S. aureus and Gram-negative strains of E. coli. Significantly, due to the strong binding between GCP and the bacterial membrane, GCPCB can effectively eradicate the bacteria encapsulated within. Furthermore, the formation of a host–guest complex between GCPCB and berberine hydrochloride (BH) not only enhances synergistic destructive activity against both species of bacteria but also provides a potential supramolecular platform for effective bacterial membrane destruction.
Global public health is significantly threatened by the escalating antimicrobial resistance (AMR),1 which not only imposes a substantial financial burden on healthcare systems, but also leads to increased levels of illness and mortality.2–4 Exposure to antimicrobial drugs prompts bacteria to adjust and develop resistance, resulting in decreased effectiveness of these medications over time. This reduced efficacy facilitates the persistence of infections within the body and increases the likelihood of transmission to others.5,6 Consequently, there is an urgent need for the development of new antimicrobial agents capable of effectively disrupting bacterial membranes in order to eradicate these infections.7–9 In recent years, there has been a significant focus on exploring supramolecular strategies for the development of innovative antimicrobial agents due to their simplicity and adaptability.10–19 Among these strategies, cucurbit[n]uril (CB[n]), an important class of macrocyclic compounds, has garnered attention due to its highly symmetric hydrophobic cavity and polar port composed of carbonyl groups, facilitating efficient accommodation of guest molecules.20–22 However, the facile modification challenge has hindered the potential application of cucurbit[7]uril (CB[7]). Therefore, researchers have directed their efforts towards improving CB[7] by designing derivatives with remarkable luminescent properties while preserving its host–guest recognition capability. This modification offers the potential for detecting and recognizing non-fluorescent compounds, thereby expanding the application of CB[7] in imaging, drug delivery, and other fields. This advancement represents a compelling new avenue for the evolution of CB[7].23–28
The exceptional fluorescence properties of aggregation-induced emission (AIE) fluorophores have recently sparked a growing interest within the scientific community, leading to an increased focus on their biomedical applications.29,30 Through meticulous design strategies, AIE fluorophores can be intricately linked with a diverse range of functional groups through either covalent or non-covalent bonding, resulting in the development of multifunctional hybrid materials. These materials, based on AIE fluorophores, exhibit enhanced and adjustable AIE properties, making them suitable for various applications.31–34 Additionally, the weakly basic guanidiniocarbonyl-pyrrole (GCP) is widely recognized for its ability to bind to and permeate cell membranes to facilitate the delivery of different gene carriers.35,36 Our previous research has demonstrated the synthesis of amphiphilic peptides incorporating various quantities of GCP moieties based on pillar[5]arene. These compounds can self-assemble into nanoparticles in an aqueous medium and exhibit effective cell internalization through strong binding between GCP and the cell membrane.37,38
Herein, as shown in Scheme 1, we propose utilizing the tetraphenylethylene (TPE) motif as an AIE-type bridging unit, integrating CB[7] as a functional macrocyclic host to encapsulate antibacterial agent at one end and GCP as the recognition and binding site to the bacterial membrane at the other end. By designing and synthesizing this structurally distinctive amphiphilic CB[7] derivative (GCPCB) with AIE properties, our primary aim is to achieve superior antibacterial properties. We carefully selected berberine hydrochloride (BH), an antibacterial drug, as the guest molecule to fabricate a distinctive supramolecular nano-antimicrobial material through the host–guest interaction between CB[7] and BH. Importantly, the positive charge in the GCP molecule at the hydrophilic end of GCPCB enhances its ability to firmly bind to negatively charged bacterial membranes, leading to the destruction of membrane structure and integrity through electrostatic adsorption, ultimately inhibiting bacterial activity.
 | ||
| Scheme 1 Schematic illustration of the formation of supramolecular nanoparticles and their synergistic antimicrobial effect on bacterial membrane disruption. | ||
Furthermore, we found that the aggregation of GCPCB can induce the emission of TPE, thereby endowing the molecule with excellent optical properties. Additionally, the cavity of GCPCB can accommodate BH to form a host–guest complex (GCPCB⊃BH), which can assemble into nanoparticles in water. These hydrophilic nanoparticles surface-modified with GCP moieties, exhibit remarkable efficacy in eradicating bacterial membranes. Upon subsequent disassembly, GCPCB⊃BH synergistically achieves imaging-guided antimicrobial therapy, further exemplifying the potential of our research.
Scheme 2 shows a simplified synthetic route of the host molecule (GCPCB). The TPE moiety acts as an AIE-type bridging unit, with one end covalently attached to CB[7] as a functional macrocyclic host capable of encapsulating antimicrobial agents. And the other end is covalently attached to GCP, which acts as the recognition and the binding site for the bacterial membrane, ultimately yielding the amphiphilic host molecule (GCPCB). The detailed design and synthesis of GCPCB are illustrated in Scheme S1 (ESI†), while the GCP moiety was synthesized according to the reported procedure.39
To facilitate the investigation of host–guest interaction, CB[7] was initially employed as a representative host model. The interaction between CB[7] and BH in D2O was examined using 1H NMR spectroscopy, and the results revealed that the proton signals of BH experienced significant upfield chemical shifts due to the shielding effect (Fig. 1). The stoichiometric ratio of CB[7] and BH was determined as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using UV-Vis spectroscopy (Fig. S24, ESI†), and based on this ratio, the binding constant for the host–guest complex was calculated to be (3.7 ± 0.4) × 105 M−1 (Fig. S25, ESI†).
1 using UV-Vis spectroscopy (Fig. S24, ESI†), and based on this ratio, the binding constant for the host–guest complex was calculated to be (3.7 ± 0.4) × 105 M−1 (Fig. S25, ESI†).
 | ||
| Fig. 1 1H NMR (400 MHz, D2O, 298 K) spectra of (a) BH (10 mM), (b) CB[7]⊃BH (10 mM), and (c) CB[7] (10 mM). | ||
Subsequently, the formation of nanoparticles by GCPCB was confirmed through the observation of a notable Tyndall effect (Fig. S26, ESI†). Moreover, the optical properties of GCPCB were examined, revealing a significant increase in fluorescence intensity, indicating a successful assembly process (Fig. S27, ESI†). The critical aggregation concentration of GCPCB was determined to be 0.036 mM, and upon addition of BH to GCPCB, the critical aggregation concentration decreased to 0.015 mM, thereby indicating that BH facilitated the aggregation of GCPCB (Fig. S28, ESI†). To further clarify the assembly process before and after adding BH to GCPCB, dynamic light scattering (DLS), transmission electron microscopy (TEM), and zeta potential measurements were conducted. The DLS results revealed that the aggregates formed by GCPCB exhibited a narrow size distribution, with an average hydrodynamic diameter of 229 nm. Upon the addition of BH, the average hydrodynamic diameter increased to 265 nm. TEM images of these assemblies showed spherical structures with similar diameters, indicating the formation of homogeneous nanoparticles (Fig. 2). Upon analyzing the results, it becomes evident that the simultaneous formation of nanoparticles from the GCPCB⊃BH complex is attributed to their strong host–guest interaction. After being stored at room temperature for 10 days, the zeta potential measurements of GCPCB and GCPCB⊃BH nanoparticles ranged from +8.33 mV to +7.76 mV and from +10.91 mV to +10.77 mV, respectively (Fig. S29, ESI†), confirming the stability of the formed nanoparticles in solution due to the positively charged structure of GCP. We hypothesized that the supramolecular nanoparticles formed from the GCPCB⊃BH amphiphile would exhibit pH sensitivity. As expected, upon adjusting the solution pH to 6.0, both the Tyndall effect disappeared (Fig. S26, ESI†) and no nanoparticles could be observed in the TEM images anymore (Fig. S30, ESI†). These findings collectively indicate the disassembly of the aforementioned supramolecular nanoparticles by pH adjustment. Therefore, considering the stability of these nanoparticles and the strong host–guest interaction between CB[7] and BH, further investigation into their potential application in synergistic antimicrobials is warranted.
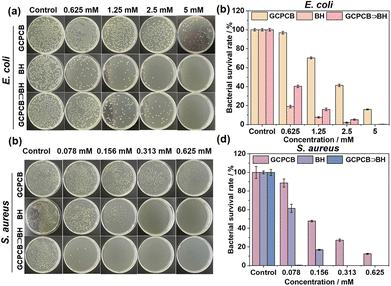
In the following study, functionalized TPE derivatives (H-9 and H-10) were synthesized to compare their antibacterial activities with GCPCB. The minimum inhibitory concentration (MIC) was measured to evaluate the antibacterial activities of GCPCB, as well as control compounds H-9 and H-10 against S. aureus and E. coli. Notably, GCPCB exhibited the most potent antimicrobial activity against S. aureus and E. coli, particularly with a MIC value of 0.625 mM against S. aureus, in comparison to H-9 and H-10 (Table S1, ESI†). In order to investigate the underlying reason for this disparity, we observed that there was no significant Tyndall phenomenon at the same concentration for H-9 and H-10 when compared to GCPCB (Fig. S26, ESI†). This suggests that these two compounds did not form proper nanoparticles, which likely explains their lack of significant results in the antimicrobial experiments.
The presence of a GCP moiety at the end of the GCPCB molecule facilitates the efficient delivery of positive charges, enabling cooperative electrostatic adsorption and hydrogen bonding with the bacterial membrane, thereby exerting antimicrobial effects. Additionally, berberine hydrochloride (BH), an alkaloid known for its antimicrobial and anti-inflammatory properties,40,41 can synergistically enhance the antimicrobial effects. Moreover, due to the existence of a cage-like cavity in GCPCB, it has the capability to self-assemble with BH in an aqueous medium and form collaborative antimicrobial nanoparticles. The in vitro antimicrobial activity of the GCPCB⊃BH nanoparticles, as well as the control compounds, was evaluated using the plate counting method for colony counting. As depicted in Fig. 3, GCPCB⊃BH nanoparticles, GCPCB, and BH demonstrated superior antimicrobial properties against S. aureus compared to E. coli, probably due to structural differences between the two bacteria. And their minimum inhibitory concentrations for S. aureus were significantly lower than those for E. coli, possibly due to the comparatively thin peptidoglycan cell wall of E. coli, which contains an outer and inner membrane layer with lipopolysaccharides that presents a higher barrier for compound penetration, resulting in increased bacterial survival rates.42 Notably, the GCPCB⊃BH nanoparticles exhibited enhanced antimicrobial activity, indicating that BH could effectively reduce the bactericidal concentration of GCPCB while synergistically enhancing its antimicrobial effect.
Live and dead staining of bacteria exhibited consistent results (Fig. 4a and b). The morphology of S. aureus and E. coli in different sample groups was examined using a scanning electron microscope (SEM). As shown in Fig. 4c, the bacterial membranes in the control group exhibited smooth and intact surfaces. In contrast to the control group, the bacterial cells in the sample group adhered together and displayed rough surfaces with leaked contents. Subsequently, nucleic acid and protein leakage from the bacteria were assessed (Fig. 4d and e), revealing that GCPCB⊃BH nanoparticle-treated bacterial supernatant displayed similar absorption values at 260 nm as well as protein content to those treated with polymyxin B antibiotic. These findings demonstrate that GCPCB⊃BH nanoparticles effectively induce the destruction of bacterial membranes, resulting in the leakage of nucleic acids and proteins. The biocompatibility of GCPCB, BH, and GCPCB⊃BH was assessed in vitro using methyl tetrazolium (MTT) assay. 3T3 cells were exposed to varying concentrations (ranging from 19 to 626 μM) for a duration of 24 hours. As illustrated in Fig. 5, GCPCB exhibited minimal cytotoxicity and also mitigated the cytotoxic effects of BH. Moreover, the cell survival rate following treatment with different concentrations of GCPCB⊃BH was significantly high, indicating the favorable biocompatibility of GCPCB⊃BH nanoparticles.
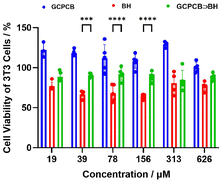 | ||
| Fig. 5 Cell viability of 3T3 cells treated with different concentrations of GCPCB, BH, and GCPCB⊃BH for 24 h. | ||
Conclusions
In summary, we have successfully designed and synthesized a supramolecular system comprising guanidiniocarbonyl-pyrrole functionalized cucurbit[7]uril (GCPCB) for highly efficient antibacterial activity. GCPCB has demonstrated significant antimicrobial efficacy against two foodborne microorganisms, with S. aureus displaying greater sensitivity to GCPCB than E. coli. The mechanism of action involves the disruption of bacterial membranes by the GCPCB⊃BH nanoparticles, as well as the electrostatic interaction between the GCP unit in GCPCB and the bacterial membrane, resulting in membrane permeation, disintegration, and bacterial lysis. Furthermore, the addition of berberine hydrochloride has been found to enhance the antimicrobial effect of GCPCB, and their host–guest complex demonstrates synergistic enhancement in bacterial membrane-disrupting activity. Therefore, both GCPCB and its complexes with the antimicrobial drug GCPCB⊃BH hold great potential as innovative therapeutic agents and strategies against drug-resistant bacteria.Author contributions
The project was conceived by X.-Y. Hu and R. Han, who also drafted the manuscript. The experiments were performed by R. Han, K. Du, S. Li, and M. Zuo. The manuscript underwent revisions from X.-Y. Hu, L. Wang, and H. Jiang while P. Jeyakkumar drew the illustrations. All authors contributed to data analysis, result discussion, and manuscript review.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Innovation Support Program of Jiangsu Province (No. BZ2023055), the National Natural Science Foundation of China (No. 22271154 and M-0411), the Science Fund for Distinguished Young Scholars of Jiangsu Province (No. BK20240078), and the China Postdoctoral Science Foundation (No. 2022M721601).Notes and references
- F. Prestinaci, P. Pezzotti and A. Pantosti, Pathog. Global Health, 2015, 109, 309–318 CrossRef PubMed.
- S. I. Ahmad, H. A. Malak and H. H. Abulreesh, J. Global Antimicrob. Resist., 2021, 27, 101–111 CrossRef PubMed.
- B. Khameneh, R. Diab, K. Ghazvini and B. S. Fazly Bazzaz, Microb. Pathog., 2016, 95, 32–42 CrossRef CAS PubMed.
- A. F. Read and R. J. Woods, Evol. Med. Public Health, 2014, 2014, 147 CrossRef PubMed.
- D. A. Relman and M. Lipsitch, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 12902–12910 CrossRef CAS PubMed.
- M. Lobanovska and G. Pilla, Yale J. Biol. Med., 2017, 90, 135–145 CAS.
- K. Du, Z.-R. Yang, H. Qin, T. Ma, J. Tang, J. Xia, Z. Zhou, H. Jiang and J. Zhu, Macromol. Biosci., 2023, 24, 2300451 CrossRef PubMed.
- E. M. Darby, E. Trampari, P. Siasat, M. S. Gaya, I. Alav, M. A. Webber and J. M. A. Blair, Nat. Rev. Microbiol., 2023, 21, 280–295 CrossRef CAS PubMed.
- K. Sharma and M. Sharma, Nat. Microbiol., 2024, 9, 584 CrossRef CAS PubMed.
- H. Sun, S. Li, Q. Liu, M. Zuo, X. Tian, K. Wang and X.-Y. Hu, Chin. Chem. Lett., 2024, 109999 CrossRef.
- X. Li, H. Bai, Y. Yang, J. Yoon, S. Wang and X. Zhang, Adv. Mater., 2019, 31, 1805092 CrossRef PubMed.
- H. Hu, Y.-Y. Zhang, H. Ma, Y. Yang, S. Mei, J. Li, J.-F. Xu and X. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202308513 CrossRef CAS PubMed.
- X. Tian, S. Li, K. Velmurugan, Z. Bai, Q. Liu, K. Wang, M. Zuo and X.-Y. Hu, Mater. Chem. Front., 2023, 7, 2484–2492 RSC.
- H. Peng, B. Xie, X. Yang, J. Dai, G. Wei and Y. He, Chem. Commun., 2020, 56, 8115–8118 RSC.
- J. Song, C. Yuan, T. Jiao, R. Xing, M. Yang, D. J. Adams and X. Yan, Small, 2020, 16, 1907309 CrossRef CAS PubMed.
- Q. Li, Y. Wu, H. Lu, X. Wu, S. Chen, N. Song, Y.-W. Yang and H. Gao, ACS Appl. Mater. Interfaces, 2017, 9, 10180–10189 CrossRef CAS PubMed.
- Y. Yang, H. Hu, L. Chen, H. Bai, S. Wang, J.-F. Xu and X. Zhang, Mater. Chem. Front., 2019, 3, 806–811 RSC.
- S. Guo, Q. Huang, Y. Chen, J. Wei, J. Zheng, L. Wang, Y. Wang and R. Wang, Angew. Chem., Int. Ed., 2021, 60, 618–623 CrossRef CAS PubMed.
- Z. Pei, X. Ying, Y. Tang, L. Liu, H. Zhang, S. Liu, D. Zhang, K. Wang, D. Zhang, L. Kong, Y. Gao and H. Ma, Pol. J. Vet. Sci., 2018, 21, 533–542 CAS.
- J. Lagona, P. Mukhopadhyay, S. Chakrabarti and L. Isaacs, Angew. Chem., Int. Ed., 2005, 44, 4844–4870 CrossRef CAS PubMed.
- S. J. Barrow, S. Kasera, M. J. Rowland, J. Del Barrio and O. A. Scherman, Chem. Rev., 2015, 115, 12320–12406 CrossRef CAS PubMed.
- D. Shetty, J. K. Khedlkar, K. M. Park and K. Kim, Chem. Soc. Rev., 2015, 44, 8747–8761 RSC.
- J. Chen, Q. Huang, Q. Wang, Y. Ding, S. Lu, L.-H. Wang, S. Li and R. Wang, ACS Appl. Nano Mater., 2022, 5, 5993–6000 CrossRef CAS.
- H. Yin, Q. Cheng, D. Bardelang and R. Wang, JACS Au, 2023, 3, 2356–2377 CrossRef CAS PubMed.
- W.-L. Zhou, Y. Chen, Q. Yu, H. Zhang, Z.-X. Liu, X.-Y. Dai, J.-J. Li and Y. Liu, Nat. Commun., 2020, 11, 4655 CrossRef CAS PubMed.
- J. Chen, S. Li, Z. Wang, Y. Pan, J. Wei, S. Lu, Q.-W. Zhang, L.-H. Wang and R. Wang, Chem. Sci., 2021, 12, 7727–7734 RSC.
- H.-J. Wang, W.-W. Xing, Z.-H. Yu, Y.-X. Li, H.-Y. Zhang, Q. Yu, H. Zhu, Y.-Y. Wang and Y. Liu, Chin. Chem. Lett., 2024, 35, 109183 CrossRef CAS.
- S. Pandey, D. V. D. W. Kankanamalage, X. Zhou, C. Hu, L. Isaacs, J. Jayawickramarajah and H. Mao, J. Am. Chem. Soc., 2019, 141, 18385–18389 CrossRef CAS PubMed.
- F.-C. Bin, M. Guo, T. Li, Y.-C. Zheng, X.-Z. Dong, J. Liu, F. Jin and M.-L. Zheng, Adv. Funct. Mater., 2023, 33, 2300293 CrossRef CAS.
- D. Zhao, H.-H. Han, L. Zhu, F.-Z. Xu, X.-Y. Ma, J. Li, T. D. James, Y. Zang, X.-P. He and C. Wang, ACS Appl. Bio Mater., 2021, 4, 7016–7024 CrossRef CAS PubMed.
- N. Song, Z. Zhang, P. Liu, Y.-W. Yang, L. Wang, D. Wang and B. Z. Tang, Adv. Mater., 2020, 32, e2004208 CrossRef PubMed.
- N. Yan, Y. Hu, B. Z. Tang and W.-X. Wang, ACS Sens., 2021, 6, 4206–4216 CrossRef CAS PubMed.
- F. Oroojalian, F. Azizollahi, P. Kesharwain and A. Sahebkar, J. Controlled Release, 2024, 373, 766–802 CrossRef CAS PubMed.
- Z. Li, B. Z. Tang and D. Wang, Adv. Mater., 2024, 36, 2406047 CrossRef CAS PubMed.
- X. Liu, K. Wang, M. Externbrink, J. Niemeyer, M. Giese and X.-Y. Hu, Chin. Chem. Lett., 2020, 31, 1239–1242 CrossRef CAS.
- H. Jiang, X.-Y. Hu, S. Mosel, S. K. Knauer, C. Hirschhäeuser and C. Schmuck, ChemBioChem, 2019, 20, 1410–1416 CrossRef CAS PubMed.
- K. Wang, M. Zuo, T. Zhang, H. Yue and X.-Y. Hu, Chin. Chem. Lett., 2023, 34, 107848 CrossRef CAS.
- X.-Y. Hu, M. Ehlers, T. Wang, E. Zellermann, S. Mosel, H. Jiang, J.-E. Ostwaldt, S. K. Knauer, L. Wang and C. Schmuck, Chem. – Eur. J., 2018, 24, 9754–9759 CrossRef CAS PubMed.
- C. Schmuck and M. Schwegmann, J. Am. Chem. Soc., 2005, 127, 3373–3379 CrossRef CAS PubMed.
- F. Gan, Z. Yao, Y. Zeng, Q. Zhang and Y. Zeng, Sci. Adv. Mater., 2023, 15, 1560–1574 CrossRef CAS.
- Z. Tang, J. Luo, Y. Faqir, Y. Zhang, W. Xue, H. Zhao, A. M. Jakhar, C. Tan and J. Ma, Int. J. Biol. Macromol., 2024, 255, 128219 CrossRef CAS PubMed.
- F. F. Sperandio, Y.-Y. Huang and M. R. Hamblin, Recent Pat. Anti-Infect. Drug Discovery, 2013, 8, 108–120 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and NMR spectra etc. See DOI: https://doi.org/10.1039/d4tb01840k |
| ‡ Equally contributing authors: RH and KD. |
| This journal is © The Royal Society of Chemistry 2024 |