Ferrocene conjugated Os(II) complex for photo-catalytic cancer therapy of triple-negative breast cancer cells†
Apurba
Mandal
a,
Virendra
Singh
b,
Silda
Peters
c,
Arif Ali
Mandal
a,
Tumpa
Sadhukhan
*c,
Biplob
Koch
 *b and
Samya
Banerjee
*b and
Samya
Banerjee
 *a
*a
aDepartment of Chemistry, Indian Institute of Technology (BHU), Varanasi, Uttar Pradesh 221005, India. E-mail: samya.chy@itbhu.ac.in
bDepartment of Zoology, Institute of Science, Banaras Hindu University, Varanasi, Uttar Pradesh 221005, India. E-mail: biplob@bhu.ac.in
cDepartment of Chemistry, SRM Institute of Science and Technology, Kattankulathur, Tamil Nadu 603203, India. E-mail: tumpas@srmist.edu.in
First published on 7th April 2025
Abstract
A novel ferrocene-conjugated bimetallic Os(II) photocatalyst (OsFe) showed micromolar photocatalytic anticancer activity against triple-negative breast cancer cells via NADH oxidation and caspase 3 activation under visible light.
Recently, “photocatalytic cancer therapy” (PCT) has emerged as an alternative to chemotherapy with a novel mode of action and target site to address the drawbacks of platins.1–4 PCT provides spatiotemporal control over activating catalytic amounts of drugs at the target cancer site.1–4 PCT is reported to have minimal harmful effects on normal cells in vitro and in vivo.1–4 In PCT, catalysts induce ROS (reactive oxygen species) generation and photo-oxidation of NADH at catalytic concentrations to create redox and metabolic disorders, leading to cell death.1–4 NADH is the main target of catalytic cancer drug development because of its essential role in cellular processes.1–4 NADH is the cofactor for >400 oxidoreductase enzymes and is the primary electron source in the mitochondrial ETC (electron transfer chain).1–4 Consequently, intracellular oxidation of NADH disrupts redox homeostasis and metabolic equilibrium and causes cell death.1–4
Several Ru(II) and Ir(III) photocatalysts have been explored for PCT.1–8 To generalize this new concept of cancer therapy and for its rapid progress, other metal-based photocatalysts with enhanced photophysical properties need to be developed and tested. The polypyridyl Os(II) complexes are known for high kinetic inertness, efficient ISC, and longer triplet excited state lifetime.9–11 In recent years, Gasser, McFarland, Chen, and others have reported ROS-mediated anticancer activity of polypyridyl Os(II) complexes.9–11 However, only one report is available for Os(II)-photocatalyst showing NADH photo-oxidation-mediated anticancer activity.12 Zhang et al. reported an Os(II)-peroxo complex that induced ROS generation and NADH oxidation-mediated anticancer activity against HeLa cells under 465 nm light.12 Thus, the efficacy of Os(II) photocatalysts in PCT remains largely unexplored.
TNBC (triple-negative breast cancer) is one of the most aggressive and resistant forms of breast cancer, with the absence of HER2 receptors, progesterone, and estrogen.13 So far, the effectiveness of PCT against TNBC is totally unexplored.
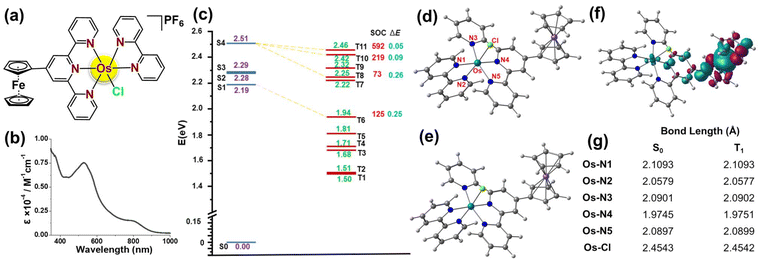
The above-discussed unsolved questions in PCT, i.e., (i) scope of Os(II) photocatalysts in PCT, and (ii) treatment scope of TNBC with PCT, promoted us to develop a novel bi-metallic polypyridyl Os(II) photocatalyst, i.e., [Os(Fc-tpy)(bpy)Cl]PF6 (OsFe) (Fig. 1a). Ferrocenyl terpyridine (Fc-tpy) was employed to enhance the visible light absorption ability.14 Additionally, ferrocene moiety is known for its antitumor properties via ROS production.14 A detailed investigation with OsFe revealed its efficient PCT activities against MDA-MB-231 TNBC cells under 10 J cm−2 (400–700 nm) light. DFT/TDDFT further supported the observed anticancer activity attributed to intracellular ROS production and photo-oxidation of NADH under visible light.
The ferrocene appended Os(II) photocatalyst viz., [Os(Fc-tpy)(bpy)Cl]PF6 (OsFe), where Fc-tpy = 4′-ferrocenyl-2,2′:6′,2′′-terpyridine, bpy = 2,2′-bipyridine (bipyridine), (Fig. 1a) was synthesized by reacting 1.0 eq. of (NH4)2OsCl6 with 1.0 eq. of Fc-tpy, followed by adding 1.1 eq. of bpy in ethylene glycol (Fig. S1, ESI†). OsFe was isolated as PF6− salt after adding excess aq. NH4PF6. OsFe was characterized by HRMS, NMR, and UV-Vis. spectral analysis, and elemental analysis. The HRMS spectra of OsFe in MeCN showed an m/z peak corresponding to the [M]+ ion (Fig. S2, ESI†). In 1H NMR, the aromatic protons were between 6.7–10.0 ppm. The ferrocene-based characteristic peaks were at 4.24 ppm (5H), 4.67 ppm (2H), and 5.45 ppm (2H) (Fig. S3, ESI†). OsFe showed strong absorption at 530 nm due to Os(dπ) to π*Fc-tpy and bpy MLCT and a weak band at 750–900 nm attributed to spin-forbidden MLCT, arising from the direct singlet–triplet transition of the photocatalyst in PBS-DMSO (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (Fig. 1b).9–11 Interestingly, OsFe displayed a greater shift towards the red light region than previously reported polypyridyl Os(II) complexes.9,11 Overall, the absorption in the visible region (green and red) might be useful for PCT via in-cell NADH oxidation and ROS generation.1–8
1, v/v) (Fig. 1b).9–11 Interestingly, OsFe displayed a greater shift towards the red light region than previously reported polypyridyl Os(II) complexes.9,11 Overall, the absorption in the visible region (green and red) might be useful for PCT via in-cell NADH oxidation and ROS generation.1–8
OsFe exhibited excellent photo-stability in the PBS-DMSO (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) mixture and also in DMEM-DMSO (9
1, v/v) mixture and also in DMEM-DMSO (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), as evident from the minimal spectral changes over time (Fig. S4 and S5, ESI†). High light stability is essential for a photocatalyst to avoid off-target toxicity and photobleaching.3OsFe exhibited a log
1, v/v), as evident from the minimal spectral changes over time (Fig. S4 and S5, ESI†). High light stability is essential for a photocatalyst to avoid off-target toxicity and photobleaching.3OsFe exhibited a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Po/w (partition coefficient in water and octanol mixture) value of +0.44 ± 0.12 (Fig. S6, ESI†), indicating its lipophilic nature, facilitating good cellular uptake.7,15
Po/w (partition coefficient in water and octanol mixture) value of +0.44 ± 0.12 (Fig. S6, ESI†), indicating its lipophilic nature, facilitating good cellular uptake.7,15
The optimized structure with the range-separated hybrid ω-B97XD functional and the def2-SVP basis set16a (Fig. S7, ESI†) confirmed a distorted octahedral coordination around the Os(II) center with Fc-tpy, bipyridine, and chloride as ligands. The fourth excited state (S4) exhibited the highest oscillator strength, with an energy of 2.51 eV, while the lowest excited state (S1) appears at 2.19 eV. As shown in Fig. S7, ESI,† the HOMO–LUMO transition corresponds to charge transfer from the Os(II) to the LUMO, which is delocalized over the Fc-tpy and bipyridine. This confirmed that Fc-tpy subunit influences the properties of OsFe. Natural Transition Orbitals (NTOs) (Fig. S8, ESI†) indicated that the lowest energy transition (S0 → S1) could be attributed to MLCT, where the occupied NTO was on the Os(II) center, and the virtual NTO was on Fc-tpy. Similarly, the brightest transition (S0 → S4) is a combination of MLCT from the Os(II) center to both Fc-tpy and bipyridine.
The photosensitizing potential of OsFe was assessed by examining its ability to undergo Intersystem Spin Crossing (ISC) from a singlet to a triplet state. Effective ISC requires strong spin–orbit coupling (SOC) between excited singlet and triplet states, enabling energy transfer to 3O2 or electron transfer to biomolecules. ISC probability depends on SOC strength and the energy gap (ΔE) between coupled states.16b Several triplet states with energies above 0.98 eV (required for 1O2 generation from 3O2),16c located below the lowest-energy singlet states associated with the first absorption band, have been identified. These results are summarized in Fig. 1c, where both SOC values and ΔE are provided. In this study, singlet and triplet states with energy differences of less than 0.30 eV are considered. The calculated SOC value for the bright S4 state and T11 state is 592 cm−1, which is notably large, confirming the efficient population of the triplet states. This enables the production of 1O2via radiation-less transitions between the singlet states and the lower-lying triplet states. Moreover, considering the energy gap between the interacting singlet and triplet states, the most efficient nonradiative decay pathway would be S0 → S4 → T11 → T1. The adiabatic T1 → S0 energy gap of OsFe was 1.71 eV, which is higher than the energy required for the conversion of 3O2 to 1O2, facilitating 1O2 generation.16cFig. 1d–g confirm similar S0 and T1 geometry, suggesting minimal structural distortion upon excitation.
In PCT, ROS are crucial in inducing apoptosis in cancer cells selectively, while unexposed healthy ones remain unaffected.1–31O2 is a highly reactive and metastable state of molecular oxygen.17 Previously, several polypyridyl Os(II) complexes are reported for potential 1O2 generation.9–11,15 As OsFe is polypyridyl-based and showed absorption in the visible region, the photo-triggered 1O2 generation efficacy of OsFe was studied using DPBF, a well-established 1O2 probe (Fig. 2a).18 Under light (10 J cm−2, 400–700 nm), OsFe displayed significant 1O2 generation efficacy, evidenced by a notable decrease in the absorption band of DPBF at 417 nm in PBS-DMSO (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The control experiments confirmed that the decrease in the absorption band of DPBF was due to the generation of 1O2 in the presence of OsFe (Fig. S9, ESI†). The 1O2 generation quantum yield of OsFe was 0.06 with [Ru(bpy)3]Cl2 as standard.4
1, v/v). The control experiments confirmed that the decrease in the absorption band of DPBF was due to the generation of 1O2 in the presence of OsFe (Fig. S9, ESI†). The 1O2 generation quantum yield of OsFe was 0.06 with [Ru(bpy)3]Cl2 as standard.4
NADH is a key coenzyme supplying electrons to the ETC, and its oxidation can disrupt the ETC in cancerous cells, causing cell death.1–8 The light-triggered (10 J cm−2, 400–700 nm) oxidation of NADH (175 μM) by OsFe (5 μM) was analyzed using UV-Vis. spectroscopy in PBS-DMSO (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). A gradual decrease in NADH's characteristic absorbance at 339 nm, accompanied by an increase in the NAD+ absorbance at 256 nm, confirmed the oxidation of NADH to NAD+ upon OsFe + light treatment (Fig. 2b). The observed turnover frequency (TOF) for NADH oxidation showed that OsFe (TOF = 12.82 h−1) exhibited much higher efficiency than the Os(II)-peroxo complex.12 Further, during NADH photo-oxidation, OsFe generated H2O2 (Fig. S10, ESI†). The above finding revealed that OsFe could be used as an efficient photo-catalyst for NADH photo-oxidation and might disrupt the ETC, resulting in mitochondrial depolarization and cell death.
1, v/v). A gradual decrease in NADH's characteristic absorbance at 339 nm, accompanied by an increase in the NAD+ absorbance at 256 nm, confirmed the oxidation of NADH to NAD+ upon OsFe + light treatment (Fig. 2b). The observed turnover frequency (TOF) for NADH oxidation showed that OsFe (TOF = 12.82 h−1) exhibited much higher efficiency than the Os(II)-peroxo complex.12 Further, during NADH photo-oxidation, OsFe generated H2O2 (Fig. S10, ESI†). The above finding revealed that OsFe could be used as an efficient photo-catalyst for NADH photo-oxidation and might disrupt the ETC, resulting in mitochondrial depolarization and cell death.
The 1O2 generation and NADH photo-oxidation ability of OsFe led to explore its photo-triggered anticancer activity against MDA-MB-231 TNBC and HEK-293 (Human embryonic kidney) normal cells under both light (10 J cm−2, 400–700 nm) and dark conditions. Against MDA-MB-231 cells, under dark, OsFe did not show any notable activity (IC50 > 50 μM), but cytotoxicity was markedly improved upon light exposure (IC50ca. 1.0 μM). OsFe showed an impressive phototoxicity index (dark IC50/light IC50) of >50 (Fig. S11, ESI†). To the best of our knowledge, this is the first report of PCT against TNBC cells, increasing the scope of PCT for TNBC treatment. Importantly, OsFe exhibited no significant cytotoxic effect against normal HEK-293 cell lines under light or dark conditions (Fig. S11, ESI†). It is worth noting that only cancer cells are exposed to light during PCT, leaving healthy cells unexposed.1–8 Thus, OsFe can induce cell death of TNBC cells selectively, keeping unexposed normal cells unaffected.
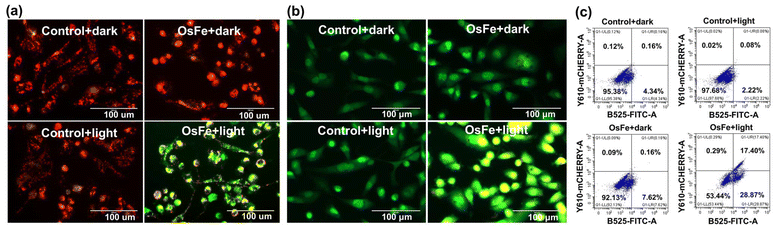
Light activation of OsFe enabled the generation of 1O2, suggesting its potential to generate ROS in solution. Further, DCFH-DA was used as a fluorescent probe to assess the ROS generation by OsFe in MDA-MB-231 cells.7,16,19 Upon oxidation by ROS, DCFH-DA (nonfluorescent) converted to fluorescent DCF, emitting a green fluorescent signal.7,16,19 As displayed in Fig. 2c, S12, ESI,† in MDA-MB-231 cells, the OsFe + light treatment enhanced the intracellular ROS significantly, as evidenced by intense green fluorescence. Interestingly, in the dark, OsFe did not induce any notable ROS. Thus, OsFe under light significantly enhanced ROS production while showing negligible ROS production in the dark (Fig. 2c, S12, ESI†).
Metal photocatalysts can induce ROS generation and NADH photo-oxidation to effectively influence mitochondrial membrane potential (MMP) and trigger apoptosis in cancer cells.1,4,7,16 Therefore, the effect of OsFe + light on the MMP of MDA-MB-231 cells was visualized by the JC-1 dye.4,7,16 Under dark, OsFe-treated MDA-MB-231 cells showed red fluorescence, indicating no significant change in MMP (Fig. 3a).4,7,16 However, the fluorescence shifted to green after light (10 J cm−2, 400–700 nm) treatment, indicating a drop and change in the MMP (Fig. 3a).4,7,16 This observation suggested that OsFe + light effectively altered MMP, most likely due to effective NADH photo-oxidation and ROS generation, thereby contributing to its anticancer potential.4,7,16
The significant cytotoxicity, ROS generation, and NADH oxidation ability of OsFe under light promoted further investigation into the cell death mechanism. The mode of cell death was investigated by AO (Acridine Orange)/EtBr (Ethidium Bromide) and Hoechst33342/PI (Propidium Iodide) staining.4,20 As demonstrated in Fig. 3b, MDA-MB-231 cells treated with OsFe or light only displayed organized cytoplasm with intact green nuclei, indicating the nontoxic behavior of OsFe in the absence of light.4,20 In contrast, OsFe + light-treated MDA-MB-231 cells exhibited bright green and yellowish green nuclei with membrane blebbing, indicating early and late apoptotic cells.4,20 Further, as shown in Fig. S13, ESI,† after OsFe (IC50 concentration) + light treatment, MDA-MB-231 cells showed nuclei with blue emission in the presence of Hoechst33342 and red fluorescence with PI, indicating apoptotic cell death mechanism.4,20 The condensed nuclei were noted to be bright. Therefore, it can be implied that OsFe induced apoptosis in MDA-MB-231 cells under light. A similar observation was found with polypyridyl Os(II) complexes, as reported by Gasser and Chen et al.9,11 Further flow cytometry was performed for quantitative apoptosis analysis using Annexin-V/FITC and PI assay.20 The Annexin-V/FITC is the dye that binds to the phosphatidyl serine of the plasma membrane during apoptosis and stains early apoptotic cells.20 In contrast, necrotic and late apoptotic cells are stained by PI with disintegrated plasma and nuclear membranes.20 As demonstrated in Fig. S14, ESI,† control experiments and OsFe + dark showed negligible cell death. However, upon treatment with OsFe + light, cell death was enhanced significantly, with ca. 29.86% early apoptosis and 18.29% late apoptosis. Caspase 3 is the final executor of apoptosis, and its activation leads to cell death.20,21 Caspase activation in MDA-MB-231 cells in the presence of OsFe + light was studied using caspase 3/7 and SYTOX red assay.20,21 The results showed that OsFe + light activated caspase 3 (Fig. 3c), highlighting its crucial role in triggering apoptosis. Overall, these findings indicated that OsFe, under visible light, induced apoptotic cell death via ROS generation and NADH photo-oxidation mediated mitochondrial depolarization and caspase 3 activation.
In summary, a novel bimetallic Os(II) photocatalyst (OsFe) was developed as a PCT agent. The photostable OsFe displayed green-red light absorption ability, beneficial for photo-induced (10 J cm−2, 400–700 nm) NADH oxidation and ROS generation. The observed NADH photo-oxidation TOF was much higher than the earlier reported Os(II)-catalyst.12 The SOC result confirmed that the triplet state is highly populated, which is crucial for interacting with NADH in photocatalytic oxidation. Additionally, the calculated adiabatic singlet–triplet energy gap of 1.71 eV indicates that OsFe can effectively generate 1O2. OsFe showed no notable dark toxicity but was highly cytotoxic against MDA-MB-231 TNBC under light, marking the first proof of the successful use of PCT for TNBC treatment. Mechanistic studies confirmed that OsFe induced apoptotic cell death through NADH photo-oxidation and ROS-mediated mitochondrial depolarization and caspase 3 activations. Interestingly, OsFe did not display any significant toxicity against HEK-293 normal cell lines, making OsFe a promising candidate for targeted PCT of TNBC. Overall, in conclusion, this is the first report on treating TNBC by PCT, expanding the scope of PCT. A key challenge ahead is the successful transition from in vitro to in vivo studies, which will be the focus of our future investigations.
Data availability
The data supporting this article have been included as part of the ESI.† The raw data are available from the corresponding authors upon reasonable request.Conflicts of interest
S. B., A. M., and A. A. M. have filed an Indian patent application (202511011489) lodged with the IIT (BHU) based on the novel composition and chemical structures reported here.Acknowledgements
This work was supported by SERB (now ANRF) (SRG/2022/000030) and the Prime Minister's Research Fellowship, the Government of India.References
- A. K. Yadav, R. Kushwaha, A. A. Mandal, A. Mandal and S. Banerjee, J. Am. Chem. Soc., 2025, 147, 7161–7181 CrossRef CAS PubMed.
- H. Huang, S. Banerjee, K. Qiu, P. Zhang, O. Blacque, T. Malcomson, M. J. Paterson, G. J. Clarkson, M. Staniforth, V. G. Stavros, G. Gasser, H. Chao and P. J. Sadler, Nat. Chem., 2019, 11, 1041–1048 CrossRef CAS PubMed.
- L. Wei, R. Kushwaha, A. Dao, Z. Fan, S. Banerjee and H. Huang, Chem. Commun., 2023, 59, 3083–3086 RSC.
- A. K. Yadav, A. Upadhyay, A. Bera, R. Kushwaha, A. A. Mandal, S. Acharjee, A. Kunwar and S. Banerjee, Inorg. Chem. Front., 2024, 11, 5435–5448 RSC.
- J. Kasparkova, A. H. García, H. Kostrhunova, M. Goicuría, V. Novohradsky, D. Bautista, L. Markova, M. D. Santana, V. Brabec and J. Ruiz, J. Med. Chem., 2024, 67, 691–708 CrossRef CAS PubMed.
- J. Liu, A. W. Prentice, G. J. Clarkson, J. M. Woolley, V. G. Stavros, M. J. Paterson and P. J. Sadler, Adv. Mater., 2023, 35, 2210363 CrossRef CAS PubMed.
- A. A. Mandal, V. Singh, S. Saha, S. Peters, T. Sadhukhan, R. Kushwaha, A. K. Yadav, A. Mandal, A. Upadhyay, A. Bera, A. Dutta, B. Koch and S. Banerjee, Inorg. Chem., 2024, 63, 7493–7503 CrossRef CAS PubMed.
- Z. Fan, J. Xie, R. Kushwaha, S. Liang, W. Li, A. A. Mandal, L. Wei, S. Banerjee and H. Huang, Chem. – Asian J., 2023, 18, e202300047 CrossRef CAS PubMed.
- A. Mani, T. Feng, A. Gandioso, R. Vinck, A. Notaro, L. Gourdon, P. Burckel, B. Saubamea, O. Blacque, K. Cariou, J.-E. Belgaied, H. Chao and G. Gasser, Angew. Chem., Int. Ed., 2023, 62, e202218347 CrossRef CAS PubMed.
- J. A. Roque III, P. C. Barrett, H. D. Cole, L. M. Lifshits, G. Shi, S. Monro, D. V. Dohlen, S. Kim, N. Russo, G. Deep, C. G. Cameron, M. E. Alberto and S. A. McFarland, Chem. Sci., 2020, 11, 9784–9806 RSC.
- Y. Lu and F. Chen, Chem. – Eur. J., 2024, 30, e202402861 CrossRef CAS PubMed.
- N. Lu, Z. Deng, J. Gao, C. Liang, H. Xia and P. Zhang, Nat. Commun., 2022, 13, 2245 CrossRef CAS PubMed.
- (a) G. Bianchini, J. M. Balko, I. A. Mayer, M. E. Sanders and L. Gianni, Nat. Rev. Clin. Oncol., 2016, 13, 674–690 CrossRef CAS PubMed; (b) U. Das, U. Basu and P. Paira, Dalton Trans., 2024, 53, 15113–15157 RSC.
- M. Patra and G. Gasser, Nat. Rev. Chem., 2017, 1, 0066 CrossRef CAS.
- F. Chen, K. Cui, S. Si, Y. Liu, S. Xue, G. Wang, X. Liang, C. Zhu and Q. Y. Chen, Inorg. Chim. Acta, 2025, 578, 122537 CrossRef CAS.
- (a) R. Kushwaha, V. Singh, S. Peters, A. K. Yadav, T. Sadhukhan, B. Koch and S. Banerjee, J. Med. Chem., 2024, 67, 6537–6548 CrossRef CAS PubMed; (b) C. M. Marian, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 187 CAS; (c) S. Noimark, E. Salvadori, R. G. Bombarelli, A. J. MacRobert, I. P. Parkin and C. W. M. Kay, Phys. Chem. Chem. Phys., 2016, 18, 28101–28109 RSC.
- J. Karges, Angew. Chem., Int. Ed., 2022, 61, e202112236 CrossRef CAS PubMed.
- (a) A. Marco, J. Kasparkova, D. Bautista, H. Kostrhunova, N. Cutillas, L. Markova, V. Novohradsky, J. Ruiz and V. Brabec, J. Med. Chem., 2024, 67, 21470–21485 CrossRef CAS PubMed; (b) U. Das and P. Paira, Dalton Trans., 2024, 53, 6459–6471 RSC.
- V. Venkatesh, R. B. Martin, C. J. Wedge, I. R. Canelón, C. S. Cano, J. I. Song, J. P. C. Coverdale, P. Zhang, G. J. Clarkson, A. Habtemariam, S. W. Magennis, R. J. Deeth and P. J. Sadler, Chem. Sci., 2017, 8, 8271–8278 RSC.
- A. Kumar Yadav, V. Singh, S. Acharjee, S. Saha, R. Kushwaha, A. Dutta, B. Koch and S. Banerjee, Chem. – Eur. J., 2025, 31, e202403454 CrossRef CAS PubMed.
- H. Zhou, D. Tang, Y. Yu, L. Zhang, B. Wang, J. Karges and H. Xiao, Nat. Commun., 2023, 14, 5350 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5dt00515a |
| This journal is © The Royal Society of Chemistry 2025 |